Mouse IL-12 p70 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant mouse IL-12 p70. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
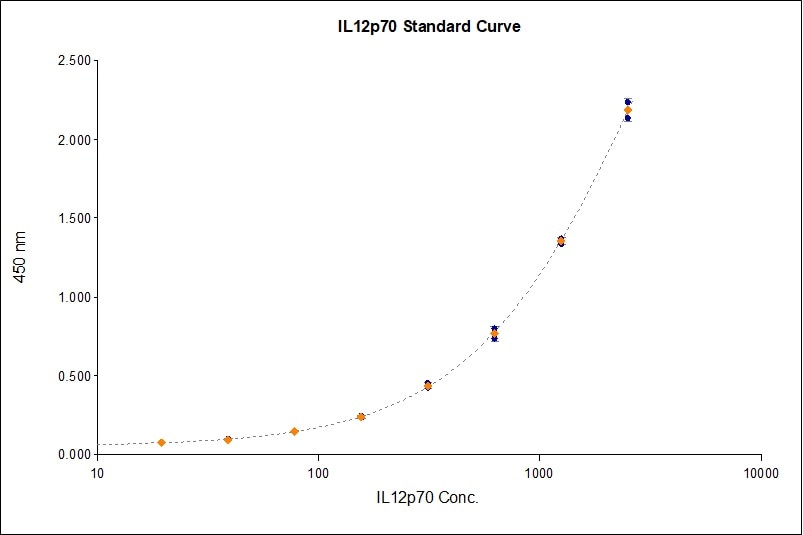
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-12 p70
Interleukin 12 (IL-12), also known as natural killer cell stimulatory factor (NKSF) or cytotoxic lymphocyte maturation factor (CLMF), is a heterodimeric pleiotropic cytokine made up of a 40 kDa (p40) subunit and a 35 kDa (p35) subunit. The IL-12 p40 subunit is shared by IL-23, another heterodimeric cytokine that has biological activities similar to, as well as distinct from, IL-12. IL-12 is produced by macrophages and B cells and has been shown to have multiple effects on T cells and natural killer (NK) cells. While mouse IL-12 is active on both human and mouse cells, human IL-12 is not active on mouse cells.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse IL-12 p70 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
87
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Antioxidant and anti-inflammatory activity of Euterpe oleracea Mart. (Açaí) seed bioproducts
Authors: Previtalli-Silva, H;Hardoim, DJ;Banaggia, RL;Moragas-Tellis, CJ;Chagas, MDSDS;Behrens, MD;Dias-Silva, TS;Calabrese, KDS;Cardoso, FO;
Heliyon
Species: Mouse
Sample Types: Serum
-
Sphinganine recruits TLR4 adaptors in macrophages and promotes inflammation in murine models of sepsis and melanoma
Authors: Hering, M;Madi, A;Sandhoff, R;Ma, S;Wu, J;Mieg, A;Richter, K;Mohr, K;Knabe, N;Stichling, D;Poschet, G;Bestvater, F;Frank, L;Utikal, J;Umansky, V;Cui, G;
Nature communications
Species: Mouse
Sample Types: Cell Culture Supernates, Plasma
-
The Mucus-Binding Factor Mediates Lacticaseibacillus rhamnosus CRL1505 Adhesion but Not Immunomodulation in the Respiratory Tract
Authors: Zhou, B;Elean, M;Arce, L;Fukuyama, K;Tomotsune, K;Dentice Maidana, S;Saha, S;Namai, F;Nishiyama, K;Vizoso-Pinto, MG;Villena, J;Kitazawa, H;
Microorganisms
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immunobiotic Ligilactobacillus salivarius FFIG58 Confers Long-Term Protection against Streptococcus pneumoniae
Authors: Elean, M;Raya Tonetti, F;Fukuyama, K;Arellano-Arriagada, L;Namai, F;Suda, Y;Gobbato, N;Nishiyama, K;Villena, J;Kitazawa, H;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates, BALF
-
Protein Concentration Affects the Food Allergen ?-Conglutin Uptake and Bacteria-Induced Cytokine Production in Dendritic Cells
Authors: Heinzl, GC;Eriksen, DB;Johnsen, PR;Scarafoni, A;Frøkiær, H;
Biomolecules
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of the IL-33/ST2 Activation Pathway in the Development of the Hepatic Fibrosis Induced by Schistosoma mansoni Granulomas in Mice
Authors: Maggi, L;Camelo, GMA;Rocha, IC;Pereira Alves, W;Moreira, JMP;Almeida Pereira, T;Tafuri, WL;Rabelo, ÉML;Correa, A;Ecco, R;Negrão-Corrêa, DA;
International journal of molecular sciences
Species: Mouse
Sample Types: Serum
-
Metabololipidomic and proteomic profiling reveals aberrant macrophage activation and interrelated immunomodulatory mediator release during aging
Authors: P Schädel, A Czapka, N Gebert, ID Jacobsen, A Ori, O Werz
Aging Cell, 2023-04-26;0(0):e13856.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Investigation of the Effects of Monomeric and Dimeric Stilbenoids on Bacteria-Induced Cytokines and LPS-Induced ROS Formation in Bone Marrow-Derived Dendritic Cells
Authors: PR Johnsen, C Pinna, L Mattio, MB Strube, M Di Nunzio, S Iametti, S Dallavalle, A Pinto, H Frøkiær
International Journal of Molecular Sciences, 2023-02-01;24(3):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TRPC absence induces pro-inflammatory macrophages and gut microbe disorder, sensitizing mice to colitis
Authors: Y Lin, X Cui, Q Cao, R Bi, Y Liu, D Jing, C Yue, Q Zhao, Y Wang, S Liu, Y Su, K Formoso, S Susperregu, L Birnbaumer, M Freichel, Y Yang, L You, X Gao
International immunopharmacology, 2022-12-31;115(0):109655.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Modulation of Alveolar Macrophages by Postimmunobiotics: Impact on TLR3-Mediated Antiviral Respiratory Immunity
Authors: M Tomokiyo, FR Tonetti, H Yamamuro, R Shibata, K Fukuyama, N Gobbato, L Albarracin, MSR Rajoka, AKMH Kober, W Ikeda-Ohts, J Villena, H Kitazawa
Cells, 2022-09-25;11(19):.
Species: Mouse
Sample Types: BALF
-
Tubule-mitophagic secretion of SerpinG1 reprograms macrophages to instruct anti-septic acute kidney injury efficacy of high-dose ascorbate mediated by NRF2 transactivation
Authors: Y Ni, GH Wu, JJ Cai, R Zhang, Y Zheng, JQ Liu, XH Yang, X Yang, Y Shen, JM Lai, XM Ye, SJ Mo
International journal of biological sciences, 2022-08-08;18(13):5168-5184.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lithocholic acid inhibits dendritic cell activation by reducing intracellular glutathione via TGR5 signaling
Authors: J Hu, Y Zhang, S Yi, C Wang, X Huang, S Pan, J Yang, G Yuan, S Tan, H Li
International journal of biological sciences, 2022-07-11;18(11):4545-4559.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The phosphoinositide-3-kinase (PI3K)-delta inhibitor seletalisib impairs monocyte-derived dendritic cells maturation, APC function, and promotes their migration to CCR7 and CXCR4 ligands
Authors: F Scopelliti, L Mercurio, C Cattani, V Dimartino, C Albanesi, G Costanzo, C Mirisola, S Madonna, A Cavani
Journal of leukocyte biology, 2022-02-24;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-2/JES6-1 mAb complexes dramatically increase sensitivity to LPS through IFN-gamma production by CD25+Foxp3- T cells
Authors: J Tomala, P Weberova, B Tomalova, Z Jiraskova, L Sivak, J Kovarova, M Kovar
Elife, 2021-12-21;10(0):.
Species: Mouse
Sample Types: Serum
-
Endangered Lymphocytes: The Effects of Alloxan and Streptozotocin on Immune Cells in Type 1 Induced Diabetes
Authors: LAD Queiroz, JB Assis, JPT Guimarães, ESA Sousa, AC Milhomem, KKS Sunahara, A Sá-Nunes, JO Martins
Mediators of Inflammation, 2021-10-19;2021(0):9940009.
Species: Mouse
Sample Types: Tissue Homogenates
-
Enhancing adoptive CD8 T cell therapy by systemic delivery of tumor associated antigens
Authors: DE Jæhger, ML Hübbe, MK Kræmer, G Clergeaud, AV Olsen, C Stavnsbjer, MN Wiinholt, A Kjær, JR Henriksen, AE Hansen, TL Andresen
Scientific Reports, 2021-10-05;11(1):19794.
Species: Mouse
Sample Types: Serum
-
Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling
Authors: J Hu, C Wang, X Huang, S Yi, S Pan, Y Zhang, G Yuan, Q Cao, X Ye, H Li
Cell Reports, 2021-09-21;36(12):109726.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Water-soluble polysaccharides from Pleurotus eryngii fruiting bodies, their activity and affinity for Toll-like receptor 2 and dectin-1
Authors: CF Ellefsen, CW Wold, AL Wilkins, F Rise, ABC Samuelsen
Carbohydrate polymers, 2021-03-26;264(0):117991.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Hepatoprotective Effect of Mixture of Dipropyl Polysulfides in Concanavalin A-Induced Hepatitis
Authors: D Arsenijevi, B Stojanovic, J Milovanovi, A Arsenijevi, M Simic, M Pergal, I Kodranov, O Cvetkovic, D Vojvodic, E Ristanovic, D Manojlovic, M Milovanovi, N Arsenijevi
Nutrients, 2021-03-22;13(3):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Protein Digests and Pure Peptides from Chia Seed Prevented Adipogenesis and Inflammation by Inhibiting PPAR&gamma and NF-&kappaB Pathways in 3T3L-1 Adipocytes
Authors: M Grancieri, HSD Martino, E Gonzalez d
Nutrients, 2021-01-08;13(1):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lactococcus lactis subsp. Cremoris C60 restores T Cell Population in Small Intestinal Lamina Propria in Aged Interleukin-18 Deficient Mice
Authors: S Saito, N Kakizaki, A Okuno, T Maekawa, NM Tsuji
Nutrients, 2020-10-27;12(11):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immunomodulatory Effect of a Salvia plebeia R. Aqueous Extract in Forced Swimming Exercise-induced Mice
Authors: J Shin, OK Kim, S Kim, D Bae, J Lee, J Park, W Jun
Nutrients, 2020-07-28;12(8):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Role of Alveolar Macrophages in the Improved Protection against Respiratory Syncytial Virus and Pneumococcal Superinfection Induced by the Peptidoglycan of Lactobacillus rhamnosus CRL1505
Authors: P Clua, M Tomokiyo, F Raya Tonet, MA Islam, V García Cas, G Marcial, S Salva, S Alvarez, H Takahashi, S Kurata, H Kitazawa, J Villena
Cells, 2020-07-09;9(7):.
Species: Mouse
Sample Types: BALF
-
Gut Microbiota Modulate CD8�T Cell Responses to Influence Colitis-Associated Tumorigenesis
Authors: AI Yu, L Zhao, KA Eaton, S Ho, J Chen, S Poe, J Becker, A Gonzalez, D McKinstry, M Hasso, J Mendoza-Ca, J Whitfield, C Koumpouras, PD Schloss, EC Martens, GY Chen
Cell Rep, 2020-04-07;31(1):107471.
Species: Mouse
Sample Types: Stool Homogenate
-
Nucleic Acid-Sensing Toll-Like Receptors Play a Dominant Role in Innate Immune Recognition of Pneumococci
Authors: A Famà, A Midiri, G Mancuso, C Biondo, G Lentini, R Galbo, MM Giardina, GV De Gaetano, L Romeo, G Teti, C Beninati
MBio, 2020-03-24;11(2):.
Species: Mouse
Sample Types: Tissue Lysates
-
Genetically Modified Mouse Mesenchymal Stem Cells Expressing Non-Structural Proteins of Hepatitis C Virus Induce Effective Immune Response
Authors: OV Masalova, EI Lesnova, RR Klimova, ED Momotyuk, VV Kozlov, AM Ivanova, OV Payushina, NN Butorina, NF Zakirova, AN Narovlyans, AV Pronin, AV Ivanov, AA Kushch
Vaccines (Basel), 2020-02-02;8(1):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Enzyme Treatment Alters the Anti-Inflammatory Activity of the Water Extract of Wheat Germ In Vitro and In Vivo
Authors: Y Song, HY Jeong, JK Lee, YS Choi, DO Kim, D Jang, CS Park, S Maeng, H Kang
Nutrients, 2019-10-16;11(10):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Syk-dependent glycolytic reprogramming in dendritic cells regulates IL-1 β production to β-glucan ligands in a TLR-independent manner
Authors: PM Thwe, DI Fritz, JP Snyder, PR Smith, KD Curtis, A O'Donnell, NA Galasso, LA Sepaniac, BJ Adamik, LR Hoyt, PD Rodriguez, TC Hogan, AF Schmidt, ME Poynter, E Amiel
J. Leukoc. Biol., 2019-09-11;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Discovery of a Novel Microtubule Targeting Agent as an Adjuvant for Cancer Immunotherapy
Authors: F Sato-Kanek, X Wang, S Yao, T Hosoya, FS Lao, K Messer, M Pu, NM Shukla, HB Cottam, M Chan, DA Carson, M Corr, T Hayashi
Biomed Res Int, 2018-10-10;2018(0):8091283.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Regulation of S100A8 Stability by RNF5 in Intestinal Epithelial Cells Determines Intestinal Inflammation and Severity of Colitis
Authors: Y Fujita, A Khateb, Y Li, R Tinoco, T Zhang, H Bar-Yoseph, MA Tam, Y Chowers, E Sabo, S Gerassy-Va, E Starosvets, B James, K Brown, SS Shen-Orr, LM Bradley, PA Tessier, ZA Ronai
Cell Rep, 2018-09-18;24(12):3296-3311.e6.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Improving therapeutic efficacy of IL-12 intratumoral gene electrotransfer through novel plasmid design and modified parameters
Authors: C Burkart, A Mukhopadhy, SA Shirley, RJ Connolly, JH Wright, A Bahrami, JS Campbell, RH Pierce, DA Canton
Gene Ther., 2018-03-09;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Activation of macrophage mediated host defense againstSalmonella typhimuriumbyMorus albaL
Authors: B Chang, B Koo, H Lee, JS Oh, S Kim
Food Nutr Res, 2018-02-21;62(0):.
Species: Mouse
Sample Types: Serum
-
Natural killer cell-intrinsic type I IFN signaling controls Klebsiella pneumoniae growth during lung infection
Authors: M Ivin, A Dumigan, FN de Vasconc, F Ebner, M Borroni, A Kavirayani, KN Przybyszew, RJ Ingram, S Lienenklau, U Kalinke, D Stoiber, JA Bengoechea, P Kovarik
PLoS Pathog., 2017-11-07;13(11):e1006696.
Species: Mouse
Sample Types: Tissue Homogenates
-
Erythroid Suppressor Cells Compromise Neonatal Immune Response against Bordetella pertussis
Authors: G Dunsmore, N Bozorgmehr, C Delyea, P Koleva, A Namdar, S Elahi
J. Immunol., 2017-08-04;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
TLR7 and TLR3 Sense Brucella abortus RNA to Induce Proinflammatory Cytokine Production but They Are Dispensable for Host Control of Infection
Authors: PC Campos, MT Gomes, ES Guimarães, G Guimarães, SC Oliveira
Front Immunol, 2017-01-23;8(0):28.
Species: Mouse
Sample Types: Cell Culture Supernates
-
N-Dihydrogalactochitosan as a potent immune activator for dendritic cells
Authors: Ahmed El-Hussein
J Biomed Mater Res A, 2017-01-10;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
NLRP12 negatively regulates proinflammatory cytokine production and host defense against Brucella abortus
Eur. J. Immunol., 2016-12-05;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Polarization of macrophages towards M2 phenotype is favored by reduction in iPLA2?
J Biol Chem, 2016-09-20;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Proinflammatory M1 macrophages inhibit RANKL-induced osteoclastogenesis
Infect Immun, 2016-09-19;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Poly-N-acetyl glucosamine production by Staphylococcus epidermidis cells increases their in vivo pro-inflammatory effect
Infect Immun, 2016-09-19;0(0):.
Species: Mouse
Sample Types: Tissue Secretion
-
Eriobotrya japonica Water Extract Characterization: An Inducer of Interferon-Gamma Production Mainly by the JAK-STAT Pathway
Authors: Khalid Z Matalka
Molecules, 2016-06-02;21(6):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Memory Th1 Cells Are Protective in Invasive Staphylococcus aureus Infection.
Authors: Brown A, Murphy A, Lalor S, Leech J, O'Keeffe K, Mac Aogain M, O'Halloran D, Lacey K, Tavakol M, Hearnden C, Fitzgerald-Hughes D, Humphreys H, Fennell J, van Wamel W, Foster T, Geoghegan J, Lavelle E, Rogers T, McLoughlin R
PLoS Pathog, 2015-11-05;11(11):e1005226.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-10 Production in Macrophages Is Regulated by a TLR-Driven CREB-Mediated Mechanism That Is Linked to Genes Involved in Cell Metabolism.
Authors: Sanin D, Prendergast C, Mountford A
J Immunol, 2015-06-26;195(3):1218-32.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Simultaneous and dose dependent melanoma cytotoxic and immune stimulatory activity of betulin.
Authors: Pfarr K, Danciu C, Arlt O, Neske C, Dehelean C, Pfeilschifter J, Radeke H
PLoS ONE, 2015-03-10;10(3):e0118802.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Consistent inhibition of cyclooxygenase drives macrophages towards the inflammatory phenotype.
Authors: Na Y, Yoon Y, Son D, Jung D, Gu G, Seok S
PLoS ONE, 2015-02-13;10(2):e0118203.
Species: Mouse
Sample Types: Cell Culture Supernates
-
MicroRNA-29b modulates innate and antigen-specific immune responses in mouse models of autoimmunity.
Authors: Salama A, Fichou N, Allard M, Dubreil L, De Beaurepaire L, Viel A, Jegou D, Bosch S, Bach J
PLoS ONE, 2014-09-09;9(9):e106153.
Species: Mouse
Sample Types: Cell Culture Supernates
-
No Impairment in host defense against Streptococcus pneumoniae in obese CPEfat/fat mice.
Authors: Mancuso P, O Brien E, Prano J, Goel D, Aronoff D
PLoS ONE, 2014-09-09;9(9):e106420.
Species: Mouse
Sample Types: BALF
-
Lifetime-dependent effects of bisphenol A on asthma development in an experimental mouse model.
Authors: Petzold, Susanne, Averbeck, Marco, Simon, Jan C, Lehmann, Irina, Polte, Tobias
PLoS ONE, 2014-06-20;9(6):e100468.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The glycosylated Rv1860 protein of mycobacterium tuberculosis inhibits dendritic cell mediated TH1 and TH17 polarization of T cells and abrogates protective immunity conferred by BCG.
Authors: Satchidanandam V, Kumar N, Jumani R, Challu V, Elangovan S, Khan N
PLoS Pathog, 2014-06-12;10(6):e1004176.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of the aryl hydrocarbon receptor in the immune response profile and development of pathology during Plasmodium berghei Anka infection.
Authors: Brant F, Miranda A, Esper L, Rodrigues D, Kangussu L, Bonaventura D, Soriani F, Pinho V, Souza D, Rachid M, Weiss L, Tanowitz H, Teixeira M, Teixeira A, Machado F
Infect Immun, 2014-05-12;82(8):3127-40.
Species: Mouse
Sample Types: Tissue Homogenates
-
Assessing the role of STAT3 in DC differentiation and autologous DC immunotherapy in mouse models of GBM.
Authors: Assi H, Espinosa J, Suprise S, Sofroniew M, Doherty R, Zamler D, Lowenstein P, Castro M
PLoS ONE, 2014-05-07;9(5):e96318.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dichloroacetate modulates cytokines toward T helper 1 function via induction of the interleukin-12-interferon-gamma pathway.
Authors: Badr M, Qinna N, Qadan F, Matalka K
Onco Targets Ther, 2014-02-07;7(0):193-201.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Endogenous oils derived from human adipocytes are potent adjuvants that promote IL-1?-dependent inflammation.
Authors: Tynan, Graham A, Hearnden, Claire H, Oleszycka, Ewa, Lyons, Claire L, Coutts, Graham, O'Connell, Jean, Corrigan, Michelle, Lynch, Lydia, Campbell, Matthew, Callanan, John J, Mok, Kenneth, Geoghegan, Justin, O'Farrelly, Cliona, Allan, Stuart M, Roche, Helen M, O'Shea, Donal B, Lavelle, Ed C
Diabetes, 2014-01-23;63(6):2037-50.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Intraperitoneal prophylaxis with CpG oligodeoxynucleotides protects neutropenic mice against intracerebral Escherichia coli K1 infection.
Authors: Ribes S, Meister T, Ott M, Redlich S, Janova H, Hanisch U, Nessler S, Nau R
J Neuroinflammation, 2014-01-23;11(0):14.
Species: Mouse
Sample Types: Tissue Homogenates
-
Transcriptional analysis of murine macrophages infected with different Toxoplasma strains identifies novel regulation of host signaling pathways.
Authors: Melo M, Nguyen Q, Cordeiro C, Hassan M, Yang N, McKell R, Rosowski E, Julien L, Butty V, Darde M, Ajzenberg D, Fitzgerald K, Young L, Saeij J
PLoS Pathog, 2013-12-19;9(12):e1003779.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Effects of Lactobacillus kefiranofaciens M1 isolated from kefir grains on germ-free mice.
Authors: Chen Y, Chen M
PLoS ONE, 2013-11-11;8(11):e78789.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Nod1 and Nod2 enhance TLR-mediated invariant NKT cell activation during bacterial infection.
Authors: Selvanantham T, Escalante N, Cruz Tleugabulova M, Fieve S, Girardin S, Philpott D, Mallevaey T
J Immunol, 2013-10-25;191(11):5646-54.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages.
Authors: Koscso B, Csoka B, Kokai E, Nemeth Z, Pacher P, Virag L, Leibovich S, Hasko G
J Leukoc Biol, 2013-08-06;94(6):1309-15.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Toll-like receptor 6 plays an important role in host innate resistance to Brucella abortus infection in mice.
Authors: de Almeida L, Macedo G, Marinho F, Gomes M, Corsetti P, Silva A, Cassataro J, Giambartolomei G, Oliveira S
Infect Immun, 2013-03-04;81(5):1654-62.
Species: Mouse
Sample Types: Cell Culture Supernates
-
UNC93B1 and nucleic acid-sensing Toll-like receptors mediate host resistance to infection with Leishmania major.
Authors: Schamber-Reis B, Petritus P, Caetano B, Martinez E, Okuda K, Golenbock D, Scott P, Gazzinelli R
J Biol Chem, 2013-01-16;288(10):7127-36.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of OGR1 in myeloid-derived cells in prostate cancer.
Authors: Yan, L, Singh, L S, Zhang, L, Xu, Y
Oncogene, 2012-12-10;33(2):157-64.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response.
Authors: Oh J, Lee R, Yu J, Ko J, Lee H, Ko A, Roddy G, Prockop D
Mol Ther, 2012-08-28;20(11):2143-52.
Species: Mouse
Sample Types: Tissue Homogenates
-
OX40 ligand and programmed cell death 1 ligand 2 expression on inflammatory dendritic cells regulates CD4 T cell cytokine production in the lung during viral disease.
Authors: Wythe SE, Dodd JS, Openshaw PJ, Schwarze J
J. Immunol., 2012-01-20;188(4):1647-55.
Species: Mouse
Sample Types: BALF
-
Intestinal epithelial cell-derived semaphorin 7A negatively regulates development of colitis via alphavbeta1 integrin.
Authors: Kang S, Okuno T, Takegahara N, Takamatsu H, Nojima S, Kimura T, Yoshida Y, Ito D, Ohmae S, You DJ, Toyofuku T, Jang MH, Kumanogoh A
J. Immunol., 2011-12-23;188(3):1108-16.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection.
Authors: Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, Riva F, Garlanda C
Infect. Immun., 2011-10-24;80(1):100-9.
Species: Mouse
Sample Types: Serum
-
A critical role for C5L2 in the pathogenesis of experimental allergic asthma.
Authors: Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, Konig P, Gerard NP, Gerard C, Wills-Karp M, Kohl J
J. Immunol., 2010-10-25;185(11):6741-52.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity.
Authors: Sellge G, Magalhaes JG, Konradt C, Fritz JH, Salgado-Pabon W, Eberl G, Bandeira A, Di Santo JP, Sansonetti PJ, Phalipon A
J. Immunol., 2010-01-20;184(4):2076-85.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Critical role of the IFN-stimulated gene factor 3 complex in TLR-mediated IL-27p28 gene expression revealing a two-step activation process.
Authors: Molle C, Goldman M, Goriely S
J. Immunol., 2010-01-18;184(4):1784-92.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A conjugated linoleic acid-enriched beef diet attenuates lipopolysaccharide-induced inflammation in mice in part through PPARgamma-mediated suppression of toll-like receptor 4.
Authors: Reynolds CM, Draper E, Keogh B, Rahman A, Moloney AP, Mills KH, Loscher CE, Roche HM
J. Nutr., 2009-10-21;139(12):2351-7.
Species: Mouse
Sample Types: Cell Culture Supernates
-
E-prostanoid 3 receptor deletion improves pulmonary host defense and protects mice from death in severe Streptococcus pneumoniae infection.
Authors: Aronoff DM, Lewis C, Serezani CH, Eaton KA, Goel D, Phipps JC, Peters-Golden M, Mancuso P
J. Immunol., 2009-07-27;183(4):2642-9.
Species: Mouse
Sample Types: Tissue Homogenates
-
The IFN regulatory factor 7-dependent type I IFN response is not essential for early resistance against murine cytomegalovirus infection.
Authors: Steinberg C, Eisenacher K, Gross O, Reindl W, Schmitz F, Ruland J, Krug A
Eur. J. Immunol., 2009-04-01;39(4):1007-18.
Species: Mouse
Sample Types: Serum
-
CD40L-expressing CD8 T cells prime CD8alpha(+) DC for IL-12p70 production.
Authors: Wong KL, Lew FC, MacAry PA, Kemeny DM
Eur. J. Immunol., 2008-08-01;38(8):2251-62.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Direct B cell stimulation by dendritic cells in a mouse model of lupus.
Authors: Wan S, Zhou Z, Duan B, Morel L
Arthritis Rheum., 2008-06-01;8(0):1741-50.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Expression of bioactive single-chain murine IL-12 in transgenic plants.
Authors: Liu J, Dolan MC, Reidy M, Cramer CL
J. Interferon Cytokine Res., 2008-06-01;28(0):381-92.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Administration of a synthetic TLR4 agonist protects mice from pneumonic tularemia.
Authors: Lembo A, Pelletier M, Iyer R, Timko M, Dudda JC, West TE, Wilson CB, Hajjar AM, Skerrett SJ
J. Immunol., 2008-06-01;180(11):7574-81.
Species: Mouse
Sample Types: Tissue Homogenates
-
Mycobacterium tuberculosis antigens specifically modulate CCR2 and MCP-1/CCL2 on lymphoid cells from human pulmonary hilar lymph nodes.
Authors: Arias MA, Jaramillo G, Lopez YP, Mejia N, Mejia C, Pantoja AE, Shattock RJ, Garcia LF, Griffin GE
J. Immunol., 2007-12-15;179(12):8381-91.
Species: Human
Sample Types: Cell Culture Supernates
-
Lung dendritic cells elicited by Fms-like tyrosine 3-kinase ligand amplify the lung inflammatory response to lipopolysaccharide.
Authors: von Wulffen W, Steinmueller M, Herold S, Marsh LM, Bulau P, Seeger W, Welte T, Lohmeyer J, Maus UA
Am. J. Respir. Crit. Care Med., 2007-08-09;176(9):892-901.
Species: Mouse
Sample Types: BALF
-
Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis.
Authors: Hwang SA, Wilk KM, Budnicka M, Olsen M, Bangale YA, Hunter RL, Kruzel ML, Actor JK
Vaccine, 2007-07-27;25(37):6730-43.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immunotherapy for neuroblastoma using syngeneic fibroblasts transfected with IL-2 and IL-12.
Authors: Barker SE, Grosse SM, Siapati EK, Kritz A, Kinnon C, Thrasher AJ, Hart SL
Br. J. Cancer, 2007-06-26;97(2):210-7.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity.
Authors: Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, Collins C, Viala J, Ferrero RL, Girardin SE, Philpott DJ
Immunity, 2007-04-12;26(4):445-59.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of Toll-like receptor 2 in recognition of Legionella pneumophila in a murine pneumonia model.
Authors: Fuse ET, Tateda K, Kikuchi Y, Matsumoto T, Gondaira F, Azuma A, Kudoh S, Standiford TJ, Yamaguchi K
J. Med. Microbiol., 2007-03-01;56(0):305-12.
Species: Mouse
Sample Types: Tissue Homogenates
-
Glucose-6-phosphate dehydrogenase deficiency and the inflammatory response to endotoxin and polymicrobial sepsis.
Authors: Wilmanski J, Villanueva E, Deitch EA, Spolarics Z
Crit. Care Med., 2007-02-01;35(2):510-8.
Species: Mouse
Sample Types: Plasma
-
Immunosuppressive effects of CCL17 on pulmonary antifungal responses during pulmonary invasive aspergillosis.
Authors: Carpenter KJ, Hogaboam CM
Infect. Immun., 2005-11-01;73(11):7198-207.
Species: Mouse
Sample Types: Tissue Homogenates
-
Leukemia inhibitory factor is linked to regulatory transplantation tolerance.
Authors: Metcalfe SM, Watson TJ, Shurey S, Adams E, Green CJ
Transplantation, 2005-03-27;79(6):726-30.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Cytokine production in association with phagocytosis of apoptotic cells by immature dendritic cells.
Authors: Takahashi M, Kobayashi Y
Cell. Immunol., 2003-12-01;226(2):105-15.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Intracellular thiols contribute to Th2 function via a positive role in IL-4 production.
Authors: Monick MM, Samavati L, Butler NS, Mohning M, Powers LS, Yarovinsky T, Spitz DR, Hunninghake GW
J. Immunol., 2003-11-15;171(10):5107-15.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody.
Authors: O'Garra A
J. Exp. Med., 2002-08-19;196(4):541-9.
Species: Mouse
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse IL-12 p70 DuoSet ELISA
Average Rating: 4.7 (Based on 6 Reviews)
Have you used Mouse IL-12 p70 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: