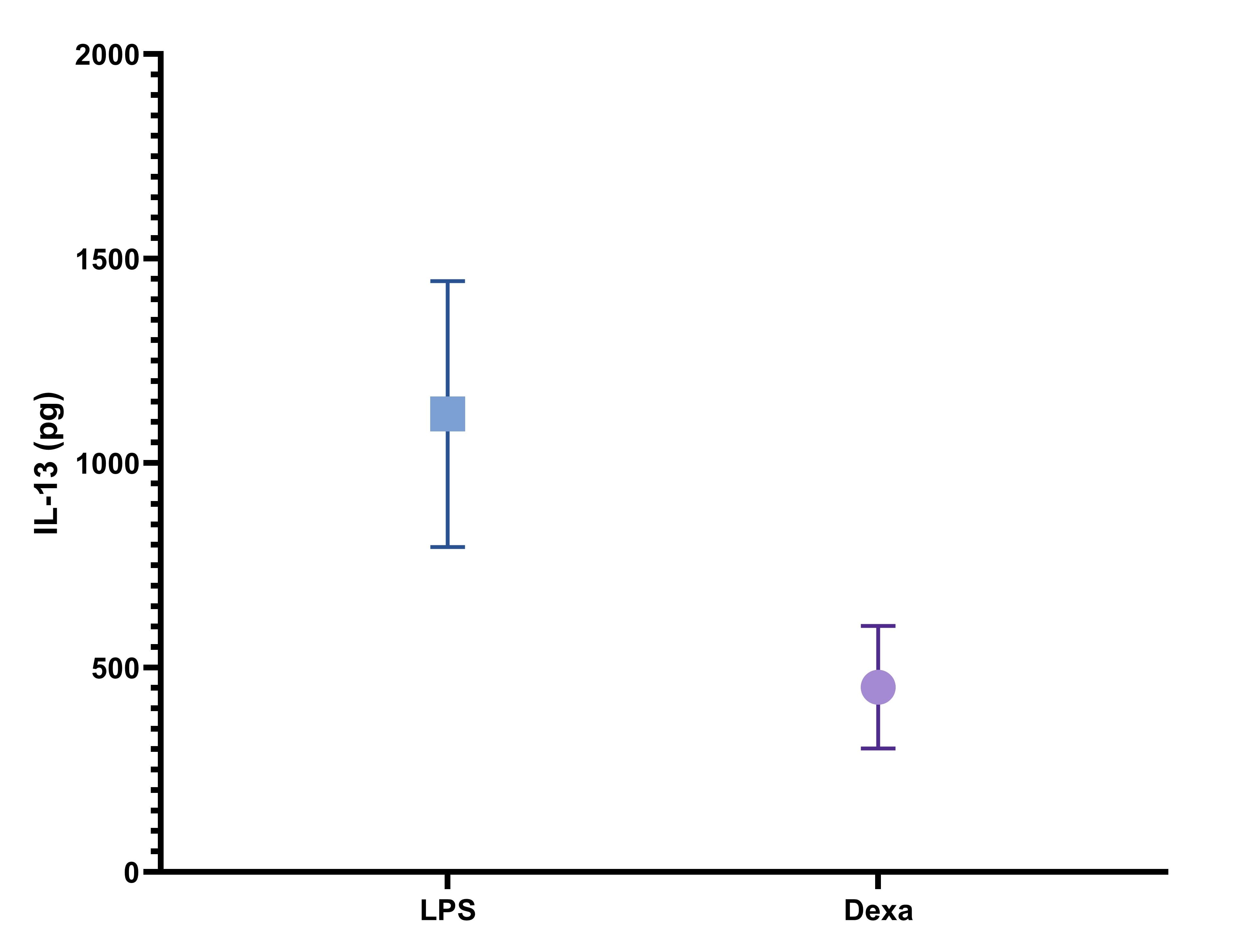
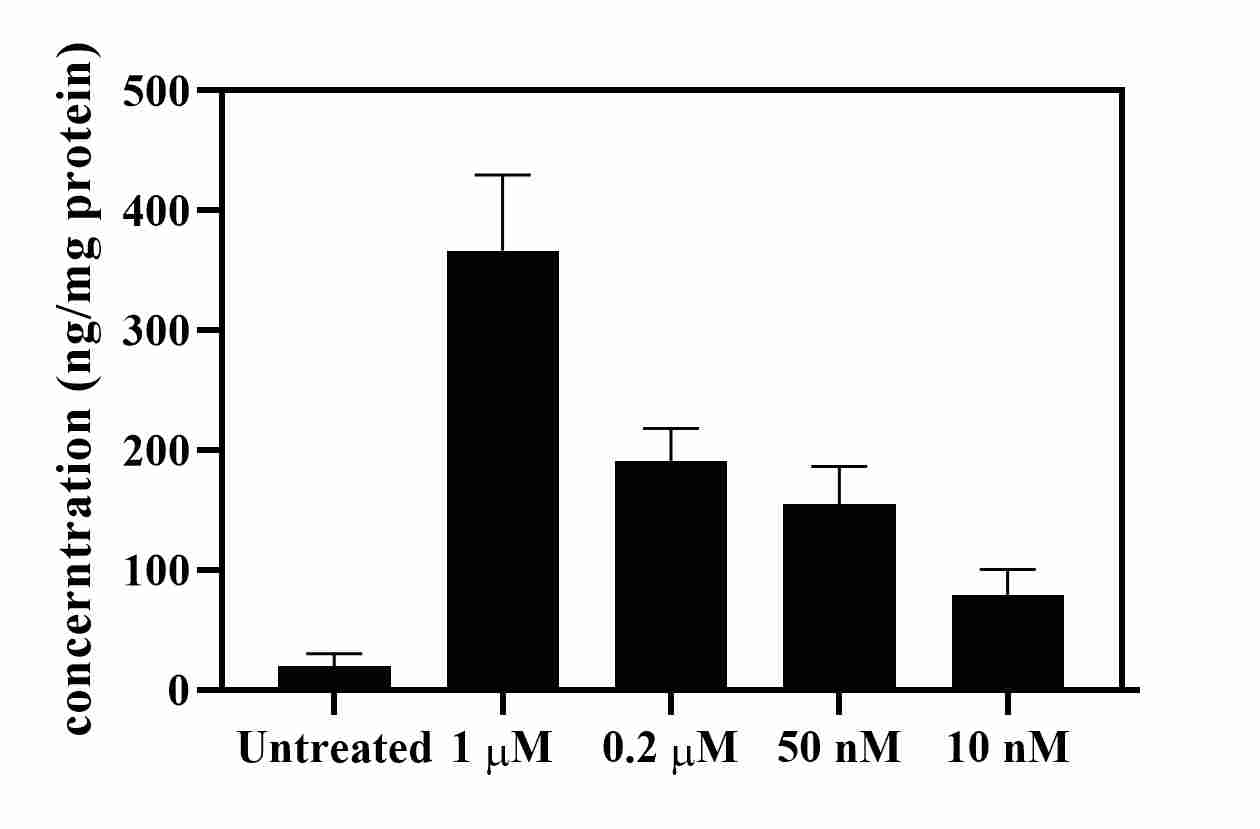
Mouse IL-13 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant mouse IL-13. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-13
IL-13 (Interleukin-13) plays a key role in the pathogenesis of allergy, cancer, and tissue fibrosis. It is secreted by several helper T cell subsets, NK cells, mast cells, eosinophils, basophils, and visceral smooth muscle cells. IL-13 suppresses the production of proinflammatory cytokines and other cytotoxic substances by macrophages, fibroblasts, and endothelial cells. It promotes B cell activation, immunoglobulin class switching to IgE, and the upregulation of CD23/Fc epsilon RII. IL-13 signals through a receptor complex containing IL-13 R alpha 1 and IL-4 R alpha. This complex also functions as the type 2 IL-4 receptor. IL-13 R alpha 2 binds IL-13 and prevents IL-13 from signaling through the IL-13 R alpha 1/IL-4 R alpha complex. IL-13-bound IL-13 R alpha 2 can directly promote tumor cell invasiveness and the development of tissue fibrosis.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse IL-13 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
152
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Eosinophil-Airway Epithelial Cells crosstalk reveals the eosinophils-mediated DUOX1 up-regulation in a murine allergic inflammation setting
Authors: Raggi, C;Spadaro, F;Mattei, F;Gambardella, AR;Noto, F;Andreone, S;Signore, M;Schiavoni, G;Parolini, I;Afferni, C;
Journal of leukocyte biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Intermittent ozone inhalation during house dust mite-induced sensitization primes for adverse asthma phenotype
Authors: Hussain, S;Majumder, N;Mazumder, MHH;Lewis, SE;Olapeju, O;Velayutham, M;Amin, MS;Brundage, K;Kelley, EE;Vanoirbeek, J;
Redox biology
Species: Mouse
Sample Types: Tissue Homogenates, BALF
-
The integrin receptor beta7 subunit mediates airway remodeling and hyperresponsiveness in allergen exposed mice
Authors: Assayag, M;Obedeyah, T;Abutbul, A;Berkman, N;
Respiratory research
Species: Mouse
Sample Types: Tissue Homogenates, BALF
-
Host gastric corpus microenvironment facilitates Ascaris suum larval hatching and infection in a murine model
Authors: Wu, Y;Adeniyi-Ipadeola, G;Adkins-Threats, M;Seasock, M;Suarez-Reyes, C;Fujiwara, R;Bottazzi, ME;Song, L;Mills, JC;Weatherhead, JE;
PLoS neglected tropical diseases
Species: Mouse
Sample Types: Tissue Homogenates
-
Microbiota encoded fatty-acid metabolism expands tuft cells to protect tissues homeostasis during Clostridioides difficile infection in the large intestine
Authors: Kellogg, TD;Ceglia, S;Mortzfeld, BM;Zeamer, AL;Foley, SE;Ward, DV;Bhattarai, SK;McCormick, BA;Reboldi, A;Bucci, V;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Tissue Homogenates
-
Cullin5 drives experimental asthma exacerbations by modulating alveolar macrophage antiviral immunity
Authors: Zhang, H;Xue, K;Li, W;Yang, X;Gou, Y;Su, X;Qian, F;Sun, L;
Nature communications
Species: Mouse, Transgenic Mouse
Sample Types: BALF
-
Interleukin-13 Mediates Non-Steroidal Anti-Inflammatory-Drug-Induced Small Intestinal Mucosal Injury with Ulceration
Authors: Kawashima, R;Tamaki, S;Hara, Y;Maekawa, T;Kawakami, F;Ichikawa, T;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Acrylamide, an Air Pollutant, Enhances Allergen-Induced Eosinophilic Lung Inflammation via Group 2 Innate Lymphoid Cells
Authors: Su, HH;Cheng, CM;Yang, YN;Chang, YW;Li, CY;Wu, ST;Lin, CC;Wu, HE;Suen, JL;
Mucosal immunology
Species: Mouse
Sample Types: BALF, Cell Culture Supernates
-
Therapeutic potential of ozone water treatment in alleviating atopic dermatitis symptoms in mouse models: Exploring its bactericidal and direct anti-inflammatory properties
Authors: Kaneki, M;Ohira, C;Takahashi, M;Iwashita, N;Takagi, Y;Nagane, M;Uchiyama, J;Fukuyama, T;
International immunopharmacology
Species: Mouse
Sample Types: Cell Culture Supernates
-
TRAF4 is crucial for ST2+ memory Th2 cell expansion in IL-33-driven airway inflammation
Authors: Xiao, J;Chen, X;Liu, W;Qian, W;Bulek, K;Hong, L;Miller-Little, W;Li, X;Liu, C;
JCI insight
Species: Transgenic Mouse
Sample Types: Cell Culture Supernates, Tissue Homogenates
-
Role of the IL-33/ST2 Activation Pathway in the Development of the Hepatic Fibrosis Induced by Schistosoma mansoni Granulomas in Mice
Authors: Maggi, L;Camelo, GMA;Rocha, IC;Pereira Alves, W;Moreira, JMP;Almeida Pereira, T;Tafuri, WL;Rabelo, ÉML;Correa, A;Ecco, R;Negrão-Corrêa, DA;
International journal of molecular sciences
Species: Mouse
Sample Types: Serum, Tissue Homogenates
-
Obesity impairs cardiolipin-dependent mitophagy and therapeutic intercellular mitochondrial transfer ability of mesenchymal stem cells
Authors: Sagar, S;Faizan, MI;Chaudhary, N;Singh, V;Singh, P;Gheware, A;Sharma, K;Azmi, I;Singh, VP;Kharya, G;Mabalirajan, U;Agrawal, A;Ahmad, T;Sinha Roy, S;
Cell death & disease
Species: Mouse
Sample Types: Tissue Homogenates
-
Kaempferol Suppresses the Activation of Mast Cells by Modulating the Expression of FcepsilonRI and SHIP1
Authors: K Nagata, S Araumi, D Ando, N Ito, M Ando, Y Ikeda, M Takahashi, S Noguchi, Y Yasuda, N Nakano, T Ando, M Hara, T Yashiro, M Hachisu, C Nishiyama
International Journal of Molecular Sciences, 2023-03-22;24(6):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Increased hepatic interleukin-1, arachidonic acid, and reactive oxygen species mediate the protective potential of peptides shared by gut cysteine peptidases against Schistosoma mansoni infection in mice
Authors: H Tallima, R El Ridi
PloS Neglected Tropical Diseases, 2023-03-15;17(3):e0011164.
Species: Mouse
Sample Types: Tissue Homogenates
-
TRAF4 is crucial for the propagation of ST2+ memory Th2 cells in IL-33-mediated type 2 airway inflammation
Authors: J Xiao, X Chen, W Liu, W Qian, K Bulek, L Hong, W Miller-Lit, X Li, C Liu
bioRxiv : the preprint server for biology, 2023-02-12;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Downregulation of deubiquitinating enzyme USP25 promotes the development of allergic rhinitis by enhancing TSLP signaling in the nasal epithelium
Authors: W Chang, H Lv, L Tan, Z Gao, P Liu, D Qin, W Zhang, Y Xu
Molecular Medicine Reports, 2022-09-30;26(5):.
Species: Mouse, Transgenic Mouse
Sample Types: Serum
-
Liver group 2 innate lymphoid cells regulate blood glucose levels through IL-13 signaling and suppression of gluconeogenesis
Authors: M Fujimoto, M Yokoyama, M Kiuchi, H Hosokawa, A Nakayama, N Hashimoto, I Sakuma, H Nagano, K Yamagata, F Kudo, I Manabe, E Lee, R Hatano, A Onodera, K Hirahara, K Yokote, T Miki, T Nakayama, T Tanaka
Nature Communications, 2022-09-15;13(1):5408.
Species: Mouse
Sample Types: Serum
-
Sirtuin 6 maintains epithelial STAT6 activity to support intestinal tuft cell development and type 2 immunity
Authors: X Xiong, C Yang, WQ He, J Yu, Y Xin, X Zhang, R Huang, H Ma, S Xu, Z Li, J Ma, L Xu, Q Wang, K Ren, XS Wu, CR Vakoc, J Zhong, G Zhong, X Zhu, Y Song, HB Ruan, Q Wang
Nature Communications, 2022-09-03;13(1):5192.
Species: Mouse
Sample Types: Tissue Homogenates
-
Inhaled delivery of recombinant interferon-lambda restores allergic inflammation after development of asthma by controlling Th2- and Th17-cell-mediated immune responses
Authors: J Won, A Jo, CH Gil, S Kim, H Shin, H Jik Kim
International immunopharmacology, 2022-08-27;112(0):109180.
Species: Mouse
Sample Types: BALF
-
Extracellular matrix of early pulmonary fibrosis modifies the polarization of alveolar macrophage
Authors: Y Zhang, L Zhu, J Hong, C Chen
International immunopharmacology, 2022-08-24;111(0):109179.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Salicylaldehyde Suppresses IgE-Mediated Activation of Mast Cells and Ameliorates Anaphylaxis in Mice
Authors: T Ashikari, M Hachisu, K Nagata, D Ando, Y Iizuka, N Ito, K Ito, Y Ikeda, H Matsubara, T Yashiro, K Kasakura, C Nishiyama
International Journal of Molecular Sciences, 2022-08-08;23(15):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Macrophage-intrinsic DUOX1 contributes to type 2 inflammation and mucus metaplasia during allergic airway disease
Authors: CR Morris, A Habibovic, CM Dustin, C Schiffers, MC Lin, JL Ather, YMW Janssen-He, ME Poynter, O Utermohlen, M Krönke, A van der Vl
Mucosal Immunology, 2022-06-02;0(0):.
Species: Mouse
Sample Types: BALF
-
Interleukin 13 promotes long-term recovery after ischemic stroke by inhibiting the activation of STAT3
Authors: D Chen, J Li, Y Huang, P Wei, W Miao, Y Yang, Y Gao
Journal of Neuroinflammation, 2022-05-16;19(1):112.
Species: Mouse
Sample Types: Tissue Homogenates
-
Lipoteichoic Acid from Lacticaseibacillus rhamnosus GG Modulates Dendritic Cells and T Cells in the Gut
Authors: AD Friedrich, J Leoni, ML Paz, DH González M
Nutrients, 2022-02-08;14(3):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Resveratrol attenuates inflammation and apoptosis through alleviating endoplasmic reticulum stress via Akt/mTOR pathway in fungus-induced allergic airways inflammation
Authors: S Wang, L Gong, Y Mo, J Zhang, Z Jiang, Z Tian, C Shao
International immunopharmacology, 2021-12-27;103(0):108489.
Species: Mouse
Sample Types: BALF
-
Transient Ascaris suum larval migration induces intractable chronic pulmonary disease and anemia in mice
Authors: Y Wu, E Li, M Knight, G Adeniyi-Ip, LZ Song, AR Burns, AC Gazzinelli, R Fujiwara, ME Bottazzi, JE Weatherhea
PloS Neglected Tropical Diseases, 2021-12-16;15(12):e0010050.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Oxidized carbon black nanoparticles induce endothelial damage through C-X-C chemokine receptor 3-mediated pathway
Authors: N Majumder, M Velayutham, D Bitounis, VK Kodali, MH Hasan Mazu, J Amedro, VV Khramtsov, A Erdely, T Nurkiewicz, P Demokritou, EE Kelley, S Hussain
Redox Biology, 2021-10-04;47(0):102161.
Species: Mouse
Sample Types: BALF
-
Ding Chuan Tang Attenuates Airway Inflammation and Eosinophil Infiltration in Ovalbumin-Sensitized Asthmatic Mice
Authors: J Ma, MX Liu, LC Chen, JJ Shen, ML Kuo
BioMed Research International, 2021-09-20;2021(0):6692772.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Fasting impairs type 2 helper T cell infiltration in the lung of an eosinophilic asthma mouse model
Authors: Y Suzuki, T Hayashi, R Yokoyama, F Nakagawa, J Inoue, T Higashi, R Onodera, K Motoyama
FEBS Open Bio, 2021-08-20;0(0):.
Species: Mouse
Sample Types: BALF
-
Neutrophils promote T-cell activation through the regulated release of CD44-bound Galectin-9 from the cell surface during HIV infection
Authors: G Dunsmore, EP Rosero, S Shahbaz, DM Santer, J Jovel, P Lacy, S Houston, S Elahi
PloS Biology, 2021-08-19;19(8):e3001387.
Species: Human
Sample Types: Plasma
-
Oxidant-induced epithelial alarmin pathway mediates lung inflammation and functional decline following ultrafine carbon and ozone inhalation co-exposure
Authors: N Majumder, WT Goldsmith, VK Kodali, M Velayutham, SA Friend, VV Khramtsov, TR Nurkiewicz, A Erdely, PC Zeidler-Er, V Castranova, JR Harkema, EE Kelley, S Hussain
Redox Biology, 2021-08-05;46(0):102092.
Species: Mouse
Sample Types: BALF
-
Crucial role of stimulator of interferon genes-dependent signaling in house dust mite extract-induced IgE production
Authors: H Nunokawa, Y Murakami, T Ishii, T Narita, H Ishii, H Takizawa, N Yamashita
Scientific Reports, 2021-06-23;11(1):13157.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The role of Sp140 revealed in IgE and mast cell responses in Collaborative Cross mice
Authors: K Matsushita, X Li, Y Nakamura, D Dong, K Mukai, M Tsai, SB Montgomery, SJ Galli
JCI Insight, 2021-06-22;6(12):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Allergen Exposure in Murine Neonates Promoted the Development of Asthmatic Lungs
Authors: JC Chen, CC Chan, NC Ting, ML Kuo
Biomedicines, 2021-06-18;9(6):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Ameliorative effects of eosinophil deficiency on immune response, endoplasmic reticulum stress, apoptosis, and autophagy in fungus-induced allergic lung inflammation
Authors: S Wang, Z Jiang, L Li, J Zhang, C Zhang, C Shao
Respiratory Research, 2021-06-07;22(1):173.
Species: Mouse
Sample Types: BALF
-
Short and dysfunctional telomeres protect from allergen-induced airway inflammation
Authors: S Piñeiro-He, P Martínez, MA Blasco
Aging Cell, 2021-05-04;20(5):e13352.
Species: Mouse
Sample Types: Tissue Homogenates
-
STAT6-Dependent Exacerbation of House Dust Mite-Induced Allergic Airway Disease in Mice by Multi-Walled Carbon Nanotubes
Authors: MD Ihrie, KS Duke, KA Shipkowski, DJ You, HY Lee, AJ Taylor-Jus, JC Bonner
NanoImpact, 2021-03-13;22(0):.
Species: Mouse
Sample Types: BALF
-
Sprouty2 positively regulates T cell function and airway inflammation through regulation of CSK and LCK kinases
Authors: A Sripada, K Sirohi, L Michalec, L Guo, JT McKay, S Yadav, M Verma, J Good, D Rollins, MM Gorska, R Alam
PloS Biology, 2021-03-08;19(3):e3001063.
Species: Mouse
Sample Types: BALF
-
Wood emissions and asthma development: Results from an experimental mouse model and a prospective cohort study
Authors: KM Junge, L Buchenauer, E Elter, K Butter, T Kohajda, G Herberth, S Röder, M Borte, W Kiess, M von Bergen, JC Simon, UE Rolle-Kamp, I Lehmann, R Gminski, M Ohlmeyer, T Polte
Environment international, 2021-02-18;151(0):106449.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin (IL)-33 is dispensable for Schistosoma mansoni worm maturation and the maintenance of egg-induced pathology in intestines of infected mice
Authors: JPK Mukendi, R Nakamura, S Uematsu, S Hamano
Parasites & vectors, 2021-01-22;14(1):70.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Specific Antibodies and Arachidonic Acid Mediate the Protection Induced by the Schistosoma mansoni Cysteine Peptidase-Based Vaccine in Mice
Authors: H Tallima, M Abou El Da, R El Ridi
Vaccines (Basel), 2020-11-16;8(4):.
Species: Mouse
Sample Types: Serum
-
Antileishmanial activity of a new chloroquine analog in an animal model of Leishmania panamensis infection
Authors: L Herrera, A Llanes, J Álvarez, K Degracia, CM Restrepo, R Rivera, DE Stephens, HT Dang, OV Larionov, R Lleonart, PL Fernández
Int J Parasitol Drugs Drug Resist, 2020-08-15;14(0):56-61.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Chinese herbal medicine SS-1 inhibits T cell activation and abrogates TH responses in Sj�gren's syndrome
Authors: GA Lee, CM Chang, YC Wu, RY Ma, CY Chen, YT Hsue, NS Liao, HH Chang
J. Formos. Med. Assoc., 2020-07-30;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
High-dose intranasal application of titanium dioxide nanoparticles induces the systemic uptakes and allergic airway inflammation in asthmatic mice
Authors: S Abdulnasse, M Hannig, DD Le, S Heck, M Leitner, AJ Omlor, I Tavernaro, A Kraegeloh, R Kautenburg, G Kickelbick, A Beilhack, M Bischoff, J Nguyen, M Sester, R Bals, QT Dinh
Respir. Res., 2020-07-02;21(1):168.
Species: Mouse
Sample Types: Serum
-
Lactobacillus reuteri attenuated allergic inflammation induced by HDM in the mouse and modulated gut microbes
Authors: L Li, Z Fang, X Liu, W Hu, W Lu, YK Lee, J Zhao, H Zhang, W Chen
PLoS ONE, 2020-04-21;15(4):e0231865.
Species: Mouse
Sample Types: BALF
-
Regnase-1 degradation is crucial for interleukin-33- and interleukin-25-mediated ILC2 activation
Authors: K Matsushita, H Tanaka, K Yasuda, T Adachi, A Fukuoka, S Akasaki, A Koida, E Kuroda, S Akira, T Yoshimoto
JCI Insight, 2020-02-27;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
BHLHE40 Promotes TH2 Cell-Mediated Antihelminth Immunity and Reveals Cooperative CSF2RB Family Cytokines
Authors: NN Jarjour, TR Bradstreet, EA Schwarzkop, ME Cook, CW Lai, SC Huang, R Taneja, TS Stappenbec, SJ Van Dyken, JF Urban, BT Edelson
J. Immunol., 2020-01-03;204(4):923-932.
Species: Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Strongyloides venezuelensis-infection alters the profile of cytokines and liver inflammation in mice co-infected with Schistosoma mansoni
Authors: MC de Rezende, JMP Moreira, LLM Fernandes, VF Rodrigues, D Negrão-Cor
Cytokine, 2019-11-26;127(0):154931.
Species: Mouse
Sample Types: Tissue Homogenates
-
SOCS2 modulates adipose tissue inflammation and expansion in mice
Authors: CH Val, MC de Oliveir, DR Lacerda, A Barroso, NV Batista, Z Menezes-Ga, DRR de Assis, AT Cramer, F Brant, MM Teixeira, D Glória Sou, AM Ferreira, FS Machado
J. Nutr. Biochem., 2019-11-23;76(0):108304.
Species: Mouse
Sample Types: Tissue Homogenates
-
Pyrrothiogatain acts as an inhibitor of GATA family proteins and inhibits Th2 cell differentiation in vitro
Authors: S Nomura, H Takahashi, J Suzuki, M Kuwahara, M Yamashita, T Sawasaki
Sci Rep, 2019-11-22;9(1):17335.
Species: Mouse
Sample Types: Cell Cultue Supernates
-
Targeted deletion of NFAT-Interacting-Protein-(NIP) 45 resolves experimental asthma by inhibiting Innate Lymphoid Cells group 2 (ILC2)
Authors: S Koch, L Knipfer, J Kölle, H Mirzakhani, A Graser, T Zimmermann, A Kiefer, VO Melichar, W Rascher, NG Papadopoul, RJ Rieker, BA Raby, ST Weiss, S Wirtz, S Finotto
Sci Rep, 2019-10-30;9(1):15695.
Species: Mouse
Sample Types: Tissue Culture Supernates
-
Non-hematopoietic STAT6 induces epithelial tight junction dysfunction and promotes intestinal inflammation and tumorigenesis
Authors: Y Lin, B Li, X Yang, T Liu, T Shi, B Deng, Y Zhang, L Jia, Z Jiang, R He
Mucosal Immunol, 2019-09-18;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Ablation of RhoA impairs Th17 cell differentiation and alleviates house dust mite-triggered allergic airway inflammation
Authors: JQ Yang, KW Kalim, Y Li, Y Zheng, F Guo
J. Leukoc. Biol., 2019-07-01;0(0):.
Species: Mouse
Sample Types: BALF
-
TET1 contributes to allergic airway inflammation and regulates interferon and aryl hydrocarbon receptor signaling pathways in bronchial epithelial cells
Authors: JD Burleson, D Siniard, VK Yadagiri, X Chen, MT Weirauch, BP Ruff, EB Brandt, GKK Hershey, H Ji
Sci Rep, 2019-05-14;9(1):7361.
Species: Mouse
Sample Types: BALF
-
Inhalation of the prodrug PI3K inhibitor CL27c improves lung function in asthma and fibrosis
Authors: CC Campa, RL Silva, JP Margaria, T Pirali, MS Mattos, LR Kraemer, DC Reis, G Grosa, F Copperi, EM Dalmarco, RCP Lima-Júnio, S Aprile, V Sala, F Dal Bello, DS Prado, JC Alves-Filh, C Medana, GD Cassali, GC Tron, MM Teixeira, E Ciraolo, RC Russo, E Hirsch
Nat Commun, 2018-12-12;9(1):5232.
Species: Mouse
Sample Types: Tissue Homogenates
-
Basophils are dispensable for the establishment of protective adaptive immunity against primary and challenge infection with the intestinal helminth parasite Strongyloides ratti
Authors: M Reitz, ML Brunn, D Voehringer, M Breloer
PLoS Negl Trop Dis, 2018-11-29;12(11):e0006992.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A20-binding inhibitor of NF-kappa B (ABIN) 2 negatively regulates allergic airway inflammation
Authors: Sonia Ventura, Florencia Cano, Yashaswini Kannan, Felix Breyer, Michael J. Pattison, Mark S. Wilson et al.
Journal of Experimental Medicine
Species: Mouse
Sample Types: Cell Lysates
-
PrtA immunization fails to protect against pulmonary and invasive infection by Streptococcus pneumoniae
Authors: CF Hsu, CH Hsiao, SF Tseng, JR Chen, YJ Liao, SJ Chen, CS Lin, HK Sytwu, YP Chuang
Respir. Res., 2018-09-25;19(1):187.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Influenza A Virus Infection Causes Chronic Lung Disease Linked to Sites of Active Viral RNA Remnants
Authors: SP Keeler, EV Agapov, ME Hinojosa, AN Letvin, K Wu, MJ Holtzman
J. Immunol., 2018-09-12;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Airway Epithelial Cell-Derived Colony Stimulating Factor-1 Promotes Allergen Sensitization
Authors: Hyung-Geun Moon, Seung-jae Kim, Jong Jin Jeong, Seon-Sook Han, Nizar N. Jarjour, Hyun Lee et al.
Immunity
Species: Mouse
Sample Types: Tissue Homogenates, BALF
-
Repeated Allergen Exposure in A/J Mice Causes Steroid-Insensitive Asthma via a Defect in Glucocorticoid Receptor Bioavailability
Authors: MF Serra, AC Cotias, CRR Pão, JB Daleprane, PB Jurgilas, GC Couto, EA Anjos-Valo, RSB Cordeiro, VF Carvalho, PMR Silva, MA Martins
J. Immunol., 2018-06-18;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Increases in helminth-induced IL-21 protein levels disappear upon sample freezing
Authors: A Ariyaratne, EK Szabo, J Bowron, CAM Finney
Cytokine, 2018-04-21;108(0):179-181.
Species: Mouse
Sample Types: Tissue Homogenates
-
IL-33/ST2 axis mediates hyperplasia of intrarenal urothelium in obstructive renal injury
Authors: WY Chen, JL Yang, YH Wu, LC Li, RF Li, YT Chang, LH Dai, WC Wang, YJ Chang
Exp. Mol. Med., 2018-04-20;50(4):36.
Species: Mouse
Sample Types: Serum
-
Probiotic treatment during neonatal age provides optimal protection against experimental asthma through the modulation of microbiota and T cells: Neonatal exposure to probiotics prevents asthma
Authors: CFCG Nunes, JS Nogueira, FB Canto, PHO Vianna, BT Ciambarell, PMRE Silva, KR Miranda, LA Lobo, RMCP Domingues, M Busch, GC Atella, AM Vale, M Bellio, A Nóbrega, R Fucs
Int. Immunol., 2018-04-03;0(0):.
Species: Mouse
Sample Types: BALF
Applications: ELISA Capture -
Analogous corticosteroids, 9A and EK100, derived from solid-state-cultured mycelium of Antrodia camphorata inhibit proinflammatory cytokine expression in macrophages
Authors: ST Kao, YH Kuo, SD Wang, HJ Hong, LJ Lin
Cytokine, 2018-03-30;108(0):136-144.
Species: Mouse
Sample Types: BALF
-
Bcl11b, a novel GATA3-interacting protein, suppresses Th1 while limiting Th2 cell differentiation
Authors: D Fang, K Cui, G Hu, RK Gurram, C Zhong, AJ Oler, R Yagi, M Zhao, S Sharma, P Liu, B Sun, K Zhao, J Zhu
J. Exp. Med., 2018-03-07;0(0):.
Species: Mouse
Sample Types: BALF
-
Respiratory Disease following Viral Lung Infection Alters the Murine Gut Microbiota
Authors: HT Groves, L Cuthbertso, P James, MF Moffatt, MJ Cox, JS Tregoning
Front Immunol, 2018-02-12;9(0):182.
Species: Mouse
Sample Types: Airway Lavage Fluid
-
Characterization of the acute inflammatory profile and resolution of airway inflammation after Igf1r-gene targeting in a murine model of HDM-induced asthma
Authors: S Piñeiro-He, E Alfaro-Arn, JA Gregory, R Torrens, C Ruíz-Martí, M Adner, IP López, JG Pichel
PLoS ONE, 2017-12-22;12(12):e0190159.
Species: Mouse
Sample Types: Tissue Homogenates
-
Experimental asthma persists in IL-33 receptor knockout mice because of the emergence of thymic stromal lymphopoietin-driven IL-9<sup>+</sup> and IL-13<sup>+</sup> type 2 innate lymphoid cell subpopulations.
Authors: Verma M, Liu S, Michalec L, Sripada A, Gorska M, Alam R
J Allergy Clin Immunol, 2017-11-10;0(0):.
Species: Mouse
Sample Types: BALF
-
TPL-2 restricts Ccl24-dependent immunity to Heligmosomoides polygyrus
Authors: Y Kannan, LJ Entwistle, VS Pelly, J Perez-Llor, AW Walker, SC Ley, MS Wilson
PLoS Pathog., 2017-07-31;13(7):e1006536.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Menin Controls the Memory Th2 Cell Function by Maintaining the Epigenetic Integrity of Th2 Cells
Authors: A Onodera, M Kiuchi, K Kokubo, M Kato, T Ogino, S Horiuchi, U Kanai, K Hirahara, T Nakayama
J. Immunol., 2017-06-28;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IGF1R deficiency attenuates acute inflammatory response in a bleomycin-induced lung injury mouse model
Authors: S Piñeiro-He, IP López, E Alfaro-Arn, R Torrens, M Iñiguez, L Alvarez-Er, C Ruíz-Martí, JG Pichel
Sci Rep, 2017-06-27;7(1):4290.
Species: Mouse
Sample Types: Tissue Homogenates
-
Schistosoma japonicum infection downregulates house dust mite-induced allergic airway inflammation in mice
Authors: S Qiu, X Fan, Y Yang, P Dong, W Zhou, Y Xu, Y Zhou, F Guo, Y Zheng, JQ Yang
PLoS ONE, 2017-06-14;12(6):e0179565.
Species: Mouse
Sample Types: BALF
-
IRF5 distinguishes severe asthma in humans and drives Th1 phenotype and airway hyperreactivity in mice
Authors: TB Oriss, M Raundhal, C Morse, RE Huff, S Das, R Hannum, MC Gauthier, KL Scholl, K Chakrabort, SM Nouraie, SE Wenzel, P Ray, A Ray
JCI Insight, 2017-05-18;2(10):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Somatic extracts of Marshallagia marshalli downregulate the Th2 associated immune responses in ovalbumin-induced airway inflammation in BALB/c mice
Authors: S Parande Sh, A Ebrahimby, A Dousty, M Maleki, A Movassaghi, H Borji, A Haghparast
Parasit Vectors, 2017-05-12;10(1):233.
Species: Mouse
Sample Types: BALF
-
Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation
Authors: M Toussaint, DJ Jackson, D Swieboda, A Guedán, TD Tsouroukts, YM Ching, C Radermecke, H Makrinioti, J Aniscenko, MR Edwards, R Solari, F Farnir, V Papayannop, F Bureau, T Marichal, SL Johnston
Nat. Med., 2017-05-01;23(6):681-691.
Species: Mouse
Sample Types: Tissue Homogenates
-
Water-soluble chitosan inhibits nerve growth factor and attenuates allergic inflammation in mite allergen-induced allergic rhinitis
Authors: PC Chen, MH Hsieh, P Wen-ShuoKu, HF Kao, CL Hsu, JY Wang
J. Allergy Clin. Immunol., 2017-04-12;0(0):.
Species: Mouse
Sample Types: NLF
-
Experimental atopic dermatitis depends on IL-33R signaling via MyD88 in dendritic cells
Authors: C Li, I Maillet, C Mackowiak, C Viala, F Di Padova, M Li, D Togbe, V Quesniaux, Y Lai, B Ryffel
Cell Death Dis, 2017-04-06;8(4):e2735.
Species: Mouse
Sample Types: Tissue Homogenates
-
Protective immune responses against Schistosoma mansoni infection by immunization with functionally active gut-derived cysteine peptidases alone and in combination with glyceraldehyde 3-phosphate dehydrogenase
Authors: H Tallima, J Dvo?ák, S Kareem, M Abou El Da, N Abdel Aziz, JP Dalton, R El Ridi
PLoS Negl Trop Dis, 2017-03-27;11(3):e0005443.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Attenuated airway hyperresponsiveness and mucus secretion in HDM exposed Igf1r-deficient mice
Authors: S Piñeiro-He, JA Gregory, IP López, R Torrens, C Ruíz-Martí, M Adner, JG Pichel
Allergy, 2017-03-20;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Mitochondrial CaMKII inhibition in airway epithelium protects against allergic asthma
Authors: SC Sebag, OM Koval, JD Paschke, CJ Winters, OA Jaffer, R Dworski, FS Sutterwala, ME Anderson, IM Grumbach
JCI Insight, 2017-02-09;2(3):e88297.
Species: Mouse
Sample Types: Tissue Homogenates
-
Notch regulates Th17 differentiation and controls trafficking of IL-17 and metabolic regulators within Th17 cells in a context-dependent manner
Sci Rep, 2016-12-15;6(0):39117.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Brazilian red propolis improves cutaneous wound healing suppressing inflammation-associated transcription factor NF?B
Authors: Flavia Regina Sobreira Corrêa
Biomed. Pharmacother, 2016-12-12;86(0):162-171.
Species: Mouse
Sample Types: Plasma
-
Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung
Authors: Ismé M de Kleer
Immunity, 2016-12-06;45(6):1285-1298.
Species: Mouse
Sample Types: Tissue Homogenates
-
Advax, a Delta Inulin Microparticle, Potentiates In-built Adjuvant Property of Co-administered Vaccines
EBioMedicine, 2016-12-01;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Acid-Sensing Ion Channel 1a Contributes to Airway Hyperreactivity in Mice
PLoS ONE, 2016-11-07;11(11):e0166089.
Species: Mouse
Sample Types: BALF
-
DUOX1 mediates persistent epithelial EGFR activation, mucous cell metaplasia, and airway remodeling during allergic asthma
JCI Insight, 2016-11-03;1(18):e88811.
Species: Mouse
Sample Types: BALF
-
IL-27 Is Essential for Suppression of Experimental Allergic Asthma by the TLR7/8 Agonist R848 (Resiquimod)
Authors: Gesine Hansen
J. Immunol., 2016-10-31;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Adenovirus-mediated Foxp3 expression in lung epithelial cells reduces airway inflammation in ovalbumin and cockroach-induced asthma model
Exp Mol Med, 2016-09-16;48(9):e259.
Species: Mouse
Sample Types: Serum
-
Allergen-Induced IL-6 Regulates IL-9/IL-17A Balance in CD4+ T Cells in Allergic Airway Inflammation
J Immunol, 2016-08-29;197(7):2653-64.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Editor's Highlight: Subvisible Aggregates of Immunogenic Proteins Promote a Th1-Type Response
Authors: Jeremy P Derrick
Toxicol. Sci., 2016-06-30;153(2):258-70.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Paired Ig-like Receptor B Inhibits IL-13-Driven Eosinophil Accumulation and Activation in the Esophagus
Authors: Netali Ben Baruch
J Immunol, 2016-06-20;0(0):.
Species: Mouse
Sample Types: BALF
-
Exposure to Aedes aegypti Bites Induces a Mixed-Type Allergic Response following Salivary Antigens Challenge in Mice
PLoS ONE, 2016-05-20;11(5):e0155454.
Species: Mouse
Sample Types: BALF
-
Impact of Histone H1 on the Progression of Allergic Rhinitis and Its Suppression by Neutralizing Antibody in Mice
Authors: T Nakano, R Kamei, T Fujimura, Y Takaoka, A Hori, CY Lai, KC Chiang, Y Shimada, N Ohmori, T Goto, K Ono, CL Chen, S Goto, S Kawamoto
PLoS ONE, 2016-04-18;11(4):e0153630.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-22 Restrains Tapeworm-Mediated Protection against Experimental Colitis via Regulation of IL-25 Expression
Authors: JL Reyes, MR Fernando, F Lopes, G Leung, NL Mancini, CE Matisz, A Wang, DM McKay
PLoS Pathog, 2016-04-07;12(4):e1005481.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Repeated Activation of Lung Invariant NKT Cells Results in Chronic Obstructive Pulmonary Disease-Like Symptoms.
Authors: Tsao C, Tsao P, Chen Y, Chuang Y
PLoS ONE, 2016-01-26;11(1):e0147710.
Species: Mouse
Sample Types: BALF
-
CD252 regulates mast cell mediated, CD1d-restricted NKT-cell activation in mice.
Authors: Gonzalez Roldan N, Orinska Z, Ewers H, Bulfone-Paus S
Eur J Immunol, 2015-12-09;46(2):432-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat Feeding.
Authors: Jovicic N, Jeftic I, Jovanovic I, Radosavljevic G, Arsenijevic N, Lukic M, Pejnovic N
PLoS ONE, 2015-07-28;10(7):e0134089.
Species: Mouse
Sample Types: Tissue Homogenates
-
Superior Suppressive Capacity of Skin Tregs Compared with Lung Tregs in a Model of Epicutaneous Priming.
Authors: Mahapatra S, Albrecht M, Baru A, Sparwasser T, Herrick C, Dittrich A
J Invest Dermatol, 2015-05-22;135(10):2418-26.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Hydroxypropyl-beta-cyclodextrin spikes local inflammation that induces Th2 cell and T follicular helper cell responses to the coadministered antigen.
Authors: Onishi M, Ozasa K, Kobiyama K, Ohata K, Kitano M, Taniguchi K, Homma T, Kobayashi M, Sato A, Katakai Y, Yasutomi Y, Wijaya E, Igarashi Y, Nakatsu N, Ise W, Inoue T, Yamada H, Vandenbon A, Standley D, Kurosaki T, Coban C, Aoshi T, Kuroda E, Ishii K
J Immunol, 2015-02-13;194(6):2673-82.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IRAK-M promotes alternative macrophage activation and fibroproliferation in bleomycin-induced lung injury.
Authors: Ballinger M, Newstead M, Zeng X, Bhan U, Mo X, Kunkel S, Moore B, Flavell R, Christman J, Standiford T
J Immunol, 2015-01-16;194(4):1894-904.
Species: Mouse
Sample Types: BALF
-
Cyclooxygenase inhibition abrogates aeroallergen-induced immune tolerance by suppressing prostaglandin I2 receptor signaling.
Authors: Zhou W, Goleniewska K, Zhang J, Dulek D, Toki S, Lotz M, Newcomb D, Boswell M, Polosukhin V, Milne G, Wu P, Moore M, FitzGerald G, Peebles R
J Allergy Clin Immunol, 2014-07-18;134(3):698-705.e5.
Species: Mouse
Sample Types: BALF
-
Lifetime-dependent effects of bisphenol A on asthma development in an experimental mouse model.
Authors: Petzold, Susanne, Averbeck, Marco, Simon, Jan C, Lehmann, Irina, Polte, Tobias
PLoS ONE, 2014-06-20;9(6):e100468.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-13 orchestrates resolution of chronic intestinal inflammation via phosphorylation of glycogen synthase kinase-3beta.
Authors: Fichtner-Feigl S, Kesselring R, Martin M, Obermeier F, Ruemmele P, Kitani A, Brunner S, Haimerl M, Geissler E, Strober W, Schlitt H
J Immunol, 2014-03-14;192(8):3969-80.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Unique macrophages different from M1/M2 macrophages inhibit T cell mitogenesis while upregulating Th17 polarization.
Authors: Tatano Y, Shimizu T, Tomioka H
Sci Rep, 2014-02-20;4(0):4146.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Foxp3(+) regulatory T cells delay expulsion of intestinal nematodes by suppression of IL-9-driven mast cell activation in BALB/c but not in C57BL/6 mice.
Authors: Blankenhaus B, Reitz M, Brenz Y, Eschbach M, Hartmann W, Haben I, Sparwasser T, Huehn J, Kuhl A, Feyerabend T, Rodewald H, Breloer M
PLoS Pathog, 2014-02-06;10(2):e1003913.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Delineating the role of histamine-1- and -4-receptors in a mouse model of Th2-dependent antigen-specific skin inflammation.
Authors: Mahapatra S, Albrecht M, Behrens B, Jirmo A, Behrens G, Hartwig C, Neumann D, Raap U, Bahre H, Herrick C, Dittrich A
PLoS ONE, 2014-02-04;9(2):e87296.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis.
Authors: Hams, Emily, Armstrong, Michelle, Barlow, Jillian, Saunders, Sean P, Schwartz, Christia, Cooke, Gordon, Fahy, Ruairi J, Crotty, Thomas B, Hirani, Nikhil, Flynn, Robin J, Voehringer, David, McKenzie, Andrew N, Donnelly, Seamas C, Fallon, Padraic
Proc Natl Acad Sci U S A, 2013-12-16;111(1):367-72.
Species: Mouse
Sample Types: Tissue Homogenates
-
The activating protein 1 transcription factor basic leucine zipper transcription factor, ATF-like (BATF), regulates lymphocyte- and mast cell-driven immune responses in the setting of allergic asthma.
Authors: Ubel C, Sopel N, Graser A, Hildner K, Reinhardt C, Zimmermann T, Rieker R, Maier A, Neurath M, Murphy K, Finotto S
J Allergy Clin Immunol, 2013-11-28;133(1):198-206.e1-9.
Species: Mouse
Sample Types: BALF
-
Epithelial NF-kappaB orchestrates house dust mite-induced airway inflammation, hyperresponsiveness, and fibrotic remodeling.
Authors: Tully J, Hoffman S, Lahue K, Nolin J, Anathy V, Lundblad L, Daphtary N, Aliyeva M, Black K, Dixon A, Poynter M, Irvin C, Janssen-Heininger Y
J Immunol, 2013-11-13;191(12):5811-21.
Species: Mouse
Sample Types: Tissue Homogenates
-
Retinoic acid prevents mesenteric lymph node dendritic cells from inducing IL-13-producing inflammatory Th2 cells.
Authors: Yokota-Nakatsuma A, Takeuchi H, Ohoka Y, Kato C, Song S, Hoshino T, Yagita H, Ohteki T, Iwata M
Mucosal Immunol, 2013-11-13;7(4):786-801.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis.
Authors: McHedlidze T, Waldner M, Zopf S, Walker J, Rankin A, Schuchmann M, Voehringer D, McKenzie A, Neurath M, Pflanz S, Wirtz S
Immunity, 2013-08-15;39(2):357-71.
Species: Mouse
Sample Types: Serum
-
Mice deficient in the St3gal3 gene product alpha2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation.
Authors: Kiwamoto T, Brummet M, Wu F, Motari M, Smith D, Schnaar R, Zhu Z, Bochner B
J Allergy Clin Immunol, 2013-07-02;133(1):240-7.e1-3.
Species: Mouse
Sample Types: BALF
-
Nanogel-based PspA intranasal vaccine prevents invasive disease and nasal colonization by Streptococcus pneumoniae.
Authors: Kong, Il Gyu, Sato, Ayuko, Yuki, Yoshikaz, Nochi, Tomonori, Takahashi, Haruko, Sawada, Shinichi, Mejima, Mio, Kurokawa, Shiho, Okada, Kazunari, Sato, Shintaro, Briles, David E, Kunisawa, Jun, Inoue, Yusuke, Yamamoto, Masafumi, Akiyoshi, Kazunari, Kiyono, Hiroshi
Infect Immun, 2013-03-04;81(5):1625-34.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immune Modulation Mediated by Cryptococcal Laccase Promotes Pulmonary Growth and Brain Dissemination of Virulent Cryptococcus neoformans in Mice.
Authors: Qiu Y, Davis M, Dayrit J, Hadd Z, Meister D, Osterholzer J, Williamson P, Olszewski M
PLoS ONE, 2012-10-22;7(10):e47853.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Alveolar Macrophages Play a Key Role in Cockroach-Induced Allergic Inflammation via TNF-alpha Pathway.
Authors: Kim J, Sohn J, Choi J, Lee J, Hong C, Lee J, Park J
PLoS ONE, 2012-10-19;7(10):e47971.
Species: Mouse
Sample Types: Tissue Homogenates
-
Endogenous retinoids in the pathogenesis of alopecia areata.
Authors: Duncan F, Silva K, Johnson C, King B, Szatkiewicz J, Kamdar S, Ong D, Napoli J, Wang J, King L, Whiting D, McElwee K, Sundberg J, Everts H
J Invest Dermatol, 2012-09-27;133(2):334-43.
Species: Mouse
Sample Types: Tissue Homogenates
-
T1/ST2 promotes T helper 2 cell activation and polyfunctionality in bronchopulmonary mycosis.
Authors: Piehler D, Grahnert A, Eschke M, Richter T, Kohler G, Stenzel W, Alber G
Mucosal Immunol, 2012-09-19;6(2):405-14.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Control of allergen-induced inflammation and hyperresponsiveness by the metalloproteinase ADAMTS-12.
Authors: Paulissen G, El Hour M, Rocks N, Gueders M, Bureau F, Foidart J, Lopez-Otin C, Noel A, Cataldo D
J Immunol, 2012-09-07;189(8):4135-43.
Species: Mouse
Sample Types: Tissue Homogenates
-
Epithelial cell-derived IL-25, but not Th17 cell-derived IL-17 or IL-17F, Is crucial for murine asthma.
Authors: Suzukawa M, Morita H, Nambu A, Arae K, Shimura E, Shibui A, Yamaguchi S, Suzukawa K, Nakanishi W, Oboki K, Kajiwara N, Ohno T, Ishii A, Korner H, Cua D, Suto H, Yoshimoto T, Iwakura Y, Yamasoba T, Ohta K, Sudo K, Saito H, Okumura K, Broide D, Matsumoto K, Nakae S
J Immunol, 2012-08-31;189(7):3641-52.
Species: Mouse
Sample Types: BALF
-
Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33.
J. Exp. Med., 2012-07-16;209(8):1505-17.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs.
Authors: Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H
J. Immunol., 2011-12-23;188(3):1503-13.
Species: Mouse
Sample Types: Cell Culture Supernates
-
High expression of IL-22 suppresses antigen-induced immune responses and eosinophilic airway inflammation via an IL-10-associated mechanism.
Authors: Nakagome K, Imamura M, Kawahata K
J. Immunol., 2011-10-12;187(10):5077-89.
Species: Mouse
Sample Types: BALF
-
T cell-derived Act1 is necessary for IL-25-mediated Th2 responses and allergic airway inflammation.
Authors: Swaidani S, Bulek K, Kang Z
J. Immunol., 2011-08-19;187(6):3155-64.
Species: Mouse
Sample Types: BALF
-
The Therapeutic Potential of the Humoral Pattern Recognition Molecule PTX3 in Chronic Lung Infection Caused by Pseudomonas aeruginosa.
Authors: Moalli F, Paroni M, Veliz Rodriguez T, Riva F, Polentarutti N, Bottazzi B, Valentino S, Mantero S, Nebuloni M, Mantovani A, Bragonzi A, Garlanda C
J. Immunol., 2011-03-25;186(9):5425-34.
Species: Mouse
Sample Types: Tissue Homogenates
-
Impaired basophil induction leads to an age-dependent innate defect in type 2 immunity during helminth infection in mice.
Authors: Nel HJ, Hams E, Saunders SP
J. Immunol., 2011-03-11;186(8):4631-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Alpha-Galactosylceramide-induced airway eosinophilia is mediated through the activation of NKT cells.
Authors: Chuang YH, Wang TC, Jen HY, Yu AL, Chiang BL
J. Immunol., 2011-03-07;186(8):4687-92.
Species: Mouse
Sample Types: BALF
-
A critical role for C5L2 in the pathogenesis of experimental allergic asthma.
Authors: Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, Konig P, Gerard NP, Gerard C, Wills-Karp M, Kohl J
J. Immunol., 2010-10-25;185(11):6741-52.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mucosal delivery of human papillomavirus pseudovirus-encapsidated plasmids improves the potency of DNA vaccination.
Authors: Graham BS, Kines RC, Corbett KS, Nicewonger J, Roberts JN, Schiller JT, Buck CB
Mucosal Immunol, 2010-06-16;3(5):475-86.
Species: Mouse
Sample Types: BALF
-
Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity.
Authors: Sellge G, Magalhaes JG, Konradt C, Fritz JH, Salgado-Pabon W, Eberl G, Bandeira A, Di Santo JP, Sansonetti PJ, Phalipon A
J. Immunol., 2010-01-20;184(4):2076-85.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Effects of umbelliferone in a murine model of allergic airway inflammation.
Authors: Vasconcelos JF, Teixeira MM, Barbosa-Filho JM, Agra MF, Nunes XP, Giulietti AM, Ribeiro-Dos-Santos R, Soares MB
Eur. J. Pharmacol., 2009-03-14;609(1):126-31.
Species: Mouse
Sample Types: BALF
-
Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway.
Authors: Kearley J, Buckland KF, Mathie SA, Lloyd CM
Am. J. Respir. Crit. Care Med., 2009-01-29;179(9):772-81.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis.
Authors: Kaya Z, Goser S, Buss SJ, Leuschner F, Ottl R, Li J, Volkers M, Zittrich S, Pfitzer G, Rose NR, Katus HA
Circulation, 2008-10-27;118(20):2063-72.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities.
Authors: Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL
J. Immunol., 2008-09-15;181(6):4299-310.
Species: Mouse
Sample Types: BALF
-
Elevated asymmetric dimethylarginine alters lung function and induces collagen deposition in mice.
Authors: Wells SM, Buford MC, Migliaccio CT, Holian A
Am. J. Respir. Cell Mol. Biol., 2008-08-14;40(2):179-88.
Species: Mouse
Sample Types: BALF
-
Induction of IL-33 expression and activity in central nervous system glia.
Authors: Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT
J. Leukoc. Biol., 2008-06-13;84(3):631-43.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis.
Authors: Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME
Proc. Natl. Acad. Sci. U.S.A., 2008-05-14;105(20):7240-5.
Species: Mouse
Sample Types: BALF
-
Surfactant protein D alters allergic lung responses in mice and human subjects.
Authors: Brandt EB, Mingler MK, Stevenson MD, Wang N, Khurana Hershey GK, Whitsett JA, Rothenberg ME
J. Allergy Clin. Immunol., 2008-03-19;121(5):1140-1147.e2.
Species: Mouse
Sample Types: BALF
-
Transforming growth factor-beta regulates house dust mite-induced allergic airway inflammation but not airway remodeling.
Authors: Fattouh R, Midence NG, Arias K, Johnson JR, Walker TD, Goncharova S, Souza KP, Gregory RC, Lonning S, Gauldie J, Jordana M
Am. J. Respir. Crit. Care Med., 2008-01-03;177(6):593-603.
Species: Mouse
Sample Types: BALF
-
Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo.
Authors: Jenkins SJ, Perona-Wright G, Worsley AG, Ishii N, MacDonald AS
J. Immunol., 2007-09-15;179(6):3515-23.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells.
Authors: Nakae S, Iikura M, Suto H, Akiba H, Umetsu DT, DeKruyff RH, Saito H, Galli SJ
Blood, 2007-07-09;110(7):2565-8.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Therapeutic targeting of CC ligand 21 or CC chemokine receptor 7 abrogates pulmonary fibrosis induced by the adoptive transfer of human pulmonary fibroblasts to immunodeficient mice.
Authors: Pierce EM, Carpenter K, Jakubzick C, Kunkel SL, Flaherty KR, Martinez FJ, Hogaboam CM
Am. J. Pathol., 2007-04-01;170(4):1152-64.
Species: Mouse
Sample Types: Tissue Homogenates
-
IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production.
Authors: Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI
J. Immunol., 2007-03-01;178(5):2827-34.
Species: Mouse
Sample Types: Cell Culture Supernates
-
An alternative pathway of NF-kappaB activation results in maturation and T cell priming activity of dendritic cells overexpressing a mutated IkappaBalpha.
Authors: Moore F, Buonocore S, Aksoy E, Ouled-Haddou N, Goriely S, Lazarova E, Paulart F, Heirman C, Vaeremans E, Thielemans K, Goldman M, Flamand V
J. Immunol., 2007-02-01;178(3):1301-11.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin-17 is a negative regulator of established allergic asthma.
Authors: Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B
J. Exp. Med., 2006-11-13;203(12):2715-25.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation.
Authors: Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME
Proc. Natl. Acad. Sci. U.S.A., 2006-10-23;103(44):16418-23.
Species: Mouse
Sample Types: Tissue Homogenates
-
Induced trefoil factor family 1 expression by trans-differentiating Clara cells in a murine asthma model.
Authors: Kouznetsova I, Chwieralski CE, Balder R, Hinz M, Braun A, Krug N, Hoffmann W
Am. J. Respir. Cell Mol. Biol., 2006-09-21;36(3):286-95.
Species: Mouse
Sample Types: BALF
-
Induction of prolonged infiltration of T lymphocytes and transient T lymphocyte-dependent collagen deposition in mouse lungs following adenoviral gene transfer of CCL18.
Authors: Luzina IG, Papadimitriou JC, Anderson R, Pochetuhen K, Atamas SP
Arthritis Rheum., 2006-08-01;54(8):2643-55.
Species: Mouse
Sample Types: BALF
-
Inhibition of angiogenesis by interleukin-4 gene therapy in rat adjuvant-induced arthritis.
Authors: Haas CS, Amin MA, Allen BB, Ruth JH, Haines GK, Woods JM, Koch AE
Arthritis Rheum., 2006-08-01;54(8):2402-14.
Species: Rat
Sample Types: Tissue Homogenates
-
Pulmonary distribution, regulation, and functional role of Trk receptors in a murine model of asthma.
Authors: Nassenstein C, Dawbarn D, Pollock K, Allen SJ, Erpenbeck VJ, Spies E, Krug N, Braun A
J. Allergy Clin. Immunol., 2006-07-03;118(3):597-605.
Species: Mouse
Sample Types: BALF
-
Hierarchical suppression of asthma-like responses by mucosal tolerance.
Authors: Keller AC, Mucida D, Gomes E, Faquim-Mauro E, Faria AM, Rodriguez D, Russo M
J. Allergy Clin. Immunol., 2006-02-01;117(2):283-90.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Leukemia inhibitory factor is linked to regulatory transplantation tolerance.
Authors: Metcalfe SM, Watson TJ, Shurey S, Adams E, Green CJ
Transplantation, 2005-03-27;79(6):726-30.
Species: Mouse
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse IL-13 DuoSet ELISA
Average Rating: 4.9 (Based on 7 Reviews)
Have you used Mouse IL-13 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: