Human Flt-3/Flk-2 Antibody Summary
Asn27-Asn541
Accession # AAA18947
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Flt‑3/Flk‑2 in THP-1 cells by Flow Cytometry. THP-1 cells were stained with Mouse Anti-Human Flt‑3/Flk‑2 Monoclonal Antibody (Catalog # MAB812, filled histogram) or isotype control antibody (Catalog # MAB002, open histogram), followed by Phycoerythrin-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # F0102B). View our protocol for Staining Membrane-associated Proteins.
 View Larger
View Larger
Detection of Flt‑3/Flk‑2 in PBMC monocytes by Flow Cytometry. PBMC monocytes were stained with Mouse Anti-Human Flt‑3/Flk‑2 Monoclonal Antibody (Catalog # MAB812, filled histogram) or isotype control antibody (Catalog # MAB002, open histogram), followed by Phycoerythrin-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # F0102B). View our protocol for Staining Membrane-associated Proteins.
 View Larger
View Larger
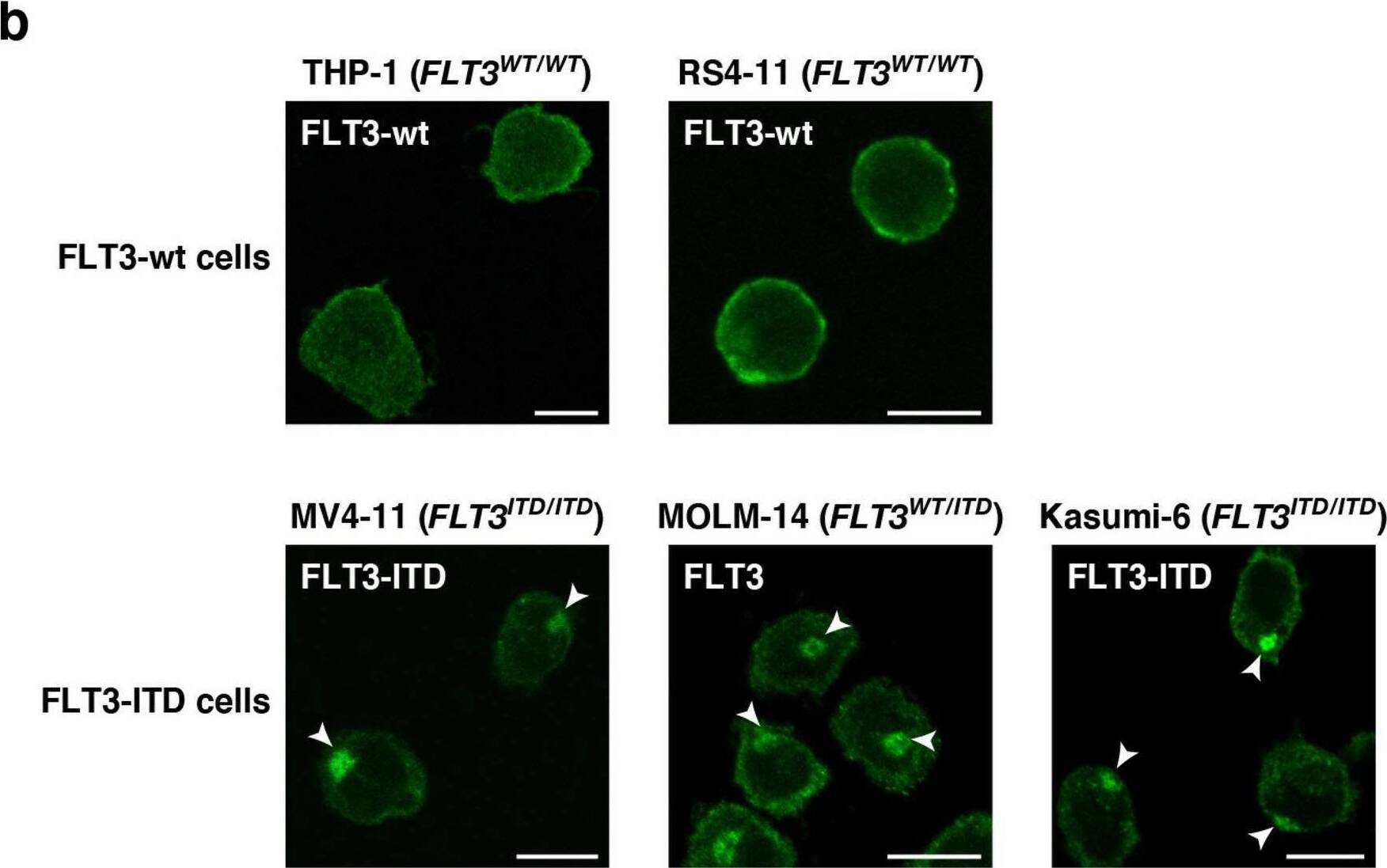
Detection of Human Flt-3/Flk-2 by Immunocytochemistry/ Immunofluorescence FLT3-ITD mislocalizes to the perinuclear region in AML cells. (a) Schematic representations of wild-type FLT3 (FLT3-wt) and an FLT3 internal tandem duplication (FLT3-ITD) mutant showing the extracellular domain (ECD, blue), the transmembrane domain (TM, yellow), the kinase domain (pink), and the ITD (green). (b) Fixed THP-1, RS4-11, MV4-11, MOLM-14, or Kasumi-6 cells were permeabilized and subsequently immunostained with anti-FLT3 ECD antibody. Arrowheads indicate the perinuclear region. Bars, 10 µm. Note that FLT3-wt localized to the plasma membrane, whereas FLT3-ITD accumulated in the perinuclear region. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/34811450), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
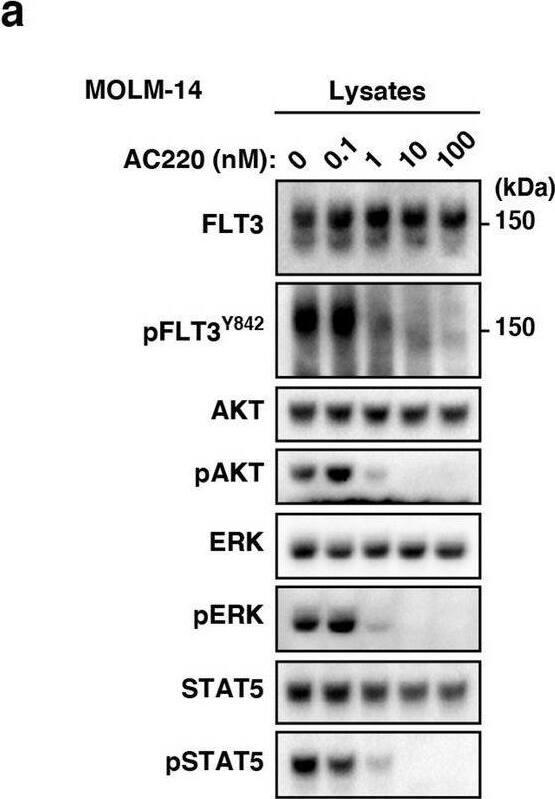
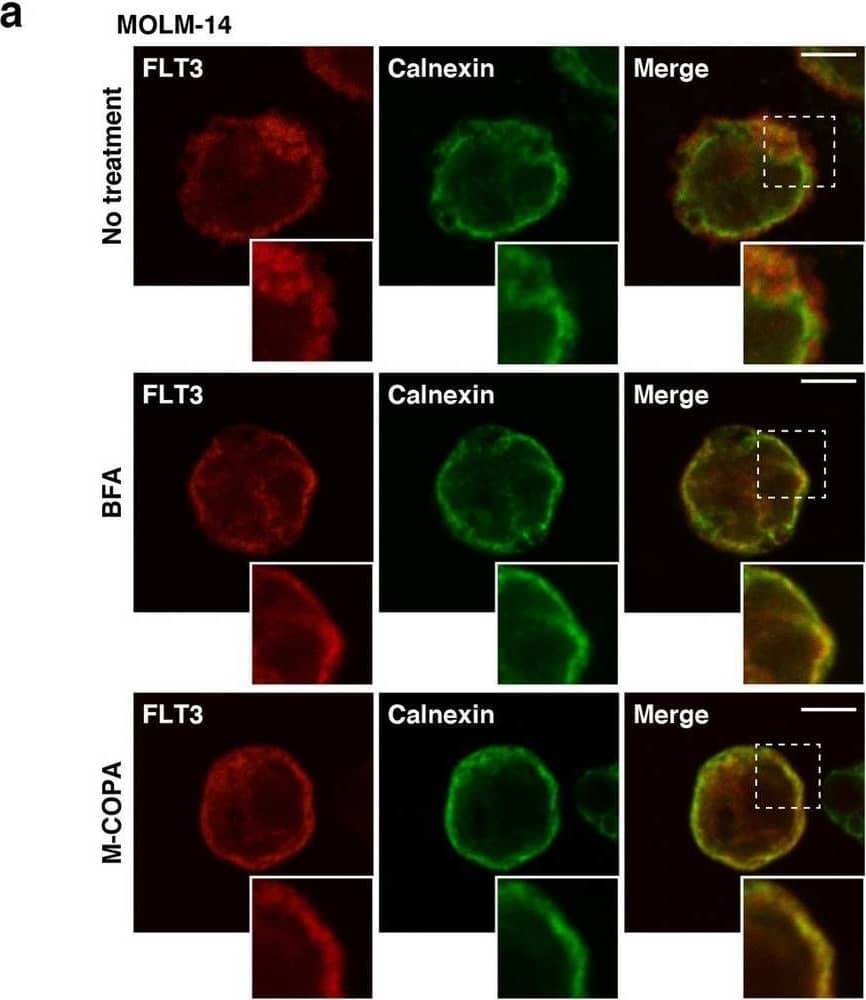
Detection of Human Flt-3/Flk-2 by Western Blot FLT3-ITD retention in the Golgi region is dependent on its tyrosine kinase activity. (a, b) MOLM-14 cells were treated for 4 h with AC220 (a) or PKC412 (b). Lysates were immunoblotted for FLT3, phospho-FLT3 Tyr842 (pFLT3Y842), AKT, pAKT, ERK, pERK, STAT5, and pSTAT5. Full length blots are presented in Supplementary Fig. 5. (c) MOLM-14 cells were treated with AC220 (upper graph) or PKC412 (lower graph) for 48 h. Cell proliferation was assessed by ATP production. Results are means ± s.d. (n = 3). (d) Lysates from MOLM-14 were treated with peptide N-glycosidase F (PNGase F) or endoglycosidase H (endo H) then immunoblotted with anti-FLT3 antibody. CG complex-glycosylated form, HM high mannose form, DG deglycosylated form. Full length blots are presented in Supplementary Fig. 5. (e, f) MOLM-14 cells were treated with 10 nM AC220 or 100 nM PKC412 for 8 h (e) or 16 h (f). (e) Fixed cells were permeabilized, then immunostained with anti-FLT3 (red) and anti-calnexin (ER marker, green). Insets show the magnified images of the boxed area. Bars, 10 µm. (f) Non-permeabilized cells were immunostained with an anti-FLT3 extracellular domain (ECD) antibody. Bars, 10 µm. Note that FLT3 tyrosine kinase inhibitors inactivated FLT3, then released the receptor from the Golgi region for localization to the PM. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/34811450), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Flt-3/Flk-2 by Immunocytochemistry/ Immunofluorescence FLT3-ITD retention in the Golgi region is dependent on its tyrosine kinase activity. (a, b) MOLM-14 cells were treated for 4 h with AC220 (a) or PKC412 (b). Lysates were immunoblotted for FLT3, phospho-FLT3 Tyr842 (pFLT3Y842), AKT, pAKT, ERK, pERK, STAT5, and pSTAT5. Full length blots are presented in Supplementary Fig. 5. (c) MOLM-14 cells were treated with AC220 (upper graph) or PKC412 (lower graph) for 48 h. Cell proliferation was assessed by ATP production. Results are means ± s.d. (n = 3). (d) Lysates from MOLM-14 were treated with peptide N-glycosidase F (PNGase F) or endoglycosidase H (endo H) then immunoblotted with anti-FLT3 antibody. CG complex-glycosylated form, HM high mannose form, DG deglycosylated form. Full length blots are presented in Supplementary Fig. 5. (e, f) MOLM-14 cells were treated with 10 nM AC220 or 100 nM PKC412 for 8 h (e) or 16 h (f). (e) Fixed cells were permeabilized, then immunostained with anti-FLT3 (red) and anti-calnexin (ER marker, green). Insets show the magnified images of the boxed area. Bars, 10 µm. (f) Non-permeabilized cells were immunostained with an anti-FLT3 extracellular domain (ECD) antibody. Bars, 10 µm. Note that FLT3 tyrosine kinase inhibitors inactivated FLT3, then released the receptor from the Golgi region for localization to the PM. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/34811450), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
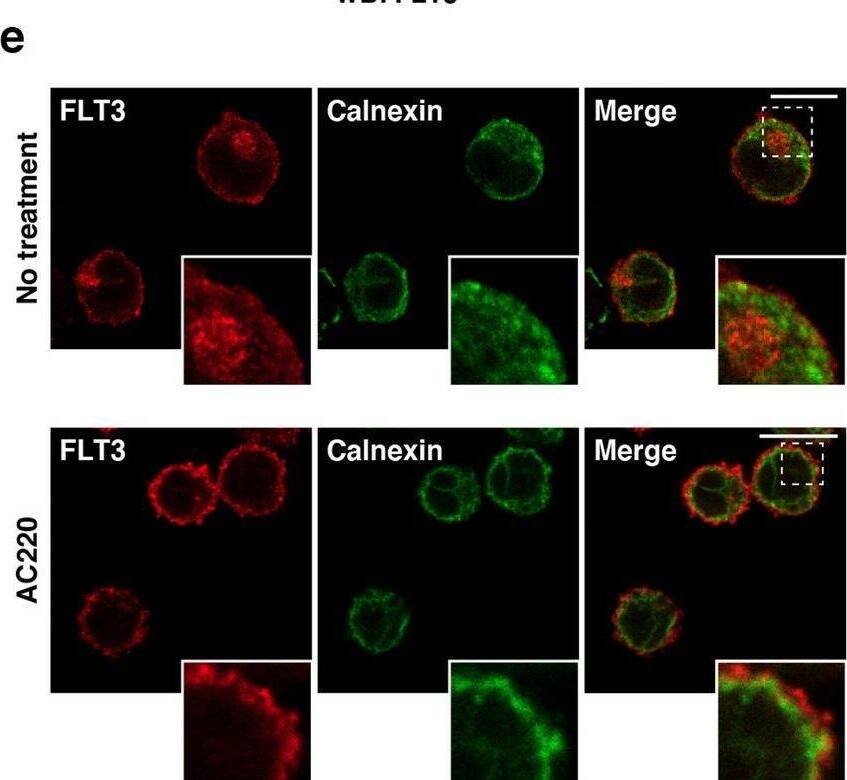
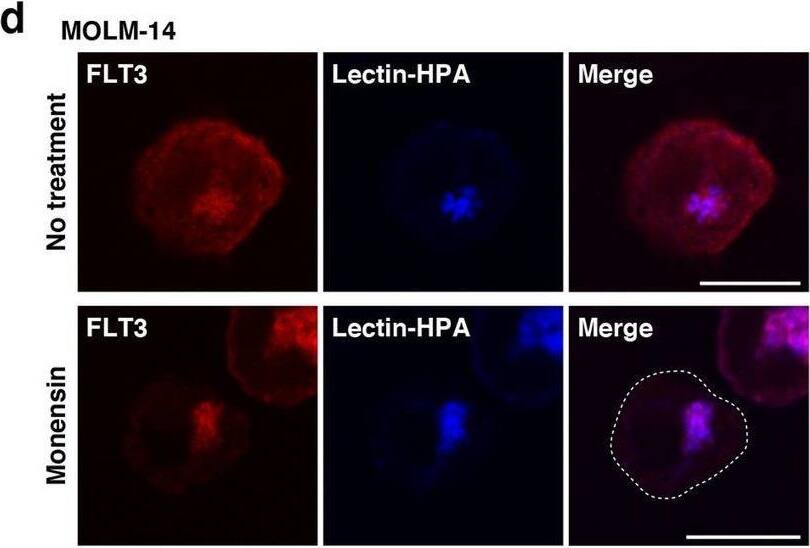
Detection of Human Flt-3/Flk-2 by Immunocytochemistry/ Immunofluorescence In AML cells, FLT3-ITD can activate AKT, ERK, and STAT5 before it reaches the PM. (a–c) MOLM-14 (a, b), MV4-11, or Kasumi-6 cells (c) were treated with inhibitors of ER export (BFA or M-COPA) for 8 h. (a) MOLM-14 cells treated with 1 µM BFA (middle panels) or 1 µM M-COPA (bottom panels) were stained with anti-FLT3 (red) and calnexin (ER marker, green). Insets show the magnified images of the boxed area. Bars, 10 µm. (b) Lysates were immunoblotted for FLT3, phospho-FLT3 Tyr842 (pFLT3Y842), AKT, pAKT, ERK, pERK, STAT5, and pSTAT5. To examine pFLT3Y591, FLT3 was immunoprecipitated, then immunoblotted. (c) MV4-11 (left) or Kasumi-6 cells (right) were treated with M-COPA for 8 h, then immunoblotted. Full length blots are presented in Supplementary Fig. 5. Note that BFA and M-COPA inhibited the activation of AKT and ERK but not that of STAT5 through blocking FLT3-ITD trafficking from the ER to the Golgi apparatus. (d, e) MOLM-14 cells were treated with monensin (inhibitor of Golgi export) for 8 h. (d) Cells treated with 100 nM monensin were stained with anti-FLT3 (red) and lectin-HPA (Golgi marker, blue). Dashed line, cell border. Bars, 10 µm. (e) Lysates were immunoblotted with the indicated antibody. To examine pFLT3Y591, FLT3 was immunoprecipitated, then immunoblotted. Full length blots are presented in Supplementary Fig. 5. (f) MV4-11 (left) or Kasumi-6 cells (right) were treated with monensin for 8 h, then immunoblotted. Full length blots are presented in Supplementary Fig. 6. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/34811450), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Flt-3/Flk-2 by Immunocytochemistry/ Immunofluorescence In AML cells, FLT3-ITD can activate AKT, ERK, and STAT5 before it reaches the PM. (a–c) MOLM-14 (a, b), MV4-11, or Kasumi-6 cells (c) were treated with inhibitors of ER export (BFA or M-COPA) for 8 h. (a) MOLM-14 cells treated with 1 µM BFA (middle panels) or 1 µM M-COPA (bottom panels) were stained with anti-FLT3 (red) and calnexin (ER marker, green). Insets show the magnified images of the boxed area. Bars, 10 µm. (b) Lysates were immunoblotted for FLT3, phospho-FLT3 Tyr842 (pFLT3Y842), AKT, pAKT, ERK, pERK, STAT5, and pSTAT5. To examine pFLT3Y591, FLT3 was immunoprecipitated, then immunoblotted. (c) MV4-11 (left) or Kasumi-6 cells (right) were treated with M-COPA for 8 h, then immunoblotted. Full length blots are presented in Supplementary Fig. 5. Note that BFA and M-COPA inhibited the activation of AKT and ERK but not that of STAT5 through blocking FLT3-ITD trafficking from the ER to the Golgi apparatus. (d, e) MOLM-14 cells were treated with monensin (inhibitor of Golgi export) for 8 h. (d) Cells treated with 100 nM monensin were stained with anti-FLT3 (red) and lectin-HPA (Golgi marker, blue). Dashed line, cell border. Bars, 10 µm. (e) Lysates were immunoblotted with the indicated antibody. To examine pFLT3Y591, FLT3 was immunoprecipitated, then immunoblotted. Full length blots are presented in Supplementary Fig. 5. (f) MV4-11 (left) or Kasumi-6 cells (right) were treated with monensin for 8 h, then immunoblotted. Full length blots are presented in Supplementary Fig. 6. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/34811450), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Flt-3/Flk-2
The Flt-3 (fms-like tyrosine kinase) receptor, also named Flk-2 (fetal liver kinase) and Stk-1(stem cell tyrosine kinase) is a member of the class III subfamily of receptor tyrosine kinases that also includes KIT, the receptor for SCF and FMS, the receptor for M-CSF. The extracellular region of these receptors contains five immunoglobulin-like domains and the intracellular region contains a split kinase domain. Human Flt-3 cDNA encodes a 993 amino acid (aa) residue type I membrane protein with a 26 aa residue signal peptide, a 515 aa extracellular domain with 10 potential N-linked glycosylation sites, a 21 aa residue transmembrane domain and a 431 aa residue cytoplasmic domain. Mouse Flt-3 has also been cloned and shown to share 85% amino acid sequence identity with human Flt-3. Flt-3 expression has been detected in various tissues, including placenta, gonads, and tissues of nervous and hematopoietic origin. Among hematopoietic cells, the expression of Flt-3 was found to be restricted to the highly enriched stem/progenitor cell populations. The ligand for Flt-3 (FL) has been identified to be a transmembrane protein with structural homology to M-CSF and SCF. Recombinant soluble Flt-3/Fc chimeric protein has been shown to bind FL with high affinity and is a potent FL antagonist.
Product Datasheets
Citations for Human Flt-3/Flk-2 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
4
Citations: Showing 1 - 4
Filter your results:
Filter by:
-
FLT3-ITD transduces autonomous growth signals during its biosynthetic trafficking in acute myelogenous leukemia cells
Authors: K Yamawaki, I Shiina, T Murata, S Tateyama, Y Maekawa, M Niwa, M Shimonaka, K Okamoto, T Suzuki, T Nishida, R Abe, Y Obata
Scientific Reports, 2021-11-22;11(1):22678.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Immunoprecipitation, Western Blot -
Proteasome inhibitors induce FLT3-ITD degradation through autophagy in AML cells
Authors: Clément Larrue, Estelle Saland, Héléna Boutzen, François Vergez, Marion David, Carine Joffre et al.
Blood
-
Features of Ras activation by a mislocalized oncogenic tyrosine kinase: FLT3 ITD signals through K-Ras at the plasma membrane of acute myeloid leukemia cells
Authors: Susanne Köthe, Jörg P. Müller, Sylvia-Annette Böhmer, Todor Tschongov, Melanie Fricke, Sina Koch et al.
Journal of Cell Science
-
Fluvastatin inhibits FLT3 glycosylation in human and murine cells and prolongs survival of mice with FLT3/ITD leukemia.
Authors: Williams A, Li L, Nguyen B, Brown P, Levis M, Small D
Blood, 2012-08-27;120(15):3069-79.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human Flt-3/Flk-2 Antibody
There are currently no reviews for this product. Be the first to review Human Flt-3/Flk-2 Antibody and earn rewards!
Have you used Human Flt-3/Flk-2 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image


