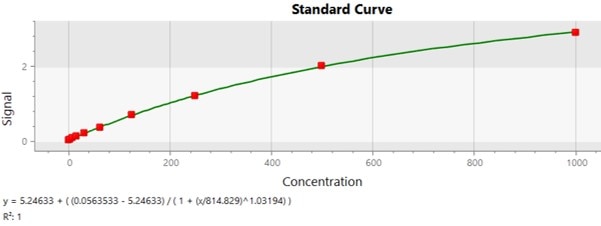
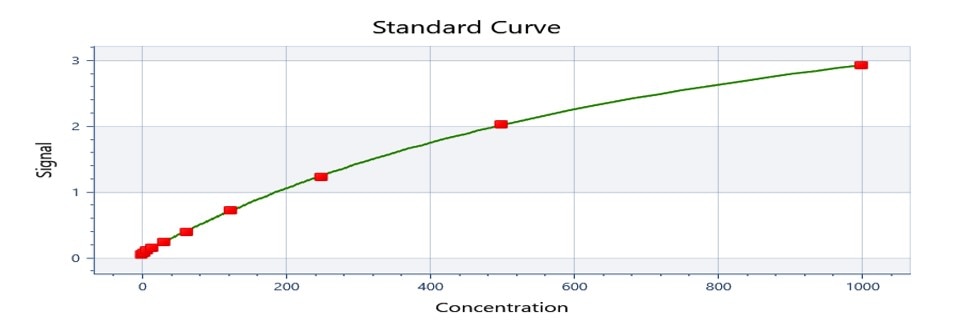
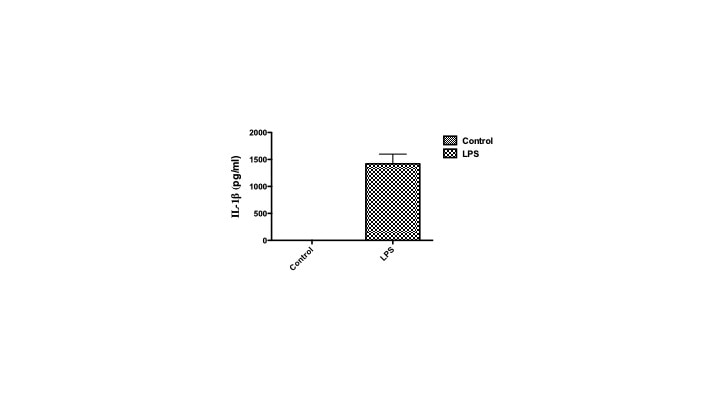
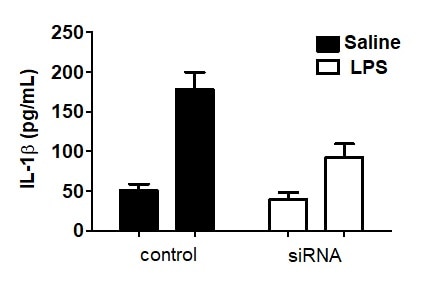
Mouse IL-1 beta/IL-1F2 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant mouse IL-1 beta/IL-1F2. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
 View Larger
View Larger
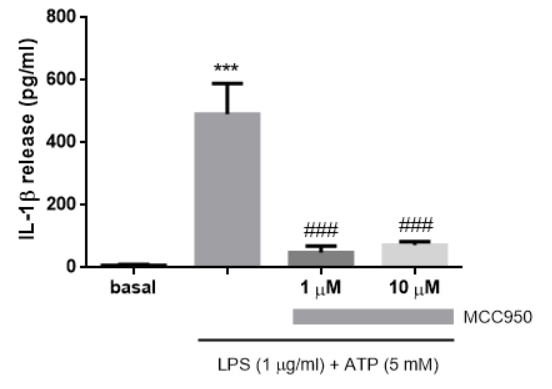
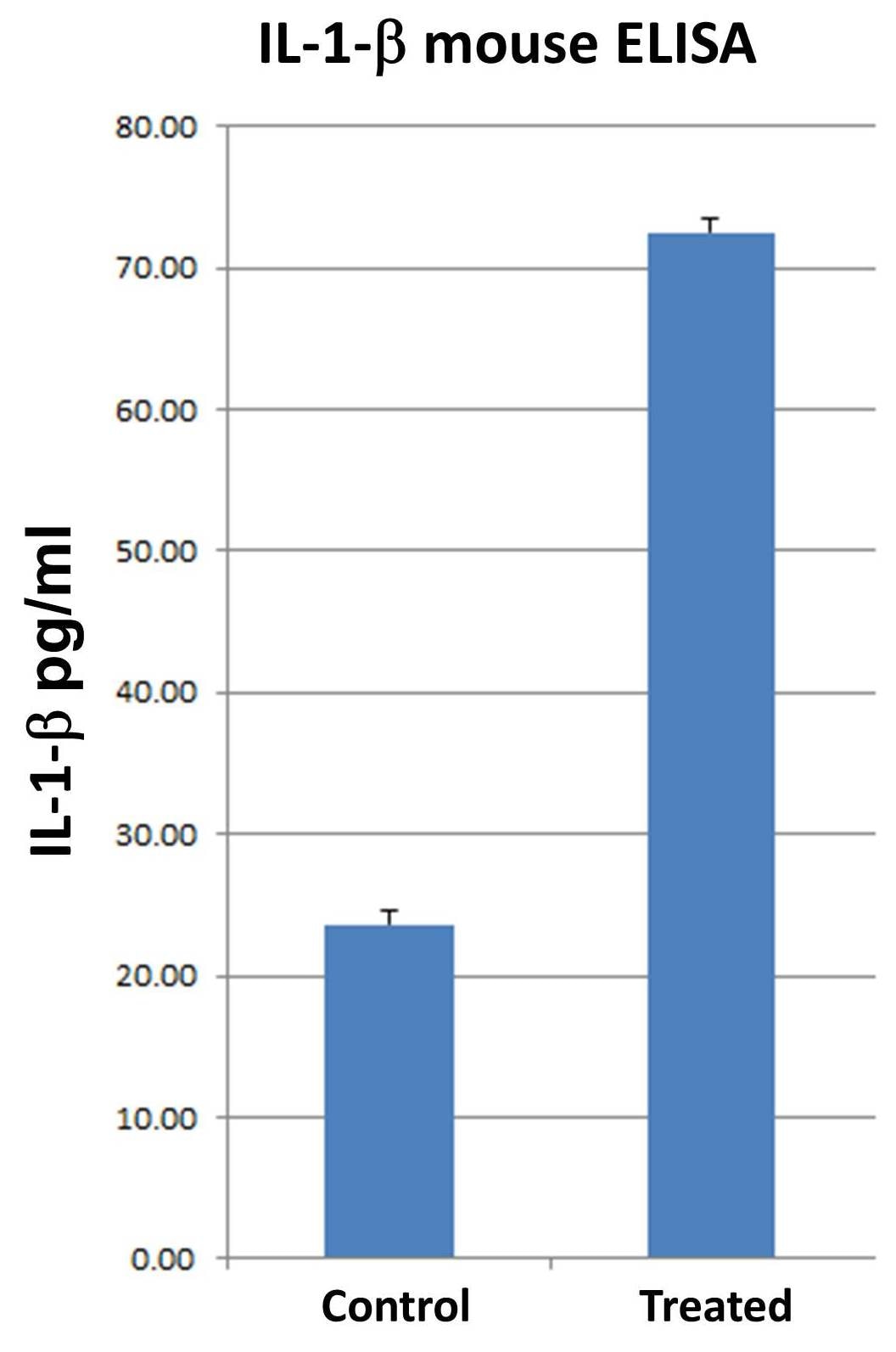
Detection of Mouse IL-1 beta/IL-1F2 by ELISA Exosomal release of IL-1 alpha by neutrophils(A) Representative confocal images of peritoneal neutrophils stimulated with LPS or curdlan for 6 h.(B) Quantification of IL-1 alpha and CD63 co-localization using ImageJ (each data point represents a single cell).(C) NTA of EV size distribution and concentration.(D–G) Neutrophils were stimulated in the presence of exosome inhibitor GW4869, and IL-1 alpha and IL-1 beta were quantified by ELISA in isolated EVs following lysis (D and F) and in total cell-free supernatants (E and G).(H) Inhibition of EV secretion shown by NTA.(I) Bioactive IL-1 signaling through IL-1R1 reporter cells was measured in isolated exosomes in the absence of detergent lysis (n = 4). Neutralizing antibodies (Abs) to IL-1 alpha, IL-1 beta, or both cytokines were included in the reporter assay, and bioactive cytokine concentration was calculated based on a standard curve using recombinant IL-1 alpha and IL-1 beta.Two-way ANOVA with Tukey’s multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Experiments in (A) and (B) were repeated three times; (C)–(G) are biological replicates from repeat experiments. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/34010648), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse IL-1 beta/IL-1F2 by ELISA Exosomal release of IL-1 alpha by neutrophils(A) Representative confocal images of peritoneal neutrophils stimulated with LPS or curdlan for 6 h.(B) Quantification of IL-1 alpha and CD63 co-localization using ImageJ (each data point represents a single cell).(C) NTA of EV size distribution and concentration.(D–G) Neutrophils were stimulated in the presence of exosome inhibitor GW4869, and IL-1 alpha and IL-1 beta were quantified by ELISA in isolated EVs following lysis (D and F) and in total cell-free supernatants (E and G).(H) Inhibition of EV secretion shown by NTA.(I) Bioactive IL-1 signaling through IL-1R1 reporter cells was measured in isolated exosomes in the absence of detergent lysis (n = 4). Neutralizing antibodies (Abs) to IL-1 alpha, IL-1 beta, or both cytokines were included in the reporter assay, and bioactive cytokine concentration was calculated based on a standard curve using recombinant IL-1 alpha and IL-1 beta.Two-way ANOVA with Tukey’s multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Experiments in (A) and (B) were repeated three times; (C)–(G) are biological replicates from repeat experiments. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/34010648), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse IL-1 beta/IL-1F2 by ELISA Exosomal release of IL-1 alpha by neutrophils(A) Representative confocal images of peritoneal neutrophils stimulated with LPS or curdlan for 6 h.(B) Quantification of IL-1 alpha and CD63 co-localization using ImageJ (each data point represents a single cell).(C) NTA of EV size distribution and concentration.(D–G) Neutrophils were stimulated in the presence of exosome inhibitor GW4869, and IL-1 alpha and IL-1 beta were quantified by ELISA in isolated EVs following lysis (D and F) and in total cell-free supernatants (E and G).(H) Inhibition of EV secretion shown by NTA.(I) Bioactive IL-1 signaling through IL-1R1 reporter cells was measured in isolated exosomes in the absence of detergent lysis (n = 4). Neutralizing antibodies (Abs) to IL-1 alpha, IL-1 beta, or both cytokines were included in the reporter assay, and bioactive cytokine concentration was calculated based on a standard curve using recombinant IL-1 alpha and IL-1 beta.Two-way ANOVA with Tukey’s multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Experiments in (A) and (B) were repeated three times; (C)–(G) are biological replicates from repeat experiments. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/34010648), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse IL-1 beta/IL-1F2 by ELISA Exosomal release of IL-1 alpha by neutrophils(A) Representative confocal images of peritoneal neutrophils stimulated with LPS or curdlan for 6 h.(B) Quantification of IL-1 alpha and CD63 co-localization using ImageJ (each data point represents a single cell).(C) NTA of EV size distribution and concentration.(D–G) Neutrophils were stimulated in the presence of exosome inhibitor GW4869, and IL-1 alpha and IL-1 beta were quantified by ELISA in isolated EVs following lysis (D and F) and in total cell-free supernatants (E and G).(H) Inhibition of EV secretion shown by NTA.(I) Bioactive IL-1 signaling through IL-1R1 reporter cells was measured in isolated exosomes in the absence of detergent lysis (n = 4). Neutralizing antibodies (Abs) to IL-1 alpha, IL-1 beta, or both cytokines were included in the reporter assay, and bioactive cytokine concentration was calculated based on a standard curve using recombinant IL-1 alpha and IL-1 beta.Two-way ANOVA with Tukey’s multiple comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Experiments in (A) and (B) were repeated three times; (C)–(G) are biological replicates from repeat experiments. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/34010648), licensed under a CC-BY license. Not internally tested by R&D Systems.
Product Datasheets
Preparation and Storage
Background: IL-1 beta/IL-1F2
The Interleukin 1 (IL-1) family of proteins consists of IL-1 alpha, IL-1 beta, and the IL-1 receptor antagonist (IL-1ra). IL-1 alpha and IL-1 beta bind to the same cell surface receptors and share biological functions (1). IL-1 is not produced by unstimulated cells of healthy individuals with the exception of skin keratinocytes, some epithelial cells, and certain cells of the central nervous system. However, in response to inflammatory agents, infections, or microbial endotoxins, a dramatic increase in the production of IL-1 by macrophages and various other cell types is seen. IL-1 beta plays a central role in immune and inflammatory responses, bone remodeling, fever, carbohydrate metabolism, and GH/IGF-I physiology. Inappropriate or prolonged production of IL-1 has been implicated in a variety of pathological conditions including sepsis, rheumatoid arthritis, inflammatory bowel disease, acute and chronic myelogenous leukemia, insulindependent diabetes mellitus, atherosclerosis, neuronal injury, and aging-related diseases (2-5).
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL of Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse IL-1 beta/IL-1F2 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
557
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Immune subversion by Leishmania infantum parasites suppresses NLRP3-driven inflammatory responses in amyloid-?-activated microglia
Authors: Calvo Alvarez, E;Sfogliarini, C;La Rosa, F;Saresella, M;Dolci, M;Vegeto, E;Taramelli, D;Basilico, N;Clerici, M;
Journal of neuroinflammation
Species: Mouse
Sample Types: Cell Culture Supernates
-
Adipocyte FMO3-derived TMAO induces WAT dysfunction and metabolic disorders by promoting inflammasome activation in ageing
Authors: Ganapathy, T;Yuan, J;Ho, MY;Wu, KK;Hoque, MM;Wang, B;Li, X;Wang, K;Wabitsch, M;Feng, X;Niu, Y;Long, K;Lian, Q;Zhu, Y;Cheng, KK;
Nature communications
Species: Mouse
Sample Types: Cell Culture Supernates, Cell Lysates, Tissue Homogenates
-
Macrophage expression of P2X7 controls autoimmune uveitis
Authors: Déchelle-Marquet, PA;Che, Y;Roux, C;Blond, F;Ronning, KE;Augustin, S;Lagouge-Roussey, P;Nous, C;Touhami, S;Bodaghi, B;Kanellopoulos, J;Adriouch, S;Guillonneau, X;Sennlaub, F;Delarasse, C;
Journal of neuroinflammation
Species: Mouse
Sample Types: Cell Culture Supernates
-
Characterizing the mechanisms underpinning interleukin-15R?-mediated protection against sepsis and candidiasis
Authors: Yang, J;Xu, B;Cao, J;Liu, Y;Tang, L;Zhao, P;Li, S;Li, X;Liu, J;Yu, R;Tang, Y;Tan, W;Ding, H;Li, J;Liu, Y;
Cytokine
Species: Mouse, Transgenic Mouse
Sample Types: Serum, Cell Culture Supernates
-
SIRT7 inhibits cerebral ischemic injury by inhibiting microglia M1 polarization via desuccinylation of NAMPT
Authors: Cheng, Y;Zhao, K;Li, J;Lei, Q;Zhang, G;Gao, X;
Brain research bulletin
Species: Mouse
Sample Types: Tissue Homogenates
-
Glutamate utilization fuels rapid production of mitochondrial ROS in dendritic cells and drives systemic inflammation during tularemia
Authors: Fabrik, I;Spidlova, P;Prchal, L;Fabrikova, D;Viduka, I;Marecic, V;Filimonenko, V;Sleha, R;Vajrychova, M;Kupcik, R;Soukup, O;Rousar, T;Härtlova, A;Santic, M;Stulik, J;
Science advances
Species: Mouse
Sample Types: Cell Culture Supernates
-
Hydroxyapatite microspheres induce durable pleurodesis and are rapidly cleared by pleural osteoclasts
Authors: Tanaka, Y;Takahashi, Y;Shindo, Y;Pitstick, LB;Teitelbaum, SL;Zou, W;Wang, X;Woods, J;Wikenheiser-Brokamp, KA;McCormack, FX;
JCI insight
Species: Mouse
Sample Types: Serum, Pleural Lavage Fluid
-
The Influence of polycyclic aromatic hydrocarbons exposure on the gut microbiome composition and inflammatory responses
Authors: Jeong, SH;Jung, J;Park, YJ;Lee, SJ;Lee, SJ;
Ecotoxicology and environmental safety
Species: Mouse
Sample Types: Serum, Tissue Homogenates
-
Caspase-11 regulates systemic inflammation and cell death in a cell-specific manner after trauma with shock
Authors: Mulla, J;Gregory, A;Liao, H;Al Matour, B;Li, Y;Abdullah, A;Billiar, TR;Scott, MJ;
Journal of leukocyte biology
Species: Mouse
Sample Types: Plasma
-
Gasdermin E is Dispensable for H1N1 Influenza Virus Pathogenesis in Mice
Authors: Speaks, S;Papa, J;McFadden, M;Roettger, JE;Liu, BD;Mohan, S;Reznik, BM;Leumi, S;Cable, JM;Forero, A;Yount, JS;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Tissue Homogenates
-
Developing a Novel Fermented Milk with Anti-Aging and Anti-Oxidative Properties Using Lactobacillus kefiranofaciens HL1 and Lactococcus lactis APL015
Authors: Wang, SY;Yen, WC;Chen, YP;Shiu, JS;Chen, MJ;
Nutrients
Species: Mouse
Sample Types: Serum, Tissue Homogenates
-
Macrophage-T cell interactions promote SLAMF1 expression for enhanced TB defense
Authors: Krishna Prasad, GVR;Grigsby, SJ;Erkenswick, GA;Portal-Celhay, C;Mittal, E;Yang, G;Fallon, SM;Chen, F;Klevorn, T;Jain, N;Li, Y;Mitreva, M;Martinot, AJ;Ernst, JD;Philips, JA;
Nature communications
Species: Mouse
Sample Types: Tissue Homogenates
-
Whole-body inhalation study of nanoparticle-enhanced vegetable oil metalworking fluids in mice for assessing occupational health risks
Authors: Vardhanapu, M;Chaganti, PK;Ghosh, A;Mahale, A;Kulkarni, OP;
Scientific reports
Species: Mouse
Sample Types: Tissue Homogenates, BALF
-
Roflumilast reduces the number of lung adenocarcinomas, inflammation, and emphysema in a smoking-induced mouse model
Authors: Sakurai, K;Nakayama, S;Chubachi, S;Otake, S;Shimada, T;Irie, H;Tsutsumi, A;Kameyama, N;Hegab, AE;Shimoda, M;Hamamoto, J;Terai, H;Yasuda, H;Kanai, Y;Fukunaga, K;
BMC pulmonary medicine
Species: Mouse
Sample Types: Tissue Homogenates
-
Acidosis Licenses the NLRP3 Inflammasome-Inhibiting Effects of Beta-Hydroxybutyrate and Short-Chain Carboxylic Acids
Authors: Mank, MM;Zoller, KA;Fastiggi, VA;Ather, JL;Poynter, ME;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Polystyrene nanoplastics exposure trigger cognitive impairment mitigated by luteolin modulated glucose-6-phosphate dehydrogenase/glutathione-dependent pathway
Authors: Tan, C;Kang, C;Liu, P;Sun, Y;Jin, H;
Journal of hazardous materials
Species: Mouse
Sample Types: Tissue Homogenates
-
PAD4 inhibition impacts immune responses in SARS-CoV-2 infection
Authors: Bonilha, CS;Veras, FP;Dos Santos Ramos, A;Gomes, GF;Rodrigues Lemes, RM;Arruda, E;Alves-Filho, JC;Cunha, TM;Cunha, FQ;
Mucosal immunology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Phytochemical Profile and Biological Activities of the Hydroethanolic Extract of Pouteria guianensis: A Pharmacological Investigation
Authors: de Matos, RC;Bitencourt, AFA;Santana, PAL;de Oliveira, ADM;Inoue, TT;Machado, BC;Caldeira, ASP;Cunha Júnior, ADS;de Souza Moreira, CP;Scopel, M;Machado, RR;
Journal of ethnopharmacology
Species: Mouse
Sample Types: Tissue Homogenates
-
HIF-1 regulates mitochondrial function in bone marrow-derived macrophages but not in tissue-resident alveolar macrophages
Authors: Woods, PS;Cetin-Atalay, R;Meliton, AY;Sun, KA;Shamaa, OR;Shin, KWD;Tian, Y;Haugen, B;Hamanaka, RB;Mutlu, GM;
Scientific reports
Species: Mouse
Sample Types: Cell Culture Supernates
-
Human breast milk-derived exosomes attenuate lipopolysaccharide-induced activation in microglia
Authors: Akinduro, O;Kumar, S;Chen, Y;Thomas, B;Hassan, Q;Sims, B;
Journal of neuroinflammation
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interferon-? production in response to the cytotoxic T lymphocyte epitope within an antigen incorporated in simian virus 40 virus-like particles
Authors: Watanabe, T;Hayashi, M;Arai, M;Matsushita, S;Handa, H;Kawano, M;
Heliyon
Species: Mouse
Sample Types: Cell Culture Supernates
-
FGF21 and APOA1 mRNA-based therapies for the treatment of experimental acute pancreatitis
Authors: Lopez-Pascual, A;Santamaria, E;Ardaiz, N;Uriarte, I;Palmer, T;Graham, AR;Gomar, C;Barbero, RC;Latasa, MU;Arechederra, M;Urman, JM;Berasain, C;Fontanellas, A;Del Rio, CL;Fernandez-Barrena, MG;Martini, PGV;Schultz, JR;Berraondo, P;Avila, MA;
Journal of translational medicine
Species: Human
Sample Types: Serum
-
Mgl2+ cDC2s coordinate fungal allergic airway type 2, but not type 17, inflammation in mice
Authors: Cook, PC;Brown, SL;Houlder, EL;Furlong-Silva, J;Conn, DP;Colombo, SAP;Baker, S;Svedberg, FR;Howell, G;Bertuzzi, M;Boon, L;Konkel, JE;Thornton, CR;Allen, JE;MacDonald, AS;
Nature communications
Species: Mouse
Sample Types: BALF
-
Plasticity of cell death pathways ensures GSDMD activation during Yersinia pseudotuberculosis infection
Authors: Chan, FHM;Yeap, HW;Liu, Z;Rosli, SN;Low, KE;Bonne, I;Wu, Y;Chong, SZ;Chen, KW;
Cell reports
Species: Mouse
Sample Types: Cell Lysates
-
Chronic Exposure to Two Regimens of Waterpipe Smoke Elicits Lung Injury, Genotoxicity, and Mitochondrial Impairment with the Involvement of MAPKs Activation in Mice
Authors: Hamadi, N;Al?Salam, S;Beegam, S;Zaaba, N;Elzaki, O;Nemmar, A;
International journal of molecular sciences
Species: Mouse
Sample Types: Tissue Homogenates
-
Gasdermin D-mediated neutrophil pyroptosis drives inflammation in psoriasis
Authors: Liu, J;Jiang, Y;Diao, Z;Chen, D;Xia, R;Wang, B;Yang, S;Yin, Z;
eLife
Species: Mouse, Transgenic Mouse
Sample Types: Tissue Homogenates
-
Inhibition of RhoA-mediated secretory autophagy in megakaryocytes mitigates myelofibrosis in mice
Authors: Becker, IC;Barrachina, MN;Lykins, J;Camacho, V;Stone, AP;Chua, BA;Signer, RAJ;Machlus, KR;Whiteheart, SW;Roweth, HG;Italiano, JE;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates, Bone Marrow Fluid
-
Inhibition of IRE1?/XBP1 axis alleviates LPS-induced acute lung injury by suppressing TXNIP/NLRP3 inflammasome activation and ERK/p65 signaling pathway
Authors: Wang, S;Hu, L;Fu, Y;Xu, F;Shen, Y;Liu, H;Zhu, L;
Respiratory research
Species: Mouse
Sample Types: BALF
-
Precision-cut lung slices in air-liquid interface (PCLS-ALI): A novel ex-vivo model for the study of Pulmonary Aspergillosis
Authors: Gonzales-Huerta, LE;Williams, TJ;Aljohani, R;Robertson, BD;Evans, CA;Armstrong-James, D;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Tissue Culture Supernates
-
Cav3.2 channel regulates cerebral ischemia/reperfusion injury: a promising target for intervention
Authors: Dai, F;Hu, C;Li, X;Zhang, Z;Wang, H;Zhou, W;Wang, J;Geng, Q;Dong, Y;Tang, C;
Neural regeneration research
Species: Mouse
Sample Types: Tissue Homogenates
-
Inhibition of PIM kinase in tumor associated macrophages suppresses inflammasome activation and sensitizes prostate cancer to immunotherapy
Authors: Clements, AN;Casillas, AL;Flores, CE;Liou, H;Toth, RK;Chauhan, SS;Sutterby, K;Deshmukh, SK;Wu, S;Xiu, J;Farrell, A;Radovich, M;Nabhan, C;Heath, EI;McKay, RR;Subah, N;Centuori, S;Wheeler, TJ;Cress, AE;Rogers, GC;Wilson, JE;Recio-Boiles, A;Warfel, NA;
bioRxiv : the preprint server for biology
Species: Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Bothrops atrox snake venom decreased MHC-II and CD86 expression in bone marrow-derived dendritic cells
Authors: da Silva, CP;Silva, MDS;Santana, HM;Paloschi, MV;Ferreira E Ferreira, AA;Brilhante, LMV;Cruz, LF;Serrath, SN;Eulálio, MMC;Setúbal, SDS;Vallochi, AL;Nery, NM;Zuliani, JP;
Acta tropica
Species: Mouse
Sample Types: Cell Culture Supernates
-
25-hydroxycholesterol promotes brain cytokine production and leukocyte infiltration in a mouse model of lipopolysaccharide-induced neuroinflammation
Authors: Romero, J;Toral-Rios, D;Yu, J;Paul, SM;Cashikar, AG;
Journal of neuroinflammation
Species: Mouse, Transgenic Mouse
Sample Types: Tissue Homogenates
-
Purified CDT toxins and a clean deletion within the CDT locus provide novel insights into the contribution of binary toxin in cellular inflammation and Clostridioides difficile infection
Authors: Nabukhotna, K;Kordus, SL;Shupe, JA;Cano Rodríguez, R;Smith, A;Bohannon, JK;Washington, MK;Lacy, DB;
PLoS pathogens
Species: Mouse
Sample Types: Tissue Homogenates
-
Characterization of the Immune-Modulating Properties of Different ?-Glucans on Myeloid Dendritic Cells
Authors: Rainer, H;Goretzki, A;Lin, YJ;Schiller, HR;Krause, M;Döring, S;Strecker, D;Junker, AC;Wolfheimer, S;Toda, M;Scheurer, S;Schülke, S;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Local administration of regulatory T cells promotes tissue healing
Authors: Nayer, B;Tan, JL;Alshoubaki, YK;Lu, YZ;Legrand, JMD;Lau, S;Hu, N;Park, AJ;Wang, XN;Amann-Zalcenstein, D;Hickey, PF;Wilson, T;Kuhn, GA;Müller, R;Vasanthakumar, A;Akira, S;Martino, MM;
Nature communications
Species: Mouse
Sample Types: Tissue Homogenates
-
Modulating NLRP3 splicing with antisense oligonucleotides to control pathological inflammation
Authors: Klein, R;Onyuru, J;Viera, EM;Putnam, CD;Hoffman, HM;Hastings, ML;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Serum, Cell Culture Supernates, Plasma
-
Mitigated toxicity of polystyrene nanoplastics in combination exposure with copper ions by transformation into copper (I) oxide: Inhibits the oxidative potential of nanoplastics
Authors: Maruthupandy, M;Jeon, JH;Noh, J;Yang, SI;Cho, WS;
Chemosphere
Species: Mouse
Sample Types: BALF
-
In Vitro Evaluation of Sodium Hypochlorite, Chlorhexidine, Propolis, and Calcium Hydroxide Effect on Lipoteichoic-Acid-Induced Proinflammatory Cytokines Production
Authors: de Oliveira, LD;de Carvalho, LS;Xavier, ACC;de Oliveira, FE;Leão, MVP;Diamantino, MGG;Khoury, RD;Valera, MC;Carvalho, CAT;Abu Hasna, A;
Dentistry journal
Species: Mouse
Sample Types: Cell Culture Supernates
-
Aspergillus fumigatus conidial surface-associated proteome reveals factors for fungal evasion and host immunity modulation
Authors: Pinzan, CF;Valero, C;de Castro, PA;da Silva, JL;Earle, K;Liu, H;Horta, MAC;Kniemeyer, O;Krüger, T;Pschibul, A;Cömert, DN;Heinekamp, T;Brakhage, AA;Steenwyk, JL;Mead, ME;Hermsdorf, N;Filler, SG;da Rosa-Garzon, NG;Delbaje, E;Bromley, MJ;Cabral, H;Diehl, C;Angeli, CB;Palmisano, G;Ibrahim, AS;Rinker, DC;Sauters, TJC;Steffen, K;Gumilang, A;Rokas, A;Gago, S;Dos Reis, TF;Goldman, GH;
Nature microbiology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Atg16l2 augments Nlrc4 inflammasome activation by facilitating NAIPs-NLRC4 association
Authors: Wen, Z;Yuan, T;Liu, J;Wang, D;Ni, J;Yan, X;Tang, J;Tang, J;Wu, X;Wang, Z;
European journal of immunology
Species: Mouse, Transgenic Mouse
Sample Types: Serum, Cell Culture Supernates
-
The potential therapeutic role of itaconate and mesaconate on the detrimental effects of LPS-induced neuroinflammation in the brain
Authors: Ohm, M;Hosseini, S;Lonnemann, N;He, W;More, T;Goldmann, O;Medina, E;Hiller, K;Korte, M;
Journal of neuroinflammation
Species: Mouse
Sample Types: Cell Culture Supernates
-
Oxysterol binding protein regulates the resolution of TLR-induced cytokine production in macrophages
Authors: Diercks, AH;Podolskaia, IS;Murray, TA;Jahn, AN;Mai, D;Liu, D;Amon, LM;Nakagawa, Y;Shimano, H;Aderem, A;Gold, ES;
Proceedings of the National Academy of Sciences of the United States of America
Species: Mouse
Sample Types: Cell Culture Supernates
-
TFEB-Mediated Pro-inflammatory Response in Murine Macrophages Induced by Acute Alpha7 Nicotinic Receptor Activation
Authors: Honwad, HH;Najibi, M;Koscso, B;Bogunovic, M;Irazoqui, JE;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
FDA-approved disulfiram inhibits the NLRP3 inflammasome by regulating NLRP3 palmitoylation
Authors: Xu, J;Pickard, JM;Núñez, G;
Cell reports
Species: Mouse
Sample Types: Cell Culture Supernates
-
H7N7 viral infection elicits pronounced, sex-specific neuroinflammatory responses in vitro
Authors: Gabele, L;Bochow, I;Rieke, N;Sieben, C;Michaelsen-Preusse, K;Hosseini, S;Korte, M;
Frontiers in cellular neuroscience
Species: Mouse
Sample Types: Cell Culture Supernates
-
A Natural deep eutectic solvent as an effective material for dual debridement and antibiofilm effects in chronic wound treatment
Authors: Map Schuh, C;Ezquer, F;Mamani, S;Campodónico, PR;Cárcamo, C;Martinez-Gómez, F;Aburto, I;Ezquer, M;Morales, B;Olivares, B;
International journal of pharmaceutics
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dihydrothiazolo ring-fused 2-pyridone antimicrobial compounds treat Streptococcus pyogenes skin and soft tissue infection
Authors: Zou, Z;Singh, P;Pinkner, JS;Obernuefemann, CLP;Xu, W;Nye, TM;Dodson, KW;Almqvist, F;Hultgren, SJ;Caparon, MG;
Science advances
Species: Mouse
Sample Types: Serum
-
Acute effects of atrazine on the immunoexpressions of Sertoli and germ cells molecular markers, cytokines, chemokines, and sex hormones levels in mice testes and epididymides
Authors: Abarikwu, SO;Coimbra, JLP;Campolina-Silva, G;Rocha, ST;Costa, VV;Lacerda, SMSN;Costa, GMJ;
Chemosphere
Species: Mouse
Sample Types: Tissue Homogenates
-
Neutrophil extracellular traps promote immunopathogenesis of virus-induced COPD exacerbations
Authors: Katsoulis, O;Toussaint, M;Jackson, MM;Mallia, P;Footitt, J;Mincham, KT;Meyer, GFM;Kebadze, T;Gilmour, A;Long, M;Aswani, AD;Snelgrove, RJ;Johnston, SL;Chalmers, JD;Singanayagam, A;
Nature communications
Species: Mouse
Sample Types: BALF
-
Differential signalling requirements for RIPK1-dependent pyroptosis in neutrophils and macrophages
Authors: Yow, SJ;Rosli, SN;Hutchinson, PE;Chen, KW;
Cell death & disease
Species: Mouse, Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Poly(allylamine)-tripolyphosphate polymeric nanoparticle as an NLRP3-dependent systemic adjuvant for the vaccine development
Authors: Rizzo, GP;Sanches, RC;Chavero, C;Bianchi, DS;Apuzzo, E;Herrera, SE;Agazzi, ML;Keitelman, IA;Trevani, AS;Oliveira, SC;Azzaroni, O;Smaldini, PL;Docena, GH;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
ATG16L1 in myeloid cells limits colorectal tumor growth in ApcMin/+ mice infected with colibactin-producing Escherichia coli via decreasing inflammasome activation
Authors: Salesse, L;Duval, A;Sauvanet, P;Da Silva, A;Barnich, N;Godfraind, C;Dalmasso, G;Nguyen, HTT;
Autophagy
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-10 inhibition during immunization improves vaccine-induced protection against Staphylococcus aureus infection
Authors: Kelly, AM;McCarthy, KN;Claxton, TJ;Carlile, SR;O'Brien, EC;Vozza, EG;Mills, KH;McLoughlin, RM;
JCI insight
Species: Mouse
Sample Types: Cell Culture Supernates, Tissue Homogenates
-
Opposing effects of the purinergic P2X7 receptor on seizures in neurons and microglia in male mice
Authors: Alves, M;Gil, B;Villegas-Salmerón, J;Salari, V;Martins-Ferreira, R;Arribas Blázquez, M;Menéndez Méndez, A;Da Rosa Gerbatin, R;Smith, J;de Diego-Garcia, L;Conte, G;Sierra-Marquez, J;Merino Serrais, P;Mitra, M;Fernandez Martin, A;Wang, Y;Kesavan, J;Melia, C;Parras, A;Beamer, E;Zimmer, B;Heiland, M;Cavanagh, B;Parcianello Cipolat, R;Morgan, J;Teng, X;Prehn, JHM;Fabene, PF;Bertini, G;Artalejo, AR;Ballestar, E;Nicke, A;Olivos-Oré, LA;Connolly, NMC;Henshall, DC;Engel, T;
Brain, behavior, and immunity
Species: Transgenic Mouse
Sample Types: Tissue Homogenates
-
Impaired autophagy in myeloid cells aggravates psoriasis-like skin inflammation through the IL-1?/CXCL2/neutrophil axis
Authors: Lee, J;Kim, MY;Kim, HJ;Choi, WS;Kim, HS;
Cell & bioscience
Species: Transgenic Mouse
Sample Types: Cell Culture Supernates
-
TLR2 Supports ?? T cell IL-17A Response to ocular surface commensals by Metabolic Reprogramming
Authors: Zhu, W;Xu, X;Nagarajan, V;Guo, J;Peng, Z;Zhang, A;Liu, J;Mattapallil, MJ;Jittayasothorn, Y;Horai, R;Leger, AJS;Caspi, RR;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
A palmitoylation-depalmitoylation relay spatiotemporally controls GSDMD activation in pyroptosis
Authors: Zhang, N;Zhang, J;Yang, Y;Shan, H;Hou, S;Fang, H;Ma, M;Chen, Z;Tan, L;Xu, D;
Nature cell biology
Species: Mouse
Sample Types: Serum, Cell Culture Supernates
-
LRRK2 G2019S Promotes Colon Cancer Potentially via LRRK2-GSDMD Axis-Mediated Gut Inflammation
Authors: Wang, Y;Gao, JZ;Sakaguchi, T;Maretzky, T;Gurung, P;Narayanan, NS;Short, S;Xiong, Y;Kang, Z;
Cells
Species: Transgenic Mouse
Sample Types: Cell Culture Supernates
-
The effects of urolithin A on poly I:C-induced microglial activation
Authors: Mingo, YB;Gabele, L;Lonnemann, N;Brône, B;Korte, M;Hosseini, S;
Frontiers in cellular neuroscience
Species: Mouse
Sample Types: Cell Culture Supernates
-
Targeting terminal pathway reduces brain complement activation, amyloid load and synapse loss, and improves cognition in a mouse model of dementia
Authors: Zelek, WM;Bevan, RJ;Morgan, BP;
Brain, behavior, and immunity
Species: Mouse
Sample Types: Serum
-
Species-specific NLRP3 regulation and its role in CNS autoinflammatory diseases
Authors: Koller, BH;Nguyen, M;Snouwaert, JN;Gabel, CA;Ting, JP;
Cell reports
Species: Mouse
Sample Types: Cell Culture Supernates, BALF
-
Osteostatin Mitigates Gouty Arthritis through the Inhibition of Caspase-1 Activation and Upregulation of Nrf2 Expression
Authors: Catalán, L;Carceller, M;Terencio, M;Alcaraz, M;Ferrándiz, M;Montesinos, M;
International Journal of Molecular Sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Panaxydol extracted from Panax ginseng inhibits NLRP3 inflammasome activation to ameliorate NASH-induced liver injury
Authors: Kim, MY;Jeong, B;Lee, GS;Jeon, H;Yang, YM;Yang, H;Han, YH;
International immunopharmacology
Species: Mouse
Sample Types: Cell Culture Supernates
-
IFN-?3 is induced by Leishmania donovani and can inhibit parasite growth in cell line models but not in the mouse model, while it shows a significant association with leishmaniasis in humans
Authors: De, M;Sukla, S;Bharatiya, S;Keshri, S;Roy, DG;Roy, S;Dutta, D;Saha, S;Ejazi, SA;Ravichandiran, V;Ali, N;Chatterjee, M;Chinnaswamy, S;
Infection and immunity
Species: Mouse
Sample Types: Cell Culture Supernates
-
GM-CSF-mediated epithelial-immune cell crosstalk orchestrates pulmonary immunity to Aspergillus fumigatus
Authors: Mills, KAM;Westermann, F;Espinosa, V;Rosiek, E;Desai, JV;Aufiero, MA;Guo, Y;Mitchell, KA;Tuzlak, S;De Feo, D;Lionakis, MS;Rivera, A;Becher, B;Hohl, TM;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Tissue Homogenates
-
Dihydrothiazolo ring-fused 2-pyridone antimicrobial compounds treat Streptococcus pyogenes skin and soft tissue infection
Authors: Zou, Z;Obernuefemann, CLP;Singh, P;Pinkner, JS;Xu, W;Nye, TM;Dodson, KW;Almqvist, F;Hultgren, SJ;Caparon, MG;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Serum
-
CpsA mediates infection of recruited lung myeloid cells by Mycobacterium tuberculosis
Authors: Grigsby, SJ;Prasad, GVRK;Wallach, JB;Mittal, E;Hsu, FF;Schnappinger, D;Philips, JA;
Cell reports
Species: Mouse
Sample Types: Tissue Homogenates
-
Central activation of the fatty acid sensor GPR120 suppresses microglia reactivity and alleviates sickness- and anxiety-like behaviors
Authors: Nakajima, S;Demers, G;Machuca-Parra, AI;Pour, ZD;Bairamian, D;Bouyakdan, K;Fisette, A;Kabahizi, A;Robb, J;Rodaros, D;Laurent, C;Ferreira, G;Arbour, N;Alquier, T;Fulton, S;
Journal of neuroinflammation
Species: Mouse
Sample Types: Cell Culture Supernates
-
Metformin inhibits paclitaxel-induced mechanical allodynia by activating opioidergic pathways and reducing cytokines production in the dorsal root ganglia and thalamus
Authors: Morais, MÍ;Braga, AV;Silva, RRL;Barbosa, BCM;Costa, SOAM;Rodrigues, FF;Melo, ISF;Matos, RC;Carobin, NV;Sabino, AP;Coelho, MM;Machado, RR;
Cytokine
Species: Mouse
Sample Types: Tissue Homogenates
-
Keratin 15 protects against cigarette smoke-induced epithelial mesenchymal transformation by MMP-9
Authors: Zhu, W;Han, L;Wu, Y;Tong, L;He, L;Wang, Q;Yan, Y;Pan, T;Shen, J;Song, Y;Shen, Y;Zhu, Q;Zhou, J;
Respiratory research
Species: Mouse, Transgenic Mouse
Sample Types: BALF
-
Discovery of anti-neuroinflammatory agents from 1,4,5,6-tetrahydrobenzo[2,3]oxepino[4,5-d]pyrimidin-2-amine derivatives by regulating microglia polarization
Authors: Yang, Y;Gao, ZF;Hou, GG;Meng, QG;Hou, Y;
European journal of medicinal chemistry
-
Discovery of anti-neuroinflammatory agents from 1,4,5,6-tetrahydrobenzo[2,3]oxepino[4,5-d]pyrimidin-2-amine derivatives by regulating microglia polarization
Authors: Yang, Y;Gao, ZF;Hou, GG;Meng, QG;Hou, Y;
European journal of medicinal chemistry
Species: Mouse
Sample Types: Cell Culture Supernates
-
A 5-Lipoxygenase Inhibitor, Zileuton, Modulates Host Immune Responses and Improves Lung Function in a Model of Severe Acute Respiratory Syndrome (SARS) Induced by Betacoronavirus
Authors: Pereira, RDD;Rabelo, RAN;Oliveira, NFM;Porto, SLT;Andrade, ACDSP;Queiroz-Junior, CM;Barbosa, CLN;de Souza-Costa, LP;Santos, FRDS;Oliveira, FBR;da Silva, BLV;Umezu, HL;Ferreira, R;da Silva, GSF;Cruz, JS;Teixeira, MM;Costa, VV;Machado, FS;
Viruses
Species: Mouse
Sample Types: Tissue Homogenates
-
Loss of polarity regulators initiates gasdermin-E-mediated pyroptosis in syncytiotrophoblasts
Authors: Khushali Patel, Jasmine Nguyen, Sumaiyah Shaha, Amy Brightwell, Wendy Duan, Ashley Zubkowski et al.
Life Science Alliance
Species: Human
Sample Types: Cell Culture Supernates
-
Chronic hyperpalatable diet induces impairment of hippocampal-dependent memories and alters glutamatergic and fractalkine axis signaling
Authors: Ribeiro, R;Silva, EG;Moreira, FC;Gomes, GF;Cussat, GR;Silva, BSR;da Silva, MCM;de Barros Fernandes, H;de Sena Oliveira, C;de Oliveira Guarnieri, L;Lopes, V;Ferreira, CN;de Faria, AMC;Maioli, TU;Ribeiro, FM;de Miranda, AS;Moraes, GSP;de Oliveira, ACP;Vieira, LB;
Scientific reports
Species: Mouse
Sample Types: Serum, Tissue Homogenates
-
Effector memory T cells induce innate inflammation by triggering DNA damage and a non-canonical STING pathway in dendritic cells
Authors: Meibers, HE;Warrick, KA;VonHandorf, A;Vallez, CN;Kawarizadeh, K;Saha, I;Donmez, O;Jain, VG;Kottyan, LC;Weirauch, MT;Pasare, C;
Cell reports
Species: Mouse
Sample Types: Serum
-
Inflammasome activation occurs in CD4+ and CD8+ T cells during graft-versus-host disease
Authors: Talley, S;Rademacher, DJ;Campbell, EM;
Cell death & disease
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Inflammasome Pathway is Activated by Dengue Virus Non-structural Protein 1 and is Protective During Dengue Virus Infection
Authors: Wong, MP;Juan, EYW;Chelluri, SS;Wang, P;Pahmeier, F;Castillo-Rojas, B;Blanc, SF;Biering, SB;Vance, RE;Harris, E;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Cefaclor causes vagus nerve-mediated depression-like symptoms with gut dysbiosis in mice
Authors: Joo, MK;Shin, YJ;Kim, DH;
Scientific reports
Species: Mouse
Sample Types: Tissue Homogenates
-
A phylogenetic approach to explore the Aspergillus fumigatus conidial surface-associated proteome and its role in pathogenesis
Authors: Goldman, G;Valero, C;Pinzan, C;de Castro, P;van Rhijn, N;Earle, K;Liu, H;Horta, MA;Kniemeyer, O;Kruger, T;Pschibul, A;Coemert, D;Heinekamp, T;Brakhage, A;Steenwyk, J;Mead, M;Rokas, A;Filler, S;da Rosa-Garzon, N;Delbaje, E;Bromley, M;Angeli, C;Palmisano, G;Ibrahim, A;Gago, S;Reis, TD;
Research square
Species: Mouse
Sample Types: Cell Culture Supernates
-
P2X7R influences tau aggregate burden in human tauopathies and shows distinct signalling in microglia and astrocytes
Authors: Beltran-Lobo, P;Hughes, MM;Troakes, C;Croft, CL;Rupawala, H;Jutzi, D;Ruepp, MD;Jimenez-Sanchez, M;Perkinton, MS;Kassiou, M;Golde, TE;Hanger, DP;Verkhratsky, A;Perez-Nievas, BG;Noble, W;
Brain, behavior, and immunity
Species: Mouse
Sample Types: Cell Culture Supernates
-
3,3-dimethyl-1-butanol and its metabolite 3,3-dimethylbutyrate ameliorate collagen-induced arthritis independent of choline trimethylamine lyase activity
Authors: Fechtner, S;Allen, BE;Chriswell, ME;Jubair, WK;Robertson, CE;Kofonow, JN;Frank, DN;Holers, VM;Kuhn, KA;
Research square
Species: Mouse
Sample Types: Cell Culture Supernates
-
Deficient P. aeruginosa in MlaA/VacJ outer membrane lipoprotein shows decrease in rhamnolipids secretion, motility, and biofilm formation, and increase in fluoroquinolones susceptibility and innate immune response
Authors: Kaur, M;Buyck, JM;Goormaghtigh, F;Decout, JL;Mozaheb, N;Mingeot-Leclercq, MP;
Research in microbiology
Species: Human
Sample Types: Cell Culture Supernates
-
A phylogenetic approach to explore the Aspergillus fumigatus conidial surface-associated proteome and its role in pathogenesis
Authors: Valero, C;Pinzan, CF;de Castro, PA;van Rhijn, N;Earle, K;Liu, H;Horta, MAC;Kniemeyer, O;Krüger, T;Pschibul, A;Coemert, DN;Heinekamp, T;Brakhage, AA;Steenwyk, JL;Mead, ME;Rokas, A;Filler, SG;da Rosa-Garzon, NG;Cabral, H;Deljabe, E;Bromley, MJ;Angeli, CB;Palmisano, G;Ibrahim, AS;Gago, S;Dos Reis, TF;Goldman, GH;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Short-Term Caloric Restriction and Subsequent Re-Feeding Compromise Liver Health and Associated Lipid Mediator Signaling in Aged Mice
Authors: Schädel, P;Wichmann-Costaganna, M;Czapka, A;Gebert, N;Ori, A;Werz, O;
Nutrients
Species: Mouse
Sample Types: Tissue Homogenates
-
Genetic Downregulation of the Metabotropic Glutamate Receptor Type 5 Dampens the Reactive and Neurotoxic Phenotype of Adult ALS Astrocytes
Authors: Torazza, C;Provenzano, F;Gallia, E;Cerminara, M;Balbi, M;Bonifacino, T;Tessitore, S;Ravera, S;Usai, C;Musante, I;Puliti, A;Van Den Bosch, L;Jafar-Nejad, P;Rigo, F;Milanese, M;Bonanno, G;
Cells
Species: Mouse
Sample Types: Cell Culture Supernates
-
Caudal DMN neurons innervate the spleen and release CART peptide to regulate neuroimmune function
Authors: Kobori, N;Moore, AN;Redell, JB;Dash, PK;
Journal of neuroinflammation
Species: Mouse
Sample Types: Plasma
-
LRRK2 G2019S promotes the development of colon cancer via modulating intestinal inflammation
Authors: Wang, Y;Gao, JZ;Sakaguchi, T;Maretzky, T;Gurung, P;Short, S;Xiong, Y;Kang, Z;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Pharmacologic inhibition of NLRP3 reduces the levels of ?-synuclein and protects dopaminergic neurons in a model of Parkinson's disease
Authors: Amo-Aparicio, J;Daly, J;Højen, JF;Dinarello, CA;
Journal of neuroinflammation
Species: Mouse
Sample Types: Tissue Homogenates
-
Polysialylation controls immune function of myeloid cells in murine model of pneumococcal pneumonia
Authors: Shinde, P;Kiepas, A;Zhang, L;Sudhir, S;Konstantopoulos, K;Stamatos, NM;
Cell reports
Species: Mouse
Sample Types: Tissue Homogenates
-
Poly-?-caprolactone nanocapsules loaded with copaiba essential oil reduce inflammation and pain in mice
Authors: Pinto, EP;da Costa, SOAM;D'Haese, C;Nysten, B;Machado, FP;Rocha, LM;de Souza, TM;Beloqui, A;Machado, RR;Araújo, RS;
International journal of pharmaceutics
Species: Mouse
Sample Types: Tissue Homogenates
-
The herbicides glyphosate and glufosinate and the cyanotoxin ?-N-methylamino-l-alanine induce long-term motor disorders following postnatal exposure: the importance of prior asymptomatic maternal inflammatory sensitization
Authors: Oummadi, A;Menuet, A;Méresse, S;Laugeray, A;Guillemin, G;Mortaud, S;
Frontiers in neuroscience
Species: Mouse
Sample Types: Peritoneal Lavage Fluid
-
Modified Xiaoyao San reverses lipopolysaccharide-induced depression-like behavior through suppressing microglia M1 polarization via enhancing autophagy involved in PI3K/Akt/mTOR pathway in mice
Authors: Su, P;Wu, M;Yin, X;Li, M;Li, Y;Bai, M;Wang, B;Xu, E;
Journal of ethnopharmacology
Species: Mouse
Sample Types: Tissue Homogenates
-
Soluble epoxide hydrolase inhibitor blockage microglial cell activation in subnucleus caudalis in a persistent model of arthritis
Authors: Tarkany Basting, R;Henrique Napimoga, M;Ant�nio Trindade Silva, C;Ballassini Abdalla, H;Campos Durso, B;Henrique Barboza Martins, L;de Abreu Cavalcanti, H;Hammock, BD;Trindade Clemente-Napimoga, J;
International immunopharmacology
Species: Mouse
Sample Types: Tissue Homogenates
-
Fisetin, an Anti-Inflammatory Agent, Overcomes Radioresistance by Activating the PERK-ATF4-CHOP Axis in Liver Cancer
Authors: Kim, TW;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Discovery of an inhibitor of DNA-driven inflammation that preferentially targets the AIM2 inflammasome
Authors: Jack P. Green, Lina Y. El-Sharkawy, Stefan Roth, Jie Zhu, Jiayu Cao, Andrew G. Leach et al.
iScience
Species: Mouse
Sample Types: Cell Culture Supernates
-
Candida auris uses metabolic strategies to escape and kill macrophages while avoiding robust activation of the NLRP3 inflammasome response
Authors: Weerasinghe, H;Simm, C;Djajawi, TM;Tedja, I;Lo, TL;Simpson, DS;Shasha, D;Mizrahi, N;Olivier, FAB;Speir, M;Lawlor, KE;Ben-Ami, R;Traven, A;
Cell reports
Species: Mouse
Sample Types: Cell Culture Supernates
-
Salmonella Enteritidis T1SS protein SiiD inhibits NLRP3 inflammasome activation via repressing the mtROS-ASC dependent pathway
Authors: Guo, Y;Gu, D;Huang, T;Li, A;Zhou, Y;Kang, X;Meng, C;Xiong, D;Song, L;Jiao, X;Pan, Z;
PLoS pathogens
Species: Transgenic Mouse, Mouse
Sample Types: Cell Culture Supernates, Serum
-
Deletion of p75NTR rescues the synaptic but not the inflammatory status in the brain of a mouse model for Alzheimer’s disease
Authors: Hendrik Demuth, Shirin Hosseini, Henning Peter Düsedeau, Ildiko Rita Dunay, Martin Korte, Marta Zagrebelsky
Frontiers in Molecular Neuroscience
Species: Mouse
Sample Types: Tissue Homogenates
-
Metabololipidomic and proteomic profiling reveals aberrant macrophage activation and interrelated immunomodulatory mediator release during aging
Authors: P Schädel, A Czapka, N Gebert, ID Jacobsen, A Ori, O Werz
Aging Cell, 2023-04-26;0(0):e13856.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Collagen-Derived Dipeptides and Amino Acids Have Immunomodulatory Effects in M1-Differentiated RAW264.7 Cells and PBMC
Authors: T Tominaga, J Huang, S Wang, M Noguchi, Y Tong, M Asano-Orit, K Suzuki
International Journal of Molecular Sciences, 2023-04-08;24(8):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Neuroinflammation, Oxidative Stress, Apoptosis, Microgliosis and Astrogliosis in the Cerebellum of Mice Chronically Exposed to Waterpipe Smoke
Authors: Hamadi, N;Beegam, S;Zaaba, NE;Elzaki, O;Altamimi, MA;Nemmar, A;
Biomedicines
Species: Mouse
Sample Types: Tissue Homogenates
-
ATP-releasing SWELL1 channel in spinal microglia contributes to neuropathic pain
Authors: J Chu, J Yang, Y Zhou, J Chen, KH Chen, C Zhang, HY Cheng, N Koylass, JO Liu, Y Guan, Z Qiu
Science Advances, 2023-03-29;9(13):eade9931.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Macrophage fumarate hydratase restrains mtRNA-mediated interferon production
Authors: A Hooftman, CG Peace, DG Ryan, EA Day, M Yang, AF McGettrick, M Yin, EN Montano, L Huo, JE Toller-Kaw, V Zecchini, TAJ Ryan, A Bolado-Car, AM Casey, HA Prag, ASH Costa, G De Los San, M Ishimori, DJ Wallace, S Venuturupa, E Nikitopoul, N Frizzell, C Johansson, A Von Kriegs, MP Murphy, C Jefferies, C Frezza, LAJ O'Neill
Nature, 2023-03-08;615(7952):490-498.
Species: Mouse
Sample Types: Cell Culture Supernates
-
SIRT6 ameliorates LPS-induced apoptosis and tight junction injury in ARDS through the ERK1/2 pathway and autophagy
Authors: H Liu, S Wang, L Gong, Y Shen, F Xu, Y Wang, L Hu, L Zhu
International Journal of Medical Sciences, 2023-03-05;20(5):581-594.
Species: Mouse
Sample Types: BALF
-
The DNA adenine methylase of Salmonella Enteritidis promotes their intracellular replication by inhibiting arachidonic acid metabolism pathway in macrophages
Authors: M Wang, D Xiong, X Wang, D Gu, C Meng, X Jiao, Z Pan
Frontiers in Microbiology, 2023-03-02;14(0):1080851.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Identifying a selective inhibitor of autophagy that targets ATG12-ATG3 protein-protein interaction
Authors: Nuta, GC;Gilad, Y;Goldberg, N;Meril, S;Bahlsen, M;Carvalho, S;Kozer, N;Barr, H;Fridmann Sirkis, Y;Herc�k, K;B?ehov�, P;Nencka, R;Bialik, S;Eisenstein, M;Kimchi, A;
Autophagy
Species: Mouse
Sample Types: Cell Culture Supernates
-
SARS-CoV-2 Spike protein induces TLR4-mediated long-term cognitive dysfunction recapitulating post-COVID-19 syndrome in mice
Authors: FL Fontes-Dan, GG Fernandes, EG Gutman, EV De Lima, LS Antonio, MB Hammerle, HP Mota-Arauj, LC Colodeti, SMB Araújo, GM Froz, TN da Silva, LA Duarte, AL Salvio, KL Pires, LAA Leon, CCF Vasconcelo, L Romão, LEB Savio, JL Silva, R da Costa, JR Clarke, AT Da Poian, SV Alves-Leon, GF Passos, CP Figueiredo
Cell Reports, 2023-02-17;42(3):112189.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Small molecule inhibiting microglial nitric oxide release could become a potential treatment for neuroinflammation
Authors: P Jordan, A Costa, E Specker, O Popp, A Volkamer, R Piske, T Obrusnik, S Kleissle, K Stuke, A Rex, M Neuenschwa, JP von Kries, M Nazare, P Mertins, H Kettenmann, SA Wolf
PLoS ONE, 2023-02-06;18(2):e0278325.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Definition of the contribution of an Osteopontin-producing CD11c+ microglial subset to Alzheimer's disease
Authors: Y Qiu, X Shen, O Ravid, D Atrakchi, D Rand, AE Wight, HJ Kim, S Liraz-Zalt, I Cooper, M Schnaider, H Cantor
Proceedings of the National Academy of Sciences of the United States of America, 2023-02-02;120(6):e2218915120.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The NLRP3 Inflammasome Is Required for Protection Against Pseudomonas Keratitis
Authors: A Ramadan, Z Cao, M Gadjeva, TS Zaidi, VA Rathinam, N Panjwani
Investigative Ophthalmology & Visual Science, 2023-02-01;64(2):11.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Antimicrobial overproduction sustains intestinal inflammation by inhibiting Enterococcus colonization
Authors: KK Jang, T Heaney, M London, Y Ding, F Yeung, D Ercelen, YH Chen, J Axelrad, S Gurunathan, A Marijke Ke, ME Griffin, HC Hang, K Cadwell
bioRxiv : the preprint server for biology, 2023-02-01;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A hierarchy of cell death pathways confers layered resistance to shigellosis in mice
Authors: JL Roncaioli, JP Babirye, RA Chavez, FL Liu, EA Turcotte, AY Lee, CF Lesser, RE Vance
Elife, 2023-01-16;12(0):.
Species: Mouse
Sample Types: Fecal Supernates
-
Phenolic and quinone methide nor-triterpenes as selective NLRP3 inflammasome inhibitors
Authors: L González-C, J P Green, I Cuadrado, Á Amesty, S Oramas-Roy, David Brou, A Estévez-Br, S Hortelano, B de Las Her
Bioorganic chemistry, 2023-01-14;132(0):106362.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TRPC absence induces pro-inflammatory macrophages and gut microbe disorder, sensitizing mice to colitis
Authors: Y Lin, X Cui, Q Cao, R Bi, Y Liu, D Jing, C Yue, Q Zhao, Y Wang, S Liu, Y Su, K Formoso, S Susperregu, L Birnbaumer, M Freichel, Y Yang, L You, X Gao
International immunopharmacology, 2022-12-31;115(0):109655.
Species: Mouse
Sample Types: Tissue Homogenates
-
Dysregulated systemic metabolism in a Down syndrome mouse model
Authors: DC Sarver, C Xu, LM Velez, S Aja, AE Jaffe, MM Seldin, RH Reeves, GW Wong
Molecular Metabolism, 2022-12-29;68(0):101666.
Species: Mouse
Sample Types: Serum
-
JC2-11, a benzylideneacetophenone derivative, attenuates inflammasome activation
Authors: G Lee, H Ahn, JH Yun, J Park, E Lee, S Oh, GS Lee
Scientific Reports, 2022-12-28;12(1):22484.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Distinct changes in endosomal composition promote NLRP3 inflammasome activation
Authors: Z Zhang, R Venditti, L Ran, Z Liu, K Vivot, A Schürmann, JS Bonifacino, MA De Matteis, R Ricci
Nature Immunology, 2022-11-28;0(0):.
Species: Mouse
Sample Types: Serum
-
Neutrophil inflammasomes sense the subcellular delivery route of translocated bacterial effectors and toxins
Authors: C Oh, L Li, A Verma, AD Reuven, EA Miao, JB Bliska, Y Aachoui
Cell Reports, 2022-11-22;41(8):111688.
Species: Mouse, Transgenic Mouse
Sample Types: Cell Culture Supernates
-
EC-18 prevents autoimmune arthritis by suppressing inflammatory cytokines and osteoclastogenesis
Authors: JS Park, SC Yang, HY Jeong, SY Lee, JG Ryu, JW Choi, HY Kang, SM Kim, SH Hwang, ML Cho, SH Park
Arthritis Research & Therapy, 2022-11-17;24(1):254.
Species: Mouse
Sample Types: Serum
-
Consequences of the Lack of TNFR1 in Ouabain Response in the Hippocampus of C57BL/6J Mice
Authors: PF Kinoshita, AM Orellana, DZ Andreotti, GA de Souza, NP de Mello, L de Sá Lima, EM Kawamoto, C Scavone
Biomedicines, 2022-11-15;10(11):.
Species: Mouse
Sample Types: Serum
-
Involvement of glycoinositolphospholipid from Trypanosoma cruzi and macrophage migration inhibitory factor in proinflammatory mechanisms promoting cardiovascular injury mechanisms promoting cardiovascular inflammation tThe combined action of glycoinositolphospholipid from Trypanosoma cruzi and macrophage migration inhibitory factor increases proinflammatory mediator production by cardiomyocytes and vascular endothelial cells
Authors: CS Rigazio, N Mariz-Pont, EP Caballero, FN Penas, NB Goren, MH Santamaría, RS Corral
Microbial pathogenesis, 2022-11-12;0(0):105881.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Involvement of the p38 MAPK-NLRC4-Caspase-1 Pathway in Ionizing Radiation-Enhanced Macrophage IL-1beta Production
Authors: JS Baik, YN Seo, YC Lee, JM Yi, MH Rhee, MT Park, SD Kim
International Journal of Molecular Sciences, 2022-11-09;23(22):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Transcriptional licensing is required for Pyrin inflammasome activation in human macrophages and bypassed by mutations causing familial Mediterranean fever
Authors: MSJ Mangan, F Gorki, K Krause, A Heinz, A Pankow, T Ebert, D Jahn, K Hiller, V Hornung, M Maurer, FI Schmidt, R Gerhard, E Latz
PloS Biology, 2022-11-07;20(11):e3001351.
Species: Human
Sample Types: Cell Culture Supernates
-
Only Acute but Not Chronic Thrombocytopenia Protects Mice against Left Ventricular Dysfunction after Acute Myocardial Infarction
Authors: F Reusswig, A Polzin, M Klier, MA Dille, A Ayhan, M Benkhoff, C Lersch, A Prinz, S Gorressen, JW Fischer, M Kelm, M Elvers
Cells, 2022-11-04;11(21):.
Species: Mouse
Sample Types: Plasma
-
Postbiotics Prepared Using Lactobacillus paracasei CCFM1224 Prevent Nonalcoholic Fatty Liver Disease by Modulating the Gut Microbiota and Liver Metabolism
Authors: Z Pan, B Mao, Q Zhang, X Tang, B Yang, J Zhao, S Cui, H Zhang
International Journal of Molecular Sciences, 2022-11-04;23(21):.
Species: Mouse
Sample Types: Serum
-
PD-1/PD-L1 inhibition enhances chemotherapy-induced neuropathic pain by suppressing neuroimmune antinociceptive signaling
Authors: CWS Wanderley, A Maganin, B Adjafre, AS Mendes, CE Anibal Sil, AU Quadros, JPM Luiz, CMS Silva, NR Silva, FF Oliveira, FIF Gomes, JLJ Restrepo, CA Speck-Hern, F Turaça, GVL Silva, GR Pigatto, HI Nakaya, JM Mota, R Barroso-So, JC Alves-Filh, TM Cunha, FQ Cunha
Cancer Immunology Research, 2022-11-02;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Blockage of KHSRP-NLRP3 by MCC950 Can Reverse the Effect of Manganese-Induced Neuroinflammation in N2a Cells and Rat Brain
Authors: S Singh, IA Shaikh, SS More, MH Mahnashi, HM Almohaimee, M El-Sherbin, MM Ghoneim, A Umar, HK Soni, H Agrawal, BA Mannasaheb, AA Khan, UM Muddapur, SMS Iqubal
International Journal of Molecular Sciences, 2022-10-30;23(21):.
Species: Mouse, Rat
Sample Types: Cell Culture Supernates, Tissue Homogenates
-
Microglial NLRP3 inflammasome activates neurotoxic astrocytes in depression-like mice
Authors: S Li, Y Fang, Y Zhang, M Song, X Zhang, X Ding, H Yao, M Chen, Y Sun, J Ding, Q Wang, M Lu, G Wu, G Hu
Cell Reports, 2022-10-25;41(4):111532.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Gasdermin D restricts anti-tumor immunity during PD-L1 checkpoint blockade
Authors: Y Jiang, Y Yang, Y Hu, R Yang, J Huang, Y Liu, Y Wu, S Li, C Ma, F Humphries, B Wang, X Wang, Z Hu, S Yang
Cell Reports, 2022-10-25;41(4):111553.
Species: Mouse
Sample Types: Tissue Homogenates
-
Combinatorial microRNA Loading into Extracellular Vesicles for Increased Anti-Inflammatory Efficacy
Authors: AE Pottash, D Levy, A Jeyaram, L Kuo, SM Kronstadt, W Chao, SM Jay
Non-coding RNA, 2022-10-21;8(5):.
Species: Mouse
Sample Types: Plasma
-
YAP1 protects against septic liver injury via ferroptosis resistance
Authors: J Wang, Q Zhu, R Li, J Zhang, X Ye, X Li
Cell & bioscience, 2022-10-01;12(1):163.
Species: Mouse
Sample Types: Tissue Homogenates
-
Homoharringtonine Attenuates Dextran Sulfate Sodium-Induced Colitis by Inhibiting NF-kappaB Signaling
Authors: J Liu, L Shi, W Huang, Z Zheng, X Huang, Y Su
Mediators of Inflammation, 2022-09-29;2022(0):3441357.
Species: Mouse
Sample Types: Cell Lysates
-
In vivo peptide-based delivery of a gene-modifying enzyme into cells of the central nervous system
Authors: JK Allen, TC Sutherland, AR Prater, CG Geoffroy, JP Pellois
Science Advances, 2022-09-28;8(39):eabo2954.
Species: Mouse
Sample Types: Tissue Homogenates
-
Bone marrow adipocytes drive the development of tissue invasive Ly6Chigh monocytes during obesity
Authors: P Boroumand, DC Prescott, T Mukherjee, PJ Bilan, M Wong, J Shen, I Tattoli, Y Zhou, A Li, T Sivasubram, N Shi, LY Zhu, Z Liu, C Robbins, DJ Philpott, SE Girardin, A Klip
Elife, 2022-09-20;11(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The escape of Candida albicans from macrophages is enabled by the fungal toxin candidalysin and two host cell death pathways
Authors: FAB Olivier, V Hilsenstei, H Weerasingh, A Weir, S Hughes, S Crawford, JE Vince, MJ Hickey, A Traven
Cell Reports, 2022-09-20;40(12):111374.
Species: Mouse
Sample Types: Cell Culture Supernates
-
FAAH served a key membrane-anchoring and stabilizing role for NLRP3 protein independently of the endocannabinoid system
Authors: Y Zhu, H Zhang, H Mao, S Zhong, Y Huang, S Chen, K Yan, Z Zhao, X Hao, Y Zhang, H Yao, X Huang, M Wang, W Zhang, J Li, G Meng, X Qin, Z Ye, J Shen, Y Song, Y Xu, Z Yang, L Wang, Y Zhang, L Wen
Cell Death and Differentiation, 2022-09-14;0(0):.
Species: Mouse
Sample Types: Serum
-
Keratinocyte-derived defensins activate neutrophil-specific receptors Mrgpra2a/b to prevent skin dysbiosis and bacterial infection
Authors: Xintong Dong, Nathachit Limjunyawong, Elizabeth I. Sypek, Gaofeng Wang, Roger V. Ortines, Christine Youn et al.
Immunity
Species: Mouse
Sample Types: Tissue Homogenates
-
Context-dependent function of TSLP and IL-1beta in skin allergic sensitization and atopic march
Authors: J Segaud, W Yao, P Marschall, F Daubeuf, C Lehalle, B German, P Meyer, P Hener, C Hugel, E Flatter, M Guivarch, L Clauss, SF Martin, M Oulad-Abde, M Li
Nature Communications, 2022-09-01;13(1):4703.
Species: Mouse
Sample Types: Tissue Homogenates
-
Dynamics of action of a Lys-49 and an Asp-49 PLA2s on inflammasome NLRP3 activation in murine macrophages
Authors: CN Boeno, MV Paloschi, JA Lopes, MD Souza Silv, JR Evangelist, VP Dos Reis, S da S Setúb, AM Soares, JP Zuliani
International immunopharmacology, 2022-08-27;112(0):109194.
Species: Mouse
Sample Types: Cell Supernates
-
Extracellular matrix of early pulmonary fibrosis modifies the polarization of alveolar macrophage
Authors: Y Zhang, L Zhu, J Hong, C Chen
International immunopharmacology, 2022-08-24;111(0):109179.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Tubule-mitophagic secretion of SerpinG1 reprograms macrophages to instruct anti-septic acute kidney injury efficacy of high-dose ascorbate mediated by NRF2 transactivation
Authors: Y Ni, GH Wu, JJ Cai, R Zhang, Y Zheng, JQ Liu, XH Yang, X Yang, Y Shen, JM Lai, XM Ye, SJ Mo
International journal of biological sciences, 2022-08-08;18(13):5168-5184.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Concerted regulation of OPG/RANKL/ NF?kappaB/MMP-13 trajectories contribute to ameliorative capability of prodigiosin and/or low dose gamma-radiation against adjuvant- induced arthritis in rats
Authors: MK Abdel-Rafe, NM Thabet, MM Amin
International immunopharmacology, 2022-08-06;111(0):109068.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The role of PP2A /NLRP3 signaling pathway in ambient particulate matter 2.5 induced lung injury
Authors: B Han, Q Liu, X Su, L Zhou, B Zhang, H Kang, J Ning, C Li, B Zhao, Y Niu, W Chen, L Chen, R Zhang
Chemosphere, 2022-08-01;307(0):135794.
Species: Mouse
Sample Types: BALF
-
Understanding the Role of Nitronate Monooxygenases in Virulence of the Human Fungal Pathogen Aspergillus fumigatus
Authors: Phuong Tuyen Nguyen, Theresa Wacker, Alistair J. P. Brown, Alessandra da Silva Dantas, Elena Shekhova
Journal of Fungi
Species: Mouse
Sample Types: Cell Culture Supernates
-
HIF-1alpha induces glycolytic reprograming in tissue-resident alveolar macrophages to promote cell survival during acute lung injury
Authors: PS Woods, LM Kimmig, KA Sun, AY Meliton, OR Shamaa, Y Tian, R Cetin-Atal, WW Sharp, RB Hamanaka, GM Mutlu
Elife, 2022-07-13;11(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lithocholic acid inhibits dendritic cell activation by reducing intracellular glutathione via TGR5 signaling
Authors: J Hu, Y Zhang, S Yi, C Wang, X Huang, S Pan, J Yang, G Yuan, S Tan, H Li
International journal of biological sciences, 2022-07-11;18(11):4545-4559.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Allergen protease-activated stress granule assembly and gasdermin D fragmentation control interleukin-33 secretion
Authors: W Chen, S Chen, C Yan, Y Zhang, R Zhang, M Chen, S Zhong, W Fan, S Zhu, D Zhang, X Lu, J Zhang, Y Huang, L Zhu, X Li, D Lv, Y Fu, H Iv, Z Ling, L Ma, H Jiang, G Long, J Zhu, D Wu, B Wu, B Sun
Nature Immunology, 2022-07-06;23(7):1021-1030.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Artemisinin inhibits neutrophil and macrophage chemotaxis, cytokine production and NET release
Authors: HOJ Morad, S Luqman, LG Pinto, KP Cunningham, B Vilar, G Clayton, M Shankar-Ha, PA McNaughton
Scientific Reports, 2022-06-30;12(1):11078.
Species: Mouse
Sample Types: BALF
-
Synthetic cannabinoids reduce the inflammatory activity of microglia and subsequently improve neuronal survival in vitro
Authors: AP Young, EM Denovan-Wr
Brain, Behavior, and Immunity, 2022-06-25;105(0):29-43.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Phosphatidylinositol 3-Kinase (PI3K) Orchestrates Aspergillus fumigatus-Induced Eosinophil Activation Independently of Canonical Toll-Like Receptor (TLR)/C-Type-Lectin Receptor (CLR) Signaling
Authors: A Dietschman, S Schruefer, S Westermann, F Henkel, K Castiglion, R Willebrand, J Adam, J Ruland, R Lang, DC Sheppard, J Esser-von-, D Radtke, S Krappmann, D Voehringer
MBio, 2022-06-13;0(0):e0123922.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Neuroprotective effects of ex vivo-expanded regulatory T cells on trimethyltin-induced neurodegeneration in mice
Authors: SY Park, H Yang, M Ye, X Liu, I Shim, YT Chang, H Bae
Journal of Neuroinflammation, 2022-06-11;19(1):143.
Species: Mouse
Sample Types: Serum
-
Acinetobacter quorum sensing contributes to inflammation-induced inhibition of orthopaedic implant osseointegration
Authors: H Choe, BS Hausman, KM Hujer, O Akkus, PN Rather, Z Lee, RA Bonomo, EM Greenfield
Oncogene, 2022-06-09;43(0):267-276.
Species: Mouse
Sample Types: Tissue Homogenates
-
ZBP1 promotes inflammatory responses downstream of TLR3/TLR4 via timely delivery of RIPK1 to TRIF
Authors: HI Muendlein, WM Connolly, Z Magri, D Jetton, I Smirnova, A Degterev, S Balachandr, A Poltorak
Proceedings of the National Academy of Sciences of the United States of America, 2022-06-06;119(24):e2113872119.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Asymmetric synthesis of flavanols via Cu-catalyzed kinetic resolution of chromenes and their anti-inflammatory activity
Authors: Q Yang, Z Wang, CHH Hor, H Xiao, Z Bian, JJ Wang
Science Advances, 2022-06-03;8(22):eabm9603.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Macrophage-intrinsic DUOX1 contributes to type 2 inflammation and mucus metaplasia during allergic airway disease
Authors: CR Morris, A Habibovic, CM Dustin, C Schiffers, MC Lin, JL Ather, YMW Janssen-He, ME Poynter, O Utermohlen, M Krönke, A van der Vl
Mucosal Immunology, 2022-06-02;0(0):.
Species: Mouse
Sample Types: BALF
-
The Therapeutic Treatment with the GAG-Binding Chemokine Fragment CXCL9(74-103) Attenuates Neutrophilic Inflammation and Lung Dysfunction during Klebsiella pneumoniae Infection in Mice
Authors: D Boff, RC Russo, H Crijns, VLS de Oliveir, MS Mattos, PE Marques, GB Menezes, AT Vieira, MM Teixeira, P Proost, FA Amaral
International Journal of Molecular Sciences, 2022-06-02;23(11):.
Species: Mouse
Sample Types: BALF
-
NLRP3 Inflammasome Negatively Regulates RANKL-Induced Osteoclastogenesis of Mouse Bone Marrow Macrophages but Positively Regulates It in the Presence of Lipopolysaccharides
Authors: MI Alam, M Mae, F Farhana, M Oohira, Y Yamashita, Y Ozaki, E Sakai, A Yoshimura
Oncogene, 2022-05-29;23(11):.
Species: Mouse
Sample Types: Cell Lysates
-
The antioxidant enzyme Peroxiredoxin-1 controls stroke-associated microglia against acute ischemic stroke
Authors: S Kim, W Lee, H Jo, SK Sonn, SJ Jeong, S Seo, J Suh, J Jin, HY Kweon, TK Kim, SH Moon, S Jeon, JW Kim, YR Kim, EW Lee, HK Shin, SH Park, GT Oh
Redox Biology, 2022-05-25;54(0):102347.
Species: Mouse
Sample Types: Serum
-
Preclinical Efficacy of a Capsid Virus-like Particle-Based Vaccine Targeting IL-1beta for Treatment of Allergic Contact Dermatitis
Authors: L Goksøyr, AB Funch, AK Okholm, TG Theander, WA de Jongh, CM Bonefeld, AF Sander
Vaccines, 2022-05-23;10(5):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Development of sulfonamide-based NLRP3 inhibitors: Further modifications and optimization through structure-activity relationship studies
Authors: Y Xu, Y Xu, H Blevins, C Guo, S Biby, XY Wang, C Wang, S Zhang
European Journal of Medicinal Chemistry, 2022-05-21;238(0):114468.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation
Authors: LK Billingham, JS Stoolman, K Vasan, AE Rodriguez, TA Poor, M Szibor, HT Jacobs, CR Reczek, A Rashidi, P Zhang, J Miska, NS Chandel
Nature Immunology, 2022-04-28;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Salmonella Enteritidis GalE Protein Inhibits LPS-Induced NLRP3 Inflammasome Activation
Authors: T Huang, D Gu, Y Guo, A Li, X Kang, X Jiao, Z Pan
Microorganisms, 2022-04-26;10(5):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Bladder epithelial cell phosphate transporter inhibition protects mice against uropathogenic Escherichia coli infection
Authors: Y Pang, Z Cheng, S Zhang, S Li, X Li, X Li, X Zhang, X Li, Y Feng, H Cui, Z Chen, L Liu, Q Li, J Huang, M Zhang, S Zhu, L Wang, L Feng
Cell Reports, 2022-04-19;39(3):110698.
Species: Mouse
Sample Types: Serum
-
ADP-ribosylating adjuvant reveals plasticity in cDC1 cells that drive mucosal Th17 cell development and protection against influenza virus infection
Authors: M Arabpour, C Lebrero-Fe, K Schön, A Strömberg, V Börjesson, K Lahl, M Ballegeer, X Saelens, D Angeletti, W Agace, N Lycke
Mucosal Immunology, 2022-04-13;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Liposomal Dexamethasone Reduces A/H1N1 Influenza-Associated Morbidity in Mice
Authors: JW Kwon, H Quan, J Song, H Chung, D Jung, JJ Hong, YR Na, SH Seok
Frontiers in Microbiology, 2022-04-12;13(0):845795.
Species: Mouse
Sample Types: BALF
-
Effects of mango and mint pod-based e-cigarette aerosol inhalation on inflammatory states of the brain, lung, heart, and colon in mice
Authors: A Moshensky, CS Brand, H Alhaddad, J Shin, JA Masso-Silv, I Advani, D Gunge, A Sharma, S Mehta, A Jahan, S Nilaad, J Olay, W Gu, T Simonson, D Almarghala, J Pham, S Perera, K Park, R Al-Kolla, H Moon, S Das, M Byun, Z Shah, Y Sari, J Heller Bro, LE Crotty Ale
Elife, 2022-04-12;11(0):.
Species: Mouse
Sample Types: BALF
-
Menthone Inhalation Alleviates Local and Systemic Allergic Inflammation in Ovalbumin-Sensitized and Challenged Asthmatic Mice
Authors: YH Su, JY Lin
International Journal of Molecular Sciences, 2022-04-04;23(7):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Mechanism of Pertussis Cough Revealed by the Mouse-Coughing Model
Authors: Y Hiramatsu, K Suzuki, T Nishida, N Onoda, T Satoh, S Akira, M Ikawa, H Ikeda, J Kamei, S Derouiche, M Tominaga, Y Horiguchi
MBio, 2022-03-31;0(0):e0319721.
Species: Mouse
Sample Types: Cell culture supernate
-
Disulfiram inhibits neutrophil extracellular trap formation protecting rodents from acute lung injury and SARS-CoV-2 infection
Authors: JM Adrover, L Carrau, J Da beta ler-Ple, Y Bram, V Chandar, S Houghton, D Redmond, JR Merrill, M Shevik, BR tenOever, SK Lyons, RE Schwartz, M Egeblad
JCI Insight, 2022-03-08;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Nociceptor-derived Reg3gamma prevents endotoxic death by targeting kynurenine pathway in microglia
Authors: E Sugisawa, T Kondo, Y Kumagai, H Kato, Y Takayama, K Isohashi, E Shimosegaw, N Takemura, Y Hayashi, T Sasaki, MM Martino, M Tominaga, K Maruyama
Cell Reports, 2022-03-08;38(10):110462.
Species: Mouse
Sample Types: Plasma
-
Acute exposure to phthalates during recovery from a myocardial infarction induces greater inflammasome activation in male C57bl/6N mice
Authors: A Schwendt, JB Chammas, LE Chalifour
Toxicology and Applied Pharmacology, 2022-03-01;440(0):115954.
Species: Mouse
Sample Types: Plasma
-
The phosphoinositide-3-kinase (PI3K)-delta inhibitor seletalisib impairs monocyte-derived dendritic cells maturation, APC function, and promotes their migration to CCR7 and CXCR4 ligands
Authors: F Scopelliti, L Mercurio, C Cattani, V Dimartino, C Albanesi, G Costanzo, C Mirisola, S Madonna, A Cavani
Journal of leukocyte biology, 2022-02-24;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Fibrotic lung disease inhibits innate immune responses to Staphylococcal pneumonia via impaired neutrophil and macrophage function
Authors: HI Warheit-Ni, SJ Edwards, S SenGupta, CA Parent, X Zhou, DN O'Dwyer, BB Moore
JCI Insight, 2022-02-22;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Inhibition of mitoNEET attenuates LPS-induced inflammation and oxidative stress
Authors: S Lee, BG Seok, SJ Lee, SW Chung
Cell Death & Disease, 2022-02-08;13(2):127.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Recombinant BCG Expressing the Subunit 1 of Pertussis Toxin Induces Innate Immune Memory and Confers Protection against Non-Related Pathogens
Authors: AI Kanno, D Boraschi, LCC Leite, D Rodriguez
Vaccines, 2022-02-03;10(2):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Recruitment and activation of type 3 innate lymphoid cells promote antitumor immune responses
Authors: M Bruchard, M Geindreau, A Perrichet, C Truntzer, E Ballot, R Boidot, C Racoeur, E Barsac, F Chalmin, C Hibos, T Baranek, C Paget, B Ryffel, C Rébé, C Paul, F Végran, F Ghiringhel
Nature Immunology, 2022-01-31;23(2):262-274.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Langerhans cells and cDC1s play redundant roles in mRNA-LNP induced protective anti-influenza and anti-SARS-CoV-2 immune responses
Authors: S Ndeupen, A Bouteau, C Herbst, Z Qin, S Jacobsen, NE Powers, Z Hutchins, D Kurup, LZ Diba, M Watson, H Ramage, BZ Igyártó
PloS Pathogens, 2022-01-24;18(1):e1010255.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Clinical-like cryotherapy in acute knee arthritis of the knee improves inflammation signs, pain, joint swelling, and motor performance in mice
Authors: PATS Castro, GM Barbosa, DH Machanocke, RS Peres, TM Cunha, JE Cunha, FFB Oliveira, FS Ramalho, TL Russo, FQ Cunha, TF Salvini
PLoS ONE, 2022-01-21;17(1):e0261667.
Species: Mouse
Sample Types: Tissue Homogenates
-
The Compound (E)-2-Cyano-N,3-diphenylacrylamide (JMPR-01): A Potential Drug for Treatment of Inflammatory Diseases
Authors: PR da Silva, RF do Espírit, CO Melo, FE Pachú Cava, TB Costa, YV Barbosa, YMSM E Silva, NF de Sousa, CF Villarreal, RO de Moura, VL Dos Santos
Pharmaceutics, 2022-01-13;14(1):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Intense Acute Swimming Induces Delayed-Onset Muscle Soreness Dependent on Spinal Cord Neuroinflammation
Authors: SM Borghi, SKD Bussulo, FA Pinho-Ribe, V Fattori, TT Carvalho, FS Rasquel-Ol, TH Zaninelli, CR Ferraz, AMB Casella, FQ Cunha, TM Cunha, R Casagrande, WA Verri
Frontiers in Pharmacology, 2022-01-07;12(0):734091.
Species: Mouse
Sample Types: Tissue Homogenates
-
The Salutary Effects of Catalpol on Diesel Exhaust Particles-Induced Thrombogenic Changes and Cardiac Oxidative Stress, Inflammation and Apoptosis
Authors: A Nemmar, S Beegam, NE Zaaba, S Alblooshi, S Alseiari, BH Ali
Biomedicines, 2022-01-04;10(1):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Ephedrine ameliorates chronic obstructive pulmonary disease (COPD) through restraining endoplasmic reticulum (ER) stress in vitro and in vivo
Authors: HL Wang, FQ Chen, LJ Wu
International immunopharmacology, 2021-12-22;103(0):107842.
Species: Mouse
Sample Types: BALF
-
Metabolic Regulation of Macrophages by SIRT1 Determines Activation During Cholestatic Liver Disease in Mice
Authors: A Isaacs-Ten, M Moreno-Gon, C Bone, A Martens, F Bernuzzi, T Ludwig, C Hellmich, K Hiller, SA Rushworth, N Beraza
Cellular and Molecular Gastroenterology and Hepatology, 2021-12-22;0(0):.
Species: Mouse
Sample Types: Cell Lysates
-
IL-2/JES6-1 mAb complexes dramatically increase sensitivity to LPS through IFN-gamma production by CD25+Foxp3- T cells
Authors: J Tomala, P Weberova, B Tomalova, Z Jiraskova, L Sivak, J Kovarova, M Kovar
Elife, 2021-12-21;10(0):.
Species: Mouse
Sample Types: Serum
-
Heterocellular OSM-OSMR signalling reprograms fibroblasts to promote pancreatic cancer growth and metastasis
Authors: BY Lee, EKJ Hogg, CR Below, A Kononov, A Blanco-Gom, F Heider, J Xu, C Hutton, X Zhang, T Scheidt, K Beattie, A Lamarca, M McNamara, JW Valle, C Jørgensen
Nature Communications, 2021-12-17;12(1):7336.
Species: Mouse
Sample Types: Tissue Homogenates
-
Differences in Cell-Intrinsic Inflammatory Programs of Yolk Sac and Bone Marrow Macrophages
Authors: S Elhag, C Stremmel, A Zehrer, J Plocke, R Hennel, M Keuper, C Knabe, J Winterhalt, V Gölling, L Tomas, T Weinberger, M Fischer, L Liu, F Wagner, M Lorenz, K Stark, H Häcker, M Schmidt-Su, U Völker, M Jastroch, K Lauber, T Straub, B Walzog, E Hammer, C Schulz
Cells, 2021-12-17;10(12):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Identification of an ASC oligomerization inhibitor for the treatment of inflammatory diseases
Authors: PM Soriano-Te, G García-Laí, M Marco-Salv, J Pardo, M Arias, C DeFord, I Merfort, MJ Vicent, P Pelegrín, M Sancho, M Orzáez
Cell Death & Disease, 2021-12-13;12(12):1155.
Species: Mouse
Sample Types: Peritoneal Lavage Fluid
-
Hepatocytes are resistant to cell death from canonical and non-canonical inflammasome-activated pyroptosis
Authors: P Sun, J Zhong, H Liao, P Loughran, J Mulla, G Fu, D Tang, J Fan, TR Billiar, W Gao, MJ Scott
Cellular and Molecular Gastroenterology and Hepatology, 2021-12-07;0(0):.
Species: Mouse
Sample Types: Plasma
-
The Role of Cytokines Produced via the NLRP3 Inflammasome in Mouse Macrophages Stimulated with Dental Calculus in Osteoclastogenesis
Authors: M Mae, MI Alam, Y Yamashita, Y Ozaki, K Higuchi, SM Ziauddin, JL Montenegro, E Sakai, T Tsukuba, A Yoshimura
International Journal of Molecular Sciences, 2021-11-18;22(22):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The APPL1-Rab5 axis restricts NLRP3 inflammasome activation through early endosomal-dependent mitophagy in macrophages
Authors: KKL Wu, K Long, H Lin, PMF Siu, RLC Hoo, D Ye, A Xu, KKY Cheng
Nature Communications, 2021-11-17;12(1):6637.
Species: Mouse
Sample Types: Tissue Homogenates
-
Anti-IAPP Monoclonal Antibody Improves Clinical Symptoms in a Mouse Model of Type 2 Diabetes
Authors: AS Vogt, ES Roesti, MO Mohsen, A Leonchiks, M Vogel, MF Bachmann
Vaccines, 2021-11-12;9(11):.
Species: Mouse
Sample Types: Serum
-
Cooling and Sterile Inflammation in an Oxygen-Glucose-Deprivation/Reperfusion Injury Model in BV-2 Microglia
Authors: J Lücht, N Rolfs, SJ Wowro, F Berger, KRL Schmitt, G Tong
Mediators of Inflammation, 2021-11-05;2021(0):8906561.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Endangered Lymphocytes: The Effects of Alloxan and Streptozotocin on Immune Cells in Type 1 Induced Diabetes
Authors: LAD Queiroz, JB Assis, JPT Guimarães, ESA Sousa, AC Milhomem, KKS Sunahara, A Sá-Nunes, JO Martins
Mediators of Inflammation, 2021-10-19;2021(0):9940009.
Species: Mouse
Sample Types: Tissue Homogenates
-
Sulforaphane ameliorates non-alcoholic fatty liver disease in mice by promoting FGF21/FGFR1 signaling pathway
Authors: YK Wu, ZN Ren, SL Zhu, YZ Wu, G Wang, H Zhang, W Chen, Z He, XL Ye, QX Zhai
Acta pharmacologica Sinica, 2021-10-15;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Sambucus nigra: A traditional medicine effective in reducing inflammation in mice
Authors: JR Santin, L Benvenutti, MF Broering, R Nunes, FC Goldoni, YBK Patel, JA de Souza, MAT Kopp, P de Souza, RCV da Silva, MVD Pastor, AB de Souza, LD Testoni, AG Couto, TMB Bresolin, NLM Quintão
Journal of ethnopharmacology, 2021-10-11;283(0):114736.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Oxidized carbon black nanoparticles induce endothelial damage through C-X-C chemokine receptor 3-mediated pathway
Authors: N Majumder, M Velayutham, D Bitounis, VK Kodali, MH Hasan Mazu, J Amedro, VV Khramtsov, A Erdely, T Nurkiewicz, P Demokritou, EE Kelley, S Hussain
Redox Biology, 2021-10-04;47(0):102161.
Species: Mouse
Sample Types: BALF
-
Parabens disrupt non-canonical inflammasome activation
Authors: H Ahn, H Lee, G Lee, J Park, HW Sung, E Lee, GS Lee
International immunopharmacology, 2021-09-30;101(0):108196.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Anti-inflammatory activity of lefamulin versus azithromycin and dexamethasone in vivo and in vitro in a lipopolysaccharide-induced lung neutrophilia mouse model
Authors: M Hafner, S Paukner, WW Wicha, B Hrva?i?, M Cedilak, I Faraho, SP Gelone
PLoS ONE, 2021-09-29;16(9):e0237659.
Species: Mouse
Sample Types: Tissue Homogenates
-
Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling
Authors: J Hu, C Wang, X Huang, S Yi, S Pan, Y Zhang, G Yuan, Q Cao, X Ye, H Li
Cell Reports, 2021-09-21;36(12):109726.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Coronavirus induces diabetic macrophage-mediated inflammation via SETDB2
Authors: WJ Melvin, CO Audu, FM Davis, SB Sharma, A Joshi, A DenDekker, S Wolf, E Barrett, K Mangum, X Zhou, M Bame, A Ruan, A Obi, SL Kunkel, BB Moore, KA Gallagher
Proceedings of the National Academy of Sciences of the United States of America, 2021-09-21;118(38):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Proteopathic tau primes and activates interleukin-1beta via myeloid-cell-specific MyD88- and NLRP3-ASC-inflammasome pathway
Authors: S Jiang, NM Maphis, J Binder, D Chisholm, L Weston, W Duran, C Peterson, A Zimmerman, MA Mandell, SD Jett, E Bigio, C Geula, N Mellios, JP Weick, GA Rosenberg, E Latz, MT Heneka, K Bhaskar
Cell Reports, 2021-09-21;36(12):109720.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The SNX-482 peptide from Hysterocrates gigas spider acts as an immunomodulatory molecule activating macrophages
Authors: J Munhoz, R Thomé, A Rostami, LLW Ishikawa, L Verinaud, C Rapôso
Peptides, 2021-09-16;146(0):170648.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Peony seed oil ameliorates neuroinflammation-mediated cognitive deficits by suppressing microglial activation through inhibition of NF-kappaB pathway in presenilin 1/2 conditional double knockout mice
Authors: J Gao, L Wang, C Zhao, Y Wu, Z Lu, Y Gu, Z Ba, X Wang, J Wang, Y Xu
Journal of leukocyte biology, 2021-09-08;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Oxidant-induced epithelial alarmin pathway mediates lung inflammation and functional decline following ultrafine carbon and ozone inhalation co-exposure
Authors: N Majumder, WT Goldsmith, VK Kodali, M Velayutham, SA Friend, VV Khramtsov, TR Nurkiewicz, A Erdely, PC Zeidler-Er, V Castranova, JR Harkema, EE Kelley, S Hussain
Redox Biology, 2021-08-05;46(0):102092.
Species: Mouse
Sample Types: BALF
-
Correction: Type I IFNs facilitate innate immune control of the opportunistic bacteria Burkholderia cenocepacia in the macrophage cytosol
Authors: MG Dorrington, CJ Bradfield, JB Lack, B Lin, SP John, JJ Liang, T Starr, O Ernst, JL Gross, J Sun, AH Miller, O Steele-Mor, IDC Fraser
PloS Pathogens, 2021-08-04;17(8):e1009821.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Pertactin contributes to shedding and transmission of Bordetella bronchiseptica
Authors: L Ma, KK Dewan, DL Taylor-Mul, SM Wagner, B Linz, I Rivera, Y Su, AD Caulfield, U Blas-Macha, ET Harvill
PloS Pathogens, 2021-08-04;17(8):e1009735.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A phenotypic high-content, high-throughput screen identifies inhibitors of NLRP3 inflammasome activation
Authors: S Nizami, V Millar, K Arunasalam, T Zarganes-T, D Brough, G Tresadern, PE Brennan, JB Davis, D Ebner, E Di Daniel
Scientific Reports, 2021-07-28;11(1):15319.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Anti-Inflammatory and Healing Activity of the Hydroalcoholic Fruit Extract of Solanum diploconos (Mart.) Bohs
Authors: L Benvenutti, R Nunes, I Venturi, SA Ramos, MF Broering, FC Goldoni, SE Pavan, MVD Pastor, A Malheiros, NLM Quintão, ES Fernandes, JR Santin
Journal of Immunology Research, 2021-07-19;2021(0):9957451.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Peroxiredoxin AhpC1 protects Pseudomonas aeruginosa against the inflammatory oxidative burst and confers virulence
Authors: LS Rocha, BPD Silva, TML Correia, RPD Silva, DA Meireles, R Pereira, LES Netto, FC Meotti, RF Queiroz
Redox Biology, 2021-07-19;46(0):102075.
Species: Mouse
Sample Types: Serum
-
Acute Lung Injury in Cholinergic-Deficient Mice Supports Anti-Inflammatory Role of alpha7 Nicotinic Acetylcholine Receptor
Authors: NM Pinheiro, R Banzato, I Tibério, MAM Prado, VF Prado, AK Hamouda, CM Prado
International Journal of Molecular Sciences, 2021-07-14;22(14):.
Species: Mouse
Sample Types: Tissue Homogenates
-
PB1-F2 amyloid-like fibers correlate with pro-inflammatory signaling and respiratory distress in influenza-infected mice
Authors: C Chevalier, O Leymarie, L Sedano, B Da Costa, CA Richard, P Maisonnass, M Réfregiers, F Jamme, R Le Goffic
The Journal of Biological Chemistry, 2021-06-17;0(0):100885.
Species: Mouse
Sample Types: BALF
-
The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity
Authors: C Xu, S Sun, T Johnson, R Qi, S Zhang, J Zhang, K Yang
Cell Reports, 2021-06-15;35(11):109235.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Anti-inflammatory Effects of Empagliflozin and Gemigliptin on LPS-Stimulated Macrophage via the IKK/NF-&kappaB, MKK7/JNK, and JAK2/STAT1 Signalling Pathways
Authors: N Lee, YJ Heo, SE Choi, JY Jeon, SJ Han, DJ Kim, Y Kang, KW Lee, HJ Kim
Journal of Immunology Research, 2021-06-02;2021(0):9944880.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Clostridioides difficile spores stimulate inflammatory cytokine responses and induce cytotoxicity in macrophages
Authors: PJ Chiu, J Rathod, YP Hong, PJ Tsai, YP Hung, WC Ko, JW Chen, D Paredes-Sa, IH Huang
Anaerobe, 2021-05-31;70(0):102381.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Probiotic and Functional Properties of Limosilactobacillus reuteri INIA P572
Authors: P Diez-Echav, I Martín-Cab, J Garrido-Me, S Langa, T Vezza, JM Landete, L Hidalgo-Ga, F Algieri, MJ Mayer, A Narbad, A García-Laf, M Medina, A Rodríguez-, ME Rodríguez-, J Gálvez, JL Arqués
Nutrients, 2021-05-29;13(6):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
d-enantiomers of CATH-2 enhance the response of macrophages against Streptococcus suis serotype 2
Authors: RM van Harten, JLM Tjeerdsma-, A de Greeff, MD Balhuizen, A van Dijk, EJA Veldhuizen, HP Haagsman, MR Scheenstra
Journal of advanced research, 2021-05-26;36(0):101-112.
Species: Mouse
Sample Types: Cell Culture Supernates
-
NLRP1 acts as a negative regulator of Th17 cell programming in mice and humans with autoimmune diabetes
Authors: FRC Costa, JA Leite, DM Rassi, JF da Silva, J Elias-Oliv, JB Guimarães, MC Foss-Freit, NOS Câmara, A Pontillo, RC Tostes, JS Silva, D Carlos
Cell Reports, 2021-05-25;35(8):109176.
Species: Mouse
Sample Types: Tissue Homogenates
-
Serum Amyloid A1/Toll-Like Receptor-4 Axis, an Important Link between Inflammation and Outcome of TBI Patients
Authors: V Farré-Alin, A Palomino-A, P Narros-Fer, AB Lopez-Rodr, C Decouty-Pe, A Muñoz-Mont, J Zamorano-F, B Mansilla-F, J Giner-Garc, P García-Fei, M Sáez-Alegr, AJ Palpán-Flo, JM Roda-Frade, CS Carabias, JM Rosa, B Civantos-M, S Yus-Teruel, L Gandía, A Lagares, BJ Hernández-, J Egea
Biomedicines, 2021-05-25;9(6):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
&beta-Glucan-stimulated neutrophil secretion of IL-1&alpha is independent of GSDMD and mediated through extracellular vesicles
Authors: B Ratitong, M Marshall, E Pearlman
Cell Reports, 2021-05-18;35(7):109139.
Species: Mouse, Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Studies of involvement of G-protein coupled receptor-3 in cannabidiol effects on inflammatory responses of mouse primary astrocytes and microglia
Authors: J Wu, N Chen, Y Liu, G Godlewski, HJ Kaplan, SH Shrader, ZH Song, H Shao
PLoS ONE, 2021-05-13;16(5):e0251677.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Topical administration of the kappa opioid receptor agonist nalfurafine suppresses corneal neovascularization and inflammation
Authors: H Shokirova, T Inomata, T Saitoh, J Zhu, K Fujio, Y Okumura, A Yanagawa, K Fujimoto, J Sung, A Eguchi, M Miura, K Nagino, K Hirosawa, M Kuwahara, Y Akasaki, H Nagase, A Murakami
Scientific Reports, 2021-04-21;11(1):8647.
Species: Mouse
Sample Types: Tissue Homogenates
-
4R-cembranoid confers neuroprotection against LPS-induced hippocampal inflammation in mice
Authors: LA Rojas-Coló, PK Dash, FA Morales-Ví, M Lebrón-Dáv, PA Ferchmin, JB Redell, G Maldonado-, WI Vélez-Torr
Journal of Neuroinflammation, 2021-04-19;18(1):95.
Species: Mouse
Sample Types: Plasma
-
Thymoquinone, a Dietary Bioactive Compound, Exerts Anti-Inflammatory Effects in Colitis by Stimulating Expression of the Colonic Epithelial PPAR-&gamma Transcription Factor
Authors: B Venkataram, S Almarzooqi, V Raj, AT Alhassani, AS Alhassani, KJ Ahmed, VS Subramania, SK Ojha, S Attoub, TE Adrian, SB Subramanya
Nutrients, 2021-04-17;13(4):.
Species: Mouse
Sample Types: Tissue Homogenates
-
NLRP3-Inflammasome Inhibition during Respiratory Virus Infection Abrogates Lung Immunopathology and Long-Term Airway Disease Development
Authors: CA Malinczak, CF Schuler, AJ Duran, AJ Rasky, MM Mire, G Núñez, NW Lukacs, W Fonseca
Viruses, 2021-04-16;13(4):.
Species: Mouse
Sample Types: BALF
-
A NOVEL NOX/PHOX-CD38-NAADP-TFEB AXIS IMPORTANT FOR MACROPHAGE ACTIVATION DURING BACTERIAL PHAGOCYTOSIS
Authors: M Najibi, HH Honwad, JA Moreau, SM Becker, JE Irazoqui
Autophagy, 2021-04-13;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Generation and utilization of a HEK-293T murine GM-CSF expressing cell line
Authors: EK Robinson, S Covarrubia, S Zhou, S Carpenter
PLoS ONE, 2021-04-09;16(4):e0249117.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Exploiting pyocyanin to treat mitochondrial disease due to respiratory complex III dysfunction
Authors: R Peruzzo, S Corrà, R Costa, M Brischigli, T Varanita, L Biasutto, C Rampazzo, D Ghezzi, L Leanza, M Zoratti, M Zeviani, C De Pittà, C Viscomi, R Costa, I Szabò
Nature Communications, 2021-04-08;12(1):2103.
Species: Mouse
Sample Types: Plasma
-
The anti-inflammatory cytokine interleukin-37 is an inhibitor of trained immunity
Authors: G Cavalli, IW Tengesdal, M Gresnigt, T Nemkov, RJW Arts, J Domínguez-, R Molteni, D Stefanoni, E Cantoni, L Cassina, S Giugliano, K Schraa, TS Mills, EM Pietras, EZ Eisenmenss, L Dagna, A Boletta, A D'Alessand, LAB Joosten, MG Netea, CA Dinarello
Cell Reports, 2021-04-06;35(1):108955.
Species: Mouse
Sample Types: Tissue Homogenates
-
Combined Effects of Exercise and Phytoanabolic Extracts in Castrated Male and Female Mice
Authors: JP Martins, LC Silva, MS Nunes, G Rübensam, JR Oliveira, RBM Silva, MM Campos
Nutrients, 2021-04-02;13(4):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Discovery and characterization of small molecule inhibitors of NLRP3 and NLRC4 inflammasomes
Authors: M Sebastian-, H Wu, M Al Rahim, R Sanchez, K Kumar, RJ De Vita, GM Pasinetti
The Journal of Biological Chemistry, 2021-03-26;0(0):100597.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Hepatoprotective Effect of Mixture of Dipropyl Polysulfides in Concanavalin A-Induced Hepatitis
Authors: D Arsenijevi, B Stojanovic, J Milovanovi, A Arsenijevi, M Simic, M Pergal, I Kodranov, O Cvetkovic, D Vojvodic, E Ristanovic, D Manojlovic, M Milovanovi, N Arsenijevi
Nutrients, 2021-03-22;13(3):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Roles of lung-recruited monocytes and pulmonary Vascular Endothelial Growth Factor (VEGF) in resolving Ventilator-Induced Lung Injury (VILI)
Authors: CK Lin, TH Huang, CT Yang, CS Shi
PLoS ONE, 2021-03-19;16(3):e0248959.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis
Authors: TP Fidler, C Xue, M Yalcinkaya, B Hardaway, S Abramowicz, T Xiao, W Liu, DG Thomas, MA Hajebrahim, J Pircher, C Silvestre-, AG Kotini, LL Luchsinger, Y Wei, M Westerterp, HW Snoeck, EP Papapetrou, C Schulz, S Massberg, O Soehnlein, B Ebert, RL Levine, MP Reilly, P Libby, N Wang, AR Tall
Nature, 2021-03-17;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
&alpha-Phellandrene exhibits antinociceptive and tumor-reducing effects in a mouse model of oncologic pain
Authors: FR Pinheiro-N, EM Lopes, BT Acha, LDS Gomes, WA Dias, ACD Reis Filho, BS Leal, DCDN Rodrigues, JDN Silva, D Dittz, PMP Ferreira, FRC Almeida
Toxicology and Applied Pharmacology, 2021-03-17;418(0):115497.
Species: Mouse
Sample Types: Serum
-
STAT6-Dependent Exacerbation of House Dust Mite-Induced Allergic Airway Disease in Mice by Multi-Walled Carbon Nanotubes
Authors: MD Ihrie, KS Duke, KA Shipkowski, DJ You, HY Lee, AJ Taylor-Jus, JC Bonner
NanoImpact, 2021-03-13;22(0):.
Species: Mouse
Sample Types: BALF
-
Itaconate confers tolerance to late NLRP3 inflammasome activation
Authors: M Bambouskov, L Potuckova, T Paulenda, M Kerndl, DA Mogilenko, K Lizotte, A Swain, S Hayes, RD Sheldon, H Kim, U Kapadnis, AE Ellis, C Isaguirre, S Burdess, A Laha, GK Amarasingh, V Chubukov, TP Roddy, MS Diamond, RG Jones, DM Simons, MN Artyomov
Cell Reports, 2021-03-09;34(10):108756.
Species: Mouse
Sample Types: Peritoneal Fluid
-
Proteomics reveals distinct mechanisms regulating the release of cytokines and alarmins during pyroptosis
Authors: K Phulphagar, LI Kühn, S Ebner, A Frauenstei, JJ Swietlik, J Rieckmann, F Meissner
Cell Reports, 2021-03-09;34(10):108826.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Type I IFNs facilitate innate immune control of the opportunistic bacteria Burkholderia cenocepacia in the macrophage cytosol
Authors: MG Dorrington, CJ Bradfield, JB Lack, B Lin, JJ Liang, T Starr, O Ernst, JL Gross, J Sun, AH Miller, O Steele-Mor, IDC Fraser
PloS Pathogens, 2021-03-08;17(3):e1009395.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mitochondrial arginase-2 is essential for IL-10 metabolic reprogramming of inflammatory macrophages
Authors: JK Dowling, R Afzal, LJ Gearing, MP Cervantes-, S Annett, GM Davis, C De Santi, N Assmann, K Dettmer, DJ Gough, GR Bantug, FI Hamid, FK Nally, CP Duffy, AL Gorman, AM Liddicoat, EC Lavelle, C Hess, PJ Oefner, DK Finlay, GP Davey, T Robson, AM Curtis, PJ Hertzog, BRG Williams, CE McCoy
Nature Communications, 2021-03-05;12(1):1460.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Small Immunomodulatory Molecules as Potential Therapeutics in Experimental Murine Models of Acute Lung Injury (ALI)/Acute Respiratory Distress Syndrome (ARDS)
Authors: D Shah, P Das, S Acharya, B Agarwal, DJ Christense, SM Robertson, V Bhandari
International Journal of Molecular Sciences, 2021-03-04;22(5):.
Species: Mouse
Sample Types: BALF
-
Physalis angulata reduces the progression of chronic experimental periodontitis by immunomodulatory mechanisms
Authors: PS Vieceli, PJ Lima Juiz, PS Sales Laur, RD Couto, TC Barbosa To, IM Ribeiro, MB Pereira So, CF Villarreal
Journal of ethnopharmacology, 2021-03-03;0(0):113986.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Sevoflurane preconditioning activates HGF/Met-mediated autophagy to attenuate hepatic ischemia-reperfusion injury in mice
Authors: X Xiao, D Liu, S Chen, X Li, M Ge, W Huang
Cellular Signalling, 2021-02-25;0(0):109966.
Species: Mouse
Sample Types: Tissue Homogenates
-
Inflammatory, synaptic, motor, and behavioral alterations induced by gestational sepsis on the offspring at different stages of life
Authors: MG Granja, LP Alves, M Leardini-T, ME Saul, LC Bortoni, FM de Moraes, EC Ferreira, BPT de Moraes, VZ da Silva, AFR Dos Santos, AR Silva, CF Gonçalves-, V Bambini-Ju, AS Weyrich, MT Rondina, GA Zimmerman, HC de Castro-
Journal of Neuroinflammation, 2021-02-25;18(1):60.
Species: Mouse
Sample Types: Tissue Homogenates
-
Sex-dependent differences in the gut microbiota following chronic nasal inflammation in adult mice
Authors: Y Mishima, T Osaki, A Shimada, S Kamiya, S Hasegawa-I
Scientific Reports, 2021-02-25;11(1):4640.
Species: Mouse
Sample Types: Serum
-
NLRP6-associated host microbiota composition impacts in the intestinal barrier to systemic dissemination of Brucella abortus
Authors: M Rungue, V Melo, D Martins, PC Campos, G Leles, I Galvão, V Mendes, M Aganetti, Á Pedersen, NRG Assis, R Santos, GD Cassali, ALB Godard, FS Martins, SC Oliveira, AT Vieira
PloS Neglected Tropical Diseases, 2021-02-22;15(2):e0009171.
Species: Mouse
Sample Types: Tissue Homogenates
-
Tim4 recognizes carbon nanotubes and mediates phagocytosis leading to granuloma formation
Authors: S Omori, M Tsugita, Y Hoshikawa, M Morita, F Ito, SI Yamaguchi, Q Xie, O Noyori, T Yamaguchi, A Takada, T Saitoh, S Toyokuni, H Akiba, S Nagata, K Kinoshita, M Nakayama
Cell Reports, 2021-02-09;34(6):108734.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dual Role of Interleukin-10 in Murine NZB/W F1 Lupus
Authors: A Amend, N Wickli, AL Schäfer, DTL Sprenger, RA Manz, RE Voll, N Chevalier
International Journal of Molecular Sciences, 2021-01-29;22(3):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Fatty Acids and a High-Fat Diet Induce Epithelial-Mesenchymal Transition by Activating TGF&beta and &beta-Catenin in Liver Cells
Authors: O Kwapisz, J Górka, A Korlatowic, J Kotlinowsk, A Waligórska, P Marona, N Pydyn, JW Dobrucki, J Jura, K Miekus
International Journal of Molecular Sciences, 2021-01-28;22(3):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Hypoxia-Induced S100A8 Expression Activates Microglial Inflammation and Promotes Neuronal Apoptosis
Authors: JS Ha, HR Choi, IS Kim, EA Kim, SW Cho, SJ Yang
International Journal of Molecular Sciences, 2021-01-26;22(3):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Gasdermin D restricts Burkholderia cenocepacia infection in vitro and in vivo
Authors: S Estfanous, K Krause, MNK Anne, M Eltobgy, K Caution, A Abu Khweek, K Hamilton, A Badr, K Daily, C Carafice, D Baetzhold, X Zhang, T Li, H Wen, MA Gavrilin, H Haffez, S Soror, AO Amer
Scientific Reports, 2021-01-13;11(1):855.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Disrupted Iron Metabolism and Mortality during Co-infection with Malaria and an Intestinal Gram-Negative Extracellular Pathogen
Authors: LI Dos Santos, TA Torres, SQ Diniz, R Gonçalves, G Caballero-, G Núñez, RT Gazzinelli, KJ Maloy, L Ribeiro do
Cell Reports, 2021-01-12;34(2):108613.
Species: Mouse
Sample Types: Serum
-
Pra-C exerts analgesic effect through inhibiting microglial activation in anterior cingulate cortex in complete Freund's adjuvant-induced mouse model
Authors: DJ Su, LF Li, SY Wang, Q Yang, YJ Wu, MG Zhao, L Yang
Molecular pain, 2021-01-01;17(0):1744806921990.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Severity of Experimental Autoimmune Uveitis Is Reduced by Pretreatment with Live Probiotic Escherichia coli Nissle 1917
Authors: O Dusek, A Fajstova, A Klimova, P Svozilkova, T Hrncir, M Kverka, S Coufal, J Slemin, H Tlaskalova, JV Forrester, J Heissigero
Cells, 2020-12-25;10(1):.
Species: Mouse
Sample Types: Tissue Culture Supernates
-
Diet Rich in Simple Sugars Promotes Pro-Inflammatory Response via Gut Microbiota Alteration and TLR4 Signaling
Authors: A Fajstova, N Galanova, S Coufal, J Malkova, M Kostovcik, M Cermakova, H Pelantova, M Kuzma, B Sediva, T Hudcovic, T Hrncir, H Tlaskalova, M Kverka, K Kostovciko
Cells, 2020-12-16;9(12):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TXNIP Regulates Natural Killer Cell-Mediated Innate Immunity by Inhibiting IFN-&gamma Production during Bacterial Infection
Authors: DO Kim, JE Byun, WS Kim, MJ Kim, JH Choi, H Kim, E Choi, TD Kim, SR Yoon, JY Noh, YJ Park, J Lee, HJ Cho, HG Lee, SH Min, I Choi, H Jung
International Journal of Molecular Sciences, 2020-12-14;21(24):.
Species: Mouse
Sample Types: Serum
-
Active food ingredients production from cold pressed processing residues of Camellia oleifera and Camellia sinensis seeds for regulation of blood pressure and vascular function
Authors: SS Chiang, LS Chen, CY Chu
Chemosphere, 2020-12-11;267(0):129267.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Ubiquitination of RIPK1 regulates its activation mediated by TNFR1 and TLRs signaling in distinct manners
Authors: X Li, M Zhang, X Huang, W Liang, G Li, X Lu, Y Li, H Pan, L Shi, H Zhu, L Qian, B Shan, J Yuan
Nature Communications, 2020-12-11;11(1):6364.
Species: Mouse
Sample Types: Serum
-
Olive Leaf Extract, from Olea europaea L., Reduces Palmitate-Induced Inflammation via Regulation of Murine Macrophages Polarization
Authors: P De Cicco, M Maisto, GC Tenore, A Ianaro
Nutrients, 2020-11-28;12(12):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dual chemotherapy with benznidazole at suboptimal dose plus curcumin nanoparticles mitigates Trypanosoma cruzi-elicited chronic cardiomyopathy
Authors: M Hernández, S Wicz, E Pérez Caba, MH Santamaría, RS Corral
Parasitol Int, 2020-11-22;81(0):102248.
Species: Mouse
Sample Types: Tissue Homogenates
-
Unique maternal immune and functional microbial profiles during prenatal stress
Authors: AM Antonson, MV Evans, JD Galley, HJ Chen, TA Rajasekera, SM Lammers, VL Hale, MT Bailey, TL Gur
Sci Rep, 2020-11-20;10(1):20288.
Species: Mouse
Sample Types: Tissue Homogenates
-
Different experimental multiple trauma models induce comparable inflammation and organ injury
Authors: B Relja, B Yang, K Bundkirche, B Xu, K Köhler, C Neunaber
Sci Rep, 2020-11-19;10(1):20185.
Species: Mouse
Sample Types: Plasma
-
Disrupting Bordetella Immunosuppression Reveals a Role for Eosinophils in Coordinating the Adaptive Immune Response in the Respiratory Tract
Authors: MC Gestal, U Blas-Macha, HM Johnson, LN Rubin, KK Dewan, C Bryant, M Tiemeyer, ET Harvill
Microorganisms, 2020-11-17;8(11):.
Species: Mouse
Sample Types: Tissue Homogenates
-
RACK1 Mediates NLRP3 Inflammasome Activation by Promoting NLRP3 Active Conformation and Inflammasome Assembly
Authors: Y Duan, L Zhang, D Angosto-Ba, P Pelegrín, G Núñez, Y He
Cell Rep, 2020-11-17;33(7):108405.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Inducing regulated necrosis and shifting macrophage polarization with anti-EMMPRIN antibody (161-pAb) and complement factors
Authors: N Hijaze, M Ledersnaid, E Simanovich, S Kassem, MA Rahat
J Leukoc Biol, 2020-11-17;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Tau Pathology Drives Dementia Risk-Associated Gene Networks toward Chronic Inflammatory States and Immunosuppression
Authors: JE Rexach, D Polioudaki, A Yin, V Swarup, TS Chang, T Nguyen, A Sarkar, L Chen, J Huang, LC Lin, W Seeley, JQ Trojanowsk, D Malhotra, DH Geschwind
Cell Rep, 2020-11-17;33(7):108398.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Riboflavin, vitamin B2, attenuates NLRP3, NLRC4, AIM2, and non-canonical inflammasomes by the inhibition of caspase-1 activity
Authors: H Ahn, GS Lee
Sci Rep, 2020-11-05;10(1):19091.
Species: Mouse
Sample Types: Peritoneal Lavage Fluid
-
Anti-Axl antibody treatment reduces the severity of experimental autoimmune encephalomyelitis
Authors: JC DuBois, AK Ray, P Davies, B Shafit-Zag
J Neuroinflammation, 2020-10-29;17(1):324.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Human recombinant interleukin-38 suppresses inflammation in mouse models of local and systemic disease
Authors: DM de Graaf, RJA Maas, SP Smeekens, E Eisenmesse, JS Redzic, MM Helsen, NE Powers, S Li, V Kalabokis, MS Gresnigt, LAB Joosten, CA Dinarello, FL van de Vee
Cytokine, 2020-10-28;137(0):155334.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TNF Signaling Dictates Myeloid and Non-Myeloid Cell Crosstalk to Execute MCMV-Induced Extrinsic Apoptosis
Authors: P Mandal, AL McCormick, ES Mocarski
Viruses, 2020-10-28;12(11):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Repeated dermal application of the common preservative methylisothiazolinone triggers local inflammation, T cell influx, and prolonged mast cell-dependent tactile sensitivity in mice
Authors: JM Kline, E Arriaga-Go, T Yangdon, B Boo, J Landry, M Saldías-Mo, N Neamonitak, H Mengistu, S Silverio, H Zacheis, D Pasha, T Martinov, BT Fife, D Chatterjea
PLoS ONE, 2020-10-26;15(10):e0241218.
Species: Mouse
Sample Types: Cell Lysates
-
The overexpression of TDP-43 in astrocytes causes neurodegeneration via a PTP1B-mediated inflammatory response
Authors: S Lee, S Kim, HY Kang, HR Lim, Y Kwon, M Jo, YM Jeon, SR Kim, K Kim, CM Ha, S Lee, HJ Kim
J Neuroinflammation, 2020-10-14;17(1):299.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Simvastatin Posttreatment Controls Inflammation and Improves Bacterial Clearance in Experimental Sepsis
Authors: FM de Jesus O, CF Gonçalves-, IMM de Moraes, PA Reis, VN Rocha, PT Bozza, AR Silva, HC de Castro
Mediators Inflamm, 2020-10-14;2020(0):1839762.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The protective role of proton-sensing TDAG8 in the brain injury in a mouse ischemia reperfusion model
Authors: K Sato, A Tobo, C Mogi, M Tobo, N Yamane, M Tosaka, H Tomura, DS Im, F Okajima
Sci Rep, 2020-10-14;10(1):17193.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Staphylococcus aureus-Derived &alpha-Hemolysin Evokes Generation of Specialized Pro-resolving Mediators Promoting Inflammation Resolution
Authors: PM Jordan, J Gerstmeier, S Pace, R Bilancia, Z Rao, F Börner, L Miek, Ó Gutiérrez-, V Arakandy, A Rossi, A Ialenti, C González-E, B Löffler, L Tuchscherr, CN Serhan, O Werz
Cell Rep, 2020-10-13;33(2):108247.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Pseudomonas aeruginosa protease LasB directly activates IL-1&beta
Authors: J Sun, DL LaRock, EA Skowronski, JM Kimmey, J Olson, Z Jiang, AJ O'Donoghue, V Nizet, CN LaRock
EBioMedicine, 2020-09-23;60(0):102984.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Deficiency of peroxiredoxin 2 exacerbates angiotensin II-induced abdominal aortic aneurysm
Authors: SJ Jeong, MJ Cho, NY Ko, S Kim, IH Jung, JK Min, SH Lee, JG Park, GT Oh
Exp. Mol. Med., 2020-09-14;0(0):.
Species: Mouse
Sample Types: Cell Lysates
-
Standardized herbal extract PM014 alleviates fine dust-induced lung inflammation in mice
Authors: YS Lee, D Min, SY Park, J Lee, H Bae
BMC Complement Med Ther, 2020-09-07;20(1):270.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Activation of the NLRP3 Inflammasome by Particles from the Echinococcus granulosus Laminated Layer
Authors: C Casaravill, Á Pittini, D Rückerl, JE Allen, Á Díaz
Infect. Immun., 2020-08-19;88(9):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Neutrophil Extracellular Traps Impair Intestinal Barrier Function during Experimental Colitis
Authors: EY Lin, HJ Lai, YK Cheng, KQ Leong, LC Cheng, YC Chou, YC Peng, YH Hsu, HS Chiang
Biomedicines, 2020-08-05;8(8):.
Species: Mouse
Sample Types: Cell Lysates, Tissue Homogenates
-
Metabolic competition between host and pathogen dictates inflammasome responses to fungal infection
Authors: TM Tucey, J Verma, FAB Olivier, TL Lo, AAB Robertson, T Naderer, A Traven
PLoS Pathog., 2020-08-04;16(8):e1008695.
Species: Mouse
Sample Types: Cell Culture Supernates, Tissue Homogenates
-
A Clinical Metabolite of Azidothymidine Inhibits Experimental Choroidal Neovascularization and Retinal Pigmented Epithelium Degeneration
Authors: S Narendran, F Pereira, P Yerramothu, I Apicella, SB Wang, A Varshney, KL Baker, KM Marion, M Ambati, VL Ambati, K Ambati, SR Sadda, BD Gelfand, J Ambati
Invest. Ophthalmol. Vis. Sci., 2020-08-03;61(10):4.
Species: Mouse
Sample Types: Cell Culture Supernates
-
STAT3 serine phosphorylation is required for TLR4 metabolic reprogramming and IL-1&beta expression
Authors: JJ Balic, H Albargy, K Luu, FJ Kirby, WSN Jayasekara, F Mansell, DJ Garama, D De Nardo, N Baschuk, C Louis, F Humphries, K Fitzgerald, E Latz, DJ Gough, A Mansell
Nat Commun, 2020-07-30;11(1):3816.
Species: Mouse
Sample Types: Cell Lysate
-
Essential role of Salmonella Enteritidis DNA adenine methylase in modulating inflammasome activation
Authors: Y Guo, D Gu, T Huang, L Cao, X Zhu, Y Zhou, K Wang, X Kang, C Meng, X Jiao, Z Pan
BMC Microbiol., 2020-07-28;20(1):226.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Neutrophil Caspase-11 Is Essential to Defend against a Cytosol-Invasive Bacterium
Authors: SB Kovacs, C Oh, VI Maltez, BD McGlaughon, A Verma, EA Miao, Y Aachoui
Cell Rep, 2020-07-28;32(4):107967.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immunomodulatory Effect of a Salvia plebeia R. Aqueous Extract in Forced Swimming Exercise-induced Mice
Authors: J Shin, OK Kim, S Kim, D Bae, J Lee, J Park, W Jun
Nutrients, 2020-07-28;12(8):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
MG53 suppresses interferon-&beta and inflammation via regulation of ryanodine receptor-mediated intracellular calcium signaling
Authors: M Sermershei, AD Kenney, PH Lin, TM McMichael, C Cai, K Gumpper, TMA Adesanya, H Li, X Zhou, KH Park, JS Yount, J Ma
Nat Commun, 2020-07-17;11(1):3624.
Species: Human
Sample Types: Cell Culture Supernates
-
Zinc Finger Protein St18 Protects against Septic Death by Inhibiting VEGF-A from Macrophages
Authors: K Maruyama, H Kidoya, N Takemura, E Sugisawa, O Takeuchi, T Kondo, MMA Eid, H Tanaka, MM Martino, N Takakura, Y Takayama, S Akira, A Vandenbon, Y Kumagai
Cell Rep, 2020-07-14;32(2):107906.
Species: Mouse
Sample Types: Serum
-
Regorafenib Regulates AD Pathology, Neuroinflammation, and Dendritic Spinogenesis in Cells and a Mouse Model of AD
Authors: KM Han, RJ Kang, H Jeon, HJ Lee, JS Lee, H Park, S Gak Jeon, K Suk, J Seo, HS Hoe
Cells, 2020-07-09;9(7):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
GM-CSF Calibrates Macrophage Defense and Wound Healing Programs during Intestinal Infection and Inflammation
Authors: T Castro-Dop, A Fleming, TW Dennison, JR Ferdinand, K Harcourt, BJ Stewart, Z Cader, ZK Tuong, C Jing, LSC Lok, RJ Mathews, A Portet, A Kaser, S Clare, MR Clatworthy
Cell Rep, 2020-07-07;32(1):107857.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Induction of ASC pyroptosis requires gasdermin D or caspase-1/11-dependent mediators and IFNbeta from pyroptotic macrophages
Authors: C Zhang, C Zhao, X Chen, R Tao, S Wang, G Meng, X Liu, C Shao, X Su
Cell Death Dis, 2020-06-18;11(6):470.
Species: Mouse, Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Enhancing the regenerative effectiveness of growth factors by local inhibition of interleukin-1 receptor signaling
Authors: Z Julier, R Karami, B Nayer, YZ Lu, AJ Park, K Maruyama, GA Kuhn, R Müller, S Akira, MM Martino
Sci Adv, 2020-06-12;6(24):eaba7602.
Species: Mouse
Sample Types: Tissue Homogenates
-
TRPM5 Negatively Regulates Calcium-Dependent Responses in Lipopolysaccharide-Stimulated B Lymphocytes
Authors: T Sakaguchi, R Okumura, C Ono, D Okuzaki, T Kawai, Y Okochi, N Tanimura, M Murakami, H Kayama, E Umemoto, H Kioka, T Ohtani, Y Sakata, K Miyake, Y Okamura, Y Baba, K Takeda
Cell Rep, 2020-06-09;31(10):107755.
Species: Mouse
Sample Types: Serum
-
Antitumor and Anti-Metastatic Effects of Citral-Loaded Nanostructured Lipid Carrier in 4T1-Induced Breast Cancer Mouse Model
Authors: N Nordin, SK Yeap, HS Rahman, NR Zamberi, NE Mohamad, N Abu, MJ Masarudin, R Abdullah, NB Alitheen
Molecules, 2020-06-09;25(11):.
Species: Mouse
Sample Types: Serum
-
Sulforaphane elicts dual therapeutic effects on Renal Inflammatory Injury and crystal deposition in Calcium Oxalate Nephrocalcinosis
Authors: H Liu, X Yang, K Tang, T Ye, C Duan, P Lv, L Yan, X Wu, Z Chen, J Liu, Y Deng, G Zeng, J Xing, Z Ye, H Xu
Theranostics, 2020-06-05;10(16):7319-7334.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The TSPO-NOX1 axis controls phagocyte-triggered pathological angiogenesis in the eye
Authors: A Wolf, M Herb, M Schramm, T Langmann
Nat Commun, 2020-06-01;11(1):2709.
Species: Mouse
Sample Types: Tissue Homogenates
-
Progesterone Attenuates Stress-Induced NLRP3 Inflammasome Activation and Enhances Autophagy following Ischemic Brain Injury
Authors: C Espinosa-G, F Atif, S Yousuf, I Sayeed, GN Neigh, DG Stein
Int J Mol Sci, 2020-05-26;21(11):.
Species: Mouse
Sample Types: Cell Supernatants
-
Mitophagy deficiency increases NLRP3 to induce brown fat dysfunction in mice
Authors: MS Ko, JY Yun, IJ Baek, JE Jang, JJ Hwang, SE Lee, SH Heo, DA Bader, CH Lee, J Han, JS Moon, JM Lee, EG Hong, IK Lee, SW Kim, JY Park, SM Hartig, UJ Kang, DD Moore, EH Koh, KU Lee
Autophagy, 2020-05-13;0(0):1-17.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TNF-&alpha Pretreatment Improves the Survival and Function of Transplanted Human Neural Progenitor Cells Following Hypoxic-Ischemic Brain Injury
Authors: M Kim, K Jung, Y Ko, IS Kim, K Hwang, JH Jang, JE Shin, KI Park
Cells, 2020-05-11;9(5):.
Species: Mouse
Sample Types: Tissue Homogenates
-
N-GSDMD trafficking to neutrophil organelles facilitates IL-1&beta release independently of plasma membrane pores and pyroptosis
Authors: M Karmakar, M Minns, EN Greenberg, J Diaz-Apont, K Pestonjama, JL Johnson, JK Rathkey, DW Abbott, K Wang, F Shao, SD Catz, GR Dubyak, E Pearlman
Nat Commun, 2020-05-05;11(1):2212.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Region-Specific Proteome Changes of the Intestinal Epithelium during Aging and Dietary Restriction
Authors: N Gebert, CW Cheng, JM Kirkpatric, D Di Fraia, J Yun, P Schädel, S Pace, GB Garside, O Werz, KL Rudolph, H Jasper, ÖH Yilmaz, A Ori
Cell Rep, 2020-04-28;31(4):107565.
Species: Mouse
Sample Types: Tissue Homogenates
-
The Role of ST2 Receptor in the Regulation of Brucella abortus Oral Infection
Authors: R Santos, PC Campos, M Rungue, V Rocha, D Santos, V Mendes, FV Marinho, F Martins, MF Ricci, DCD Reis, GD Cassali, JC Alves-Filh, AT Vieira, SC Oliveira
Pathogens, 2020-04-28;9(5):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TLR2 and caspase-1 signaling are critical for bacterial containment but not clearance during craniotomy-associated biofilm infection
Authors: AL Aldrich, CE Heim, W Shi, RW Fallet, B Duan, T Kielian
J Neuroinflammation, 2020-04-14;17(1):114.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The C5a/C5aR2 axis promotes renal inflammation and tissue damage
Authors: T Zhang, KY Wu, N Ma, LL Wei, M Garstka, W Zhou, K Li
JCI Insight, 2020-04-09;5(7):.
Species: Mouse
Sample Types: Tissue Lysates
-
(E)-2-Cyano-3-(1H-Indol-3-yl)-N-Phenylacrylamide, a Hybrid Compound Derived from Indomethacin and Paracetamol: Design, Synthesis and Evaluation of the Anti-Inflammatory Potential
Authors: P Silva, M de Almeida, J Silva, S Albino, R Espírito-S, M Lima, C Villarreal, R Moura, V Santos
Int J Mol Sci, 2020-04-08;21(7):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
M1 Polarization but Anti-LPS-Induced Inflammation and Anti-MCF-7 Breast Cancer Cell Growth Effects of Five Selected Polysaccharides
Authors: HC Lin, JY Lin
Evid Based Complement Alternat Med, 2020-03-25;2020(0):9450246.
Species: Mouse
Sample Types: Cell Culture Supernates
-
&beta-Amyloid Clustering around ASC Fibrils Boosts Its Toxicity in Microglia
Authors: LL Friker, H Scheiblich, IV Hochheiser, R Brinkschul, D Riedel, E Latz, M Geyer, MT Heneka
Cell Rep, 2020-03-17;30(11):3743-3754.e6.
Species: Mouse
Sample Types: Cell Culture Supernates
-
SIRT1 Alleviates LPS-Induced IL-1&beta Production by Suppressing NLRP3 Inflammasome Activation and ROS Production in Trophoblasts
Authors: S Park, J Shin, J Bae, D Han, SR Park, J Shin, SK Lee, HW Park
Cells, 2020-03-16;9(3):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Angiotensin II induces RAW264.7 macrophage polarization to the M1?type through the connexin 43/NF?&kappaB pathway
Authors: L Wu, K Chen, J Xiao, J Xin, L Zhang, X Li, L Li, J Si, L Wang, K Ma
Mol Med Rep, 2020-03-12;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin-1 Receptor-Induced Nitric Oxide Production in the Pancreas Controls Hyperglycemia Caused by Scorpion Envenomation
Authors: MB Reis, J Elias-Oliv, MR Pastore, SG Ramos, LG Gardinassi, LH Faccioli
Toxins (Basel), 2020-03-05;12(3):.
Species: Mouse
Sample Types: Serum
-
Chronic oral administration of adipoRon reverses cognitive impairments and ameliorates neuropathology in an Alzheimer's disease mouse model
Authors: RC Ng, M Jian, OK Ma, M Bunting, JS Kwan, GJ Zhou, K Senthilkum, A Iyaswamy, PK Chan, M Li, KM Leung, SS Kumar Dura, KS Lam, LW Chu, R Festenstei, SK Chung, KH Chan
Mol. Psychiatry, 2020-03-04;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Pharmacological LRH-1/Nr5a2 inhibition limits pro-inflammatory cytokine production in macrophages and associated experimental hepatitis
Authors: J Schwaderer, TS Phan, A Glöckner, J Delp, M Leist, T Brunner, ME Delgado
Cell Death Dis, 2020-02-28;11(2):154.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Waterpipe Tobacco Smoke Inhalation Triggers Thrombogenicity, Cardiac Inflammation and Oxidative Stress in Mice: Effects of Flavouring
Authors: A Nemmar, S Al-Salam, S Beegam, P Yuvaraju, NE Zaaba, J Yasin, BH Ali
Int J Mol Sci, 2020-02-14;21(4):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Nose-Only Water-Pipe Smoke Exposure in Mice Elicits Renal Histopathological Alterations, Inflammation, Oxidative Stress, DNA Damage, and Apoptosis
Authors: A Nemmar, S Beegam, P Yuvaraju, J Yasin, BH Ali, E Adeghate
Front Physiol, 2020-02-11;11(0):46.
Species: Mouse
Sample Types: Tissue Homogenates
-
Uric acid pathway activation during respiratory virus infection promotes Th2 immune response via innate cytokine production and ILC2 accumulation
Authors: W Fonseca, CA Malinczak, CF Schuler, SKK Best, AJ Rasky, SB Morris, TX Cui, AP Popova, NW Lukacs
Mucosal Immunol, 2020-02-11;0(0):.
Species: Mouse
Sample Types: BALF
-
ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer's disease.
Authors: Evans C, Miners J, Piva G, Willis C, Heard D, Kidd E, Good M, Kehoe P
Acta Neuropathol, 2020-01-25;139(3):485-502.
Species: Mouse
Sample Types: Tissue Homogenates
-
Periostin Promotes Colorectal Tumorigenesis through Integrin-FAK-Src Pathway-Mediated YAP/TAZ Activation
Authors: H Ma, J Wang, X Zhao, T Wu, Z Huang, D Chen, Y Liu, G Ouyang
Cell Rep, 2020-01-21;30(3):793-806.e6.
Species: Mouse
Sample Types: Tissue Homogenates
-
Detrimental Role of Nerve Injury-Induced Protein 1 in Myeloid Cells under Intestinal Inflammatory Conditions
Authors: HJ Jung, JH Kang, S Pak, K Lee, JK Seong, SH Oh
Int J Mol Sci, 2020-01-17;21(2):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Maternal high fat diet consumption reduces liver alpha7 nicotinic cholinergic receptor expression and impairs insulin signalling in the offspring
Authors: SO Costa, CM Souza, PG Lanza, JO Sartori, LM Ignacio-So, T Candreva, HG Rodrigues, AS Torsoni, M Milanski, MA Torsoni
Sci Rep, 2020-01-08;10(1):48.
Species: Mouse
Sample Types: Tissue Homogenates
-
I&kappaB? controls NLRP3 inflammasome activation via upregulation of the Nlrp3 gene
Authors: J Kim, H Ahn, S Yu, JH Ahn, HJ Ko, MN Kweon, EJ Hong, BS An, E Lee, GS Lee
Cytokine, 2020-01-07;127(0):154983.
Species: Mouse
Sample Types: Serum
-
Eupatorin Suppressed Tumor Progression and Enhanced Immunity in a 4T1 Murine Breast Cancer Model
Authors: N Abd Razak, SK Yeap, NB Alitheen, WY Ho, CY Yong, SW Tan, WS Tan, K Long
Integr Cancer Ther, 2020-01-01;19(0):1534735420935.
Species: Mouse
Sample Types: Serum
-
Enhancing the regenerative effectiveness of growth factors by local inhibition of interleukin-1 receptor signaling
Authors: Z Julier, R Karami, B Nayer, YZ Lu, AJ Park, K Maruyama, GA Kuhn, R Müller, S Akira, MM Martino
Sci Adv, 2020;6(24):eaba7602.
Species: Mouse
Sample Types: Tissue Homogenates
-
Caveolin-1 gene therapy inhibits inflammasome activation to protect from bleomycin-induced pulmonary fibrosis
Authors: X Lin, M Barravecch, R Matthew Ko, P Sime, DA Dean
Sci Rep, 2019-12-23;9(1):19643.
Species: Mouse
Sample Types: BALF
-
Sphingosine kinase-2 prevents macrophage cholesterol accumulation and atherosclerosis by stimulating autophagic lipid degradation
Authors: K Ishimaru, K Yoshioka, K Kano, M Kurano, D Saigusa, J Aoki, Y Yatomi, N Takuwa, Y Okamoto, RL Proia, Y Takuwa
Sci Rep, 2019-12-04;9(1):18329.
Species: Mouse
Sample Types: Plasma
-
Maternal Obesity in Mice Exacerbates the Allergic Inflammatory Response in the Airways of Male Offspring
Authors: RR E-Lacerda, CJ Teixeira, S Bordin, E Antunes, GF Anhê
Nutrients, 2019-12-01;11(12):.
Species: Mouse
Sample Types: BALF
-
Adiponectin Reverses the Hypothalamic Microglial Inflammation during Short-Term Exposure to Fat-Rich Diet
Authors: H Lee, TH Tu, BS Park, S Yang, JG Kim
Int J Mol Sci, 2019-11-15;20(22):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The effects of oxygen concentration on cell death, anti-oxidant transcription, acute inflammation, and cell proliferation in precision-cut lung slices
Authors: MJR Ruigrok, J Tomar, HW Frijlink, BN Melgert, WLJ Hinrichs, P Olinga
Sci Rep, 2019-11-07;9(1):16239.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A single systemic inflammatory insult causes acute motor deficits and accelerates disease progression in a mouse model of human tauopathy
Authors: M Torvell, DW Hampton, P Connick, AMJ MacLullich, C Cunningham, S Chandran
Alzheimers Dement (N Y), 2019-10-09;5(0):579-591.
Species: Mouse
Sample Types: Serum
-
Relative Contributions of Extracellular and Internalized Bacteria to Early Macrophage Proinflammatory Responses to Streptococcus pneumoniae
Authors: J Periselner, G Ercoli, T Pollard, S Chimalapat, E Camberlein, G Szylar, C Hyams, G Tomlinson, FC Petersen, RA Floto, M Noursadegh, JS Brown
MBio, 2019-09-24;10(5):.
Species: Mouse
Sample Types: BALF
-
PTP1B negatively regulates nitric oxide-mediated Pseudomonas aeruginosa killing by neutrophils
Authors: L Yue, M Yan, ML Tremblay, TJ Lin, H Li, T Yang, X Song, T Xie, Z Xie
PLoS ONE, 2019-09-18;14(9):e0222753.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Pyroptotic Cell Death Effector Gasdermin D Is Activated by Gout-Associated Uric Acid Crystals but Is Dispensable for Cell Death and IL-1 beta Release
Authors: Maryam Rashidi, Daniel S. Simpson, Anne Hempel, Daniel Frank, Emma Petrie, Angelina Vince et al.
The Journal of Immunology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Nitric Oxide Modulates Metabolic Remodeling in Inflammatory Macrophages through TCA Cycle Regulation and Itaconate Accumulation
Authors: JD Bailey, M Diotallevi, T Nicol, E McNeill, A Shaw, S Chuaiphich, A Hale, A Starr, M Nandi, E Stylianou, H McShane, S Davis, R Fischer, BM Kessler, J McCullagh, KM Channon, MJ Crabtree
Cell Rep, 2019-07-02;28(1):218-230.e7.
Species: Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Oral administration of EPA-rich oil impairs collagen reorganization due to elevated production of IL-10 during skin wound healing in mice
Authors: B Burger, CMC Kühl, T Candreva, RDS Cardoso, JR Silva, BG Castelucci, SR Consonni, HL Fisk, PC Calder, MAR Vinolo, HG Rodrigues
Sci Rep, 2019-06-24;9(1):9119.
Species: Mouse
Sample Types: Tissue Homogenates
-
Role of adipose tissue inflammation in fat pad loss induced by fasting in lean and mildly obese mice
Authors: DR Lacerda, KA Costa, ALM Silveira, DF Rodrigues, AN Silva, JL Sabino, V Pinho, GB Menezes, DD Soares, MM Teixeira, AVM Ferreira
J. Nutr. Biochem., 2019-06-21;72(0):108208.
Species: Mouse
Sample Types: Tissue Homogenates
-
IL-33 drives group 2 innate lymphoid cell-mediated protection during Clostridium difficile infection
Authors: AL Frisbee, MM Saleh, MK Young, JL Leslie, ME Simpson, MM Abhyankar, CA Cowardin, JZ Ma, P Pramoonjag, SD Turner, AP Liou, EL Buonomo, WA Petri
Nat Commun, 2019-06-20;10(1):2712.
Species: Mouse
Sample Types: Tissue Homogenates
-
Epigallocatechin-3-Gallate Prevents Acute Gout by Suppressing NLRP3 Inflammasome Activation and Mitochondrial DNA Synthesis
Authors: HE Lee, G Yang, YB Park, HC Kang, YY Cho, HS Lee, JY Lee
Molecules, 2019-06-06;24(11):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Frontline Science: Acyl-CoA synthetase 1 exacerbates lipotoxic inflammasome activation in primary macrophages
Authors: G Kalugotla, L He, KJ Weber, S Daemen, A Reller, B Razani, JD Schilling
J. Leukoc. Biol., 2019-06-05;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
In-depth characterization of congenital Zika syndrome in immunocompetent mice: Antibody-dependent enhancement and an antiviral peptide therapy
Authors: VN Camargos, G Foureaux, DC Medeiros, VT da Silveir, CM Queiroz-Ju, ALB Matosinhos, AFA Figueiredo, CDF Sousa, TP Moreira, VF Queiroz, ACF Dias, KTO Santana, I Passos, ALCV Real, LC Silva, FAG Mourão, NT Wnuk, MAP Oliveira, S Macari, T Silva, GP Garlet, JA Jackman, FM Soriani, MFD Moraes, EMAM Mendes, FM Ribeiro, GMJ Costa, AL Teixeira, NJ Cho, ACP Oliveira, MM Teixeira, VV Costa, DG Souza
EBioMedicine, 2019-05-23;0(0):.
Species: Mouse
Sample Types: Plasma
-
Innate immune memory through TLR2 and NOD2 contributes to the control of Leptospira interrogans infection
Authors: I Santecchia, F Vernel-Pau, O Rasid, J Quintin, M Gomes-Sole, IG Boneca, C Werts
PLoS Pathog., 2019-05-20;15(5):e1007811.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Newly defined ABCB5+ dermal mesenchymal stem cells promote healing of chronic iron overload wounds via secretion of interleukin-1 receptor antagonist
Authors: S Vander Bek, JC de Vries, B Meier-Schi, P Meyer, D Jiang, A Sindrilaru, FF Ferreira, A Hainzl, S Schatz, J Muschhamme, NJ Scheurmann, P Kampilafko, AM Seitz, L Dürselen, A Ignatius, MA Kluth, C Ganss, M Wlaschek, K Singh, P Maity, NY Frank, MH Frank, K Scharffett
Stem Cells, 2019-05-13;0(0):.
Species: Mouse
Sample Types: Cell Lysates
-
Context-specific switch from anti- to pro-epileptogenic function of the P2Y1 receptor in experimental epilepsy
Authors: M Alves, L De Diego G, G Conte, EM Jimenez-Ma, B D'Orsi, A Sanz-Rodri, JH Prehn, DC Henshall, T Engel
J. Neurosci., 2019-05-02;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Butyrate Protects Mice from Clostridium difficile-Induced Colitis through an HIF-1-Dependent Mechanism
Authors: JL Fachi, JS Felipe, LP Pral, BK da Silva, RO Corrêa, MCP de Andrade, DM da Fonseca, PJ Basso, NOS Câmara, ÉL de Sales E, F Dos Santos, SES Guima, AM Thomas, JC Setubal, YT Magalhães, FL Forti, T Candreva, HG Rodrigues, MB de Jesus, SR Consonni, ADS Farias, P Varga-Weis, MAR Vinolo
Cell Rep, 2019-04-16;27(3):750-761.e7.
Species: Mouse
Sample Types: Tissue Homogenates
-
Human metapneumovirus activates NOD-like receptor protein 3 inflammasome via its small hydrophobic protein which plays a detrimental role during infection in mice
Authors: VB Lê, J Dubois, C Couture, MH Cavanagh, O Uyar, A Pizzorno, M Rosa-Calat, MÈ Hamelin, G Boivin
PLoS Pathog., 2019-04-09;15(4):e1007689.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The three cytokines IL-1?, IL-18, and IL-1? share related but distinct secretory routes
Authors: VS Tapia, MJD Daniels, P Palazón-Ri, M Dewhurst, NM Luheshi, J Rivers-Aut, J Green, E Redondo-Ca, P Kaldis, G Lopez-Cast, D Brough
J. Biol. Chem., 2019-04-02;294(21):8325-8335.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Elevated circulating TGF?1 during acute liver failure activates TGF?R2 on cortical neurons and exacerbates neuroinflammation and hepatic encephalopathy in mice
Authors: M McMillin, S Grant, G Frampton, AD Petrescu, E Williams, B Jefferson, A Thomas, A Brahmarout, S DeMorrow
J Neuroinflammation, 2019-04-02;16(1):69.
Species: Mouse
Sample Types: Tissue Homogenates
-
Exercise-induced AMPK activation and IL-6 muscle production are disturbed in adiponectin knockout mice
Authors: TA Diniz, JCJ Aquino Jún, FC Mosele, C Cabral-San, EA Lima Junio, AAS Teixeira, FS Lira, JC Rosa Neto
Cytokine, 2019-03-20;119(0):71-80.
Species: Mouse
Sample Types: Tissue Homogenates
-
HSP70 is a negative regulator of NLRP3 inflammasome activation
Authors: P Martine, A Chevriaux, V Derangère, L Apetoh, C Garrido, F Ghiringhel, C Rébé
Cell Death Dis, 2019-03-15;10(4):256.
Species: Mouse
Sample Types: Lavage Fluid
-
Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice
Authors: Z Gong, J Huang, B Xu, Z Ou, L Zhang, X Lin, X Ye, X Kong, D Long, X Sun, X He, L Xu, Q Li, A Xuan
J Neuroinflammation, 2019-03-14;16(1):62.
Species: Mouse
Sample Types: Tissue Homogenates
-
The NLRP3 inflammasome modulates glycolysis by increasing PFKFB3 in an IL-1?-dependent manner in macrophages
Authors: OM Finucane, J Sugrue, A Rubio-Arai, MV Guillot-Se, MA Lynch
Sci Rep, 2019-03-11;9(1):4034.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Epithelium-specific MyD88 signaling, but not DCs or macrophages, control acute intestinal infection with Clostridium difficile
Authors: P Mamareli, F Kruse, C Friedrich, N Smit, T Strowig, T Sparwasser, M Lochner
Eur. J. Immunol., 2019-03-10;0(0):.
Species: Mouse
Sample Types: Serum
-
Combinatory FK506 and Minocycline Treatment Alleviates Prion-Induced Neurodegenerative Events via Caspase-Mediated MAPK-NRF2 Pathway
Authors: SZA Shah, D Zhao, G Taglialate, T Hussain, H Dong, N Sabir, MH Mangi, W Wu, M Lai, X Zhang, Y Duan, L Wang, X Zhou, L Yang
Int J Mol Sci, 2019-03-06;20(5):.
Species: Hamster
Sample Types: Tissue Homogenates
-
Interleukin-37 monomer is the active form for reducing innate immunity
Authors: EZ Eisenmesse, A Gottschlic, JS Redzic, N Paukovich, JC Nix, T Azam, L Zhang, R Zhao, JS Kieft, E The, X Meng, CA Dinarello
Proc. Natl. Acad. Sci. U.S.A., 2019-02-28;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Gum Arabic Ameliorates Impaired Coagulation and Cardiotoxicity Induced by Water-Pipe Smoke Exposure in Mice
Authors: A Nemmar, S Al-Salam, S Beegam, P Yuvaraju, BH Ali
Front Physiol, 2019-02-25;10(0):53.
Species: Mouse
Sample Types: Tissue Homogenates
-
Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host
Authors: M Ahn, DE Anderson, Q Zhang, CW Tan, BL Lim, K Luko, M Wen, WN Chia, S Mani, LC Wang, JHJ Ng, RM Sobota, CA Dutertre, F Ginhoux, ZL Shi, AT Irving, LF Wang
Nat Microbiol, 2019-02-25;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Honokiol-Mediated Mitophagy Ameliorates Postoperative Cognitive Impairment Induced by Surgery/Sevoflurane via Inhibiting the Activation of NLRP3 Inflammasome in the Hippocampus
Authors: JS Ye, L Chen, YY Lu, SQ Lei, M Peng, ZY Xia
Oxid Med Cell Longev, 2019-02-24;2019(0):8639618.
Species: Mouse
Sample Types: Tissue Secretion
-
Structural basis for species-selective targeting of Hsp90 in a pathogenic fungus
Authors: L Whitesell, N Robbins, DS Huang, CA McLellan, T Shekhar-Gu, EV LeBlanc, CS Nation, R Hui, A Hutchinson, C Collins, S Chatterjee, R Trilles, JL Xie, DJ Krysan, S Lindquist, JA Porco, U Tatu, LE Brown, J Pizarro, LE Cowen
Nat Commun, 2019-01-24;10(1):402.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Leishmania Lipophosphoglycan Triggers Caspase-11 and the Non-canonical Activation of the NLRP3 Inflammasome
Authors: RVH de Carvalh, WA Andrade, DS Lima-Junio, M Dilucca, CV de Oliveir, K Wang, PM Nogueira, JN Rugani, RP Soares, SM Beverley, F Shao, DS Zamboni
Cell Rep, 2019-01-08;26(2):429-437.e5.
Species: Mouse
Sample Types: Cell Culture Supernates
-
In�Vitro Macrophage Assay Predicts the In�Vivo Anti-inflammatory Potential of Exosomes from Human Mesenchymal Stromal Cells
Authors: N Pacienza, RH Lee, EH Bae, DK Kim, Q Liu, DJ Prockop, G Yannarelli
Mol Ther Methods Clin Dev, 2018-12-15;13(0):67-76.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of a fluid-phase PRR in fighting an intracellular pathogen: PTX3 in Shigella infection
Authors: V Ciancarell, L Lembo-Fazi, I Paciello, AK Bruno, S Jaillon, S Berardi, M Barbagallo, S Meron-Suda, D Cohen, A Molinaro, G Rossi, C Garlanda, ML Bernardini
PLoS Pathog., 2018-12-07;14(12):e1007469.
Species: Mouse
Sample Types: Tissue Homogenates
-
Genetically Engineered Resveratrol-Enriched Rice Inhibits Neuroinflammation in Lipopolysaccharide-Activated BV2 Microglia Via Downregulating Mitogen-Activated Protein Kinase-Nuclear Factor Kappa B Signaling Pathway
Authors: L Subedi, SH Baek, SY Kim
Oxid Med Cell Longev, 2018-12-03;2018(0):8092713.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Enzymatic Activity Is Not Required for Phospholipase D Mediated TNF-? Regulation and Myocardial Healing
Authors: M Klier, S Gorressen, MA Urbahn, D Barbosa, M Ouwens, JW Fischer, M Elvers
Front Physiol, 2018-11-29;9(0):1698.
Species: Mouse
Sample Types: Plasma
-
The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1? Activation
Authors: JE Vince, D De Nardo, W Gao, AJ Vince, C Hall, K McArthur, D Simpson, S Vijayaraj, LM Lindqvist, P Bouillet, MA Rizzacasa, SM Man, J Silke, SL Masters, G Lessene, DCS Huang, DHD Gray, BT Kile, F Shao, KE Lawlor
Cell Rep, 2018-11-27;25(9):2339-2353.e4.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A Metabolic Checkpoint for the Yeast-to-Hyphae Developmental Switch Regulated by Endogenous Nitric Oxide Signaling
Authors: B Koch, AA Barugahare, TL Lo, C Huang, RB Schittenhe, DR Powell, TH Beilharz, A Traven
Cell Rep, 2018-11-20;25(8):2244-2258.e7.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Omega-9 Oleic Acid, the Main Compound of Olive Oil, Mitigates Inflammation during Experimental Sepsis
Authors: IM Medeiros-d, CF Gonçalves-, ARM Kurz, FMJ Oliveira, VHP de Abreu, RC Torres, VF Carvalho, V Estato, PT Bozza, M Sperandio, HC de Castro-, AR Silva
Oxid Med Cell Longev, 2018-11-13;2018(0):6053492.
Species: Mouse
Sample Types: Peritoneal Lavage Fluid
-
A non-canonical autophagy-dependent role of the ATG16L1T300A variant in urothelial vesicular trafficking and uropathogenic Escherichia coli persistence
Authors: C Wang, KA Bauckman, ASB Ross, JW Symington, MM Ligon, G Scholtes, A Kumar, HW Chang, J Twentyman, BE Fashemi, RJ Xavier, IU Mysorekar
Autophagy, 2018-11-08;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Ibuprofen prevents progression of ataxia telangiectasia symptoms in ATM-deficient mice
Authors: CW Hui, X Song, F Ma, X Shen, K Herrup
J Neuroinflammation, 2018-11-06;15(1):308.
Species: Mouse
Sample Types: Tissue Homogenates
-
Human Defensin-5 Blocks Ethanol and Colitis-Induced Dysbiosis, Tight Junction Disruption and Inflammation in Mouse Intestine
Authors: PK Shukla, AS Meena, V Rao, RG Rao, L Balazs, R Rao
Sci Rep, 2018-11-02;8(1):16241.
Species: Mouse
Sample Types: Plasma
-
Carbon monoxide-induced TFEB nuclear translocation enhances mitophagy/mitochondrial biogenesis in hepatocytes and ameliorates inflammatory liver injury
Authors: HJ Kim, Y Joe, SY Rah, SK Kim, SU Park, J Park, J Kim, J Ryu, GJ Cho, YJ Surh, SW Ryter, UH Kim, HT Chung
Cell Death Dis, 2018-10-17;9(11):1060.
Species: Mouse
Sample Types: Serum
-
Discovery of a Novel Microtubule Targeting Agent as an Adjuvant for Cancer Immunotherapy
Authors: F Sato-Kanek, X Wang, S Yao, T Hosoya, FS Lao, K Messer, M Pu, NM Shukla, HB Cottam, M Chan, DA Carson, M Corr, T Hayashi
Biomed Res Int, 2018-10-10;2018(0):8091283.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Protective Effects of Anti-IL17 on Acute Lung Injury Induced by LPS in Mice
Authors: RF Righetti, TM Dos Santos, LDN Camargo, LRCRB Aristótele, S Fukuzaki, FCR de Souza, FPR Santana, MVR de Agrela, MM Cruz, MIC Alonso-Val, IS Genaro, BM Saraiva-Ro, EA Leick, MA Martins, CM Prado, IFLC Tibério
Front Pharmacol, 2018-10-04;9(0):1021.
Species: Mouse
Sample Types: Tissue Homogenates
-
Regulation of S100A8 Stability by RNF5 in Intestinal Epithelial Cells Determines Intestinal Inflammation and Severity of Colitis
Authors: Y Fujita, A Khateb, Y Li, R Tinoco, T Zhang, H Bar-Yoseph, MA Tam, Y Chowers, E Sabo, S Gerassy-Va, E Starosvets, B James, K Brown, SS Shen-Orr, LM Bradley, PA Tessier, ZA Ronai
Cell Rep, 2018-09-18;24(12):3296-3311.e6.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Arhalofenate acid inhibits monosodium urate crystal-induced inflammatory responses through activation of AMP-activated protein kinase (AMPK) signaling
Authors: C McWherter, YJ Choi, RL Serrano, SK Mahata, R Terkeltaub, R Liu-Bryan
Arthritis Res. Ther., 2018-09-06;20(1):204.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Disabled-2 (DAB2) Overexpression Inhibits Monocyte-Derived Dendritic Cells' Function in Vogt-Koyanagi-Harada Disease
Authors: S Yi, R Chang, J Hu, Y Qiu, Q Wang, Q Cao, G Yuan, G Su, C Zhou, Y Wang, A Kijlstra, P Yang
Invest. Ophthalmol. Vis. Sci., 2018-09-04;59(11):4662-4669.
Species: Human
Sample Types: Cell Culture Supernates
-
The histamine H3R antagonist DL77 attenuates autistic behaviors in a prenatal valproic acid-induced mouse model of autism
Authors: N Eissa, P Jayaprakas, S Azimullah, SK Ojha, M Al-Houqani, FY Jalal, D ?a?ewska, K Kie?-Konon, B Sadek
Sci Rep, 2018-08-30;8(1):13077.
Species: Mouse
Sample Types:
-
NADPH Oxidase and Guanylate Binding Protein 5 Restrict Survival of Avirulent Type III Strains of Toxoplasma gondii in Naive Macrophages
Authors: SK Matta, K Patten, Q Wang, BH Kim, JD MacMicking, LD Sibley
MBio, 2018-08-28;9(4):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Myeloperoxidase deficiency attenuates systemic and dietary iron-induced adverse effects
Authors: X Xiao, P Saha, BS Yeoh, JA Hipp, V Singh, M Vijay-Kuma
J. Nutr. Biochem., 2018-08-21;62(0):28-34.
Species: Mouse
Sample Types: Tissue Homogenates
-
Helminths-based bi-functional molecule, tuftsin-phosphorylcholine (TPC), ameliorates an established murine arthritis
Authors: M Blank, T Bashi, J Lachnish, D Ben-Ami-Sh, O Shovman, M Fridkin, M Eisenstein, A Volkov, I Barshack, Y Shoenfeld
PLoS ONE, 2018-08-08;13(8):e0200615.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Extracellular ATP is a danger signal activating P2X7 receptor in a LPS mediated inflammation (ARDS/ALI)
Authors: S Cicko, TC Köhler, CK Ayata, T Müller, N Ehrat, A Meyer, M Hossfeld, A Zech, F Di Virgili, M Idzko
Oncotarget, 2018-07-17;9(55):30635-30648.
Species: Mouse
Sample Types: BALF
-
Apigenin Retards Atherogenesis by Promoting ABCA1-Mediated Cholesterol Efflux and Suppressing Inflammation
Authors: K Ren, T Jiang, HF Zhou, Y Liang, GJ Zhao
Cell. Physiol. Biochem., 2018-07-05;47(5):2170-2184.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Targeting the IL33-NLRP3 axis improves therapy for experimental cerebral malaria
Authors: P Strangward, MJ Haley, MG Albornoz, J Barrington, T Shaw, R Dookie, L Zeef, SM Baker, E Winter, TC Tzeng, DT Golenbock, SM Cruickshan, SM Allan, A Craig, FY Liew, D Brough, KN Couper
Proc. Natl. Acad. Sci. U.S.A., 2018-06-28;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Induction of endoplasmic reticulum stress under endotoxin tolerance increases inflammatory responses and decreases Pseudomonas aeruginosa pneumonia
Authors: S Kim, Y Joe, SU Park, SO Jeong, JK Kim, SH Park, HO Pae, YJ Surh, J Shin, HT Chung
J. Leukoc. Biol., 2018-06-20;0(0):.
Species: Mouse
Sample Types: BALF
-
Choline transport links macrophage phospholipid metabolism and inflammation
Authors: SA Snider, KD Margison, P Ghorbani, ND LeBlond, C O'Dwyer, JR Nunes, T Nguyen, H Xu, SA Bennett, MD Fullerton
J. Biol. Chem., 2018-06-07;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice
Authors: AP Perera, R Fernando, T Shinde, R Gundamaraj, B Southam, SS Sohal, AAB Robertson, K Schroder, D Kunde, R Eri
Sci Rep, 2018-06-05;8(1):8618.
Species: Mouse
Sample Types: Tissue Homogenates
-
Glucose Homeostasis Is Important for Immune Cell Viability during Candida Challenge and Host Survival of Systemic Fungal Infection
Authors: Timothy M. Tucey, Jiyoti Verma, Paul F. Harrison, Sarah L. Snelgrove, Tricia L. Lo, Allison K. Scherer et al.
Cell Metabolism
Species: Mouse
Sample Types: Tissue Homogenates
-
Activation of M1 macrophages play a critical role in the initiation of acute lung injury
Authors: HL Lu, XY Huang, YF Luo, WP Tan, PF Chen, YB Guo
Biosci. Rep., 2018-04-27;0(0):.
Species: Mouse
Sample Types: Serum
-
Parkin deficiency modulates NLRP3 inflammasome activation by attenuating an A20-dependent negative feedback loop
Authors: F Mouton-Lig, T Rosazza, J Sepulveda-, A Ieang, SM Hassoun, E Claire, G Mangone, A Brice, PP Michel, JC Corvol, O Corti
Glia, 2018-04-17;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Jugular arginine infusion relieves lipopolysaccharide-triggered inflammatory stress and improves immunity status of lactating dairy cows
Authors: FF Zhao, TY Wu, HR Wang, LY Ding, G Ahmed, HW Li, W Tian, YZ Shen
J. Dairy Sci., 2018-04-05;0(0):.
Species: Bovine
Sample Types: Serum
-
Analogous corticosteroids, 9A and EK100, derived from solid-state-cultured mycelium of Antrodia camphorata inhibit proinflammatory cytokine expression in macrophages
Authors: ST Kao, YH Kuo, SD Wang, HJ Hong, LJ Lin
Cytokine, 2018-03-30;108(0):136-144.
Species: Mouse
Sample Types: BALF
-
HMGB1/IL-1? complexes in plasma microvesicles modulate immune responses to burn injury
Authors: LG Coleman, R Maile, SW Jones, BA Cairns, FT Crews
PLoS ONE, 2018-03-30;13(3):e0195335.
Species: Mouse
Sample Types: Plasma
-
Artemisia Extract Suppresses NLRP3 and AIM2 Inflammasome Activation by Inhibition of ASC Phosphorylation
Authors: SB Kwak, S Koppula, EJ In, X Sun, YK Kim, MK Kim, KH Lee, TB Kang
Mediators Inflamm., 2018-03-04;2018(0):6054069.
Species: Mouse
Sample Types: Cell Culture Supernates
-
FOXO3-dependent apoptosis limits alcohol-induced liver inflammation by promoting infiltrating macrophage differentiation
Authors: Z Li, J Zhao, S Zhang, SA Weinman
Cell Death Discov, 2018-02-13;4(0):16.
Species: Mouse
Sample Types: Serum
-
Saturated Fatty Acids Undergo Intracellular Crystallization and Activate the NLRP3 Inflammasome in Macrophages
Authors: T Karasawa, A Kawashima, F Usui-Kawan, S Watanabe, H Kimura, R Kamata, K Shirasuna, Y Koyama, A Sato-Tomit, T Matsuzaka, H Tomoda, SY Park, N Shibayama, H Shimano, T Kasahara, M Takahashi
Arterioscler. Thromb. Vasc. Biol., 2018-02-08;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Age-related cognitive impairment is associated with long-term neuroinflammation and oxidative stress in a mouse model of episodic systemic inflammation
Authors: JC d'Avila, LD Siqueira, A Mazeraud, EP Azevedo, D Foguel, HC Castro-Far, T Sharshar, F Chrétien, FA Bozza
J Neuroinflammation, 2018-01-30;15(1):28.
Species: Mouse
Sample Types: Tissue Homogenates
-
Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11
Authors: B Lagrange, S Benaoudia, P Wallet, F Magnotti, A Provost, F Michal, A Martin, F Di Lorenzo, BF Py, A Molinaro, T Henry
Nat Commun, 2018-01-16;9(1):242.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses
Authors: L Boutens, GJ Hooiveld, S Dhingra, RA Cramer, MG Netea, R Stienstra
Diabetologia, 2018-01-14;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Necroptosis promotes autophagy-dependent upregulation of DAMP and results in immunosurveillance
Authors: SY Lin, SY Hsieh, YT Fan, WC Wei, PW Hsiao, DH Tsai, TS Wu, NS Yang
Autophagy, 2017-12-31;0(0):0.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Nlrp12 Mediates Adverse Neutrophil Recruitment during Influenza Virus Infection
Authors: EE Hornick, B Banoth, AM Miller, ZR Zacharias, N Jain, ME Wilson, KN Gibson-Cor, KL Legge, GA Bishop, FS Sutterwala, SL Cassel
J. Immunol., 2017-12-27;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Neuronal ICAM-5 Inhibits Microglia Adhesion and Phagocytosis and Promotes an Anti-inflammatory Response in LPS Stimulated Microglia
Authors: S Paetau, T Rolova, L Ning, CG Gahmberg
Front Mol Neurosci, 2017-12-22;10(0):431.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Absence of NOD1 Enhances Killing of Aspergillus fumigatus Through Modulation of Dectin-1 Expression
Authors: MS Gresnigt, M Jaeger, RK Subbarao M, O Rasid, G Jouvion, C Fitting, WJG Melchers, TD Kanneganti, A Carvalho, O Ibrahim-Gr, FL van de Vee
Front Immunol, 2017-12-13;8(0):1777.
Species: Mouse
Sample Types: BALF
-
CRISPR/Cas9 mediated mutation of mouse IL-1? nuclear localisation sequence abolishes expression
Authors: MJD Daniels, AD Adamson, N Humphreys, D Brough
Sci Rep, 2017-12-06;7(1):17077.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Boron-Based Inhibitors of the NLRP3 Inflammasome
Authors: Alex G. Baldwin, Jack Rivers-Auty, Michael J.D. Daniels, Claire S. White, Carl H. Schwalbe, Tom Schilling et al.
Cell Chemical Biology
Species: Mouse
Sample Types: Cell Culture Supernates, Plasma, Peritoneal Lavage Fluid
-
Natural killer cell-intrinsic type I IFN signaling controls Klebsiella pneumoniae growth during lung infection
Authors: M Ivin, A Dumigan, FN de Vasconc, F Ebner, M Borroni, A Kavirayani, KN Przybyszew, RJ Ingram, S Lienenklau, U Kalinke, D Stoiber, JA Bengoechea, P Kovarik
PLoS Pathog., 2017-11-07;13(11):e1006696.
Species: Mouse
Sample Types: Tissue Homogenates
-
Respiratory syncytial virus infection of newborn CX3CR1-deficent mice induces a pathogenic pulmonary innate immune response
Authors: S Das, M Raundhal, J Chen, TB Oriss, R Huff, JV Williams, A Ray, P Ray
JCI Insight, 2017-09-07;2(17):.
Species: Mouse
Sample Types: Tissue Homogenates
-
CLEC5A is a critical receptor in innate immunity against Listeria infection
Authors: ST Chen, FJ Li, TY Hsu, SM Liang, YC Yeh, WY Liao, TY Chou, NJ Chen, M Hsiao, WB Yang, SL Hsieh
Nat Commun, 2017-08-21;8(1):299.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Nitric Oxide Modulates Macrophage Responses to Mycobacterium tuberculosis Infection through Activation of HIF-1? and Repression of NF-?B
Authors: J Braverman, SA Stanley
J. Immunol., 2017-07-28;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The dataset describes: HIF-1 ? expression and LPS mediated cytokine production in MKP-1 deficient bone marrow derived murine macrophages
Authors: H Talwar, C Bauerfeld, Y Liu, L Samavati
Data Brief, 2017-07-20;14(0):56-61.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Cathelicidins Inhibit Escherichia coli-Induced TLR2 and TLR4 Activation in a Viability-Dependent Manner
Authors: M Coorens, VAF Schneider, AM de Groot, A van Dijk, M Meijerink, JM Wells, MR Scheenstra, EJA Veldhuizen, HP Haagsman
J. Immunol., 2017-07-14;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Intramembrane attenuation of the TLR4-TLR6 dimer impairs receptor assembly and reduces microglia mediated neurodegeneration
Authors: L Shmuel-Gal, Y Klug, Z Porat, M Charni, B Zarmi, Y Shai
J. Biol. Chem., 2017-06-27;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
MyD88-dependent pro-interleukin-1? induction in dendritic cells exposed to food-grade synthetic amorphous silica
Authors: HC Winkler, J Kornprobst, P Wick, LM von Moos, I Trantakis, EM Schraner, B Bathke, H Hochrein, M Suter, H Naegeli
Part Fibre Toxicol, 2017-06-23;14(1):21.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Combination of a high-fat diet with sweetened condensed milk exacerbates inflammation and insulin resistance induced by each separately in mice
Authors: LN Masi, AR Martins, AR Crisma, CL do Amaral, MR Davanso, TDA Serdan, RDC da Cunha d, MM Cruz, MIC Alonso-Val, RP Torres, J Mancini-Fi, JNB Pereira, MM da Silva R, EA Liberti, SM Hirabara, R Curi
Sci Rep, 2017-06-21;7(1):3937.
Species: Mouse
Sample Types: Tissue Homogenates
-
The anti-inflammatory and immunomodulatory potential of braylin: Pharmacological properties and mechanisms by in silico, in vitro and in vivo approaches
Authors: RF Espírito-S, CS Meira, RDS Costa, OP Souza Filh, AF Evangelist, GHG Trossini, GM Ferreira, EDS Velozo, CF Villarreal, MB Pereira So
PLoS ONE, 2017-06-08;12(6):e0179174.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Elevated prostaglandin E2 post-bone marrow transplant mediates interleukin-1?-related lung injury
Authors: GJ Martínez-C, QM Taylor, CA Wilke, AB Podsiad, BB Moore
Mucosal Immunol, 2017-06-07;0(0):.
Species: Mouse
Sample Types: BALF
-
Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome
Authors: V Neudecker, M Haneklaus, O Jensen, L Khailova, JC Masterson, H Tye, K Biette, P Jedlicka, KS Brodsky, ME Gerich, M Mack, AAB Robertson, MA Cooper, GT Furuta, CA Dinarello, LA O'Neill, HK Eltzschig, SL Masters, EN McNamee
J. Exp. Med., 2017-05-09;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
IL-24 Promotes Pseudomonas aeruginosa Keratitis in C57BL/6 Mouse Corneas
Authors: BX Ross, N Gao, X Cui, TJ Standiford, J Xu, FX Yu
J. Immunol, 2017-03-22;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Cerium Oxide Nanoparticles in Lung Acutely Induce Oxidative Stress, Inflammation, and DNA Damage in Various Organs of Mice
Authors: A Nemmar, P Yuvaraju, S Beegam, MA Fahim, BH Ali
Oxid Med Cell Longev, 2017-03-14;2017(0):9639035.
Species: Mouse
Sample Types: Tissue Homogenates
-
The Carbohydrate Lectin Receptor, Dectin-1, Mediates Immune Response to Exserohilum rostratum
Infect. Immun, 2017-02-23;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner
Authors: SA Conos, KW Chen, D De Nardo, H Hara, L Whitehead, G N£¤ez, SL Masters, JM Murphy, K Schroder, DL Vaux, KE Lawlor, LM Lindqvist, JE Vince
Proc. Natl. Acad. Sci. U.S.A, 2017-01-17;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
LRP1 modulates the microglial immune response via regulation of JNK and NF-?B signaling pathways
J Neuroinflammation, 2016-12-08;13(1):304.
Species: Mouse
Sample Types: Cell Culture Supernates
-
NLRP12 negatively regulates proinflammatory cytokine production and host defense against Brucella abortus
Eur. J. Immunol., 2016-12-05;0(0):.
Species: Mouse
Sample Types: Serum
-
Differential humoral and cellular immunity induced by vaccination using plasmid DNA and protein recombinant expressing the NS3 protein of dengue virus type 3
J. Biomed. Sci, 2016-12-01;23(1):85.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Advax, a Delta Inulin Microparticle, Potentiates In-built Adjuvant Property of Co-administered Vaccines
EBioMedicine, 2016-12-01;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immunogenic Dendritic Cell Generation from Pluripotent Stem Cells by Ectopic Expression of Runx3
Authors: Istvan Szatmari
J. Immunol., 2016-11-16;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Palmitate promotes inflammatory responses and cellular senescence in cardiac fibroblasts
Authors: Arne Yndestad
Biochim. Biophys. Acta, 2016-11-12;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Indoor secondary organic aerosols formation from ozonolysis of monoterpene: An example of d-limonene with ammonia and potential impacts on pulmonary inflammations
Authors: Rujin Huang
Sci. Total Environ., 2016-11-11;0(0):.
Species: Mouse
Sample Types: BALF
-
Carvacrol reduces irinotecan-induced intestinal mucositis through inhibition of inflammation and oxidative damage via TRPA1 receptor activation
Authors: Jand Venes R Medeiros
Chem. Biol. Interact., 2016-11-09;260(0):129-140.
Species: Mouse
Sample Types: Tissue Homogenates
-
Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice
Authors: Silvana Peterini Boeira
Chem. Biol. Interact., 2016-11-03;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Molecular Basis of Acute Cystitis Reveals Susceptibility Genes and Immunotherapeutic Targets.
Authors: Ines Ambite, Manoj Puthia, Karoly Nagy, Caterina Cafaro, Aftab Nadeem, Daniel S C Butler, Gustav Rydström, Nina A Filenko, Björn Wullt, Thomas Miethke, Catharina Svanborg
PloS Pathogens, 2016-10-12;0(0):1553-7374.
Species: Mouse
Sample Types: Urine
-
Polarization of macrophages towards M2 phenotype is favored by reduction in iPLA2?
J Biol Chem, 2016-09-20;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dental Calculus Stimulates Interleukin-1? Secretion by Activating NLRP3 Inflammasome in Human and Mouse Phagocytes
Authors: JL Montenegro, A Yoshimura, Z Sm, T Kaneko, Y Ozaki, T Ukai, T Miyazaki, E Latz, Y Hara
PLoS ONE, 2016-09-15;11(9):e0162865.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Klebsiella pneumoniae Siderophores Induce Inflammation, Bacterial Dissemination, and HIF-1? Stabilization during Pneumonia
MBio, 2016-09-13;7(5):.
Species: Mouse
Sample Types: Tissue Homogenates
-
P2X7 receptor-dependent tuning of gut epithelial responses to infection
Authors: SW Huang, C Walker, J Pennock, K Else, W Muller, MJ Daniels, C Pellegrini, D Brough, G Lopez-Cast, SM Cruickshan
Immunol. Cell Biol, 2016-08-25;95(2):178-188.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Caspase-2 deficiency accelerates chemically induced liver cancer in mice
Cell Death Differ, 2016-08-12;0(0):.
Species: Mouse
Sample Types: Serum
-
The circadian clock regulates inflammatory arthritis
FASEB J, 2016-08-03;0(0):.
Species: Mouse
Sample Types: Serum
-
Transcriptional and functional characterization of CD137L-dendritic cells identifies a novel dendritic cell phenotype
Sci Rep, 2016-07-19;6(0):29712.
Species: Human
Sample Types: Cell Culture Supernates
-
HIF-1? Is an Essential Mediator of IFN-?-Dependent Immunity to Mycobacterium tuberculosis
J Immunol, 2016-07-18;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Cell death is not essential for caspase-1-mediated interleukin-1? activation and secretion
Cell Death Differ, 2016-07-15;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A Simple Screening Approach To Prioritize Genes for Functional Analysis Identifies a Role for Interferon Regulatory Factor 7 in the Control of Respiratory Syncytial Virus Disease
Authors: John S Tregoning
mSystems, 2016-06-28;1(3):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Host Inflammatory Response to Mosquito Bites Enhances the Severity of Arbovirus Infection
Authors: Marieke Pingen
Immunity, 2016-06-21;44(6):1455-69.
Species: Mouse
Sample Types: Tissue Homogenates
-
Plasminogen activator inhibitor-1 stimulates macrophage activation through Toll-like Receptor-4
Authors: Kamlesh K Gupta
Biochem Biophys Res Commun, 2016-06-15;0(0):.
Species: Mouse
Sample Types: Whole Cells
-
Sphingosine-1-Phosphate Receptor 2 Regulates Proinflammatory Cytokine Production and Osteoclastogenesis
PLoS ONE, 2016-05-25;11(5):e0156303.
Species: Mouse
Sample Types: Cell Lysates
-
Reducing hypoxia and inflammation during invasive pulmonary aspergillosis by targeting the Interleukin-1 receptor
Sci Rep, 2016-05-24;6(0):26490.
Species: Mouse
Sample Types: Tissue Homogenates
-
Uropathogenic Escherichia coli strain CFT073 disrupts NLRP3 inflammasome activation
J Clin Invest, 2016-05-23;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Hemocyanins Stimulate Innate Immunity by Inducing Different Temporal Patterns of Proinflammatory Cytokine Expression in Macrophages
J Immunol, 2016-04-22;196(11):4650-62.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Infection-Mediated Priming of Phagocytes Protects against Lethal Secondary Aspergillus fumigatus Challenge
Authors: A Savers, O Rasid, M Parlato, M Brock, G Jouvion, B Ryffel, JM Cavaillon, G Eberl, O Ibrahim-Gr
PLoS ONE, 2016-04-14;11(4):e0153829.
Species: Mouse
Sample Types: Tissue Homogenates
-
A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet
Authors: G Yang, HE Lee, JY Lee
Sci Rep, 2016-04-14;6(0):24399.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Manipulation of Interleukin-1? and Interleukin-18 Production by Yersinia pestis Effectors YopJ and YopM and Redundant Impact on Virulence
Authors: D Ratner, MP Orning, KK Starheim, R Marty-Roix, MK Proulx, JD Goguen, E Lien
J. Biol. Chem., 2016-02-16;291(19):9894-905.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1 beta secretion in response to ATP
Authors: Mausita Karmakar, Michael A. Katsnelson, George R. Dubyak, Eric Pearlman
Nature Communications
Species: Human
Sample Types: Cell Culture Supernates
-
C1P Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Preventing NF-?B Activation in Neutrophils
J Immunol, 2016-01-22;196(5):2319-26.
Species: Mouse
Sample Types: BALF
-
Memory Th1 Cells Are Protective in Invasive Staphylococcus aureus Infection.
Authors: Brown A, Murphy A, Lalor S, Leech J, O'Keeffe K, Mac Aogain M, O'Halloran D, Lacey K, Tavakol M, Hearnden C, Fitzgerald-Hughes D, Humphreys H, Fennell J, van Wamel W, Foster T, Geoghegan J, Lavelle E, Rogers T, McLoughlin R
PLoS Pathog, 2015-11-05;11(11):e1005226.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Effects of High-Intensity Swimming on Lung Inflammation and Oxidative Stress in a Murine Model of DEP-Induced Injury.
Authors: Avila L, Bruggemann T, Bobinski F, da Silva M, Oliveira R, Martins D, Mazzardo-Martins L, Duarte M, de Souza L, Dafre A, Vieira R, Santos A, Bonorino K, Hizume Kunzler D
PLoS ONE, 2015-09-02;10(9):e0137273.
Species: Mouse
Sample Types: Tissue Homogenates
-
Cellular FLIP Inhibits Myeloid Cell Activation by Suppressing Selective Innate Signaling.
Authors: Wu Y, Wu Y, Mo S, Hsiao H, He Y, Lai M
J Immunol, 2015-08-03;195(6):2612-23.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Inflammatory Caspases-1 and -11 Mediate the Pathogenesis of Dermatitis in Sharpin-Deficient Mice.
Authors: Douglas T, Champagne C, Morizot A, Lapointe J, Saleh M
J Immunol, 2015-07-27;195(5):2365-73.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Deficient NLRP3 and AIM2 Inflammasome Function in Autoimmune NZB Mice.
Authors: Sester D, Sagulenko V, Thygesen S, Cridland J, Loi Y, Cridland S, Masters S, Genske U, Hornung V, Andoniou C, Sweet M, Degli-Esposti M, Schroder K, Stacey K
J Immunol, 2015-06-26;195(3):1233-41.
Species: Mouse
Sample Types: Cell Culture Supernates
-
An Allergic Lung Microenvironment Suppresses Carbon Nanotube-Induced Inflammasome Activation via STAT6-Dependent Inhibition of Caspase-1.
Authors: Shipkowski K, Taylor A, Thompson E, Glista-Baker E, Sayers B, Messenger Z, Bauer R, Jaspers I, Bonner J
PLoS ONE, 2015-06-19;10(6):e0128888.
Species: Mouse
Sample Types: BALF
-
The generation of macrophages with anti-inflammatory activity in the absence of STAT6 signaling.
Authors: Fleming B, Chandrasekaran P, Dillon L, Dalby E, Suresh R, Sarkar A, El-Sayed N, Mosser D
J Leukoc Biol, 2015-06-05;98(3):395-407.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Endoplasmic reticulum stress-induced IRE1alpha activation mediates cross-talk of GSK-3beta and XBP-1 to regulate inflammatory cytokine production.
Authors: Kim S, Joe Y, Kim H, Kim Y, Jeong S, Pae H, Ryter S, Surh Y, Chung H
J Immunol, 2015-03-27;194(9):4498-506.
Species: Mouse
Sample Types: Serum
-
Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida.
Authors: Meunier E, Wallet P, Dreier R, Costanzo S, Anton L, Ruhl S, Dussurgey S, Dick M, Kistner A, Rigard M, Degrandi D, Pfeffer K, Yamamoto M, Henry T, Broz P
2015-03-16;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases.
Authors: Coll R, Robertson A, Chae J, Higgins S, Munoz-Planillo R, Inserra M, Vetter I, Dungan L, Monks B, Stutz A, Croker D, Butler M, Haneklaus M, Sutton C, Nunez G, Latz E, Kastner D, Mills K, Masters S, Schroder K, Cooper M, O'Neill L
Nat Med, 2015-02-16;21(3):248-55.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Liver X receptor-dependent inhibition of microglial nitric oxide synthase 2.
Authors: Secor McVoy J, Oughli H, Oh U
J Neuroinflammation, 2015-02-10;12(0):27.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Necroptosis suppresses inflammation via termination of TNF- or LPS-induced cytokine and chemokine production.
Authors: Kearney C, Cullen S, Tynan G, Henry C, Clancy D, Lavelle E, Martin S
Cell Death Differ, 2015-01-23;22(8):1313-27.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mitochondrial damage contributes to Pseudomonas aeruginosa activation of the inflammasome and is downregulated by autophagy.
Authors: Jabir M, Hopkins L, Ritchie N, Ullah I, Bayes H, Li D, Tourlomousis P, Lupton A, Puleston D, Simon A, Bryant C, Evans T
Autophagy, 2015-01-01;11(1):166-82.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A critical role for the TLR signaling adapter Mal in alveolar macrophage-mediated protection against Bordetella pertussis.
Authors: Bernard N, Finlay C, Tannahill G, Cassidy J, O'Neill L, Mills K
Mucosal Immunol, 2014-12-17;8(5):982-92.
Species: Mouse
Sample Types: Tissue Homogenates
-
Role of IL-1β in experimental cystic fibrosis upon P. aeruginosa infection.
PLoS ONE, 2014-12-12;9(12):e114884.
Species: Mouse
Sample Types: Tissue Homogenates
-
Dendritic cell IL-1alpha and IL-1beta are polyubiquitinated and degraded by the proteasome.
Authors: Ainscough J, Frank Gerberick G, Zahedi-Nejad M, Lopez-Castejon G, Brough D, Kimber I, Dearman R
J Biol Chem, 2014-11-04;289(51):35582-92.
Species: Mouse
Sample Types: Cell Lysates
-
No Impairment in host defense against Streptococcus pneumoniae in obese CPEfat/fat mice.
Authors: Mancuso P, O Brien E, Prano J, Goel D, Aronoff D
PLoS ONE, 2014-09-09;9(9):e106420.
Species: Mouse
Sample Types: Serum
-
Murine lung injury caused by Leptospira interrogans glycolipoprotein, a specific Na/K-ATPase inhibitor.
Authors: Goncalves-de-Albuquerque C, Burth P, Silva A, de Moraes I, Oliveira F, Santelli R, Freire A, de Lima G, da Silva E, da Silva C, Morandi V, Bozza P, Younes-Ibrahim M, de Castro Faria Neto H, de Castro Faria M
Respir Res, 2014-08-14;15(0):93.
Species: Mouse
Sample Types: BALF
-
Micheliolide, a new sesquiterpene lactone that inhibits intestinal inflammation and colitis-associated cancer.
Authors: Viennois E, Xiao B, Ayyadurai S, Wang L, Wang P, Zhang Q, Chen Y, Merlin D
Lab Invest, 2014-07-28;94(9):950-65.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of lysosomes in silica-induced inflammasome activation and inflammation in absence of MARCO.
Authors: Biswas R, Hamilton R, Holian A
J Immunol Res, 2014-06-26;2014(0):304180.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Palmitoylethanolamide stimulates phagocytosis of Escherichia coli K1 by macrophages and increases the resistance of mice against infections.
Authors: Redlich S, Ribes S, Schutze S, Nau R
J Neuroinflammation, 2014-06-14;11(0):108.
Species: Mouse
Sample Types: Tissue Homogenates
-
Role of the aryl hydrocarbon receptor in the immune response profile and development of pathology during Plasmodium berghei Anka infection.
Authors: Brant F, Miranda A, Esper L, Rodrigues D, Kangussu L, Bonaventura D, Soriani F, Pinho V, Souza D, Rachid M, Weiss L, Tanowitz H, Teixeira M, Teixeira A, Machado F
Infect Immun, 2014-05-12;82(8):3127-40.
Species: Mouse
Sample Types: Tissue Homogenates
-
Heat shock protein 60: an endogenous inducer of dopaminergic cell death in Parkinson disease.
Authors: Noelker C, Morel L, Osterloh A, Alvarez-Fischer D, Lescot T, Breloer M, Gold M, Oertel W, Henze C, Michel P, Dodel R, Lu L, Hirsch E, Hunot S, Hartmann A
J Neuroinflammation, 2014-05-08;11(0):86.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The effect of size on Ag nanosphere toxicity in macrophage cell models and lung epithelial cell lines is dependent on particle dissolution.
Authors: Hamilton, Raymond, Buckingham, Sarah, Holian, Andrij
Int J Mol Sci, 2014-04-22;15(4):6815-30.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Peripheral inflammation is associated with remote global gene expression changes in the brain.
Authors: Thomson, Carolyn, McColl, Alison, Cavanagh, Jonathan, Graham, Gerard J
J Neuroinflammation, 2014-04-08;11(0):73.
Species: Mouse
Sample Types: Plasma
-
Staphylococcus aureus infection of mice expands a population of memory gammadelta T cells that are protective against subsequent infection.
Authors: Murphy A, O'Keeffe K, Lalor S, Maher B, Mills K, McLoughlin R
J Immunol, 2014-03-12;192(8):3697-708.
Species: Mouse
Sample Types: Peritoneal Lavage Fluid
-
ASC in renal collecting duct epithelial cells contributes to inflammation and injury after unilateral ureteral obstruction.
Authors: Komada T, Usui F, Shirasuna K, Kawashima A, Kimura H, Karasawa T, Nishimura S, Sagara J, Noda T, Taniguchi S, Muto S, Nagata D, Kusano E, Takahashi M
Am J Pathol, 2014-03-05;184(5):1287-98.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Toll-like receptor 9 deficiency protects mice against Pseudomonas aeruginosa lung infection.
Authors: Benmohamed F, Medina M, Wu Y, Maschalidi S, Jouvion G, Guillemot L, Chignard M, Manoury B, Touqui L
PLoS ONE, 2014-03-04;9(3):e90466.
Species: Mouse
Sample Types: BALF
-
Lipopolysaccharide induces inflammatory hyperalgesia triggering a TLR4/MyD88-dependent cytokine cascade in the mice paw.
Authors: Calil I, Zarpelon A, Guerrero A, Alves-Filho J, Ferreira S, Cunha F, Cunha T, Verri W
PLoS ONE, 2014-03-03;9(3):e90013.
Species: Mouse
Sample Types: Tissue Homogenates
-
The role of Nox2-derived ROS in the development of cognitive impairment after sepsis.
Authors: Hernandes M, D'Avila J, Trevelin S, Reis P, Kinjo E, Lopes L, Castro-Faria-Neto H, Cunha F, Britto L, Bozza F
J Neuroinflammation, 2014-02-27;11(0):36.
Species: Mouse
Sample Types: Peritoneal Fluid
-
Unique macrophages different from M1/M2 macrophages inhibit T cell mitogenesis while upregulating Th17 polarization.
Authors: Tatano Y, Shimizu T, Tomioka H
Sci Rep, 2014-02-20;4(0):4146.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation.
Authors: Weber K, Schilling J
J Biol Chem, 2014-02-15;289(13):9158-71.
Species: Mouse
Sample Types: Cell Culture Supernates
-
MyD88 signalling in myeloid cells is sufficient to prevent chronic mycobacterial infection.
Authors: Berod L, Stuve P, Swallow M, Arnold-Schrauf C, Kruse F, Gentilini M, Freitag J, Holzmann B, Sparwasser T
Eur J Immunol, 2014-02-13;44(5):1399-409.
Species: Mouse
Sample Types: Serum
-
Protective or deleterious role of scavenger receptors SR-A and CD36 on host resistance to Staphylococcus aureus depends on the site of infection.
Authors: Blanchet C, Jouvion G, Fitting C, Cavaillon J, Adib-Conquy M
PLoS ONE, 2014-01-31;9(1):e87927.
Species: Mouse
Sample Types: Plasma
-
PPAR-gamma/IL-10 axis inhibits MyD88 expression and ameliorates murine polymicrobial sepsis.
Authors: Ferreira A, Sisti F, Sonego F, Wang S, Filgueiras L, Brandt S, Serezani A, Du H, Cunha F, Alves-Filho J, Serezani C
J Immunol, 2014-01-31;192(5):2357-65.
Species: Mouse
Sample Types: Serum
-
Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection.
Authors: Ataide M, Andrade W, Zamboni D, Wang D, Souza M, Franklin B, Elian S, Martins F, Pereira D, Reed G, Fitzgerald K, Golenbock D, Gazzinelli R
PLoS Pathog, 2014-01-16;10(1):e1003885.
Species: Mouse
Sample Types: Whole Blood
-
Promyelocytic leukemia protein interacts with the apoptosis-associated speck-like protein to limit inflammasome activation.
Authors: Dowling J, Becker C, Bourke N, Corr S, Connolly D, Quinn S, Pandolfi P, Mansell A, O'Neill L
J Biol Chem, 2014-01-09;289(10):6429-37.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Sustained Na+/H+ exchanger activation promotes gliotransmitter release from reactive hippocampal astrocytes following oxygen-glucose deprivation.
Authors: Cengiz P, Kintner D, Chanana V, Yuan H, Akture E, Kendigelen P, Begum G, Fidan E, Uluc K, Ferrazzano P, Sun D
PLoS ONE, 2014-01-02;9(1):e84294.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A pseudopterane diterpene isolated from the octocoral Pseudopterogorgia acerosa inhibits the inflammatory response mediated by TLR-ligands and TNF-alpha in macrophages.
Authors: Gonzalez Y, Doens D, Santamaria R, Ramos M, Restrepo C, Barros de Arruda L, Lleonart R, Gutierrez M, Fernandez P
PLoS ONE, 2013-12-16;8(12):e84107.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis.
Authors: Hams, Emily, Armstrong, Michelle, Barlow, Jillian, Saunders, Sean P, Schwartz, Christia, Cooke, Gordon, Fahy, Ruairi J, Crotty, Thomas B, Hirani, Nikhil, Flynn, Robin J, Voehringer, David, McKenzie, Andrew N, Donnelly, Seamas C, Fallon, Padraic
Proc Natl Acad Sci U S A, 2013-12-16;111(1):367-72.
Species: Mouse
Sample Types: Tissue Homogenates
-
Lack of platelet-activating factor receptor protects mice against diet-induced adipose inflammation and insulin-resistance despite fat pad expansion.
Authors: Menezes-Garcia Z, Oliveira M, Lima R, Soriani F, Cisalpino D, Botion L, Teixeira M, Souza D, Ferreira A
Obesity (Silver Spring), 2013-12-14;22(3):663-72.
Species: Mouse
Sample Types: Tissue Homogenates
-
NLRP3 inflammasome activation by Paracoccidioides brasiliensis.
Authors: Tavares A, Magalhaes K, Almeida R, Correa R, Burgel P, Bocca A
PLoS Negl Trop Dis, 2013-12-05;7(12):e2595.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Rift Valley fever virus infection induces activation of the NLRP3 inflammasome.
Authors: Ermler M, Traylor Z, Patel K, Schattgen S, Vanaja S, Fitzgerald K, Hise A
Virology, 2013-12-03;449(0):174-80.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Defining the range of pathogens susceptible to Ifitm3 restriction using a knockout mouse model.
Authors: Everitt, Aaron R, Clare, Simon, McDonald, Jacqueli, Kane, Leanne, Harcourt, Katherin, Ahras, Malika, Lall, Amar, Hale, Christin, Rodgers, Angela, Young, Douglas, Haque, Ashraful, Billker, Oliver, Tregoning, John S, Dougan, Gordon, Kellam, Paul
PLoS ONE, 2013-11-21;8(11):e80723.
Species: Mouse
Sample Types: BALF
-
The lung inflammation and skeletal muscle wasting induced by subchronic cigarette smoke exposure are not altered by a high-fat diet in mice.
Authors: Hansen, Michelle, Chen, Hui, Jones, Jessica, Langenbach, Shenna Y, Vlahos, Ross, Gualano, Rosa C, Morris, Margaret, Anderson, Gary P
PLoS ONE, 2013-11-19;8(11):e80471.
Species: Mouse
Sample Types: Tissue Homogenates
-
A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation.
Authors: Csoka B, Koscso B, Toro G, Kokai E, Virag L, Nemeth Z, Pacher P, Bai P, Hasko G
Diabetes, 2013-11-05;63(3):850-66.
Species: Mouse
Sample Types: Plasma
-
Further evidence for an anti-inflammatory role of artesunate in experimental cerebral malaria.
Authors: Miranda A, Brant F, Rocha N, Cisalpino D, Rodrigues D, Souza D, Machado F, Rachid M, Teixeira A, Campos A
Malar J, 2013-11-02;12(0):388.
Species: Mouse
Sample Types: Tissue Homogenates
-
TXNIP deficiency exacerbates endotoxic shock via the induction of excessive nitric oxide synthesis.
Authors: Park Y, Yoon S, Suh H, Kim D, Park J, Jung H, Kim T, Yoon S, Min J, Na H, Lee S, Lee H, Lee Y, Lee H, Choi I
PLoS Pathog, 2013-10-03;9(10):e1003646.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation.
Authors: Horton J, Hudak K, Chung E, White A, Scroggins B, Burkeen J, Citrin D
Stem Cells, 2013-10-01;31(10):2231-41.
Species: Mouse
Sample Types: Tissue Homogenates
-
Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model.
Authors: Alvarez-Fischer D, Noelker C, Vulinovic F, Grunewald A, Chevarin C, Klein C, Oertel W, Hirsch E, Michel P, Hartmann A
PLoS ONE, 2013-04-18;8(4):e61700.
Species: Mouse
Sample Types: Tissue Homogenates
-
Intestinal CCL11 and eosinophilic inflammation is regulated by myeloid cell-specific RelA/p65 in mice.
Authors: Waddell A, Ahrens R, Tsai Y, Sherrill J, Denson L, Steinbrecher K, Hogan S
J Immunol, 2013-04-05;190(9):4773-85.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A novel murine model of rhinoscleroma identifies Mikulicz cells, the disease signature, as IL-10 dependent derivatives of inflammatory monocytes.
Authors: Fevre C, Almeida A, Taront S, Pedron T, Huerre M, Prevost M, Kieusseian A, Cumano A, Brisse S, Sansonetti P, Tournebize R
EMBO Mol Med, 2013-04-01;5(4):516-30.
Species: Mouse
Sample Types: Tissue Homogenates
-
Ghrelin inhibits LPS-induced release of IL-6 from mouse dopaminergic neurones.
Authors: Beynon A, Brown M, Wright R, Rees M, Sheldon I, Davies J
J Neuroinflammation, 2013-03-19;10(0):40.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TLR2 deletion promotes arthritis through reduction of IL-10.
Authors: Huang Q, Koessler R, Birkett R, Perlman H, Xing L, Pope R
J Leukoc Biol, 2013-02-27;93(5):751-9.
Species: Mouse
Sample Types: Tissue Homogenates
-
Milano summer particulate matter (PM10) triggers lung inflammation and extra pulmonary adverse events in mice.
Authors: Farina F, Sancini G, Battaglia C, Tinaglia V, Mantecca P, Camatini M, Palestini P
PLoS ONE, 2013-02-25;8(2):e56636.
Species: Mouse
Sample Types: BALF
-
Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response.
Authors: Oh J, Lee R, Yu J, Ko J, Lee H, Ko A, Roddy G, Prockop D
Mol Ther, 2012-08-28;20(11):2143-52.
Species: Mouse
Sample Types: Tissue Homogenates
-
Macrophage Dectin-1 Expression Is Controlled by Leukotriene B4 via a GM-CSF/PU.1 Axis.
Authors: Serezani CH, Kane S, Collins L
J. Immunol., 2012-06-13;189(2):906-15.
Species: Mouse
Sample Types: BALF
-
Dok2 mediates the CD200Fc attenuation of Abeta-induced changes in glia.
Authors: Lyons A, Downer E, Costello D, Murphy N, Lynch M
J Neuroinflammation, 2012-05-29;9(0):107.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Influence of hypoxia-inducible factor 1-alpha on dendritic cell differentiation and migration.
Authors: Kohler T, Reizis B, Johnson RS, Weighardt H, Forster I
Eur. J. Immunol., 2012-05-01;42(5):1226-36.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TLR9 and MyD88 are crucial for the development of protective immunity to malaria.
Authors: Gowda NM, Wu X, Gowda DC
J. Immunol., 2012-04-18;188(10):5073-85.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin-1 participates in the classical and alternative activation of microglia/macrophages after spinal cord injury.
Authors: Sato A, Ohtaki H, Tsumuraya T, Song D, Ohara K, Asano M, Iwakura Y, Atsumi T, Shioda S
J Neuroinflammation, 2012-04-07;9(0):65.
Species: Mouse
Sample Types: Tissue Homogenates
-
Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection.
Authors: Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, Riva F, Garlanda C
Infect. Immun., 2011-10-24;80(1):100-9.
Species: Mouse
Sample Types: Serum
-
The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF.
Authors: El-Behi M, Ciric B, Dai H
Nat. Immunol., 2011-04-24;12(6):568-75.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Therapeutic Potential of the Humoral Pattern Recognition Molecule PTX3 in Chronic Lung Infection Caused by Pseudomonas aeruginosa.
Authors: Moalli F, Paroni M, Veliz Rodriguez T, Riva F, Polentarutti N, Bottazzi B, Valentino S, Mantero S, Nebuloni M, Mantovani A, Bragonzi A, Garlanda C
J. Immunol., 2011-03-25;186(9):5425-34.
Species: Mouse
Sample Types: Tissue Homogenates
-
IL-27 mediates the response to IFN-beta therapy in multiple sclerosis patients by inhibiting Th17 cells.
Authors: Sweeney CM, Lonergan R, Basdeo SA, Kinsella K, Dungan LS, Higgins SC, Kelly PJ, Costelloe L, Tubridy N, Mills KH, Fletcher JM
Brain Behav. Immun., 2011-03-21;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition.
Authors: Barber DL, Mayer-Barber KD, Feng CG
J. Immunol., 2010-12-20;186(3):1598-607.
Species: Mouse
Sample Types: BALF
-
A critical role for C5L2 in the pathogenesis of experimental allergic asthma.
Authors: Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, Konig P, Gerard NP, Gerard C, Wills-Karp M, Kohl J
J. Immunol., 2010-10-25;185(11):6741-52.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Toll-like receptor 4 mediates the response of epithelial and stromal cells to lipopolysaccharide in the endometrium.
Authors: Sheldon IM, Roberts MH
PLoS ONE, 2010-09-22;5(9):e12906.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Tyrosine kinase 2 controls IL-1beta production at the translational level.
Authors: Radwan M, Stiefvater R, Grunert T, Sharif O, Miller I, Marchetti-Deschmann M, Allmaier G, Gemeiner M, Knapp S, Kovarik P, Muller M, Strobl B
J. Immunol., 2010-08-16;185(6):3544-53.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Importance of TLR2 in the direct response of T lymphocytes to Schistosoma mansoni antigens.
Authors: Burton OT, Gibbs S, Miller N, Jones FM, Wen L, Dunne DW, Cooke A, Zaccone P
Eur. J. Immunol., 2010-08-01;40(8):2221-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
P2X(7) receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases.
Authors: Lopez-Castejon G, Theaker J, Pelegrin P
J. Immunol., 2010-07-16;185(4):2611-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Distinct roles of hepatocyte- and myeloid cell-derived IL-1 receptor antagonist during endotoxemia and sterile inflammation in mice.
Authors: Lamacchia C, Palmer G, Bischoff L, Rodriguez E, Talabot-Ayer D, Gabay C
J. Immunol., 2010-07-16;185(0):2516-24.
Species: Mouse
Sample Types: Serum
-
Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5.
Authors: Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT
Science, 2010-03-04;328(5975):228-31.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity.
Authors: Sellge G, Magalhaes JG, Konradt C, Fritz JH, Salgado-Pabon W, Eberl G, Bandeira A, Di Santo JP, Sansonetti PJ, Phalipon A
J. Immunol., 2010-01-20;184(4):2076-85.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-1R1/MyD88 signaling is critical for elastase-induced lung inflammation and emphysema.
Authors: Couillin I, Vasseur V, Charron S, Gasse P, Tavernier M, Guillet J, Lagente V, Fick L, Jacobs M, Coelho FR, Moser R, Ryffel B
J. Immunol., 2009-12-15;183(12):8195-202.
Species: Mouse
Sample Types: BALF
-
A conjugated linoleic acid-enriched beef diet attenuates lipopolysaccharide-induced inflammation in mice in part through PPARgamma-mediated suppression of toll-like receptor 4.
Authors: Reynolds CM, Draper E, Keogh B, Rahman A, Moloney AP, Mills KH, Loscher CE, Roche HM
J. Nutr., 2009-10-21;139(12):2351-7.
Species: Mouse
Sample Types: Serum
-
E-prostanoid 3 receptor deletion improves pulmonary host defense and protects mice from death in severe Streptococcus pneumoniae infection.
Authors: Aronoff DM, Lewis C, Serezani CH, Eaton KA, Goel D, Phipps JC, Peters-Golden M, Mancuso P
J. Immunol., 2009-07-27;183(4):2642-9.
Species: Mouse
Sample Types: BALF
-
TREM-1 activation alters the dynamics of pulmonary IRAK-M expression in vivo and improves host defense during pneumococcal pneumonia.
Authors: Lagler H, Sharif O, Haslinger I, Matt U, Stich K, Furtner T, Doninger B, Schmid K, Gattringer R, de Vos AF, Knapp S
J. Immunol., 2009-07-13;183(3):2027-36.
Species: Mouse
Sample Types: Tissue Homogenates
-
The CDK domain of p21 is a suppressor of IL-1beta-mediated inflammation in activated macrophages.
Authors: Scatizzi JC, Mavers M, Hutcheson J, Young B, Shi B, Pope RM, Ruderman EM, Samways DS, Corbett JA, Egan TM, Perlman H
Eur. J. Immunol., 2009-03-01;39(3):820-5.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy.
Authors: Jaffre F, Bonnin P, Callebert J, Debbabi H, Setola V, Doly S, Monassier L, Mettauer B, Blaxall BC, Launay JM, Maroteaux L
Circ. Res., 2008-11-20;104(1):113-23.
Species: Mouse
Sample Types: Plasma
-
Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis.
Authors: Kaya Z, Goser S, Buss SJ, Leuschner F, Ottl R, Li J, Volkers M, Zittrich S, Pfitzer G, Rose NR, Katus HA
Circulation, 2008-10-27;118(20):2063-72.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mutual modulation between interleukin-10 and interleukin-6 induced by Rhodococcus aurantiacus infection in mice.
Authors: Yimin, Kohanawa M, Ozaki M, Haga S, Fujikawa K, Zhao S, Kuge Y, Tamaki N
Microbes Infect., 2008-09-13;10(14):1450-8.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Divergent effects of hypoxia on dendritic cell functions.
Authors: Mancino A, Schioppa T, Larghi P, Pasqualini F, Nebuloni M, Chen IH, Sozzani S, Austyn JM, Mantovani A, Sica A
Blood, 2008-08-11;112(9):3723-34.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Induction of IL-33 expression and activity in central nervous system glia.
Authors: Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT
J. Leukoc. Biol., 2008-06-13;84(3):631-43.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection.
Authors: Bozza S, Zelante T, Moretti S, Bonifazi P, DeLuca A, D'Angelo C, Giovannini G, Garlanda C, Boon L, Bistoni F, Puccetti P, Mantovani A, Romani L
J. Immunol., 2008-03-15;180(6):4022-31.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent.
Authors: Doz E, Noulin N, Boichot E, Guenon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, Couillin I
J. Immunol., 2008-01-15;180(2):1169-78.
Species: Mouse
Sample Types: Tissue Homogenates
-
Gas6 promotes inflammation by enhancing interactions between endothelial cells, platelets, and leukocytes.
Authors: Tjwa M, Bellido-Martin L, Lin Y, Lutgens E, Plaisance S, Bono F, Delesque-Touchard N, Herve C, Moura R, Billiau AD, Aparicio C, Levi M, Daemen M, Dewerchin M, Lupu F, Arnout J, Herbert JM, Waer M, Garcia de Frutos P, Dahlback B, Carmeliet P, Hoylaerts MF, Moons L
Blood, 2007-12-21;111(8):4096-105.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Deletion of TLR5 results in spontaneous colitis in mice.
Authors: Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, Gewirtz AT
J. Clin. Invest., 2007-12-01;117(12):3909-21.
Species: Mouse
Sample Types: Serum
-
IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice.
Authors: Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF, Lagente V, Ryffel B, Couillin I
J. Clin. Invest., 2007-12-01;117(12):3786-99.
Species: Mouse
Sample Types: BALF
-
Involvement of vitronectin in lipopolysaccaride-induced acute lung injury.
Authors: Tsuruta Y, Park YJ, Siegal GP, Liu G, Abraham E
J. Immunol., 2007-11-15;179(10):7079-86.
Species: Mouse
Sample Types: Tissue Homogenates
-
CD40L disruption enhances Abeta vaccine-mediated reduction of cerebral amyloidosis while minimizing cerebral amyloid angiopathy and inflammation.
Authors: Obregon D, Hou H, Bai Y, Nikolic WV, Mori T, Luo D, Zeng J, Ehrhart J, Fernandez F, Morgan D, Giunta B, Town T, Tan J
Neurobiol. Dis., 2007-10-16;29(2):336-53.
Species: Mouse
Sample Types: Plasma
-
Differential ex vivo nitric oxide production by acutely isolated neonatal and adult microglia.
Authors: Schell JB, Crane CA, Smith MF, Roberts MR
J. Neuroimmunol., 2007-08-15;189(1):75-87.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis.
Authors: Hwang SA, Wilk KM, Budnicka M, Olsen M, Bangale YA, Hunter RL, Kruzel ML, Actor JK
Vaccine, 2007-07-27;25(37):6730-43.
Species: Mouse
Sample Types: Cell Culture Supernates
-
ABCC transporter inhibition reduces zymosan-induced peritonitis.
Authors: Leite DF, Echevarria-Lima J, Ferreira SC, Calixto JB, Rumjanek VM
J. Leukoc. Biol., 2007-06-18;82(3):630-7.
Species: Mouse
Sample Types: Tissue Secretion
-
Neurokinin-1 receptor antagonist treatment protects mice against lung injury in polymicrobial sepsis.
Authors: Hegde A, Zhang H, Moochhala SM, Bhatia M
J. Leukoc. Biol., 2007-06-12;82(3):678-85.
Species: Mouse
Sample Types: Plasma
-
Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity.
Authors: Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, Collins C, Viala J, Ferrero RL, Girardin SE, Philpott DJ
Immunity, 2007-04-12;26(4):445-59.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Glucose-6-phosphate dehydrogenase deficiency and the inflammatory response to endotoxin and polymicrobial sepsis.
Authors: Wilmanski J, Villanueva E, Deitch EA, Spolarics Z
Crit. Care Med., 2007-02-01;35(2):510-8.
Species: Mouse
Sample Types: Plasma
-
TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells.
Authors: Higgins SC, Jarnicki AG, Lavelle EC, Mills KH
J. Immunol., 2006-12-01;177(11):7980-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals.
Authors: Scott P, Ma H, Viriyakosol S, Terkeltaub R, Liu-Bryan R
J. Immunol., 2006-11-01;177(9):6370-8.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Anti-tumor necrosis factor alpha F(ab')2 antibody fragments protect in murine polymicrobial sepsis: concentration and early intervention are fundamental to the outcome.
Authors: Marquez-Velasco R, Bojalil R, Buelna A, Flores-Guzman F, Estevez-Ramirez J, Laguna J, Hernandez AM, Diaz-Quinonez A, Paniagua-Solis JF
Inflamm. Res., 2006-09-01;55(9):378-84.
Species: Mouse
Sample Types: Serum
-
Choosing the frequency of deep inflation in mice: balancing recruitment against ventilator-induced lung injury.
Authors: Allen GB, Suratt BT, Rinaldi L, Petty JM, Bates JH
Am. J. Physiol. Lung Cell Mol. Physiol., 2006-05-12;291(4):L710-7.
Species: Mouse
Sample Types: BALF, Tissue Homogenates
-
The effects of dehydroepiandrosterone (DHEA) on in vitro spleen cell proliferation and cytokine production.
Authors: Powell JM, Sonnenfeld G
J. Interferon Cytokine Res., 2006-01-01;26(1):34-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
An essential role for non-bone marrow-derived cells in control of Pseudomonas aeruginosa pneumonia.
Authors: Hajjar AM, Harowicz H, Liggitt HD, Fink PJ, Wilson CB, Skerrett SJ
Am. J. Respir. Cell Mol. Biol., 2005-08-11;33(5):470-5.
Species: Mouse
Sample Types: Tissue Homogenates
-
Anti-inflammatory activity in the aqueous crude extract of the leaves of Nidularium procerum: A bromeliaceae from the Brazilian coastal rain forest.
Authors: Amendoeira FC, Frutuoso VS, Zanon C, Chedier LM, Figueiredo MR, Kaplan MA, Bandeira-Melo C, Bozza PT, Castro-Faria-Neto HC
Biol. Pharm. Bull., 2005-06-01;28(6):1010-5.
Species: Mouse
Sample Types: Pleural Fluid
-
Attenuation of primary nonfunction for syngeneic islet graft using sodium 4-phenylbutyrate.
Authors: Fu SH, Chen ST, Hsu BR
Transplant. Proc., 2005-05-01;37(4):1830-1.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Active hexose correlated compound enhances the immune function of mice in the hindlimb-unloading model of spaceflight conditions.
Authors: Aviles H, Belay T, Vance M, Sun B, Sonnenfeld G
J. Appl. Physiol., 2004-06-11;97(4):1437-44.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus.
Authors: Esen N, Tanga FY, DeLeo JA, Kielian T
J. Neurochem., 2004-02-01;88(3):746-58.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Membrane-bound Interleukin-1 alpha mediates leukocyte adhesion during atherogenesis
Species: Mouse
Sample Types: Serum
FAQs
-
Does the Mouse IL-1 beta/IL-1F2 DuoSet ELISA (Catalog DY401) crosss-react with the pro-form of Mouse IL-1 beta?
The Mouse IL-1 beta/IL-1F2 DuoSet ELISA is designed to detect mature mouse IL-1 beta and the assay is calibrated against the mature form of mouse IL-1 beta. It is possible that the kit also detects the pro-form, however, R&D Systems has not performed this characterization.
Reviews for Mouse IL-1 beta/IL-1F2 DuoSet ELISA
Average Rating: 4.7 (Based on 36 Reviews)
Have you used Mouse IL-1 beta/IL-1F2 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Optimisation of cytokine response and cell numbers
Except for the highest standard all other standards worked well
we have used this kit for LPS stimulated in vivo and in vitro experiments. It works very well and efficiently.
Mouse IL-1b ELISA kit was used to assess IL-1b levels in serum after 12-weeks treatment of adult C57BL7/6J mice with a high-fat diet treatment. Results were accurate and showed low intra-experimental variation (less than 6 %). The picture shows the standard diet (SD), high-fat diet (HFD), and two concentrations of corn pericarp extract (200 and 500 parts per million of extract).
We needed to calibrate the dilutions of our samples.
lungs were homogenized in PBS-T, and sup was filtered through 0.22um filter before use. Homogenate was diluted 1:3 for analysis by ELISA.
50ul of murine IL-1beta Capture antibody (4ug/mL) was coated per well of a 96 well plate at 4 degrees overnight. Purified IL-1beta standard was diluted (7 fold) and assayed to ensure the quality of the ELISA. Mouse IL-1beta Detector antibody was added (2h @ RT) followed by a 20 min incubation with Streptavidin-HRP. Substrate solution was added to measure IL-1beta concentration according to the manufactures protocol. The standard curve looked great as can be seen by calculation of the R- squared value. Highly recommend doing ELISA's using kits from R&D.
Buffer: 7-point standard curve