Mouse CCL2/JE/MCP-1 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant mouse CCL2/JE. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
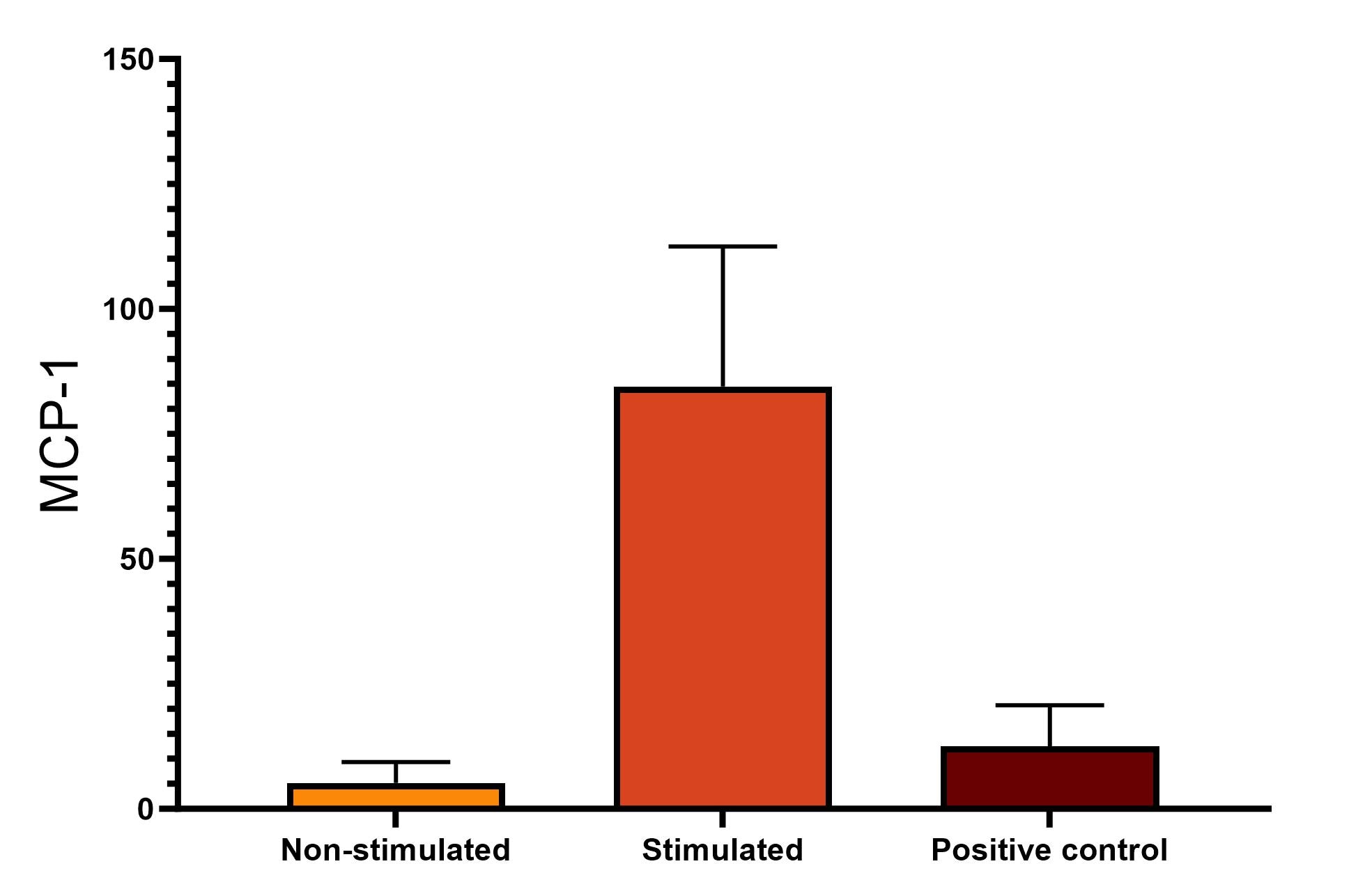
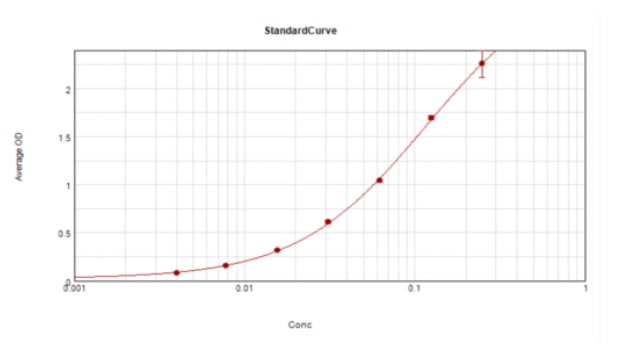
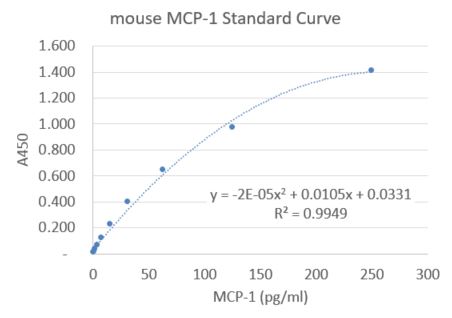
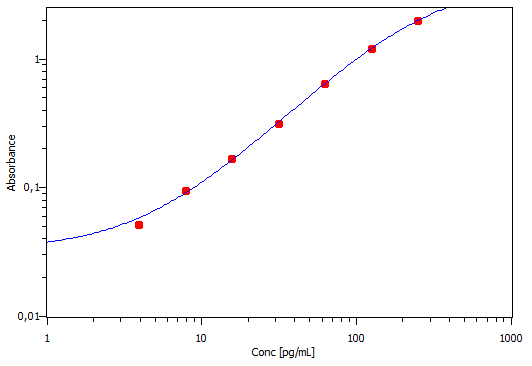
Scientific Data
Product Datasheets
Preparation and Storage
Background: CCL2/JE/MCP-1
CCL2/JE/MCP-1, is a chemokine that binds the receptor CCR2 and induces the chemoattraction of mononuclear cells. It induces the activation of monocytes, NK cells, lymphocytes, and basophils. Additionally, CCL2 promotes Th2 polarization in CD4+ T cells, and CCL2-mediated recruitment of monocytes to sites of inflammation contributes to disease severity in atherosclerosis, multiple sclerosis, and allergic asthma. Endogenous proteolytic trimming of CCL2 at the N-terminus, including the N-terminal pyrrolidone carboxylic acid-modified glutamine, downregulates activity but not receptor binding.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse CCL2/JE/MCP-1 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
178
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Adipocyte FMO3-derived TMAO induces WAT dysfunction and metabolic disorders by promoting inflammasome activation in ageing
Authors: Ganapathy, T;Yuan, J;Ho, MY;Wu, KK;Hoque, MM;Wang, B;Li, X;Wang, K;Wabitsch, M;Feng, X;Niu, Y;Long, K;Lian, Q;Zhu, Y;Cheng, KK;
Nature communications
Species: Mouse
Sample Types: Cell Culture Supernates, Cell Lysates, Tissue Homogenates
-
Characterizing the mechanisms underpinning interleukin-15R?-mediated protection against sepsis and candidiasis
Authors: Yang, J;Xu, B;Cao, J;Liu, Y;Tang, L;Zhao, P;Li, S;Li, X;Liu, J;Yu, R;Tang, Y;Tan, W;Ding, H;Li, J;Liu, Y;
Cytokine
Species: Mouse, Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Microplastic exposure aggravates pneumococcus-induced inflammation in macrophages by activating ferroptosis
Authors: Chang, KW;Chen, JT;Chuang, CN;Thi Thanh Thao, D;Huang, YT;Wu, HY;Kuo, ML;Kao, KC;Chiu, CH;Lai, CH;
Journal of hazardous materials
Species: Mouse
Sample Types: Cell Culture Supernates
-
Spectral immune cell profiling reveals modulations in immune cell response to repetitive inhaled organic dust exposure in a high omega-3 fatty acid mouse model
Authors: Dean, LS;Wahl, M;Threatt, AN;Pauly, M;Barahona, M;Oyewole, EO;Nordgren, TM;
Lipids in health and disease
Species: Mouse
Sample Types: BALF
-
Single-cell RNA sequencing identifies accumulation of Fcgr2b+ virtual memory like CD8 T cells with cytotoxic and inflammatory potential in aged mouse white adipose tissue
Authors: Kumar, A;O'Brien, M;Young, VB;Yung, R;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Bidirectional Interaction Between Chronic Kidney Disease and Porphyromonas gingivalis Infection Drives Inflammation and Immune Dysfunction
Authors: Adamowicz, K;Lima Ribeiro, AS;Golda, A;Wadowska, M;Potempa, J;Schmaderer, C;Anders, HJ;Koziel, J;Lech, M;
Journal of immunology research
Species: Mouse
Sample Types: Serum, Cell Culture Supernates
-
Combination treatment with anti-RANKL and antibiotics for preventing joint destruction in septic arthritis
Authors: Hu, Z;Deshmukh, M;Jarneborn, A;Bollmann, M;Corciulo, C;Kopparapu, PK;Ali, A;Svensson, MND;Engdahl, C;Pullerits, R;Mohammad, M;Jin, T;
JCI insight
Species: Mouse
Sample Types: Tissue Homogenates
-
Chronic Exposure to Two Regimens of Waterpipe Smoke Elicits Lung Injury, Genotoxicity, and Mitochondrial Impairment with the Involvement of MAPKs Activation in Mice
Authors: Hamadi, N;Al?Salam, S;Beegam, S;Zaaba, N;Elzaki, O;Nemmar, A;
International journal of molecular sciences
Species: Mouse
Sample Types: Tissue Homogenates
-
Genetic diversity leads to differential inflammatory responses to cigarette smoke in mice
Authors: Faizan, MI;Kaur, G;Shaikh, SB;Effah, F;Unwalla, H;Rahman, I;
Physiological reports
Species: Mouse
Sample Types: Tissue Homogenates, BALF
-
PGE2 and HCN2 ion channels are critical mediators of pain initiated by angiotensin II
Authors: Pinto, LG;Vilar, B;McNaughton, PA;
Brain, behavior, and immunity
Species: Mouse
Sample Types: Tissue Homogenates
-
Regulation of ROS signaling by TIGAR induces cancer-modulating responses in the tumor microenvironment
Authors: Cheung, EC;Strathdee, D;Stevenson, D;Coomes, J;Blyth, K;Vousden, KH;
Proceedings of the National Academy of Sciences of the United States of America
Species: Mouse
Sample Types: Cell Culture Supernates
-
Inhibiting endothelial cell Mst1 attenuates acute lung injury in mice
Authors: Guo, ZF;Tongmuang, N;Li, C;Zhang, C;Hu, L;Capreri, D;Zuo, MX;Summer, R;Sun, J;
JCI insight
Species: Mouse, Transgenic Mouse
Sample Types: Tissue Homogenates, BALF
-
Local administration of regulatory T cells promotes tissue healing
Authors: Nayer, B;Tan, JL;Alshoubaki, YK;Lu, YZ;Legrand, JMD;Lau, S;Hu, N;Park, AJ;Wang, XN;Amann-Zalcenstein, D;Hickey, PF;Wilson, T;Kuhn, GA;Müller, R;Vasanthakumar, A;Akira, S;Martino, MM;
Nature communications
Species: Mouse
Sample Types: Tissue Homogenates
-
Gestational administration of Bifidobacterium dentium results in intergenerational modulation of inflammatory, metabolic, and social behavior
Authors: Galley, JD;King, MK;Rajasekera, TA;Batabyal, A;Woodke, ST;Gur, TL;
Brain, behavior, and immunity
Species: Mouse
Sample Types: Plasma
-
H7N7 viral infection elicits pronounced, sex-specific neuroinflammatory responses in vitro
Authors: Gabele, L;Bochow, I;Rieke, N;Sieben, C;Michaelsen-Preusse, K;Hosseini, S;Korte, M;
Frontiers in cellular neuroscience
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-10 Enhances the Inhibitory Effect of Adipose-Derived Stromal Cells on Insulin Resistance/Liver Gluconeogenesis by Treg Cell Induction
Authors: Lai, HC;Chen, PH;Tang, CH;Chen, LW;
International journal of molecular sciences
Species: Mouse
Sample Types: Tissue Homogenates
-
Anti-Neuroinflammatory Effects of Ginkgo biloba Extract EGb 761 in LPS-Activated BV2 Microglial Cells
Authors: Sun, L;Apweiler, M;Tirkey, A;Klett, D;Normann, C;Dietz, GPH;Lehner, MD;Fiebich, BL;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Rujin Jiedu decoction protects against influenza virus infection by modulating gut microbiota
Authors: Huang, Q;Yang, G;Tang, C;Dou, B;Hu, Y;Liu, H;Wu, X;Zhang, H;Wang, H;Xu, L;Yang, XD;Xu, Y;Zheng, Y;
Heliyon
Species: Mouse
Sample Types: Cell Culture Supernates
-
Anti-Neuroinflammatory Effects of a Macrocyclic Peptide-Peptoid Hybrid in Lipopolysaccharide-Stimulated BV2 Microglial Cells
Authors: Sun, L;Wilke Saliba, S;Apweiler, M;Akmermer, K;Herlan, C;Grathwol, C;de Oliveira, ACP;Normann, C;Jung, N;Bräse, S;Fiebich, BL;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
The effects of urolithin A on poly I:C-induced microglial activation
Authors: Mingo, YB;Gabele, L;Lonnemann, N;Brône, B;Korte, M;Hosseini, S;
Frontiers in cellular neuroscience
Species: Mouse
Sample Types: Cell Culture Supernates
-
Myeloid PHD2 Conditional Knockout Improves Intraplaque Angiogenesis and Vascular Remodeling in a Murine Model of Venous Bypass Grafting
Authors: Sluiter, TJ;Tillie, RJHA;de Jong, A;de Bruijn, JBG;Peters, HAB;van de Leijgraaf, R;Halawani, R;Westmaas, M;Starink, LIW;Quax, PHA;Sluimer, JC;de Vries, MR;
Journal of the American Heart Association
Species: Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Central activation of the fatty acid sensor GPR120 suppresses microglia reactivity and alleviates sickness- and anxiety-like behaviors
Authors: Nakajima, S;Demers, G;Machuca-Parra, AI;Pour, ZD;Bairamian, D;Bouyakdan, K;Fisette, A;Kabahizi, A;Robb, J;Rodaros, D;Laurent, C;Ferreira, G;Arbour, N;Alquier, T;Fulton, S;
Journal of neuroinflammation
Species: Mouse
Sample Types: Cell Culture Supernates
-
A 5-Lipoxygenase Inhibitor, Zileuton, Modulates Host Immune Responses and Improves Lung Function in a Model of Severe Acute Respiratory Syndrome (SARS) Induced by Betacoronavirus
Authors: Pereira, RDD;Rabelo, RAN;Oliveira, NFM;Porto, SLT;Andrade, ACDSP;Queiroz-Junior, CM;Barbosa, CLN;de Souza-Costa, LP;Santos, FRDS;Oliveira, FBR;da Silva, BLV;Umezu, HL;Ferreira, R;da Silva, GSF;Cruz, JS;Teixeira, MM;Costa, VV;Machado, FS;
Viruses
Species: Mouse
Sample Types: Tissue Homogenates
-
Inhibiting endothelial cell Mst1 attenuates acute lung injury in mice
Authors: Guo, ZF;Tongmuang, N;Li, C;Zhang, C;Hu, L;Capreri, D;Zuo, MX;Summer, R;Sun, J;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Tissue Homogenates, BALF
-
Xinmaikang (XMK) tablets alleviate atherosclerosis by regulating the SREBP2-mediated NLRP3/ASC/Caspase-1 signaling pathway
Authors: Hou, C;Jiang, X;Sheng, W;Zhang, Y;Lin, Q;Hong, S;Zhao, J;Wang, T;Ye, X;
Journal of ethnopharmacology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Pharmacological Inhibition and Genetic Deletion of Cystathionine Gamma-Lyase in Mice Protects against Organ Injury in Sepsis: A Key Role of Adhesion Molecules on Endothelial Cells
Authors: Manandhar, S;Chambers, S;Miller, A;Ishii, I;Bhatia, M;
International journal of molecular sciences
Species: Mouse, Transgenic Mouse
Sample Types: Tissue Homogenates
-
Investigating Chemokine-Matrix Networks in Breast Cancer: Tenascin-C Sets the Tone for CCL2
Authors: Gschwandtner, M;Gammage, AN;Deligne, C;Mies, LFM;Domaingo, A;Murdamoothoo, D;Loustau, T;Schwenzer, A;Derler, R;Carapito, R;Koch, M;M�rgelin, M;Orend, G;Kungl, AJ;Midwood, KS;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Deletion of p75NTR rescues the synaptic but not the inflammatory status in the brain of a mouse model for Alzheimer’s disease
Authors: Hendrik Demuth, Shirin Hosseini, Henning Peter Düsedeau, Ildiko Rita Dunay, Martin Korte, Marta Zagrebelsky
Frontiers in Molecular Neuroscience
Species: Mouse
Sample Types: Tissue Homogenates
-
PIMT is a novel and potent suppressor of endothelial activation
Authors: C Zhang, ZF Guo, W Liu, K Kazama, L Hu, X Sun, L Wang, H Lee, L Lu, XF Yang, R Summer, J Sun
Elife, 2023-04-18;12(0):.
Species: Mouse
Sample Types: Bronchoalveolar lavage fluid
-
Cardiac Myocyte-Specific Overexpression of FASTKD1 Prevents Ventricular Rupture After Myocardial Infarction
Authors: KD Marshall, PJ Klutho, L Song, R Roy, M Krenz, CP Baines
Journal of the American Heart Association, 2023-02-15;12(4):e025867.
Species: Mouse, Transgenic Mouse
Sample Types: Tissue Homogenates
-
Tryptophan-dependent and -independent secretions of tryptophanyl- tRNA synthetase mediate innate inflammatory responses
Authors: TTT Nguyen, YH Choi, WK Lee, Y Ji, E Chun, YH Kim, JE Lee, HS Jung, JH Suh, S Kim, M Jin
Cell Reports, 2022-12-30;42(1):111905.
Species: Mouse
Sample Types: Lavage Fluid
-
Dysregulated systemic metabolism in a Down syndrome mouse model
Authors: DC Sarver, C Xu, LM Velez, S Aja, AE Jaffe, MM Seldin, RH Reeves, GW Wong
Molecular Metabolism, 2022-12-29;68(0):101666.
Species: Mouse
Sample Types: Serum
-
Adipose-targeted triiodothyronine therapy counteracts obesity-related metabolic complications and atherosclerosis with negligible side effects
Authors: K Chen, LY Cheong, Y Gao, Y Zhang, T Feng, Q Wang, L Jin, E Honoré, KSL Lam, W Wang, X Hui, A Xu
Nature Communications, 2022-12-20;13(1):7838.
Species: Mouse
Sample Types: Serum
-
Involvement of glycoinositolphospholipid from Trypanosoma cruzi and macrophage migration inhibitory factor in proinflammatory mechanisms promoting cardiovascular injury mechanisms promoting cardiovascular inflammation tThe combined action of glycoinositolphospholipid from Trypanosoma cruzi and macrophage migration inhibitory factor increases proinflammatory mediator production by cardiomyocytes and vascular endothelial cells
Authors: CS Rigazio, N Mariz-Pont, EP Caballero, FN Penas, NB Goren, MH Santamaría, RS Corral
Microbial pathogenesis, 2022-11-12;0(0):105881.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Oral administration of human carbonic anhydrase I suppresses colitis in a murine inflammatory bowel disease model
Authors: K Tange, S Yagi, E Takeshita, M Abe, Y Yamamoto, H Tomida, T Kawamura, M Hanayama, B Matsuura, Y Ikeda, Y Hiasa
Scientific Reports, 2022-10-26;12(1):17983.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The effects of intermittent hypoxia on hepatic expression of fatty acid translocase CD36 in lean and diet-induced obese mice
Authors: Y Ji, Y Liang, PH Chu, M Ge, SC Yeung, MS Man Ip, JC Wo Mak
Biomedical journal, 2022-10-13;0(0):.
Species: Mouse
Sample Types: Serum
-
A neutrophil-B-cell axis impacts tissue damage control in a mouse model of intraabdominal bacterial infection via Cxcr4
Authors: R Gawish, B Maier, G Obermayer, ML Watzenboec, AD Gorki, F Quattrone, A Farhat, K Lakovits, A Hladik, A Korosec, A Alimohamma, I Mesteri, F Oberndorfe, F Oakley, J Brain, L Boon, I Lang, CJ Binder, S Knapp
Elife, 2022-09-30;11(0):.
Species: Mouse
Sample Types: Plasma
-
Homoharringtonine Attenuates Dextran Sulfate Sodium-Induced Colitis by Inhibiting NF-kappaB Signaling
Authors: J Liu, L Shi, W Huang, Z Zheng, X Huang, Y Su
Mediators of Inflammation, 2022-09-29;2022(0):3441357.
Species: Mouse
Sample Types: Cell Lysates
-
Bone marrow adipocytes drive the development of tissue invasive Ly6Chigh monocytes during obesity
Authors: P Boroumand, DC Prescott, T Mukherjee, PJ Bilan, M Wong, J Shen, I Tattoli, Y Zhou, A Li, T Sivasubram, N Shi, LY Zhu, Z Liu, C Robbins, DJ Philpott, SE Girardin, A Klip
Elife, 2022-09-20;11(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Protective and vaccine dose-sparing efficacy of Poly I:C-functionalized calcium phosphate nanoparticle adjuvants in inactivated influenza vaccination
Authors: J Lee, SY Ahn, CTT Le, DH Lee, J Jung, EJ Ko
International immunopharmacology, 2022-09-14;112(0):109240.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Therapeutic Intervention with Anti-Complement Component 5 Antibody Does Not Reduce NASH but Does Attenuate Atherosclerosis and MIF Concentrations in Ldlr-/-.Leiden Mice
Authors: F Seidel, R Kleemann, W van Duyven, N van Trigt, N Keijzer, S van der Ko, C van Kooten, L Verschuren, A Menke, AJ Kiliaan, J Winter, TR Hughes, BP Morgan, F Baas, K Fluiter, MC Morrison
International Journal of Molecular Sciences, 2022-09-14;23(18):.
Species: Mouse
Sample Types: Plasma
-
Novel Anti-Neuroinflammatory Properties of a Thiosemicarbazone-Pyridylhydrazone Copper(II) Complex
Authors: XY Choo, LE McInnes, A Grubman, JM Wasielewsk, I Belaya, E Burrows, H Quek, JC Martín, S Loppi, A Sorvari, D Rait, A Powell, C Duncan, JR Liddell, H Tanila, JM Polo, T Malm, KM Kanninen, PS Donnelly, AR White
International Journal of Molecular Sciences, 2022-09-14;23(18):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Smoking status impacts treatment efficacy in smoke-induced lung inflammation: A pre-clinical study
Authors: N Milad, M Pineault, F Tremblay, J Routhier, A Lechasseur, MJ Beaulieu, S Aubin, MC Morissette
Frontiers in Pharmacology, 2022-09-07;13(0):971238.
Species: Mouse
Sample Types: BALF
-
A novel mechanism of regulation of the oncogenic transcription factor GLI3 by toll-like receptor signaling
Authors: SJ Matissek, M Karbalivan, W Han, A Boutilier, E Yzar-Garci, LL Kehoe, DS Gardner, A Hage, K Fleck, V Jeffers, R Rajsbaum, SF Elsawa
Oncotarget, 2022-08-03;13(0):944-959.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Evaluation and Characterization of Post-Stroke Lung Damage in a Murine Model of Cerebral Ischemia
Authors: J Faura, L Ramiro, A Simats, F Ma, A Penalba, T Gasull, A Rosell, J Montaner, A Bustamante
International Journal of Molecular Sciences, 2022-07-22;23(15):.
Species: Mouse
Sample Types: Tissue Homogenates
-
HIF-1alpha induces glycolytic reprograming in tissue-resident alveolar macrophages to promote cell survival during acute lung injury
Authors: PS Woods, LM Kimmig, KA Sun, AY Meliton, OR Shamaa, Y Tian, R Cetin-Atal, WW Sharp, RB Hamanaka, GM Mutlu
Elife, 2022-07-13;11(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Unravelling the sex-specific diversity and functions of adrenal gland macrophages
Authors: B Dolfi, A Gallerand, MM Firulyova, Y Xu, J Merlin, A Dumont, A Castiglion, N Vaillant, S Quemener, H Gerke, MI Stunault, PR Schrank, SH Kim, A Zhu, J Ding, J Gilleron, V Magnone, P Barbry, D Dombrowicz, C Duranton, A Wakkach, C Blin-Wakka, B Becher, S Pagnotta, RJ Argüello, P Rantakari, S Chakarov, F Ginhoux, K Zaitsev, KW Kim, L Yvan-Charv, RR Guinamard, JW Williams, S Ivanov
Cell Reports, 2022-06-14;39(11):110949.
Species: Mouse
Sample Types: Serum, Tissue Homogenates
-
Acinetobacter quorum sensing contributes to inflammation-induced inhibition of orthopaedic implant osseointegration
Authors: H Choe, BS Hausman, KM Hujer, O Akkus, PN Rather, Z Lee, RA Bonomo, EM Greenfield
Oncogene, 2022-06-09;43(0):267-276.
Species: Mouse
Sample Types: Tissue Homogenates
-
The antioxidant enzyme Peroxiredoxin-1 controls stroke-associated microglia against acute ischemic stroke
Authors: S Kim, W Lee, H Jo, SK Sonn, SJ Jeong, S Seo, J Suh, J Jin, HY Kweon, TK Kim, SH Moon, S Jeon, JW Kim, YR Kim, EW Lee, HK Shin, SH Park, GT Oh
Redox Biology, 2022-05-25;54(0):102347.
Species: Mouse
Sample Types: Serum
-
Effects of mango and mint pod-based e-cigarette aerosol inhalation on inflammatory states of the brain, lung, heart, and colon in mice
Authors: A Moshensky, CS Brand, H Alhaddad, J Shin, JA Masso-Silv, I Advani, D Gunge, A Sharma, S Mehta, A Jahan, S Nilaad, J Olay, W Gu, T Simonson, D Almarghala, J Pham, S Perera, K Park, R Al-Kolla, H Moon, S Das, M Byun, Z Shah, Y Sari, J Heller Bro, LE Crotty Ale
Elife, 2022-04-12;11(0):.
Species: Mouse
Sample Types: BALF
-
Fibrotic lung disease inhibits innate immune responses to Staphylococcal pneumonia via impaired neutrophil and macrophage function
Authors: HI Warheit-Ni, SJ Edwards, S SenGupta, CA Parent, X Zhou, DN O'Dwyer, BB Moore
JCI Insight, 2022-02-22;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
CCL2-mediated monocytes regulate immune checkpoint blockade resistance in pancreatic cancer
Authors: X Li, G He, J Liu, M Yan, M Shen, L Xu, M An, J Huang, Z Gao
International immunopharmacology, 2022-02-17;106(0):108598.
Species: Mouse
Sample Types: Serum
-
PD-L1 signaling in reactive astrocytes counteracts neuroinflammation and ameliorates neuronal damage after traumatic brain injury
Authors: X Gao, W Li, F Syed, F Yuan, P Li, Q Yu
Journal of Neuroinflammation, 2022-02-08;19(1):43.
Species: Mouse
Sample Types: Tissue Homogenates
-
Chemerin Overexpression in the Liver Protects against Inflammation in Experimental Non-Alcoholic Steatohepatitis
Authors: R Pohl, S Feder, EM Haberl, L Rein-Fisch, TS Weiss, M Spirk, A Bruckmann, N McMullen, CJ Sinal, C Buechler
Biomedicines, 2022-01-07;10(1):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
B7-H3 Suppresses Antitumor Immunity via the CCL2–CCR2–M2 Macrophage Axis and Contributes to Ovarian Cancer Progression
Authors: Taito Miyamoto, Ryusuke Murakami, Junzo Hamanishi, Kenji Tanigaki, Yuko Hosoe, Nathan Mise et al.
Cancer Immunology Research
Species: Mouse
Sample Types: Cell Culture Supernates, Tissue Homogenates
-
The APPL1-Rab5 axis restricts NLRP3 inflammasome activation through early endosomal-dependent mitophagy in macrophages
Authors: KKL Wu, K Long, H Lin, PMF Siu, RLC Hoo, D Ye, A Xu, KKY Cheng
Nature Communications, 2021-11-17;12(1):6637.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Sulforaphane ameliorates non-alcoholic fatty liver disease in mice by promoting FGF21/FGFR1 signaling pathway
Authors: YK Wu, ZN Ren, SL Zhu, YZ Wu, G Wang, H Zhang, W Chen, Z He, XL Ye, QX Zhai
Acta pharmacologica Sinica, 2021-10-15;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
SYK-3BP2 pathway activity in parenchymal and myeloid cells is a key pathogenic factor in metabolic steatohepatitis
Authors: C Luci, E Vieira, M Bourinet, D Rousseau, S Bonnafous, S Patouraux, L Lefevre, F Larbret, V Prod'homme, A Iannelli, A Tran, R Anty, B Bailly-Mai, M Deckert, P Gual
Cellular and Molecular Gastroenterology and Hepatology, 2021-08-16;0(0):.
Species: Mouse
Sample Types: Serum
-
NOTUM promotes thermogenic capacity and protects against diet-induced obesity in male mice
Authors: F Guo, M Seldin, M Péterfy, S Charugundl, Z Zhou, SD Lee, A Mouton, P Rajbhandar, W Zhang, M Pellegrini, P Tontonoz, AJ Lusis, DM Shih
Scientific Reports, 2021-08-12;11(1):16409.
Species: Mouse
Sample Types: Tissue Homogenates
-
Role of the inhibitor of serine peptidase 2 (ISP2) of Trypanosoma brucei rhodesiense in parasite virulence and modulation of the inflammatory responses of the host
Authors: DJ Levy, A Goundry, RSS Laires, TFR Costa, CM Novo, DJ Grab, JC Mottram, APCA Lima
PloS Neglected Tropical Diseases, 2021-06-21;15(6):e0009526.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Tumor microenvironmental cytokines bound to cancer exosomes determine uptake by cytokine receptor-expressing cells and biodistribution
Authors: LG Lima, S Ham, H Shin, EPZ Chai, ESH Lek, RJ Lobb, AF Müller, S Mathivanan, B Yeo, Y Choi, BS Parker, A Möller
Nature Communications, 2021-06-10;12(1):3543.
Species: Mouse
Sample Types: Exosomes
-
DA-DRD5 signaling controls colitis by regulating colonic M1/M2 macrophage polarization
Authors: L Liu, Y Wu, B Wang, Y Jiang, L Lin, X Li, S Yang
Cell Death & Disease, 2021-05-17;12(6):500.
Species: Mouse
Sample Types: Serum
-
Resveratrol-Loaded Lipid-Core Nanocapsules Modulate Acute Lung Inflammation and Oxidative Imbalance Induced by LPS in Mice
Authors: MTP de Oliveir, DS Coutinho, SS Guterres, AR Pohlmann, PMRE Silva, MA Martins, A Bernardi
Pharmaceutics, 2021-05-10;13(5):.
Species: Mouse
Sample Types: Tissue Homogenates
-
AIM2 controls microglial inflammation to prevent experimental autoimmune encephalomyelitis
Authors: Chunmei Ma, Sheng Li, Yingchao Hu, Yan Ma, Yuqing Wu, Chunyan Wu et al.
Journal of Experimental Medicine
Species: Mouse
Sample Types: Cell Culture Supernates
-
Protein Digests and Pure Peptides from Chia Seed Prevented Adipogenesis and Inflammation by Inhibiting PPAR&gamma and NF-&kappaB Pathways in 3T3L-1 Adipocytes
Authors: M Grancieri, HSD Martino, E Gonzalez d
Nutrients, 2021-01-08;13(1):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Respiratory Epithelial Cells Respond to Lactobacillus plantarum but Provide No Cross-Protection against Virus-Induced Inflammation
Authors: E Mai, CM Percopo, AR Limkar, AC Sek, M Ma, HF Rosenberg
Viruses, 2020-12-22;13(1):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Tumor derived UBR5 promotes ovarian cancer growth and metastasis through inducing immunosuppressive macrophages
Authors: M Song, OO Yeku, S Rafiq, T Purdon, X Dong, L Zhu, T Zhang, H Wang, Z Yu, J Mai, H Shen, B Nixon, M Li, RJ Brentjens, X Ma
Nature Communications, 2020-12-08;11(1):6298.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dapagliflozin Does Not Modulate Atherosclerosis in Mice with Insulin Resistance
Authors: A Taberner-C, Á Vinué, A Herrero-Ce, M Aguilar-Ba, JT Real, DJ Burks, S Martínez-H, H González-N
International Journal of Molecular Sciences, 2020-12-03;21(23):.
Species: Mouse
Sample Types: Plasma
-
Unique maternal immune and functional microbial profiles during prenatal stress
Authors: AM Antonson, MV Evans, JD Galley, HJ Chen, TA Rajasekera, SM Lammers, VL Hale, MT Bailey, TL Gur
Sci Rep, 2020-11-20;10(1):20288.
Species: Mouse
Sample Types: Tissue Homogenates
-
Indole-3-carbinol regulates microglia homeostasis and protects the retina from degeneration
Authors: AS Khan, T Langmann
J Neuroinflammation, 2020-11-03;17(1):327.
Species: Mouse
Sample Types: Tissue Homogenates
-
Recombinant Myxoma Virus-Derived Immune Modulator M-T7 Accelerates Cutaneous Wound Healing and Improves Tissue Remodeling
Authors: JR Yaron, L Zhang, Q Guo, EA Awo, M Burgin, LN Schutz, N Zhang, J Kilbourne, J Daggett-Vo, KM Lowe, AR Lucas
Pharmaceutics, 2020-10-22;12(11):.
Species: Mouse
Sample Types: Tissue Homogenates
-
The Small RNA ErsA Plays a Role in the Regulatory Network of Pseudomonas aeruginosa Pathogenicity in Airway Infections
Authors: S Ferrara, A Rossi, S Ranucci, I De Fino, A Bragonzi, C Cigana, G Bertoni
mSphere, 2020-10-14;5(5):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mental stress promotes the proliferation of endometriotic lesions in mice
Authors: T Kawakita, T Kato, T Iwasa, O Erdenebaya, Y Kadota, K Kasai, K Yoshida, M Irahara
Cytokine, 2020-08-06;135(0):155222.
Species: Mouse
Sample Types: Plasma
-
Biological Characterization of Commercial Recombinantly Expressed Immunomodulating Proteins Contaminated with Bacterial Products in the Year 2020: The SAA3 Case
Authors: S Abouelasra, M De Bondt, N Berghmans, M Gouwy, VLS de Oliveir, SC Oliveira, FA Amaral, P Proost, J Van Damme, S Struyf, M De Buck
Mediators Inflamm., 2020-07-06;2020(0):6087109.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Tofacitinib treatment aggravates Staphylococcus aureus septic arthritis, but attenuates sepsis and enterotoxin induced shock in mice
Authors: A Jarneborn, M Mohammad, C Engdahl, Z Hu, M Na, A Ali, T Jin
Sci Rep, 2020-07-02;10(1):10891.
Species: Mouse
Sample Types: Serum
-
Mast Cell Activation, Neuroinflammation, and Tight Junction Protein Derangement in Acute Traumatic Brain Injury
Authors: D Kempuraj, ME Ahmed, GP Selvakumar, R Thangavel, SP Raikwar, SA Zaheer, SS Iyer, C Burton, D James, A Zaheer
Mediators Inflamm., 2020-06-24;2020(0):4243953.
Species: Mouse
Sample Types: Tissue Homogenates
-
The TSPO-NOX1 axis controls phagocyte-triggered pathological angiogenesis in the eye
Authors: A Wolf, M Herb, M Schramm, T Langmann
Nat Commun, 2020-06-01;11(1):2709.
Species: Mouse
Sample Types: Tissue Homogenates
-
The role of Staphylococcus aureus lipoproteins in hematogenous septic arthritis
Authors: M Mohammad, Z Hu, A Ali, PK Kopparapu, M Na, A Jarneborn, MDN Stroparo, MT Nguyen, A Karlsson, F Götz, R Pullerits, T Jin
Sci Rep, 2020-05-13;10(1):7936.
Species: Mouse
Sample Types: Serum
-
Divergent Roles for Macrophage C-type Lectin Receptors, Dectin-1 and Mannose Receptors, in the Intestinal Inflammatory Response
Authors: M Rahabi, G Jacquemin, M Prat, E Meunier, M AlaEddine, B Bertrand, L Lefèvre, K Benmoussa, P Batigne, A Aubouy, J Auwerx, S Kirzin, D Bonnet, M Danjoux, B Pipy, L Alric, H Authier, A Coste
Cell Rep, 2020-03-31;30(13):4386-4398.e5.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Exopolysaccharide of Lactobacillus fermentum UCO-979C Is Partially Involved in Its Immunomodulatory Effect and Its Ability to Improve the Resistance against Helicobacter pylori Infection
Authors: V Garcia-Cas, G Marcial, L Albarracín, M Tomokiyo, P Clua, H Takahashi, H Kitazawa, A Garcia-Can, J Villena
Microorganisms, 2020-03-27;8(4):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin-1 Receptor-Induced Nitric Oxide Production in the Pancreas Controls Hyperglycemia Caused by Scorpion Envenomation
Authors: MB Reis, J Elias-Oliv, MR Pastore, SG Ramos, LG Gardinassi, LH Faccioli
Toxins (Basel), 2020-03-05;12(3):.
Species: Mouse
Sample Types: Serum
-
Fish oil reverses metabolic syndrome, adipocyte dysfunction, and altered adipokines secretion triggered by high-fat diet-induced obesity
Authors: RDC da Cunha d, MM Cruz, TM de Farias, VS da Silva, J de Jesus S, MM Telles, MIC Alonso-Val
Physiol Rep, 2020-02-01;8(4):e14380.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Role of MKP-5 in Adipocyte-Macrophage Interactions during Obesity
Authors: Y Lu, J Ma, J Zhao, Z Song, C Zhou, X Liu, W Teng, W Wang, Q Zhang, W Yan, P Jiao
Obes Facts, 2020-01-21;0(0):1-16.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Rupintrivir reduces RV-induced TH-2 cytokine IL-4 in precision-cut lung slices (PCLS) of HDM-sensitized mice ex vivo
Authors: O Danov, L Lasswitz, H Obernolte, C Hesse, A Braun, S Wronski, K Sewald
Respir. Res., 2019-10-22;20(1):228.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Targeting a therapeutic LIF transgene to muscle via the immune system ameliorates muscular dystrophy
Authors: SS Welc, I Flores, M Wehling-He, J Ramos, Y Wang, C Bertoni, JG Tidball
Nat Commun, 2019-06-26;10(1):2788.
Species: Mouse, Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Immature Low-Density Neutrophils Exhibit Metabolic Flexibility that Facilitates Breast Cancer Liver Metastasis
Authors: BE Hsu, S Tabariès, RM Johnson, S Andrzejews, J Senecal, C Lehuédé, MG Annis, EH Ma, S Völs, L Ramsay, R Froment, A Monast, IR Watson, Z Granot, RG Jones, J St-Pierre, PM Siegel
Cell Rep, 2019-06-25;27(13):3902-3915.e6.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The YIN and YANG of lipoproteins in developing and preventing infectious arthritis by Staphylococcus aureus
Authors: M Mohammad, MT Nguyen, C Engdahl, M Na, A Jarneborn, Z Hu, A Karlsson, R Pullerits, A Ali, F Götz, T Jin
PLoS Pathog., 2019-06-21;15(6):e1007877.
Species: Mouse
Sample Types: Cell Culture Supernates
-
LIF regulates CXCL9 in tumor-associated macrophages and prevents CD8+ T cell tumor-infiltration impairing anti-PD1 therapy
Authors: M Pascual-Ga, E Bonfill-Te, E Planas-Rig, C Rubio-Pere, R Iurlaro, A Arias, I Cuartas, A Sala-Hojma, L Escudero, F Martínez-R, I Huber-Ruan, P Nuciforo, L Pedrosa, C Marques, I Braña, E Garralda, M Vieito, M Squatrito, E Pineda, F Graus, C Espejo, J Sahuquillo, J Tabernero, J Seoane
Nat Commun, 2019-06-11;10(1):2416.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Tumour-associated macrophages exhibit anti-tumoural properties in Sonic Hedgehog medulloblastoma
Authors: V Maximov, Z Chen, Y Wei, MH Robinson, CJ Herting, NS Shanmugam, VA Rudneva, KC Goldsmith, TJ MacDonald, PA Northcott, D Hambardzum, AM Kenney
Nat Commun, 2019-06-03;10(1):2410.
Species: Mouse
Sample Types: Tissue Homogenates
-
In-depth characterization of congenital Zika syndrome in immunocompetent mice: Antibody-dependent enhancement and an antiviral peptide therapy
Authors: VN Camargos, G Foureaux, DC Medeiros, VT da Silveir, CM Queiroz-Ju, ALB Matosinhos, AFA Figueiredo, CDF Sousa, TP Moreira, VF Queiroz, ACF Dias, KTO Santana, I Passos, ALCV Real, LC Silva, FAG Mourão, NT Wnuk, MAP Oliveira, S Macari, T Silva, GP Garlet, JA Jackman, FM Soriani, MFD Moraes, EMAM Mendes, FM Ribeiro, GMJ Costa, AL Teixeira, NJ Cho, ACP Oliveira, MM Teixeira, VV Costa, DG Souza
EBioMedicine, 2019-05-23;0(0):.
Species: Mouse
Sample Types: Plasma
-
Elevated circulating TGF?1 during acute liver failure activates TGF?R2 on cortical neurons and exacerbates neuroinflammation and hepatic encephalopathy in mice
Authors: M McMillin, S Grant, G Frampton, AD Petrescu, E Williams, B Jefferson, A Thomas, A Brahmarout, S DeMorrow
J Neuroinflammation, 2019-04-02;16(1):69.
Species: Mouse
Sample Types: Tissue Homogenates
-
NLRX1 does not play a role in diabetes nor the development of diabetic nephropathy induced by multiple low doses of streptozotocin
Authors: AML Scantleber, M Uil, LM Butter, R Poelman, N Claessen, SE Girardin, S Florquin, JJTH Roelofs, JC Leemans
PLoS ONE, 2019-03-25;14(3):e0214437.
Species: Mouse
Sample Types: Tissue Homogenates
-
EphB2-dependent signaling promotes neuronal excitotoxicity and inflammation in the acute phase of ischemic stroke
Authors: AS Ernst, LI Böhler, AM Hagenston, A Hoffmann, S Heiland, C Sticht, M Bendszus, M Hecker, H Bading, HH Marti, T Korff, R Kunze
Acta Neuropathol Commun, 2019-02-05;7(1):15.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation
Authors: Y Pan, X Hui, RLC Hoo, D Ye, CYC Chan, T Feng, Y Wang, KSL Lam, A Xu
J. Clin. Invest., 2019-01-22;129(2):834-849.
Species: Mouse
Sample Types: Serum
-
Inhalation of the prodrug PI3K inhibitor CL27c improves lung function in asthma and fibrosis
Authors: CC Campa, RL Silva, JP Margaria, T Pirali, MS Mattos, LR Kraemer, DC Reis, G Grosa, F Copperi, EM Dalmarco, RCP Lima-Júnio, S Aprile, V Sala, F Dal Bello, DS Prado, JC Alves-Filh, C Medana, GD Cassali, GC Tron, MM Teixeira, E Ciraolo, RC Russo, E Hirsch
Nat Commun, 2018-12-12;9(1):5232.
Species: Mouse
Sample Types:
-
CCR2 mediates increased susceptibility to post-H1N1 bacterial pneumonia by limiting dendritic cell induction of IL-17
Authors: SJ Gurczynski, N Nathani, HI Warheit-Ni, EM Hult, A Podsiad, J Deng, RL Zemans, U Bhan, BB Moore
Mucosal Immunol, 2018-11-29;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Microphthalmia-Associated Transcription Factor (MITF) Regulates Immune Cell Migration into Melanoma
Authors: GM Wiedemann, C Aithal, A Kraechan, C Heise, BL Cadilha, J Zhang, P Duewell, R Ballotti, S Endres, C Bertolotto, S Kobold
Transl Oncol, 2018-11-28;12(2):350-360.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Impact of immunization against OxLDL on the pulmonary response to cigarette smoke exposure in mice
Authors: M Talbot, M Hamel-Auge, MJ Beaulieu, M Gazzola, A Lechasseur, S Aubin, MÈ Paré, D Marsolais, Y Bossé, MC Morissette
Respir. Res., 2018-07-03;19(1):131.
Species: Mouse
Sample Types: BALF
-
Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms
Authors: DT Skelly, ÉW Griffin, CL Murray, S Harney, C O'Boyle, E Hennessy, MA Dansereau, A Nazmi, L Tortorelli, JN Rawlins, DM Bannerman, C Cunningham
Mol. Psychiatry, 2018-06-06;24(10):1533-1548.
Species: Mouse
Sample Types: Plasma
-
Factor XIIIA-expressing inflammatory monocytes promote lung squamous cancer through fibrin cross-linking
Authors: A Porrello, PL Leslie, EB Harrison, BK Gorentla, S Kattula, SK Ghosh, SH Azam, A Holtzhause, YL Chao, MC Hayward, TA Waugh, S Bae, V Godfrey, SH Randell, C Oderup, L Makowski, J Weiss, MD Wilkerson, DN Hayes, HS Earp, AS Baldwin, AS Wolberg, CV Pecot
Nat Commun, 2018-05-18;9(1):1988.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Classical and alternative complement activation on photoreceptor outer segments drives monocyte-dependent retinal atrophy
Authors: KJ Katschke, H Xi, C Cox, T Truong, Y Malato, WP Lee, B McKenzie, R Arceo, J Tao, L Rangell, M Reichelt, L Diehl, J Elstrott, RM Weimer, MVL Campagne
Sci Rep, 2018-05-09;8(1):7348.
Species: Mouse
Sample Types: Tissue Homogenates
-
Antibiotics with Interleukin-15 inhibition reduces joint inflammation and bone erosions but not cartilage destruction ininduced arthritis
Authors: B Bergmann, P Jirholt, P Henning, C Lindholm, C Ohlsson, IB McInnes, UH Lerner, I Gjertsson
Infect. Immun., 2018-04-23;0(0):.
Species: Mouse
Sample Types: Serum
-
Parkin deficiency modulates NLRP3 inflammasome activation by attenuating an A20-dependent negative feedback loop
Authors: F Mouton-Lig, T Rosazza, J Sepulveda-, A Ieang, SM Hassoun, E Claire, G Mangone, A Brice, PP Michel, JC Corvol, O Corti
Glia, 2018-04-17;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Alternaria alternata challenge at the nasal mucosa results in eosinophilic inflammation and increased susceptibility to influenza virus infection
Authors: M Ma, JL Redes, CM Percopo, KM Druey, HF Rosenberg
Clin. Exp. Allergy, 2018-04-15;0(0):.
Species: Mouse
Sample Types: Nasal Lavage Fluid
-
Genetic ablation of histone deacetylase 2 leads to lung cellular senescence and lymphoid follicle formation in COPD/emphysema
Authors: IK Sundar, K Rashid, J Gerloff, J Rangel-Mor, D Li, I Rahman
FASEB J., 2018-04-09;0(0):fj201701518R.
Species: Mouse
Sample Types: Tissue Homogenates
-
p53 in Bronchial Club Cells Facilitates Chronic Lung Inflammation by Promoting Senescence
Authors: A Sagiv, A Bar-Shai, N Levi, M Hatzav, L Zada, Y Ovadya, L Roitman, G Manella, O Regev, J Majewska, E Vadai, R Eilam, SW Feigelson, M Tsoory, M Tauc, R Alon, V Krizhanovs
Cell Rep, 2018-03-27;22(13):3468-3479.
Species: Mouse
Sample Types: BALF
-
Identification of periplakin as a major regulator of lung injury and repair in mice
Authors: V Besnard, R Dagher, T Madjer, A Joannes, M Jaillet, M Kolb, P Bonniaud, LA Murray, MA Sleeman, B Crestani
JCI Insight, 2018-03-08;3(5):.
Species: Mouse
Sample Types: BALF
-
The Long Pentraxin 3 Plays a Role in Bone Turnover and Repair
Authors: D Gr?evi?, M Sironi, S Valentino, L Deban, H Cvija, A Inforzato, N Kova?i?, V Katavi?, T Kelava, I Kalajzi?, A Mantovani, B Bottazzi
Front Immunol, 2018-03-05;9(0):417.
Species: Mouse
Sample Types: Serum
-
FOXO3-dependent apoptosis limits alcohol-induced liver inflammation by promoting infiltrating macrophage differentiation
Authors: Z Li, J Zhao, S Zhang, SA Weinman
Cell Death Discov, 2018-02-13;4(0):16.
Species: Mouse
Sample Types: Serum
-
Nlrp12 Mediates Adverse Neutrophil Recruitment during Influenza Virus Infection
Authors: EE Hornick, B Banoth, AM Miller, ZR Zacharias, N Jain, ME Wilson, KN Gibson-Cor, KL Legge, GA Bishop, FS Sutterwala, SL Cassel
J. Immunol., 2017-12-27;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Intramembrane attenuation of the TLR4-TLR6 dimer impairs receptor assembly and reduces microglia mediated neurodegeneration
Authors: L Shmuel-Gal, Y Klug, Z Porat, M Charni, B Zarmi, Y Shai
J. Biol. Chem., 2017-06-27;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
NLRX1 dampens oxidative stress and apoptosis in tissue injury via control of mitochondrial activity
Authors: G Stokman, L Kors, PJ Bakker, E Rampanelli, N Claessen, GJD Teske, L Butter, H van Andel, MA van den Be, PWB Larsen, MC Dessing, CJ Zuurbier, SE Girardin, S Florquin, JC Leemans
J. Exp. Med., 2017-06-16;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
The GLP-1 analogue lixisenatide decreases atherosclerosis in insulin-resistant mice by modulating macrophage phenotype
Authors: Á Vinué, J Navarro, A Herrero-Ce, M García-Cub, I Andrés-Bla, S Martínez-H, JT Real, JF Ascaso, H González-N
Diabetologia, 2017-06-12;0(0):.
Species: Mouse
Sample Types: Plasma
-
Fetuin-A aggravates lipotoxicity in podocytes via interleukin-1 signaling
Authors: JM Orellana, K Kampe, F Schulze, J Sieber, AW Jehle
Physiol Rep, 2017-05-01;5(10):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Contributions of CD8 T cells to the pathogenesis of mouse adenovirus type 1 respiratory infection
Authors: CT Molloy, JS Andonian, HM Seltzer, MC Procario, ME Watson, JB Weinberg
Virology, 2017-04-11;507(0):64-74.
Species: Mouse
Sample Types: BALF
-
Surface layer proteins from virulent Clostridium difficile ribotypes exhibit signatures of positive selection with consequences for innate immune response
Authors: M Lynch, TA Walsh, I Marszalows, AE Webb, M MacAogain, TR Rogers, H Windle, D Kelleher, MJ O'Connell, CE Loscher
BMC Evol. Biol, 2017-03-23;17(1):90.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Biglycan- and Sphingosine Kinase-1 Signaling Crosstalk Regulates the Synthesis of Macrophage Chemoattractants
Authors: LT Hsieh, MV Nastase, H Roedig, J Zeng-Brouw, C Poluzzi, S Schwalm, C Fork, C Tredup, RP Brandes, M Wygrecka, A Huwiler, J Pfeilschif, L Schaefer
Int J Mol Sci, 2017-03-09;18(3):.
Species: Mouse
Sample Types: Plasma
-
Both anti-TNF and CTLA4 Ig treatments attenuate the disease severity of staphylococcal dermatitis in mice
Authors: M Na, W Wang, Y Fei, E Josefsson, A Ali, T Jin
PLoS ONE, 2017-03-06;12(3):e0173492.
Species: Mouse
Sample Types: Tissue Homogenates
-
Impacts of allergic airway inflammation on lung pathology in a mouse model of influenza A virus infection
Authors: A Kawaguchi, T Suzuki, Y Ohara, K Takahashi, Y Sato, A Ainai, N Nagata, M Tashiro, H Hasegawa
PLoS ONE, 2017-02-28;12(2):e0173008.
Species: Mouse
Sample Types: BALF
-
Antibodies against nonstructural protein 1 protect mice from dengue virus-induced mast cell activation
Authors: YT Chu, SW Wan, YC Chang, CK Lee, BA Wu-Hsieh, R Anderson, YS Lin
Lab. Invest, 2017-02-27;0(0):.
Species: Mouse
Sample Types: Serum
-
The TRAIL-Induced Cancer Secretome Promotes a Tumor-Supportive Immune Microenvironment via CCR2
Authors: T Hartwig, A Montinaro, S von Karste, A Sevko, S Surinova, A Chakravart, L Taraborrel, P Draber, E Lafont, F Arce Varga, MA El-Bahrawy, SA Quezada, H Walczak
Mol. Cell, 2017-02-16;65(4):730-742.e5.
Species: Mouse
Sample Types: Tissue Homogenates
-
APPL1 prevents pancreatic beta cell death and inflammation by dampening NF?B activation in a mouse model of type 1 diabetes
Authors: Xue Jiang
Diabetologia, 2016-12-23;0(0):.
Species: Mouse
Sample Types: Whole Cells
-
NADPH oxidase 4 regulates vascular inflammation in aging and atherosclerosis
Authors: Andrey Lozhkin
J. Mol. Cell. Cardiol, 2016-12-14;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
PGI2 Controls Pulmonary NK Cells That Prevent Airway Sensitization to House Dust Mite Allergen
Authors: Bryan Simons
J. Immunol, 2016-11-28;0(0):.
Species: Mouse
Sample Types: BALF
-
Influenza A virus-dependent remodeling of pulmonary clock function in a mouse model of COPD.
Authors: Sundar, Isaac K, Ahmad, Tanveer, Yao, Hongwei, Hwang, Jae-woon, Gerloff, Janice, Lawrence, B Paige, Sellix, Michael, Rahman, Irfan
Sci Rep, 2015-04-29;4(0):9927.
Species: Mouse
Sample Types: BALF
-
Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes.
Authors: Goritzka M, Makris S, Kausar F, Durant L, Pereira C, Kumagai Y, Culley F, Mack M, Akira S, Johansson C
J Exp Med, 2015-04-20;212(5):699-714.
Species: Mouse
Sample Types: Tissue Homogenates
-
Autoprocessing of neutrophil elastase near its active site reduces the efficiency of natural and synthetic elastase inhibitors.
Authors: Dau T, Sarker R, Yildirim A, Eickelberg O, Jenne D
Nat Commun, 2015-04-10;6(0):6722.
Species: Mouse
Sample Types: BALF
-
IL-1 receptor type 2 suppresses collagen-induced arthritis by inhibiting IL-1 signal on macrophages.
Authors: Shimizu K, Nakajima A, Sudo K, Liu Y, Mizoroki A, Ikarashi T, Horai R, Kakuta S, Watanabe T, Iwakura Y
J Immunol, 2015-02-27;194(7):3156-68.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Modulation of the mevalonate pathway by akt regulates macrophage survival and development of pulmonary fibrosis.
Authors: Larson-Casey J, Murthy S, Ryan A, Carter A
J Biol Chem, 2014-11-05;289(52):36204-19.
Species: Mouse
Sample Types: BALF
-
Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis.
Authors: Bonapace L, Coissieux M, Wyckoff J, Mertz K, Varga Z, Junt T, Bentires-Alj M
Nature, 2014-10-22;515(7525):130-3.
Species: Mouse
Sample Types: Cell Culture Supernates
-
No Impairment in host defense against Streptococcus pneumoniae in obese CPEfat/fat mice.
Authors: Mancuso P, O Brien E, Prano J, Goel D, Aronoff D
PLoS ONE, 2014-09-09;9(9):e106420.
Species: Mouse
Sample Types: Serum
-
Animal model of respiratory syncytial virus: CD8+ T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy.
Authors: Walsh K, Teijaro J, Brock L, Fremgen D, Collins P, Rosen H, Oldstone M
J Virol, 2014-03-26;88(11):6281-93.
Species: Mouse
Sample Types: BALF
-
Hypothalamic gene transfer of BDNF inhibits breast cancer progression and metastasis in middle age obese mice.
Authors: Liu, Xianglan, McMurphy, Travis, Xiao, Run, Slater, Andrew, Huang, Wei, Cao, Lei
Mol Ther, 2014-03-18;22(7):1275-84.
Species: Mouse
Sample Types: Serum
-
The role of Nox2-derived ROS in the development of cognitive impairment after sepsis.
Authors: Hernandes M, D'Avila J, Trevelin S, Reis P, Kinjo E, Lopes L, Castro-Faria-Neto H, Cunha F, Britto L, Bozza F
J Neuroinflammation, 2014-02-27;11(0):36.
Species: Mouse
Sample Types: Peritoneal Fluid
-
Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection.
Authors: Teijaro J, Walsh K, Rice S, Rosen H, Oldstone M
Proc Natl Acad Sci U S A, 2014-02-26;111(10):3799-804.
Species: Mouse
Sample Types: BALF
-
A TNF-alpha-CCL20-CCR6 axis regulates Nod1-induced B cell responses.
Authors: Paradis M, Mindt B, Duerr C, Rojas O, Ng D, Boulianne B, McCarthy D, Yu M, Summers deLuca L, Ward L, Waldron J, Philpott D, Gommerman J, Fritz J
J Immunol, 2014-02-17;192(6):2787-99.
Species: Mouse
Sample Types: Serum
-
Bone marrow-specific knock-in of a non-activatable Ikkalpha kinase mutant influences haematopoiesis but not atherosclerosis in Apoe-deficient mice.
Authors: Tilstam P, Gijbels M, Habbeddine M, Cudejko C, Asare Y, Theelen W, Zhou B, Doring Y, Drechsler M, Pawig L, Simsekyilmaz S, Koenen R, de Winther M, Lawrence T, Bernhagen J, Zernecke A, Weber C, Noels H
PLoS ONE, 2014-02-03;9(2):e87452.
Species: Mouse
Sample Types: Cell Culture Supernates
-
MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung.
Authors: Malmhall C, Alawieh S, Lu Y, Sjostrand M, Bossios A, Eldh M, Radinger M
J Allergy Clin Immunol, 2013-12-24;133(5):1429-38, 1438.
Species: Mouse
Sample Types: Tissue Homogenates
-
Munc18-2 and syntaxin 3 control distinct essential steps in mast cell degranulation.
Authors: Brochetta C, Suzuki R, Vita F, Soranzo M, Claver J, Madjene L, Attout T, Vitte J, Varin-Blank N, Zabucchi G, Rivera J, Blank U
J Immunol, 2013-12-09;192(1):41-51.
Species: Rat
Sample Types: Cell Culture Supernates
-
Defining the range of pathogens susceptible to Ifitm3 restriction using a knockout mouse model.
Authors: Everitt, Aaron R, Clare, Simon, McDonald, Jacqueli, Kane, Leanne, Harcourt, Katherin, Ahras, Malika, Lall, Amar, Hale, Christin, Rodgers, Angela, Young, Douglas, Haque, Ashraful, Billker, Oliver, Tregoning, John S, Dougan, Gordon, Kellam, Paul
PLoS ONE, 2013-11-21;8(11):e80723.
Species: Mouse
Sample Types: BALF
-
A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation.
Authors: Csoka B, Koscso B, Toro G, Kokai E, Virag L, Nemeth Z, Pacher P, Bai P, Hasko G
Diabetes, 2013-11-05;63(3):850-66.
Species: Mouse
Sample Types: Plasma
-
Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway.
Authors: Hwang J, Sundar I, Yao H, Sellix M, Rahman I
FASEB J, 2013-09-11;28(1):176-94.
Species: Mouse
Sample Types: Tissue Homogenates
-
Deficiency of C5L2 increases macrophage infiltration and alters adipose tissue function in mice.
Authors: Gauvreau D, Gupta A, Fisette A, Tom F, Cianflone K
PLoS ONE, 2013-04-22;8(4):e60795.
Species: Mouse
Sample Types: Plasma
-
A novel murine model of rhinoscleroma identifies Mikulicz cells, the disease signature, as IL-10 dependent derivatives of inflammatory monocytes.
Authors: Fevre C, Almeida A, Taront S, Pedron T, Huerre M, Prevost M, Kieusseian A, Cumano A, Brisse S, Sansonetti P, Tournebize R
EMBO Mol Med, 2013-04-01;5(4):516-30.
Species: Mouse
Sample Types: Tissue Homogenates
-
Syntaxin binding protein 1 is not required for allergic inflammation via IgE-mediated mast cell activation.
Authors: Wu Z, MacNeil A, Berman J, Lin T
PLoS ONE, 2013-03-06;8(3):e58560.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The novel cytokine interleukin-33 activates acinar cell proinflammatory pathways and induces acute pancreatic inflammation in mice.
Authors: Kempuraj D, Twait E, Williard D, Yuan Z, Meyerholz D, Samuel I
PLoS ONE, 2013-02-13;8(2):e56866.
Species: Mouse
Sample Types: Tissue Homogenates
-
Effect of secretin on preadipocyte, differentiating and mature adipocyte functions.
Authors: Miegueu P, Cianflone K, Richard D, St-Pierre D
Int J Obes (Lond), 2012-05-08;37(3):366-74.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection.
Authors: Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, Riva F, Garlanda C
Infect. Immun., 2011-10-24;80(1):100-9.
Species: Mouse
Sample Types: Serum
-
Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus.
Authors: Walsh KB, Teijaro JR, Wilker PR
Proc. Natl. Acad. Sci. U.S.A., 2011-06-29;108(29):12018-23.
Species: Mouse
Sample Types: BALF
-
Lung-targeted overexpression of the NF-kappaB member RelB inhibits cigarette smoke-induced inflammation.
Authors: McMillan DH, Baglole CJ, Thatcher TH, Maggirwar S, Sime PJ, Phipps RP
Am. J. Pathol., 2011-05-05;179(1):125-33.
Species: Mouse
Sample Types: BALF
-
The Therapeutic Potential of the Humoral Pattern Recognition Molecule PTX3 in Chronic Lung Infection Caused by Pseudomonas aeruginosa.
Authors: Moalli F, Paroni M, Veliz Rodriguez T, Riva F, Polentarutti N, Bottazzi B, Valentino S, Mantero S, Nebuloni M, Mantovani A, Bragonzi A, Garlanda C
J. Immunol., 2011-03-25;186(9):5425-34.
Species: Mouse
Sample Types: Tissue Homogenates
-
Phosphoinositide 3-kinase gamma plays a critical role in bleomycin-induced pulmonary inflammation and fibrosis in mice.
Authors: Russo RC, Garcia CC, Barcelos LS, Rachid MA, Guabiraba R, Roffe E, Souza AL, Sousa LP, Mirolo M, Doni A, Cassali GD, Pinho V, Locati M, Teixeira MM
J. Leukoc. Biol., 2010-11-02;89(2):269-82.
Species: Mouse
Sample Types: Tissue Homogenates
-
Inflammatory chemokine release of astrocytes in vitro is reduced by all-trans retinoic acid.
Authors: van Neerven S, Regen T, Wolf D, Nemes A, Johann S, Beyer C, Hanisch UK, Mey J
J. Neurochem., 2010-06-16;114(5):1511-26.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Latent herpesvirus infection augments experimental pulmonary fibrosis.
Authors: Vannella KM, Luckhardt TR, Wilke CA, van Dyk LF, Toews GB, Moore BB
Am. J. Respir. Crit. Care Med., 2009-12-10;181(5):465-77.
Species: Mouse
Sample Types: Tissue Homogenates
-
E-prostanoid 3 receptor deletion improves pulmonary host defense and protects mice from death in severe Streptococcus pneumoniae infection.
Authors: Aronoff DM, Lewis C, Serezani CH, Eaton KA, Goel D, Phipps JC, Peters-Golden M, Mancuso P
J. Immunol., 2009-07-27;183(4):2642-9.
Species: Mouse
Sample Types: Tissue Homogenates
-
Oxidized alpha1-antitrypsin stimulates the release of monocyte chemotactic protein-1 from lung epithelial cells: potential role in emphysema.
Authors: Li Z, Alam S, Wang J, Sandstrom CS, Janciauskiene S, Mahadeva R
Am. J. Physiol. Lung Cell Mol. Physiol., 2009-06-12;297(2):L388-400.
Species: Mouse
Sample Types: BALF
-
Matrix metalloproteinase-9 deficiency worsens lung injury in a model of bronchopulmonary dysplasia.
Authors: Lukkarinen H, Hogmalm A, Lappalainen U, Bry K
Am. J. Respir. Cell Mol. Biol., 2008-12-18;41(1):59-68.
Species: Mouse
Sample Types: Tissue Homogenates
-
Modulation of macrophage infiltration and inflammatory activity by the phosphatase SHP-1 in virus-induced demyelinating disease.
Authors: Christophi GP, Hudson CA, Panos M, Gruber RC, Massa PT
J. Virol., 2008-11-05;83(2):522-39.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Reduction in dietary omega-6 polyunsaturated fatty acids: eicosapentaenoic acid plus docosahexaenoic acid ratio minimizes atherosclerotic lesion formation and inflammatory response in the LDL receptor null mouse.
Authors: Wang S, Wu D, Matthan NR, Lamon-Fava S, Lecker JL, Lichtenstein AH
Atherosclerosis, 2008-09-02;204(1):147-55.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Neurokinin A engages neurokinin-1 receptor to induce NF-kappaB-dependent gene expression in murine macrophages: implications of ERK1/2 and PI 3-kinase/Akt pathways.
Authors: Sun J, Ramnath RD, Tamizhselvi R, Bhatia M
Am. J. Physiol., Cell Physiol., 2008-07-02;295(3):C679-91.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis.
Authors: Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME
Proc. Natl. Acad. Sci. U.S.A., 2008-05-14;105(20):7240-5.
Species: Mouse
Sample Types: BALF
-
Expanded-polyglutamine huntingtin protein suppresses the secretion and production of a chemokine (CCL5/RANTES) by astrocytes.
Authors: Chou SY, Weng JY, Lai HL, Liao F, Sun SH, Tu PH, Dickson DW, Chern Y
J. Neurosci., 2008-03-26;28(13):3277-90.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Surfactant protein D alters allergic lung responses in mice and human subjects.
Authors: Brandt EB, Mingler MK, Stevenson MD, Wang N, Khurana Hershey GK, Whitsett JA, Rothenberg ME
J. Allergy Clin. Immunol., 2008-03-19;121(5):1140-1147.e2.
Species: Mouse
Sample Types: BALF
-
Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues.
Authors: Cardona AE, Sasse ME, Liu L, Cardona SM, Mizutani M, Savarin C, Hu T, Ransohoff RM
Blood, 2008-03-17;112(2):256-63.
Species: Mouse
Sample Types: Tissue Homogenates
-
Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection.
Authors: Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC
J. Immunol., 2008-01-15;180(2):1080-7.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mycobacterium tuberculosis antigens specifically modulate CCR2 and MCP-1/CCL2 on lymphoid cells from human pulmonary hilar lymph nodes.
Authors: Arias MA, Jaramillo G, Lopez YP, Mejia N, Mejia C, Pantoja AE, Shattock RJ, Garcia LF, Griffin GE
J. Immunol., 2007-12-15;179(12):8381-91.
Species: Human
Sample Types: Cell Culture Supernates
-
Osteopontin regulates hindlimb-unloading-induced lymphoid organ atrophy and weight loss by modulating corticosteroid production.
Authors: Wang KX, Shi Y, Denhardt DT
Proc. Natl. Acad. Sci. U.S.A., 2007-09-04;104(37):14777-82.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CD8+ T Cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice.
Authors: Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD
J. Immunol., 2007-06-15;178(12):8090-6.
Species: Mouse
Sample Types: BALF
-
Neurokinin-1 receptor antagonist treatment protects mice against lung injury in polymicrobial sepsis.
Authors: Hegde A, Zhang H, Moochhala SM, Bhatia M
J. Leukoc. Biol., 2007-06-12;82(3):678-85.
Species: Mouse
Sample Types: Plasma
-
Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity.
Authors: Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, Collins C, Viala J, Ferrero RL, Girardin SE, Philpott DJ
Immunity, 2007-04-12;26(4):445-59.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Infiltrating neutrophils induce allospecific CTL in response to immunization with apoptotic cells via MCP-1 production.
Authors: Shiratsuchi Y, Iyoda T, Tanimoto N, Kegai D, Nagata K, Kobayashi Y
J. Leukoc. Biol., 2006-11-09;81(2):412-20.
Species: Mouse
Sample Types: Peritoneal Lavage Fluid
-
Leishmania-derived murine monocyte chemoattractant protein 1 enhances the recruitment of a restrictive population of CC chemokine receptor 2-positive macrophages.
Authors: Conrad SM, Strauss-Ayali D, Field AE, Mack M, Mosser DM
Infect. Immun., 2006-11-06;75(2):653-65.
Species: Parasite - Leishmania
Sample Types: Cell Culture Supernates
-
A functional comparison of canine and murine bone marrow derived cultured mast cells.
Authors: Lin TY, London CA
Vet. Immunol. Immunopathol., 2006-10-06;114(3):320-34.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Exogenous platelet-activating factor acetylhydrolase reduces mortality in mice with systemic inflammatory response syndrome and sepsis.
Authors: Gomes RN, Bozza FA, Amancio RT, Japiassu AM, Vianna RC, Larangeira AP, Gouvea JM, Bastos MS, Zimmerman GA, Stafforini DM, Prescott SM, Bozza PT, Castro-Faria-Neto HC
Shock, 2006-07-01;26(1):41-9.
Species: Mouse
Sample Types: Peritoneal Fluid
-
Differential involvement of TLR2 and TLR4 in host survival during pulmonary infection with Chlamydia pneumoniae.
Authors: Rodriguez N, Wantia N, Fend F, Durr S, Wagner H, Miethke T
Eur. J. Immunol., 2006-05-01;36(5):1145-55.
Species: Mouse
Sample Types: Tissue Homogenates
-
Increased pulmonary responses to acute ozone exposure in obese db/db mice.
Authors: Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA
Am. J. Physiol. Lung Cell Mol. Physiol., 2005-12-22;290(5):L856-65.
Species: Mouse
Sample Types: Serum
-
Immunosuppressive effects of CCL17 on pulmonary antifungal responses during pulmonary invasive aspergillosis.
Authors: Carpenter KJ, Hogaboam CM
Infect. Immun., 2005-11-01;73(11):7198-207.
Species: Mouse
Sample Types: Tissue Homogenates
-
Impaired inflammatory angiogenesis, but not leukocyte influx, in mice lacking TNFR1.
Authors: Barcelos LS, Talvani A, Teixeira AS, Vieira LQ, Cassali GD, Andrade SP, Teixeira MM
J. Leukoc. Biol., 2005-05-13;78(2):352-8.
Species: Mouse
Sample Types: Complex Sample Type
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse CCL2/JE/MCP-1 DuoSet ELISA
Average Rating: 4.6 (Based on 19 Reviews)
Have you used Mouse CCL2/JE/MCP-1 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Used this kit for quantifying mouse MCP-1 in serum samples, could see the detection but not strong in our samples. Standard curve looks good.
stable and precise
We used this kit for the quantification of CCL2 in human serum and plasma. Works very well and well-described protocol.