Human, Mouse, & Rat GDF-8/Myostatin ELISA Kit - Quantikine
GDF-8/Myostatin Quantikine ELISA Kit Summary
Product Summary
Precision
Cell Culture Supernates, Tissue Homogenates, Serum, EDTA Plasma, Heparin Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 171 | 583 | 1147 | 146 | 491 | 1058 |
| Standard Deviation | 9.2 | 14.8 | 20.9 | 8.8 | 17.7 | 32.3 |
| CV% | 5.4 | 2.5 | 1.8 | 6 | 3.6 | 3.1 |
Recovery
The recovery of GDF-8 spiked to levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Samples (n=4) | 110 | 100-118 |
| Serum and Plasma (n=12) | 91 | 80-117 |
| Tissue Homogenates (n=4) | 100 | 90-120 |
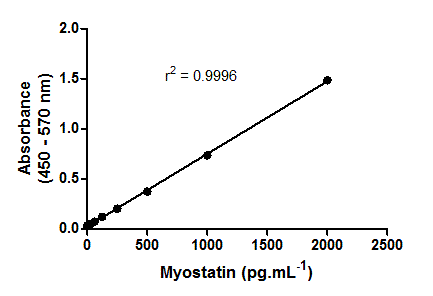
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: GDF-8/Myostatin
Growth/differentiation factors (GDF-1 to GDF-15) are members of the BMP family of TGF-beta superfamily proteins. They are produced as inactive preproproteins which are then cleaved and assembled into active secreted homodimers. GDF dimers are disulfide-linked with the exception of GDF-3 and -9. GDF proteins are important during embryonic development, particularly in the skeletal, nervous, and muscular systems.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 50 µL of Assay Diluent to each well.
- Add 50 µL of Standard, control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours on a horizontal orbital microplate shaker.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes.
- Add 200 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours on the shaker.
- Aspirate and wash 4 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes on the benchtop. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for GDF-8/Myostatin Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
65
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Characterizing sarcopenia and sarcopenic obesity in patients aged 65 years and over, at risk of mobility disability: a multicenter observational trial (SARA-OBS)
Authors: Fielding, RA;Rolland, Y;Bruyere, O;Desvarieux, M;Donini, LM;Incalzi, RA;Muscaritoli, M;Tchalla, A;Bonnefoy, M;Rondanelli, M;Van Maanen, R;Mariani, J;Margalef, C;Del Signore, S;Tourette, C;Dioh, W;Veillet, S;
BMC geriatrics
Species: Human
Sample Types: Plasma
-
Correlations of serum myostatin and irisin with sarcopenia and osteoporosis in rheumatoid arthritis patients: a cross-sectional study
Authors: Li, WJ;Yin, RY;Xia, XX;Xing, QX;Xu, SQ;
Scientific reports
Species: Human
Sample Types: Serum
-
Correlation between physiological and biochemical variables during short term adequate protein intake combined with resistance exercise in sedentary adults
Authors: Baek, KW;Won, JH;Kim, CB;Park, JJ;
Scientific reports
Species: Human
Sample Types: Serum
-
Myostatin as a plausible biomarker for early stage of sarcopenic obesity
Authors: Ishibashi, C;Nakanishi, K;Nishida, M;Shinomiya, H;Shinzawa, M;Kanayama, D;Yamamoto, R;Kudo, T;Nagatomo, I;Yamauchi-Takihara, K;
Scientific reports
Species: Human
Sample Types: Serum
-
Exploring the Impact of Resistance Training at Moderate Altitude on Metabolic Cytokines in Humans: Implications for Adipose Tissue Dynamics
Authors: Pérez-Regalado, S;Leon, J;Padial, P;Benavente, C;Almeida, F;Bonitch-Góngora, J;de la Fuente, B;Feriche, B;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
ORAI1 inhibition as an efficient preclinical therapy for tubular aggregate myopathy and Stormorken syndrome
Authors: Silva-Rojas, R;Pérez-Guàrdia, L;Simon, A;Djeddi, S;Treves, S;Ribes, A;Silva-Hernández, L;Tard, C;Laporte, J;Böhm, J;
JCI insight
Species: Human, Mouse
Sample Types: Plasma
-
Salbutamol ameliorates skeletal muscle wasting and inflammatory markers in streptozotocin (STZ)-induced diabetic rats
Authors: Kumar, A;Prajapati, P;Raj, V;Kim, SC;Mishra, V;Raorane, CJ;Raj, R;Kumar, D;Kushwaha, S;
International immunopharmacology
Species: Rat
Sample Types: Serum
-
Changes in Choline Metabolites and Ceramides in Response to a DASH-Style Diet in Older Adults
Authors: Tate, BN;Van Guilder, GP;Aly, M;Spence, LA;Diaz-Rubio, ME;Le, HH;Johnson, EL;McFadden, JW;Perry, CA;
Nutrients
Species: Human
Sample Types: Serum
-
Salbutamol Attenuates Diabetic Skeletal Muscle Atrophy by Reducing Oxidative Stress, Myostatin/GDF-8, and Pro-Inflammatory Cytokines in Rats
Authors: Kumar, A;Prajapati, P;Singh, G;Kumar, D;Mishra, V;Kim, SC;Raorane, CJ;Raj, V;Kushwaha, S;
Pharmaceutics
Species: Rat
Sample Types: Serum
-
GDF8 Contributes to Liver Fibrogenesis and Concomitant Skeletal Muscle Wasting
Authors: Culver, A;Hamang, M;Wang, Y;Jiang, H;Yanum, J;White, E;Gawrieh, S;Vuppalanchi, RK;Chalasani, NP;Dai, G;Yaden, BC;
Biomedicines
Species: Mouse
Sample Types: Tissue Homogenates
-
Determinants of bone mass in older adults with normal- and overweight derived from the crosstalk with muscle and adipose tissue
Authors: Walowski, CO;Herpich, C;Enderle, J;Braun, W;Both, M;Hasler, M;Müller, MJ;Norman, K;Bosy-Westphal, A;
Scientific reports
Species: Human
Sample Types: Serum
-
Hepatic follistatin increases basal metabolic rate and attenuates diet-induced obesity during hepatic insulin resistance
Authors: R Tao, O Stöhr, C Wang, W Qiu, KD Copps, MF White
Molecular Metabolism, 2023-03-10;71(0):101703.
Species: Mouse
Sample Types: Serum
-
Astaxanthin Supplemented with High-Intensity Functional Training Decreases Adipokines Levels and Cardiovascular Risk Factors in Men with Obesity
Authors: A Saeidi, A Nouri-Haba, O Razi, A Ataeinosra, H Rahmani, SS Mollabashi, B Bagherzade, SM Aghdam, L Khalajzade, MH Al Kiyumi, AC Hackney, I Laher, KM Heinrich, H Zouhal
Nutrients, 2023-01-06;15(2):.
Species: Human
Sample Types: Plasma
-
Indoxyl Sulfate Might Play a Role in Sarcopenia, While Myostatin Is an Indicator of Muscle Mass in Patients with Chronic Kidney Disease: Analysis from the RECOVERY Study
Authors: S Lee, M Han, S Kim, R Cha, S Kang, J Kim, W An
Toxins, 2022-09-23;0(0):.
Species: Human
Sample Types: Serum
-
High serum concentrations of growth differentiation factor-15 and their association with Crohn's disease and a low skeletal muscle index
Authors: H Yamamoto, F Takeshima, M Haraguchi, Y Akazawa, K Matsushima, M Kitayama, K Ogihara, M Tabuchi, K Hashiguchi, N Yamaguchi, H Miyaaki, H Kondo, K Nakao
Scientific Reports, 2022-04-21;12(1):6591.
Species: Human
Sample Types: Serum
-
Decreased myostatin in response to a controlled DASH diet is associated with improved body composition and cardiometabolic biomarkers in older adults: results from a controlled-feeding diet intervention study
Authors: CA Perry, GP Van Guilde, TA Butterick
BMC nutrition, 2022-03-15;8(1):24.
Species: Human
Sample Types: EDTA Plasma
-
Effects of Resistance Training Intervention along with Leucine-Enriched Whey Protein Supplementation on Sarcopenia and Frailty in Post-Hospitalized Older Adults: Preliminary Findings of a Randomized Controlled Trial
Authors: M Amasene, C Cadenas-Sa, I Echeverria, B Sanz, C Alonso, I Tobalina, J Irazusta, I Labayen, A Besga
Journal of Clinical Medicine, 2021-12-24;11(1):.
Species: Human
Sample Types: Serum
-
Effects of resistance exercise and whey protein supplementation on skeletal muscle strength, mass, physical function, and hormonal and inflammatory biomarkers in healthy active older men: A randomised, double-blind, placebo-controlled trial
Authors: C Griffen, M Duncan, J Hattersley, MO Weickert, A Dallaway, D Renshaw
Experimental gerontology, 2021-12-09;0(0):111651.
Species: Human
Sample Types: Plasma
-
Nordic Walking Rather Than High Intensity Interval Training Reduced Myostatin Concentration More Effectively in Elderly Subjects and the Range of This Drop Was Modified by Metabolites of Vitamin D
Authors: K Micielska, M Flis, JA Kortas, E Rodziewicz, J Antosiewic, K Wochna, G Lombardi, E Ziemann
Nutrients, 2021-12-08;13(12):.
Species: Human
Sample Types: Serum
-
Differences in the Biomarker Profile of De Novo Acute Heart Failure versus Decompensation of Chronic Heart Failure
Authors: S Nawrocka-M, J Biegus, M Hurkacz, M Guzik, M Rosiek-Bie, EA Jankowska, P Ponikowski, R Zymli?ski
Biomolecules, 2021-11-16;11(11):.
Species: Human
Sample Types: Plasma
-
Serum concentrations of oxytocin, DHEA and follistatin are associated with osteoporosis or sarcopenia in community-dwelling postmenopausal women
Authors: Y Du, C Xu, H Shi, X Jiang, W Tang, X Wu, M Chen, H Li, X Zhang, Q Cheng
BMC geriatrics, 2021-10-12;21(1):542.
Species: Human
Sample Types: Serum
-
Skeletal muscle-targeted delivery of Fgf6 protects mice from diet-induced obesity and insulin resistance
Authors: B Xu, C Liu, H Zhang, R Zhang, M Tang, Y Huang, L Jin, L Xu, C Hu, W Jia
JCI Insight, 2021-10-08;6(19):.
Species: Mouse
Sample Types: Serum
-
The association between sarcopenia and endotoxin in patients with alcoholic cirrhosis
Authors: S Sato, T Namisaki, K Murata, Y Fujimoto, S Takeda, M Enomoto, A Shibamoto, K Ishida, H Ogawa, H Takagi, Y Tsuji, D Kaya, Y Fujinaga, M Furukawa, T Inoue, Y Sawada, N Nishimura, K Kitagawa, T Ozutsumi, H Takaya, K Kaji, N Shimozato, H Kawaratani, K Moriya, T Akahane, A Mitoro, H Yoshiji
Medicine, 2021-09-10;100(36):e27212.
Species: Human
Sample Types: Serum
-
Local versus systemic control of bone and skeletal muscle mass by components of the transforming growth factor-beta signaling pathway
Authors: Y Liu, A Lehar, R Rydzik, H Chandok, YS Lee, DW Youngstrom, J George, MM Matzuk, EL Germain-Le, SJ Lee
Proceedings of the National Academy of Sciences of the United States of America, 2021-08-17;118(33):.
Species: Mouse
Sample Types: Serum
-
Myokine secretion following moderate-intensity endurance exercise under different environmental temperatures
Authors: Y Tsuchiya, K Goto
Cytokine, 2021-05-29;144(0):155553.
Species: Human
Sample Types: Plasma
-
Prevention of non-alcoholic steatohepatitis by long-term exercise via the induction of phenotypic changes in Kupffer cells of hyperphagic obese mice
Authors: I Miura, S Komine, K Okada, S Wada, E Warabi, F Uchida, S Oh, H Suzuki, Y Mizokami, J Shoda
Physiological Reports, 2021-05-01;9(9):e14859.
Species: Mouse
Sample Types: Serum
-
Regulation of circulating CTRP-2/CTRP-9 and GDF-8/GDF-15 by intralipids and insulin in healthy control and polycystic ovary syndrome women following chronic exercise training
Authors: J Jerobin, M Ramanjaney, I Bettahi, R Parammal, KS Siveen, M Alkasem, M Aye, T Sathyapala, M Skarulis, SL Atkin, AB Abou-Samra
Lipids in Health and Disease, 2021-04-19;20(1):34.
Species: Human
Sample Types: Plasma
-
Beneficial effects of whole-body cryotherapy on glucose homeostasis and amino acid profile are associated with a reduced myostatin serum concentration
Authors: M Koz?owska, J Kortas, M ?ychowska, J Antosiewic, K ?uczek, S Perego, G Lombardi, E Ziemann
Scientific Reports, 2021-03-29;11(1):7097.
Species: Human
Sample Types: Serum
-
Association of serum adiponectin and myostatin levels with skeletal muscle in patients with obesity: A cross-sectional study
Authors: S Kurose, K Onishi, N Takao, T Miyauchi, K Takahashi, Y Kimura
PLoS ONE, 2021-01-19;16(1):e0245678.
Species: Human
Sample Types: Serum
-
Functional redundancy of type I and type II receptors in the regulation of skeletal muscle growth by myostatin and activin A
Authors: SJ Lee, A Lehar, Y Liu, CH Ly, QM Pham, M Michaud, R Rydzik, DW Youngstrom, MM Shen, V Kaartinen, EL Germain-Le, TA Rando
Proc Natl Acad Sci U S A, 2020-11-20;0(0):.
Species: Mouse
Sample Types: Serum
-
Beige fat is dispensable for the metabolic benefits associated with myostatin deletion
Authors: F Marchildon, J Chi, S O'Connor, H Bediako, P Cohen
Molecular Metabolism, 2020-11-18;0(0):101120.
Species: Mouse
Sample Types: Plasma
-
Muscle and serum myostatin expression in type 1 diabetes
Authors: AG Dial, CMF Monaco, GK Grafham, N Romanova, JA Simpson, MA Tarnopolsk, CGR Perry, E Kalaitzogl, TJ Hawke
Physiol Rep, 2020-07-01;8(13):e14500.
Species: Human
Sample Types: Serum
-
Myostatin Is a Quantifiable Biomarker for Monitoring Pharmaco-gene Therapy in Duchenne Muscular Dystrophy
Authors: V Mariot, C Le Guiner, I Barthélémy, M Montus, S Blot, S Torelli, J Morgan, F Muntoni, T Voit, J Dumonceaux
Mol Ther Methods Clin Dev, 2020-06-24;18(0):415-421.
Species: Canine
Sample Types: Serum
-
Myostatin: a Circulating Biomarker Correlating with Disease in Myotubular Myopathy Mice and Patients
Authors: C Koch, S Buono, A Menuet, A Robé, S Djeddi, C Kretz, R Gomez-Oca, M Depla, A Monseur, L Thielemans, L Servais, J Laporte, BS Cowling
Mol Ther Methods Clin Dev, 2020-05-04;17(0):1178-1189.
Species: Mouse, Transgenic Mouse
Sample Types: Plasma
-
Iron Status in Elderly Women Impacts Myostatin, Adiponectin and Osteocalcin Levels Induced by Nordic Walking Training
Authors: J Kortas, E Ziemann, D Juszczak, K Micielska, M Koz?owska, K Prusik, K Prusik, J Antosiewic
Nutrients, 2020-04-17;12(4):.
Species: Human
Sample Types: Serum
-
Fortetropin inhibits disuse muscle atrophy in dogs after tibial plateau leveling osteotomy
Authors: DA White, KR Harkin, JK Roush, WC Renberg, D Biller
PLoS ONE, 2020-04-09;15(4):e0231306.
Species: Canine
Sample Types: Serum
-
Prostate tumor-derived GDF11 accelerates androgen deprivation therapy-induced sarcopenia
Authors: C Pan, N Jaiswal Ag, Y Zulia, S Singh, K Sha, JL Mohler, KH Eng, J Chakkalaka, JJ Krolewski, KL Nastiuk
JCI Insight, 2020-03-26;0(0):.
Species: Mouse
Sample Types: Serum
-
Inhibition of Myostatin Reduces Collagen Deposition in a Mouse Model of Oculopharyngeal Muscular Dystrophy (OPMD) With Established Disease
Authors: P Harish, L Forrest, S Herath, G Dickson, A Malerba, L Popplewell
Front Physiol, 2020-03-05;11(0):184.
Species: Mouse
Sample Types: Serum
-
Significance of serum Myostatin in hemodialysis patients
Authors: P Esposito, Y Battaglia, E La Porta, MA Grignano, E Caramella, A Avella, S Peressini, N Sessa, R Albertini, G Di Natali, C Lisi, M Gregorini, T Rampino
BMC Nephrol, 2019-12-11;20(1):462.
Species: Human
Sample Types: Serum
-
Effects of dapagliflozin on the serum levels of fibroblast growth factor 21 and myokines and muscle mass in Japanese patients with type 2 diabetes: a randomized, controlled trial
Authors: H Yamakage, M Tanaka, T Inoue, S Odori, T Kusakabe, N Satoh-Asah
J Diabetes Investig, 2019-12-10;0(0):.
Species: Human
Sample Types: Serum
-
Randomised trial on clinical performances and biocompatibility of four high-flux hemodialyzers in two mode treatments: hemodialysis vs post dilution hemodiafiltration
Authors: M Morena, C Creput, M Bouzernidj, A Rodriguez, L Chalabi, B Seigneuric, C Lauret, AS Bargnoux, AM Dupuy, JP Cristol
Sci Rep, 2019-12-04;9(1):18265.
Species: Human
Sample Types: Serum
-
Sex differences in body composition but not neuromuscular function following long-term, doxycycline-induced reduction in circulating levels of myostatin in mice
Authors: D Tavoian, WD Arnold, SC Mort, S de Lacalle
PLoS ONE, 2019-11-21;14(11):e0225283.
Species: Mouse
Sample Types: Serum
-
Down-regulation of the mitochondrial i-AAA protease Yme1L induces muscle atrophy via FoxO3a and myostatin activation
Authors: YJ Lee, GH Kim, SI Park, JH Lim
J. Cell. Mol. Med., 2019-11-14;0(0):.
Species: Mouse
Sample Types: Serum
-
miR-27a regulates vascular remodeling by targeting endothelial cells' apoptosis and interaction with vascular smooth muscle cells in aortic dissection
Authors: Y Sun, Y Xiao, H Sun, Z Zhao, J Zhu, L Zhang, J Dong, T Han, Q Jing, J Zhou, Z Jing
Theranostics, 2019-10-18;9(25):7961-7975.
Species: Human
Sample Types: Cell Culture Supernates
-
Myokine/Adipokine Response to "Aerobic" Exercise: Is It Just a Matter of Exercise Load?
Authors: Z He, Y Tian, PL Valenzuela, C Huang, J Zhao, P Hong, Z He, S Yin, A Lucia
Front Physiol, 2019-05-29;10(0):691.
Species: Human
Sample Types: Serum
-
Myokine Response to High-Intensity Interval vs. Resistance Exercise: An Individual Approach
Authors: Z He, Y Tian, PL Valenzuela, C Huang, J Zhao, P Hong, Z He, S Yin, A Lucia
Front Physiol, 2018-12-03;9(0):1735.
Species: Human
Sample Types: Serum
-
A 2-Week Specific Volleyball Training Supported by the Whole Body Cryostimulation Protocol Induced an Increase of Growth Factors and Counteracted Deterioration of Physical Performance
Authors: J Jaworska, K Micielska, M Koz?owska, K Wnorowski, J Skrobecki, L Radzimi?sk, A Babi?ska, E Rodziewicz, G Lombardi, E Ziemann
Front Physiol, 2018-11-28;9(0):1711.
Species: Human
Sample Types: Serum
-
Activin subfamily peptides predict chronological age in humans
Authors: LV Barrios-Si, M Parnell, ZB Shinwari, GA Chaudhary, T Xenofontos, A van Bekhov, S McArthur, BT Elliott
Physiol Rep, 2018-09-01;6(17):e13823.
Species: Human
Sample Types: Plasma
-
Interconnected Microphysiological Systems for Quantitative Biology and Pharmacology Studies
Authors: CD Edington, WLK Chen, E Geishecker, T Kassis, LR Soenksen, BM Bhushan, D Freake, J Kirschner, C Maass, N Tsamandour, J Valdez, CD Cook, T Parent, S Snyder, J Yu, E Suter, M Shockley, J Velazquez, JJ Velazquez, L Stockdale, JP Papps, I Lee, N Vann, M Gamboa, ME LaBarge, Z Zhong, X Wang, LA Boyer, DA Lauffenbur, RL Carrier, C Communal, SR Tannenbaum, CL Stokes, DJ Hughes, G Rohatgi, DL Trumper, M Cirit, LG Griffith
Sci Rep, 2018-03-14;8(1):4530.
-
Differential response of adipose tissue gene and protein expressions to 4- and 8-week administration of ?-guanidinopropionic acid in mice
Authors: H Kato, S Masuda, T Ohira, L Ohira, H Takakura, Y Ohira, T Izawa
Physiol Rep, 2018-03-01;6(5):.
Species: Mouse
Sample Types: Serum
-
Supraphysiologic Administration of GDF11 Induces Cachexia in Part by Upregulating GDF15
Authors: JE Jones, SM Cadena, C Gong, X Wang, Z Chen, SX Wang, C Vickers, H Chen, E Lach-Trifi, JR Hadcock, DJ Glass
Cell Rep, 2018-02-06;22(6):1522-1530.
Species: Mouse
Sample Types: Plasma
-
Plasma Levels of Myonectin But Not Myostatin or Fibroblast-Derived Growth Factor 21 Are Associated with Insulin Resistance in Adult Humans without Diabetes Mellitus
Authors: FJK Toloza, JO Mantilla-R, MC Pérez-Mato, ML Ricardo-Si, MC Morales-Al, JA Pinzón-Cor, M Pérez-Mayo, ML Arévalo-Ga, G Tolosa-Gon, CO Mendivil
Front Endocrinol (Lausanne), 2018-01-31;9(0):5.
Species: Human
Sample Types: Plasma
-
Relative abundance of mature myostatin rather than total myostatin is negatively associated with bone mineral density in Chinese
Authors: LF Wu, DC Zhu, BH Wang, YH Lu, P He, YH Zhang, HQ Gao, XW Zhu, W Xia, H Zhu, XB Mo, X Lu, L Zhang, YH Zhang, FY Deng, SF Lei
J. Cell. Mol. Med., 2017-12-16;0(0):.
Species: Human
Sample Types: Plasma
-
Myostatin and adipokines: The role of the metabolically unhealthy obese phenotype in muscle function and aerobic capacity in young adults
Authors: LP Carvalho, RP Basso-Vane, L Di Thommaz, RG Mendes, MC Oliveira-J, RP Vieira, JC Bonjorno-J, CR Oliveira, R Luporini, A Borghi-Sil
Cytokine, 2017-12-13;0(0):.
Species: Human
Sample Types: Plasma
-
Alterations in Adiposity and Glucose Homeostasis in Adult Gasp-1 Overexpressing Mice
Authors: L Périè, A Parenté, F Baraige, L Magnol, V Blanquet
Cell. Physiol. Biochem., 2017-12-08;44(5):1896-1911.
Species: Mouse
Sample Types: Plasma
-
Lifelong exercise, but not short-term high-intensity interval training, increases GDF11, a marker of successful aging: a�preliminary investigation
Authors: BT Elliott, P Herbert, N Sculthorpe, FM Grace, D Stratton, LD Hayes
Physiol Rep, 2017-07-11;5(13):.
Species: Human
Sample Types: Serum
-
Activin A more prominently regulates muscle mass in primates than does GDF8
Authors: E Latres, J Mastaitis, W Fury, L Miloscio, J Trejos, J Pangilinan, H Okamoto, K Cavino, E Na, A Papatheodo, T Willer, Y Bai, J Hae Kim, A Rafique, S Jaspers, T Stitt, AJ Murphy, GD Yancopoulo, J Gromada
Nat Commun, 2017-04-28;8(0):15153.
Species: Human, Mouse, Primate - Macaca fascicularis (Crab-eating Monkey or Cynomolgus Macaque), Rat
Sample Types: Serum
-
Serum Biomarkers of Myocardial Remodeling and Coronary Dysfunction in Early Stages of Hypertrophic Cardiomyopathy in the Young
Authors: E Fernlund, T Gyllenhamm, R Jablonowsk, M Carlsson, A Larsson, J Ärnlöv, P Liuba
Pediatr Cardiol, 2017-03-30;0(0):.
Species: Human
Sample Types: Serum
-
Decreasing maternal myostatin programs adult offspring bone strength in a mouse model of osteogenesis imperfecta
Proc. Natl. Acad. Sci. U.S.A., 2016-11-07;0(0):.
Species: Mouse
Sample Types: Serum
-
Serum GDF-8 levels change dynamically during controlled ovarian hyperstimulation in patients undergoing IVF/ICSI-ET
Authors: Lanlan Fang
Sci Rep, 2016-06-22;6(0):28036.
Species: Human
Sample Types: Serum
-
Supplementation of Magnolol Attenuates Skeletal Muscle Atrophy in Bladder Cancer-Bearing Mice Undergoing Chemotherapy via Suppression of FoxO3 Activation and Induction of IGF-1.
Authors: Chen M, Chen Y, Lee C, Hung C, Chou T
PLoS ONE, 2015-11-24;10(11):e0143594.
Species: Mouse
Sample Types: Tissue Homogenates
-
Efficient modification of the myostatin gene in porcine somatic cells and generation of knockout piglets.
Authors: Rao S, Fujimura T, Matsunari H, Sakuma T, Nakano K, Watanabe M, Asano Y, Kitagawa E, Yamamoto T, Nagashima H
Mol Reprod Dev, 2015-11-09;83(1):61-70.
Species: Porcine
Sample Types: Tissue Homogenates
-
Relationship between Blood Myostatin Levels and Kidney Function:Shimane CoHRE Study.
Authors: Yano S, Nagai A, Isomura M, Yamasaki M, Kijima T, Takeda M, Hamano T, Nabika T
PLoS ONE, 2015-10-26;10(10):e0141035.
Species: Human
Sample Types: Plasma
-
Preliminary investigation into a potential role for myostatin and its receptor (ActRIIB) in lean and obese horses and ponies.
Authors: Morrison P, Bing C, Harris P, Maltin C, Grove-White D, Argo C
PLoS ONE, 2014-11-12;9(11):e112621.
Species: Equine
Sample Types: Serum
-
Myostatin regulates energy homeostasis in the heart and prevents heart failure.
Authors: Biesemann N, Mendler L, Wietelmann A, Hermann S, Schafers M, Kruger M, Boettger T, Borchardt T, Braun T
Circ Res, 2014-05-07;115(2):296-310.
Species: Mouse
Sample Types: Serum
FAQs
-
What form of GDF-8/Myostatin does this kit recognize?
This ELISA is specific for the mature active protein.
Reviews for GDF-8/Myostatin Quantikine ELISA Kit
Average Rating: 4.8 (Based on 4 Reviews)
Have you used GDF-8/Myostatin Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Great kit overall. Standard curve is very reproducible (R2>0.999), and good above suggested limits. Easy to follow protocol. Great, detailed suggestion on sample dilutions and expected ranges for different samples.
Ran assay successfully with human plasma (Heparin and EDTA) and human serum (SST) samples, with and without binding protein separation step. High linearity of standard curve (example shown produced by undergrad researchers) when run in triplicate.