Mouse IL-2 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant mouse IL-2. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Block Buffer: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Reagent Diluent: 0.1% BSA, 0.05% Tween 20 in Tris-buffered Saline (20 mM Trizma base, 150 mM NaCI) pH 7.2-7.4, 0.2 μm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Preparation and Storage
Background: IL-2
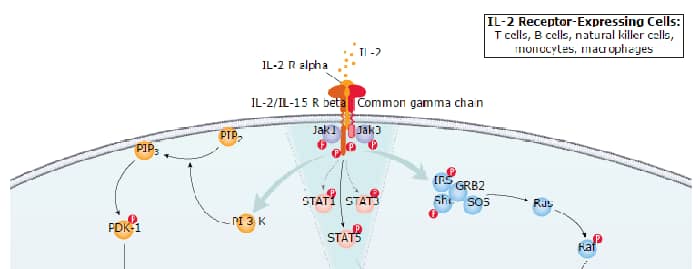
IL-2 (Interleukin 2) is a critical cytokine in T cell biology. It promotes T cell activation and expansion, the development, maintenance and function of regulatory T cells (Treg), and the differentiation of CD8+ T cells into terminal effector cells and memory cells. IL-2 signals through a receptor complex composed of CD25/IL-2 R alpha, IL-2 R beta, and the Common gamma Chain (gamma c). IL-2 R beta is also a component of the IL-15 receptor complex. Gamma c is also a signaling subunit in the receptors for IL-4, -7, -9, -15, and -21.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL of Block Buffer to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse IL-2 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
90
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
The Influence of polycyclic aromatic hydrocarbons exposure on the gut microbiome composition and inflammatory responses
Authors: Jeong, SH;Jung, J;Park, YJ;Lee, SJ;Lee, SJ;
Ecotoxicology and environmental safety
Species: Mouse
Sample Types: Serum, Tissue Homogenates
-
Substituent-based Modulation of Self-Assembly and Immunogenicity of Amphipathic Peptides
Authors: Das, A;Pramanik, U;Brown, EM;Liu, CY;Gong, H;Fascetti, J;Gibson, M;Stealey, S;Zustiak, SP;Berkland, C;Jackrel, ME;White, MA;Rudra, JS;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Laquinimod treatment attenuates EAU by inhibiting both the inductive and effector phases in an APC-dependent manner
Authors: Xu, B;Mattapallil, MJ;Jia, X;Tang, J;Horai, R;Caspi, RR;Gery, I;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Bidirectional Interaction Between Chronic Kidney Disease and Porphyromonas gingivalis Infection Drives Inflammation and Immune Dysfunction
Authors: Adamowicz, K;Lima Ribeiro, AS;Golda, A;Wadowska, M;Potempa, J;Schmaderer, C;Anders, HJ;Koziel, J;Lech, M;
Journal of immunology research
Species: Mouse
Sample Types: Serum, Cell Culture Supernates
-
Lung and liver editing by lipid nanoparticle delivery of a stable CRISPR-Cas9 ribonucleoprotein
Authors: Chen, K;Han, H;Zhao, S;Xu, B;Yin, B;Lawanprasert, A;Trinidad, M;Burgstone, BW;Murthy, N;Doudna, JA;
Nature biotechnology
Species: Mouse
Sample Types: Plasma
-
The Tyrosine Phosphatase Activity of PTPN22 Is Involved in T Cell Development via the Regulation of TCR Expression
Authors: Bai, B;Li, T;Zhao, J;Zhao, Y;Zhang, X;Wang, T;Zhang, N;Wang, X;Ba, X;Xu, J;Yu, Y;Wang, B;
International journal of molecular sciences
Species: Mouse
Sample Types: Whole Blood
-
Activation and differentiation of cognate T cells by a dextran-based antigen-presenting system for cancer immunotherapy
Authors: Mahata, D;Mukherjee, D;Biswas, D;Basak, S;Basak, AJ;Jamir, I;Pandey, N;Khatoon, H;Samanta, D;Basak, A;Mukherjee, G;
European journal of immunology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Ligand-based adoptive T cell targeting CA125 in ovarian cancer
Authors: Zhao, H;Wu, L;Dai, J;Sun, K;Zi, Z;Guan, J;Zhang, L;
Journal of translational medicine
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-7R licenses a population of epigenetically poised memory CD8+ T cells with superior antitumor efficacy that are critical for melanoma memory
Authors: Micevic, G;Daniels, A;Flem-Karlsen, K;Park, K;Talty, R;McGeary, M;Mirza, H;Blackburn, HN;Sefik, E;Cheung, JF;Hornick, NI;Aizenbud, L;Joshi, NS;Kluger, H;Iwasaki, A;Bosenberg, MW;Flavell, RA;
Proceedings of the National Academy of Sciences of the United States of America
Species: Mouse
Sample Types: Cell Culture Supernates
-
Modulation of tumor immune microenvironment by TAS-115, a multi-receptor tyrosine kinase inhibitor, promotes antitumor immunity and contributes anti-PD-1 antibody therapy
Authors: Shibutani, T;Goto, R;Miyazaki, I;Hashimoto, A;Suzuki, T;Ishida, K;Haruma, T;Osada, T;Harada, T;Fujita, H;Ohkubo, S;
Scientific reports
Species: Mouse
Sample Types: Cell Culture Supernates
-
Diversity-Oriented Synthesis of a Molecular Library of Immunomodulatory alpha-Galactosylceramides with Fluorous-Tag-Assisted Purification and Evaluation of Their Bioactivities in Regard to IL-2 Secretion
Authors: YN Chen, JT Hung, FD Jan, YY Su, JR Hwu, AL Yu, AK Adak, CC Lin
Oncogene, 2022-11-02;23(21):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Arginine-based cationic liposomes accelerate T cell activation and differentiation in vitro
Authors: T Li, F Tolksdorf, W Sung, H Sato, FJ Eppler, M Hotta, W Kolanus, S Takeoka
International journal of pharmaceutics, 2022-06-15;623(0):121917.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Menthone Inhalation Alleviates Local and Systemic Allergic Inflammation in Ovalbumin-Sensitized and Challenged Asthmatic Mice
Authors: YH Su, JY Lin
International Journal of Molecular Sciences, 2022-04-04;23(7):.
Species: Mouse
Sample Types: BALF
-
Anti-CD80/86 antibodies inhibit inflammatory reaction and improve graft survival in a high-risk murine corneal transplantation rejection model
Authors: J Zhu, T Inomata, M Nakamura, K Fujimoto, Y Akasaki, K Fujio, A Yanagawa, K Uchida, J Sung, N Negishi, K Nagino, Y Okumura, M Miura, H Shokirova, M Kuwahara, K Hirosawa, A Midorikawa, A Eguchi, T Huang, H Yagita, S Habu, K Okumura, A Murakami
Scientific Reports, 2022-03-22;12(1):4853.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Target modulation and pharmacokinetics/pharmacodynamics translation of the BTK inhibitor poseltinib for model-informed phase II dose selection
Authors: JY Byun, YT Koh, SY Jang, JW Witcher, JR Chan, A Pustilnik, MJ Daniels, YH Kim, KH Suh, MD Linnik, YM Lee
Scientific Reports, 2021-09-21;11(1):18671.
Species: Mouse
Sample Types: Cell Culture Supernates
-
NR4A family members regulate T cell tolerance to preserve immune homeostasis and suppress autoimmunity
Authors: R Hiwa, HV Nielsen, JL Mueller, R Mandla, J Zikherman
JCI Insight, 2021-09-08;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Bifidobacterium breve Exopolysaccharide Blocks Dendritic Cell Maturation and Activation of CD4+ T Cells
Authors: A Hickey, P Stamou, S Udayan, A Ramón-Vázq, M Esteban-To, F Bottacini, JA Woznicki, O Hughes, S Melgar, M Ventura, D Van Sinder, V Rossini, K Nally
Frontiers in Microbiology, 2021-06-16;12(0):653587.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Ex Vivo-Induced Bone Marrow-Derived Myeloid Suppressor Cells Prevent Corneal Allograft Rejection in Mice
Authors: J Zhu, T Inomata, K Fujimoto, K Uchida, K Fujio, K Nagino, M Miura, N Negishi, Y Okumura, Y Akasaki, K Hirosawa, M Kuwahara, A Eguchi, H Shokirova, A Yanagawa, A Midorikawa, A Murakami
Investigative Ophthalmology & Visual Science, 2021-06-01;62(7):3.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Coordination of asparagine uptake and asparagine synthetase expression modulates CD8+ T cell activation
Authors: HC Hope, RJ Brownlie, CM Fife, L Steele, M Lorger, RJ Salmond
JCI Insight, 2021-05-10;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Programmed Death-Ligand 2 Deficiency Exacerbates Experimental Autoimmune Myocarditis in Mice
Authors: S Li, K Tajiri, N Murakoshi, D Xu, S Yonebayash, Y Okabe, Z Yuan, D Feng, K Inoue, K Aonuma, Y Shimoda, Z Song, H Mori, H Huang, K Aonuma, M Ieda
International Journal of Molecular Sciences, 2021-01-31;22(3):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Potent antitumor effects of cell-penetrating peptides targeting STAT3 axis
Authors: M Aftabizade, YJ Li, Q Zhao, C Zhang, N Ambaye, J Song, T Nagao, C Lahtz, M Fakih, DK Ann, H Yu, A Herrmann
JCI Insight, 2021-01-25;6(2):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Modifying bacterial flagellin to evade Nod-like Receptor CARD 4 recognition enhances protective immunity against Salmonella
Authors: P Tourlomous, JA Wright, AS Bittante, LJ Hopkins, SJ Webster, OJ Bryant, P Mastroeni, DJ Maskell, CE Bryant
Nat Microbiol, 2020-10-26;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
An Antibody Targeting ICOS Increases Intratumoral Cytotoxic to Regulatory T-cell Ratio and Induces Tumor Regression
Authors: RCA Sainson, AK Thotakura, M Kosmac, G Borhis, N Parveen, R Kimber, J Carvalho, SJ Henderson, KL Pryke, T Okell, S O'Leary, S Ball, C Van Krinks, L Gamand, E Taggart, EJ Pring, H Ali, H Craig, VWY Wong, Q Liang, RJ Rowlands, M Lecointre, J Campbell, I Kirby, D Melvin, V Germaschew, E Oelmann, S Quaratino, M McCourt
Cancer Immunology Research, 2020-09-30;8(12):1568-1582.
Species: Mouse
Sample Types: Cell Culture Supernates
-
WAS Promoter-Driven Lentiviral Vectors Mimic Closely the Lopsided WASP Expression during Megakaryocytic Differentiation
Authors: P Muñoz, M Tristán-Ma, A Sánchez-Gi, G Santilli, A Galy, AJ Thrasher, F Martin
Mol Ther Methods Clin Dev, 2020-09-16;19(0):220-235.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Aptamer targeted therapy potentiates immune checkpoint blockade in triple-negative breast cancer
Authors: S Camorani, M Passariell, L Agnello, S Esposito, F Collina, M Cantile, M Di Bonito, IV Ulasov, M Fedele, A Zannetti, C De Lorenzo, L Cerchia
J. Exp. Clin. Cancer Res., 2020-09-07;39(1):180.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Isolation of Two Novel Human Anti-CTLA-4 mAbs with Intriguing Biological Properties on Tumor and NK Cells
Authors: M Passariell, C Vetrei, E Sasso, G Froechlich, C Gentile, AM D'Alise, N Zambrano, E Scarselli, A Nicosia, C De Lorenzo
Cancers (Basel), 2020-08-06;12(8):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immunomodulatory Effect of a Salvia plebeia R. Aqueous Extract in Forced Swimming Exercise-induced Mice
Authors: J Shin, OK Kim, S Kim, D Bae, J Lee, J Park, W Jun
Nutrients, 2020-07-28;12(8):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Combination therapy using human papillomavirus L1/E6/E7 genes and archaeosome: a nanovaccine confer immuneadjuvanting effects to fight cervical cancer
Authors: H Karimi, H Soleimanja, A Abdoli, RS Banijamali
Sci Rep, 2020-04-01;10(1):5787.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Acorus gramineusand and Euodia ruticarpa Steam Distilled Essential Oils Exert Anti-Inflammatory Effects Through Decreasing Th1/Th2 and Pro-/Anti-Inflammatory Cytokine Secretion Ratios In Vitro
Authors: TH Yeh, JY Lin
Biomolecules, 2020-02-19;10(2):.
Species: Mouse
Sample Types: Cell Lysates
-
Antitumor and antioxidant effects of Clinacanthus nutans Lindau in 4?T1 tumor-bearing mice
Authors: NMA Nik Abd Ra, MY Nurliyana, MNFNN Afiqah, MA Osman, M Hamid, MAM Lila
BMC Complement Altern Med, 2019-11-29;19(1):340.
Species: Mouse
Sample Types: Serum
-
Quantitative interactomics in primary T cells unveils TCR signal diversification extent and dynamics
Authors: G Voisinne, K Kersse, K Chaoui, L Lu, J Chaix, L Zhang, M Goncalves, L Girard, Y Ounoughene, H Wang, O Burlet-Sch, H Luche, F Fiore, M Malissen, A Gonzalez d, Y Liang, R Roncagalli, B Malissen
Nat. Immunol., 2019-10-07;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Nano spray dryer for vectorizing ?-galactosylceramide in polymeric nanoparticles: A single step process to enhance invariant Natural Killer T lymphocyte responses
Authors: MB Gonzatti, MEP Sousa, AS Tunissi, RA Mortara, AM de Oliveir, NN Pereira Ce, AC Keller
Int J Pharm, 2019-05-07;565(0):123-132.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Rapid synthesis and immunogenicity of mycobacterial (1?5)-?-d-arabinofuranan
Authors: H Leelayuwap, S Ruchirawat, S Boonyaratt
Carbohydr Polym, 2018-10-24;206(0):262-272.
Species: Mouse
Sample Types: Cell Culture Supernates
-
ATX-MS-1467 Induces Long-Term Tolerance to Myelin Basic Protein in (DR2?�?Ob1)F1 Mice by Induction of IL-10-Secreting iTregs
Authors: ALS De Souza, S Rudin, R Chang, K Mitchell, T Crandall, S Huang, JK Choi, SL Okitsu, DL Graham, B Tomkinson, T Dellovade
Neurol Ther, 2018-03-14;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A Restricted Role for Fc?R in the Regulation of Adaptive Immunity
Authors: MF Fransen, H Benonisson, WW van Maren, HS Sow, C Breukel, MM Linssen, JWC Claassens, C Brouwers, J van der Ka, M Camps, JW Kleinovink, KK Vonk, S van Heinin, N Klar, L van Beek, V van Harmel, L Daxinger, KS Nandakumar, R Holmdahl, C Coward, Q Lin, S Hirose, D Salvatori, T van Hall, C van Kooten, P Mastroeni, F Ossendorp, JS Verbeek
J. Immunol., 2018-03-09;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain
Authors: AT Brini, G Amodeo, LM Ferreira, A Milani, S Niada, G Moschetti, S Franchi, E Borsani, LF Rodella, AE Panerai, P Sacerdote
Sci Rep, 2017-08-29;7(1):9904.
Species: Mouse
Sample Types: Tissue Homogenates
-
Lamtor1 Is Critically Required for CD4(+) T Cell Proliferation and Regulatory T Cell Suppressive Function
Authors: T Hosokawa, T Kimura, S Nada, T Okuno, D Ito, S Kang, S Nojima, K Yamashita, T Nakatani, Y Hayama, Y Kato, Y Kinehara, M Nishide, N Mikami, S Koyama, H Takamatsu, D Okuzaki, N Ohkura, S Sakaguchi, M Okada, A Kumanogoh
J. Immunol., 2017-08-02;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Inducing maternal inflammation promotes leptin production in offspring but does not improve allergic symptoms in a mouse model of allergic rhinitis
Authors: A Imai, K Satoi, E Fujimoto, K Sato
Heliyon, 2017-06-22;3(6):e00327.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Synthetic Cannabinoid-Induced Immunosuppression Augments Cerebellar Dysfunction in Tetanus-Toxin Treated Mice
Authors: J Yun, SM Gu, TH Lee, YJ Song, S Seong, YH Kim, HJ Cha, KM Han, J Shin, H Oh, K Jung, C Ahn, HK Park, HS Kim
Biomol Ther (Seoul), 2017-05-01;25(3):266-271.
Species: Mouse
Sample Types: Tissue Homogenates
-
Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses
Authors: S Kang, S Ahn, J Lee, JY Kim, M Choi, V Gujrati, H Kim, J Kim, EC Shin, S Jon
J Control Release, 2017-04-18;256(0):56-67.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Th17 cells are refractory to senescence and retain robust antitumor activity after long-term ex vivo expansion
Authors: JS Bowers, MH Nelson, K Majchrzak, SR Bailey, B Rohrer, AD Kaiser, C Atkinson, L Gattinoni, CM Paulos
JCI Insight, 2017-03-09;2(5):e90772.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CD74 Deficiency Mitigates Systemic Lupus Erythematosus-like Autoimmunity and Pathological Findings in Mice
Authors: Y Zhou, H Chen, L Liu, X Yu, GK Sukhova, M Yang, L Zhang, VC Kyttaris, GC Tsokos, IE Stillman, T Ichimura, JV Bonventre, P Libby, GP Shi
J. Immunol, 2017-02-20;0(0):.
Species: Mouse
Sample Types: Serum
-
NK cells require antigen-specific memory CD4+ T cells to mediate superior effector functions during HSV-2 recall responses in vitro
J. Leukoc. Biol, 2016-12-14;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Comparison of potential protection conferred by three immunization strategies (protein/protein, DNA/DNA, and DNA/protein) against Brucella infection using Omp2b in BALB/c Mice
Authors: Saeid Bouzari
Vet. Microbiol., 2016-11-05;197(0):47-52.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Tuberculosis Susceptibility and Vaccine Protection Are Independently Controlled by Host Genotype
MBio, 2016-09-20;7(5):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Testosterone-Mediated Endocrine Function and TH1/TH2 Cytokine Balance after Prenatal Exposure to Perfluorooctane Sulfonate: By Sex Status
Int J Mol Sci, 2016-09-12;17(9):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dendritic Cell-Like Cells Accumulate in Regenerating Murine Skeletal Muscle after Injury and Boost Adaptive Immune Responses Only upon a Microbial Challenge
PLoS ONE, 2016-05-19;11(5):e0155870.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Gene Therapy Induces Antigen-Specific Tolerance in Experimental Collagen-Induced Arthritis
PLoS ONE, 2016-05-09;11(5):e0154630.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immune Cell-Conditioned Media Suppress Prostate Cancer PC-3 Cell Growth Correlating With Decreased Proinflammatory/Anti-inflammatory Cytokine Ratios in the Media Using 5 Selected Crude Polysaccharides
Integr Cancer Ther, 2016-04-29;15(4):NP13-NP25.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TARM1 Is a Novel Leukocyte Receptor Complex-Encoded ITAM Receptor That Costimulates Proinflammatory Cytokine Secretion by Macrophages and Neutrophils.
Authors: Radjabova V, Mastroeni P, Skjodt K, Zaccone P, de Bono B, Goodall J, Chilvers E, Juss J, Jones D, Trowsdale J, Barrow A
J Immunol, 2015-08-26;195(7):3149-59.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Cellular FLIP Inhibits Myeloid Cell Activation by Suppressing Selective Innate Signaling.
Authors: Wu Y, Wu Y, Mo S, Hsiao H, He Y, Lai M
J Immunol, 2015-08-03;195(6):2612-23.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TAGLN2 regulates T cell activation by stabilizing the actin cytoskeleton at the immunological synapse.
Authors: Na B, Kim H, Piragyte I, Oh H, Kwon M, Akber U, Lee H, Park D, Song W, Park Z, Im S, Rho M, Hyun Y, Kim M, Jun C
J Cell Biol, 2015-04-13;209(1):143-62.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Simultaneous and dose dependent melanoma cytotoxic and immune stimulatory activity of betulin.
Authors: Pfarr K, Danciu C, Arlt O, Neske C, Dehelean C, Pfeilschifter J, Radeke H
PLoS ONE, 2015-03-10;10(3):e0118802.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The glycosylated Rv1860 protein of mycobacterium tuberculosis inhibits dendritic cell mediated TH1 and TH17 polarization of T cells and abrogates protective immunity conferred by BCG.
Authors: Satchidanandam V, Kumar N, Jumani R, Challu V, Elangovan S, Khan N
PLoS Pathog, 2014-06-12;10(6):e1004176.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dynamic motile T cells highly respond to the T cell stimulation via PI3K-Akt and NF-kappaB pathways.
Authors: Kim H, Na B, Kwon M, Ko Y, Han W, Jun C
PLoS ONE, 2013-03-26;8(3):e59793.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Genetic deletion of the HIF-1alpha isoform I.1 in T cells enhances antibacterial immunity and improves survival in a murine peritonitis model.
Authors: Georgiev P, Belikoff B, Hatfield S, Ohta A, Sitkovsky M, Lukashev D
Eur J Immunol, 2013-01-31;43(3):655-66.
Species: Mouse
Sample Types: Serum
-
Role of OGR1 in myeloid-derived cells in prostate cancer.
Authors: Yan, L, Singh, L S, Zhang, L, Xu, Y
Oncogene, 2012-12-10;33(2):157-64.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection.
Authors: Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, Riva F, Garlanda C
Infect. Immun., 2011-10-24;80(1):100-9.
Species: Mouse
Sample Types: Serum
-
Api6/AIM/Sp{alpha}/CD5L Overexpression in Alveolar Type II Epithelial Cells Induces Spontaneous Lung Adenocarcinoma.
Authors: Li Y, Qu P, Wu L, Li B, Du H, Yan C
Cancer Res., 2011-06-22;71(16):5488-99.
Species: Mouse
Sample Types: Serum
-
Mucosal immunity in mice induced by orally administered transgenic rice.
Authors: Zhang X, Yuan Z, Duan Q, Zhu H, Yu H, Wang Q
Vaccine, 2009-01-13;27(10):1596-600.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CD69+ CD4+ CD25- T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1.
Authors: Han Y, Guo Q, Zhang M, Chen Z, Cao X
J. Immunol., 2009-01-01;182(1):111-20.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis.
Authors: Kaya Z, Goser S, Buss SJ, Leuschner F, Ottl R, Li J, Volkers M, Zittrich S, Pfitzer G, Rose NR, Katus HA
Circulation, 2008-10-27;118(20):2063-72.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation.
Authors: Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C
J. Immunol., 2008-08-01;181(3):1835-48.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function.
Authors: Csoka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, Deitch EA, Spolarics Z, Nemeth ZH, Hasko G
FASEB J., 2008-07-14;22(10):3491-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Amelioration of delayed-type hypersensitivity responses by IL-27 administration.
Authors: Miyazaki Y, Shimanoe Y, Wang S, Yoshida H
Biochem. Biophys. Res. Commun., 2008-06-20;373(3):397-402.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Streptococcus equi antigens adsorbed onto surface modified poly-epsilon-caprolactone microspheres induce humoral and cellular specific immune responses.
Authors: Florindo HF, Pandit S, Goncalves LM, Alpar HO, Almeida AJ
Vaccine, 2008-06-17;26(33):4168-77.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Effect of intestinal microbiota on the induction of regulatory CD25+ CD4+ T cells.
Authors: Ishikawa H, Tanaka K, Maeda Y, Aiba Y, Hata A, Tsuji NM, Koga Y, Matsumoto T
Clin. Exp. Immunol., 2008-05-05;153(1):127-35.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells.
Authors: Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A
Nat. Immunol., 2007-11-11;8(12):1372-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CD40L disruption enhances Abeta vaccine-mediated reduction of cerebral amyloidosis while minimizing cerebral amyloid angiopathy and inflammation.
Authors: Obregon D, Hou H, Bai Y, Nikolic WV, Mori T, Luo D, Zeng J, Ehrhart J, Fernandez F, Morgan D, Giunta B, Town T, Tan J
Neurobiol. Dis., 2007-10-16;29(2):336-53.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Osteopontin regulates hindlimb-unloading-induced lymphoid organ atrophy and weight loss by modulating corticosteroid production.
Authors: Wang KX, Shi Y, Denhardt DT
Proc. Natl. Acad. Sci. U.S.A., 2007-09-04;104(37):14777-82.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The related adaptors, adaptor in lymphocytes of unknown function X and Rlk/Itk-binding protein, have nonredundant functions in lymphocytes.
Authors: Perchonock CE, Pajerowski AG, Nguyen C, Shapiro MJ, Shapiro VS
J. Immunol., 2007-08-01;179(3):1768-75.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis.
Authors: Hwang SA, Wilk KM, Budnicka M, Olsen M, Bangale YA, Hunter RL, Kruzel ML, Actor JK
Vaccine, 2007-07-27;25(37):6730-43.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CD8+ T cells are not required for vaccine-induced immunity against Leishmania amazonensis in IL-12/23P40(-/-) C57BL/6 mice.
Authors: Hernandez Sanabria MX, Afonso LC, Golgher D, Tafuri WL, Vieira LQ
Microbes Infect., 2007-05-18;9(9):1124-34.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity.
Authors: Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, Collins C, Viala J, Ferrero RL, Girardin SE, Philpott DJ
Immunity, 2007-04-12;26(4):445-59.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Differing effects of clarithromycin and azithromycin on cytokine production by murine dendritic cells.
Authors: Sugiyama K, Shirai R, Mukae H, Ishimoto H, Nagata T, Sakamoto N, Ishii H, Nakayama S, Yanagihara K, Mizuta Y, Kohno S
Clin. Exp. Immunol., 2007-03-01;147(3):540-6.
Species: Mouse
Sample Types: Cell Culture Supernates
-
An alternative pathway of NF-kappaB activation results in maturation and T cell priming activity of dendritic cells overexpressing a mutated IkappaBalpha.
Authors: Moore F, Buonocore S, Aksoy E, Ouled-Haddou N, Goriely S, Lazarova E, Paulart F, Heirman C, Vaeremans E, Thielemans K, Goldman M, Flamand V
J. Immunol., 2007-02-01;178(3):1301-11.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Transgenic expression of a CD83-immunoglobulin fusion protein impairs the development of immune-competent CD4-positive T cells.
Authors: Luthje K, Cramer SO, Ehrlich S, Veit A, Steeg C, Fleischer B, Bonin A, Breloer M
Eur. J. Immunol., 2006-08-01;36(8):2035-45.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Negative regulation of interleukin-2 and p38 mitogen-activated protein kinase during T-cell activation by the adaptor ALX.
Authors: Perchonock CE, Fernando MC, Quinn WJ, Nguyen CT, Sun J, Shapiro MJ, Shapiro VS
Mol. Cell. Biol., 2006-08-01;26(16):6005-15.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Exogenous IL-12 suppresses experimental autoimmune encephalomyelitis (EAE) by tuning IL-10 and IL-5 levels in an IFN-gamma-dependent way.
Authors: Berghmans N, Dillen C, Heremans H
J. Neuroimmunol., 2006-06-09;176(1):63-75.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Effects of dexamethazone on LPS-induced activationand migration of mouse dendritic cells revealed by a genome-wide transcriptional analysis.
Authors: Vizzardelli C, Pavelka N, Luchini A, Zanoni I, Bendickson L, Pelizzola M, Beretta O, Foti M, Granucci F, Nilsen-Hamilton M, Ricciardi-Castagnoli P
Eur. J. Immunol., 2006-06-01;36(6):1504-15.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Induction of CTLA-4-mediated anergy contributes to persistent colonization in the murine model of gastric Helicobacter pylori infection.
Authors: Anderson KM, Czinn SJ, Redline RW, Blanchard TG
J. Immunol., 2006-05-01;176(9):5306-13.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The effects of dehydroepiandrosterone (DHEA) on in vitro spleen cell proliferation and cytokine production.
Authors: Powell JM, Sonnenfeld G
J. Interferon Cytokine Res., 2006-01-01;26(1):34-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Leukemia inhibitory factor is linked to regulatory transplantation tolerance.
Authors: Metcalfe SM, Watson TJ, Shurey S, Adams E, Green CJ
Transplantation, 2005-03-27;79(6):726-30.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Comparative immunomodulatory properties of a chitosan-MDP adjuvant combination following intranasal or intramuscular immunisation.
Authors: Moschos SA, Bramwell VW, Somavarapu S, Alpar HO
Vaccine, 2005-03-14;23(16):1923-30.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Leukaemia inhibitory factor (LIF) is functionally linked to axotrophin and both LIF and axotrophin are linked to regulatory immune tolerance.
Authors: Metcalfe SM, Muthukumarana PA, Thompson HL, Haendel MA, Lyons GE
FEBS Lett., 2005-01-31;579(3):609-14.
Species: Human
Sample Types: Cell Culture Supernates
-
Subcellular fractions of Brucella ovis distinctively induce the production of interleukin-2, interleukin-4, and interferon-gamma in mice.
Authors: Salas-Tellez E, Nunez del Arco A, Tenorio V, Diaz-Aparicio E, de la Garza M, Suarez-Guemes F
Can. J. Vet. Res., 2005-01-01;69(1):53-7.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Comparison of immunogenicities of recombinant Plasmodium vivax merozoite surface protein 1 19- and 42-kiloDalton fragments expressed in Escherichia coli.
Authors: Sachdeva S, Ahmad G, Malhotra P, Mukherjee P, Chauhan VS
Infect. Immun., 2004-10-01;72(10):5775-82.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Active hexose correlated compound enhances the immune function of mice in the hindlimb-unloading model of spaceflight conditions.
Authors: Aviles H, Belay T, Vance M, Sun B, Sonnenfeld G
J. Appl. Physiol., 2004-06-11;97(4):1437-44.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Intracellular thiols contribute to Th2 function via a positive role in IL-4 production.
Authors: Monick MM, Samavati L, Butler NS, Mohning M, Powers LS, Yarovinsky T, Spitz DR, Hunninghake GW
J. Immunol., 2003-11-15;171(10):5107-15.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Transcutaneous immunization with cholera toxin B subunit adjuvant suppresses IgE antibody responses via selective induction of Th1 immune responses.
Authors: Anjuere F, George-Chandy A, Audant F, Rousseau D, Holmgren J, Czerkinsky C
J. Immunol., 2003-02-01;170(3):1586-92.
Species: Mouse
Sample Types:
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse IL-2 DuoSet ELISA
Average Rating: 4 (Based on 2 Reviews)
Have you used Mouse IL-2 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: