Human IL‑6 Antibody
R&D Systems | Catalog # MAB95402

Key Product Details
Species Reactivity
Applications
Label
Antibody Source
Product Specifications
Immunogen
Pro29-Met212
Accession # Q75MH2
Specificity
Clonality
Host
Isotype
Scientific Data Images for Human IL‑6 Antibody
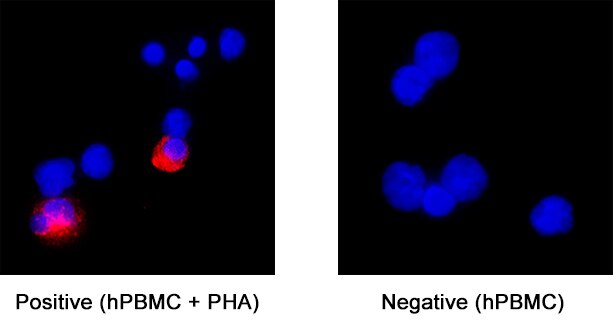
IL‑6 in Human PBMCs.
IL‑6 was detected in immersion fixed human peripheral blood mononuclear cells (PBMCs) treated with PHA (positive staining) and untreated PBMCs (negative control) using Rabbit Anti-Human IL‑6 Monoclonal Antibody (Catalog # MAB95402) at 8 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Rabbit IgG Secondary Antibody (red; NL004) and counterstained with DAPI (blue). Specific staining was localized to cell cytoplasm. Staining was performed using our protocol for Fluorescent ICC Staining of Non-adherent Cells.
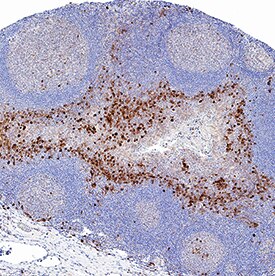
IL‑6 in Human Tonsil.
IL‑6 was detected in immersion fixed paraffin-embedded sections of human tonsil using Rabbit Anti-Human IL‑6 Monoclonal Antibody (Catalog # MAB95402) at 3 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Rabbit IgG VisUCyte™ HRP Polymer Antibody (VC003). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to lymphocytes. Staining was performed using our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
Applications for Human IL‑6 Antibody
Immunocytochemistry
Sample: Immersion fixed human peripheral blood mononuclear cells (PBMCs) treated with PHA
Immunohistochemistry
Sample: Immersion fixed paraffin-embedded sections of human tonsil
Formulation, Preparation, and Storage
Purification
Reconstitution
Reconstitute at 0.5 mg/mL in sterile PBS. For liquid material, refer to CoA for concentration.
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Calculators
Background: IL-6
References
- Mansell, A. and B.J. Jenkins (2013) Cytokine Growth Factor Rev. 24:249.
- Schuett, H. et al. (2009) Thromb. Haemost. 102:215.
- Erta, M. et al. (2012) Int. J. Biol. Sci. 8:1254.
- Garbers, C. et al. (2012) Cytokine Growth Factor Rev. 23:85.
- Mihara, M. et al. (2012) Clin. Sci. (Lond.) 122:143.
- Hirano, T. et al. (1986) Nature 324:73.
- Kestler, D.P. et al. (1995) Blood 86:4559.
- Kestler, D.P. et al. (1999) Am. J. Hematol. 61:169.
- Bihl, M.P. et al. (2002) Am. J. Respir. Cell Mol. Biol. 27:48.
- Alberti, L. et al. (2005) Cancer Res. 65:2.
- Murakami, M. et al. (1993) Science 260:1808.
- Muller-Newen, G. (2003) Sci. STKE 2003:PE40.
- Mitsuyama, K. et al. (2006) Clin. Exp. Immunol. 143:125.
- Cerutti, A. et al. (1998) J. Immunol. 160:2145.
Long Name
Alternate Names
Entrez Gene IDs
Gene Symbol
UniProt
Additional IL-6 Products
Product Documents for Human IL‑6 Antibody
Product Specific Notices for Human IL‑6 Antibody
For research use only
Related Research Areas
Customer Reviews for Human IL‑6 Antibody
There are currently no reviews for this product. Be the first to review Human IL‑6 Antibody and earn rewards!
Have you used Human IL‑6 Antibody?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Protocols
Find general support by application which include: protocols, troubleshooting, illustrated assays, videos and webinars.
- Antigen Retrieval Protocol (PIER)
- Antigen Retrieval for Frozen Sections Protocol
- Appropriate Fixation of IHC/ICC Samples
- Cellular Response to Hypoxia Protocols
- Chromogenic IHC Staining of Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Protocol
- Chromogenic Immunohistochemistry Staining of Frozen Tissue
- Detection & Visualization of Antibody Binding
- Fluorescent IHC Staining of Frozen Tissue Protocol
- Graphic Protocol for Heat-induced Epitope Retrieval
- Graphic Protocol for the Preparation and Fluorescent IHC Staining of Frozen Tissue Sections
- Graphic Protocol for the Preparation and Fluorescent IHC Staining of Paraffin-embedded Tissue Sections
- Graphic Protocol for the Preparation of Gelatin-coated Slides for Histological Tissue Sections
- ICC Cell Smear Protocol for Suspension Cells
- ICC Immunocytochemistry Protocol Videos
- ICC for Adherent Cells
- IHC Sample Preparation (Frozen sections vs Paraffin)
- Immunocytochemistry (ICC) Protocol
- Immunocytochemistry Troubleshooting
- Immunofluorescence of Organoids Embedded in Cultrex Basement Membrane Extract
- Immunofluorescent IHC Staining of Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Protocol
- Immunohistochemistry (IHC) and Immunocytochemistry (ICC) Protocols
- Immunohistochemistry Frozen Troubleshooting
- Immunohistochemistry Paraffin Troubleshooting
- Preparing Samples for IHC/ICC Experiments
- Preventing Non-Specific Staining (Non-Specific Binding)
- Primary Antibody Selection & Optimization
- Protocol for Heat-Induced Epitope Retrieval (HIER)
- Protocol for Making a 4% Formaldehyde Solution in PBS
- Protocol for VisUCyte™ HRP Polymer Detection Reagent
- Protocol for the Fluorescent ICC Staining of Cell Smears - Graphic
- Protocol for the Fluorescent ICC Staining of Cultured Cells on Coverslips - Graphic
- Protocol for the Preparation & Fixation of Cells on Coverslips
- Protocol for the Preparation and Chromogenic IHC Staining of Frozen Tissue Sections
- Protocol for the Preparation and Chromogenic IHC Staining of Frozen Tissue Sections - Graphic
- Protocol for the Preparation and Chromogenic IHC Staining of Paraffin-embedded Tissue Sections
- Protocol for the Preparation and Chromogenic IHC Staining of Paraffin-embedded Tissue Sections - Graphic
- Protocol for the Preparation and Fluorescent ICC Staining of Cells on Coverslips
- Protocol for the Preparation and Fluorescent ICC Staining of Non-adherent Cells
- Protocol for the Preparation and Fluorescent ICC Staining of Stem Cells on Coverslips
- Protocol for the Preparation and Fluorescent IHC Staining of Frozen Tissue Sections
- Protocol for the Preparation and Fluorescent IHC Staining of Paraffin-embedded Tissue Sections
- Protocol for the Preparation of Gelatin-coated Slides for Histological Tissue Sections
- Protocol for the Preparation of a Cell Smear for Non-adherent Cell ICC - Graphic
- TUNEL and Active Caspase-3 Detection by IHC/ICC Protocol
- The Importance of IHC/ICC Controls
- Troubleshooting Guide: Immunohistochemistry
- View all Protocols, Troubleshooting, Illustrated assays and Webinars
Associated Pathways
 Dendritic Cell Lineage Development Pathways
Dendritic Cell Lineage Development Pathways
 Embryonic and Induced Pluripotent Stem Cell Differentiation Pathways & Lineage-specific Markers
Embryonic and Induced Pluripotent Stem Cell Differentiation Pathways & Lineage-specific Markers
 Hematopoietic Stem Cell Differentiation Pathways & Lineage-specific Markers
Hematopoietic Stem Cell Differentiation Pathways & Lineage-specific Markers
 IL-9 Signaling Pathways and their Primary Biological Effects in Different Immune Cell Types
IL-9 Signaling Pathways and their Primary Biological Effects in Different Immune Cell Types
 Jak/STAT Signaling Pathway
Jak/STAT Signaling Pathway
 Mesenchymal Stem Cell Differentiation Pathways & Lineage-specific Markers
Mesenchymal Stem Cell Differentiation Pathways & Lineage-specific Markers
 NOD-like Receptor Signaling Pathways
NOD-like Receptor Signaling Pathways
 Th17 Differentiation Pathway
Th17 Differentiation Pathway
 Toll-Like Receptor Signaling Pathways
Toll-Like Receptor Signaling Pathways