Recombinant Human/Mouse Wnt-5a Protein Summary
- R&D Systems CHO-derived Recombinant Human/Mouse Wnt-5a Protein (645-WN)
- Quality control testing to verify active proteins with lot specific assays by in-house scientists
- All R&D Systems proteins are covered with a 100% guarantee
Product Specifications
Optimal concentrations should be determined by each laboratory for each application.
Gln38-Lys380
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
645-WN
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS, EDTA and CHAPS with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
645-WN/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS, EDTA and CHAPS. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
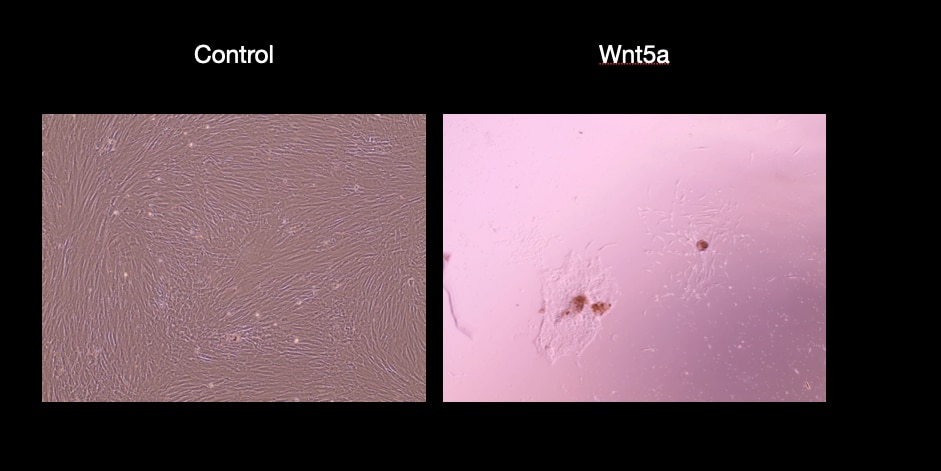
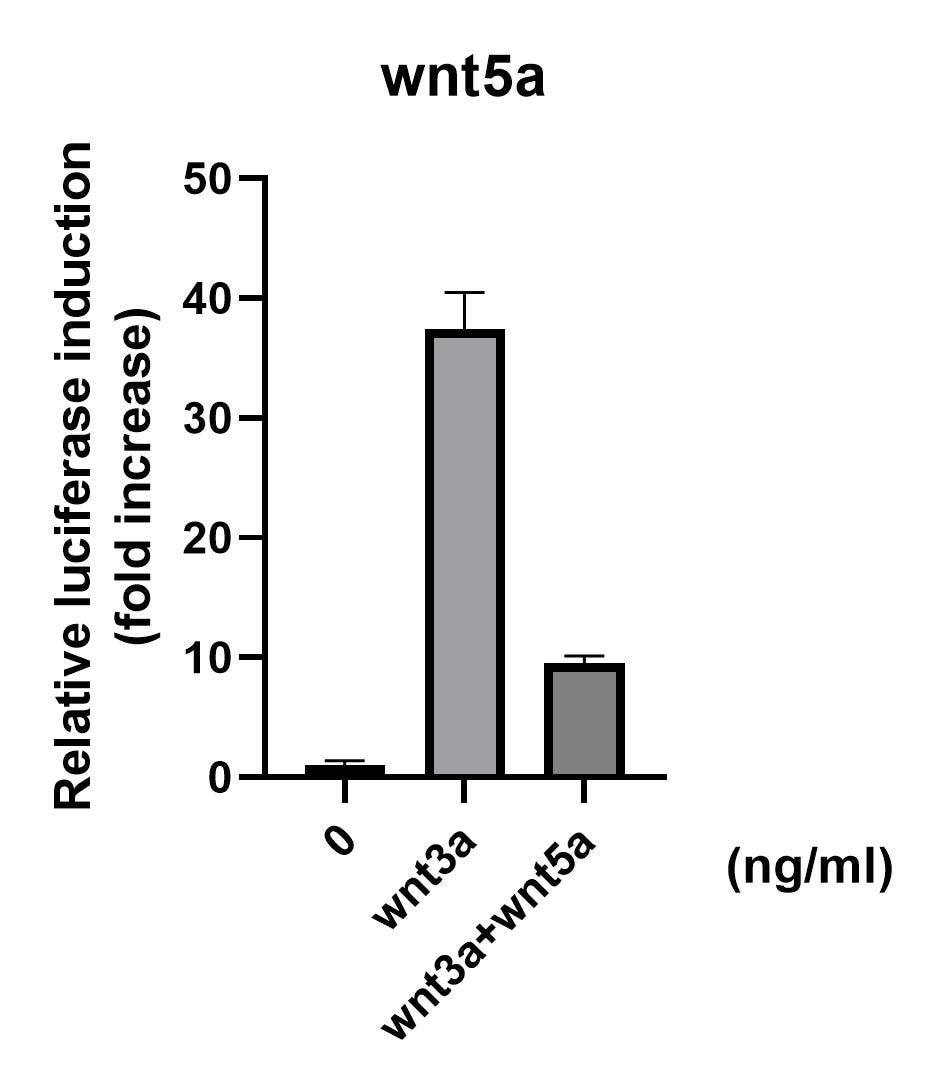
 View Larger
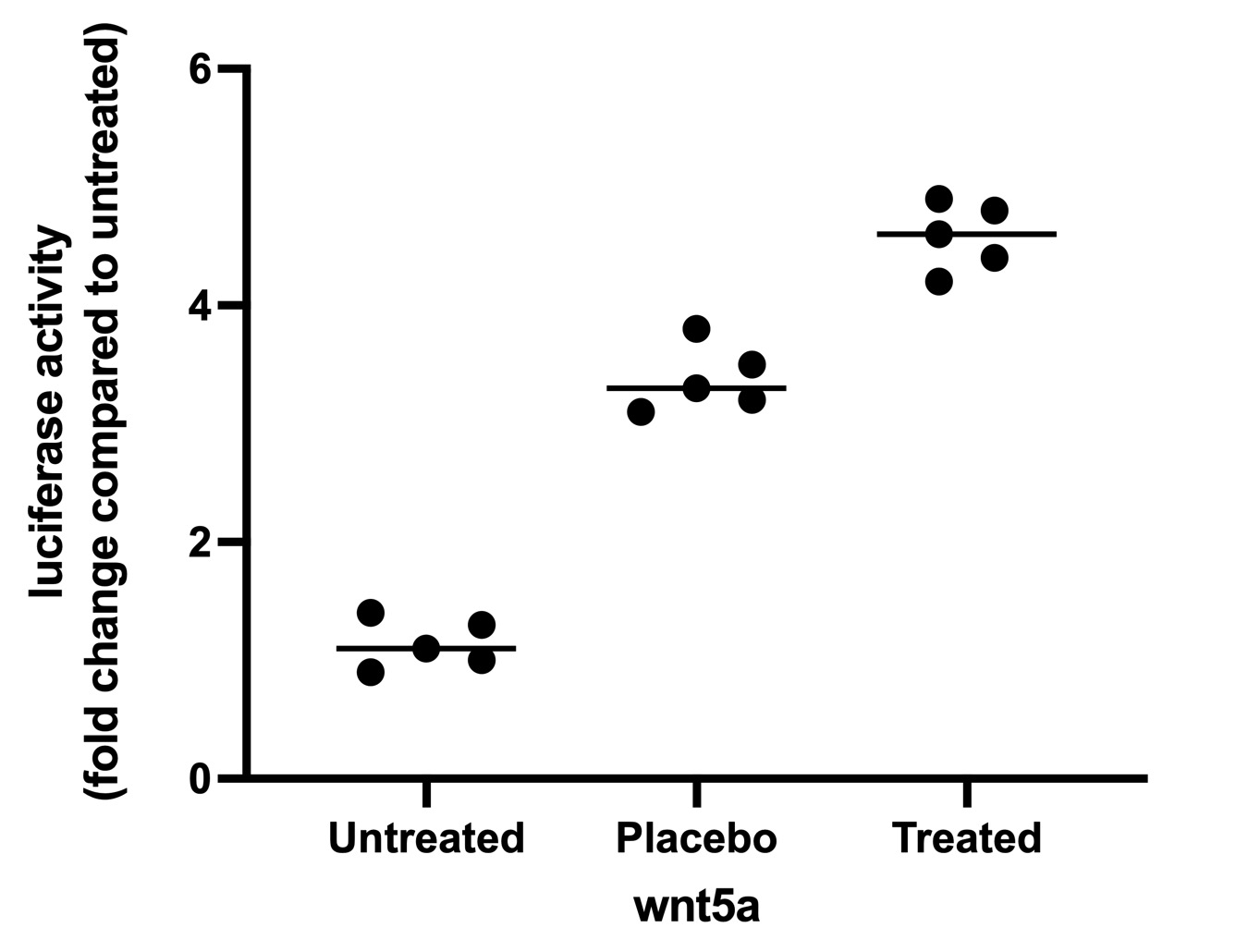
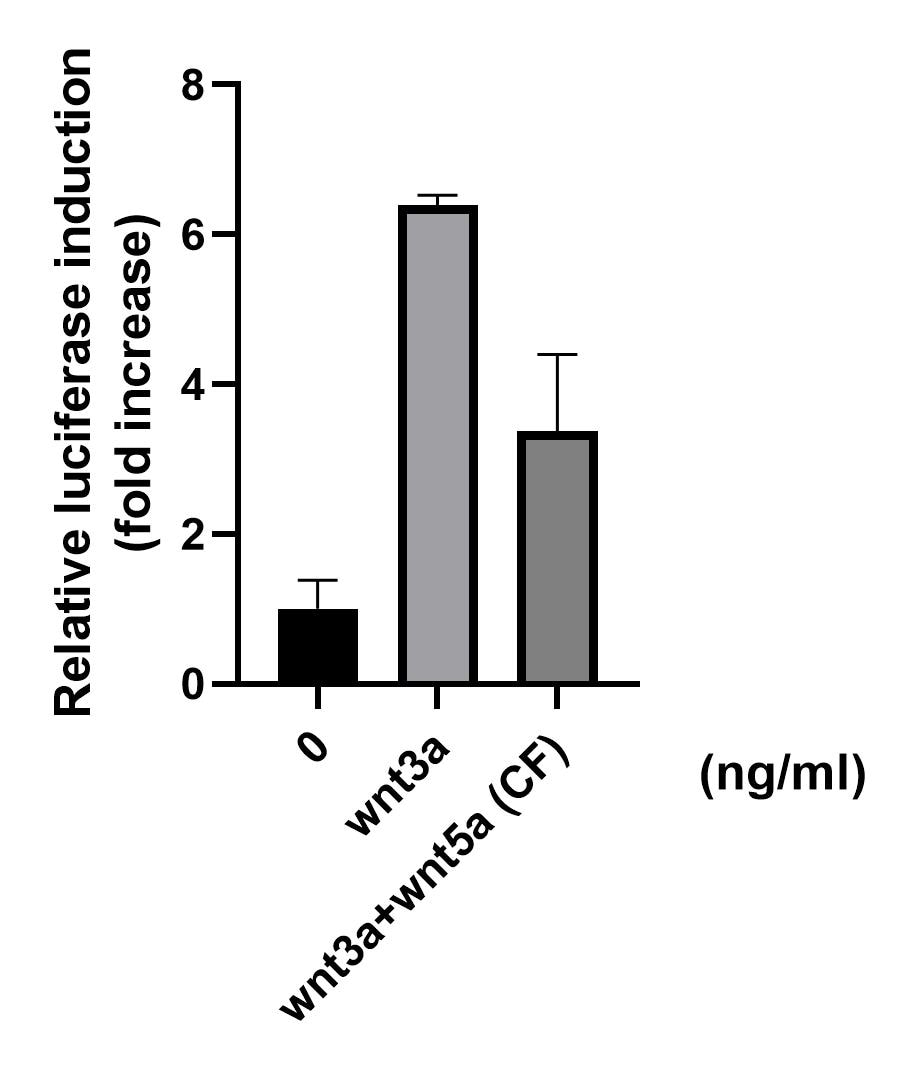
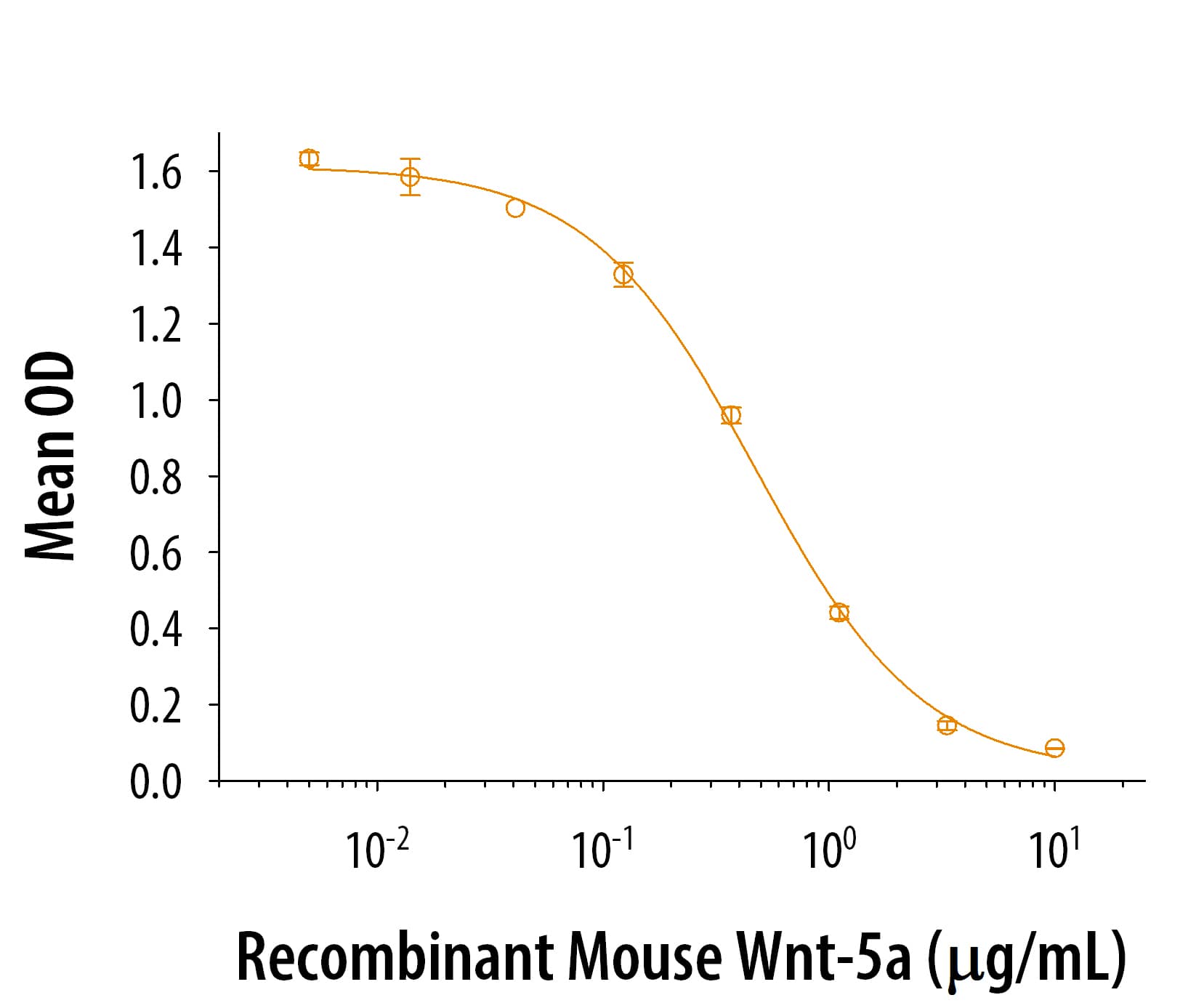
View Larger
Reconstitution Calculator
Background: Wnt-5a
Wnt-5a is a 44‑50 kDa member of the Wnt family of proteins (1‑6). Based on its activity towards C57Mg mammary epithelium, it is classified as a nontransforming Wnt. Human Wnt‑5a is synthesized as a 380 amino acid (aa) precursor that contains a 37 aa signal sequence, a 25 aa prosegment, and a 319 aa mature region (1, 2, 3). The mature region has 24 cysteine residues that form multiple intrachain disulfide bonds, plus four N‑linked glycosylation sites that are utilized for proper secretion (3, 5, 7). There is also a palmitate adduct at Cys104 that is essential for activity, and a potential palmitoleic acid modification at Ser244 that may also contribute to secretion (7‑9). One alternative start site is reported at Met16. Over aa 38‑380, human and mouse Wnt‑5a are identical in amino acid sequence (1, 10). Cells known to express Wnt‑5a include brainstem astrocytes (11), mammary epithelium (12), CD34+ primitive progenitor stem cells (13), chondrocytes (14), CD34- pericytes and vascular smooth muscle cells (15), plus mesenchymal cells at various sites (16, 17). There are multiple receptors for Wnt‑5a. These include Fzd-1, -2,
-3, -4, -5, and -7 (3, 18‑22), Ror2 (3), LRP6 (23), Ryk (24) and sFRP1 (25). All these molecules function within the context of a larger number of “co‑factors” that regulate signaling by the Wnts. Initially, it was suggested that there were three pathways for Wnt signaling; a beta -catenin-mediated canonical pathway, and two noncanonical pathways described as the Wnt/JNK (PCP) pathway and the Wnt/Ca++ pathway (26, 27). And it was assumed that various Wnts could be accommodated by these classifications. At present, it is now recognized that individual Wnts, through various combinations of receptor complex subunits, can have diverse effects, perhaps even within the same cell (3, 6, 27). Further complexity is introduced by the fact that Xenopus Wnt‑5a and Wnt‑11 are known to form bioactive heterodimers following Tyr sulfation (28). Thus, predicting the activity of Wnt‑5a, or any other Wnt, on any cell type will require substantial insight into the interaction between all the extracellular, cell surface and intracellular components of the Wnt signaling system.
- Clark, C.C. et al. (1993) Genomics 18:249.
- LeJeune, S. et al. (1995) Clin. Cancer Res. 1:215.
- Mikels, A.J. & R. Nusse (2006) PLoS Biol. 4:e115.
- Nishita, M. et al. (2010) Trends Cell Biol. 20:346.
- Mikels, A.J. & R. Nusse (2006) Oncogene 25:7461.
- van Amerongen, R. & R. Nusse (2009) Development 136:3205.
- Kurayoshi, M. et al. (2007) Biochem. J. 402:515.
- Takada, R. et al. (2006) Dev. Cell 11:791.
- Port, F. & K. Basler (2010) Traffic May 3. [Epub ahead of print].
- Gavin, B.J. et al. (1990) Genes Dev. 4:2319.
- Castelo-Branco, G. et al. (2006) Mol. Cell. Neurosci. 31:251.
- Jonsson, M. et al. (1998) Br. J. Cancer 78:430.
- van Den Berg, D.J. et al. (1998) Blood 92:3189.
- Kruger, C. & C. Kappen (2010) PLoS One 5:e8978.
- Lin, G. et al. (2008) Stem Cells Dev. 17:1053.
- Lickert, H. et al. (2001) Mech. Dev. 105:181.
- Danielson, K.G. et al. (1995) J. Biol. Chem. 270:31225.
- Gazit, A. et al. (1999) Oncogene 18:5959.
- Bazhin, A. V. et al. (2010) Cell. Mol. Life Sci. 67:817.
- Kawasaki, A. et al. (2007) Cell. Signal. 19:2498.
- Blumenthal, A. et al. (2006) Blood 108:965.
- Umbhauer, M. et al. (2000) EMBO J. 19:4944.
- Bryja, V. et al. (2009) Mol. Biol. Cell 20:924.
- Keeble, T.R. et al. (2006) J. Neurosci. 26:5840.
- Lin, K. et al. (1997) Proc. Natl. Acad. Sci. USA 94:11196.
- Rao, T.P. & M. Kuhl (2010) Circ. Res. 106:1798.
- McDonald, S.L. & A. Silver (2009) Br. J. Cancer 101:209.
- Cha, S-W. et al. (2009) Curr. Biol. 19:1573.
Citations for Recombinant Human/Mouse Wnt-5a Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
191
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Intestinal tuft cell subtypes represent successive stages of maturation driven by crypt-villus signaling gradients
Authors: Buissant des Amorie, JR;Betjes, MA;Bernink, JH;Hageman, JH;Geurts, VE;Begthel, H;Laskaris, D;Heinz, MC;Jordens, I;Vinck, T;Houtekamer, RM;Verlaan-Klink, I;Brunner, SR;van Rheenen, J;Gloerich, M;Clevers, H;Tans, SJ;van Zon, JS;Snippert, HJG;
Nature communications
Species: Mouse
Sample Types: Organoid
Applications: Bioassay -
WNT-induced association of Frizzled and LRP6 is not sufficient for the initiation of WNT/?-catenin signaling
Authors: Voss, JH;Koszegi, Z;Yan, Y;Shorter, E;Grätz, L;Lanner, JT;Calebiro, D;Schulte, G;
Nature communications
Species: Human
Sample Types: Transfected Whole Cells
Applications: Bioassay -
Non-canonical Wnt signaling promotes epithelial fluidization in the repairing airway
Authors: Hu, DJ;Cai, XT;Simons, J;Yun, J;Elstrott, J;Jasper, H;
Nature communications
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
Wnt signaling drives stromal inflammation in inflammatory arthritis
Authors: Mueller, AA;Zou, AE;Marsh, LJ;Kemble, S;Nayar, S;Watts, GFM;Murphy, CL;Taylor, E;Major, T;Gardner, D;Buckley, CD;Wei, K;Raychaudhuri, S;Korsunsky, I;Filer, A;Croft, AP;Brenner, MB;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
DPP an extracellular matrix molecule induces Wnt5a mediated signaling to promote the differentiation of adult stem cells into odontogenic lineage
Authors: Chen, Y;Petho, A;Ganapathy, A;George, A;
Scientific reports
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
SLMAP3 is crucial for organogenesis through mechanisms involving primary cilia formation
Authors: Dias, AP;Rehmani, T;Applin, BD;Salih, M;Tuana, B;
Open biology
Species: Transgenic Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Structural insights into Frizzled3 through nanobody modulators
Authors: Hillier, J;Zhao, Y;Carrique, L;Malinauskas, T;Ruza, RR;Chang, TH;Yi, G;Duyvesteyn, HME;Yu, J;Lu, W;Pardon, E;Steyaert, J;Zhu, Y;Ni, T;Jones, EY;
Nature communications
Species: N/A
Sample Types: Nanobody, Recombinant Protein
Applications: Biolayer Interferometry (BLI) -
Wnt5a Promotes Axon Elongation in Coordination with the Wnt-Planar Cell Polarity Pathway
Authors: Ahmad, S;Attisano, L;
Cells
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Craniofacial studies in chicken embryos confirm the pathogenicity of human FZD2 variants associated with Robinow syndrome
Authors: Tophkhane, SS;Fu, K;Verheyen, EM;Richman, JM;
Disease models & mechanisms
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Structure and function of the ROR2 cysteine-rich domain in vertebrate noncanonical WNT5A signaling
Authors: Griffiths, SC;Tan, J;Wagner, A;Blazer, LL;Adams, JJ;Srinivasan, S;Moghisaei, S;Sidhu, SS;Siebold, C;Ho, HH;
eLife
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Dysregulation of Wnt/?-catenin signaling contributes to intestinal inflammation through regulation of group 3 innate lymphoid cells
Authors: Hao, J;Liu, C;Gu, Z;Yang, X;Lan, X;Guo, X;
Nature communications
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Intestinal Paneth cell differentiation relies on asymmetric regulation of Wnt signaling by Daam1/2
Authors: Colozza, G;Lee, H;Merenda, A;Wu, SS;Català-Bordes, A;Radaszkiewicz, TW;Jordens, I;Lee, JH;Bamford, AD;Farnhammer, F;Low, TY;Maurice, MM;Bryja, V;Kim, J;Koo, BK;
Science advances
Species: Human, Transgenic Mouse
Sample Types: Whole Cells, Organoid, Transfected Whole Cells
Applications: Bioassay -
Tumor Explants Elucidate a Cascade of Paracrine SHH, WNT, and VEGF Signals Driving Pancreatic Cancer Angiosuppression
Authors: Hasselluhn, MC;Decker-Farrell, AR;Vlahos, L;Thomas, DH;Curiel-Garcia, A;Maurer, HC;Wasko, UN;Tomassoni, L;Sastra, SA;Palermo, CF;Dalton, TC;Ma, A;Li, F;Tolosa, EJ;Hibshoosh, H;Fernandez-Zapico, ME;Muir, A;Califano, A;Olive, KP;
Cancer discovery
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Single-cell RNA sequencing of intestinal crypts reveals vital events in damage repair and the double-edged sword effect of the Wnt3/?-catenin pathway in irradiated mice
Authors: Yuan, T;Zhang, J;Zhao, Y;Guo, Y;Fan, S;
Redox biology
Species: Mouse
Sample Types: Organoids
Applications: Bioassay -
ROR2 homodimerization is sufficient to activate a neuronal Wnt/calcium signaling pathway
Authors: Riquelme, R;Li, L;Gambrill, A;Barria, A;
The Journal of biological chemistry
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
ASCL1 is activated downstream of the ROR2/CREB signaling pathway to support lineage plasticity in prostate cancer
Authors: Tabrizian, N;Nouruzi, S;Cui, CJ;Kobelev, M;Namekawa, T;Lodhia, I;Talal, A;Sivak, O;Ganguli, D;Zoubeidi, A;
Cell reports
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Ehrlichia Wnt SLiM ligand mimic deactivates the Hippo pathway to engage the anti-apoptotic Yap-GLUT1-BCL-xL axis
Authors: Byerly, CD;Patterson, LL;Pittner, NA;Solomon, RN;Patel, JG;Rogan, MR;McBride, JW;
Infection and immunity
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Adult Multipotent Cardiac Progenitor-Derived Spheroids: A Reproducible Model of In Vitro Cardiomyocyte Commitment and Specification
Authors: Scalise, M;Marino, F;Salerno, L;Amato, N;Quercia, C;Siracusa, C;Filardo, A;Chiefalo, A;Pagano, L;Misdea, G;Salerno, N;De Angelis, A;Urbanek, K;Viglietto, G;Torella, D;Cianflone, E;
Cells
Species: Mouse
Sample Types: Spheroid
Applications: Bioassay -
Dissecting specific Wnt components governing osteogenic differentiation potential by human periodontal ligament stem cells through interleukin-6
Authors: Purwaningrum, M;Giachelli, CM;Osathanon, T;Rattanapuchpong, S;Sawangmake, C;
Scientific reports
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
WNT5a Signaling through ROR2 Activates the Hippo Pathway to Suppress YAP1 Activity and Tumor Growth
Authors: K Wang, F Ma, S Arai, Y Wang, A Varkaris, L Poluben, O Voznesensk, F Xie, X Zhang, X Yuan, SP Balk
Cancer Research, 2023-04-04;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Convergent deployment of ancestral functions during the evolution of mammalian flight membranes
Authors: CY Feigin, JA Moreno, R Ramos, SA Mereby, A Alivisatos, W Wang, R van Ameron, J Camacho, JJ Rasweiler, RR Behringer, B Ostrow, MV Plikus, R Mallarino
Science Advances, 2023-03-24;9(12):eade7511.
Species: Sugar Glider
Sample Types: Protein
Applications: Bioassay -
Ehrlichia Wnt short linear motif ligand mimetic deactivates the Hippo pathway to engage the anti-apoptotic Yap-GLUT1-BCL-xL axis
Authors: CD Byerly, LL Patterson, NA Pittner, RN Solomon, JG Patel, MR Rogan, JW McBride
bioRxiv : the preprint server for biology, 2023-03-07;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A DLG1-ARHGAP31-CDC42 axis is essential for the intestinal stem cell response to fluctuating niche Wnt signaling
Authors: D Castillo-A, T Wald, EA Reyes, A Gallagher, J Schanin, S Vlachos, N Lamarche-V, C Bomidi, S Blutt, MK Estes, T Nystul, OD Klein
Cell Stem Cell, 2023-01-13;30(2):188-206.e6.
Species: Mouse
Sample Types: Organoids
Applications: Bioassay -
WNT5A-RHOA signaling is a driver of tumorigenesis and represents a therapeutically actionable vulnerability in small cell lung cancer
Authors: KB Kim, DW Kim, Y Kim, J Tang, N Kirk, Y Gan, B Kim, B Fang, JL Park, Y Zheng, KS Park
Cancer Research, 2022-11-15;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Pharmacological clearance of senescent cells improves cardiac remodeling and function after myocardial infarction in female aged mice
Authors: N Salerno, F Marino, M Scalise, L Salerno, C Molinaro, A Filardo, A Chiefalo, G Panuccio, A De Angelis, K Urbanek, D Torella, E Cianflone
Mechanisms of Ageing and Development, 2022-09-20;208(0):111740.
Species: Mouse
Sample Types: Whole Cells
Applications: Differentiation, Differentiation -
Human PSCs determine the competency of cerebral organoid differentiation via FGF signaling and epigenetic mechanisms.
Authors: Ideno H, Imaizumi K, Shimada H, Sanosaka T, Nemoto A, Kohyama J, Okano H
iScience, 2022-09-16;25(10):105140.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
Transient and Prolonged Activation of Wnt Signaling Contribute Oppositely to the Pathogenesis of Asherman's Syndrome
Authors: X Xue, X Li, J Yao, X Zhang, X Ren, S Xu
International Journal of Molecular Sciences, 2022-08-08;23(15):.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Hormone regulation of thrombospondin-1 mRNA in porcine granulosa cells in vitro
Authors: LJ Spicer, JR Evans, NB Schreiber
Oncogene, 2022-07-26;244(0):107048.
Species: Porcine
Sample Types: Whole Cells
Applications: Bioassay -
Single-cell analysis of human basal cell carcinoma reveals novel regulators of tumor growth and the tumor microenvironment
Authors: CF Guerrero-J, GH Lee, Y Liu, S Wang, M Karikomi, Y Sha, RY Chow, TTL Nguyen, VS Iglesias, S Aasi, ML Drummond, Q Nie, K Sarin, SX Atwood
Science Advances, 2022-06-10;8(23):eabm7981.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Non-canonical Wnt signaling participates in Jagged1-induced osteo/odontogenic differentiation in human dental pulp stem cells
Authors: C Kornsuthis, A Chansaenro, J Manokawinc, KA Tompkins, N Pirarat, T Osathanon
Scientific Reports, 2022-05-09;12(1):7583.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
High expression level of ROR1 and ROR1-signaling associates with venetoclax resistance in chronic lymphocytic leukemia
Authors: EM Ghia, LZ Rassenti, MY Choi, M Quijada-Ál, E Chu, GF Widhopf, TJ Kipps
Leukemia, 2022-04-13;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Pseudohypoxic HIF pathway activation dysregulates collagen structure-function in human lung fibrosis
Authors: CJ Brereton, L Yao, ER Davies, Y Zhou, M Vukmirovic, JA Bell, S Wang, RA Ridley, LSN Dean, OG Andriotis, F Conforti, L Brewitz, S Mohammed, T Wallis, A Tavassoli, RM Ewing, A Alzetani, BG Marshall, SV Fletcher, PJ Thurner, A Fabre, N Kaminski, L Richeldi, A Bhaskar, CJ Schofield, M Loxham, DE Davies, Y Wang, MG Jones
Elife, 2022-02-21;11(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Cadmium exposure reprograms energy metabolism of hematopoietic stem cells to promote myelopoiesis at the expense of lymphopoiesis in mice
Authors: Y Zhao, J He, T Zhu, Y Zhang, Y Zhai, P Xue, Y Yao, Z Zhou, M He, W Qu, Y Zhang
Oncogene, 2022-01-17;231(0):113208.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Wnt5A and TGFbeta1 Converges through YAP1 Activity and Integrin Alpha v Up-Regulation Promoting Epithelial to Mesenchymal Transition in Ovarian Cancer Cells and Mesothelial Cell Activation
Authors: Z Dehghani-G, S Sheikh Has, E Arefian, G Hossein
Cells, 2022-01-11;11(2):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
MicroRNA Expression in the Infarcted Heart Following Neonatal Cardiovascular Progenitor Cell Transplantation in a Sheep Model of Stem Cell-Based Repair
Authors: LV Lopez, V Camberos, LL Bailey, N Hasaniya, C Ramos, L Hughes, C Knox, MK Kearns-Jon
Cell Transplantation, 2022-01-01;31(0):9636897221136.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Frizzled BRET sensors based on bioorthogonal labeling of unnatural amino acids reveal WNT-induced dynamics of the cysteine-rich domain
Authors: M Kowalski-J, H Schihada, A Turku, T Huber, TP Sakmar, G Schulte
Science Advances, 2021-11-10;7(46):eabj7917.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Immunocytochemical Analysis of Endogenous Frizzled-(Co-)Receptor Interactions and Rapid Wnt Pathway Activation in Mammalian Cells
Authors: J Neuhaus, A Weimann, M Berndt-Pae
International Journal of Molecular Sciences, 2021-11-08;22(21):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
RNF43 inhibits WNT5A-driven signaling and suppresses melanoma invasion and resistance to the targeted therapy
Authors: T Radaszkiew, M Nosková, K Gömöryová, O Vondálová, KA Radaszkiew, M Picková, R Víchová, T Gybe?, K Kaiser, L Demková, L Ku?erová, T Bárta, D Pot?šil, Z Zdráhal, K Sou?ek, V Bryja
Elife, 2021-10-27;10(0):.
Species: Human
Sample Types: Transduced Whole Cells, Transfected Whole Cells
Applications: Bioassay -
IgD promotes pannus formation by activating Wnt5A-Fzd5-CTHRC1-NF-kappaB signaling pathway in FLS of CIA rats and the regulation of IgD-Fc-Ig fusion protein
Authors: Y Tai, Y Zhu, D Mei, H Wang, Q Yu, C Hong, X Cai, L Xu, J Ge, F Liang, C Jiang, Z Xue, L Hu, R Liu, T Zhang, P Wang, X Zhang, F Zhang, W Wei, L Zhang
International immunopharmacology, 2021-10-20;101(0):108261.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
FSH stimulated bovine granulosa cell steroidogenesis involves both canonical and noncanonical WNT signaling
Authors: M Ashry, JK Folger, SK Rajput, J Baroni, GW Smith
Domestic animal endocrinology, 2021-09-05;78(0):106678.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Transcriptional Regulation of Drug Metabolizing CYP Enzymes by Proinflammatory Wnt5A Signaling in Human Coronary Artery Endothelial Cells
Authors: T Skaria, E Bachli, G Schoedon
Frontiers in Pharmacology, 2021-05-17;12(0):619588.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Regulation of Wnt/PCP signaling through p97/VCP-KBTBD7-mediated Vangl ubiquitination and endoplasmic reticulum-associated degradation
Authors: D Feng, J Wang, W Yang, J Li, X Lin, F Zha, X Wang, L Ma, NT Choi, Y Mii, S Takada, MSY Huen, Y Guo, L Zhang, B Gao
Science Advances, 2021-05-14;7(20):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Pharmacological Wnt ligand inhibition overcomes key tumor-mediated resistance pathways to anti-PD-1 immunotherapy
Authors: NC DeVito, M Sturdivant, B Thievanthi, C Xiao, MP Plebanek, AKS Salama, GM Beasley, A Holtzhause, V Novotny-Di, JH Strickler, BA Hanks
Cell Reports, 2021-05-04;35(5):109071.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Wnt5a promotes hippocampal postsynaptic development and GluN2B-induced expression via the eIF2&alpha HRI kinase
Authors: E Ramos-Fern, MS Arrázola, CA Oliva, SB Arredondo, L Varela-Nal, NC Inestrosa
Scientific Reports, 2021-04-01;11(1):7395.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
FOSL2 promotes VEGF-independent angiogenesis by transcriptionnally activating Wnt5a in breast cancer-associated fibroblasts
Authors: X Wan, S Guan, Y Hou, Y Qin, H Zeng, L Yang, Y Qiao, S Liu, Q Li, T Jin, Y Qiu, M Liu
Theranostics, 2021-03-05;11(10):4975-4991.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Phosphorylation-induced changes in the PDZ domain of Dishevelled 3
Authors: M Jurásek, J Kumar, P Paclíková, A Kumari, K Tripsianes, V Bryja, R Vácha
Scientific Reports, 2021-01-15;11(1):1484.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Non-canonical Wnt/PCP signalling regulates intestinal stem cell lineage priming towards enteroendocrine and Paneth cell fates
Authors: A Böttcher, M Büttner, S Tritschler, M Sterr, A Aliluev, L Oppenlände, I Burtscher, S Sass, M Irmler, J Beckers, C Ziegenhain, W Enard, AC Schamberge, FM Verhamme, O Eickelberg, FJ Theis, H Lickert
Nature Cell Biology, 2021-01-04;23(1):23-31.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
Regulation of ABCG2 expression by Wnt5a through FZD7 in human pancreatic cancer cells
Authors: Z Zhang, S Gao, Y Xu, C Zhao
Mol Med Rep, 2020-11-17;23(1):.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
A Zic2-regulated switch in a noncanonical Wnt/&betacatenin pathway is essential for the formation of bilateral circuits
Authors: C Morenilla-, MT López-Casc, JP López-Atal, D Baeza, L Calvo-Díaz, A Barco, E Herrera
Sci Adv, 2020-11-13;6(46):.
Species: Mouse
Sample Types: Whole Tissue
Applications: Tissue Culture -
Egr-1 mediates low-dose arecoline induced human oral mucosa fibroblast proliferation via transactivation of Wnt5a expression
Authors: Q Chen, J Jiao, Y Wang, Z Mai, J Ren, S He, X Li, Z Chen
BMC Mol Cell Biol, 2020-11-10;21(1):80.
Species: Human
Sample Types: Whole Cells
Applications: Control -
Glucocorticoids induce differentiation and chemoresistance in ovarian cancer by promoting ROR1-mediated stemness
Authors: H Karvonen, M Arjama, L Kaleva, W Niininen, H Barker, R Koivisto-K, J Tapper, P Pakarinen, H Lassus, M Loukovaara, R Bützow, O Kallioniem, A Murumägi, D Ungureanu
Cell Death Dis, 2020-09-23;11(9):790.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Salt causes aging-associated hypertension via vascular Wnt5a under Klotho deficiency
Authors: W Kawarazaki, R Mizuno, M Nishimoto, N Ayuzawa, D Hirohama, K Ueda, F Kawakami-M, S Oba, T Marumo, T Fujita
J. Clin. Invest., 2020-08-03;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Ryk modulates the niche activity of mesenchymal stromal cells by fine-tuning canonical Wnt signaling
Authors: SY Jeong, J Lyu, JA Kim, IH Oh
Exp. Mol. Med., 2020-07-28;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Wnt5a regulates Ameloblastoma Cell Migration by modulating Mitochondrial and Cytoskeletal Dynamics
Authors: X Qiao, X Niu, J Shi, L Chen, X Wang, J Liu, L Zhu, M Zhong
J Cancer, 2020-07-11;11(18):5490-5502.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay, Cell Culture -
Rlip Depletion Suppresses Growth of Breast Cancer
Authors: C Bose, S Yadav, SS Singhal, J Singhal, A Hindle, J Lee, NKS Cheedella, S Rehman, RL Rahman, C Jones, M Darden, PT Palade, D Berz, SP Singh, S Awasthi
Cancers (Basel), 2020-06-02;12(6):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Adipogenesis of skeletal muscle fibro/adipogenic progenitors is affected by the WNT5a/GSK3/beta-catenin axis
Authors: A Reggio, M Rosina, A Palma, A Cerquone P, LL Petrilli, C Gargioli, C Fuoco, E Micarelli, G Giuliani, M Cerretani, A Bresciani, F Sacco, L Castagnoli, G Cesareni
Cell Death Differ., 2020-05-07;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A tumor-intrinsic PD-L1-NLRP3 inflammasome signaling pathway drives resistance to anti-PD-1 immunotherapy
Authors: B Theivanthi, KS Evans, NC DeVito, MP Plebanek, M Sturdivant, LP Wachsmuth, AK Salama, Y Kang, D Hsu, JM Balko, DB Johnson, M Starr, AB Nixon, A Holtzhause, BA Hanks
J. Clin. Invest., 2020-05-01;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Notch signalling drives synovial fibroblast identity and arthritis pathology
Authors: K Wei, I Korsunsky, JL Marshall, A Gao, GFM Watts, T Major, AP Croft, J Watts, PE Blazar, JK Lange, TS Thornhill, A Filer, K Raza, LT Donlin, CW Siebel, CD Buckley, S Raychaudhu, MB Brenner
Nature, 2020-04-22;582(7811):259-264.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Canonical and non-canonical Wnt signaling are simultaneously activated by Wnts in colon cancer cells
Authors: E Flores-Her, DM Velázquez, MC Castañeda-, G Fuentes-Ga, G Fonseca-Ca, JK Yamamoto-F, MT Romero-Avi, JA García-Sái, M Robles-Flo
Cell. Signal., 2020-04-10;72(0):109636.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Involvement of Wnt7a in the role of M2c microglia in neural stem cell oligodendrogenesis
Authors: M Mecha, N Yanguas-Ca, A Feliú, L Mestre, FJ Carrillo-S, K Riecken, D Gomez-Nico, C Guaza
J Neuroinflammation, 2020-03-19;17(1):88.
Species: Rat
Sample Types:
Applications: Cell Culture -
Norrin restores blood-retinal barrier properties after vascular endothelial growth factor-induced permeability
Authors: M Díaz-Corán, CM Lin, S Liebner, DA Antonetti
J. Biol. Chem., 2020-02-21;0(0):.
Species: Bovine
Sample Types: Whole Cells
Applications: Bioassay -
Inhibition of colony stimulating factor 1 receptor corrects maternal inflammation-induced microglial and synaptic dysfunction and behavioral abnormalities
Authors: S Ikezu, H Yeh, JC Delpech, ME Woodbury, AA Van Enoo, Z Ruan, S Sivakumara, Y You, C Holland, T Guillamon-, A Yoshii-Kit, MB Botros, C Madore, PH Chao, A Desani, S Manimaran, SV Kalavai, WE Johnson, O Butovsky, M Medalla, JI Luebke, T Ikezu
Mol. Psychiatry, 2020-02-18;0(0):.
Species: Mouse
Sample Types: Reference Standard
Applications: ELISA Standard -
Wnt5a/CaMKII/ERK/CCL2 axis is required for tumor-associated macrophages to promote colorectal cancer progression
Authors: Q Liu, J Song, Y Pan, D Shi, C Yang, S Wang, B Xiong
Int. J. Biol. Sci., 2020-02-04;16(6):1023-1034.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
H3K27me3-mediated PGC1&alpha gene silencing promotes melanoma invasion through WNT5A and YAP
Authors: C Luo, E Balsa, EA Perry, J Liang, CD Tavares, F Vazquez, HR Widlund, P Puigserver
J. Clin. Invest., 2020-02-03;130(2):853-862.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Structural insight into small molecule action on Frizzleds
Authors: P Kozielewic, A Turku, CF Bowin, J Petersen, J Valnohova, MCA Cañizal, Y Ono, A Inoue, C Hoffmann, G Schulte
Nat Commun, 2020-01-21;11(1):414.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Divergent effects of Wnt5b on IL-3- and GM-CSF-induced myeloid differentiation
Authors: MM de Rezende, JP Ng-Blichfe, GZ Justo, EJ Paredes-Ga, R Gosens
Cell. Signal., 2019-12-17;67(0):109507.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Construction of a vascularized bladder with autologous adipose-derived stromal vascular fraction cells combined with bladder acellular matrix via tissue engineering
Authors: F Zhao, L Zhou, J Liu, Z Xu, W Ping, H Li, L Xu, Z Xu, C Zhou, M Wang, R Jia
J Tissue Eng, 2019-11-29;10(0):2041731419891.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Mesenchymal WNT-5A/5B Signaling Represses Lung Alveolar Epithelial Progenitors
Authors: X Wu, EM van Dijk, JP Ng-Blichfe, IST Bos, C Ciminieri, M Königshoff, LEM Kistemaker, R Gosens
Cells, 2019-09-25;8(10):.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Bioassay -
An Autocrine Wnt5a Loop Promotes NF-kappaB Pathway Activation and Cytokine/Chemokine Secretion in Melanoma
Authors: G Barbero, MV Castro, MB Villanueva, MJ Quezada, NB Fernández, S DeMorrow, P Lopez-Berg
Cells, 2019-09-10;8(9):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Systematically understanding the immunity leading to CRPC progression
Authors: Z Ji, W Zhao, HK Lin, X Zhou
PLoS Comput. Biol., 2019-09-10;15(9):e1007344.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
WNT5A is transported via lipoprotein particles in the cerebrospinal fluid to regulate hindbrain morphogenesis
Authors: K Kaiser, D Gyllborg, J Procházka, A Salašová, P Kompaníkov, FL Molina, R Laguna-Goy, T Radaszkiew, J Harnoš, M Procházkov, D Pot?šil, RA Barker, ÁG Casado, Z Zdráhal, R Sedlá?ek, E Arenas, JC Villaescus, V Bryja
Nat Commun, 2019-04-02;10(1):1498.
Species: Mouse
Sample Types: Recombinant Protein, Whole Cells
Applications: Bioassay -
Non-canonical WNT-signaling controls differentiation of lymphatics and extension lymphangiogenesis via RAC and JNK signaling
Authors: G Lutze, A Haarmann, JA Demanou To, K Buttler, J Wilting, J Becker
Sci Rep, 2019-03-18;9(1):4739.
Species: Human, Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Bioassay -
Wnt5a and ROR1 activate non-canonical Wnt signaling via RhoA in TCF3-PBX1 acute lymphoblastic leukemia and highlight new treatment strategies via Bcl-2 co-targeting
Authors: H Karvonen, R Perttilä, W Niininen, V Hautanen, H Barker, A Murumägi, CA Heckman, D Ungureanu
Oncogene, 2019-01-10;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody
Authors: S Zhang, H Zhang, EM Ghia, J Huang, L Wu, J Zhang, S Lam, Y Lei, J He, B Cui, GF Widhopf, J Yu, R Schwab, K Messer, W Jiang, BA Parker, DA Carson, TJ Kipps
Proc. Natl. Acad. Sci. U.S.A., 2019-01-08;116(4):1370-1377.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Transcriptome 3'end organization by PCF11 links alternative polyadenylation to formation and neuronal differentiation of neuroblastoma
Authors: A Ogorodniko, M Levin, S Tattikota, S Tokalov, M Hoque, D Scherzinge, F Marini, A Poetsch, H Binder, S Macher-Göp, HC Probst, B Tian, M Schaefer, KJ Lackner, F Westermann, S Danckwardt
Nat Commun, 2018-12-14;9(1):5331.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Aging differentially modulates the Wnt pro-survival signalling pathways in vascular smooth muscle cells
Authors: BA Brown, GM Connolly, CEJ Mill, H Williams, GD Angelini, JL Johnson, SJ George
Aging Cell, 2018-12-12;0(0):e12844.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Receptor tyrosine kinase profiling of ischemic heart identifies ROR1 as a potential therapeutic target
Authors: J Heliste, A Jokilammi, I Paatero, D Chakrobort, C Stark, T Savunen, M Laaksonen, K Elenius
BMC Cardiovasc Disord, 2018-10-20;18(1):196.
-
Identification of genes which regulate stroma-dependent in vitro hematopoiesis
Authors: P Periasamy, V Tran, HC O'Neill
PLoS ONE, 2018-10-11;13(10):e0205583.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Reciprocal control of excitatory synapse numbers by Wnt and Wnt inhibitor PRR7 secreted on exosomes
Authors: SH Lee, SM Shin, P Zhong, HT Kim, DI Kim, JM Kim, W Do Heo, DW Kim, CY Yeo, CH Kim, QS Liu
Nat Commun, 2018-08-24;9(1):3434.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Precardiac organoids form two heart fields via Bmp/Wnt signaling
Authors: P Andersen, E Tampakakis, DV Jimenez, S Kannan, M Miyamoto, HK Shin, A Saberi, S Murphy, E Sulistio, SP Chelko, C Kwon
Nat Commun, 2018-08-07;9(1):3140.
Species: Mouse
Sample Types:
-
Intracellular Calcium Determines the Adipogenic Differentiation Potential of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells via the Wnt5a/?-Catenin Signaling Pathway
Authors: YK Bae, JH Kwon, M Kim, GH Kim, SJ Choi, W Oh, YS Yang, HJ Jin, HB Jeon
Stem Cells Int, 2018-07-11;2018(0):6545071.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Role of Wnt11 during Osteogenic Differentiation of Human Mesenchymal Stem Cells on Microstructured Titanium Surfaces
Authors: BD Boyan, R Olivares-N, MB Berger, SL Hyzy, Z Schwartz
Sci Rep, 2018-06-05;8(1):8588.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Retinoid-Sensitive Epigenetic Regulation of the Hoxb Cluster Maintains Normal Hematopoiesis and Inhibits Leukemogenesis
Authors: P Qian, B De Kumar, XC He, C Nolte, M Gogol, Y Ahn, S Chen, Z Li, H Xu, JM Perry, D Hu, F Tao, M Zhao, Y Han, K Hall, A Peak, A Paulson, C Zhao, A Venkatrama, A Box, A Perera, JS Haug, T Parmely, H Li, R Krumlauf, L Li
Cell Stem Cell, 2018-05-03;22(5):740-754.e7.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A20 regulates canonical wnt-signaling through an interaction with RIPK4
Authors: BN Nakamura, A Glazier, MG Kattah, B Duong, Y Jia, D Campo, L Shao
PLoS ONE, 2018-05-02;13(5):e0195893.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Wnt5a induces ROR1 to recruit DOCK2 to activate Rac1/2 in chronic lymphocytic leukemia
Authors: MK Hasan, J Yu, GF Widhopf, LZ Rassenti, L Chen, Z Shen, SP Briggs, DS Neuberg, TJ Kipps
Blood, 2018-04-20;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Effect of SFRP5 (Secreted Frizzled-Related Protein 5) on the WNT5A (Wingless-Type Family Member 5A)-Induced Endothelial Dysfunction and Its Relevance With Arterial Stiffness in Human Subjects
Authors: YK Cho, YM Kang, SE Lee, Y Lee, SM Seol, WJ Lee, JY Park, CH Jung
Arterioscler. Thromb. Vasc. Biol., 2018-04-19;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Wnt5a suppresses inflammation-driven intervertebral disc degeneration via a TNF-α/NF-κB -Wnt5a negative-feedback loop
Authors: Z Li, K Zhang, X Li, H Pan, S Li, F Chen, J Zhang, Z Zheng, J Wang, H Liu
Osteoarthr. Cartil., 2018-04-12;0(0):.
Species: Rat
Sample Types: In Vivo, Whole Cells
Applications: Bioassay, In Vivo -
The Coordinated Activities of nAChR and Wnt Signaling Regulate Intestinal Stem Cell Function in Mice
Authors: T Takahashi, A Shiraishi, J Murata
Int J Mol Sci, 2018-03-05;19(3):.
Species: Mouse
Sample Types: Complex Sample Type
Applications: Bioassay -
Caveolin-1 down-regulation is required for Wnt5a-Frizzled 2 signalling in Ha-RasV12-induced cell transformation
Authors: HK Lin, HH Lin, YW Chiou, CL Wu, WT Chiu, MJ Tang
J. Cell. Mol. Med., 2018-03-04;0(0):.
Applications: Bioassay -
Blocking Wnt5a signaling decreases CD36 expression and foam cell formation in atherosclerosis
Authors: I Ackers, C Szymanski, KJ Duckett, LA Consitt, MJ Silver, R Malgor
Cardiovasc. Pathol., 2018-02-21;34(0):1-8.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Population snapshots predict early haematopoietic and erythroid hierarchies
Authors: BK Tusi, SL Wolock, C Weinreb, Y Hwang, D Hidalgo, R Zilionis, A Waisman, JR Huh, AM Klein, M Socolovsky
Nature, 2018-02-21;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Spaceflight Activates Protein Kinase C Alpha Signaling and Modifies the Developmental Stage of Human Neonatal Cardiovascular Progenitor Cells
Authors: J Baio, AF Martinez, LL Bailey, N Hasaniya, M Pecaut, M Kearns-Jon
Stem Cells Dev., 2018-02-12;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Reduced Cdc42 Activity Compromises Hematopoiesis-Supportive Function Of Fanconi Anemia Mesenchymal Stromal Cells
Authors: J Xu, X Li, A Cole, Z Sherman, W Du
Stem Cells, 2018-02-09;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
TSC1 and TSC2 regulate cilia length and canonical Hedgehog signaling via different mechanisms
Authors: T Rosengren, LJ Larsen, LB Pedersen, ST Christense, LB Møller
Cell. Mol. Life Sci., 2018-02-02;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
miR-613 inhibits cell migration and invasion by downregulating Daam1 in triple-negative breast cancer
Authors: H Xiong, T Yan, W Zhang, F Shi, X Jiang, X Wang, S Li, Y Chen, C Chen, Y Zhu
Cell. Signal., 2018-01-12;44(0):33-42.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Wnt inhibition promotes vascular specification of embryonic cardiac progenitors
Authors: DE Reichman, L Park, L Man, D Redmond, K Chao, RP Harvey, MM Taketo, Z Rosenwaks, D James
Development, 2018-01-08;145(1):.
Species: Human
Sample Types: Whole Cells
Applications: Differentiation, Differentiation -
Generation of iPSC-derived limb progenitor-like cells for stimulating phalange regeneration in the adult mouse
Authors: Y Chen, H Xu, G Lin
Cell Discov, 2017-12-19;3(0):17046.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
BMP3 expression by osteoblast lineage cells is regulated by canonical Wnt signaling
Authors: S Kokabu, V Rosen
FEBS Open Bio, 2017-12-16;8(2):168-176.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Kinesin superfamily protein Kif26b links Wnt5a-Ror signaling to the control of cell and tissue behaviors in vertebrates
Authors: MW Susman, EP Karuna, RC Kunz, TS Gujral, AV Cantú, SS Choi, BY Jong, K Okada, MK Scales, J Hum, LS Hu, MW Kirschner, R Nishinakam, S Yamada, DJ Laird, LE Jao, SP Gygi, ME Greenberg, HH Ho
Elife, 2017-09-08;6(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
ATG5 mediates a positive feedback loop between Wnt signaling and autophagy in melanoma
Authors: A Ndoye, A Budina-Kol, CH Kugel, MR Webster, A Kaur, R Behera, VW Rebecca, L Li, PA Brafford, Q Liu, YN Vashisht G, MA Davies, GB Mills, X Xu, H Wu, M Herlyn, MC Nicastri, JD Winkler, MS Soengas, RK Amaravadi, ME Murphy, AT Weeraratna
Cancer Res., 2017-09-08;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
N-terminal part of Dishevelled DEP domain is required for Wnt/?-catenin signaling in mammalian cells
Authors: P Paclíková, O Bernatík, TW Radaszkiew, V Bryja
Mol. Cell. Biol., 2017-08-28;0(0):.
Species: Human
Sample Types: Whole Cells
-
Agonist-induced dimer dissociation as a macromolecular step in G protein-coupled receptor signaling
Authors: J Petersen, SC Wright, D Rodríguez, P Matricon, N Lahav, A Vromen, A Friedler, J Strömqvist, S Wennmalm, J Carlsson, G Schulte
Nat Commun, 2017-08-09;8(1):226.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Expression of RMRP RNA is regulated in chondrocyte hypertrophy and determines chondrogenic differentiation
Authors: MMF Steinbusch, MMJ Caron, DAM Surtel, F Friedrich, E Lausch, GJM Pruijn, W Verhesen, BLM Schroen, LW van Rhijn, B Zabel, TJM Welting
Sci Rep, 2017-07-25;7(1):6440.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The tyrosine Y250(2.39) in Frizzled 4 defines a conserved motif important for structural integrity of the receptor and recruitment of Disheveled
Authors: K Strakova, P Matricon, C Yokota, E Arthofer, O Bernatik, D Rodriguez, E Arenas, J Carlsson, V Bryja, G Schulte
Cell. Signal., 2017-06-29;38(0):85-96.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Higher expression of WNT5A protein in oral squamous cell carcinoma compared with dysplasia and oral mucosa with a normal appearance
Authors: Z Prgomet, T Andersson, P Lindberg
Eur. J. Oral Sci., 2017-06-12;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Rear-polarized Wnt5a-receptor-actin-myosin-polarity (WRAMP) structures promote the speed and persistence of directional cell migration
Authors: MK Connacher, JW Tay, NG Ahn
Mol. Biol. Cell, 2017-06-07;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
WIF1 prevents Wnt5A mediated LIMK/CFL phosphorylation and adherens junction disruption in human vascular endothelial cells
Authors: T Skaria, E Bachli, G Schoedon
J Inflamm (Lond), 2017-05-19;14(0):10.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Beta-catenin inhibitor BC2059 is efficacious as monotherapy or in combination with proteasome inhibitor bortezomib in multiple myeloma
Authors: I Savvidou, T Khong, A Cuddihy, C McLean, S Horrigan, A Spencer
Mol. Cancer Ther., 2017-05-12;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A Novel Role for the BMP Antagonist Noggin in Sensitizing Cells to Non-canonical Wnt-5a/Ror2/Disheveled Pathway Activation
Authors: O Bernatik, T Radaszkiew, M Behal, Z Dave, F Witte, A Mahl, NH Cernohorsk, P Krejci, S Stricker, V Bryja
Front Cell Dev Biol, 2017-05-04;5(0):47.
Species: Mouse, Rat
Sample Types: Whole Cells
Applications: Bioassay -
Ring finger protein 43 associates with gastric cancer progression and attenuates the stemness of gastric cancer stem-like cells via the Wnt-?/catenin signaling pathway
Authors: Y Gao, A Cai, H Xi, J Li, W Xu, Y Zhang, K Zhang, J Cui, X Wu, B Wei, L Chen
Stem Cell Res Ther, 2017-04-26;8(1):98.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
ABCB1 and ABCG2 drug transporters are differentially expressed in non-small cell lung cancers (NSCLC) and expression is modified by cisplatin treatment via altered Wnt signaling
Authors: M Vesel, J Rapp, D Feller, E Kiss, L Jaromi, M Meggyes, G Miskei, B Duga, G Smuk, T Laszlo, I Karner, JE Pongracz
Respir. Res, 2017-03-24;18(1):52.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A Wnt5 Activity Asymmetry and Intercellular Signaling via PCP Proteins Polarize Node Cells for Left-Right Symmetry Breaking
Authors: K Minegishi, M Hashimoto, R Ajima, K Takaoka, K Shinohara, Y Ikawa, H Nishimura, AP McMahon, K Willert, Y Okada, H Sasaki, D Shi, T Fujimori, T Ohtsuka, Y Igarashi, TP Yamaguchi, A Shimono, H Shiratori, H Hamada
Dev. Cell, 2017-03-13;40(5):439-452.e4.
Species: Mouse
Sample Types: Whole Embryo
Applications: Bioassay -
Diverse regulation of mammary epithelial growth and branching morphogenesis through noncanonical Wnt signaling
Authors: K Kessenbroc, P Smith, SC Steenbeek, N Pervolarak, R Kumar, Y Minami, A Goga, L Hinck, Z Werb
Proc. Natl. Acad. Sci. U.S.A, 2017-03-07;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Heterozygous Vangl2(Looptail) mice reveal novel roles for the planar cell polarity pathway in adult lung homeostasis and repair
Authors: T Poobalasin, LL Yates, SA Walker, M Pereira, NY Gross, A Ali, M Kolatsi-Jo, MR Jarvelin, J Pekkanen, E Papakrivop, DA Long, M Griffiths, D Wagner, M Königshoff, M Hind, C Minelli, CM Lloyd, CH Dean
Dis Model Mech, 2017-02-24;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Inhibition of age-related therapy resistance in melanoma by rosiglitazone-mediated induction of Klotho
Authors: R Behera, A Kaur, MR Webster, S Kim, A Ndoye, CH Kugel, GM Alicea, JX Wang, K Ghosh, PF Cheng, S Lisanti, K Marchbank, V Dang, MP Levesque, R Dummer, X Xu, M Heryn, AE Aplin, A Roesch, MC Caino, DC Altieri, AT Weeraratna
Clin. Cancer Res, 2017-02-23;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
KIF13B establishes a CAV1-enriched microdomain at the ciliary transition zone to promote Sonic hedgehog signalling
Authors: KB Schou, JB Mogensen, SK Morthorst, BS Nielsen, A Aleliunait, A Serra-Marq, N Frstenber, S Saunier, AA Bizet, IR Veland, A Akhmanova, ST Christense, LB Pedersen
Nat Commun, 2017-01-30;8(0):14177.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
WNT/?-catenin pathway modulates the TNF-?-induced inflammatory response in bronchial epithelial cells
Authors: J Jang, Y Jung, S Chae, SI Chung, SM Kim, Y Yoon
Biochem. Biophys. Res. Commun, 2017-01-28;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
LPS-induced inflammatory response is suppressed by Wnt inhibitors, Dickkopf-1 and LGK974
Authors: J Jang, Y Jung, Y Kim, EH Jho, Y Yoon
Sci Rep, 2017-01-27;7(0):41612.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD
Authors: Hoeke A Baarsma
J. Exp. Med, 2016-12-15;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Porcupine-dependent Wnt signaling controls stromal proliferation and endometrial gland maintenance through the action of distinct WNTs
Authors: Omar Farah
Dev. Biol, 2016-12-10;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Dickkopf-1 promotes hematopoietic regeneration via direct and niche-mediated mechanisms
Nat. Med, 2016-12-05;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Wnt5a induces renal AQP2 expression by activating calcineurin signalling pathway
Nat Commun, 2016-11-28;7(0):13636.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Increased Wnt5a in squamous cell lung carcinoma inhibits endothelial cell motility
BMC Cancer, 2016-11-23;16(1):915.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Epigenetic Activation of WNT5A Drives Glioblastoma Stem Cell Differentiation and Invasive Growth
Authors: Ronald A DePinho
Cell, 2016-11-17;167(5):1281-1295.e18.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
WNT5A signaling impairs breast cancer cell migration and invasion via mechanisms independent of the epithelial-mesenchymal transition
J Exp Clin Cancer Res, 2016-09-13;35(1):144.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Identification of proliferative and mature ?-cells in the islets of Langerhans
Nature, 2016-07-11;535(7612):430-4.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Wnt-5a/Frizzled9 receptor signaling through the G?o/G?? complex regulates dendritic spine formation
J Biol Chem, 2016-07-11;0(0):.
Applications: Bioassay -
IL-4 Causes Hyperpermeability of Vascular Endothelial Cells through Wnt5A Signaling
PLoS ONE, 2016-05-23;11(5):e0156002.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
KLF4 transcriptionally activates non-canonical WNT5A to control epithelial stratification
Sci Rep, 2016-05-17;6(0):26130.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Wnt5A/Ryk signaling critically affects barrier function in human vascular endothelial cells
Authors: T Skaria, E Bachli, G Schoedon
Cell Adh Migr, 2016-05-09;11(1):24-38.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Dysregulation of WNT5A/ROR2 Signaling Characterizes the Progression of Barrett's-associated Esophageal Adenocarcinoma
Authors: O Lyros, L Nie, T Moore, R Medda, M Otterson, B Behmaram, A Mackinnon, I Gockel, R Shaker, P Rafiee
Mol Cancer Res, 2016-04-15;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
FRIZZLED7 Is Required for Tumor Inititation and Metastatic Growth of Melanoma Cells.
Authors: Tiwary S, Xu L
PLoS ONE, 2016-01-25;11(1):e0147638.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Facilitation of axon outgrowth via a Wnt5a-CaMKK-CaMKIalpha pathway during neuronal polarization.
Authors: Horigane S, Ageta-Ishihara N, Kamijo S, Fujii H, Okamura M, Kinoshita M, Takemoto-Kimura S, Bito H
Mol Brain, 2016-01-16;9(1):8.
Species: Mouse, Rat
Sample Types: Whole Cells
Applications: Bioassay -
Regeneration of Articular Cartilage by Human ESC-Derived Mesenchymal Progenitors Treated Sequentially With BMP-2 and Wnt5a
Stem Cells Transl Med, 2016-01-01;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Wnt5a induces ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and proliferation
Authors: Jian Yu, Liguang Chen, Bing Cui, George F. Widhopf, Zhouxin Shen, Rongrong Wu et al.
Journal of Clinical Investigation
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
WNT5A transforms intestinal CD8alphaalpha(+) IELs into an unconventional phenotype with pro-inflammatory features.
Authors: Zhao D, Xu A, Dai Z, Peng J, Zhu M, Shen J, Zheng Q, Ran Z
BMC Gastroenterol, 2015-12-10;15(0):173.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Targeting the ROR1 and ROR2 receptors in epithelial ovarian cancer inhibits cell migration and invasion.
Authors: Henry C, Llamosas E, Knipprath-Meszaros A, Schoetzau A, Obermann E, Fuenfschilling M, Caduff R, Fink D, Hacker N, Ward R, Heinzelmann-Schwarz V, Ford C
Oncotarget, 2015-11-24;6(37):40310-26.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Uterine RAC1 via Pak1-ERM signaling directs normal luminal epithelial integrity conducive to on-time embryo implantation in mice.
Authors: Tu Z, Wang Q, Cui T, Wang J, Ran H, Bao H, Lu J, Wang B, Lydon J, DeMayo F, Zhang S, Kong S, Wu X, Wang H
Cell Death Differ, 2015-07-17;23(1):169-81.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Canonical wnt signaling in dendritic cells regulates Th1/Th17 responses and suppresses autoimmune neuroinflammation.
Authors: Suryawanshi A, Manoharan I, Hong Y, Swafford D, Majumdar T, Taketo M, Manicassamy B, Koni P, Thangaraju M, Sun Z, Mellor A, Munn D, Manicassamy S
J Immunol, 2015-02-20;194(7):3295-304.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Endogenously produced Indian Hedgehog regulates TGFbeta-driven chondrogenesis of human bone marrow stromal/stem cells.
Authors: Handorf A, Chamberlain C, Li W
Stem Cells Dev, 2015-01-26;24(8):995-1007.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Partial maintenance and long-term expansion of murine skin epithelial stem cells by Wnt-3a in vitro.
Authors: Ouji Y, Ishizaka S, Nakamura-Uchiyama F, Okuzaki D, Yoshikawa M
J Invest Dermatol, 2014-12-01;135(6):1598-608.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Wnt-5a ligand modulates mitochondrial fission-fusion in rat hippocampal neurons.
Authors: Godoy J, Arrazola M, Ordenes D, Silva-Alvarez C, Braidy N, Inestrosa N
J Biol Chem, 2014-10-21;289(52):36179-93.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Thyroid hormone-regulated Wnt5a/Ror2 signaling is essential for dedifferentiation of larval epithelial cells into adult stem cells in the Xenopus laevis intestine.
Authors: Ishizuya-Oka, Atsuko, Kajita, Mitsuko, Hasebe, Takashi
PLoS ONE, 2014-09-11;9(9):e107611.
Species: Xenopus
Sample Types: N/A, Whole Tissue
Applications: Bioassay, Western Blot -
Wnts enhance neurotrophin-induced neuronal differentiation in adult bone-marrow-derived mesenchymal stem cells via canonical and noncanonical signaling pathways.
Authors: Tsai H, Deng W, Lai W, Chiu W, Yang C, Tsai Y, Hwang S, Renshaw P
PLoS ONE, 2014-08-29;9(8):e104937.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Wnt5a promotes inflammatory responses via nuclear factor kappaB (NF-kappaB) and mitogen-activated protein kinase (MAPK) pathways in human dental pulp cells.
Authors: Zhao Y, Wang C, Li R, Hui T, Su Y, Yuan Q, Zhou X, Ye L
J Biol Chem, 2014-07-25;289(30):21028-39.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Evolutionary divergence in the catalytic activity of the CAM-1, ROR1 and ROR2 kinase domains.
Authors: Bainbridge T, DeAlmeida V, Izrael-Tomasevic A, Chalouni C, Pan B, Goldsmith J, Schoen A, Quinones G, Kelly R, Lill J, Sandoval W, Costa M, Polakis P, Arnott D, Rubinfeld B, Ernst J
PLoS ONE, 2014-07-16;9(7):e102695.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Both canonical and non-canonical Wnt signaling independently promote stem cell growth in mammospheres.
Authors: Many A, Brown A
PLoS ONE, 2014-07-14;9(7):e101800.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-1beta-induced Wnt5a enhances human corneal endothelial cell migration through regulation of Cdc42 and RhoA.
Authors: Lee J, Heur M
Mol Cell Biol, 2014-07-14;34(18):3535-45.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
IL-1beta mediates MCP-1 induction by Wnt5a in gastric cancer cells.
Authors: Li S, Wang W, Zhang N, Ma T, Zhao C
BMC Cancer, 2014-07-03;14(0):480.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Isolation and characterization of resident endogenous c-Kit+ cardiac stem cells from the adult mouse and rat heart.
Authors: Smith A, Lewis F, Aquila I, Waring C, Nocera A, Agosti V, Nadal-Ginard B, Torella D, Ellison G
Nat Protoc, 2014-06-19;9(7):1662-81.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Switch from canonical to noncanonical Wnt signaling mediates high glucose-induced adipogenesis.
Authors: Keats E, Dominguez J, Grant M, Khan Z
Stem Cells, 2014-06-01;32(6):1649-60.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Assessment of Frizzled 6 membrane mobility by FRAP supports G protein coupling and reveals WNT-Frizzled selectivity.
Authors: Kilander M, Dahlstrom J, Schulte G
Cell Signal, 2014-05-27;26(9):1943-9.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-6 drives melanoma cell motility through p38alpha-MAPK-dependent up-regulation of WNT5A expression.
Authors: Linnskog R, Jonsson G, Axelsson L, Prasad C, Andersson T
Mol Oncol, 2014-05-27;8(8):1365-78.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells.
Authors: Ekstrom E, Bergenfelz C, von Bulow V, Serifler F, Carlemalm E, Jonsson G, Andersson T, Leandersson K
Mol Cancer, 2014-04-26;13(0):88.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Wnt7a stimulates myogenic stem cell motility and engraftment resulting in improved muscle strength.
Authors: Bentzinger C, von Maltzahn J, Dumont N, Stark D, Wang Y, Nhan K, Frenette J, Cornelison D, Rudnicki M
J Cell Biol, 2014-04-07;205(1):97-111.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Noncanonical Wnt5a enhances Wnt/beta-catenin signaling during osteoblastogenesis.
Authors: Okamoto M, Udagawa N, Uehara S, Maeda K, Yamashita T, Nakamichi Y, Kato H, Saito N, Minami Y, Takahashi N, Kobayashi Y
Sci Rep, 2014-03-27;4(0):4493.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Wnt5a through noncanonical Wnt/JNK or Wnt/PKC signaling contributes to the differentiation of mesenchymal stem cells into type II alveolar epithelial cells in vitro.
Authors: Liu, Airan, Chen, Song, Cai, Shixia, Dong, Liang, Liu, Le, Yang, Yi, Guo, Fengmei, Lu, Xiaomin, He, Hongli, Chen, Qihong, Hu, Shuling, Qiu, Haibo
PLoS ONE, 2014-03-21;9(3):e90229.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Recombinant Wnt3a and Wnt5a elicit macrophage cytokine production and tolerization to microbial stimulation via Toll-like receptor 4.
Authors: Yu C, Nguyen T, Irvine K, Sweet M, Frazer I, Blumenthal A
Eur J Immunol, 2014-03-07;44(5):1480-90.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Wnt5a promotes migration of human osteosarcoma cells by triggering a phosphatidylinositol-3 kinase/Akt signals.
Authors: Zhang A, He S, Sun X, Ding L, Bao X, Wang N
Cancer Cell Int, 2014-02-14;14(1):15.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Prostate cancer bone metastases acquire resistance to androgen deprivation via WNT5A-mediated BMP-6 induction.
Authors: Lee G, Kang D, Ha Y, Jung Y, Chung J, Min K, Kim T, Moon K, Chung J, Lee D, Kim W, Kim I
Br J Cancer, 2014-02-11;110(6):1634-44.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
GEC-derived SFRP5 inhibits Wnt5a-induced macrophage chemotaxis and activation.
Authors: Zhao C, Bu X, Wang W, Ma T, Ma H
PLoS ONE, 2014-01-08;9(1):e85058.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Wnt5a regulates hematopoietic stem cell proliferation and repopulation through the Ryk receptor.
Authors: Povinelli B, Nemeth M
Stem Cells, 2014-01-01;32(1):105-15.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interactions between lens epithelial and fiber cells reveal an intrinsic self-assembly mechanism.
Authors: Dawes L, Sugiyama Y, Lovicu F, Harris C, Shelley E, McAvoy J
Dev Biol, 2013-11-08;385(2):291-303.
Species: Rat
Sample Types: Tissue Homogenates
Applications: Bioassay -
Activation of intracellular calcium by multiple Wnt ligands and translocation of beta-catenin into the nucleus: a convergent model of Wnt/Ca2+ and Wnt/beta-catenin pathways.
Authors: Thrasivoulou C, Millar M, Ahmed A
J Biol Chem, 2013-10-24;288(50):35651-9.
Species: Human, Primate - Chlorocebus pygerythrus (Vervet Monkey)
Sample Types: Whole Cells
Applications: Bioassay -
A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing.
Authors: Florian M, Nattamai K, Dorr K, Marka G, Uberle B, Vas V, Eckl C, Andra I, Schiemann M, Oostendorp R, Scharffetter-Kochanek K, Kestler H, Zheng Y, Geiger H
Nature, 2013-10-20;503(7476):392-6.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Wingless-type mammary tumor virus integration site family, member 5A (Wnt5a) regulates human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein 120 (gp120)-induced expression of pro-inflammatory cytokines via the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and c-Jun N-terminal kinase (JNK) signaling pathways.
Authors: Li B, Shi Y, Shu J, Gao J, Wu P, Tang S
J Biol Chem, 2013-03-28;288(19):13610-9.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Wnt5a uses CD146 as a receptor to regulate cell motility and convergent extension.
Authors: Ye Z, Zhang C, Tu T, Sun M, Liu D, Lu D, Feng J, Yang D, Liu F, Yan X
Nat Commun, 2013-01-01;4(0):2803.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Tiam1 regulates the Wnt/Dvl/Rac1 signaling pathway and the differentiation of midbrain dopaminergic neurons.
Authors: Cajanek L, Ganji R, Henriques-Oliveira C, Theofilopoulos S, Konik P, Bryja V, Arenas E
Mol Cell Biol, 2012-10-29;33(1):59-70.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors.
Authors: Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, Ribas A, Li J, Moffat J, Sutherlin DP, Koeppen H, Merchant M, Neve R, Settleman J
Nature, 2012-07-26;487(7408):505-9.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Reversible modification of adenomatous polyposis coli (APC) with K63-linked polyubiquitin regulates the assembly and activity of the beta-catenin destruction complex.
Authors: Tran H, Polakis P
J Biol Chem, 2012-07-03;287(34):28552-63.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Wnt5a and Wnt11 are essential for second heart field progenitor development.
Authors: Cohen E, Miller M, Wang Z, Moon R, Morrisey E
Development, 2012-06-01;139(11):1931-40.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Both LRP5 and LRP6 receptors are required to respond to physiological Wnt ligands in mammary epithelial cells and fibroblasts.
Authors: Goel S, Chin EN, Fakhraldeen SA
J. Biol. Chem., 2012-03-20;287(20):16454-66.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Ror family receptor tyrosine kinases regulate the maintenance of neural progenitor cells in the developing neocortex.
J. Cell. Sci., 2012-02-10;125(0):2017-29.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Separate and distinctive roles for Wnt5a in tongue, lingual tissue and taste papilla development
Authors: Hong-Xiang Liu, Ann S. Grosse, Ken Iwatsuki, Yuji Mishina, Deborah L. Gumucio, Charlotte M. Mistretta
Developmental Biology
Species: Mouse, Rat
Sample Types: Whole Cells
Applications: Bioassay -
WNT5A Signaling Contributes to Abeta-Induced Neuroinflammation and Neurotoxicity.
Authors: Li B, Zhong L, Yang X, Andersson T, Huang M, Tang SJ
PLoS ONE, 2011-08-17;6(8):e22920.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast.
Authors: Scheel C, Eaton E, Li S, Chaffer C, Reinhardt F, Kah K, Bell G, Guo W, Rubin J, Richardson A, Weinberg R
Cell, 2011-06-10;145(6):926-40.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Wnt5a regulates midbrain dopaminergic axon growth and guidance.
Authors: Blakely BD, Bye CR, Fernando CV
PLoS ONE, 2011-03-31;6(3):e18373.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
WNT-5A stimulates the GDP/GTP exchange at pertussis toxin-sensitive heterotrimeric G proteins.
Authors: Kilander MB, Dijksterhuis JP, Ganji RS, Bryja V, Schulte G
Cell. Signal., 2010-11-08;23(3):550-4.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Effects of Wnt5a protein on proliferation and apoptosis in JAR choriocarcinoma cells
Authors: Sha Peng, Junlin Zhang, Jiahuan Chen, Huayan Wang
Molecular Medicine Reports
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Evidence for altered Wnt signaling in psoriatic skin.
Authors: Gudjonsson JE, Johnston A, Stoll SW
J. Invest. Dermatol., 2010-04-08;130(7):1849-59.
Species: Human
Sample Types: Whole Cells
-
Reconstitution of a frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6.
Authors: Bourhis E, Tam C, Franke Y, Bazan JF, Ernst J, Hwang J, Costa M, Cochran AG, Hannoush RN
J. Biol. Chem., 2010-01-21;285(12):9172-9.
Species: Human
Sample Types: Recombinant Protein
Applications: Binding Assay -
Canonical Wnt signaling negatively regulates platelet function.
Authors: Steele BM, Harper MT, Macaulay IC, Morrell CN, Perez-Tamayo A, Foy M, Habas R, Poole AW, Fitzgerald DJ, Maguire PB
Proc. Natl. Acad. Sci. U.S.A., 2009-11-09;106(47):19836-41.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma.
Authors: O'Connell MP, Fiori JL, Xu M
Oncogene, 2009-10-05;29(1):34-44.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus.
Authors: Gogolla N, Galimberti I, Deguchi Y, Caroni P
Neuron, 2009-05-28;62(4):510-25.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Wnt5A activates the calpain-mediated cleavage of filamin A.
Authors: O'Connell MP, Fiori JL, Baugher KM, Indig FE, French AD, Camilli TC, Frank BP, Earley R, Hoek KS, Hasskamp JH, Elias EG, Taub DD, Bernier M, Weeraratna AT
J. Invest. Dermatol., 2009-01-29;129(7):1782-9.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Establishment of a transitory dorsal-biased window of localized Ca2+ signaling in the superficial epithelium following the mid-blastula transition in zebrafish embryos.
Authors: Ma LH, Webb SE, Chan CM, Zhang J, Miller AL
Dev. Biol., 2008-12-24;327(1):143-57.
Species: Zebrafish
Sample Types: Whole Cells
Applications: Bioassay -
The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility.
Authors: Safholm A, Tuomela J, Rosenkvist J, Dejmek J, Harkonen P, Andersson T
Clin. Cancer Res., 2008-10-15;14(20):6556-63.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Stably overexpressed human Frizzled-2 signals through the beta-catenin pathway and does not activate Ca2+-mobilization in Human Embryonic Kidney 293 cells.
Authors: Verkaar F, van Rosmalen JW, Smits JF, Blankesteijn WM, Zaman GJ
Cell. Signal., 2008-09-25;21(1):22-33.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3- and c-Jun N-terminal kinase-dependent mechanism.
Authors: Endo Y, Beauchamp E, Woods D, Taylor WG, Toretsky JA, Uren A, Rubin JS
Mol. Cell. Biol., 2008-01-22;28(7):2368-79.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Derivation of sarcomas from mesenchymal stem cells via inactivation of the Wnt pathway.
Authors: Matushansky I, Hernando E, Socci ND, Mills JE, Matos TA, Edgar MA, Singer S, Maki RG, Cordon-Cardo C
J. Clin. Invest., 2007-11-01;117(11):3248-57.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2.
Authors: Masckauchan TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, Khoo A, Tycko B, Brown AM, Kitajewski J
Mol. Biol. Cell, 2006-10-11;17(12):5163-72.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human/Mouse Wnt-5a Protein
Average Rating: 5 (Based on 8 Reviews)
Have you used Recombinant Human/Mouse Wnt-5a Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Reason for Rating: wnt5a was used to test if it an promote stem cell proliferation.
put it in -80 freezer
Kept in -80 freezer
avoid freezing and refreezing, and kept in -80
Reason for Rating: very good product
Try to avoid thaw and freeze many times.
Reason for Rating: it worked very good.
preserve it under -20 after reconstitution
Reason for Rating: Works great with invitro studies