Human TGF-beta 1 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and Recombinant TGF-ß1. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 1 (5 plates): (Catalog # DY007) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 1.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY997), or 1.4% delipidized bovine serum, 0.05% Tween 20 in PBS, pH 7.2-7.4, 0.2 μm filtered.
Blocking Buffer: (Catalog # DY004), or 5% Tween 20 in PBS, pH 7.2-7.4, 0.2 μm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Activation Reagents Required:
Sample Activation Kit 1: 3 vials (10 mL/vial) of 1N HCL and 3 vials (10 mL/vial) of 1.2 N NaOH/1M HEPES (R&D Systems, Catalog # DY010).
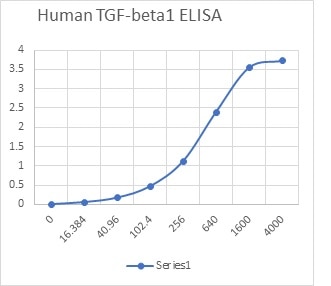
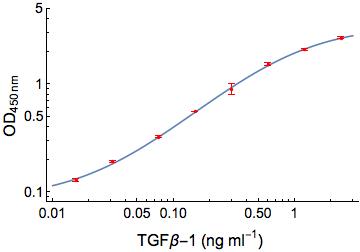
Scientific Data
Product Datasheets
Preparation and Storage
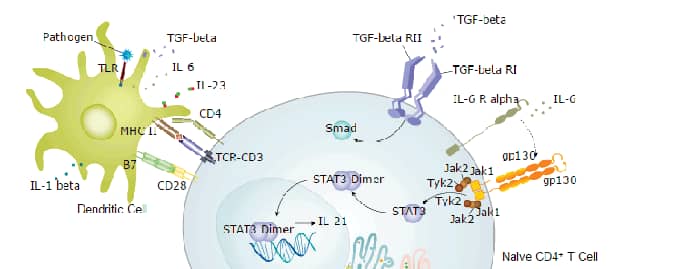
Background: TGF-beta 1
Transforming Growth Factor Beta 1, 2, and 3 (TGF-beta 1, TGF-beta 2, and TGF-beta 3) are highly pleiotropic cytokines that virtually all cell types secrete. TGF-beta molecules are proposed to act as cellular switches that regulate processes such as immune function, proliferation, and epithelial-mesenchymal transition. Targeted deletions of these genes in mice show that each TGF-beta isoform has some non-redundant functions: TGF-beta 1 is involved in hematopoiesis and endothelial differentiation; TGF-beta 2 affects development of cardiac, lung, craniofacial, limb, eye, ear, and urogenital systems; and TGF-beta 3 influences palatogenesis and pulmonary development. The full range of in vitro biological activities of TGF-beta 5 has not yet been explored. However, TGF-beta 1, TGF-beta 2, TGF-beta 3, and TGF-beta 5 have been found to be largely interchangeable in an inhibitory bioassay, and it is anticipated that TGF-beta 5 will show a spectrum of activities similar to the other TGF-beta family members. To date, the production of TGF-beta 5 has only been demonstrated in Xenopus.
TGF-beta ligands are initially synthesized as precursor proteins that undergo proteolytic cleavage. The mature segments form active ligand dimers via a disulfide-rich core consisting of the characteristic 'cysteine knot'. TGF-beta signaling begins with binding to a complex of the accessory receptor betaglycan (also known as TGF-beta RIII) and a type II serine/threonine kinase receptor termed TGF-beta RII. This receptor then phosphorylates and activates a type I serine/threonine kinase receptor, either ALK-1 or TGF-beta RI (also called ALK-5). The activated type I receptor phosphorylates and activates Smad proteins that regulate transcription. Use of other signaling pathways that are Smad-independent allows for distinct actions observed in response to TGF-beta in different contexts.
Assay Procedure
ACTIVATION REAGENT PREPARATION
To activate latent TGF-ß1 to the immunoreactive form, prepare the following solutions for acid activation and neutralization. The solutions may be stored in polypropylene bottles at room temperature for up to one month.
Caution: Wear protective clothing and safety glasses during preparation or use of these reagents. Refer to the appropriate MSDS before use.
1 N HCl (100 mL) - To 91.67 mL of deionized water, slowly add 8.33 mL of 12 N HCl. Mix well.
1.2 N NaOH/0.5 M HEPES (100 mL) - To 75 mL of deionized water, slowly add 12 mL of 10 N NaOH. Mix well. Add 11.9 g of HEPES. Mix well. Bring final volume to 100 mL with deionized water.
TGF-β1 SAMPLE ACTIVATION
To activate latent TGF-β1 to immunoreactive TGF-β1, follow the activation procedure outlined below. Assay samples after neutralization (pH 7.2-7.6). Use polypropylene test tubes.
Note: Do not activate the kit standards. The kit standards contain active recombinant TGF-β1.
| Cell Culture Supernates | Serum/Plasma |
|---|---|
| To 100 μL of cell culture supernate, add 20 μL of 1 N HCI. | To 40 μL of serum/plasma, add 20 μL of 1 N HCI. |
| Mix well. | Mix well. |
| Incubate 10 minutes at room temperature. | Incubate 10 minutes at room temperature. |
| Neutralize the acidified sample by adding 20 μL of 1.2 N NaOH/0.5 M HEPES. | Neutralize the acidified sample by adding 20 μL of 1.2 N NaOH/0.5 M HEPES. |
| Mix well. | Mix well. |
| Assay immediately. | Prior to the assay, dilute the activated sample 20-fold with Reagent Diluent.* |
| The concentration read off the standard curve must be multiplied by the dilution factor, 1.4. | The concentration read off the standard curve must be multiplied by the appropriate dilution factor, 40. |
*A suggested 20-fold dilution is 10 μL of activated sample + 190 μL of Reagent Diluent.
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody (to the working concentration stated in the product datasheet ) in PBS without carrier protein. Immediately coat a 96-well microplate with 100 µL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 µL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block each well of the microplate as recommended in the product datasheet. Incubate at room temperature for a minimum of 1 hour.
Note: The recommended Reagent Diluent typically contains 1% BSA. Some DuoSet Development Kits require alternative blocking agents, or for plates to be blocked overnight with a higher percentage of BSA, please see the product datasheet for details.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
PRECAUTION
The Stop Solution suggested for use with this kit is an acid solution. Wear eye, hand, face and clothing protection when using this material.
Assay Procedure
- Add 100 µL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 µL of the Detection Antibody, diluted in Reagent Diluent (as recommended in the product datasheet), to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 µL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 µL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 µL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human TGF-beta 1 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
204
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Mesenchymal stem cell activity across a graded scaffold-hydrogel composite biomaterial for tendon-to-bone enthesis repair
Authors: Timmer, KB;Killian, ML;Harley, BAC;
Bioactive materials
Species: Human
Sample Types: Cell Culture Supernates
-
Telomerase modRNA Offers a Novel RNA-Based Approach to Treat Human Pulmonary Fibrosis
Authors: Ye, JL;Grieger, K;Lu, D;Brandenberger, C;Juchem, M;Jordan, M;Oehlsen, L;Zardo, P;Werlein, C;Hesse, C;Sewald, K;Tretbar, S;Thum, T;Chatterjee, S;Bär, C;
Aging cell
Species: Human
Sample Types: Cell Culture Supernates
-
Modulation of IRAK4 as a Therapeutic Strategy Against Monosodium Urate- and Xanthine-Induced Inflammation in Macrophages and HepG2 Cells
Authors: Umar, S;Chang, HT;Maienschein-Cline, M;Ravindran, S;
Research square
Species: Human
Sample Types: Cell Culture Supernates
-
Compound K-enriched Korean red ginseng prevents lung cancer progression by targeting cancer cells and fibroblasts
Authors: Hwang, JH;Park, SY;Kang, JH;Jung, HJ;Park, J;Maeng, HJ;Choi, MK;Song, HS;Song, IS;Oh, SH;
Journal of ginseng research
Species: Human
Sample Types: Cell Culture Supernates
-
Mechanostimulatory cues determine intestinal fibroblast fate and profibrotic remodeling in a physiodynamic human gut-on-a-chip
Authors: Min, S;Than, N;Shin, YC;Ertugral, EG;Kothapalli, CR;Awoniyi, O;Kim, HJ;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Estrogen induces the alternative activation of macrophages through binding to estrogen receptor-alpha
Authors: Belboul, A;Ashworth, J;Fadel, A;Mcloughlin, J;Mahmoud, A;El Mohtadi, M;
Experimental and molecular pathology
Species: Human
Sample Types: Cell Culture Supernates
-
Altered miRNA Signatures in Follicular Fluid: Insights into Infertility Etiologies
Authors: Braicu, C;Ciocan, C;Bica, C;Zanoaga, O;Pop, LA;Strilciuc, S;Staicu, A;Goidescu, I;Muresan, D;Surcel, M;Berindan-Neagoe, I;
Genes
Species: Human
Sample Types: Cell Culture Supernates
-
Transforming growth factor beta induces interleukin-11 expression in osteoarthritis
Authors: Lokau, J;Bollmann, M;Garbers, Y;Feist, E;Lohmann, CH;Bertrand, J;Garbers, C;
Cytokine
Species: Human
Sample Types: Cell Culture Supernates
-
The cellular response of lipopolysaccharide-induced inflammation in keratoconus human corneal fibroblasts to RB-PDT: Insights into cytokines, chemokines and related signaling pathways
Authors: Chai, N;Stachon, T;Häcker, S;Berger, T;Li, Z;Amini, M;Suiwal, S;Seitz, B;Langenbucher, A;Szentmáry, N;
PloS one
Species: Human
Sample Types: Cell Culture Supernates
-
TGF? signaling sensitizes MEKi-resistant human melanoma to targeted therapy-induced apoptosis
Authors: Loos, B;Salas-Bastos, A;Nordin, A;Debbache, J;Stierli, S;Cheng, PF;Rufli, S;Wyss, C;Levesque, MP;Dummer, R;Wong, WW;Pascolo, S;Cantù, C;Sommer, L;
Cell death & disease
Species: Human
Sample Types: Cell Culture Supernates
-
Dynamic changes in immune cells in humanized liver metastasis and subcutaneous xenograft mouse models
Authors: Bang, HJ;Lee, KH;Park, MS;Sun, EG;Cho, SH;Chung, IJ;Shim, HJ;Bae, WK;
Scientific reports
Species: Xenograft
Sample Types: Serum
-
The TGF? type I receptor kinase inhibitor vactosertib in combination with pomalidomide in relapsed/refractory multiple myeloma: a phase 1b trial
Authors: Malek, E;Rana, PS;Swamydas, M;Daunov, M;Miyagi, M;Murphy, E;Ignatz-Hoover, JJ;Metheny, L;Kim, SJ;Driscoll, JJ;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Toxicity and efficacy of type I interferons on the ocular surface: in vitro, animal, and clinical studies
Authors: Yun, YI;Ko, JH;Ryu, JS;Kim, S;Jeon, HS;Kim, N;Kim, MK;Oh, JY;
The ocular surface
Species: Human
Sample Types: Cell Culture Supernates
-
Identification and characterization of plasma proteins associated with intra-amniotic inflammation and/or infection in women with preterm labor.
Authors: Cho, HY;Lee, JE;Park, KH;Choi, BY;Lee, MJ;Jeong, DE;Shin, S;
Scientific reports
Species: Human
Sample Types: Plasma
-
Repeat controlled human Plasmodium falciparum infections delay bloodstream patency and reduce symptoms
Authors: Ferrer, P;Berry, AA;Bucsan, AN;Prajapati, SK;Krishnan, K;Barbeau, MC;Rickert, DM;Guerrero, SM;Usui, M;Abebe, Y;Patil, A;Chakravarty, S;Billingsley, PF;Pa'ahana-Brown, F;Strauss, K;Shrestha, B;Nomicos, E;Deye, GA;Sim, BKL;Hoffman, SL;Williamson, KC;Lyke, KE;
Nature communications
Species: Human
Sample Types: Plasma
-
Novel paired CD13-negative (MT-50.1) and CD13-positive (MT-50.4) HTLV-1-infected T-cell lines with differential regulatory T cell-like activity
Authors: Egawa, Y;Higuchi, T;Hashida, Y;Ueno, K;Kojima, K;Daibata, M;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Nuclear factor-?B activation by transforming growth factor-?1 drives tumour microenvironment-mediated drug resistance in neuroblastoma
Authors: Louault, K;Blavier, L;Lee, MH;Kennedy, RJ;Fernandez, GE;Pawel, BR;Asgharzadeh, S;DeClerck, YA;
British journal of cancer
Species: Human
Sample Types: Cell Culture Supernates
-
Macrophage MCT4 inhibition activates reparative genes and protects from atherosclerosis by histone H3 lysine 18 lactylation
Authors: Zhang, Y;Jiang, H;Dong, M;Min, J;He, X;Tan, Y;Liu, F;Chen, M;Chen, X;Yin, Q;Zheng, L;Shao, Y;Li, X;Chen, H;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
ADAMTSL2 mutations determine the phenotypic severity in Geleophysic Dysplasia
Authors: Camarena, V;Williams, MM;Morales, AA;Zafeer, MF;Kilic, OV;Kamiar, A;Abad, C;Rasmussen, MA;Briski, LM;Peart, L;Bademci, G;Barbouth, DS;Smithson, S;Wang, G;Shehadeh, LA;Walz, K;Tekin, M;
JCI insight
Species: Mouse
Sample Types: Cell Culture Supernates, Cell Lysates
-
Characterization of platelet rich plasma in feline immunodeficiency virus-infected cats: Cell, and PDGF-BB and TGF-beta 1 growth factor analysis
Authors: Miguel-Pastor, L;Satué, K;Chicharro, D;Damiá, E;Cuervo, B;Torres-Torrillas, M;Martins, E;Velasco-Martínez, MG;Carrillo, JM;Sopena, JJ;Cerón, JJ;Rubio, M;
Research in veterinary science
Species: Feline
Sample Types: Platelet-Poor Plasma, Platelet-Rich Plasma
-
Effects of calcitriol upon TGF-?s and their receptors in trophoblast cells
Authors: Noyola-Martínez, N;Chirinos, M;Ramírez-Camacho, I;Escamilla-Bucio, JE;García-Olivares, M;Aragón-Hernández, JP;Segovia-Mendoza, M;Halhali, A;Barrera, D;
Journal of reproductive immunology
Species: Human
Sample Types: Cell Culture Supernates
-
Neutralizing tumor-related inflammation and reprogramming of cancer-associated fibroblasts by Curcumin in breast cancer therapy
Authors: Jalilian, E;Abolhasani-Zadeh, F;Afgar, A;Samoudi, A;Zeinalynezhad, H;Langroudi, L;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Enhanced Intervertebral Disc Repair via Genetically Engineered Mesenchymal Stem Cells with Tetracycline Regulatory System
Authors: Kim, Y;An, SB;Lee, SH;Lee, JJ;Kim, SB;Ahn, JC;Hwang, DY;Han, I;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Unrevealed roles of extracellular enolase?1 (ENO1) in promoting glycolysis and pro?cancer activities in multiple myeloma via hypoxia?inducible factor 1?
Authors: Chung, IC;Huang, WC;Huang, YT;Chen, ML;Tsai, AW;Wu, PY;Yuan, TT;
Oncology reports
Species: Human, Mouse
Sample Types: Cell Culture Supernates
-
Serum interleukin-6, procalcitonin, and C-reactive protein at hospital admission can identify patients at low risk for severe COVID-19 progression
Authors: Zobel, CM;Wenzel, W;Krüger, JP;Baumgarten, U;Wagelöhner, T;Neumann, N;Foroutan, B;Müller, R;Müller, A;Rauschning, D;Schü beta ler, M;Scheit, L;Weinreich, F;Oltmanns, K;Keidel, F;Koch, M;Spethmann, S;Schreiner, M;
Frontiers in microbiology
Species: Human
Sample Types: Serum
-
Cullin 4B Ubiquitin Ligase Is Important for Cell Survival and Regulates TGF-?1 Expression in Pleural Mesothelioma
Authors: Kreienbühl, J;Changkhong, S;Orlowski, V;Kirschner, MB;Opitz, I;Meerang, M;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
The influence of calcitriol and methylprednisolone on podocytes function in minimal change disease in vitro model
Authors: Grubczak, K;Starosz, A;Makowska, B;Parfienowicz, Z;Kr?towska, M;Naumnik, B;Moniuszko, M;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernatants
-
Different concentrations of fetal bovine serum affect cytokine modulation in Lipopolysaccharide-activated apical papilla cells in vitro*
Authors: Letícia Martins SANTOS, Patricia e Silva CARDOSO, Elisa Abreu DINIZ, Juliana Garuba RAHHAL, Carla Renata SIPERT
Journal of Applied Oral Science
Species: Human
Sample Types: Cell Culture Supernates
-
Hypoxia Preconditioned Serum (HPS) Promotes Proliferation and Chondrogenic Phenotype of Chondrocytes In Vitro
Authors: Jiang, J;Altammar, J;Cong, X;Ramsauer, L;Steinbacher, V;Dornseifer, U;Schilling, AF;Machens, HG;Moog, P;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
Serum TGF-?1 and CD14 Predicts Response to Anti-TNF-? Therapy in IBD
Authors: Coufal, S;Kverka, M;Kreisinger, J;Thon, T;Rob, F;Kolar, M;Reiss, Z;Schierova, D;Kostovcikova, K;Roubalova, R;Bajer, L;Jackova, Z;Mihula, M;Drastich, P;Tresnak Hercogova, J;Novakova, M;Vasatko, M;Lukas, M;Tlaskalova-Hogenova, H;Jiraskova Zakostelska, Z;
Journal of immunology research
Species: Human
Sample Types: Serum
-
Mild Cognitive Impairment Is Associated with Enhanced Activation of Th17 Lymphocytes in Non-Alcoholic Fatty Liver Disease
Authors: Fiorillo, A;Gallego, JJ;Casanova-Ferrer, F;Giménez-Garzó, C;Urios, A;Ballester, MP;Durbán, L;Rios, MP;Megías, J;San Miguel, T;Kosenko, E;Escudero-García, D;Benlloch, S;Felipo, V;Montoliu, C;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
A Reconstructed Human Melanoma-in-Skin Model to Study Immune Modulatory and Angiogenic Mechanisms Facilitating Initial Melanoma Growth and Invasion
Authors: Michielon, E;López González, M;Stolk, DA;Stolwijk, JGC;Roffel, S;Waaijman, T;Lougheed, SM;de Gruijl, TD;Gibbs, S;
Cancers
Species: Human
Sample Types: Cell Culture Supernates
-
Targeting the PAI-1 Mechanism with a Small Peptide Increases the Efficacy of Alteplase in a Rabbit Model of Chronic Empyema
Authors: Florova, G;De Vera, CJ;Emerine, RL;Girard, RA;Azghani, AO;Sarva, K;Jacob, J;Morris, DE;Chamiso, M;Idell, S;Komissarov, AA;
Pharmaceutics
Species: Rabbit
Sample Types: Pleural Fluid
-
Periodontal Disease in Young Adults as a Risk Factor for Subclinical Atherosclerosis: A Clinical, Biochemical and Immunological Study
Authors: S Cicmil, A Cicmil, V Pavlic, J Kruni?, D Sladoje Pu, D Bokonji?, M ?oli?
Journal of Clinical Medicine, 2023-03-12;12(6):.
Species: Human
Sample Types: Serum
-
TGF-beta is elevated in hyperuricemic individuals and mediates urate-induced hyperinflammatory phenotype in human mononuclear cells
Authors: V Klück, G Cab?u, L Mies, F Bukkems, L van Emst, R Bakker, A van Caam, HINT conso, TO Cri?an, LAB Joosten
Arthritis Research & Therapy, 2023-02-27;25(1):30.
Species: Human
Sample Types: Cell Culture Supernates
-
In vitro and in vivo applications of a universal and synthetic thermo-responsive drug delivery hydrogel platform
Authors: H Gholizadeh, E Landh, DM Silva, A Granata, D Traini, P Young, A Fathi, S Maleknia, T Abrams, F Dehghani, H Xin Ong
International journal of pharmaceutics, 2023-02-25;635(0):122777.
Species: N/A
Sample Types: Hydrogel Supernates
-
In vitro and in vivo applications of a universal and synthetic thermo-responsive drug delivery hydrogel platform
Authors: H Gholizadeh, E Landh, DM Silva, A Granata, D Traini, P Young, A Fathi, S Maleknia, T Abrams, F Dehghani, H Xin Ong
International journal of pharmaceutics, 2023;635(0):122777.
Species: N/A
Sample Types: Hydrogel Supernates
-
Rhinovirus Suppresses TGF-beta-GARP Presentation by Peripheral NK Cells
Authors: S Krammer, Z Yang, H Mitländer, JC Grund, S Trump, S Mittler, S Zirlik, S Finotto
Cells, 2022-12-28;12(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of Potential microRNA Panels for Male Non-Small Cell Lung Cancer Identification Using Microarray Datasets and Bioinformatics Methods
Authors: A Harangu?, R Lajos, L Budisan, O Zanoaga, C Ciocan, C Bica, R Pirlog, I Simon, M Simon, C Braicu, I Berindan-N
Journal of personalized medicine, 2022-12-13;12(12):.
Species: Human
Sample Types: Serum
-
SARS-CoV-2 Spike triggers barrier dysfunction and vascular leak via integrins and TGF-beta signaling
Authors: SB Biering, FT Gomes de S, LV Tjang, F Pahmeier, C Zhu, R Ruan, SF Blanc, TS Patel, CM Worthingto, DR Glasner, B Castillo-R, V Servellita, NTN Lo, MP Wong, CM Warnes, DR Sandoval, TM Clausen, YA Santos, DM Fox, V Ortega, AM Näär, RS Baric, SA Stanley, HC Aguilar, JD Esko, CY Chiu, JE Pak, PR Beatty, E Harris
Nature Communications, 2022-12-09;13(1):7630.
Species: Human
Sample Types: Cell Culture Supernates
-
Eribulin normalizes pancreatic cancer-associated fibroblasts by simulating selected features of TGFbeta inhibition
Authors: T Luong, E Cukierman
BMC Cancer, 2022-12-02;22(1):1255.
Species: Human
Sample Types: Cell Culture Supernates
-
Substrate stiffness engineered to replicate disease conditions influence senescence and fibrotic responses in primary lung fibroblasts
Authors: KEC Blokland, M Nizamoglu, H Habibie, T Borghuis, M Schuliga, BN Melgert, DA Knight, CA Brandsma, SD Pouwels, JK Burgess
Frontiers in Pharmacology, 2022-11-03;13(0):989169.
Species: Human
Sample Types: Cell Culture Supernates
-
Utilization of ex vivo tissue model to study skin regeneration following microneedle stimuli
Authors: X Liu, R Barresi, M Kaminer, K Qian, F Thillou, M Bataillon, IC Liao, Q Zheng, C Bouez
Scientific Reports, 2022-10-27;12(1):18115.
Species: Human
Sample Types: Cell Culture Supernates
-
Hepatocyte phosphatase DUSP22 mitigates NASH-HCC progression by targeting FAK
Authors: C Ge, J Tan, X Dai, Q Kuang, S Zhong, L Lai, C Yi, Y Sun, J Luo, C Zhang, L Zhu, B Wang, M Xu
Oncogene, 2022-10-08;13(1):5945.
Species: Human
Sample Types: Serum
-
SerpinB3 drives cancer stem cell survival in glioblastoma
Authors: A Lauko, J Volovetz, SM Turaga, D Bayik, DJ Silver, K Mitchell, EE Mulkearns-, DC Watson, K Desai, M Midha, J Hao, K McCortney, A Steffens, U Naik, MS Ahluwalia, S Bao, C Horbinski, JS Yu, JD Lathia
Cell Reports, 2022-09-13;40(11):111348.
Species: Human
Sample Types: Cell Culture Supernates
-
Immortalised canine buccal epithelial cells' CXCL8 secretion is affected by allergen extracts, Toll-like receptor ligands, IL-17A and calcitriol
Authors: M Pelst, C Höbart, H de Rooster, B Devriendt, E Cox
Veterinary research, 2022-09-13;53(1):72.
Species: Canine
Sample Types: Cell Culture Supernates
-
Minimal Development of Liver Fibrosis in Adult Tolerant Liver Transplant Recipients Late After Immunosuppressive Drug Weaning and Transplantation
Authors: AA Duizendstr, RJ De Knegt, NMA Nagtzaam, MGH Betjes, WA Dik, NHR Litjens, J Kwekkeboom
Transplantation Proceedings, 2022-09-11;0(0):.
Species: Human
Sample Types: Serum
-
A TGFbetaR inhibitor represses keratin-7 expression in 3D cultures of human salivary gland progenitor cells
Authors: EW Fowler, EJ van Venroo, RL Witt, X Jia
Scientific Reports, 2022-09-02;12(1):15008.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-17A in Human Liver: Significant Source of Inflammation and Trigger of Liver Fibrosis Initiation.
Authors: Kartasheva-Ebertz D, Gaston J, Lair-Mehiri L, Mottez E, Buivan T, Massault P, Scatton O, Gaujoux S, Vaillant J, Pol S, Lagaye S
Int J Mol Sci, 2022-08-29;23(17):.
Species: Human
Sample Types: Tissue Culture Supernates
-
Growth Factor Release within Liquid and Solid PRF
Authors: K Zwittnig, B Kirnbauer, N Jakse, P Schlenke, I Mischak, S Ghanaati, S Al-Maawi, D Végh, M Payer, TA Zrnc
Journal of Clinical Medicine, 2022-08-29;11(17):.
Species: Human
Sample Types: Serum
-
Extracellular matrix of early pulmonary fibrosis modifies the polarization of alveolar macrophage
Authors: Y Zhang, L Zhu, J Hong, C Chen
International immunopharmacology, 2022-08-24;111(0):109179.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Effects of estradiol on the virulence traits of Porphyromonas gingivalis
Authors: KJ Demirel, AN Guimaraes, I Demirel
Scientific Reports, 2022-08-16;12(1):13881.
Species: Human
Sample Types: Cell Culture Supernates
-
Development of an Electrochemical CCL5 Chemokine Immunoplatform for Rapid Diagnosis of Multiple Sclerosis
Authors: S Guerrero, E Sánchez-Ti, L Agüí, A González-C, P Yáñez-Sede, JM Pingarrón
Biosensors, 2022-08-07;12(8):.
Species: Human
Sample Types: Serum
-
Pro-cancerogenic effects of spontaneous and drug-induced senescence of ovarian cancer cells in vitro and in vivo: a comparative analysis
Authors: S Rutecki, P Szulc, M Paku?a, P Uruski, A Radziemski, E Naumowicz, R Moszy?ski, A Tykarski, J Miku?a-Pie, K Ksi??ek
Journal of ovarian research, 2022-07-26;15(1):87.
Species: Human
Sample Types: Cell Culture Supernates
-
Melanoma Stem Cells Educate Neutrophils to Support Cancer Progression
Authors: M Anselmi, F Fontana, M Marzagalli, N Gagliano, M Sommariva, P Limonta
Cancers, 2022-07-13;14(14):.
Species: Human
Sample Types: Cell Culture Supernates
-
Plasma proteomic analysis to identify potential biomarkers of histologic chorioamnionitis in women with preterm premature rupture of membranes
Authors: JE Lee, K Dan, HJ Kim, YM Kim, KH Park
PLoS ONE, 2022-07-07;17(7):e0270884.
Species: Human
Sample Types: Plasma
-
Prediction of emergency cerclage outcomes in women with cervical insufficiency: The role of inflammatory, angiogenic, and extracellular matrix-related proteins in amniotic fluid
Authors: KN Lee, KH Park, YM Kim, I Cho, TE Kim
PLoS ONE, 2022-05-10;17(5):e0268291.
Species: Human
Sample Types: Amniotic Fluid
-
Cytokine Profile and Anti-Inflammatory Activity of a Standardized Conditioned Medium Obtained by Coculture of Monocytes and Mesenchymal Stromal Cells (PRS CK STORM)
Authors: JP Lapuente, A Blázquez-M, J Marco-Brua, G Gómez, P Desportes, J Sanz, P Fernández, M García-Gil, F Bermejo, JV San Martín, A Algaba, JC De Gregori, D Lapuente, A De Gregori, B Lapuente, MV Andrés, A Anel
Biomolecules, 2022-03-31;12(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
Parathyroid Hormone Induces Human Valvular Endothelial Cells Dysfunction That Impacts the Osteogenic Phenotype of Valvular Interstitial Cells
Authors: M Vadana, S Cecoltan, L Ciortan, RD Macarie, AC Mihaila, MM Tucureanu, AM Gan, M Simionescu, I Manduteanu, I Droc, E Butoi
International Journal of Molecular Sciences, 2022-03-29;23(7):.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of Betaglycan in TGF-beta Signaling and Wound Healing in Human Endometriotic Epithelial Cells and in Endometriosis
Authors: AN Mwaura, MA Riaz, JB Maoga, E Mecha, COA Omwandho, G Scheiner-B, I Meinhold-H, L Konrad
Biology, 2022-03-26;11(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
Optimization of loading protocols for tissue engineering experiments
Authors: YD Ladner, AR Armiento, EJ Kubosch, JG Snedeker, MJ Stoddart
Scientific Reports, 2022-03-24;12(1):5094.
Species: Human
Sample Types: Cell Culture Supernates
-
Therapeutic Effects of Platelet-Derived Extracellular Vesicles in a Bioengineered Tendon Disease Model
Authors: AL Graça, RMA Domingues, I Calejo, M Gómez-Flor, ME Gomes
International Journal of Molecular Sciences, 2022-03-09;23(6):.
Species: Human
Sample Types: Extracellular Vesicles
-
Increased serum LOXL2 concentration in pelvic inflammatory disease with pelvic adhesion
Authors: C Xie, B Tang, K Wu, Q Meng, F Wang
Oncogene, 2022-03-04;22(1):59.
Species: Human
Sample Types: Serum
-
Anti-inflammatory effects of an autologous gold-based serum therapy in osteoarthritis patients
Authors: J Feldt, AJ Donaubauer, J Welss, U Schneider, US Gaipl, F Paulsen
Scientific Reports, 2022-03-03;12(1):3560.
Species: Human
Sample Types: Serum
-
Nucleoporin-93 reveals a common feature of aggressive breast cancers: robust nucleocytoplasmic transport of transcription factors
Authors: NB Nataraj, A Noronha, JS Lee, S Ghosh, HR Mohan Raju, A Sekar, B Zuckerman, M Lindzen, E Tarcitano, S Srivastava, M Selitrenni, I Livneh, D Drago-Garc, O Rueda, C Caldas, S Lev, T Geiger, A Ciechanove, I Ulitsky, R Seger, E Ruppin, Y Yarden
Cell Reports, 2022-02-22;38(8):110418.
Species: Human
Sample Types: Cell Culture Supernates
-
Tethered TGF-beta1 in a Hyaluronic Acid-Based Bioink for Bioprinting Cartilaginous Tissues
Authors: J Hauptstein, L Forster, A Nadernezha, J Groll, J Te beta mar, T Blunk
International Journal of Molecular Sciences, 2022-01-15;23(2):.
Species: Human
Sample Types: Recombinant Protein
-
Impact and Possible Mechanism(s) of Adipose Tissue-Derived Mesenchymal Stem Cells on T-Cell Proliferation in Patients With Rheumatic Disease
Authors: E Kuca-Warna, M Olesi?ska, P Szcz?sny, E Kontny
Frontiers in Physiology, 2022-01-13;12(0):749481.
Species: Human
Sample Types: Cell Culture Supernates
-
Skin Substitute Preparation Method Induces Immunomodulatory Changes in Co-Incubated Cells through Collagen Modification
Authors: J Holl, C Pawlukiani, J Corton Rui, D Groth, K Grubczak, HR Hady, J Dadan, J Reszec, S Czaban, C Kowalewski, M Moniuszko, A Eljaszewic
Pharmaceutics, 2021-12-15;13(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
SARS-CoV-2 Spike triggers barrier dysfunction and vascular leak via integrins and TGF-beta signaling
Authors: SB Biering, FTG de Sousa, LV Tjang, F Pahmeier, R Ruan, SF Blanc, TS Patel, CM Worthingto, DR Glasner, B Castillo-R, V Servellita, NTN Lo, MP Wong, CM Warnes, DR Sandoval, TM Clausen, YA Santos, V Ortega, HC Aguilar, JD Esko, CY Chui, JE Pak, PR Beatty, E Harris
bioRxiv : the preprint server for biology, 2021-12-13;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Intramammary treatment using allogeneic pure platelet-rich plasma in cows with subclinical mastitis caused by Gram-positive bacteria
Authors: PC Duque-Madr, J Velasco-Bo, A Ceballos-M, C López, JU Carmona
Scientific Reports, 2021-12-09;11(1):23737.
Species: Bovine
Sample Types: Plasma
-
Longitudinal relationships between polycyclic aromatic hydrocarbons exposure and heart rate variability: Exploring the role of transforming�growth factor-beta in a general Chinese population
Authors: J Ma, Q Tan, X Nie, M Zhou, B Wang, X Wang, M Cheng, Z Ye, Y Xie, D Wang, W Chen
Journal of Hazardous Materials, 2021-11-15;0(0):127770.
Species: Human
Sample Types: Plasma
-
Inhibition of miR-155 Promotes TGF-beta Mediated Suppression of HIV Release in the Cervical Epithelial Cells
Authors: J Gokavi, S Sadawarte, A Shelke, U Kulkarni-K, M Thakar, V Saxena
Viruses, 2021-11-12;13(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Preparation and growth factor characterization of cord blood-derived plasma, serum, growth factor-rich plasma and induced serum
Authors: ME Rhéaume, J Perreault, D Fournier, P Trépanier
Cytokine, 2021-11-08;149(0):155756.
Species: Human
Sample Types: Serum
-
Intestinal CD11b+ B Cells Ameliorate Colitis by Secreting Immunoglobulin A
Authors: Ying Fu, Zhiming Wang, Baichao Yu, Yuli Lin, Enyu Huang, Ronghua Liu et al.
Frontiers in Immunology
Species: Mouse
Sample Types: Tissue Homogenates, Fecal Extract, Mucus
-
Proteomic identification of novel plasma biomarkers associated with spontaneous preterm birth in women with preterm labor without infection/inflammation
Authors: JE Lee, KH Park, HJ Kim, YM Kim, JW Choi, S Shin, KN Lee
PLoS ONE, 2021-10-28;16(10):e0259265.
Species: Human
Sample Types: Plasma
-
Latent TGFbeta-binding proteins regulate UCP1 expression and function via TGFbeta2
Authors: D Halbgebaue, J Roos, JB Funcke, H Neubauer, BS Hamilton, E Simon, EZ Amri, KM Debatin, M Wabitsch, P Fischer-Po, D Tews
Molecular Metabolism, 2021-09-01;53(0):101336.
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophils promote T-cell activation through the regulated release of CD44-bound Galectin-9 from the cell surface during HIV infection
Authors: G Dunsmore, EP Rosero, S Shahbaz, DM Santer, J Jovel, P Lacy, S Houston, S Elahi
PloS Biology, 2021-08-19;19(8):e3001387.
Species: Human
Sample Types: Plasma
-
Severe COVID-19 Patients Show an Increase in Soluble TNFR1 and ADAM17, with a Relationship to Mortality
Authors: Y Palacios, A Ruiz, LA Ramón-Luin, R Ocaña-Guzm, O Barreto-Ro, A Sánchez-Mo, B Tecuatzi-C, AG Regalado-G, RD Pineda-Gud, A García-Mar, F Juárez-Her, JP Farias-Con, I Fricke-Gal, G Pérez-Rubi, R Falfán-Val, I Buendia-Ro, K Medina-Que, L Chavez-Gal
International Journal of Molecular Sciences, 2021-08-05;22(16):.
Species: Human
Sample Types: Serum
-
An In Vitro System to Study the Effect of Subchondral Bone Health on Articular Cartilage Repair in Humans
Authors: T Hopkins, KT Wright, NJ Kuiper, S Roberts, P Jermin, P Gallacher, JH Kuiper
Cells, 2021-07-27;10(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Regulation of Cellular Senescence Is Independent from Profibrotic Fibroblast-Deposited ECM
Authors: KEC Blokland, H Habibie, T Borghuis, GJ Teitsma, M Schuliga, BN Melgert, DA Knight, CA Brandsma, SD Pouwels, JK Burgess
Cells, 2021-06-29;10(7):.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum protein triplet TGF-&beta1, TIMP-1, and YKL-40 serve as diagnostic and prognostic profile for astrocytoma
Authors: R Urbanavi?i, R Zabitait?, A Kriš?iukai, VP Deltuva, D Skiriut?
Scientific Reports, 2021-06-23;11(1):13100.
Species: Human
Sample Types: Serum
-
Th17/Treg-Related Intracellular Signaling in Patients with Chronic Obstructive Pulmonary Disease: Comparison between Local and Systemic Responses
Authors: JD Lourenço, WR Teodoro, DF Barbeiro, APP Velosa, LEF Silva, JB Kohler, AR Moreira, MV Aun, IC da Silva, FLA Fernandes, EM Negri, JL Gross, IFLC Tibério, JT Ito, FDTQS Lopes
Cells, 2021-06-22;10(7):.
Species: Human
Sample Types: Tissue Homogenates
-
Changes in liver steatosis in HIV-positive women are associated with the BMI, but not with biomarkers
Authors: R Fernandez-, MW Plankey, D Ware, J Bordon
Cytokine, 2021-05-12;0(0):155573.
Species: Human
Sample Types: Plasma
-
The noncoding MIR100HG RNA enhances the autocrine function of transforming growth factor &beta signaling
Authors: P Papoutsogl, DM Rodrigues-, A Morén, A Bergman, F Pontén, C Coulouarn, L Caja, CH Heldin, A Moustakas
Oncogene, 2021-05-04;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Precision targeting of the plasminogen activator inhibitor-1 mechanism increases efficacy of fibrinolytic therapy in empyema
Authors: G Florova, RA Girard, AO Azghani, K Sarva, A Buchanan, S Karandasho, CJ DeVera, D Morris, M Chamiso, K Koenig, DB Cines, S Idell, AA Komissarov
Physiological Reports, 2021-05-01;9(9):e14861.
Species: Rabbit
Sample Types: Pleural Fluid
-
TGF&beta1 induces in-vitro and ex-vivo angiogenesis through VEGF production in human ovarian follicular fluid-derived granulosa cells during in-vitro fertilization cycle
Authors: TH Lai, HT Chen, WB Wu
Journal of reproductive immunology, 2021-03-20;145(0):103311.
Species: Human
Sample Types: Cell Culture Supernates
-
The effects of resveratrol on the expression of VEGF, TGF-&beta, and MMP-9 in endometrial stromal cells of women with endometriosis
Authors: T Arablou, N Aryaeian, S Khodaverdi, R Kolahdouz-, Z Moradi, N Rashidi, AA Delbandi
Scientific Reports, 2021-03-15;11(1):6054.
Species: Human
Sample Types: Cell Culture Supernates
-
Palladin isoforms 3 and 4 regulate cancer-associated fibroblast pro-tumor functions in pancreatic ductal adenocarcinoma
Authors: JI Alexander, DB Vendramini, R Francescon, T Luong, J Franco-Bar, N Shah, JC Gardiner, E Nicolas, KS Raghavan, E Cukierman
Scientific Reports, 2021-02-15;11(1):3802.
Species: Human
Sample Types: Cell Culture Supernates
-
Downregulation of epithelial DUOX1 in Chronic Obstructive Pulmonary Disease contributes to disease pathogenesis
Authors: C Schiffers, C van de Wet, RA Bauer, A Habibovic, M Hristova, CM Dustin, S Lambrichts, PM Vacek, EF Wouters, NL Reynaert, A van der Vl
JCI Insight, 2021-01-25;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Regulation of Fibroblast Activation Protein by Transforming Growth Factor Beta-1 in Glioblastoma Microenvironment
Authors: E Krepela, Z Vanickova, P Hrabal, M Zubal, B Chmielova, E Balaziova, P Vymola, I Matrasova, P Busek, A Sedo
International Journal of Molecular Sciences, 2021-01-21;22(3):.
Species: Human
Sample Types: Tissue Homogenates
-
Human Adipose Tissue-Derived Mesenchymal Stromal Cells Inhibit CD4+ T Cell Proliferation and Induce Regulatory T Cells as Well as CD127 Expression on CD4+CD25+ T Cells
Authors: A Fiori, S Uhlig, H Klüter, K Bieback
Cells, 2021-01-01;10(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Exploring the Role of C-C Motif Chemokine Ligand-2 Single Nucleotide Polymorphism in Pulmonary Tuberculosis: A Genetic Association Study from North India
Authors: SK Biswas, M Mittal, E Sinha, V Singh, N Arela, B Bajaj, PK Tiwari, VM Katoch, KK Mohanty
Journal of Immunology Research, 2020-12-16;2020(0):1019639.
Species: Human
Sample Types: Serum
-
Patient-derived and artificial ascites have minor effects on MeT-5A mesothelial cells and do not facilitate ovarian cancer cell adhesion
Authors: M Estermann, YL Huang, D Septiadi, D Ritz, CY Liang, F Jacob, B Drasler, A Petri-Fink, V Heinzelman, B Rothen-Rut
PLoS ONE, 2020-12-03;15(12):e0241500.
Species: Human
Sample Types: Abdominal Fluid
-
TGF-&beta in the Secretome of Irradiated Peripheral Blood Mononuclear Cells Supports In Vitro Osteoclastogenesis
Authors: L Panahipour, Z Kargarpour, M Laggner, M Mildner, HJ Ankersmit, R Gruber
Int J Mol Sci, 2020-11-13;21(22):.
Species: Human
Sample Types: Cell Culture Supernates
-
Circulating biomarkers and outcomes from a randomised phase 2 trial of gemcitabine versus capecitabine-based chemoradiotherapy for pancreatic cancer
Authors: F Willenbroc, CM Cox, EE Parkes, CS Wilhelm-Be, AG Abraham, R Owens, A Sabbagh, CM Jones, DLI Hughes, T Maughan, CN Hurt, EE O'Neill, S Mukherjee
Br J Cancer, 2020-10-26;0(0):.
Species: Human
Sample Types: Plasma
-
GLP-1 receptor agonist ameliorates experimental lung fibrosis
Authors: J Fandiño, L Toba, LC González-M, Y Diz-Chaves, F Mallo
Sci Rep, 2020-10-22;10(1):18091.
Species: Rat
Sample Types: Plasma
-
Regional hyperthermia enhances mesenchymal stem cell recruitment to tumor stroma: Implications for mesenchymal stem cell-based tumor therapy
Authors: M Tutter, C Schug, KA Schmohl, S Urnauer, C Kitzberger, N Schwenk, M Petrini, C Zach, S Ziegler, P Bartenstei, WA Weber, G Multhoff, E Wagner, LH Lindner, PJ Nelson, C Spitzweg
Mol Ther, 2020-10-15;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
The role of EMMPRIN/CD147 in regulating angiogenesis in patients with psoriatic arthritis
Authors: MA Rahat, M Safieh, E Simanovich, E Pasand, T Gazitt, A Haddad, M Elias, D Zisman
Arthritis Res Ther, 2020-10-14;22(1):240.
Species: Human
Sample Types: Serum
-
Liposomes loaded with transforming growth factor &beta1 promote odontogenic differentiation of dental pulp stem cells
Authors: L Jiang, WN Ayre, GE Melling, B Song, X Wei, AJ Sloan, X Chen
J Dent, 2020-10-14;103(0):103501.
Species: Human
Sample Types: Cell Culture Supernates
-
The NRF2 stimulating agent, tin protoporphyrin, activates protective cytokine pathways in healthy human subjects and in patients with chronic kidney disease
Authors: RA Zager, ACM Johnson
Physiol Rep, 2020-09-01;8(18):e14566.
Species: Human
Sample Types: Plasma
-
Renal cell tumors convert natural killer cells to a proangiogenic phenotype
Authors: Y Guan, CB Chambers, T Tabatabai, H Hatley, KR Delfino, K Robinson, SR Alanee, S Ran, DS Torry, A Wilber
Oncotarget, 2020-06-30;11(26):2571-2585.
Species: Human
Sample Types: Serum
-
Human interleukin-4-treated regulatory macrophages promote epithelial wound healing and reduce colitis in a mouse model
Authors: TS Jayme, G Leung, A Wang, ML Workentine, S Rajeev, A Shute, BE Callejas, N Mancini, PL Beck, R Panaccione, DM McKay
Sci Adv, 2020-06-05;6(23):eaba4376.
Species: Human
Sample Types: Cell Culture Supernates
-
Immune modulation by complement receptor 3-dependent human monocyte TGF-beta1-transporting vesicles
Authors: LD Halder, EAH Jo, MZ Hasan, M Ferreira-G, T Krüger, M Westermann, DI Palme, G Rambach, N Beyersdorf, C Speth, ID Jacobsen, O Kniemeyer, B Jungnickel, PF Zipfel, C Skerka
Nat Commun, 2020-05-11;11(1):2331.
Species: Human, Mouse
Sample Types: Extracellular Vesicles
-
Mesenchymal Stem and Stromal Cells Harness Macrophage-Derived Amphiregulin to Maintain Tissue Homeostasis
Authors: JH Ko, HJ Kim, HJ Jeong, HJ Lee, JY Oh
Cell Rep, 2020-03-17;30(11):3806-3820.e6.
Species: Human
Sample Types: Cell Culture Supernates
-
Impaired Release of Neutrophil Extracellular Traps and Anemia-Associated T Cell Deficiency in Hereditary Hemorrhagic Telangiectasia
Authors: F Droege, E Pylaeva, E Siakaeva, S Bordbari, I Spyra, K Thangavelu, C Lueb, M Domnich, S Lang, U Geisthoff, J Jablonska
J Clin Med, 2020-03-12;9(3):.
Species: Human
Sample Types: Plasma
-
Odontogenic infection by Porphyromonas gingivalis exacerbates fibrosis in NASH via hepatic stellate cell activation
Authors: A Nagasaki, S Sakamoto, C Chea, E Ishida, H Furusho, M Fujii, T Takata, M Miyauchi
Sci Rep, 2020-03-05;10(1):4134.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-fibrotic effect of decorin in peritoneal dialysis and PD-associated peritonitis
Authors: N Jiang, Q Zhang, MK Chau, MS Yip, SL Lui, S Liu, KM Chu, HY Ngan, TM Chan, S Yung
EBioMedicine, 2020-02-12;52(0):102661.
Species: Human
Sample Types: Dialysate
-
Are the Immune Properties of Mesenchymal Stem Cells from Wharton's Jelly Maintained during Chondrogenic Differentiation?
Authors: C Voisin, G Cauchois, L Reppel, C Laroye, L Louarn, C Schenowitz, P Sonon, I Poras, V Wang, E D Carosell, N Benkirane-, P Moreau, N Rouas-Frei, D Bensoussan, C Huselstein
J Clin Med, 2020-02-04;9(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Growth Factors Delivery System for Skin Regeneration: An Advanced Wound Dressing
Authors: M Nardini, S Perteghell, L Mastracci, F Grillo, G Marrubini, E Bari, M Formica, C Gentili, R Cancedda, ML Torre, M Mastrogiac
Pharmaceutics, 2020-02-03;12(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
A-Kinase Anchoring Proteins Diminish TGF-&beta1/Cigarette Smoke-Induced Epithelial-To-Mesenchymal Transition
Authors: H Zuo, M Trombetta-, IH Heijink, CHTJ van der Ve, L Hesse, KN Faber, WJ Poppinga, H Maarsingh, VO Nikolaev, AM Schmidt
Cells, 2020-02-03;9(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
The Phenotype and Secretory Activity of Adipose-Derived Mesenchymal Stem Cells (ASCs) of Patients with Rheumatic Diseases
Authors: E Kuca-Warna, U Skalska, I Janicka, U Musia?owic, K Bonek, P G?uszko, P Szcz?sny, M Olesi?ska, E Kontny
Cells, 2019-12-17;8(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Tensin1 expression and function in chronic obstructive pulmonary disease
Authors: P Stylianou, K Clark, B Gooptu, D Smallwood, CE Brightling, Y Amrani, KM Roach, P Bradding
Sci Rep, 2019-12-12;9(1):18942.
Species: Human
Sample Types: Cell Culture Supernates
-
Blood cytokine, chemokine and growth factor profiling in a cohort of pregnant women from tropical countries
Authors: C Dobaño, A Bardají, S Kochar, SK Kochar, N Padilla, M López, HW Unger, M Ome-Kaius, ME Castellano, M Arévalo-He, D Hans, FE Martínez-E, C Bôtto-Mene, A Malheiros, M Desai, A Casellas, CE Chitnis, S Rogerson, I Mueller, C Menéndez, P Requena
Cytokine, 2019-09-09;125(0):154818.
Species: Human
Sample Types: Plasma
-
Changes on the Structural Architecture and Growth Factor Release, and Degradation in Equine Platelet-Rich Fibrin Clots Cultured Over Time
Authors: RF Jiménez-Ar, JU Carmona, M Prades
J. Equine Vet. Sci., 2019-08-09;82(0):102789.
Species: Equine
Sample Types: Serum
-
Inflammatory Bowel Disease Types Differ in Markers of Inflammation, Gut Barrier and in Specific Anti-Bacterial Response
Authors: S Coufal, N Galanova, L Bajer, Z Gajdarova, D Schierova, Z Jiraskova, K Kostovciko, Z Jackova, Z Stehlikova, P Drastich, H Tlaskalova, M Kverka
Cells, 2019-07-13;8(7):.
Species: Human
Sample Types: Serum
-
Type 1 diabetic mellitus patients with increased atherosclerosis risk display decreased CDKN2A/2B/2BAS gene expression in leukocytes
Authors: S Martínez-H, V Sánchez-Ga, A Herrero-Ce, Á Vinué, JT Real, JF Ascaso, DJ Burks, H González-N
J Transl Med, 2019-07-12;17(1):222.
Species: Human
Sample Types: Plasma
-
A Unique Pattern of Mesothelial-Mesenchymal Transition Induced in the Normal Peritoneal Mesothelium by High-Grade Serous Ovarian Cancer
Authors: M Paku?a, P Uruski, A Niklas, A Wo?niak, D Szpurek, A Tykarski, J Miku?a-Pie, K Ksi??ek
Cancers (Basel), 2019-05-13;11(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Plasma Rich in Growth Factors (PRGF) Disrupt the Blood-Brain Barrier Integrity and Elevate Amyloid Pathology in the Brains of 5XFAD Mice
Authors: QV Duong, ML Kintzing, WE Kintzing, IM Abdallah, AD Brannen, A Kaddoumi
Int J Mol Sci, 2019-03-25;20(6):.
Species: Human
Sample Types: Plasma
-
Effect of Propofol on the Production of Inflammatory Cytokines by Human Polarized Macrophages
Authors: T Kochiyama, X Li, H Nakayama, M Kage, Y Yamane, K Takamori, K Iwabuchi, E Inada
Mediators Inflamm., 2019-03-17;2019(0):1919538.
Species: Human
Sample Types: Cell Culture Supernates
-
Large-scale secretome analyses unveil the superior immunosuppressive phenotype of umbilical cord stromal cells as compared to other adult mesenchymal stromal cells
Authors: A Islam, I Urbarova, JA Bruun, I Martinez-Z
Eur Cell Mater, 2019-02-20;37(0):153-174.
Species: Human
Sample Types: Cell Culture Supernates
-
Inhalation of the prodrug PI3K inhibitor CL27c improves lung function in asthma and fibrosis
Authors: CC Campa, RL Silva, JP Margaria, T Pirali, MS Mattos, LR Kraemer, DC Reis, G Grosa, F Copperi, EM Dalmarco, RCP Lima-Júnio, S Aprile, V Sala, F Dal Bello, DS Prado, JC Alves-Filh, C Medana, GD Cassali, GC Tron, MM Teixeira, E Ciraolo, RC Russo, E Hirsch
Nat Commun, 2018-12-12;9(1):5232.
-
PDGF enhances the protective effect of adipose stem cell-derived extracellular vesicles in a model of acute hindlimb ischemia
Authors: T Lopatina, E Favaro, C Grange, M Cedrino, A Ranghino, S Occhipinti, S Fallo, F Buffolo, DA Gaykalova, MM Zanone, R Romagnoli, G Camussi
Sci Rep, 2018-12-04;8(1):17458.
Species: Human
Sample Types: Cell Culture Supernates
-
Four types of human platelet lysate, including one virally inactivated by solvent-detergent, can be used to propagate Wharton Jelly mesenchymal stromal cells
Authors: MS Chen, TJ Wang, HC Lin, B Thierry
New biotechnology, 2018-11-20;0(0):.
Species: Human
Sample Types: Platelet Lysates
-
Combining Calcium Phosphates with Polysaccharides: A Bone-Inspired Material Modulating Monocyte/Macrophage Early Inflammatory Response
Authors: H Rammal, C Bour, M Dubus, L Entz, L Aubert, SC Gangloff, S Audonnet, NB Bercu, F Boulmedais, C Mauprivez, H Kerdjoudj
Int J Mol Sci, 2018-11-03;19(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Inhibition of profibrotic microRNA-21 affects platelets and their releasate
Authors: T Barwari, S Eminaga, U Mayr, R Lu, PC Armstrong, MV Chan, M Sahraei, M Fernández-, T Moreau, J Barallobre, M Lynch, X Yin, C Schulte, F Baig, R Pechlaner, SR Langley, A Zampetaki, P Santer, M Weger, R Plasenzott, M Schosserer, J Grillari, S Kiechl, J Willeit, AM Shah, C Ghevaert, TD Warner, C Fernández-, Y Suárez, M Mayr
JCI Insight, 2018-11-02;3(21):.
Species: Human
Sample Types: Plasma
-
LINK-A lncRNA promotes migration and invasion of ovarian carcinoma cells by activating TGF-? pathway
Authors: J Ma, M Xue
Biosci. Rep., 2018-09-13;0(0):.
Species: Human
Sample Types: Plasma
-
Plasmodium falciparum Treated with Artemisinin-based Combined Therapy Exhibits Enhanced Mutation, Heightened Cortisol and TNF-? Induction
Authors: AO Idowu, S Bhattachar, S Gradus, W Oyibo, Z George, C Black, J Igietseme, AA Azenabor
Int J Med Sci, 2018-09-07;15(13):1449-1457.
Species: Human
Sample Types: Plasma
-
Tumor associated macrophages induce epithelial to mesenchymal transition via the EGFR/ERK1/2 pathway in head and neck squamous cell carcinoma
Authors: L Gao, W Zhang, WQ Zhong, ZJ Liu, HM Li, ZL Yu, YF Zhao
Oncol. Rep., 2018-08-17;40(5):2558-2572.
-
Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells
Authors: C Martin-Gal, F Béguet-Cre, L Marinelli, A Jamet, F Ledue, HM Blottière, N Lapaque
Sci Rep, 2018-06-27;8(1):9742.
Species: Human
Sample Types: Cell Culture Supernates
-
Crude leaf extracts of Piperaceae species downmodulate inflammatory responses by human monocytes
Authors: AC Finato, TF Fraga-Silv, AUC Prati, AA de Souza J, BF Mazzeu, LG Felippe, RA Pinto, MA Golim, MSP Arruda, M Furlan, J Venturini
PLoS ONE, 2018-06-20;13(6):e0198682.
Species: Human
Sample Types: Cell Culture Supernates
-
Helicobacter pylori-derived heat shock protein 60 increases the induction of regulatory T-cells associated with persistent infection
Authors: WT Hsu, SY Ho, TY Jian, HN Huang, YL Lin, CH Chen, TH Lin, MS Wu, CJ Wu, YL Chan, KW Liao
Microb. Pathog., 2018-04-13;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Exosomes Induce Fibroblast Differentiation into Cancer-associated Fibroblasts through TGF? Signaling
Authors: C R Goulet, G Bernard, S Tremblay, S Chabaud, S Bolduc, F Pouliot
Mol. Cancer Res., 2018-04-10;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
??CD4effector/effector memory T cells differentiate into productively and latently infected central memory T cells by TGF?1 during HIV-1 infection
Authors: KW Cheung, T Wu, SF Ho, YC Wong, L Liu, H Wang, Z Chen
J. Virol., 2018-03-28;0(0):.
Species: Bovine
Sample Types: Media
-
A model combining age, equivalent uniform dose and IL-8 may predict radiation esophagitis in patients with non-small cell lung cancer
Authors: S Wang, J Campbell, MH Stenmark, P Stanton, J Zhao, MM Matuszak, RK Ten Haken, FM Kong
Radiother Oncol, 2018-03-01;0(0):.
Species: Human
Sample Types: Plasma
-
Comparative Analysis of Different Platelet Lysates and Platelet Rich Preparations to Stimulate Tendon Cell Biology: An In Vitro Study
Authors: F Klatte-Sch, T Schmidt, M Uckert, S Scheffler, U Kalus, M Rojewski, H Schrezenme, A Pruss, B Wildemann
Int J Mol Sci, 2018-01-10;19(1):.
Species: Human
Sample Types: Plasma
-
Human M2 Macrophages Limit NK Cell Effector Functions through Secretion of TGF-? and Engagement of CD85j
Authors: SY Nuñez, A Ziblat, F Secchiari, NI Torres, JM Sierra, XL Raffo Irao, RE Araya, CI Domaica, MB Fuertes, NW Zwirner
J. Immunol., 2017-12-27;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Rheumatoid arthritis bone marrow environment supports Th17 response.
Authors: Ewa Kuca-Warna, Weronika Kurowska, Monika Prochorec, Anna Radzikows, Tomasz Burakowsk, Urszula Skalska, Magdalena Massalska, Magdalena Pleba?czy, Barbara Ma?dyk-No, Iwona S?owi?ska, Robert Gasik, W?odzimierz Ma?li?ski
Arthritis Research & Therapy, 2017-12-08;0(0):1478-6362.
Species: Human
Sample Types: Plasma
-
A Central Bioactive Region of LTBP-2 Stimulates the Expression of TGF-?1 in Fibroblasts via Akt and p38 Signalling Pathways
Authors: MA Sideek, J Smith, C Menz, JRJ Adams, AJ Cowin, MA Gibson
Int J Mol Sci, 2017-10-09;18(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
Activated Tissue-Resident Mesenchymal Stromal Cells Regulate Natural Killer Cell Immune and Tissue-Regenerative Function
Authors: RM Petri, A Hackel, K Hahnel, CA Dumitru, K Bruderek, SB Flohe, A Paschen, S Lang, S Brandau
Stem Cell Reports, 2017-08-03;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Composition and functionality of the intrahepatic innate lymphoid cell-compartment in human non-fibrotic and fibrotic livers
Authors: M Forkel, L Berglin, E Kekäläinen, A Carlsson, E Svedin, J Michaëlsso, M Nagasawa, JS Erjefält, M Mori, M Flodström-, A Bergquist, HG Ljunggren, M Westgren, U Lindforss, D Friberg, C Jorns, E Ellis, NK Björkström, J Mjösberg
Eur. J. Immunol., 2017-07-11;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Long-term cytokine and growth factor release from equine platelet-rich fibrin clots obtained with two different centrifugation protocols
Authors: RF Jiménez-Ar, C López, ME Álvarez, C Giraldo, M Prades, JU Carmona
Cytokine, 2017-06-23;97(0):149-155.
Species: Equine
Sample Types: Plasma
-
Neutrophil Extracellular Traps Reprogram IL-4/GM-CSF-Induced Monocyte Differentiation to Anti-inflammatory Macrophages
Authors: AB Guimarães-, NC Rochael, F Oliveira, J Echevarria, EM Saraiva
Front Immunol, 2017-05-17;8(0):523.
Species: Human
Sample Types: Cell Culture Supernates
-
In vitro and in vivo antagonistic activity of new probiotic culture against Clostridium difficile and Clostridium perfringens
Authors: N Goli?, K Veljovi?, N Popovi?, J Djoki?, I Strahini?, I Mrvaljevi?, A Terzi?-Vid
BMC Microbiol., 2017-05-06;17(1):108.
Species: Human
Sample Types: Cell Culture Supernates
-
A nonsense mutation in TLR5 is associated with survival and reduced IL-10 and TNF-? levels in human Melioidosis
Authors: P Chaichana, N Chantratit, F Brod, S Koosakulni, K Jenjaroen, S Chumseng, M Sumonwiriy, MN Burtnick, PJ Brett, P Teparrukku, D Limmathuro, NPJ Day, SJ Dunachie, TE West
PLoS Negl Trop Dis, 2017-05-05;11(5):e0005587.
Species: Human
Sample Types: Plasma
-
Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: a proof of concept of LSCC (low speed centrifugation concept)
Authors: K El Bagdadi, A Kubesch, X Yu, S Al-Maawi, A Orlowska, A Dias, P Booms, E Dohle, R Sader, CJ Kirkpatric, J Choukroun, S Ghanaati
Eur J Trauma Emerg Surg, 2017-03-21;0(0):.
Species: Human
Sample Types: Complex Sample Type
-
Extracellular Hsp90 and TGF? regulate adhesion, migration and anchorage independent growth in a paired colon cancer cell line model
Authors: JA de la Mare, T Jurgens, AL Edkins
BMC Cancer, 2017-03-16;17(1):202.
Species: Human
Sample Types: Cell Culture Supernates
-
On the cytokine/chemokine network during Plasmodium vivax malaria: new insights to understand the disease
Authors: NS Hojo-Souza, DB Pereira, FS de Souza, TA de Oliveir, MS Cardoso, MS Tada, GM Zanini, DC Bartholome, RT Fujiwara, LL Bueno
Malar. J, 2017-01-24;16(1):42.
Species: Human
Sample Types: Plasma
-
The differential expression of protease activated receptors contributes to functional differences between dark and fair keratinocytes
Authors: Meilang Xue
J. Dermatol. Sci, 2016-12-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Tumor-Activated Mesenchymal Stromal Cells Promote Osteosarcoma Stemness and Migratory Potential via IL-6 Secretion
PLoS ONE, 2016-11-16;11(11):e0166500.
Species: Human
Sample Types: Cell Culture Supernates
-
A novel immunomodulatory function of neutrophils on rhinovirus-activated monocytes in vitro
Authors: Brian G Oliver
Thorax, 2016-06-10;71(11):1039-1049.
Species: Human
Sample Types: Cell Culture Supernates
-
Differences in human mesenchymal stem cell secretomes during chondrogenic induction
Authors: OF Gardner, N Fahy, M Alini, MJ Stoddart
Eur Cell Mater, 2016-04-10;31(0):221-35.
Species: Human
Sample Types: Cell Culture Supernates
-
Regulating VEGF signaling in platelet concentrates via specific VEGF sequestering
Authors: DG Belair, NN Le, WL Murphy
Biomater Sci, 2016-03-24;4(5):819-25.
Species: Human
Sample Types: Plasma
-
Human Articular Cartilage Progenitor Cells Are Responsive to Mechanical Stimulation and Adenoviral-Mediated Overexpression of Bone-Morphogenetic Protein 2.
Authors: Neumann A, Gardner O, Williams R, Alini M, Archer C, Stoddart M
PLoS ONE, 2015-08-20;10(8):e0136229.
Species: Human
Sample Types: Cell Culture Supernates
-
The TNF Family Molecules LIGHT and Lymphotoxin alphabeta Induce a Distinct Steroid-Resistant Inflammatory Phenotype in Human Lung Epithelial Cells.
Authors: da Silva Antunes R, Madge L, Soroosh P, Tocker J, Croft M
J Immunol, 2015-07-24;195(5):2429-41.
Species: Human
Sample Types: Cell Culture Supernates
-
Lysosomal-associated Transmembrane Protein 4B (LAPTM4B) Decreases Transforming Growth Factor beta1 (TGF-beta1) Production in Human Regulatory T Cells.
Authors: Huygens C, Lienart S, Dedobbeleer O, Stockis J, Gauthy E, Coulie P, Lucas S
J Biol Chem, 2015-06-30;290(33):20105-16.
Species: Human
Sample Types: Cell Culture Supernates
-
Pluripotency gene expression and growth control in cultures of peripheral blood monocytes during their conversion into programmable cells of monocytic origin (PCMO): evidence for a regulatory role of autocrine activin and TGF-beta.
Authors: Ungefroren H, Hyder A, Hinz H, Groth S, Lange H, El-Sayed K, Ehnert S, Nussler A, Fandrich F, Gieseler F
PLoS ONE, 2015-02-23;10(2):e0118097.
Species: Human
Sample Types: Cell Culture Supernates
-
Antimony resistant Leishmania donovani but not sensitive ones drives greater frequency of potent T-regulatory cells upon interaction with human PBMCs: role of IL-10 and TGF-beta in early immune response.
Authors: Guha R, Das S, Ghosh J, Sundar S, Dujardin J, Roy S
PLoS Negl Trop Dis, 2014-07-17;8(7):e2995.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunomodulatory effects of bone marrow-derived mesenchymal stem cells on pro-inflammatory cytokine-stimulated human corneal epithelial cells.
Authors: Wen L, Zhu M, Madigan M, You J, King N, Billson F, McClellan K, Sutton G, Petsoglou C
PLoS ONE, 2014-07-08;9(7):e101841.
Species: Human
Sample Types: Cell Culture Supernates
-
Asthmatic airway epithelial cells differentially regulate fibroblast expression of extracellular matrix components.
Authors: Reeves S, Kolstad T, Lien T, Elliott M, Ziegler S, Wight T, Debley J
J Allergy Clin Immunol, 2014-05-27;134(3):663-670.e1.
Species: Human
Sample Types: Cell Culture Supernates
-
Diet-induced increase in plasma oxidized LDL promotes early fibrosis in a renal porcine auto-transplantation model.
Authors: Chatauret N, Favreau F, Giraud S, Thierry A, Rossard L, Le Pape S, Lerman L, Hauet T
J Transl Med, 2014-03-22;12(0):76.
Species: Human
Sample Types: Cell Culture Supernates
-
Loss of WISP2/CCN5 in estrogen-dependent MCF7 human breast cancer cells promotes a stem-like cell phenotype.
Authors: Ferrand N, Gnanapragasam A, Dorothee G, Redeuilh G, Larsen A, Sabbah M
PLoS ONE, 2014-02-03;9(2):e87878.
Species: Human
Sample Types: Cell Culture Supernates
-
TGF-beta-induced (TGFBI) protein in melanoma: a signature of high metastatic potential.
Authors: Lauden L, Siewiera J, Boukouaci W, Ramgolam K, Mourah S, Lebbe C, Charron D, Aoudjit F, Jabrane-Ferrat N, Al-Daccak R
J Invest Dermatol, 2014-01-17;134(6):1675-85.
Species: Human
Sample Types: Cell Culture Supernates
-
Activin A as a mediator of NK-dendritic cell functional interactions.
Authors: Seeger P, Bosisio D, Parolini S, Badolato R, Gismondi A, Santoni A, Sozzani S
J Immunol, 2014-01-06;192(3):1241-8.
Species: Human
Sample Types: Cell Culture Supernates
-
TLR ligands stimulation protects MSC from NK killing.
Authors: Giuliani M, Bennaceur-Griscelli A, Nanbakhsh A, Oudrhiri N, Chouaib S, Azzarone B, Durrbach A, Lataillade J
Stem Cells, 2014-01-01;32(1):290-300.
Species: Human
Sample Types: Cell Culture Supernates
-
Human neutrophil peptide-1 (HNP-1): a new anti-leishmanial drug candidate.
Authors: Dabirian S, Taslimi Y, Zahedifard F, Gholami E, Doustdari F, Motamedirad M, Khatami S, Azadmanesh K, Nylen S, Rafati S
PLoS Negl Trop Dis, 2013-10-17;7(10):e2491.
Species: Human
Sample Types: Cell Culture Supernates
-
Differential chemokine and cytokine production by neonatal bovine gammadelta T-cell subsets in response to viral toll-like receptor agonists and in vivo respiratory syncytial virus infection.
Authors: McGill J, Nonnecke B, Lippolis J, Reinhardt T, Sacco R
Immunology, 2013-06-01;139(2):227-44.
Species: Bovine
Sample Types: Cell Culture Supernates
-
Increase of O-glycosylated oncofetal fibronectin in high glucose-induced epithelial-mesenchymal transition of cultured human epithelial cells.
Authors: Alisson-Silva F, Freire-de-Lima L, Donadio J, Lucena M, Penha L, Sa-Diniz J, Dias W, Todeschini A
PLoS ONE, 2013-04-12;8(4):e60471.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of the breed, sex and age on cellular content and growth factor release from equine pure-platelet rich plasma and pure-platelet rich gel.
Authors: Giraldo C, Lopez C, Alvarez M, Samudio I, Prades M, Carmona J
BMC Vet Res, 2013-02-12;9(0):29.
Species: Equine
Sample Types: Plasma
-
Interaction with colon cancer cells hyperactivates TGF-beta signaling in cancer-associated fibroblasts.
Authors: Hawinkels L, Paauwe M, Verspaget H, Wiercinska E, van der Zon J, van der Ploeg K, Koelink P, Lindeman J, Mesker W, ten Dijke P, Sier C
Oncogene, 2012-12-03;33(1):97-107.
Species: Human
Sample Types: Cell Culture Supernates
-
Pentraxin 3 (PTX3) expression in allergic asthmatic airways: role in airway smooth muscle migration and chemokine production.
Authors: Zhang J, Shan L, Koussih L, Redhu NS, Halayko AJ, Chakir J, Gounni AS
PLoS ONE, 2012-04-18;7(4):e34965.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated adaptive immune responses are associated with latent infections of Wuchereria bancrofti.
Authors: Arndts K, Deininger S, Specht S, Klarmann U, Mand S, Adjobimey T, Debrah AY, Batsa L, Kwarteng A, Epp C, Taylor M, Adjei O, Layland LE, Hoerauf A
PLoS Negl Trop Dis, 2012-04-03;6(4):e1611.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin (IL)-4, IL-13, and IL-17A differentially affect the profibrotic and proinflammatory functions of fibrocytes from asthmatic patients.
Authors: Bellini A, Marini MA, Bianchetti L, Barczyk M, Schmidt M, Mattoli S
Mucosal Immunol, 2011-12-21;5(2):140-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased secretion of Gas6 by smooth muscle cells in human atherosclerotic carotid plaques.
Authors: Clauser S, Meilhac O, Bieche I, Raynal P, Bruneval P, Michel JB, Borgel D
Thromb. Haemost., 2011-11-10;107(1):140-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity.
Authors: Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Goncalves A, Andre P, Romagne F, Thibault G, Viens P, Birnbaum D, Bertucci F, Moretta A, Olive D
J. Clin. Invest., 2011-08-15;121(9):3609-22.
Species: Human
Sample Types: Cell Culture Supernates
-
Enrichment of Foxp3+ CD4 regulatory T cells in migrated T cells to IL-6- and IL-8-expressing tumors through predominant induction of CXCR1 by IL-6.
Authors: Eikawa S, Ohue Y, Kitaoka K
J. Immunol., 2010-11-03;185(11):6734-40.
Species: Human
Sample Types: Pleural Effusion
-
Soluble forms of VEGF receptor-1 and -2 promote vascular maturation via mural cell recruitment.
Authors: Lorquet S, Berndt S, Blacher S, Gengoux E, Peulen O, Maquoi E, Noel A, Foidart JM, Munaut C, Pequeux C
FASEB J., 2010-05-19;24(10):3782-95.
Species: Human
Sample Types: Cell Culture Supernates
-
Secretion of angiogenic growth factors by villous cytotrophoblast and extravillous trophoblast in early human pregnancy.
Authors: Lash GE, Naruse K, Innes BA, Robson SC, Searle RF, Bulmer JN
Placenta, 2010-03-24;31(6):545-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-programmed cell death 1 antibody reduces CD4+PD-1+ T cells and relieves the lupus-like nephritis of NZB/W F1 mice.
Authors: Kasagi S, Kawano S, Okazaki T, Honjo T, Morinobu A, Hatachi S, Shimatani K, Tanaka Y, Minato N, Kumagai S
J. Immunol., 2010-02-05;184(5):2337-47.
Species: Human
Sample Types: Serum
-
The roles of Wnt signaling modulators Dickkopf-1 (Dkk1) and Dickkopf-2 (Dkk2) and cell maturation state in osteogenesis on microstructured titanium surfaces.
Authors: Olivares-Navarrete R, Hyzy S, Wieland M, Boyan BD, Schwartz Z
Biomaterials, 2009-12-09;31(8):2015-24.
Species: Human
Sample Types: Cell Lysates
-
Helminth antigens modulate immune responses in cells from multiple sclerosis patients through TLR2-dependent mechanisms.
Authors: Correale J, Farez M
J. Immunol., 2009-10-07;183(9):5999-6012.
Species: Human
Sample Types: Cell Culture Supernates
-
TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23.
Authors: Aliahmadi E, Gramlich R, Grutzkau A, Hitzler M, Kruger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M
Eur. J. Immunol., 2009-05-01;39(5):1221-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Normally occurring NKG2D+CD4+ T cells are immunosuppressive and inversely correlated with disease activity in juvenile-onset lupus.
Authors: Dai Z, Turtle CJ, Booth GC, Riddell SR, Gooley TA, Stevens AM, Spies T, Groh V
J. Exp. Med., 2009-03-16;206(4):793-805.
Species: Human
Sample Types: Cell Culture Supernates
-
Growth factor regulation of growth factors in articular chondrocytes.
Authors: Shi S, Mercer S, Eckert GJ, Trippel SB
J. Biol. Chem., 2009-01-09;284(11):6697-704.
Species: Human
Sample Types: Whole Cells
-
Detuning CD8+ T lymphocytes by down-regulation of the activating receptor NKG2D: role of NKG2D ligands released by activated T cells.
Authors: Cerboni C, Ardolino M, Santoni A, Zingoni A
Blood, 2009-01-05;113(13):2955-64.
Species: Human
Sample Types: Cell Culture Supernates
-
Hyperexpression of NOD2 in intestinal mast cells of Crohn's disease patients: preferential expression of inflammatory cell-recruiting molecules via NOD2 in mast cells.
Authors: Okumura S, Yuki K, Kobayashi R, Okamura S, Ohmori K, Saito H, Ra C, Okayama Y
Clin. Immunol., 2008-10-19;130(2):175-85.
Species: Human
Sample Types: Cell Culture Supernates
-
Glioblastoma-secreted factors induce IGFBP7 and angiogenesis by modulating Smad-2-dependent TGF-beta signaling.
Authors: Pen A, Moreno MJ, Durocher Y, Deb-Rinker P, Stanimirovic DB
Oncogene, 2008-08-18;27(54):6834-44.
Species: Human
Sample Types: Cell Culture Supernates
-
Helminth infections associated with multiple sclerosis induce regulatory B cells.
Authors: Correale J, Farez M, Razzitte G
Ann. Neurol., 2008-08-01;64(2):187-99.
Species: Human
Sample Types: Cell Culture Supernates
-
Human CD4(+) T cells recognize an epitope within alpha-fetoprotein sequence and develop into TGF-beta-producing CD4(+) T cells.
Authors: Alisa A, Boswell S, Pathan AA, Ayaru L, Williams R, Behboudi S
J. Immunol., 2008-04-01;180(7):5109-17.
Species: Human
Sample Types: Cell Culture Supernates
-
Opposite regulation of transforming growth factors-beta2 and -beta3 expression in the human endometrium.
Authors: Gaide Chevronnay HP, Cornet PB, Delvaux D, Lemoine P, Courtoy PJ, Henriet P, Marbaix E
Endocrinology, 2007-11-26;149(3):1015-25.
Species: Human
Sample Types: Tissue Homogenates
-
Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells.
Authors: Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N, Descamps P, Gamelin E, Gascan H, Hebbar M, Jeannin P
Blood, 2007-09-11;110(13):4319-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Transforming growth factor-beta1 suppresses airway hyperresponsiveness in allergic airway disease.
Authors: Alcorn JF, Rinaldi LM, Jaffe EF, van Loon M, Bates JH, Janssen-Heininger YM, Irvin CG
Am. J. Respir. Crit. Care Med., 2007-08-29;176(10):974-82.
Species: Mouse
Sample Types: BALF
-
Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2.
Authors: Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z
Cancer Res., 2007-08-01;67(15):7458-66.
Species: Human
Sample Types: Cell Culture Supernates
-
Airway remodelling in children with cystic fibrosis.
Authors: Hilliard TN, Regamey N, Shute JK, Nicholson AG, Alton EW, Bush A, Davies JC
Thorax, 2007-05-25;62(12):1074-80.
Species: Human
Sample Types: BALF
-
Cancer immunoediting by GITR (glucocorticoid-induced TNF-related protein) ligand in humans: NK cell/tumor cell interactions.
Authors: Baltz KM, Krusch M, Bringmann A, Brossart P, Mayer F, Kloss M, Baessler T, Kumbier I, Peterfi A, Kupka S, Kroeber S, Menzel D, Radsak MP, Rammensee HG, Salih HR
FASEB J., 2007-03-14;21(10):2442-54.
Species: Human
Sample Types: Cell Culture Supernates
-
Coordinated functions of E-cadherin and transforming growth factor beta receptor II in vitro and in vivo.
Authors: Andl CD, Fargnoli BB, Okawa T, Bowser M, Takaoka M, Nakagawa H, Klein-Szanto A, Hua X, Herlyn M, Rustgi AK
Cancer Res., 2006-10-15;66(20):9878-85.
Species: Human
Sample Types: Cell Culture Supernates
-
Activation of transforming growth factor-beta by the integrin alphavbeta8 delays epithelial wound closure.
Authors: Neurohr C, Nishimura SL, Sheppard D
Am. J. Respir. Cell Mol. Biol., 2006-03-30;35(2):252-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of a short-course of amoxicillin/clavulanic acid on systemic and mucosal immunity in healthy adult humans.
Authors: Dufour V, Millon L, Faucher JF, Bard E, Robinet E, Piarroux R, Vuitton DA, Meillet D
Int. Immunopharmacol., 2005-05-01;5(5):917-28.
Species: Human
Sample Types: Feces
-
Leukemia inhibitory factor is linked to regulatory transplantation tolerance.
Authors: Metcalfe SM, Watson TJ, Shurey S, Adams E, Green CJ
Transplantation, 2005-03-27;79(6):726-30.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Association of type 2 cytokines with hepatic fibrosis in human Schistosoma mansoni infection.
Authors: de Jesus AR, Magalhaes A, Miranda DG, Miranda RG, Araujo MI, de Jesus AA, Silva A, Santana LB, Pearce E, Carvalho EM
Infect. Immun., 2004-06-01;72(6):3391-7.
Species: Human
Sample Types: Cell Culture Supernates
-
The selective estrogen receptor modulator arzoxifene and the rexinoid LG100268 cooperate to promote transforming growth factor beta-dependent apoptosis in breast cancer.
Authors: Rendi MH, Suh N, Lamph WW, Krajewski S, Reed JC, Heyman RA, Berchuck A, Liby K, Risingsong R, Royce DB, Williams CR, Sporn MB
Cancer Res., 2004-05-15;64(10):3566-71.
Species: Rat
Sample Types: Cell Culture Supernates
-
Effect of neutralizing transforming growth factor beta1 on the immune response against Mycobacterium tuberculosis in guinea pigs.
Authors: Allen SS, Cassone L, Lasco TM
Infect. Immun., 2004-03-01;72(3):1358-63.
Species: Guinea Pig
Sample Types: Pleural Effusion
-
Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue.
Authors: Henkel JS, Engelhardt JI, Siklos L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH
Ann. Neurol., 2004-02-01;55(2):221-35.
Species: Human
Sample Types: CSF
-
Role of TGF-beta1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue.
Authors: Heinemeier K, Langberg H, Olesen JL, Kjaer M
J. Appl. Physiol., 2003-08-15;95(6):2390-7.
Species: Human
Sample Types: Plasma
-
Coordinate cytokine gene expression in vivo following induction of tuberculous pleurisy in guinea pigs.
Authors: Allen SS, McMurray DN
Infect. Immun., 2003-08-01;71(8):4271-7.
Species: Guinea Pig
Sample Types: Pleural Fluid
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human TGF-beta 1 DuoSet ELISA
Average Rating: 4.1 (Based on 14 Reviews)
Have you used Human TGF-beta 1 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
we used this kit to quantify human TGF beta 1 in human healthy controls sera, it produces a very good standard curve, levels of TGF beta in in healthy controls are very low.
Very good performance of the kit, we diluted our EDTA plasma samples 1:20 and applied the kit on a 384-well format. Very reliable and replicates are tight.
Should mention this kit detects active TGF beta1 only in the description.
Need buy activation kit for total TGF beta1 (including the latent form)
I differentiated my THP-1 cells to macrophages and polarized using IL-4 and IL-13. Then I checked the TGF-beta and i have in trouble to detect the concentration for some of my samples. Then I always verify my experiments, and for the other assays I detected tgf-ß