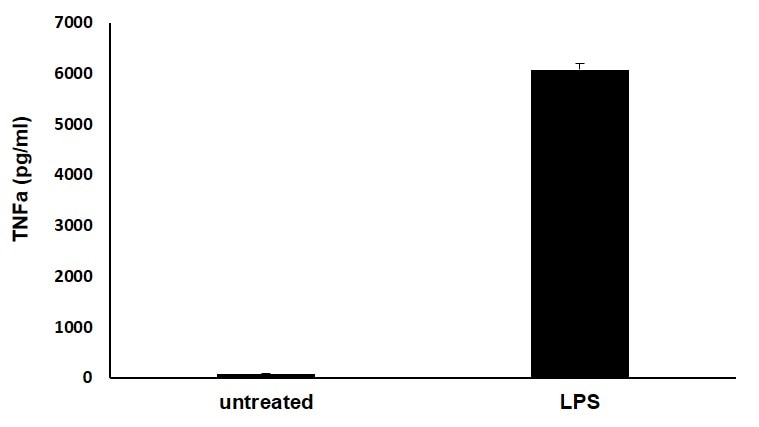
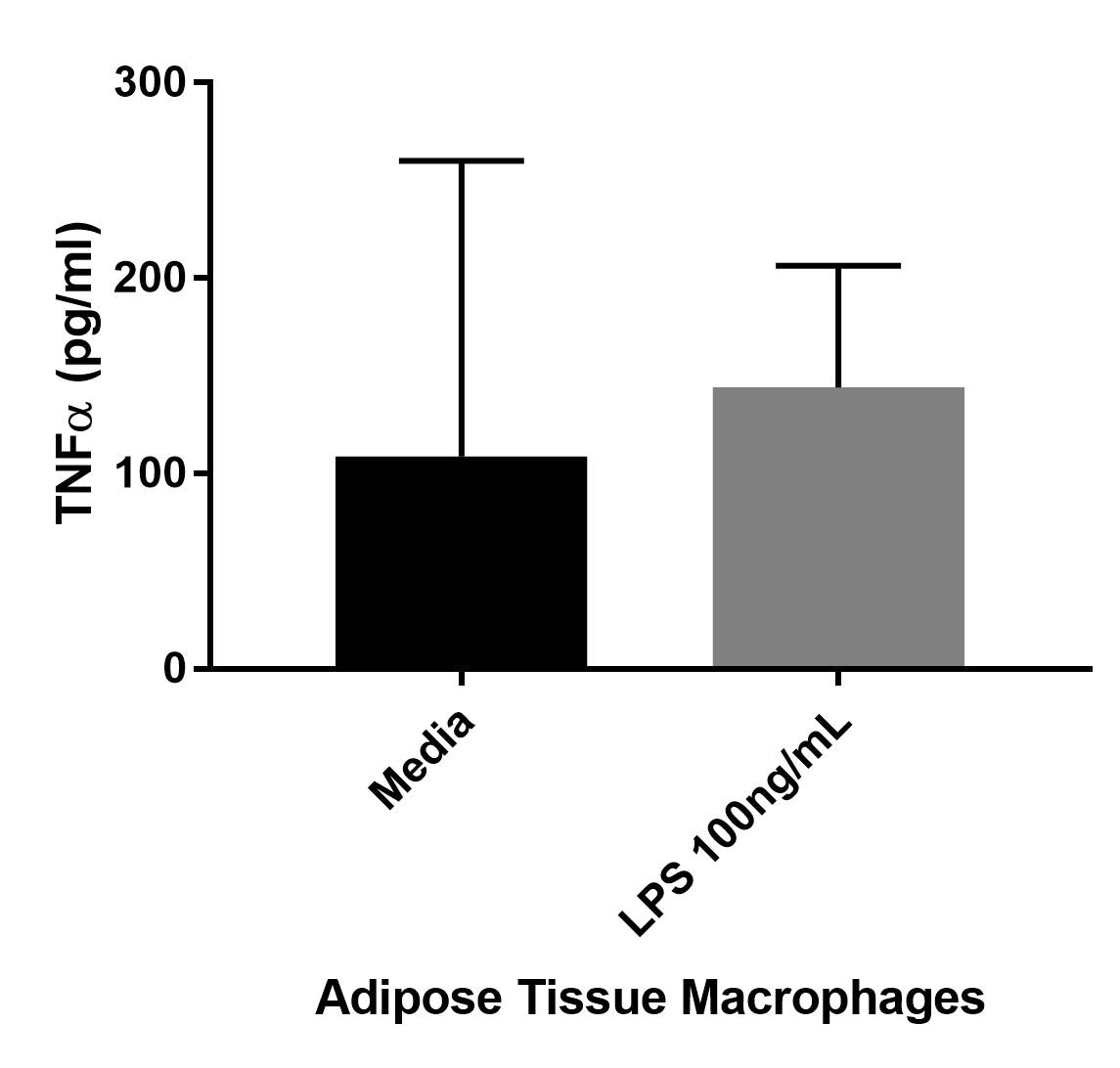
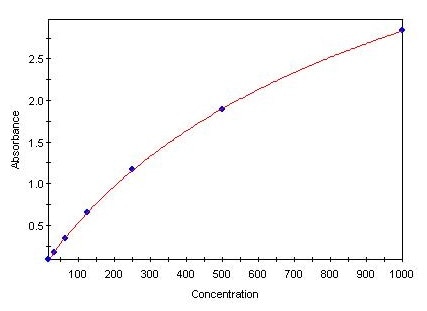
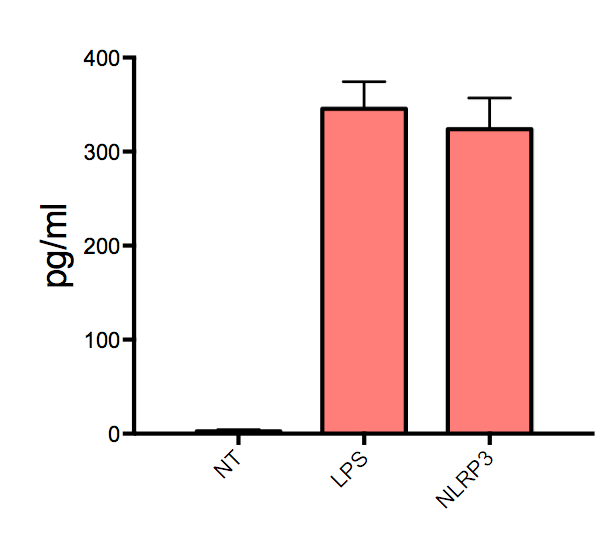
Human TNF-alpha DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human TNF-alpha. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: TNF-alpha
Tumor Necrosis Factor alpha (TNF-alpha ), also known as cachectin and TNFSF1A, is the prototypic ligand of the TNF superfamily (1). It is a pleiotropic molecule that plays a central role in inflammation, immune system development, apoptosis, and lipid metabolism (2-5). TNF-alpha is also involved in a number of pathological conditions including asthma, Crohn's disease, rheumatoid arthritis, neuropathic pain, obesity, type 2 diabetes, septic shock, autoimmunity, and cancer (5-11).
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human TNF-alpha DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
533
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Variable steady inflammation and inflammatory responses in precision-cut lung slices from various IPF lung Regions
Authors: Machahua, C;Marti, TM;Kewalramani, N;Dorn, P;Neubert, L;Petzold-Mügge, C;Funke-Chambour, M;
Respiratory research
Species: Human
Sample Types: Cell Culture Supernates
-
Polystyrene nanoplastics and lung cancer: Insights from network toxicology and mechanistic in vitro studies
Authors: Le, TK;Kuo, CH;Wu, CC;Chen, YT;Hsieh, SL;
Toxicology and applied pharmacology
Species: Human
Sample Types: Cell Culture Supernates
-
HO-1197 as a Multifaceted Therapeutic: Targeting the Cell Cycle, Angiogenesis, Metastasis, and Tumor Immunity in Hepatocellular Carcinoma
Authors: Song, Y;Lee, S;Heo, SW;Spohn, J;Schmiedel, D;Heo, T;Kim, S;Park, J;Seo, HR;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Amino acid-equipped poly(ester amide) nanoparticles for improved encapsulation efficiency of lipophilic anti-inflammatory 6BIGOE based on molecular dynamic simulations
Authors: Westhoff, J;Weber, C;Bachmann, V;Göppert, NE;Peric, M;George, A;Sierka, M;Hoeppener, S;Vilotijevic, I;Schubert, US;Werz, O;Fischer, D;
International journal of pharmaceutics
Species: Human
Sample Types: Cell Culture Supernates
-
Houttuynia cordata Exhibits Anti-Inflammatory Activity Against Interleukin-1?-Induced Inflammation in Human Gingival Epithelial Cells: An In Vitro Study
Authors: Kunimatsu, R;Ikeoka, S;Koizumi, Y;Odo, A;Tanabe, I;Kawashima, Y;Kiso, A;Hashii, Y;Tsuka, Y;Tanimoto, K;
Dentistry journal
Species: Human
Sample Types: Cell Culture Supernates
-
Modulation of IRAK4 as a Therapeutic Strategy Against Monosodium Urate- and Xanthine-Induced Inflammation in Macrophages and HepG2 Cells
Authors: Umar, S;Chang, HT;Maienschein-Cline, M;Ravindran, S;
Research square
Species: Human
Sample Types: Cell Culture Supernates
-
Reciprocal effects of conditioned medium on gene and protein expression of limbal epithelial cells and limbal fibroblasts in congenital aniridia
Authors: Berger, M;Szentmáry, N;Berger, T;Zimmermann, J;Trusen, S;Seitz, B;Fries, FN;Suiwal, S;Amini, M;Stachon, T;
PloS one
Species: Human
Sample Types: Cell Culture Supernates
-
Engineering and in vitro evaluation of semi-dissolving, hydrogel-forming polymeric microneedles for sustained-release drug delivery
Authors: Abdelghany, TM;Vo, N;Vukajlovic, D;Smith, E;Wong, JZ;Jackson, E;Hilkens, CMU;Lau, WM;Ng, KW;Novakovic, K;
International journal of pharmaceutics
Species: Human
Sample Types: Cell Culture Supernates
-
Estrogen induces the alternative activation of macrophages through binding to estrogen receptor-alpha
Authors: Belboul, A;Ashworth, J;Fadel, A;Mcloughlin, J;Mahmoud, A;El Mohtadi, M;
Experimental and molecular pathology
Species: Human
Sample Types: Cell Culture Supernates
-
Ginsenoside Rh2 Mitigates Endoplasmic Reticulum Stress-Induced Apoptosis and Inflammation and Through Inhibition of Hepatocyte-Macrophage Inflammatory Crosstalk
Authors: Park, S;Jeong, I;Kim, OK;
Nutrients
Species: Human
Sample Types: Cell Culture Supernates
-
Acidosis Licenses the NLRP3 Inflammasome-Inhibiting Effects of Beta-Hydroxybutyrate and Short-Chain Carboxylic Acids
Authors: Mank, MM;Zoller, KA;Fastiggi, VA;Ather, JL;Poynter, ME;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Cytokine profiles dynamics in COVID-19 patients: a longitudinal analysis of disease severity and outcomes
Authors: Ghaffarpour, S;Ghazanfari, T;Ardestani, SK;Naghizadeh, MM;Vaez Mahdavi, MR;Salehi, M;Majd, AMM;Rashidi, A;Chenary, MR;Mostafazadeh, A;Rezaei, A;Khodadadi, A;Iranparast, S;Khazaei, HA;
Scientific reports
Species: Human
Sample Types: Serum
-
Bidirectional Interaction Between Chronic Kidney Disease and Porphyromonas gingivalis Infection Drives Inflammation and Immune Dysfunction
Authors: Adamowicz, K;Lima Ribeiro, AS;Golda, A;Wadowska, M;Potempa, J;Schmaderer, C;Anders, HJ;Koziel, J;Lech, M;
Journal of immunology research
Species: Human
Sample Types: Cell Culture Supernates
-
Enhancing human NK cell antitumor function by knocking out SMAD4 to counteract TGF? and activin A suppression
Authors: Rea, A;Santana-Hernández, S;Villanueva, J;Sanvicente-García, M;Cabo, M;Suarez-Olmos, J;Quimis, F;Qin, M;Llorens, E;Blasco-Benito, S;Torralba-Raga, L;Perez, L;Bhattarai, B;Alari-Pahissa, E;Georgoudaki, AM;Balaguer, F;Juan, M;Pardo, J;Celià-Terrassa, T;Rovira, A;Möker, N;Zhang, C;Colonna, M;Spanholtz, J;Malmberg, KJ;Montagut, C;Albanell, J;Güell, M;López-Botet, M;Muntasell, A;
Nature immunology
Species: Human
Sample Types: Cell Culture Supernates
-
Ex Vivo Overactivation of Lymphocyte Subsets in Fibrotic Hypersensitivity Pneumonitis Is Blunted by a Sphingosine-1-Phosphate Receptor Ligand
Authors: Courtemanche, O;Huppé, CA;Blais-Lecours, P;Maranda, C;Morissette, MC;Blanchet, MR;Dion, G;Marsolais, D;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Effect of Free Long-Chain Fatty Acids on Anagen Induction: Metabolic or Inflammatory Aspect?
Authors: Pan, X;Vishnyakova, KS;Chermnykh, ES;Jasko, MV;Zhuravlev, AD;Verkhova, SS;Chegodaev, YS;Popov, MA;Nikiforov, NG;Yegorov, YE;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immunophenotypic Implications of Reverse-Circadian Glucocorticoid Treatment in Congenital Adrenal Hyperplasia
Authors: Nowotny, HF;Choi, H;Ziegler, S;Doll, N;Bäuerle, A;Welp, AC;Dubinski, I;Schiergens, K;Neumann, U;Tschaidse, L;Auer, MK;Rothenfusser, S;Schmidt, H;Reisch, N;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Bruton's tyrosine kinase inhibition re-sensitizes multidrug-resistant DLBCL tumors driven by BCL10 gain-of-function mutants to venetoclax
Authors: Coughlin, CA;Chahar, D;Lekakis, M;Youssfi, AA;Li, L;Roberts, E;Gallego, NC;Volmar, CH;Landgren, O;Brothers, S;Griswold, AJ;Amador, C;Bilbao, D;Maura, F;Schatz, JH;
Blood cancer journal
Species: Human
Sample Types: Cell Culture Supernates
-
Zn-doped CaP coating equips Ti implants with corrosion resistance, biomineralization, antibacterial and immunotolerant activities
Authors: Parau, AC;Büyüksungur, S;Li, G;Liu, Q;Badillo, E;Blum, L;Schmidt, J;Pana, I;Vitelaru, C;Marinescu, IM;Dinu, M;Smuglov, M;Schmuttermaier, C;Tanir, TE;Klüter, H;Hasirci, N;Kzhyshkowska, J;Dragomir, AV;
Journal of advanced research
Species: Human
Sample Types: Cell Culture Supernates
-
Alleviating symptoms of paediatric acute rhinosinusitis and acute otitis media with otorrhea using nasal-spraying Bacillus probiotics: a randomized controlled trial
Authors: Khieu, TH;Le, DP;Nguyen, BT;Ngo, BT;Chu, HT;Truong, DM;Nguyen, HM;Nguyen, AH;Pham, TD;Van Nguyen, AT;
Scientific reports
Species: Human
Sample Types: Middle Ear Lavage Fluid (MELF), Nasal Fluid
-
Cystatin C 3 (CST3) drives pathological progression in recurrent spontaneous abortion
Authors: Li, M;Xie, H;Du, X;
Journal of reproductive immunology
Species: Mouse
Sample Types: Cell Culture Supernates
-
A Novel Approach for In Vitro Testing and Hazard Evaluation of Nanoformulated RyR2-Targeting siRNA Drugs Using Human PBMCs
Authors: Bettinsoli, V;Melzi, G;Crea, A;Esposti, L;Iafisco, M;Catalucci, D;Ciana, P;Corsini, E;
Life
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of general versus spinal anesthesia on perioperative innate immune function in patients undergoing total hip arthroplasty
Authors: Jacobs, LMC;Bijkerk, V;van Eijk, LT;Joosten, LAB;Keijzer, C;Visser, J;Warlé, MC;
BMC anesthesiology
Species: Human
Sample Types: Plasma
-
Trabectedin enhances the antitumor effects of IL-12 in triple-negative breast cancer
Authors: Schwarz, E;Savardekar, H;Zelinskas, S;Mouse, A;Lapurga, G;Lyberger, J;Rivaldi, A;Ringwalt, EM;Miller, KE;Yu, L;Behbehani, GK;Cripe, TP;Carson, WE;
Cancer immunology research
Species: Human
Sample Types: Cell Culture Supernates
-
Calcitriol prevents SARS-CoV spike-induced inflammation in human trophoblasts through downregulating ACE2 and TMPRSS2 expression
Authors: Vargas-Castro, R;García-Quiroz, J;Olmos-Ortiz, A;Avila, E;Larrea, F;Díaz, L;
The Journal of steroid biochemistry and molecular biology
Species: Human
Sample Types: Cell Culture Supernates
-
Distinct inflammatory profiles in mustard lung: A study of sulfur mustard-exposed patients with serious pulmonary complications
Authors: Pourfarzam, S;Ardestani, SK;Jamali, T;Ghazanfari, H;Naghizadeh, MM;Faghihzadeh, S;Yaraee, R;Ghazanfari, Z;Ghazanfari, T;
International immunopharmacology
Species: Human
Sample Types: Serum, Sputum
-
SARS-CoV-2 Spike Protein Amplifies the Immunogenicity of Healthy Renal Epithelium in the Presence of Renal Cell Carcinoma
Authors: Somova, M;Simm, S;Ehrhardt, J;Schoon, J;Burchardt, M;Pinto, PC;
Cells
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA -
?-1,6-Glucan plays a central role in the structure and remodeling of the bilaminate fungal cell wall
Authors: Bekirian, C;Valsecchi, I;Bachellier-Bassi, S;Scandola, C;Guijarro, JI;Chauvel, M;Mourer, T;Gow, NAR;Aimanianda, VK;d'Enfert, C;Fontaine, T;
eLife
Species: Human
Sample Types: Cell Culture Supernates
-
Exploring the innate immune response in polycystic liver disease
Authors: Duijzer, R;Dalloyaux, D;Boerrigter, MM;Lemmers, H;Dijkstra, H;van Emst, L;Te Morsche, RHM;Jaeger, M;Joosten, LAB;Drenth, JPH;
Cytokine
Species: Human
Sample Types: Cell Culture Supernates
-
Commonly Used Dose of Montmorency Tart Cherry Powder Does Not Improve Sleep or Inflammation Outcomes in Individuals with Overweight or Obesity
Authors: Tucker, RM;Kim, N;Gurzell, E;Mathi, S;Chavva, S;Senthilkumar, D;Bartunek, O;Fenton, KC;Herndon-Fenton, SJ;Cardino, VN;Cooney, GM;Young, S;Fenton, JI;
Nutrients
Species: Human
Sample Types: Serum
-
Pseudomonas aeruginosa- mediated cardiac dysfunction is driven by extracellular vesicles released during infection
Authors: Kumar, N;Mattoo, SS;Sanghvi, S;Ellendula, MP;Mahajan, S;Planner, C;Bednash, JS;Khan, M;Ganesan, LP;Singh, H;Lafuse, WP;Wozniak, DJ;Rajaram, MVS;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Low Caloric Intake Confers Cardiovascular Protection and Improves Functional Capacity Without Affecting Immunological Response in Sedentary Older Adults
Authors: Moura-Maia, MS;Brill, B;Paula-Vieira, RHR;Ramos-Gomes, NV;Melamed, D;Silva-Reis, A;Wolpp, ETR;Moreira-Silva, NN;Bella, YF;Vieira, RP;
Nutrients
Species: Human
Sample Types: Plasma
-
Pancreatic Adenocarcinoma Up-Regulated Factor (PAUF) Transforms Human Monocytes into Alternative M2 Macrophages with Immunosuppressive Action
Authors: Kim, YJ;Nanda, SS;Jiang, F;Pyo, SY;Han, JY;Koh, SS;Kang, TH;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-1? induces trained innate immunity in human hematopoietic progenitor cells in vitro
Authors: Flores-Gomez, D;Hobo, W;van Ens, D;Kessler, EL;Novakovic, B;Schaap, NPM;Rijnen, WHC;Joosten, LAB;Netea, MG;Riksen, NP;Bekkering, S;
Stem cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of immunogenic HLA class I and II neoantigens using surrogate immunopeptidomes
Authors: Tokita, S;Fusagawa, M;Matsumoto, S;Mariya, T;Umemoto, M;Hirohashi, Y;Hata, F;Saito, T;Kanaseki, T;Torigoe, T;
Science advances
Species: Human
Sample Types: Cell Culture Supernates
-
Krebs cycle derivatives, dimethyl fumarate and itaconate, control metabolic reprogramming in inflammatory human microglia cell line
Authors: Sangineto, M;Ciarnelli, M;Moola, A;Naik Bukke, V;Cassano, T;Villani, R;Romano, AD;Di Gioia, G;Avolio, C;Serviddio, G;
Mitochondrion
Species: Human
Sample Types: Cell Culture Supernates
-
Modulating NLRP3 splicing with antisense oligonucleotides to control pathological inflammation
Authors: Klein, R;Onyuru, J;Viera, EM;Putnam, CD;Hoffman, HM;Hastings, ML;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Mitigated toxicity of polystyrene nanoplastics in combination exposure with copper ions by transformation into copper (I) oxide: Inhibits the oxidative potential of nanoplastics
Authors: Maruthupandy, M;Jeon, JH;Noh, J;Yang, SI;Cho, WS;
Chemosphere
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-inflammatory effects of phytocannabinoids and terpenes on inflamed Tregs and Th17 cells in vitro
Authors: Tan, KBC;Alexander, HD;Linden, J;Murray, EK;Gibson, DS;
Experimental and molecular pathology
Species: Human
Sample Types: Cell Culture Supernates
-
Trained immunity is regulated by T cell-induced CD40-TRAF6 signaling
Authors: Jacobs, MME;Maas, RJF;Jonkman, I;Negishi, Y;Tielemans Zamora, W;Yanginlar, C;van Heck, J;Matzaraki, V;Martens, JHA;Baltissen, M;Vermeulen, M;Morla-Folch, J;Ranzenigo, A;Wang, W;Umali, M;Ochando, J;van der Vlag, J;Hilbrands, LB;Joosten, LAB;Netea, MG;Mulder, WJM;van Leent, MMT;Mhlanga, MM;Teunissen, AJP;Rother, N;Duivenvoorden, R;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Interplay between host humoral pattern recognition molecules controls undue immune responses against Aspergillus fumigatus
Authors: Dellière, S;Chauvin, C;Wong, SSW;Gressler, M;Possetti, V;Parente, R;Fontaine, T;Krüger, T;Kniemeyer, O;Bayry, J;Carvalho, A;Brakhage, AA;Inforzato, A;Latgé, JP;Aimanianda, V;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Modulation of galectin-9 mediated responses in monocytes and T-cells by pregnancy-specific glycoprotein 1
Authors: Mendoza, M;Ballesteros, A;Rendon-Correa, E;Tonk, R;Warren, J;Snow, AL;Stowell, SR;Blois, SM;Dveksler, G;
The Journal of biological chemistry
Species: Human
Sample Types: Cell Culture Supernates
-
Human microglia-derived proinflammatory cytokines facilitate human retinal ganglion cell development and regeneration
Authors: Subramani, M;Lambrecht, B;Ahmad, I;
Stem cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of progestin-based contraceptives on HIV-associated vaginal immune biomarkers and microbiome in adolescent girls
Authors: Nasr, MA;Aldous, A;Daniels, J;Joy, C;Capozzi, E;Yang, M;Moriarty, P;Emmanuel-Baker, V;Malcolm, S;Green, SJ;Gomez-Lobo, V;Ghosh, M;
PloS one
Species: Human
Sample Types: Vaginal Swab
-
Long-Lasting Enhanced Cytokine Responses Following SARS-CoV-2 BNT162b2 mRNA Vaccination
Authors: Cab?u, G;Badii, M;Mirea, AM;Gaal, OI;van Emst, L;Popp, RA;Cri?an, TO;Joosten, LAB;
Vaccines
Species: Human
Sample Types: Cell Culture Supernates
-
A Synthetic Small Molecule, LGM2605: A Promising Modulator of Increased Pro-Inflammatory Cytokine and Osteoclast Differentiation by Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin
Authors: Kim, TJ;MacElroy, AS;Defreitas, A;Shenker, BJ;Boesze-Battaglia, K;
Dentistry journal
Species: Human
Sample Types: Cell Culture Supernates
-
Structural characterization of a proanthocyanidin-rich fraction from Hancornia speciosa leaves and its effect on the release of pro-inflammatory cytokines and oxidative stress in THP-1 cells
Authors: Carneiro Junior, WO;Guimarães, MLR;Freitas, KM;Pereira, RS;Pádua, RM;Campana, PRV;Braga, FC;
Journal of ethnopharmacology
Species: Human
Sample Types: Cell Culture Supernates
-
Bromo-substituted indirubins for inhibition of protein kinase-mediated signalling involved in inflammatory mediator release in human monocytes
Authors: Bachmann, V;Schädel, P;Westhoff, J;Peri?, M;Schömberg, F;Skaltsounis, AL;Höppener, S;Pantsar, T;Fischer, D;Vilotijevi?, I;Werz, O;
Bioorganic chemistry
Species: Human
Sample Types: Cell Culture Supernates
-
Cognitive function is associated with performance in time up and go test and with leptin blood levels in community-dwelling older women
Authors: da Costa Teixeira, LA;Soares, LA;Lima, LP;Avelar, NCP;de Moura, JA;Leopoldino, AAO;Figueiredo, PHS;Parentoni, AN;Mendonça, VA;Lacerda, ACR;
Scientific reports
Species: Human
Sample Types: Plasma
-
SARS-CoV-2 superinfection in CD14+ monocytes with latent human cytomegalovirus (HCMV) promotes inflammatory cascade
Authors: Payen, SH;Adhikari, K;Petereit, J;Uppal, T;Rossetto, CC;Verma, SC;
Virus research
Species: Human
Sample Types: Cell Culture Supernates
-
TAK1 inhibition leads to RIPK1-dependent apoptosis in immune-activated cancers
Authors: Damhofer, H;Tatar, T;Southgate, B;Scarneo, S;Agger, K;Shlyueva, D;Uhrbom, L;Morrison, GM;Hughes, PF;Haystead, T;Pollard, SM;Helin, K;
Cell death & disease
Species: Human
Sample Types: Cell Culture Supernates
-
Soluble CD27 is an intrathecal biomarker of T-cell-mediated lesion activity in multiple sclerosis
Authors: Cencioni, MT;Magliozzi, R;Palmisano, I;Suwan, K;Mensi, A;Fuentes-Font, L;Villar, LM;Fernández-Velasco, JI;Migallón, NV;Costa-Frossard, L;Monreal, E;Ali, R;Romozzi, M;Mazarakis, N;Reynolds, R;Nicholas, R;Muraro, PA;
Journal of neuroinflammation
Species: Human
Sample Types: CSF
-
Empowering Naringin's Anti-Inflammatory Effects through Nanoencapsulation
Authors: Marinho, A;Seabra, CL;Lima, SAC;Lobo-da-Cunha, A;Reis, S;Nunes, C;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Leishmania braziliensis enhances monocyte responses to promote anti-tumor activity
Authors: Dos Santos, JC;Moreno, M;Teufel, LU;Chilibroste, S;Keating, ST;Groh, L;Domínguez-Andrés, J;Williams, DL;Ma, Z;Lowman, DW;Ensley, HE;Novakovic, B;Ribeiro-Dias, F;Netea, MG;Chabalgoity, JA;Joosten, LAB;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Integration of Kupffer cells into human iPSC-derived liver organoids for modeling liver dysfunction in sepsis
Authors: Li, Y;Nie, Y;Yang, X;Liu, Y;Deng, X;Hayashi, Y;Plummer, R;Li, Q;Luo, N;Kasai, T;Okumura, T;Kamishibahara, Y;Komoto, T;Ohkuma, T;Okamoto, S;Isobe, Y;Yamaguchi, K;Furukawa, Y;Taniguchi, H;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
The Prolonged Activation of the p65 Subunit of the NF-Kappa-B Nuclear Factor Sustains the Persistent Effect of Advanced Glycation End Products on Inflammatory Sensitization in Macrophages
Authors: Assis, SIS;Amendola, LS;Okamoto, MM;Ferreira, GDS;Iborra, RT;Santos, DR;Santana, MFM;Santana, KG;Correa-Giannella, ML;Barbeiro, DF;Soriano, FG;Machado, UF;Passarelli, M;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Protective Effects of Inulin on Stress-Recurrent Inflammatory Bowel Disease
Authors: Du, Y;Kusama, K;Hama, K;Chen, X;Tahara, Y;Kajiwara, S;Shibata, S;Orihara, K;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Delayed CCL23 response is associated with poor outcomes after cardiac arrest
Authors: deKay, JT;Chepurko, E;Chepurko, V;Knudsen, L;Lord, C;Searight, M;Tsibulnikov, S;Robich, MP;Sawyer, DB;Gagnon, DJ;May, T;Riker, R;Seder, DB;Ryzhov, S;
Cytokine
Species: Human
Sample Types: Plasma
-
Efficacy of Lactiplantibacillus plantarum PBS067, Bifidobacterium animalis subsp. lactis BL050, and Lacticaseibacillus rhamnosus LRH020 in the Amelioration of Vaginal Microbiota in Post-Menopausal Women: A Prospective Observational Clinical Trial
Authors: Vicariotto, F;Malfa, P;Viciani, E;Dell'Atti, F;Squarzanti, DF;Marcante, A;Castagnetti, A;Ponchia, R;Governini, L;De Leo, V;
Nutrients
Species: Human
Sample Types: Vaginal Swab
-
Anti-inflammatory and urease inhibitory iridoid glycosides from Nyctanthes arbor-tristis Linn
Authors: Sana, T;Khan, M;Siddiqui, BS;Baig, TA;Jabeen, A;Begum, S;Hadda, TB;Shah, L;
Journal of ethnopharmacology
Species: Human
Sample Types: Cell Culture Supernates
-
The strong inverse association between plasma concentrations of soluble tumor necrosis factor receptors type 1 with adiponectin/leptin ratio in older women
Authors: Augusto da Costa Teixeira, L;Rocha-Vieira, E;Aparecida Soares, L;Mota de Oliveira, F;Aparecida Oliveira Leopoldino, A;Netto Parentoni, A;Amaral Mendonça, V;Cristina Rodrigues Lacerda, A;
Cytokine
Species: Human
Sample Types: Plasma
-
Direct comparison of canine and human immune responses using transcriptomic and functional analyses
Authors: Chow, L;Wheat, W;Ramirez, D;Impastato, R;Dow, S;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Circulating Adipocytokines and Insulin Like-Growth Factors and Their Modulation in Obesity-Associated Endometrial Cancer
Authors: Ray, I;Möller-Levet, CS;Michael, A;Butler-Manuel, S;Chatterjee, J;Tailor, A;Ellis, PE;Meira, LB;
Cancers
Species: Human
Sample Types: Plasma
-
Effects of Combinatory In Vitro Treatment with Immune Checkpoint Inhibitors and Cytarabine on the Anti-Cancer Immune Microenvironment in De Novo AML Patients
Authors: Bo?kun, ?;Starosz, A;Kr?towska-Grunwald, A;Wasiluk, T;Walewska, A;Wierzbowska, A;Moniuszko, M;Grubczak, K;
Cancers
Species: Human
Sample Types: Cell Culture Supernates
-
Resveratrol attenuates high glucose-induced inflammation and improves glucose metabolism in HepG2 cells
Authors: Tshivhase, AM;Matsha, T;Raghubeer, S;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
IFN-?3 is induced by Leishmania donovani and can inhibit parasite growth in cell line models but not in the mouse model, while it shows a significant association with leishmaniasis in humans
Authors: De, M;Sukla, S;Bharatiya, S;Keshri, S;Roy, DG;Roy, S;Dutta, D;Saha, S;Ejazi, SA;Ravichandiran, V;Ali, N;Chatterjee, M;Chinnaswamy, S;
Infection and immunity
Species: Human
Sample Types: Cell Culture Supernates
-
Highly secreted tryptophanyl tRNA synthetase 1 as a potential theranostic target for hypercytokinemic severe sepsis
Authors: Kim, YT;Huh, JW;Choi, YH;Yoon, HK;Nguyen, TT;Chun, E;Jeong, G;Park, S;Ahn, S;Lee, WK;Noh, YW;Lee, KS;Ahn, HS;Lee, C;Lee, SM;Kim, KS;Suh, GJ;Jeon, K;Kim, S;Jin, M;
EMBO molecular medicine
Species: Mouse
Sample Types: Plasma, Peritoneal Lavage Fluid
-
Oral Supplementation of Phosphatidylcholine Attenuates the Onset of a Diet-Induced Metabolic Dysfunction-Associated Steatohepatitis in Female C57BL/6J Mice
Authors: Sánchez, V;Baumann, A;Brandt, A;Wodak, M;Staltner, R;Bergheim, I;
Cellular and Molecular Gastroenterology and Hepatology
Species: Human
Sample Types: Cell Culture Supernates
-
?-endorphin suppresses ultraviolet B irradiation-induced epidermal barrier damage by regulating inflammation-dependent mTORC1 signaling
Authors: Kim, HS;Kim, HJ;Hong, YD;Son, ED;Cho, SY;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Investigation of the structural and immunomodulatory properties of alkali-soluble ?-glucans from Pleurotus eryngii fruiting bodies
Authors: Ellefsen, CF;Lindstad, L;Klau, LJ;Aachmann, FL;Hiorth, M;Samuelsen, ABC;
Carbohydrate polymers
Species: Human
Sample Types: Cell Culture Supernates
-
Macrophages derived from LPS-stimulated monocytes from individuals with subclinical atherosclerosis were characterized by increased pro-inflammatory activity
Authors: Nikiforov, NG;Kirichenko, TV;Kubekina, MV;Chegodaev, YS;Zhuravlev, AD;Ilchuk, LA;Nikolaeva, MA;Arefieva, AS;Popov, MA;Verkhova, SS;Bagheri Ekta, M;Orekhov, AN;
Cytokine
Species: Human
Sample Types: Cell Culture Supernates
-
Acid ceramidase regulates innate immune memory
Authors: Rother, N;Yanginlar, C;Prévot, G;Jonkman, I;Jacobs, M;van Leent, MMT;van Heck, J;Matzaraki, V;Azzun, A;Morla-Folch, J;Ranzenigo, A;Wang, W;van der Meel, R;Fayad, ZA;Riksen, NP;Hilbrands, LB;Lindeboom, RGH;Martens, JHA;Vermeulen, M;Joosten, LAB;Netea, MG;Mulder, WJM;van der Vlag, J;Teunissen, AJP;Duivenvoorden, R;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Functional genome analysis and anti-Helicobacter pylori activity of a novel bacteriocinogenic Lactococcus sp. NH2-7C from Thai fermented pork (Nham)
Authors: Kingkaew, E;Woraprayote, W;Booncharoen, A;Niwasabutra, K;Janyaphisan, T;Vilaichone, RK;Yamaoka, Y;Visessanguan, W;Tanasupawat, S;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
RNA m6A methylation modulates airway inflammation in allergic asthma via PTX3-dependent macrophage homeostasis
Authors: Han, X;Liu, L;Huang, S;Xiao, W;Gao, Y;Zhou, W;Zhang, C;Zheng, H;Yang, L;Xie, X;Liang, Q;Tu, Z;Yu, H;Fu, J;Wang, L;Zhang, X;Qian, L;Zhou, Y;
Nature communications
-
Ventilatory support and inflammatory peptides in hospitalised patients with COVID-19: A prospective cohort trial
Authors: Gysan, MR;Milacek, C;Bal, C;Zech, A;Brugger, J;Milos, RI;Antoniewicz, L;Idzko, M;Gompelmann, D;
PloS one
Species: Human
Sample Types: Serum
-
Human PBMCs Form Lipid Droplets in Response to Spike Proteins
Authors: Sivaraman, K;Pino, P;Raussin, G;Anchisi, S;Metayer, C;Dagany, N;Held, J;Wrenger, S;Welte, T;Wurm, MJ;Wurm, FM;Olejnicka, B;Janciauskiene, S;
Microorganisms
Species: Human
Sample Types: Cell Culture Supernates
-
Human interleukin-12? and EBI3 are cytokines with anti-inflammatory functions
Authors: Hildenbrand, K;Bohnacker, S;Menon, PR;Kerle, A;Prodjinotho, UF;Hartung, F;Strasser, PC;Catici, DAM;Rührnö beta l, F;Haslbeck, M;Schumann, K;Müller, SI;da Costa, CP;Esser-von Bieren, J;Feige, MJ;
Science advances
Species: Human
Sample Types: Cell Culture Supernates
-
Stratification of Amniotic Fluid Cells and Amniotic Fluid by Sex Opens Up New Perspectives on Fetal Health
Authors: Campesi, I;Capobianco, G;Cano, A;Lodde, V;Cruciani, S;Maioli, M;Sotgiu, G;Idda, ML;Puci, MV;Ruoppolo, M;Costanzo, M;Caterino, M;Cambosu, F;Montella, A;Franconi, F;
Biomedicines
Species: Human
Sample Types: Amniotic Fluid
-
Endoplasmic Reticulum Stress Promotes the Expression of TNF-? in THP-1 Cells by Mechanisms Involving ROS/CHOP/HIF-1? and MAPK/NF-?B Pathways
Authors: Akhter, N;Wilson, A;Arefanian, H;Thomas, R;Kochumon, S;Al-Rashed, F;Abu-Farha, M;Al-Madhoun, A;Al-Mulla, F;Ahmad, R;Sindhu, S;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of caatinga propolis from Mimosa tenuiflora and its constituents (santin, sakuranetin and kaempferide) on human immune cells
Authors: Sartori, AA;Son, NT;da Silva Honorio, M;Ripari, N;Santiago, KB;Gomes, AM;Zambuzzi, WF;Bastos, JK;Sforcin, JM;
Journal of ethnopharmacology
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of leptin on trained innate immunity and on systemic inflammation in subjects with obesity
Authors: Flores Gomez, D;Bekkering, S;Horst, RT;Cossins, B;van den Munckhof, ICL;Rutten, JHW;Joosten, LAB;Netea, MG;Riksen, NP;
Journal of leukocyte biology
Species: Human
Sample Types: Cell Culture Supernates
-
Targeting Toll-like receptor-driven systemic inflammation by engineering an innate structural fold into drugs
Authors: Petruk, G;Puthia, M;Samsudin, F;Petrlova, J;Olm, F;Mittendorfer, M;Hyllén, S;Edström, D;Strömdahl, AC;Diehl, C;Ekström, S;Walse, B;Kjellström, S;Bond, PJ;Lindstedt, S;Schmidtchen, A;
Nature communications
Species: Human
Sample Types: Plasma
-
Cytotoxic immune cells do not affect TDP-43 and p62 sarcoplasmic aggregation but influence TDP-43 localisation
Authors: McCord, B;Day, RM;
Scientific reports
Species: Human
Sample Types: Cell Culture Sueprnates
-
Social Determinants modulate NK cell activity via obesity, LDL, and DUSP1 signaling
Authors: Baumer, Y;Singh, K;Baez, AS;Gutierrez-Huerta, CA;Chen, L;Igboko, M;Turner, BS;Yeboah, JA;Reger, RN;Ortiz-Whittingham, LR;Bleck, CKE;Mitchell, VM;Collins, BS;Pirooznia, M;Dagur, PK;Allan, DSJ;Muallem-Schwartz, D;Childs, RW;Powell-Wiley, TM;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Efficient symptomatic treatment and viral load reduction for children with influenza virus infection by nasal-spraying Bacillus spore probiotics
Authors: Tran, TT;Phung, TTB;Tran, DM;Bui, HT;Nguyen, PTT;Vu, TT;Ngo, NTP;Nguyen, MT;Nguyen, AH;Nguyen, ATV;
Scientific reports
Species: Human
Sample Types: Nasopharyngeal aspirate
-
TNF? induced by DNA-sensing in macrophage compromises retinal pigment epithelial (RPE) barrier function
Authors: Twarog, M;Schustak, J;Xu, Y;Coble, M;Dolan, K;Esterberg, R;Huang, Q;Saint-Geniez, M;Bao, Y;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates, Cell Lysates
-
Deficient P. aeruginosa in MlaA/VacJ outer membrane lipoprotein shows decrease in rhamnolipids secretion, motility, and biofilm formation, and increase in fluoroquinolones susceptibility and innate immune response
Authors: Kaur, M;Buyck, JM;Goormaghtigh, F;Decout, JL;Mozaheb, N;Mingeot-Leclercq, MP;
Research in microbiology
Species: Human
Sample Types: Cell Culture Supernates
-
An oral cholera vaccine in the prevention and/or treatment of inflammatory bowel disease
Authors: Meunier, M;Spillmann, A;Rousseaux, C;Schwamborn, K;Hanson, M;
PloS one
Species: Human
Sample Types: Cell Culture Supernates
-
Adipose-Tumor Crosstalk contributes to CXCL5 Mediated Immune Evasion in PDAC
Authors: Walsh, RM;Ambrose, J;Jack, JL;Eades, AE;Bye, B;Ruckert, MT;Olou, AA;Messaggio, F;Chalise, P;Pei, D;VanSaun, MN;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Size- and oxidative potential-dependent toxicity of environmentally relevant expanded polystyrene styrofoam microplastics to macrophages
Authors: Jeon, S;Jeon, JH;Jeong, J;Kim, G;Lee, S;Kim, S;Maruthupandy, M;Lee, K;Yang, SI;Cho, WS;
Journal of hazardous materials
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of bile salt-stimulated lipase on blood cells and associations with disease activity in human inflammatory joint disorders
Authors: Lindquist, S;Wang, Y;Andersson, EL;Tsuji Grebe, S;Alenius, GM;Rantapää-Dahlqvist, S;Lundberg, L;Hernell, O;
PloS one
Species: Human
Sample Types: Serum
-
The influence of calcitriol and methylprednisolone on podocytes function in minimal change disease in vitro model
Authors: Grubczak, K;Starosz, A;Makowska, B;Parfienowicz, Z;Kr?towska, M;Naumnik, B;Moniuszko, M;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernatants
-
Diclofenac prodrugs nanoparticles: An alternative and efficient treatment for rheumatoid arthritis?
Authors: Hussain, S;Ur-Rehman, M;Arif, A;Cailleau, C;Gillet, C;Saleem, R;Noor, H;Naqvi, F;Jabeen, A;Atta-Ur-Rahman, ;Iqbal Choudhary, M;Fattal, E;Tsapis, N;
International journal of pharmaceutics
Species: Human
Sample Types: Cell Culture Supernates
-
Comparison of the effects of high-intensity interval and moderate-intensity continuous training on inflammatory markers, cardiorespiratory fitness, and quality of life in breast cancer patients
Authors: Isanejad, A;Nazari, S;Gharib, B;Motlagh, AG;
Journal of sport and health science
Species: Human
Sample Types: Serum
-
Natural mutations in the sensor kinase of the PhoPR two-component regulatory system modulate virulence of ancestor-like tuberculosis bacilli
Authors: Malaga, W;Payros, D;Meunier, E;Frigui, W;Sayes, F;Pawlik, A;Orgeur, M;Berrone, C;Moreau, F;Mazères, S;Gonzalo-Asensio, J;Rengel, D;Martin, C;Astarie-Dequeker, C;Mourey, L;Brosch, R;Guilhot, C;
PLoS pathogens
Species: Human
Sample Types: Cell Culture Supernates
-
Inflammatory biomarkers at different stages of Sarcopenia in older women
Authors: da Costa Teixeira, LA;Avelar, NCP;Peixoto, MFD;Parentoni, AN;Santos, JMD;Pereira, FSM;Danielewicz, AL;Leopoldino, AAO;Costa, SP;Arrieiro, AN;Soares, LA;da Silva Lage, VK;Prates, ACN;Taiar, R;de Carvalho Bastone, A;Oliveira, VC;Oliveira, MX;Costa, HS;Nobre, JNP;Brant, FP;Duarte, TC;Figueiredo, PHS;Mendonça, VA;Lacerda, ACR;
Scientific reports
Species: Human
Sample Types: Plasma
-
Prevention of M2 polarization and temporal limitation of differentiation in monocytes by extracellular ATP
Authors: Scherr, BF;Reiner, MF;Baumann, F;Höhne, K;Müller, T;Ayata, K;Müller-Quernheim, J;Idzko, M;Zissel, G;
BMC immunology
Species: Human
Sample Types: Cell Culture Supernates
-
Mild Cognitive Impairment Is Associated with Enhanced Activation of Th17 Lymphocytes in Non-Alcoholic Fatty Liver Disease
Authors: Fiorillo, A;Gallego, JJ;Casanova-Ferrer, F;Giménez-Garzó, C;Urios, A;Ballester, MP;Durbán, L;Rios, MP;Megías, J;San Miguel, T;Kosenko, E;Escudero-García, D;Benlloch, S;Felipo, V;Montoliu, C;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Serum TGF-?1 and CD14 Predicts Response to Anti-TNF-? Therapy in IBD
Authors: Coufal, S;Kverka, M;Kreisinger, J;Thon, T;Rob, F;Kolar, M;Reiss, Z;Schierova, D;Kostovcikova, K;Roubalova, R;Bajer, L;Jackova, Z;Mihula, M;Drastich, P;Tresnak Hercogova, J;Novakova, M;Vasatko, M;Lukas, M;Tlaskalova-Hogenova, H;Jiraskova Zakostelska, Z;
Journal of immunology research
Species: Human
Sample Types: Serum
-
Dimethyl itaconate induces long-term innate immune responses and confers protection against infection
Authors: Ferreira, AV;Kostidis, S;Groh, LA;Koeken, VACM;Bruno, M;Baydemir, I;Kilic, G;Bulut, Ö;Andriopoulou, T;Spanou, V;Synodinou, KD;Gkavogianni, T;Moorlag, SJCFM;Charlotte de Bree, L;Mourits, VP;Matzaraki, V;Koopman, WJH;van de Veerdonk, FL;Renieris, G;Giera, M;Giamarellos-Bourboulis, EJ;Novakovic, B;Domínguez-Andrés, J;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Omega-3 polyunsaturated fatty acids (n-3 PUFAs), somatic and fatigue symptoms in cardiovascular diseases comorbid major depressive disorder (MDD): A randomized controlled trial
Authors: Chang, JP;Chang, SS;Chen, HT;Chien, YC;Yang, HT;Huang, SY;Tseng, PT;Chang, CH;Galecki, P;Su, KP;
Brain, behavior, and immunity
Species: Human
Sample Types: Plasma
-
T Cells Expressing a Modified FcgammaRI Exert Antibody-Dependent Cytotoxicity and Overcome the Limitations of CAR T-cell Therapy against Solid Tumors
Authors: D Rasoulouni, N Santana-Ma, A Gutwillig, L Farhat-You, L Tal, S Amar, M Milyavsky, SSNA Muddineni, N Solomon, H Shpilt, S Dotan, N Pilpel, C Waskow, M Feinmesser, P Rider, Y Carmi
Cancer Immunology Research, 2023-06-02;0(0):OF1-OF18.
Species: Human
Sample Types: Cell Culture Supernates
-
Efficacy of Cooling Centers for Mitigating Physiological Strain in Older Adults during Daylong Heat Exposure: A Laboratory-Based Heat Wave Simulation
Authors: Meade, RD;Notley, SR;Akerman, AP;McCormick, JJ;King, KE;Sigal, RJ;Kenny, GP;
Environmental health perspectives
Species: Human
Sample Types: Serum
-
Functional IgG Autoantibodies against Autonomic Nervous System Receptors in Symptomatic Women with Silicone Breast Implants
Authors: Talalai, E;Gorobets, D;Halpert, G;Tsur, AM;Heidecke, H;Levy, Y;Watad, A;Blank, M;Michaelevski, I;Shoenfeld, Y;Amital, H;
Cells
Species: Human
Sample Types: Cell Culture Supernates
-
Preliminary Study on the Effect of a Night Shift on Blood Pressure and Clock Gene Expression
Authors: Toffoli, B;Tonon, F;Giudici, F;Ferretti, T;Ghirigato, E;Contessa, M;Francica, M;Candido, R;Puato, M;Grillo, A;Fabris, B;Bernardi, S;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Unsaturated oxidated fatty acid 12(S)-HETE attenuates TNF-? expression in TNF-?/IFN-?-stimulated human keratinocytes
Authors: Jeon, KB;Kim, J;Lim, CM;Park, JY;Kim, NY;Lee, J;Oh, DK;Yoon, DY;
International immunopharmacology
Species: Human
Sample Types: Cell Culture Supernates
-
Thrombocytopenia Independently Leads to Monocyte Immune Dysfunction
Authors: Li, C;Ture, SK;Nieves-Lopez, B;Blick-Nitko, SK;Maurya, P;Livada, AC;Stahl, TJ;Kim, M;Pietropaoli, AP;Morrell, CN;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Inhibition of BET Proteins Regulates Fcgamma Receptor Function and Reduces Inflammation in Rheumatoid Arthritis
Authors: D Shankar, G Merchand-R, NJ Buteyn, R Santhanam, H Fang, K Kumar, X Mo, LP Ganesan, W Jarjour, JP Butchar, S Tridandapa
International Journal of Molecular Sciences, 2023-04-21;24(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Induced pluripotent stem cell-derived extracellular vesicles promote wound repair in a diabetic mouse model via an anti-inflammatory immunomodulatory mechanism
Authors: D Levy, SN Abadchi, N Shababi, MR Ravari, NH Pirolli, C Bergeron, A Obiorah, F Mokhtari-E, S Gheshlaghi, JM Abraham, IM Smith, E Powsner, T Solomon, JW Harmon, SM Jay
bioRxiv : the preprint server for biology, 2023-03-23;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
CARD-only proteins regulate in�vivo inflammasome responses and ameliorate gout
Authors: S Devi, M Indramohan, E Jäger, J Carriere, LH Chu, L de Almeida, DR Greaves, C Stehlik, A Dorfleutne
Cell Reports, 2023-03-16;42(3):112265.
Species: Human
Sample Types: Cell Culture Supernates
-
Molecular Analysis of SARS-CoV-2 Spike Protein-Induced Endothelial Cell Permeability and vWF Secretion
Authors: Y Guo, V Kanamarlap
International Journal of Molecular Sciences, 2023-03-16;24(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
High Glucose Promotes Inflammation and Weakens Placental Defenses against E. coli and S. agalactiae Infection: Protective Role of Insulin and Metformin
Authors: R Jiménez-Es, D Vargas-Alc, P Flores-Esp, AC Helguera-R, O Villavicen, I Mancilla-H, C Irles, YD Torres-Ram, MY Valdespino, P Velázquez-, R Zamora-Esc, M Islas-Lópe, C Carranco-S, L Díaz, V Zaga-Clave, A Olmos-Orti
International Journal of Molecular Sciences, 2023-03-09;24(6):.
Species: Human
Sample Types: Placental Supernates
-
ApoM binds endotoxin contributing to neutralization and clearance by High Density Lipoprotein
Authors: H Mousa, A Thanassoul, SM Zughaier
Biochemistry and Biophysics Reports, 2023-02-28;34(0):101445.
Species: Human
Sample Types: Cell Culture Supernates
-
Inhibition of poly (ADP-ribose) Polymerase-1 (PARP-1) improves endothelial function in pulmonary hypertension
Authors: M Shafiq, ZR Lone, AO Abdulkaree, G Kaur, S Navya, H Singh, K Jagavelu, K Hanif
Pulmonary pharmacology & therapeutics, 2023-02-25;0(0):102200.
Species: Human
Sample Types: Cell Culture Supernates
-
In vitro and in vivo applications of a universal and synthetic thermo-responsive drug delivery hydrogel platform
Authors: H Gholizadeh, E Landh, DM Silva, A Granata, D Traini, P Young, A Fathi, S Maleknia, T Abrams, F Dehghani, H Xin Ong
International journal of pharmaceutics, 2023-02-25;635(0):122777.
Species: N/A
Sample Types: Hydrogel Supernates
-
Metabolic Reprogramming of Macrophages upon In Vitro Incubation with Aluminum-Based Adjuvant
Authors: R Danielsson, N Ferey, I Mile, H Eriksson
International Journal of Molecular Sciences, 2023-02-23;24(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Agricultural dust derived bacterial extracellular vesicle mediated inflammation is attenuated by DHA
Authors: AJ Heires, D Samuelson, D Villageliu, TM Nordgren, DJ Romberger
Scientific Reports, 2023-02-16;13(1):2767.
Species: Human
Sample Types: Cell Culture Supernates
-
Opposing Effects of Interleukin-36gamma and Interleukin-38 on Trained Immunity
Authors: LU Teufel, MG Netea, FL van de Vee, CA Dinarello, LAB Joosten, RJW Arts
International Journal of Molecular Sciences, 2023-01-24;24(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
ADS024, a Bacillus velezensis strain, protects human colonic epithelial cells against C. difficile toxin-mediated apoptosis
Authors: Y Xie, A Chupina Es, B Nelson, H Feng, C Pothoulaki, L Chesnel, HW Koon
Frontiers in Microbiology, 2023-01-10;13(0):1072534.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-inflammatory activities of Coleus forsteri (formerly Plectranthus forsteri) extracts on human macrophages and chemical characterization
Authors: M Nicolas, M Lasalo, S Chow, C Antheaume, K Huet, E Hnawia, GJ Guillemin, M Nour, M Matsui
Frontiers in Pharmacology, 2023-01-09;13(0):1081310.
Species: Human
Sample Types: Cell culture supernate
-
In vitro and in vivo applications of a universal and synthetic thermo-responsive drug delivery hydrogel platform
Authors: H Gholizadeh, E Landh, DM Silva, A Granata, D Traini, P Young, A Fathi, S Maleknia, T Abrams, F Dehghani, H Xin Ong
International journal of pharmaceutics, 2023;635(0):122777.
Species: N/A
Sample Types: Hydrogel Supernates
-
Tryptophan-dependent and -independent secretions of tryptophanyl- tRNA synthetase mediate innate inflammatory responses
Authors: TTT Nguyen, YH Choi, WK Lee, Y Ji, E Chun, YH Kim, JE Lee, HS Jung, JH Suh, S Kim, M Jin
Cell Reports, 2022-12-30;42(1):111905.
Species: Human
Sample Types: Cell Culture Supernates
-
Hepcidin as a Diagnostic Biomarker in Anaemic Lung Cancer Patients
Authors: K Wadowska, P B?asiak, A Rzechonek, I Bil-Lula, M ?liwi?ska-
Cancers, 2022-12-30;15(1):.
Species: Human
Sample Types: Serum
-
Pyruvate kinase M2 mediates IL-17 signaling in keratinocytes driving psoriatic skin inflammation
Authors: FP Veras, GA Publio, BM Melo, DS Prado, T Norbiato, NT Cecilio, C Hiroki, LEA Damasceno, R Jung, JE Toller-Kaw, TV Martins, SF Assunção, D Lima, MG Alves, GV Vieira, LA Tavares, ALR Alves-Reze, SH Karbach, HI Nakaya, TM Cunha, CS Souza, FQ Cunha, KU Sales, A Waisman, JC Alves-Filh
Cell Reports, 2022-12-27;41(13):111897.
Species: Human
Sample Types: Cell Culture Supernates
-
Asprosin Exerts Pro-Inflammatory Effects in THP-1 Macrophages Mediated via the Toll-like Receptor 4 (TLR4) Pathway
Authors: K Shabir, S Gharanei, S Orton, V Patel, P Chauhan, E Karteris, HS Randeva, JE Brown, I Kyrou
International Journal of Molecular Sciences, 2022-12-23;24(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Shrunken Pore Syndrome Is Frequently Occurring in Severe COVID-19
Authors: AO Larsson, M Hultström, R Frithiof, M Lipcsey, MB Eriksson
International Journal of Molecular Sciences, 2022-12-10;23(24):.
Species: Human
Sample Types: Plasma
-
Anti-tumor necrosis factor-alpha is potentially better than tumor necrosis factor-alpha as the biomarker for sarcopenia: Results from the I-Lan longitudinal aging study
Authors: WJ Lin, WJ Lee, LN Peng, YL Huang, CY Tung, CH Lin, TF Tsai, LK Chen
Experimental gerontology, 2022-12-09;172(0):112053.
Species: Human
Sample Types: Plasma
-
Phytohustil� and root extract of Althaea officinalis L. exert anti-inflammatory and anti-oxidative properties and improve the migratory capacity of endothelial cells in vitro
Authors: GA Bonaterra, J Schmitt, K Schneider, H Schwarzbac, H Aziz-Kalbh, O Kelber, J Müller, R Kinscherf
Frontiers in Pharmacology, 2022-12-08;13(0):948248.
Species: Human
Sample Types: Cell Culture Supernates
-
Genome-wide gain-of-function screening characterized lncRNA regulators for tumor immune response
Authors: Y Wang, Y Zhao, W Guo, GS Yadav, C Bhaskarla, Z Wang, X Wang, S Li, Y Wang, Y Chen, D Pattarayan, W Xie, S Li, B Lu, US Kammula, M Zhang, D Yang
Science Advances, 2022-12-07;8(49):eadd0005.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of exogenous IL-37 on immune cells from a patient carrying a potential IL37 loss-of-function variant: A case study
Authors: LU Teufel, CI van der Ma, V Klück, A Simons, A Hoischen, V Vernimmen, LAB Joosten, RJW Arts
Cytokine, 2022-12-05;162(0):156102.
Species: Human
Sample Types: Cell Culture Supernates
-
Adiponectin Is a Contributing Factor of Low Appendicular Lean Mass in Older Community-Dwelling Women: A Cross-Sectional Study
Authors: LAC Teixeira, JM Dos Santos, AN Parentoni, LP Lima, TC Duarte, FP Brant, CDC Neves, FSM Pereira, NCP Avelar, AL Danielewic, AAO Leopoldino, SP Costa, AN Arrieiro, LA Soares, ACN Prates, JNP Nobre, A de Carvalh, VC de Oliveir, MX Oliveira, PH Scheidt Fi, HS Costa, V Amaral Men, R Taiar, AC Rodrigues
Journal of Clinical Medicine, 2022-12-02;11(23):.
Species: Human
Sample Types: Plasma
-
A Single Chain Fragment Variant Binding Misfolded Alpha-Synuclein Exhibits Neuroprotective and Antigen-Specific Anti-Inflammatory Properties
Authors: M Fassler, C Benaim, J George
Cells, 2022-11-29;11(23):.
Species: Human
Sample Types: Whole Cells
-
Distinct changes in endosomal composition promote NLRP3 inflammasome activation
Authors: Z Zhang, R Venditti, L Ran, Z Liu, K Vivot, A Schürmann, JS Bonifacino, MA De Matteis, R Ricci
Nature Immunology, 2022-11-28;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Blood orange juice intake modulates plasma and PBMC microRNA expression in overweight and insulin resistance women: impact on MAPK and NFkappaB signaling pathways
Authors: VC Capetini, BJ Quintanilh, DC de Oliveir, AH Nishioka, LA de Matos, LRP Ferreira, FM Ferreira, GR Sampaio, NMA Hassimotto, FM Lajolo, RA Fock, MM Rogero
The Journal of nutritional biochemistry, 2022-11-26;0(0):109240.
Species: Human
Sample Types: Plasma
-
Large libraries of single-chain trimer peptide-MHCs enable rapid antigen-specific CD8+ T cell discovery and analysis
Authors: J Heath, W Chour, J Choi, J Xie, M Chaffee, T Schmitt, K Finton, D Delucia, A Xu, Y Su, D Chen, R Zhang, D Yuan, S Hong, A Ng, J Butler, R Edmark, L Jones, K Murray, S Peng, G Li, R Strong, J Lee, J Goldman, P Greenberg
Research square, 2022-11-18;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
EC-18 prevents autoimmune arthritis by suppressing inflammatory cytokines and osteoclastogenesis
Authors: JS Park, SC Yang, HY Jeong, SY Lee, JG Ryu, JW Choi, HY Kang, SM Kim, SH Hwang, ML Cho, SH Park
Arthritis Research & Therapy, 2022-11-17;24(1):254.
Species: Human
Sample Types: Serum
-
Involvement of glycoinositolphospholipid from Trypanosoma cruzi and macrophage migration inhibitory factor in proinflammatory mechanisms promoting cardiovascular injury mechanisms promoting cardiovascular inflammation tThe combined action of glycoinositolphospholipid from Trypanosoma cruzi and macrophage migration inhibitory factor increases proinflammatory mediator production by cardiomyocytes and vascular endothelial cells
Authors: CS Rigazio, N Mariz-Pont, EP Caballero, FN Penas, NB Goren, MH Santamaría, RS Corral
Microbial pathogenesis, 2022-11-12;0(0):105881.
Species: Human
Sample Types: Cell Culture Supernates
-
CD169+ Monocyte and Regulatory T Cell Subsets Are Associated with Disease Activity in Rheumatoid Arthritis
Authors: AJ Eakin, T Ahmed, CM McGeough, S Drain, HD Alexander, GD Wright, PV Gardiner, D Small, AJ Bjourson, DS Gibson
Journal of personalized medicine, 2022-11-09;12(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Transcriptional licensing is required for Pyrin inflammasome activation in human macrophages and bypassed by mutations causing familial Mediterranean fever
Authors: MSJ Mangan, F Gorki, K Krause, A Heinz, A Pankow, T Ebert, D Jahn, K Hiller, V Hornung, M Maurer, FI Schmidt, R Gerhard, E Latz
PloS Biology, 2022-11-07;20(11):e3001351.
Species: Human
Sample Types: Cell Culture Supernates
-
On the Bioactivity of Echinacea purpurea Extracts to Modulate the Production of Inflammatory Mediators
Authors: SF Vieira, VMF Gonçalves, CP Llaguno, F Macías, ME Tiritan, RL Reis, H Ferreira, NM Neves
Oncogene, 2022-11-06;23(21):.
Species: Human
Sample Types: Cell Culture Supernates
-
Enzyme-Treated Caviar Prevents UVB Irradiation-Induced Skin Photoaging
Authors: J Park, D Kim, M Lee, S Han, W Jun, HM Jung, YK Koo, GH Na, SH Han, J Han, OK Kim
Oncogene, 2022-10-30;20(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Negative Magnetic Sorting Preserves the Functionality of Ex Vivo Cultivated Non-Adherent Human Monocytes
Authors: M Hornschuh, V Haas, PP Winkel, MY Gökyildiri, CS Mullins, IM Wrobel, C Manteuffel, E Wirthgen
Biology, 2022-10-27;11(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Signal strength of STING activation determines cytokine plasticity and cell death in human monocytes
Authors: D Kabelitz, M Zarobkiewi, M Heib, R Serrano, M Kunz, G Chitadze, D Adam, C Peters
Scientific Reports, 2022-10-24;12(1):17827.
Species: Human
Sample Types: Cell Culture Supernates
-
Adipose-Derived Stem Cell Exosomes Inhibit Hypertrophic Scaring Formation by Regulating Th17/Treg Cell Balance
Authors: H Wang, Y Li, Z Yue, Y Liu, Q Chen, D Hu, J Han
BioMed Research International, 2022-10-12;2022(0):9899135.
Species: Human
Sample Types: Tissue Homogenates
-
Hybrid Mineral/Organic Material Induces Bone Bridging and Bone Volume Augmentation in Rat Calvarial Critical Size Defects
Authors: M Dubus, L Scomazzon, C Ledouble, J Braux, A Beljebbar, L Van Gulick, A Baldit, C Gorin, H Alem, N Bouland, M Britton, J Schiavi, TJ Vaughan, C Mauprivez, H Kerdjoudj
Cells, 2022-09-14;11(18):.
Species: Human
Sample Types: Cell Culture Supernates
-
Human Allogeneic Liver-Derived Progenitor Cells Significantly Improve NAFLD Activity Score and Fibrosis in Late-Stage NASH Animal Model
Authors: M Najimi, S Michel, MM Binda, K Gellynck, N Belmonte, G Mazza, N Gordillo, Y Vainilovic, E Sokal
Cells, 2022-09-13;11(18):.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-Fibrotic Effect of SDF-1beta Overexpression in Bleomycin-Injured Rat Lung
Authors: K Fytianos, R Schliep, S Mykoniati, P Khan, KE Hostettler, M Tamm, A Gazdhar, L Knudsen, T Geiser
Pharmaceutics, 2022-08-27;14(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Pyrroloquinoline quinone (PQQ) improves pulmonary hypertension by regulating mitochondrial and metabolic functions
Authors: M Shafiq, ZR Lone, P Bharati, S Mahapatra, P Rai, N Khandelwal, AN Gaikwad, K Jagavelu, K Hanif
Pulmonary pharmacology & therapeutics, 2022-08-25;76(0):102156.
Species: Rat
Sample Types: Tissue Homogenates
-
Nicotine in Combination with SARS-CoV-2 Affects Cells Viability, Inflammatory Response and Ultrastructural Integrity
Authors: L Sansone, A de Iure, M Cristina, M Belli, L Vitiello, F Marcolongo, A Rosellini, L Macera, PG Spezia, C Tomino, S Bonassi, MA Russo, F Maggi, P Russo
International Journal of Molecular Sciences, 2022-08-22;23(16):.
Species: Human
Sample Types: Cell Culture Supernates
-
SARS-CoV-2 Spike Does Not Possess Intrinsic Superantigen-like Inflammatory Activity
Authors: C Amormino, V Tedeschi, G Paldino, S Arcieri, MT Fiorillo, A Paiardini, L Tuosto, M Kunkl
Cells, 2022-08-15;11(16):.
Species: Human
Sample Types: Cell Culture Supernates
-
Development of an Electrochemical CCL5 Chemokine Immunoplatform for Rapid Diagnosis of Multiple Sclerosis
Authors: S Guerrero, E Sánchez-Ti, L Agüí, A González-C, P Yáñez-Sede, JM Pingarrón
Biosensors, 2022-08-07;12(8):.
Species: Human
Sample Types: Serum
-
The effect of sex, menstrual cycle phase and oral contraceptive use on intestinal permeability and ex-vivo monocyte TNFalpha release following treatment with lipopolysaccharide and hyperthermia
Authors: TR Flood, MR Kuennen, SD Blacker, SD Myers, EF Walker, BJ Lee
Cytokine, 2022-08-06;158(0):155991.
Species: Human
Sample Types: Plasma
-
Cluster of Differentiation 147 (CD147) serves as a promoter of atherosclerosis in patients with cerebral infarction
Authors: GJ Wang, X Dong, Y Li
European review for medical and pharmacological sciences, 2022-08-01;26(16):5710-5717.
Species: Human
Sample Types: Serum
-
Development of a rapid in vitro pre-screen for distinguishing effective liposome-adjuvant delivery systems
Authors: LAJ Feather, V Nadella, E Kastner, Y Perrie, AC Hilton, A Devitt
Scientific Reports, 2022-07-20;12(1):12448.
Species: Human
Sample Types: Cell Culture Supernates
-
Nasal-spraying Bacillus spores as an effective symptomatic treatment for children with acute respiratory syncytial virus infection
Authors: DM Tran, TT Tran, TTB Phung, HT Bui, PTT Nguyen, TT Vu, NTP Ngo, MT Nguyen, AH Nguyen, ATV Nguyen
Scientific Reports, 2022-07-20;12(1):12402.
Species: Human
Sample Types: Nasal Lavage Fluid
-
Melanoma Stem Cells Educate Neutrophils to Support Cancer Progression
Authors: M Anselmi, F Fontana, M Marzagalli, N Gagliano, M Sommariva, P Limonta
Cancers, 2022-07-13;14(14):.
Species: Human
Sample Types: Cell Culture Supernates
-
Lithocholic acid inhibits dendritic cell activation by reducing intracellular glutathione via TGR5 signaling
Authors: J Hu, Y Zhang, S Yi, C Wang, X Huang, S Pan, J Yang, G Yuan, S Tan, H Li
International journal of biological sciences, 2022-07-11;18(11):4545-4559.
Species: Human
Sample Types: Cell Culture Supernates
-
Commercially Available Flavonols Are Better SARS-CoV-2 Inhibitors than Isoflavone and Flavones
Authors: OA Chaves, N Fintelman-, X Wang, CQ Sacramento, JR Temerozo, AC Ferreira, M Mattos, F Pereira-Du, PT Bozza, HC Castro-Far, JJ Russo, J Ju, TML Souza
Viruses, 2022-06-30;14(7):.
Species: Human
Sample Types: Cell Culture Supernates
-
Inhibition of Recruitment and Activation of Neutrophils by Pyridazinone-Scaffold-Based Compounds
Authors: A Moniot, J Braux, R Siboni, C Guillaume, S Audonnet, I Allart-Sim, J Sapi, R Tirouvanzi, S Gérard, SC Gangloff, F Velard
International Journal of Molecular Sciences, 2022-06-29;23(13):.
Species: Human
Sample Types: Cell Culture Supernates
-
TIRAP/Mal Positively Regulates TLR8-Mediated Signaling via IRF5 in Human Cells
Authors: KE Nilsen, A Skjesol, J Frengen Ko, T Espevik, J Stenvik, M Yurchenko
Oncogene, 2022-06-22;10(7):.
Species: Human
Sample Types: Cell Culture Supernates
-
From EGFR kinase inhibitors to anti-inflammatory drugs: Optimization and biological evaluation of (4-(phenylamino)quinazolinyl)-phenylthiourea derivatives as novel NF-kappaB inhibitors
Authors: RA Wagdy, PJ Chen, MM Hamed, SS Darwish, SH Chen, AH Abadi, M Abdel-Hali, TL Hwang, M Engel
Bioorganic chemistry, 2022-06-18;127(0):105977.
Species: Human
Sample Types: Cell Culture Supernates
-
Antibacterial and Anti-Inflammatory Effects of Apolipoprotein E
Authors: M Puthia, JK Marzinek, G Petruk, G Ertürk Ber, PJ Bond, J Petrlova
Biomedicines, 2022-06-17;10(6):.
Species: Human
Sample Types: Plasma
-
Sirtuin 1 Induces Choroidal Neovascularization and Triggers Age-Related Macular Degeneration by Promoting LCN2 through SOX9 Deacetylation
Authors: S Zhao, Z Huang, H Jiang, J Xiu, L Zhang, Q Long, Y Yang, L Yu, L Lu, H Gu
Oncogene, 2022-06-09;2022(0):1671438.
Species: Human
Sample Types: Cell Culture Supernates
-
Quality assessment of a serum and xenofree medium for the expansion of human GMP-grade mesenchymal stromal cells
Authors: C Aussel, E Busson, H Vantomme, J Peltzer, C Martinaud
PeerJ, 2022-05-30;10(0):e13391.
Species: Human
Sample Types: Cell Culture Supernates
-
Decreased expression of ErbB2 on left ventricular epicardial cells in patients with diabetes mellitus
Authors: JT de Kay, J Carver, B Shevenell, AM Kosta, S Tsibulniko, E Certo, DB Sawyer, S Ryzhov, MP Robich
Cellular Signalling, 2022-05-21;96(0):110360.
Species: Human
Sample Types: Plasma
-
Toxoplasma gondii Rhoptry Protein 7 (ROP7) Interacts with NLRP3 and Promotes Inflammasome Hyperactivation in THP-1-Derived Macrophages
Authors: L Zhu, W Qi, G Yang, Y Yang, Y Wang, L Zheng, Y Fu, X Cheng
Cells, 2022-05-12;11(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
Green and Roasted Coffee Extracts Inhibit Interferon-beta Release in LPS-Stimulated Human Macrophages
Authors: V Artusa, C Ciaramelli, A D'Aloia, FA Facchini, N Gotri, A Bruno, B Costa, A Palmioli, C Airoldi, F Peri
Frontiers in Pharmacology, 2022-05-05;13(0):806010.
Species: Human
Sample Types: Cell Culture Supernates
-
Mycobacterium avium-intracellulare complex promote release of pro-inflammatory enzymes matrix metalloproteinases by inducing neutrophil extracellular trap formation
Authors: K Nakamura, H Nakayama, S Sasaki, K Takahashi, K Iwabuchi
Scientific Reports, 2022-04-11;12(1):5181.
Species: Human
Sample Types: Cell Culture Supernates
-
The Effects of Freshwater Clam (Corbicula fluminea) Extract on Serum Tumor Necrosis Factor-Alpha (TNF-alpha) in Prediabetic Patients in Taiwan
Authors: TH Huang, CH Ke, CC Chen, CH Chuang, KW Liao, YH Shiao, CS Lin
Marine Drugs, 2022-04-10;20(4):.
Species: Human
Sample Types: Serum
-
Isolation and characterization of anti-inflammatory and anti-proliferative compound, for B-cell Non-Hodgkin lymphoma, from Nyctanthes arbor-tristis Linn
Authors: T Sana, S Qayyum, A Jabeen, BS Siddiqui, S Begum, RA Siddiqui, TB Hadda
Journal of ethnopharmacology, 2022-04-07;0(0):115267.
Species: Human
Sample Types: Cell Culture Supernates
-
The TLR-chaperone CNPY3 is a critical regulator of NLRP3-Inflammasome activation
Authors: M Ghait, RA Husain, SN Duduskar, TB Haack, M Rooney, B Göhrig, M Bauer, I Rubio, SD Deshmukh
European Journal of Immunology, 2022-04-07;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Discovery of a novel GRPR antagonist for protection against cisplatin-induced acute kidney injury
Authors: MJ Yu, C Li, SS Deng, XM Meng, RS Yao
Bioorganic chemistry, 2022-04-06;124(0):105794.
Species: Human
Sample Types: Cell Culture Supernates
-
Immune Modulating Brevetoxins: Monocyte Cytotoxicity, Apoptosis, and Activation of M1/M2 Response Elements Is Dependent on Reactive Groups
Authors: JR McCall, KT Sausman, DM Keeler, AP Brown, SL Turrise
Marine Drugs, 2022-03-29;20(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
Sulforaphane Reduces Pro-Inflammatory Response To Palmitic Acid In Monocytes And Adipose Tissue Macrophages
Authors: EJ Williams, L Guillemina, BS Berthon, S Eslick, T Wright, C Karihaloo, M Gately, KJ Baines, LG Wood
The Journal of nutritional biochemistry, 2022-03-07;0(0):108978.
Species: Human
Sample Types: Cell Culture Supernates
-
A novel Streptococcus pneumoniae human challenge model demonstrates Treg lymphocyte recruitment to the infection site
Authors: G Szylar, R Wysoczansk, H Marshall, DJB Marks, R José, ME Ehrenstein, JS Brown
Scientific Reports, 2022-03-07;12(1):3990.
Species: Human
Sample Types: Blister Fluid
-
Anti-inflammatory effects of an autologous gold-based serum therapy in osteoarthritis patients
Authors: J Feldt, AJ Donaubauer, J Welss, U Schneider, US Gaipl, F Paulsen
Scientific Reports, 2022-03-03;12(1):3560.
Species: Human
Sample Types: Serum
-
Poly epsilon-Caprolactone Nanoparticles for Sustained Intra-Articular Immune Modulation in Adjuvant-Induced Arthritis Rodent Model
Authors: E Singh, RAM Osmani, R Banerjee, AS Abu Lila, A Moin, K Almansour, HH Arab, HF Alotaibi, ES Khafagy
Pharmaceutics, 2022-02-26;14(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
E-Cigarette Aerosols Promote Oral S. aureus Colonization by Delaying an Immune Response and Bacterial Clearing
Authors: AR Cátala-Val, J Almeda, JN Bernard, AM Cole, AL Cole, SD Moore, CD Andl
Cells, 2022-02-23;11(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Oral administration of bovine lactoferrin suppresses the progression of rheumatoid arthritis in an SKG mouse model
Authors: S Yanagisawa, K Nagasaki, C Chea, T Ando, NF Ayuningtya, T Inubushi, A Ishikado, H Imanaka, E Sugiyama, I Takahashi, M Miyauchi, T Takata
PLoS ONE, 2022-02-11;17(2):e0263254.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Weight Loss and Short-Chain Fatty Acids Reduce Systemic Inflammation in Monocytes and Adipose Tissue Macrophages from Obese Subjects
Authors: S Eslick, EJ Williams, BS Berthon, T Wright, C Karihaloo, M Gately, LG Wood
Nutrients, 2022-02-11;14(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
Tumour cell apoptosis modulates the colorectal cancer immune microenvironment via interleukin-8-dependent neutrophil recruitment
Authors: V Schimek, K Strasser, A Beer, S Göber, N Walterskir, C Brostjan, C Müller, T Bachleitne, M Bergmann, H Dolznig, R Oehler
Cell Death & Disease, 2022-02-04;13(2):113.
Species: Human
Sample Types: Cell Culture Supernates
-
The effects of sulfated secondary bile acids on intestinal barrier function and immune response in an inflammatory in vitro human intestinal model
Authors: B van der Lu, MCP Vos, M Grootte Br, N Ijssennagg, F Vrieling, J Meijerink, WT Steegenga
Heliyon, 2022-02-02;8(2):e08883.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of Different Parameters of In Vitro Static Tensile Strain on Human Periodontal Ligament Cells Simulating the Tension Side of Orthodontic Tooth Movement
Authors: C Sun, M Janjic Ran, M Folwaczny, T Stocker, S Otto, A Wichelhaus, U Baumert
International Journal of Molecular Sciences, 2022-01-28;23(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Trained immunity induction by the inactivated mucosal vaccine MV130 protects against experimental viral respiratory infections
Authors: P Brandi, L Conejero, FJ Cueto, S Martínez-C, G Dunphy, MJ Gómez, C Relaño, P Saz-Leal, M Enamorado, A Quintas, A Dopazo, J Amores-Ini, C Del Fresno, E Nistal-Vil, C Ardavín, A Nieto, M Casanovas, JL Subiza, D Sancho
Cell Reports, 2022-01-04;38(1):110184.
Species: Human
Sample Types: Cell Culture Supernates
-
LncRNA NEAT1 is upregulated in recurrent aphthous stomatitis (RAS) and has predictive values
Authors: Y Han, L Wang, Q Li, H Chen, X Ma
BMC Oral Health, 2021-12-31;21(1):673.
Species: Human
Sample Types: Plasma
-
IL-25 Induced ROS-Mediated M2 Macrophage Polarization via AMPK-Associated Mitophagy
Authors: ML Tsai, YG Tsai, YC Lin, YL Hsu, YT Chen, MK Tsai, WT Liao, YC Lin, CH Hung
International Journal of Molecular Sciences, 2021-12-21;23(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Reduced Interleukin-17-Expressing Cells in Cutaneous Melanoma
Authors: A Tosi, L Nardinocch, ML Carbone, L Capriotti, E Pagani, S Mastroeni, C Fortes, F Scopelliti, C Cattani, F Passarelli, A Rosato, S D'Atri, CM Failla, A Cavani
Biomedicines, 2021-12-16;9(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Skin Substitute Preparation Method Induces Immunomodulatory Changes in Co-Incubated Cells through Collagen Modification
Authors: J Holl, C Pawlukiani, J Corton Rui, D Groth, K Grubczak, HR Hady, J Dadan, J Reszec, S Czaban, C Kowalewski, M Moniuszko, A Eljaszewic
Pharmaceutics, 2021-12-15;13(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
CRISPR/Cas9-mediated TGFbetaRII disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells in vitro
Authors: K Alishah, M Birtel, E Masoumi, L Jafarzadeh, HR Mirzaee, J Hadjati, RH Voss, M Diken, S Asad
Journal of Translational Medicine, 2021-11-27;19(1):482.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of RIPK1 in SMAC mimetics-induced apoptosis in primary human HIV-infected macrophages
Authors: RE Caballero, SXM Dong, N Gajanayaka, H Ali, E Cassol, WD Cameron, R Korneluk, MJ Tremblay, J Angel, A Kumar
Scientific Reports, 2021-11-25;11(1):22901.
Species: Human
Sample Types: Cell Culture Supernates
-
Defactinib inhibits PYK2 phosphorylation of IRF5 and reduces intestinal inflammation
Authors: G Ryzhakov, H Almuttaqi, AL Corbin, DL Berthold, T Khoyratty, HL Eames, S Bullers, C Pearson, Z Ai, K Zec, S Bonham, R Fischer, L Jostins-De, SPL Travis, BM Kessler, IA Udalova
Nature Communications, 2021-11-18;12(1):6702.
Species: Human
Sample Types: Cell Culture Supernates
-
Mitochondrial respiration contributes to the interferon gamma response in antigen-presenting cells
Authors: MC Kiritsy, K McCann, D Mott, SM Holland, SM Behar, CM Sassetti, AJ Olive
Elife, 2021-11-02;10(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
ADAM17 Mediates Hypoxia-Induced Keratinocyte Migration via the p38/MAPK Pathway
Authors: G Zhu, J Liu, Y Wang, N Jia, W Wang, J Zhang, Y Liang, H Tian, J Zhang
BioMed Research International, 2021-10-28;2021(0):8328216.
Species: Human
Sample Types: Cell Culture Supernates
-
The effects of the dietary compound L-sulforaphane against respiratory pathogens
Authors: N Mazarakis, RA Higgins, J Anderson, ZQ Toh, RB Luwor, KJ Snibson, TC Karagianni, LAH Do, PV Licciardi
International journal of antimicrobial agents, 2021-10-22;0(0):106460.
Species: Human
Sample Types: Cell Culture Supernates
-
A prospective cohort study providing insights for markers of adverse pregnancy outcome in older mothers
Authors: SC Lean, RL Jones, SA Roberts, AEP Heazell
BMC pregnancy and childbirth, 2021-10-20;21(1):706.
Species: Human
Sample Types: Serum
-
Persistent Effect of Advanced Glycated Albumin Driving Inflammation and Disturbances in Cholesterol Efflux in Macrophages
Authors: CA Minanni, A Machado-Li, RT Iborra, LS Okuda, R de Souza P, MFM Santana, ALA Lira, ER Nakandakar, MLC Côrrea-Gia, M Passarelli
Nutrients, 2021-10-17;13(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
Tofacitinib-Induced Modulation of Intestinal Adaptive and Innate Immunity and Factors Driving Cellular and Systemic Pharmacokinetics
Authors: B Texler, A Zollner, V Reinstadle, SJ Reider, S Macheiner, B Jelusic, A Pfister, C Watschinge, N Przysiecki, H Tilg, H Oberacher, AR Moschen
Cellular and Molecular Gastroenterology and Hepatology, 2021-10-06;0(0):.
Species: Human
Sample Types: Cell culture supernate
-
PLCgamma2 regulates TREM2 signalling and integrin-mediated adhesion and migration of human iPSC-derived macrophages
Authors: J Obst, HL Hall-Rober, TB Smith, M Kreuzer, L Magno, E Di Daniel, JB Davis, E Mead
Scientific Reports, 2021-10-06;11(1):19842.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-6 promotes drug resistance through formation of polyploid giant cancer cells and stromal fibroblast reprogramming
Authors: N Niu, J Yao, RC Bast, AK Sood, J Liu
Oncogenesis, 2021-09-29;10(9):65.
Species: Human
Sample Types: Cell Culture Supernates
-
The influence of the gut microbiome on BCG-induced trained immunity
Authors: M Stražar, VP Mourits, VACM Koeken, LCJ de Bree, SJCFM Moorlag, LAB Joosten, R van Crevel, H Vlamakis, MG Netea, RJ Xavier
Genome Biology, 2021-09-22;22(1):275.
Species: Human
Sample Types: Cell Culture Supernates
-
Endothelial-Derived Extracellular Vesicles Induce Cerebrovascular Dysfunction in Inflammation
Authors: D Roig-Carle, E Willms, RD Fontijn, S Martinez-P, I Mäger, HE de Vries, M Hirst, B Sharrack, DK Male, CA Hawkes, IA Romero
Pharmaceutics, 2021-09-21;13(9):.
Species: Human
Sample Types: Extracellular Vesicles
-
Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling
Authors: J Hu, C Wang, X Huang, S Yi, S Pan, Y Zhang, G Yuan, Q Cao, X Ye, H Li
Cell Reports, 2021-09-21;36(12):109726.
Species: Human
Sample Types: Cell Culture Supernates
-
Functional role of galectin-9 in directing human innate immune reactions to Gram-negative bacteria and T cell apoptosis
Authors: S Schlichtne, NH Meyer, IM Yasinska, N Aliu, SM Berger, BF Gibbs, E Fasler-Kan, VV Sumbayev
International immunopharmacology, 2021-09-17;100(0):108155.
Species: Human
Sample Types: Cell Culture Supernates
-
Ascorbic acid supports ex vivo generation of plasmacytoid dendritic cells from circulating hematopoietic stem cells
Authors: A Laustsen, RM van der Sl, A Gris-Olive, SS Hernández, E Cemalovic, HQ Tang, LH Pedersen, N Uldbjerg, MR Jakobsen, RO Bak
Elife, 2021-09-02;10(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Pulmonary function changes in older adults with and without metabolic syndrome
Authors: MAR Brandao-Ra, R Moraes-Fer, MC Oliveira-J, A Santos-Dia, ALL Bachi, G Gabriela-P, S de Oliveir, AC Araújo-Ros, LVF Oliveira, CR Frison, WL do Prado, RP Raju, PB Balagopal, RP Vieira
Scientific Reports, 2021-08-30;11(1):17337.
Species: Human
Sample Types: Exhaled Breath Condensate (EBC)
-
Neutrophil function and bactericidal activity against Staphylococcus aureus after cardiac surgery with cardiopulmonary bypass
Authors: M Lesouhaiti, M Gregoire, A Gacouin, V Coirier, A Frerou, C Piau, V Cattoir, E Dumontet, M Revest, P Tattevin, A Roisne, JP Verhoye, E Flecher, Y Le Tulzo, K Tarte, JM Tadié
Journal of leukocyte biology, 2021-08-23;0(0):.
Species: Human
Sample Types: Plasma
-
In Vitro Priming of Human T Cells by Dendritic Cells Provides a Screening Tool for Candidate Vaccines for Burkholderia pseudomallei
Authors: D Reddi, L Durant, D Bernardo, A Noble, NR English, P Hendy, GC Clark, JL Prior, ED Williamson, SC Knight
Vaccines, 2021-08-22;9(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophils promote T-cell activation through the regulated release of CD44-bound Galectin-9 from the cell surface during HIV infection
Authors: G Dunsmore, EP Rosero, S Shahbaz, DM Santer, J Jovel, P Lacy, S Houston, S Elahi
PloS Biology, 2021-08-19;19(8):e3001387.
Species: Human
Sample Types: Plasma
-
New Insights on Old Biomarkers Involved in Tumor Microenvironment Changes and Their Diagnostic Relevance in Non-Small Cell Lung Carcinoma
Authors: K Wadowska, P B?asiak, A Rzechonek, I Bil-Lula, M ?liwi?ska-
Biomolecules, 2021-08-13;11(8):.
Species: Human
Sample Types: Serum
-
Mimicking of Blood Flow Results in a Distinct Functional Phenotype in Human Non-Adherent Classical Monocytes
Authors: E Wirthgen, M Hornschuh, IM Wrobel, C Manteuffel, J Däbritz
Biology, 2021-08-04;10(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Home-based exercise training influences gut bacterial levels in multiple sclerosis
Authors: M Mokhtarzad, M Molanouri, M Abolhasani, B Bakhshi, MA Sahraian, LS Quinn, R Negaresh
Complementary therapies in clinical practice, 2021-07-30;45(0):101463.
Species: Human
Sample Types: Serum
-
Kras-driven intratumoral heterogeneity triggers infiltration of M2 polarized macrophages via the circHIPK3/PTK2 immunosuppressive circuit
Authors: T Katopodi, S Petanidis, K Domvri, P Zarogoulid, D Anestakis, C Charalampi, D Tsavlis, C Bai, H Huang, L Freitag, W Hohenforst, D Matthaios, K Porpodis
Scientific Reports, 2021-07-29;11(1):15455.
Species: Human
Sample Types: Cell Culture Supernates
-
Jellyfish Collagen: A Biocompatible Collagen Source for 3D Scaffold Fabrication and Enhanced Chondrogenicity
Authors: Z Ahmed, LC Powell, N Matin, A Mearns-Spr, CA Thornton, IM Khan, LW Francis
Marine Drugs, 2021-07-22;19(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Structural Deformation of MTX Induced by Nanodrug Conjugation Dictate Intracellular Drug Transport and Drug Efficacy
Authors: JY Park, JS Hyun, JG Jee, SJ Park, D Khang
International Journal of Nanomedicine, 2021-07-22;16(0):4943-4957.
Species: Human
Sample Types: Cell Culture Supernates
-
Bioengineered Nanoparticles Loaded-Hydrogels to Target TNF Alpha in Inflammatory Diseases
Authors: IM Oliveira, DC Fernandes, FR Maia, RF Canadas, RL Reis, JM Oliveira
Pharmaceutics, 2021-07-21;13(8):.
Species: Human
Sample Types: Nanoparticle Supernates
-
Deep-Sea Coral Garden Invertebrates and Their Associated Fungi Are Genetic Resources for Chronic Disease Drug Discovery
Authors: P Marchese, R Young, E O'Connell, S Afoullouss, BJ Baker, AL Allcock, F Barry, JM Murphy
Marine Drugs, 2021-07-13;19(7):.
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of moderate wine consumption on cytokine secretion by peripheral blood mononuclear cells: A randomized clinical study in coronary heart disease patients
Authors: E Fragopoulo, C Argyrou, M Detopoulou, S Tsitsou, S Seremeti, M Yannakouli, S Antonopoul, G Kolovou, P Kalogeropo
Cytokine, 2021-07-08;146(0):155629.
Species: Human
Sample Types: Cell Culture Supernates
-
Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial
Authors: V Mocanu, Z Zhang, EC Deehan, DH Kao, N Hotte, S Karmali, DW Birch, KK Samarasing, J Walter, KL Madsen
Nature Medicine, 2021-07-05;27(7):1272-1279.
Species: Human
Sample Types: Serum
-
Biomarkers for Comorbidities Modulate the Activity of T-Cells in COPD
Authors: K Jamal Jame, WJ Gallert, SD Yanik, S Panek, J Kronsbein, D Jungck, A Koch, J Knobloch
International Journal of Molecular Sciences, 2021-07-02;22(13):.
Species: Human
Sample Types: Cell Culture Supernates
-
An inverted in vitro triple culture model of the healthy and inflamed intestine: Adverse effects of polyethylene particles
Authors: M Busch, AAM Kämpfer, RPF Schins
Chemosphere, 2021-06-28;284(0):131345.
Species: Human
Sample Types: Cell Culture Supernates
-
DHX15 is required to control RNA virus-induced intestinal inflammation
Authors: J Xing, X Zhou, M Fang, E Zhang, LJ Minze, Z Zhang
Cell Reports, 2021-06-22;35(12):109205.
Species: Human
Sample Types: Cell Culture Supernates
-
The dynamics of human bone marrow adipose tissue in response to feeding and fasting
Authors: PK Fazeli, MA Bredella, OG Pachon-Peñ, W Zhao, X Zhang, AT Faje, M Resulaj, SP Polineni, TM Holmes, H Lee, EK O'Donnell, OA MacDougald, MC Horowitz, CJ Rosen, A Klibanski
JCI Insight, 2021-06-22;0(0):.
Species: Human
Sample Types: Serum
-
Timing of Tributyrin Supplementation Differentially Modulates Gastrointestinal Inflammation and Gut Microbial Recolonization Following Murine Ileocecal Resection
Authors: V Mocanu, H Park, J Dang, N Hotte, A Thiesen, M Laffin, H Wang, D Birch, K Madsen
Nutrients, 2021-06-17;13(6):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Bifidobacterium breve Exopolysaccharide Blocks Dendritic Cell Maturation and Activation of CD4+ T Cells
Authors: A Hickey, P Stamou, S Udayan, A Ramón-Vázq, M Esteban-To, F Bottacini, JA Woznicki, O Hughes, S Melgar, M Ventura, D Van Sinder, V Rossini, K Nally
Frontiers in Microbiology, 2021-06-16;12(0):653587.
Species: Human
Sample Types: Cell Culture Supernates
-
Cancer Cells Shuttle Extracellular Vesicles Containing Oncogenic Mutant p53 Proteins to the Tumor Microenvironment
Authors: B Bhatta, I Luz, C Krueger, FX Teo, DP Lane, K Sabapathy, T Cooks
Cancers, 2021-06-15;13(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance
Authors: CM Yamazaki, A Yamaguchi, Y Anami, W Xiong, Y Otani, J Lee, NT Ueno, N Zhang, Z An, K Tsuchikama
Nature Communications, 2021-06-10;12(1):3528.
Species: Human
Sample Types: Cell Culture Supernates
-
Lipopolysaccharide stimulation test on cultured PBMCs assists the discrimination of cryopyrin-associated periodic syndrome from systemic juvenile idiopathic arthritis
Authors: CY Wu, WL Fan, YM Chiu, HY Yang, WI Lee, JL Huang
Scientific Reports, 2021-06-07;11(1):11903.
Species: Human
Sample Types: Plasma
-
Plasma-derived DNA containing-extracellular vesicles induce STING-mediated proinflammatory responses in dermatomyositis
Authors: Y Li, C Bax, J Patel, T Vazquez, A Ravishanka, MM Bashir, M Grinnell, D Diaz, VP Werth
Theranostics, 2021-05-21;11(15):7144-7158.
Species: Human
Sample Types: Cell Culture Supernates
-
Decreased microRNA-155 in Behcet's disease leads to defective control of autophagy thereby stimulating excessive proinflammatory cytokine production
Authors: L Liang, Q Zhou, L Feng
Arthritis Research & Therapy, 2021-05-06;23(1):135.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased TNF-&alpha Initiates Cytoplasmic Vacuolization in Whole Blood Coculture with Dengue Virus
Authors: RD Satria, TW Huang, MK Jhan, TJ Shen, PC Tseng, YT Wang, ZY Yang, CH Hsing, CF Lin
Journal of Immunology Research, 2021-05-06;2021(0):6654617.
Species: Human
Sample Types: Plasma
-
Isoflavanquinones from Abrus precatorius roots with their antiproliferative and anti-inflammatory effects
Authors: EE Okoro, R Maharjan, A Jabeen, MS Ahmad, M Azhar, N Shehla, W Zaman, S Shams, OR Osoniyi, FD Onajobi, MI Choudhary
Phytochemistry, 2021-05-04;187(0):112743.
Species: Human
Sample Types: Cell Culture Supernates
-
Galectin-9, a Player in Cytokine Release Syndrome and a Surrogate Diagnostic Biomarker in SARS-CoV-2 Infection
Authors: N Bozorgmehr, S Mashhouri, E Perez Rose, L Xu, S Shahbaz, W Sligl, M Osman, DJ Kutsogiann, E MacIntyre, CR O'Neil, S Elahi
MBio, 2021-05-04;12(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
IRF1 governs the differential interferon-stimulated gene responses in human monocytes and macrophages by regulating chromatin accessibility
Authors: R Song, Y Gao, I Dozmorov, V Malladi, I Saha, MM McDaniel, S Parameswar, C Liang, C Arana, B Zhang, B Wakeland, J Zhou, MT Weirauch, LC Kottyan, EK Wakeland, C Pasare
Cell Reports, 2021-03-23;34(12):108891.
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophil extracellular trap clearance by synovial macrophages in gout
Authors: JH Jeong, SJ Choi, SM Ahn, JS Oh, YG Kim, CK Lee, B Yoo, S Hong
Arthritis Research & Therapy, 2021-03-19;23(1):88.
Species: Human
Sample Types: Cell Culture Supernates
-
Skin Sensitization Evaluation of Carbon-Based Graphene Nanoplatelets
Authors: SH Kim, SH Hong, JH Lee, DH Lee, K Jung, JY Yang, HS Shin, J Lee, J Jeong, JH Oh
Toxics, 2021-03-17;9(3):.
Species: Human
Sample Types: cell culture supernatant
-
Proteomics reveals distinct mechanisms regulating the release of cytokines and alarmins during pyroptosis
Authors: K Phulphagar, LI Kühn, S Ebner, A Frauenstei, JJ Swietlik, J Rieckmann, F Meissner
Cell Reports, 2021-03-09;34(10):108826.
Species: Human
Sample Types: Cell Culture Supernates
-
Impairment in inflammasome signaling by the chronic Pseudomonas aeruginosa isolates from cystic fibrosis patients results in an increase in inflammatory response
Authors: MS Phuong, RE Hernandez, DJ Wolter, LR Hoffman, S Sad
Cell Death & Disease, 2021-03-04;12(3):241.
Species: Human
Sample Types: Cell Culture Supernates
-
Fructose reprogrammes glutamine-dependent oxidative metabolism to support LPS-induced inflammation
Authors: N Jones, J Blagih, F Zani, A Rees, DG Hill, BJ Jenkins, CJ Bull, D Moreira, AIM Bantan, JG Cronin, D Avancini, GW Jones, DK Finlay, KH Vousden, EE Vincent, CA Thornton
Nature Communications, 2021-02-22;12(1):1209.
Species: Human
Sample Types: Cell Culture Supernates
-
Development and evaluation of inhalable composite niclosamide-lysozyme particles: A broad-spectrum, patient-adaptable treatment for coronavirus infections and sequalae
Authors: AD Brunaugh, H Seo, Z Warnken, L Ding, SH Seo, HDC Smyth
PLoS ONE, 2021-02-11;16(2):e0246803.
Species: Human
Sample Types: Cell Culture Supernates
-
High levels of interleukin-6 in rheumatoid arthritis joint fluids can stimulate local production of C-reactive protein resulting in elevated circulating levels
Authors: J Rönnelid, A Knight, J Lysholm, VA Manivel, A Sohrabian, A Larsson, T Weitoft
Joint Bone Spine, 2021-02-06;88(3):105159.
Species: Human
Sample Types: Serum
-
Anti-inflammatory activity of soluble chito-oligosaccharides (CHOS) on VitD3-induced human THP-1 monocytes
Authors: P Jitprasert, M Khamphio, P Petsrichua, VGH Eijsink, W Poolsri, C Muanprasat, K Rangnoi, M Yamabhai
PLoS ONE, 2021-02-03;16(2):e0246381.
Species: Human
Sample Types: Cell Culture Supernates
-
Downregulation of miR-126-3p expression contributes to increased inflammatory response in placental trophoblasts in preeclampsia
Authors: X Chu, Y Gu, W Sheng, J Sun, JA Morgan, DF Lewis, DB Cooper, CE McCathran, Y Wang
Journal of reproductive immunology, 2021-02-01;144(0):103281.
Species: Human
Sample Types: Cell Culture Supernates
-
Polyphenol-rich extract and fractions of Terminalia phaeocarpa Eichler possess hypoglycemic effect, reduce the release of cytokines, and inhibit lipase, &alpha-glucosidase, and &alpha-amilase enzymes
Authors: JHS Gomes, UC Mbiakop, RL Oliveira, JR Stehmann, RM Pádua, SF Cortes, FC Braga
Journal of ethnopharmacology, 2021-01-27;271(0):113847.
Species: Human
Sample Types: Cell Culture Supernates
-
Low levels of PCSK9 are associated with remission in patients with rheumatoid arthritis treated with anti-TNF-&alpha: potential underlying mechanisms
Authors: J Frostegård, S Ahmed, I Hafström, S Ajeganova, M Rahman
Arthritis Research & Therapy, 2021-01-19;23(1):32.
Species: Human
Sample Types: Cell Culture Supernates
-
New Biomarker in Chagas Disease: Extracellular Vesicles Isolated from Peripheral Blood in Chronic Chagas Disease Patients Modulate the Human Immune Response
Authors: RP Madeira, LM Dal'Mas Ro, P de Cássia, C Mady, BM Ianni, AC Torrecilha
Journal of Immunology Research, 2021-01-11;2021(0):6650670.
Species: Human
Sample Types: Cell Culture Supernates
-
Engagement of TREM2 by a novel monoclonal antibody induces activation of microglia and improves cognitive function in Alzheimer's disease models
Authors: M Fassler, MS Rappaport, CB Cuño, J George
Journal of Neuroinflammation, 2021-01-09;18(1):19.
Species: Human
Sample Types: Cell Lysates
-
Sintered S53P4 bioactive glass scaffolds have anti-inflammatory properties and stimulate osteogenesis in vitro
Authors: R Björkenhei, E Jämsen, E Eriksson, P Uppstu, L Aalto-Setä, L Hupa, KK Eklund, M Ainola, NC Lindfors, J Pajarinen
European cells & materials, 2021-01-03;41(0):15-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Iron Oxide Particles Alter Bacterial Uptake and the LPS-Induced Inflammatory Response in Macrophages
Authors: LJ Williams, SG Tristram, GR Zosky
International Journal of Environmental Research and Public Health, 2020-12-28;18(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
A new cytokine-based dynamic stratification during induction is highly predictive of survivals in acute myeloid leukemia
Authors: P Peterlin, J Gaschet, T Guillaume, A Garnier, M Eveillard, A Le Bourgeo, M Cherel, C Debord, Y Le Bris, O Theisen, C Godon, B Mahé, V Dubruille, S Wuilleme, C Touzeau, T Gastinne, N Blin, A Lok, B Tessoulin, S Le Gouill, P Moreau, MC Béné, P Chevallier
Cancer Medicine, 2020-12-25;0(0):.
Species: Human
Sample Types: Serum
-
Acute Conditioning of Antigen-Expanded CD8+ T Cells via the GSK3&beta-mTORC Axis Differentially Dictates Their Immediate and Distal Responses after Antigen Rechallenge
Authors: P Taborska, D Stakheev, H Svobodova, Z Strizova, J Bartunkova, D Smrz
Cancers, 2020-12-14;12(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
SARS-CoV-2-specific IgA and limited inflammatory cytokines are present in the stool of select patients with acute COVID-19
Authors: GJ Britton, A Chen-Liaw, F Cossarini, AE Livanos, MP Spindler, T Plitt, J Eggers, I Mogno, A Gonzalez-R, S Siu, M Tankelevic, L Grinspan, RE Dixon, D Jha, G Martinez-D, F Amanat, DA Hoagland, B tenOever, MC Dubinsky, M Merad, H van Bakel, F Krammer, G Bongers, S Mehandru, JJ Faith
medRxiv : the preprint server for health sciences, 2020-12-09;0(0):.
Species: Human
Sample Types: Fecal Supernates
-
Clinical Relevance of the Anti-inflammatory Effects of Roflumilast on Human Bronchus: Potentiation by a Long-Acting Beta-2-Agonist
Authors: H Salvator, A Buenestado, M Brollo, E Naline, T Victoni, E Longchamp, H Tenor, S Grassin-De, P Devillier
Frontiers in Pharmacology, 2020-12-08;11(0):598702.
Species: Human
Sample Types: Cell Culture Supernates
-
HMGB1 as a potential biomarker and therapeutic target for severe COVID-19
Authors: R Chen, Y Huang, J Quan, J Liu, H Wang, TR Billiar, MT Lotze, HJ Zeh, R Kang, D Tang
Heliyon, 2020-12-07;6(12):e05672.
Species: Human
Sample Types: Cell Culture Supernates
-
Patient-derived and artificial ascites have minor effects on MeT-5A mesothelial cells and do not facilitate ovarian cancer cell adhesion
Authors: M Estermann, YL Huang, D Septiadi, D Ritz, CY Liang, F Jacob, B Drasler, A Petri-Fink, V Heinzelman, B Rothen-Rut
PLoS ONE, 2020-12-03;15(12):e0241500.
Species: Human
Sample Types: Abdominal Fluid
-
BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils
Authors: SJCFM Moorlag, YA Rodriguez-, J Gillard, S Fanucchi, K Theunissen, B Novakovic, CM de Bont, Y Negishi, ET Fok, L Kalafati, P Verginis, VP Mourits, VACM Koeken, LCJ de Bree, GJM Pruijn, C Fenwick, R van Crevel, LAB Joosten, I Joosten, H Koenen, MM Mhlanga, DA Diavatopou, T Chavakis, MG Netea
Cell Rep, 2020-11-17;33(7):108387.
Species: Human
Sample Types: Cell Culture Supernates
-
Monocytes differentiated into macrophages and dendritic cells in the presence of human IFN-&lambda3 or IFN-&lambda4 show distinct phenotypes
Authors: M De, A Bhushan, S Chinnaswam
J Leukoc Biol, 2020-11-17;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
New insights in gene expression alteration as effect of doxorubicin drug resistance in triple negative breast cancer cells
Authors: CA Ciocan-Car, A Jurj, O Zanoaga, R Cojocneanu, LA Pop, A Moldovan, C Moldovan, AA Zimta, L Raduly, C Pop-Bica, M Buse, L Budisan, P Virag, A Irimie, SMG Diaz, I Berindan-N, C Braicu
J Exp Clin Cancer Res, 2020-11-13;39(1):241.
Species: Human
Sample Types: Cell Culture Supernates
-
Pathogenic, glycolytic PD-1+ B cells accumulate in the hypoxic RA joint
Authors: A Floudas, N Neto, V Marzaioli, K Murray, B Moran, MG Monaghan, C Low, RH Mullan, N Rao, V Krishna, S Nagpal, DJ Veale, U Fearon
JCI Insight, 2020-11-05;5(21):.
Species: Human
Sample Types: Cell Culture Supernates
-
Evaluation of in vitro and in vivo Efficacy of a Novel Amphotericin B-Loaded Nanostructured Lipid Carrier in the Treatment of Leishmania braziliensis Infection
Authors: J Rebouças-S, MC Tadini, D Devequi-Nu, AL Mansur, P S Silveira, C I de Olive, F R Formiga, AA Berretta, F Marquele-O, VM Borges
Int J Nanomedicine, 2020-11-05;15(0):8659-8672.
Species: Human
Sample Types: Cell Culture Supernates
-
CXCL9, CXCL10, and CXCL11; biomarkers of pulmonary inflammation associated with autoimmunity in patients with collagen vascular diseases-associated interstitial lung disease and interstitial pneumonia with autoimmune features
Authors: M Kameda, M Otsuka, H Chiba, K Kuronuma, T Hasegawa, H Takahashi, H Takahashi
PLoS ONE, 2020-11-02;15(11):e0241719.
Species: Human
Sample Types: Serum
-
Anti-Inflammatory Properties of Injectable Betamethasone-Loaded Tyramine-Modified Gellan Gum/Silk Fibroin Hydrogels
Authors: IM Oliveira, C Gonçalves, ME Shin, S Lee, RL Reis, G Khang, JM Oliveira
Biomolecules, 2020-10-17;10(10):.
Species: Rabbit
Sample Types: Cell Culture Supernates
-
Calcium Ionophore-Induced Extracellular Vesicles Mediate Cytoprotection against Simulated Ischemia/Reperfusion Injury in Cardiomyocyte-Derived Cell Lines by Inducing Heme Oxygenase 1
Authors: P Pe?an, S Hambalkó, VT Ha, CT Nagy, C Pelyhe, D Lainš?ek, B Kenyeres, GB Brenner, A Görbe, Á Kittel, M Barteková, P Ferdinandy, M Man?ek-Keb, Z Giricz
Int J Mol Sci, 2020-10-16;21(20):.
Species: Human
Sample Types: Cell Culture Supernates
-
The role of EMMPRIN/CD147 in regulating angiogenesis in patients with psoriatic arthritis
Authors: MA Rahat, M Safieh, E Simanovich, E Pasand, T Gazitt, A Haddad, M Elias, D Zisman
Arthritis Res Ther, 2020-10-14;22(1):240.
Species: Human
Sample Types: Serum
-
Interleukin-26 activates macrophages and facilitates killing of Mycobacterium tuberculosis
Authors: HC Hawerkamp, L van Geelen, J Korte, J Di Domizio, M Swidergall, AA Momin, FJ Guzmán-Veg, ST Arold, J Ernst, M Gilliet, R Kalscheuer, B Homey, S Meller
Sci Rep, 2020-10-14;10(1):17178.
Species: Human
Sample Types: Cell Culture Supernates
-
Staphylococcus aureus-Derived &alpha-Hemolysin Evokes Generation of Specialized Pro-resolving Mediators Promoting Inflammation Resolution
Authors: PM Jordan, J Gerstmeier, S Pace, R Bilancia, Z Rao, F Börner, L Miek, Ó Gutiérrez-, V Arakandy, A Rossi, A Ialenti, C González-E, B Löffler, L Tuchscherr, CN Serhan, O Werz
Cell Rep, 2020-10-13;33(2):108247.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lentiviral delivery of combinatorial CAR/CRISPRi circuit into human primary T cells is enhanced by TBK1/IKK? complex inhibitor BX795
Authors: L Li, Y Gao, R Srivastava, W Wang, Q Xiong, Z Fang, A Pelayo, C Denson, A Goswami, R Harari-Ste, Z Yang, L Weng, LS Qi, FM Marincola
Journal of Translational Medicine, 2020-09-23;18(1):363.
Species: Human
Sample Types: Cell Culture Supernates
-
Human Mesenchymal Stromal Cell Secretome Promotes the Immunoregulatory Phenotype and Phagocytosis Activity in Human Macrophages
Authors: M Holopainen, U Impola, P Lehenkari, S Laitinen, E Kerkelä
Cells, 2020-09-22;9(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Design and characterization of Squalene-Gusperimus nanoparticles for modulation of innate immunity
Authors: CE Navarro Ch, BJ de Haan, MM Faas, AM Smink, L Sierra, P de Vos, BL López
Int J Pharm, 2020-09-19;590(0):119893.
Species: Human
Sample Types: Cell Culture Supernates
-
Modeling HIV-1 neuropathogenesis using three-dimensional human brain organoids (hBORGs) with HIV-1 infected microglia
Authors: RS Dos Reis, S Sant, H Keeney, MCE Wagner, V Ayyavoo
Scientific Reports, 2020-09-16;10(1):15209.
Species: Human
Sample Types: Organoid
-
Enrichment of apolipoprotein A-IV and apolipoprotein D in the HDL proteome is associated with HDL functions in diabetic kidney disease without dialysis
Authors: MFM Santana, ALA Lira, RS Pinto, CA Minanni, ARM Silva, MIBAC Sawada, ER Nakandakar, MLC Correa-Gia, MS Queiroz, GE Ronsein, M Passarelli
Lipids Health Dis, 2020-09-14;19(1):205.
Species: Human
Sample Types: Cell Culture Supernates
-
The NRF2 stimulating agent, tin protoporphyrin, activates protective cytokine pathways in healthy human subjects and in patients with chronic kidney disease
Authors: RA Zager, ACM Johnson
Physiol Rep, 2020-09-01;8(18):e14566.
Species: Human
Sample Types: Plasma
-
The Influence of Hypertensive Therapies on Circulating Factors: Clinical Implications for SCFAs, FGF21, TNFSF14 and TNF-&alpha
Authors: AL Magno, LY Herat, MG Kiuchi, MP Schlaich, NC Ward, VB Matthews
Journal of Clinical Medicine, 2020-08-26;9(9):.
Species: Human
Sample Types: Serum
-
The effect of intraoperative goal-directed crystalloid versus colloid administration on perioperative inflammatory markers - a substudy of a randomized controlled trial
Authors: M Obradovic, A Kurz, B Kabon, G Roth, O Kimberger, O Zotti, A Bayoumi, C Reiterer, A Stift, E Fleischman
BMC Anesthesiol, 2020-08-21;20(1):210.
Species: Human
Sample Types: Plasma, Serum
-
Gene expression network analyses during infection with virulent and avirulent Trypanosoma cruzi�strains unveil a role for fibroblasts in neutrophil recruitment and activation
Authors: AER Oliveira, MCA Pereira, AT Belew, LRP Ferreira, LMN Pereira, EGA Neves, MDCP Nunes, BA Burleigh, WO Dutra, NM El-Sayed, RT Gazzinelli, SMR Teixeira
PLoS Pathog., 2020-08-18;16(8):e1008781.
Species: Human
Sample Types: Cell Culture Supernates
-
Targeting PD-L1 in non-small cell lung cancer using CAR T cells
Authors: M Liu, X Wang, W Li, X Yu, P Flores-Vil, ZY Xu-Monette, L Li, M Zhang, KH Young, X Ma, Y Li
Oncogenesis, 2020-08-13;9(8):72.
Species: Human
Sample Types: Cell Culture Supernates
-
Erythromycin inhibits neutrophilic inflammation and mucosal disease by upregulating DEL-1
Authors: T Maekawa, H Tamura, H Domon, T Hiyoshi, T Isono, D Yonezawa, N Hayashi, N Takahashi, K Tabeta, T Maeda, M Oda, A Ziogas, V? Alexaki, T Chavakis, Y Terao, G Hajishenga
JCI Insight, 2020-08-06;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Standardized ethanol extract of Tinospora crispa upregulates pro-inflammatory mediators release in LPS-primed U937 human macrophages through stimulation of MAPK, NF-kappaB and PI3K-Akt signaling networks
Authors: MA Haque, I Jantan, H Harikrishn, W Ahmad
BMC Complement Med Ther, 2020-08-06;20(1):245.
Species: Human
Sample Types: Cell Culture Supernates
-
Modified citrus pectins by UV/H2O2 oxidation at acidic and basic conditions: Structures and in vitro anti-inflammatory, anti-proliferative activities
Authors: J Cao, J Yang, Z Wang, M Lu, K Yue
Carbohydr Polym, 2020-07-09;247(0):116742.
Species: Human
Sample Types: Cell Culture Supernates
-
Loss of function of the mitochondrial peptidase PITRM1 induces proteotoxic stress and Alzheimer's disease-like pathology in human cerebral organoids
Authors: MJ Pérez, D Ivanyuk, V Panagiotak, G Di Napoli, S Kalb, D Brunetti, R Al-Shaana, SA Kaeser, SA Fraschka, M Jucker, M Zeviani, C Viscomi, M Deleidi
Mol. Psychiatry, 2020-07-07;0(0):.
Species: Human
Sample Types: Cell Culture Lysates
-
The Delivery of &alpha1-Antitrypsin Therapy Through Transepidermal Route: Worthwhile to Explore
Authors: S Tumpara, B Martinez-D, G Gomez-Mari, B Liu, DS DeLuca, E Korenbaum, D Jonigk, F Jugert, FM Wurm, MJ Wurm, T Welte, S Janciauski
Front Pharmacol, 2020-07-03;11(0):983.
Species: Human
Sample Types: Cell Culture Supernates
-
Altered iron metabolism in cystic fibrosis macrophages: the impact of CFTR modulators and implications for Pseudomonas aeruginosa survival
Authors: HF Hazlett, TH Hampton, DS Aridgides, DA Armstrong, JA Dessaint, DL Mellinger, AB Nymon, A Ashare
Sci Rep, 2020-07-02;10(1):10935.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-Inflammatory Principles from Tamarix aphylla L.: A Bioassay-Guided Fractionation Study
Authors: AS Gadallah, Mujeeb-Ur-, Atta-Ur-Ra, S Yousuf, Atia-Tul-W, A Jabeen, MM Swilam, SAM Khalifa, HR El-Seedi, MI Choudhary
Molecules, 2020-06-30;25(13):.
Species: Human
Sample Types: Cell Culture Supernates
-
Lysophosphatidylcholine acyltransferase 2 (LPCAT2) co-localises with TLR4 and regulates macrophage inflammatory gene expression in response to LPS
Authors: W Abate, H Alrammah, M Kiernan, AJ Tonks, SK Jackson
Sci Rep, 2020-06-25;10(1):10355.
Species: Human
Sample Types: Cell Culture Supernates
-
Vibration synergistically enhances IL-1beta and TNF-alpha in compressed human periodontal ligament cells in the frequency-dependent manner
Authors: S Benjakul, B Unat, P Thammanich, C Leethanaku
J Oral Biol Craniofac Res, 2020-06-22;10(4):412-416.
Species: Human
Sample Types: Cell Culture Supernates
-
The tumour microenvironment of the upper and lower gastrointestinal tract differentially influences dendritic cell maturation
Authors: ME Morrissey, R Byrne, C Nulty, NH McCabe, N Lynam-Lenn, CT Butler, S Kennedy, D O'Toole, J Larkin, P McCormick, B Mehigan, MC Cathcart, J Lysaght, JV Reynolds, EJ Ryan, MR Dunne, J O'Sullivan
BMC Cancer, 2020-06-17;20(1):566.
Species: Human
Sample Types: Cell Culture Supernates
-
Human interleukin-4-treated regulatory macrophages promote epithelial wound healing and reduce colitis in a mouse model
Authors: TS Jayme, G Leung, A Wang, ML Workentine, S Rajeev, A Shute, BE Callejas, N Mancini, PL Beck, R Panaccione, DM McKay
Sci Adv, 2020-06-05;6(23):eaba4376.
Species: Human
Sample Types: Cell Culture Supernates
-
Single residue in CD28-costimulated CAR-T cells limits long-term persistence and antitumor durability
Authors: S Guedan, A Madar, V Casado-Med, C Shaw, A Wing, F Liu, RM Young, CH June, AD Posey
J. Clin. Invest., 2020-06-01;130(6):3087-3097.
Species: Human
Sample Types: Cell Culture Supernates
-
Sex Differences in Proatherogenic Cytokine Levels
Authors: S Bernardi, B Toffoli, F Tonon, M Francica, E Campagnolo, T Ferretti, S Comar, F Giudici, E Stenner, B Fabris
Int J Mol Sci, 2020-05-29;21(11):.
Species: Human
Sample Types:
-
Persistent elevation of intrathecal pro-inflammatory cytokines leads to multiple sclerosis-like cortical demyelination and neurodegeneration
Authors: RE James, R Schalks, E Browne, I Eleftheria, CP Munoz, ND Mazarakis, R Reynolds
Acta Neuropathol Commun, 2020-05-12;8(1):66.
Species: Human
Sample Types: Cell Culutre Supernatant
-
Influence of Indoleamine-2,3-Dioxygenase and Its Metabolite Kynurenine on gammadelta T Cell Cytotoxicity against Ductal Pancreatic Adenocarcinoma Cells
Authors: H Jonescheit, HH Oberg, D Gonnermann, M Hermes, V Sulaj, C Peters, D Kabelitz, D Wesch
Cells, 2020-05-06;9(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Gaps in Study Design for Immune Parameter Research for Latent Tuberculosis Infection: A Systematic Review
Authors: M Herrera, C Vera, Y Keynan, ZV Rueda
J Immunol Res, 2020-04-21;2020(0):8074183.
Species: Human
Sample Types: Cell Culture Supernates, Plasma
-
The Set7 Lysine Methyltransferase Regulates Plasticity in Oxidative Phosphorylation Necessary for Trained Immunity Induced by &beta-Glucan
Authors: ST Keating, L Groh, CDCC van der He, H Rodriguez, JC Dos Santos, S Fanucchi, J Okabe, H Kaipananic, JH van Puffel, L Helder, MP Noz, V Matzaraki, Y Li, LCJ de Bree, VACM Koeken, SJCFM Moorlag, VP Mourits, J Domínguez-, M Oosting, EP Bulthuis, WJH Koopman, M Mhlanga, A El-Osta, LAB Joosten, MG Netea, NP Riksen
Cell Rep, 2020-04-21;31(3):107548.
Species: Human
Sample Types: Cell Culture Supernates
-
Antifibrotic Effects of a Barbituric Acid Derivative on Liver Fibrosis by Blocking the NF-&kappaB Signaling Pathway in Hepatic Stellate Cells
Authors: YH Wang, FM Suk, CL Liu, TL Chen, YC Twu, MH Hsu, YJ Liao
Front Pharmacol, 2020-03-31;11(0):388.
Species: Human
Sample Types: Cell Culture Supernates
-
Cerebrospinal fluid of chronic osteoarthritic patients induced interleukin-6 release in human glial cell-line T98G
Authors: W Liu, C Li, FCK Tan, HJ Neo, YH Chan, CM Low, TL Lee
BMC Anesthesiol, 2020-03-25;20(1):69.
Species: Human
Sample Types: Cell Culture Supernates
-
Activation of Human &gamma&delta T Cells: Modulation by Toll-Like Receptor 8 Ligands and Role of Monocytes
Authors: R Serrano, D Wesch, D Kabelitz
Cells, 2020-03-13;9(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Theophylline and dexamethasone in combination reduce inflammation and prevent the decrease in HDAC2 expression seen in monocytes exposed to cigarette smoke extract
Authors: Xue‑Jiao Sun, Zhan‑Hua Li, Yang Zhang, Xiao‑Ning Zhong, Zhi‑Yi He, Ji‑Hong Zhou et al.
Experimental and Therapeutic Medicine
Species: Human
Sample Types: Cell Culture Supernates
-
CD4+FOXP3+ T Cells in Rheumatoid Arthritis Bone Marrow Are Partially Impaired
Authors: M Massalska, A Radzikowsk, E Kuca-Warna, M Plebanczyk, M Prochorec-, U Skalska, W Kurowska, P Maldyk, E Kontny, HJ Gober, W Maslinski
Cells, 2020-02-26;9(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Monocytes from neonates and adults have a similar capacity to adapt their cytokine production after previous exposure to BCG and beta-glucan
Authors: R Namakula, LCJ de Bree, TH A Tvedt, MG Netea, S Cose, K Hanevik
PLoS ONE, 2020-02-21;15(2):e0229287.
Species: Human
Sample Types: Serum
-
1,25-dihydroxy Vitamin D3 and Interleukin-6 Blockade Synergistically Regulate Rheumatoid Arthritis by Suppressing Interleukin-17 Production and Osteoclastogenesis
Authors: H Kim, S Baek, SM Hong, J Lee, SM Jung, J Lee, M Cho, SK Kwok, SH Park
J. Korean Med. Sci., 2020-02-17;35(6):e40.
Species: Human
Sample Types: Cell Culture Supernates
-
Whole-cell fungal-mediated structural transformation of anabolic drug metenolone acetate into potent anti-inflammatory metabolites
Authors: M Siddiqui, Atia-Tul-W, A Jabeen, Y Wang, W Wang, Atta-Ur-Ra, MI Choudhary
J Adv Res, 2020-02-15;24(0):69-78.
Species: Aspergillus alliaceus, Caenorhabditis elegans, Fusarium lini, Rhizopus stolonifer
Sample Types: Cell Culture Supernates
-
Lung Surfactant Accelerates Skin Wound Healing: A Translational Study with a Randomized Clinical Phase I Study
Authors: U Mirastschi, I Schwab, V Coger, U Zier, C Rianna, W He, K Maedler, S Kelm, A Radtke, G Belge, P Lindner, F Stahl, M Scharpenbe, L Lasota, J Timm
Sci Rep, 2020-02-13;10(1):2581.
Species: Human
Sample Types: Tissue Homogenates
-
Regulation of Transcription Factor E2-2 in Human Plasmacytoid Dendritic Cells by Monocyte-Derived TNF&alpha
Authors: HK Dewald, HJ Hurley, P Fitzgerald
Viruses, 2020-01-31;12(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Pancreatic cancer-derived small extracellular vesical Ezrin regulates macrophage polarization and promotes metastasis
Authors: YT Chang, HY Peng, CM Hu, SC Huang, SC Tien, YM Jeng
Am J Cancer Res, 2020-01-01;10(1):12-37.
Species: Human
Sample Types: Cell Culture Supernates
-
The non-saponin fraction of Korean Red Ginseng (KGC05P0) decreases glucose uptake and transport in�vitro and modulates glucose production via down-regulation of the PI3K/AKT pathway in�vivo
Authors: SJ Park, D Lee, D Kim, M Lee, G In, ST Han, SW Kim, MH Lee, OK Kim, J Lee
J Ginseng Res, 2019-12-17;44(2):362-372.
Species: Human
Sample Types: Serum
-
Human immune cells infiltrate the spinal cord and impair recovery after spinal cord injury in humanized mice
Authors: RS Carpenter, RR Jiang, FH Brennan, JCE Hall, MK Gottipati, S Niewiesk, PG Popovich
Sci Rep, 2019-12-13;9(1):19105.
Species: Transgenic Mouse
Sample Types: Serum
-
Physiologic intestinal 18F-FDG uptake is associated with alteration of gut microbiota and proinflammatory cytokine levels in breast cancer
Authors: HJ Yoon, HN Kim, JI Bang, W Lim, BI Moon, NS Paik, BS Kim, HL Kim
Sci Rep, 2019-12-04;9(1):18273.
Species: Human
Sample Types: Serum
-
C13 Megastigmane Derivatives From Epipremnum pinnatum: beta-Damascenone Inhibits the Expression of Pro-Inflammatory Cytokines and Leukocyte Adhesion Molecules as Well as NF-kappaB Signaling
Authors: SP Pan, T Pirker, O Kunert, N Kretschmer, S Hummelbrun, SL Latkolik, J Rappai, VM Dirsch, V Bochkov, R Bauer
Front Pharmacol, 2019-11-28;10(0):1351.
Species: Human
Sample Types: Cell Culture Supernates
-
Replication of Genome-Wide Association Analysis Identifies New Susceptibility Loci at Long Noncoding RNA Regions for Vogt-Koyanagi-Harada Disease
Authors: J Qi, L Du, J Deng, Y Qin, G Su, S Hou, M Lv, Q Zhang, A Kijlstra, P Yang
Invest. Ophthalmol. Vis. Sci., 2019-11-01;60(14):4820-4829.
Species: Human
Sample Types: Cell Culture Supernates
-
Echinochrome A Reduces Colitis in Mice and Induces In Vitro Generation of Regulatory Immune Cells
Authors: SJ Oh, Y Seo, JS Ahn, YY Shin, JW Yang, HK Kim, J Han, NP Mishchenko, SA Fedoreyev, VA Stonik, HS Kim
Mar Drugs, 2019-10-31;17(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Disrupting prolonged sitting reduces IL-8 and lower leg swell in active young adults
Authors: S Dogra, M Wolf, MP Jeffrey, RCA Foley, H Logan-Spre, H Jones-Tagg, JM Green-John
BMC Sports Sci Med Rehabil, 2019-10-18;11(0):23.
Species: Human
Sample Types: Saliva
-
Comparative Investigation of Frankincense Nutraceuticals: Correlation of Boswellic and Lupeolic Acid Contents with Cytokine Release Inhibition and Toxicity against Triple-Negative Breast Cancer Cells
Authors: M Schmiech, SJ Lang, J Ulrich, K Werner, LJ Rashan, T Syrovets, T Simmet
Nutrients, 2019-10-02;11(10):.
Species: Human
Sample Types: Plasma
-
Relative Contributions of Extracellular and Internalized Bacteria to Early Macrophage Proinflammatory Responses to Streptococcus pneumoniae
Authors: J Periselner, G Ercoli, T Pollard, S Chimalapat, E Camberlein, G Szylar, C Hyams, G Tomlinson, FC Petersen, RA Floto, M Noursadegh, JS Brown
MBio, 2019-09-24;10(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
β-Glucan-Induced Trained Immunity Protects against Leishmania braziliensis Infection: a Crucial Role for IL-32
Authors: JC Dos Santos, AM Barroso de, MV Teodoro Si, B Cirovic, LCJ de Bree, MSMA Damen, SJCFM Moorlag, RS Gomes, MM Helsen, M Oosting, ST Keating, A Schlitzer, MG Netea, F Ribeiro-Di, LAB Joosten
Cell Rep, 2019-09-03;28(10):2659-2672.e6.
Species: Human
Sample Types: Cell Culture Supernates
-
Matrix metalloproteinases inactivate the proinflammatory functions of secreted moonlighting tryptophanyl-tRNA synthetase
Authors: PG Jobin, N Solis, Y Machado, PA Bell, NH Kwon, S Kim, CM Overall, GS Butler
J. Biol. Chem., 2019-07-19;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Mitochondrial function in liver cells is resistant to perturbations in NAD+ salvage capacity
Authors: M Dall, SAJ Trammell, M Asping, AS Hassing, M Agerholm, SG Vienberg, MP Gillum, S Larsen, JT Treebak
J. Biol. Chem., 2019-07-18;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Type 1 diabetic mellitus patients with increased atherosclerosis risk display decreased CDKN2A/2B/2BAS gene expression in leukocytes
Authors: S Martínez-H, V Sánchez-Ga, A Herrero-Ce, Á Vinué, JT Real, JF Ascaso, DJ Burks, H González-N
J Transl Med, 2019-07-12;17(1):222.
Species: Human
Sample Types: Plasma
-
Correction of bleeding in experimental severe hemophilia A by systemic delivery of factor VIII-encoding mRNA
Authors: J Russick, S Delignat, P Milanov, O Christophe, G Boros, CV Denis, PJ Lenting, SV Kaveri, S Lacroix-De
Haematologica, 2019-07-09;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
The human glomerular endothelial cells are potent pro-inflammatory contributors in an in vitro model of lupus nephritis
Authors: P Dimou, RD Wright, KL Budge, A Midgley, SC Satchell, M Peak, MW Beresford
Sci Rep, 2019-06-06;9(1):8348.
Species: Human
Sample Types: Cell Culture Supernates
-
Newly defined ABCB5+ dermal mesenchymal stem cells promote healing of chronic iron overload wounds via secretion of interleukin-1 receptor antagonist
Authors: S Vander Bek, JC de Vries, B Meier-Schi, P Meyer, D Jiang, A Sindrilaru, FF Ferreira, A Hainzl, S Schatz, J Muschhamme, NJ Scheurmann, P Kampilafko, AM Seitz, L Dürselen, A Ignatius, MA Kluth, C Ganss, M Wlaschek, K Singh, P Maity, NY Frank, MH Frank, K Scharffett
Stem Cells, 2019-05-13;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Flagellin-independent effects of a Toll-like receptor 5 polymorphism in the inflammatory response to Burkholderia pseudomallei
Authors: AK Dickey, N Chantratit, S Tandhavana, D Ducken, L Lovelace-M, S Seal, J Robertson, ND Myers, S Schwarz, MM Wurfel, S Kosamo, TE West
PLoS Negl Trop Dis, 2019-05-08;13(5):e0007354.
Species: Human
Sample Types: Cell Culture Supernates
-
Evolving understanding of cardiovascular, cerebrovascular and peripheral arterial disease in people living with HIV and role of novel biomarkers. A study of the Spanish CoRIS cohort, 2004-2015
Authors: M Masiá, S Padilla, JA García, J García-Abe, M Fernández, I Bernardino, M Montero, J Peraire, B Pernas, F Gutiérrez
PLoS ONE, 2019-04-26;14(4):e0215507.
Species: Human
Sample Types: Plasma
-
25(OH)D3 and 1.25(OH)2D3 inhibits TNF-? expression in human monocyte derived macrophages
Authors: A Rafique, L Rejnmark, L Heickendor, HJ Møller
PLoS ONE, 2019-04-12;14(4):e0215383.
Species: Human
Sample Types: Cell Culture Supernates
-
Human metapneumovirus activates NOD-like receptor protein 3 inflammasome via its small hydrophobic protein which plays a detrimental role during infection in mice
Authors: VB Lê, J Dubois, C Couture, MH Cavanagh, O Uyar, A Pizzorno, M Rosa-Calat, MÈ Hamelin, G Boivin
PLoS Pathog., 2019-04-09;15(4):e1007689.
Species: Human
Sample Types: Cell Culture Supernates
-
Secondhand smoke exposure and sleep-related breathing problems in toddlers
Authors: JA Groner, L Nicholson, H Huang, JA Bauer
Acad Pediatr, 2019-04-05;0(0):.
Species: Human
Sample Types: Serum
-
Role of vimentin in modulating immune cell apoptosis and inflammatory responses in sepsis
Authors: L Su, P Pan, P Yan, Y Long, X Zhou, X Wang, R Zhou, B Wen, L Xie, D Liu
Sci Rep, 2019-04-05;9(1):5747.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-6 released from differentiating human beige adipocytes improves browning
Authors: Endre Kristóf, Ágnes Klusóczki, Roland Veress, Abhirup Shaw, Zsolt Sándor Combi, Klára Varga et al.
Experimental Cell Research
Species: Human
Sample Types: Cell Culture Supernates
-
Calcineurin inhibitors reduce NFAT-dependent expression of antifungal pentraxin-3 by human monocytes
Authors: K Bendí?ková, F Tidu, M De Zuani, MH Kohoutková, I Andrej?ino, A Pompeiano, S B?lášková, G Forte, T Zelante, J Fri?
J. Leukoc. Biol., 2019-04-01;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis
Authors: C Wei, C Yang, S Wang, D Shi, C Zhang, X Lin, Q Liu, R Dou, B Xiong
Mol. Cancer, 2019-03-30;18(1):64.
Species: Human
Sample Types: Cell Culture Supernates
-
Historical BCG vaccination combined with drug treatment enhances inhibition of mycobacterial growth ex vivo in human peripheral blood cells
Authors: SA Prabowo, A Zelmer, L Stockdale, U Ojha, SG Smith, K Seifert, HA Fletcher
Sci Rep, 2019-03-19;9(1):4842.
Species: Human
Sample Types: Cell Culture Supernates
-
The TLR4 adaptor TRAM controls the phagocytosis of Gram-negative bacteria by interacting with the Rab11-family interacting protein 2
Authors: A Skjesol, M Yurchenko, K Bösl, C Gravastran, KE Nilsen, LM Grøvdal, F Agliano, F Patane, G Lentini, H Kim, G Teti, A Kumar Shar, RK Kandasamy, B Sporsheim, KK Starheim, DT Golenbock, H Stenmark, M McCaffrey, T Espevik, H Husebye
PLoS Pathog., 2019-03-18;15(3):e1007684.
Species: Human
Sample Types: Cell Culture Supernates
-
Engulfment, persistence and fate of Bdellovibrio bacteriovorus predators inside human phagocytic cells informs their future therapeutic potential
Authors: D Raghunatha, PM Radford, C Gell, D Negus, C Moore, R Till, PJ Tighe, SP Wheatley, L Martinez-P, RE Sockett, J Tyson
Sci Rep, 2019-03-12;9(1):4293.
Species: Human
Sample Types: Cell Culture Supernates
-
Large-scale secretome analyses unveil the superior immunosuppressive phenotype of umbilical cord stromal cells as compared to other adult mesenchymal stromal cells
Authors: A Islam, I Urbarova, JA Bruun, I Martinez-Z
Eur Cell Mater, 2019-02-20;37(0):153-174.
Species: Human
Sample Types: Cell Culture Supernates
-
Human Blood and Tonsil Plasmacytoid Dendritic Cells Display Similar Gene Expression Profiles but Exhibit Differential Type I IFN Responses to Influenza A Virus Infection
Authors: S Vangeti, J Gertow, M Yu, S Liu, F Baharom, S Scholz, D Friberg, M Starkhamma, A Ahlberg, A Smed-Sören
J. Immunol., 2019-02-13;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
D-2-Hydroxyglutarate and L-2-Hydroxyglutarate Inhibit IL-12 Secretion by Human Monocyte-Derived Dendritic Cells
Authors: I Ugele, ZE Cárdenas-C, K Hammon, M Wehrstein, C Bruss, K Peter, K Singer, E Gottfried, J Boesch, P Oefner, K Dettmer, K Renner, M Kreutz
Int J Mol Sci, 2019-02-10;20(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Phenotypic and functional stability of leukocytes from human peripheral blood samples: considerations for the design of immunological studies
Authors: A Navas, L Giraldo-Pa, MD Prieto, J Cabrera, MA Gómez
BMC Immunol., 2019-01-18;20(1):5.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-CD137 monoclonal antibody enhances trastuzumab-induced, natural killer cell-mediated cytotoxicity against pancreatic cancer cell lines with low human epidermal growth factor-like receptor 2 expression
Authors: T Masu, M Atsukawa, K Nakatsuka, M Shimizu, D Miura, T Arai, H Harimoto, C Kondo, K Kaneko, S Futagami, C Kawamoto, H Takahashi, K Iwakiri
PLoS ONE, 2018-12-31;13(12):e0200664.
Species: Human
Sample Types: Cell Culture Supernates
-
High expression of S100 calgranulin genes in peripheral blood mononuclear cells from patients with Takayasu arteritis
Authors: J Kabeerdoss, M Thomas, R Goel, H Mohan, S Danda, J L, D Danda
Cytokine, 2018-12-26;114(0):61-66.
Species: Human
Sample Types: Cell Culture Supernates
-
Sulphamethazine derivatives as immunomodulating agents: New therapeutic strategies for inflammatory diseases
Authors: H Siddiqui, HM Haniffa, A Jabeen, AU -Rahman, MI Choudhary
PLoS ONE, 2018-12-19;13(12):e0208933.
Species: Human
Sample Types: Cell Culture Supernates
-
PDGF enhances the protective effect of adipose stem cell-derived extracellular vesicles in a model of acute hindlimb ischemia
Authors: T Lopatina, E Favaro, C Grange, M Cedrino, A Ranghino, S Occhipinti, S Fallo, F Buffolo, DA Gaykalova, MM Zanone, R Romagnoli, G Camussi
Sci Rep, 2018-12-04;8(1):17458.
Species: Human
Sample Types: Cell Culture Supernates
-
Streptococcus agalactiae Induces Placental Macrophages To Release Extracellular Traps Loaded with Tissue Remodeling Enzymes via an Oxidative Burst-Dependent Mechanism
Authors: RS Doster, JA Sutton, LM Rogers, DM Aronoff, JA Gaddy
MBio, 2018-11-20;9(6):.
Species: Human
Sample Types:
-
A low-gluten diet induces changes in the intestinal microbiome of healthy Danish adults
Authors: LBS Hansen, HM Roager, NB Søndertoft, RJ Gøbel, M Kristensen, M Vallès-Col, S Vieira-Sil, S Ibrügger, MV Lind, RB Mærkedahl, MI Bahl, ML Madsen, J Havelund, G Falony, I Tetens, T Nielsen, KH Allin, HL Frandsen, B Hartmann, JJ Holst, MH Sparholt, J Holck, A Blennow, JM Moll, AS Meyer, C Hoppe, JH Poulsen, V Carvalho, D Sagnelli, MD Dalgaard, AF Christense, MC Lydolph, AB Ross, S Villas-Bôa, S Brix, T Sicheritz-, K Buschard, A Linneberg, JJ Rumessen, CT Ekstrøm, C Ritz, K Kristianse, HB Nielsen, H Vestergaar, NJ Færgeman, J Raes, H Frøkiær, T Hansen, L Lauritzen, R Gupta, TR Licht, O Pedersen
Nat Commun, 2018-11-13;9(1):4630.
Species: Human
Sample Types: Cell Culture Supernates
-
Modulation of salivary cytokines in response to alcohol, tobacco and caffeine consumption: a pilot study
Authors: CC Sheth, RM López-Pedr, MDM Jovani-San, R González-M, V Veses
Sci Rep, 2018-11-12;8(1):16687.
Species: Human
Sample Types: Saliva
-
Combining Calcium Phosphates with Polysaccharides: A Bone-Inspired Material Modulating Monocyte/Macrophage Early Inflammatory Response
Authors: H Rammal, C Bour, M Dubus, L Entz, L Aubert, SC Gangloff, S Audonnet, NB Bercu, F Boulmedais, C Mauprivez, H Kerdjoudj
Int J Mol Sci, 2018-11-03;19(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Overproduction of IL-6 and Type-I IFN in a Lethal Case of Chikungunya Virus Infection in an Elderly Man During the 2017 Italian Outbreak
Authors: F Colavita, S Vita, E Lalle, F Carletti, L Bordi, D Vincenti, I Pozzetto, M Aiuti, F Vairo, MR Capobianch, M Lichtner, C Castillett
Open Forum Infect Dis, 2018-10-25;5(11):ofy276.
Species: Human
Sample Types: Plasma
-
Haplosaurus computes protein haplotypes for use in precision drug design
Authors: W Spooner, W McLaren, T Slidel, DK Finch, R Butler, J Campbell, L Eghobamien, D Rider, CM Kiefer, MJ Robinson, C Hardman, F Cunningham, T Vaughan, P Flicek, CC Huntington
Nat Commun, 2018-10-08;9(1):4128.
Species: Human
Sample Types: Cell Culture Supernates
-
S100B regulates inflammatory response during osteoarthritis via fibroblast growth factor receptor 1 signaling
Authors: L Zhu, Z Weng, P Shen, J Zhou, J Zeng, F Weng, X Zhang, H Yang
Mol Med Rep, 2018-10-01;18(6):4855-4864.
Species: Human
Sample Types: Cell Culture Supernates
-
Augmentation of Human Monocyte Responses to Lipopolysaccharide by the Protein S and Mer/Tyro3 Receptor Tyrosine Kinase Axis
Authors: ND Barth, JA Marwick, MJ Heeb, AJ Gale, AG Rossi, I Dransfield
J. Immunol., 2018-09-24;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
The novel immunoglobulin super family receptor SLAMF9 identified in TAM of murine and human melanoma influences pro-inflammatory cytokine secretion and migration
Authors: C Dollt, J Michel, L Kloss, S Melchers, K Schledzews, K Becker, A Sauer, A Krewer, F Koll, A Schmieder
Cell Death Dis, 2018-09-19;9(10):939.
Species: Human
Sample Types: Cell Culture Supernates
-
Plasmodium falciparum Treated with Artemisinin-based Combined Therapy Exhibits Enhanced Mutation, Heightened Cortisol and TNF-? Induction
Authors: AO Idowu, S Bhattachar, S Gradus, W Oyibo, Z George, C Black, J Igietseme, AA Azenabor
Int J Med Sci, 2018-09-07;15(13):1449-1457.
Species: Human
Sample Types: Plasma
-
Disabled-2 (DAB2) Overexpression Inhibits Monocyte-Derived Dendritic Cells' Function in Vogt-Koyanagi-Harada Disease
Authors: S Yi, R Chang, J Hu, Y Qiu, Q Wang, Q Cao, G Yuan, G Su, C Zhou, Y Wang, A Kijlstra, P Yang
Invest. Ophthalmol. Vis. Sci., 2018-09-04;59(11):4662-4669.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum metabolomic profiling predicts synovial gene expression in rheumatoid arthritis
Authors: R Narasimhan, R Coras, SB Rosenthal, SR Sweeney, A Lodi, S Tiziani, D Boyle, A Kavanaugh, M Guma
Arthritis Res. Ther., 2018-08-03;20(1):164.
Species: Human
Sample Types: Serum
-
Comparison of pro- and anti-inflammatory responses in paired human primary airway epithelial cells and alveolar macrophages
Authors: RA Mubarak, N Roberts, RJ Mason, S Alper, HW Chu
Respir. Res., 2018-06-25;19(1):126.
Species: Human
Sample Types: Cell Culture Supernates
-
iTAP, a novel iRhom interactor, controls TNF secretion by policing the stability of iRhom/TACE
Authors: I Oikonomidi, E Burbridge, M Cavadas, G Sullivan, B Collis, H Naegele, D Clancy, J Brezinova, T Hu, A Bileck, C Gerner, A Bolado, A von Kriegs, SJ Martin, F Steinberg, K Strisovsky, C Adrain
Elife, 2018-06-13;7(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Electrophilic properties of itaconate and derivatives regulate the�I?B?-ATF3 inflammatory axis
Authors: M Bambouskov, L Gorvel, V Lampropoul, A Sergushich, E Loginichev, K Johnson, D Korenfeld, ME Mathyer, H Kim, LH Huang, D Duncan, H Bregman, A Keskin, A Santeford, RS Apte, R Sehgal, B Johnson, GK Amarasingh, MP Soares, T Satoh, S Akira, T Hai, C de Guzman, K Auclair, TP Roddy, SA Biller, M Jovanovic, E Klechevsky, KM Stewart, GJ Randolph, MN Artyomov
Nature, 2018-04-18;556(7702):501-504.
Species: Human
Sample Types: Cell Culture Supernates
-
CD47 as a potential prognostic marker for oral leukoplakia and oral squamous cell carcinoma
Authors: X Ye, X Wang, R Lu, J Zhang, X Chen, G Zhou
Oncol Lett, 2018-04-17;15(6):9075-9080.
Species: Human
Sample Types: Cell Culture Supernates
-
STAG2 deficiency induces interferon responses via cGAS-STING pathway and restricts virus infection
Authors: S Ding, J Diep, N Feng, L Ren, B Li, YS Ooi, X Wang, KF Brulois, LL Yasukawa, X Li, CJ Kuo, DA Solomon, JE Carette, HB Greenberg
Nat Commun, 2018-04-16;9(1):1485.
Species: Human
Sample Types: Cell Culture Supernates
-
Fecal Galectin-3: A New Promising Biomarker for Severity and Progression of Colorectal Carcinoma
Authors: M Jovanovic, N Gajovic, N Zdravkovic, M Jovanovic, M Jurisevic, D Vojvodic, V Maric, A Arsenijevi, I Jovanovic
Mediators Inflamm., 2018-04-04;2018(0):8031328.
Species: Human
Sample Types: Feces
-
Effects of agricultural organic dusts on human lung-resident mesenchymal stem (stromal) cell function
Authors: TM Nordgren, KL Bailey, AJ Heires, D Katafiasz, DJ Romberger
Toxicol. Sci., 2018-04-01;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
TET-2 up-regulation is associated with the anti-inflammatory action of Vicenin-2
Authors: N Hassan, A Ali, C Withycombe, M Ahluwalia, RH Al-Nasseri, A Tonks, K Morris
Cytokine, 2018-03-19;108(0):37-42.
Species: Human
Sample Types: Cell Culture Supernates
-
Analysis of the association between Fc receptor family gene polymorphisms and ocular Beh�et's disease in Han Chinese
Authors: D Zhang, J Qin, L Li, G Su, G Huang, Q Cao, A Kijlstra, P Yang
Sci Rep, 2018-03-19;8(1):4850.
Species: Human
Sample Types: Cell Culture Supernates
-
Clinically compliant spatial and temporal imaging of chimeric antigen receptor T-cells
Authors: N Emami-Shah, J Foster, R Kashani, P Gazinska, C Cook, J Sosabowski, J Maher, S Papa
Nat Commun, 2018-03-14;9(1):1081.
Species: Human
Sample Types: Cell Culture Supernates
-
p63 is a key regulator of iRHOM2 signalling in the keratinocyte stress response
Authors: P Arcidiacon, CM Webb, MA Brooke, H Zhou, PJ Delaney, KE Ng, DC Blaydon, A Tinker, DP Kelsell, A Chikh
Nat Commun, 2018-03-09;9(1):1021.
Species: Human
Sample Types: Cell Culture Supernates
-
Epicutaneous administration of the pattern recognition receptor agonist polyinosinic-polycytidylic acid activates the MDA5/MAVS pathway in Langerhans cells
Authors: P Tajpara, C Schuster, E Schön, P Kienzl, M Vierhapper, M Mildner, A Elbe-Bürge
FASEB J., 2018-03-06;0(0):fj201701090R.
Species: Human
Sample Types: Cell Culture Supernates
-
Association of genetic variations in PTPN2 and CD122 with ocular Behcet's disease
Authors: Q Zhang, H Li, S Hou, H Yu, G Su, B Deng, J Qi, C Zhou, A Kijlstra, P Yang
Br J Ophthalmol, 2018-03-03;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
The effects of repeated Toll-like receptors 2 and 4 stimulation in COPD alveolar macrophages
Authors: SR Lea, SL Reynolds, M Kaur, KD Simpson, SR Hall, EM Hessel, D Singh
Int J Chron Obstruct Pulmon Dis, 2018-03-02;13(0):771-780.
Species: Human
Sample Types: Cell Culture Supernates
-
Microsomal prostaglandin E synthase-1 gene deletion impairs neuro-immune circuitry of the cholinergic anti-inflammatory pathway in endotoxaemic mouse spleen
Authors: P Revathikum, J Estelius, U Karmakar, E Le Maître, M Korotkova, PJ Jakobsson, J Lampa
PLoS ONE, 2018-02-22;13(2):e0193210.
Species: Human
Sample Types: Cell Culture Supernates
-
Respiratory hazard assessment of combined exposure to complete gasoline exhaust and respirable volcanic ash in a multicellular human lung model at the air-liquid interface
Authors: I Tomašek, CJ Horwell, C Bisig, DE Damby, P Comte, J Czerwinski, A Petri-Fink, MJD Clift, B Drasler, B Rothen-Rut
Environ. Pollut., 2018-02-16;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
SLAMF1 is required for TLR4-mediated TRAM-TRIF-dependent signaling in human macrophages
Authors: M Yurchenko, A Skjesol, L Ryan, GM Richard, RK Kandasamy, N Wang, C Terhorst, H Husebye, T Espevik
J. Cell Biol., 2018-02-12;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Capture -
Association of Long Noncoding RNAs Polymorphisms With Ankylosing Spondylitis, Vogt-Koyanagi-Harada Disease, and Behcet's Disease
Authors: Y Yue, J Zhang, L Yang, S Liu, J Qi, Q Cao, C Zhou, Y Wang, A Kijlstra, P Yang, S Hou
Invest. Ophthalmol. Vis. Sci., 2018-02-01;59(2):1158-1166.
Species: Human
Sample Types: Cell Culture Supernates
-
Allergic Conjunctivitis-induced Retinal Inflammation Promotes Myopia Progression
Authors: CC Wei, YJ Kung, CS Chen, CY Chang, CJ Lin, PT Tien, HY Chang, HJ Chen, YS Huang, HJ Lin, L Wan
EBioMedicine, 2018-01-31;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
The Effects of Varying Degree of MWCNT Carboxylation on Bioactivity in Various In Vivo and In Vitro Exposure Models
Authors: RF Hamilton, Z Wu, S Mitra, A Holian
Int J Mol Sci, 2018-01-25;19(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb� sorbent porous polymer beads
Authors: MC Gruda, KG Ruggeberg, P O'Sullivan, T Guliashvil, AR Scheirer, TD Golobish, VJ Capponi, PP Chan
PLoS ONE, 2018-01-25;13(1):e0191676.
Species: Human
Sample Types: Complex Sample Type
-
TiO2 nanoparticles can selectively bind CXCL8 impacting on neutrophil chemotaxis
Authors: J Batt, M Milward, I Chapple, M Grant, H Roberts, O Addison
Eur Cell Mater, 2018-01-19;35(0):13-24.
-
Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11
Authors: B Lagrange, S Benaoudia, P Wallet, F Magnotti, A Provost, F Michal, A Martin, F Di Lorenzo, BF Py, A Molinaro, T Henry
Nat Commun, 2018-01-16;9(1):242.
Species: Human
Sample Types: Cell Culture Supernates
-
Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation.
Authors: Guedan S, Posey A, Shaw C, Wing A, Da T, Patel P, McGettigan S, Casado-Medrano V, Kawalekar O, Uribe-Herranz M, Song D, Melenhorst J, Lacey S, Scholler J, Keith B, Young R, June C
JCI Insight, 2018-01-11;3(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Does Transcranial Direct Current Stimulation Combined with Peripheral Electrical Stimulation Have an Additive Effect in the Control of Hip Joint Osteonecrosis Pain Associated with Sickle Cell Disease? A Protocol for a One-Session Double Blind, Block-Randomized Clinical Trial
Authors: TDS Lopes, WDS Silva, SB Ribeiro, CA Figueiredo, FQ Campbell, GC Daltro, A Valenzuela, P Montoya, RCS Lucena, AF Baptista
Front Hum Neurosci, 2017-12-20;11(0):633.
Species: Human
Sample Types: Serum
-
Immunoregulatory Activity of the Natural Product Laminarin Varies Widely as a Result of Its Physical Properties
Authors: AJ Smith, B Graves, R Child, PJ Rice, Z Ma, DW Lowman, HE Ensley, KT Ryter, JT Evans, DL Williams
J. Immunol., 2017-12-15;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of Vitamin D in Maintaining Renal Epithelial Barrier Function in Uremic Conditions
Authors: M Mihajlovic, M Fedecostan, MJ Oost, SKP Steenhuis, EGWM Lentjes, I Maitimu-Sm, MJ Janssen, LB Hilbrands, R Masereeuw
Int J Mol Sci, 2017-11-26;18(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Associations between TNFSF4, TNFSF8 and TNFSF15 and Beh�et's disease but not VKH syndrome in Han Chinese
Authors: Y Jiang, L Cheng, X Li, W Zhou, L Zhang
Oncotarget, 2017-10-23;8(62):105037-105046.
Species: Human
Sample Types: Cell Culture Supernates
-
Cross-Tissue Transcriptomic Analysis of Human Secondary Lymphoid Organ-Residing ILC3s Reveals a Quiescent State in the Absence of Inflammation
Authors: YE Bar-Ephrai, F Cornelisse, N Papazian, T Konijn, RM Hoogenboez, MA Sanders, BA Westerman, M Gönültas, J Kwekkeboom, JMM Den Haan, RM Reijmers, RE Mebius, T Cupedo
Cell Rep, 2017-10-17;21(3):823-833.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased expression of Siglec-9 in chronic obstructive pulmonary disease
Authors: Z Zeng, M Li, M Wang, X Wu, Q Li, Q Ning, J Zhao, Y Xu, J Xie
Sci Rep, 2017-08-31;7(1):10116.
Species: Human
Sample Types: Cell Culture Supernates
-
CLEC5A is a critical receptor in innate immunity against Listeria infection
Authors: ST Chen, FJ Li, TY Hsu, SM Liang, YC Yeh, WY Liao, TY Chou, NJ Chen, M Hsiao, WB Yang, SL Hsieh
Nat Commun, 2017-08-21;8(1):299.
Species: Human
Sample Types: Cell Culture Supernates
-
Vomocytosis of live pathogens from macrophages is regulated by the atypical MAP kinase ERK5
Authors: AS Gilbert, PI Seoane, P Sephton-Cl, A Bojarczuk, R Hotham, E Giurisato, AR Sarhan, A Hillen, GV Velde, NS Gray, DR Alessi, DL Cunningham, C Tournier, SA Johnston, RC May
Sci Adv, 2017-08-16;3(8):e1700898.
Species: Human
Sample Types: Cell Culture Supernates
-
Allostimulatory capacity of conditionally immortalized proximal tubule cell lines for bioartificial kidney application
Authors: M Mihajlovic, LP van den He, JG Hoenderop, J Jansen, MJ Wilmer, AJF Westheim, WA Allebes, D Stamatiali, LB Hilbrands, R Masereeuw
Sci Rep, 2017-08-02;7(1):7103.
Species: Human
Sample Types: Cell Culture Supernates
-
Minocycline modulates NF?B phosphorylation and enhances antimicrobial activity against Staphylococcus aureus in mesenchymal stromal/stem cells
Authors: AD Guerra, WE Rose, P Hematti, WJ Kao
Stem Cell Res Ther, 2017-07-21;8(1):171.
Species: Human
Sample Types: Cell Culture Supernates
-
Specific Biomarkers Associated With Neurological Complications and Congenital Central Nervous System Abnormalities From Zika Virus-Infected Patients in Brazil
Authors: YW Kam, JA Leite, FM Lum, JJL Tan, B Lee, CC Judice, DAT Teixeira, R Andreata-S, MA Vinolo, R Angerami, MR Resende, ARR Freitas, E Amaral, RP Junior, ML Costa, JP Guida, CW Arns, LCS Ferreira, L Rénia, JL Proença-Mo, LFP Ng, FTM Costa
J. Infect. Dis., 2017-07-15;216(2):172-181.
Species: Human
Sample Types: Serum
-
Dexamethasone induced inhibition of Dectin-1 activation of antigen presenting cells is mediated via STAT-3 and NF-?B signaling pathways.
Authors: Philipp Kotthoff, Annkristin Heine, Stefanie Andrea Erika Held, Peter Brossart
Scientific Reports, 2017-07-03;0(0):2045-2322.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-18/IL-15/IL-12 synergy induces elevated and prolonged IFN-? production by ex vivo expanded NK cells which is not due to enhanced STAT4 activation
Authors: E Lusty, SM Poznanski, K Kwofie, TS Mandur, DA Lee, CD Richards, AA Ashkar
Mol. Immunol., 2017-06-20;88(0):138-147.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of pre-emptive analgesia on clinical parameters and tissue levels of TNF-? and IL-1? in third molar surgery: a triple-blind, randomized, placebo-controlled study
Authors: AFM Albuquerqu, CSR Fonteles, DR do Val, HV Chaves, MM Bezerra, KMA Pereira, PG de Barros, BB de Lima, ECS Soares, TR Ribeiro, FWG Costa
Int J Oral Maxillofac Surg, 2017-06-10;0(0):.
Species: Human
Sample Types: Tissue Homogenates
-
Altered distribution of peripheral blood dendritic cell subsets in patients with pulmonary paracoccidioidomycosis
Authors: J Venturini, RS Cavalcante, DV Moris, M de Assis G, AD Levorato, KH Dos Reis, MSP de Arruda, RP Mendes
Acta Trop., 2017-06-09;0(0):.
Species: Human
Sample Types: Serum
-
Assessment of long-term cultivated human precision-cut lung slices as an ex vivo system for evaluation of chronic cytotoxicity and functionality
Authors: V Neuhaus, D Schaudien, T Golovina, UA Temann, C Thompson, T Lippmann, C Bersch, O Pfennig, D Jonigk, P Braubach, HG Fieguth, G Warnecke, V Yusibov, K Sewald, A Braun
J Occup Med Toxicol, 2017-05-26;12(0):13.
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophil Extracellular Traps Reprogram IL-4/GM-CSF-Induced Monocyte Differentiation to Anti-inflammatory Macrophages
Authors: AB Guimarães-, NC Rochael, F Oliveira, J Echevarria, EM Saraiva
Front Immunol, 2017-05-17;8(0):523.
Species: Human
Sample Types: Cell Culture Supernates
-
Analgesic and anti-inflammatory properties of novel, selective, and potent EP4 receptor antagonists
Authors: S Chandrasek, XP Yu, AK Harvey, JL Oskins, C Lin, X Wang, MJ Blanco, MJ Fisher, SL Kuklish, MA Schiffler, T Vetman, AM Warshawsky, JS York, AM Bendele, MG Chambers
Pharmacol Res Perspect, 2017-05-14;5(3):e00316.
Species: Human
Sample Types: Plasma
-
TB lymphadenitis is associated with enhanced baseline and antigen - specific induction of Type 1 and Type 17 cytokines and reduced IL-1? and IL-18 at the site of infection
Authors: GR Kathamuthu, K Moideen, D Baskaran, VV Banurekha, D Nair, G Sekar, R Sridhar, B Vidyajayan, G Gajendrara, DK Parandhama, A Srinivasan, S Babu
Clin. Vaccine Immunol, 2017-05-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors
Authors: A Taracanova, M Alevizos, A Karagkouni, Z Weng, E Norwitz, P Conti, SE Leeman, TC Theoharide
Proc. Natl. Acad. Sci. U.S.A., 2017-05-01;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro
Authors: E Redondo-Ca, C Cunningham, J Miller, L Martuscell, S Aoulad-Ali, NJ Rothwell, CM Kielty, SM Allan, E Pinteaux
Stem Cell Res Ther, 2017-04-17;8(1):79.
Species: Human
Sample Types: Cell Culture Supernates
-
Pre- and post-surgical evaluation of the inflammatory response in patients with aortic stenosis treated with different types of prosthesis
Authors: ME Soto, JL Salas, J Vargas-Bar, R Marquez, A Rodriguez-, R Bojalil-Pa, I Pérez-Torr, V Guarner-La
BMC Cardiovasc Disord, 2017-04-14;17(1):100.
Species: Human
Sample Types: Serum
-
Methoxyluteolin Inhibits Neuropeptide-stimulated Proinflammatory Mediator Release via mTOR Activation from Human Mast Cells.
Authors: Patel A, Theoharides T
J Pharmacol Exp Ther, 2017-04-12;361(3):462-471.
Species: Human
Sample Types: Cell Culture Supernates
-
Vitamin D both facilitates and attenuates the cellular response to lipopolysaccharide
Authors: L Chen, MS Eapen, GR Zosky
Sci Rep, 2017-03-27;7(0):45172.
Species: Human
Sample Types: Cell Culture Supernates
-
TSG-6 Downregulates IFN-Alpha and TNF-Alpha Expression by Suppressing IRF7 Phosphorylation in Human Plasmacytoid Dendritic Cells
Authors: L Kui, GC Chan, PP Lee
Mediators Inflamm., 2017-03-06;2017(0):7462945.
Species: Human
Sample Types: Cell Culture Supernates
-
Periodontitis and type 2 diabetes among women with previous gestational diabetes: epidemiological and immunological aspects in a follow-up of three years
Authors: RP Esteves Li, LO Cota, TA Silva, SC Cortelli, JR Cortelli, FO Costa
J Appl Oral Sci, 2017-03-01;25(2):130-139.
Species: Human
Sample Types: Saliva
-
Changes in peripheral immune cell numbers and functions in octogenarian walkers - an acute exercise study
Authors: KS van der Ge, Q Wang, TM Eijsvogels, HJ Koenen, I Joosten, E Brouwer, MT Hopman, JF Jacobs, AM Boots
Immun Ageing, 2017-02-22;14(0):5.
Species: Human
Sample Types: Cell Culture Supernates
-
On the cytokine/chemokine network during Plasmodium vivax malaria: new insights to understand the disease
Authors: NS Hojo-Souza, DB Pereira, FS de Souza, TA de Oliveir, MS Cardoso, MS Tada, GM Zanini, DC Bartholome, RT Fujiwara, LL Bueno
Malar. J, 2017-01-24;16(1):42.
Species: Human
Sample Types: Plasma
-
MODELING STROMA-INDUCED DRUG RESISTANCE IN A TISSUE-ENGINEERED TUMOR MODEL OF EWING SARCOMA
Authors: Marco Santoro
Tissue Eng Part A, 2017-01-01;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Differential IL-1? secretion by monocyte subsets is regulated by Hsp27 through modulating mRNA stability
Sci Rep, 2016-12-15;6(0):39035.
Species: Human
Sample Types: Cell Culture Supernates
-
DHA-derived oxylipins, neuroprostanes and protectins, differentially and dose-dependently modulate the inflammatory response in human macrophages: putative mechanisms through PPAR activation
Authors: Rémy Bosviel
Free Radic. Biol. Med, 2016-12-14;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Combined exposure of diesel exhaust particles and respirable Soufri�re Hills volcanic ash causes a (pro-)inflammatory response in an in vitro multicellular epithelial tissue barrier model
Authors: Ines Tomašek
Part Fibre Toxicol, 2016-12-12;13(1):67.
Species: Human
Sample Types: Cell Culture Supernates
-
17-oxo-DHA displays additive anti-inflammatory effects with fluticasone propionate and inhibits the NLRP3 inflammasome
Sci Rep, 2016-11-24;6(0):37625.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum from Varicose Patients Induces Senescence-Related Dysfunction of Vascular Endothelium Generating Local and Systemic Proinflammatory Conditions
Oxid Med Cell Longev, 2016-11-23;2016(0):2069290.
Species: Human
Sample Types: Serum
-
?-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance
Authors: Hendrik G Stunnenber
Cell, 2016-11-17;167(5):1354-1368.e14.
Species: Human
Sample Types: Cell Culture Supernates
-
Biocompatibility of primers and an adhesive used for implant-retained maxillofacial prostheses: An in�vitro analysis
Authors: Daniela Micheline Dos Santos
J Prosthet Dent, 2016-11-09;0(0):.
-
CD36-Mediated Uptake of Surfactant Lipids by Human Macrophages Promotes Intracellular Growth of Mycobacterium tuberculosis
J. Immunol., 2016-11-09;197(12):4727-4735.
Species: Human
Sample Types: Cell Culture Supernates
-
Reduced systemic and mycobacterial antigen-stimulated concentrations of IL-1? and IL-18 in tuberculous lymphadenitis
Cytokine, 2016-10-26;90(0):66-72.
Species: Human
Sample Types: Cell Culture Supernates
-
CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes
Sci Rep, 2016-09-27;6(0):34310.
Species: Human
Sample Types: Whole Cells
-
Temperature responsive porous silicon nanoparticles for cancer therapy - spatiotemporal triggering through infrared and radiofrequency electromagnetic heating
J Control Release, 2016-09-26;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Rational design of a Kv1.3 channel-blocking antibody as a selective immunosuppressant
Proc Natl Acad Sci USA, 2016-09-23;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Activated monocytes resist elimination by retinal pigment epithelium and downregulate their OTX2 expression via TNF-?
Aging Cell, 2016-09-22;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Bortezomib treatment sensitizes oncolytic HSV-1 treated tumors to NK cell immunotherapy
Clin Cancer Res, 2016-07-07;0(0):.
Species: Mouse
Sample Types: Serum
-
Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma
Authors: Avery D Posey
Immunity, 2016-06-21;44(6):1444-54.
Species: Human
Sample Types: Cell Culture Supernates
-
Analysis of receptor tyrosine kinase genetics identifies two novel risk loci in GAS6 and PROS1 in Beh�et's disease
Sci Rep, 2016-05-25;6(0):26662.
Species: Human
Sample Types: Cell Culture Supernates
-
vIL-10-overexpressing human MSCs modulate na�ve and activated T lymphocytes following induction of collagenase-induced osteoarthritis
Stem Cell Res Ther, 2016-05-18;7(1):74.
Species: Human
Sample Types: Cell Culture Supernates
-
Genetic polymorphisms of cell adhesion molecules in Behcet's disease in a Chinese Han population
Authors: M Zheng, L Zhang, H Yu, J Hu, Q Cao, G Huang, Y Huang, G Yuan, A Kijlstra, P Yang
Sci Rep, 2016-04-25;6(0):24974.
Species: Human
Sample Types: Cell Culture Supernates
-
New Anti-Inflammatory Metabolites by Microbial Transformation of Medrysone
Authors: S Bano, AT Wahab, S Yousuf, A Jabeen, MA Mesaik, AU Rahman, MI Choudhary
PLoS ONE, 2016-04-22;11(4):e0153951.
Species: Human
Sample Types: Cell Culture Supernates
-
Stromal fibroblasts support dendritic cells to maintain IL-23/Th17 responses after exposure to ionizing radiation
Authors: A Malecka, Q Wang, S Shah, RV Sutavani, I Spendlove, JM Ramage, J Greensmith, HA Franks, MJ Gough, A Saalbach, PM Patel, AM Jackson
J Leukoc Biol, 2016-04-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of the MMP/TIMP Imbalance and Collagen Production Induced by IL-1? or TNF-? Release from Human Hepatic Stellate Cells
Authors: S Robert, T Gicquel, A Bodin, V Lagente, E Boichot
PLoS ONE, 2016-04-05;11(4):e0153118.
Species: Human
Sample Types: Cell Culture Supernates
-
Soluble alpha-enolase activates monocytes by CD14-dependent TLR4 signalling pathway and exhibits a dual function
Authors: C Guillou, M Fréret, E Fondard, C Derambure, G Avenel, ML Golinski, M Verdet, O Boyer, F Caillot, P Musette, T Lequerré, O Vittecoq
Sci Rep, 2016-03-30;6(0):23796.
Species: Human
Sample Types: Cell Culture Supernates
-
Sensor Function for Butyrophilin 3A1 in Prenyl Pyrophosphate Stimulation of Human Vgamma2Vdelta2 T Cells.
Authors: Wang H, Morita C
J Immunol, 2015-10-16;195(10):4583-94.
Species: Human
Sample Types: Cell Culture Supernates
-
In vitro progesterone modulation on bacterial endotoxin-induced production of IL-1beta, TNFalpha, IL-6, IL-8, IL-10, MIP-1alpha, and MMP-9 in pre-labor human term placenta.
Authors: Garcia-Ruiz G, Flores-Espinosa P, Preciado-Martinez E, Bermejo-Martinez L, Espejel-Nunez A, Estrada-Gutierrez G, Maida-Claros R, Flores-Pliego A, Zaga-Clavellina V
Reprod Biol Endocrinol, 2015-10-07;13(0):115.
Species: Human
Sample Types: Cell Culture Supernates
-
Vpr Enhances Tumor Necrosis Factor Production by HIV-1-Infected T Cells.
Authors: Roesch F, Richard L, Rua R, Porrot F, Casartelli N, Schwartz O
J Virol, 2015-09-23;89(23):12118-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Transcriptomic Analyses of the Biological Effects of Airborne PM2.5 Exposure on Human Bronchial Epithelial Cells.
Authors: Zhou Z, Liu Y, Duan F, Qin M, Wu F, Sheng W, Yang L, Liu J, He K
PLoS ONE, 2015-09-18;10(9):e0138267.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-23 Receptor Gene Polymorphism May Enhance Expression of the IL-23 Receptor, IL-17, TNF-alpha and IL-6 in Behcet's Disease.
Authors: Jiang Z, Hennein L, Tao Y, Tao L
PLoS ONE, 2015-07-29;10(7):e0134632.
Species: Human
Sample Types: Cell Culture Supernates
-
An Autocrine Cytokine/JAK/STAT-Signaling Induces Kynurenine Synthesis in Multidrug Resistant Human Cancer Cells.
Authors: Campia I, Buondonno I, Castella B, Rolando B, Kopecka J, Gazzano E, Ghigo D, Riganti C
PLoS ONE, 2015-05-08;10(5):e0126159.
Species: Human
Sample Types: Cell Culture Supernates
-
Innate immune response to Streptococcus pyogenes depends on the combined activation of TLR13 and TLR2.
Authors: Fieber C, Janos M, Koestler T, Gratz N, Li X, Castiglia V, Aberle M, Sauert M, Wegner M, Alexopoulou L, Kirschning C, Chen Z, von Haeseler A, Kovarik P
PLoS ONE, 2015-03-10;10(3):e0119727.
Species: Human
Sample Types: Cell Culture Supernates
-
Amyloid precursor protein mediated changes in intestinal epithelial phenotype in vitro.
Authors: Puig K, Manocha G, Combs C
PLoS ONE, 2015-03-05;10(3):e0119534.
Species: Human
Sample Types: Cell Culture Supernates
-
Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus.
Authors: Herbst S, Shah A, Mazon Moya M, Marzola V, Jensen B, Reed A, Birrell M, Saijo S, Mostowy S, Shaunak S, Armstrong-James D
EMBO Mol Med, 2015-03-01;7(3):240-58.
Species: Human
Sample Types: Cell Culture Supernates
-
Exposure to diesel exhaust particle extracts (DEPe) impairs some polarization markers and functions of human macrophages through activation of AhR and Nrf2.
Authors: Jaguin M, Fardel O, Lecureur V
PLoS ONE, 2015-02-24;10(2):e0116560.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model.
Authors: Guirado E, Mbawuike U, Keiser T, Arcos J, Azad A, Wang S, Schlesinger L
MBio, 2015-02-17;6(1):e02537-14.
Species: Human
Sample Types: Cell Culture Supernates
-
SRSF1 facilitates cytosolic DNA-induced production of type I interferons recognized by RIG-I.
Authors: Xue F, Li X, Zhao X, Wang L, Liu M, Shi R, Zheng J
PLoS ONE, 2015-02-06;10(2):e0115354.
Species: Human
Sample Types: Cell Culture Supernates
-
Phenotypic profiling of CD8(+) T cells during Plasmodium vivax blood-stage infection.
Authors: Hojo-Souza N, Pereira D, Passos L, Gazzinelli-Guimaraes P, Cardoso M, Tada M, Zanini G, Bartholomeu D, Fujiwara R, Bueno L
BMC Infect Dis, 2015-01-31;15(0):35.
Species: Human
Sample Types: Plasma
-
Macrophage activation in acute exacerbation of idiopathic pulmonary fibrosis.
Authors: Schupp J, Binder H, Jager B, Cillis G, Zissel G, Muller-Quernheim J, Prasse A
PLoS ONE, 2015-01-15;10(1):e0116775.
Species: Human
Sample Types: BALF
-
Sourcing of an alternative pericyte-like cell type from peripheral blood in clinically relevant numbers for therapeutic angiogenic applications.
Authors: Blocki A, Wang Y, Koch M, Goralczyk A, Beyer S, Agarwal N, Lee M, Moonshi S, Dewavrin J, Peh P, Schwarz H, Bhakoo K, Raghunath M
Mol Ther, 2014-12-12;23(3):510-22.
Species: Human
Sample Types: Cell Culture Supernates
-
Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal.
Authors: Salzano S, Checconi P, Hanschmann E, Lillig C, Bowler L, Chan P, Vaudry D, Mengozzi M, Coppo L, Sacre S, Atkuri K, Sahaf B, Herzenberg L, Herzenberg L, Mullen L, Ghezzi P
Proc Natl Acad Sci U S A, 2014-08-05;111(33):12157-62.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Technical advance: live-imaging analysis of human dendritic cell migrating behavior under the influence of immune-stimulating reagents in an organotypic model of lung.
Authors: Nguyen Hoang A, Chen P, Bjornfot S, Hogstrand K, Lock J, Grandien A, Coles M, Svensson M
J Leukoc Biol, 2014-06-04;96(3):481-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of the flagellin glycosylation system in Burkholderia cenocepacia and the contribution of glycosylated flagellin to evasion of human innate immune responses.
Authors: Hanuszkiewicz A, Pittock P, Humphries F, Moll H, Rosales A, Molinaro A, Moynagh P, Lajoie G, Valvano M
J Biol Chem, 2014-05-19;289(27):19231-44.
Species: Human
Sample Types: Cell Culture Supernates
-
A functional variant of PTPN22 confers risk for Vogt-Koyanagi-Harada syndrome but not for ankylosing spondylitis.
Authors: Zhang Q, Qi J, Hou S, DU L, Yu H, Cao Q, Zhou Y, Liao D, Kijlstra A, Yang P
PLoS ONE, 2014-05-09;9(5):e96943.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-inflammatory effects of green soybean extract irradiated with visible light.
Authors: Tanaka K, Ohgo Y, Katayanagi Y, Yasui K, Hiramoto S, Ikemoto H, Nakata Y, Miyoshi N, Isemura M, Ohashi N, Imai S
Sci Rep, 2014-04-22;4(0):4732.
Species: Human
Sample Types: Cell Culture Supernates
-
Engagement of SLAMF2/CD48 prolongs the time frame of effective T cell activation by supporting mature dendritic cell survival.
Authors: Kis-Toth K, Tsokos G
J Immunol, 2014-03-26;192(9):4436-42.
Species: Human
Sample Types: Cell Culture Supernates
-
Separately or combined, LukG/LukH is functionally unique compared to other staphylococcal bicomponent leukotoxins.
Authors: Yanai M, Rocha M, Matolek A, Chintalacharuvu A, Taira Y, Chintalacharuvu K, Beenhouwer D
PLoS ONE, 2014-02-20;9(2):e89308.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunoepidemiological profiling of onchocerciasis patients reveals associations with microfilaria loads and ivermectin intake on both individual and community levels.
Authors: Arndts, Kathrin, Specht, Sabine, Debrah, Alexande, Tamarozzi, Francesc, Klarmann Schulz, Ute, Mand, Sabine, Batsa, Linda, Kwarteng, Alexande, Taylor, Mark, Adjei, Ohene, Martin, Coralie, Layland, Laura E, Hoerauf, Achim
PLoS Negl Trop Dis, 2014-02-20;8(2):e2679.
Species: Human
Sample Types: Cell Culture Supernates
-
The aryl hydrocarbon receptor is functionally upregulated early in the course ofvhuman T-cell activation.
Authors: Prigent L, Robineau M, Jouneau S, Morzadec C, Louarn L, Vernhet L, Fardel O, Sparfel L
Eur J Immunol, 2014-02-18;44(5):1330-40.
Species: Human
Sample Types: Cell Culture Supernates
-
Low adiposity during early infancy is associated with a low risk for developing dengue hemorrhagic fever: a preliminary model.
Authors: Libraty D, Zhang L, Woda M, Giaya K, Kathivu C, Acosta L, Tallo V, Segubre-Mercado E, Bautista A, Obcena A, Brion J, Capeding R
PLoS ONE, 2014-02-12;9(2):e88944.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased prevalence of Methanosphaera stadtmanae in inflammatory bowel diseases.
Authors: Blais Lecours P, Marsolais D, Cormier Y, Berberi M, Hache C, Bourdages R, Duchaine C
PLoS ONE, 2014-02-03;9(2):e87734.
Species: Human
Sample Types: Cell Culture Supernates
-
Neisseria gonorrhoeae modulates iron-limiting innate immune defenses in macrophages.
Authors: Zughaier, Susu M, Kandler, Justin L, Shafer, William
PLoS ONE, 2014-01-28;9(1):e87688.
Species: Human
Sample Types: Cell Culture Supernates
-
Smac mimetics and innate immune stimuli synergize to promote tumor death.
Authors: Beug S, Tang V, Lacasse E, Cheung H, Beauregard C, Brun J, Nuyens J, Earl N, St-Jean M, Holbrook J, Dastidar H, Mahoney D, Ilkow C, Le Boeuf F, Bell J, Korneluk R
Nat Biotechnol, 2014-01-26;32(2):182-90.
Species: Human
Sample Types: Cell Culture Supernates
-
CD137 ligand signalling induces differentiation of primary acute myeloid leukaemia cells.
Authors: Cheng K, Wong S, Linn Y, Ho L, Chng W, Schwarz H
Br J Haematol, 2014-01-15;165(1):134-44.
Species: Human
Sample Types: Cell Culture Supernates
-
TRAF5 and TRAF3IP2 gene polymorphisms are associated with Behcet's disease and Vogt-Koyanagi-Harada syndrome: a case-control study.
Authors: Xiang Q, Chen L, Hou S, Fang J, Zhou Y, Bai L, Liu Y, Kijlstra A, Yang P
PLoS ONE, 2014-01-08;9(1):e84214.
Species: Human
Sample Types: Cell Culture Supernates
-
Activin A as a mediator of NK-dendritic cell functional interactions.
Authors: Seeger P, Bosisio D, Parolini S, Badolato R, Gismondi A, Santoni A, Sozzani S
J Immunol, 2014-01-06;192(3):1241-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Association of TLR2 gene polymorphisms with ocular Behcet's disease in a Chinese Han population.
Authors: Fang J, Hu R, Hou S, Ye Z, Xiang Q, Qi J, Zhou Y, Kijlstra A, Yang P
Invest Ophthalmol Vis Sci, 2013-12-30;54(13):8384-92.
Species: Human
Sample Types: Cell Culture Supernates
-
The small GTPase Arf6 is essential for the Tram/Trif pathway in TLR4 signaling.
Authors: Van Acker T, Eyckerman S, Vande Walle L, Gerlo S, Goethals M, Lamkanfi M, Bovijn C, Tavernier J, Peelman F
J Biol Chem, 2013-12-02;289(3):1364-76.
Species: Human
Sample Types: Cell Culture Supernates
-
Survey of innate immune responses to Burkholderia pseudomallei in human blood identifies a central role for lipopolysaccharide.
Authors: Chantratita N, Tandhavanant S, Myers N, Seal S, Arayawichanont A, Kliangsa-Ad A, Hittle L, Ernst R, Emond M, Wurfel M, Day N, Peacock S, West T
PLoS ONE, 2013-11-26;8(11):e81617.
Species: Human
Sample Types: Cell Culture Supernates
-
Human neutrophil peptide-1 (HNP-1): a new anti-leishmanial drug candidate.
Authors: Dabirian S, Taslimi Y, Zahedifard F, Gholami E, Doustdari F, Motamedirad M, Khatami S, Azadmanesh K, Nylen S, Rafati S
PLoS Negl Trop Dis, 2013-10-17;7(10):e2491.
Species: Human
Sample Types: Cell Culture Supernates
-
Glycosylation in a mammalian expression system is critical for the production of functionally active leukocyte immunoglobulin-like receptor A3 protein.
Authors: Lee T, Mitchell A, Liu Lau S, An H, Rajeaskariah P, Wasinger V, Raftery M, Bryant K, Tedla N
J Biol Chem, 2013-09-30;288(46):32873-85.
Species: Human
Sample Types: Cell Culture Supernates
-
Fcgamma receptor-induced soluble vascular endothelial growth factor receptor-1 (VEGFR-1) production inhibits angiogenesis and enhances efficacy of anti-tumor antibodies.
Authors: Justiniano S, Elavazhagan S, Fatehchand K, Shah P, Mehta P, Roda J, Mo X, Cheney C, Hertlein E, Eubank T, Marsh C, Muthusamy N, Butchar J, Byrd J, Tridandapani S
J Biol Chem, 2013-07-31;288(37):26800-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Activated factor X signaling via protease-activated receptor 2 suppresses pro-inflammatory cytokine production from lipopolysaccharide-stimulated myeloid cells.
Authors: Gleeson E, O'Donnell J, Hams E, Ni Ainle F, Kenny B, Fallon P, Preston R
Haematologica, 2013-07-19;99(1):185-93.
Species: Human
Sample Types: Cell Culture Supernates
-
Tumor necrosis factor receptor 1 associates with CD137 ligand and mediates its reverse signaling.
Authors: Moh M, Lorenzini P, Gullo C, Schwarz H
FASEB J, 2013-04-25;27(8):2957-66.
Species: Human
Sample Types: Cell Culture Supernates
-
S100A12 suppresses pro-inflammatory, but not pro-thrombotic functions of serum amyloid A.
Authors: Chung Y, Goyette J, Tedla N, Hsu K, Geczy C
PLoS ONE, 2013-04-24;8(4):e62372.
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of binding sites for myeloid differentiation primary response gene 88 (MyD88) and Toll-like receptor 4 in MyD88 adapter-like (Mal).
Authors: Bovijn C, Desmet A, Uyttendaele I, Van Acker T, Tavernier J, Peelman F
J Biol Chem, 2013-03-04;288(17):12054-66.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of high-dose cholecalciferol on serum markers of inflammation and immunity in patients with early chronic kidney disease.
Authors: Alvarez J, Zughaier S, Law J, Hao L, Wasse H, Ziegler T, Tangpricha V
Eur J Clin Nutr, 2013-01-30;67(3):264-9.
Species: Human
Sample Types: Serum
-
Leptin up-regulates TLR2 in human monocytes.
Authors: Jaedicke K, Roythorne A, Padget K, Todryk S, Preshaw P, Taylor J
J Leukoc Biol, 2013-01-22;93(4):561-71.
Species: Human
Sample Types: Cell Culture Supernates
-
Thrombin-cleaved fragments of osteopontin are overexpressed in malignant glial tumors and provide a molecular niche with survival advantage.
Authors: Yamaguchi Y, Shao Z, Sharif S, Du X, Myles T, Merchant M, Harsh G, Glantz M, Recht L, Morser J, Leung L
J Biol Chem, 2012-11-30;288(5):3097-111.
Species: Human
Sample Types: CSF
-
Age-related changes in expression and function of Toll-like receptors in human skin.
Authors: Iram N, Mildner M, Prior M, Petzelbauer P, Fiala C, Hacker S, Schoppl A, Tschachler E, Elbe-Burger A
Development, 2012-10-03;139(22):4210-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse.
J Neuroinflammation, 2012-08-18;9(0):203.
Species: Human
Sample Types: Cell Culture Supernates
-
TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation.
Authors: Jouan-Lanhouet S, Arshad M, Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van Herreweghe F, Takahashi N, Sergent O, Lagadic-Gossmann D, Vandenabeele P, Samson M, Dimanche-Boitrel M
Cell Death Differ, 2012-07-20;19(12):2003-14.
Species: Human
Sample Types: Cell Culture Supernates
-
Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation.
Eur J Clin Nutr, 2012-07-18;66(9):1072-4.
Species: Human
Sample Types: Plasma
-
Anti-Tumour Effects of a Specific Anti-ADAM17 Antibody in an Ovarian Cancer Model In Vivo.
Authors: Richards FM, Tape CJ, Jodrell DI, Murphy G
PLoS ONE, 2012-07-11;7(7):e40597.
Species: Human
Sample Types: Tissue Homogenates
-
Trappin-2/elafin modulate innate immune responses of human endometrial epithelial cells to PolyI:C.
Authors: Drannik AG, Nag K, Yao XD, Henrick BM, Sallenave JM, Rosenthal KL
PLoS ONE, 2012-04-24;7(4):e35866.
Species: Human
Sample Types: Cell Culture Supernates
-
Regulation of progranulin expression in human microglia and proteolysis of progranulin by matrix metalloproteinase-12 (MMP-12).
Authors: Suh HS, Choi N, Tarassishin L, Lee SC
PLoS ONE, 2012-04-11;7(4):e35115.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated adaptive immune responses are associated with latent infections of Wuchereria bancrofti.
Authors: Arndts K, Deininger S, Specht S, Klarmann U, Mand S, Adjobimey T, Debrah AY, Batsa L, Kwarteng A, Epp C, Taylor M, Adjei O, Layland LE, Hoerauf A
PLoS Negl Trop Dis, 2012-04-03;6(4):e1611.
Species: Human
Sample Types: Cell Culture Supernates
-
Transglutaminase is essential for IgA nephropathy development acting through IgA receptors.
Authors: Berthelot L, Papista C, Maciel TT, Biarnes-Pelicot M, Tissandie E, Wang PH, Tamouza H, Jamin A, Bex-Coudrat J, Gestin A, Boumediene A, Arcos-Fajardo M, England P, Pillebout E, Walker F, Daugas E, Vrtosvnik F, Flamant M, Benhamou M, Cogne M, Moura IC, Monteiro RC
J. Exp. Med., 2012-03-26;209(4):793-806.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin (IL)-4, IL-13, and IL-17A differentially affect the profibrotic and proinflammatory functions of fibrocytes from asthmatic patients.
Authors: Bellini A, Marini MA, Bianchetti L, Barczyk M, Schmidt M, Mattoli S
Mucosal Immunol, 2011-12-21;5(2):140-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased secretion of Gas6 by smooth muscle cells in human atherosclerotic carotid plaques.
Authors: Clauser S, Meilhac O, Bieche I, Raynal P, Bruneval P, Michel JB, Borgel D
Thromb. Haemost., 2011-11-10;107(1):140-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Natural killer cell-produced IFN-gamma and TNF-alpha induce target cell cytolysis through up-regulation of ICAM-1.
Authors: Wang R, Jaw JJ, Jaw J, Stutzman NC, Stutzman N, Zou Z, Sun PD
J Leukoc Biol, 2011-11-01;91(2):299-309.
Species: Human
Sample Types: Cell Culture Supernates
-
Inhibition of IL-32 activation by alpha-1 antitrypsin suppresses alloreactivity and increases survival in an allogeneic murine marrow transplantation model.
Authors: Marcondes AM, Li X, Tabellini L, Bartenstein M, Kabacka J, Sale GE, Hansen JA, Dinarello CA, Deeg HJ
Blood, 2011-09-06;118(18):5031-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Monocytic microparticles activate endothelial cells in an IL-1{beta}-dependent manner.
Authors: Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JP, Mackman N
Blood, 2011-06-23;118(8):2366-74.
Species: Human
Sample Types: Cell Lysates
-
Adipokine resistin predicts anti-inflammatory effect of glucocorticoids in asthma.
Authors: Leivo-Korpela S, Lehtimaki L, Vuolteenaho K, Nieminen R, Kankaanranta H, Saarelainen S, Moilanen E
J Inflamm (Lond), 2011-05-26;8(0):12.
Species: Human
Sample Types: Cell Culture Supernates
-
Plasma levels of procalcitonin and eight additional inflammatory molecules in febrile neutropenic patients.
Authors: Neuenschwander LC, Bittencourt H, Ribeiro AF, Teixeira AL, Teixeira MM, Teixeira JC, Nobre V
Clinics (Sao Paulo), 2011-01-01;66(10):1699-705.
Species: Human
Sample Types: Plasma
-
IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes.
Authors: Pennino D, Eyerich K, Scarponi C, Carbone T, Eyerich S, Nasorri F, Garcovich S, Traidl-Hoffmann C, Albanesi C, Cavani A
J. Immunol., 2010-03-31;184(9):4880-8.
Species: Human
Sample Types: Cell Culture Supernates
-
KR-003048, a potent, orally active inhibitor of p38 mitogen-activated protein kinase.
Authors: Montalban AG, Boman E, Chang CD, Ceide SC, Dahl R, Dalesandro D, Delaet NG, Erb E, Ernst J, Gibbs A, Kahl J, Kessler L, Lundstrom J, Miller S, Nakanishi H, Roberts E, Saiah E, Sullivan R, Wang Z, Larson CJ
Eur. J. Pharmacol., 2010-02-02;632(1):93-102.
Species: Human
Sample Types: Cell Culture Supernates
-
Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease.
Authors: van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, Van der Meer JW, Netea MG
Proc. Natl. Acad. Sci. U.S.A., 2010-01-26;107(7):3030-3.
Species: Human
Sample Types: Cell Culture Supernates
-
Activation of platelets by Aspergillus fumigatus and potential role of platelets in the immunopathogenesis of Aspergillosis.
Authors: Rodland EK, Ueland T, Pedersen TM, Halvorsen B, Muller F, Aukrust P, Froland SS
Infect. Immun., 2009-12-14;78(3):1269-75.
Species: Human
Sample Types: Cell Culture Supernates
-
Helminth antigens modulate immune responses in cells from multiple sclerosis patients through TLR2-dependent mechanisms.
Authors: Correale J, Farez M
J. Immunol., 2009-10-07;183(9):5999-6012.
Species: Human
Sample Types: Cell Culture Supernates
-
Survival and migration of human dendritic cells are regulated by an IFN-alpha-inducible Axl/Gas6 pathway.
Authors: Scutera S, Fraone T, Musso T, Cappello P, Rossi S, Pierobon D, Orinska Z, Paus R, Bulfone-Paus S, Giovarelli M
J. Immunol., 2009-08-05;183(5):3004-13.
Species: Human
Sample Types: Cell Culture Supernates
-
Interferon-beta treatment in multiple sclerosis attenuates inflammatory gene expression through inducible activity of the phosphatase SHP-1.
Authors: Christophi GP, Panos M, Hudson CA, Tsikkou C, Mihai C, Mejico LJ, Jubelt B, Massa PT
Clin. Immunol., 2009-06-25;133(1):27-44.
Species: Human
Sample Types: Plasma
-
Reduced ex vivo interleukin-6 production by dietary fish oil is not modified by linoleic acid intake in healthy men.
Authors: Damsgaard CT, Lauritzen L, Calder PC, Kjaer TR, Frokiaer H
J. Nutr., 2009-06-03;139(7):1410-4.
Species: Human
Sample Types: Cell Culture Supernates
-
Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling.
Authors: Huang QQ, Sobkoviak R, Jockheck-Clark AR, Shi B, Mandelin AM, Tak PP, Haines GK, Nicchitta CV, Pope RM
J. Immunol., 2009-04-15;182(8):4965-73.
Species: Human
Sample Types: Cell Culture Supernates
-
The role of RGD-tagged helical rosette nanotubes in the induction of inflammation and apoptosis in human lung adenocarcinoma cells through the P38 MAPK pathway.
Authors: Suri SS, Rakotondradany F, Myles AJ, Fenniri H, Singh B
Biomaterials, 2009-02-27;30(17):3084-90.
Species: Human
Sample Types: Cell Culture Supernates
-
Divergent effects of hypoxia on dendritic cell functions.
Authors: Mancino A, Schioppa T, Larghi P, Pasqualini F, Nebuloni M, Chen IH, Sozzani S, Austyn JM, Mantovani A, Sica A
Blood, 2008-08-11;112(9):3723-34.
Species: Human
Sample Types: Cell Culture Supernates
-
Helminth infections associated with multiple sclerosis induce regulatory B cells.
Authors: Correale J, Farez M, Razzitte G
Ann. Neurol., 2008-08-01;64(2):187-99.
Species: Human
Sample Types: Cell Culture Supernates
-
Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12.
Authors: Lamhamedi-Cherradi SE, Martin RE, Ito T, Kheradmand F, Corry DB, Liu YJ, Moyle M
J. Immunol., 2008-05-01;180(9):6000-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Inorganic arsenic activates reduced NADPH oxidase in human primary macrophages through a Rho kinase/p38 kinase pathway.
Authors: Lemarie A, Bourdonnay E, Morzadec C, Fardel O, Vernhet L
J. Immunol., 2008-05-01;180(9):6010-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Impact of tobacco-smoke on key signaling pathways in the innate immune response in lung macrophages.
Authors: Birrell MA, Wong S, Catley MC, Belvisi MG
J. Cell. Physiol., 2008-01-01;214(1):27-37.
Species: Human
Sample Types: Cell Culture Supernates
-
Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling.
Authors: Weindl G, Naglik JR, Kaesler S, Biedermann T, Hube B, Korting HC, Schaller M
J. Clin. Invest., 2007-12-01;117(12):3664-3672.
Species: Human
Sample Types: Cell Culture Supernates
-
The differential effect of Eriobotrya japonica hydrophilic leaf extract on cytokines production and modulation.
Authors: Matalka KZ, Ali D, Khawad AE, Qa'dan F
Cytokine, 2007-11-26;40(3):235-40.
Species: Human
Sample Types: Cell Culture Supernates
-
Cytokine response to strenuous exercise in athletes and non-athletes--an adaptive response.
Authors: Gokhale R, Chandrashekara S, Vasanthakumar KC
Cytokine, 2007-10-22;40(2):123-7.
Species: Human
Sample Types: Plasma
-
Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells.
Authors: Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N, Descamps P, Gamelin E, Gascan H, Hebbar M, Jeannin P
Blood, 2007-09-11;110(13):4319-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Novel role for the liver X nuclear receptor in the suppression of lung inflammatory responses.
Authors: Birrell MA, Catley MC, Hardaker E, Wong S, Willson TM, McCluskie K, Leonard T, Farrow SN, Collins JL, Haj-Yahia S, Belvisi MG
J. Biol. Chem., 2007-08-31;282(44):31882-90.
Species: Human
Sample Types: Cell Culture Supernates
-
Bronchial epithelial cell growth regulation in fibroblast cocultures: the role of hepatocyte growth factor.
Authors: Skibinski G, Elborn JS, Ennis M
Am. J. Physiol. Lung Cell Mol. Physiol., 2007-03-23;293(1):L69-76.
Species: Human
Sample Types: Cell Culture Supernates
-
The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity.
Authors: Lauzon NM, Mian F, MacKenzie R, Ashkar AA
Cell. Immunol., 2006-10-17;241(2):102-12.
Species: Human
Sample Types: Cell Culture Supernates
-
Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging.
Authors: Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I
Am. J. Physiol. Lung Cell Mol. Physiol., 2006-10-13;292(2):L567-76.
Species: Human
Sample Types: Cell Culture Supernates
-
Splice variants of human FOXP3 are functional inhibitors of human CD4+ T-cell activation.
Authors: Smith EL, Finney HM, Nesbitt AM, Ramsdell F, Robinson MK
Immunology, 2006-10-01;119(2):203-11.
Species: Human
Sample Types: Cell Culture Supernates
-
Early cytokine responses after percutaneous magnetic resonance imaging guided laser thermoablation of malignant liver tumors.
Authors: Kallio R, Sequeiros R, Surcel HM, Ohtonen P, Kiviniemi H, Syrjala H
Cytokine, 2006-07-25;34(5):278-83.
Species: Human
Sample Types: Serum
-
A cyanobacterial LPS antagonist prevents endotoxin shock and blocks sustained TLR4 stimulation required for cytokine expression.
Authors: Macagno A, Molteni M, Rinaldi A
J. Exp. Med., 2006-05-22;203(6):1481-92.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of matrix metalloproteinases in the inflammatory response in human airway cell-based assays and in rodent models of airway disease.
Authors: Birrell MA, Wong S, Dekkak A, De Alba J, Haj-Yahia S, Belvisi MG
J. Pharmacol. Exp. Ther., 2006-05-11;318(2):741-50.
Species: Human
Sample Types: Cell Culture Supernates
-
A combination of E. coli DNA fragments and modified lipopolysaccharides as a cancer immunotherapy.
Authors: Cho YJ, Ahn BY, Lee NG, Lee DH, Kim DS
Vaccine, 2006-05-06;24(31):5862-71.
Species: Human
Sample Types: Cell Culture Supernates
-
Dynamic changes of apoptosis-inducing ligands and Th1/Th2 like subpopulations in Hantaan virus-induced hemorrhagic fever with renal syndrome.
Authors: Liu JM, Zhu Y, Xu ZW, Ouyang WM, Wang JP, Liu XS, Cao YX, Li Q, Fang L, Zhuang R, Yang AG, Jin BQ
Clin. Immunol., 2006-04-17;119(3):245-51.
Species: Human
Sample Types: Plasma
-
Modulation of neonatal microbial recognition: TLR-mediated innate immune responses are specifically and differentially modulated by human milk.
Authors: LeBouder E, Rey-Nores JE, Raby AC, Affolter M, Vidal K, Thornton CA, Labeta MO
J. Immunol., 2006-03-15;176(6):3742-52.
Species: Human
Sample Types: Cell Culture Supernates
-
Transmission of Mycobacterium tuberculosis undetected by tuberculin skin testing.
Authors: Anderson ST, Williams AJ, Brown JR, Newton SM, Simsova M, Nicol MP, Sebo P, Levin M, Wilkinson RJ, Wilkinson KA
Am. J. Respir. Crit. Care Med., 2006-02-02;173(9):1038-42.
Species: Human
Sample Types: Cell Culture Supernates
-
Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists.
Authors: Fritz JH, Girardin SE, Fitting C, Werts C, Mengin-Lecreulx D, Caroff M, Cavaillon JM, Philpott DJ, Adib-Conquy M
Eur. J. Immunol., 2005-08-01;35(8):2459-70.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of a short-course of amoxicillin/clavulanic acid on systemic and mucosal immunity in healthy adult humans.
Authors: Dufour V, Millon L, Faucher JF, Bard E, Robinet E, Piarroux R, Vuitton DA, Meillet D
Int. Immunopharmacol., 2005-05-01;5(5):917-28.
Species: Human
Sample Types: Feces
-
Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: implications for Burkitt's lymphoma.
Authors: Pound JD, Batth BK, Johannessen I, Wood K
J. Immunol., 2005-03-01;174(5):3015-23.
Species: Human
Sample Types: Cell Culture Supernates
-
Microbial exposure, symptoms and inflammatory mediators in nasal lavage fluid of kitchen and clerical personnel in schools.
Authors: Lignell U, Meklin T, Putus T, Vepsalainen A, Roponen M, Torvinen E, Reeslev M, Pennanen S, Hirvonen MR, Kalliokoski P, Nevalainen A
Int J Occup Med Environ Health, 2005-01-01;18(2):139-50.
Species: Human
Sample Types: Nasal Lavage Fluid
-
Stimulation of toll-like receptor 2 by Coxiella burnetii is required for macrophage production of pro-inflammatory cytokines and resistance to infection.
Authors: Zamboni DS, Campos MA, Torrecilhas AC, Kiss K, Samuel JE, Golenbock DT, Lauw FN, Roy CR, Almeida IC, Gazzinelli RT
J. Biol. Chem., 2004-10-13;279(52):54405-15.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate.
Authors: Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez de Ayala C, Martin-Mola E
J. Immunol., 2004-07-15;173(2):1463-76.
Species: Human
Sample Types: Cell Culture Supernates
-
Association of type 2 cytokines with hepatic fibrosis in human Schistosoma mansoni infection.
Authors: de Jesus AR, Magalhaes A, Miranda DG, Miranda RG, Araujo MI, de Jesus AA, Silva A, Santana LB, Pearce E, Carvalho EM
Infect. Immun., 2004-06-01;72(6):3391-7.
Species: Human
Sample Types: Cell Culture Supernates
-
The Toll-like receptor-2/6 agonist macrophage-activating lipopeptide-2 cooperates with IFN-gamma to reverse the Th2 skew in an in vitro allergy model.
Authors: Weigt H, Muhlradt PF, Larbig M, Krug N, Braun A
J. Immunol., 2004-05-15;172(10):6080-6.
Species: Human
Sample Types: Cell Culture Supernates
-
Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain.
Authors: Finney HM, Akbar AN, Lawson AD
J. Immunol., 2004-01-01;172(1):104-13.
Species: Human
Sample Types: Cell Culture Supernates
-
Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk.
Authors: LeBouder E, Rey-Nores J, Rushmere N, Grigorov M, Lawn S, Affolter M, Griffin G, Ferrara P, Schiffrin E, Morgan B, Labeta M
J Immunol, 2003-12-15;171(12):6680-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis.
Authors: Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T
Proc. Natl. Acad. Sci. U.S.A., 2003-07-23;100(16):9452-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Comparison of inflammatory elements in nasal lavage and induced sputum following occupational exposure to moldy-building microbes.
Authors: Purokivi M, Hirvonen MR, Roponen M, Randell J, Vahteristo M, Tukiainen H
Inhal Toxicol, 2002-06-01;14(6):653-62.
Species: Human
Sample Types: Sputum
-
Inflammatory mediators in nasal lavage, induced sputum and serum of employees with rheumatic and respiratory disorders.
Authors: Roponen M, Kiviranta J, Seuri M, Tukiainen H, Myllykangas-Luosujarvi R, Hirvonen MR
Eur. Respir. J., 2001-09-01;18(3):542-8.
Species: Human
Sample Types: Serum
-
Reproducibility of measurements of exhaled NO, and cell count and cytokine concentrations in induced sputum.
Authors: Purokivi M, Randell J, Hirvonen MR, Tukiainen H
Eur. Respir. J., 2000-08-01;16(2):242-6.
Species: Human
Sample Types: Sputum
-
Monocyte bioenergetics: An immunometabolic perspective in metabolic dysfunction-associated steatohepatitis
Authors: Moris Sangineto, Martina Ciarnelli, Tommaso Colangelo, Archana Moola, Vidyasagar Naik Bukke, Loren Duda, Rosanna Villani, Antonino Romano, Stefania Giandomenico, Hina Kanwal, Gaetano Serviddio
Cell Rep Med
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human TNF-alpha DuoSet ELISA
Average Rating: 5 (Based on 31 Reviews)
Have you used Human TNF-alpha DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
PBMC stimulated with LPS
Kit is detecting TNF-a in M1-like macrophages in an excellent way. See the picture.
24-hour stimulation of THP-1 cells to rotenone with or without TLR inhibitor
R-squared value of 0.98 using sigmoidal 4-parameter logistic plot
TNFa was measured in the supernatant of human kidney tissue homogenate stimulated with LPS for 24 hours.
This is a new lot of the Human TNF-alpha DuoSet ELISA kit. The results are reproducible and the quality of the kit consistent. Monocyte-derived human macrophages were treated with 20 μg/ml zymosan for 24 hours and TNFa was assessed in the supernatant.
TNFa secretion was measured in the supernatant of LPS-stimulated (100 ng/ml) cultured human dendritic cells after 18 hours.
Easy to use, good results
Monocytes were stimulated with LPS (100 ng/ml) for 24 hours. After 24 hours, TNFa was assessed in the supernatant.
TNFa levels in PBM supernatants after dIgG treatment.