Mouse CXCL10/IP-10/CRG-2 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant mouse CXCL10/IP-10/CRG-2. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: CXCL10/IP-10/CRG-2
IP-10 was originally identified as an IFN-gamma-inducible gene in monocytes, fibroblasts and endothelial cells. The mouse homolog of human IP-10, CRG-2, shares approximately 67% amino acid sequence identity with human IP-10. The amino acid sequence of IP-10 identified the protein as a member of the CXC chemokine subfamily.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse CXCL10/IP-10/CRG-2 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
109
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Microplastic exposure aggravates pneumococcus-induced inflammation in macrophages by activating ferroptosis
Authors: Chang, KW;Chen, JT;Chuang, CN;Thi Thanh Thao, D;Huang, YT;Wu, HY;Kuo, ML;Kao, KC;Chiu, CH;Lai, CH;
Journal of hazardous materials
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dissecting FAP+ Cell Diversity in Pancreatic Cancer Uncovers an Interferon-Response Subtype of Cancer-Associated Fibroblasts with Tumor-Restraining Properties
Authors: Cumming, J;Maneshi, P;Dongre, M;Alsaed, T;Dehghan-Nayeri, MJ;Ling, A;Pietras, K;Patthey, C;Öhlund, D;
Cancer research
Species: Mouse
Sample Types: Cell Culture Supernates
-
Therapeutic intervention with anti-TNF alleviates colonic and hepatic toxicity induced by perfluorooctanoic acid (PFOA)
Authors: Li, W;Qian, Y;Cai, X;He, Y;Meng, X;Zhang, L;
Ecotoxicology and environmental safety
Species: Mouse
Sample Types: Serum
-
Development of a novel complex inflammatory bowel disease mouse model: Reproducing human inflammatory bowel disease etiologies in mice
Authors: Seo, SM;Kim, NW;Yoo, ES;Lee, JH;Kang, AR;Jeong, HB;Shim, WY;Kim, DH;Park, YJ;Bae, K;Yoon, KA;Choi, YK;
PloS one
Species: Mouse
Sample Types: Serum
-
Baricitinib inhibits the activation of innate immune cells and exerts therapeutic effects on acute peritonitis and systemic inflammatory response syndrome
Authors: Hao, D;Luo, Y;Liao, H;Lu, Z;Huang, M;Du, M;Zhu, Z;Wu, Q;
International immunopharmacology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Monocyte-regulated interleukin 12 production drives clearance of Staphylococcus aureus
Authors: Peignier, A;Kim, J;Lemenze, A;Parker, D;
PLoS pathogens
Species: Mouse
Sample Types: BALF
-
TGF? primes alveolar-like macrophages to induce type I IFN following TLR2 activation
Authors: Thomas, SM;Ankley, LM;Conner, KN;Rapp, AW;McGee, AP;LeSage, F;Tanner, CD;Vielma, TE;Scheeres, EC;Obar, JJ;Olive, AJ;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
In Vitro Evaluation of Sodium Hypochlorite, Chlorhexidine, Propolis, and Calcium Hydroxide Effect on Lipoteichoic-Acid-Induced Proinflammatory Cytokines Production
Authors: de Oliveira, LD;de Carvalho, LS;Xavier, ACC;de Oliveira, FE;Leão, MVP;Diamantino, MGG;Khoury, RD;Valera, MC;Carvalho, CAT;Abu Hasna, A;
Dentistry journal
Species: Mouse
Sample Types: Cell Culture Supernates
-
An in situ depot for the sustained release of a TLR7/8 agonist in combination with a TGF? inhibitor promotes anti-tumor immune responses
Authors: Jensen, SB;Jæhger, DE;Serrano-Chávez, E;Halldórsdóttir, HR;Engel, TB;Jørgensen, JS;Björgvinsdóttir, UJ;Kostrikov, S;Scheeper, MJ;Ringgaard, L;Bruun, LM;Stavnsbjerg, C;Christensen, E;Bak, M;Thuroczy, J;Balogh, L;Jensen, ATI;Melander, F;Kjaer, A;Henriksen, JR;Hansen, AE;Andresen, TL;
Nature communications
Species: Mouse, Transgenic Mouse
Sample Types: Tissue Homogenates
-
Poly I:C vaccination drives transient CXCL9 expression near B cell follicles in the lymph node through type-I and type-II interferon signaling
Authors: Ball, AG;Morgaenko, K;Anbaei, P;Ewald, SE;Pompano, RR;
Cytokine
Species: Mouse
Sample Types: Tissue Supernates
-
Anti-Neuroinflammatory Effects of Ginkgo biloba Extract EGb 761 in LPS-Activated BV2 Microglial Cells
Authors: Sun, L;Apweiler, M;Tirkey, A;Klett, D;Normann, C;Dietz, GPH;Lehner, MD;Fiebich, BL;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Anti-Neuroinflammatory Effects of a Macrocyclic Peptide-Peptoid Hybrid in Lipopolysaccharide-Stimulated BV2 Microglial Cells
Authors: Sun, L;Wilke Saliba, S;Apweiler, M;Akmermer, K;Herlan, C;Grathwol, C;de Oliveira, ACP;Normann, C;Jung, N;Bräse, S;Fiebich, BL;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
The exonuclease TREX1 constitutes an innate immune checkpoint limiting cGAS/STING-mediated antitumor immunity
Authors: Lim, J;Rodriguez, R;Williams, K;Silva, J;Gutierrez, AG;Tyler, P;Baharom, F;Sun, T;Lin, E;Martin, S;Kayser, BD;Johnston, RJ;Mellman, I;Delamarre, L;West, NR;Müller, S;Qu, Y;Heger, K;
Cancer immunology research
Species: Mouse
Sample Types: Serum, Cell Lysates, Tissue Homogenates
-
The HIF transcription network exerts innate antiviral activity in neurons and limits brain inflammation
Authors: Farahani, E;Reinert, LS;Narita, R;Serrero, MC;Skouboe, MK;van der Horst, D;Assil, S;Zhang, B;Iversen, MB;Gutierrez, E;Hazrati, H;Johannsen, M;Olagnier, D;Kunze, R;Denham, M;Mogensen, TH;Lappe, M;Paludan, SR;
Cell reports
Species: Mouse
Sample Types: Tissue Homogenates
-
Development of cyclopeptide inhibitors of cGAS targeting protein-DNA interaction and phase separation
Authors: Wang, X;Wang, Y;Cao, A;Luo, Q;Chen, D;Zhao, W;Xu, J;Li, Q;Bu, X;Quan, J;
Nature communications
Species: Mouse
Sample Types: Serum
-
Mammalian cells internalize bacteriophages and use them as a resource to enhance cellular growth and survival
Authors: Bichet, MC;Adderley, J;Avellaneda-Franco, L;Magnin-Bougma, I;Torriero-Smith, N;Gearing, LJ;Deffrasnes, C;David, C;Pepin, G;Gantier, MP;Lin, RC;Patwa, R;Moseley, GW;Doerig, C;Barr, JJ;
PLoS biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Epigallocatechin Gallate-Modified Silver Nanoparticles Show Antiviral Activity against Herpes Simplex Type 1 and 2
Authors: Krzyzowska, M;Janicka, M;Chodkowski, M;Patrycy, M;Obuch-Woszczaty?ska, O;Tomaszewska, E;Ranoszek-Soliwoda, K;Celichowski, G;Grobelny, J;
Viruses
Species: Mouse
Sample Types: Vaginal Lavage Fluid
-
Development of LB244, an Irreversible STING Antagonist
Authors: Barasa, L;Chaudhuri, S;Zhou, JY;Jiang, Z;Choudhary, S;Green, RM;Wiggin, E;Cameron, M;Humphries, F;Fitzgerald, KA;Thompson, PR;
Journal of the American Chemical Society
Species: Mouse
Sample Types: Serum
-
Pharmacological inhibition of TBK1/IKK? blunts immunopathology in a murine model of SARS-CoV-2 infection
Authors: Ullah, TR;Johansen, MD;Balka, KR;Ambrose, RL;Gearing, LJ;Roest, J;Vivian, JP;Sapkota, S;Jayasekara, WSN;Wenholz, DS;Aldilla, VR;Zeng, J;Miemczyk, S;Nguyen, DH;Hansbro, NG;Venkatraman, R;Kang, JH;Pang, ES;Thomas, BJ;Alharbi, AS;Rezwan, R;O'Keeffe, M;Donald, WA;Ellyard, JI;Wong, W;Kumar, N;Kile, BT;Vinuesa, CG;Kelly, GE;Laczka, OF;Hansbro, PM;De Nardo, D;Gantier, MP;
Nature communications
Species: Mouse
Sample Types: Cell Culture Supernates
-
CXCL9 inhibition does not ameliorate disease in murine models of both primary and secondary hemophagocytic lymphohistiocytosis
Authors: Diamond, T;Lau, M;Morrissette, J;Chu, N;Behrens, EM;
Scientific reports
Species: Transgenic Mouse
Sample Types: Serum
-
Potential of Nucleic Acid Receptor Ligands to Improve Vaccination Efficacy against the Filarial Nematode Litomosoides sigmodontis
Authors: Scheunemann, JF;Risch, F;Reichwald, JJ;Lenz, B;Neumann, AL;Garbe, S;Frohberger, SJ;Koschel, M;Ajendra, J;Rothe, M;Latz, E;Coch, C;Hartmann, G;Schumak, B;Hoerauf, A;H�bner, MP;
Vaccines
Species: Mouse
Sample Types: Cell Culture Supernates, Pleural Lavage Fluid
-
STING-dependent interferon signatures restrict osteoclast differentiation and bone loss in mice
Authors: S MacLauchla, P Kushwaha, A Tai, J Chen, C Manning, G Swarnkar, Y Abu-Amer, KA Fitzgerald, S Sharma, EM Gravallese
Proceedings of the National Academy of Sciences of the United States of America, 2023-04-06;120(15):e2210409120.
Species: Mouse
Sample Types: Cell Culture Supernates
-
PYHIN protein IFI207 regulates cytokine transcription and IRF7 and contributes to the establishment of K. pneumoniae infection
Authors: M Baran, C Feriotti, A McGinley, SR Carlile, Z Jiang, R Calderon-G, A Dumigan, J Sá-Pessoa, CE Sutton, J Kearney, RM McLoughlin, KHG Mills, KA Fitzgerald, JA Bengeochea, AG Bowie
Cell Reports, 2023-04-04;42(4):112341.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Ionizing Radiation Selectively Increases CXC Ligand 10 Level via the DNA-Damage-Induced p38 MAPK-STAT1 Pathway in Murine J774A.1 Macrophages
Authors: YN Seo, JS Baik, SM Lee, JE Lee, HR Ahn, MS Lim, MT Park, SD Kim
Cells, 2023-03-25;12(7):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Epigenetic state determines the in vivo efficacy of STING agonist therapy
Authors: R Falahat, A Berglund, P Perez-Vill, RM Putney, I Hamaidi, S Kim, S Pilon-Thom, GN Barber, JJ Mulé
Nature Communications, 2023-03-22;14(1):1573.
Species: Mouse
Sample Types: Cell Culture Supernates
-
An Engineered IFNgamma-Antibody Fusion Protein with Improved Tumor-Homing Properties
Authors: C Di Nitto, E Gilardoni, J Mock, L Nadal, T Weiss, M Weller, F Seehusen, C Libbra, E Puca, D Neri, R De Luca
Pharmaceutics, 2023-01-22;15(2):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
DNA damage independent inhibition of NF-kappaB transcription by anthracyclines
Authors: AF Chora, D Pedroso, E Kyriakou, N Pejanovic, H Colaço, R Gozzelino, A Barros, K Willmann, T Velho, CF Moita, I Santos, P Pereira, S Carvalho, FB Martins, JA Ferreira, SF de Almeida, V Benes, J Anrather, S Weis, MP Soares, A Geerlof, J Neefjes, M Sattler, AC Messias, A Neves-Cost, LF Moita
Elife, 2022-12-07;11(0):.
Species: Mouse
Sample Types: Serum
-
MYC promotes immune-suppression in triple-negative breast cancer via inhibition of interferon signaling
Authors: D Zimmerli, CS Brambillas, F Talens, J Bhin, R Linstra, L Romanens, A Bhattachar, SEP Joosten, AM Da Silva, N Padrao, MD Wellenstei, K Kersten, M de Boo, M Roorda, L Henneman, R de Bruijn, S Annunziato, E van der Bu, AP Drenth, C Lutz, T Endres, M van de Ven, M Eilers, L Wessels, KE de Visser, W Zwart, RSN Fehrmann, MATM van Vugt, J Jonkers
Nature Communications, 2022-11-02;13(1):6579.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin-10 Deficiency Impacts on TNF-Induced NFkappaB Regulated Responses In Vivo
Authors: S Papoutsopo, L Pollock, JM Williams, MMLF Abdul-Mahd, R Dobbash, CA Duckworth, BJ Campbell
Biology, 2022-09-20;11(10):.
Species: Mouse, Transgenic Mouse
Sample Types: Serum
-
STING mediates neurodegeneration and neuroinflammation in nigrostriatal alpha-synucleinopathy
Authors: JT Hinkle, J Patel, N Panicker, SS Karuppagou, D Biswas, B Belingon, R Chen, S Brahmachar, O Pletnikova, JC Troncoso, VL Dawson, TM Dawson
Proceedings of the National Academy of Sciences of the United States of America, 2022-04-08;119(15):e2118819119.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dual G9A/EZH2 Inhibition Stimulates Antitumor Immune Response in Ovarian High-Grade Serous Carcinoma
Authors: P Spiliopoul, S Spear, H Mirza, I Garner, L McGarry, F Grundland-, Z Cheng, DP Ennis, N Iyer, S McNamara, M Natoli, S Mason, K Blyth, PD Adams, P Roxburgh, MJ Fuchter, B Brown, IA McNeish
Molecular Cancer Therapeutics, 2022-04-01;21(4):522-534.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CXCL9 inhibits tumour growth and drives anti-PD-L1 therapy in ovarian cancer
Authors: S Seitz, TF Dreyer, C Stange, K Steiger, R Bräuer, L Scheutz, G Multhoff, W Weichert, M Kiechle, V Magdolen, H Bronger
British Journal of Cancer, 2022-03-21;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
DDX50 Is a Viral Restriction Factor That Enhances IRF3 Activation
Authors: MA Pallett, Y Lu, GL Smith
Viruses, 2022-02-03;14(2):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Cisplatin and gemcitabine exert opposite effects on immunotherapy with PD-1 antibody in K-ras-driven cancer
Authors: C Glorieux, X Xia, X You, Z Wang, Y Han, J Yang, G Noppe, C Meester, J Ling, A Robert, H Zhang, SP Li, H Wang, PJ Chiao, L Zhang, X Li, P Huang
Journal of advanced research, 2021-12-21;40(0):109-124.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A noncanonical autophagy is involved in the transfer of Plasmodium-microvesicles to astrocytes
Authors: I Leleu, D Genete, SS Desnoulez, N Saidi, P Brodin, F Lafont, S Tomavo, S Pied
Autophagy, 2021-11-06;0(0):1-16.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Enhancing adoptive CD8 T cell therapy by systemic delivery of tumor associated antigens
Authors: DE Jæhger, ML Hübbe, MK Kræmer, G Clergeaud, AV Olsen, C Stavnsbjer, MN Wiinholt, A Kjær, JR Henriksen, AE Hansen, TL Andresen
Scientific Reports, 2021-10-05;11(1):19794.
Species: Mouse
Sample Types: Serum
-
Irf1- and Egr1-activated transcription plays a key role in macrophage polarization: A multiomics sequencing study with partial validation
Authors: YB Chu, J Li, P Jia, J Cui, R Zhang, X Kang, M Lv, S Zhang
International immunopharmacology, 2021-08-16;99(0):108072.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Tbx21 and Foxp3 Are Epigenetically Stabilized in T-Bet+ Tregs That Transiently Accumulate in Influenza A Virus-Infected Lungs
Authors: Y Elfaki, J Yang, J Boehme, K Schultz, D Bruder, CS Falk, J Huehn, S Floess
International Journal of Molecular Sciences, 2021-07-14;22(14):.
Species: Mouse
Sample Types: BALF
-
Cell type-specific roles of PAR1 in Coxsackievirus B3 infection
Authors: MF Bode, CM Schmedes, GJ Egnatz, V Bharathi, YM Hisada, D Martinez, T Kawano, A Weithauser, L Rosenfeldt, U Rauch, JS Palumbo, S Antoniak, N Mackman
Scientific Reports, 2021-07-12;11(1):14264.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Ligase Pellino3 Regulates Macrophage Action and Survival in Response to VSV Infection in RIG-I-Dependent Path
Authors: P Reniewicz, A Kula, E Makuch, M Ochnik, T Lipi?ski, J Siednienko
Oxidative Medicine and Cellular Longevity, 2021-07-01;2021(0):6668463.
Species: Mouse
Sample Types: Cell Culture Supernates
-
AIM2 controls microglial inflammation to prevent experimental autoimmune encephalomyelitis
Authors: Chunmei Ma, Sheng Li, Yingchao Hu, Yan Ma, Yuqing Wu, Chunyan Wu et al.
Journal of Experimental Medicine
Species: Mouse
Sample Types: Cell Culture Supernates
-
Serial transplantation unmasks galectin-9 contribution to tumor immune escape in the MB49 murine model
Authors: V Baloche, J Rivière, TBT Tran, A Gelin, O Bawa, N Signolle, MBK Diop, P Dessen, S Beq, M David, P Busson
Scientific Reports, 2021-03-04;11(1):5227.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Advanced glycation end-products reduce lipopolysaccharide uptake by macrophages
Authors: A Kitaura, T Nishinaka, S Hamasaki, OF Hatipoglu, H Wake, M Nishibori, S Mori, S Nakao, H Takahashi
PLoS ONE, 2021-01-25;16(1):e0245957.
Species: Mouse
Sample Types: Cell Culture Supenates
-
A New Mouse Model of Chronic Myocarditis Induced by Recombinant Bacille Calmette-Gu�rin Expressing a T-Cell Epitope of Cardiac Myosin Heavy Chain-&alpha
Authors: K Tajiri, K Imanaka-Yo, Y Tsujimura, K Matsuo, M Hiroe, K Aonuma, M Ieda, Y Yasutomi
International Journal of Molecular Sciences, 2021-01-14;22(2):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination
Authors: J Yu, MD Green, S Li, Y Sun, SN Journey, JE Choi, SM Rizvi, A Qin, JJ Waninger, X Lang, Z Chopra, I El Naqa, J Zhou, Y Bian, L Jiang, A Tezel, J Skvarce, RK Achar, M Sitto, BS Rosen, F Su, SP Narayanan, X Cao, S Wei, W Szeliga, L Vatan, C Mayo, MA Morgan, CA Schonewolf, K Cuneo, I Kryczek, VT Ma, CD Lao, TS Lawrence, N Ramnath, F Wen, AM Chinnaiyan, M Cieslik, A Alva, W Zou
Nature Medicine, 2021-01-04;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Respiratory Epithelial Cells Respond to Lactobacillus plantarum but Provide No Cross-Protection against Virus-Induced Inflammation
Authors: E Mai, CM Percopo, AR Limkar, AC Sek, M Ma, HF Rosenberg
Viruses, 2020-12-22;13(1):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Inducing regulated necrosis and shifting macrophage polarization with anti-EMMPRIN antibody (161-pAb) and complement factors
Authors: N Hijaze, M Ledersnaid, E Simanovich, S Kassem, MA Rahat
J Leukoc Biol, 2020-11-17;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CSF1R signaling is a regulator of pathogenesis in progressive MS
Authors: N Hagan, JL Kane, D Grover, L Woodworth, C Madore, J Saleh, J Sancho, J Liu, Y Li, J Proto, M Zelic, A Mahan, M Kothe, AA Scholte, M Fitzgerald, B Gisevius, A Haghikia, O Butovsky, D Ofengeim
Cell Death Dis, 2020-10-23;11(10):904.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The effects of glucose and fatty acids on CXCL10 expression in skeletal muscle cells
Authors: Y Ishiuchi-S, E Hiraiwa, A Shinozaki, T Nedachi
Biosci. Biotechnol. Biochem., 2020-09-02;0(0):1-10.
Species: Mouse
Sample Types: Serum
-
Head-to-head comparisons of Toxoplasma gondii and its near relative Hammondia hammondi reveal dramatic differences in the host response and effectors with species-specific functions
Authors: ZS Wong, SL Sokol-Borr, P Olias, JP Dubey, JP Boyle
PLoS Pathog., 2020-06-23;16(6):e1008528.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TRPM5 Negatively Regulates Calcium-Dependent Responses in Lipopolysaccharide-Stimulated B Lymphocytes
Authors: T Sakaguchi, R Okumura, C Ono, D Okuzaki, T Kawai, Y Okochi, N Tanimura, M Murakami, H Kayama, E Umemoto, H Kioka, T Ohtani, Y Sakata, K Miyake, Y Okamura, Y Baba, K Takeda
Cell Rep, 2020-06-09;31(10):107755.
Species: Mouse
Sample Types: Serum
-
Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti-PD-1/PD-L1 immunotherapy
Authors: MZ Noman, S Parpal, K Van Moer, M Xiao, Y Yu, J Viklund, A De Milito, M Hasmim, M Andersson, RK Amaravadi, J Martinsson, G Berchem, B Janji
Sci Adv, 2020-04-29;6(18):eaax7881.
Species: Mouse
Sample Types: Tissue Homogenates
-
Metastasis-initiating cells induce and exploit a fibroblast niche to fuel malignant colonization of the lungs
Authors: M Pein, J Insua-Rodr, T Hongu, A Riedel, J Meier, L Wiedmann, K Decker, MAG Essers, HP Sinn, S Spaich, M Sütterlin, A Schneeweis, A Trumpp, T Oskarsson
Nat Commun, 2020-03-20;11(1):1494.
Species: Mouse
Sample Types: Tissue Lysates
-
Oral flavonoid fisetin treatment protects against prolonged high-fat-diet-induced cardiac dysfunction by regulation of multicombined signaling
Authors: LF Hu, J Feng, X Dai, Y Sun, M Xiong, L Lai, S Zhong, C Yi, G Chen, H Li, Q Yang, Q Kuang, T Long, J Zhan, T Tang, C Ge, J Tan, M Xu
J. Nutr. Biochem., 2019-11-25;77(0):108253.
Species: Mouse
Sample Types: Serum
-
Rupintrivir reduces RV-induced TH-2 cytokine IL-4 in precision-cut lung slices (PCLS) of HDM-sensitized mice ex vivo
Authors: O Danov, L Lasswitz, H Obernolte, C Hesse, A Braun, S Wronski, K Sewald
Respir. Res., 2019-10-22;20(1):228.
Species: Mouse
Sample Types: Cell Culture Supernates
-
PTP1B negatively regulates nitric oxide-mediated Pseudomonas aeruginosa killing by neutrophils
Authors: L Yue, M Yan, ML Tremblay, TJ Lin, H Li, T Yang, X Song, T Xie, Z Xie
PLoS ONE, 2019-09-18;14(9):e0222753.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin 1 Receptor-Like 1 (IL1RL1) Promotes Airway Bacterial and Viral Infection and Inflammation
Authors: N Schaunaman, A Sanchez, KG Dimasuay, N Pavelka, M Numata, R Alam, RJ Martin, HW Chu
Infect. Immun., 2019-06-20;87(7):.
Species: Mouse
Sample Types: BALF
-
Targeting Lymph Node Sinus Macrophages to Inhibit Lymph Node Metastasis
Authors: Junqing Hu, Jinhao Xu, Mingyue Li, Yanping Zhang, Huaiqiang Yi, Jiangning Chen et al.
Molecular Therapy - Nucleic Acids
Species: Mouse
Sample Types: Cell Culture Supernates
-
Sterile Lung Inflammation Induced by Silica Exacerbates Mycobacterium tuberculosis Infection via STING-Dependent Type 2 Immunity
Authors: S Benmerzoug, B Bounab, S Rose, D Gosset, F Biet, T Cochard, A Xavier, N Rouxel, L Fauconnier, WGC Horsnell, B Ryffel, D Togbe, VFJ Quesniaux
Cell Rep, 2019-05-28;27(9):2649-2664.e5.
Species: Mouse
Sample Types: Tissue Homogenates
-
In-depth characterization of congenital Zika syndrome in immunocompetent mice: Antibody-dependent enhancement and an antiviral peptide therapy
Authors: VN Camargos, G Foureaux, DC Medeiros, VT da Silveir, CM Queiroz-Ju, ALB Matosinhos, AFA Figueiredo, CDF Sousa, TP Moreira, VF Queiroz, ACF Dias, KTO Santana, I Passos, ALCV Real, LC Silva, FAG Mourão, NT Wnuk, MAP Oliveira, S Macari, T Silva, GP Garlet, JA Jackman, FM Soriani, MFD Moraes, EMAM Mendes, FM Ribeiro, GMJ Costa, AL Teixeira, NJ Cho, ACP Oliveira, MM Teixeira, VV Costa, DG Souza
EBioMedicine, 2019-05-23;0(0):.
Species: Mouse
Sample Types: Plasma
-
Translational repression of Ccl5 and Cxcl10 by 4E-BP1 and 4E-BP2 restrains the ability of mouse macrophages to induce migration of activated T�cells
Authors: M William, LP Leroux, V Chaparro, TE Graber, T Alain, M Jaramillo
Eur. J. Immunol., 2019-05-06;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
PtdIns3P phosphatases MTMR3 and MTMR4 negatively regulate innate immune responses to DNA through modulating STING trafficking
Authors: DDP Putri, T Kawasaki, M Murase, T Sueyoshi, T Deguchi, D Ori, S Suetsugu, T Kawai
J. Biol. Chem., 2019-04-03;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Combination of sphingosine-1-phosphate receptor 1 (S1PR1) agonist and antiviral drug: a potential therapy against pathogenic influenza virus
Authors: J Zhao, M Zhu, H Jiang, S Shen, X Su, Y Shi
Sci Rep, 2019-03-27;9(1):5272.
Species: Mouse
Sample Types: BALF
-
Brucella abortus Cyclic Dinucleotides Trigger STING-Dependent Unfolded Protein Response That Favors Bacterial Replication
Authors: ES Guimarães, MTR Gomes, PC Campos, DS Mansur, AA Dos Santos, J Harms, G Splitter, JA Smith, GN Barber, SC Oliveira
J. Immunol., 2019-03-20;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Phagocytosis of Leptospira by leukocytes from mice with different susceptibility to leptospirosis and possible role of chemokines
Authors: PLD Silva, F Lauretti-F, M Caldas de, SS Lima, AE Covarrubia, M De Franco, E Carvalho, PL Ho, RMA da Costa, EAL Martins, JB Da Silva
BMC Microbiol., 2019-01-07;19(1):4.
Species: Mouse
Sample Types: Cell Culture Supernates
-
STING-dependent sensing of self-DNA drives silica-induced lung inflammation
Authors: S Benmerzoug, S Rose, B Bounab, D Gosset, L Duneau, P Chenuet, L Mollet, M Le Bert, C Lambers, S Geleff, M Roth, L Fauconnier, D Sedda, C Carvalho, O Perche, D Laurenceau, B Ryffel, L Apetoh, A Kiziltunc, H Uslu, FS Albez, M Akgun, D Togbe, VFJ Quesniaux
Nat Commun, 2018-12-06;9(1):5226.
Species: Mouse
Sample Types: Tissue Homogenates
-
Microphthalmia-Associated Transcription Factor (MITF) Regulates Immune Cell Migration into Melanoma
Authors: GM Wiedemann, C Aithal, A Kraechan, C Heise, BL Cadilha, J Zhang, P Duewell, R Ballotti, S Endres, C Bertolotto, S Kobold
Transl Oncol, 2018-11-28;12(2):350-360.
Species: Mouse
Sample Types: Cell Culture Supernates
-
DNA-PK Is Targeted by Multiple Vaccinia Virus Proteins to Inhibit DNA Sensing
Authors: SR Scutts, SW Ember, H Ren, C Ye, CA Lovejoy, M Mazzon, DL Veyer, RP Sumner, GL Smith
Cell Rep, 2018-11-13;25(7):1953-1965.e4.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lipoteichoic acid anchor triggers Mincle to drive protective immunity against invasive group A Streptococcus infection
Authors: T Imai, T Matsumura, S Mayer-Lamb, CA Wells, E Ishikawa, SK Butcher, TC Barnett, MJ Walker, A Imamura, H Ishida, T Ikebe, T Miyamoto, M Ato, S Ohga, B Lepenies, NM van Sorge, S Yamasaki
Proc. Natl. Acad. Sci. U.S.A., 2018-10-23;0(0):.
-
Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations
Authors: A Singanayag, N Glanville, JL Girkin, YM Ching, A Marcellini, JD Porter, M Toussaint, RP Walton, LJ Finney, J Aniscenko, J Zhu, MB Trujillo-T, MA Calderazzo, C Grainge, SL Loo, PC Veerati, PS Pathinayak, KS Nichol, AT Reid, PL James, R Solari, PAB Wark, DA Knight, MF Moffatt, WO Cookson, MR Edwards, P Mallia, NW Bartlett, SL Johnston
Nat Commun, 2018-06-08;9(1):2229.
Species: Mouse
Sample Types: BALF
-
Hu Antigen R Regulates Antiviral Innate Immune Responses through the Stabilization of mRNA for Polo-like Kinase 2
Authors: T Sueyoshi, T Kawasaki, Y Kitai, D Ori, S Akira, T Kawai
J. Immunol., 2018-04-20;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3
Authors: Moritz M. Gaidt, Thomas S. Ebert, Dhruv Chauhan, Katharina Ramshorn, Francesca Pinci, Sarah Zuber et al.
Cell
Species: Mouse
Sample Types: Cell Culture Supernates
-
SSeCKS/AKAP12 scaffolding functions suppress B16F10-induced peritoneal metastasis by attenuating CXCL9/10 secretion by resident fibroblasts
Authors: M Muramatsu, L Gao, J Peresie, B Balderman, S Akakura, IH Gelman
Oncotarget, 2017-08-09;8(41):70281-70298.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Cathelicidins Inhibit Escherichia coli-Induced TLR2 and TLR4 Activation in a Viability-Dependent Manner
Authors: M Coorens, VAF Schneider, AM de Groot, A van Dijk, M Meijerink, JM Wells, MR Scheenstra, EJA Veldhuizen, HP Haagsman
J. Immunol., 2017-07-14;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Effects of fumarates on inflammatory human astrocyte responses and oligodendrocyte differentiation
Authors: DA Galloway, JB Williams, CS Moore
Ann Clin Transl Neurol, 2017-05-04;4(6):381-391.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Optimal CD4 T cell priming after LPS-based adjuvanticity with CD134 costimulation relies on CXCL9 production
Authors: P Shinde, W Liu, A Ménoret, AD Luster, AT Vella
J. Leukoc. Biol., 2017-04-21;0(0):.
Species: Mouse
Sample Types: Serum
-
Repurposed JAK1/JAK2 Inhibitor Reverses Established Autoimmune Insulitis in Non-Obese Diabetic Mice
Authors: PM Trivedi, KL Graham, NA Scott, MR Jenkins, S Majaw, RM Sutherland, S Fynch, AM Lew, CJ Burns, B Krishnamur, TC Brodnicki, SI Mannering, TW Kay, HE Thomas
Diabetes, 2017-03-14;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
NF-?B signaling regulates cell-autonomous regulation of CXCL10 in breast cancer 4T1 cells
Authors: WJ Jin, B Kim, D Kim, HY Park Choo, HH Kim, H Ha, ZH Lee
Exp. Mol. Med, 2017-02-17;49(2):e295.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TLR7 and TLR3 Sense Brucella abortus RNA to Induce Proinflammatory Cytokine Production but They Are Dispensable for Host Control of Infection
Authors: PC Campos, MT Gomes, ES Guimarães, G Guimarães, SC Oliveira
Front Immunol, 2017-01-23;8(0):28.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interspecies cathelicidin comparison reveals divergence in antimicrobial activity, TLR modulation, chemokine induction and regulation of phagocytosis
Authors: M Coorens, MR Scheenstra, EJ Veldhuizen, HP Haagsman
Sci Rep, 2017-01-19;7(0):40874.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Allogeneic Compact Bone-Derived Mesenchymal Stem Cell Transplantation Attenuates the Severity of Idiopathic Pneumonia Syndrome in a Murine Bone Marrow Transplantation Model
Authors: Shu-Kai Qiao
Cell. Physiol. Biochem, 2016-12-23;40(6):1656-1669.
Species: Mouse
Sample Types: BALF
-
The cardiac maladaptive ATF3-dependent cross-talk between cardiomyocytes and macrophages is mediated by the IFN?-CXCL10-CXCR3 axis
Authors: A Aronheim
Int. J. Cardiol., 2016-11-10;228(0):394-400.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Protease-Activated Receptor 1 Enhances Poly I:C Induction of the Antiviral Response in Macrophages and Mice
Authors: Nigel Mackman
J Innate Immun, 2016-11-08;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Human beta-D-3 Exacerbates MDA5 but Suppresses TLR3 Responses to the Viral Molecular Pattern Mimic Polyinosinic:Polycytidylic Acid.
Authors: Semple F, MacPherson H, Webb S, Kilanowski F, Lettice L, McGlasson S, Wheeler A, Chen V, Millhauser G, Melrose L, Davidson D, Dorin J
PLoS Genet, 2015-12-08;11(12):e1005673.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The transcription factor ATF7 mediates lipopolysaccharide-induced epigenetic changes in macrophages involved in innate immunological memory.
Authors: Yoshida K, Maekawa T, Zhu Y, Renard-Guillet C, Chatton B, Inoue K, Uchiyama T, Ishibashi K, Yamada T, Ohno N, Shirahige K, Okada-Hatakeyama M, Ishii S
Nat Immunol, 2015-08-31;16(10):1034-43.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy.
Authors: Barreira da Silva R, Laird M, Yatim N, Fiette L, Ingersoll M, Albert M
Nat Immunol, 2015-06-15;16(8):850-8.
Species: Mouse
Sample Types: Plasma
-
Th1-Like ICOS+ Foxp3+ Treg Cells Preferentially Express CXCR3 and Home to beta-Islets during Pre-Diabetes in BDC2.5 NOD Mice.
Authors: Kornete M, Mason E, Girouard J, Lafferty E, Qureshi S, Piccirillo C
PLoS ONE, 2015-05-06;10(5):e0126311.
Species: Mouse
Sample Types: Tissue Homogenates
-
CXCR3 signaling in BRAFWT melanoma increases IL-8 expression and tumorigenicity.
Authors: Jenkins, Molly H, Brinckerhoff, Constanc, Mullins, David W
PLoS ONE, 2015-03-23;10(3):e0121140.
Species: Mouse
Sample Types: Tissue Homogenates
-
IRGM3 contributes to immunopathology and is required for differentiation of antigen-specific effector CD8+ T cells in experimental cerebral malaria.
Authors: Guo J, McQuillan J, Yau B, Tullo G, Long C, Bertolino P, Roediger B, Weninger W, Taylor G, Hunt N, Ball H, Mitchell A
Infect Immun, 2015-02-02;83(4):1406-17.
Species: Mouse
Sample Types: Tissue Homogenates
-
Negative regulation of melanoma differentiation-associated gene 5 (MDA5)-dependent antiviral innate immune responses by Arf-like protein 5B.
Authors: Kitai Y, Takeuchi O, Kawasaki T, Ori D, Sueyoshi T, Murase M, Akira S, Kawai T
J Biol Chem, 2014-12-01;290(2):1269-80.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Toll-like receptor 9 mediated responses in cardiac fibroblasts.
Authors: Ohm I, Alfsnes K, Belland Olsen M, Ranheim T, Sandanger O, Dahl T, Aukrust P, Finsen A, Yndestad A, Vinge L
PLoS ONE, 2014-08-15;9(8):e104398.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Animal model of respiratory syncytial virus: CD8+ T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy.
Authors: Walsh K, Teijaro J, Brock L, Fremgen D, Collins P, Rosen H, Oldstone M
J Virol, 2014-03-26;88(11):6281-93.
Species: Mouse
Sample Types: BALF
-
Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection.
Authors: Teijaro J, Walsh K, Rice S, Rosen H, Oldstone M
Proc Natl Acad Sci U S A, 2014-02-26;111(10):3799-804.
Species: Mouse
Sample Types: BALF
-
Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis
Authors: L van Bon, AJ Affandi, J Broen, RB Christmann, RJ Marijnisse, L Stawski, GA Farina, G Stifano, AL Mathes, M Cossu, M York, C Collins, M Wenink, R Huijbens, R Hesselstra, T Saxne, M DiMarzio, D Wuttge, SK Agarwal, JD Reveille, S Assassi, M Mayes, Y Deng, JP Drenth, J de Graaf, M den Heijer, CG Kallenberg, M Bijl, A Loof, WB van den Be, LA Joosten, V Smith, F de Keyser, R Scorza, C Lunardi, PL van Riel, M Vonk, W van Heerde, S Meller, B Homey, L Beretta, M Roest, M Trojanowsk, R Lafyatis, TR Radstake
N. Engl. J. Med, 2013-12-18;370(5):433-43.
Species: Human
Sample Types: Plasma
-
A pseudopterane diterpene isolated from the octocoral Pseudopterogorgia acerosa inhibits the inflammatory response mediated by TLR-ligands and TNF-alpha in macrophages.
Authors: Gonzalez Y, Doens D, Santamaria R, Ramos M, Restrepo C, Barros de Arruda L, Lleonart R, Gutierrez M, Fernandez P
PLoS ONE, 2013-12-16;8(12):e84107.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway.
Authors: Hwang J, Sundar I, Yao H, Sellix M, Rahman I
FASEB J, 2013-09-11;28(1):176-94.
Species: Mouse
Sample Types: Tissue Homogenates
-
Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing.
Authors: Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S, Tuting T, Hartmann G, Barchet W
Immunity, 2013-08-29;39(3):482-95.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The role of alveolar epithelial cells in initiating and shaping pulmonary immune responses: communication between innate and adaptive immune systems.
Authors: Chuquimia OD, Petursdottir DH, Rahman MJ, Hartl K, Singh M, Fernandez C
PLoS ONE, 2012-02-29;7(2):e32125.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection.
Authors: Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, Riva F, Garlanda C
Infect. Immun., 2011-10-24;80(1):100-9.
Species: Mouse
Sample Types: Serum
-
Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection.
Authors: Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MB, Rosen H
Cell, 2011-09-16;146(6):980-91.
Species: Mouse
Sample Types: BALF
-
Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus.
Authors: Walsh KB, Teijaro JR, Wilker PR
Proc. Natl. Acad. Sci. U.S.A., 2011-06-29;108(29):12018-23.
Species: Mouse
Sample Types: BALF
-
Phosphoinositide 3-kinase gamma plays a critical role in bleomycin-induced pulmonary inflammation and fibrosis in mice.
Authors: Russo RC, Garcia CC, Barcelos LS, Rachid MA, Guabiraba R, Roffe E, Souza AL, Sousa LP, Mirolo M, Doni A, Cassali GD, Pinho V, Locati M, Teixeira MM
J. Leukoc. Biol., 2010-11-02;89(2):269-82.
Species: Mouse
Sample Types: Tissue Homogenates
-
Systemic inhibition of transforming growth factor-beta in glioma-bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines.
Authors: Ueda R, Fujita M, Zhu X, Sasaki K, Kastenhuber ER, Kohanbash G, McDonald HA, Harper J, Lonning S, Okada H
Clin. Cancer Res., 2009-10-27;15(21):6551-9.
Species: Mouse
Sample Types: Plasma
-
Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues.
Authors: Cardona AE, Sasse ME, Liu L, Cardona SM, Mizutani M, Savarin C, Hu T, Ransohoff RM
Blood, 2008-03-17;112(2):256-63.
Species: Mouse
Sample Types: Tissue Homogenates
-
CXCR3-dependent recruitment of antigen-specific T lymphocytes to the liver during murine cytomegalovirus infection.
Authors: Hokeness KL, Deweerd ES, Munks MW, Lewis CA, Gladue RP, Salazar-Mather TP
J. Virol., 2006-11-15;81(3):1241-50.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CXCR3 and IFN protein-10 in Pneumocystis pneumonia.
Authors: McAllister F, Ruan S, Steele C, Zheng M, McKinley L, Ulrich L, Marrero L, Shellito JE, Kolls JK
J. Immunol., 2006-08-01;177(3):1846-54.
Species: Mouse
Sample Types: Tissue Homogenates
-
Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes.
Authors: Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM
J. Virol., 2006-06-01;80(11):5283-91.
Species: Mouse
Sample Types: Tissue Homogenates
-
MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo.
Authors: Delale T, Paquin A, Asselin-Paturel C, Dalod M, Brizard G, Bates EE, Kastner P, Chan S, Akira S, Vicari A, Biron CA, Trinchieri G, Briere F
J. Immunol., 2005-11-15;175(10):6723-32.
Species: Mouse
Sample Types: Serum
-
Orally administered CpG oligodeoxynucleotide induces production of CXC and CC chemokines in the gastric mucosa and suppresses bacterial colonization in a mouse model of Helicobacter pylori infection.
Authors: Raghavan S, Nystrom J, Fredriksson M, Holmgren J, Harandi AM
Infect. Immun., 2003-12-01;71(12):7014-22.
Species: Mouse
Sample Types: Serum
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse CXCL10/IP-10/CRG-2 DuoSet ELISA
Average Rating: 4.7 (Based on 9 Reviews)
Have you used Mouse CXCL10/IP-10/CRG-2 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Mouse Bone Marrow Derived Macrophages
The protocol was clear to follow and all the reagents were marked, but I wish the resuspension volumes were on the protocol sheet and not the Certificate of Analysis.
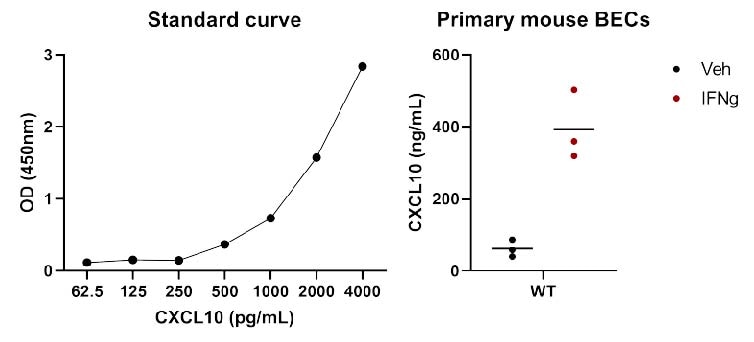
50ul of murine IL-10 Capture antibody (2ug/mL) was coated per well of a 96 well plate at 4 degrees overnight. Purified mIP-10 standard was diluted (7 fold) and assayed to ensure the quality of the ELISA. Mouse IP-10 Detector antibody was added (2h @ RT) followed by a 20 min incubation with Streptavidin-HRP. Substrate solution was added to measure mIP-10 concentration according to the manufactures protocol. The standard curve looked great as can be seen by calculation of the R- squared value. Highly recommend doing ELISA's using kits from R&D.
Buffer: 7-point standard curve