Human IL-2 Quantikine ELISA Kit
R&D Systems | Catalog # D2050


Key Product Details
Sample Type & Volume Required Per Well
Sensitivity
Assay Range
Product Summary for Human IL-2 Quantikine ELISA Kit
Product Specifications
Assay Type
Format
Measurement
Detection Method
Conjugate
Species
Specificity
Cross-reactivity
Interference
Sample Values
Precision
Intra-Assay Precision (Precision within an assay) Three samples of known concentration were tested twenty times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays) The recovery of human IL-2 spiked to three levels in samples throughout the range of the assay was evaluated.
Cell Culture Supernates
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 67.5 | 438 | 851 | 232 | 620 | 1148 |
| Standard Deviation | 2.9 | 14.4 | 26.9 | 23.2 | 34.4 | 45.5 |
| CV% | 4.3 | 3.3 | 3.2 | 10.0 | 5.5 | 4.0 |
Citrate Plasma, EDTA Plasma, Heparin Plasma, Serum
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 98.0 | 619 | 1183 | 250 | 755 | 1470 |
| Standard Deviation | 4.2 | 18.0 | 24.0 | 12.6 | 27.8 | 74.1 |
| CV% | 4.3 | 2.9 | 2.0 | 5.0 | 3.7 | 5.0 |
Recovery for Human IL-2 Quantikine ELISA Kit
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=5) | 101 | 96-107 |
| Citrate Plasma (n=5) | 105 | 98-111 |
| EDTA Plasma (n=5) | 118 | 104-139 |
| Heparin Plasma (n=5) | 109 | 94-129 |
| Serum (n=5) | 99 | 89-114 |
Linearity
To assess the linearity of the assay, samples were spiked with high concentrations of human IL-2 in various matrices and diluted with the appropriate calibrator diluent to produce samples with values within the dynamic range of the assay.

Scientific Data Images for Human IL-2 Quantikine ELISA Kit
Human IL-2 ELISA Standard Curve for Cell Culture Supernate Assay
Human IL-2 ELISA Standard Curve for Serum/Plasma Assay
Preparation and Storage
Shipping
Stability & Storage
Background: IL-2
Long Name
Alternate Names
Entrez Gene IDs
Gene Symbol
Additional IL-2 Products
Product Documents for Human IL-2 Quantikine ELISA Kit
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Note: Certificate of Analysis not available for kit components.
Product Specific Notices for Human IL-2 Quantikine ELISA Kit
For research use only
⚠ WARNING: This product can expose you to chemicals including N,N-Dimethylforamide, which is known to the State of California to cause cancer. For more information, go to www.P65Warnings.ca.gov.Related Research Areas
Citations for Human IL-2 Quantikine ELISA Kit
Customer Reviews for Human IL-2 Quantikine ELISA Kit (25)
Have you used Human IL-2 Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Customer Images
-
Sample Tested: Cell Culture SamplesVerified Customer | Posted 04/03/2023
-
Sample Tested: Serum and PlasmaVerified Customer | Posted 02/27/2023
-
Sample Tested: Whole blood T cellsVerified Customer | Posted 02/25/2023
-
Sample Tested: SerumVerified Customer | Posted 06/14/2022
-
Sample Tested: SerumVerified Customer | Posted 06/07/2022
-
Sample Tested: Cell culture supernatantVerified Customer | Posted 09/28/2021
-
Sample Tested: Peripheral blood mononuclear cells (PBMCs)Verified Customer | Posted 03/16/2021
-
Sample Tested: PlasmaVerified Customer | Posted 12/20/2020
-
Sample Tested: SerumVerified Customer | Posted 12/12/2020
-
Sample Tested: Serum-free Cell Culture SupernatesVerified Customer | Posted 11/24/2020This ELISA performed as expected with low % CV's
-
Sample Tested: Product medium matrixVerified Customer | Posted 10/19/2020
-
Sample Tested: Blood monocytesVerified Customer | Posted 10/18/2020
-
Sample Tested: Serum and PlasmaVerified Customer | Posted 10/12/2020Great assay that is easy to use and provides correct results. The typical range for healthy patients is very low, this test shows that accurately.
-
Sample Tested: Serum-free Cell Culture MediaVerified Customer | Posted 01/06/2020
-
Sample Tested: Cell culture supernatantVerified Customer | Posted 12/08/2019
-
Sample Tested: Cell culture supernatantVerified Customer | Posted 12/08/2019
-
Sample Tested: Jurkat human acute T cell leukemia cell lineVerified Customer | Posted 04/04/2019
-
Sample Tested: MOLT-4 human acute lymphoblastic leukemia cell lineVerified Customer | Posted 04/04/2019
-
Sample Tested: Jurkat human acute T cell leukemia cell line and MOLT-4 human acute lymphoblastic leukemia cell lineVerified Customer | Posted 03/21/2019
-
Sample Tested: A375 human melanoma cell lineVerified Customer | Posted 03/21/2019
-
Sample Tested: SerumVerified Customer | Posted 11/02/2018
-
Sample Tested: Serum and PlasmaVerified Customer | Posted 04/04/2018
-
Sample Tested: SerumVerified Customer | Posted 03/30/2018
-
Sample Tested: Human PBMC after stimulation in cultureVerified Customer | Posted 03/22/2018
-
Sample Tested: mouse serumVerified Customer | Posted 11/03/2017Used mice treated with human IL-2 product to test the IL-2 clearance in the blood. Were able to use our own IL-2 construct to make a trustable standard curve and comparable with the kit's products. We use this kit daily.
There are no reviews that match your criteria.
Protocols
View specific protocols for Human IL-2 Quantikine ELISA Kit (D2050):
- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 100 µL of Assay Diluent to each well.
- Add 100 µL of Standard, control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process twice for a total of 3 washes.
- Add 200 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours.
- Aspirate and wash 3 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Find general support by application which include: protocols, troubleshooting, illustrated assays, videos and webinars.
- ELISA Sample Preparation & Collection Guide
- ELISA Troubleshooting Guide
- How to Run an R&D Systems DuoSet ELISA
- How to Run an R&D Systems Quantikine ELISA
- How to Run an R&D Systems Quantikine™ QuicKit™ ELISA
- Quantikine HS ELISA Kit Assay Principle, Alkaline Phosphatase
- Quantikine HS ELISA Kit Principle, Streptavidin-HRP Polymer
- Sandwich ELISA (Colorimetric) – Biotin/Streptavidin Detection Protocol
- Sandwich ELISA (Colorimetric) – Direct Detection Protocol
- Troubleshooting Guide: ELISA
- View all Protocols, Troubleshooting, Illustrated assays and Webinars
Associated Pathways
 Hematopoietic Stem Cell Differentiation Pathways & Lineage-specific Markers
Hematopoietic Stem Cell Differentiation Pathways & Lineage-specific Markers
 IL-2 Signaling Pathways
IL-2 Signaling Pathways
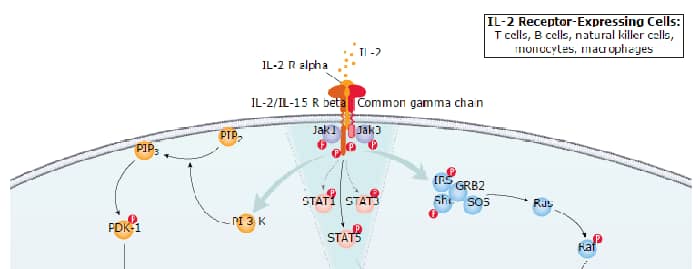 IL-2 Signaling Pathways and their Primary Biological Effects in Different Immune Cell Types
IL-2 Signaling Pathways and their Primary Biological Effects in Different Immune Cell Types
 IL-15 Signaling Pathways and their Primary Biological Effects in Different Immune Cell Types
IL-15 Signaling Pathways and their Primary Biological Effects in Different Immune Cell Types

 Jak/STAT Signaling Pathway
Jak/STAT Signaling Pathway
 Th1 Differentiation Pathway
Th1 Differentiation Pathway
 Th2 Differentiation Pathway
Th2 Differentiation Pathway












