Human IL-1 beta/IL-1F2 Quantikine ELISA Kit Summary
Sample Values
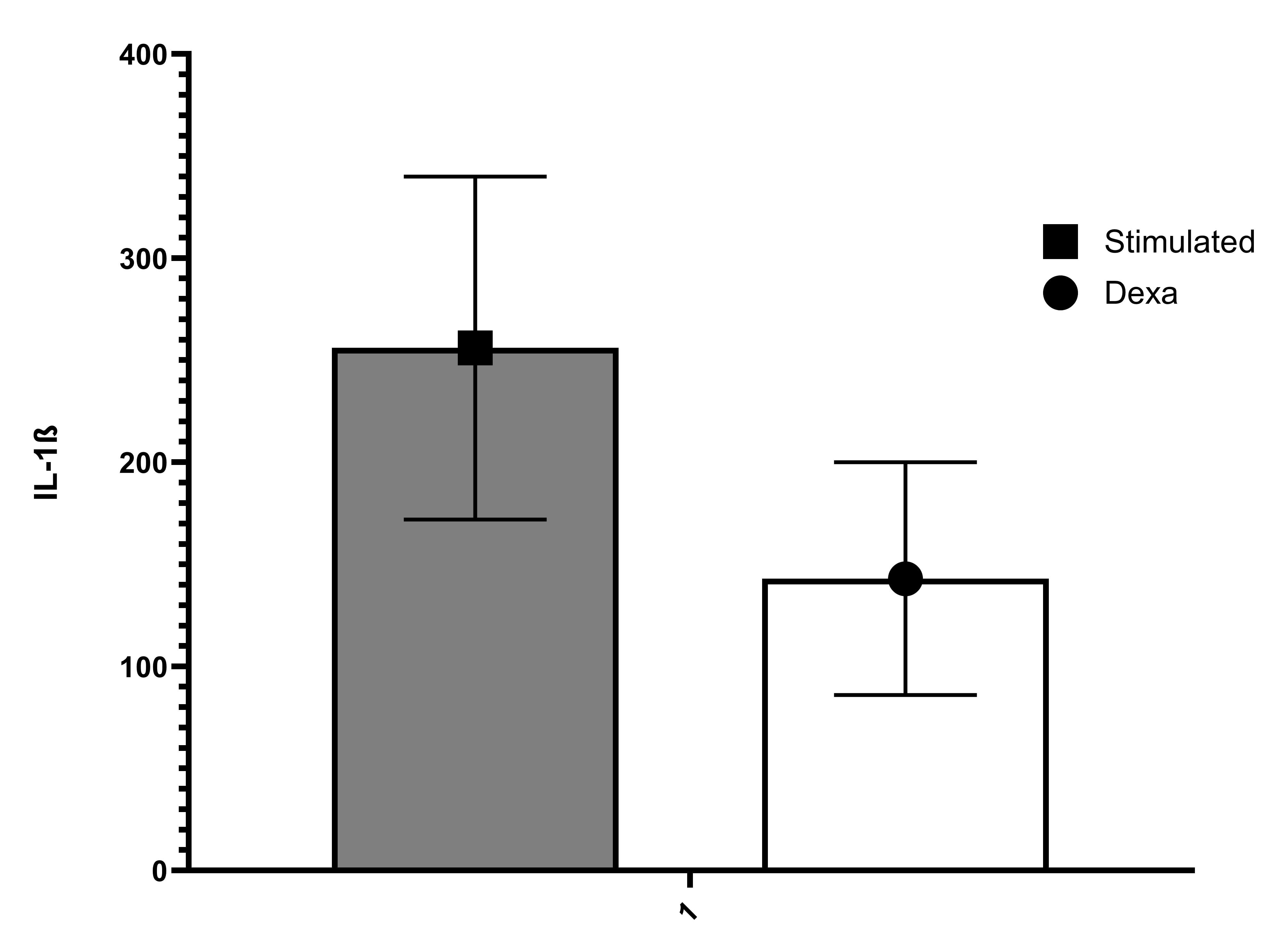
Serum/Plasma - Forty serum and plasma samples from apparently healthy volunteers were evaluated for the presence of human IL-1 beta in this assay. No medical histories were available for the donors used in this study. All samples measured less than the lowest IL-1 beta standard, 3.9 pg/mL.| Stimulant | Day 1 (pg/mL) | Day 3 (pg/mL) | Day 5 (pg/mL) |
| 10 μg/mL PHA | 2185 | 2004 | 2383 |
| 10 μg/mL PHA+10 ng/mL rhIL-2 | 1938 | 1973 | 2839 |
| 50 ng/mL PMA | 1767 | 1027 | 1159 |
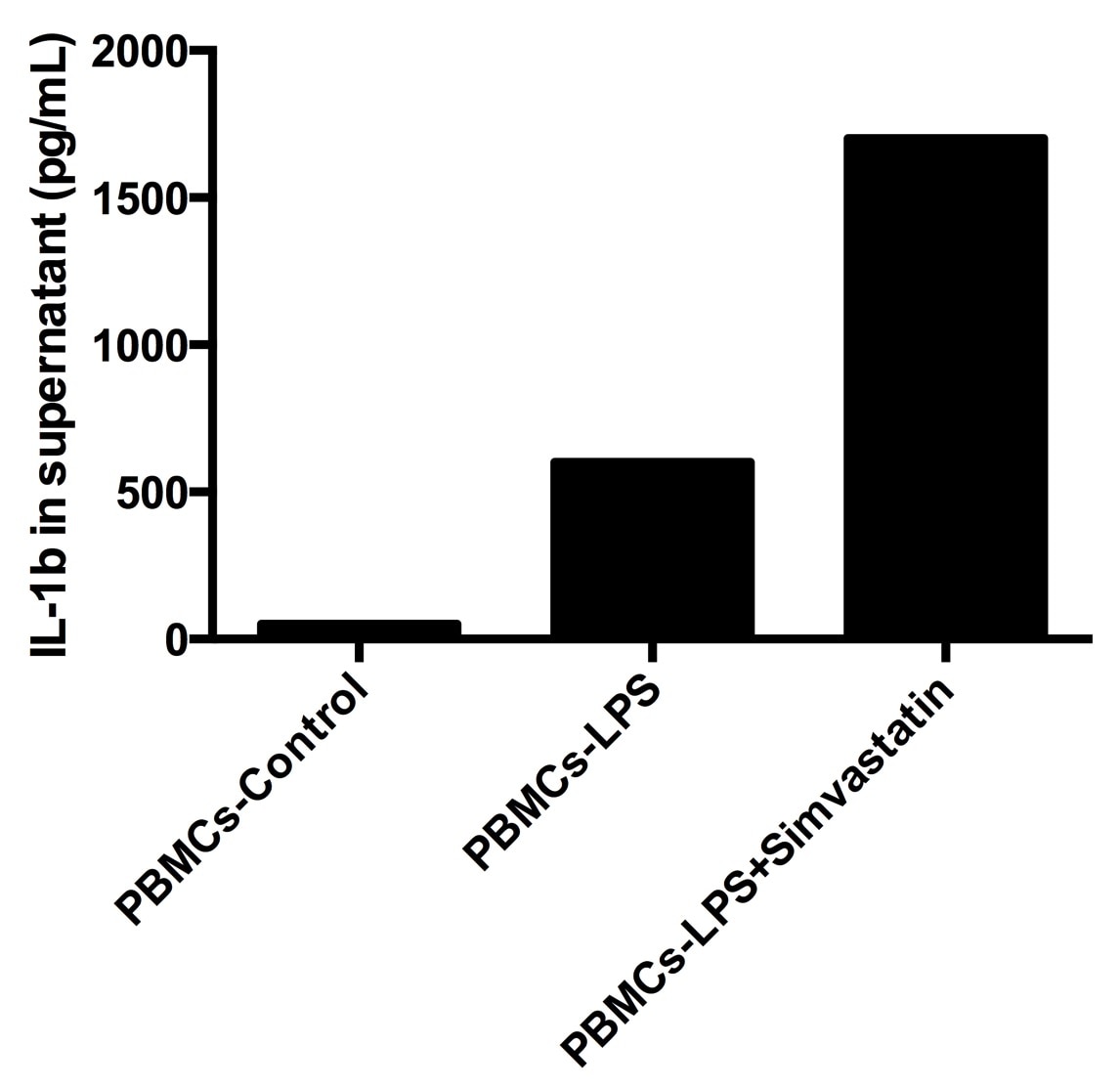
| 50 ng/mL LPS | 4158 | 2145 | 1308 |
Product Summary
Precision
Cell Culture Supernates
| Intra-Assay Precision | Inter-Assay Precision | ||||||
|---|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 4 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 12.6 | 30.4 | 69.2 | 192 | 30.8 | 120 | 194 |
| Standard Deviation | 0.3 | 1 | 1.6 | 6.5 | 2.2 | 4.1 | 7.9 |
| CV% | 2.4 | 3.3 | 2.3 | 3.4 | 7.1 | 3.4 | 4.1 |
Serum, EDTA Plasma, Heparin Plasma, Citrate Plasma
| Intra-Assay Precision | Inter-Assay Precision | ||||||
|---|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 4 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 18.9 | 30.2 | 122 | 181 | 29.8 | 118 | 193 |
| Standard Deviation | 1.6 | 1 | 5.4 | 5 | 2.5 | 5 | 8 |
| CV% | 8.5 | 3.3 | 4.4 | 2.8 | 8.4 | 4.2 | 4.1 |
Recovery
The recovery of IL-1 beta spiked to different levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=4) | 97 | 80-111 |
| Citrate Plasma (n=10) | 93 | 83-110 |
| EDTA Plasma (n=10) | 86 | 81-100 |
| Heparin Plasma (n=10) | 82 | 76-100 |
| Serum (n=10) | 95 | 87-110 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-1 beta/IL-1F2
The Interleukin 1 (IL-1) family of proteins consists of the classic members IL-1 alpha, IL-1 beta, and IL-1ra, plus IL-18, IL-33 and IL-1F5-F10. IL-1 alpha and IL-1 beta bind to the same cell surface receptors and share biological functions. IL-1 is not produced by unstimulated cells of healthy individuals with the exception of skin keratinocytes, some epithelial cells, and certain cells of the central nervous system. However, in response to inflammatory agents, infections, or microbial endotoxins, a dramatic increase in the production of IL-1 by macrophages and various other cell types is observed. IL-1 beta plays a central role in immune and inflammatory responses, bone remodeling, fever, carbohydrate metabolism, and GH/IGF-I physiology. Inappropriate or prolonged production of IL-1 has been implicated in a variety of pathological conditions including sepsis, rheumatoid arthritis, inflammatory bowel disease, acute and chronic myelogenous leukemia, insulin dependent diabetes mellitus, atherosclerosis, neuronal injury, and aging-related diseases.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- For Serum & Plasma Samples: Add 50 µL of Assay Diluent to each well.
- Add 200 µL of Standard, control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process twice for a total of 3 washes.
- Add 200 µL of Conjugate to each well.
- For Cell Culture Supernate Samples: Cover with a new plate sealer, and incubate at room temperature for 1 hour.
For Serum & Plasma Samples: Cover with a new plate sealer, and incubate at room temperature for 2 hours. - Aspirate and wash 3 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 20 minutes. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Human IL-1 beta/IL-1F2 Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
356
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Loss of NKCC1 activates the NLRP3 inflammasome in intestinal epithelia
Authors: Koumangoye, RB;Ferdaus, MZ;Davis, X;Bohannon, JK;Delpire, E;
Cellular and molecular gastroenterology and hepatology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Peroxiredoxin 1 inhibits tumorigenesis by activating the NLRP3/GSDMD pathway to induce pyroptosis of colorectal cancer cells
Authors: He, Y;Liu, J;Zhou, N;Xie, LX;Jiang, YF;Chen, CL;
World journal of gastroenterology
Species: Human
Sample Types: Cell Culture Supernates
-
Baricitinib and Infliximab Mitigate the Endothelial-to-Mesenchymal Transition (EndMT) Induced by Cytokines in HUVECs
Authors: Barilli, A;Visigalli, R;Recchia Luciani, G;Crescini, E;Dall'Asta, V;Rotoli, BM;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Midkine promotes PDGF-BB-induced proliferation, migration, and glycolysis of airway smooth muscle cells via the PI3K Akt pathway
Authors: Xu, Y;Li, W;Shi, J;Guo, Y;
Physiological reports
Species: Human
Sample Types: Serum
-
Molecular Insights into the Nociceptive Modulation by Palmitoylethanolamide and Equisetum arvense Extract: An In Vitro Study Across the Blood-Brain Barrier
Authors: Mulè, S;Galla, R;Ferrari, S;Invernizzi, M;Uberti, F;
Nutrients
Species: Human
Sample Types: Cell Culture Supernates
-
Atractylenolide I Inhibits Nicotine-Induced Macrophage Pyroptosis and Alleviates Atherogenesis by Suppressing the TLR4/ROS/TXNIP/NLRP3 Pathway
Authors: Li, HH;Liu, X;Wang, YP;Xu, X;Zhu, L;Zhang, W;Ren, K;
Metabolites
Species: Human
Sample Types: Cell Culture Supernates
-
Engagement of CD300c by a Novel Monoclonal Antibody Ameliorates Behavioral Deficits in a 5xFAD Mouse Model of Alzheimer's Disease
Authors: Lee, S;Lim, CK;Kim, J;Kim, J;Jin, HK;Bae, JS;Jeon, JW;
Biomedicines
Species: Human
Sample Types: Cell Culture Supernates
-
Investigating the relationship between sleep disturbances and cortical thickness, brainstem volume, amyloid accumulation, and inflammatory markers in Parkinson's disease patients
Authors: Chen, M;Guo, G;Liu, S;Cai, J;Tong, X;Liu, X;Zhang, Y;Chen, Y;Huo, J;
Experimental gerontology
Species: Human
Sample Types: Serum
-
Generation of induced alveolar assembloids with functional alveolar-like macrophages
Authors: Kang, JS;Lee, Y;Lee, Y;Gil, D;Kim, MJ;Wood, C;Delorme, V;Lee, JM;Ko, KC;Kim, JH;Lee, MO;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Therapeutic Potential of Cannabidiol Cyclodextrin Complex in Polymeric Micelle and Tetrahydrocurcumin Cyclodextrin Complex Loaded in Hydrogel to Treat Lymphedema
Authors: Srakhao, W;Nakpheng, T;Mohd Amin, MCI;Srichana, T;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Therapeutic potential of AAV2-shmTOR gene therapy in reducing retinal inflammation and preserving endothelial Integrity in age-related macular degeneration
Authors: Kim, J;Moon, SY;Kang, HG;Kim, HJ;Choi, JS;Lee, SHS;Park, K;Won, SY;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Increasing the concentration of plasma molecules improves the biological activity of platelet-rich plasma for tissue regeneration
Authors: Sánchez, M;Mercader Ruiz, J;Marijuán Pinel, D;Sánchez, P;Fiz, N;Guadilla, J;Azofra, J;Beitia, M;Delgado, D;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
B cells enhance IL-1 beta driven invasiveness in triple negative breast cancer
Authors: Toney, NJ;Opdenaker, LM;Frerichs, L;Modarai, SR;Ma, A;Archinal, H;Ajayi, GO;Sims-Mourtada, J;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Cytokine Storm among Bangladeshi adults with COVID-19: A prospective cohort study
Authors: Sarmin, M;Akter, F;Islam, AN;Mahfuz, M;Das, S;Sharifuzzaman, ;Hasan, SMT;Bhuiyan, TR;Rahman, M;Gazi, A;Matin, FB;Tariqujjaman, M;Shahrin, L;Islam, M;Mahmud, AM;Banu, S;Chisti, MJ;Qadri, F;Clemens, JD;Ahmed, T;
Heliyon
Species: Human
Sample Types: Plasma
-
?-hydroxybutyrate suppresses pathological changes of blood-induced arthropathy in rats
Authors: Kawasaki, R;Sakata, A;Tatsumi, K;Mitani, S;Takeda, M;Kasuda, S;Matsumoto, N;Harada, S;Soeda, T;Nishida, Y;Yoshimura, Y;Shima, M;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
PTPN23-dependent ESCRT machinery functions as a cell death checkpoint
Authors: Song, D;Cen, Y;Qian, Z;Wu, XS;Rivera, K;Wee, TL;Demerdash, OE;Chang, K;Pappin, D;Vakoc, CR;Tonks, NK;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
The link between osteoporosis and frozen shoulder: exploring the therapeutic effect of TAK715 on reversing fibrosis and protecting against osteoporosis via the p38 MAPK signaling pathway
Authors: Li, X;Yan, Y;Wang, Z;Hou, J;Meng, Y;Cui, D;Long, Y;Li, M;Yang, R;
BMC musculoskeletal disorders
Species: Human
Sample Types: Cell Culture Supernates, Joint Lavage Fluid
-
Serum proinflammatory cytokines, receptor activator of nuclear factor kappa-? ligand (RANKL), osteoprotegerin (OPG) and RANKL/OPG ratio in mild and severe COVID-19
Authors: Kazemi-Sufi, S;Alipour, S;Rabieepour, M;Roshan-Milani, S;Naderi, R;
BMC infectious diseases
Species: Human
Sample Types: Plasma
-
Neonatal neutrophils exhibit reduced NLRP3 inflammasome activation
Authors: Wackerbarth, LM;Seifert, SB;Napoli, M;Rohwedder, I;Vogl, T;Scheiermann, C;Kolben, T;Nussbaum, C;Pruenster, M;Immler, R;Sperandio, M;
Journal of leukocyte biology
Species: Human
Sample Types: Cell Culture Supernates
-
DGAT1 and DGAT2 Inhibitors for Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Management: Benefits for Their Single or Combined Application
Authors: Longo, M;Paolini, E;Di Benedetto, P;Tomassini, E;Meroni, M;Dongiovanni, P;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Granulocyte colony-stimulating factor mediates bone loss via the activation of IL-1?/JNK signaling pathway in murine Staphylococcus aureus-induced osteomyelitis
Authors: Song, M;Deng, M;Peng, Z;Dai, F;Wang, Y;Shu, W;Zhou, X;Zhang, J;Hou, Y;Yu, B;
International immunopharmacology
Species: Mouse
Sample Types: Serum, Bone Marrow Fluid
-
Caspase-4/11 promotes hyperlipidemia and chronic kidney disease-accelerated vascular inflammation by enhancing trained immunity
Authors: Sun, Y;Lu, Y;Liu, L;Saaoud, F;Shao, Y;Xu, K;Drummer Iv, C;Cueto, R;Shan, H;Jiang, X;Zhao, H;Wang, H;Yang, X;
JCI insight
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukins IL33/ST2 and IL1-? in Intrauterine Growth Restriction and Seropositivity of Anti-Toxoplasma gondii Antibodies
Authors: Franco-De León, K;Camarena, EE;Pereira-Suárez, AL;Barrios-Prieto, E;Soto-Venegas, A;Hernández-Nazara, ZH;Luna Rojas, YG;Galván-Ramírez, ML;
Microorganisms
Species: Human
Sample Types: Serum
-
Cytokine expression profiles in children and adolescents with tic disorders
Authors: Kutuk, MO;Tufan, AE;Kilicaslan, F;Gokcen, C;Aksu, GG;Yektas, C;Kandemir, H;Celik, F;Mutluer, T;Buber, A;Karadag, M;Coban, N;Coskun, S;Hangul, Z;Altintas, E;Acikbas, U;Giray, A;Aka, Y;Basturk, B;Kutuk, O;
Scientific reports
Species: Human
Sample Types: Serum
-
The TOX-RAGE axis mediates inflammatory activation and lung injury in severe pulmonary infectious diseases
Authors: Kim, H;Park, HH;Kim, HN;Seo, D;Hong, KS;Jang, JG;Seo, EU;Kim, IY;Jeon, SY;Son, B;Cho, SW;Kim, W;Ahn, JH;Lee, W;
Proceedings of the National Academy of Sciences of the United States of America
Species: Human
Sample Types: Plasma
-
Imeglimin attenuates NLRP3 inflammasome activation by restoring mitochondrial functions in macrophages
Authors: Lee, JY;Kang, Y;Jeon, JY;Kim, HJ;Kim, DJ;Lee, KW;Han, SJ;
Journal of pharmacological sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Modeling of solar UV-induced photodamage on the hair follicles in human skin organoids
Authors: Kim, MJ;Ahn, HJ;Kong, D;Lee, S;Kim, DH;Kang, KS;
Journal of tissue engineering
Species: Human
Sample Types: Cell Culture Supernates
-
Autonomous circadian rhythms in the human hepatocyte regulate hepatic drug metabolism and inflammatory responses
Authors: March, S;Nerurkar, N;Jain, A;Andrus, L;Kim, D;Whittaker, CA;Tan, EKW;Thiberge, S;Fleming, HE;Mancio-Silva, L;Rice, CM;Bhatia, SN;
Science advances
Species: Human
Sample Types: Cell Culture Supernates
-
Differential Response of Human Dendritic Cells upon Stimulation with Encapsulated or Non-Encapsulated Isogenic Strains of Porphyromonas gingivalis
Authors: Melgar-Rodríguez, S;Polanco, A;Ríos-Muñoz, J;García, M;Sierra-Cristancho, A;González-Osuna, L;Díaz-Zúñiga, J;Carvajal, P;Vernal, R;Bravo, D;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
The alterations in nerve growth factor concentration in plasma and synovial fluid before and after total knee arthroplasty
Authors: Onodera, T;Iwasaki, K;Matsuoka, M;Morioka, Y;Matsubara, S;Kondo, E;Iwasaki, N;
Scientific reports
Species: Human
Sample Types: Synovial Fluid
Applications: ELISA -
A Comparative Analysis of Innate Immune Responses and the Structural Characterization of Spike from SARS-CoV-2 Gamma Variants and Subvariants
Authors: Scovino, AM;Dahab, EC;Diniz-Lima, I;de Senna Silveira, E;Barroso, SPC;Cardoso, KM;Nico, D;Makhoul, GJ;da Silva-Junior, EB;Freire-de-Lima, CG;Freire-de-Lima, L;Fonseca, LMD;Valente, N;Nacife, V;Machado, A;Araújo, M;Vieira, GF;Pauvolid-Corrêa, A;Siqueira, M;Morrot, A;
Microorganisms
Species: Human
Sample Types: Cell Culture Supernates
-
Alternative autophagy dampens UVB-induced NLRP3 inflammasome activation in human keratinocytes
Authors: Hasegawa, T;Noguchi, S;Nakashima, M;Miyai, M;Goto, M;Matsumoto, Y;Torii, S;Honda, S;Shimizu, S;
The Journal of biological chemistry
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophil Gelatinase-Associated Lipocalin for the Differentiation of Mucinous Pancreatic Cystic Lesions
Authors: Olar, MP;Iacobescu, M;Bolboac?, SD;Pojoga, C;Mo?teanu, O;Seicean, R;Rusu, I;Banc, O;Iuga, CA;Seicean, A;
International journal of molecular sciences
Species: Human
Sample Types: Serum, Cyst Fluid
-
Hepatitis B virus e antigen induces atypical metabolism and differentially regulates programmed cell deaths of macrophages
Authors: Li, Y;Wu, C;Lee, J;Ning, Q;Lim, J;Eoh, H;Wang, S;Hurrell, BP;Akbari, O;Ou, JJ;
PLoS pathogens
Species: Human
Sample Types: Cell Culture Supernates
-
NF?B and NLRP3/NLRC4 inflammasomes regulate differentiation, activation and functional properties of monocytes in response to distinct SARS-CoV-2 proteins
Authors: Tsukalov, I;Sánchez-Cerrillo, I;Rajas, O;Avalos, E;Iturricastillo, G;Esparcia, L;Buzón, MJ;Genescà, M;Scagnetti, C;Popova, O;Martin-Cófreces, N;Calvet-Mirabent, M;Marcos-Jimenez, A;Martínez-Fleta, P;Delgado-Arévalo, C;de Los Santos, I;Muñoz-Calleja, C;Calzada, MJ;González Álvaro, I;Palacios-Calvo, J;Alfranca, A;Ancochea, J;Sánchez-Madrid, F;Martin-Gayo, E;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
SOD2 orchestrates redox homeostasis in intervertebral discs: A novel insight into oxidative stress-mediated degeneration and therapeutic potential
Authors: Tamagawa, S;Sakai, D;Nojiri, H;Nakamura, Y;Warita, T;Matsushita, E;Schol, J;Soma, H;Ogasawara, S;Munesada, D;Koike, M;Shimizu, T;Sato, M;Ishijima, M;Watanabe, M;
Redox biology
Species: Human
Sample Types: Cell Culture Supernates
-
VEXAS syndrome is characterized by inflammasome activation and monocyte dysregulation
Authors: Kosmider, O;Possémé, C;Templé, M;Corneau, A;Carbone, F;Duroyon, E;Breillat, P;Chirayath, TW;Oules, B;Sohier, P;Luka, M;Gobeaux, C;Lazaro, E;Outh, R;Le Guenno, G;Lifermann, F;Berleur, M;Le Mene, M;Friedrich, C;Lenormand, C;Weitten, T;Guillotin, V;Burroni, B;Boussier, J;Willems, L;Aractingi, S;Dionet, L;Tharaux, PL;Vergier, B;Raynaud, P;Ea, HK;Ménager, M;Duffy, D;Terrier, B;
Nature communications
Species: Human
Sample Types: Plasma
-
AIM2 promotes irradiation resistance, migration ability and PD-L1 expression through STAT1/NF-?B activation in oral squamous cell carcinoma
Authors: Chiu, HW;Lee, HL;Lee, HH;Lu, HW;Lin, KY;Lin, YF;Lin, CH;
Journal of translational medicine
Species: Human
Sample Types: Cell Culture Supernates
-
Pyrogenic and inflammatory mediators are produced by polarized M1 and M2 macrophages activated with D-dimer and SARS-CoV-2 spike immune complexes
Authors: Park, YJ;Acosta, D;Rubel Hoq, M;Khurana, S;Golding, H;Zaitseva, M;
Cytokine
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated IL-1? and Comparable IL-1 Receptor Antagonist Levels Are Characteristic Features of L-PRP in Female College Athletes Compared to Male Professional Soccer Players
Authors: Mochizuki, T;Ushiki, T;Suzuki, K;Sato, M;Ishiguro, H;Suwabe, T;Watanabe, S;Edama, M;Omori, G;Yamamoto, N;Kawase, T;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Role of the cAMP-PKA-NF-?B pathway in Mucin1 over-expression in A549 cells during Respiratory syncytial virus infection
Authors: Jin, Y;Zhang, D;Deng, K;Wu, P;Yang, D;Xie, Z;Qiu, W;Yu, G;
BMC infectious diseases
Species: Human
Sample Types: Sputum Plug Supernate
-
Synthesis and characterization of piperic acid conjugates with homochiral and heterochiral dipeptides containing phenylalanine and their application in skin cancer
Authors: Ur Rahim, J;Faheem, MM;Nawaz, S;Goswami, A;Rai, R;
Peptides
Species: Mouse
Sample Types: Cell Culture Supernates
-
Increased Levels of Caspase-1 and IL-1? Among Adults With Persistent Immune Activation After 12 Years of Suppressive Antiretroviral Therapy in the Infectious Diseases Institute HIV Treatment Cohort
Authors: Nabatanzi, R;Ssekamatte, P;Castelnuovo, B;Kambugu, A;Nakanjako, D;
Open forum infectious diseases
Species: Human
Sample Types: Plasma
-
Insensitive Effects of Inflammatory Cytokines on the Reference Genes of Synovial Fluid Resident-Mesenchymal Stem Cells Derived from Rheumatoid Arthritis Patients
Authors: Bok, EY;Kim, SB;Thakur, G;Choe, YH;Oh, SJ;Hwang, SC;Ock, SA;Rho, GJ;Lee, SI;Lee, WJ;Lee, SL;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Dialysis Patients Respond Adequately to Influenza Vaccination Irrespective of Dialysis Modality and Chronic Inflammation
Authors: Pleros, C;Adamidis, K;Kantartzi, K;Griveas, I;Baltsavia, I;Moustakas, A;Kalliaropoulos, A;Fraggedaki, E;Petra, C;Damianakis, N;Mentis, A;Drosataki, E;Petrakis, I;Passadakis, P;Panagopoulos, P;Stylianou, K;Panagoutsos, S;
Journal of clinical medicine
Species: Human
Sample Types: Serum
-
Deiodinase Types 1 and 3 and Proinflammatory Cytokine Values May Discriminate Depressive Disorder Patients from Healthy Controls
Authors: Ma?uj?o-Balcerska, E;Pietras, T;
Journal of clinical medicine
Species: Human
Sample Types: Serum
-
Circulating Soluble EPCR Levels Are Reduced in Patients with Ischemic Peripheral Artery Disease and Associated with Markers of Endothelial and Vascular Function
Authors: Krug, J;Bochenek, ML;Gogiraju, R;Laubert-Reh, D;Lackner, KJ;Münzel, T;Wild, PS;Espinola-Klein, C;Schäfer, K;
Biomedicines
Species: Human
Sample Types: Plasma
-
Characterization of Leukocyte- and Platelet-Rich Plasma Derived from Female Collage Athletes: A Cross-Sectional Cohort Study Focusing on Growth Factor, Inflammatory Cytokines, and Anti-Inflammatory Cytokine Levels
Authors: Mochizuki, T;Ushiki, T;Suzuki, K;Sato, M;Ishiguro, H;Suwabe, T;Edama, M;Omori, G;Yamamoto, N;Kawase, T;
International journal of molecular sciences
Species: Human
Sample Types: Platelet-Rich Plasma
-
Role of NLRP3 inflammasome activation in HCC cell progression
Authors: Dai, B;Cao, H;Hu, Y;Gong, Z;Huang, X;Chen, Y;Liu, F;Peng, X;Zhang, Y;Lei, X;
Heliyon
Species: Human
Sample Types: Cell Culture Supernates
-
Understanding the Immunomodulatory Effects of Bovine Colostrum: Insights into IL-6/IL-10 Axis-Mediated Inflammatory Control
Authors: Grigalevi?i?t?, R;Matusevi?ius, P;Plan?i?nien?, R;Stankevi?ius, R;Radzevi?i?t?-Val?iuk?, E;Balevi?i?t?, A;elvys, A;Zinkevi?ien?, A;Zigmantait?, V;Ku?inskas, A;Kavaliauskas, P;
Veterinary sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Three-dimensional human neural culture on a chip recapitulating neuroinflammation and neurodegeneration
Authors: Kang, YJ;Diep, YN;Tran, M;Tran, VTA;Ambrin, G;Ngo, H;Cho, H;
Nature protocols
Species: Human
Sample Types: Cell Culture Supernates
-
Targeting polyploid giant cancer cells potentiates a therapeutic response and overcomes resistance to PARP inhibitors in ovarian cancer
Authors: Zhang, X;Yao, J;Li, X;Niu, N;Liu, Y;Hajek, RA;Peng, G;Westin, S;Sood, AK;Liu, J;
Science advances
Species: Human
Sample Types: Cell Culture Supernates
-
Sulforaphane Attenuates Neutrophil ROS Production, MPO Degranulation and Phagocytosis, but Does Not Affect NET Formation Ex Vivo and In Vitro
Authors: Wakasugi-Onogi, S;Ma, S;Ruhee, RT;Tong, Y;Seki, Y;Suzuki, K;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Efficacy of indocyanine green and methylene blue mediated-photodynamic therapy on peri-implant outcomes among diabetics with peri-implant mucositis
Authors: H Alsayed, IA Bukhari, R Alsaif, F Vohra
Photodiagnosis and photodynamic therapy, 2023-02-24;42(0):103344.
Species: Human
Sample Types: Sulcular Fluid
-
Analysis of Selected Salivary Adipokines and Cytokines in Patients with Obesity-A Pilot Study
Authors: L Ostrowska, J Smarkusz-Z, A Gornowicz, K Lendzion, B Zy?k, D Pogodzi?sk
International Journal of Molecular Sciences, 2023-02-18;24(4):.
Species: Human
Sample Types: Saliva
-
The NLRP3 inflammasome - Interleukin 1beta axis in uveal melanoma
Authors: VSMC Correa, NE Efstathiou, DP Ntentakis, Z Yu, T Narimatsu, E Gragoudas, IK Kim, DG Vavvas
FEBS Open Bio, 2023-02-12;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Affective neural circuits and inflammatory markers linked to depression and anxiety symptoms in patients with comorbid obesity
Authors: H Hallihan, P Tsai, N Lv, L Xiao, B Peñalver B, Y Wu, GN Pandey, LM Williams, OA Ajilore, J Ma
Journal of psychiatric research, 2023-02-03;160(0):9-18.
Species: Human
Sample Types: Plasma
-
Pro-Inflammatory Adipokine and Cytokine Profiles in the Saliva of Obese Patients with Non-Alcoholic Fatty Liver Disease (NAFLD)-A Pilot Study
Authors: B Zy?k, L Ostrowska, J Smarkusz-Z, K Witczak-Sa, A Gornowicz, A Bielawska
International Journal of Molecular Sciences, 2023-02-02;24(3):.
Species: Human
Sample Types: Saliva
-
Increased Angiopoietin-1 and -2 levels in human vitreous are associated with proliferative diabetic retinopathy
Authors: T Tsai, M Alwees, MA Asaad, J Theile, V Kakkassery, HB Dick, T Schultz, SC Joachim
PLoS ONE, 2023-01-20;18(1):e0280488.
Species: Human
Sample Types: Vitreous Humor
-
Expansion of interferon inducible gene pool via USP18 inhibition promotes cancer cell pyroptosis
Authors: KI Arimoto, S Miyauchi, TD Troutman, Y Zhang, M Liu, SA Stoner, AG Davis, JB Fan, YJ Huang, M Yan, CK Glass, DE Zhang
Nature Communications, 2023-01-17;14(1):251.
Species: Human
Sample Types: Cell Culture Supernates
-
Protein folding stress potentiates NLRP1 and CARD8 inflammasome activation
Authors: EL Orth-He, HC Huang, SD Rao, Q Wang, Q Chen, CM O'Mara, AJ Chui, M Saoi, AR Griswold, A Bhattachar, DP Ball, JR Cross, DA Bachovchin
Cell Reports, 2023-01-16;0(0):111965.
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of Nutritional Factors to Evaluate Periodontal Clinical Parameters in Patients with Systemic Diseases
Authors: Y Nakayama, S Tabe, A Yamaguchi, Y Tsuruya, R Kobayashi, K Oyama, D Kitano, K Kojima, R Kogawa, Y Okumura, J Ogihara, H Senpuku, Y Ogata
Nutrients, 2023-01-11;15(2):.
Species: Human
Sample Types: Saliva
-
High p62 expression suppresses the NLRP1 inflammasome and increases stress resistance in cutaneous SCC cells
Authors: P Hennig, M Di Filippo, G Bilfeld, M Mellett, HD Beer
Cell Death & Disease, 2022-12-29;13(12):1077.
Species: Human
Sample Types: Cell Culture Supernates
-
Lysosomal cathepsins act in concert with Gasdermin-D during NAIP/NLRC4-dependent IL-1beta secretion
Authors: LM Branco, MP Amaral, H Boekhoff, ABF de Lima, IS Farias, SL Lage, GJS Pereira, BS Franklin, KR Bortoluci
Cell Death & Disease, 2022-12-08;13(12):1029.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of Apamin on MPP+-Induced Calcium Overload and Neurotoxicity by Targeting CaMKII/ERK/p65/STAT3 Signaling Pathways in Dopaminergic Neuronal Cells
Authors: J Park, KM Jang, KK Park
International Journal of Molecular Sciences, 2022-12-03;23(23):.
Species: Human
Sample Types: Cell Culture Supernates
-
N-acetylcysteine Reduces Inflammasome Activation Induced by SARS-CoV-2 Proteins In Vitro
Authors: J Milara, F Martínez-E, P Montero, I Roger, MA Bayarri, P Ribera, MN Oishi-Kona, JR Alba-Garcí, E Zapater, J Cortijo
International Journal of Molecular Sciences, 2022-11-22;23(23):.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of Propofol versus Sevoflurane Anesthesia on Acute Kidney Injury after Lung Transplantation Surgery: A Prospective Randomized Controlled Trial
Authors: Y Song, HC Paik, N Kim, H Jung, JG Lee, YC Yoo
Journal of Clinical Medicine, 2022-11-21;11(22):.
Species: Human
Sample Types: Serum
-
Bovine lactoferrin suppresses inflammatory cytokine expression in endometrial stromal cells in chronic endometritis
Authors: A Nakamura, F Kimura, S Tsuji, T Hanada, A Takebayash, A Takahashi, J Kitazawa, A Morimune, T Amano, R Kushima, T Murakami
Journal of reproductive immunology, 2022-11-08;154(0):103761.
Species: Human
Sample Types: Cell Culture Supernates
-
Inflammaging and body composition: New insights in diabetic and hypertensive elderly men
Authors: CV Gonçalves, IS Ribeiro, MPL Galantini, IPR Muniz, PHB Lima, GS Santos, RAA da Silva
Experimental gerontology, 2022-10-28;170(0):112005.
Species: Human
Sample Types: Serum
-
Macrophage Phenotypes and Gene Expression Patterns Are Unique in Naturally Occurring Metabolically Healthy Obesity
Authors: AD Ruggiero, R Vemuri, M Block, D DeStephani, M Davis, J Chou, A Williams, A Brock, SK Das, K Kavanagh
International Journal of Molecular Sciences, 2022-10-21;23(20):.
Species: Primate - Chlorocebus aethiops (African Green Monkey)
Sample Types: Plasma
-
Resveratrol Mitigates Oxygen and Glucose Deprivation-Induced Inflammation, NLRP3 Inflammasome, and Oxidative Stress in 3D Neuronal Culture
Authors: MC Chiang, CJB Nicol, SS Lo, SW Hung, CJ Wang, CH Lin
International Journal of Molecular Sciences, 2022-10-02;23(19):.
Species: Human
Sample Types: Cell Culture Supernates
-
Breed and Feeding System Impact the Bioactive Anti-Inflammatory Properties of Bovine Milk
Authors: A Salzano, MC Di Meo, N D'Onofrio, G Bifulco, A Cotticelli, F Licitra, A Iraci Fuin, G Cascone, ML Balestrier, E Varricchio, G Campanile
International Journal of Molecular Sciences, 2022-09-21;23(19):.
Species: Human
Sample Types: Cell Culture Supernates
-
FASN inhibition targets multiple drivers of NASH by reducing steatosis, inflammation and fibrosis in preclinical models
Authors: M O'Farrell, G Duke, R Crowley, D Buckley, EB Martins, D Bhattachar, SL Friedman, G Kemble
Scientific Reports, 2022-09-19;12(1):15661.
Species: Human
Sample Types: Cell Culture Supernates
-
FAAH served a key membrane-anchoring and stabilizing role for NLRP3 protein independently of the endocannabinoid system
Authors: Y Zhu, H Zhang, H Mao, S Zhong, Y Huang, S Chen, K Yan, Z Zhao, X Hao, Y Zhang, H Yao, X Huang, M Wang, W Zhang, J Li, G Meng, X Qin, Z Ye, J Shen, Y Song, Y Xu, Z Yang, L Wang, Y Zhang, L Wen
Cell Death and Differentiation, 2022-09-14;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
The Effect of Dietary Methyl-Donor Intake and Other Lifestyle Factors on Cancer Patients in Hungary
Authors: E Kiss, A Hajdu, G Forika, M Dank, T Krenacs, Z Nemeth
Cancers, 2022-09-13;14(18):.
Species: Human
Sample Types: Plasma
-
Lipopolysaccharide Inhibits Autophagy and Promotes Inflammatory Responses via p38 MAPK-Induced Proteasomal Degradation of Atg13 in Hepatic Stellate Cells
Authors: Y Wu, Y He, F Wang, N Yao, Y Zhao, Z Tian
Mediators of Inflammation, 2022-09-13;2022(0):9603989.
Species: Human
Sample Types: Cell Culture Supernates
-
Platyconic acid A?induced PPM1A upregulation inhibits the proliferation, inflammation and extracellular matrix deposition of TGF?beta1?induced lung fibroblasts
Authors: C Su, Y Tang, C Wang, H Huang, H Hou
Molecular Medicine Reports, 2022-09-07;26(5):.
Species: Human
Sample Types:
-
Effects of resveratrol therapy on glucose metabolism, insulin resistance, inflammation, and renal function in the elderly patients with type 2 diabetes mellitus: A randomized controlled clinical trial protocol
Authors: N Ma, Y Zhang
Medicine, 2022-08-12;101(32):e30049.
Species: Human
Sample Types: Serum
-
Modulation of Neuroendocrine and Immunological Biomarkers Following Rehabilitation in Sarcopenic Patients
Authors: F Piancone, F La Rosa, I Marventano, A Hernis, R Miglioli, F Trecate, M Saresella, M Clerici
Cells, 2022-08-10;11(16):.
Species: Human
Sample Types: Plasma
-
The sweet fuel of inflammation: New perspectives on the complex web that interconnects diabetes
Authors: MPL Galantini, IS Ribeiro, CV Gonçalves, IPR Muniz, PHB Lima, GS Santos, RAA da Silva
Experimental gerontology, 2022-07-30;167(0):111905.
Species: Human
Sample Types: Serum
-
The Mechanism of Inflammatory Factors and Soluble Vascular Cell Adhesion Molecule-1 Regulated by Nuclear Transcription Factor NF-kappaB in Unstable Angina Pectoris
Authors: Q Su, L Zhang, Z Qi, B Huang
Journal of Immunology Research, 2022-07-30;2022(0):6137219.
Species: Human
Sample Types: Serum
-
IL-6 is involved in thoracic ossification of the ligamentum flavum
Authors: AY Huang, L Shu, Z Chen, C Zhang
PLoS ONE, 2022-07-29;17(7):e0272357.
Species: Human
Sample Types: Cell Culture Supernates
-
Modulation of MAPK- and PI3/AKT-Dependent Autophagy Signaling by Stavudine (D4T) in PBMC of Alzheimer's Disease Patients
Authors: F La Rosa, CP Zoia, C Bazzini, A Bolognini, M Saresella, E Conti, C Ferrarese, F Piancone, I Marventano, D Galimberti, C Fenoglio, E Scarpini, M Clerici
Cells, 2022-07-12;11(14):.
Species: Human
Sample Types: Cell Culture Supernates
-
Differential contribution of estrogen receptors to the intestinal therapeutic effects of 17beta-estradiol in a murine model of Parkinson's disease
Authors: AA Poirier, M Côté, M Bourque, H Jarras, J Lamontagne, M Morissette, TD Paolo, D Soulet
Brain research bulletin, 2022-07-01;187(0):85-97.
Species: Human
Sample Types: Cell Culture Supernates
-
Inflammatory adipose activates a nutritional immunity pathway leading to retinal dysfunction
Authors: JK Sterling, B Baumann, S Foshe, A Voigt, S Guttha, A Alnemri, SJ McCright, M Li, RJ Zauhar, SR Montezuma, RJ Kapphahn, VRM Chavali, DA Hill, DA Ferrington, D Stambolian, RF Mullins, D Merrick, JL Dunaief
Cell Reports, 2022-06-14;39(11):110942.
Species: Human
Sample Types: Serum
-
Adipokine human Resistin promotes obesity-associated inflammatory intervertebral disc degeneration via pro-inflammatory cytokine cascade activation
Authors: JH Shin, S Park, H Cho, JH Kim, H Choi
Scientific Reports, 2022-05-27;12(1):8936.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-1beta-Triggered Long Non-coding RNA CHRF Induces Non-Small Cell Lung Cancer by Modulating the microRNA-489/Myd88 Axis
Authors: Y Zhang, Y Zhang, Q Zeng, C Li, H Zhou, J Liu, Z Shi, L Ma
Journal of Cancer, 2022-05-16;13(8):2620-2630.
Species: Human
Sample Types: Cell Culture Supernates
-
Dexmedetomidine attenuates oxygen-glucose deprivation/ reperfusion-induced inflammation through the miR-17-5p/ TLR4/ NF-kappaB axis
Authors: L Suo, M Wang
BMC anesthesiology, 2022-04-29;22(1):126.
Species: Rat
Sample Types:
-
Cannabinoids Alleviate the LPS-Induced Cytokine Storm via Attenuating NLRP3 Inflammasome Signaling and TYK2-Mediated STAT3 Signaling Pathways In Vitro
Authors: SV Suryavansh, M Zaiachuk, N Pryimak, I Kovalchuk, O Kovalchuk
Cells, 2022-04-20;11(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
KIF4A promotes tumor progression of bladder cancer via CXCL5 dependent myeloid-derived suppressor cells recruitment
Authors: N Lin, L Chen, Y Zhang, Y Yang, L Zhang, L Chen, P Zhang, H Su, M Yin
Scientific Reports, 2022-04-10;12(1):6015.
Species: Human
Sample Types: Cell Culture Supernates
-
Circ_0050908 up-regulates TRAF3 by sponging miR-324-5p to aggravate myocardial ischemia-reperfusion injury
Authors: A Jin, Q Zhang, H Cheng, C Yang, X Wang
International immunopharmacology, 2022-04-09;108(0):108740.
Species: Human
Sample Types: Cell Culture Supernates
-
D-dimer and CoV-2 spike-immune complexes contribute to the production of PGE2 and proinflammatory cytokines in monocytes
Authors: YJ Park, D Acosta, R Vassell, J Tang, S Khurana, CD Weiss, H Golding, M Zaitseva
PloS Pathogens, 2022-04-06;18(4):e1010468.
Species: Human
Sample Types: Cell Culture Supernates
-
Blood KL-6 predicts prognosis in primary Sj�gren's syndrome-associated interstitial lung disease
Authors: YJ Kim, J Choe, SJ Moon, JW Song
Scientific Reports, 2022-03-29;12(1):5343.
Species: Human
Sample Types: Cell Culture Supernates
-
NaCl exposure results in increased expression and processing of IL-1beta in Meniere's disease patients
Authors: S Pathak, A Vambutas
Scientific Reports, 2022-03-23;12(1):4957.
Species: Human
Sample Types: Cell Culture Supernates
-
Calystegines Improve the Metabolic Activity of Human Adipose Derived Stromal Stem Cells (ASCs) under Hyperglycaemic Condition through the Reduction of Oxidative/ER Stress, Inflammation, and the Promotion of the AKT/PI3K/mTOR Pathway
Authors: A Kowalczuk, N Bourebaba, J Panchuk, K Marycz, L Bourebaba
Biomolecules, 2022-03-16;12(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Povidone iodine suppresses LPS-induced inflammation by inhibiting TLR4/MyD88 formation in airway epithelial cells
Authors: SH Lee, MR Choi, J Chung, SH Choi, SK Park, YM Kim
Scientific Reports, 2022-03-07;12(1):3681.
Species: Human
Sample Types: Cell Culture Supernates
-
The NLRP1 Inflammasome Induces Pyroptosis in Human Corneal Epithelial Cells
Authors: AR Griswold, HC Huang, DA Bachovchin
Investigative Ophthalmology & Visual Science, 2022-03-02;63(3):2.
Species: Human
Sample Types: Cell Culture Supernates
-
Liensinine alleviates high fat diet (HFD)-induced non-alcoholic fatty liver disease (NAFLD) through suppressing oxidative stress and inflammation via regulating TAK1/AMPK signaling
Authors: L Liang, S Ye, R Jiang, X Zhou, J Zhou, S Meng
International immunopharmacology, 2022-01-06;104(0):108306.
Species: Mouse
Sample Types: Serum
-
LncRNA NEAT1 is upregulated in recurrent aphthous stomatitis (RAS) and has predictive values
Authors: Y Han, L Wang, Q Li, H Chen, X Ma
BMC Oral Health, 2021-12-31;21(1):673.
Species: Human
Sample Types: Plasma
-
Salivary gland dysfunction and salivary redox imbalance in patients with Alzheimer's disease
Authors: A Zalewska, A Klimiuk, S Zi?ba, O Wnorowska, M Rusak, N Waszkiewic, I Szarmach, K Dzier?anow, M Maciejczyk
Scientific Reports, 2021-12-13;11(1):23904.
Species: Human
Sample Types: Saliva
-
1,25-Dihydroxyvitamin D3 attenuates IL-1beta secretion by suppressing NLRP1 inflammasome activation by upregulating the NRF2-HO-1 pathway in epidermal keratinocytes
Authors: T Nakajo, T Katayoshi, N Kitajima, K Tsuji-Nait
Redox Biology, 2021-12-01;48(0):102203.
Species: Human
Sample Types: Cell Culture Supernates
-
Design, synthesis and biological evaluation of 1,5-disubstituted alpha-amino tetrazole derivatives as non-covalent inflammasome-caspase-1 complex inhibitors with potential application against immune and inflammatory disorders
Authors: F Ulgheri, P Spanu, F Deligia, G Loriga, MP Fuggetta, I de Haan, A Chandgudge, M Groves, A Domling
European Journal of Medicinal Chemistry, 2021-11-18;0(0):114002.
Species: Human
Sample Types: Cell Culture Supernates
-
Apoptosis-associated speck-like protein containing a CARD regulates the growth of pancreatic ductal adenocarcinoma
Authors: M Koizumi, T Watanabe, J Masumoto, K Sunago, Y Imamura, K Kanemitsu, T Kumagi, Y Hiasa
Scientific Reports, 2021-11-16;11(1):22351.
Species: Human
Sample Types: Cell Culture Supernates
-
Regular physical activity reduces the effects of inflammaging in diabetic and hypertensive men
Authors: IS Ribeiro, ÍS Pereira, MPL Galantini, DP Santos, MF Teles, IPR Muniz, GS Santos, RAA Silva
Experimental gerontology, 2021-09-20;155(0):111558.
Species: Human
Sample Types: Plasma
-
Interruption of neutrophil extracellular traps formation dictates host defense and tubular HOXA5 stability to augment efficacy of anti-Fn14 therapy against septic AKI
Authors: Y Ni, BC Hu, GH Wu, ZQ Shao, Y Zheng, R Zhang, J Jin, J Hong, XH Yang, RH Sun, JQ Liu, SJ Mo
Theranostics, 2021-09-13;11(19):9431-9451.
Species: Mouse
Sample Types: Plasma
-
High glucose?induced upregulation of CD36 promotes inflammation stress via NF?kappaB in H9c2 cells
Authors: B Han, J Wang, J Wu, F Yan, Y Wang, J Li
Molecular Medicine Reports, 2021-09-07;24(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Normalizing HIF-1alpha Signaling Improves Cellular Glucose Metabolism and Blocks the Pathological Pathways of Hyperglycemic Damage
Authors: C Iacobini, M Vitale, G Pugliese, S Menini
Biomedicines, 2021-09-02;9(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Generation and functional characterization of recombinant Porphyromonas gingivalis W83 FimA
Authors: S Groeger, M Hudel, S Zechel, T Chakrabort, G Lochnit, J Meyle, E Domann
Journal of biotechnology, 2021-08-31;340(0):22-29.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum levels of SIRT3 and other inflammatory factors are associated with clinical outcomes and prognosis in severe community-acquired pneumonia in adults: A prospective study
Authors: W Zhu, P Chen, L Hu, L Deng
Medicine, 2021-08-13;100(32):e26721.
Species: Human
Sample Types: Serum
-
ELK4 promotes the development of gastric cancer by inducing M2 polarization of macrophages through regulation of the KDM5A-PJA2-KSR1 axis
Authors: L Zheng, H Xu, Y Di, L Chen, J Liu, L Kang, L Gao
Journal of Translational Medicine, 2021-08-09;19(1):342.
Species: Human
Sample Types: Cell Culture Supernates
-
DNA-Aptamer Raised against Receptor for Advanced Glycation End Products Improves Survival Rate in Septic Mice
Authors: Y Koga, A Sotokawauc, Y Higashimot, Y Nishino, N Hashizume, T Kakuma, J Akiba, Y Tanaka, T Matsui, M Yagi, SI Yamagishi
Oxidative Medicine and Cellular Longevity, 2021-08-07;2021(0):9932311.
Species: Human
Sample Types: Cell Culture Supernates
-
HIF-1alpha-Mediated miR-623 Regulates Apoptosis and Inflammatory Responses of Nucleus Pulposus Induced by Oxidative Stress via Targeting TXNIP
Authors: X Bao, Z Wang, Q Jia, S Shen, L Wu, Q Jiang, C Li, G Xu
Oxidative Medicine and Cellular Longevity, 2021-08-03;2021(0):6389568.
Species: Human
Sample Types: Cell Culture Supernates
-
Polymorphisms of Pro-Inflammatory IL-6 and IL-1&beta Cytokines in Ascending Aortic Aneurysms as Genetic Modifiers and Predictive and Prognostic Biomarkers
Authors: L Scola, RM Giarratana, V Marinello, V Cancila, C Pisano, G Ruvolo, G Frati, D Lio, CR Balistreri
Biomolecules, 2021-06-25;11(7):.
Species: Human
Sample Types: Plasma
-
Regular physical activity reduces the proinflammatory response in older women with diabetes and hypertension in the postmenopausal phase
Authors: DPS Lopes, IS Ribeiro, DC Santos, FMS Lima, AA Santos, DSP Souza, DN Lopes, AO Prado, ÍS Pereira, DP Santos, GS Santos, RAA Silva
Experimental gerontology, 2021-06-11;152(0):111449.
Species: Human
Sample Types: Plasma
-
Down-regulation of SNHG16 alleviates the acute lung injury in sepsis rats through miR-128-3p/HMGB3 axis
Authors: J Sun, K Xin, C Leng, J Ge
BMC pulmonary medicine, 2021-06-06;21(1):191.
Species: Rat
Sample Types: Tissue Homogenates
-
CD36 promotes NLRP3 inflammasome activation via the mtROS pathway in renal tubular epithelial cells of diabetic kidneys
Authors: Y Hou, Q Wang, B Han, Y Chen, X Qiao, L Wang
Cell Death & Disease, 2021-05-21;12(6):523.
Species: Human
Sample Types: Cell Culture Supernates
-
Salidroside protects endothelial cells against LPS-induced inflammatory injury by inhibiting NLRP3 and enhancing autophagy
Authors: L You, D Zhang, H Geng, F Sun, M Lei
BMC complementary medicine and therapies, 2021-05-19;21(1):146.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-1&beta Impaired Diabetic Wound Healing by Regulating MMP-2 and MMP-9 through the p38 Pathway
Authors: J Dai, J Shen, Y Chai, H Chen
Mediators of Inflammation, 2021-05-18;2021(0):6645766.
Species: Human
Sample Types: Serum
-
The Blockade of Tumoral IL1&beta-Mediated Signaling in Normal Colonic Fibroblasts Sensitizes Tumor Cells to Chemotherapy and Prevents Inflammatory CAF Activation
Authors: NG Díaz-Marot, G Garcia-Vic, G Polcaro, M Bañuls, N Albert, A Villanueva, DG Molleví
International Journal of Molecular Sciences, 2021-05-07;22(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunological response of fallopian tube epithelial cells to spermatozoa through modulating cytokines and chemokines
Authors: SO Mousavi, R Mohammadi, F Amjadi, Z Zandieh, S Aghajanpou, K Aflatoonia, M Sabbaghian, M Eslami, T Madani, R Aflatoonia
Journal of reproductive immunology, 2021-05-02;146(0):103327.
Species: Human
Sample Types: Cell Culture Supernates
-
Circ_0004104 knockdown alleviates oxidized low-density lipoprotein-induced dysfunction in vascular endothelial cells through targeting miR-328-3p/TRIM14 axis in atherosclerosis
Authors: C Zhang, L Wang, Y Shen
BMC cardiovascular disorders, 2021-04-23;21(1):207.
Species: Human
Sample Types: Cell Culture Supernates
-
Porphyromonas gingivalis lipopolysaccharide promotes T-hel per17 cell differentiation by upregulating Delta-like ligand 4 expression on CD14+ monocytes
Authors: C Zhang, C Xu, L Gao, X Li, C Zhao
PeerJ, 2021-04-23;9(0):e11094.
Species: Human
Sample Types: Cell Culture Supernates
-
Functional disability is related to serum chemerin levels in rheumatoid arthritis
Authors: ML Vazquez-Vi, JI Gamez-Nava, AM Saldaña-Cr, A Celis, EN Sanchez-Ro, EE Perez-Guer, M Ramirez-Vi, CA Nava-Valdi, B Contreras-, JC Vasquez-Ji, JM Ponce-Guar, AK Barocio-Ra, S Cerpa-Cruz, MF Alcaraz-Lo, L Gonzalez-L
Scientific Reports, 2021-04-16;11(1):8360.
Species: Human
Sample Types: Serum
-
Can Coagulation System Disorders and Cytokine and Inflammatory Marker Levels Predict the Temporary Clinical Deterioration or Improvement of Septic Patients on ICU Admission?
Authors: GA Lavranou, S Mentzelopo, P Katsaounou, I Siempos, I Kalomenidi, A Geranaki, C Routsi, S Zakynthino
Journal of Clinical Medicine, 2021-04-07;10(8):.
Species: Human
Sample Types: Serum
-
Analysis of IL-1&beta, CXCL8, and TNF-&alpha levels in the crevicular fluid of patients with periodontitis or healthy implants
Authors: P Aleksandro, E Brzezi?ska, E Koz?owska, P ?elechowsk, AE Borgonovo, J Agier
BMC Oral Health, 2021-03-16;21(1):120.
Species: Human
Sample Types: Gingival Crevicular Fluid
-
Repurposing of Pirfenidone (Anti-Pulmonary Fibrosis Drug) for Treatment of Rheumatoid Arthritis
Authors: D Gan, W Cheng, L Ke, AR Sun, Q Jia, J Chen, J Lin, J Li, Z Xu, P Zhang
Frontiers in Pharmacology, 2021-03-05;12(0):631891.
Species: Rat
Sample Types: Cell Culture Supernates
-
Circulating inflammatory markers may mediate the relationship between low carbohydrate diet and circadian rhythm in overweight and obese women
Authors: A Tavakoli, A Mirzababae, F Sajadi, K Mirzaei
Oncogene, 2021-03-01;21(1):87.
Species: Human
Sample Types: Serum
-
SARS-CoV-2 Nonstructural Proteins 1 and 13 Suppress Caspase-1 and the NLRP3 Inflammasome Activation
Authors: NE Kim, DK Kim, YJ Song
Microorganisms, 2021-02-26;9(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Progesterone and vitamin D downregulate the activation of the NLRP1/NLRP3 inflammasomes and TLR4-MyD88-NF-&kappaB pathway in monocytes from pregnant women with preeclampsia
Authors: ML Matias, M Romao-Veig, VR Ribeiro, PR Nunes, VJ Gomes, AC Devides, VT Borges, GG Romagnoli, JC Peracoli, MT Peracoli
Journal of reproductive immunology, 2021-02-05;144(0):103286.
Species: Human
Sample Types: Cell Culture Supernates
-
Intravenous allogeneic umbilical cord blood-derived mesenchymal stem cell therapy in recessive dystrophic epidermolysis bullosa patients
Authors: SE Lee, SJ Lee, SE Kim, K Kim, B Cho, K Roh, SC Kim
JCI Insight, 2021-01-25;6(2):.
Species: Human
Sample Types: Serum
-
Impact of Anesthetic Agents on Endothelial Glycocalyx Injury during Total Knee Arthroplasty: Desflurane- vs. Propofol-Based Anesthesia-A Prospective Randomized Controlled Trial
Authors: CS Oh, JM Choi, EH Park, L Piao, HJ Park, KY Rhee, SH Kim
BioMed Research International, 2021-01-23;2021(0):8880267.
Species: Human
Sample Types: Serum
-
The anti-tumor effects of cetuximab in combination with VTX-2337 are T cell dependent
Authors: Y Cheng, N Borcherdin, A Ogunsakin, CD Lemke-Milt, KN Gibson-Cor, A Rajan, AB Choi, W Wongpattar, CHF Chan, AK Salem, GJ Weiner, AL Simons
Scientific Reports, 2021-01-15;11(1):1535.
Species: Human
Sample Types: Cell Culture Supernates
-
Familial Mediterranean fever-related miR-197-3p targets IL1R1 gene and modulates inflammation in monocytes and synovial fibroblasts
Authors: YZ Akkaya-Ulu, TH Akbaba, Z Tavukcuogl, JJ Chae, E Yilmaz, S Ozen, B Balci-Peyn
Scientific Reports, 2021-01-12;11(1):685.
Species: Human
Sample Types: Cell Culture Supernates
-
Relationships between inflammatory markers and suicide risk status in major depression
Authors: L Ganança, HC Galfalvy, S Cisneros-T, Z Basseda, TB Cooper, X Ren, ML Figueira, MA Oquendo, JJ Mann, ME Sublette
Journal of psychiatric research, 2020-12-17;134(0):192-199.
Species: Human
Sample Types: Serum
-
Reduced intracellular antioxidant capacity in platelets contributes to primary immune thrombocytopenia via ROS-NLRP3-caspase-1 pathway
Authors: S Wang, Y Liu, G Li, Q Feng, M Hou, J Peng
Thrombosis Research, 2020-12-16;199(0):1-9.
Species: Human
Sample Types: Cell Culture Supernates
-
The Role of Soluble Uric Acid in Modulating Autophagy Flux and Inflammasome Activation during Bacterial Infection in Macrophages
Authors: D Al-Awad, N Al-Emadi, M Abu-Madi, AA Al-Thani, SM Zughaier
Biomedicines, 2020-12-12;8(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
LMWF5A suppresses cytokine release by modulating select inflammatory transcription factor activity in stimulated PBMC
Authors: G Thomas, E Frederick, L Thompson, R Bar-Or, Y Mulugeta, M Hausburg, M Roshon, C Mains, D Bar-Or
Journal of Translational Medicine, 2020-11-30;18(1):452.
Species: Human
Sample Types: Cell Cultue Supernates
-
Proteomics of extracellular vesicles produced by Granulicatella adiacens, which causes infective endocarditis
Authors: SA Alkandari, RG Bhardwaj, A Ellepola, M Karched
PLoS ONE, 2020-11-20;15(11):e0227657.
Species: Human
Sample Types: Cell Culture Supernates
-
The SGLT2 inhibitor Empagliflozin attenuates interleukin-17A-induced human aortic smooth muscle cell proliferation and migration by targeting TRAF3IP2/ROS/NLRP3/Caspase-1-dependent IL-1&beta and IL-18 secretion
Authors: S Sukhanov, Y Higashi, T Yoshida, S Mummidi, AR Aroor, J Jeffrey Ru, SB Bender, VG DeMarco, B Chandrasek
Cell Signal, 2020-11-04;77(0):109825.
Species: Human
Sample Types: Cell Culture Supernates
-
Pistacia lentiscus Hydrosol: Untargeted Metabolomic Analysis and Anti-Inflammatory Activity Mediated by NF-&kappaB and the Citrate Pathway
Authors: A Santarsier, A Onzo, R Pascale, MA Acquavia, M Coviello, P Convertini, S Todisco, M Marsico, C Pifano, P Iannece, C Gaeta, S D'Angelo, MC Padula, G Bianco, V Infantino, G Martelli
Oxid Med Cell Longev, 2020-11-01;2020(0):4264815.
Species: Human
Sample Types: Cell Culture Supernates
-
Knockdown of exosome?mediated lnc?PVT1 alleviates lipopolysaccharide?induced osteoarthritis progression by mediating the HMGB1/TLR4/NF?&kappaB pathway via miR?93?5p
Authors: Y Meng, S Qiu, L Sun, J Zuo
Molecular Medicine Reports, 2020-10-14;22(6):5313-5325.
Species: Human
Sample Types: Cell Culture Supernates
-
Expression of trophoblast derived prostaglandin E2 receptor 2 (EP2) is reduced in patients with recurrent miscarriage and EP2 regulates cell proliferation and expression of inflammatory cytokines
Authors: L Peng, Y Ye, H Mullikin, L Lin, C Kuhn, M Rahmeh, S Mahner, U Jeschke, V von Schönf
J Reprod Immunol, 2020-10-01;142(0):103210.
Species: Human
Sample Types: Cell Culture Supernates
-
Short-chain fatty acids bind to apoptosis-associated speck-like protein to activate inflammasome complex to prevent Salmonella infection
Authors: H Tsugawa, Y Kabe, A Kanai, Y Sugiura, S Hida, S Taniguchi, T Takahashi, H Matsui, Z Yasukawa, H Itou, K Takubo, H Suzuki, K Honda, H Handa, M Suematsu
PLoS Biol, 2020-09-29;18(9):e3000813.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Cognitive functional therapy (CFT) compared with core training exercise (CTE) in patients with failed back surgery syndrome (FBSS): A study protocol for a randomized controlled trial
Authors: L Avila, ML Neves, AR Abreu, CR Fiuza, L Fukusawa, N Meziat-Fil, AR Soares San
Journal of bodywork and movement therapies, 2020-09-14;26(0):428-434.
Species: Human
Sample Types: Serum
-
MicroRNA-4485 ameliorates severe influenza pneumonia via inhibition of the STAT3/PI3K/AKT signaling pathway
Authors: L Guo, Q Wang, D Zhang
Oncology Letters, 2020-09-09;20(5):215.
Species: Human
Sample Types: Serum
-
Flurbiprofen suppresses the inflammation, proliferation, invasion and migration of colorectal cancer cells via COX2
Authors: X Wang, X Ye, Y Zhang, F Ji
Oncology Letters, 2020-08-20;20(5):132.
Species: Human
Sample Types: Cell Culture Supernates
-
DPP8/9 inhibitors activate the CARD8 inflammasome in resting lymphocytes
Authors: DC Johnson, MC Okondo, EL Orth, SD Rao, HC Huang, DP Ball, DA Bachovchin
Cell Death Dis, 2020-08-14;11(8):628.
Species: Human
Sample Types: Cell Culture Supernates
-
Relaxin Can Mediate Its Anti-Fibrotic Effects by Targeting the Myofibroblast NLRP3 Inflammasome at the Level of Caspase-1
Authors: AA Pinar, A Yuferov, TA Gaspari, CS Samuel
Front Pharmacol, 2020-08-04;11(0):1201.
Species: Human
Sample Types: Cell Culture Supernates, Whole Cells
-
Neonatal hand, foot, and mouth disease due to coxsackievirus A6 in Shanghai
Authors: S Xu, H Li, P Qiao, G Xu, D Zhao, X Lin, Y Qin, H Yu, X Zhang, W Zhang, L Huang
BMC Pediatr, 2020-08-03;20(1):364.
Species: Human
Sample Types: Plasma, Serum
-
Acetylated K676 TGFBIp as a severity diagnostic blood biomarker for SARS-CoV-2 pneumonia
Authors: HH Park, HN Kim, H Kim, Y Yoo, H Shin, EY Choi, JS Bae, W Lee
Sci Adv, 2020-07-31;6(31):.
Species: Human
Sample Types: Plasma
-
IL-1beta suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury
Authors: S Xiong, Z Hong, LS Huang, Y Tsukasaki, S Nepal, A Di, M Zhong, W Wu, Z Ye, X Gao, GN Rao, D Mehta, J Rehman, AB Malik
J. Clin. Invest., 2020-07-01;130(7):3684-3698.
Species: Human
Sample Types: Cell Culture Supernates
-
Cytokine expression profiles in Autism spectrum disorder: A multi-center study from Turkey
Authors: MO Kutuk, E Tufan, C Gokcen, F Kilicaslan, M Karadag, T Mutluer, C Yektas, N Coban, H Kandemir, A Buber, S Coskun, U Acikbas, G Guler, Z Topal, F Celik, E Altintas, A Giray, Y Aka, O Kutuk
Cytokine, 2020-06-18;133(0):155152.
Species: Human
Sample Types: Serum
-
Pro-inflammatory markers and fatigue in patients with depression: A case-control study
Authors: B Pedraz-Pet, E Neumann, G Sammer
Sci Rep, 2020-06-11;10(1):9494.
Species: Human
Sample Types: Plasma
-
IL-33 and its decoy sST2 in patients with Alzheimer's disease and mild cognitive impairment
Authors: M Saresella, I Marventano, F Piancone, F La Rosa, D Galimberti, C Fenoglio, E Scarpini, M Clerici
J Neuroinflammation, 2020-06-06;17(1):174.
Species: Human
Sample Types: CSF
-
Pharmacological inhibition of P2RX7 ameliorates liver injury by reducing inflammation and fibrosis
Authors: B Baeza-Raja, A Goodyear, X Liu, K Lam, L Yamamoto, Y Li, GS Dodson, T Takeuchi, T Kisseleva, DA Brenner, K Dabbagh
PLoS ONE, 2020-06-03;15(6):e0234038.
Species: Human
Sample Types: Cell Culture Supernates
-
Javamide-II Inhibits IL-6 without Significant Impact on TNF-alpha and IL-1beta in Macrophage-Like Cells
Authors: JB Park, R Peters, Q Pham, TTY Wang
Biomedicines, 2020-05-29;8(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
Dysfunctional Nurr1 promotes high glucose-induced M�ller cell activation by up-regulating the NF-?B/NLRP3 inflammasome axis
Authors: W Li, X Liu, Y Tu, D Ding, Q Yi, X Sun, Y Wang, K Wang, M Zhu, J Mao
Neuropeptides, 2020-05-20;0(0):102057.
Species: Human
Sample Types: Cell Culture Supernates
-
LINC01116 promotes tumor proliferation and neutrophil recruitment via DDX5-mediated regulation of IL-1&beta in glioma cell
Authors: T Wang, L Cao, X Dong, F Wu, W De, L Huang, Q Wan
Cell Death Dis, 2020-05-01;11(5):302.
Species: Human
Sample Types: Cell Culture Supernates
-
Gaps in Study Design for Immune Parameter Research for Latent Tuberculosis Infection: A Systematic Review
Authors: M Herrera, C Vera, Y Keynan, ZV Rueda
J Immunol Res, 2020-04-21;2020(0):8074183.
Species: Human
Sample Types: Plasma
-
Interleukin-31 interaction with inflammasome: A promising diagnostic and prognostic panel for early sepsis identification in critically ill patients
Authors: MM Watany, MI Elmazny, EM Nasif
Cytokine, 2020-04-16;131(0):155102.
Species: Human
Sample Types: Serum
-
Tumor-neuroglia interaction promotes pancreatic cancer metastasis
Authors: D Su, X Guo, L Huang, H Ye, Z Li, L Lin, R Chen, Q Zhou
Theranostics, 2020-04-06;10(11):5029-5047.
Species: Human
Sample Types: Cell Culture Supernants
-
Metastasis-initiating cells induce and exploit a fibroblast niche to fuel malignant colonization of the lungs
Authors: M Pein, J Insua-Rodr, T Hongu, A Riedel, J Meier, L Wiedmann, K Decker, MAG Essers, HP Sinn, S Spaich, M Sütterlin, A Schneeweis, A Trumpp, T Oskarsson
Nat Commun, 2020-03-20;11(1):1494.
Species: Human
Sample Types: Tissue Lysate
-
Activity of fibroblast-like synoviocytes in rheumatoid arthritis was impaired by dickkopf-1 targeting siRNA
Authors: YY Liu, SY Wang, YN Li, WJ Bian, LQ Zhang, YH Li, L Long, X Liu, XW Zhang, ZG Li
Chin. Med. J., 2020-03-20;0(6):679-686.
Species: Human
Sample Types: Cell Culture Supernates
-
Network between Cytokines, Cortisol and Occupational Stress in Gas and Oilfield Workers
Authors: M Reale, E Costantini, C D'Angelo, L Coppeta, R Mangifesta, S Jagarlapoo, M Di Nicola, L Di Giampao
Int J Mol Sci, 2020-02-07;21(3):.
Species: Human
Sample Types: Plasma
-
Antagonizing binding of cell cycle and apoptosis regulatory protein 1 (CARP-1) to the NEMO/IKKgamma protein enhances the anticancer effect of chemotherapy
Authors: J Venkatesh, SC Sekhar, VT Cheriyan, M Muthu, P Meister, E Levi, S Dzinic, JW Gauld, LA Polin, AK Rishi
J. Biol. Chem., 2020-02-04;295(11):3532-3552.
Species: Human
Sample Types: Cell Culture Supernates
-
Glycated albumin stimulates expression of inflammatory cytokines in muscle cells
Authors: IV Kravchenko, VA Furalyov, VO Popov
Cytokine, 2020-01-27;128(0):154991.
Species: Human
Sample Types: Cell Culture Supernates
-
Evaluation of immunological, inflammatory, and oxidative stress biomarkers in gasoline station attendants
Authors: AM Moro, E Sauer, N Brucker, MF Charão, B Gauer, SN do Nascime, G Goethel, MMMF Duarte, SC Garcia
BMC Pharmacol Toxicol, 2019-12-19;20(0):75.
Species: Human
Sample Types: Serum
-
Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease
Authors: N Lalaoui, SE Boyden, H Oda, GM Wood, DL Stone, D Chau, L Liu, M Stoffels, T Kratina, KE Lawlor, KJM Zaal, PM Hoffmann, N Etemadi, K Shield-Art, C Biben, WL Tsai, MD Blake, HS Kuehn, D Yang, H Anderton, N Silke, L Wachsmuth, L Zheng, NS Moura, DB Beck, G Gutierrez-, AK Ombrello, GP Pinto-Pata, AJ Kueh, MJ Herold, C Hall, H Wang, JJ Chae, NI Dmitrieva, M McKenzie, A Light, BK Barham, A Jones, TM Romeo, Q Zhou, I Aksentijev, JC Mullikin, AJ Gross, AK Shum, ED Hawkins, SL Masters, MJ Lenardo, M Boehm, SD Rosenzweig, M Pasparakis, AK Voss, M Gadina, DL Kastner, J Silke
Nature, 2019-12-11;577(7788):103-108.
Species: Human
Sample Types: Serum
-
Inhibition of Triggering Receptor Expressed on Myeloid Cell-1 Alleviates Acute Gouty Inflammation
Authors: Y He, Q Yang, X Wang, A Jia, W Xie, J Zhou
Mediators Inflamm., 2019-12-06;2019(0):5647074.
Species: Human
Sample Types: Plasma
-
Visualization of epithelial-mesenchymal transition in an inflammatory microenvironment-colorectal cancer network
Authors: T Ieda, H Tazawa, H Okabayashi, S Yano, K Shigeyasu, S Kuroda, T Ohara, K Noma, H Kishimoto, M Nishizaki, S Kagawa, Y Shirakawa, T Saitou, T Imamura, T Fujiwara
Sci Rep, 2019-11-08;9(1):16378.
Species: Human
Sample Types: Cell Culture Supernates
-
Stimulation of Phagocytic Activity in Cultured Human Corneal Fibroblasts by Plasminogen
Authors: T Sato, K Sugioka, A Kodama-Tak, J Murakami, A Saito, H Mishima, T Nishida, S Kusaka
Invest. Ophthalmol. Vis. Sci., 2019-10-01;60(13):4205-4214.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum IL-1 β can be a biomarker in children with severe persistent allergic rhinitis
Authors: MW Han, SH Kim, I Oh, YH Kim, J Lee
Allergy Asthma Clin Immunol, 2019-09-18;15(0):58.
Species: Human
Sample Types: Serum
-
Epigenetic downregulation of STAT6 increases HIF-1α expression via mTOR/S6K/S6, leading to enhanced hypoxic viability of glioma cells
Authors: SJ Park, H Kim, SH Kim, EH Joe, I Jou
Acta Neuropathol Commun, 2019-09-17;7(1):149.
Species: Human
Sample Types: Cell Culture Supernates
-
Association between body composition and inflammation: A central role of IL-17 and IL-10 in diabetic and hypertensive elderly women
Authors: IS Ribeiro, ÍS Pereira, DP Santos, DS Lopes, AO Prado, SPM Calado, CV Gonçalves, MPL Galantini, IPR Muniz, GS Santos, RAA Silva
Exp. Gerontol., 2019-09-10;127(0):110734.
Species: Human
Sample Types: Plasma
-
Cancer-associated fibroblast stimulates cancer cell invasion in an interleukin-1 receptor (IL-1R)-dependent manner
Authors: X Zhang, YS Hwang
Oncol Lett, 2019-08-27;18(5):4645-4650.
Species: Human
Sample Types: Cell Culture Supernates
-
Multilevel defects in the hematopoietic niche in essential thrombocythemia
Authors: T Sun, M Ju, X Dai, H Dong, W Gu, Y Gao, R Fu, X Liu, Y Huang, W Liu, Y Chi, W Wang, H Li, Y Zhou, L Shi, R Yang, L Zhang
Haematologica, 2019-07-09;0(0):.
Species: Human
Sample Types: Bone Marrow Fluid
-
Immunoglobulin light chains generate proinflammatory and profibrotic kidney injury
Authors: WZ Ying, X Li, S Rangarajan, W Feng, LM Curtis, PW Sanders
J. Clin. Invest., 2019-06-17;129(7):2792-2806.
Species: Human
Sample Types: Cell Culture Supernates
-
cAMP metabolism controls caspase-11 inflammasome activation and pyroptosis in sepsis
Authors: R Chen, L Zeng, S Zhu, J Liu, HJ Zeh, G Kroemer, H Wang, TR Billiar, J Jiang, D Tang, R Kang
Sci Adv, 2019-05-22;5(5):eaav5562.
Species: Human
Sample Types: Cell Culture Supernates
-
Whole blood assay as a model for in vitro evaluation of inflammasome activation and subsequent caspase-mediated interleukin-1 beta release
Authors: TAT Tran, HW Grievink, K Lipinska, C Kluft, J Burggraaf, M Moerland, D Tasev, KE Malone
PLoS ONE, 2019-04-08;14(4):e0214999.
Species: Human
Sample Types: Cell Culture Supernates
-
Circulating Cell-Free mtDNA Contributes to AIM2 Inflammasome-Mediated Chronic Inflammation in Patients with Type 2 Diabetes
Authors: JH Bae, SI Jo, SJ Kim, JM Lee, JH Jeong, JS Kang, NJ Cho, SS Kim, EY Lee, JS Moon
Cells, 2019-04-08;8(4):.
Species: Human
Sample Types: Plasma
-
Genome-wide association study in Turkish and Iranian populations identify rare familial Mediterranean fever gene (MEFV) polymorphisms associated with ankylosing spondylitis
Authors: Z Li, S Akar, H Yarkan, SK Lee, P Çetin, G Can, G Kenar, F Çapa, ON Pamuk, Y Pehlivan, K Cremin, E De Guzman, J Harris, L Wheeler, A Jamshidi, M Vojdanian, E Farhadi, N Ahmadzadeh, Z Yüce, E Dalk?l?ç, D Solmaz, B Ak?n, S Dönmez, ? Sar?, PJ Leo, TJ Kenna, F Önen, M Mahmoudi, MA Brown, N Akkoc
PLoS Genet., 2019-04-04;15(4):e1008038.
Species: Human
Sample Types: Serum
-
Interplay between pro-inflammatory cytokines, childhood trauma, and executive function in depressed adolescents
Authors: AT Peters, X Ren, KL Bessette, BI Goldstein, AE West, SA Langenecke, GN Pandey
J Psychiatr Res, 2019-04-01;114(0):1-10.
Species: Human
Sample Types: Serum
-
Dental pulp cell-derived powerful inducer of TNF-? comprises PKR containing stress granule rich microvesicles
Authors: S Suzuki, T Fukuda, S Nagayasu, J Nakanishi, K Yoshida, S Hirata-Tsu, Y Nakao, T Sano, A Yamashita, S Yamada, K Ohta, H Shiba, F Nishimura
Sci Rep, 2019-03-07;9(1):3825.
Species: Human
Sample Types: Cell Culture Supernates
-
Sulodexide counteracts endothelial dysfunction induced by metabolic or non-metabolic stresses through activation of the autophagic program
Authors: F De Felice, F Megiorni, I Pietranton, P Tini, G Lessiani, D Mastroiaco, P Mattana, C Antinozzi, L Di Luigi, S Delle Mona, A Angelucci, C Festuccia, A Fanzani, R Maggio, V Tombolini, GL Gravina, F Marampon
Eur Rev Med Pharmacol Sci, 2019-03-01;23(6):2669-2680.
Species: Human
Sample Types: Cell Culture Supernates
-
Stimulated hepatic stellate cell promotes progression of hepatocellular carcinoma due to protein kinase R activation
Authors: Y Imai, O Yoshida, T Watanabe, A Yukimoto, Y Koizumi, Y Ikeda, Y Tokumoto, M Hirooka, M Abe, Y Hiasa
PLoS ONE, 2019-02-22;14(2):e0212589.
Species: Human
Sample Types: Cell Lysates
-
The Pros1/Tyro3 axis protects against periodontitis by modulating STAT/SOCS signalling
Authors: L Jiang, XQ Chen, MJ Gao, W Lee, J Zhou, YF Zhao, GD Wang
J. Cell. Mol. Med., 2019-02-07;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of high temperature on pandemic and seasonal human influenza viral replication and infection-induced damage in primary human tracheal epithelial cell cultures
Authors: M Yamaya, H Nishimura, N Lusamba Ka, X Deng, H Momma, Y Shimotai, R Nagatomi
Heliyon, 2019-02-05;5(2):e01149.
Species: Human
Sample Types: Cell Culture Supernates
-
Fluid restriction reduces pulmonary edema in a model of acute lung injury in mechanically ventilated rats
Authors: SA Ingelse, J Juschten, MAW Maas, G Matute-Bel, NP Juffermans, JBM van Woense, RA Bem
PLoS ONE, 2019-01-17;14(1):e0210172.
Species: Human
Sample Types: Tissue Homogenates
-
Downregulated Caveolin-1 expression in circulating monocytes may contribute to the pathogenesis of psoriasis
Authors: N Takamura, Y Yamaguchi, Y Watanabe, M Asami, N Komitsu, M Aihara
Sci Rep, 2019-01-15;9(1):125.
Species: Human
Sample Types: Cell Culture Supernates
-
TRAF3-interacting JNK-activating modulator promotes inflammation by stimulating� translocation of Toll-like receptor 4 to lipid rafts
Authors: Y Li, J Guan, W Wang, C Hou, L Zhou, J Ma, Y Cheng, S Jiao, Z Zhou
J. Biol. Chem., 2018-12-20;0(0):.
Species: Human
Sample Types: Serum
-
Particulate matter induces inflammatory cytokine production via activation of NF?B by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin
Authors: YS Ryu, KA Kang, MJ Piao, MJ Ahn, JM Yi, YM Hyun, SH Kim, MK Ko, CO Park, JW Hyun
Redox Biol, 2018-12-15;21(0):101080.
Species: Human
Sample Types: Cell Culture Supernates
-
Theaflavins prevent cartilage degeneration via AKT/FOXO3 signaling in�vitro
Authors: J Li, J Zheng
Mol Med Rep, 2018-12-12;19(2):821-830.
Species: Human
Sample Types: Cell Culture Supernates
-
Comparative Analysis of Peptide Composition and Bioactivity of Different Collagen Hydrolysate Batches on Human Osteoarthritic Synoviocytes
Authors: VS Simons, G Lochnit, J Wilhelm, B Ishaque, M Rickert, J Steinmeyer
Sci Rep, 2018-12-07;8(1):17733.
Species: Human
Sample Types: Cell Culture Supernates
-
Downregulation of signal transduction and STAT3 expression exacerbates oxidative stress mediated by NLRP3 inflammasome
Authors: H Bai, QF Zhang, JJ Duan, DJ Yu, LJ Liu
Neural Regen Res, 2018-12-01;13(12):2147-2155.
Species: Human
Sample Types: Cell Culture Supernates
-
Tryptophan Photoproduct FICZ Upregulates IL1A, IL1B, and IL6 Expression via Oxidative Stress in Keratinocytes
Authors: Y Tanaka, H Uchi, A Hashimoto-, M Furue
Oxid Med Cell Longev, 2018-11-25;2018(0):9298052.
Species: Human
Sample Types: Cell Culture Supernates
-
Bioengineering Human Neurological Constructs Using Decellularized Meningeal Scaffolds for Application in Spinal Cord Injury.
Authors: Sandeep Kumar Vishwakarm, Avinash Bardia, Chandrakala Lakkiredd, Syed Ameer Basha Paspala, Aleem Ahmed Khan
Frontiers in Bioengineering and Biotechnology, 2018-11-01;0(0):2296-4185.
Species: Rat
Sample Types: Serum
-
Dysfunctional endothelial-derived microparticles promote inflammatory macrophage formation via NF-?B and IL-1? signal pathways
Authors: Y Wang, J Liu, X Chen, H Sun, S Peng, Y Kuang, J Pi, T Zhuang, L Zhang, Z Yu, B Tomlinson, P Chan, Y Chen, Y Zhang, Y Li
J. Cell. Mol. Med., 2018-10-18;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Aggression, impulsivity and inflammatory markers as risk factors for suicidal behavior
Authors: W Coryell, H Wilcox, SJ Evans, GN Pandey, L Jones-Bran, F Dickerson, R Yolken
J Psychiatr Res, 2018-09-11;106(0):38-42.
Species: Human
Sample Types: Plasma
-
Second generation atypical antipsychotics olanzapine and aripiprazole reduce expression and secretion of inflammatory cytokines in human immune cells
Authors: B Stapel, I Sieve, CS Falk, S Bleich, D Hilfiker-K, KG Kahl
J Psychiatr Res, 2018-09-04;105(0):95-102.
Species: Human
Sample Types: Cell Culture Supernates
-
NT-proBNP and CA 125 levels are associated with increased pro-inflammatory cytokines in coronary sinus serum of patients with chronic heart failure
Authors: AE Stanciu, MM Stanciu, RG Vatasescu
Cytokine, 2018-08-08;111(0):13-19.
Species: Human
Sample Types: Serum
-
Nanoparticle decoration impacts airborne fungal pathobiology
Authors: D Westmeier, D Solouk-Sar, C Vallet, S Siemer, D Docter, H Götz, L Männ, A Hasenberg, A Hahlbrock, K Erler, C Reinhardt, O Schilling, S Becker, M Gunzer, M Hasenberg, SK Knauer, RH Stauber
Proc. Natl. Acad. Sci. U.S.A., 2018-06-20;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of loxoprofen on the apical root resorption during orthodontic tooth movement in rats
Authors: T Yamamoto, M Kaku, H Sumi, Y Yashima, J Izumino, K Tanimoto
PLoS ONE, 2018-04-25;13(4):e0194453.
Species: Human
Sample Types: Cell Culture Supernates
-
?-Nicotinamide Adenine Dinucleotide (?-NAD) Inhibits ATP-Dependent IL-1? Release from Human Monocytic Cells
Authors: SD Hiller, S Heldmann, K Richter, I Jurastow, M Küllmar, A Hecker, S Wilker, G Fuchs-Moll, I Manzini, G Schmalzing, W Kummer, W Padberg, JM McIntosh, J Damm, A Zakrzewicz, V Grau
Int J Mol Sci, 2018-04-10;19(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
HMGB1/IL-1? complexes in plasma microvesicles modulate immune responses to burn injury
Authors: LG Coleman, R Maile, SW Jones, BA Cairns, FT Crews
PLoS ONE, 2018-03-30;13(3):e0195335.
Species: Human
Sample Types: Serum
-
Anti-inflammatory cytokine and angiogenic factors levels in vitreous samples of diabetic retinopathy patients
Authors: T Tsai, S Kuehn, N Tsiampalis, MK Vu, V Kakkassery, G Stute, HB Dick, SC Joachim
PLoS ONE, 2018-03-27;13(3):e0194603.
Species: Human
Sample Types: Vitreous Humor
-
Wheat amylase/trypsin inhibitors exacerbate intestinal and airway allergic immune responses in humanized mice
Authors: I Bellinghau, B Weigmann, V Zevallos, J Maxeiner, S Rei beta ig, A Waisman, D Schuppan, J Saloga
J. Allergy Clin. Immunol., 2018-03-21;0(0):.
Species: Human
Sample Types: Serum
Applications: ELISA Capture -
Adipose-derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1-macrophage population through prostaglandin E2
Authors: HJ Park, J Kim, FT Saima, KJ Rhee, S Hwang, MY Kim, SK Baik, YW Eom, HS Kim
Biochem. Biophys. Res. Commun., 2018-03-16;0(0):.
Species: Human
Sample Types: THP-1 cells
-
Specific Interactions Measured by AFM on Living Cells between Peroxiredoxin-5 and TLR4: Relevance for Mechanisms of Innate Immunity
Authors: B Knoops, S Becker, MA Poncin, J Glibert, S Derclaye, A Clippe, D Alsteens
Cell Chem Biol, 2018-03-15;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Does the Use of a "Walking Bleaching" Technique Increase Bone Resorption Markers?
Authors: C Bersezio, P Vildósola, M Sáez, F Sánchez, R Vernal, OB Oliveira, G Jorquera, J Basualdo, A Loguercio, E Fernández
Oper Dent, 2018-03-13;0(0):.
Species: Human
Sample Types: Gingival Crevicular Fluid
-
ABCG2 contributes to the development of gout and hyperuricemia in a genome-wide association study
Authors: CJ Chen, CC Tseng, JH Yen, JG Chang, WC Chou, HW Chu, SJ Chang, WT Liao
Sci Rep, 2018-02-16;8(1):3137.
Species: Human
Sample Types: Cell Culture Supernates
-
Changes in the Anti-Allergic Activities of Sesame by Bioconversion
Authors: TD Jung, SI Choi, SH Choi, BY Cho, WS Sim, SJ Lee, SJ Park, DB Kim, YC Kim, JH Lee, OH Lee
Nutrients, 2018-02-14;10(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Celecoxib-mediated reduction of prostanoid release in Hoffa's fat pad from donors with cartilage pathology results in an attenuated inflammatory phenotype
Authors: UT Timur, MMJ Caron, YM Bastiaanse, TJM Welting, LW van Rhijn, GJVM van Osch, PJ Emans
Osteoarthr. Cartil., 2018-02-07;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Capture -
Elucidating the Role of CD84 and AHR in Modulation of LPS-Induced Cytokines Production by Cruciferous Vegetable-Derived Compounds Indole-3-Carbinol and 3,3'-Diindolylmethane
Authors: TTY Wang, Q Pham, YS Kim
Int J Mol Sci, 2018-01-24;19(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity
Authors: RJW Arts, SJCFM Moorlag, B Novakovic, Y Li, SY Wang, M Oosting, V Kumar, RJ Xavier, C Wijmenga, LAB Joosten, CBEM Reusken, CS Benn, P Aaby, MP Koopmans, HG Stunnenber, R van Crevel, MG Netea
Cell Host Microbe, 2018-01-10;23(1):89-100.e5.
Species: Human
Sample Types: Cell Culture Supernates
-
Reduced-HMGB1 suppresses poly(I:C)-induced inflammation in keratinocytes
Authors: H Mori, M Murakami, T Tsuda, K Kameda, R Utsunomiya, K Masuda, K Shiraishi, X Dai, M Tohyama, H Nakaoka, K Sayama
J. Dermatol. Sci., 2018;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Capture -
Extracellular signal regulated kinase 5 and inflammasome in progression of mesothelioma
Authors: JK Thompson, A Shukla, AL Leggett, PB Munson, JM Miller, MB MacPherson, SL Beuschel, HI Pass, A Shukla
Oncotarget, 2017-12-06;9(1):293-305.
Species: Human
Sample Types: Cell Culture Supernates
-
Immune and Imaging Correlates of Mild Cognitive Impairment Conversion to Alzheimer's Disease
Authors: F La Rosa, M Saresella, F Baglio, F Piancone, I Marventano, E Calabrese, R Nemni, E Ripamonti, M Cabinio, M Clerici
Sci Rep, 2017-12-01;7(1):16760.
Species: Human
Sample Types: Cell Culture Supernates
-
A comparison of blood and cerebrospinal fluid cytokines (IL-1?, IL-6, IL-18, TNF-?) in neonates with perinatal hypoxia
Authors: D Šumanovi?-, F ?ulo, MI ?ulo, P Konjevoda, M Jerkovi?-R
Bosn J Basic Med Sci, 2017-08-20;0(0):.
Species: Human
Sample Types: Serum
-
Elastin?derived peptides are involved in the processes of human temporomandibular disorder by inducing inflammatory responses in synovial cells
Authors: K Kobayashi, R Jokaji, M Miyazawa-H, S Takatsuka, A Tanaka, K Ooi, H Nakamura, S Kawashiri
Mol Med Rep, 2017-07-15;16(3):3147-3154.
Species: Human
Sample Types: Synovial Fluid
-
P2X7 receptor antagonism modulates IL-1? and MMP9 in human atherosclerotic vessels
Authors: M Lombardi, ME Mantione, D Baccellier, D Ferrara, R Castellano, R Chiesa, O Alfieri, C Foglieni
Sci Rep, 2017-07-07;7(1):4872.
Species: Human
Sample Types: Tissue Culture Supernates
-
Canonical and Novel Non-Canonical Cholinergic Agonists Inhibit ATP-Induced Release of Monocytic Interleukin-1? via Different Combinations of Nicotinic Acetylcholine Receptor Subunits ?7, ?9 and ?10
Authors: A Zakrzewicz, K Richter, A Agné, S Wilker, K Siebers, B Fink, G Krasteva-C, M Althaus, W Padberg, AJ Hone, JM McIntosh, V Grau
Front Cell Neurosci, 2017-07-05;11(0):189.
Species: Human
Sample Types: Cell Culture Supernates
-
Stress fibers, autophagy and necrosis by persistent exposure to PM2.5 from biomass combustion
Authors: R Dornhof, C Maschowski, A Osipova, R Gieré, M Seidl, I Merfort, M Humar
PLoS ONE, 2017-07-03;12(7):e0180291.
Species: Human
Sample Types: Cell Culture Supernates
-
The increased concentration of macrophage migration inhibitory factor in serum and cerebrospinal fluid of patients with tick-borne encephalitis
Authors: S Grygorczuk, M Parczewski, R ?wierzbi?s, P Czupryna, A Moniuszko, J Dunaj, M Kondrusik, S Pancewicz
J Neuroinflammation, 2017-06-24;14(1):126.
Species: Human
Sample Types: Serum
-
Type II Endometrial Cancer Overexpresses NILCO: A Preliminary Evaluation
Authors: D Daley-Brow, G Oprea-Iles, KT Vann, V Lanier, R Lee, PV Candelaria, A Quarshie, R Pattillo, RR Gonzalez-P
Dis. Markers, 2017-06-04;2017(0):8248175.
Species: Human
Sample Types: Tissue Homogenates
-
Abnormal gene and protein expression of inflammatory cytokines in the postmortem brain of schizophrenia patients
Authors: GN Pandey, HS Rizavi, H Zhang, X Ren
Schizophr. Res., 2017-05-02;0(0):.
Species: Human
Sample Types: Tissue Homogenates
-
Age-related increase in Wnt inhibitor causes a senescence-like phenotype in human cardiac stem cells
Authors: T Nakamura, T Hosoyama, J Murakami, M Samura, K Ueno, H Kurazumi, R Suzuki, A Mikamo, K Hamano
Biochem. Biophys. Res. Commun., 2017-04-21;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
The long non-coding RNA TP73-AS1 modulates HCC cell proliferation through miR-200a-dependent HMGB1/RAGE regulation
Authors: S Li, Y Huang, Y Huang, Y Fu, D Tang, R Kang, R Zhou, XG Fan
J. Exp. Clin. Cancer Res., 2017-04-12;36(1):51.
Species: Human
Sample Types: Cell Culture Supernates
-
Surfactant protein A is expressed in the central nervous system of rats with experimental autoimmune encephalomyelitis, and suppresses inflammation in human astrocytes and microglia
Authors: X Yang, J Yan, J Feng
Mol Med Rep, 2017-04-07;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Epstein-Barr virus infection-induced inflammasome activation in human monocytes
Authors: Y Torii, JI Kawada, T Murata, H Yoshiyama, H Kimura, Y Ito
PLoS ONE, 2017-04-03;12(4):e0175053.
Species: Human
Sample Types: Cell Culture Supernates
-
Fucoidan modulates cytokine production and migration of THP-1-derived macrophages via colony-stimulating factor-1
Authors: Peng Li, Huayang Wang, Qianqian Shao, Beihua Kong, Xun Qu
Molecular Medicine Reports
Species: Human
Sample Types: Cell Culture Supernates
-
Impaired Surface Expression of HLA-DR, TLR2, TLR4, and TLR9 in Ex Vivo-In Vitro Stimulated Monocytes from Severely Injured Trauma Patients
Authors: D Heftrig, R Sturm, E Oppermann, K Kontradowi, K Jurida, L Schimunek, M Woschek, I Marzi, B Relja
Mediators Inflamm, 2017-02-01;2017(0):2608349.
Species: Human
Sample Types: Complex Sample Type
-
High serum resistin associates with intrahepatic inflammation and necrosis: an index of disease severity for patients with chronic HBV infection
Authors: Z Meng, Y Zhang, Z Wei, P Liu, J Kang, Y Zhang, D Ma, C Ke, Y Chen, J Luo, Z Gong
BMC Gastroenterol, 2017-01-07;17(1):6.
Species: Human
Sample Types: Serum
-
The NLRP3 inflammasome and bruton's tyrosine kinase in platelets co-regulate platelet activation, aggregation, and in�vitro thrombus formation
Authors: Pranav Murthy
Biochem. Biophys. Res. Commun, 2016-12-26;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of Gingival Crevicular Fluid Sampling, Analytical Methods, and Oral Biomarkers for the Diagnosis and Monitoring of Periodontal Diseases: A Systematic Review
Authors: Z Nazar Maje, K Philip, AM Alabsi, S Pushparaja, D Swaminatha
Dis. Markers, 2016-12-15;2016(0):1804727.
Species: Human
Sample Types: Saliva
-
Glucagon-like peptide 1 improves insulin resistance in vitro through anti-inflammation of macrophages
Authors: X Gu
Braz. J. Med. Biol. Res., 2016-11-21;49(12):e5826.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Pre-treatment serum inflammatory cytokines as survival predictors of patients with nasopharyngeal carcinoma receiving chemoradiotherapy
Authors: AF Al-Kholy, OA Abdullah, MZ Abadier, MM Hassaan, MF Shindy, DM Nor El-Die, A Hasaneen
Mol Clin Oncol, 2016-09-30;5(6):811-816.
Species: Human
Sample Types: Serum
-
Clarithromycin Decreases IL-6 Concentration in Serum and BAL Fluid in Patients with Cryptogenic Organizing Pneumonia
Authors: El?bieta Radzikowsk
Adv Clin Exp Med, 2016-09-01;25(5):871-878.
Species: Human
Sample Types: Serum
-
Cell surface-anchored syndecan-1 ameliorates intestinal inflammation and neutrophil transmigration in ulcerative colitis
J Cell Mol Med, 2016-08-25;0(0):.
Species: Human
Sample Types: Serum
-
Association between mannose-binding lectin variants, haplotypes and risk of hepatocellular carcinoma: A case-control study
Sci Rep, 2016-08-25;6(0):32147.
Species: Human
Sample Types: Plasma
-
Innovative Flow Cytometry Allows Accurate Identification of Rare Circulating Cells Involved in Endothelial Dysfunction
PLoS ONE, 2016-08-25;11(8):e0160153.
Species: Human
Sample Types: Plasma
-
NLRP3 Inflammasome Activation in THP-1 Target Cells Triggered by Pathogenic Naegleria fowleri
Authors: JH Kim, HJ Sohn, JK Yoo, H Kang, GS Seong, YJ Chwae, K Kim, S Park, HJ Shin
Infect. Immun, 2016-08-19;84(9):2422-8.
Species: Human
Sample Types: Cell Culture Supernates
-
A point mutation in AgrC determines cytotoxic or colonizing properties associated with phenotypic variants of ST22 MRSA strains
Authors: S Mairpady S, N Siemens, IR Monk, DB Mohan, S Mukundan, KC Krishnan, S Prabhakara, J Snäll, A Kearns, F Vandenesch, M Svensson, M Kotb, B Gopal, G Arakere, A Norrby-Teg
Sci Rep, 2016-08-11;6(0):31360.
Species: Human
Sample Types: Tissue Culture Supernates
-
Activation and IL-1? secretion of human peripheral phagocytes infected with Actinomadura madurae, Nocardia asteroides and Candida albicans
Asian Pac J Trop Med, 2016-08-10;9(10):962-967.
Species: Human
Sample Types: Cell Culture Supernates
-
Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease
Sci Rep, 2016-07-15;6(0):29783.
Species: Human
Sample Types: Cell Culture Supernates
-
Nuclear DNA damage-triggered NLRP3 inflammasome activation promotes UVB-induced inflammatory responses in human keratinocytes
Authors: Tatsuya Hasegawa
Biochem Biophys Res Commun, 2016-06-23;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Circulating Fibrocytes Are Increased in Neonates with Bronchopulmonary Dysplasia
Authors: Chun Li
PLoS ONE, 2016-06-16;11(6):e0157181.
Species: Human
Sample Types: Plasma
-
Novel high throughput pooled shRNA screening identifies NQO1 as a potential drug target for host directed therapy for tuberculosis
Authors: Qing Li
Sci Rep, 2016-06-14;6(0):27566.
Species: Human
Sample Types: Cell Culture Supernates
-
Simple Plex(�) : A Novel Multi-Analyte, Automated Microfluidic Immunoassay Platform for the Detection of Human and Mouse Cytokines and Chemokines
Authors: Gil Mor
Am. J. Reprod. Immunol., 2016-05-11;75(6):678-93.
Species: Human
Sample Types: Plasma, Serum
-
Identification of tumour-reactive lymphatic endothelial cells capable of inducing progression of gastric cancer.
Authors: Tokumoto M, Tanaka H, Tauchi Y, Kasashima H, Kurata K, Yashiro M, Sakurai K, Toyokawa T, Kubo N, Amano R, Kimura K, Muguruma K, Maeda K, Ohira M, Hirakawa K
Br J Cancer, 2015-09-10;113(7):1046-54.
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophil elastase promotes interleukin-1beta secretion from human coronary endothelium.
Authors: Alfaidi M, Wilson H, Daigneault M, Burnett A, Ridger V, Chamberlain J, Francis S
J Biol Chem, 2015-08-12;290(40):24067-78.
Species: Human
Sample Types: Cell Culture Supernates
-
Phosphocholine-Modified Macromolecules and Canonical Nicotinic Agonists Inhibit ATP-Induced IL-1beta Release.
Authors: Hecker A, Kullmar M, Wilker S, Richter K, Zakrzewicz A, Atanasova S, Mathes V, Timm T, Lerner S, Klein J, Kaufmann A, Bauer S, Padberg W, Kummer W, Janciauskiene S, Fronius M, Schweda E, Lochnit G, Grau V
J Immunol, 2015-07-22;195(5):2325-34.
Species: Human
Sample Types: Cell Culture Supernates
-
An Allergic Lung Microenvironment Suppresses Carbon Nanotube-Induced Inflammasome Activation via STAT6-Dependent Inhibition of Caspase-1.
Authors: Shipkowski K, Taylor A, Thompson E, Glista-Baker E, Sayers B, Messenger Z, Bauer R, Jaspers I, Bonner J
PLoS ONE, 2015-06-19;10(6):e0128888.
Species: Human
Sample Types: Cell Culture Supernates
-
Prostaglandin E2 Inhibits NLRP3 Inflammasome Activation through EP4 Receptor and Intracellular Cyclic AMP in Human Macrophages.
Authors: Sokolowska M, Chen L, Liu Y, Martinez-Anton A, Qi H, Logun C, Alsaaty S, Park Y, Kastner D, Chae J, Shelhamer J
J Immunol, 2015-04-27;194(11):5472-87.
Species: Human
Sample Types: Cell Culture Supernates
-
Cross talk between neuroregulatory molecule and monocyte: nerve growth factor activates the inflammasome.
Authors: Datta-Mitra A, Kundu-Raychaudhuri S, Mitra A, Raychaudhuri S
PLoS ONE, 2015-04-15;10(4):e0121626.
Species: Human
Sample Types: Cell Culture Supernates
-
MyD88-Dependent Signaling Decreases the Antitumor Efficacy of Epidermal Growth Factor Receptor Inhibition in Head and Neck Cancer Cells.
Authors: Koch A, Love-Homan L, Espinosa-Cotton M, Stanam A, Simons A
Cancer Res, 2015-02-20;75(8):1657-67.
Species: Human
Sample Types: Cell Culture Supernates
-
Aggregatibacter actinomycetemcomitans cytolethal distending toxin activates the NLRP3 inflammasome in human macrophages, leading to the release of proinflammatory cytokines.
Authors: Shenker B, Ojcius D, Walker L, Zekavat A, Scuron M, Boesze-Battaglia K
Infect Immun, 2015-02-02;83(4):1487-96.
Species: Human
Sample Types: Cell Culture Supernates
-
A novel carboline derivative inhibits nitric oxide formation in macrophages independent of effects on tumor necrosis factor alpha and interleukin-1beta expression.
Authors: Grodzki A, Poola B, Pasupuleti N, Nantz M, Lein P, Gorin F
J Pharmacol Exp Ther, 2014-12-23;352(3):438-47.
Species: Human
Sample Types: Cell Culture Supernates
-
The impact of concomitant pulmonary infection on immune dysregulation in Pneumocystis jirovecii pneumonia.
Authors: Chou C, Lin F, Tsai H, Chang S
BMC Pulm Med, 2014-11-19;14(0):182.
Species: Human
Sample Types: BALF
-
Preventive effect of Daiokanzoto (TJ-84) on 5-fluorouracil-induced human gingival cell death through the inhibition of reactive oxygen species production.
Authors: Yoshida K, Yoshioka M, Okamura H, Moriyama S, Kawazoe K, Grenier D, Hinode D
PLoS ONE, 2014-11-12;9(11):e112689.
Species: Human
Sample Types: Cell Culture Supernates
-
Small molecules dorsomorphin and LDN-193189 inhibit myostatin/GDF8 signaling and promote functional myoblast differentiation.
Authors: Horbelt D, Boergermann J, Chaikuad A, Alfano I, Williams E, Lukonin I, Timmel T, Bullock A, Knaus P
J Biol Chem, 2014-11-03;290(6):3390-404.
Species: Human
Sample Types: Cell Culture Supernates
-
beta-glucan induces reactive oxygen species production in human neutrophils to improve the killing of Candida albicans and Candida glabrata isolates from vulvovaginal candidiasis.
Authors: Bonfim-Mendonca P, Ratti B, Godoy J, Negri M, Lima N, Fiorini A, Hatanaka E, Consolaro M, de Oliveira Silva S, Svidzinski T
PLoS ONE, 2014-09-17;9(9):e107805.
Species: Human
Sample Types: Cell Culture Supernates
-
Looking beyond endotoxin: a comparative study of pyrogen retention by ultrafilters used for the preparation of sterile dialyis fluid.
Authors: Glorieux, Griet, Hulko, Michael, Speidel, Rose, Brodbeck, Katrin, Krause, Bernd, Vanholder, Raymond
Sci Rep, 2014-09-17;4(0):6390.
Species: Human
Sample Types: Cell Culture Supernates
-
Early mucosal sensing of SIV infection by paneth cells induces IL-1beta production and initiates gut epithelial disruption.
Authors: Hirao L, Grishina I, Bourry O, Hu W, Somrit M, Sankaran-Walters S, Gaulke C, Fenton A, Li J, Crawford R, Chuang F, Tarara R, Marco M, Baumler A, Cheng H, Dandekar S
PLoS Pathog, 2014-08-28;10(8):e1004311.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Tissue Homogenates
-
Adipocyte hypertrophy, inflammation and fibrosis characterize subcutaneous adipose tissue of healthy, non-obese subjects predisposed to type 2 diabetes.
Authors: Henninger A, Eliasson B, Jenndahl L, Hammarstedt A
PLoS ONE, 2014-08-22;9(8):e105262.
Species: Human
Sample Types: Serum
-
Early efficacy trial of anakinra in corticosteroid-resistant autoimmune inner ear disease.
Authors: Vambutas A, Lesser M, Mullooly V, Pathak S, Zahtz G, Rosen L, Goldofsky E
J Clin Invest, 2014-08-18;124(9):4115-22.
Species: Human
Sample Types: Plasma
-
CREB-induced inflammation is important for malignant mesothelioma growth.
Authors: Westbom C, Shukla A, MacPherson M, Yasewicz E, Miller J, Beuschel S, Steele C, Pass H, Vacek P, Shukla A
Am J Pathol, 2014-08-08;184(10):2816-27.
Species: Human
Sample Types: Cell Culture Supernates
-
Improving membrane based multiplex immunoassays for semi-quantitative detection of multiple cytokines in a single sample.
Authors: Altara, Raffaele, Manca, Marco, Hessel, Marleen, Janssen, Ben J, Struijker-Boudier, Harry H, Hermans, Rob J J, Blankesteijn, W Matthi
BMC Biotechnol, 2014-07-15;14(0):63.
Species: Human
Sample Types: Serum
-
Expression and functional characterization of NOD2 in decidual stromal cells isolated during the first trimester of pregnancy.
Authors: Zhang Y, Chen H, Sun C, Wang H, Liu M, Li Y, Nie X, Du M, Li D, Zhang J
PLoS ONE, 2014-06-16;9(6):e99612.
Species: Human
Sample Types: Cell Culture Supernates
-
Differences in PGE2 production between primary human monocytes and differentiated macrophages: role of IL-1beta and TRIF/IRF3.
Authors: Endo Y, Blinova K, Romantseva T, Golding H, Zaitseva M
PLoS ONE, 2014-05-28;9(5):e98517.
Species: Human
Sample Types: Cell Culture Supernates
-
Oxidative and pro-inflammatory impact of regular and denicotinized cigarettes on blood brain barrier endothelial cells: is smoking reduced or nicotine-free products really safe?
Authors: Naik, Pooja, Fofaria, Neel, Prasad, Shikha, Sajja, Ravi K, Weksler, Babette, Couraud, Pierre-O, Romero, Ignacio, Cucullo, Luca
BMC Neurosci, 2014-04-23;15(0):51.
Species: Human
Sample Types: Cell Culture Supernates
-
Chlorogenic acid inhibits human platelet activation and thrombus formation.
Authors: Fuentes E, Caballero J, Alarcon M, Rojas A, Palomo I
PLoS ONE, 2014-03-05;9(3):e90699.
Species: Human
Sample Types: Cell Culture Supernates
-
Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke with and without metabolic syndrome.
Authors: Tuttolomondo A, Pecoraro R, Di Raimondo D, Di Sciacca R, Canino B, Arnao V, Butta C, Della Corte V, Maida C, Licata G, Pinto A
Diabetol Metab Syndr, 2014-02-27;6(1):28.
Species: Human
Sample Types: Plasma
-
Effect of renal impairment on cognitive function during a 3-year follow up in elderly patients with type 2 diabetes: Association with microinflammation.
Authors: Kawamura T, Umemura T, Umegaki H, Imamine R, Kawano N, Tanaka C, Kawai M, Minatoguchi M, Kusama M, Kouchi Y, Watarai A, Kanai A, Nakashima E, Hotta N
J Diabetes Investig, 2014-02-04;5(5):597-605.
Species: Human
Sample Types: Plasma
-
Absence of a role for interleukin-13 in inflammatory bowel disease.
Authors: Biancheri P, Di Sabatino A, Ammoscato F, Facciotti F, Caprioli F, Curciarello R, Hoque S, Ghanbari A, Joe-Njoku I, Giuffrida P, Rovedatti L, Geginat J, Corazza G, MacDonald T
Eur J Immunol, 2014-02-01;44(2):370-85.
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of FHL1 as a therapeutic target for Duchenne muscular dystrophy.
Authors: D'Arcy, Colleen, Feeney, Sandra J, McLean, Catriona, Gehrig, Stefan M, Lynch, Gordon S, Smith, Jaclyn E, Cowling, Belinda, Mitchell, Christin, McGrath, Meagan J
Hum Mol Genet, 2013-09-18;23(3):618-36.
Species: Human
Sample Types: Whole Cells
-
Unsaturated fatty acids prevent activation of NLRP3 inflammasome in human monocytes/macrophages.
Authors: L'homme L, Esser N, Riva L, Scheen A, Paquot N, Piette J, Legrand-Poels S
J Lipid Res, 2013-09-03;54(11):2998-3008.
Species: Human
Sample Types: Cell Culture Supernates
-
Multiple sclerosis lymphocytes upregulate A2A adenosine receptors that are antiinflammatory when stimulated.
Authors: Vincenzi F, Corciulo C, Targa M, Merighi S, Gessi S, Casetta I, Gentile M, Granieri E, Borea P, Varani K
Eur J Immunol, 2013-06-03;43(8):2206-16.
Species: Human
Sample Types: Cell Culture Supernates
-
An autoinflammatory neurological disease due to interleukin 6 hypersecretion.
Authors: Salsano, Ettore, Rizzo, Ambra, Bedini, Gloria, Bernard, Loris, Dall'olio, Valentin, Volorio, Sara, Lazzaroni, Monica, Ceccherini, Isabella, Lazarevic, Dejan, Cittaro, Davide, Stupka, Elia, Paterra, Rosina, Farina, Laura, Savoiardo, Mario, Pareyson, Davide, Sciacca, Francesc
J Neuroinflammation, 2013-02-21;10(0):29.
Species: Human
Sample Types: CSF
-
Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis.
Authors: Ho I, Toh H, Ng W, Teo Y, Guo C, Hui K, Lam P
Stem Cells, 2013-01-01;31(1):146-55.
Species: Human
Sample Types: Tissue Homogenates
-
CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages.
Authors: Wu M, Chen S, Yang A, Lin W, Lin Y, Chen N, Tsai I, Li L, Hsieh S
Blood, 2012-11-14;121(1):95-106.
Species: Human
Sample Types: Cell Culture Supernates
-
The anti-tumorigenic mushroom Agaricus blazei Murill enhances IL-1beta production and activates the NLRP3 inflammasome in human macrophages.
Authors: Huang T, Ojcius D, Young J, Wu Y, Ko Y, Wong T, Wu C, Lu C, Lai H
PLoS ONE, 2012-07-23;7(7):e41383.
Species: Human
Sample Types: Cell Culture Supernates
-
A novel small molecule, HK-156, inhibits lipopolysaccharide-induced activation of NF-kappaB signaling and improves survival in mouse models of sepsis.
Acta Pharmacol. Sin., 2012-06-11;33(9):1204-16.
Species: Human
Sample Types: Cell Culture Supernates
-
Validation and quality control of ELISAs for the use with human saliva samples.
Authors: Jaedicke KM, Taylor JJ, Preshaw PM
J. Immunol. Methods, 2012-01-28;377(1):62-5.
Species: Human
Sample Types: Saliva
-
High levels of beta-catenin signaling reduce osteogenic differentiation of stem cells in inflammatory microenvironments through inhibition of the noncanonical Wnt pathway.
Authors: Liu N, Shi S, Deng M, Tang L, Zhang G, Liu N, Ding B, Liu W, Liu Y, Shi H, Liu L, Jin Y
J. Bone Miner. Res., 2011-09-01;26(9):2082-95.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of IL-1alpha and the Nlrp3/caspase-1/IL-1beta axis in cigarette smoke-induced pulmonary inflammation and COPD.
Authors: Pauwels NS, Bracke KR, Dupont LL, Van Pottelberge GR, Provoost S, Vanden Berghe T, Vandenabeele P, Lambrecht BN, Joos GF, Brusselle GG
Eur. Respir. J., 2011-05-26;38(5):1019-28.
Species: Human
Sample Types: Sputum
-
IL-1beta is overexpressed and aberrantly regulated in corticosteroid nonresponders with autoimmune inner ear disease.
Authors: Pathak S, Goldofsky E, Vivas EX, Bonagura VR, Vambutas A
J. Immunol., 2011-01-03;186(3):1870-9.
Species: Human
Sample Types: Plasma
-
Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma.
Authors: Bachert C, Zhang N, Holtappels G, De Lobel L, Van Cauwenberge P, Liu S, Lin P, Bousquet J, Van Steen K
J. Allergy Clin. Immunol., 2010-11-01;126(5):962.
Species: Human
Sample Types: Tissue Homogenates
-
The detrimental effects of IFN-alpha on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction.
Authors: Thacker SG, Berthier CC, Mattinzoli D, Rastaldi MP, Kretzler M, Kaplan MJ
J. Immunol., 2010-08-30;185(7):4457-69.
Species: Human
Sample Types: Serum
-
RAS mutations affect tumor necrosis factor-induced apoptosis in colon carcinoma cells via ERK-modulatory negative and positive feedback circuits along with non-ERK pathway effects.
Authors: Kreeger PK, Mandhana R, Alford SK, Haigis KM, Lauffenburger DA
Cancer Res., 2009-09-29;69(20):8191-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Validity of multiplex-based assays for cytokine measurements in serum and plasma from "non-diseased" subjects: comparison with ELISA.
Authors: Dossus L, Becker S, Achaintre D, Kaaks R, Rinaldi S
J. Immunol. Methods, 2009-09-10;350(1):125-32.
Species: Human
Sample Types: Serum
-
Bacillus anthracis capsule activates caspase-1 and induces interleukin-1beta release from differentiated THP-1 and human monocyte-derived dendritic cells.
Authors: Cho MH, Ahn HJ, Ha HJ, Park J, Chun JH, Kim BS, Oh HB, Rhie GE
Infect. Immun., 2009-09-08;78(1):387-92.
Species: Human
Sample Types: Cell Culture Supernates
-
Characteristics of dengue virus-infected peripheral blood mononuclear cell death that correlates with the severity of illness.
Authors: Jaiyen Y, Masrinoul P, Kalayanarooj S, Pulmanausahakul R, Ubol S
Microbiol. Immunol., 2009-08-01;53(8):442-50.
Species: Human
Sample Types: Plasma
-
Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome.
Authors: Duncan JA, Gao X, Huang MT, O'Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP
J. Immunol., 2009-05-15;182(10):6460-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Immuno-inflammatory predictors of stroke at follow-up in patients with chronic non-valvular atrial fibrillation (NVAF).
Authors: Pinto A, Tuttolomondo A, Casuccio A, Di Raimondo D, Di Sciacca R, Arnao V, Licata G
Clin. Sci., 2009-05-01;116(10):781-9.
Species: Human
Sample Types: Plasma
-
Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling.
Authors: Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, McKenzie BS, Kastelein RA, Cua DJ, de Waal Malefyt R
J. Exp. Med., 2009-03-09;206(3):535-48.
Species: Human
Sample Types: Cell Culture Supernates
-
Toll-like receptor-mediated production of IL-1Ra is negatively regulated by GSK3 via the MAPK ERK1/2.
Authors: Rehani K, Wang H, Garcia CA, Kinane DF, Martin M
J. Immunol., 2009-01-01;182(1):547-53.
Species: Human
Sample Types: Cell Culture Supernates
-
Escherichia coli type 1 pili trigger late IL-8 production by neutrophil-like differentiated PLB-985 cells through a Src family kinase- and MAPK-dependent mechanism.
Authors: Semiramoth N, Gleizes A, Turbica I, Sandre C, Gorges R, Kansau I, Servin A, Chollet-Martin S
J. Leukoc. Biol., 2008-11-17;85(2):310-21.
Species: Human
Sample Types: Cell Culture Supernates
-
Direct and indirect impairment of human dendritic cell function by virulent Francisella tularensis Schu S4.
Authors: Chase JC, Celli J, Bosio CM
Infect. Immun., 2008-11-03;77(1):180-95.
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of interleukin-7 as a candidate disease mediator in spondylarthritis.
Authors: Rihl M, Kellner H, Kellner W, Barthel C, Yu DT, Tak PP, Zeidler H, Baeten D
Arthritis Rheum., 2008-11-01;58(11):3430-5.
Species: Human
Sample Types: Synovial Fluid
-
Immuno-inflammatory and thrombotic/fibrinolytic variables associated with acute ischemic stroke diagnosis.
Authors: Tuttolomondo A, Pinto A, Corrao S, Di Raimondo D, Fernandez P, Di Sciacca R, Arnao V, Licata G
Atherosclerosis, 2008-07-09;203(2):503-8.
Species: Human
Sample Types: Plasma
-
The role of nerve growth factor in hyperosmolar stress induced apoptosis.
Authors: Chang EJ, Im YS, Kay EP
J. Cell. Physiol., 2008-07-01;216(1):69-77.
Species: Human
Sample Types: Cell Culture Supernates
-
Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury.
Authors: Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, Shalvoy R, Jay GD
Arthritis Rheum., 2008-06-01;58(6):1707-15.
Species: Human
Sample Types: Synovial Fluid
-
Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study.
Authors: Fichorova RN, Richardson-Harman N, Alfano M, Belec L, Carbonneil C, Chen S, Cosentino L, Curtis K, Dezzutti CS, Donoval B, Doncel GF, Donaghay M, Grivel JC, Guzman E, Hayes M, Herold B, Hillier S, Lackman-Smith C, Landay A, Margolis L, Mayer KH, Pasicznyk JM, Pallansch-Cokonis M, Poli G, Reichelderfer P, Roberts P, Rodriguez I, Saidi H, Sassi RR, Shattock R, Cummins JE
Anal. Chem., 2008-05-17;80(12):4741-51.
Species: Human
Sample Types: Reference Standard
-
In vitro induction of a dendritic cell phenotype in primary human acute myelogenous leukemia (AML) blasts alters the chemokine release profile and increases the levels of T cell chemotactic CCL17 and CCL22.
Authors: Olsnes AM, Ryningen A, Ersvaer E, Bruserud Ø
J. Interferon Cytokine Res., 2008-05-01;28(5):297-310.
Species: Human
Sample Types: Cell Culture Supernates
-
TREM-1 expression in tumor-associated macrophages and clinical outcome in lung cancer.
Authors: Ho CC, Liao WY, Wang CY, Lu YH, Huang HY, Chen HY, Chan WK, Chen HW, Yang PC
Am. J. Respir. Crit. Care Med., 2007-12-20;177(7):763-70.
Species: Human
Sample Types: Whole Cells
-
Regulation of hepatocyte growth factor secretion by fibroblasts in patients with acute lung injury.
Authors: Quesnel C, Marchand-Adam S, Fabre A, Marchal-Somme J, Philip I, Lasocki S, Lecon V, Crestani B, Dehoux M
Am. J. Physiol. Lung Cell Mol. Physiol., 2007-12-07;294(2):L334-43.
Species: Human
Sample Types: BALF
-
IL-1alpha and IL-1beta are endogenous mediators linking cell injury to the adaptive alloimmune response.
Authors: Rao DA, Tracey KJ, Pober JS
J. Immunol., 2007-11-15;179(10):6536-46.
Species: Human
Sample Types: Cell Culture Supernates
-
The levels of steroid hormones and cytokines in individual follicles are not associated with the fertilization outcome after intracytoplasmic sperm injection.
Authors: Asimakopoulos B, Abu-Hassan D, Metzen E, Al-Hasani S, Diedrich K, Nikolettos N
Fertil. Steril., 2007-11-05;90(1):60-4.
Species: Human
Sample Types: Follicular Fluid
-
Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling.
Authors: Liotta F, Angeli R, Cosmi L, Fili L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, Santarlasci V, Consoloni L, Angelotti ML, Romagnani P, Parronchi P, Krampera M, Maggi E, Romagnani S, Annunziato F
Stem Cells, 2007-10-25;26(1):279-89.
Species: Human
Sample Types: Cell Culture Supernates
-
Expression and gene polymorphisms of the chemokine CXCL5 in colorectal cancer patients.
Authors: Dimberg J, Dienus O, Lofgren S, Hugander A, Wagsater D
Int. J. Oncol., 2007-07-01;31(1):97-102.
Species: Human
Sample Types:
-
C-reactive protein induces endothelial cell apoptosis and matrix metalloproteinase-9 production in human mononuclear cells: Implications for the destabilization of atherosclerotic plaque.
Authors: Nabata A, Kuroki M, Ueba H, Hashimoto S, Umemoto T, Wada H, Yasu T, Saito M, Momomura S, Kawakami M
Atherosclerosis, 2007-05-24;196(1):129-35.
Species: Human
Sample Types: Cell Culture Supernates
-
Advanced glycation end products increases matrix metalloproteinase-1, -3, and -13, and TNF-alpha in human osteoarthritic chondrocytes.
Authors: Nah SS, Choi IY, Yoo B, Kim YG, Moon HB, Lee CK
FEBS Lett., 2007-04-09;581(9):1928-32.
Species: Human
Sample Types: Cell Culture Supernates
-
Modulation of cytokine secretion by pentacyclic triterpenes from olive pomace oil in human mononuclear cells.
Authors: Marquez-Martin A, De La Puerta R, Fernandez-Arche A, Ruiz-Gutierrez V, Yaqoob P
Cytokine, 2007-02-09;36(5):211-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Microvascular endothelial cells increase proliferation and inhibit apoptosis of native human acute myelogenous leukemia blasts.
Authors: Hatfield K, Ryningen A, Corbascio M, Bruserud O
Int. J. Cancer, 2006-11-15;119(10):2313-21.
Species: Human
Sample Types: Cell Culture Supernates
-
Humoral and cellular factors responsible for coronary collateral formation.
Authors: Sherman JA, Hall A, Malenka DJ, De Muinck ED, Simons M
Am. J. Cardiol., 2006-08-31;98(9):1194-7.
Species: Human
Sample Types: Plasma
-
Dental plaque, gingival inflammation, and elevated levels of interleukin-6 and cortisol in gingival crevicular fluid from women with stress-related depression and exhaustion.
Authors: Johannsen A, Rylander G, Soder B, Asberg M
J. Periodontol., 2006-08-01;77(8):1403-9.
Species: Human
Sample Types:
-
The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production.
Authors: Chae JJ, Wood G, Masters SL, Richard K, Park G, Smith BJ, Kastner DL
Proc. Natl. Acad. Sci. U.S.A., 2006-06-19;103(26):9982-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Kinin receptor expression during Staphylococcus aureus infection.
Authors: Bengtson SH, Phagoo SB, Norrby-Teglund A, Pahlman L, Morgelin M, Zuraw BL, Leeb-Lundberg LM, Herwald H
Blood, 2006-05-30;108(6):2055-63.
Species: Human
Sample Types: Cell Culture Supernates
-
Statin-induced proinflammatory response in mitogen-activated peripheral blood mononuclear cells through the activation of caspase-1 and IL-18 secretion in monocytes.
Authors: Coward WR, Marei A, Yang A, Vasa-Nicotera MM, Chow SC
J. Immunol., 2006-05-01;176(9):5284-92.
Species: Human
Sample Types: Cell Culture Supernates
-
The apoptotic-cell receptor CR3, but not alphavbeta5, is a regulator of human dendritic-cell immunostimulatory function.
Authors: Skoberne M, Somersan S, Almodovar W, Truong T, Petrova K, Henson PM, Bhardwaj N
Blood, 2006-04-13;108(3):947-55.
Species: Human
Sample Types: Cell Culture Supernates
-
The potential of adiponectin in driving arthritis.
Authors: Ehling A, Schaffler A, Herfarth H, Tarner IH, Anders S, Distler O, Paul G, Distler J, Gay S, Scholmerich J, Neumann E, Muller-Ladner U
J. Immunol., 2006-04-01;176(7):4468-78.
Species: Human
Sample Types: Cell Culture Supernates
-
Celecoxib up-regulates the expression of the zeta chain of T cell receptor complex in tumor-infiltrating lymphocytes in human cervical cancer.
Authors: Ferrandina G, Ranelletti FO, Legge F, Salutari V, Martinelli E, Fattorossi A, Lorusso D, Zannoni G, Vellone V, Paglia A, Scambia G
Clin. Cancer Res., 2006-04-01;12(7):2055-60.
Species: Human
Sample Types: Plasma
-
The response of human epithelial cells to TNF involves an inducible autocrine cascade.
Authors: Janes KA, Gaudet S, Albeck JG, Nielsen UB, Lauffenburger DA, Sorger PK
Cell, 2006-03-24;124(6):1225-39.
Species: Human
Sample Types: Cell Culture Supernates
-
Glycerol monolaurate inhibits the effects of Gram-positive select agents on eukaryotic cells.
Authors: Peterson ML, Schlievert PM
Biochemistry, 2006-02-21;45(7):2387-97.
Species: Human
Sample Types: Cell Culture Supernates
-
C-reactive protein and vascular cell adhesion molecule-1 as markers of severity in sickle cell disease.
Authors: Makis AC, Hatzimichael EC, Stebbing J, Bourantas KL
Arch. Intern. Med., 2006-02-13;166(3):366-8.
Species: Human
Sample Types: Plasma
-
Non-esterified fatty acid concentrations are independently associated with hepatic steatosis in obese subjects.
Authors: Holt HB, Wild SH, Wood PJ, Zhang J, Darekar AA, Dewbury K, Poole RB, Holt RI, Phillips DI, Byrne CD
Diabetologia, 2005-12-02;49(1):141-8.
Species: Human
Sample Types: Serum
-
Secretory IgA induces antigen-independent eosinophil survival and cytokine production without inducing effector functions.
Authors: Bartemes KR, Cooper KM, Drain KL, Kita H
J. Allergy Clin. Immunol., 2005-10-01;116(4):827-35.
Species: Human
Sample Types: Cell Culture Supernates
-
Keratinocyte-fibroblast paracrine interaction: the effects of substrate and culture condition.
Authors: Witte RP, Kao WJ
Biomaterials, 2005-06-01;26(17):3673-82.
Species: Human
Sample Types: Whole Cells
-
Relationship between cytokines and the embryotoxicity of hydrosalpingeal fluid.
Authors: Bedaiwy MA, Falcone T, Goldberg JM, Attaran M, Sharma R, Miller K, Nelson DR, Agarwal A
J. Assist. Reprod. Genet., 2005-04-01;22(4):161-5.
Species: Human
Sample Types: Hypersalpingeal Fluid (HSF)
-
Circulating and synovial levels of IGF-I, cytokines, physical function and anthropometry differ in women awaiting total knee arthroplasty when compared to men.
Authors: Pagura SM, Thomas SG, Woodhouse LJ, Ezzat S, Marks P
J. Orthop. Res., 2005-03-01;23(2):397-405.
Species: Human
Sample Types: Synovial Fluid
-
Analysis of tumor necrosis factor-alpha, interleukin-6, interleukin-1beta, soluble tumor necrosis factor receptors I and II, interleukin-6 soluble receptor, interleukin-1 soluble receptor type II, interleukin-1 receptor antagonist, and protein in the synovial fluid of patients with temporomandibular joint disorders.
Authors: Kaneyama K, Segami N, Sun W, Sato J, Fujimura K
Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2005-03-01;99(3):276-84.
Species: Human
Sample Types: Synovial Fluid
-
Aseptic peritonitis due to peptidoglycan contamination of pharmacopoeia standard dialysis solution.
Authors: Martis L, Patel M, Giertych J, Mongoven J, Taminne M, Perrier MA, Mendoza O, Goud N, Costigan A, Denjoy N, Verger C, Owen WF
Lancet, 2005-02-01;365(9459):588-94.
Species: Human
Sample Types: Complex Sample Type
-
Mucosal wound healing in a rabbit model of subglottic stenosis: biochemical analysis of secretions.
Authors: Branski RC, Sandulache VC, Dohar JE, Hebda PA
Arch. Otolaryngol. Head Neck Surg., 2005-02-01;131(2):153-7.
Species: Rabbit
Sample Types: Tissue Secretion
-
Interleukin-1beta tear concentration in glaucomatous and ocular hypertensive patients treated with preservative-free nonselective beta-blockers.
Authors: Manni G, Centofanti M, Oddone F, Parravano M, Bucci MG
Am. J. Ophthalmol., 2005-01-01;139(1):72-7.
Species: Human
Sample Types: Tears
-
Guiding the vaginal microbicide trials with biomarkers of inflammation.
Authors: Fichorova RN
J. Acquir. Immune Defic. Syndr., 2004-10-01;37(0):S184-93.
Species: Human
Sample Types: Vaginal Lavage Fluid
-
The investigation of glutathione peroxidase, lactoferrin, myeloperoxidase and interleukin-1beta in gingival crevicular fluid: implications for oxidative stress in human periodontal diseases.
Authors: Wei PF, Ho KY, Ho YP, Wu YM, Yang YH, Tsai CC
J. Periodont. Res., 2004-10-01;39(5):287-93.
Species: Human
Sample Types:
-
Changes in CSF S100B and cytokine concentrations in early-phase severe traumatic brain injury.
Authors: Hayakata T, Shiozaki T, Tasaki O, Ikegawa H, Inoue Y, Toshiyuki F, Hosotubo H, Kieko F, Yamashita T, Tanaka H, Shimazu T, Sugimoto H
Shock, 2004-08-01;22(2):102-7.
Species: Human
Sample Types: Serum
-
Effects of scaling and root planing on the amounts of interleukin-1 and interleukin-1 receptor antagonist and the mRNA expression of interleukin-1beta in gingival crevicular fluid and gingival tissues.
Authors: Yoshinari N, Kawase H, Mitani A, Ito M, Sugiishi S, Matsuoka M, Shirozu N, Ishihara Y, Bito B, Hiraga M, Arakawa K, Noguchi T
J. Periodont. Res., 2004-06-01;39(3):158-67.
Species: Human
Sample Types:
-
Inflammatory cytokine concentrations are elevated in seminal plasma of men with spinal cord injuries.
Authors: Basu S, Aballa TC, Ferrell SM, Lynne CM, Brackett NL
J. Androl., 2004-03-01;25(2):250-4.
Species: Human
Sample Types: Seminal Plasma
-
Tissue adhesiveness and host response of in situ photopolymerizable interpenetrating networks containing methylprednisolone acetate.
Authors: Zilinski JL, Kao WJ
J Biomed Mater Res A, 2004-02-01;68(2):392-400.
Species: Human
Sample Types: Tissue Perfusion Fluid
-
Determinants of plasma leptin concentration in postmenopausal women.
Authors: Brooks-Asplund EM, Tupper CE, Daun JM, Kenney WL, Cannon JG
Mech. Ageing Dev., 2004-02-01;125(2):117-9.
Species: Human
Sample Types: Whole Cells
-
Markers of wound healing in vocal fold secretions from patients with laryngeal pathology.
Authors: Branski RC, Rosen CA, Verdolini K, Hebda PA
Ann. Otol. Rhinol. Laryngol., 2004-01-01;113(1):23-9.
Species: Human
Sample Types: Tissue Secretion
-
Shifts in biochemical markers associated with wound healing in laryngeal secretions following phonotrauma: a preliminary study.
Authors: Verdolini K, Rosen CA, Branski RC, Hebda PA
Ann. Otol. Rhinol. Laryngol., 2003-12-01;112(12):1021-5.
Species: Human
Sample Types: Tissue Secretion
-
T lymphocyte-mediated changes in airway smooth muscle responsiveness are attributed to induced autocrine release and actions of IL-5 and IL-1beta.
Authors: Hakonarson H, Whelan R, Leiter J, Kim C, Chen M, Campbell D, Grunstein MM
J. Allergy Clin. Immunol., 2002-10-01;110(4):624-33.
Species: Human
Sample Types: Cell Culture Supernates
-
Intraperitoneal bispecific antibody (HEA125xOKT3) therapy inhibits malignant ascites production in advanced ovarian carcinoma.
Authors: Marme A, Strauss G, Bastert G, Grischke EM, Moldenhauer G
Int. J. Cancer, 2002-09-10;101(2):183-9.
Species: Human
Sample Types: Ascites Fluid
-
Cytotoxicity and interleukin-1beta processing following Shigella flexneri infection of human monocyte-derived dendritic cells.
Authors: Edgeworth JD, Spencer J, Phalipon A, Griffin GE, Sansonetti PJ
Eur. J. Immunol., 2002-05-01;32(5):1464-71.
Species: Human
Sample Types: Cell Culture Supernates
-
Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies.
Authors: Jotwani R, 175, Palucka AK, Al-Quotub M, Nouri-Shirazi M, Kim J, Bell D, Banchereau J, Cutler CW
2001-10-15;167(8):4693-700.
Species: Human
Sample Types:
-
Interleukin-6 is a major effector molecule of short-term G-CSF treatment inducing bone metabolism and an acute-phase response.
Authors: Carstanjen D, Regenfus M, Muller C, Salama A
Exp. Hematol., 2001-07-01;29(7):812-21.
Species: Human
Sample Types: Plasma
-
Interleukin-11 induces Th2 polarization of human CD4(+) T cells.
Authors: Curti A, Ratta M, Corinti S, Girolomoni G, Ricci F, Tazzari P, Siena M, Grande A, Fogli M, Tura S, Lemoli RM
Blood, 2001-05-01;97(9):2758-63.
Species: Human
Sample Types: Cell Culture Supernates
-
Natural interferon alpha/beta-producing cells link innate and adaptive immunity.
Authors: Kadowaki N, Antonenko S, Lau JY, Liu YJ
J. Exp. Med., 2000-07-17;192(2):219-26.
Species: Human
Sample Types: Cell Culture Supernates
-
Proinflammatory cytokines increase in sepsis after anti-adhesion molecule therapy.
Authors: Welty-Wolf KE, 2016, Carraway MS, Ghio A, Kantrow SP, Huang YC, Piantadosi CA
1-23, 2000-05-01;13(5):404-9.
Species: Primate - Papio cynocephalus (Yellow Baboon)
Sample Types: Serum
-
Luminal neutrophil migration in ileoanal pouches studied by whole gut lavage.
Authors: Evgenikos NL, Bartolo DC, Hamer-Hodges DW, Ghosh S
Eur J Gastroenterol Hepatol, 2000-05-01;12(5):553-7.
Species: Human
Sample Types: Gut Lavage Fluid
-
Functional genomic analysis in arthritis-affected cartilage: yin-yang regulation of inflammatory mediators by alpha 5 beta 1 and alpha V beta 3 integrins.
Authors: Attur MG, Dave MN, Clancy RM, Patel IR, Abramson SB, Amin AR
J. Immunol., 2000-03-01;164(5):2684-91.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of bone resorbing activity in gingival crevicular fluid from patients with periodontitis.
Authors: Rasmussen L, Hanstrom L, Lerner UH
J. Clin. Periodontol., 2000-01-01;27(1):41-52.
Species: Human
Sample Types:
-
Gingival crevicular fluid from patients with periodontitis contains bone resorbing activity.
Authors: Lerner UH, Modeer T, Krekmanova L, Claesson R, Rasmussen L
Eur. J. Oral Sci., 1998-06-01;106(3):778-87.
Species: Human
Sample Types:
-
Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF.
Authors: Fadok, V A, Bratton, D L, Konowal, A, Freed, P W, Westcott, J Y, Henson, P M
J Clin Invest, 1998-02-15;101(4):890-8.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
-
Does the Human IL-1 beta/IL-1F2 Quantikine® ELISA kit detect pro or mature IL-1 beta?
The kit has been designed to measure mature human IL-1 beta. Recombinant human Pro-IL-1 beta does not interfere with the assay but displays 6.1% cross-reactivity.
Reviews for Human IL-1 beta/IL-1F2 Quantikine ELISA Kit
Average Rating: 4.8 (Based on 13 Reviews)
Have you used Human IL-1 beta/IL-1F2 Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Matrix effect detected for the lowest standard in urine diluted 2-fold. Was able to detect low concentrations in urine at 2-fold dilution.
Among best ELISAs I've worked with. Great consistency lot to lot, high sensitivity, easy to work with small amount of samples (I've diluted samples 1:100 in the past). Appropriate for concentration response curves. The only thing that can be improved is expanding the dynamic range of the assay (highest standard is at 250pg/ml and in our case we sometimes have 20,000pg/ml so need to make significant dilution to fit within the standard curve).
The Human IL-1beta Quantikine ELISA kit is used for diagnostic use.
Freshly isolated PBMCs from buffy coats stimulated with LPS (1ug/mL) for 3h together with Simvastatin. Total amount of 3 million cells/well. Volume supernatant used: 100uL. Two donors. Great production of IL1B in supernatant. Increased when statins are present as expected. Experiment used as a positive control