Mouse IL-4 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant mouse IL-4. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
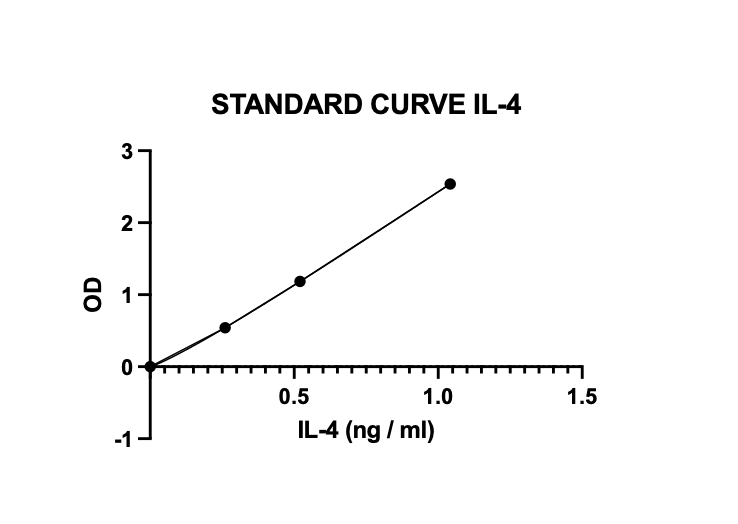
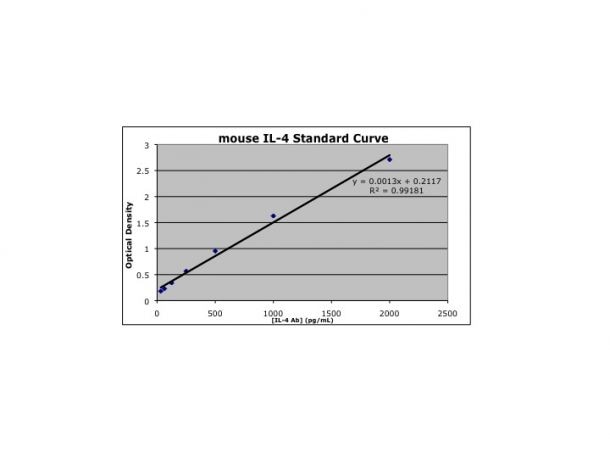
Scientific Data
Preparation and Storage
Background: IL-4
IL-4 (Interleukin-4) is a cytokine that is primarily expressed by Th2-biased CD4+ T cells, mast cells, basophils, and eosinophils. It promotes cell proliferation, survival, and immunoglobulin class switch to IgG4 and IgE in human B cells, acquisition of the Th2 phenotype by naïve CD4+ T cells, priming and chemotaxis of mast cells, eosinophils, and basophils, and the proliferation and activation of epithelial cells. IL-4 plays a dominant role in the development of allergic inflammation and asthma. The type I receptor for IL-4, which is expressed on hematopoietic cells, is a heterodimer of the ligand binding IL-4 R? and the common ? chain (a shared subunit of the receptors for IL-2, -7, -9, -15, and -21). The type II receptor on nonhematopoietic cells consists of IL-4 R? and IL-13 R?1. The type II receptor also transduces IL-13 mediated signals.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse IL-4 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
146
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Caffeic Acid Inhibits Degranulation, Cytokine Secretions, and IP3 Receptor 2 Gene Expression in Compound 48/80-Stimulated Mouse P815 Mast Cells
Authors: Chang, KC;Lin, JY;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Laquinimod treatment attenuates EAU by inhibiting both the inductive and effector phases in an APC-dependent manner
Authors: Xu, B;Mattapallil, MJ;Jia, X;Tang, J;Horai, R;Caspi, RR;Gery, I;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
TLR7 Promotes Acute Inflammatory-Driven Lung Dysfunction in Influenza-Infected Mice but Prevents Late Airway Hyperresponsiveness
Authors: Miles, MA;Liong, S;Liong, F;Trollope, GS;Wang, H;Brooks, RD;Bozinovski, S;O'Leary, JJ;Brooks, DA;Selemidis, S;
International journal of molecular sciences
Species: Mouse, Transgenic Mouse
Sample Types: BALF
-
Eosinophil-Airway Epithelial Cells crosstalk reveals the eosinophils-mediated DUOX1 up-regulation in a murine allergic inflammation setting
Authors: Raggi, C;Spadaro, F;Mattei, F;Gambardella, AR;Noto, F;Andreone, S;Signore, M;Schiavoni, G;Parolini, I;Afferni, C;
Journal of leukocyte biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Intermittent ozone inhalation during house dust mite-induced sensitization primes for adverse asthma phenotype
Authors: Hussain, S;Majumder, N;Mazumder, MHH;Lewis, SE;Olapeju, O;Velayutham, M;Amin, MS;Brundage, K;Kelley, EE;Vanoirbeek, J;
Redox biology
Species: Mouse
Sample Types: Tissue Homogenates, BALF
-
The potential of mesenchymal stem cell coexpressing cytosine deaminase and secretory IL18-FC chimeric cytokine in suppressing glioblastoma recurrence
Authors: Taheri, M;Tehrani, HA;Farzad, SA;Korourian, A;Arefian, E;Ramezani, M;
International immunopharmacology
Species: Mouse
Sample Types: Serum
-
Oral and topical administration of a geranyl acetophenone attenuates DNCB-induced atopic dermatitis-like skin lesions in BALB/c mice
Authors: Mohd Kasim, VNK;Lee, YZ;Bakrin, IH;Hussain, MK;Israf, DA;Shaari, K;Tan, JW;Lee, MT;Tham, CL;
Scientific reports
Species: Mouse
Sample Types: Tissue Homogenates
-
Malaria transmission blocking activity of Anopheles stephensi alanyl aminopeptidase N antigen formulated with MPL, CpG, and QS21 adjuvants
Authors: Pourhashem, Z;Nourani, L;Pirahmadi, S;Yousefi, H;J Sani, J;Raz, A;Zakeri, S;Dinparast Djadid, N;Abouie Mehrizi, A;
PloS one
Species: Mouse
Sample Types: Cell Culture Supernates
-
Host gastric corpus microenvironment facilitates Ascaris suum larval hatching and infection in a murine model
Authors: Wu, Y;Adeniyi-Ipadeola, G;Adkins-Threats, M;Seasock, M;Suarez-Reyes, C;Fujiwara, R;Bottazzi, ME;Song, L;Mills, JC;Weatherhead, JE;
PLoS neglected tropical diseases
Species: Mouse
Sample Types: Tissue Homogenates
-
IFN-?3 is induced by Leishmania donovani and can inhibit parasite growth in cell line models but not in the mouse model, while it shows a significant association with leishmaniasis in humans
Authors: De, M;Sukla, S;Bharatiya, S;Keshri, S;Roy, DG;Roy, S;Dutta, D;Saha, S;Ejazi, SA;Ravichandiran, V;Ali, N;Chatterjee, M;Chinnaswamy, S;
Infection and immunity
Species: Mouse
Sample Types: Serum
-
Cullin5 drives experimental asthma exacerbations by modulating alveolar macrophage antiviral immunity
Authors: Zhang, H;Xue, K;Li, W;Yang, X;Gou, Y;Su, X;Qian, F;Sun, L;
Nature communications
Species: Mouse, Transgenic Mouse
Sample Types: BALF
-
Design and fabrication of a vaccine candidate based on rOmpA from Klebsiella pneumoniae encapsulated in silk fibroin-sodium alginate nanoparticles against pneumonia infection
Authors: Shahbazi, S;Habibi, M;Badmasti, F;Sabzi, S;Farokhi, M;Asadi Karam, MR;
International immunopharmacology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Acrylamide, an Air Pollutant, Enhances Allergen-Induced Eosinophilic Lung Inflammation via Group 2 Innate Lymphoid Cells
Authors: Su, HH;Cheng, CM;Yang, YN;Chang, YW;Li, CY;Wu, ST;Lin, CC;Wu, HE;Suen, JL;
Mucosal immunology
Species: Mouse
Sample Types: BALF, Cell Culture Supernates
-
Camellia sinensis (L.) Kuntze Extract Attenuates Ovalbumin-Induced Allergic Asthma by Regulating Airway Inflammation and Mucus Hypersecretion
Authors: Kang, S;Kim, HY;Lee, AY;Kim, HS;Park, JH;Moon, BC;Nam, HH;Chae, SW;Jung, B;Moon, C;Shin, IS;Kim, JS;Seo, YS;
Pharmaceutics
Species: Mouse
Sample Types: BALF, Plasma
-
Role of the IL-33/ST2 Activation Pathway in the Development of the Hepatic Fibrosis Induced by Schistosoma mansoni Granulomas in Mice
Authors: Maggi, L;Camelo, GMA;Rocha, IC;Pereira Alves, W;Moreira, JMP;Almeida Pereira, T;Tafuri, WL;Rabelo, ÉML;Correa, A;Ecco, R;Negrão-Corrêa, DA;
International journal of molecular sciences
Species: Mouse
Sample Types: Tissue Homogenates
-
cis interaction of CD153 with TCR/CD3 is crucial for the pathogenic activation of senescence-associated T�cells
Authors: Y Fukushima, K Sakamoto, M Matsuda, Y Yoshikai, H Yagita, D Kitamura, M Chihara, N Minato, M Hattori
Cell Reports, 2022-09-20;40(12):111373.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Protective and vaccine dose-sparing efficacy of Poly I:C-functionalized calcium phosphate nanoparticle adjuvants in inactivated influenza vaccination
Authors: J Lee, SY Ahn, CTT Le, DH Lee, J Jung, EJ Ko
International immunopharmacology, 2022-09-14;112(0):109240.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Inhaled delivery of recombinant interferon-lambda restores allergic inflammation after development of asthma by controlling Th2- and Th17-cell-mediated immune responses
Authors: J Won, A Jo, CH Gil, S Kim, H Shin, H Jik Kim
International immunopharmacology, 2022-08-27;112(0):109180.
Species: Mouse
Sample Types: BALF
-
Extracellular matrix of early pulmonary fibrosis modifies the polarization of alveolar macrophage
Authors: Y Zhang, L Zhu, J Hong, C Chen
International immunopharmacology, 2022-08-24;111(0):109179.
Species: Mouse
Sample Types: Cell Culture Supernates
-
In vitro and in vivo Characterization of Host-Pathogen Interactions of the L3881 Candida albicans Clinical Isolate
Authors: PHF Sucupira, TR Moura, ILS Gurgel, TTP Pereira, ACB Padovan, MM Teixeira, D Bahia, FM Soriani
Frontiers in Microbiology, 2022-07-11;13(0):901442.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Menthone Inhalation Alleviates Local and Systemic Allergic Inflammation in Ovalbumin-Sensitized and Challenged Asthmatic Mice
Authors: YH Su, JY Lin
International Journal of Molecular Sciences, 2022-04-04;23(7):.
Species: Mouse
Sample Types: BALF
-
Immunogenic properties of empty pcDNA3 plasmid against zoonotic cutaneous leishmaniasis in mice
Authors: H Montakhab-, R Shafiei, M Najm, L Masoori, A Aspatwar, A Badirzadeh
PLoS ONE, 2022-02-15;17(2):e0263993.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Resveratrol attenuates inflammation and apoptosis through alleviating endoplasmic reticulum stress via Akt/mTOR pathway in fungus-induced allergic airways inflammation
Authors: S Wang, L Gong, Y Mo, J Zhang, Z Jiang, Z Tian, C Shao
International immunopharmacology, 2021-12-27;103(0):108489.
Species: Mouse
Sample Types: BALF
-
DNA-based artificial dendritic cells for in situ cytotoxic T cell stimulation and immunotherapy
Authors: QV Le, J Lee, J Byun, G Shim, YK Oh
Bioactive materials, 2021-12-23;15(0):160-172.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Metabolic Regulation of Macrophages by SIRT1 Determines Activation During Cholestatic Liver Disease in Mice
Authors: A Isaacs-Ten, M Moreno-Gon, C Bone, A Martens, F Bernuzzi, T Ludwig, C Hellmich, K Hiller, SA Rushworth, N Beraza
Cellular and Molecular Gastroenterology and Hepatology, 2021-12-22;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Transient Ascaris suum larval migration induces intractable chronic pulmonary disease and anemia in mice
Authors: Y Wu, E Li, M Knight, G Adeniyi-Ip, LZ Song, AR Burns, AC Gazzinelli, R Fujiwara, ME Bottazzi, JE Weatherhea
PloS Neglected Tropical Diseases, 2021-12-16;15(12):e0010050.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Endangered Lymphocytes: The Effects of Alloxan and Streptozotocin on Immune Cells in Type 1 Induced Diabetes
Authors: LAD Queiroz, JB Assis, JPT Guimarães, ESA Sousa, AC Milhomem, KKS Sunahara, A Sá-Nunes, JO Martins
Mediators of Inflammation, 2021-10-19;2021(0):9940009.
Species: Mouse
Sample Types: Tissue Homogenates
-
Ding Chuan Tang Attenuates Airway Inflammation and Eosinophil Infiltration in Ovalbumin-Sensitized Asthmatic Mice
Authors: J Ma, MX Liu, LC Chen, JJ Shen, ML Kuo
BioMed Research International, 2021-09-20;2021(0):6692772.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Diesel exhaust particles increase nasal symptoms and IL-17A in house dust mite-induced allergic mice
Authors: HJ Jung, YK Ko, WS Shim, HJ Kim, DY Kim, CS Rhee, MK Park, DH Han
Scientific Reports, 2021-08-11;11(1):16300.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The role of Sp140 revealed in IgE and mast cell responses in Collaborative Cross mice
Authors: K Matsushita, X Li, Y Nakamura, D Dong, K Mukai, M Tsai, SB Montgomery, SJ Galli
JCI Insight, 2021-06-22;6(12):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Allergen Exposure in Murine Neonates Promoted the Development of Asthmatic Lungs
Authors: JC Chen, CC Chan, NC Ting, ML Kuo
Biomedicines, 2021-06-18;9(6):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Ameliorative effects of eosinophil deficiency on immune response, endoplasmic reticulum stress, apoptosis, and autophagy in fungus-induced allergic lung inflammation
Authors: S Wang, Z Jiang, L Li, J Zhang, C Zhang, C Shao
Respiratory Research, 2021-06-07;22(1):173.
Species: Mouse
Sample Types: BALF
-
Opisthorchis viverrini antigens up-regulates the expression of CD80 and MHC class II in JAWSII mouse dendritic cells and promotes IL-10 and TGF-&beta secretions
Authors: S Jittimanee, S Wongratana, C Kaewraemru, J Jittimanee
Parasitology international, 2021-05-31;84(0):102401.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Adjuvants Polyphosphazene (PCEP) and a Combination of Curdlan Plus Leptin Promote a Th17-Type Immune Response to an Intramuscular Vaccine in Mice
Authors: A Chaffey, G Hamonic, D Chand, GK Mutwiri, HL Wilson
Vaccines, 2021-05-14;9(5):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
&alpha-Phellandrene exhibits antinociceptive and tumor-reducing effects in a mouse model of oncologic pain
Authors: FR Pinheiro-N, EM Lopes, BT Acha, LDS Gomes, WA Dias, ACD Reis Filho, BS Leal, DCDN Rodrigues, JDN Silva, D Dittz, PMP Ferreira, FRC Almeida
Toxicology and Applied Pharmacology, 2021-03-17;418(0):115497.
Species: Mouse
Sample Types: Serum
-
Neuroinflammaging underlies emotional disturbances and circadian rhythm disruption in young male senescence-accelerated mouse prone 8 mice
Authors: N Ito, H Takemoto, A Hasegawa, C Sugiyama, K Honma, T Nagai, Y Kobayashi, H Odaguchi
Exp Gerontol, 2020-10-16;0(0):111109.
Species: Mouse
Sample Types: Serum
-
Combination of Innate Immune Modulators as Vaccine Adjuvants in Mice
Authors: A Haddadi, A Chaffey, SH Ng, D Yalamati, HL Wilson
Vaccines (Basel), 2020-10-01;8(4):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
In silico analysis and in vivo assessment of a novel epitope-based vaccine candidate against uropathogenic Escherichia coli
Authors: S Hasanzadeh, M Habibi, MA Shokrgozar, R Ahangari C, K Ahmadi, MR Asadi Kara, S Bouzari
Sci Rep, 2020-10-01;10(1):16258.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Antileishmanial activity of a new chloroquine analog in an animal model of Leishmania panamensis infection
Authors: L Herrera, A Llanes, J Álvarez, K Degracia, CM Restrepo, R Rivera, DE Stephens, HT Dang, OV Larionov, R Lleonart, PL Fernández
Int J Parasitol Drugs Drug Resist, 2020-08-15;14(0):56-61.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immunomodulatory Effect of a Salvia plebeia R. Aqueous Extract in Forced Swimming Exercise-induced Mice
Authors: J Shin, OK Kim, S Kim, D Bae, J Lee, J Park, W Jun
Nutrients, 2020-07-28;12(8):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
High-dose intranasal application of titanium dioxide nanoparticles induces the systemic uptakes and allergic airway inflammation in asthmatic mice
Authors: S Abdulnasse, M Hannig, DD Le, S Heck, M Leitner, AJ Omlor, I Tavernaro, A Kraegeloh, R Kautenburg, G Kickelbick, A Beilhack, M Bischoff, J Nguyen, M Sester, R Bals, QT Dinh
Respir. Res., 2020-07-02;21(1):168.
Species: Mouse
Sample Types: Serum
-
Evaluation of M2-like macrophage enrichment after diffuse traumatic brain injury through transient interleukin-4 expression from engineered mesenchymal stromal cells
Authors: SF Enam, SR Kader, N Bodkin, JG Lyon, M Calhoun, C Azrak, PM Tiwari, D Vanover, H Wang, PJ Santangelo, RV Bellamkond
J Neuroinflammation, 2020-06-20;17(1):197.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Strain-Specific Differences in House Dust Mite (Dermatophagoides farinae)-Induced Mouse Models of Allergic Rhinitis
Authors: KI Lee, JS Bae, EH Kim, JH Kim, L Lyu, YJ Chung, JH Mo
Clin Exp Otorhinolaryngol, 2020-05-15;0(0):.
Species: Mouse
Sample Types: cell culture supernatant
-
Combination therapy using human papillomavirus L1/E6/E7 genes and archaeosome: a nanovaccine confer immuneadjuvanting effects to fight cervical cancer
Authors: H Karimi, H Soleimanja, A Abdoli, RS Banijamali
Sci Rep, 2020-04-01;10(1):5787.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Strongyloides venezuelensis-infection alters the profile of cytokines and liver inflammation in mice co-infected with Schistosoma mansoni
Authors: MC de Rezende, JMP Moreira, LLM Fernandes, VF Rodrigues, D Negrão-Cor
Cytokine, 2019-11-26;127(0):154931.
Species: Mouse
Sample Types: Tissue Homogenates
-
SOCS2 modulates adipose tissue inflammation and expansion in mice
Authors: CH Val, MC de Oliveir, DR Lacerda, A Barroso, NV Batista, Z Menezes-Ga, DRR de Assis, AT Cramer, F Brant, MM Teixeira, D Glória Sou, AM Ferreira, FS Machado
J. Nutr. Biochem., 2019-11-23;76(0):108304.
Species: Mouse
Sample Types: Tissue Homogenates
-
Non-hematopoietic STAT6 induces epithelial tight junction dysfunction and promotes intestinal inflammation and tumorigenesis
Authors: Y Lin, B Li, X Yang, T Liu, T Shi, B Deng, Y Zhang, L Jia, Z Jiang, R He
Mucosal Immunol, 2019-09-18;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
IL-33 drives group 2 innate lymphoid cell-mediated protection during Clostridium difficile infection
Authors: AL Frisbee, MM Saleh, MK Young, JL Leslie, ME Simpson, MM Abhyankar, CA Cowardin, JZ Ma, P Pramoonjag, SD Turner, AP Liou, EL Buonomo, WA Petri
Nat Commun, 2019-06-20;10(1):2712.
Species: Mouse
Sample Types: Tissue Homogenates
-
Anti-leishmanial activity of Brevinin 2R and its Lauric acid conjugate type against L. major: In vitro mechanism of actions and in vivo treatment potentials
Authors: F Zahedifard, H Lee, JH No, M Salimi, N Seyed, A Asoodeh, S Rafati
PLoS Negl Trop Dis, 2019-02-27;13(2):e0007217.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Basophils are dispensable for the establishment of protective adaptive immunity against primary and challenge infection with the intestinal helminth parasite Strongyloides ratti
Authors: M Reitz, ML Brunn, D Voehringer, M Breloer
PLoS Negl Trop Dis, 2018-11-29;12(11):e0006992.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Brief Exposure of Skin to Near-Infrared Laser Modulates Mast Cell Function and Augments the Immune Response
Authors: Y Kimizuka, W Katagiri, JJ Locascio, A Shigeta, Y Sasaki, M Shibata, K Morse, RF Sîrbulescu, M Miyatake, P Reeves, M Suematsu, J Gelfand, T Brauns, MC Poznansky, K Tsukada, S Kashiwagi
J. Immunol., 2018-11-12;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Single-cell RNA-Seq analysis identifies a noncoding interleukin 4 (IL-4) RNA that post-transcriptionally up-regulates IL-4 production in T helper cells
Authors: W Yin, Y Song, X Chang
J. Biol. Chem., 2018-11-07;294(1):290-298.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Plant Proteinase Inhibitor CrataBL Plays a Role in Controlling Asthma Response in Mice
Authors: ASS Bortolozzo, APD Rodrigues, FM Arantes-Co, BM Saraiva-Ro, FCR de Souza, TR Brüggemann, MV de Brito, RDS Ferreira, MTDS Correia, PMG Paiva, CM Prado, EA Leick, MLV Oliva, MA Martins, VC Ruiz-Schut, RF Righetti, IFLC Tibério
Biomed Res Int, 2018-10-01;2018(0):9274817.
Species: Mouse
Sample Types: Tissue Homogenates
-
PrtA immunization fails to protect against pulmonary and invasive infection by Streptococcus pneumoniae
Authors: CF Hsu, CH Hsiao, SF Tseng, JR Chen, YJ Liao, SJ Chen, CS Lin, HK Sytwu, YP Chuang
Respir. Res., 2018-09-25;19(1):187.
Species: Mouse
Sample Types: Whole Cells
-
T cell microvilli constitute immunological synaptosomes that carry messages to antigen-presenting cells
Authors: HR Kim, Y Mun, KS Lee, YJ Park, JS Park, JH Park, BN Jeon, CH Kim, Y Jun, YM Hyun, M Kim, SM Lee, CS Park, SH Im, CD Jun
Nat Commun, 2018-09-07;9(1):3630.
Species: Mouse
Sample Types: Cell Lysates
-
Low dose of chlorine exposure exacerbates nasal and pulmonary allergic inflammation in mice
Authors: IS de Genaro, FM de Almeida, DC Hizume-Kun, HT Moriya, RA Silva, JCG Cruz, RB Lopes, RF Righetti, R de Paula V, M Saiki, MA Martins, IFLC Tibério, FM Arantes-Co, BM Saraiva-Ro
Sci Rep, 2018-08-22;8(1):12636.
Species: Mouse
Sample Types: Tissue Homogenates
-
Airway Epithelial Cell-Derived Colony Stimulating Factor-1 Promotes Allergen Sensitization
Authors: Hyung-Geun Moon, Seung-jae Kim, Jong Jin Jeong, Seon-Sook Han, Nizar N. Jarjour, Hyun Lee et al.
Immunity
Species: Mouse
Sample Types: Cell Culture Supernates
-
Repeated Allergen Exposure in A/J Mice Causes Steroid-Insensitive Asthma via a Defect in Glucocorticoid Receptor Bioavailability
Authors: MF Serra, AC Cotias, CRR Pão, JB Daleprane, PB Jurgilas, GC Couto, EA Anjos-Valo, RSB Cordeiro, VF Carvalho, PMR Silva, MA Martins
J. Immunol., 2018-06-18;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Canonical PI3K? signaling in myeloid cells restricts Trypanosoma cruzi infection and dampens chagasic myocarditis
Authors: MC Silva, M Davoli-Fer, TS Medina, R Sesti-Cost, GK Silva, CD Lopes, LE Cardozo, FN Gava, K Lyroni, FC Dias, AF Frade, M Baron, HI Nakaya, F Figueiredo, JC Alves-Filh, FQ Cunha, C Tsatsanis, C Chevillard, E Cunha-Neto, E Hirsch, JS Silva, TM Cunha
Nat Commun, 2018-04-17;9(1):1513.
Species: Mouse
Sample Types: Serum
-
A novel multi-peptide subunit vaccine admixed with AddaVax adjuvant produces significant immunogenicity and protection against Proteus mirabilis urinary tract infection in mice model
Authors: E Choubini, M Habibi, A Khorshidi, A Ghasemi, MR Asadi Kara, S Bouzari
Mol. Immunol., 2018-03-09;96(0):88-97.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Standard -
Age-related cognitive impairment is associated with long-term neuroinflammation and oxidative stress in a mouse model of episodic systemic inflammation
Authors: JC d'Avila, LD Siqueira, A Mazeraud, EP Azevedo, D Foguel, HC Castro-Far, T Sharshar, F Chrétien, FA Bozza
J Neuroinflammation, 2018-01-30;15(1):28.
Species: Mouse
Sample Types: Tissue Homogenates
-
Human Neutrophil Peptide 1 as immunotherapeutic agent against Leishmania infected BALB/c mice
Authors: Z Abdossamad, N Seyed, F Zahedifard, T Taheri, Y Taslimi, H Montakhab-, A Badirzadeh, M Vasei, S Gharibzade, S Rafati
PLoS Negl Trop Dis, 2017-12-18;11(12):e0006123.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain
Authors: AT Brini, G Amodeo, LM Ferreira, A Milani, S Niada, G Moschetti, S Franchi, E Borsani, LF Rodella, AE Panerai, P Sacerdote
Sci Rep, 2017-08-29;7(1):9904.
Species: Mouse
Sample Types: Tissue Homogenates
-
Erythroid Suppressor Cells Compromise Neonatal Immune Response against Bordetella pertussis
Authors: G Dunsmore, N Bozorgmehr, C Delyea, P Koleva, A Namdar, S Elahi
J. Immunol., 2017-08-04;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Inducing maternal inflammation promotes leptin production in offspring but does not improve allergic symptoms in a mouse model of allergic rhinitis
Authors: A Imai, K Satoi, E Fujimoto, K Sato
Heliyon, 2017-06-22;3(6):e00327.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Somatic extracts of Marshallagia marshalli downregulate the Th2 associated immune responses in ovalbumin-induced airway inflammation in BALB/c mice
Authors: S Parande Sh, A Ebrahimby, A Dousty, M Maleki, A Movassaghi, H Borji, A Haghparast
Parasit Vectors, 2017-05-12;10(1):233.
Species: Mouse
Sample Types: BALF
-
Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation
Authors: M Toussaint, DJ Jackson, D Swieboda, A Guedán, TD Tsouroukts, YM Ching, C Radermecke, H Makrinioti, J Aniscenko, MR Edwards, R Solari, F Farnir, V Papayannop, F Bureau, T Marichal, SL Johnston
Nat. Med., 2017-05-01;23(6):681-691.
Species: Mouse
Sample Types: Tissue Homogenates
-
Water-soluble chitosan inhibits nerve growth factor and attenuates allergic inflammation in mite allergen-induced allergic rhinitis
Authors: PC Chen, MH Hsieh, P Wen-ShuoKu, HF Kao, CL Hsu, JY Wang
J. Allergy Clin. Immunol., 2017-04-12;0(0):.
Species: Mouse
Sample Types: NLF
-
Experimental atopic dermatitis depends on IL-33R signaling via MyD88 in dendritic cells
Authors: C Li, I Maillet, C Mackowiak, C Viala, F Di Padova, M Li, D Togbe, V Quesniaux, Y Lai, B Ryffel
Cell Death Dis, 2017-04-06;8(4):e2735.
Species: Mouse
Sample Types: Tissue Homogenates
-
Semiconductor diode laser device adjuvanting intradermal vaccine
Authors: Y Kimizuka, JJ Callahan, Z Huang, K Morse, W Katagiri, A Shigeta, R Bronson, S Takeuchi, Y Shimaoka, MP Chan, Y Zeng, B Li, H Chen, RY Tan, C Dwyer, T Mulley, P Leblanc, C Goudie, J Gelfand, K Tsukada, T Brauns, MC Poznansky, D Bean, S Kashiwagi
Vaccine, 2017-03-30;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mitochondrial CaMKII inhibition in airway epithelium protects against allergic asthma
Authors: SC Sebag, OM Koval, JD Paschke, CJ Winters, OA Jaffer, R Dworski, FS Sutterwala, ME Anderson, IM Grumbach
JCI Insight, 2017-02-09;2(3):e88297.
Species: Mouse
Sample Types: Tissue Homogenates
-
Suppression of colitis by adoptive transfer of helminth antigen-treated dendritic cells requires interleukin-4 receptor-� signaling
Authors: CE Matisz, B Faz-L¢pez, E Thomson, A Al Rajabi, F Lopes, LI Terrazas, A Wang, KA Sharkey, DM McKay
Sci Rep, 2017-01-17;7(0):40631.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Acid-Sensing Ion Channel 1a Contributes to Airway Hyperreactivity in Mice
PLoS ONE, 2016-11-07;11(11):e0166089.
Species: Mouse
Sample Types: BALF
-
Comparison of potential protection conferred by three immunization strategies (protein/protein, DNA/DNA, and DNA/protein) against Brucella infection using Omp2b in BALB/c Mice
Authors: Saeid Bouzari
Vet. Microbiol., 2016-11-05;197(0):47-52.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The soluble guanylyl cyclase activator BAY 60-2770 inhibits murine allergic airways inflammation and human eosinophil chemotaxis
Authors: Edson Antunes
Pulm Pharmacol Ther, 2016-11-03;0(0):.
Species: Mouse
Sample Types: BALF
-
Testosterone-Mediated Endocrine Function and TH1/TH2 Cytokine Balance after Prenatal Exposure to Perfluorooctane Sulfonate: By Sex Status
Int J Mol Sci, 2016-09-12;17(9):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Allergen-Induced IL-6 Regulates IL-9/IL-17A Balance in CD4+ T Cells in Allergic Airway Inflammation
J Immunol, 2016-08-29;197(7):2653-64.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Alternatively Activated Mononuclear Phagocytes from the Skin Site of Infection and the Impact of IL-4R? Signalling on CD4+T Cell Survival in Draining Lymph Nodes after Repeated Exposure to Schistosoma mansoni Cercariae
PLoS Negl Trop Dis, 2016-08-09;10(8):e0004911.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Editor's Highlight: Subvisible Aggregates of Immunogenic Proteins Promote a Th1-Type Response
Authors: Jeremy P Derrick
Toxicol. Sci., 2016-06-30;153(2):258-70.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Impact of Histone H1 on the Progression of Allergic Rhinitis and Its Suppression by Neutralizing Antibody in Mice
Authors: T Nakano, R Kamei, T Fujimura, Y Takaoka, A Hori, CY Lai, KC Chiang, Y Shimada, N Ohmori, T Goto, K Ono, CL Chen, S Goto, S Kawamoto
PLoS ONE, 2016-04-18;11(4):e0153630.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-22 Restrains Tapeworm-Mediated Protection against Experimental Colitis via Regulation of IL-25 Expression
Authors: JL Reyes, MR Fernando, F Lopes, G Leung, NL Mancini, CE Matisz, A Wang, DM McKay
PLoS Pathog, 2016-04-07;12(4):e1005481.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Repeated Activation of Lung Invariant NKT Cells Results in Chronic Obstructive Pulmonary Disease-Like Symptoms.
Authors: Tsao C, Tsao P, Chen Y, Chuang Y
PLoS ONE, 2016-01-26;11(1):e0147710.
Species: Mouse
Sample Types: BALF
-
CD252 regulates mast cell mediated, CD1d-restricted NKT-cell activation in mice.
Authors: Gonzalez Roldan N, Orinska Z, Ewers H, Bulfone-Paus S
Eur J Immunol, 2015-12-09;46(2):432-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Prostaglandin E2 Production and T Cell Function in Mouse Adenovirus Type 1 Infection following Allogeneic Bone Marrow Transplantation.
Authors: McCarthy M, Procario M, Wilke C, Moore B, Weinberg J
PLoS ONE, 2015-09-25;10(9):e0139235.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Superior Suppressive Capacity of Skin Tregs Compared with Lung Tregs in a Model of Epicutaneous Priming.
Authors: Mahapatra S, Albrecht M, Baru A, Sparwasser T, Herrick C, Dittrich A
J Invest Dermatol, 2015-05-22;135(10):2418-26.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CD4+ T cell hyporesponsiveness after repeated exposure to Schistosoma mansoni larvae is dependent upon interleukin-10.
Authors: Prendergast C, Sanin D, Cook P, Mountford A
Infect Immun, 2015-01-26;83(4):1418-30.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Intestinal helminths regulate lethal acute graft-versus-host disease and preserve the graft-versus-tumor effect in mice.
Authors: Li Y, Chen H, Bannick N, Henry M, Holm A, Metwali A, Urban J, Rothman P, Weiner G, Blazar B, Elliott D, Ince M
J Immunol, 2014-12-19;194(3):1011-20.
Species: Mouse
Sample Types: Cell Culture Supernates
-
SLy2 controls the antibody response to pneumococcal vaccine through an IL-5Ralpha-dependent mechanism in B-1 cells.
Authors: Schmitt F, Schall D, Bucher K, Schindler T, Hector A, Biedermann T, Zemlin M, Hartl D, Beer-Hammer S
Eur J Immunol, 2014-11-13;45(1):60-70.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A ThPOK-LRF transcriptional node maintains the integrity and effector potential of post-thymic CD4+ T cells.
Authors: Vacchio M, Wang L, Bouladoux N, Carpenter A, Xiong Y, Williams L, Wohlfert E, Song K, Belkaid Y, Love P, Bosselut R
Nat Immunol, 2014-08-17;15(10):947-56.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lifetime-dependent effects of bisphenol A on asthma development in an experimental mouse model.
Authors: Petzold, Susanne, Averbeck, Marco, Simon, Jan C, Lehmann, Irina, Polte, Tobias
PLoS ONE, 2014-06-20;9(6):e100468.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The glycosylated Rv1860 protein of mycobacterium tuberculosis inhibits dendritic cell mediated TH1 and TH17 polarization of T cells and abrogates protective immunity conferred by BCG.
Authors: Satchidanandam V, Kumar N, Jumani R, Challu V, Elangovan S, Khan N
PLoS Pathog, 2014-06-12;10(6):e1004176.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-17 contributes to neutrophil recruitment but not to control of viral replication during acute mouse adenovirus type 1 respiratory infection.
Authors: McCarthy M, Zhu L, Procario M, Weinberg J
Virology, 2014-04-19;456(0):259-67.
Species: Mouse
Sample Types: Cell Culture Supernates
-
An iron-acquisition-deficient mutant of Corynebacterium pseudotuberculosis efficiently protects mice against challenge.
Authors: Ribeiro D, Rocha F, Leite K, Soares S, Silva A, Portela R, Meyer R, Miyoshi A, Oliveira S, Azevedo V, Dorella F
Vet Res, 2014-03-06;45(0):28.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Signaling via tumor necrosis factor receptor 1 but not Toll-like receptor 2 contributes significantly to hydrosalpinx development following Chlamydia muridarum infection.
Authors: Dong X, Liu Y, Chang X, Lei L, Zhong G
Infect Immun, 2014-02-18;82(5):1833-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Foxp3(+) regulatory T cells delay expulsion of intestinal nematodes by suppression of IL-9-driven mast cell activation in BALB/c but not in C57BL/6 mice.
Authors: Blankenhaus B, Reitz M, Brenz Y, Eschbach M, Hartmann W, Haben I, Sparwasser T, Huehn J, Kuhl A, Feyerabend T, Rodewald H, Breloer M
PLoS Pathog, 2014-02-06;10(2):e1003913.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Delineating the role of histamine-1- and -4-receptors in a mouse model of Th2-dependent antigen-specific skin inflammation.
Authors: Mahapatra S, Albrecht M, Behrens B, Jirmo A, Behrens G, Hartwig C, Neumann D, Raap U, Bahre H, Herrick C, Dittrich A
PLoS ONE, 2014-02-04;9(2):e87296.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis.
Authors: Hams, Emily, Armstrong, Michelle, Barlow, Jillian, Saunders, Sean P, Schwartz, Christia, Cooke, Gordon, Fahy, Ruairi J, Crotty, Thomas B, Hirani, Nikhil, Flynn, Robin J, Voehringer, David, McKenzie, Andrew N, Donnelly, Seamas C, Fallon, Padraic
Proc Natl Acad Sci U S A, 2013-12-16;111(1):367-72.
Species: Transgenic Rat
Sample Types: Tissue Homogenates
-
Bifidobacterium longum alleviates dextran sulfate sodium-induced colitis by suppressing IL-17A response: involvement of intestinal epithelial costimulatory molecules.
Authors: Miyauchi, Eiji, Ogita, Tasuku, Miyamoto, Junki, Kawamoto, Seiji, Morita, Hidetosh, Ohno, Hiroshi, Suzuki, Takuya, Tanabe, Soichi
PLoS ONE, 2013-11-08;8(11):e79735.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Rac2-deficiency leads to exacerbated and protracted colitis in response to Citrobacter rodentium infection.
Authors: Fattouh R, Guo C, Lam G, Gareau M, Ngan B, Glogauer M, Muise A, Brumell J
PLoS ONE, 2013-04-16;8(4):e61629.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Nanogel-based PspA intranasal vaccine prevents invasive disease and nasal colonization by Streptococcus pneumoniae.
Authors: Kong, Il Gyu, Sato, Ayuko, Yuki, Yoshikaz, Nochi, Tomonori, Takahashi, Haruko, Sawada, Shinichi, Mejima, Mio, Kurokawa, Shiho, Okada, Kazunari, Sato, Shintaro, Briles, David E, Kunisawa, Jun, Inoue, Yusuke, Yamamoto, Masafumi, Akiyoshi, Kazunari, Kiyono, Hiroshi
Infect Immun, 2013-03-04;81(5):1625-34.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Genetic deletion of the HIF-1alpha isoform I.1 in T cells enhances antibacterial immunity and improves survival in a murine peritonitis model.
Authors: Georgiev P, Belikoff B, Hatfield S, Ohta A, Sitkovsky M, Lukashev D
Eur J Immunol, 2013-01-31;43(3):655-66.
Species: Mouse
Sample Types: Serum
-
Immune Modulation Mediated by Cryptococcal Laccase Promotes Pulmonary Growth and Brain Dissemination of Virulent Cryptococcus neoformans in Mice.
Authors: Qiu Y, Davis M, Dayrit J, Hadd Z, Meister D, Osterholzer J, Williamson P, Olszewski M
PLoS ONE, 2012-10-22;7(10):e47853.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor.
J. Exp. Med., 2012-09-10;209(10):1753-67, S1.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Control of allergen-induced inflammation and hyperresponsiveness by the metalloproteinase ADAMTS-12.
Authors: Paulissen G, El Hour M, Rocks N, Gueders M, Bureau F, Foidart J, Lopez-Otin C, Noel A, Cataldo D
J Immunol, 2012-09-07;189(8):4135-43.
Species: Mouse
Sample Types: Tissue Homogenates
-
TLR9 and MyD88 are crucial for the development of protective immunity to malaria.
Authors: Gowda NM, Wu X, Gowda DC
J. Immunol., 2012-04-18;188(10):5073-85.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs.
Authors: Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H
J. Immunol., 2011-12-23;188(3):1503-13.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection.
Authors: Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, Riva F, Garlanda C
Infect. Immun., 2011-10-24;80(1):100-9.
Species: Mouse
Sample Types: Serum
-
T cell-derived Act1 is necessary for IL-25-mediated Th2 responses and allergic airway inflammation.
Authors: Swaidani S, Bulek K, Kang Z
J. Immunol., 2011-08-19;187(6):3155-64.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Api6/AIM/Sp{alpha}/CD5L Overexpression in Alveolar Type II Epithelial Cells Induces Spontaneous Lung Adenocarcinoma.
Authors: Li Y, Qu P, Wu L, Li B, Du H, Yan C
Cancer Res., 2011-06-22;71(16):5488-99.
Species: Mouse
Sample Types: Serum
-
The Therapeutic Potential of the Humoral Pattern Recognition Molecule PTX3 in Chronic Lung Infection Caused by Pseudomonas aeruginosa.
Authors: Moalli F, Paroni M, Veliz Rodriguez T, Riva F, Polentarutti N, Bottazzi B, Valentino S, Mantero S, Nebuloni M, Mantovani A, Bragonzi A, Garlanda C
J. Immunol., 2011-03-25;186(9):5425-34.
Species: Mouse
Sample Types: Tissue Homogenates
-
Alpha-Galactosylceramide-induced airway eosinophilia is mediated through the activation of NKT cells.
Authors: Chuang YH, Wang TC, Jen HY, Yu AL, Chiang BL
J. Immunol., 2011-03-07;186(8):4687-92.
Species: Mouse
Sample Types: BALF
-
A critical role for C5L2 in the pathogenesis of experimental allergic asthma.
Authors: Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, Konig P, Gerard NP, Gerard C, Wills-Karp M, Kohl J
J. Immunol., 2010-10-25;185(11):6741-52.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mucosal delivery of human papillomavirus pseudovirus-encapsidated plasmids improves the potency of DNA vaccination.
Authors: Graham BS, Kines RC, Corbett KS, Nicewonger J, Roberts JN, Schiller JT, Buck CB
Mucosal Immunol, 2010-06-16;3(5):475-86.
Species: Mouse
Sample Types: BALF
-
Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity.
Authors: Sellge G, Magalhaes JG, Konradt C, Fritz JH, Salgado-Pabon W, Eberl G, Bandeira A, Di Santo JP, Sansonetti PJ, Phalipon A
J. Immunol., 2010-01-20;184(4):2076-85.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Effects of umbelliferone in a murine model of allergic airway inflammation.
Authors: Vasconcelos JF, Teixeira MM, Barbosa-Filho JM, Agra MF, Nunes XP, Giulietti AM, Ribeiro-Dos-Santos R, Soares MB
Eur. J. Pharmacol., 2009-03-14;609(1):126-31.
Species: Mouse
Sample Types: BALF
-
A chlamydial type III-secreted effector protein (Tarp) is predominantly recognized by antibodies from humans infected with Chlamydia trachomatis and induces protective immunity against upper genital tract pathologies in mice.
Authors: Wang J, Chen L, Chen F, Zhang X, Zhang Y, Baseman J, Perdue S, Yeh IT, Shain R, Holland M, Bailey R, Mabey D, Yu P, Zhong G
Vaccine, 2009-03-10;27(22):2967-80.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Decoy Receptor 3 (DcR3, TNFRSF6B) suppresses Th17 immune responses and is abundant in human cerebrospinal fluid.
Authors: Mueller AM, Pedre X, Killian S, David M, Steinbrecher A
J. Neuroimmunol., 2009-03-06;209(1):57-64.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mucosal immunity in mice induced by orally administered transgenic rice.
Authors: Zhang X, Yuan Z, Duan Q, Zhu H, Yu H, Wang Q
Vaccine, 2009-01-13;27(10):1596-600.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis.
Authors: Kaya Z, Goser S, Buss SJ, Leuschner F, Ottl R, Li J, Volkers M, Zittrich S, Pfitzer G, Rose NR, Katus HA
Circulation, 2008-10-27;118(20):2063-72.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function.
Authors: Csoka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, Deitch EA, Spolarics Z, Nemeth ZH, Hasko G
FASEB J., 2008-07-14;22(10):3491-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Streptococcus equi antigens adsorbed onto surface modified poly-epsilon-caprolactone microspheres induce humoral and cellular specific immune responses.
Authors: Florindo HF, Pandit S, Goncalves LM, Alpar HO, Almeida AJ
Vaccine, 2008-06-17;26(33):4168-77.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Amelioration of experimental autoimmune encephalomyelitis in IL-4Ralpha-/- mice implicates compensatory up-regulation of Th2-type cytokines.
Authors: Gaupp S, Cannella B, Raine CS
Am. J. Pathol., 2008-06-05;173(1):119-29.
Species: Mouse
Sample Types: Cell Culture Supernates
-
VAMP-8 segregates mast cell-preformed mediator exocytosis from cytokine trafficking pathways.
Authors: Tiwari N, Wang CC, Brochetta C, Ke G, Vita F, Qi Z, Rivera J, Soranzo MR, Zabucchi G, Hong W, Blank U
Blood, 2008-01-18;111(7):3665-74.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection.
Authors: Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC
J. Immunol., 2008-01-15;180(2):1080-7.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Transforming growth factor-beta regulates house dust mite-induced allergic airway inflammation but not airway remodeling.
Authors: Fattouh R, Midence NG, Arias K, Johnson JR, Walker TD, Goncharova S, Souza KP, Gregory RC, Lonning S, Gauldie J, Jordana M
Am. J. Respir. Crit. Care Med., 2008-01-03;177(6):593-603.
Species: Mouse
Sample Types: BALF
-
CD40L disruption enhances Abeta vaccine-mediated reduction of cerebral amyloidosis while minimizing cerebral amyloid angiopathy and inflammation.
Authors: Obregon D, Hou H, Bai Y, Nikolic WV, Mori T, Luo D, Zeng J, Ehrhart J, Fernandez F, Morgan D, Giunta B, Town T, Tan J
Neurobiol. Dis., 2007-10-16;29(2):336-53.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The related adaptors, adaptor in lymphocytes of unknown function X and Rlk/Itk-binding protein, have nonredundant functions in lymphocytes.
Authors: Perchonock CE, Pajerowski AG, Nguyen C, Shapiro MJ, Shapiro VS
J. Immunol., 2007-08-01;179(3):1768-75.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis.
Authors: Hwang SA, Wilk KM, Budnicka M, Olsen M, Bangale YA, Hunter RL, Kruzel ML, Actor JK
Vaccine, 2007-07-27;25(37):6730-43.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism.
Authors: Levy BD, Lukacs NW, Berlin AA, Schmidt B, Guilford WJ, Serhan CN, Parkinson JF
FASEB J., 2007-07-11;21(14):3877-84.
Species: Mouse
Sample Types: Tissue Homogenates
-
Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity.
Authors: Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, Collins C, Viala J, Ferrero RL, Girardin SE, Philpott DJ
Immunity, 2007-04-12;26(4):445-59.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Differing effects of clarithromycin and azithromycin on cytokine production by murine dendritic cells.
Authors: Sugiyama K, Shirai R, Mukae H, Ishimoto H, Nagata T, Sakamoto N, Ishii H, Nakayama S, Yanagihara K, Mizuta Y, Kohno S
Clin. Exp. Immunol., 2007-03-01;147(3):540-6.
Species: Mouse
Sample Types: Cell Culture Supernates
-
An alternative pathway of NF-kappaB activation results in maturation and T cell priming activity of dendritic cells overexpressing a mutated IkappaBalpha.
Authors: Moore F, Buonocore S, Aksoy E, Ouled-Haddou N, Goriely S, Lazarova E, Paulart F, Heirman C, Vaeremans E, Thielemans K, Goldman M, Flamand V
J. Immunol., 2007-02-01;178(3):1301-11.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Pulmonary distribution, regulation, and functional role of Trk receptors in a murine model of asthma.
Authors: Nassenstein C, Dawbarn D, Pollock K, Allen SJ, Erpenbeck VJ, Spies E, Krug N, Braun A
J. Allergy Clin. Immunol., 2006-07-03;118(3):597-605.
Species: Mouse
Sample Types: BALF
-
Exogenous IL-12 suppresses experimental autoimmune encephalomyelitis (EAE) by tuning IL-10 and IL-5 levels in an IFN-gamma-dependent way.
Authors: Berghmans N, Dillen C, Heremans H
J. Neuroimmunol., 2006-06-09;176(1):63-75.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immunity induced with a Salmonella enterica serovar Enteritidis live vaccine is regulated by Th1-cell-dependent cellular and humoral effector mechanisms in susceptible BALB/c mice.
Authors: Lehmann J, Springer S, Werner CE, Lindner T, Bellmann S, Straubinger RK, Selbitz HJ, Alber G
Vaccine, 2006-03-29;24(22):4779-93.
Species: Mouse
Sample Types: Serum
-
Hierarchical suppression of asthma-like responses by mucosal tolerance.
Authors: Keller AC, Mucida D, Gomes E, Faquim-Mauro E, Faria AM, Rodriguez D, Russo M
J. Allergy Clin. Immunol., 2006-02-01;117(2):283-90.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The effects of dehydroepiandrosterone (DHEA) on in vitro spleen cell proliferation and cytokine production.
Authors: Powell JM, Sonnenfeld G
J. Interferon Cytokine Res., 2006-01-01;26(1):34-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Leukemia inhibitory factor is linked to regulatory transplantation tolerance.
Authors: Metcalfe SM, Watson TJ, Shurey S, Adams E, Green CJ
Transplantation, 2005-03-27;79(6):726-30.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Comparative immunomodulatory properties of a chitosan-MDP adjuvant combination following intranasal or intramuscular immunisation.
Authors: Moschos SA, Bramwell VW, Somavarapu S, Alpar HO
Vaccine, 2005-03-14;23(16):1923-30.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Leukaemia inhibitory factor (LIF) is functionally linked to axotrophin and both LIF and axotrophin are linked to regulatory immune tolerance.
Authors: Metcalfe SM, Muthukumarana PA, Thompson HL, Haendel MA, Lyons GE
FEBS Lett., 2005-01-31;579(3):609-14.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The IgE adjuvant effect of particles: characterisation of the primary cellular response in the draining lymph node.
Authors: Nygaard UC, Ormstad H, Aase A, Lovik M
Toxicology, 2005-01-15;206(2):181-93.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Subcellular fractions of Brucella ovis distinctively induce the production of interleukin-2, interleukin-4, and interferon-gamma in mice.
Authors: Salas-Tellez E, Nunez del Arco A, Tenorio V, Diaz-Aparicio E, de la Garza M, Suarez-Guemes F
Can. J. Vet. Res., 2005-01-01;69(1):53-7.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Comparison of immunogenicities of recombinant Plasmodium vivax merozoite surface protein 1 19- and 42-kiloDalton fragments expressed in Escherichia coli.
Authors: Sachdeva S, Ahmad G, Malhotra P, Mukherjee P, Chauhan VS
Infect. Immun., 2004-10-01;72(10):5775-82.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Active hexose correlated compound enhances the immune function of mice in the hindlimb-unloading model of spaceflight conditions.
Authors: Aviles H, Belay T, Vance M, Sun B, Sonnenfeld G
J. Appl. Physiol., 2004-06-11;97(4):1437-44.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Intracellular thiols contribute to Th2 function via a positive role in IL-4 production.
Authors: Monick MM, Samavati L, Butler NS, Mohning M, Powers LS, Yarovinsky T, Spitz DR, Hunninghake GW
J. Immunol., 2003-11-15;171(10):5107-15.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Transcutaneous immunization with cholera toxin B subunit adjuvant suppresses IgE antibody responses via selective induction of Th1 immune responses.
Authors: Anjuere F, George-Chandy A, Audant F, Rousseau D, Holmgren J, Czerkinsky C
J. Immunol., 2003-02-01;170(3):1586-92.
Species: Mouse
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse IL-4 DuoSet ELISA
Average Rating: 4.8 (Based on 9 Reviews)
Have you used Mouse IL-4 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Great kit, easy to use and very reliable.
We used this kit to detect the IL-4 production from mice immunized by our vaccine candidate, the kit really works well and convenient, and cheaper compared to other kits. We highly recommended it
I have used DuoSet ELISAs for years and appreciate the simplicity and ease of use for the kits. We don't perform ELISAs at the same rate we used to, but when we needed to do a quick IL-4 test of some sera. I purchased this kit, the accessory kit and was able to do all I needed quickly. I recommend DuoSet ELISA kits to everyone.