Human IL-10 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
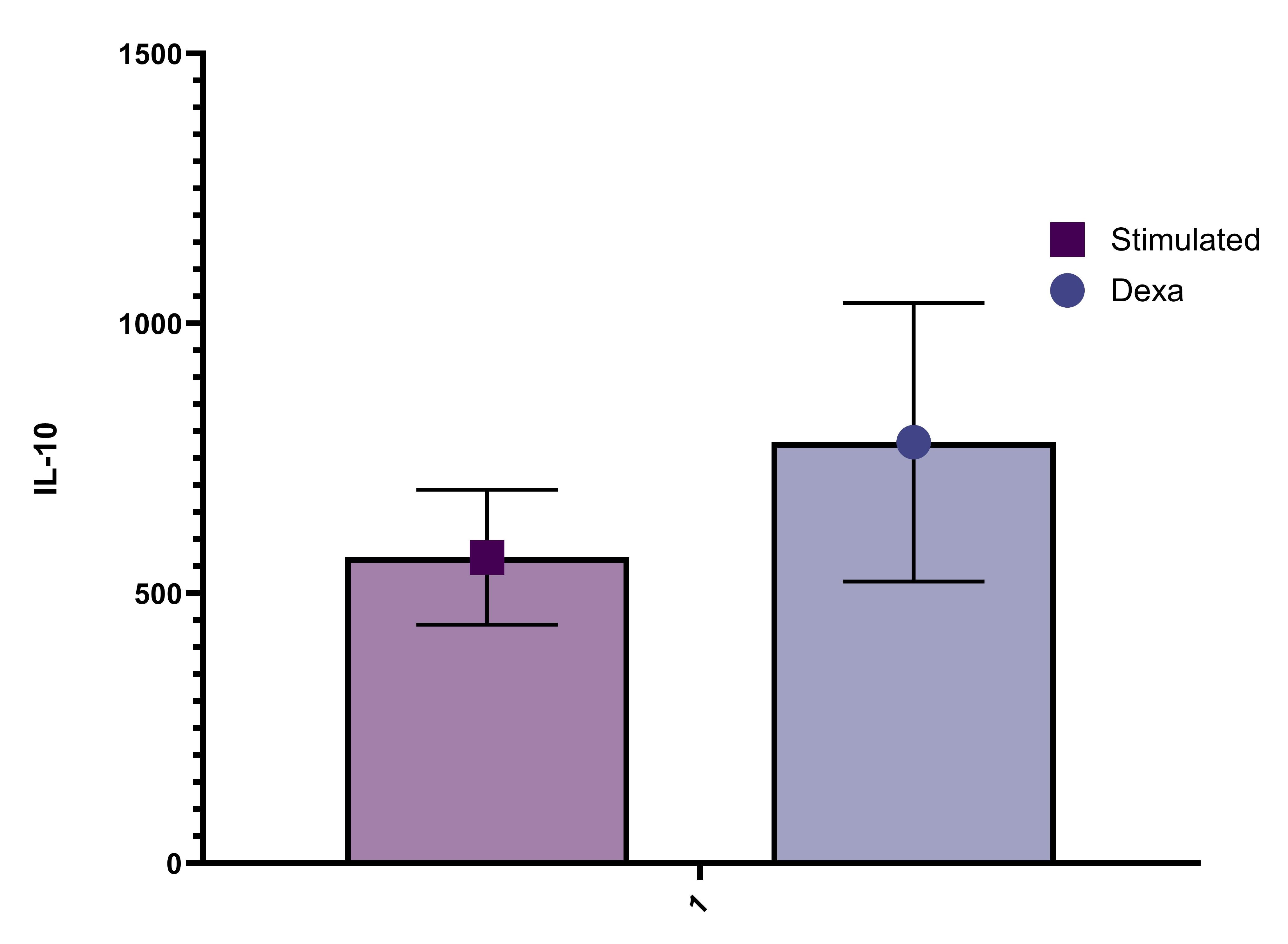
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human IL-10. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-10
Interleukin-10 (IL-10), also known as cytokine synthesis inhibitory factor (CSIF), is the charter member of the IL-10 alpha -helical cytokine family that also includes IL-19, IL-20, IL-22, IL-24, and IL-26/AK155 (1-3). IL-10 is secreted by many activated hematopoietic cell types as well as hepatic stellate cells, keratinocytes, and placental cytotrophoblasts. Whereas human IL-10 is active on mouse cells, mouse IL-10 does not act on human cells (4, 5). Mature human IL-10 shares 86% amino acid sequence identity with equine IL-10 and 72% - 80% with bovine, canine, feline, guinea pig, mouse, ovine, porcine, and rat IL-10. It contains two intrachain disulfide bridges and is expressed as a 36 kDa noncovalently-associated homodimer (4, 6, 7).
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human IL-10 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
225
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Amino acid-equipped poly(ester amide) nanoparticles for improved encapsulation efficiency of lipophilic anti-inflammatory 6BIGOE based on molecular dynamic simulations
Authors: Westhoff, J;Weber, C;Bachmann, V;Göppert, NE;Peric, M;George, A;Sierka, M;Hoeppener, S;Vilotijevic, I;Schubert, US;Werz, O;Fischer, D;
International journal of pharmaceutics
Species: Human
Sample Types: Cell Culture Supernates
-
DNA 5-Hydroxymethylcytosine Landscape and Transcriptional Profile Highlight the TUBB4B-Mediated Th17/Th1/Treg Imbalance in Behçet's Uveitis
Authors: Zhang, W;Zhang, P;Pu, Y;Chen, Z;Su, G;Deng, Y;Zhang, Y;Ji, Y;Huang, Z;Zhou, Q;Luo, X;Lai, Y;Yang, P;
Investigative ophthalmology & visual science
Species: Human
Sample Types: Cell Culture Supernates
-
CD4+ differentiated T regulatory cells is modified by physical fitness and visceral adipose tissue in young adults-A cross-sectional study
Authors: Padilha, CS;Olean-Oliveira, T;Figueiredo, C;Dos Santos, VR;Dorneles, GP;Ribeiro, JPJ;Deminice, R;Krüger, K;Rosa-Neto, JC;Lira, FS;
Physiological reports
Species: Human
Sample Types: Cell Culture Supernates, Whole Blood
-
Modulation of IRAK4 as a Therapeutic Strategy Against Monosodium Urate- and Xanthine-Induced Inflammation in Macrophages and HepG2 Cells
Authors: Umar, S;Chang, HT;Maienschein-Cline, M;Ravindran, S;
Research square
Species: Human
Sample Types: Cell Culture Supernates
-
ECDD-S16, a synthetic derivative of cleistanthin A, suppresses pyroptosis in Burkholderia pseudomallei-infected U937 macrophages
Authors: Khongpraphan, S;Sanongkiet, S;Luangjindarat, C;Thairat, S;Munyoo, B;Chabang, N;Charoensutthivarakul, S;Borwornpinyo, S;Tuchinda, P;Ponpuak, M;Utaisincharoen, P;Pudla, M;
PloS one
Species: Human
Sample Types: Cell Culture Supernates
-
Estrogen induces the alternative activation of macrophages through binding to estrogen receptor-alpha
Authors: Belboul, A;Ashworth, J;Fadel, A;Mcloughlin, J;Mahmoud, A;El Mohtadi, M;
Experimental and molecular pathology
Species: Human
Sample Types: Cell Culture Supernates
-
A single amino acid in the Salmonella effector SarA/SteE triggers supraphysiological activation of STAT3 for anti-inflammatory gene expression
Authors: Gaggioli, MR;Jones, AG;Panagi, I;Washington, EJ;Loney, RE;Muench, JH;Foster, MW;Brennan, RG;Thurston, TLM;Ko, DC;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of Free Long-Chain Fatty Acids on Anagen Induction: Metabolic or Inflammatory Aspect?
Authors: Pan, X;Vishnyakova, KS;Chermnykh, ES;Jasko, MV;Zhuravlev, AD;Verkhova, SS;Chegodaev, YS;Popov, MA;Nikiforov, NG;Yegorov, YE;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Effect of gut microbiota changes on cytokines IL-10 and IL-17 levels in liver transplantation patients
Authors: Abdel-Raoof Fouda, M;Abdel-Wahhab, M;Abdelkader, AE;Ibrahim, ME;Elsheikh, TA;Aldeweik, HM;Elfeky, N;
BMC infectious diseases
Species: Human
Sample Types: Serum
-
Plasma Proteomic Signature as a Predictor of Age Advancement in People Living With HIV
Authors: Navas, A;Matzaraki, V;van Eekeren, LE;Blaauw, MJT;Groenendijk, AL;Vos, WAJW;Jacobs-Cleophas, M;Dos Santos, JC;van der Ven, AJAM;Joosten, LAB;Netea, MG;
Aging cell
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of general versus spinal anesthesia on perioperative innate immune function in patients undergoing total hip arthroplasty
Authors: Jacobs, LMC;Bijkerk, V;van Eijk, LT;Joosten, LAB;Keijzer, C;Visser, J;Warlé, MC;
BMC anesthesiology
Species: Human
Sample Types: Plasma
-
?-1,6-Glucan plays a central role in the structure and remodeling of the bilaminate fungal cell wall
Authors: Bekirian, C;Valsecchi, I;Bachellier-Bassi, S;Scandola, C;Guijarro, JI;Chauvel, M;Mourer, T;Gow, NAR;Aimanianda, VK;d'Enfert, C;Fontaine, T;
eLife
Species: Human
Sample Types: Cell Culture Supernates
-
Commonly Used Dose of Montmorency Tart Cherry Powder Does Not Improve Sleep or Inflammation Outcomes in Individuals with Overweight or Obesity
Authors: Tucker, RM;Kim, N;Gurzell, E;Mathi, S;Chavva, S;Senthilkumar, D;Bartunek, O;Fenton, KC;Herndon-Fenton, SJ;Cardino, VN;Cooney, GM;Young, S;Fenton, JI;
Nutrients
Species: Human
Sample Types: Serum
-
Pseudomonas aeruginosa- mediated cardiac dysfunction is driven by extracellular vesicles released during infection
Authors: Kumar, N;Mattoo, SS;Sanghvi, S;Ellendula, MP;Mahajan, S;Planner, C;Bednash, JS;Khan, M;Ganesan, LP;Singh, H;Lafuse, WP;Wozniak, DJ;Rajaram, MVS;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Low Caloric Intake Confers Cardiovascular Protection and Improves Functional Capacity Without Affecting Immunological Response in Sedentary Older Adults
Authors: Moura-Maia, MS;Brill, B;Paula-Vieira, RHR;Ramos-Gomes, NV;Melamed, D;Silva-Reis, A;Wolpp, ETR;Moreira-Silva, NN;Bella, YF;Vieira, RP;
Nutrients
Species: Human
Sample Types: Plasma
-
Interleukin-1? induces trained innate immunity in human hematopoietic progenitor cells in vitro
Authors: Flores-Gomez, D;Hobo, W;van Ens, D;Kessler, EL;Novakovic, B;Schaap, NPM;Rijnen, WHC;Joosten, LAB;Netea, MG;Riksen, NP;Bekkering, S;
Stem cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-inflammatory effects of phytocannabinoids and terpenes on inflamed Tregs and Th17 cells in vitro
Authors: Tan, KBC;Alexander, HD;Linden, J;Murray, EK;Gibson, DS;
Experimental and molecular pathology
Species: Human
Sample Types: Cell Culture Supernates
-
Interplay between host humoral pattern recognition molecules controls undue immune responses against Aspergillus fumigatus
Authors: Dellière, S;Chauvin, C;Wong, SSW;Gressler, M;Possetti, V;Parente, R;Fontaine, T;Krüger, T;Kniemeyer, O;Bayry, J;Carvalho, A;Brakhage, AA;Inforzato, A;Latgé, JP;Aimanianda, V;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Novel paired CD13-negative (MT-50.1) and CD13-positive (MT-50.4) HTLV-1-infected T-cell lines with differential regulatory T cell-like activity
Authors: Egawa, Y;Higuchi, T;Hashida, Y;Ueno, K;Kojima, K;Daibata, M;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Bromo-substituted indirubins for inhibition of protein kinase-mediated signalling involved in inflammatory mediator release in human monocytes
Authors: Bachmann, V;Schädel, P;Westhoff, J;Peri?, M;Schömberg, F;Skaltsounis, AL;Höppener, S;Pantsar, T;Fischer, D;Vilotijevi?, I;Werz, O;
Bioorganic chemistry
Species: Human
Sample Types: Cell Culture Supernates
-
Reduced Tolerogenic Program Death-Ligand 1-Expressing Conventional Type 1 Dendritic Cells Are Associated with Rapid Decline in Chronic Obstructive Pulmonary Disease
Authors: Chen, KY;Sun, WL;Wu, SM;Feng, PH;Lin, CF;Chen, TT;Lu, YH;Ho, SC;Chen, YH;Lee, KY;
Cells
Species: Human
Sample Types: Cell Culture Supernates
-
Cognitive function is associated with performance in time up and go test and with leptin blood levels in community-dwelling older women
Authors: da Costa Teixeira, LA;Soares, LA;Lima, LP;Avelar, NCP;de Moura, JA;Leopoldino, AAO;Figueiredo, PHS;Parentoni, AN;Mendonça, VA;Lacerda, ACR;
Scientific reports
Species: Human
Sample Types: Serum
-
Using the Traditional Ex Vivo Whole Blood Model to Discriminate Bacteria by Their Inducible Host Responses
Authors: Chick, HM;Rees, ME;Lewis, ML;Williams, LK;Bodger, O;Harris, LG;Rushton, S;Wilkinson, TS;
Biomedicines
Species: Human
Sample Types: Whole Blood
-
Leishmania braziliensis enhances monocyte responses to promote anti-tumor activity
Authors: Dos Santos, JC;Moreno, M;Teufel, LU;Chilibroste, S;Keating, ST;Groh, L;Domínguez-Andrés, J;Williams, DL;Ma, Z;Lowman, DW;Ensley, HE;Novakovic, B;Ribeiro-Dias, F;Netea, MG;Chabalgoity, JA;Joosten, LAB;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
A single amino acid in the Salmonella effector SarA/SteE triggers supraphysiological activation of STAT3 for anti-inflammatory target gene expression
Authors: Gaggioli, MR;Jones, AG;Panagi, I;Washington, EJ;Loney, RE;Muench, JH;Brennan, RG;Thurston, TLM;Ko, DC;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
The strong inverse association between plasma concentrations of soluble tumor necrosis factor receptors type 1 with adiponectin/leptin ratio in older women
Authors: Augusto da Costa Teixeira, L;Rocha-Vieira, E;Aparecida Soares, L;Mota de Oliveira, F;Aparecida Oliveira Leopoldino, A;Netto Parentoni, A;Amaral Mendonça, V;Cristina Rodrigues Lacerda, A;
Cytokine
Species: Human
Sample Types: Plasma
-
Effects of Combinatory In Vitro Treatment with Immune Checkpoint Inhibitors and Cytarabine on the Anti-Cancer Immune Microenvironment in De Novo AML Patients
Authors: Bo?kun, ?;Starosz, A;Kr?towska-Grunwald, A;Wasiluk, T;Walewska, A;Wierzbowska, A;Moniuszko, M;Grubczak, K;
Cancers
Species: Human
Sample Types: Cell Culture Supernates
-
IFN-?3 is induced by Leishmania donovani and can inhibit parasite growth in cell line models but not in the mouse model, while it shows a significant association with leishmaniasis in humans
Authors: De, M;Sukla, S;Bharatiya, S;Keshri, S;Roy, DG;Roy, S;Dutta, D;Saha, S;Ejazi, SA;Ravichandiran, V;Ali, N;Chatterjee, M;Chinnaswamy, S;
Infection and immunity
Species: Human
Sample Types: Cell Culture Supernates
-
Downregulation of Salt-Inducible Kinase 3 Enhances CCL24 Activation in the Placental Environment with Preeclampsia
Authors: Tsai, HF;Tseng, CF;Liang, YL;Wu, PY;Huang, LY;Lin, YH;Lin, LH;Lin, CN;Hsu, KF;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates, Cord Blood Serum
-
Suppression of NK Cell Activation by JAK3 Inhibition: Implication in the Treatment of Autoimmune Diseases
Authors: Wu, WC;Shiu, C;Tong, TK;Leung, SO;Hui, CW;
Journal of immunology research
Species: Human
Sample Types: Cell Culture Supernates
-
Macrophages derived from LPS-stimulated monocytes from individuals with subclinical atherosclerosis were characterized by increased pro-inflammatory activity
Authors: Nikiforov, NG;Kirichenko, TV;Kubekina, MV;Chegodaev, YS;Zhuravlev, AD;Ilchuk, LA;Nikolaeva, MA;Arefieva, AS;Popov, MA;Verkhova, SS;Bagheri Ekta, M;Orekhov, AN;
Cytokine
Species: Human
Sample Types: Cell Culture Supernates
-
Neutralizing tumor-related inflammation and reprogramming of cancer-associated fibroblasts by Curcumin in breast cancer therapy
Authors: Jalilian, E;Abolhasani-Zadeh, F;Afgar, A;Samoudi, A;Zeinalynezhad, H;Langroudi, L;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
RNA m6A methylation modulates airway inflammation in allergic asthma via PTX3-dependent macrophage homeostasis
Authors: Han, X;Liu, L;Huang, S;Xiao, W;Gao, Y;Zhou, W;Zhang, C;Zheng, H;Yang, L;Xie, X;Liang, Q;Tu, Z;Yu, H;Fu, J;Wang, L;Zhang, X;Qian, L;Zhou, Y;
Nature communications
Species: Human
Sample Types: Serum, Cell Culture Supernates, Plasma
-
Ventilatory support and inflammatory peptides in hospitalised patients with COVID-19: A prospective cohort trial
Authors: Gysan, MR;Milacek, C;Bal, C;Zech, A;Brugger, J;Milos, RI;Antoniewicz, L;Idzko, M;Gompelmann, D;
PloS one
Species: Human
Sample Types: Serum
-
An oral cholera vaccine in the prevention and/or treatment of inflammatory bowel disease
Authors: Meunier, M;Spillmann, A;Rousseaux, C;Schwamborn, K;Hanson, M;
PloS one
Species: Human
Sample Types:
-
The Potential Use of THP-1, a Monocytic Leukemia Cell Line, to Predict Immune-Suppressive Potency of Human Bone-Marrow Stromal Cells (BMSCs) In Vitro: A Pilot Study
Authors: Ren, J;Szombath, G;Vitale-Cross, L;Stroncek, DF;Robey, PG;Hajdara, A;Szalayova, I;Mayer, B;Martin, D;Mezey, E;Nemeth, K;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
The influence of calcitriol and methylprednisolone on podocytes function in minimal change disease in vitro model
Authors: Grubczak, K;Starosz, A;Makowska, B;Parfienowicz, Z;Kr?towska, M;Naumnik, B;Moniuszko, M;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernatants
-
Significance of Th17 and Treg in Treatment Efficacy and Outcome in Pediatric Acute Lymphoblastic Leukemia
Authors: Kr?towska-Grunwald, A;Sawicka-?ukowska, M;Kowalska, M;Basaj, A;Krawczuk-Rybak, M;Moniuszko, M;Grubczak, K;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Transplantation of adipose tissue-derived microvascular fragments promotes therapy of critical limb ischemia
Authors: Park, GT;Lim, JK;Choi, EB;Lim, MJ;Yun, BY;Kim, DK;Yoon, JW;Hong, YG;Chang, JH;Bae, SH;Ahn, JY;Kim, JH;
Biomaterials research
Species: Human
Sample Types: Cell Culture Supernates
-
Comparison of the effects of high-intensity interval and moderate-intensity continuous training on inflammatory markers, cardiorespiratory fitness, and quality of life in breast cancer patients
Authors: Isanejad, A;Nazari, S;Gharib, B;Motlagh, AG;
Journal of sport and health science
Species: Human
Sample Types: Serum
-
Natural mutations in the sensor kinase of the PhoPR two-component regulatory system modulate virulence of ancestor-like tuberculosis bacilli
Authors: Malaga, W;Payros, D;Meunier, E;Frigui, W;Sayes, F;Pawlik, A;Orgeur, M;Berrone, C;Moreau, F;Mazères, S;Gonzalo-Asensio, J;Rengel, D;Martin, C;Astarie-Dequeker, C;Mourey, L;Brosch, R;Guilhot, C;
PLoS pathogens
Species: Human
Sample Types: Cell Culture Supernates
-
Inflammatory biomarkers at different stages of Sarcopenia in older women
Authors: da Costa Teixeira, LA;Avelar, NCP;Peixoto, MFD;Parentoni, AN;Santos, JMD;Pereira, FSM;Danielewicz, AL;Leopoldino, AAO;Costa, SP;Arrieiro, AN;Soares, LA;da Silva Lage, VK;Prates, ACN;Taiar, R;de Carvalho Bastone, A;Oliveira, VC;Oliveira, MX;Costa, HS;Nobre, JNP;Brant, FP;Duarte, TC;Figueiredo, PHS;Mendonça, VA;Lacerda, ACR;
Scientific reports
Species: Human
Sample Types: Plasma
-
Mild Cognitive Impairment Is Associated with Enhanced Activation of Th17 Lymphocytes in Non-Alcoholic Fatty Liver Disease
Authors: Fiorillo, A;Gallego, JJ;Casanova-Ferrer, F;Giménez-Garzó, C;Urios, A;Ballester, MP;Durbán, L;Rios, MP;Megías, J;San Miguel, T;Kosenko, E;Escudero-García, D;Benlloch, S;Felipo, V;Montoliu, C;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Dimethyl itaconate induces long-term innate immune responses and confers protection against infection
Authors: Ferreira, AV;Kostidis, S;Groh, LA;Koeken, VACM;Bruno, M;Baydemir, I;Kilic, G;Bulut, Ö;Andriopoulou, T;Spanou, V;Synodinou, KD;Gkavogianni, T;Moorlag, SJCFM;Charlotte de Bree, L;Mourits, VP;Matzaraki, V;Koopman, WJH;van de Veerdonk, FL;Renieris, G;Giera, M;Giamarellos-Bourboulis, EJ;Novakovic, B;Domínguez-Andrés, J;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
COVID-19 vaccination exacerbates ex vivo IL-6 release from isolated PBMCs
Authors: Langgartner, D;Winkler, R;Brunner-Weisser, J;Rohleder, N;Jarczok, MN;Gündel, H;Weimer, K;Reber, SO;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
The Endogenous Dual Retinoid Receptor Agonist Alitretinoin Exhibits Immunoregulatory Functions on Antigen-Presenting Cells
Authors: Kislat, A;Olah, P;Kuchner, M;Gerber, PA;Schrader, J;Meller, S;Homey, B;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Functional IgG Autoantibodies against Autonomic Nervous System Receptors in Symptomatic Women with Silicone Breast Implants
Authors: Talalai, E;Gorobets, D;Halpert, G;Tsur, AM;Heidecke, H;Levy, Y;Watad, A;Blank, M;Michaelevski, I;Shoenfeld, Y;Amital, H;
Cells
Species: Human
Sample Types: Cell Culture Supernates
-
Inhibition of poly (ADP-ribose) Polymerase-1 (PARP-1) improves endothelial function in pulmonary hypertension
Authors: M Shafiq, ZR Lone, AO Abdulkaree, G Kaur, S Navya, H Singh, K Jagavelu, K Hanif
Pulmonary pharmacology & therapeutics, 2023-02-25;0(0):102200.
Species: Human
Sample Types: Cell Culture Supernates
-
Agricultural dust derived bacterial extracellular vesicle mediated inflammation is attenuated by DHA
Authors: AJ Heires, D Samuelson, D Villageliu, TM Nordgren, DJ Romberger
Scientific Reports, 2023-02-16;13(1):2767.
Species: Human
Sample Types: Cell Culture Supernates
-
Rare Phytocannabinoids Exert Anti-Inflammatory Effects on Human Keratinocytes via the Endocannabinoid System and MAPK Signaling Pathway
Authors: D Tortolani, C Di Meo, S Standoli, F Ciaramella, S Kadhim, E Hsu, C Rapino, M Maccarrone
International Journal of Molecular Sciences, 2023-02-01;24(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-inflammatory activities of Coleus forsteri (formerly Plectranthus forsteri) extracts on human macrophages and chemical characterization
Authors: M Nicolas, M Lasalo, S Chow, C Antheaume, K Huet, E Hnawia, GJ Guillemin, M Nour, M Matsui
Frontiers in Pharmacology, 2023-01-09;13(0):1081310.
Species: Human
Sample Types: Cell culture supernate
-
Human TH17 cells engage gasdermin E pores to release IL-1alpha on NLRP3 inflammasome activation
Authors: YY Chao, A Puhach, D Frieser, M Arunkumar, L Lehner, T Seeholzer, A Garcia-Lop, M van der Wa, S Fibi-Smeta, A Dietschman, T Sommermann, T ?ikovi?, L Taher, MS Gresnigt, SJ Vastert, F van Wijk, G Panagiotou, D Krappmann, O Gro beta, CE Zielinski
Nature Immunology, 2023-01-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
PD-1highCXCR5-CD4+ peripheral helper T�cells promote CXCR3+ plasmablasts in human acute viral infection
Authors: H Asashima, S Mohanty, M Comi, WE Ruff, KB Hoehn, P Wong, J Klein, C Lucas, I Cohen, S Coffey, N Lele, L Greta, K Raddassi, O Chaudhary, A Unterman, B Emu, SH Kleinstein, RR Montgomery, A Iwasaki, CS Dela Cruz, N Kaminski, AC Shaw, DA Hafler, TS Sumida
Cell Reports, 2023-01-02;0(0):111895.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of exogenous IL-37 on immune cells from a patient carrying a potential IL37 loss-of-function variant: A case study
Authors: LU Teufel, CI van der Ma, V Klück, A Simons, A Hoischen, V Vernimmen, LAB Joosten, RJW Arts
Cytokine, 2022-12-05;162(0):156102.
Species: Human
Sample Types: Cell Culture Supernates
-
Adiponectin Is a Contributing Factor of Low Appendicular Lean Mass in Older Community-Dwelling Women: A Cross-Sectional Study
Authors: LAC Teixeira, JM Dos Santos, AN Parentoni, LP Lima, TC Duarte, FP Brant, CDC Neves, FSM Pereira, NCP Avelar, AL Danielewic, AAO Leopoldino, SP Costa, AN Arrieiro, LA Soares, ACN Prates, JNP Nobre, A de Carvalh, VC de Oliveir, MX Oliveira, PH Scheidt Fi, HS Costa, V Amaral Men, R Taiar, AC Rodrigues
Journal of Clinical Medicine, 2022-12-02;11(23):.
Species: Human
Sample Types: Plasma
-
Blood orange juice intake modulates plasma and PBMC microRNA expression in overweight and insulin resistance women: impact on MAPK and NFkappaB signaling pathways
Authors: VC Capetini, BJ Quintanilh, DC de Oliveir, AH Nishioka, LA de Matos, LRP Ferreira, FM Ferreira, GR Sampaio, NMA Hassimotto, FM Lajolo, RA Fock, MM Rogero
The Journal of nutritional biochemistry, 2022-11-26;0(0):109240.
Species: Human
Sample Types: Plasma
-
EC-18 prevents autoimmune arthritis by suppressing inflammatory cytokines and osteoclastogenesis
Authors: JS Park, SC Yang, HY Jeong, SY Lee, JG Ryu, JW Choi, HY Kang, SM Kim, SH Hwang, ML Cho, SH Park
Arthritis Research & Therapy, 2022-11-17;24(1):254.
Species: Human
Sample Types: Serum
-
CD169+ Monocyte and Regulatory T Cell Subsets Are Associated with Disease Activity in Rheumatoid Arthritis
Authors: AJ Eakin, T Ahmed, CM McGeough, S Drain, HD Alexander, GD Wright, PV Gardiner, D Small, AJ Bjourson, DS Gibson
Journal of personalized medicine, 2022-11-09;12(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
In Vitro Characterization of a Tissue Renin-Angiotensin System in Human Nucleus Pulposus Cells
Authors: B Saravi, Z Li, V Basoli, S Grad, S Häckel, CE Albers, M Alini, H Schmal, P Obid, G Lang
Cells, 2022-10-28;11(21):.
Species: Human
Sample Types: Cell Culture Supernates
-
Signal strength of STING activation determines cytokine plasticity and cell death in human monocytes
Authors: D Kabelitz, M Zarobkiewi, M Heib, R Serrano, M Kunz, G Chitadze, D Adam, C Peters
Scientific Reports, 2022-10-24;12(1):17827.
Species: Human
Sample Types: Cell Culture Supernates
-
Adipose-Derived Stem Cell Exosomes Inhibit Hypertrophic Scaring Formation by Regulating Th17/Treg Cell Balance
Authors: H Wang, Y Li, Z Yue, Y Liu, Q Chen, D Hu, J Han
BioMed Research International, 2022-10-12;2022(0):9899135.
Species: Human
Sample Types: Tissue Homogenates
-
T Lymphocyte Serotonin 5-HT7 Receptor Is Dysregulated in Natalizumab-Treated Multiple Sclerosis Patients
Authors: F Reverchon, C Guillard, L Mollet, P Auzou, D Gosset, F Madouri, A Valéry, A Menuet, C Ozsancak, M Pallix-Guy, S Morisset-L
Biomedicines, 2022-09-27;10(10):.
Species: Human
Sample Types: Plasma
-
Hybrid Mineral/Organic Material Induces Bone Bridging and Bone Volume Augmentation in Rat Calvarial Critical Size Defects
Authors: M Dubus, L Scomazzon, C Ledouble, J Braux, A Beljebbar, L Van Gulick, A Baldit, C Gorin, H Alem, N Bouland, M Britton, J Schiavi, TJ Vaughan, C Mauprivez, H Kerdjoudj
Cells, 2022-09-14;11(18):.
Species: Human
Sample Types: Cell Culture Supernates
-
Combined resistance and aerobic training improves lung function and mechanics and fibrotic biomarkers in overweight and obese women
Authors: A Silva-Reis, MA Rodrigues, R Moraes-Fer, TG Gonçalves-, VH Souza-Palm, HC Aquino-San, ALL Bachi, LVF de Oliveir, RÁB Lopes-Mart, I Oliveira-S, R Albertini, CR Frison, RP Vieira
Frontiers in Physiology, 2022-09-07;13(0):946402.
Species: Human
Sample Types: Plasma
-
Pyrroloquinoline quinone (PQQ) improves pulmonary hypertension by regulating mitochondrial and metabolic functions
Authors: M Shafiq, ZR Lone, P Bharati, S Mahapatra, P Rai, N Khandelwal, AN Gaikwad, K Jagavelu, K Hanif
Pulmonary pharmacology & therapeutics, 2022-08-25;76(0):102156.
Species: Rat
Sample Types: Tissue Homogenates
-
Nicotine in Combination with SARS-CoV-2 Affects Cells Viability, Inflammatory Response and Ultrastructural Integrity
Authors: L Sansone, A de Iure, M Cristina, M Belli, L Vitiello, F Marcolongo, A Rosellini, L Macera, PG Spezia, C Tomino, S Bonassi, MA Russo, F Maggi, P Russo
International Journal of Molecular Sciences, 2022-08-22;23(16):.
Species: Human
Sample Types: Cell Culture Supernates
-
Gentle Touch Therapy, Pain Relief and Neuroplasticity at Baseline in Fibromyalgia Syndrome: A Randomized, Multicenter Trial with Six-Month Follow-Up
Authors: ASI Salgado, MH Takemoto, CFTC de Souza, DC Salm, D da Rosa, GC Cardoso, DD Ludtke, SFC Soares, JK Ferreira, AR Dutra, YC Szeremeta, G Mazzardo, J Mayra, DDL Sheffer, W Caumo, EB Bittencour, R Schleip, A Latini, F Bobinski, DF Martins
Journal of Clinical Medicine, 2022-08-20;11(16):.
Species: Human
Sample Types: Serum
-
Development of a rapid in vitro pre-screen for distinguishing effective liposome-adjuvant delivery systems
Authors: LAJ Feather, V Nadella, E Kastner, Y Perrie, AC Hilton, A Devitt
Scientific Reports, 2022-07-20;12(1):12448.
Species: Human
Sample Types: Cell Culture Supernates
-
Onchocerca volvulus-specific antibody and cellular responses in onchocerciasis patients treated annually with ivermectin for 30 years and exposed to parasite transmission in central Togo
Authors: SI Johanns, RG Gantin, B Wangala, K Komlan, WA Halatoko, M Banla, P Karabou, AJ Luty, H Schulz-Key, C Köhler, PT Soboslay
PloS Neglected Tropical Diseases, 2022-05-03;16(5):e0010340.
Species: Human
Sample Types: Cell Culture Supernates
-
Repeated exposure of bronchial epithelial cells to particular matter increases allergen-induced cytokine release and permeability
Authors: H Janbazacya, J van Bergen, S Varasteh, J Garssen, G Folkerts, S Braber
Cytokine, 2022-04-08;154(0):155878.
Species: Human
Sample Types: Cell Culture Supernates
-
A novel Streptococcus pneumoniae human challenge model demonstrates Treg lymphocyte recruitment to the infection site
Authors: G Szylar, R Wysoczansk, H Marshall, DJB Marks, R José, ME Ehrenstein, JS Brown
Scientific Reports, 2022-03-07;12(1):3990.
Species: Human
Sample Types: Blister Fluid
-
Comparative Analysis of Circulating Levels of SARS-CoV-2 Antibodies and Inflammatory Mediators in Healthcare Workers and COVID-19 Patients
Authors: LM de-Oliveir, VE Fiestas So, M de Lourdes, C Fernandes-, PH Damasco, MAMT de Siqueir, HG Dias, A Pauvolid-C, PV Damasco, EL de Azeredo
Viruses, 2022-02-23;14(3):.
Species: Human
Sample Types: Plasma
-
Echinococcus multilocularis specific antibody, systemic cytokine, and chemokine levels, as well as antigen-specific cellular responses in patients with progressive, stable, and cured alveolar echinococcosis: A 10-year follow-up
Authors: B Grüner, L Peters, A Hillenbran, P Vo beta berg, J Schweiker, EG Rollmann, LH Rodriguez, J Blumhardt, S Burkert, P Kern, C Köhler, PT Soboslay
PloS Neglected Tropical Diseases, 2022-02-02;16(2):e0010099.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of Different Parameters of In Vitro Static Tensile Strain on Human Periodontal Ligament Cells Simulating the Tension Side of Orthodontic Tooth Movement
Authors: C Sun, M Janjic Ran, M Folwaczny, T Stocker, S Otto, A Wichelhaus, U Baumert
International Journal of Molecular Sciences, 2022-01-28;23(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
A New Perspective for Bone Tissue Engineering: Human Mesenchymal Stromal Cells Well-Survive Cryopreservation on beta-TCP Scaffold and Show Increased Ability for Osteogenic Differentiation
Authors: L Leppik, A Gempp, Z Kuçi, S Kuçi, P Bader, H Bönig, I Marzi, D Henrich
International Journal of Molecular Sciences, 2022-01-26;23(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Pluripotent Stem Cell-Derived Hepatocytes Inhibit T Cell Proliferation In Vitro through Tryptophan Starvation
Authors: M Romano, R Elgueta, D McCluskey, AM Ortega-Pri, E Stolarczyk, F Dazzi, B Lucendo-Vi, J Meseguer-R, J Williams, G Fanelli, DC Hay, FM Watt, G Lombardi
Cells, 2021-12-22;11(1):.
Species: Human
Sample Types: Serum
-
Skin Substitute Preparation Method Induces Immunomodulatory Changes in Co-Incubated Cells through Collagen Modification
Authors: J Holl, C Pawlukiani, J Corton Rui, D Groth, K Grubczak, HR Hady, J Dadan, J Reszec, S Czaban, C Kowalewski, M Moniuszko, A Eljaszewic
Pharmaceutics, 2021-12-15;13(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Defactinib inhibits PYK2 phosphorylation of IRF5 and reduces intestinal inflammation
Authors: G Ryzhakov, H Almuttaqi, AL Corbin, DL Berthold, T Khoyratty, HL Eames, S Bullers, C Pearson, Z Ai, K Zec, S Bonham, R Fischer, L Jostins-De, SPL Travis, BM Kessler, IA Udalova
Nature Communications, 2021-11-18;12(1):6702.
Species: Human
Sample Types: Tissue Homogenates
-
Hypovitaminosis D Is Associated with Higher Levels of Inflammatory Cytokines and with HAM/TSP in HTLV-Infected Patients
Authors: EC Netto, AC Silva, C Pedroso, C Brites
Viruses, 2021-11-04;13(11):.
Species: Human
Sample Types: Plasma
-
A prospective cohort study providing insights for markers of adverse pregnancy outcome in older mothers
Authors: SC Lean, RL Jones, SA Roberts, AEP Heazell
BMC pregnancy and childbirth, 2021-10-20;21(1):706.
Species: Human
Sample Types: Serum
-
Targeting of Janus Kinases Limits Pro-Inflammatory but Also Immunosuppressive Circuits in the Crosstalk between Synovial Fibroblasts and Lymphocytes
Authors: N Yao, T Tretter, P Kvacskay, W Merkt, N Blank, HM Lorenz, LO Tykocinski
Biomedicines, 2021-10-08;9(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
Tofacitinib-Induced Modulation of Intestinal Adaptive and Innate Immunity and Factors Driving Cellular and Systemic Pharmacokinetics
Authors: B Texler, A Zollner, V Reinstadle, SJ Reider, S Macheiner, B Jelusic, A Pfister, C Watschinge, N Przysiecki, H Tilg, H Oberacher, AR Moschen
Cellular and Molecular Gastroenterology and Hepatology, 2021-10-06;0(0):.
Species: Human
Sample Types: Cell culture supernate
-
mRNA-Based Nanomedicinal Products to Address Corneal Inflammation by Interleukin-10 Supplementation
Authors: I Gómez-Agua, J Rodríguez-, M Beraza-Mil, M Vicente-Pa, A Rodríguez-, S Garelli, L Battaglia, A Del Pozo-R, MÁ Solinís
Pharmaceutics, 2021-09-15;13(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophil function and bactericidal activity against Staphylococcus aureus after cardiac surgery with cardiopulmonary bypass
Authors: M Lesouhaiti, M Gregoire, A Gacouin, V Coirier, A Frerou, C Piau, V Cattoir, E Dumontet, M Revest, P Tattevin, A Roisne, JP Verhoye, E Flecher, Y Le Tulzo, K Tarte, JM Tadié
Journal of leukocyte biology, 2021-08-23;0(0):.
Species: Human
Sample Types: Plasma
-
Gut Microbiota and Development of Vibrio cholerae-Specific Long-Term Memory B Cells in Adults after Whole-Cell Killed Oral Cholera Vaccine
Authors: D Chac, TR Bhuiyan, A Saha, MM Alam, U Salma, N Jahan, F Chowdhury, AI Khan, ET Ryan, R LaRocque, JB Harris, F Qadri, AA Weil
Infection and Immunity, 2021-08-16;89(9):e0021721.
Species: Human
Sample Types: Cell Culture Supernates
-
Mimicking of Blood Flow Results in a Distinct Functional Phenotype in Human Non-Adherent Classical Monocytes
Authors: E Wirthgen, M Hornschuh, IM Wrobel, C Manteuffel, J Däbritz
Biology, 2021-08-04;10(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Home-based exercise training influences gut bacterial levels in multiple sclerosis
Authors: M Mokhtarzad, M Molanouri, M Abolhasani, B Bakhshi, MA Sahraian, LS Quinn, R Negaresh
Complementary therapies in clinical practice, 2021-07-30;45(0):101463.
Species: Human
Sample Types: Serum
-
Kras-driven intratumoral heterogeneity triggers infiltration of M2 polarized macrophages via the circHIPK3/PTK2 immunosuppressive circuit
Authors: T Katopodi, S Petanidis, K Domvri, P Zarogoulid, D Anestakis, C Charalampi, D Tsavlis, C Bai, H Huang, L Freitag, W Hohenforst, D Matthaios, K Porpodis
Scientific Reports, 2021-07-29;11(1):15455.
Species: Human
Sample Types: Cell Culture Supernates
-
Jellyfish Collagen: A Biocompatible Collagen Source for 3D Scaffold Fabrication and Enhanced Chondrogenicity
Authors: Z Ahmed, LC Powell, N Matin, A Mearns-Spr, CA Thornton, IM Khan, LW Francis
Marine Drugs, 2021-07-22;19(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial
Authors: V Mocanu, Z Zhang, EC Deehan, DH Kao, N Hotte, S Karmali, DW Birch, KK Samarasing, J Walter, KL Madsen
Nature Medicine, 2021-07-05;27(7):1272-1279.
Species: Human
Sample Types: Serum
-
Novel immunomodulatory properties of low dose cytarabine entrapped in a mannosylated cationic liposome
Authors: AL Martel, NL Fraleigh, E Picard, JD Lewicky, G Pawelec, H Lee, GW Ma, L Mousavifar, R Roy, HT Le
International journal of pharmaceutics, 2021-07-01;0(0):120849.
Species: Human
Sample Types: Cell Culture Supernates
-
Th17/Treg-Related Intracellular Signaling in Patients with Chronic Obstructive Pulmonary Disease: Comparison between Local and Systemic Responses
Authors: JD Lourenço, WR Teodoro, DF Barbeiro, APP Velosa, LEF Silva, JB Kohler, AR Moreira, MV Aun, IC da Silva, FLA Fernandes, EM Negri, JL Gross, IFLC Tibério, JT Ito, FDTQS Lopes
Cells, 2021-06-22;10(7):.
Species: Human
Sample Types: Tissue Homogenates
-
Bacterial genotoxins induce T�cell senescence
Authors: SL Mathiasen, L Gall-Mas, IS Pateras, SDP Theodorou, MRJ Namini, MB Hansen, OCB Martin, CK Vadivel, K Ntostoglou, D Butter, M Givskov, C Geisler, AN Akbar, VG Gorgoulis, T Frisan, N Ødum, T Krejsgaard
Cell Reports, 2021-06-08;35(10):109220.
Species: Human
Sample Types: Cell Culture Supernates
-
Psychological symptoms and salivary inflammatory biomarkers in patients with dentofacial deformities: a case-control study
Authors: MCC Volkweis, GW Neculqueo, RDS Freitas, APA Dagnino, GG Fritscher, TQ Irigaray, MM Campos
Scientific Reports, 2021-05-26;11(1):11083.
Species: Human
Sample Types: Saliva
-
Severe T cell hyporeactivity in ventilated COVID-19 patients correlates with prolonged virus persistence and poor outcomes
Authors: K Renner, T Schwittay, S Chaabane, J Gottschlin, C Müller, C Tiefenböck, JN Salewski, F Winter, S Buchtler, S Balam, MV Malferthei, M Lubnow, D Lunz, B Graf, F Hitzenbich, F Hanses, H Poeck, M Kreutz, E Orsó, R Burkhardt, T Niedermair, C Brochhause, A Gessner, B Salzberger, M Mack
Nature Communications, 2021-05-21;12(1):3006.
Species: Human
Sample Types: Cell Culture Supernates
-
Impact of the Uncoupling Protein 1 on Cardiovascular Risk in Patients with Rheumatoid Arthritis
Authors: LI Lyngfelt, MC Erlandsson, M Nadali, S Hedjazifar, R Pullerits, KM Andersson, P Brembeck, ST Silfverswä, U Smith, MI Bokarewa
Cells, 2021-05-07;10(5):.
Species: Human
Sample Types: Serum
-
Macrophage miR-210 induction and metabolic reprogramming in response to pathogen interaction boost life-threatening inflammation
Authors: F Virga, F Cappelless, B Stijlemans, AT Henze, R Trotta, J Van Audena, AS Mirchandan, MA Sanchez-Ga, J Vandewalle, F Orso, C Riera-Domi, A Griffa, C Ivan, E Smits, D Laoui, F Martelli, L Langouche, G Van den Be, O Feron, B Ghesquière, H Prenen, C Libert, SR Walmsley, C Corbet, JA Van Ginder, M Ivan, D Taverna, M Mazzone
Science Advances, 2021-05-07;7(19):.
Species: Mouse
Sample Types: Plasma
-
Plasma markers in pulmonary hypertension subgroups correlate with patient survival
Authors: T Koudstaal, D van Uden, JAC van Hulst, P Heukels, IM Bergen, LW Geenen, VJM Baggen, AE van den Bo, LM van den To, PP Chandoesin, M Kool, E Boersma, RW Hendriks, KA Boomars
Respiratory Research, 2021-05-04;22(1):137.
Species: Human
Sample Types: Plasma
-
Neutrophil extracellular trap clearance by synovial macrophages in gout
Authors: JH Jeong, SJ Choi, SM Ahn, JS Oh, YG Kim, CK Lee, B Yoo, S Hong
Arthritis Research & Therapy, 2021-03-19;23(1):88.
Species: Human
Sample Types: Cell Culture Supernates
-
NF&kapppaB-Activated COX2/PGE2/EP4 Axis Controls the Magnitude and Selectivity of BCG-Induced Inflammation in Human Bladder Cancer Tissues
Authors: OM Ibrahim, PH Basse, W Jiang, K Guru, G Chatta, P Kalinski
Cancers, 2021-03-16;13(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
Fructose reprogrammes glutamine-dependent oxidative metabolism to support LPS-induced inflammation
Authors: N Jones, J Blagih, F Zani, A Rees, DG Hill, BJ Jenkins, CJ Bull, D Moreira, AIM Bantan, JG Cronin, D Avancini, GW Jones, DK Finlay, KH Vousden, EE Vincent, CA Thornton
Nature Communications, 2021-02-22;12(1):1209.
Species: Human
Sample Types: Cell Culture Supernates
-
Ex vivo modelling of PD-1/PD-L1 immune checkpoint blockade under acute, chronic, and exhaustion-like conditions of T-cell stimulation
Authors: A Roberts, L Bentley, T Tang, F Stewart, C Pallini, J Juvvanapud, GR Wallace, AJ Cooper, A Scott, D Thickett, ST Lugg, H Bancroft, B Hemming, C Ferris, G Langman, A Robinson, J Chapman, B Naidu, T Pinkney, GS Taylor, K Brock, Z Stamataki, CA Brady, SJ Curnow, J Gordon, O Qureshi, NM Barnes
Scientific Reports, 2021-02-17;11(1):4030.
Species: Human
Sample Types: Cell Culture Supernates
-
New Biomarker in Chagas Disease: Extracellular Vesicles Isolated from Peripheral Blood in Chronic Chagas Disease Patients Modulate the Human Immune Response
Authors: RP Madeira, LM Dal'Mas Ro, P de Cássia, C Mady, BM Ianni, AC Torrecilha
Journal of Immunology Research, 2021-01-11;2021(0):6650670.
Species: Human
Sample Types: Cell Culture Supernates
-
A new cytokine-based dynamic stratification during induction is highly predictive of survivals in acute myeloid leukemia
Authors: P Peterlin, J Gaschet, T Guillaume, A Garnier, M Eveillard, A Le Bourgeo, M Cherel, C Debord, Y Le Bris, O Theisen, C Godon, B Mahé, V Dubruille, S Wuilleme, C Touzeau, T Gastinne, N Blin, A Lok, B Tessoulin, S Le Gouill, P Moreau, MC Béné, P Chevallier
Cancer Medicine, 2020-12-25;0(0):.
Species: Human
Sample Types: Serum
-
SARS-CoV-2-specific IgA and limited inflammatory cytokines are present in the stool of select patients with acute COVID-19
Authors: GJ Britton, A Chen-Liaw, F Cossarini, AE Livanos, MP Spindler, T Plitt, J Eggers, I Mogno, A Gonzalez-R, S Siu, M Tankelevic, L Grinspan, RE Dixon, D Jha, G Martinez-D, F Amanat, DA Hoagland, B tenOever, MC Dubinsky, M Merad, H van Bakel, F Krammer, G Bongers, S Mehandru, JJ Faith
medRxiv : the preprint server for health sciences, 2020-12-09;0(0):.
Species: Human
Sample Types: Fecal Supernates
-
Monocytes differentiated into macrophages and dendritic cells in the presence of human IFN-&lambda3 or IFN-&lambda4 show distinct phenotypes
Authors: M De, A Bhushan, S Chinnaswam
J Leukoc Biol, 2020-11-17;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
An observational study of innate immune responses in patients with acute appendicitis
Authors: T Peeters, S Martens, V D'Onofrio, MHT Stappers, JCH van der Hi, B Houben, R Achten, LAB Joosten, IC Gyssens
Sci Rep, 2020-10-15;10(1):17352.
Species: Human
Sample Types: Cell Culture Supernates
-
Human Mesenchymal Stromal Cell Secretome Promotes the Immunoregulatory Phenotype and Phagocytosis Activity in Human Macrophages
Authors: M Holopainen, U Impola, P Lehenkari, S Laitinen, E Kerkelä
Cells, 2020-09-22;9(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Design and characterization of Squalene-Gusperimus nanoparticles for modulation of innate immunity
Authors: CE Navarro Ch, BJ de Haan, MM Faas, AM Smink, L Sierra, P de Vos, BL López
Int J Pharm, 2020-09-19;590(0):119893.
Species: Human
Sample Types: Cell Culture Supernates
-
Erythromycin inhibits neutrophilic inflammation and mucosal disease by upregulating DEL-1
Authors: T Maekawa, H Tamura, H Domon, T Hiyoshi, T Isono, D Yonezawa, N Hayashi, N Takahashi, K Tabeta, T Maeda, M Oda, A Ziogas, V? Alexaki, T Chavakis, Y Terao, G Hajishenga
JCI Insight, 2020-08-06;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Cholesterol metabolism drives regulatory B cell IL-10 through provision of geranylgeranyl pyrophosphate
Authors: JA Bibby, HA Purvis, T Hayday, A Chandra, K Okkenhaug, S Rosenzweig, I Aksentijev, M Wood, HJ Lachmann, C Kemper, AP Cope, E Perucha
Nat Commun, 2020-07-08;11(1):3412.
Species: Human
Sample Types: Cell Culture Supernates
-
Topical Administration of SLN-Based Gene Therapy for the Treatment of Corneal Inflammation by De Novo IL-10 Production
Authors: M Vicente-Pa, I Gómez-Agua, J Rodríguez-, A Rodríguez-, E Muntoni, L Battaglia, A Del Pozo-R, MÁ Solinís As
Pharmaceutics, 2020-06-23;12(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
FTY720 ameliorates GvHD by blocking T lymphocyte migration to target organs and by skin fibrosis inhibition
Authors: J Ryu, J Jhun, MJ Park, JA Baek, SY Kim, KH Cho, JW Choi, SH Park, JY Choi, ML Cho
J Transl Med, 2020-06-06;18(1):225.
Species: Human
Sample Types: Cell Culture Supernates
-
Human interleukin-4-treated regulatory macrophages promote epithelial wound healing and reduce colitis in a mouse model
Authors: TS Jayme, G Leung, A Wang, ML Workentine, S Rajeev, A Shute, BE Callejas, N Mancini, PL Beck, R Panaccione, DM McKay
Sci Adv, 2020-06-05;6(23):eaba4376.
Species: Human
Sample Types: Cell Culture Supernates
-
Gaps in Study Design for Immune Parameter Research for Latent Tuberculosis Infection: A Systematic Review
Authors: M Herrera, C Vera, Y Keynan, ZV Rueda
J Immunol Res, 2020-04-21;2020(0):8074183.
Species: Human
Sample Types: Cell Culture Supernates
-
Narciclasine, an isocarbostyril alkaloid, has preferential activity against primary effusion lymphoma
Authors: R Gopalakris, H Matta, S Choi, PM Chaudhary
Sci Rep, 2020-03-31;10(1):5712.
Species: Mouse
Sample Types: Plasma
-
Monocytes from neonates and adults have a similar capacity to adapt their cytokine production after previous exposure to BCG and beta-glucan
Authors: R Namakula, LCJ de Bree, TH A Tvedt, MG Netea, S Cose, K Hanevik
PLoS ONE, 2020-02-21;15(2):e0229287.
Species: Human
Sample Types: Serum
-
Are the Immune Properties of Mesenchymal Stem Cells from Wharton's Jelly Maintained during Chondrogenic Differentiation?
Authors: C Voisin, G Cauchois, L Reppel, C Laroye, L Louarn, C Schenowitz, P Sonon, I Poras, V Wang, E D Carosell, N Benkirane-, P Moreau, N Rouas-Frei, D Bensoussan, C Huselstein
J Clin Med, 2020-02-04;9(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Pancreatic cancer-derived small extracellular vesical Ezrin regulates macrophage polarization and promotes metastasis
Authors: YT Chang, HY Peng, CM Hu, SC Huang, SC Tien, YM Jeng
Am J Cancer Res, 2020-01-01;10(1):12-37.
Species: Human
Sample Types: Cell Culture Supernates
-
Human immune cells infiltrate the spinal cord and impair recovery after spinal cord injury in humanized mice
Authors: RS Carpenter, RR Jiang, FH Brennan, JCE Hall, MK Gottipati, S Niewiesk, PG Popovich
Sci Rep, 2019-12-13;9(1):19105.
Species: Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Replication of Genome-Wide Association Analysis Identifies New Susceptibility Loci at Long Noncoding RNA Regions for Vogt-Koyanagi-Harada Disease
Authors: J Qi, L Du, J Deng, Y Qin, G Su, S Hou, M Lv, Q Zhang, A Kijlstra, P Yang
Invest. Ophthalmol. Vis. Sci., 2019-11-01;60(14):4820-4829.
Species: Human
Sample Types: Cell Culture Supernates
-
Echinochrome A Reduces Colitis in Mice and Induces In Vitro Generation of Regulatory Immune Cells
Authors: SJ Oh, Y Seo, JS Ahn, YY Shin, JW Yang, HK Kim, J Han, NP Mishchenko, SA Fedoreyev, VA Stonik, HS Kim
Mar Drugs, 2019-10-31;17(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Comparative Investigation of Frankincense Nutraceuticals: Correlation of Boswellic and Lupeolic Acid Contents with Cytokine Release Inhibition and Toxicity against Triple-Negative Breast Cancer Cells
Authors: M Schmiech, SJ Lang, J Ulrich, K Werner, LJ Rashan, T Syrovets, T Simmet
Nutrients, 2019-10-02;11(10):.
Species: Human
Sample Types: Plasma
-
β-Glucan-Induced Trained Immunity Protects against Leishmania braziliensis Infection: a Crucial Role for IL-32
Authors: JC Dos Santos, AM Barroso de, MV Teodoro Si, B Cirovic, LCJ de Bree, MSMA Damen, SJCFM Moorlag, RS Gomes, MM Helsen, M Oosting, ST Keating, A Schlitzer, MG Netea, F Ribeiro-Di, LAB Joosten
Cell Rep, 2019-09-03;28(10):2659-2672.e6.
Species: Human
Sample Types: Cell Culture Supernates
-
LIF regulates CXCL9 in tumor-associated macrophages and prevents CD8+ T cell tumor-infiltration impairing anti-PD1 therapy
Authors: M Pascual-Ga, E Bonfill-Te, E Planas-Rig, C Rubio-Pere, R Iurlaro, A Arias, I Cuartas, A Sala-Hojma, L Escudero, F Martínez-R, I Huber-Ruan, P Nuciforo, L Pedrosa, C Marques, I Braña, E Garralda, M Vieito, M Squatrito, E Pineda, F Graus, C Espejo, J Sahuquillo, J Tabernero, J Seoane
Nat Commun, 2019-06-11;10(1):2416.
Species: Human
Sample Types: Tissue Homogenates
-
The human glomerular endothelial cells are potent pro-inflammatory contributors in an in vitro model of lupus nephritis
Authors: P Dimou, RD Wright, KL Budge, A Midgley, SC Satchell, M Peak, MW Beresford
Sci Rep, 2019-06-06;9(1):8348.
Species: Human
Sample Types: Cell Culture Supernates
-
Newly defined ABCB5+ dermal mesenchymal stem cells promote healing of chronic iron overload wounds via secretion of interleukin-1 receptor antagonist
Authors: S Vander Bek, JC de Vries, B Meier-Schi, P Meyer, D Jiang, A Sindrilaru, FF Ferreira, A Hainzl, S Schatz, J Muschhamme, NJ Scheurmann, P Kampilafko, AM Seitz, L Dürselen, A Ignatius, MA Kluth, C Ganss, M Wlaschek, K Singh, P Maity, NY Frank, MH Frank, K Scharffett
Stem Cells, 2019-05-13;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of vimentin in modulating immune cell apoptosis and inflammatory responses in sepsis
Authors: L Su, P Pan, P Yan, Y Long, X Zhou, X Wang, R Zhou, B Wen, L Xie, D Liu
Sci Rep, 2019-04-05;9(1):5747.
Species: Human
Sample Types: Cell Culture Supernates
-
Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis
Authors: C Wei, C Yang, S Wang, D Shi, C Zhang, X Lin, Q Liu, R Dou, B Xiong
Mol. Cancer, 2019-03-30;18(1):64.
Species: Human
Sample Types: Cell Culture Supernates
-
Lower levels of leptin are associated with severity parameters in visceral leishmaniasis patients
Authors: AMDC Fievez, ML Silva-Frei, AQ Sousa, JR Santos-Oli, AM Da-Cruz
PLoS ONE, 2019-03-26;14(3):e0214413.
Species: Human
Sample Types: Serum
-
Effect of Propofol on the Production of Inflammatory Cytokines by Human Polarized Macrophages
Authors: T Kochiyama, X Li, H Nakayama, M Kage, Y Yamane, K Takamori, K Iwabuchi, E Inada
Mediators Inflamm., 2019-03-17;2019(0):1919538.
Species: Human
Sample Types: Cell Culture Supernates
-
Large-scale secretome analyses unveil the superior immunosuppressive phenotype of umbilical cord stromal cells as compared to other adult mesenchymal stromal cells
Authors: A Islam, I Urbarova, JA Bruun, I Martinez-Z
Eur Cell Mater, 2019-02-20;37(0):153-174.
Species: Human
Sample Types: Cell Culture Supernates
-
D-2-Hydroxyglutarate and L-2-Hydroxyglutarate Inhibit IL-12 Secretion by Human Monocyte-Derived Dendritic Cells
Authors: I Ugele, ZE Cárdenas-C, K Hammon, M Wehrstein, C Bruss, K Peter, K Singer, E Gottfried, J Boesch, P Oefner, K Dettmer, K Renner, M Kreutz
Int J Mol Sci, 2019-02-10;20(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of continuous moderate exercise with partial blood flow restriction during hemodialysis: A protocol for a randomized clinical trial
Authors: RK Cardoso, AM Araujo, RB Orcy, M Bohlke, JP Oses, FB Del Vecchi, FC Barcellos, MC Gonzalez, AJ Rombaldi
MethodsX, 2019-01-23;6(0):190-198.
Species: Human
Sample Types: Serum
-
PDGF enhances the protective effect of adipose stem cell-derived extracellular vesicles in a model of acute hindlimb ischemia
Authors: T Lopatina, E Favaro, C Grange, M Cedrino, A Ranghino, S Occhipinti, S Fallo, F Buffolo, DA Gaykalova, MM Zanone, R Romagnoli, G Camussi
Sci Rep, 2018-12-04;8(1):17458.
Species: Human
Sample Types: Cell Culture Supernates
-
Plasminogen Activator Inhibitor-1 Promotes the Recruitment and Polarization of Macrophages in Cancer
Authors: MH Kubala, V Punj, VR Placencio-, H Fang, GE Fernandez, R Sposto, YA DeClerck
Cell Rep, 2018-11-20;25(8):2177-2191.e7.
Species: Human
Sample Types: Cell Culture Supernates
-
Combining Calcium Phosphates with Polysaccharides: A Bone-Inspired Material Modulating Monocyte/Macrophage Early Inflammatory Response
Authors: H Rammal, C Bour, M Dubus, L Entz, L Aubert, SC Gangloff, S Audonnet, NB Bercu, F Boulmedais, C Mauprivez, H Kerdjoudj
Int J Mol Sci, 2018-11-03;19(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Decreased allergy incidence in children supplemented with E. coli O83:K24:H31 and its possible modes of action
Authors: J Hrdý, K Vlasáková, V ?erný, L Súkeníková, O Novotná, P Petrásková, K Boráková, R Lodinová-Ž, L Kolá?ová, L Prokešová
Eur. J. Immunol., 2018-10-21;0(0):.
Species: Human
Sample Types: Plasma
-
Proteome Analysis Reveals the Conidial Surface Protein CcpA Essential for Virulence of the Pathogenic Fungus Aspergillus fumigatus
Authors: V Voltersen, MG Blango, S Herrmann, F Schmidt, T Heinekamp, M Strassburg, T Krüger, P Bacher, J Lother, E Weiss, K Hünniger, H Liu, P Hortschans, A Scheffold, J Löffler, S Krappmann, S Nietzsche, O Kurzai, H Einsele, O Kniemeyer, SG Filler, U Reichard, AA Brakhage
MBio, 2018-10-02;9(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Human Mesenchymal Stem Cell Secretome from Bone Marrow or Adipose-Derived Tissue Sources for Treatment of Hypoxia-Induced Pulmonary Epithelial Injury
Authors: N Shologu, M Scully, JG Laffey, D O'Toole
Int J Mol Sci, 2018-09-30;19(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
Insufficient IL-10 Production as a Mechanism Underlying the Pathogenesis of Systemic Juvenile Idiopathic Arthritis
Authors: M Imbrechts, A Avau, J Vandenhaut, B Malengier-, K Put, T Mitera, N Berghmans, O Burton, S Junius, A Liston, L de Somer, C Wouters, P Matthys
J. Immunol., 2018-09-28;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Plasmodium falciparum Treated with Artemisinin-based Combined Therapy Exhibits Enhanced Mutation, Heightened Cortisol and TNF-? Induction
Authors: AO Idowu, S Bhattachar, S Gradus, W Oyibo, Z George, C Black, J Igietseme, AA Azenabor
Int J Med Sci, 2018-09-07;15(13):1449-1457.
Species: Human
Sample Types: Plasma
-
Relationship of systemic IL-10 levels with proinflammatory cytokine responsiveness and lung function in agriculture workers.
Authors: Tricia D LeVan, Debra J Romberger, Mohammad Siahpush, Brandon L Grimm, Athena K Ramos, Patrik L Johansson, Tzeyu L Michaud, Art J Heires, Todd A Wyatt, Jill A Poole
Respiratory Research, 2018-09-03;0(0):1465-993X.
Species: Human
Sample Types: Cell Culture Supernates
-
Annexin A1, FPR2/ALX, and inflammatory cytokine expression in peritoneal endometriosis
Authors: LK Volpato, VV Horewicz, F Bobinski, DF Martins, AP Piovezan
J. Reprod. Immunol., 2018-08-03;129(0):30-35.
Species: Human
Sample Types: Peritoneal Fluid
-
Salmonella Activation of STAT3 Signaling by SarA Effector Promotes Intracellular Replication and Production of IL-10
Authors: SL Jaslow, KD Gibbs, WF Fricke, L Wang, KJ Pittman, MK Mammel, JT Thaden, VG Fowler, GE Hammer, JR Elfenbein, DC Ko
Cell Rep, 2018-06-19;23(12):3525-3536.
Species: Human
Sample Types: Cell Culture Supernates
-
Diminished circulating plasma and elevated lymph node culture supernatant levels of IL-10 family cytokines in tuberculous lymphadenitis
Authors: GR Kathamuthu, NP Kumar, K Moideen, D Baskaran, S Hissar, BM Shrinivasa, R Sridhar, S Babu
Cytokine, 2018-06-02;0(0):.
Species: Human
Sample Types: Plasma
-
Helicobacter pylori-derived heat shock protein 60 increases the induction of regulatory T-cells associated with persistent infection
Authors: WT Hsu, SY Ho, TY Jian, HN Huang, YL Lin, CH Chen, TH Lin, MS Wu, CJ Wu, YL Chan, KW Liao
Microb. Pathog., 2018-04-13;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Fecal Galectin-3: A New Promising Biomarker for Severity and Progression of Colorectal Carcinoma
Authors: M Jovanovic, N Gajovic, N Zdravkovic, M Jovanovic, M Jurisevic, D Vojvodic, V Maric, A Arsenijevi, I Jovanovic
Mediators Inflamm., 2018-04-04;2018(0):8031328.
Species: Human
Sample Types: Feces
-
Analysis of the association between Fc receptor family gene polymorphisms and ocular Beh�et's disease in Han Chinese
Authors: D Zhang, J Qin, L Li, G Su, G Huang, Q Cao, A Kijlstra, P Yang
Sci Rep, 2018-03-19;8(1):4850.
Species: Human
Sample Types: Cell Culture Supernates
-
Association of Long Noncoding RNAs Polymorphisms With Ankylosing Spondylitis, Vogt-Koyanagi-Harada Disease, and Behcet's Disease
Authors: Y Yue, J Zhang, L Yang, S Liu, J Qi, Q Cao, C Zhou, Y Wang, A Kijlstra, P Yang, S Hou
Invest. Ophthalmol. Vis. Sci., 2018-02-01;59(2):1158-1166.
Species: Human
Sample Types: Cell Culture Supernates
-
Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation.
Authors: Guedan S, Posey A, Shaw C, Wing A, Da T, Patel P, McGettigan S, Casado-Medrano V, Kawalekar O, Uribe-Herranz M, Song D, Melenhorst J, Lacey S, Scholler J, Keith B, Young R, June C
JCI Insight, 2018-01-11;3(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Collagen-Based Medical Device as a Stem Cell Carrier for Regenerative Medicine
Authors: L Aubert, M Dubus, H Rammal, C Bour, C Mongaret, C Boulagnon-, R Garnotel, C Schneider, R Rahouadj, C Laurent, SC Gangloff, F Velard, C Mauprivez, H Kerdjoudj
Int J Mol Sci, 2017-10-21;18(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
Dexamethasone induced inhibition of Dectin-1 activation of antigen presenting cells is mediated via STAT-3 and NF-?B signaling pathways.
Authors: Philipp Kotthoff, Annkristin Heine, Stefanie Andrea Erika Held, Peter Brossart
Scientific Reports, 2017-07-03;0(0):2045-2322.
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophil Extracellular Traps Reprogram IL-4/GM-CSF-Induced Monocyte Differentiation to Anti-inflammatory Macrophages
Authors: AB Guimarães-, NC Rochael, F Oliveira, J Echevarria, EM Saraiva
Front Immunol, 2017-05-17;8(0):523.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro
Authors: E Redondo-Ca, C Cunningham, J Miller, L Martuscell, S Aoulad-Ali, NJ Rothwell, CM Kielty, SM Allan, E Pinteaux
Stem Cell Res Ther, 2017-04-17;8(1):79.
Species: Human
Sample Types: Cell Culture Supernates
-
The role of indoleamine 2,3-dioxygenase-aryl hydrocarbon receptor pathway in the TLR4-induced tolerogenic phenotype in human DCs
Authors: F Salazar, D Awuah, OH Negm, F Shakib, AM Ghaemmagha
Sci Rep, 2017-03-03;7(0):43337.
Species: Human
Sample Types: Cell Culture Supernates
-
Periodontitis and type 2 diabetes among women with previous gestational diabetes: epidemiological and immunological aspects in a follow-up of three years
Authors: RP Esteves Li, LO Cota, TA Silva, SC Cortelli, JR Cortelli, FO Costa
J Appl Oral Sci, 2017-03-01;25(2):130-139.
Species: Human
Sample Types: Saliva
-
Serological signatures of clinical cure following successful treatment with sodium stibogluconate in Ethiopian visceral leishmaniasis
Cytokine, 2016-12-08;91(0):6-9.
Species: Human
Sample Types: Serum
-
Receptor-Type Protein-Tyrosine Phosphatase ? and Colony Stimulating Factor-1 Receptor in the Intestine: Cellular Expression and Cytokine- and Chemokine Responses by Interleukin-34 and Colony Stimulating Factor-1
PLoS ONE, 2016-11-29;11(11):e0167324.
Species: Human
Sample Types: Cell Culture Supernates
-
Reduced systemic and mycobacterial antigen-stimulated concentrations of IL-1? and IL-18 in tuberculous lymphadenitis
Cytokine, 2016-10-26;90(0):66-72.
Species: Human
Sample Types: Cell Culture Supernates
-
Expression of Tim-1 in primary CNS lymphoma
Cancer Med, 2016-10-05;5(11):3235-3245.
Species: Human
Sample Types: Cell Culture Supernates
-
Acanthamoeba castellanii (Genotype T4) Stimulates the Production of Interleukin-10 as well as Pro-inflammatory Cytokines in THP-1 Cells, Human Peripheral Blood Mononuclear Cells and Human Monocyte-Derived Macrophages
Infect Immun, 2016-09-19;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Pleural Effusions from Patients with Mesothelioma Induce Recruitment of Monocytes and Their Differentiation into M2 Macrophages.
Authors: Chene A, d'Almeida S, Blondy T, Tabiasco J, Deshayes S, Fonteneau J, Cellerin L, Delneste Y, Gregoire M, Blanquart C
J Thorac Oncol, 2016-07-12;11(10):1765-73.
Species: Human
Sample Types: Cell Culture Supernates
-
Analysis of receptor tyrosine kinase genetics identifies two novel risk loci in GAS6 and PROS1 in Beh�et's disease
Sci Rep, 2016-05-25;6(0):26662.
Species: Human
Sample Types: Cell Culture Supernates
-
Genetic polymorphisms of cell adhesion molecules in Behcet's disease in a Chinese Han population
Authors: M Zheng, L Zhang, H Yu, J Hu, Q Cao, G Huang, Y Huang, G Yuan, A Kijlstra, P Yang
Sci Rep, 2016-04-25;6(0):24974.
Species: Human
Sample Types: Cell Culture Supernates
-
Soluble alpha-enolase activates monocytes by CD14-dependent TLR4 signalling pathway and exhibits a dual function
Authors: C Guillou, M Fréret, E Fondard, C Derambure, G Avenel, ML Golinski, M Verdet, O Boyer, F Caillot, P Musette, T Lequerré, O Vittecoq
Sci Rep, 2016-03-30;6(0):23796.
Species: Human
Sample Types: Cell Culture Supernates
-
In vitro progesterone modulation on bacterial endotoxin-induced production of IL-1beta, TNFalpha, IL-6, IL-8, IL-10, MIP-1alpha, and MMP-9 in pre-labor human term placenta.
Authors: Garcia-Ruiz G, Flores-Espinosa P, Preciado-Martinez E, Bermejo-Martinez L, Espejel-Nunez A, Estrada-Gutierrez G, Maida-Claros R, Flores-Pliego A, Zaga-Clavellina V
Reprod Biol Endocrinol, 2015-10-07;13(0):115.
Species: Human
Sample Types: Cell Culture Supernates
-
LPS-Stimulated Whole Blood Cytokine Production Is Not Related to Disease Behavior in Patients with Quiescent Crohn's Disease.
Authors: Broekman M, Roelofs H, Hoentjen F, Wiegertjes R, Stoel N, Joosten L, de Jong D, Wanten G
PLoS ONE, 2015-07-24;10(7):e0133932.
Species: Human
Sample Types: Plasma
-
The BAFF receptor TACI controls IL-10 production by regulatory B cells and CLL B cells.
Authors: Saulep-Easton D, Vincent F, Quah P, Wei A, Ting S, Croce C, Tam C, Mackay F
Leukemia, 2015-07-03;30(1):163-72.
Species: Human
Sample Types: Cell Culture Supernates
-
Ligation of TLR7 on CD19(+) CD1d(hi) B cells suppresses allergic lung inflammation via regulatory T cells.
Authors: Khan A, Amu S, Saunders S, Hams E, Blackshields G, Leonard M, Weaver C, Sparwasser T, Sheils O, Fallon P
Eur J Immunol, 2015-04-14;45(6):1842-54.
Species: Human
Sample Types: Cell Culture Supernates
-
Exposure to diesel exhaust particle extracts (DEPe) impairs some polarization markers and functions of human macrophages through activation of AhR and Nrf2.
Authors: Jaguin M, Fardel O, Lecureur V
PLoS ONE, 2015-02-24;10(2):e0116560.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model.
Authors: Guirado E, Mbawuike U, Keiser T, Arcos J, Azad A, Wang S, Schlesinger L
MBio, 2015-02-17;6(1):e02537-14.
Species: Human
Sample Types: Cell Culture Supernates
-
The effects of cytokines on spontaneous hepatitis B surface antigen seroconversion in chronic hepatitis B virus infection.
Authors: Wu J, Hsu H, Chiu Y, Chen H, Ni Y, Chang M
J Immunol, 2014-12-12;194(2):690-6.
Species: Human
Sample Types: Serum
-
Sourcing of an alternative pericyte-like cell type from peripheral blood in clinically relevant numbers for therapeutic angiogenic applications.
Authors: Blocki A, Wang Y, Koch M, Goralczyk A, Beyer S, Agarwal N, Lee M, Moonshi S, Dewavrin J, Peh P, Schwarz H, Bhakoo K, Raghunath M
Mol Ther, 2014-12-12;23(3):510-22.
Species: Human
Sample Types: Cell Culture Supernates
-
Macrophages and dendritic cells as actors in the immune reaction of classical Hodgkin lymphoma.
Authors: Tudor C, Bruns H, Daniel C, Distel L, Hartmann A, Gerbitz A, Buettner M
PLoS ONE, 2014-12-03;9(12):e114345.
Species: Human
Sample Types: Cell Culture Supernates
-
Tumour microenvironment of both early- and late-stage colorectal cancer is equally immunosuppressive.
Authors: O'Toole A, Michielsen A, Nolan B, Tosetto M, Sheahan K, Mulcahy H, Winter D, Hyland J, O'Connell P, Fennelly D, O'Donoghue D, O'Sullivan J, Doherty G, Ryan E
Br J Cancer, 2014-07-24;111(5):927-32.
Species: Human
Sample Types: Cell Culture Supernates
-
Human CD4+ T cell responses to the dog major allergen Can f 1 and its human homologue tear lipocalin resemble each other.
Authors: Liukko A, Kinnunen T, Rytkonen-Nissinen M, Kailaanmaki A, Randell J, Maillere B, Virtanen T
PLoS ONE, 2014-05-29;9(5):e98461.
Species: Human
Sample Types: Cell Culture Supernates
-
A functional variant of PTPN22 confers risk for Vogt-Koyanagi-Harada syndrome but not for ankylosing spondylitis.
Authors: Zhang Q, Qi J, Hou S, DU L, Yu H, Cao Q, Zhou Y, Liao D, Kijlstra A, Yang P
PLoS ONE, 2014-05-09;9(5):e96943.
Species: Human
Sample Types: Cell Culture Supernates
-
Autocrine IL-10 promotes human B-cell differentiation into IgM- or IgG-secreting plasmablasts.
Authors: Heine G, Drozdenko G, Grun J, Chang H, Radbruch A, Worm M
Eur J Immunol, 2014-03-11;44(6):1615-21.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunoepidemiological profiling of onchocerciasis patients reveals associations with microfilaria loads and ivermectin intake on both individual and community levels.
Authors: Arndts, Kathrin, Specht, Sabine, Debrah, Alexande, Tamarozzi, Francesc, Klarmann Schulz, Ute, Mand, Sabine, Batsa, Linda, Kwarteng, Alexande, Taylor, Mark, Adjei, Ohene, Martin, Coralie, Layland, Laura E, Hoerauf, Achim
PLoS Negl Trop Dis, 2014-02-20;8(2):e2679.
Species: Human
Sample Types: Cell Culture Supernates
-
cmvIL-10 stimulates the invasive potential of MDA-MB-231 breast cancer cells.
Authors: Valle Oseguera, Cendy A, Spencer, Juliet V
PLoS ONE, 2014-02-10;9(2):e88708.
Species: Human
Sample Types: Cell Culture Supernates
-
IFN- alpha blocks IL-17 production by peripheral blood mononuclear cells in patients with chronic active hepatitis B Infection.
Authors: Cui F, Meng J, Luo P, Chen P
BMC Infect Dis, 2014-02-01;14(0):55.
Species: Human
Sample Types: Cell Culture Supernates
-
Host biomarkers distinguish dengue from leptospirosis in Colombia: a case-control study.
Authors: Conroy A, Gelvez M, Hawkes M, Rajwans N, Liles W, Villar-Centeno L, Kain K
BMC Infect Dis, 2014-01-20;14(0):35.
Species: Human
Sample Types: Serum
-
CD137 ligand signalling induces differentiation of primary acute myeloid leukaemia cells.
Authors: Cheng K, Wong S, Linn Y, Ho L, Chng W, Schwarz H
Br J Haematol, 2014-01-15;165(1):134-44.
Species: Human
Sample Types: Cell Culture Supernates
-
IFNgamma/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children.
Authors: Jagannathan P, Eccles-James I, Bowen K, Nankya F, Auma A, Wamala S, Ebusu C, Muhindo M, Arinaitwe E, Briggs J, Greenhouse B, Tappero J, Kamya M, Dorsey G, Feeney M
PLoS Pathog, 2014-01-09;10(1):e1003864.
Species: Human
Sample Types: Plasma
-
TNF-alpha blockade induces IL-10 expression in human CD4+ T cells.
Authors: Evans H, Roostalu U, Walter G, Gullick N, Frederiksen K, Roberts C, Sumner J, Baeten D, Gerwien J, Cope A, Geissmann F, Kirkham B, Taams L
Nat Commun, 2014-01-01;5(0):3199.
Species: Human
Sample Types: Cell Culture Supernates
-
Selective activation of human dendritic cells by OM-85 through a NF-kB and MAPK dependent pathway.
Authors: Parola C, Salogni L, Vaira X, Scutera S, Somma P, Salvi V, Musso T, Tabbia G, Bardessono M, Pasquali C, Mantovani A, Sozzani S, Bosisio D
PLoS ONE, 2013-12-30;8(12):e82867.
Species: Human
Sample Types: Cell Culture Supernates
-
Association of TLR2 gene polymorphisms with ocular Behcet's disease in a Chinese Han population.
Authors: Fang J, Hu R, Hou S, Ye Z, Xiang Q, Qi J, Zhou Y, Kijlstra A, Yang P
Invest Ophthalmol Vis Sci, 2013-12-30;54(13):8384-92.
Species: Human
Sample Types: Cell Culture Supernates
-
Leishmania braziliensis-reactive T cells are down-regulated in long-term cured cutaneous Leishmaniasis, but the renewal capacity of T effector memory compartments is preserved.
Authors: Pereira-Carvalho R, Mendes-Aguiar C, Oliveira-Neto M, Covas C, Bertho A, Da-Cruz A, Gomes-Silva A
PLoS ONE, 2013-11-26;8(11):e81529.
Species: Human
Sample Types: Cell Culture Supernates
-
Independent and interdependent immunoregulatory effects of IL-27, IFN-beta, and IL-10 in the suppression of human Th17 cells and murine experimental autoimmune encephalomyelitis.
Authors: Fitzgerald D, Fonseca-Kelly Z, Cullimore M, Safabakhsh P, Saris C, Zhang G, Rostami A
J Immunol, 2013-03-01;190(7):3225-34.
Species: Human
Sample Types: Cell Culture Supernates
-
Quiescent innate response to infective filariae by human Langerhans cells suggests a strategy of immune evasion.
Authors: Boyd A, Bennuru S, Wang Y, Sanprasert V, Law M, Chaussabel D, Nutman T, Semnani R
Infect Immun, 2013-02-19;81(5):1420-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse.
J Neuroinflammation, 2012-08-18;9(0):203.
Species: Human
Sample Types: Cell Culture Supernates
-
Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation.
Eur J Clin Nutr, 2012-07-18;66(9):1072-4.
Species: Human
Sample Types: Serum
-
Regulation of progranulin expression in human microglia and proteolysis of progranulin by matrix metalloproteinase-12 (MMP-12).
Authors: Suh HS, Choi N, Tarassishin L, Lee SC
PLoS ONE, 2012-04-11;7(4):e35115.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated adaptive immune responses are associated with latent infections of Wuchereria bancrofti.
Authors: Arndts K, Deininger S, Specht S, Klarmann U, Mand S, Adjobimey T, Debrah AY, Batsa L, Kwarteng A, Epp C, Taylor M, Adjei O, Layland LE, Hoerauf A
PLoS Negl Trop Dis, 2012-04-03;6(4):e1611.
Species: Human
Sample Types: Cell Culture Supernates
-
Urinary neutrophil gelatinase-associated lipocalin is a potential biomarker for renal damage in patients with systemic lupus erythematosus.
Authors: Yang CC, Hsieh SC, Li KJ, Wu CH, Lu MC, Tsai CY, Yu CL
J. Biomed. Biotechnol., 2012-02-15;2012(0):759313.
Species: Human
Sample Types: Urine
-
Possible involvement of IL-21 and IL-10 on salivary IgA levels in chronic periodontitis subjects.
Authors: Napimoga MH, Nunes LH, Maciel AA, Demasi AP, Benatti BB, Santos VR, Bastos MF, de Miranda TS, Duarte PM
Scand. J. Immunol., 2011-12-01;74(6):596-602.
Species: Human
Sample Types: Tissue Homogenates
-
IFN-alpha blocks IL-17 production by peripheral blood mononuclear cells in Behcet's disease.
Authors: Liu X, Yang P, Wang C, Li F, Kijlstra A
Rheumatology (Oxford), 2010-11-08;50(2):293-8.
Species: Human
Sample Types: Cell Culture Supernates
-
PEGylation of interleukin-10 for the mitigation of enhanced pain states.
Authors: Soderquist RG, Milligan ED, Harrison JA, Chavez RA, Johnson KW, Watkins LR, Mahoney MJ
J Biomed Mater Res A, 2010-06-01;93(3):1169-79.
Species: Human
Sample Types: CSF
-
Anemia in Hodgkin's lymphoma: the role of interleukin-6 and hepcidin.
Authors: Hohaus S, Massini G, Giachelia M, Vannata B, Bozzoli V, Cuccaro A, D'Alo' F, Larocca LM, Raymakers RA, Swinkels DW, Voso MT, Leone G
J. Clin. Oncol., 2010-04-20;28(15):2538-43.
Species: Human
Sample Types: Plasma
-
Human uterine epithelial cell secretions regulate dendritic cell differentiation and responses to TLR ligands.
Authors: Ochiel DO, Ghosh M, Fahey JV
J. Leukoc. Biol., 2010-04-12;88(3):435-44.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes.
Authors: Pennino D, Eyerich K, Scarponi C, Carbone T, Eyerich S, Nasorri F, Garcovich S, Traidl-Hoffmann C, Albanesi C, Cavani A
J. Immunol., 2010-03-31;184(9):4880-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunoregulation of autocrine prolactin: suppressing the expression of costimulatory molecules and cytokines in T lymphocytes by prolactin receptor knockdown.
Authors: Xu D, Lin L, Lin X
Cell. Immunol., 2010-03-02;263(1):71-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Investigation of functional IL-10 gene polymorphism and IL-10 levels in acute graft-versus-host disease.
Authors: Resende RG, Correia-Silva Jde F, Arao TC, Silva TA, Abreu MH, Bittencourt H, Gomez RS
J. Clin. Immunol., 2010-03-02;30(3):465-73.
Species: Human
Sample Types: Saliva
-
Helminth antigens modulate immune responses in cells from multiple sclerosis patients through TLR2-dependent mechanisms.
Authors: Correale J, Farez M
J. Immunol., 2009-10-07;183(9):5999-6012.
Species: Human
Sample Types: Cell Culture Supernates
-
Reduced ex vivo interleukin-6 production by dietary fish oil is not modified by linoleic acid intake in healthy men.
Authors: Damsgaard CT, Lauritzen L, Calder PC, Kjaer TR, Frokiaer H
J. Nutr., 2009-06-03;139(7):1410-4.
Species: Human
Sample Types: Cell Culture Supernates
-
Clinical significance of interleukin-10 gene polymorphisms and plasma levels in Hodgkin lymphoma.
Authors: Hohaus S, Giachelia M, Massini G, Vannata B, Criscuolo M, Martini M, D'Alo' F, Voso MT, Larocca LM, Leone G
Leuk. Res., 2009-02-08;33(10):1352-6.
Species: Human
Sample Types: Plasma
-
Divergent effects of hypoxia on dendritic cell functions.
Authors: Mancino A, Schioppa T, Larghi P, Pasqualini F, Nebuloni M, Chen IH, Sozzani S, Austyn JM, Mantovani A, Sica A
Blood, 2008-08-11;112(9):3723-34.
Species: Human
Sample Types: Cell Culture Supernates
-
Helminth infections associated with multiple sclerosis induce regulatory B cells.
Authors: Correale J, Farez M, Razzitte G
Ann. Neurol., 2008-08-01;64(2):187-99.
Species: Human
Sample Types: Cell Culture Supernates
-
Adenosine receptors in regulation of dendritic cell differentiation and function.
Authors: Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM
Blood, 2008-06-17;112(5):1822-31.
Species: Human
Sample Types: Cell Culture Supernates
-
Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12.
Authors: Lamhamedi-Cherradi SE, Martin RE, Ito T, Kheradmand F, Corry DB, Liu YJ, Moyle M
J. Immunol., 2008-05-01;180(9):6000-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Reduced response to adiponectin and lower abundance of adiponectin receptor proteins in type 2 diabetic monocytes.
Authors: Weigert J, Neumeier M, Wanninger J, Wurm S, Kopp A, Schober F, Filarsky M, Schaffler A, Zeitoun M, Aslanidis C, Buechler C
FEBS Lett., 2008-04-28;582(12):1777-82.
Species: Human
Sample Types: Cell Culture Supernates
-
Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells.
Authors: Spadaro M, Caorsi C, Ceruti P, Varadhachary A, Forni G, Pericle F, Giovarelli M
FASEB J., 2008-03-25;22(8):2747-57.
Species: Human
Sample Types: Cell Culture Supernates
-
The differential effect of Eriobotrya japonica hydrophilic leaf extract on cytokines production and modulation.
Authors: Matalka KZ, Ali D, Khawad AE, Qa'dan F
Cytokine, 2007-11-26;40(3):235-40.
Species: Human
Sample Types: Cell Culture Supernates
-
Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells.
Authors: Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N, Descamps P, Gamelin E, Gascan H, Hebbar M, Jeannin P
Blood, 2007-09-11;110(13):4319-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2.
Authors: Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z
Cancer Res., 2007-08-01;67(15):7458-66.
-
Airway remodelling in children with cystic fibrosis.
Authors: Hilliard TN, Regamey N, Shute JK, Nicholson AG, Alton EW, Bush A, Davies JC
Thorax, 2007-05-25;62(12):1074-80.
-
Splice variants of human FOXP3 are functional inhibitors of human CD4+ T-cell activation.
Authors: Smith EL, Finney HM, Nesbitt AM, Ramsdell F, Robinson MK
Immunology, 2006-10-01;119(2):203-11.
-
B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease.
Authors: Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA
Am. J. Pathol., 2006-09-01;169(3):987-98.
Species: Human
Sample Types: Tissue Homogenates
-
Early cytokine responses after percutaneous magnetic resonance imaging guided laser thermoablation of malignant liver tumors.
Authors: Kallio R, Sequeiros R, Surcel HM, Ohtonen P, Kiviniemi H, Syrjala H
Cytokine, 2006-07-25;34(5):278-83.
-
Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists.
Authors: Fritz JH, Girardin SE, Fitting C, Werts C, Mengin-Lecreulx D, Caroff M, Cavaillon JM, Philpott DJ, Adib-Conquy M
Eur. J. Immunol., 2005-08-01;35(8):2459-70.
Species: Human
Sample Types: Cell Culture Supernates
-
Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: implications for Burkitt's lymphoma.
Authors: Pound JD, Batth BK, Johannessen I, Wood K
J. Immunol., 2005-03-01;174(5):3015-23.
Species: Human
Sample Types: Cell Culture Supernates
-
Association of type 2 cytokines with hepatic fibrosis in human Schistosoma mansoni infection.
Authors: de Jesus AR, Magalhaes A, Miranda DG, Miranda RG, Araujo MI, de Jesus AA, Silva A, Santana LB, Pearce E, Carvalho EM
Infect. Immun., 2004-06-01;72(6):3391-7.
Species: Human
Sample Types: Cell Culture Supernates
-
The Toll-like receptor-2/6 agonist macrophage-activating lipopeptide-2 cooperates with IFN-gamma to reverse the Th2 skew in an in vitro allergy model.
Authors: Weigt H, Muhlradt PF, Larbig M, Krug N, Braun A
J. Immunol., 2004-05-15;172(10):6080-6.
Species: Human
Sample Types: Cell Culture Supernates
-
Differential effect of serotonin on cytokine production in lipopolysaccharide-stimulated human peripheral blood mononuclear cells: involvement of 5-hydroxytryptamine2A receptors.
Authors: Cloez-Tayarani I, Petit-Bertron AF, Venters HD, Cavaillon JM
Int. Immunol., 2003-02-01;15(2):233-40.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human IL-10 DuoSet ELISA
Average Rating: 4.6 (Based on 18 Reviews)
Have you used Human IL-10 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Human dermal fibroblast cell culture supernatant following stimulation with stem cell derived small extracellular vesicles
Used in both cell culture material (supernatant and lysate) and EDTA plasma. Good intense signal at 450nm corrected for signal at 550nm
I am processing different type of samples following the same protocols and in for some of the samples the results are not consistent.