Mouse CCL21/6Ckine Antibody Summary
Ser24-Gly133
Accession # P84444
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Chemotaxis Induced by CCL21/6Ckine and Neutralization by Mouse CCL21/6Ckine Antibody. Recombinant Mouse CCL21/6Ckine (Catalog # 457-6C) chemoattracts the BaF3 mouse pro-B cell line transfected with human CCR7 in a dose-dependent manner (orange line). The amount of cells that migrated through to the lower chemotaxis chamber was measured by Resazurin (Catalog # AR002). Chemotaxis elicited by Recombinant Mouse CCL21/6Ckine (50 ng/mL) is neutralized (green line) by increasing concentrations of Goat Anti-Mouse CCL21/6Ckine Antigen Affinity-purified Polyclonal Antibody (Catalog # AF457). The ND50 is typically 0.5-2.5 µg/mL.
 View Larger
View Larger
CCL21/6Ckine in Mouse Thymus. CCL21/6Ckine was detected in perfusion fixed frozen sections of mouse thymus using Goat Anti-Mouse CCL21/6Ckine Antigen Affinity-purified Polyclonal Antibody (Catalog # AF457) at 5 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). Specific staining was localized to endothelial cells. View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
Detection of CCL21/6Ckine in D3 Mouse Cell Line by Flow Cytometry. D3 mouse embryonic stem cell line was stained with Goat Anti-Mouse CCL21/6Ckine Antigen Affinity-purified Polyclonal Antibody (Catalog # AF457, filled histogram) or control antibody (Catalog # AB-108-C, open histogram), followed by Phycoerythrin-conjugated Anti-Goat IgG Secondary Antibody (Catalog # F0107). To facilitate intracellular staining, cells were fixed with paraformaldehyde and permeabilized with saponin.
 View Larger
View Larger
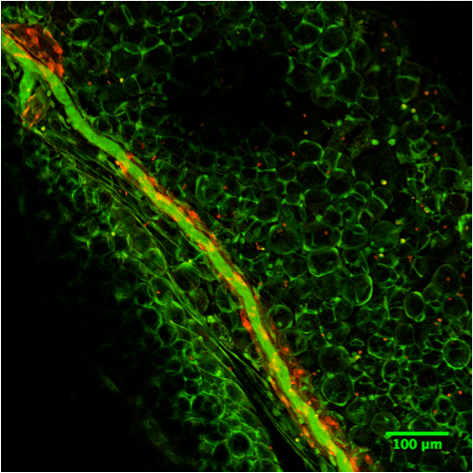
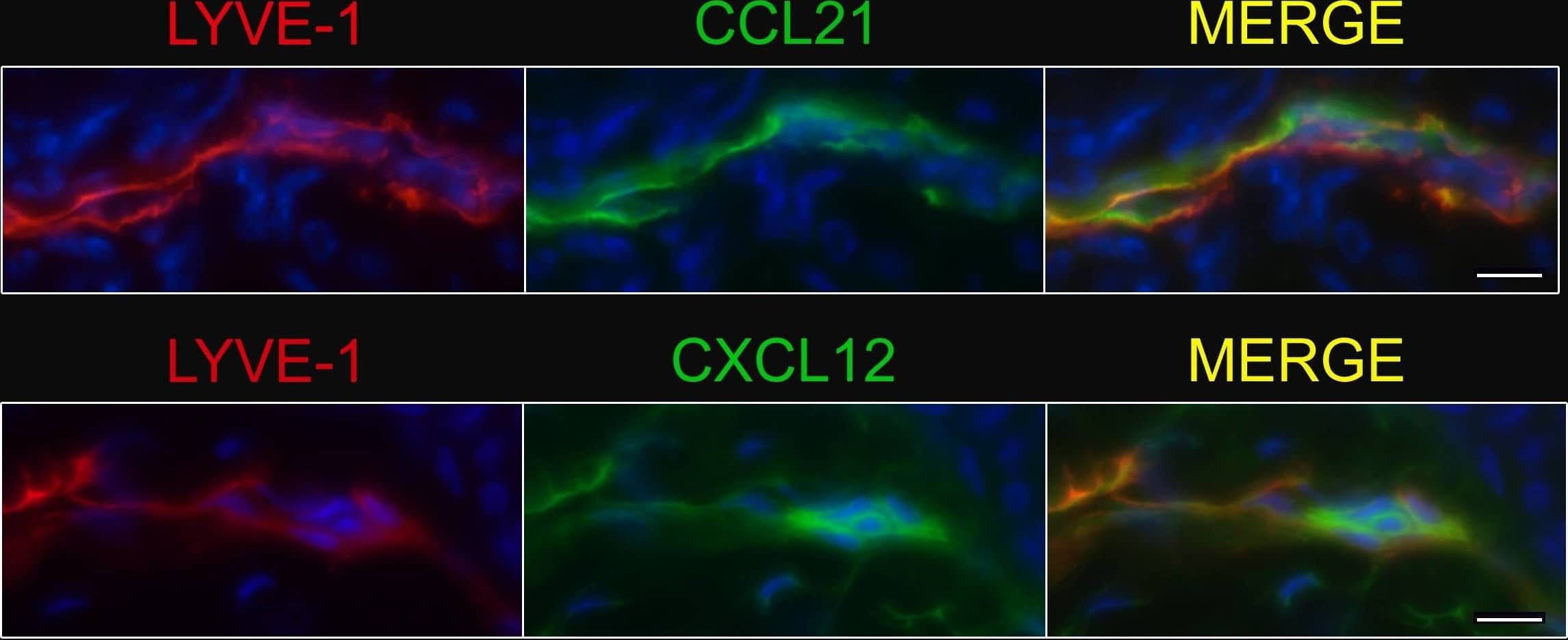
Detection of Mouse CCL21/6Ckine by Immunohistochemistry Afferent lymph vessels in chronically inflamed skin express CXCL12.Immunofluorescence staining of frozen sections of chronically inflamed skin (21 days after inflammation elicited by subcutaneous injection of CFA) for CXC12 and CCL21 by LYVE-1+ lymph vessels using DAPI counterstaining. One representative image of lymph vessels analyzed in 5 mice is shown. Scale bars, 10 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/24752354), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: CCL21/6Ckine
6Ckine is a novel CC chemokine discovered independently by three groups from the EST database. 6Ckine, also named SLC (secondary lymphoid-tissue chemokine), CCL21 and Exodus-2, shows 21-33% identity to other CC chemokines. 6Ckine contains the four conserved cysteines characteristic of beta chemokines plus two additional cysteines in its unusually long carboxyl-terminal domain. Human 6Ckine cDNA encodes a 134 amino acid residue, highly basic, precursor protein with a 23 amino acid residue signal peptide that is cleaved to form the predicted 111 amino acid residue mature protein. Mouse 6Ckine cDNA encodes a 133 amino acid residue protein with a 23 residue signal peptide that is cleaved to generate the 110 residue mature protein. Human and mouse 6Ckine are highly conserved, exhibiting 86% amino acid sequence identity. 6Ckine is constitutively expressed at high levels in lymphoid tissues such as lymph nodes, spleen and appendix. In mouse, high levels of 6Ckine mRNA are also detected in the lung. The gene for human 6Ckine has been localized at human chromosome 9p13 rather than chromosome 17 where the genes of many human CC chemokines are clustered. The 6Ckine gene location is within a region of about 100 kb from the MIP-3 beta /ELC gene, another identified novel CC chemokine. Unlike most CC chemokines, 6Ckine is not chemotactic for monocytes. 6Ckine has also been reported to inhibit hemopoietic progenitor colony formation in a dose-dependent manner. 6Ckine acts via a class of as yet unidentified CC receptors on both T cells and B cells that are not shared by any other CC chemokines. Mature rat CCL21 shares 84% aa sequence identity with mouse CCL21.
Product Datasheets
Citations for Mouse CCL21/6Ckine Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
81
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Tumor-induced stromal reprogramming drives lymph node transformation
Authors: Angela Riedel, David Shorthouse, Lisa Haas, Benjamin A Hall, Jacqueline Shields
Nature Immunology
-
Anti-CCL21 Antibody Attenuates Infarct Size and Improves Cardiac Remodeling After Myocardial Infarction
Authors: Yi Jiang, Jianwen Bai, Lunxian Tang, Pei Zhang, Jun Pu
Cellular Physiology and Biochemistry
-
Attenuation of chronic antiviral T-cell responses through constitutive COX2-dependent prostanoid synthesis by lymph node fibroblasts
Authors: Karin Schaeuble, Hélène Cannelle, Stéphanie Favre, Hsin-Ying Huang, Susanne G. Oberle, Daniel E. Speiser et al.
PLOS Biology
-
Lymph node fibroblastic reticular cells preserve a tolerogenic niche in allograft transplantation through laminin alpha 4
Authors: Lushen Li, Marina W. Shirkey, Tianshu Zhang, Wenji Piao, Xiaofei Li, Jing Zhao et al.
Journal of Clinical Investigation
-
Nonclassical monocytes potentiate anti-tumoral CD8+ T cell responses in the lungs
Authors: Lindsey E. Padgett, Paola M. Marcovecchio, Claire E. Olingy, Daniel J. Araujo, Kathleen Steel, Huy Q. Dinh et al.
Frontiers in Immunology
-
Artesunate Therapy Alleviates Fracture-Associated Chronic Pain After Orthopedic Surgery by Suppressing CCL21-Dependent TREM2/DAP12 Inflammatory Signaling in Mice
Authors: Linlin Zhang, Nan Li, Haoyue Zhang, Yigang Wang, Tianyu Gao, Yuying Zhao et al.
Frontiers in Pharmacology
-
Visualization of T Cell Migration in the Spleen Reveals a Network of Perivascular Pathways that Guide Entry into T Zones
Authors: Anne Chauveau, Gabriela Pirgova, Hung-Wei Cheng, Angelina De Martin, Felix Y. Zhou, Sarah Wideman et al.
Immunity
-
Erythropoietin ameliorates renal interstitial fibrosis via the inhibition of fibrocyte accumulation
Authors: XU CHANG GENG, ZHOU PANG HU, GUO YONG LIAN
Molecular Medicine Reports
-
Inflammation rapidly recruits mammalian GMP and MDP from bone marrow into regional lymphatics
Authors: Juana Serrano-Lopez, Shailaja Hegde, Sachin Kumar, Josefina Serrano, Jing Fang, Ashley M Wellendorf et al.
eLife
-
Structural and Functional Changes in Aged Skin Lymphatic Vessels
Authors: Raghu P. Kataru, Hyeung Ju Park, Jinyeon Shin, Jung Eun Baik, Ananta Sarker, Stav Brown et al.
Frontiers in Aging
-
Salmonella infection induces the reorganization of follicular dendritic cell networks concomitant with the failure to generate germinal centers
Authors: Edith Marcial-Juárez, Marisol Pérez-Toledo, Saba Nayar, Elena Pipi, Areej Alshayea, Ruby Persaud et al.
iScience
-
Graft Site Microenvironment Determines Dendritic Cell Trafficking Through the CCR7-CCL19/21 Axis
Authors: Jing Hua, William Stevenson, Thomas H. Dohlman, Takenori Inomata, Maryam Tahvildari, Narghes Calcagno et al.
Investigative Opthalmology & Visual Science
-
Association of T-Zone Reticular Networks and Conduits with Ectopic Lymphoid Tissues in Mice and Humans
Authors: Alexander Link, Debbie L. Hardie, Stéphanie Favre, Mirjam R. Britschgi, David H. Adams, Michael Sixt et al.
The American Journal of Pathology
-
Injury to the tunica media initiates atherogenesis in the presence of hyperlipidemia
Authors: Belhoul-Fakir H, Wu J, Yeow YL et al.
Frontiers in cardiovascular medicine
-
Mesenteric Lymphatic Alterations Observed During DSS Induced Intestinal Inflammation Are Driven in a TLR4-PAMP/DAMP Discriminative Manner
Authors: Matthew Stephens, Shan Liao, Pierre-Yves von der Weid
Frontiers in Immunology
-
Meningeal lymphatics regulate radiotherapy efficacy through modulating anti-tumor immunity
Authors: Changping Zhou, Lu Ma, Han Xu, Yingqing Huo, Jincai Luo
Cell Research
-
Molecular and spatial analysis of tertiary lymphoid structures in Sjogrens syndrome
Authors: Nayar, S;Turner, J;Asam, S;Fennell, É;Pugh, M;Colafrancesco, S;Berardicurti, O;Smith, C;Flint, J;Teodósio, A;Iannizzotto, V;Gardner, D;Roon, J;Korsunsky, I;Howdle, D;Brenner, M;Coles, M;Powrie, F;Raychaudhuri, S;Frei, A;Lassen, K;Bowman, S;Ng, W;Croft, A;Filer, A;Fisher, B;Buckley, C;Barone, F;
Nature communications
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
IFN?-dependent metabolic reprogramming restrains an immature, pro-metastatic lymphatic state in melanoma
Authors: Karakousi, T;Cristaldi, V;Lopes de Oliveira, ML;Medeiros Geraldo, LH;González-Robles, TJ;da Silva, G;Breazeale, AP;Encarnacion-Rosado, J;Pozniak, J;Kimmelman, AC;Ruggles, KV;Chris Marine, J;Chandel, NS;Lund, AW;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
ILC2-derived LIF licences progress from tissue to systemic immunity
Authors: Gogoi, M;Clark, PA;Ferreira, ACF;Rodriguez Rodriguez, N;Heycock, M;Ko, M;Murphy, JE;Chen, V;Luan, SL;Jolin, HE;McKenzie, ANJ;
Nature
Species: Transgenic Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
L-selectin-dependent and -independent homing of naïve lymphocytes through the lung draining lymph node support T cell response to pulmonary Mycobacterium tuberculosis infection
Authors: Daniel, L;Counoupas, C;Bhattacharyya, ND;Triccas, JA;Britton, WJ;Feng, CG;
PLoS pathogens
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
A2BAR Antagonism Decreases the Glomerular Expression and Secretion of Chemoattractants for Monocytes and the Pro-Fibrotic M2 Macrophages Polarization during Diabetic Nephropathy
Authors: Torres-Arévalo, Á;Nahuelpán, Y;Muñoz, K;Jara, C;Cappelli, C;Taracha-Wiśniewska, A;Quezada-Monrás, C;Martín, RS;
International journal of molecular sciences
Species: Rat
Sample Types: Whole Cells, Whole Tissue
Applications: Immunohistochemistry, Neutralization -
Glycosyltransferase Extl1 promotes CCR7-mediated dendritic cell migration to restrain infection and autoimmunity
Authors: J Liu, Y Cheng, X Zhang, Y Chen, H Zhu, K Chen, S Liu, Z Li, X Cao
Cell Reports, 2023-01-18;42(1):111991.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Lymph node fibroblastic reticular cells preserve a tolerogenic niche in allograft transplantation through laminin alpha 4
Authors: Lushen Li, Marina W. Shirkey, Tianshu Zhang, Wenji Piao, Xiaofei Li, Jing Zhao et al.
Journal of Clinical Investigation
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Artesunate Therapy Alleviates Fracture-Associated Chronic Pain After Orthopedic Surgery by Suppressing CCL21-Dependent TREM2/DAP12 Inflammatory Signaling in Mice
Authors: Linlin Zhang, Nan Li, Haoyue Zhang, Yigang Wang, Tianyu Gao, Yuying Zhao et al.
Frontiers in Pharmacology
Species: Mouse
Sample Types: In Vivo
Applications: In vivo assay -
Treg tissue stability depends on lymphotoxin beta-receptor- and adenosine-receptor-driven lymphatic endothelial cell responses
Authors: V Saxena, W Piao, L Li, C Paluskievi, Y Xiong, T Simon, R Lakhan, CC Brinkman, S Walden, KL Hippen, M WillsonShi, YS Lee, C Wagner, BR Blazar, JS Bromberg
Cell Reports, 2022-04-19;39(3):110727.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Drainage of senescent astrocytes from brain via meningeal lymphatic routes
Authors: Q Li, Y Chen, W Feng, J Cai, J Gao, F Ge, T Zhou, Z Wang, F Ding, C Marshall, C Sheng, Y Zhang, M Sun, J Shi, M Xiao
Brain, Behavior, and Immunity, 2022-04-12;103(0):85-96.
Species: Mouse
Sample Types:
Applications: In Vivo -
Mechanosensitive ACKR4 scavenges CCR7 chemokines to facilitate T�cell de-adhesion and passive transport by flow in inflamed afferent lymphatics
Authors: MC Friess, I Kritikos, P Schineis, JD Medina-San, AO Gkountidi, A Vallone, EC Sigmund, C Schwitter, M Vranova, C Matti, J Arasa, C Saygili De, E Bovay, ST Proulx, M Tomura, A Rot, DF Legler, TV Petrova, C Halin
Cell Reports, 2022-02-01;38(5):110334.
Species: Mouse
Sample Types: Whole Tissues
Applications: IHC -
Anagliptin prevents lipopolysaccharide (LPS)- induced inflammation and activation of macrophages
Authors: F Yu, W Tian, J Dong
International immunopharmacology, 2022-01-16;104(0):108514.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
A Versatile Toolkit for Semi-Automated Production of Fluorescent Chemokines to Study CCR7 Expression and Functions
Authors: M Artinger, C Matti, OJ Gerken, CT Veldkamp, DF Legler
International Journal of Molecular Sciences, 2021-04-16;22(8):.
Species: Mouse
Sample Types: Protein Lysate
Applications: Western Blot -
Meningeal lymphatic vessels regulate brain tumor drainage and immunity
Authors: X Hu, Q Deng, L Ma, Q Li, Y Chen, Y Liao, F Zhou, C Zhang, L Shao, J Feng, T He, W Ning, Y Kong, Y Huo, A He, B Liu, J Zhang, R Adams, Y He, F Tang, X Bian, J Luo
Cell Res., 2020-02-24;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
YAP/TAZ direct commitment and maturation of lymph node fibroblastic reticular cells
Authors: SY Choi, H Bae, SH Jeong, I Park, H Cho, SP Hong, DH Lee, CK Lee, JS Park, SH Suh, J Choi, MJ Yang, JY Jang, L Onder, JH Moon, HS Jeong, RH Adams, JM Kim, B Ludewig, JH Song, DS Lim, GY Koh
Nat Commun, 2020-01-24;11(1):519.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC -
Foliate Lymphoid Aggregates as Novel Forms of Serous Lymphocyte Entry Sites of Peritoneal B Cells and High-Grade B Cell Lymphomas
Authors: X Jia, F Gábris, Ó Jacobsen, G Bedics, B Botz, Z Helyes, Z Kellermaye, D Vojkovics, G Berta, N Nagy, Z Jakus, P Balogh
J. Immunol., 2019-11-25;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Attenuation of chronic antiviral T-cell responses through constitutive COX2-dependent prostanoid synthesis by lymph node fibroblasts
Authors: Karin Schaeuble, Hélène Cannelle, Stéphanie Favre, Hsin-Ying Huang, Susanne G. Oberle, Daniel E. Speiser et al.
PLOS Biology
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Origin and differentiation trajectories of fibroblastic reticular cells in the splenic white pulp
Authors: HW Cheng, L Onder, M Novkovic, C Soneson, M Lütge, N Pikor, E Scandella, MD Robinson, JI Miyazaki, A Tersteegen, U Sorg, K Pfeffer, T Rülicke, T Hehlgans, B Ludewig
Nat Commun, 2019-04-15;10(1):1739.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Single-Cell Analysis Reveals Heterogeneity of High Endothelial Venules and Different Regulation of Genes Controlling Lymphocyte Entry to Lymph Nodes
Authors: K Veerman, C Tardiveau, F Martins, J Coudert, JP Girard
Cell Rep, 2019-03-12;26(11):3116-3131.e5.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells
Authors: M Hsu, A Rayasam, JA Kijak, YH Choi, JS Harding, SA Marcus, WJ Karpus, M Sandor, Z Fabry
Nat Commun, 2019-01-16;10(1):229.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Brief Exposure of Skin to Near-Infrared Laser Modulates Mast Cell Function and Augments the Immune Response
Authors: Y Kimizuka, W Katagiri, JJ Locascio, A Shigeta, Y Sasaki, M Shibata, K Morse, RF Sîrbulescu, M Miyatake, P Reeves, M Suematsu, J Gelfand, T Brauns, MC Poznansky, K Tsukada, S Kashiwagi
J. Immunol., 2018-11-12;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Cytotoxic chemotherapy reduces T cell trafficking to the spleen by downregulating the expression of C-C motif chemokine ligand 21 and C-C motif chemokine ligand 19
Authors: L Liu, L Zhao, Y Yang, J Gao, C Hu, B Guo, B Zhu
Oncol Lett, 2018-08-09;16(4):5013-5019.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Regulation of T cell afferent lymphatic migration by targeting LT?R-mediated non-classical NF?B signaling
Authors: W Piao, Y Xiong, K Famulski, CC Brinkman, L Li, N Toney, C Wagner, V Saxena, T Simon, JS Bromberg
Nat Commun, 2018-08-01;9(1):3020.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Expression of the Atypical Chemokine Receptor ACKR4 Identifies a Novel Population of Intestinal Submucosal Fibroblasts That Preferentially Expresses Endothelial Cell Regulators
Authors: CA Thomson, SA van de Pav, M Stakenborg, E Labeeuw, G Matteoli, AM Mowat, RJB Nibbs
J. Immunol., 2018-05-14;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Role of SIRP? in Homeostatic Regulation of T Cells and Fibroblastic Reticular Cells in the Spleen
Authors: D Respatika, Y Saito, K Washio, S Komori, T Kotani, H Okazawa, Y Murata, T Matozaki
Kobe J Med Sci, 2017-08-10;63(1):E22-E29.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Increased migration of antigen presenting cells to newly-formed lymphatic vessels in transplanted kidneys by glycol-split heparin
Authors: DT Talsma, K Katta, M Boersema, S Adepu, A Naggi, G Torri, C Stegeman, G Navis, H van Goor, JL Hillebrand, S Yazdani, J van den Bo
PLoS ONE, 2017-06-30;12(6):e0180206.
Species: Human
Sample Types: Recombinant Protein
Applications: Functional Assay -
Dendritic Cells Interpret Haptotactic Chemokine Gradients in a Manner Governed by Signal-to-Noise Ratio and Dependent on GRK6
Authors: J Schwarz, V Bierbaum, K Vaahtomeri, R Hauschild, M Brown, I de Vries, A Leithner, A Reversat, J Merrin, T Tarrant, T Bollenbach, M Sixt
Curr. Biol., 2017-04-27;0(0):.
Species: Mouse
Sample Types: Complex Sample Type
Applications: IHC -
Treg engage lymphotoxin beta receptor for afferent lymphatic transendothelial migration
Authors: C Colin Brinkman
Nat Commun, 2016-06-21;7(0):12021.
Species: Mouse
Sample Types: Whole Tissue
Applications: Functional Assay -
Structural and functional features of central nervous system lymphatic vessels.
Authors: Louveau A, Smirnov I, Keyes T, Eccles J, Rouhani S, Peske J, Derecki N, Castle D, Mandell J, Lee K, Harris T, Kipnis J
Nature, 2015-06-01;523(7560):337-41.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Erythropoietin ameliorates renal interstitial fibrosis via the inhibition of fibrocyte accumulation
Authors: XU CHANG GENG, ZHOU PANG HU, GUO YONG LIAN
Molecular Medicine Reports
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells.
Authors: Kuan E, Ivanov S, Bridenbaugh E, Victora G, Wang W, Childs E, Platt A, Jakubzick C, Mason R, Gashev A, Nussenzweig M, Swartz M, Dustin M, Zawieja D, Randolph G
J Immunol, 2015-04-27;194(11):5200-10.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The Schlemm's canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel.
Authors: Aspelund A, Tammela T, Antila S, Nurmi H, Leppanen V, Zarkada G, Stanczuk L, Francois M, Makinen T, Saharinen P, Immonen I, Alitalo K
J Clin Invest, 2014-07-25;124(9):3975-86.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Deficiency of lymph node-resident dendritic cells (DCs) and dysregulation of DC chemoattractants in a malnourished mouse model of Leishmania donovani infection.
Authors: Ibrahim M, Barnes J, Osorio E, Anstead G, Jimenez F, Osterholzer J, Travi B, Ahuja S, White A, Melby P
Infect Immun, 2014-05-12;82(8):3098-112.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
CXCR4 is dispensable for T cell egress from chronically inflamed skin via the afferent lymph.
Authors: Geherin, Skye A, Wilson, R Paul, Jennrich, Silke, Debes, Gudrun F
PLoS ONE, 2014-04-21;9(4):e95626.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Preferential lymphatic growth in bronchus-associated lymphoid tissue in sustained lung inflammation.
Authors: Baluk P, Adams A, Phillips K, Feng J, Hong Y, Brown M, McDonald D
Am J Pathol, 2014-03-11;184(5):1577-92.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
TWEAK promotes peritoneal inflammation.
Authors: Sanz A, Aroeira L, Bellon T, del Peso G, Jimenez-Heffernan J, Santamaria B, Sanchez-Nino M, Blanco-Colio L, Lopez-Cabrera M, Ruiz-Ortega M, Egido J, Selgas R, Ortiz A
PLoS ONE, 2014-03-05;9(3):e90399.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
CLEC-2 is required for development and maintenance of lymph nodes.
Authors: Benezech C, Nayar S, Finney B, Withers D, Lowe K, Desanti G, Marriott C, Watson S, Caamano J, Buckley C, Barone F
Blood, 2014-02-14;123(20):3200-7.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers.
Authors: Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, Laghi L, Allavena P, Mantovani A, Marchesi F
Clin Cancer Res, 2014-02-12;20(8):2147-58.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Increased expression of the homeostatic chemokines CCL19 and CCL21 in clinical and experimental Rickettsia conorii infection.
Authors: Astrup E, Ranheim T, Damas J, Davi G, Santilli F, Jensenius M, Vitale G, Aukrust P, Olano J, Otterdal K
BMC Infect Dis, 2014-02-09;14(0):70.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Remodeling of the lymphatic vasculature during mouse mammary gland morphogenesis is mediated via epithelial-derived lymphangiogenic stimuli.
Authors: Betterman K, Paquet-Fifield S, Asselin-Labat M, Visvader J, Butler L, Stacker S, Achen M, Harvey N
Am J Pathol, 2012-10-11;181(6):2225-38.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Exposure to Ionizing Radiation Induces the Migration of Cutaneous Dendritic Cells by a CCR7-Dependent Mechanism.
Authors: Cummings, Ryan J, Gerber, Scott A, Judge, Jennifer, Ryan, Julie L, Pentland, Alice P, Lord, Edith M
J Immunol, 2012-09-21;189(9):4247-57.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
NK cells regulate CD8+ T cell priming and dendritic cell migration during influenza A infection by IFN-gamma and perforin-dependent mechanisms.
J Immunol, 2012-08-06;189(5):2099-109.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Cooperative function of CCR7 and lymphotoxin in the formation of a lymphoma-permissive niche within murine secondary lymphoid organs.
Authors: Rehm A, Mensen A, Schradi K, Gerlach K, Wittstock S, Winter S, Buchner G, Dorken B, Lipp M, Hopken UE
Blood, 2011-05-17;118(4):1020-33.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Ontogeny of stromal organizer cells during lymph node development.
Authors: Benezech C, White A, Mader E, Serre K, Parnell S, Pfeffer K, Ware CF, Anderson G, Caamano JH
J. Immunol., 2010-03-17;184(8):4521-30.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Induction of targeted cell migration by cutaneous administration of a DNA vector encoding a biologically active chemokine CCL21.
Authors: Jalili A, Pashenkov M, Kriehuber E, Wagner C, Nakano H, Stingl G, Wagner SN
J. Invest. Dermatol., 2010-02-25;130(6):1611-23.
Species: Mouse
Sample Types: Cell Lysates, Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr, Western Blot -
Comprehensive assessment of chemokine expression profiles by flow cytometry.
Authors: Eberlein J, Nguyen TT, Victorino F, Golden-Mason L, Rosen HR, Homann D
J. Clin. Invest., 2010-02-08;120(3):907-23.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Salmonella disrupts lymph node architecture by TLR4-mediated suppression of homeostatic chemokines.
Authors: St John AL, Abraham SN
Nat. Med., 2009-10-25;15(11):1259-65.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
CCR7-CCL19/CCL21-regulated dendritic cells are responsible for effectiveness of sublingual vaccination.
Authors: Song JH, Kim JI, Kwon HJ, Shim DH, Parajuli N, Cuburu N, Czerkinsky C, Kweon MN
J. Immunol., 2009-06-01;182(11):6851-60.
Species: Mouse
Sample Types: In Vivo, Whole Tissue
Applications: IHC, Neutralization -
Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE-/- mice.
Authors: Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M, Spanbroek R, Lippert B, Reardon CA, Getz GS, Fu YX, Hehlgans T, Mebius RE, van der Wall M, Kruspe D, Englert C, Lovas A, Hu D, Randolph GJ, Weih F, Habenicht AJ
J. Exp. Med., 2009-01-12;206(1):233-48.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Macrophage mannose receptor on lymphatics controls cell trafficking.
Authors: Marttila-Ichihara F, Turja R, Miiluniemi M, Karikoski M, Maksimow M, Niemela J, Martinez-Pomares L, Salmi M, Jalkanen S
Blood, 2008-04-23;112(1):64-72.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
The role of lymphoid tissue inducer cells in splenic white pulp development.
Authors: Withers DR, Kim MY, Bekiaris V, Rossi SW, Jenkinson WE, Gaspal F, McConnell F, Caamano JH, Anderson G, Lane PJ
Eur. J. Immunol., 2007-11-01;37(11):3240-5.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
The chemokine receptor CCR7 mediates corneal antigen-presenting cell trafficking.
Authors: Jin Y, Shen L, Chong EM
Mol. Vis., 2007-04-27;13(0):626-34.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo.
Authors: Worbs T, Mempel TR, Bolter J, von Andrian UH, Forster R
J. Exp. Med., 2007-02-26;204(3):489-95.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium.
Authors: Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG
J. Exp. Med., 2006-11-20;203(12):2763-77.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
The role of CCL21 in recruitment of T-precursor cells to fetal thymi.
Authors: Ueno T, Kuse S, Saito F, Nitta T, Kakiuchi T, Hollander GA, Takahama Y
Blood, 2004-09-09;105(1):31-9.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Local delivery of recombinant vaccinia virus expressing secondary lymphoid chemokine (SLC) results in a CD4 T-cell dependent antitumor response.
Authors: Flanagan K, Glover RT, Horig H, Yang W, Kaufman HL
Vaccine, 2004-07-29;22(21):2894-903.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Role of CC chemokines (macrophage inflammatory protein-1 beta, monocyte chemoattractant protein-1, RANTES) in acute lung injury in rats.
Authors: Bless NM, Huber-Lang M, Guo RF, Warner RL, Schmal H, Czermak BJ, Shanley TP, Crouch LD, Lentsch AB, Sarma V, Mulligan MS, Friedl HP, Ward PA
J. Immunol., 2000-03-01;164(5):2650-9.
Species: Rat
Sample Types: In Vivo
Applications: Neutralization -
Sphingosine 1-phosphate-regulated transcriptomes in heterogenous arterial and lymphatic endothelium of the aorta
Authors: Eric Engelbrecht, Michel V Levesque, Liqun He, Michael Vanlandewijck, Anja Nitzsche, Hira Niazi et al.
eLife
-
Immune Remodeling of the Extracellular Matrix Drives Loss of Cancer Stem Cells and Tumor Rejection.
Authors: Pires, A, Greenshields-Watson, A Et al.
Cancer Immunol Res
-
Chemokine C‐C motif ligand 21 synergized with programmed death‐ligand 1 blockade restrains tumor growth
Authors: Qiangda Chen, Hanlin Yin, Ning Pu, Jicheng Zhang, Guochao Zhao, Wenhui Lou et al.
Cancer Science
-
Heterogeneous fibroblasts underlie age-dependent tertiary lymphoid tissues in the kidney
Authors: Yuki Sato, Akiko Mii, Yoko Hamazaki, Harumi Fujita, Hirosuke Nakata, Kyoko Masuda et al.
JCI Insight
-
High-endothelial cell-derived S1P regulates dendritic cell localization and vascular integrity in the lymph node
Authors: Szandor Simmons, Naoko Sasaki, Eiji Umemoto, Yutaka Uchida, Shigetomo Fukuhara, Yusuke Kitazawa et al.
eLife
-
Smooth muscle cell recruitment to lymphatic vessels requires PDGFB and impacts vessel size but not identity
Authors: Yixin Wang, Yi Jin, Maarja Andaloussi Mäe, Yang Zhang, Henrik Ortsäter, Christer Betsholtz et al.
Development
-
Inducible Tertiary Lymphoid Structures, Autoimmunity, and Exocrine Dysfunction in a Novel Model of Salivary Gland Inflammation in C57BL/6 Mice
Authors: Michele Bombardieri, Francesca Barone, Davide Lucchesi, Saba Nayar, Wim B. van den Berg, Gordon Proctor et al.
The Journal of Immunology
-
The homeostatic chemokine CCL21 predicts mortality in aortic stenosis patients and modulates left ventricular remodeling.
Authors: Finsen Alexandra Vanessa, Ueland Thor, Sjaastad Ivar et al.
PLoS One
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse CCL21/6Ckine Antibody
Average Rating: 5 (Based on 2 Reviews)
Have you used Mouse CCL21/6Ckine Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: