Human Lysyl Oxidase Homolog 2/LOXL2 Antibody Summary
Gln26-Gln774
Accession # AAH00594
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human Lysyl Oxidase Homolog 2/LOXL2 by Western Blot. Western blot shows lysates of HEC-1-B human endometrial adenocarcinoma cell line and U-87 MG human glioblastoma/astrocytoma cell line. PVDF Membrane was probed with 1 µg/mL of Goat Anti-Human Lysyl Oxidase Homolog 2/LOXL2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF2639) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF019). A specific band was detected for Lysyl Oxidase Homolog 2/LOXL2 at approximately 105 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 8.
 View Larger
View Larger
Detection of Human Lysyl Oxidase Homolog 2/LOXL2 by Simple WesternTM. Simple Western lane view shows lysates of U-87 MG human glioblastoma/astrocytoma cell line, loaded at 0.2 mg/mL. A specific band was detected for Lysyl Oxidase Homolog 2/LOXL2 at approximately 109 kDa (as indicated) using 10 µg/mL of Goat Anti-Human Lysyl Oxidase Homolog 2/LOXL2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF2639) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
Detection of Human Lysyl Oxidase Homolog 2/LOXL2 by Knockdown Validated LOXL2 is responsible for increased collagen crosslinking activity in hypoxic EC‐derived exosomes. (A) LOXL2 mRNA expression is decreased in shLOXL2‐transfected EC (shLOXL2) compared to control‐infected EC (shCtrl) (n = 7 ± SD, Student's t‐test). (B) LOXL2 protein expression is decreased in shLOXL2‐transfected EC‐derived exosomes. (C) Analysis of lysyl oxidase activity in exosomes from control (shCtrl) and LOXL2 knock‐down (shLOXL2) (n = 3 ± SD, Student's t‐test). (D) Collagen gels comparing control buffer (Vehicle), and exosomes from control (Exosomes shCtrl) and LOXL2 knock‐down (Exosomes shLOXL2) EC, and (E) quantification of gel contraction after 36 hrs for these conditions (n = 3 ± SD, anova). Collagen crosslinking activity of exosomes from control and LOXL2 knock‐down EC at 20% (Exosomes Control and shLOXL2 Control) and 2% O2 (shLOXL2 Hypoxia) was assessed using (F) the in vitro lysyl oxidase assay and (G and H) the collagen gel contraction assay (n = 3 ± SD, anova). *P < 0.05; ***P < 0.001. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26612622), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
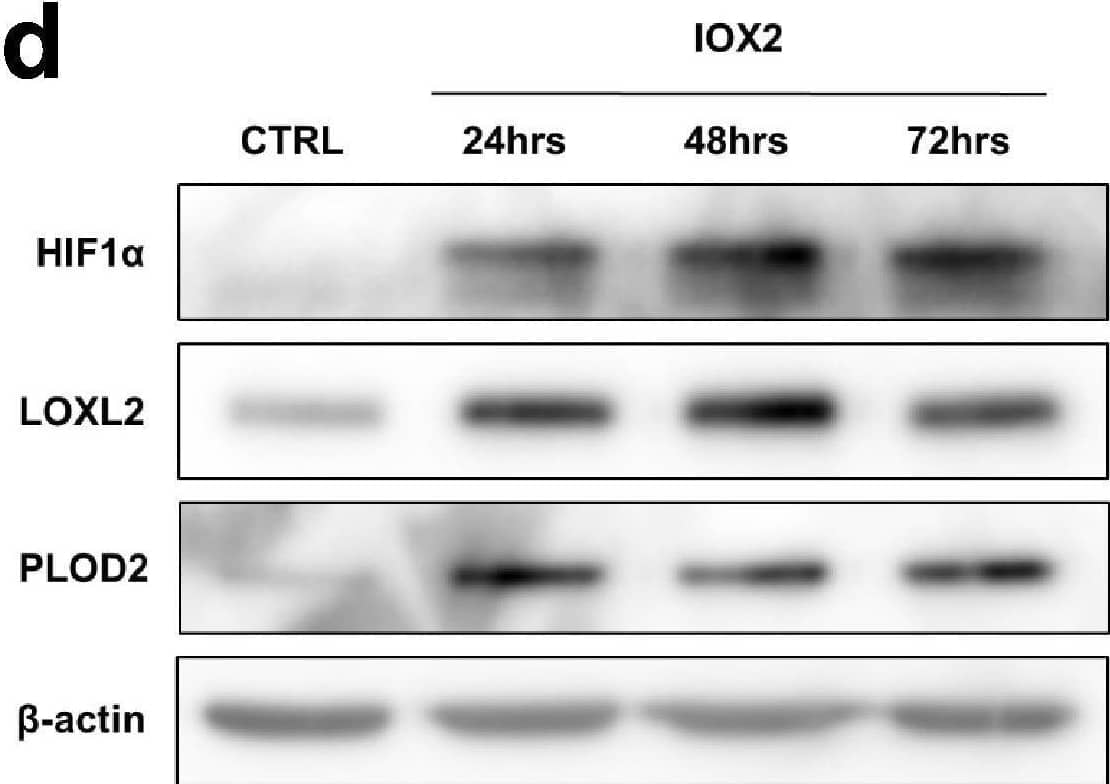
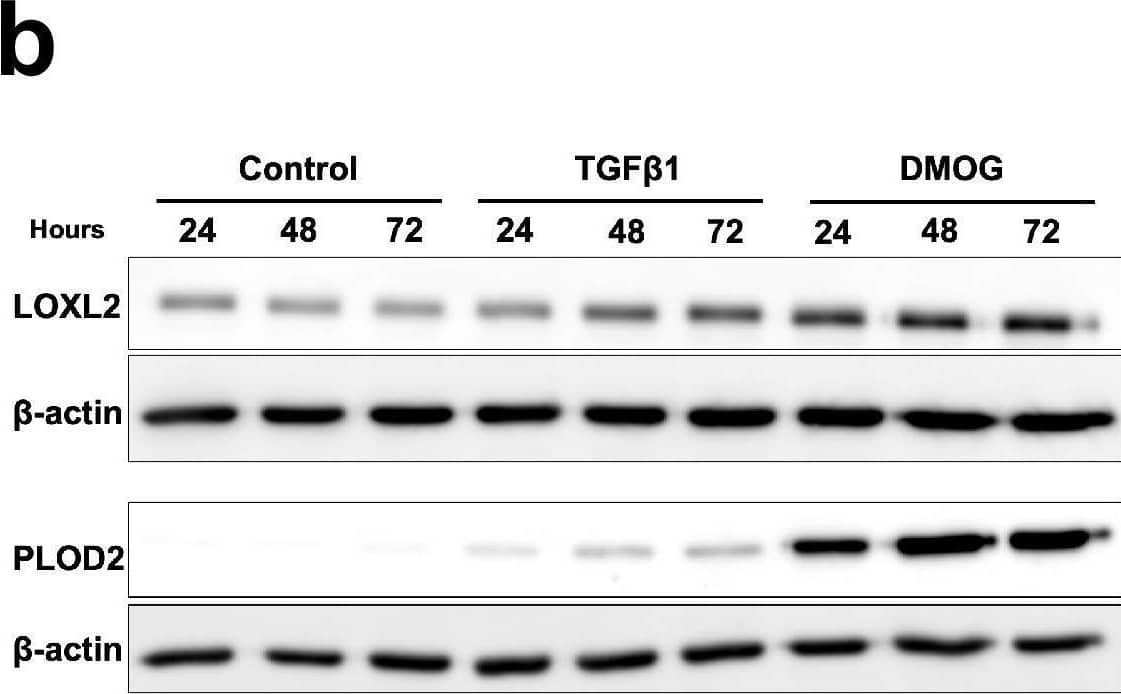
Detection of Human Lysyl Oxidase Homolog 2/LOXL2 by Western Blot Pro-fibrotic signalling pathways in human lung fibroblasts.(A–C) Healthy lung fibroblasts exposed to control, EGF, TGF beta 1, DMOG, Wnt3 alpha or Wnt5 alpha signalling for 24, 48, or 72 hr. n = 3 independent experiments. (A) Protein expression of phospho-ERK, phospho-SMAD2/3, HIF1 alpha, and active beta -catenin at 24 hr of exposure to conditions. beta -actin was used as a loading control. The full blots are shown in Figure 2—figure supplement 1—source data 1. (B) LOXL2 and PLOD2 protein levels at 24, 48, or 72 hr of exposure to conditions. beta -actin was used as a loading control. The full blots are shown in Figure 2—figure supplement 1—source data 1. (C) Expression of COL3A1 in healthy lung fibroblasts exposed to conditions for 24, 48, or 72 hr using the delta delta Ct method. Bars indicate geometric means. ****p < 0.0001 by Dunnett’s multiple comparisons test. (D) Protein expression of HIF1 alpha, LOXL2, and PLOD2 in IPF fibroblasts exposed to control media or IOX2 for 24, 48, or 72 hr. beta -actin was used as a loading control. The full blots are shown in Figure 2—figure supplement 1—source data 1. (E) Fold change in mRNA levels of LOXL2, PLOD2 and the HIF pathway activation marker gene carbonic anhydrase IX/9 (CA9) in MRC5 fibroblasts after incubation in nomoxia (21% O2) or hypoxia (1% O2) for 24 hr. beta -actin-normalised mRNA levels under nomoxia were used to set the baseline value at unity. Data are mean ± s.d. n = 3 samples per group. ****p < 0.0001 using unpaired t test. Figure 2—figure supplement 1—source data 1.Full membrane scans for western blot images for Figure 2—figure supplement 1a, b, d.Full membrane scans for western blot images for Figure 2—figure supplement 1a, b, d. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35188460), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
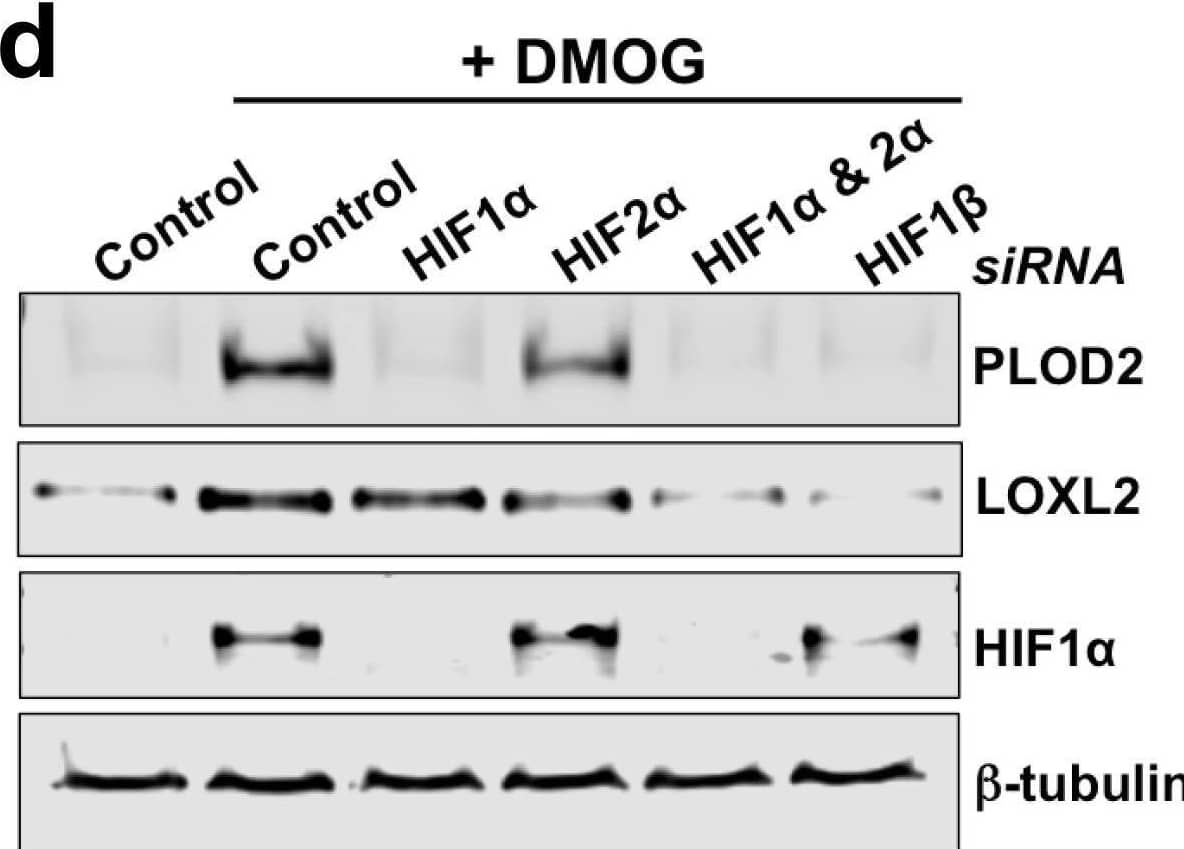
Detection of Human Lysyl Oxidase Homolog 2/LOXL2 by Western Blot HIF pathway activation regulates PLOD2 and LOXL2 expression in lung fibroblasts from patients with IPF.(A) Fold changes in mRNA levels of HIF1 alpha (HIF1A), HIF2 alpha (EPAS1), and HIF1 beta (ARNT) in primary human lung fibroblasts from patients with IPF transfected with indicated siRNA followed by treatment with DMOG. beta -actin-normalised mRNA levels in control cells were used to set the baseline value at unity. Data are mean ± s.d. n = 3 samples per group. (B, C) Fold change in mRNA levels of LOXL2 (B) and PLOD2 (C) in IPF fibroblasts transfected with indicated siRNA followed by treatment with DMOG or vehicle control. beta -actin-normalised mRNA levels in control cells were used to set the baseline value at unity. Data are mean ± s.d. n = 3 samples per group. ns (not significant, p > 0.05); *p < 0.05; ****p < 0.0001 by Dunnett’s multiple comparisons test. (D) PLOD2, LOXL2 and HIF1 alpha and beta -tubulin protein levels in IPF fibroblasts transfected with indicated siRNA followed by treatment of DMSO or DMOG. beta -tubulin was used as a loading control. The full blots are shown in Figure 3—source data 1.Figure 3—source data 1.Full membrane scans for western blot images for Figure 3d.Full membrane scans for western blot images for Figure 3d. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35188460), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Lysyl Oxidase Homolog 2/LOXL2 by Western Blot Pro-fibrotic signalling pathways in human lung fibroblasts.(A–C) Healthy lung fibroblasts exposed to control, EGF, TGF beta 1, DMOG, Wnt3 alpha or Wnt5 alpha signalling for 24, 48, or 72 hr. n = 3 independent experiments. (A) Protein expression of phospho-ERK, phospho-SMAD2/3, HIF1 alpha, and active beta -catenin at 24 hr of exposure to conditions. beta -actin was used as a loading control. The full blots are shown in Figure 2—figure supplement 1—source data 1. (B) LOXL2 and PLOD2 protein levels at 24, 48, or 72 hr of exposure to conditions. beta -actin was used as a loading control. The full blots are shown in Figure 2—figure supplement 1—source data 1. (C) Expression of COL3A1 in healthy lung fibroblasts exposed to conditions for 24, 48, or 72 hr using the delta delta Ct method. Bars indicate geometric means. ****p < 0.0001 by Dunnett’s multiple comparisons test. (D) Protein expression of HIF1 alpha, LOXL2, and PLOD2 in IPF fibroblasts exposed to control media or IOX2 for 24, 48, or 72 hr. beta -actin was used as a loading control. The full blots are shown in Figure 2—figure supplement 1—source data 1. (E) Fold change in mRNA levels of LOXL2, PLOD2 and the HIF pathway activation marker gene carbonic anhydrase IX/9 (CA9) in MRC5 fibroblasts after incubation in nomoxia (21% O2) or hypoxia (1% O2) for 24 hr. beta -actin-normalised mRNA levels under nomoxia were used to set the baseline value at unity. Data are mean ± s.d. n = 3 samples per group. ****p < 0.0001 using unpaired t test. Figure 2—figure supplement 1—source data 1.Full membrane scans for western blot images for Figure 2—figure supplement 1a, b, d.Full membrane scans for western blot images for Figure 2—figure supplement 1a, b, d. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35188460), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
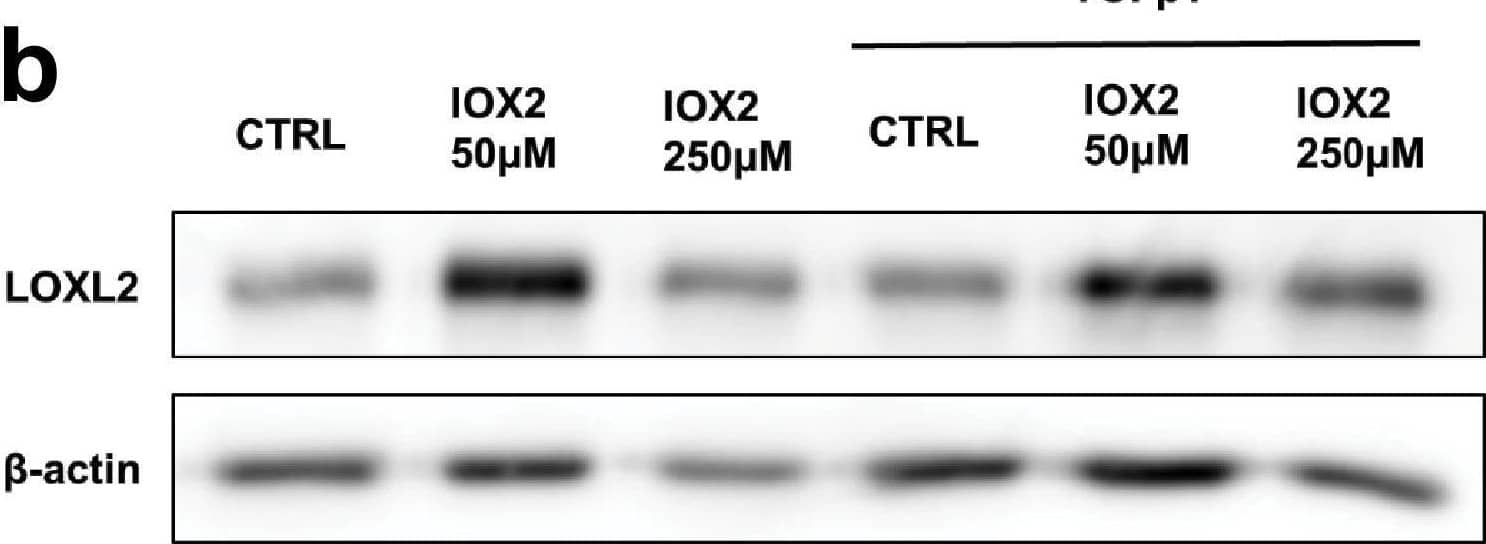
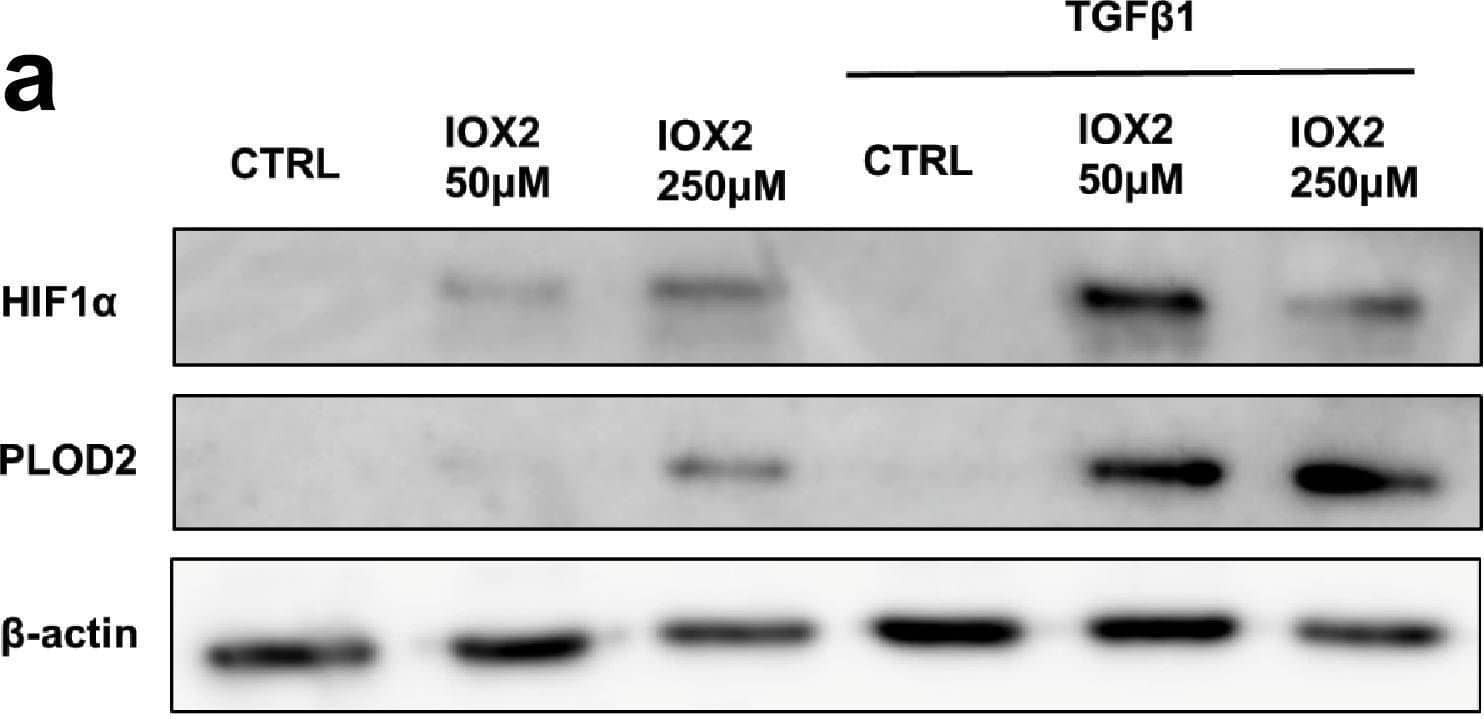
Detection of Human Lysyl Oxidase Homolog 2/LOXL2 by Western Blot IOX2-mediated HIF pathway activation promotes PLOD2 and LOXL2 expression in the 3D in vitro model of fibrosis. Lung fibroblasts from IPF patients were used in the 3D model of fibrosis in the presence of IOX2 or vehicle control as indicated. Protein expression of (A) HIF1 alpha, PLOD2, and (B) LOXL2 following 2 weeks of culture in the presence or absence of TGF beta 1 with or without IOX2 (50 μM or 250 μM) or vehicle control. beta -actin loading control. Blots representative of experiments from two separate IPF donors. The full blots are shown in Figure 5—figure supplement 1—source data 1.Figure 5—figure supplement 1—source data 1.Full membrane scans for western blot images for Figure 5—figure supplement 1a, b.Full membrane scans for western blot images for Figure 5—figure supplement 1a, b. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35188460), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Lysyl Oxidase Homolog 2/LOXL2 by Western Blot IOX2-mediated HIF pathway activation promotes PLOD2 and LOXL2 expression in the 3D in vitro model of fibrosis. Lung fibroblasts from IPF patients were used in the 3D model of fibrosis in the presence of IOX2 or vehicle control as indicated. Protein expression of (A) HIF1 alpha, PLOD2, and (B) LOXL2 following 2 weeks of culture in the presence or absence of TGF beta 1 with or without IOX2 (50 μM or 250 μM) or vehicle control. beta -actin loading control. Blots representative of experiments from two separate IPF donors. The full blots are shown in Figure 5—figure supplement 1—source data 1.Figure 5—figure supplement 1—source data 1.Full membrane scans for western blot images for Figure 5—figure supplement 1a, b.Full membrane scans for western blot images for Figure 5—figure supplement 1a, b. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35188460), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Lysyl Oxidase Homolog 2/LOXL2
Lysyl Oxidase Homolog 2 (lysyl oxidase-like protein 2, LOXL2) is a member of lysyl oxidase-like (LOXL) gene family which includes LOXL1 through LOXL4. These enzymes are secreted copper-binding amine oxidases that oxidize primary amine substrates to aldehydes (1). The N-terminal region of LOXL2 contains four scavenger receptor cysteine-rich (SRCR) domains, and the C-terminal region is a catalytic domain similar to other lysyl oxidases (1). The catalytic domain contains conserved residues required for copper binding and formation of a lysyl tyrosylquinone co-factor (2). Although some of the LOXL enzymes are known to cross-link collagen and elastin substrates, such a function has yet to be characterized for LOXL2. It has been shown that LOXL2 promotes cell migration and tumor cell invasiveness (3, 4). Elevated expression of LOXL2 is also associated with cancer progression in various tumors and carcinoma cell lines, which makes it a potential marker for prognosis of cancer (5). LOXL2 is expressed in many tissues, with elevated levels in reproductive tissues such as placenta, uterus, and prostate (6).
- Csiszar, H. (2001) Prog. Nucleic Acid Res. Mol. Biol. 70:1.
- Maki, J.M. and K.I. Kivirikko (2001) Biochem J. 355:381.
- Akiri, G. et al. (2003) Cancer Res. 63:1657.
- Hollosi, P. et al. (2009) Int. J. Cancer. 125:318.
- Peinado, H. et al. (2008) Cancer Res. 68:4541.
- Jourdan-Le Saux C. et al. (1999) J. Biol. Chem. 274:12939.
Product Datasheets
Citations for Human Lysyl Oxidase Homolog 2/LOXL2 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
7
Citations: Showing 1 - 7
Filter your results:
Filter by:
-
Lysyl oxidase like-2 contributes to renal fibrosis in Col4a3/Alport Mice.
Authors: Dominic Cosgrove, Brianna Dufek, Daniel T. Meehan, Duane Delimont, Michael Hartnett, Gina Samuelson et al.
Kidney International
-
Lysyl oxidase‐like 2 is a regulator of angiogenesis through modulation of endothelial‐to‐mesenchymal transition
Authors: Olivier G. de Jong, Lizet M. van der Waals, Farah R. W. Kools, Marianne C. Verhaar, Bas W. M. van Balkom
Journal of Cellular Physiology
-
Pan-Lysyl Oxidase Inhibitor PXS-5505 Ameliorates Multiple-Organ Fibrosis by Inhibiting Collagen Crosslinks in Rodent Models of Systemic Sclerosis
Authors: Y Yao, A Findlay, J Stolp, B Rayner, K Ask, W Jarolimek
International Journal of Molecular Sciences, 2022-05-16;23(10):.
Species: Human, Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Pseudohypoxic HIF pathway activation dysregulates collagen structure-function in human lung fibrosis
Authors: CJ Brereton, L Yao, ER Davies, Y Zhou, M Vukmirovic, JA Bell, S Wang, RA Ridley, LSN Dean, OG Andriotis, F Conforti, L Brewitz, S Mohammed, T Wallis, A Tavassoli, RM Ewing, A Alzetani, BG Marshall, SV Fletcher, PJ Thurner, A Fabre, N Kaminski, L Richeldi, A Bhaskar, CJ Schofield, M Loxham, DE Davies, Y Wang, MG Jones
Elife, 2022-02-21;11(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Lysyl oxidase‐like 2 is a regulator of angiogenesis through modulation of endothelial‐to‐mesenchymal transition
Authors: Olivier G. de Jong, Lizet M. van der Waals, Farah R. W. Kools, Marianne C. Verhaar, Bas W. M. van Balkom
Journal of Cellular Physiology
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Exosomes from hypoxic endothelial cells have increased collagen crosslinking activity through up-regulation of lysyl oxidase-like 2.
Authors: de Jong OG, van Balkom BW, Gremmels H, Verhaar MC.
J. Cell. Mol. Med.
-
Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes.
Authors: de Jong OG, Verhaar MC, Chen Y et al.
J Extracell Vesicles.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human Lysyl Oxidase Homolog 2/LOXL2 Antibody
There are currently no reviews for this product. Be the first to review Human Lysyl Oxidase Homolog 2/LOXL2 Antibody and earn rewards!
Have you used Human Lysyl Oxidase Homolog 2/LOXL2 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
