Human/Mouse SOD1/Cu-Zn SOD Antibody Summary
Met1-Gln154
Accession # P00441
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human/Mouse SOD1/Cu‑Zn SOD by Western Blot. Western blot shows lysates of HepG2 human hepatocellular carcinoma cell line and NIH-3T3 mouse embryonic fibroblast cell line. PVDF membrane was probed with 0.2 µg/mL Goat Anti-Human/Mouse SOD1/Cu-Zn SOD Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3418) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). For additional reference, recombinant human SOD1, SOD2, and SOD3 (1 ng/lane) were included. A specific band for SOD1/Cu-Zn SOD was detected at approximately 16-19 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 2.
 View Larger
View Larger
Detection of Human SOD1/Cu‑Zn SOD by Simple WesternTM. Simple Western lane view shows lysates of HepG2 human hepatocellular carcinoma cell line, loaded at 0.2 mg/mL. A specific band was detected for SOD1/Cu-Zn SOD at approximately 25 kDa (as indicated) using 10 µg/mL of Goat Anti-Human/Mouse SOD1/Cu-Zn SOD Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3418) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
Detection of Human SOD1/Cu‑Zn SOD by Simple WesternTM. Simple Western lane view shows lysates of A549 human lung carcinoma cell line and SK-BR-3 human breast cancer cell line, loaded at 0.2 mg/mL. A specific band was detected for SOD1/Cu-Zn SOD at approximately 24 kDa (as indicated) using 10 µg/mL of Goat Anti-Human/Mouse SOD1/Cu-Zn SOD Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3418) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
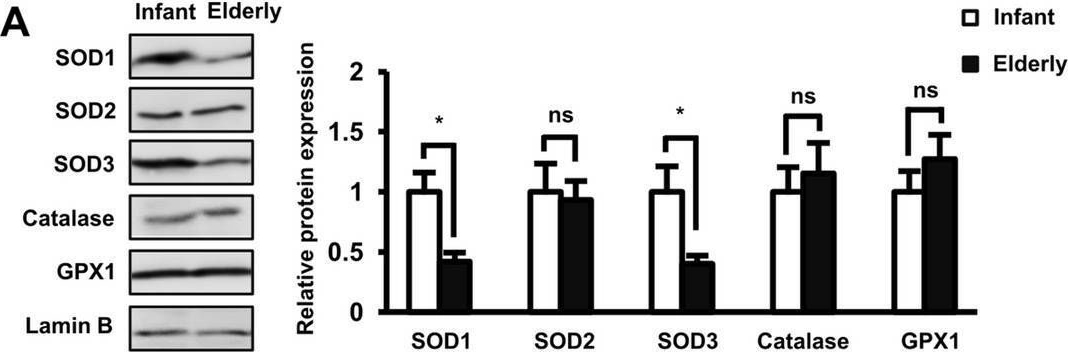
Detection of SOD1/Cu-Zn SOD by Western Blot The role of SOD1 and SOD3 antioxidant enzymes in the functions of AT-MSCs. (A) The protein expression of antioxidant enzymes in infant and elderly AT-MSCs. Infant AT-MSCs and elderly AT-MSCs were derived from 5 different donors, respectively (n = 5) and the comparison was conducted with infant AT-MSCs and elderly AT-MSCs at the same passage number. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/33057147), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
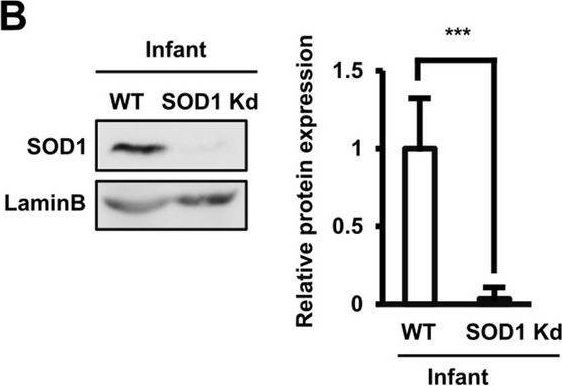
Detection of SOD1/Cu-Zn SOD by Western Blot The role of SOD1 and SOD3 antioxidant enzymes in the functions of AT-MSCs. (B) The protein expression of wild-type and SOD1 knockdown infant AT-MSCs. The knockdown experiments were conducted with infant AT-MSCs from 3 different donors (n = 3). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/33057147), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
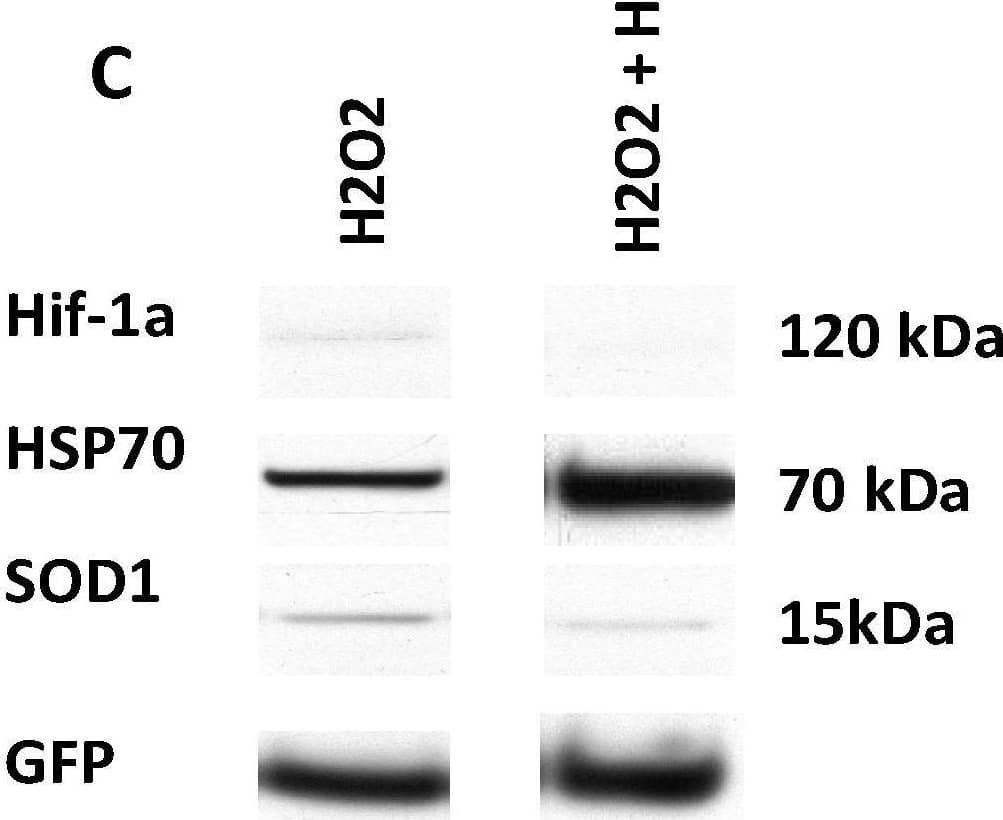
Detection of SOD1/Cu-Zn SOD by Western Blot GFP-rMSCs following in vitro stress assays. (A) Trypsinized and re-plated GFP-rMSCs at 24 h following hydrogen peroxide treatment. PSF-treated cells were much more abundant and had begun dividing, but GFP-rMSCs not treated with PSF appeared to have lasting negative effects of H2O2 exposure. (B) Lactate dehydrogenase released into medium of the hydrogen peroxide-treated cells was a means to measure cell death. (C) Changes in expression of stress-related proteins Hif-1a, HSP70, and SOD1 in GFP-rMSCs following hydrogen peroxide treatment. * Indicates p < 0.5. Experiments were repeated twice. Bar =100 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35054878), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
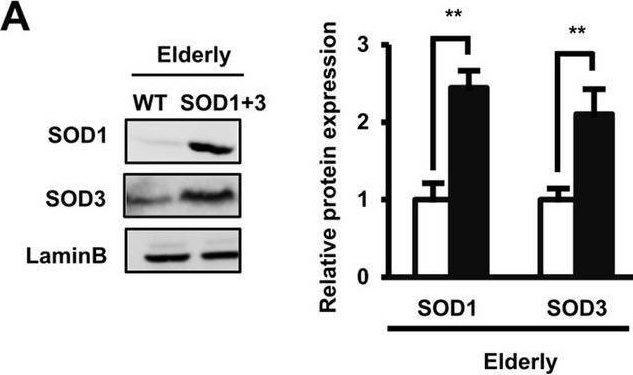
Detection of SOD1/Cu-Zn SOD by Western Blot The co-overexpression of SOD1 and SOD3 significantly improved the poor functions of elderly AT-MSCs by the activation of the pERK/ERK pathway. (A) The protein expression of wild-type elderly AT-MSCs or with the co-overexpression of SOD1 and SOD3 (n = 3). (B) The ROS expression in wild-type elderly AT-MSCs or with the co-overexpression of SOD1 and SOD3 (n = 3). (C) The cellular senescence of wild-type elderly AT-MSCs or with the co-overexpression of SOD1 and SOD3 (n = 3). (D) Transplantation of wild-type elderly AT-MSCs or with the co-overexpression of SOD1 and SOD3 to an in vivo streptozotocin-induced diabetic ischemic flap mouse model (n = 3). (E) The protein expression of pERK/ERK in infant AT-MSC, wildtype elderly AT-MSCs, elderly AT-MSCs with the individual overexpression of SOD1 or SOD3 or elderly AT-MSCs with the co-overexpression of SOD1 and SOD3 (n = 3). (F) The protein expression of pERK/ERK under the presence of a MEK inhibitor (n = 3). (G) The mRNA expression of wound healing-related growth factors in elderly AT-MSCs with the co-overexpression of SOD1 and SOD3 under the presence of a MEK inhibitor (n = 3). (H) Transplantation of elderly AT-MSCs with the co-overexpression of SOD1 and SOD3 under the presence of a MEK inhibitor to an in vivo streptozotocin-induced diabetic ischemic flap mouse model (n = 3). In all above experiments, elderly AT-MSCs were derived from 3 different donors (n = 3). SOD1 + 3: elderly AT-MSCs with co-overexpression of SOD1 and SOD3. PD098059 (PD) was used as a MEK inhibitor. The data represent the mean ± SD. ***P < 0.001, **P < 0.01, *P < 0.05, ns no significance. The experiments were performed in triplicate. Full-length Western blots are presented in Supplementary Figure S4. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/33057147), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: SOD1/Cu-Zn SOD
Superoxide Dismutases (SODs), originally identified as Indophenoloxidase (IPO), are enzymes that catalyze the converversion of naturally-occuring but harmful superoxide radicals into molecular oxygen and hydrogen peroxide. Superoxide Dismutases 1, SOD1, also known as Cu/Zn SOD, soluble SOD, and IPO-A, is a soluble, cytoplasmic 16 kDa homodimer. Each SOD1 monomer binds one Cu2+ and Zn2+ ion. Three isozymes of SOD have been identified and are functionally related but have very modest sequence homology. SOD1 shares 23% and 27% sequence identity with SOD2 and SOD3, respectively. Mutations in SOD1 have been suggested to be the cause of familial amyotrophic lateral sclerosis (ALS). The ALS-causing mutations of SOD1 are scattered throughout the protein and provide no clear functional or structural clues to the underlying disease mechanism. The oligomerization hypothesis suggests that mutant SOD1 proteins become misfolded and consequently oligomerize into high molecular weight aggregates that result in the death of motor neurons. The oxidative damage hypothesis suggests that loss of function mutations in SOD1 result in the intracellular accumulation of the superoxide radical, leading to free radical-mediated damage, the release of cytochrome c, and apoptosis.
Product Datasheets
Citations for Human/Mouse SOD1/Cu-Zn SOD Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
6
Citations: Showing 1 - 6
Filter your results:
Filter by:
-
A diacidic motif determines unconventional secretion of wild-type and ALS-linked mutant SOD1
Authors: David Cruz-Garcia, Nathalie Brouwers, Juan M. Duran, Gabriel Mora, Amy J. Curwin, Vivek Malhotra
Journal of Cell Biology
-
Chronic Restraint Stress Induces Gastric Mucosal Inflammation with Enhanced Oxidative Stress in a Murine Model
Authors: Maimaiti Yisireyili, Aziguli Alimujiang, Aikebaier Aili, Yiliang Li, Salamaiti Yisireyili, Kelimu Abudureyimu
Psychology Research and Behavior Management
-
MSC Pretreatment for Improved Transplantation Viability Results in Improved Ventricular Function in Infarcted Hearts
Authors: MF Pittenger, S Eghtesad, PG Sanchez, X Liu, Z Wu, L Chen, BP Griffith
International Journal of Molecular Sciences, 2022-01-08;23(2):.
Species: Rat
Sample Types: Cell Lysates
Applications: Western Blot -
CETSA-based target engagement of taxanes as biomarkers for efficacy and resistance
Authors: A Langebäck, S Bacanu, H Laursen, L Mout, T Seki, S Erkens-Sch, AD Ramos, A Berggren, Y Cao, J Hartman, W van Weerde, J Bergh, P Nordlund, S Lööf
Sci Rep, 2019-12-18;9(1):19384.
Species: Human, Xenograft
Sample Types: Beads
Applications: Immunoassay -
Neuroprotective and neuritogenic activities of novel multimodal iron-chelating drugs in motor-neuron-like NSC-34 cells and transgenic mouse model of amyotrophic lateral sclerosis.
Authors: Kupershmidt L, Weinreb O, Amit T, Mandel S, Carri MT, Youdim MB
FASEB J., 2009-07-28;23(11):3766-79.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Rejuvenation of mesenchymal stem cells by extracellular vesicles inhibits the elevation of reactive oxygen species
Authors: Vuong Cat Khanh, Toshiharu Yamashita, Kinuko Ohneda, Chiho Tokunaga, Hideyuki Kato, Motoo Osaka et al.
Scientific Reports
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse SOD1/Cu-Zn SOD Antibody
Average Rating: 4 (Based on 1 Review)
Have you used Human/Mouse SOD1/Cu-Zn SOD Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: