Rat IL-6 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant rat IL-6. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
Normal Goat Serum: (Catalog # DY005)
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Normal Goat Serum: (Catalog # DY005)
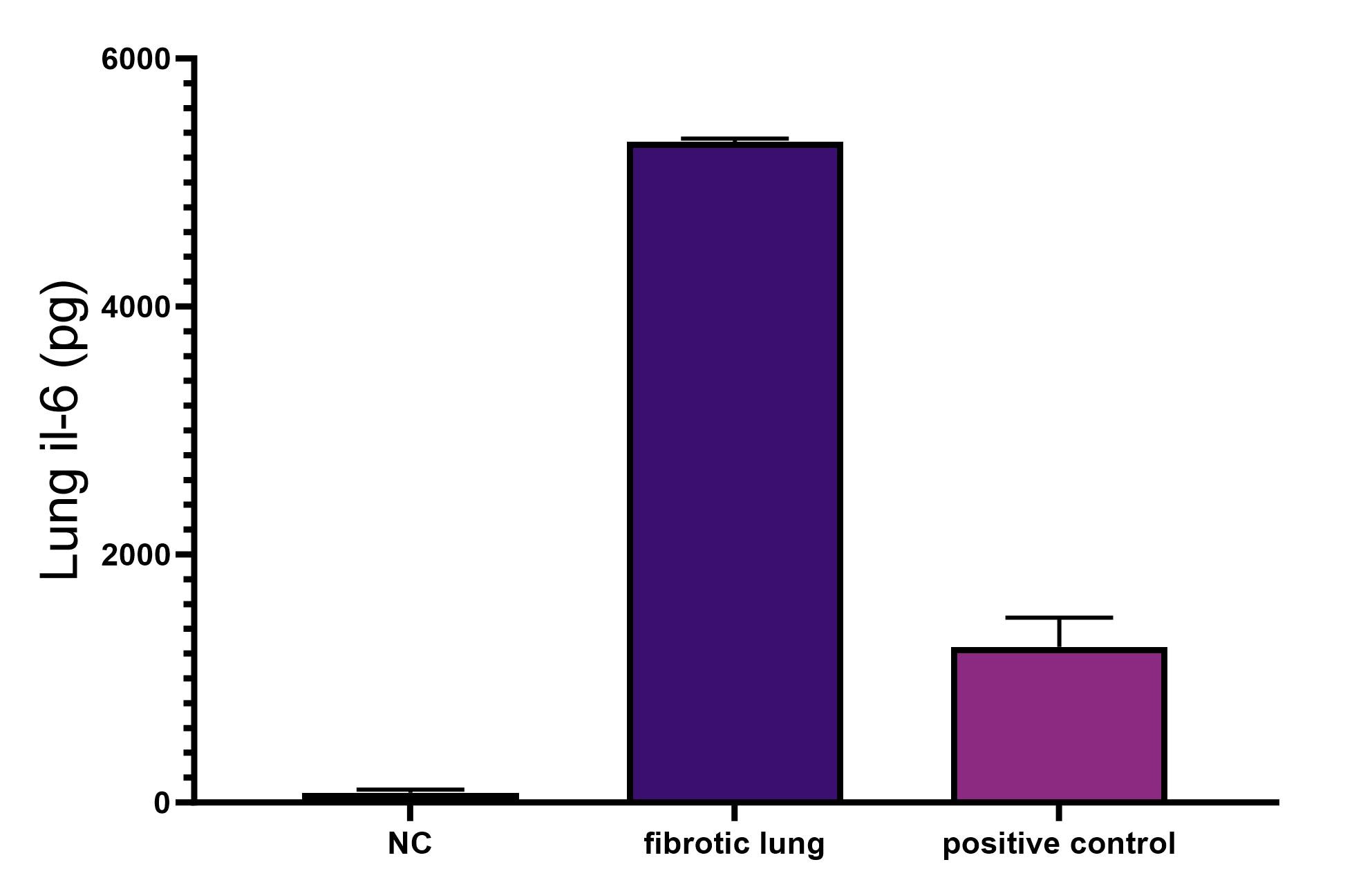
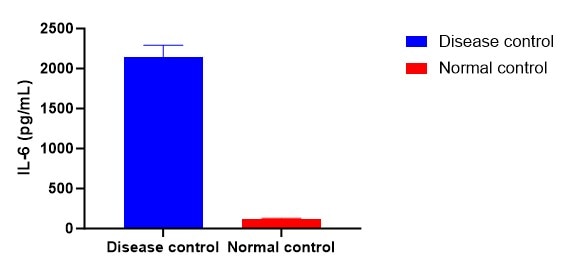
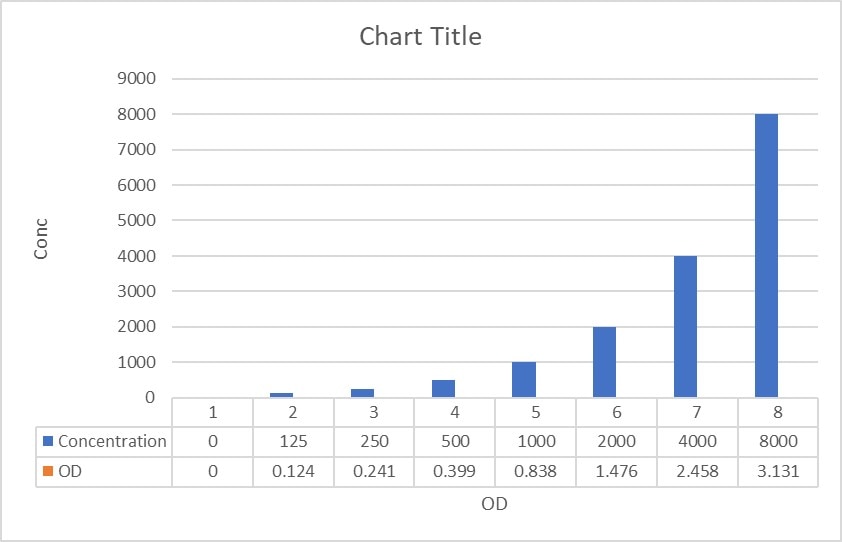
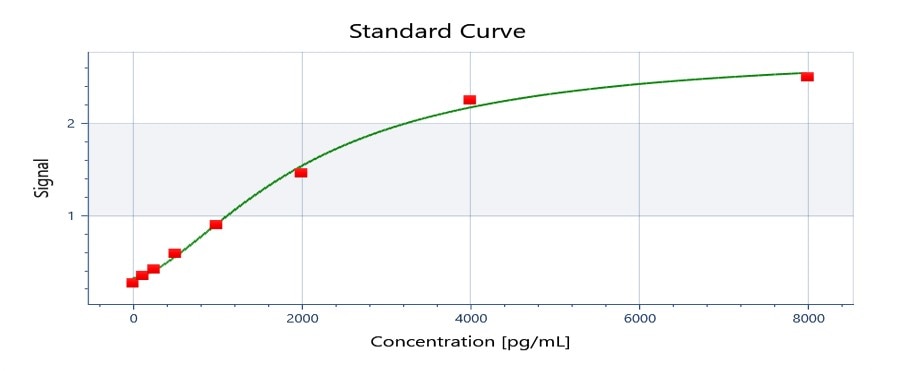
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-6
Interleukin 6 (IL-6) is a pleiotropic, a-helical, 22-28 kDa phosphorylated and variably glycosylated cytokine that plays important roles in the acute phase reaction, inflammation, hematopoiesis, bone metabolism, and cancer progression. Mature human IL-6 is 183 amino acids (aa) in length and shares 39% aa sequence identity with mouse and rat IL-6. Alternative splicing generates several isoforms with internal deletions, some of which exhibit antagonistic properties. Cells known to express IL-6 include CD8+ T cells, fibroblasts, synoviocytes, adipocytes, osteoblasts, megakaryocytes, endothelial cells (under the influence of endothelins), sympathetic neurons, cerebral cortex neurons, adrenal medulla chromaffin cells, retinal pigment cells, mast cells, keratinocytes, Langerhans cells, fetal and adult astrocytes, neutrophils, monocytes, eosinophils, colonic epithelial cells, B1 B cells and pancreatic islet beta cells. IL-6 production is generally correlated with cell activation and is normally kept in control by glucocorticoids, catecholamines, and secondary sex steroids. Normal human circulating IL-6 is in the 1 pg/mL range, with slight elevations during the menstrual cycle, modest elevations in certain cancers, and large elevations after surgery.
IL-6 induces signaling through a cell surface heterodimeric receptor complex composed of a ligand binding subunit (IL-6 R alpha) and a signal transducing subunit (gp130). IL-6 binds to IL-6 Ra, triggering IL-6 Ra association with gp130 and gp130 dimerization. gp130 is also a component of the receptors for CLC, CNTF, CT-1, IL-11, IL-27, LIF, and OSM. Soluble forms of IL-6 Ra are generated by both alternative splicing and proteolytic cleavage. In a mechanism known as trans-signaling, complexes of soluble IL-6 and IL-6 Ra elicit responses from gp130-expressing cells that lack cell surface IL-6 Ra. Trans-signaling enables a wider range of cell types to respond to IL-6, as the expression of gp130 is ubiquitous, while that of IL-6 Ra is predominantly restricted to hepatocytes, monocytes, and resting lymphocytes. Soluble splice forms of gp130 block trans-signaling from IL-6/IL-6 Ra but not from other cytokines that use gp130 as a co-receptor.
IL-6, along with TNF-a and IL-1, drives the acute inflammatory response. IL-6 is almost solely responsible for fever and the acute phase response in the liver, and it is important in the transition from acute inflammation to either acquired immunity or chronic inflammatory disease. When dysregulated, it contributes to chronic inflammation in conditions such as obesity, insulin resistance, inflammatory bowel disease, arthritis, and sepsis. IL-6 modulates bone resorption and is a major effector of inflammatory joint destruction in rheumatoid arthritis through its promotion of Th17 cell development and activity. It contributes to atherosclerotic plaque development and destabilization as well as the development of inflammation-associated carcinogenesis.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent with NGS, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Rat IL-6 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
105
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Trichosanthes kirilowii Maxim. and Bioactive Compound Cucurbitacin D Alleviate Cisplatin-Induced Peripheral Neuropathy In Vitro and In Vivo
Authors: Kang, S;Choi, G;Kim, D;Kim, H;Cheon, C;Ko, SG;
Integrative cancer therapies
Species: Rat
Sample Types: Serum
-
Cardioprotective effect of senotherapy in chronically obese middle-aged female rats may be mediated by a MERCSs/Nrf2 interaction
Authors: Silva-Palacios, A;Zúñiga-Muñoz, AM;Soria-Castro, E;Álvarez-León, E;Nieto, M;Navarrete-Anastasio, G;Carbó, R;García-Niño, WR;López-Cervantes, PS;Salas-Venegas, V;Flores-Torres, RP;Luna-López, A;Zazueta, C;Königsberg, M;
The Journal of nutritional biochemistry
Species: Rat
Sample Types: Serum
-
A high fraction of inspired oxygen does not mitigate atelectasis-induced lung tissue hypoxia or injury in experimental acute respiratory distress syndrome
Authors: Tojo, K;Yazawa, T;
Scientific reports
Species: Rat
Sample Types: BALF
-
Ginsenosides, salidroside, and syringin complex exhibits anti-fatigue in exhaustive exercise rats
Authors: Wu, YC;Lian, YZ;Zhao, H;Wang, L;Ning, D;Chao, JC;
International journal of medical sciences
Species: Rat
Sample Types: Serum, Tissue Homogenates
-
Hemoadsorption improves kidney microcirculatory oxygenation and oxygen consumption, ameliorates tubular injury, and improves kidney function in a rat model of sepsis-induced AKI
Authors: Ergin, B;Kutucu, DE;Kapucu, A;van Dam, W;Moretto, L;Heyman, P;Ince, C;
Scientific reports
Species: Rat
Sample Types: Plasma
-
Periodontitis and diabetes in pregnant rats: Maternal-fetal outcomes
Authors: Souza, SS;Lopes Cruz, L;Alves-Reis, AM;Costa, VQ;Moraes-Souza, RQ;Damasceno, DC;Volpato, GT;
Heliyon
Species: Rat
Sample Types: Serum
-
Metabolic flexibility ensures proper neuronal network function in moderate neuroinflammation
Authors: Chausse, B;Malorny, N;Lewen, A;Poschet, G;Berndt, N;Kann, O;
Scientific reports
Species: Rat
Sample Types: Cell Culture Supernates
-
Physical training attenuates systemic cytokine response and tissue damage triggered by apical periodontitis
Authors: Ferreira, RO;Pereira, MS;Souza-Monteiro, D;Frazão, DR;de Moura, JDM;Baia-da-Silva, DC;Bittencourt, LO;Balbinot, GS;Collares, FM;Lima, MLS;de Araújo, AA;Lima, RR;
Scientific reports
Species: Rat
Sample Types: Serum
-
Methamphetamine Enhancement of HIV-1 gp120-Mediated NLRP3 Inflammasome Activation and Resultant Proinflammatory Responses in Rat Microglial Cultures
Authors: Dutta, D;Liu, J;Xu, E;Xiong, H;
International Journal of Molecular Sciences
Species: Rat
Sample Types: Cell Culture Supernates
-
Oxyberberine protects middle cerebral artery occlusion triggered cerebral injury through TLR4/NLRP3 pathway in rats
Authors: Rahman, Z;Shaikh, AS;Rao, KV;Dandekar, MP;
Journal of chemical neuroanatomy
Species: Rat
Sample Types: Antibody
-
MANF protein expression is upregulated in immune cells in the ischemic human brain and systemic recombinant MANF delivery in rat ischemic stroke model demonstrates anti-inflammatory effects
Authors: Anttila, JE;Mattila, OS;Liew, HK;Mätlik, K;Mervaala, E;Lindholm, P;Lindahl, M;Lindsberg, PJ;Tseng, KY;Airavaara, M;
Acta neuropathologica communications
Species: Rat
Sample Types: Tissue Homogenates
-
Doxorubicin-Induced Cardiomyopathy: A Preliminary Study on the Cardioprotective Benefits of 7-Hydroxyflavanone
Authors: Sangweni, NF;Gabuza, K;van Aarde, R;Mabasa, L;van Vuuren, D;Huisamen, B;Barry, R;Johnson, R;
International journal of molecular sciences
Species: Rat
Sample Types: Cell Culture Supernates
-
Granulocyte differentiation of rat bone marrow resident C-kit+ hematopoietic stem cells induced by mesenchymal stem cells could be considered as new option in cell-based therapy
Authors: Farahzadi, R;Fathi, E;Mesbah-Namin, SA;Vietor, I;
Regenerative therapy
Species: Rat
Sample Types: Cell Culture Supernates
-
Potential Benefits of Epidermal Growth Factor for Inhibiting Muscle Degrative Markers in Rats with Alcoholic Liver Damage
Authors: Xiao, Q;Chen, YH;Chen, YL;Chien, YS;Hsieh, LH;Shirakawa, H;Yang, SC;
International journal of molecular sciences
Species: Rat
Sample Types: Tissue Homogenates
-
Targeting TGF-beta/periostin signaling by sesamol ameliorates pulmonary fibrosis and improves lung function and survival
Authors: SK Tirunavall, M Kuncha, R Sistla, SB Andugulapa
The Journal of nutritional biochemistry, 2023-03-21;0(0):109294.
Species: Rat
Sample Types: Tissue Homogenates
-
Investigating behavioral phenotypes related to autism spectrum disorder in a gene-environment interaction model of Cntnap2 deficiency and Poly I:C maternal immune activation
Authors: FL Haddad, C De Oliveir, S Schmid
Frontiers in Neuroscience, 2023-03-14;17(0):1160243.
Species: Rat
Sample Types: Serum
-
Efficacy and Safety Evaluation of Mometasone Furoate in Treating Ocular Inflammation
Authors: NA Lage, MRB de Paiva, DV Vasconcelo, RR Machado, SL Fialho, A Silva-Cunh
Pharmaceutics, 2023-01-05;15(1):.
Species: Rat
Sample Types: Tissue Homogenates
-
A nanofiber-hydrogel composite improves tissue repair in a rat model of Crohn's disease perianal fistulas
Authors: L Li, ZC Yao, A Parian, YH Yang, J Chao, J Yin, KJ Salimian, SK Reddy, A Zaheer, SL Gearhart, HQ Mao, FM Selaru
Science Advances, 2023-01-04;9(1):eade1067.
Species: Rat
Sample Types: Tissue Homogenates
-
Sex dimorphism in Resolvin D5-induced analgesia in rat models of trigeminal pain
Authors: DF Baggio, FMR da Luz, RV Lopes, LEN Ferreira, EI Araya, JG Chichorro
The journal of pain, 2022-12-28;0(0):.
Species: Rat
Sample Types: Whole Tissue
-
DAMPs Released from Proinflammatory Macrophages Induce Inflammation in Cardiomyocytes via Activation of TLR4 and TNFR
Authors: C Neu, Y Thiele, F Horr, C Beckers, N Frank, G Marx, L Martin, S Kraemer, E Zechendorf
International Journal of Molecular Sciences, 2022-12-08;23(24):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Mitochondrial protective effects caused by the administration of mefenamic acid in sepsis
Authors: D Dominguini, M Michels, LB Wessler, EL Streck, T Barichello, F Dal-Pizzol
Journal of Neuroinflammation, 2022-11-04;19(1):268.
Species: Rat
Sample Types: Tissue Homogenates
-
Effect of citronellol on oxidative stress, neuroinflammation and autophagy pathways in an in vivo model of Parkinson's disease
Authors: RL Jayaraj, S Azimullah, KA Parekh, SK Ojha, R Beiram
Heliyon, 2022-11-03;8(11):e11434.
Species: Rat
Sample Types: Tissue Homogenates
-
Glia from the central and peripheral nervous system are differentially affected by paclitaxel chemotherapy via modulating their neuroinflammatory and neuroregenerative properties
Authors: I Klein, J Boenert, F Lange, B Christense, MK Wassermann, MHJ Wiesen, DN Olschewski, M Rabenstein, C Müller, HC Lehmann, GR Fink, M Schroeter, MA Rueger, SU Vay
Frontiers in Pharmacology, 2022-11-02;13(0):1038285.
Species: Rat
Sample Types: Cell Culture Supernates
-
Changes in the proteome and secretome of rat liver sinusoidal endothelial cells during early primary culture and effects of dexamethasone
Authors: R Li, S Bhandari, I Martinez-Z, JA Bruun, I Urbarova, B Smedsrød, J Simón-Sant, KK Sørensen
PLoS ONE, 2022-09-02;17(9):e0273843.
Species: Rat
Sample Types: Cell Culture Supernates
-
Safety of Special Waveform of Transcranial Electrical Stimulation (TES): In Vivo Assessment
Authors: M Adeel, CC Chen, BS Lin, HC Chen, JC Liou, YT Li, CW Peng
International Journal of Molecular Sciences, 2022-06-20;23(12):.
Species: Rat
Sample Types: Tissue Homogenates
-
The superior beneficial effects of exercise training versus hormone replacement therapy on skeletal muscle of ovariectomized rats
Authors: SB Silva, K Honorato-S, SP Costa, TE Domingues, TMM da Cruz, CM Rodrigues, KB Costa, JM Dos Santos, VK da Silva L, TP Gaiad, AP Santos, MF Dias-Peixo, CC Coimbra, AM Dos Reis, RE Szawka, PHS Figueiredo, HS Costa, MX Oliveira, VA Mendonça, ACR Lacerda
Scientific Reports, 2022-05-24;12(1):8764.
Species: Rat
Sample Types: Tissue Homogenates
-
Low-Dose Aspirin Augments the Anti-Inflammatory Effects of Low-Dose Lithium in Lipopolysaccharide-Treated Rats
Authors: R Shvartsur, G Agam, S Uzzan, AN Azab
Pharmaceutics, 2022-04-20;14(5):.
Species: Rat
Sample Types: Tissue Homogenates
-
The evaluation of cytotoxicity and cytokine IL-6 production of root canal sealers with and without the incorporation of simvastatin: an invitro study
Authors: A Sharma, K Sanjeev, VMJ Selvanatha, M Sekar, N Harikrishn
BMC Oral Health, 2022-01-11;22(1):6.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Anti-inflammatory role of GM1 and other gangliosides on microglia
Authors: D Galleguill, Q Wang, N Steinberg, A Zaidi, G Shrivastav, K Dhami, GC Daskhan, EN Schmidt, Z Dworsky-Fr, F Giuliani, M Churchward, C Power, K Todd, A Taylor, MS Macauley, S Sipione
Journal of Neuroinflammation, 2022-01-06;19(1):9.
Species: Rat
Sample Types: Cell Culture Supernates
-
Exposure to Nickel Oxide Nanoparticles Induces Acute and Chronic Inflammatory Responses in Rat Lungs and Perturbs the Lung Microbiome
Authors: MJ Jeong, S Jeon, HS Yu, WS Cho, S Lee, D Kang, Y Kim, YJ Kim, SY Kim
International Journal of Environmental Research and Public Health, 2022-01-04;19(1):.
Species: Rat
Sample Types: BALF
-
Effect of Pulmonary Inflammation by Surface Functionalization of Zinc Oxide Nanoparticles
Authors: A Jung, SH Kim, JY Yang, J Jeong, JK Lee, JH Oh, JH Lee
Toxics, 2021-12-06;9(12):.
Species: Rat
Sample Types: BALF
-
Prenatal administration of IL-1Ra attenuate the neurodevelopmental impacts following non-pathogenic inflammation during pregnancy
Authors: ME Brien, K Hughes, S Girard
Scientific Reports, 2021-12-03;11(1):23404.
Species: Rat
Sample Types: Placenta
-
Moderate Physical Exercise Activates ATR2 Receptors, Improving Inflammation and Oxidative Stress in the Duodenum of 2K1C Hypertensive Rats
Authors: ACA da Silva, JS Severo, BLB Dos Santos, PHM Mendes, LMS Nobre, AP de Oliveir, FCS Ferreira, JVR Medeiros, RC Lima-Junio, A Havt, RC Palheta-Ju, AA Dos Santos, M Tolentino
Frontiers in Physiology, 2021-10-14;12(0):734038.
Species: Rat
Sample Types: Tissue Homogenates
-
Neuron-Derived Extracellular Vesicles Modulate Microglia Activation and Function
Authors: H Peng, BT Harvey, CI Richards, K Nixon
Biology, 2021-09-22;10(10):.
Species: Rat
Sample Types: Cell Culture Supernates
-
The Prophylactic Effects of Glutamine on Muscle Protein Synthesis and Degradation in Rats with Ethanol-Induced Liver Damage
Authors: Q Xiao, YH Chen, SA Pratama, YL Chen, H Shirakawa, HC Peng, SC Yang
Nutrients, 2021-08-14;13(8):.
Species: Rat
Sample Types: Tissue Homogenates
-
Programming of intestinal homeostasis in male rat offspring after maternal exposure to chlorpyrifos and/or to a high fat diet
Authors: M Guibourden, H El Khayat, N Djekkoun, H Khorsi-Cau, V Bach, PM Anton, J Gay-Quéhei
Scientific Reports, 2021-06-01;11(1):11420.
Species: Rat
Sample Types: Plasma
-
In Vitro Evaluation of the Anti-Inflammatory Effect of KMUP-1 and In Vivo Analysis of Its Therapeutic Potential in Osteoarthritis
Authors: SE Huang, E Sulistyowa, YY Chao, BN Wu, ZK Dai, JH Hsu, JL Yeh
Biomedicines, 2021-05-28;9(6):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Local Immune Changes in Early Stages of Inflammation and Carcinogenesis Correlate with the Collagen Scaffold Changes of the Colon Mucosa
Authors: F ?aja, D Stakheev, O Chernyavsk, L Kubinová, J K?ížan, J Dvo?ák, P Rossmann, R Št?pánková, P Makovický, P Makovický, V Vymetalkov, P Sou?ek, P Vodi?ka, L Vodi?ková, M Levý, LE Vannucci
Cancers, 2021-05-18;13(10):.
Species: Rat
Sample Types: Tissue Homogenates
-
TLR2- and TLR3-activated microglia induce different levels of neuronal network dysfunction in a context-dependent manner
Authors: S Schilling, B Chausse, HO Dikmen, F Almouhanna, JO Hollnagel, A Lewen, O Kann
Brain, Behavior, and Immunity, 2021-05-17;0(0):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Inflammatory Cytokine Profile and Plasticity of Brain and Spinal Microglia in Response to ATP and Glutamate
Authors: SJB Jesudasan, SJ Gupta, MA Churchward, KG Todd, IR Winship
Frontiers in Cellular Neuroscience, 2021-04-06;15(0):634020.
Species: Rat
Sample Types: Cell Culture Supernates
-
Co-Incubation with PPAR&beta/&delta Agonists and Antagonists Modeled Using Computational Chemistry: Effect on LPS Induced Inflammatory Markers in Pulmonary Artery
Authors: N Perez Diaz, LA Lione, V Hutter, LS Mackenzie
International Journal of Molecular Sciences, 2021-03-19;22(6):.
Species: Rat
Sample Types: Tissue Homogenates
-
Mast cells contribute to alveolar bone loss in Spontaneously Hypertensive Rats with periodontal disease regulating cytokines production
Authors: VGB Brito, MS Patrocinio, MCL Sousa, AEA Barreto, SCT Frasnelli, VS Lara, CF Santos, SHP Oliveira
PLoS ONE, 2021-03-04;16(3):e0247372.
Species: Rat
Sample Types: Tissue Homogenates
-
Differential and paradoxical roles of new-generation antidepressants in primary astrocytic inflammation
Authors: JH He, RP Liu, YM Peng, Q Guo, LB Zhu, YZ Lian, BL Hu, HH Fan, X Zhang, JH Zhu
Journal of Neuroinflammation, 2021-02-18;18(1):47.
Species: Rat
Sample Types: Cell Culture Supernates
-
Small intestine remodeling in male Goto-Kakizaki rats
Authors: JNB Pereira, GM Murata, FT Sato, AR Marosti, CRO Carvalho, R Curi
Physiological Reports, 2021-02-01;9(3):e14755.
Species: Rat
Sample Types: Tissue Homogenates
-
The Poststroke Peripheral Immune Response Is Differentially Regulated by Leukemia Inhibitory Factor in Aged Male and Female Rodents
Authors: SM Davis, LA Collier, SJ Messmer, KR Pennypacke
Oxidative Medicine and Cellular Longevity, 2020-12-10;2020(0):8880244.
Species: Rat
Sample Types: Tissue Homogenates
-
&alpha-Bisabolol, a Dietary Bioactive Phytochemical Attenuates Dopaminergic Neurodegeneration through Modulation of Oxidative Stress, Neuroinflammation and Apoptosis in Rotenone-Induced Rat Model of Parkinson's disease
Authors: H Javed, MFN Meeran, S Azimullah, L Bader Eddi, VD Dwivedi, NK Jha, S Ojha
Biomolecules, 2020-10-08;10(10):.
Species: Rat
Sample Types: Tissue Homogenates
-
Impacts of fish oil on the gut microbiota of rats with alcoholic liver damage
Authors: YL Chen, H Shirakawa, NS Lu, HC Peng, Q Xiao, SC Yang
J. Nutr. Biochem., 2020-09-11;86(0):108491.
Species: Rat
Sample Types: Cell Culture Supernates
-
Fecal dysbiosis associated with colonic hypersensitivity and behavioral alterations in chronically Blastocystis-infected rats
Authors: M Defaye, C Nourrisson, E Baudu, A Lashermes, M Meynier, M Meleine, I Wawrzyniak, V Bonnin, J Barbier, B Chassaing, C Godfraind, A Gelot, N Barnich, D Ardid, M Bonnet, F Delbac, FA Carvalho, P Poirier
Sci Rep, 2020-06-04;10(1):9146.
Species: Rat
Sample Types: Tissue Homogenates
-
Hepcidin-to-Ferritin Ratio Is Decreased in Astrocytes With Extracellular Alpha-Synuclein and Iron Exposure
Authors: J Cui, X Guo, Q Li, N Song, J Xie
Front Cell Neurosci, 2020-03-10;14(0):47.
Species: Rat
Sample Types: Cell Culture Supernates
-
Acute sleep deprivation during pregnancy in rats: Rapid elevation of placental and fetal inflammation and kynurenic acid
Authors: AM Baratta, NR Kanyuch, CA Cole, H Valafar, J Deslaurier, A Pocivavsek
Neurobiol Stress, 2019-12-14;12(0):100204.
Species: Rat
Sample Types: Tissue Homogenates
-
The Preventive Effects of Greenshell Mussel (Perna canaliculus) on Early-Stage Metabolic Osteoarthritis in Rats with Diet-Induced Obesity
Authors: P Siriarchav, MC Kruger, MR Miller, HS Tian, FM Wolber
Nutrients, 2019-07-15;11(7):.
Species: Rat
Sample Types: Plasma
-
A diet including xanthan gum triggers a pro-inflammatory response in Wistar rats inoculated with Walker 256 cells
Authors: AB Silva Risc, NIP Neto, K Gascho, M Carnier, DA de Miranda, FP Silva, VT Boldarine, M Seelaender, EB Ribeiro, LM Oyama, CM Oller do N
PLoS ONE, 2019-06-18;14(6):e0218567.
Species: Rat
Sample Types: Tissue Homogenates
-
Time dependent dual effect of anti-inflammatory treatments on sarin-induced brain inflammation: suggested role of prostaglandins
Authors: S Chapman, E Grauer, R Gez, I Egoz, S Lazar
Neurotoxicology, 2019-05-13;0(0):.
Species: Rat
Sample Types: Tissue Homogenates
-
Hyperbilirubinemia in Gunn Rats is Associated with Decreased Inflammatory Response in LPS-Mediated Systemic Inflammation
Authors: P Valaskova, A Dvorak, M Lenicek, K Zizalova, N Kutinova-C, J Zelenka, M Cahova, L Vitek, L Muchova
Int J Mol Sci, 2019-05-09;20(9):.
Species: Rat
Sample Types: Serum
-
Polyphenols-Rich Fruit (Euterpe edulis Mart.) Prevents Peripheral Inflammatory Pathway Activation by the Short-Term High-Fat Diet
Authors: AB Santamarin, G Jamar, LV Mennitti, DA Ribeiro, CM Cardoso, VV de Rosso, LM Oyama, LP Pisani
Molecules, 2019-04-27;24(9):.
Species: Rat
Sample Types: Tissue Homogenates
-
Matrix-bound nanovesicles prevent ischemia-induced retinal ganglion cell axon degeneration and death and preserve visual function
Authors: Y van der Me, AE Faust, ET Sakalli, CC Westrick, G Hussey, IP Conner, VLN Fu, SF Badylak, MB Steketee
Sci Rep, 2019-03-05;9(1):3482.
Species: Rat
Sample Types: Cell Culture Supernates
-
BRD4 inhibition attenuates inflammatory response in microglia and facilitates recovery after spinal cord injury in rats
Authors: J Wang, J Chen, H Jin, D Lin, Y Chen, X Chen, B Wang, S Hu, Y Wu, Y Wu, Y Zhou, N Tian, W Gao, X Wang, X Zhang
J. Cell. Mol. Med., 2019-02-26;0(0):.
Species: Rat
Sample Types: Tissue Homogenates
-
Cholinergic Stimulation by Pyridostigmine Bromide Before Myocardial Infarction Prevent Cardiac and Autonomic Dysfunction
Authors: CA Barboza, AR Fukushima, N Carrozzi, JF Machi, PMM Dourado, CT Mostarda, MC Irigoyen, L Nathanson, M Morris, EC Caperuto, B Rodrigues
Sci Rep, 2019-02-21;9(1):2481.
Species: Rat
Sample Types: Plasma
-
Metabolomics Approach Based on Multivariate Techniques for Blood Transfusion Reactions
Authors: SJ Lee, H Wang, SH Ahn, MK Son, GH Hyun, SJ Yoon, J Lee, JH Park, J Lim, SS Hong, SW Kwon
Sci Rep, 2019-02-11;9(1):1740.
Species: Rat
Sample Types: Serum
-
Elevated pulmonary arterial pressure in Zucker diabetic fatty rats
Authors: D Morales-Ca, M Callejo, B Barreira, G Mondejar-P, S Esquivel-R, S Ramos, MÁ Martín, A Cogolludo, L Moreno, F Perez-Vizc
PLoS ONE, 2019-01-28;14(1):e0211281.
Species: Rat
Sample Types: Serum
-
Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats
Authors: WS Tan, P Arulselvan, SF Ng, CN Mat Taib, MN Sarian, S Fakurazi
BMC Complement Altern Med, 2019-01-17;19(1):20.
Species: Rat
Sample Types: Tissue Homogenates
-
Characterization of the Myocardial Inflammatory Response in Acute Stress-Induced (Takotsubo) Cardiomyopathy
Authors: HM Wilson, L Cheyne, PAJ Brown, K Kerr, A Hannah, J Srinivasan, N Duniak, G Horgan, DK Dawson
JACC Basic Transl Sci, 2018-12-31;3(6):766-778.
Species: Rat
Sample Types: Serum
-
Obesity and Type 2 Diabetes mellitus induce lipopolysaccharide tolerance in rat neutrophils
Authors: WMT Kuwabara, CNF Yokota, R Curi, TC Alba-Loure
Sci Rep, 2018-12-03;8(1):17534.
Species: Rat
Sample Types: BALF
-
Activation of trigeminal ganglion satellite glial cells in CFA-induced tooth pulp pain in rats
Authors: HF Filippini, PA Scalzilli, KM Costa, RDS Freitas, MM Campos
PLoS ONE, 2018-11-12;13(11):e0207411.
Species: Rat
Sample Types: Serum
-
Leukemia inhibitory factor modulates the peripheral immune response in a rat model of emergent large vessel occlusion
Authors: SM Davis, LA Collier, ED Winford, CC Leonardo, CT Ajmo, EA Foran, TJ Kopper, JC Gensel, KR Pennypacke
J Neuroinflammation, 2018-10-15;15(1):288.
Species: Rat
Sample Types: Tissue Homogenates
-
Human Mesenchymal Stem Cell Secretome from Bone Marrow or Adipose-Derived Tissue Sources for Treatment of Hypoxia-Induced Pulmonary Epithelial Injury
Authors: N Shologu, M Scully, JG Laffey, D O'Toole
Int J Mol Sci, 2018-09-30;19(10):.
Species: Rat
Sample Types: Cell Culture Supernates
-
The plasticity of primary microglia and their multifaceted effects on endogenous neural stem cells in vitro and in vivo
Authors: SU Vay, LJ Flitsch, M Rabenstein, R Rogall, S Blaschke, J Kleinhaus, N Reinert, A Bach, GR Fink, M Schroeter, MA Rueger
J Neuroinflammation, 2018-08-13;15(1):226.
Species: Rat
Sample Types: Cell Culture Supernates
-
The Use of Ju�ara (Euterpe edulis Mart.) Supplementation for Suppression of NF-?B Pathway in the Hypothalamus after High-Fat Diet in Wistar Rats
Authors: AB Santamarin, G Jamar, LV Mennitti, VV de Rosso, HC Cesar, LM Oyama, LP Pisani
Molecules, 2018-07-21;23(7):.
Species: Rat
Sample Types: Tissue Homogenates
-
Cortical stimulation in conscious rats controls joint inflammation
Authors: GS Bassi, L Ulloa, VR Santos, F Del Vecchi, P Delfino-Pe, GJ Rodrigues, JA Castania, FQ Cunha, HC Salgado, TM Cunha, N Garcia-Cai, A Kanashiro
Prog. Neuropsychopharmacol. Biol. Psychiatry, 2018-03-06;0(0):.
Species: Rat
Sample Types: Synovial Fluid
-
Early exposure to distinct sources of lipids affects differently the development and hepatic inflammatory profiles of 21-day-old rat offspring
Authors: LV Mennitti, LM Oyama, AB Santamarin, CMDPO do Nascime, LP Pisani
J Inflamm Res, 2018-01-18;11(0):11-24.
Species: Rat
Sample Types: Tissue Homogenates
-
Lactobacillus fermentum HP3-Mediated Fermented Hericium erinaceus Juice as a Health Promoting Food Supplement to Manage Diabetes Mellitus
Authors: C Chaiyasut, S Woraharn, BS Sivamaruth, N Lailerd, P Kesika, S Peerajan
J Evid Based Integr Med, 2018-01-01;23(0):2515690X18765.
Species: Rat
Sample Types: Serum
-
Different Dietary Proportions of Fish Oil Regulate Inflammatory Factors but Do Not Change Intestinal Tight Junction ZO-1 Expression in Ethanol-Fed Rats
Authors: YW Chien, HC Peng, YL Chen, MH Pai, HY Wang, HL Chuang, SC Yang
Mediators Inflamm., 2017-12-13;2017(0):5801768.
Species: Rat
Sample Types: Tissue Homogenates
-
Cholinergic Stimulation Improves Oxidative Stress and Inflammation in Experimental Myocardial Infarction
Authors: OC Bezerra, CM França, JA Rocha, GA Neves, PRM Souza, M Teixeira G, C Malfitano, TCA Loleiro, PM Dourado, S Llesuy, K de Angelis, MCC Irigoyen, L Ulloa, FM Consolim-C
Sci Rep, 2017-10-20;7(1):13687.
Species: Rat
Sample Types: Tissue Homogenates
-
?9-Tetrahydrocannabinol Prevents Cardiovascular Dysfunction in STZ-Diabetic Wistar-Kyoto Rats
Authors: RK Vella, DJ Jackson, AS Fenning
Biomed Res Int, 2017-10-18;2017(0):7974149.
Species: Rat
Sample Types: Serum
-
Carotid sinus nerve electrical stimulation in conscious rats attenuates systemic inflammation via chemoreceptor activation
Authors: FM Santos-Alm, G Domingos-S, CA Meschiari, LC Fávaro, C Becari, JA Castania, A Lopes, TM Cunha, DJA Moraes, FQ Cunha, L Ulloa, A Kanashiro, GCSV Tezini, HC Salgado
Sci Rep, 2017-07-24;7(1):6265.
Species: Rat
Sample Types: Plasma
-
Intestinal anti-inflammatory activity of Ground Cherry (Physalis angulata L.) standardized CO2 phytopharmaceutical preparation
Authors: LD Almeida Ju, AEV Quaglio, CAR de Almeida, LC Di Stasi
World J. Gastroenterol., 2017-06-28;23(24):4369-4380.
Species: Rat
Sample Types: Tissue Homogenates
-
Impact of gestational nicotine exposure on intrauterine and fetal infection in a rodent model
Authors: M von Chamie, L Reyes, LF Hayward, MB Brown
Biol. Reprod., 2017-05-01;96(5):1071-1084.
Species: Rat
Sample Types: Whole Tissue
Applications: ELISA Capture -
The protective role of nitric oxide-dependent innate immunosuppression in the early stage of cartilage damage in rats: Role of nitric oxide in cartilage damage
Authors: CC Hsu, CL Lin, IM Jou, PH Wang, JS Lee
Bone Joint Res, 2017-04-01;6(4):253-258.
Species: Rat
Sample Types: Tissue Homogenates
-
Pyrrolidine dithiocarbamate administered during ex-vivo lung perfusion promotes rehabilitation of injured donor rat lungs obtained after prolonged warm ischemia
Authors: C Francioli, X Wang, R Parapanov, E Abdelnour, J Lugrin, F Gronchi, J Perentes, P Eckert, HB Ris, L Piquilloud, T Krueger, L Liaudet
PLoS ONE, 2017-03-21;12(3):e0173916.
Species: Rat
Sample Types: BALF
-
Incline treadmill exercise suppresses pain hypersensitivity associated with the modulation of pro-inflammatory cytokines and anti-inflammatory cytokine in rats with peripheral nerve injury
Authors: KL Tsai, PC Huang, LK Wang, CH Hung, YW Chen
Neurosci. Lett, 2017-02-12;643(0):27-31.
Species: Rat
Sample Types: Tissue Homogenates
-
Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon
Authors: S Bettini, E Boutet-Rob, C Cartier, C Com‚ra, E Gaultier, J Dupuy, N Naud, S Tach‚, P Grysan, S Reguer, N Thieriet, M R‚fr‚giers, D ThiaudiŠre, JP Cravedi, M CarriŠre, JN Audinot, FH Pierre, L Guzylack-P, E Houdeau
Sci Rep, 2017-01-20;7(0):40373.
Species: Rat
Sample Types: Cell Culture Supernates
-
Intra-articular blockade of P2X7 receptor reduces the articular hyperalgesia and inflammation in the knee joint synovitis especially in female rats
Authors: Cláudia Herrera Tambeli
J Pain, 2016-11-04;0(0):.
Species: Rat
Sample Types: Synovial Lavage Fluid
-
Impact of exercise training associated to pyridostigmine treatment on autonomic function and inflammatory profile after myocardial infarction in rats
Authors: Bruno Rodrigues
Int. J. Cardiol., 2016-10-26;0(0):.
Species: Rat
Sample Types: Plasma
-
Effect of different intensities of physical activity on cardiometabolic markers and vascular and cardiac function in adult rats fed with a high-fat high-carbohydrate diet
Authors: RB Batacan, MJ Duncan, VJ Dalbo, GL Buitrago, AS Fenning
J Sport Health Sci, 2016-08-03;7(1):109-119.
Species: Rat
Sample Types: Serum
-
Enteral sesame oil therapeutically relieves disease severity in rat experimental osteoarthritis
Authors: DZ Hsu, PY Chu, IM Jou
Food Nutr Res, 2016-03-30;60(0):29807.
Species: Rat
Sample Types: Tissue Homogenates
-
Differential activation of inflammatory pathways in testicular macrophages provides a rationale for their subdued inflammatory capacity.
Authors: Bhushan S, Tchatalbachev S, Lu Y, Frohlich S, Fijak M, Vijayan V, Chakraborty T, Meinhardt A
J Immunol, 2015-04-27;194(11):5455-64.
Species: Rat
Sample Types: Cell Culture Supernates
-
Biodistribution and Clearance of TiO2 Nanoparticles in Rats after Intravenous Injection.
Authors: Elgrabli D, Beaudouin R, Jbilou N, Floriani M, Pery A, Rogerieux F, Lacroix G
PLoS ONE, 2015-04-24;10(4):e0124490.
Species: Rat
Sample Types: Serum
-
Reduced inflammatory phenotype in microglia derived from neonatal rat spinal cord versus brain.
Authors: Baskar Jesudasan, Sam Josh, Todd, Kathryn, Winship, Ian R
PLoS ONE, 2014-06-10;9(6):e99443.
Species: Rat
Sample Types: Cell Culture Supernates
-
S1P1 receptor modulation preserves vascular function in mesenteric and coronary arteries after CPB in the rat independent of depletion of lymphocytes.
Authors: Samarska I, Bouma H, Buikema H, Mungroop H, Houwertjes M, Absalom A, Epema A, Henning R
PLoS ONE, 2014-05-12;9(5):e97196.
Species: Rat
Sample Types: Plasma
-
Oligofructose supplementation (10%) during pregnancy and lactation does not change the inflammatory effect of concurrent trans fatty acid ingestion on 21-day-old offspring.
Authors: Hachul A, Mennitti L, de Oliveira J, Moreno M, Okuda M, Dos Santos B, Oyama L, Ribeiro E, do Nascimento C, Pisani L
Lipids Health Dis, 2013-05-01;12(0):59.
Species: Rat
Sample Types: Tissue Homogenates
-
Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6.
Authors: Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, Rosa RH, Prockop DJ
Stem Cells, 2011-10-01;29(10):1572-9.
Species: Rat
Sample Types: Tissue Homogenates
-
Depot-specific modulation of adipokine levels in rat adipose tissue by diet-induced obesity: the effect of aerobic training and energy restriction.
Authors: Yamashita AS, Lira FS, Rosa JC, Paulino EC, Brum PC, Negrao CE, dos Santos RV, Batista ML, do Nascimento CO, Oyama LM, Seelaender M
Cytokine, 2010-08-21;52(3):168-74.
Species: Rat
Sample Types: Tissue Homogenates
-
Supernatant of stored platelets causes lung inflammation and coagulopathy in a novel in vivo transfusion model.
Authors: Vlaar AP, Hofstra JJ, Kulik W
Blood, 2010-05-17;116(8):1360-8.
Species: Rat
Sample Types: Tissue Homogenates
-
TNF-alpha, IL-1beta, IL-6, and cinc-1 levels in rat brain after meningitis induced by Streptococcus pneumoniae.
Authors: Barichello T, dos Santos I, Savi GD, Simoes LR, Silvestre T, Comim CM, Sachs D, Teixeira MM, Teixeira AL, Quevedo J
J. Neuroimmunol., 2010-03-03;221(1):42-5.
Species: Rat
Sample Types: Tissue Homogenates
-
Interleukin-1 mediates the anorexic and febrile actions of galanin-like Peptide.
Authors: Man PS, Lawrence CB
Endocrinology, 2008-07-10;149(11):5791-802.
Species: Rat
Sample Types: Plasma
-
Comparative studies on the interplay of testosterone, estrogen and progesterone in collagen induced arthritis in rats.
Authors: Ganesan K, Balachandran C, Manohar BM, Puvanakrishnan R
Bone, 2008-06-10;43(4):758-65.
Species: Rat
Sample Types: Serum
-
Augmentation of endogenous cannabinoid tone modulates lipopolysaccharide-induced alterations in circulating cytokine levels in rats.
Authors: Roche M, Kelly JP, O'Driscoll M, O'Driscoll M, Finn DP
Immunology, 2008-04-03;125(2):263-71.
Species: Rat
Sample Types: Plasma
-
Haemostatic imbalance following carrageenan-induced rat paw oedema.
Authors: Cicala C, Morello S, Alfieri A, Vellecco V, Marzocco S, Autore G
Eur. J. Pharmacol., 2007-08-14;577(1):156-61.
Species: Rat
Sample Types: Serum
-
MAPKs are key players in mediating cytokine release and cell death induced by unconjugated bilirubin in cultured rat cortical astrocytes.
Authors: Fernandes A, Falcao AS, Silva RF, Brito MA, Brites D
Eur. J. Neurosci., 2007-02-01;25(4):1058-68.
Species: Rat
Sample Types: Cell Culture Supernates
-
Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging.
Authors: Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I
Am. J. Physiol. Lung Cell Mol. Physiol., 2006-10-13;292(2):L567-76.
Species: Rat
Sample Types: BALF
-
The influence of kainic acid on core temperature and cytokine levels in the brain.
Authors: Oprica M, Spulber SD, Aronsson AF, Post C, Winblad B, Schultzberg M
Cytokine, 2006-09-06;35(1):77-87.
Species: Rat
Sample Types: Tissue Homogenates
-
Inhibition of angiogenesis by interleukin-4 gene therapy in rat adjuvant-induced arthritis.
Authors: Haas CS, Amin MA, Allen BB, Ruth JH, Haines GK, Woods JM, Koch AE
Arthritis Rheum., 2006-08-01;54(8):2402-14.
Species: Rat
Sample Types: Tissue Homogenates
-
Role of matrix metalloproteinases in the inflammatory response in human airway cell-based assays and in rodent models of airway disease.
Authors: Birrell MA, Wong S, Dekkak A, De Alba J, Haj-Yahia S, Belvisi MG
J. Pharmacol. Exp. Ther., 2006-05-11;318(2):741-50.
Species: Rat
Sample Types: Tissue Homogenates
-
Inflammatory signalling pathways involved in astroglial activation by unconjugated bilirubin.
Authors: Fernandes A, Falcao AS, Silva RF, Gordo AC, Gama MJ, Brito MA, Brites D
J. Neurochem., 2006-02-10;96(6):1667-79.
Species: Rat
Sample Types: Cell Culture Supernates
-
Cardiomyocyte function after burn injury and lipopolysaccharide exposure: single-cell contraction analysis and cytokine secretion profile.
Authors: Niederbichler AD, Westfall MV, Su GL, Donnerberg J, Usman A, Vogt PM, Ipaktchi KR, Arbabi S, Wang SC, Hemmila MR
Shock, 2006-02-01;25(2):176-83.
Species: Rat
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Rat IL-6 DuoSet ELISA
Average Rating: 4.3 (Based on 7 Reviews)
Have you used Rat IL-6 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: