Human IL-1 beta/IL-1F2 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human IL-1 beta/IL-1F2. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
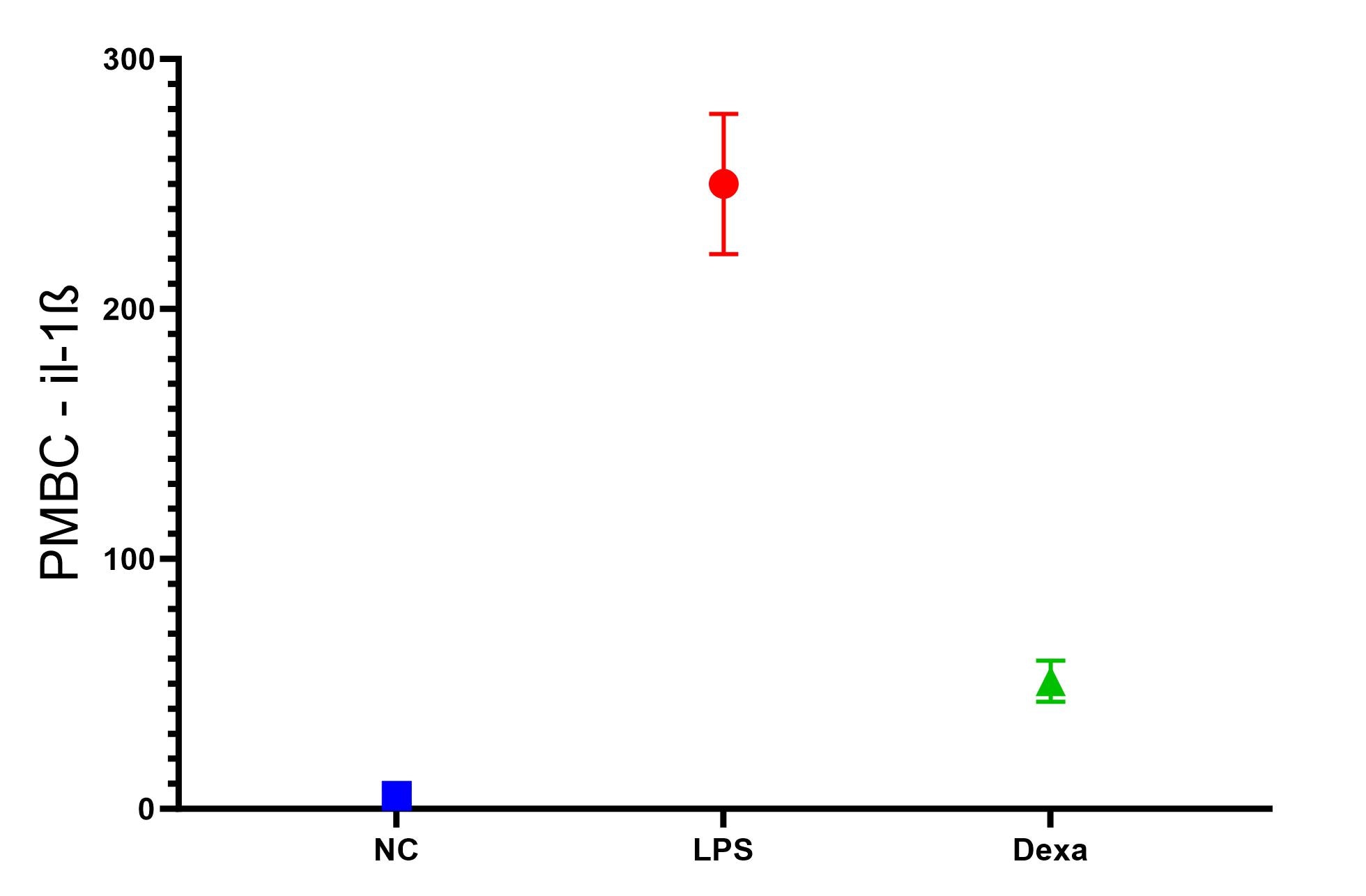
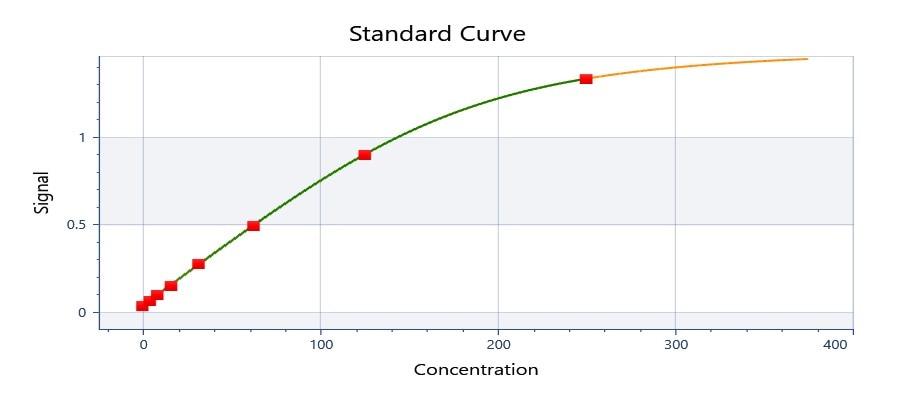
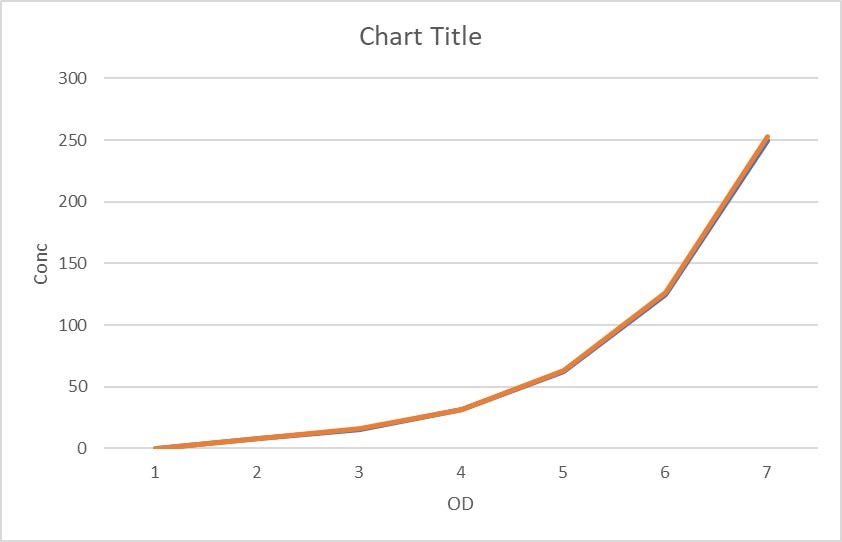
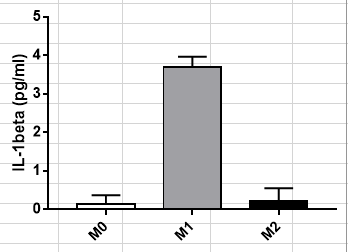
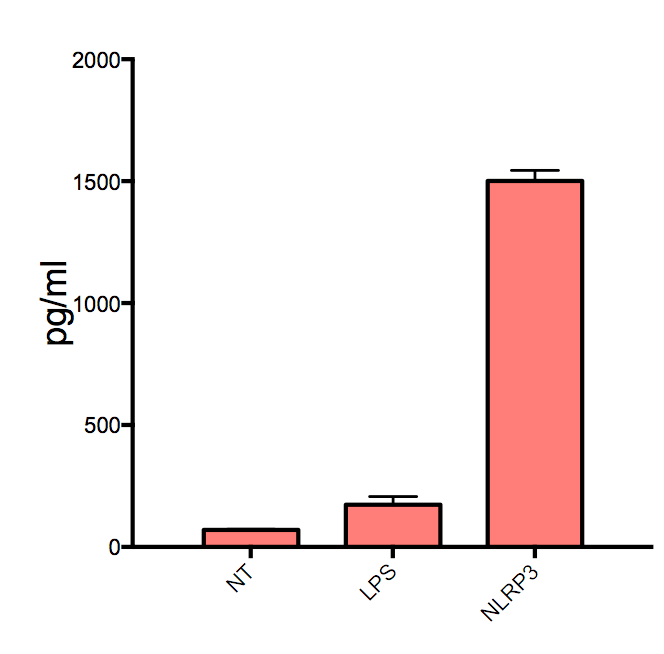
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-1 beta/IL-1F2
The Interleukin 1 (IL-1) family of proteins consists of the classic members IL-1 alpha, IL-1 beta, and IL-1ra, plus IL-18, IL-33 and IL-1F5-F10. IL-1 alpha and IL-1 beta bind to the same cell surface receptors and share biological functions. IL-1 is not produced by unstimulated cells of healthy individuals with the exception of skin keratinocytes, some epithelial cells, and certain cells of the central nervous system. However, in response to inflammatory agents, infections, or microbial endotoxins, a dramatic increase in the production of IL-1 by macrophages and various other cell types is observed. IL-1 beta plays a central role in immune and inflammatory responses, bone remodeling, fever, carbohydrate metabolism, and GH/IGF-I physiology. Inappropriate or prolonged production of IL-1 has been implicated in a variety of pathological conditions including sepsis, rheumatoid arthritis, inflammatory bowel disease, acute and chronic myelogenous leukemia, insulin dependent diabetes mellitus, atherosclerosis, neuronal injury, and aging-related diseases.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human IL-1 beta/IL-1F2 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
406
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Defining Microbiota-Derived Metabolite Butyrate as a Senomorphic: Therapeutic Potential in the Age-Related T Cell Senescence
Authors: Rees, NP;Conway, J;Dugan, B;Amir, SS;Parker, A;Carding, SR;Duggal, NA;
Aging cell
Species: Human
Sample Types: Cell Culture Supernates
-
Polystyrene nanoplastics and lung cancer: Insights from network toxicology and mechanistic in vitro studies
Authors: Le, TK;Kuo, CH;Wu, CC;Chen, YT;Hsieh, SL;
Toxicology and applied pharmacology
Species: Human
Sample Types: Cell Culture Supernates
-
HO-1197 as a Multifaceted Therapeutic: Targeting the Cell Cycle, Angiogenesis, Metastasis, and Tumor Immunity in Hepatocellular Carcinoma
Authors: Song, Y;Lee, S;Heo, SW;Spohn, J;Schmiedel, D;Heo, T;Kim, S;Park, J;Seo, HR;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Amino acid-equipped poly(ester amide) nanoparticles for improved encapsulation efficiency of lipophilic anti-inflammatory 6BIGOE based on molecular dynamic simulations
Authors: Westhoff, J;Weber, C;Bachmann, V;Göppert, NE;Peric, M;George, A;Sierka, M;Hoeppener, S;Vilotijevic, I;Schubert, US;Werz, O;Fischer, D;
International journal of pharmaceutics
Species: Human
Sample Types: Cell Culture Supernates
-
Expression of intron-containing HIV-1 RNA induces NLRP1 inflammasome activation in myeloid cells
Authors: Jalloh, S;Hughes, IK;Akiyama, H;Gojanovich, AD;Quiñones-Molina, AA;Yang, M;Henderson, AJ;Mostoslavsky, G;Gummuluru, S;
PLoS biology
Species: Human
Sample Types: Cell Culture Supernates
-
Combining mucosal microbiome and host multi-omics data shows prognostic potential in paediatric ulcerative colitis
Authors: Kulecka, M;O'Sullivan, J;Fitzgerald, R;Velikonja, A;Huseyin, CE;Laserna-Mendieta, EJ;Ruiz-Limón, P;Eckenberger, J;Vidal-Marín, M;Truppel, BA;Singh, R;Naik, S;Croft, NM;Temko, A;Zomer, A;MacSharry, J;Melgar, S;Deb, P;Sanderson, IR;Claesson, MJ;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Modulation of IRAK4 as a Therapeutic Strategy Against Monosodium Urate- and Xanthine-Induced Inflammation in Macrophages and HepG2 Cells
Authors: Umar, S;Chang, HT;Maienschein-Cline, M;Ravindran, S;
Research square
Species: Human
Sample Types: Cell Culture Supernates
-
ECDD-S16, a synthetic derivative of cleistanthin A, suppresses pyroptosis in Burkholderia pseudomallei-infected U937 macrophages
Authors: Khongpraphan, S;Sanongkiet, S;Luangjindarat, C;Thairat, S;Munyoo, B;Chabang, N;Charoensutthivarakul, S;Borwornpinyo, S;Tuchinda, P;Ponpuak, M;Utaisincharoen, P;Pudla, M;
PloS one
Species: Human
Sample Types: Cell Culture Supernates
-
Reciprocal effects of conditioned medium on gene and protein expression of limbal epithelial cells and limbal fibroblasts in congenital aniridia
Authors: Berger, M;Szentmáry, N;Berger, T;Zimmermann, J;Trusen, S;Seitz, B;Fries, FN;Suiwal, S;Amini, M;Stachon, T;
PloS one
Species: Human
Sample Types: Cell Culture Supernates
-
Ginsenoside Rh2 Mitigates Endoplasmic Reticulum Stress-Induced Apoptosis and Inflammation and Through Inhibition of Hepatocyte-Macrophage Inflammatory Crosstalk
Authors: Park, S;Jeong, I;Kim, OK;
Nutrients
Species: Human
Sample Types: Cell Culture Supernates
-
Biomechanical force-primed periodontal ligament stem cells exhibit a tolerance effect against bacterial inflammation
Authors: Nan, DN;Praneetpong, N;Bulanawichit, W;Chantarangsu, S;Everts, V;Ferreira, JN;Osathanon, T;Limjeerajarus, CN;Limjeerajarus, N;
Journal of dentistry
Species: Human
Sample Types: Cell Culture Supernates, Cell Lysates
-
Acidosis Licenses the NLRP3 Inflammasome-Inhibiting Effects of Beta-Hydroxybutyrate and Short-Chain Carboxylic Acids
Authors: Mank, MM;Zoller, KA;Fastiggi, VA;Ather, JL;Poynter, ME;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
miR-369-3p Ameliorates Inflammation and Apoptosis in Intestinal Epithelial Cells via the MEK/ERK Signaling Pathway
Authors: Scalavino, V;Piccinno, E;Giannelli, G;Serino, G;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Cytokine profiles dynamics in COVID-19 patients: a longitudinal analysis of disease severity and outcomes
Authors: Ghaffarpour, S;Ghazanfari, T;Ardestani, SK;Naghizadeh, MM;Vaez Mahdavi, MR;Salehi, M;Majd, AMM;Rashidi, A;Chenary, MR;Mostafazadeh, A;Rezaei, A;Khodadadi, A;Iranparast, S;Khazaei, HA;
Scientific reports
Species: Human
Sample Types: Serum
-
Effect of Free Long-Chain Fatty Acids on Anagen Induction: Metabolic or Inflammatory Aspect?
Authors: Pan, X;Vishnyakova, KS;Chermnykh, ES;Jasko, MV;Zhuravlev, AD;Verkhova, SS;Chegodaev, YS;Popov, MA;Nikiforov, NG;Yegorov, YE;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Human breast milk-derived exosomes attenuate lipopolysaccharide-induced activation in microglia
Authors: Akinduro, O;Kumar, S;Chen, Y;Thomas, B;Hassan, Q;Sims, B;
Journal of neuroinflammation
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-1 prevents SARS-CoV-2-induced membrane fusion to restrict viral transmission via induction of actin bundles
Authors: Zheng, X;Yu, S;Zhou, Y;Yu, K;Gao, Y;Chen, M;Duan, D;Li, Y;Cui, X;Mou, J;Yang, Y;Wang, X;Chen, M;Jiu, Y;Zhao, J;Meng, G;
eLife
Species: Human
Sample Types: Cell Culture Supernates
-
REDD1-dependent GSK3? signaling in podocytes promotes canonical NF-?B activation in diabetic nephropathy
Authors: Sunilkumar, S;Yerlikaya, EI;VanCleave, A;Subrahmanian, SM;Toro, AL;Kimball, SR;Dennis, MD;
The Journal of biological chemistry
Species: Human
Sample Types: Cell Culture Supernates
-
Zn-doped CaP coating equips Ti implants with corrosion resistance, biomineralization, antibacterial and immunotolerant activities
Authors: Parau, AC;Büyüksungur, S;Li, G;Liu, Q;Badillo, E;Blum, L;Schmidt, J;Pana, I;Vitelaru, C;Marinescu, IM;Dinu, M;Smuglov, M;Schmuttermaier, C;Tanir, TE;Klüter, H;Hasirci, N;Kzhyshkowska, J;Dragomir, AV;
Journal of advanced research
Species: Human
Sample Types: Cell Culture Supernates
-
Cystatin C 3 (CST3) drives pathological progression in recurrent spontaneous abortion
Authors: Li, M;Xie, H;Du, X;
Journal of reproductive immunology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Plasma Proteomic Signature as a Predictor of Age Advancement in People Living With HIV
Authors: Navas, A;Matzaraki, V;van Eekeren, LE;Blaauw, MJT;Groenendijk, AL;Vos, WAJW;Jacobs-Cleophas, M;Dos Santos, JC;van der Ven, AJAM;Joosten, LAB;Netea, MG;
Aging cell
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of general versus spinal anesthesia on perioperative innate immune function in patients undergoing total hip arthroplasty
Authors: Jacobs, LMC;Bijkerk, V;van Eijk, LT;Joosten, LAB;Keijzer, C;Visser, J;Warlé, MC;
BMC anesthesiology
Species: Human
Sample Types: Plasma
-
Calcitriol prevents SARS-CoV spike-induced inflammation in human trophoblasts through downregulating ACE2 and TMPRSS2 expression
Authors: Vargas-Castro, R;García-Quiroz, J;Olmos-Ortiz, A;Avila, E;Larrea, F;Díaz, L;
The Journal of steroid biochemistry and molecular biology
Species: Human
Sample Types: Cell Culture Supernates
-
Distinct inflammatory profiles in mustard lung: A study of sulfur mustard-exposed patients with serious pulmonary complications
Authors: Pourfarzam, S;Ardestani, SK;Jamali, T;Ghazanfari, H;Naghizadeh, MM;Faghihzadeh, S;Yaraee, R;Ghazanfari, Z;Ghazanfari, T;
International immunopharmacology
Species: Human
Sample Types: Serum, Sputum
-
Therapeutic Potential of a Natural Blend of Aronia melancarpa, Lonicera caerulea, and Echinacea purpurea Extracts in Treating Upper Respiratory Tract Infections: Preliminary Clinical and In Vitro Immunomodulatory Insights
Authors: Zima, K;Sochocka, M;Ochnik, M;Khaidakov, B;Lemke, K;Kowalczyk, P;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
?-1,6-Glucan plays a central role in the structure and remodeling of the bilaminate fungal cell wall
Authors: Bekirian, C;Valsecchi, I;Bachellier-Bassi, S;Scandola, C;Guijarro, JI;Chauvel, M;Mourer, T;Gow, NAR;Aimanianda, VK;d'Enfert, C;Fontaine, T;
eLife
Species: Human
Sample Types: Cell Culture Supernates
-
Exploring the innate immune response in polycystic liver disease
Authors: Duijzer, R;Dalloyaux, D;Boerrigter, MM;Lemmers, H;Dijkstra, H;van Emst, L;Te Morsche, RHM;Jaeger, M;Joosten, LAB;Drenth, JPH;
Cytokine
Species: Human
Sample Types: Cell Culture Supernates
-
Pseudomonas aeruginosa- mediated cardiac dysfunction is driven by extracellular vesicles released during infection
Authors: Kumar, N;Mattoo, SS;Sanghvi, S;Ellendula, MP;Mahajan, S;Planner, C;Bednash, JS;Khan, M;Ganesan, LP;Singh, H;Lafuse, WP;Wozniak, DJ;Rajaram, MVS;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Development of a novel complex inflammatory bowel disease mouse model: Reproducing human inflammatory bowel disease etiologies in mice
Authors: Seo, SM;Kim, NW;Yoo, ES;Lee, JH;Kang, AR;Jeong, HB;Shim, WY;Kim, DH;Park, YJ;Bae, K;Yoon, KA;Choi, YK;
PloS one
Species: Mouse
Sample Types: Serum
-
Functional identification of soluble uric acid as an endogenous inhibitor of CD38
Authors: Wen, S;Arakawa, H;Yokoyama, S;Shirasaka, Y;Higashida, H;Tamai, I;
eLife
Species: Mouse
Sample Types: Serum, Cell Culture Supernates
-
Comparison of Different Keratinocyte Cell Line Models for Analysis of NLRP1 Inflammasome Activation
Authors: Wang, T;Yazdi, AS;Panayotova-Dimitrova, D;
Biomolecules
Species: Human
Sample Types: Cell Culture Supernates
-
Characteristics of Pulmonary Inflammation in Patients with Different Forms of Active Tuberculosis
Authors: Shepelkova, GS;Evstifeev, VV;Berezovskiy, YS;Ergeshova, AE;Tarasov, RV;Bagirov, MA;Yeremeev, VV;
International journal of molecular sciences
Species: Human
Sample Types: Tissue Homogenates
-
Interleukin-1? induces trained innate immunity in human hematopoietic progenitor cells in vitro
Authors: Flores-Gomez, D;Hobo, W;van Ens, D;Kessler, EL;Novakovic, B;Schaap, NPM;Rijnen, WHC;Joosten, LAB;Netea, MG;Riksen, NP;Bekkering, S;
Stem cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Krebs cycle derivatives, dimethyl fumarate and itaconate, control metabolic reprogramming in inflammatory human microglia cell line
Authors: Sangineto, M;Ciarnelli, M;Moola, A;Naik Bukke, V;Cassano, T;Villani, R;Romano, AD;Di Gioia, G;Avolio, C;Serviddio, G;
Mitochondrion
Species: Human
Sample Types: Cell Culture Supernates
-
Mitigated toxicity of polystyrene nanoplastics in combination exposure with copper ions by transformation into copper (I) oxide: Inhibits the oxidative potential of nanoplastics
Authors: Maruthupandy, M;Jeon, JH;Noh, J;Yang, SI;Cho, WS;
Chemosphere
Species: Human
Sample Types: Cell Culture Supernates
-
The Clinical Effect of a Propolis and Mangosteen Extract Complex in Subjects with Gingivitis: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial
Authors: Jung, JS;Choi, GH;Lee, H;Ko, Y;Ji, S;
Nutrients
Species: Human
Sample Types: Gingival Crevicular Fluid (GCF)
-
Dengue NS1 interaction with lipids alters its pathogenic effects on monocyte derived macrophages
Authors: Dayarathna, S;Senadheera, B;Jeewandara, C;Dissanayake, M;Bary, F;Ogg, GS;Malavige, GN;
Journal of biomedical science
Species: Human
Sample Types: Cell Culture Supernates
-
The MondoA-dependent TXNIP/GDF15 axis predicts oxaliplatin response in colorectal adenocarcinomas
Authors: Deng, J;Pan, T;Wang, D;Hong, Y;Liu, Z;Zhou, X;An, Z;Li, L;Alfano, G;Li, G;Dolcetti, L;Evans, R;Vicencio, JM;Vlckova, P;Chen, Y;Monypenny, J;Gomes, CAC;Weitsman, G;Ng, K;McCarthy, C;Yang, X;Hu, Z;Porter, JC;Tape, CJ;Yin, M;Wei, F;Rodriguez-Justo, M;Zhang, J;Tejpar, S;Beatson, R;Ng, T;
EMBO molecular medicine
Species: Human
Sample Types: Cell Culture Supernates
-
The assembly of neutrophil inflammasomes during COVID-19 is mediated by type I interferons
Authors: Cabrera, LE;Jokiranta, ST;Mäki, S;Miettinen, S;Kant, R;Kareinen, L;Sironen, T;Pietilä, JP;Kantele, A;Kekäläinen, E;Lindgren, H;Mattila, P;Kipar, A;Vapalahti, O;Strandin, T;
PLoS pathogens
Species: Human
Sample Types: Cell Culture Supernates
-
Human microglia-derived proinflammatory cytokines facilitate human retinal ganglion cell development and regeneration
Authors: Subramani, M;Lambrecht, B;Ahmad, I;
Stem cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of progestin-based contraceptives on HIV-associated vaginal immune biomarkers and microbiome in adolescent girls
Authors: Nasr, MA;Aldous, A;Daniels, J;Joy, C;Capozzi, E;Yang, M;Moriarty, P;Emmanuel-Baker, V;Malcolm, S;Green, SJ;Gomez-Lobo, V;Ghosh, M;
PloS one
Species: Human
Sample Types: Vaginal Swab
-
Toxicity and efficacy of type I interferons on the ocular surface: in vitro, animal, and clinical studies
Authors: Yun, YI;Ko, JH;Ryu, JS;Kim, S;Jeon, HS;Kim, N;Kim, MK;Oh, JY;
The ocular surface
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophil extracellular traps promote immunopathogenesis of virus-induced COPD exacerbations
Authors: Katsoulis, O;Toussaint, M;Jackson, MM;Mallia, P;Footitt, J;Mincham, KT;Meyer, GFM;Kebadze, T;Gilmour, A;Long, M;Aswani, AD;Snelgrove, RJ;Johnston, SL;Chalmers, JD;Singanayagam, A;
Nature communications
Species: Human
Sample Types: Sputum
-
Effect on neutrophil migration and antimicrobial functions by the Bruton's tyrosine kinase inhibitors tolebrutinib, evobrutinib and fenebrutinib
Authors: De Bondt, M;Renders, J;de Prado, PP;Berghmans, N;Pörtner, N;Vanbrabant, L;de Oliveira, VLS;Duran, G;Baeten, P;Broux, B;Gouwy, M;Matthys, P;Hellings, N;Struyf, S;
Journal of leukocyte biology
Species: Human
Sample Types: Cell Culture Supernates
-
Long-Lasting Enhanced Cytokine Responses Following SARS-CoV-2 BNT162b2 mRNA Vaccination
Authors: Cab?u, G;Badii, M;Mirea, AM;Gaal, OI;van Emst, L;Popp, RA;Cri?an, TO;Joosten, LAB;
Vaccines
Species: Human
Sample Types: Cell Culture Supernates
-
A Synthetic Small Molecule, LGM2605: A Promising Modulator of Increased Pro-Inflammatory Cytokine and Osteoclast Differentiation by Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin
Authors: Kim, TJ;MacElroy, AS;Defreitas, A;Shenker, BJ;Boesze-Battaglia, K;
Dentistry journal
Species: Human
Sample Types: Cell Culture Supernates
-
Structural characterization of a proanthocyanidin-rich fraction from Hancornia speciosa leaves and its effect on the release of pro-inflammatory cytokines and oxidative stress in THP-1 cells
Authors: Carneiro Junior, WO;Guimarães, MLR;Freitas, KM;Pereira, RS;Pádua, RM;Campana, PRV;Braga, FC;
Journal of ethnopharmacology
Species: Human
Sample Types: Cell Culture Supernates
-
Dengue NS1 interaction with lipids alters its pathogenic effects on monocyte derived macrophages
Authors: Dayarathna, S;Senadheera, B;Jeewandara, C;Dissanayaka, M;Bary, F;Ogg, GS;Malavige, GN;
medRxiv : the preprint server for health sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Bromo-substituted indirubins for inhibition of protein kinase-mediated signalling involved in inflammatory mediator release in human monocytes
Authors: Bachmann, V;Schädel, P;Westhoff, J;Peri?, M;Schömberg, F;Skaltsounis, AL;Höppener, S;Pantsar, T;Fischer, D;Vilotijevi?, I;Werz, O;
Bioorganic chemistry
Species: Human
Sample Types: Cell Culture Supernates
-
Vitamin D regulates COVID-19 associated severity by suppressing the NLRP3 inflammasome pathway
Authors: Khalil, B;Sharif-Askari, NS;Hafezi, S;Sharif-Askari, FS;Al Anouti, F;Hamid, Q;Halwani, R;
PloS one
Species: Human
Sample Types: Whole Blood
-
Anti-CAMP1 IgG promotes macrophage phagocytosis of Cutibacterium acnes type II
Authors: Min, TT;Choowongkomon, K;Htoo, HH;Nonejuie, P;Haltrich, D;Yamabhai, M;
Microbiological research
Species: Human
Sample Types: Cell Culture Supernates
-
The Elevated Inflammatory Status of Neutrophils Is Related to In-Hospital Complications in Patients with Acute Coronary Syndrome and Has Important Prognosis Value for Diabetic Patients
Authors: Barbu, E;Mihaila, AC;Gan, AM;Ciortan, L;Macarie, RD;Tucureanu, MM;Filippi, A;Stoenescu, AI;Petrea, SV;Simionescu, M;Balanescu, SM;Butoi, E;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
SARS-CoV-2 superinfection in CD14+ monocytes with latent human cytomegalovirus (HCMV) promotes inflammatory cascade
Authors: Payen, SH;Adhikari, K;Petereit, J;Uppal, T;Rossetto, CC;Verma, SC;
Virus research
Species: Human
Sample Types: Cell Culture Supernates
-
An IL-1?-driven neutrophil-stromal cell axis fosters a BAFF-rich protumor microenvironment in individuals with multiple myeloma
Authors: de Jong, MME;Fokkema, C;Papazian, N;Czeti, Á;Appelman, MK;Vermeulen, M;van Heusden, T;Hoogenboezem, RM;van Beek, G;Tahri, S;Sanders, MA;van de Woestijne, PC;Gay, F;Moreau, P;Büttner-Herold, M;Bruns, H;van Duin, M;Broijl, A;Sonneveld, P;Cupedo, T;
Nature immunology
Species: Human
Sample Types: Cell Culture Supernates
-
Empowering Naringin's Anti-Inflammatory Effects through Nanoencapsulation
Authors: Marinho, A;Seabra, CL;Lima, SAC;Lobo-da-Cunha, A;Reis, S;Nunes, C;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Cell type-dependent response to benzo(a)pyrene exposure of human placental cell lines under normoxic, hypoxic, and pro-inflammatory conditions
Authors: Dai, Y;Xu, X;Huo, X;Schuitemaker, JHN;Faas, MM;
Ecotoxicology and environmental safety
Species: Human
Sample Types: Cell Culture Supernates
-
Leishmania braziliensis enhances monocyte responses to promote anti-tumor activity
Authors: Dos Santos, JC;Moreno, M;Teufel, LU;Chilibroste, S;Keating, ST;Groh, L;Domínguez-Andrés, J;Williams, DL;Ma, Z;Lowman, DW;Ensley, HE;Novakovic, B;Ribeiro-Dias, F;Netea, MG;Chabalgoity, JA;Joosten, LAB;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
The proteome of bacterial membrane vesicles in Escherichia coli-a time course comparison study in two different media
Authors: Yu, MSC;Chiang, DM;Reithmair, M;Meidert, A;Brandes, F;Schelling, G;Ludwig, C;Meng, C;Kirchner, B;Zenner, C;Muller, L;Pfaffl, MW;
Frontiers in microbiology
Species: Human
Sample Types: Cell Culture Supernates
-
Efficacy of Lactiplantibacillus plantarum PBS067, Bifidobacterium animalis subsp. lactis BL050, and Lacticaseibacillus rhamnosus LRH020 in the Amelioration of Vaginal Microbiota in Post-Menopausal Women: A Prospective Observational Clinical Trial
Authors: Vicariotto, F;Malfa, P;Viciani, E;Dell'Atti, F;Squarzanti, DF;Marcante, A;Castagnetti, A;Ponchia, R;Governini, L;De Leo, V;
Nutrients
Species: Human
Sample Types: Vaginal Swab
-
Effects of Combinatory In Vitro Treatment with Immune Checkpoint Inhibitors and Cytarabine on the Anti-Cancer Immune Microenvironment in De Novo AML Patients
Authors: Bo?kun, ?;Starosz, A;Kr?towska-Grunwald, A;Wasiluk, T;Walewska, A;Wierzbowska, A;Moniuszko, M;Grubczak, K;
Cancers
Species: Human
Sample Types: Cell Culture Supernates
-
Valorization of shrimp processing waste-derived chitosan into anti-inflammatory chitosan-oligosaccharides (CHOS)
Authors: Yamabhai, M;Khamphio, M;Min, TT;Soem, CN;Cuong, NC;Aprilia, WR;Luesukprasert, K;Teeranitayatarn, K;Maneedaeng, A;Tuveng, TR;Lorentzen, SB;Antonsen, S;Jitprasertwong, P;Eijsink, VGH;
Carbohydrate polymers
Species: Human
Sample Types: Cell Culture Supernates
-
Resveratrol attenuates high glucose-induced inflammation and improves glucose metabolism in HepG2 cells
Authors: Tshivhase, AM;Matsha, T;Raghubeer, S;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
IFN-?3 is induced by Leishmania donovani and can inhibit parasite growth in cell line models but not in the mouse model, while it shows a significant association with leishmaniasis in humans
Authors: De, M;Sukla, S;Bharatiya, S;Keshri, S;Roy, DG;Roy, S;Dutta, D;Saha, S;Ejazi, SA;Ravichandiran, V;Ali, N;Chatterjee, M;Chinnaswamy, S;
Infection and immunity
Species: Human
Sample Types: Cell Culture Supernates
-
Inactivation of the NLRP3 inflammasome mediates exosome-based prevention of atrial fibrillation
Authors: Parent, S;Vaka, R;St Amant, J;Kahn, S;Van Remortel, S;Bi, C;Courtman, D;Stewart, DJ;Davis, DR;
Theranostics
Species: Rat
Sample Types: Cell Culture Supernates, Cell Lysates
-
Investigation of the structural and immunomodulatory properties of alkali-soluble ?-glucans from Pleurotus eryngii fruiting bodies
Authors: Ellefsen, CF;Lindstad, L;Klau, LJ;Aachmann, FL;Hiorth, M;Samuelsen, ABC;
Carbohydrate polymers
Species: Human
Sample Types: Cell Culture Supernates
-
?-endorphin suppresses ultraviolet B irradiation-induced epidermal barrier damage by regulating inflammation-dependent mTORC1 signaling
Authors: Kim, HS;Kim, HJ;Hong, YD;Son, ED;Cho, SY;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Macrophages derived from LPS-stimulated monocytes from individuals with subclinical atherosclerosis were characterized by increased pro-inflammatory activity
Authors: Nikiforov, NG;Kirichenko, TV;Kubekina, MV;Chegodaev, YS;Zhuravlev, AD;Ilchuk, LA;Nikolaeva, MA;Arefieva, AS;Popov, MA;Verkhova, SS;Bagheri Ekta, M;Orekhov, AN;
Cytokine
Species: Human
Sample Types: Cell Culture Supernates
-
Acid ceramidase regulates innate immune memory
Authors: Rother, N;Yanginlar, C;Prévot, G;Jonkman, I;Jacobs, M;van Leent, MMT;van Heck, J;Matzaraki, V;Azzun, A;Morla-Folch, J;Ranzenigo, A;Wang, W;van der Meel, R;Fayad, ZA;Riksen, NP;Hilbrands, LB;Lindeboom, RGH;Martens, JHA;Vermeulen, M;Joosten, LAB;Netea, MG;Mulder, WJM;van der Vlag, J;Teunissen, AJP;Duivenvoorden, R;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Transcriptomic changes during the replicative senescence of human articular chondrocytes
Authors: Atasoy-Zeybek, A;Hawse, GP;Nagelli, CV;De Padilla, CL;Abdel, MP;Evans, CH;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Ventilatory support and inflammatory peptides in hospitalised patients with COVID-19: A prospective cohort trial
Authors: Gysan, MR;Milacek, C;Bal, C;Zech, A;Brugger, J;Milos, RI;Antoniewicz, L;Idzko, M;Gompelmann, D;
PloS one
Species: Human
Sample Types: Serum
-
Human PBMCs Form Lipid Droplets in Response to Spike Proteins
Authors: Sivaraman, K;Pino, P;Raussin, G;Anchisi, S;Metayer, C;Dagany, N;Held, J;Wrenger, S;Welte, T;Wurm, MJ;Wurm, FM;Olejnicka, B;Janciauskiene, S;
Microorganisms
Species: Human
Sample Types: Cell Culture Supernates
-
Serum interleukin-6, procalcitonin, and C-reactive protein at hospital admission can identify patients at low risk for severe COVID-19 progression
Authors: Zobel, CM;Wenzel, W;Krüger, JP;Baumgarten, U;Wagelöhner, T;Neumann, N;Foroutan, B;Müller, R;Müller, A;Rauschning, D;Schü beta ler, M;Scheit, L;Weinreich, F;Oltmanns, K;Keidel, F;Koch, M;Spethmann, S;Schreiner, M;
Frontiers in microbiology
Species: Human
Sample Types: Serum
-
The role of the Toll like receptor 4 signaling in sex-specific persistency of depression-like behavior in response to chronic stress
Authors: Yang, EJ;Frolinger, T;Iqbal, U;Estill, M;Shen, L;Trageser, KJ;Pasinetti, GM;
Brain, behavior, and immunity
Species: Mouse
Sample Types: Cell Lysates
-
Effects of caatinga propolis from Mimosa tenuiflora and its constituents (santin, sakuranetin and kaempferide) on human immune cells
Authors: Sartori, AA;Son, NT;da Silva Honorio, M;Ripari, N;Santiago, KB;Gomes, AM;Zambuzzi, WF;Bastos, JK;Sforcin, JM;
Journal of ethnopharmacology
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of leptin on trained innate immunity and on systemic inflammation in subjects with obesity
Authors: Flores Gomez, D;Bekkering, S;Horst, RT;Cossins, B;van den Munckhof, ICL;Rutten, JHW;Joosten, LAB;Netea, MG;Riksen, NP;
Journal of leukocyte biology
Species: Human
Sample Types: Cell Culture Supernates, Plasma
-
Targeting Toll-like receptor-driven systemic inflammation by engineering an innate structural fold into drugs
Authors: Petruk, G;Puthia, M;Samsudin, F;Petrlova, J;Olm, F;Mittendorfer, M;Hyllén, S;Edström, D;Strömdahl, AC;Diehl, C;Ekström, S;Walse, B;Kjellström, S;Bond, PJ;Lindstedt, S;Schmidtchen, A;
Nature communications
Species: Human
Sample Types: Plasma
-
TNF? induced by DNA-sensing in macrophage compromises retinal pigment epithelial (RPE) barrier function
Authors: Twarog, M;Schustak, J;Xu, Y;Coble, M;Dolan, K;Esterberg, R;Huang, Q;Saint-Geniez, M;Bao, Y;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates, Cell Lysates
-
TNF? is a key trigger of inflammation in diet-induced non-obese MASLD in mice
Authors: Burger, K;Jung, F;Baumann, A;Brandt, A;Staltner, R;Sánchez, V;Bergheim, I;
Redox biology
Species: Human
Sample Types: Tissue Homogenates
-
Adipose-Tumor Crosstalk contributes to CXCL5 Mediated Immune Evasion in PDAC
Authors: Walsh, RM;Ambrose, J;Jack, JL;Eades, AE;Bye, B;Ruckert, MT;Olou, AA;Messaggio, F;Chalise, P;Pei, D;VanSaun, MN;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Size- and oxidative potential-dependent toxicity of environmentally relevant expanded polystyrene styrofoam microplastics to macrophages
Authors: Jeon, S;Jeon, JH;Jeong, J;Kim, G;Lee, S;Kim, S;Maruthupandy, M;Lee, K;Yang, SI;Cho, WS;
Journal of hazardous materials
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of bile salt-stimulated lipase on blood cells and associations with disease activity in human inflammatory joint disorders
Authors: Lindquist, S;Wang, Y;Andersson, EL;Tsuji Grebe, S;Alenius, GM;Rantapää-Dahlqvist, S;Lundberg, L;Hernell, O;
PloS one
Species: Human
Sample Types: Serum
-
The influence of calcitriol and methylprednisolone on podocytes function in minimal change disease in vitro model
Authors: Grubczak, K;Starosz, A;Makowska, B;Parfienowicz, Z;Kr?towska, M;Naumnik, B;Moniuszko, M;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernatants
-
Amyloid Beta Peptides Lead to Mast Cell Activation in a Novel 3D Hydrogel Model
Authors: Liu, J;Liu, S;Zeng, L;Tsilioni, I;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Assessment of temporomandibular disorders and their relationship with life quality and salivary biomarkers in patients with dentofacial deformities: A clinical observational study
Authors: Crescente, BB;Bisatto, NV;Rübensam, G;Fritscher, GG;Campos, MM;
PloS one
Species: Human
Sample Types: Saliva
-
IL1R1+ cancer-associated fibroblasts drive tumor development and immunosuppression in colorectal cancer
Authors: Koncina, E;Nurmik, M;Pozdeev, VI;Gilson, C;Tsenkova, M;Begaj, R;Stang, S;Gaigneaux, A;Weindorfer, C;Rodriguez, F;Schmoetten, M;Klein, E;Karta, J;Atanasova, VS;Grzyb, K;Ullmann, P;Halder, R;Hengstschläger, M;Graas, J;Augendre, V;Karapetyan, YE;Kerger, L;Zuegel, N;Skupin, A;Haan, S;Meiser, J;Dolznig, H;Letellier, E;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Natural mutations in the sensor kinase of the PhoPR two-component regulatory system modulate virulence of ancestor-like tuberculosis bacilli
Authors: Malaga, W;Payros, D;Meunier, E;Frigui, W;Sayes, F;Pawlik, A;Orgeur, M;Berrone, C;Moreau, F;Mazères, S;Gonzalo-Asensio, J;Rengel, D;Martin, C;Astarie-Dequeker, C;Mourey, L;Brosch, R;Guilhot, C;
PLoS pathogens
Species: Human
Sample Types: Cell Culture Supernates
-
Prevention of M2 polarization and temporal limitation of differentiation in monocytes by extracellular ATP
Authors: Scherr, BF;Reiner, MF;Baumann, F;Höhne, K;Müller, T;Ayata, K;Müller-Quernheim, J;Idzko, M;Zissel, G;
BMC immunology
Species: Human
Sample Types: Cell Culture Supernates
-
Mild Cognitive Impairment Is Associated with Enhanced Activation of Th17 Lymphocytes in Non-Alcoholic Fatty Liver Disease
Authors: Fiorillo, A;Gallego, JJ;Casanova-Ferrer, F;Giménez-Garzó, C;Urios, A;Ballester, MP;Durbán, L;Rios, MP;Megías, J;San Miguel, T;Kosenko, E;Escudero-García, D;Benlloch, S;Felipo, V;Montoliu, C;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Dimethyl itaconate induces long-term innate immune responses and confers protection against infection
Authors: Ferreira, AV;Kostidis, S;Groh, LA;Koeken, VACM;Bruno, M;Baydemir, I;Kilic, G;Bulut, Ö;Andriopoulou, T;Spanou, V;Synodinou, KD;Gkavogianni, T;Moorlag, SJCFM;Charlotte de Bree, L;Mourits, VP;Matzaraki, V;Koopman, WJH;van de Veerdonk, FL;Renieris, G;Giera, M;Giamarellos-Bourboulis, EJ;Novakovic, B;Domínguez-Andrés, J;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Mast cells disrupt the function of the esophageal epithelial barrier
Authors: Kleuskens, MTA;Bek, MK;Al Halabi, Y;Blokhuis, BRJ;Diks, MAP;Haasnoot, ML;Garssen, J;Bredenoord, AJ;C A M van Esch, B;Redegeld, FA;
Mucosal immunology
Species: Human
Sample Types: Cell Culture Supernates
-
Omega-3 polyunsaturated fatty acids (n-3 PUFAs), somatic and fatigue symptoms in cardiovascular diseases comorbid major depressive disorder (MDD): A randomized controlled trial
Authors: Chang, JP;Chang, SS;Chen, HT;Chien, YC;Yang, HT;Huang, SY;Tseng, PT;Chang, CH;Galecki, P;Su, KP;
Brain, behavior, and immunity
Species: Human
Sample Types: Plasma
-
Preliminary Study on the Effect of a Night Shift on Blood Pressure and Clock Gene Expression
Authors: Toffoli, B;Tonon, F;Giudici, F;Ferretti, T;Ghirigato, E;Contessa, M;Francica, M;Candido, R;Puato, M;Grillo, A;Fabris, B;Bernardi, S;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Discovery of an inhibitor of DNA-driven inflammation that preferentially targets the AIM2 inflammasome
Authors: Jack P. Green, Lina Y. El-Sharkawy, Stefan Roth, Jie Zhu, Jiayu Cao, Andrew G. Leach et al.
iScience
Species: Mouse
Sample Types: Cell Culture Supernates
-
A single-cell view on host immune transcriptional response to in�vivo BCG-induced trained immunity
Authors: Li, W;Moorlag, SJCFM;Koeken, VACM;R�ring, RJ;de Bree, LCJ;Mourits, VP;Gupta, MK;Zhang, B;Fu, J;Zhang, Z;Grondman, I;van Meijgaarden, KE;Zhou, L;Alaswad, A;Joosten, LAB;van Crevel, R;Xu, CJ;Netea, MG;Li, Y;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Plasmodium falciparum-infected erythrocyte co-culture with the monocyte cell line THP-1 does not trigger production of soluble factors reducing brain microvascular barrier function
Authors: Storm, J;Camarda, G;Haley, MJ;Brough, D;Couper, KN;Craig, AG;
PloS one
Species: Human
Sample Types: Cell Culture Supernates
-
Cell-autonomous immune dysfunction driven by disrupted autophagy in C9orf72-ALS iPSC-derived microglia contributes to neurodegeneration
Authors: P Banerjee, AR Mehta, RS Nirujogi, J Cooper, OG James, J Nanda, J Longden, K Burr, K McDade, A Salzinger, E Paza, J Newton, D Story, S Pal, C Smith, DR Alessi, BT Selvaraj, J Priller, S Chandran
Science Advances, 2023-04-21;9(16):eabq0651.
Species: Human
Sample Types: Cell Culture Supernates
-
Loss of Gasdermin D is protective against influenza virus-induced inflammation and mortality
Authors: S Speaks, A Zani, A Solstad, A Kenney, L Zhang, AC Eddy, AO Amer, R Robinson, C Cai, J Ma, EA Hemann, A Forero, JS Yount
bioRxiv : the preprint server for biology, 2023-04-07;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of triggering receptor expressed on myeloid cells-1 in the mechanotransduction signaling pathways that link low shear stress with inflammation
Authors: M Liu, AN Panagopoul, UM Oguz, S Samant, CH Vasa, DK Agrawal, YS Chatzizisi
Scientific Reports, 2023-03-21;13(1):4656.
Species: Human
Sample Types: Cell Culture Supernates
-
Molecular Analysis of SARS-CoV-2 Spike Protein-Induced Endothelial Cell Permeability and vWF Secretion
Authors: Y Guo, V Kanamarlap
International Journal of Molecular Sciences, 2023-03-16;24(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
TGF-beta is elevated in hyperuricemic individuals and mediates urate-induced hyperinflammatory phenotype in human mononuclear cells
Authors: V Klück, G Cab?u, L Mies, F Bukkems, L van Emst, R Bakker, A van Caam, HINT conso, TO Cri?an, LAB Joosten
Arthritis Research & Therapy, 2023-02-27;25(1):30.
Species: Human
Sample Types: Cell Culture Supernates
-
CXCL12 and CXCR4 as Novel Biomarkers in Uric Acid-Induced Inflammation and Patients with Gouty Arthritis
Authors: SK Kim, JY Choe, KY Park
Biomedicines, 2023-02-21;11(3):.
Species: Human
Sample Types: Serum
-
Role of PARP and TRPM2 in VEGF Inhibitor-Induced Vascular Dysfunction
Authors: KB Neves, R Alves-Lope, AC Montezano, RM Touyz
Journal of the American Heart Association, 2023-02-20;0(0):e027769.
Species: Human
Sample Types: Cell Culture Supernates
-
Rare Phytocannabinoids Exert Anti-Inflammatory Effects on Human Keratinocytes via the Endocannabinoid System and MAPK Signaling Pathway
Authors: D Tortolani, C Di Meo, S Standoli, F Ciaramella, S Kadhim, E Hsu, C Rapino, M Maccarrone
International Journal of Molecular Sciences, 2023-02-01;24(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Vacuole Membrane Protein 1 (VMP1) Restricts NLRP3 Inflammasome Activation by Modulating SERCA Activity and Autophagy
Authors: SR Zack, R Nikolaienk, B Cook, R Melki, AV Zima, EM Campbell
Research square, 2023-01-27;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Co-culture models of endothelial cells, macrophages, and vascular smooth muscle cells for the study of the natural history of atherosclerosis
Authors: M Liu, S Samant, CH Vasa, RM Pedrigi, UM Oguz, S Ryu, T Wei, DR Anderson, DK Agrawal, YS Chatzizisi
PLoS ONE, 2023-01-20;18(1):e0280385.
Species: Human
Sample Types: Cell Culture Supernates
-
Hemoglobin Derived from Subarachnoid Hemorrhage-Induced Pyroptosis of Neural Stem Cells via ROS/NLRP3/GSDMD Pathway
Authors: T Yue, X Li, X Chen, T Zhu, W Li, B Wang, C Hang
Oxidative Medicine and Cellular Longevity, 2023-01-16;2023(0):4383332.
Species: Human
Sample Types: CSF
-
Inhalable Saharan dust induces oxidative stress, NLRP3 inflammasome activation, and inflammatory cytokine release
Authors: G Bredeck, M Busch, A Rossi, B Stahlmecke, KW Fomba, H Herrmann, RPF Schins
Environment international, 2023-01-11;172(0):107732.
Species: Human
Sample Types: Cell Culture Supernates
-
ADS024, a Bacillus velezensis strain, protects human colonic epithelial cells against C. difficile toxin-mediated apoptosis
Authors: Y Xie, A Chupina Es, B Nelson, H Feng, C Pothoulaki, L Chesnel, HW Koon
Frontiers in Microbiology, 2023-01-10;13(0):1072534.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-inflammatory activities of Coleus forsteri (formerly Plectranthus forsteri) extracts on human macrophages and chemical characterization
Authors: M Nicolas, M Lasalo, S Chow, C Antheaume, K Huet, E Hnawia, GJ Guillemin, M Nour, M Matsui
Frontiers in Pharmacology, 2023-01-09;13(0):1081310.
Species: Human
Sample Types: Cell culture supernate
-
Human TH17 cells engage gasdermin E pores to release IL-1alpha on NLRP3 inflammasome activation
Authors: YY Chao, A Puhach, D Frieser, M Arunkumar, L Lehner, T Seeholzer, A Garcia-Lop, M van der Wa, S Fibi-Smeta, A Dietschman, T Sommermann, T ?ikovi?, L Taher, MS Gresnigt, SJ Vastert, F van Wijk, G Panagiotou, D Krappmann, O Gro beta, CE Zielinski
Nature Immunology, 2023-01-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
JC2-11, a benzylideneacetophenone derivative, attenuates inflammasome activation
Authors: G Lee, H Ahn, JH Yun, J Park, E Lee, S Oh, GS Lee
Scientific Reports, 2022-12-28;12(1):22484.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Asprosin Exerts Pro-Inflammatory Effects in THP-1 Macrophages Mediated via the Toll-like Receptor 4 (TLR4) Pathway
Authors: K Shabir, S Gharanei, S Orton, V Patel, P Chauhan, E Karteris, HS Randeva, JE Brown, I Kyrou
International Journal of Molecular Sciences, 2022-12-23;24(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
microRNA-338-3p suppresses lipopolysaccharide-induced inflammatory response in HK-2 cells
Authors: J Wang, G Li, M Lin, S Lin, L Wu
BMC molecular and cell biology, 2022-12-23;23(1):60.
Species: Human
Sample Types: Cell Culture Supernates
-
Alpinetin Suppresses Zika Virus-Induced Interleukin-1beta Production and Secretion in Human Macrophages
Authors: N Wikan, S Potikanond, P Hankittich, P Thaklaewph, S Monkaew, DR Smith, W Nimlamool
Pharmaceutics, 2022-12-14;14(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Phytohustil� and root extract of Althaea officinalis L. exert anti-inflammatory and anti-oxidative properties and improve the migratory capacity of endothelial cells in vitro
Authors: GA Bonaterra, J Schmitt, K Schneider, H Schwarzbac, H Aziz-Kalbh, O Kelber, J Müller, R Kinscherf
Frontiers in Pharmacology, 2022-12-08;13(0):948248.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of exogenous IL-37 on immune cells from a patient carrying a potential IL37 loss-of-function variant: A case study
Authors: LU Teufel, CI van der Ma, V Klück, A Simons, A Hoischen, V Vernimmen, LAB Joosten, RJW Arts
Cytokine, 2022-12-05;162(0):156102.
Species: Human
Sample Types: Cell Culture Supernates
-
Glycine inhibits NINJ1 membrane clustering to suppress plasma membrane rupture in cell death
Authors: JP Borges, RSR Sætra, A Volchuk, M Bugge, P Devant, B Sporsheim, BR Kilburn, CL Evavold, JC Kagan, NM Goldenberg, TH Flo, BE Steinberg
Elife, 2022-12-05;11(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Distinct changes in endosomal composition promote NLRP3 inflammasome activation
Authors: Z Zhang, R Venditti, L Ran, Z Liu, K Vivot, A Schürmann, JS Bonifacino, MA De Matteis, R Ricci
Nature Immunology, 2022-11-28;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
EC-18 prevents autoimmune arthritis by suppressing inflammatory cytokines and osteoclastogenesis
Authors: JS Park, SC Yang, HY Jeong, SY Lee, JG Ryu, JW Choi, HY Kang, SM Kim, SH Hwang, ML Cho, SH Park
Arthritis Research & Therapy, 2022-11-17;24(1):254.
Species: Human
Sample Types: Serum
-
Involvement of glycoinositolphospholipid from Trypanosoma cruzi and macrophage migration inhibitory factor in proinflammatory mechanisms promoting cardiovascular injury mechanisms promoting cardiovascular inflammation tThe combined action of glycoinositolphospholipid from Trypanosoma cruzi and macrophage migration inhibitory factor increases proinflammatory mediator production by cardiomyocytes and vascular endothelial cells
Authors: CS Rigazio, N Mariz-Pont, EP Caballero, FN Penas, NB Goren, MH Santamaría, RS Corral
Microbial pathogenesis, 2022-11-12;0(0):105881.
Species: Human
Sample Types: Cell Culture Supernates
-
Transcriptional licensing is required for Pyrin inflammasome activation in human macrophages and bypassed by mutations causing familial Mediterranean fever
Authors: MSJ Mangan, F Gorki, K Krause, A Heinz, A Pankow, T Ebert, D Jahn, K Hiller, V Hornung, M Maurer, FI Schmidt, R Gerhard, E Latz
PloS Biology, 2022-11-07;20(11):e3001351.
Species: Human
Sample Types: Cell Culture Supernates
-
On the Bioactivity of Echinacea purpurea Extracts to Modulate the Production of Inflammatory Mediators
Authors: SF Vieira, VMF Gonçalves, CP Llaguno, F Macías, ME Tiritan, RL Reis, H Ferreira, NM Neves
Oncogene, 2022-11-06;23(21):.
Species: Human
Sample Types: Cell Culture Supernates
-
SARS-CoV-2 drives NLRP3 inflammasome activation in human microglia through spike protein
Authors: EA Albornoz, AA Amarilla, N Modhiran, S Parker, XX Li, DK Wijesundar, J Aguado, AP Zamora, CLD McMillan, B Liang, NYG Peng, JDJ Sng, FT Saima, JN Fung, JD Lee, D Paramitha, R Parry, MS Avumegah, A Isaacs, MW Lo, Z Miranda-Ch, D Bradshaw, C Salinas-Re, NW Rajapakse, EJ Wolvetang, TP Munro, A Rojas-Fern, PR Young, KJ Stacey, AA Khromykh, KJ Chappell, D Watterson, TM Woodruff
Molecular Psychiatry, 2022-11-01;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Enzyme-Treated Caviar Prevents UVB Irradiation-Induced Skin Photoaging
Authors: J Park, D Kim, M Lee, S Han, W Jun, HM Jung, YK Koo, GH Na, SH Han, J Han, OK Kim
Oncogene, 2022-10-30;20(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Signal strength of STING activation determines cytokine plasticity and cell death in human monocytes
Authors: D Kabelitz, M Zarobkiewi, M Heib, R Serrano, M Kunz, G Chitadze, D Adam, C Peters
Scientific Reports, 2022-10-24;12(1):17827.
Species: Human
Sample Types: Cell Culture Supernates
-
Proteins from Modern and Ancient Wheat Cultivars: Impact on Immune Cells of Healthy Individuals and Patients with NCGS
Authors: W Dieterich, C Schuster, P Gundel, KA Scherf, D Pronin, S Geisslitz, A Börner, MF Neurath, Y Zopf
Nutrients, 2022-10-12;14(20):.
Species: Human
Sample Types: Cell Culture Supernates
-
T Lymphocyte Serotonin 5-HT7 Receptor Is Dysregulated in Natalizumab-Treated Multiple Sclerosis Patients
Authors: F Reverchon, C Guillard, L Mollet, P Auzou, D Gosset, F Madouri, A Valéry, A Menuet, C Ozsancak, M Pallix-Guy, S Morisset-L
Biomedicines, 2022-09-27;10(10):.
Species: Human
Sample Types: Plasma
-
Interleukin-17, a salivary biomarker for COVID-19 severity
Authors: FS Sharif-Ask, NS Sharif-Ask, S Hafezi, B Mdkhana, HAH Alsayed, AW Ansari, B Mahboub, AM Zakeri, MH Temsah, W Zahir, Q Hamid, R Halwani
PLoS ONE, 2022-09-22;17(9):e0274841.
Species: Human
Sample Types: Saliva
-
PD-1/PD-L1 blockade abrogates a dysfunctional innate-adaptive immune axis in critical beta-coronavirus disease
Authors: M Duhalde Ve, D Olivera, G Gastão Dav, M Bertullo, V Noya, G Fabiano de, S Primon Mur, I Castro, AP Arévalo, M Crispo, G Galliussi, S Russo, D Charbonnie, F Rammauro, M Jeldres, C Alamón, V Varela, C Batthyany, M Bollati-Fo, P Oppezzo, O Pritsch, JL Proença-Mó, HI Nakaya, E Trias, L Barbeito, I Anegon, MC Cuturi, P Moraes-Vie, M Segovia, M Hill
Science Advances, 2022-09-21;8(38):eabn6545.
Species: Human
Sample Types: Cell Culture Supernates
-
FAAH served a key membrane-anchoring and stabilizing role for NLRP3 protein independently of the endocannabinoid system
Authors: Y Zhu, H Zhang, H Mao, S Zhong, Y Huang, S Chen, K Yan, Z Zhao, X Hao, Y Zhang, H Yao, X Huang, M Wang, W Zhang, J Li, G Meng, X Qin, Z Ye, J Shen, Y Song, Y Xu, Z Yang, L Wang, Y Zhang, L Wen
Cell Death and Differentiation, 2022-09-14;0(0):.
Species: Mouse
Sample Types: Serum
-
Hybrid Mineral/Organic Material Induces Bone Bridging and Bone Volume Augmentation in Rat Calvarial Critical Size Defects
Authors: M Dubus, L Scomazzon, C Ledouble, J Braux, A Beljebbar, L Van Gulick, A Baldit, C Gorin, H Alem, N Bouland, M Britton, J Schiavi, TJ Vaughan, C Mauprivez, H Kerdjoudj
Cells, 2022-09-14;11(18):.
Species: Human
Sample Types: Cell Culture Supernates
-
Assessing the NLRP3 Inflammasome Activating Potential of a Large Panel of Micro- and Nanoplastics in THP-1 Cells
Authors: M Busch, G Bredeck, F Waag, K Rahimi, H Ramachandr, T Bessel, S Barcikowsk, A Herrmann, A Rossi, RPF Schins
Biomolecules, 2022-08-09;12(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Development of an Electrochemical CCL5 Chemokine Immunoplatform for Rapid Diagnosis of Multiple Sclerosis
Authors: S Guerrero, E Sánchez-Ti, L Agüí, A González-C, P Yáñez-Sede, JM Pingarrón
Biosensors, 2022-08-07;12(8):.
Species: Human
Sample Types: Serum
-
Development of a rapid in vitro pre-screen for distinguishing effective liposome-adjuvant delivery systems
Authors: LAJ Feather, V Nadella, E Kastner, Y Perrie, AC Hilton, A Devitt
Scientific Reports, 2022-07-20;12(1):12448.
Species: Human
Sample Types: Cell Culture Supernates
-
Lithocholic acid inhibits dendritic cell activation by reducing intracellular glutathione via TGR5 signaling
Authors: J Hu, Y Zhang, S Yi, C Wang, X Huang, S Pan, J Yang, G Yuan, S Tan, H Li
International journal of biological sciences, 2022-07-11;18(11):4545-4559.
Species: Human
Sample Types: Cell Culture Supernates
-
A whole genome CRISPR screen identifies AHR loss as a mechanism of PARP7 inhibitor resistance
Authors: H Chen, ME Diolaiti, PC O'Leary, A Rojc, NJ Krogan, M Kim, A Ashworth
Molecular Cancer Therapeutics, 2022-07-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Antibacterial and Anti-Inflammatory Effects of Apolipoprotein E
Authors: M Puthia, JK Marzinek, G Petruk, G Ertürk Ber, PJ Bond, J Petrlova
Biomedicines, 2022-06-17;10(6):.
Species: Human
Sample Types: Plasma
-
Toxoplasma gondii Rhoptry Protein 7 (ROP7) Interacts with NLRP3 and Promotes Inflammasome Hyperactivation in THP-1-Derived Macrophages
Authors: L Zhu, W Qi, G Yang, Y Yang, Y Wang, L Zheng, Y Fu, X Cheng
Cells, 2022-05-12;11(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
"HIIT the Inflammation": Comparative Effects of Low-Volume Interval Training and Resistance Exercises on Inflammatory Indices in Obese Metabolic Syndrome Patients Undergoing Caloric Restriction
Authors: D Reljic, W Dieterich, HJ Herrmann, MF Neurath, Y Zopf
Nutrients, 2022-05-10;14(10):.
Species: Human
Sample Types: Serum
-
Green and Roasted Coffee Extracts Inhibit Interferon-beta Release in LPS-Stimulated Human Macrophages
Authors: V Artusa, C Ciaramelli, A D'Aloia, FA Facchini, N Gotri, A Bruno, B Costa, A Palmioli, C Airoldi, F Peri
Frontiers in Pharmacology, 2022-05-05;13(0):806010.
Species: Human
Sample Types: Cell Culture Supernates
-
IFN-gamma and TNF Induce Senescence and a Distinct Senescence-Associated Secretory Phenotype in Melanoma
Authors: L Homann, M Rentschler, E Brenner, K Böhm, M Röcken, T Wieder
Cells, 2022-04-30;11(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
NLRP3 inflammasome triggers interleukin-37 release from human monocytes
Authors: A Gritsenko, R Díaz-Pino, G Lopez-Cast
European Journal of Immunology, 2022-04-28;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Mycobacterium avium-intracellulare complex promote release of pro-inflammatory enzymes matrix metalloproteinases by inducing neutrophil extracellular trap formation
Authors: K Nakamura, H Nakayama, S Sasaki, K Takahashi, K Iwabuchi
Scientific Reports, 2022-04-11;12(1):5181.
Species: Human
Sample Types: Cell Culture Supernates
-
Discovery of a novel GRPR antagonist for protection against cisplatin-induced acute kidney injury
Authors: MJ Yu, C Li, SS Deng, XM Meng, RS Yao
Bioorganic chemistry, 2022-04-06;124(0):105794.
Species: Human
Sample Types: Cell Culture Supernates
-
Cytokine Profile and Anti-Inflammatory Activity of a Standardized Conditioned Medium Obtained by Coculture of Monocytes and Mesenchymal Stromal Cells (PRS CK STORM)
Authors: JP Lapuente, A Blázquez-M, J Marco-Brua, G Gómez, P Desportes, J Sanz, P Fernández, M García-Gil, F Bermejo, JV San Martín, A Algaba, JC De Gregori, D Lapuente, A De Gregori, B Lapuente, MV Andrés, A Anel
Biomolecules, 2022-03-31;12(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
Immune Modulating Brevetoxins: Monocyte Cytotoxicity, Apoptosis, and Activation of M1/M2 Response Elements Is Dependent on Reactive Groups
Authors: JR McCall, KT Sausman, DM Keeler, AP Brown, SL Turrise
Marine Drugs, 2022-03-29;20(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
Sulforaphane Reduces Pro-Inflammatory Response To Palmitic Acid In Monocytes And Adipose Tissue Macrophages
Authors: EJ Williams, L Guillemina, BS Berthon, S Eslick, T Wright, C Karihaloo, M Gately, KJ Baines, LG Wood
The Journal of nutritional biochemistry, 2022-03-07;0(0):108978.
Species: Human
Sample Types: Cell Culture Supernates
-
A novel Streptococcus pneumoniae human challenge model demonstrates Treg lymphocyte recruitment to the infection site
Authors: G Szylar, R Wysoczansk, H Marshall, DJB Marks, R José, ME Ehrenstein, JS Brown
Scientific Reports, 2022-03-07;12(1):3990.
Species: Human
Sample Types: Blister Fluid
-
Anti-inflammatory effects of an autologous gold-based serum therapy in osteoarthritis patients
Authors: J Feldt, AJ Donaubauer, J Welss, U Schneider, US Gaipl, F Paulsen
Scientific Reports, 2022-03-03;12(1):3560.
Species: Human
Sample Types: Serum
-
E-Cigarette Aerosols Promote Oral S. aureus Colonization by Delaying an Immune Response and Bacterial Clearing
Authors: AR Cátala-Val, J Almeda, JN Bernard, AM Cole, AL Cole, SD Moore, CD Andl
Cells, 2022-02-23;11(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of Different Parameters of In Vitro Static Tensile Strain on Human Periodontal Ligament Cells Simulating the Tension Side of Orthodontic Tooth Movement
Authors: C Sun, M Janjic Ran, M Folwaczny, T Stocker, S Otto, A Wichelhaus, U Baumert
International Journal of Molecular Sciences, 2022-01-28;23(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-inflammatory role of GM1 and other gangliosides on microglia
Authors: D Galleguill, Q Wang, N Steinberg, A Zaidi, G Shrivastav, K Dhami, GC Daskhan, EN Schmidt, Z Dworsky-Fr, F Giuliani, M Churchward, C Power, K Todd, A Taylor, MS Macauley, S Sipione
Journal of Neuroinflammation, 2022-01-06;19(1):9.
Species: Rat
Sample Types: Cell Culture Supernates
-
Skin Substitute Preparation Method Induces Immunomodulatory Changes in Co-Incubated Cells through Collagen Modification
Authors: J Holl, C Pawlukiani, J Corton Rui, D Groth, K Grubczak, HR Hady, J Dadan, J Reszec, S Czaban, C Kowalewski, M Moniuszko, A Eljaszewic
Pharmaceutics, 2021-12-15;13(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
COVID-19 Modulates Inflammatory and Renal Markers That May Predict Hospital Outcomes among African American Males
Authors: W Fonseca, N Asai, K Yagi, CA Malinczak, G Savickas, CC Johnson, S Murray, EM Zoratti, NW Lukacs, J Li, CF Schuler Iv
Viruses, 2021-12-02;13(12):.
Species: Human
Sample Types: Whole Blood
-
Nanovibrational stimulation inhibits osteoclastogenesis and enhances osteogenesis in co-cultures
Authors: JW Kennedy, PM Tsimbouri, P Campsie, S Sood, PG Childs, S Reid, PS Young, DRM Meek, CS Goodyear, MJ Dalby
Scientific Reports, 2021-11-23;11(1):22741.
Species: Human
Sample Types: Cell Culture Supernates
-
Inflammasome-mediated GSDMD activation facilitates escape of Candida albicans from macrophages
Authors: X Ding, H Kambara, R Guo, A Kanneganti, M Acosta-Zal, J Li, F Liu, T Bei, W Qi, X Xie, W Han, N Liu, C Zhang, X Zhang, H Yu, L Zhao, F Ma, JR Köhler, HR Luo
Nature Communications, 2021-11-18;12(1):6699.
Species: Human
Sample Types: Cell Culture Supernates
-
Metallothionein 3-Zinc Axis Suppresses Caspase-11 Inflammasome Activation and Impairs Antibacterial Immunity
Authors: Debabrata Chowdhury, Jason C. Gardner, Abhijit Satpati, Suba Nookala, Santhosh Mukundan, Aleksey Porollo et al.
Frontiers in Immunology
Species: Human
Sample Types: Cell Culture Supernates
-
Mitochondrial respiration contributes to the interferon gamma response in antigen-presenting cells
Authors: MC Kiritsy, K McCann, D Mott, SM Holland, SM Behar, CM Sassetti, AJ Olive
Elife, 2021-11-02;10(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
The Globular C1q Receptor Is Required for Epidermal Growth Factor Receptor Signaling during Candida albicans Infection
Authors: QT Phan, J Lin, NV Solis, M Eng, M Swidergall, F Wang, S Li, SL Gaffen, TF Chou, SG Filler
MBio, 2021-11-02;0(0):e0271621.
Species: Human
Sample Types: Cell Culture Supernates
-
A prospective cohort study providing insights for markers of adverse pregnancy outcome in older mothers
Authors: SC Lean, RL Jones, SA Roberts, AEP Heazell
BMC pregnancy and childbirth, 2021-10-20;21(1):706.
Species: Human
Sample Types: Serum
-
Cigarette Smoke Promotes Interleukin-8 Production in Alveolar Macrophages Through the Reactive Oxygen Species/Stromal Interaction Molecule 1/Ca2+ Axis
Authors: X Zhu, Y Zhan, Y Gu, Q Huang, T Wang, Z Deng, J Xie
Frontiers in Physiology, 2021-10-08;12(0):733650.
Species: Human
Sample Types: Cell Culture Supernates
-
Parabens disrupt non-canonical inflammasome activation
Authors: H Ahn, H Lee, G Lee, J Park, HW Sung, E Lee, GS Lee
International immunopharmacology, 2021-09-30;101(0):108196.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-6 promotes drug resistance through formation of polyploid giant cancer cells and stromal fibroblast reprogramming
Authors: N Niu, J Yao, RC Bast, AK Sood, J Liu
Oncogenesis, 2021-09-29;10(9):65.
Species: Human
Sample Types: Cell Culture Supernates
-
The Restorative Effect of Human Amniotic Fluid Stem Cells on Spinal Cord Injury
Authors: M Lale Ataei, M Karimipour, P Shahabi, R Pashaei-As, E Ebrahimie, M Pashaiasl
Cells, 2021-09-28;10(10):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Protein-Bound Uremic Toxins Induce Reactive Oxygen Species-Dependent and Inflammasome-Mediated IL-1beta Production in Kidney Proximal Tubule Cells
Authors: M Mihajlovic, MM Krebber, Y Yang, S Ahmed, V Lozovanu, D Andreeva, MC Verhaar, R Masereeuw
Biomedicines, 2021-09-26;9(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
The influence of the gut microbiome on BCG-induced trained immunity
Authors: M Stražar, VP Mourits, VACM Koeken, LCJ de Bree, SJCFM Moorlag, LAB Joosten, R van Crevel, H Vlamakis, MG Netea, RJ Xavier
Genome Biology, 2021-09-22;22(1):275.
Species: Human
Sample Types: Cell Culture Supernates
-
Proteopathic tau primes and activates interleukin-1beta via myeloid-cell-specific MyD88- and NLRP3-ASC-inflammasome pathway
Authors: S Jiang, NM Maphis, J Binder, D Chisholm, L Weston, W Duran, C Peterson, A Zimmerman, MA Mandell, SD Jett, E Bigio, C Geula, N Mellios, JP Weick, GA Rosenberg, E Latz, MT Heneka, K Bhaskar
Cell Reports, 2021-09-21;36(12):109720.
Species: Human
Sample Types: Tissue Homogenates
-
Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling
Authors: J Hu, C Wang, X Huang, S Yi, S Pan, Y Zhang, G Yuan, Q Cao, X Ye, H Li
Cell Reports, 2021-09-21;36(12):109726.
Species: Human
Sample Types: Cell Culture Supernates
-
Functional role of galectin-9 in directing human innate immune reactions to Gram-negative bacteria and T cell apoptosis
Authors: S Schlichtne, NH Meyer, IM Yasinska, N Aliu, SM Berger, BF Gibbs, E Fasler-Kan, VV Sumbayev
International immunopharmacology, 2021-09-17;100(0):108155.
Species: Human
Sample Types: Cell Culture Supernates
-
Differential Regulation of Circulating Soluble Receptor for Advanced Glycation End Products (sRAGEs) and Its Ligands S100A8/A9 Four Weeks Post an Exercise Intervention in a Cohort of Young Army Recruits
Authors: IA Drosatos, JN Tsoporis, S Izhar, S Gupta, G Tsirebolos, E Sakadakis, AS Triantafyl, A Rigopoulos, D Rigopoulos, LS Rallidis, I Rizos, TG Parker
Biomolecules, 2021-09-13;11(9):.
Species: Human
Sample Types: Plasma
-
In Vitro Priming of Human T Cells by Dendritic Cells Provides a Screening Tool for Candidate Vaccines for Burkholderia pseudomallei
Authors: D Reddi, L Durant, D Bernardo, A Noble, NR English, P Hendy, GC Clark, JL Prior, ED Williamson, SC Knight
Vaccines, 2021-08-22;9(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-1beta augments TGF-beta inducing epithelial-mesenchymal transition of epithelial cells and associates with poor pulmonary function improvement in neutrophilic asthmatics
Authors: S Zhang, Y Fan, L Qin, X Fang, C Zhang, J Yue, W Bai, G Wang, Z Chen, H Renz, C Skevaki, X Liu, M Xie
Respiratory Research, 2021-08-03;22(1):216.
Species: Human
Sample Types: Sputum
-
Kras-driven intratumoral heterogeneity triggers infiltration of M2 polarized macrophages via the circHIPK3/PTK2 immunosuppressive circuit
Authors: T Katopodi, S Petanidis, K Domvri, P Zarogoulid, D Anestakis, C Charalampi, D Tsavlis, C Bai, H Huang, L Freitag, W Hohenforst, D Matthaios, K Porpodis
Scientific Reports, 2021-07-29;11(1):15455.
Species: Human
Sample Types: Cell Culture Supernates
-
"Plurethosome" as Vesicular System for Cutaneous Administration of Mangiferin: Formulative Study and 3D Skin Tissue Evaluation
Authors: M Sguizzato, F Ferrara, P Mariani, A Pepe, R Cortesi, N Huang, F Simelière, P Boldrini, A Baldissero, G Valacchi, E Esposito
Pharmaceutics, 2021-07-23;13(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Structural Deformation of MTX Induced by Nanodrug Conjugation Dictate Intracellular Drug Transport and Drug Efficacy
Authors: JY Park, JS Hyun, JG Jee, SJ Park, D Khang
International Journal of Nanomedicine, 2021-07-22;16(0):4943-4957.
Species: Human
Sample Types: Cell Culture Supernates
-
Topical inflammasome inhibition with disulfiram prevents irritant contact dermatitis
Authors: H Bonnekoh, C Vera, A Abad-Perez, S Radetzki, M Neuenschwa, E Specker, NA Mahnke, S Frischbutt, E Latz, M Nazaré, JV Kries, M Maurer, J Scheffel, K Krause
Clinical and translational allergy, 2021-07-22;11(5):e12045.
Species: Human
Sample Types: Tissue Homogenates
-
Synthetic Material Abdominal Swabs Reduce Activation of Platelets and Leukocytes Compared to Cotton Materials
Authors: K Gerling, LM Herrmann, C Salewski, M Wolf, P Müllerbade, D Siegel-Axe, HP Wendel, C Schlensak, M Avci-Adali, S Stoppelkam
Biomolecules, 2021-07-13;11(7):.
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of moderate wine consumption on cytokine secretion by peripheral blood mononuclear cells: A randomized clinical study in coronary heart disease patients
Authors: E Fragopoulo, C Argyrou, M Detopoulou, S Tsitsou, S Seremeti, M Yannakouli, S Antonopoul, G Kolovou, P Kalogeropo
Cytokine, 2021-07-08;146(0):155629.
Species: Human
Sample Types: Cell Culture Supernates
-
Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial
Authors: V Mocanu, Z Zhang, EC Deehan, DH Kao, N Hotte, S Karmali, DW Birch, KK Samarasing, J Walter, KL Madsen
Nature Medicine, 2021-07-05;27(7):1272-1279.
Species: Human
Sample Types: Serum
-
Aggregated Tau-PHF6 (VQIVYK) Potentiates NLRP3 Inflammasome Expression and Autophagy in Human Microglial Cells
Authors: C Panda, C Voelz, P Habib, C Mevissen, T Pufe, C Beyer, S Gupta, A Slowik
Cells, 2021-06-30;10(7):.
Species: Human
Sample Types: Cell Culture Supernates
-
An inverted in vitro triple culture model of the healthy and inflamed intestine: Adverse effects of polyethylene particles
Authors: M Busch, AAM Kämpfer, RPF Schins
Chemosphere, 2021-06-28;284(0):131345.
Species: Human
Sample Types: Cell Culture Supernates
-
Timing of Tributyrin Supplementation Differentially Modulates Gastrointestinal Inflammation and Gut Microbial Recolonization Following Murine Ileocecal Resection
Authors: V Mocanu, H Park, J Dang, N Hotte, A Thiesen, M Laffin, H Wang, D Birch, K Madsen
Nutrients, 2021-06-17;13(6):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Identification of monoclonal antibodies against human renal glomerular endothelial cells in lupus nephritis that induce endothelial interferon-alpha production
Authors: YC Hu, IJ Tsai, HY Hsu, BL Chiang, YH Yang
Arthritis Research & Therapy, 2021-06-16;23(1):171.
Species: Human
Sample Types: Cell Culture Supernates
-
Lipopolysaccharide stimulation test on cultured PBMCs assists the discrimination of cryopyrin-associated periodic syndrome from systemic juvenile idiopathic arthritis
Authors: CY Wu, WL Fan, YM Chiu, HY Yang, WI Lee, JL Huang
Scientific Reports, 2021-06-07;11(1):11903.
Species: Human
Sample Types: Cell Culture Supernates
-
Psychological symptoms and salivary inflammatory biomarkers in patients with dentofacial deformities: a case-control study
Authors: MCC Volkweis, GW Neculqueo, RDS Freitas, APA Dagnino, GG Fritscher, TQ Irigaray, MM Campos
Scientific Reports, 2021-05-26;11(1):11083.
Species: Human
Sample Types: Saliva
-
HMGB1 downregulation in retinal pigment epithelial cells protects against diabetic retinopathy through the autophagy-lysosome pathway
Authors: L Feng, L Liang, S Zhang, J Yang, Y Yue, X Zhang
Autophagy, 2021-05-24;0(0):1-20.
Species: Rat
Sample Types: Serum
-
Inflammatory responses to metal oxide ceramic nanopowders
Authors: S Jamieson, A Mawdesley, D Deehan, J Kirby, J Holland, A Tyson-Capp
Scientific Reports, 2021-05-18;11(1):10531.
Species: Human
Sample Types: Cell Culture Supernates
-
Attenuation of High Glucose-Induced Damage in RPE Cells through p38 MAPK Signaling Pathway Inhibition
Authors: G Maugeri, C Bucolo, F Drago, S Rossi, M Di Rosa, R Imbesi, V D'Agata, S Giunta
Frontiers in Pharmacology, 2021-05-07;12(0):684680.
Species: Human
Sample Types: Cell Culture Supernates
-
Decreased microRNA-155 in Behcet's disease leads to defective control of autophagy thereby stimulating excessive proinflammatory cytokine production
Authors: L Liang, Q Zhou, L Feng
Arthritis Research & Therapy, 2021-05-06;23(1):135.
Species: Human
Sample Types: Cell Culture Supernates
-
Plasma markers in pulmonary hypertension subgroups correlate with patient survival
Authors: T Koudstaal, D van Uden, JAC van Hulst, P Heukels, IM Bergen, LW Geenen, VJM Baggen, AE van den Bo, LM van den To, PP Chandoesin, M Kool, E Boersma, RW Hendriks, KA Boomars
Respiratory Research, 2021-05-04;22(1):137.
Species: Human
Sample Types: Plasma
-
Differential recognition of HIV-stimulated IL-1&beta and IL-18 secretion through NLR and NAIP signalling in monocyte-derived macrophages
Authors: K Triantafil, CJK Ward, M Czubala, RG Ferris, E Koppe, C Haffner, V Piguet, VK Patel, H Amrine-Mad, L Modis, SL Masters, M Triantafil
PloS Pathogens, 2021-04-16;17(4):e1009417.
Species: Human
Sample Types: Cell Culture Supernates
-
IRF1 governs the differential interferon-stimulated gene responses in human monocytes and macrophages by regulating chromatin accessibility
Authors: R Song, Y Gao, I Dozmorov, V Malladi, I Saha, MM McDaniel, S Parameswar, C Liang, C Arana, B Zhang, B Wakeland, J Zhou, MT Weirauch, LC Kottyan, EK Wakeland, C Pasare
Cell Reports, 2021-03-23;34(12):108891.
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophil extracellular trap clearance by synovial macrophages in gout
Authors: JH Jeong, SJ Choi, SM Ahn, JS Oh, YG Kim, CK Lee, B Yoo, S Hong
Arthritis Research & Therapy, 2021-03-19;23(1):88.
Species: Human
Sample Types: Cell Culture Supernates
-
Skin Sensitization Evaluation of Carbon-Based Graphene Nanoplatelets
Authors: SH Kim, SH Hong, JH Lee, DH Lee, K Jung, JY Yang, HS Shin, J Lee, J Jeong, JH Oh
Toxics, 2021-03-17;9(3):.
Species: Human
Sample Types: cell culture supernatant
-
Associations between salivary cytokines and periodontal and microbiological parameters in orthodontic patients
Authors: Y Chen, WK Wong, JC Seneviratn, S Huang, C McGrath, U Hagg
Medicine, 2021-03-12;100(10):e24924.
Species: Human
Sample Types: Saliva
-
Itaconate confers tolerance to late NLRP3 inflammasome activation
Authors: M Bambouskov, L Potuckova, T Paulenda, M Kerndl, DA Mogilenko, K Lizotte, A Swain, S Hayes, RD Sheldon, H Kim, U Kapadnis, AE Ellis, C Isaguirre, S Burdess, A Laha, GK Amarasingh, V Chubukov, TP Roddy, MS Diamond, RG Jones, DM Simons, MN Artyomov
Cell Reports, 2021-03-09;34(10):108756.
Species: Human
Sample Types: Cell Culture Supernates
-
Proteomics reveals distinct mechanisms regulating the release of cytokines and alarmins during pyroptosis
Authors: K Phulphagar, LI Kühn, S Ebner, A Frauenstei, JJ Swietlik, J Rieckmann, F Meissner
Cell Reports, 2021-03-09;34(10):108826.
Species: Human
Sample Types: Cell Culture Supernates
-
Impairment in inflammasome signaling by the chronic Pseudomonas aeruginosa isolates from cystic fibrosis patients results in an increase in inflammatory response
Authors: MS Phuong, RE Hernandez, DJ Wolter, LR Hoffman, S Sad
Cell Death & Disease, 2021-03-04;12(3):241.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-1&alpha Is a Critical Mediator of the Response of Human Bronchial Fibroblasts to Eosinophilic Inflammation
Authors: K Bernau, JP Leet, H Floerke, EM Bruhn, AL Noll, IS McDermott, S Esnault, NN Jarjour, N Sandbo
Cells, 2021-03-02;10(3):.
Species: Human
Sample Types: BALF
-
Fructose reprogrammes glutamine-dependent oxidative metabolism to support LPS-induced inflammation
Authors: N Jones, J Blagih, F Zani, A Rees, DG Hill, BJ Jenkins, CJ Bull, D Moreira, AIM Bantan, JG Cronin, D Avancini, GW Jones, DK Finlay, KH Vousden, EE Vincent, CA Thornton
Nature Communications, 2021-02-22;12(1):1209.
Species: Human
Sample Types: Cell Culture Supernates
-
Development and evaluation of inhalable composite niclosamide-lysozyme particles: A broad-spectrum, patient-adaptable treatment for coronavirus infections and sequalae
Authors: AD Brunaugh, H Seo, Z Warnken, L Ding, SH Seo, HDC Smyth
PLoS ONE, 2021-02-11;16(2):e0246803.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-inflammatory activity of soluble chito-oligosaccharides (CHOS) on VitD3-induced human THP-1 monocytes
Authors: P Jitprasert, M Khamphio, P Petsrichua, VGH Eijsink, W Poolsri, C Muanprasat, K Rangnoi, M Yamabhai
PLoS ONE, 2021-02-03;16(2):e0246381.
Species: Human
Sample Types: Cell Culture Supernates
-
Propionibacterium acnes Accelerates Intervertebral Disc Degeneration by Inducing Pyroptosis of Nucleus Pulposus Cells via the ROS-NLRP3 Pathway
Authors: G Tang, X Han, Z Lin, H Qian, B Chen, C Zhou, Y Chen, W Jiang
Oxidative Medicine and Cellular Longevity, 2021-02-01;2021(0):4657014.
Species: Human
Sample Types: Cell Culture Supernates
-
Polyphenol-rich extract and fractions of Terminalia phaeocarpa Eichler possess hypoglycemic effect, reduce the release of cytokines, and inhibit lipase, &alpha-glucosidase, and &alpha-amilase enzymes
Authors: JHS Gomes, UC Mbiakop, RL Oliveira, JR Stehmann, RM Pádua, SF Cortes, FC Braga
Journal of ethnopharmacology, 2021-01-27;271(0):113847.
Species: Human
Sample Types: Cell Culture Supernates
-
Secreted KIAA1199 promotes the progression of rheumatoid arthritis by mediating hyaluronic acid degradation in an ANXA1-dependent manner
Authors: W Zhang, G Yin, H Zhao, H Ling, Z Xie, C Xiao, Y Chen, Y Lin, T Jiang, S Jin, J Wang, X Yang
Cell Death & Disease, 2021-01-20;12(1):102.
Species: Human
Sample Types: Serum
-
Low levels of PCSK9 are associated with remission in patients with rheumatoid arthritis treated with anti-TNF-&alpha: potential underlying mechanisms
Authors: J Frostegård, S Ahmed, I Hafström, S Ajeganova, M Rahman
Arthritis Research & Therapy, 2021-01-19;23(1):32.
Species: Human
Sample Types: Cell Culture Supernates
-
Sintered S53P4 bioactive glass scaffolds have anti-inflammatory properties and stimulate osteogenesis in vitro
Authors: R Björkenhei, E Jämsen, E Eriksson, P Uppstu, L Aalto-Setä, L Hupa, KK Eklund, M Ainola, NC Lindfors, J Pajarinen
European cells & materials, 2021-01-03;41(0):15-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Iron Oxide Particles Alter Bacterial Uptake and the LPS-Induced Inflammatory Response in Macrophages
Authors: LJ Williams, SG Tristram, GR Zosky
International Journal of Environmental Research and Public Health, 2020-12-28;18(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
A new cytokine-based dynamic stratification during induction is highly predictive of survivals in acute myeloid leukemia
Authors: P Peterlin, J Gaschet, T Guillaume, A Garnier, M Eveillard, A Le Bourgeo, M Cherel, C Debord, Y Le Bris, O Theisen, C Godon, B Mahé, V Dubruille, S Wuilleme, C Touzeau, T Gastinne, N Blin, A Lok, B Tessoulin, S Le Gouill, P Moreau, MC Béné, P Chevallier
Cancer Medicine, 2020-12-25;0(0):.
Species: Human
Sample Types: Serum
-
SARS-CoV-2-specific IgA and limited inflammatory cytokines are present in the stool of select patients with acute COVID-19
Authors: GJ Britton, A Chen-Liaw, F Cossarini, AE Livanos, MP Spindler, T Plitt, J Eggers, I Mogno, A Gonzalez-R, S Siu, M Tankelevic, L Grinspan, RE Dixon, D Jha, G Martinez-D, F Amanat, DA Hoagland, B tenOever, MC Dubinsky, M Merad, H van Bakel, F Krammer, G Bongers, S Mehandru, JJ Faith
medRxiv : the preprint server for health sciences, 2020-12-09;0(0):.
Species: Human
Sample Types: Fecal Supernates
-
Clinical Response to the CD95-Ligand Inhibitor Asunercept Is Defined by a Pro-Inflammatory Serum Cytokine Profile
Authors: A Radujkovic, T Boch, F Nolte, D Nowak, C Kunz, A Gieffers, C Müller-Tid, P Dreger, WK Hofmann, T Luft
Cancers, 2020-12-08;12(12):.
Species: Human
Sample Types: Serum
-
Oligodendroglial glycolytic stress triggers inflammasome activation and neuropathology in Alzheimer's disease
Authors: X Zhang, R Wang, D Hu, X Sun, H Fujioka, K Lundberg, ER Chan, Q Wang, R Xu, ME Flanagan, AA Pieper, X Qi
Science Advances, 2020-12-04;6(49):.
Species: Human
Sample Types: Cell Culture Supernates
-
Patient-derived and artificial ascites have minor effects on MeT-5A mesothelial cells and do not facilitate ovarian cancer cell adhesion
Authors: M Estermann, YL Huang, D Septiadi, D Ritz, CY Liang, F Jacob, B Drasler, A Petri-Fink, V Heinzelman, B Rothen-Rut
PLoS ONE, 2020-12-03;15(12):e0241500.
Species: Human
Sample Types: Abdominal Fluid
-
Monocytes differentiated into macrophages and dendritic cells in the presence of human IFN-&lambda3 or IFN-&lambda4 show distinct phenotypes
Authors: M De, A Bhushan, S Chinnaswam
J Leukoc Biol, 2020-11-17;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils
Authors: SJCFM Moorlag, YA Rodriguez-, J Gillard, S Fanucchi, K Theunissen, B Novakovic, CM de Bont, Y Negishi, ET Fok, L Kalafati, P Verginis, VP Mourits, VACM Koeken, LCJ de Bree, GJM Pruijn, C Fenwick, R van Crevel, LAB Joosten, I Joosten, H Koenen, MM Mhlanga, DA Diavatopou, T Chavakis, MG Netea
Cell Rep, 2020-11-17;33(7):108387.
Species: Human
Sample Types: Cell Culture Supernates
-
ROCK inhibition modulates the senescence-associated secretory phenotype (SASP) in oral keratinocytes
Authors: S Niklander, D Bandaru, DW Lambert, KD Hunter
FEBS Open Bio, 2020-11-06;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Nearly free surface silanols are the critical molecular moieties that initiate the toxicity of silica particles
Authors: C Pavan, R Santalucia, R Leinardi, M Fabbiani, Y Yakoub, F Uwambayine, P Ugliengo, M Tomatis, G Martra, F Turci, D Lison, B Fubini
Proc Natl Acad Sci U S A, 2020-10-23;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Who Is Afraid of CRP? Elevated Preoperative CRP Levels Might Attenuate the Increase in Inflammatory Parameters in Response to Lung Cancer Surgery
Authors: MM Meyer, L Brandenbur, H Hudel, A Agné, W Padberg, A Erdogan, H Nef, AL Amati, O Dörr, B Witte, V Grau
J Clin Med, 2020-10-18;9(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
An observational study of innate immune responses in patients with acute appendicitis
Authors: T Peeters, S Martens, V D'Onofrio, MHT Stappers, JCH van der Hi, B Houben, R Achten, LAB Joosten, IC Gyssens
Sci Rep, 2020-10-15;10(1):17352.
Species: Human
Sample Types: Cell Culture Supernates
-
Interferon-&gamma signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells
Authors: V Panagiotak, D Ivanyuk, S De Cicco, W Haq, A Arsi?, C Yu, D Messelodi, M Oldrati, DC Schöndorf, MJ Perez, RP Cassatella, M Jakobi, N Schneiderh, T Gasser, I Niki?-Spie, M Deleidi
Nat Commun, 2020-10-14;11(1):5163.
Species: Human
Sample Types: Whole Cells
-
Staphylococcus aureus-Derived &alpha-Hemolysin Evokes Generation of Specialized Pro-resolving Mediators Promoting Inflammation Resolution
Authors: PM Jordan, J Gerstmeier, S Pace, R Bilancia, Z Rao, F Börner, L Miek, Ó Gutiérrez-, V Arakandy, A Rossi, A Ialenti, C González-E, B Löffler, L Tuchscherr, CN Serhan, O Werz
Cell Rep, 2020-10-13;33(2):108247.
Species: Mouse
Sample Types: Cell Culture Supernates
-
NLRP3 inflammasome function and pyroptotic cell death in human placental Hofbauer cells
Authors: VM Abrahams, Z Tang, G Mor, S Guller
J Reprod Immunol, 2020-10-03;142(0):103214.
Species: Human
Sample Types: Cell Culture Supernates
-
Modeling HIV-1 neuropathogenesis using three-dimensional human brain organoids (hBORGs) with HIV-1 infected microglia
Authors: RS Dos Reis, S Sant, H Keeney, MCE Wagner, V Ayyavoo
Scientific Reports, 2020-09-16;10(1):15209.
Species: Human
Sample Types: Organoid
-
Oral Lactobacillus strains reduce cytotoxicity and cytokine release from peripheral blood mononuclear cells exposed to Aggregatibacter actinomycetemcomitans subtypes in vitro
Authors: N Pahumunto, A Basic, AK Östberg, R Teanpaisan, G Dahlen
BMC Microbiol., 2020-09-11;20(1):279.
Species: Human
Sample Types: Cell Culture Supernates
-
Amyloid Beta Peptide (A&beta1-42) Reverses the Cholinergic Control of Monocytic IL-1&beta Release
Authors: K Richter, R Ogiemwonyi, S Wilker, AI Chaveiro, A Agné, M Hecker, M Reichert, AL Amati, KD Schlüter, I Manzini, G Schmalzing, JM McIntosh, W Padberg, V Grau, A Hecker
J Clin Med, 2020-09-07;9(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Inflammasome Sensor NLRP1 Confers Acquired Drug Resistance to Temozolomide in Human Melanoma
Authors: Z Zhai, JM Samson, T Yamauchi, PK Vaddi, Y Matsumoto, CA Dinarello, D Ravindran, M Fujita
Cancers (Basel), 2020-09-04;12(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Gene expression network analyses during infection with virulent and avirulent Trypanosoma cruzi�strains unveil a role for fibroblasts in neutrophil recruitment and activation
Authors: AER Oliveira, MCA Pereira, AT Belew, LRP Ferreira, LMN Pereira, EGA Neves, MDCP Nunes, BA Burleigh, WO Dutra, NM El-Sayed, RT Gazzinelli, SMR Teixeira
PLoS Pathog., 2020-08-18;16(8):e1008781.
Species: Human
Sample Types: Cell Culture Supernates
-
Standardized ethanol extract of Tinospora crispa upregulates pro-inflammatory mediators release in LPS-primed U937 human macrophages through stimulation of MAPK, NF-kappaB and PI3K-Akt signaling networks
Authors: MA Haque, I Jantan, H Harikrishn, W Ahmad
BMC Complement Med Ther, 2020-08-06;20(1):245.
Species: Human
Sample Types: Cell Culture Supernates
-
Salivary Biomarkers of Oral Inflammation Are Associated With Cardiovascular Events and Death Among Kidney Transplant Patients
Authors: F Ortiz, KM Nylund, H Ruokonen, JH Meurman, J Furuholm, N Bostanci, T Sorsa
Transplant. Proc., 2020-08-04;0(0):.
Species: Human
Sample Types: Saliva
-
Cigarette smoke and extracellular Hsp70 induce secretion of ATP and differential activation of NLRP3 inflammasome in monocytic and bronchial epithelial cells
Authors: L Rumora, A Somborac-B, I Hlap?i?, A Hulina-Tom, MG Rajkovi?
Cytokine, 2020-07-28;135(0):155220.
Species: Human
Sample Types: Cell Culture Supernates
-
Scrodentoids H and I, a Pair of Natural Epimerides from Scrophularia dentata, Inhibit Inflammation through JNK-STAT3 Axis in THP-1 Cells
Authors: G Mao, L Sun, J Xu, Y Li, C Dunzhu, L Zhang, F Qian
Evid Based Complement Alternat Med, 2020-07-27;2020(0):1842347.
Species: Human
Sample Types: Cell Culture Supernates
-
Pro-inflammatory properties of H-ferritin on human macrophages, ex vivo and in vitro observations
Authors: P Ruscitti, P Di Benedet, O Berardicur, N Panzera, N Grazia, AR Lizzi, P Cipriani, Y Shoenfeld, R Giacomelli
Sci Rep, 2020-07-22;10(1):12232.
Species: Human
Sample Types: Cell Culture Supernates
-
MG53 suppresses interferon-&beta and inflammation via regulation of ryanodine receptor-mediated intracellular calcium signaling
Authors: M Sermershei, AD Kenney, PH Lin, TM McMichael, C Cai, K Gumpper, TMA Adesanya, H Li, X Zhou, KH Park, JS Yount, J Ma
Nat Commun, 2020-07-17;11(1):3624.
Species: Human
Sample Types: Cell Culture Supernates
-
Modified citrus pectins by UV/H2O2 oxidation at acidic and basic conditions: Structures and in vitro anti-inflammatory, anti-proliferative activities
Authors: J Cao, J Yang, Z Wang, M Lu, K Yue
Carbohydr Polym, 2020-07-09;247(0):116742.
Species: Human
Sample Types: Cell Culture Supernates
-
Protective effect of bromfenac sodium on femtosecond laser?assisted cataract surgery via modulating cyclooxygenase?2 expression
Authors: L Lu, J Zhao, J Wang, Y Qin, J Zhang
Mol Med Rep, 2020-07-08;22(3):2433-2441.
Species: Human
Sample Types: Cell Culture Supernates
-
Loss of function of the mitochondrial peptidase PITRM1 induces proteotoxic stress and Alzheimer's disease-like pathology in human cerebral organoids
Authors: MJ Pérez, D Ivanyuk, V Panagiotak, G Di Napoli, S Kalb, D Brunetti, R Al-Shaana, SA Kaeser, SA Fraschka, M Jucker, M Zeviani, C Viscomi, M Deleidi
Mol. Psychiatry, 2020-07-07;0(0):.
Species: Human
Sample Types: Cell Culture Lysates
-
Reprogramming of profibrotic macrophages for treatment of bleomycin-induced pulmonary fibrosis
Authors: F Zhang, EA Ayaub, B Wang, E Puchulu-Ca, YH Li, SU Hettiarach, SD Lindeman, Q Luo, S Rout, M Srinivasar, A Cox, K Tsoyi, C Nickerson-, IO Rosas, PS Low
EMBO Mol Med, 2020-06-29;0(0):e12034.
Species: Human
Sample Types: Cell Culture Supernates
-
Vibration synergistically enhances IL-1beta and TNF-alpha in compressed human periodontal ligament cells in the frequency-dependent manner
Authors: S Benjakul, B Unat, P Thammanich, C Leethanaku
J Oral Biol Craniofac Res, 2020-06-22;10(4):412-416.
Species: Human
Sample Types: Cell Culture Supernates
-
Sex Differences in Proatherogenic Cytokine Levels
Authors: S Bernardi, B Toffoli, F Tonon, M Francica, E Campagnolo, T Ferretti, S Comar, F Giudici, E Stenner, B Fabris
Int J Mol Sci, 2020-05-29;21(11):.
Species: Human
Sample Types:
-
PB1-F2 protein of highly pathogenic influenza A (H7N9) virus selectively suppresses RNA-induced NLRP3 inflammasome activation through inhibition of MAVS-NLRP3 interaction
Authors: PH Cheung, ZW Ye, TT Lee, H Chen, CP Chan, DY Jin
J. Leukoc. Biol., 2020-05-09;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
N-GSDMD trafficking to neutrophil organelles facilitates IL-1&beta release independently of plasma membrane pores and pyroptosis
Authors: M Karmakar, M Minns, EN Greenberg, J Diaz-Apont, K Pestonjama, JL Johnson, JK Rathkey, DW Abbott, K Wang, F Shao, SD Catz, GR Dubyak, E Pearlman
Nat Commun, 2020-05-05;11(1):2212.
Species: Human
Sample Types: Cell Culture Supernates
-
Differentially Expressed Genes of Natural Killer Cells Can Distinguish Rheumatoid Arthritis Patients from Healthy Controls
Authors: NM Elemam, MY Hachim, S Hannawi, AA Maghazachi
Genes (Basel), 2020-04-30;11(5):.
Species: Human
Sample Types: Plasma
-
Intracellular Insulin-like growth factor binding protein 2 (IGFBP2) contributes to the senescence of keratinocytes in psoriasis by stabilizing cytoplasmic p21
Authors: L Mercurio, D Lulli, F Mascia, E Dellambra, C Scarponi, M Morelli, C Valente, ML Carbone, S Pallotta, G Girolomoni, C Albanesi, S Pastore, S Madonna
Aging (Albany NY), 2020-04-17;12(8):6823-6851.
Species: Human
Sample Types: Cell Culture Supernates
-
Cerebrospinal fluid of chronic osteoarthritic patients induced interleukin-6 release in human glial cell-line T98G
Authors: W Liu, C Li, FCK Tan, HJ Neo, YH Chan, CM Low, TL Lee
BMC Anesthesiol, 2020-03-25;20(1):69.
Species: Human
Sample Types: Cell Culture Supernates
-
Activation of Human &gamma&delta T Cells: Modulation by Toll-Like Receptor 8 Ligands and Role of Monocytes
Authors: R Serrano, D Wesch, D Kabelitz
Cells, 2020-03-13;9(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Herpesvirus-infected Hofbauer cells activate endothelial cells through an IL-1 beta -dependent mechanism
Authors: Paul Hendrix, Zhonghua Tang, Michelle Silasi, Karen E. Racicot, Gil Mor, Vikki M. Abrahams et al.
Placenta
Species: Human
Sample Types: Cell Culture Supernates
-
Recombinant Alkaline Phosphatase Prevents Acute on Chronic Liver Failure
Authors: C Engelmann, D Adebayo, M Oria, F De Chiara, S Novelli, A Habtesion, N Davies, F Andreola, R Jalan
Sci Rep, 2020-01-15;10(1):389.
Species: Human
Sample Types: Cell Culture Supernates
-
Pancreatic cancer-derived small extracellular vesical Ezrin regulates macrophage polarization and promotes metastasis
Authors: YT Chang, HY Peng, CM Hu, SC Huang, SC Tien, YM Jeng
Am J Cancer Res, 2020-01-01;10(1):12-37.
Species: Human
Sample Types: Cell Culture Supernates
-
The non-saponin fraction of Korean Red Ginseng (KGC05P0) decreases glucose uptake and transport in�vitro and modulates glucose production via down-regulation of the PI3K/AKT pathway in�vivo
Authors: SJ Park, D Lee, D Kim, M Lee, G In, ST Han, SW Kim, MH Lee, OK Kim, J Lee
J Ginseng Res, 2019-12-17;44(2):362-372.
Species: Human
Sample Types: Serum
-
Physiologic intestinal 18F-FDG uptake is associated with alteration of gut microbiota and proinflammatory cytokine levels in breast cancer
Authors: HJ Yoon, HN Kim, JI Bang, W Lim, BI Moon, NS Paik, BS Kim, HL Kim
Sci Rep, 2019-12-04;9(1):18273.
Species: Human
Sample Types: Serum
-
EASIX for prediction of survival in lower-risk myelodysplastic syndromes
Authors: A Merz, U Germing, G Kobbe, J Kaivers, A Jauch, A Radujkovic, M Hummel, A Benner, M Merz, P Dreger, T Luft
Blood Cancer J, 2019-11-11;9(11):85.
Species: Human
Sample Types: Plasma
-
Replication of Genome-Wide Association Analysis Identifies New Susceptibility Loci at Long Noncoding RNA Regions for Vogt-Koyanagi-Harada Disease
Authors: J Qi, L Du, J Deng, Y Qin, G Su, S Hou, M Lv, Q Zhang, A Kijlstra, P Yang
Invest. Ophthalmol. Vis. Sci., 2019-11-01;60(14):4820-4829.
Species: Human
Sample Types: Cell Culture Supernates
-
Disrupting prolonged sitting reduces IL-8 and lower leg swell in active young adults
Authors: S Dogra, M Wolf, MP Jeffrey, RCA Foley, H Logan-Spre, H Jones-Tagg, JM Green-John
BMC Sports Sci Med Rehabil, 2019-10-18;11(0):23.
Species: Human
Sample Types: Saliva
-
IL-37 is increased in brains of children with autism spectrum disorder and inhibits human microglia stimulated by neurotensin
Authors: I Tsilioni, AB Patel, H Pantazopou, S Berretta, P Conti, SE Leeman, TC Theoharide
Proc. Natl. Acad. Sci. U.S.A., 2019-10-07;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Glutathione Transferase Omega-1 Regulates NLRP3 Inflammasome Activation through NEK7 Deglutathionylation
Authors: MM Hughes, A Hooftman, S Angiari, P Tummala, Z Zaslona, MC Runtsch, AF McGettrick, CE Sutton, C Diskin, M Rooke, S Takahashi, S Sundararaj, MG Casarotto, JE Dahlstrom, EM Palsson-Mc, SC Corr, KHG Mills, RJS Preston, N Neamati, Y Xie, JB Baell, PG Board, LAJ O'Neill
Cell Rep, 2019-10-01;29(1):151-161.e5.
Species: Human
Sample Types: Cell Culture Supernates
-
Relative Contributions of Extracellular and Internalized Bacteria to Early Macrophage Proinflammatory Responses to Streptococcus pneumoniae
Authors: J Periselner, G Ercoli, T Pollard, S Chimalapat, E Camberlein, G Szylar, C Hyams, G Tomlinson, FC Petersen, RA Floto, M Noursadegh, JS Brown
MBio, 2019-09-24;10(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
β-Glucan-Induced Trained Immunity Protects against Leishmania braziliensis Infection: a Crucial Role for IL-32
Authors: JC Dos Santos, AM Barroso de, MV Teodoro Si, B Cirovic, LCJ de Bree, MSMA Damen, SJCFM Moorlag, RS Gomes, MM Helsen, M Oosting, ST Keating, A Schlitzer, MG Netea, F Ribeiro-Di, LAB Joosten
Cell Rep, 2019-09-03;28(10):2659-2672.e6.
Species: Human
Sample Types: Cell Culture Supernates
-
Inflammasome-Driven Interleukin-1α and�Interleukin-1? Production in Atherosclerotic Plaques Relates to Hyperlipidemia and Plaque Complexity
Authors: X Jiang, F Wang, Y Wang, A Gisterå, J Roy, G Paulsson-B, U Hedin, A Lerman, GK Hansson, J Herrmann, ZQ Yan
JACC Basic Transl Sci, 2019-06-24;4(3):304-317.
Species: Human
Sample Types: Tissue Culture Supernates
-
Cytokine Diversity in Human Peripheral Blood Eosinophils: Profound Variability of IL-16
Authors: M Ma, CM Percopo, DE Sturdevant, AC Sek, HD Komarow, HF Rosenberg
J. Immunol., 2019-06-10;0(0):.
Species: Human
Sample Types: Cell Lysates
-
Expression of SAA1, SAA2 and SAA4 genes in human primary monocytes and monocyte-derived macrophages
Authors: C Jumeau, F Awad, E Assrawi, L Cobret, P Duquesnoy, I Giurgea, D Valeyre, G Grateau, S Amselem, JF Bernaudin, SA Karabina
PLoS ONE, 2019-05-17;14(5):e0217005.
Species: Human
Sample Types: Cell Culture Supernates
-
Enteropathogenic Escherichia coli Stimulates Effector-Driven Rapid Caspase-4 Activation in Human Macrophages
Authors: PJ Goddard, J Sanchez-Ga, SL Slater, M Kalyan, D Ruano-Gall, O Marchès, LÁ Fernández, G Frankel, AR Shenoy
Cell Rep, 2019-04-23;27(4):1008-1017.e6.
Species: Human
Sample Types: Cell Culture Supernates
-
Human metapneumovirus activates NOD-like receptor protein 3 inflammasome via its small hydrophobic protein which plays a detrimental role during infection in mice
Authors: VB Lê, J Dubois, C Couture, MH Cavanagh, O Uyar, A Pizzorno, M Rosa-Calat, MÈ Hamelin, G Boivin
PLoS Pathog., 2019-04-09;15(4):e1007689.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of vimentin in modulating immune cell apoptosis and inflammatory responses in sepsis
Authors: L Su, P Pan, P Yan, Y Long, X Zhou, X Wang, R Zhou, B Wen, L Xie, D Liu
Sci Rep, 2019-04-05;9(1):5747.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-6 released from differentiating human beige adipocytes improves browning
Authors: Endre Kristóf, Ágnes Klusóczki, Roland Veress, Abhirup Shaw, Zsolt Sándor Combi, Klára Varga et al.
Experimental Cell Research
Species: Human
Sample Types: Cell Culture Supernates
-
Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis
Authors: C Wei, C Yang, S Wang, D Shi, C Zhang, X Lin, Q Liu, R Dou, B Xiong
Mol. Cancer, 2019-03-30;18(1):64.
Species: Human
Sample Types: Cell Culture Supernates
-
Impact of constitutional TET2 haploinsufficiency on molecular and clinical phenotype in humans
Authors: E Kaasinen, O Kuismin, K Rajamäki, H Ristolaine, M Aavikko, J Kondelin, S Saarinen, DG Berta, R Katainen, EAM Hirvonen, A Karhu, A Taira, T Tanskanen, A Alkodsi, M Taipale, E Morgunova, K Franssila, R Lehtonen, M Mäkinen, K Aittomäki, A Palotie, MI Kurki, O Pietiläine, M Hilpert, E Saarentaus, J Niinimäki, J Junttila, K Kaikkonen, P Vahteristo, RC Skoda, MRJ Seppänen, KK Eklund, J Taipale, O Kilpivaara, LA Aaltonen
Nat Commun, 2019-03-19;10(1):1252.
Species: Human
Sample Types: Cell Culture Supernates
-
In Vitro Induction of T Helper 17 Cells by Synergistic Activation of Human Monocyte-Derived Langerhans Cell-Like Cells with Bacterial Agonists
Authors: R Gramlich, E Aliahmadi, M Peiser
Int J Mol Sci, 2019-03-19;20(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
Engulfment, persistence and fate of Bdellovibrio bacteriovorus predators inside human phagocytic cells informs their future therapeutic potential
Authors: D Raghunatha, PM Radford, C Gell, D Negus, C Moore, R Till, PJ Tighe, SP Wheatley, L Martinez-P, RE Sockett, J Tyson
Sci Rep, 2019-03-12;9(1):4293.
Species: Human
Sample Types: Cell Culture Supernates
-
Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host
Authors: M Ahn, DE Anderson, Q Zhang, CW Tan, BL Lim, K Luko, M Wen, WN Chia, S Mani, LC Wang, JHJ Ng, RM Sobota, CA Dutertre, F Ginhoux, ZL Shi, AT Irving, LF Wang
Nat Microbiol, 2019-02-25;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Cervical cancer-instructed stromal fibroblasts enhance IL-23 expression in dendritic cells to support expansion of Th17 cells
Authors: B Walch-Rück, R Ströder, L Theobald, J Pahne-Zepp, S Hegde, YJ Kim, RM Bohle, I Juhasz-Bös, EF Solomayer, S Smola
Cancer Res., 2019-01-29;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Regulation of radiosensitivity by 4-methylumbelliferone via the suppression of interleukin-1 in fibrosarcoma cells
Authors: R Saga, K Hasegawa, K Murata, M Chiba, T Nakamura, K Okumura, E Tsuruga, Y Hosokawa
Oncol Lett, 2019-01-29;17(3):3555-3561.
Species: Human
Sample Types: Cell Culture Supernates
-
Phenotypic and functional stability of leukocytes from human peripheral blood samples: considerations for the design of immunological studies
Authors: A Navas, L Giraldo-Pa, MD Prieto, J Cabrera, MA Gómez
BMC Immunol., 2019-01-18;20(1):5.
Species: Human
Sample Types: Cell Culture Supernates
-
Regulation of hemolysin in uropathogenic Escherichia coli fine-tunes killing of human macrophages
Authors: AMV Murthy, MD Phan, KM Peters, NTK Nhu, RA Welch, GC Ulett, MA Schembri, MJ Sweet
Virulence, 2018-12-31;0(0):1-32.
Species: Human
Sample Types: Cell Culture Supernates
-
A Transient Pseudosenescent Secretome Promotes Tumor Growth after Antiangiogenic Therapy Withdrawal
Authors: M Mastri, A Tracz, CR Lee, M Dolan, K Attwood, JG Christense, S Liu, JML Ebos
Cell Rep, 2018-12-26;25(13):3706-3720.e8.
Species: Human
Sample Types: Cell Culture Supernates
-
Sulphamethazine derivatives as immunomodulating agents: New therapeutic strategies for inflammatory diseases
Authors: H Siddiqui, HM Haniffa, A Jabeen, AU -Rahman, MI Choudhary
PLoS ONE, 2018-12-19;13(12):e0208933.
Species: Human
Sample Types: Cell Culture Supernates
-
PDGF enhances the protective effect of adipose stem cell-derived extracellular vesicles in a model of acute hindlimb ischemia
Authors: T Lopatina, E Favaro, C Grange, M Cedrino, A Ranghino, S Occhipinti, S Fallo, F Buffolo, DA Gaykalova, MM Zanone, R Romagnoli, G Camussi
Sci Rep, 2018-12-04;8(1):17458.
Species: Human
Sample Types: Cell Culture Supernates
-
A central role for P2X7 receptors in human microglia
Authors: L Janks, CVR Sharma, TM Egan
J Neuroinflammation, 2018-11-21;15(1):325.
Species: Human
Sample Types: Cell Culture Supernates
-
A low-gluten diet induces changes in the intestinal microbiome of healthy Danish adults
Authors: LBS Hansen, HM Roager, NB Søndertoft, RJ Gøbel, M Kristensen, M Vallès-Col, S Vieira-Sil, S Ibrügger, MV Lind, RB Mærkedahl, MI Bahl, ML Madsen, J Havelund, G Falony, I Tetens, T Nielsen, KH Allin, HL Frandsen, B Hartmann, JJ Holst, MH Sparholt, J Holck, A Blennow, JM Moll, AS Meyer, C Hoppe, JH Poulsen, V Carvalho, D Sagnelli, MD Dalgaard, AF Christense, MC Lydolph, AB Ross, S Villas-Bôa, S Brix, T Sicheritz-, K Buschard, A Linneberg, JJ Rumessen, CT Ekstrøm, C Ritz, K Kristianse, HB Nielsen, H Vestergaar, NJ Færgeman, J Raes, H Frøkiær, T Hansen, L Lauritzen, R Gupta, TR Licht, O Pedersen
Nat Commun, 2018-11-13;9(1):4630.
Species: Human
Sample Types: Cell Culture Supernates
-
Combining Calcium Phosphates with Polysaccharides: A Bone-Inspired Material Modulating Monocyte/Macrophage Early Inflammatory Response
Authors: H Rammal, C Bour, M Dubus, L Entz, L Aubert, SC Gangloff, S Audonnet, NB Bercu, F Boulmedais, C Mauprivez, H Kerdjoudj
Int J Mol Sci, 2018-11-03;19(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
The Viral Tegument Protein pp65 Impairs Transcriptional Upregulation of IL-1? by Human Cytomegalovirus through Inhibition of NF-kB Activity
Authors: M Biolatti, V Dell'Oste, S Scutera, F Gugliesi, G Griffante, M De Andrea, T Musso, S Landolfo
Viruses, 2018-10-16;10(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of compressive force combined with mechanical vibration on human alveolar bone osteoblasts
Authors: C Chatmahamo, A Pravithara, S Suttapreya, C Leethanaku
J Oral Biol Craniofac Res, 2018-10-16;9(1):81-85.
Species: Human
Sample Types: Cell Culture Supernates
-
Complement receptor CD46 co-stimulates optimal human CD8+ T cell effector function via fatty acid metabolism
Authors: G Arbore, EE West, J Rahman, G Le Friec, N Niyonzima, M Pirooznia, I Tunc, P Pavlidis, N Powell, Y Li, P Liu, A Servais, L Couzi, V Fremeaux-B, L Placais, A Ferraro, PR Walsh, D Kavanagh, B Afzali, P Lavender, HJ Lachmann, C Kemper
Nat Commun, 2018-10-10;9(1):4186.
Species: Human
Sample Types: Cell Culture Supernates
-
S100B regulates inflammatory response during osteoarthritis via fibroblast growth factor receptor 1 signaling
Authors: L Zhu, Z Weng, P Shen, J Zhou, J Zeng, F Weng, X Zhang, H Yang
Mol Med Rep, 2018-10-01;18(6):4855-4864.
Species: Human
Sample Types: Cell Culture Supernates
-
Differential bronchial epithelial response regulated by ?Np63: a functional understanding of the epithelial shedding found in asthma
Authors: T Kubo, M Tsujiwaki, Y Hirohashi, T Tsukahara, T Kanaseki, M Nakatsugaw, T Hasegawa, T Torigoe
Lab. Invest., 2018-09-25;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
GWAS for Interleukin-1? levels in gingival crevicular fluid identifies IL37 variants in periodontal inflammation
Authors: S Offenbache, Y Jiao, SJ Kim, J Marchesan, KL Moss, L Jing, K Divaris, S Bencharit, CS Agler, T Morelli, S Zhang, L Sun, WT Seaman, D Cowley, SP Barros, JD Beck, M Munz, AS Schaefer, KE North
Nat Commun, 2018-09-11;9(1):3686.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum metabolomic profiling predicts synovial gene expression in rheumatoid arthritis
Authors: R Narasimhan, R Coras, SB Rosenthal, SR Sweeney, A Lodi, S Tiziani, D Boyle, A Kavanaugh, M Guma
Arthritis Res. Ther., 2018-08-03;20(1):164.
Species: Human
Sample Types: Serum
-
Annexin A1, FPR2/ALX, and inflammatory cytokine expression in peritoneal endometriosis
Authors: LK Volpato, VV Horewicz, F Bobinski, DF Martins, AP Piovezan
J. Reprod. Immunol., 2018-08-03;129(0):30-35.
Species: Human
Sample Types: Peritoneal Fluid
-
Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis
Authors: BA McKenzie, MK Mamik, LB Saito, R Boghozian, MC Monaco, EO Major, JQ Lu, WG Branton, C Power
Proc. Natl. Acad. Sci. U.S.A., 2018-06-12;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Vaccine antigens modulate the innate response of monocytes to Al(OH)3
Authors: S Kooijman, J Brummelman, CACM van Els, F Marino, AJR Heck, E van Riet, B Metz, GFA Kersten, JLA Pennings, HD Meiring
PLoS ONE, 2018-05-29;13(5):e0197885.
Species: Human
Sample Types: Cell Culture Supernates
-
CD47 as a potential prognostic marker for oral leukoplakia and oral squamous cell carcinoma
Authors: X Ye, X Wang, R Lu, J Zhang, X Chen, G Zhou
Oncol Lett, 2018-04-17;15(6):9075-9080.
Species: Human
Sample Types: Cell Culture Supernates
-
Clinical predictors of liver fibrosis in patients with chronic hepatitis B virus infection from children to adults
Authors: JF Wu, SH Song, CS Lee, HL Chen, YH Ni, HY Hsu, TC Wu, MH Chang
J. Infect. Dis., 2018-04-11;0(0):.
Species: Human
Sample Types: Serum
-
Analysis of the association between Fc receptor family gene polymorphisms and ocular Beh�et's disease in Han Chinese
Authors: D Zhang, J Qin, L Li, G Su, G Huang, Q Cao, A Kijlstra, P Yang
Sci Rep, 2018-03-19;8(1):4850.
Species: Human
Sample Types: Cell Culture Supernates
-
TET-2 up-regulation is associated with the anti-inflammatory action of Vicenin-2
Authors: N Hassan, A Ali, C Withycombe, M Ahluwalia, RH Al-Nasseri, A Tonks, K Morris
Cytokine, 2018-03-19;108(0):37-42.
Species: Human
Sample Types: Cell Culture Supernates
-
Association of genetic variations in PTPN2 and CD122 with ocular Behcet's disease
Authors: Q Zhang, H Li, S Hou, H Yu, G Su, B Deng, J Qi, C Zhou, A Kijlstra, P Yang
Br J Ophthalmol, 2018-03-03;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Respiratory hazard assessment of combined exposure to complete gasoline exhaust and respirable volcanic ash in a multicellular human lung model at the air-liquid interface
Authors: I Tomašek, CJ Horwell, C Bisig, DE Damby, P Comte, J Czerwinski, A Petri-Fink, MJD Clift, B Drasler, B Rothen-Rut
Environ. Pollut., 2018-02-16;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Saturated Fatty Acids Undergo Intracellular Crystallization and Activate the NLRP3 Inflammasome in Macrophages
Authors: T Karasawa, A Kawashima, F Usui-Kawan, S Watanabe, H Kimura, R Kamata, K Shirasuna, Y Koyama, A Sato-Tomit, T Matsuzaka, H Tomoda, SY Park, N Shibayama, H Shimano, T Kasahara, M Takahashi
Arterioscler. Thromb. Vasc. Biol., 2018-02-08;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Association of Long Noncoding RNAs Polymorphisms With Ankylosing Spondylitis, Vogt-Koyanagi-Harada Disease, and Behcet's Disease
Authors: Y Yue, J Zhang, L Yang, S Liu, J Qi, Q Cao, C Zhou, Y Wang, A Kijlstra, P Yang, S Hou
Invest. Ophthalmol. Vis. Sci., 2018-02-01;59(2):1158-1166.
Species: Human
Sample Types: Cell Culture Supernates
-
The Effects of Varying Degree of MWCNT Carboxylation on Bioactivity in Various In Vivo and In Vitro Exposure Models
Authors: RF Hamilton, Z Wu, S Mitra, A Holian
Int J Mol Sci, 2018-01-25;19(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
TiO2 nanoparticles can selectively bind CXCL8 impacting on neutrophil chemotaxis
Authors: J Batt, M Milward, I Chapple, M Grant, H Roberts, O Addison
Eur Cell Mater, 2018-01-19;35(0):13-24.
-
Identification of genes required for Mycobacterium abscessus growth in vivo with a prominent role of the ESX-4 locus
Authors: L Laencina, V Dubois, V Le Moigne, A Viljoen, L Majlessi, J Pritchard, A Bernut, L Piel, AL Roux, JL Gaillard, B Lombard, D Loew, EJ Rubin, R Brosch, L Kremer, JL Herrmann, F Girard-Mis
Proc. Natl. Acad. Sci. U.S.A., 2018-01-17;115(5):E1002-E1011.
Species: Human
Sample Types: Cell Culture Supernates
-
Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11
Authors: B Lagrange, S Benaoudia, P Wallet, F Magnotti, A Provost, F Michal, A Martin, F Di Lorenzo, BF Py, A Molinaro, T Henry
Nat Commun, 2018-01-16;9(1):242.
Species: Human
Sample Types: Cell Culture Supernates
-
Rheumatoid arthritis bone marrow environment supports Th17 response.
Authors: Ewa Kuca-Warna, Weronika Kurowska, Monika Prochorec, Anna Radzikows, Tomasz Burakowsk, Urszula Skalska, Magdalena Massalska, Magdalena Pleba?czy, Barbara Ma?dyk-No, Iwona S?owi?ska, Robert Gasik, W?odzimierz Ma?li?ski
Arthritis Research & Therapy, 2017-12-08;0(0):1478-6362.
Species: Human
Sample Types: Plasma
-
Inhibition of the inflammatory response to stress by targeting interaction between PKR and its cellular activator PACT
Authors: S Dabo, P Maillard, M Collados R, MD Hansen, S Mazouz, DJ Bigot, M Tible, G Janvier, O Helynck, P Cassonnet, Y Jacob, J Bellalou, A Gatignol, RC Patel, J Hugon, H Munier-Leh, EF Meurs
Sci Rep, 2017-11-23;7(1):16129.
Species: Human
Sample Types: Cell Culture Supernates
-
Respiratory syncytial virus infection of newborn CX3CR1-deficent mice induces a pathogenic pulmonary innate immune response
Authors: S Das, M Raundhal, J Chen, TB Oriss, R Huff, JV Williams, A Ray, P Ray
JCI Insight, 2017-09-07;2(17):.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-18 associated with lung lymphoid aggregates drives IFN? production in severe COPD
Authors: E Briend, GJ Ferguson, M Mori, G Damera, K Stephenson, NA Karp, S Sethi, CK Ward, MA Sleeman, JS Erjefält, DK Finch
Respir. Res., 2017-08-22;18(1):159.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated CRP levels predict poor outcome and tumor recurrence in patients with thymic epithelial tumors: A pro- and retrospective analysis
Authors: S Janik, C Bekos, P Hacker, T Raunegger, B Ghanim, E Einwallner, L Beer, W Klepetko, L Müllauer, HJ Ankersmit, B Moser
Oncotarget, 2017-07-18;8(29):47090-47102.
Species: Human
Sample Types: Serum
-
Production and regulation of interleukin-1 family cytokines at the materno-fetal interface
Authors: LM Scott, AH Bryant, A Rees, B Down, RH Jones, CA Thornton
Cytokine, 2017-07-13;0(0):.
Species: Human
Sample Types: Tissue Culture Supernates
-
Effect of pre-emptive analgesia on clinical parameters and tissue levels of TNF-? and IL-1? in third molar surgery: a triple-blind, randomized, placebo-controlled study
Authors: AFM Albuquerqu, CSR Fonteles, DR do Val, HV Chaves, MM Bezerra, KMA Pereira, PG de Barros, BB de Lima, ECS Soares, TR Ribeiro, FWG Costa
Int J Oral Maxillofac Surg, 2017-06-10;0(0):.
Species: Human
Sample Types: Tissue Homogenates
-
Altered distribution of peripheral blood dendritic cell subsets in patients with pulmonary paracoccidioidomycosis
Authors: J Venturini, RS Cavalcante, DV Moris, M de Assis G, AD Levorato, KH Dos Reis, MSP de Arruda, RP Mendes
Acta Trop., 2017-06-09;0(0):.
Species: Human
Sample Types: Serum
-
Induction of immune gene expression and inflammatory mediator release by commonly used surgical suture materials: an experimental in vitro study
Authors: AM Lock, R Gao, D Naot, B Coleman, J Cornish, DS Musson
Patient Saf Surg, 2017-05-31;11(0):16.
Species: Human
Sample Types: Cell Culture Supernates
-
Penicillinase-resistant antibiotics induce non-immune-mediated cholestasis through HSP27 activation associated with PKC/P38 and PI3K/AKT signaling pathways
Authors: A Burban, A Sharanek, R Hüe, M Gay, S Routier, A Guillouzo, C Guguen-Gui
Sci Rep, 2017-05-12;7(1):1815.
Species: Human
Sample Types: Cell Culture Supernates
-
In vitro and in vivo antagonistic activity of new probiotic culture against Clostridium difficile and Clostridium perfringens
Authors: N Goli?, K Veljovi?, N Popovi?, J Djoki?, I Strahini?, I Mrvaljevi?, A Terzi?-Vid
BMC Microbiol., 2017-05-06;17(1):108.
Species: Human
Sample Types: Cell Culture Supernates
-
TB lymphadenitis is associated with enhanced baseline and antigen - specific induction of Type 1 and Type 17 cytokines and reduced IL-1? and IL-18 at the site of infection
Authors: GR Kathamuthu, K Moideen, D Baskaran, VV Banurekha, D Nair, G Sekar, R Sridhar, B Vidyajayan, G Gajendrara, DK Parandhama, A Srinivasan, S Babu
Clin. Vaccine Immunol, 2017-05-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
An adaptive signaling network in melanoma inflammatory niches confers tolerance to MAPK signaling inhibition
Authors: HL Young, EJ Rowling, M Bugatti, E Giurisato, N Luheshi, I Arozarena, JC Acosta, J Kamarashev, DT Frederick, ZA Cooper, A Reuben, J Gil, KT Flaherty, JA Wargo, W Vermi, MP Smith, C Wellbrock, A Hurlstone
J. Exp. Med., 2017-04-27;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Th17 micro-milieu regulates NLRP1-dependent caspase-5 activity in skin autoinflammation
Authors: S Zwicker, E Hattinger, D Bureik, A Batycka-Ba, A Schmidt, PA Gerber, S Rothenfuss, M Gilliet, T Ruzicka, R Wolf
PLoS ONE, 2017-04-19;12(4):e0175153.
Species: Human
Sample Types: Cell Culture Supernates
-
Differential inhibition of activity, activation and gene expression of MMP-9 in THP-1 cells by azithromycin and minocycline versus bortezomib: A comparative study
Authors: J Vandooren, S Knoops, JL Aldinucci, L Boon, E Martens, G Opdenakker, E Kolaczkows
PLoS ONE, 2017-04-03;12(4):e0174853.
Species: Human
Sample Types: Cell Culture Supernates
-
Vitamin D both facilitates and attenuates the cellular response to lipopolysaccharide
Authors: L Chen, MS Eapen, GR Zosky
Sci Rep, 2017-03-27;7(0):45172.
Species: Human
Sample Types: Cell Culture Supernates
-
Changes in peripheral immune cell numbers and functions in octogenarian walkers - an acute exercise study
Authors: KS van der Ge, Q Wang, TM Eijsvogels, HJ Koenen, I Joosten, E Brouwer, MT Hopman, JF Jacobs, AM Boots
Immun Ageing, 2017-02-22;14(0):5.
Species: Human
Sample Types: Cell Culture Supernates
-
Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner
Authors: SA Conos, KW Chen, D De Nardo, H Hara, L Whitehead, G N£¤ez, SL Masters, JM Murphy, K Schroder, DL Vaux, KE Lawlor, LM Lindqvist, JE Vince
Proc. Natl. Acad. Sci. U.S.A, 2017-01-17;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
In vitro effects of hyaluronic acid on human periodontal ligament cells
Authors: M Fujioka-Ko, HD Mller, A Mueller, A Lussi, A Sculean, PR Schmidlin, RJ Miron
BMC Oral Health, 2017-01-16;17(1):44.
Species: Human
Sample Types: Cell Culture Supernates
-
Differential IL-1? secretion by monocyte subsets is regulated by Hsp27 through modulating mRNA stability
Sci Rep, 2016-12-15;6(0):39035.
Species: Human
Sample Types: Cell Culture Supernates
-
Combined exposure of diesel exhaust particles and respirable Soufri�re Hills volcanic ash causes a (pro-)inflammatory response in an in vitro multicellular epithelial tissue barrier model
Authors: Ines Tomašek
Part Fibre Toxicol, 2016-12-12;13(1):67.
Species: Human
Sample Types: Cell Culture Supernates
-
Receptor-Type Protein-Tyrosine Phosphatase ? and Colony Stimulating Factor-1 Receptor in the Intestine: Cellular Expression and Cytokine- and Chemokine Responses by Interleukin-34 and Colony Stimulating Factor-1
PLoS ONE, 2016-11-29;11(11):e0167324.
Species: Human
Sample Types: Cell Culture Supernates
-
17-oxo-DHA displays additive anti-inflammatory effects with fluticasone propionate and inhibits the NLRP3 inflammasome
Sci Rep, 2016-11-24;6(0):37625.
Species: Human
Sample Types: Cell Culture Supernates
-
Iron nanomedicines induce Toll-like receptor activation, cytokine production and complement activation
Authors: Johan J F Verhoef
Biomaterials, 2016-11-21;119(0):68-77.
Species: Human
Sample Types: Cell Culture Supernates
-
Modulating endotoxin activity by combinatorial bioengineering of meningococcal lipopolysaccharide
Sci Rep, 2016-11-14;6(0):36575.
Species: Human
Sample Types: Cell Culture Supernates
-
Biocompatibility of primers and an adhesive used for implant-retained maxillofacial prostheses: An in�vitro analysis
Authors: Daniela Micheline Dos Santos
J Prosthet Dent, 2016-11-09;0(0):.
-
Reduced systemic and mycobacterial antigen-stimulated concentrations of IL-1? and IL-18 in tuberculous lymphadenitis
Cytokine, 2016-10-26;90(0):66-72.
Species: Human
Sample Types: Cell Culture Supernates
-
Activated monocytes resist elimination by retinal pigment epithelium and downregulate their OTX2 expression via TNF-?
Aging Cell, 2016-09-22;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Immune-Complexed Adenovirus Induce AIM2-Mediated Pyroptosis in Human Dendritic Cells
PLoS Pathog, 2016-09-16;12(9):e1005871.
Species: Human
Sample Types: Cell Culture Supernates
-
Dental Calculus Stimulates Interleukin-1? Secretion by Activating NLRP3 Inflammasome in Human and Mouse Phagocytes
Authors: JL Montenegro, A Yoshimura, Z Sm, T Kaneko, Y Ozaki, T Ukai, T Miyazaki, E Latz, Y Hara
PLoS ONE, 2016-09-15;11(9):e0162865.
Species: Human
Sample Types: Cell Culture Supernates
-
DNA Sensing via TLR-9 Constitutes a Major Innate Immunity Pathway Activated during Erythema Nodosum Leprosum
J Immunol, 2016-07-29;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
CD4+ T Cells Recognizing PE/PPE Antigens Directly or via Cross Reactivity Are Protective against Pulmonary Mycobacterium tuberculosis Infection
PLoS Pathog, 2016-07-28;12(7):e1005770.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Graphene-augmented nanofiber scaffolds demonstrate new features in cells behaviour
Sci Rep, 2016-07-22;6(0):30150.
Species: Human
Sample Types: Cell Culture Supernates
-
Dual Inhibition of Rip2 and IRAK1/4 Regulates IL-1? and IL-6 in Sarcoidosis Alveolar Macrophages and Peripheral Blood Mononuclear Cells
J Immunol, 2016-07-11;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Interaction among activated lymphocytes and mesenchymal cells through podoplanin is critical for a high IL-17 secretion
Authors: Mélissa Noack
Arthritis Res Ther, 2016-06-23;18(0):148.
Species: Human
Sample Types: Cell Culture Supernates
-
Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma
Authors: Avery D Posey
Immunity, 2016-06-21;44(6):1444-54.
Species: Human
Sample Types: Cell Culture Supernates
-
Analysis of receptor tyrosine kinase genetics identifies two novel risk loci in GAS6 and PROS1 in Beh�et's disease
Sci Rep, 2016-05-25;6(0):26662.
Species: Human
Sample Types: Cell Culture Supernates
-
Silk-based blood stabilization for diagnostics
Proc Natl Acad Sci USA, 2016-05-09;113(21):5892-7.
Species: Human
Sample Types: Serum
-
Genetic polymorphisms of cell adhesion molecules in Behcet's disease in a Chinese Han population
Authors: M Zheng, L Zhang, H Yu, J Hu, Q Cao, G Huang, Y Huang, G Yuan, A Kijlstra, P Yang
Sci Rep, 2016-04-25;6(0):24974.
Species: Human
Sample Types: Cell Culture Supernates
-
Stromal fibroblasts support dendritic cells to maintain IL-23/Th17 responses after exposure to ionizing radiation
Authors: A Malecka, Q Wang, S Shah, RV Sutavani, I Spendlove, JM Ramage, J Greensmith, HA Franks, MJ Gough, A Saalbach, PM Patel, AM Jackson
J Leukoc Biol, 2016-04-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of the MMP/TIMP Imbalance and Collagen Production Induced by IL-1? or TNF-? Release from Human Hepatic Stellate Cells
Authors: S Robert, T Gicquel, A Bodin, V Lagente, E Boichot
PLoS ONE, 2016-04-05;11(4):e0153118.
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1 beta secretion in response to ATP
Authors: Mausita Karmakar, Michael A. Katsnelson, George R. Dubyak, Eric Pearlman
Nature Communications
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-17A Induces IL-1? Secretion From RPE Cells Via the NLRP3 Inflammasome
Authors: S Zhang, N Yu, R Zhang, S Zhang, J Wu
Invest. Ophthalmol. Vis. Sci, 2016-02-01;57(2):312-9.
Species: Human
Sample Types: Cell Culture Supernates
-
The Inflammasome and the Epidermal Growth Factor Receptor (EGFR) Are Involved in the Staphylococcus aureus-Mediated Induction of IL-1alpha and IL-1beta in Human Keratinocytes.
Authors: Simanski M, Rademacher F, Schroder L, Glaser R, Harder J
PLoS ONE, 2016-01-25;11(1):e0147118.
Species: Human
Sample Types: Cell Culture Supernates
-
In vitro progesterone modulation on bacterial endotoxin-induced production of IL-1beta, TNFalpha, IL-6, IL-8, IL-10, MIP-1alpha, and MMP-9 in pre-labor human term placenta.
Authors: Garcia-Ruiz G, Flores-Espinosa P, Preciado-Martinez E, Bermejo-Martinez L, Espejel-Nunez A, Estrada-Gutierrez G, Maida-Claros R, Flores-Pliego A, Zaga-Clavellina V
Reprod Biol Endocrinol, 2015-10-07;13(0):115.
Species: Human
Sample Types: Cell Culture Supernates
-
Priming of Human Resting NK Cells by Autologous M1 Macrophages via the Engagement of IL-1beta, IFN-beta, and IL-15 Pathways.
Authors: Mattiola I, Pesant M, Tentorio P, Molgora M, Marcenaro E, Lugli E, Locati M, Mavilio D
J Immunol, 2015-08-14;195(6):2818-28.
Species: Human
Sample Types: Cell Culture Supernates
-
Inflammatory and Angiogenic Factors at Mid-Pregnancy Are Associated with Spontaneous Preterm Birth in a Cohort of Tanzanian Women.
Authors: McDonald C, Darling A, Conroy A, Tran V, Cabrera A, Liles W, Wang M, Aboud S, Urassa W, Fawzi W, Kain K
PLoS ONE, 2015-08-06;10(8):e0134619.
Species: Human
Sample Types: Plasma
-
LPS-Stimulated Whole Blood Cytokine Production Is Not Related to Disease Behavior in Patients with Quiescent Crohn's Disease.
Authors: Broekman M, Roelofs H, Hoentjen F, Wiegertjes R, Stoel N, Joosten L, de Jong D, Wanten G
PLoS ONE, 2015-07-24;10(7):e0133932.
Species: Human
Sample Types: Plasma
-
Caspase-8 as an Effector and Regulator of NLRP3 Inflammasome Signaling.
Authors: Antonopoulos C, Russo H, El Sanadi C, Martin B, Li X, Kaiser W, Mocarski E, Dubyak G
J Biol Chem, 2015-06-22;290(33):20167-84.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Transforming Growth Factor-beta and Interleukin-1beta Signaling Pathways Converge on the Chemokine CCL20 Promoter.
Authors: Brand O, Somanath S, Moermans C, Yanagisawa H, Hashimoto M, Cambier S, Markovics J, Bondesson A, Hill A, Jablons D, Wolters P, Lou J, Marks J, Baron J, Nishimura S
J Biol Chem, 2015-04-27;290(23):14717-28.
Species: Human
Sample Types: Tissue Homogenates
-
SRSF1 facilitates cytosolic DNA-induced production of type I interferons recognized by RIG-I.
Authors: Xue F, Li X, Zhao X, Wang L, Liu M, Shi R, Zheng J
PLoS ONE, 2015-02-06;10(2):e0115354.
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophil IL-1beta processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux.
Authors: Karmakar M, Katsnelson M, Malak H, Greene N, Howell S, Hise A, Camilli A, Kadioglu A, Dubyak G, Pearlman E
J Immunol, 2015-01-21;194(4):1763-75.
Species: Human
Sample Types: Cell Culture Supernates
-
Macrophage activation in acute exacerbation of idiopathic pulmonary fibrosis.
Authors: Schupp J, Binder H, Jager B, Cillis G, Zissel G, Muller-Quernheim J, Prasse A
PLoS ONE, 2015-01-15;10(1):e0116775.
Species: Human
Sample Types: BALF
-
Residual endotoxin contaminations in recombinant proteins are sufficient to activate human CD1c+ dendritic cells.
Authors: Schwarz H, Schmittner M, Duschl A, Horejs-Hoeck J
PLoS ONE, 2014-12-05;9(12):e113840.
Species: Human
Sample Types: Cell Culture Supernates
-
Integrin alphaDbeta2 (CD11d/CD18) is expressed by human circulating and tissue myeloid leukocytes and mediates inflammatory signaling.
Authors: Miyazaki Y, Vieira-de-Abreu A, Harris E, Shah A, Weyrich A, Castro-Faria-Neto H, Zimmerman G
PLoS ONE, 2014-11-21;9(11):e112770.
Species: Human
Sample Types: Cell Culture Supernates
-
Kineret(R)/IL-1ra blocks the IL-1/IL-8 inflammatory cascade during recombinant Panton Valentine Leukocidin-triggered pneumonia but not during S. aureus infection.
Authors: Labrousse D, Perret M, Hayez D, da Silva S, Badiou C, Couzon F, Bes M, Chavanet P, Lina G, Vandenesch F, Croisier-Bertin D, Henry T
PLoS ONE, 2014-06-06;9(6):e97546.
Species: Human
Sample Types: BALF
-
Identification of the flagellin glycosylation system in Burkholderia cenocepacia and the contribution of glycosylated flagellin to evasion of human innate immune responses.
Authors: Hanuszkiewicz A, Pittock P, Humphries F, Moll H, Rosales A, Molinaro A, Moynagh P, Lajoie G, Valvano M
J Biol Chem, 2014-05-19;289(27):19231-44.
Species: Human
Sample Types: Cell Culture Supernates
-
A functional variant of PTPN22 confers risk for Vogt-Koyanagi-Harada syndrome but not for ankylosing spondylitis.
Authors: Zhang Q, Qi J, Hou S, DU L, Yu H, Cao Q, Zhou Y, Liao D, Kijlstra A, Yang P
PLoS ONE, 2014-05-09;9(5):e96943.
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of size on Ag nanosphere toxicity in macrophage cell models and lung epithelial cell lines is dependent on particle dissolution.
Authors: Hamilton, Raymond, Buckingham, Sarah, Holian, Andrij
Int J Mol Sci, 2014-04-22;15(4):6815-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Angiopoietin-1 upregulates de novo expression of IL-1beta and Il1-Ra, and the exclusive release of Il1-Ra from human neutrophils.
Authors: Haddad L, Sirois M
PLoS ONE, 2014-02-12;9(2):e88980.
Species: Human
Sample Types: Cell Lysates
-
Neisseria gonorrhoeae modulates iron-limiting innate immune defenses in macrophages.
Authors: Zughaier, Susu M, Kandler, Justin L, Shafer, William
PLoS ONE, 2014-01-28;9(1):e87688.
Species: Human
Sample Types: Cell Culture Supernates
-
Activin A as a mediator of NK-dendritic cell functional interactions.
Authors: Seeger P, Bosisio D, Parolini S, Badolato R, Gismondi A, Santoni A, Sozzani S
J Immunol, 2014-01-06;192(3):1241-8.
Species: Human
Sample Types: Cell Culture Supernates
-
TLR ligands stimulation protects MSC from NK killing.
Authors: Giuliani M, Bennaceur-Griscelli A, Nanbakhsh A, Oudrhiri N, Chouaib S, Azzarone B, Durrbach A, Lataillade J
Stem Cells, 2014-01-01;32(1):290-300.
Species: Human
Sample Types: Cell Culture Supernates
-
Association of TLR2 gene polymorphisms with ocular Behcet's disease in a Chinese Han population.
Authors: Fang J, Hu R, Hou S, Ye Z, Xiang Q, Qi J, Zhou Y, Kijlstra A, Yang P
Invest Ophthalmol Vis Sci, 2013-12-30;54(13):8384-92.
Species: Human
Sample Types: Cell Culture Supernates
-
Inflammasome induction in Rasmussen's encephalitis: cortical and associated white matter pathogenesis.
Authors: Ramaswamy V, Walsh J, Sinclair D, Johnson E, Tang-Wai R, Wheatley B, Branton W, Maingat F, Snyder T, Gross D, Power C
J Neuroinflammation, 2013-12-13;10(0):152.
Species: Human
Sample Types: Cell Culture Supernates
-
Ex vivo cytokine release and pattern recognition receptor expression of subjects exposed to dampness: pilot study to assess the outcome of mould exposure to the innate immune system.
Authors: Punsmann S, Liebers V, Lotz A, Bruning T, Raulf M
PLoS ONE, 2013-12-10;8(12):e82734.
Species: Human
Sample Types: Cell Culture Supernates
-
The small GTPase Arf6 is essential for the Tram/Trif pathway in TLR4 signaling.
Authors: Van Acker T, Eyckerman S, Vande Walle L, Gerlo S, Goethals M, Lamkanfi M, Bovijn C, Tavernier J, Peelman F
J Biol Chem, 2013-12-02;289(3):1364-76.
Species: Human
Sample Types: Cell Culture Supernates
-
Leptin enhances the secretion of interleukin (IL)-18, but not IL-1beta, from human monocytes via activation of caspase-1.
Authors: Jitprasertwong P, Jaedicke K, Nile C, Preshaw P, Taylor J
Cytokine, 2013-11-23;65(2):222-30.
Species: Human
Sample Types: Cell Culture Supernates
-
S100A12 suppresses pro-inflammatory, but not pro-thrombotic functions of serum amyloid A.
Authors: Chung Y, Goyette J, Tedla N, Hsu K, Geczy C
PLoS ONE, 2013-04-24;8(4):e62372.
Species: Human
Sample Types: Cell Culture Supernates
-
Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation.
Eur J Clin Nutr, 2012-07-18;66(9):1072-4.
Species: Human
Sample Types: Serum
-
Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33.
J. Exp. Med., 2012-07-16;209(8):1505-17.
Species: Human
Sample Types: Cell Culture Supernates
-
Regulation of progranulin expression in human microglia and proteolysis of progranulin by matrix metalloproteinase-12 (MMP-12).
Authors: Suh HS, Choi N, Tarassishin L, Lee SC
PLoS ONE, 2012-04-11;7(4):e35115.
Species: Human
Sample Types: Cell Culture Supernates
-
Monocytic microparticles activate endothelial cells in an IL-1{beta}-dependent manner.
Authors: Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JP, Mackman N
Blood, 2011-06-23;118(8):2366-74.
Species: Human
Sample Types: Cell Lysates
-
Temporal interleukin-1beta secretion from primary human peripheral blood monocytes by P2X7-independent and P2X7-dependent mechanisms
Authors: Jon R. Ward, Peter W. West, Mark P. Ariaans, Lisa C. Parker, Sheila E. Francis, David C. Crossman et al.
Journal of Biological Chemistry
Species: Human
Sample Types: Cell Culture Supernates
-
Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease.
Authors: van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, Van der Meer JW, Netea MG
Proc. Natl. Acad. Sci. U.S.A., 2010-01-26;107(7):3030-3.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin (IL)-31 induces pro-inflammatory cytokines in human monocytes and macrophages following stimulation with staphylococcal exotoxins.
Authors: Kasraie S, Niebuhr M, Werfel T
Allergy, 2009-11-04;65(6):712-21.
Species: Human
Sample Types: Cell Culture Supernates
-
Helminth antigens modulate immune responses in cells from multiple sclerosis patients through TLR2-dependent mechanisms.
Authors: Correale J, Farez M
J. Immunol., 2009-10-07;183(9):5999-6012.
Species: Human
Sample Types: Cell Culture Supernates
-
TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23.
Authors: Aliahmadi E, Gramlich R, Grutzkau A, Hitzler M, Kruger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M
Eur. J. Immunol., 2009-05-01;39(5):1221-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Maternal IL-1beta production prevents lung injury in a mouse model of bronchopulmonary dysplasia.
Authors: Backstrom E, Lappalainen U, Bry K
Am. J. Respir. Cell Mol. Biol., 2009-05-01;42(2):149-60.
Species: Human
Sample Types: Tissue Homogenates
-
Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells.
Authors: Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G
Circulation, 2009-03-02;119(10):1424-32.
Species: Human
Sample Types: Plasma
-
The CDK domain of p21 is a suppressor of IL-1beta-mediated inflammation in activated macrophages.
Authors: Scatizzi JC, Mavers M, Hutcheson J, Young B, Shi B, Pope RM, Ruderman EM, Samways DS, Corbett JA, Egan TM, Perlman H
Eur. J. Immunol., 2009-03-01;39(3):820-5.
Species: Human
Sample Types: Cell Culture Supernates
-
Matrix metalloproteinase-9 deficiency worsens lung injury in a model of bronchopulmonary dysplasia.
Authors: Lukkarinen H, Hogmalm A, Lappalainen U, Bry K
Am. J. Respir. Cell Mol. Biol., 2008-12-18;41(1):59-68.
Species: Human
Sample Types: Tissue Homogenates
-
Divergent effects of hypoxia on dendritic cell functions.
Authors: Mancino A, Schioppa T, Larghi P, Pasqualini F, Nebuloni M, Chen IH, Sozzani S, Austyn JM, Mantovani A, Sica A
Blood, 2008-08-11;112(9):3723-34.
Species: Human
Sample Types: Cell Culture Supernates
-
Impact of tobacco-smoke on key signaling pathways in the innate immune response in lung macrophages.
Authors: Birrell MA, Wong S, Catley MC, Belvisi MG
J. Cell. Physiol., 2008-01-01;214(1):27-37.
Species: Human
Sample Types: Cell Culture Supernates
-
Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling.
Authors: Weindl G, Naglik JR, Kaesler S, Biedermann T, Hube B, Korting HC, Schaller M
J. Clin. Invest., 2007-12-01;117(12):3664-3672.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis.
Authors: Wu T, Xie C, Wang HW, Zhou XJ, Schwartz N, Calixto S, Mackay M, Aranow C, Putterman C, Mohan C
J. Immunol., 2007-11-15;179(10):7166-75.
Species: Human
Sample Types: Urine
-
Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells.
Authors: Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N, Descamps P, Gamelin E, Gascan H, Hebbar M, Jeannin P
Blood, 2007-09-11;110(13):4319-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Novel role for the liver X nuclear receptor in the suppression of lung inflammatory responses.
Authors: Birrell MA, Catley MC, Hardaker E, Wong S, Willson TM, McCluskie K, Leonard T, Farrow SN, Collins JL, Haj-Yahia S, Belvisi MG
J. Biol. Chem., 2007-08-31;282(44):31882-90.
Species: Human
Sample Types: Cell Culture Supernates
-
B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease.
Authors: Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA
Am. J. Pathol., 2006-09-01;169(3):987-98.
Species: Human
Sample Types: Tissue Homogenates
-
Sepsis and pathophysiology of anthrax in a nonhuman primate model.
Authors: Stearns-Kurosawa DJ, Lupu F, Taylor FB, Kinasewitz G, Kurosawa S
Am. J. Pathol., 2006-08-01;169(2):433-44.
Species: Primate - Papio cynocephalus (Yellow Baboon)
Sample Types: Plasma
-
Early cytokine responses after percutaneous magnetic resonance imaging guided laser thermoablation of malignant liver tumors.
Authors: Kallio R, Sequeiros R, Surcel HM, Ohtonen P, Kiviniemi H, Syrjala H
Cytokine, 2006-07-25;34(5):278-83.
Species: Human
Sample Types: Serum
-
Role of matrix metalloproteinases in the inflammatory response in human airway cell-based assays and in rodent models of airway disease.
Authors: Birrell MA, Wong S, Dekkak A, De Alba J, Haj-Yahia S, Belvisi MG
J. Pharmacol. Exp. Ther., 2006-05-11;318(2):741-50.
Species: Human
Sample Types: Cell Culture Supernates
-
Transmission of Mycobacterium tuberculosis undetected by tuberculin skin testing.
Authors: Anderson ST, Williams AJ, Brown JR, Newton SM, Simsova M, Nicol MP, Sebo P, Levin M, Wilkinson RJ, Wilkinson KA
Am. J. Respir. Crit. Care Med., 2006-02-02;173(9):1038-42.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased ENA-78 in the follicular fluid of patients with endometriosis.
Authors: Wunder DM, Mueller MD, Birkhauser MH, Bersinger NA
Acta Obstet Gynecol Scand, 2006-01-01;85(3):336-42.
Species: Human
Sample Types: Follicular Fluid
-
ADAM-9 (MDC-9/meltrin-gamma), a member of the a disintegrin and metalloproteinase family, regulates myeloma-cell-induced interleukin-6 production in osteoblasts by direct interaction with the alpha(v)beta5 integrin.
Authors: Karadag A, Zhou M, Croucher PI
Blood, 2005-12-22;107(8):3271-8.
-
Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists.
Authors: Fritz JH, Girardin SE, Fitting C, Werts C, Mengin-Lecreulx D, Caroff M, Cavaillon JM, Philpott DJ, Adib-Conquy M
Eur. J. Immunol., 2005-08-01;35(8):2459-70.
Species: Human
Sample Types: Cell Culture Supernates
-
Differential effect of serotonin on cytokine production in lipopolysaccharide-stimulated human peripheral blood mononuclear cells: involvement of 5-hydroxytryptamine2A receptors.
Authors: Cloez-Tayarani I, Petit-Bertron AF, Venters HD, Cavaillon JM
Int. Immunol., 2003-02-01;15(2):233-40.
Species: Human
Sample Types: Cell Culture Supernates
-
Adherence influences monocyte responsiveness to interleukin-10.
Authors: Petit-Bertron AF, Fitting C, Cavaillon JM, Adib-Conquy M
J. Leukoc. Biol., 2003-01-01;73(1):145-54.
Species: Human
Sample Types: Cell Culture Supernates
-
Comparison of inflammatory elements in nasal lavage and induced sputum following occupational exposure to moldy-building microbes.
Authors: Purokivi M, Hirvonen MR, Roponen M, Randell J, Vahteristo M, Tukiainen H
Inhal Toxicol, 2002-06-01;14(6):653-62.
Species: Human
Sample Types: Sputum
-
The correlation of adhesions and peritoneal fluid cytokine concentrations: a pilot study.
Authors: Cheong YC, Laird SM, Shelton JB, Ledger WL, Li TC, Cooke ID
Hum. Reprod., 2002-04-01;17(4):1039-45.
Species: Human
Sample Types: Peritoneal Fluid
-
IL-1, IL-6 and TNF-alpha concentrations in the peritoneal fluid of women with pelvic adhesions.
Authors: Cheong YC, Shelton JB, Laird SM, Richmond M, Kudesia G, Li TC, Ledger WL
Hum. Reprod., 2002-01-01;17(1):69-75.
Species: Human
Sample Types: Peritoneal Fluid
-
Inflammatory mediators in nasal lavage, induced sputum and serum of employees with rheumatic and respiratory disorders.
Authors: Roponen M, Kiviranta J, Seuri M, Tukiainen H, Myllykangas-Luosujarvi R, Hirvonen MR
Eur. Respir. J., 2001-09-01;18(3):542-8.
Species: Human
Sample Types: Serum
-
Membrane-bound Interleukin-1 alpha mediates leukocyte adhesion during atherogenesis
Species: Human
Sample Types: Serum
-
Monocyte bioenergetics: An immunometabolic perspective in metabolic dysfunction-associated steatohepatitis
Authors: Moris Sangineto, Martina Ciarnelli, Tommaso Colangelo, Archana Moola, Vidyasagar Naik Bukke, Loren Duda, Rosanna Villani, Antonino Romano, Stefania Giandomenico, Hina Kanwal, Gaetano Serviddio
Cell Rep Med
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human IL-1 beta/IL-1F2 DuoSet ELISA
Average Rating: 4.8 (Based on 36 Reviews)
Have you used Human IL-1 beta/IL-1F2 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Supernatants of HLF cells exposed to LPS for 6-hrs
THP-1 cells were incubated with 0.1-10ug/ml PAM3CSK4 for 24-hours and culture supernatant was collected. IL-1b standard was fit to a sigmoidal 4-paramater logistic curve. Max IL-1b production was found at the 1ug/ml dosage.
3D ciliated airway tissues (cat.# 502-3D-24) were from Cell Applications (San Diego, CA) and were cultured in maintenance medium (cat.# 511M-3D-100, Cell Applications).
Twenty four hours after pre-incubation with test substances, tissues were exposed to 1µg/ml endotoxin LPS and IL-1b was was quantified after 48 additional hours. Worked very well.
Detection of IL-1b secreted by human CAR-T cells engineered to express IL-1b (PMC658), compared to normal CAR-T cells (PMC650).
Not compatible with all substrates.