Kainic acid
Tocris Bioscience | Catalog # 0222

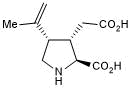
Key Product Details
Description
Product Description
Kainic acid, also known as kainate, is a selective agonist of kainate ionotropic glutamate receptors (EC50 = 0.6 - 7.4 μM) and a partial agonist at AMPA receptors (EC50 = 31 μM - 170 μM). Kainic acid is used to model epilepsy in vivo and to study the mechanisms of neurodegeneration and neurocytosis induced by excess stimulation by kainic acid. Kainate is shown to be involved in amyloidogenic processing of amyloid precursor protein and Aβ peptides in Alzheimer's disease.
Related compounds include synthetic Kainic acid (Cat. No. 7065), the kainate receptor agonist Domoic acid (Cat. No. 0269) and the antagonist NBQX (Cat. No. 0373).
Product Specifications for Kainic acid
Molecular Weight
Formula
Storage
Purity
Chemical Name
CAS Number
PubChem ID
InChI Key
SMILES
The technical data provided above is for guidance only. For batch specific data refer to the Certificate of Analysis.
Solubility
| Solvent | Max Conc. mg/mL | Max Conc. mM | |
|---|---|---|---|
| Solubility | |||
| water | 5.33 | 25 with gentle warming |
Preparing Stock Solutions for Kainic acid
The following data is based on the product molecular weight 213.23.
Batch specific molecular weights may vary from batch to batch due to the degree of hydration, which all affect the solvent volumes required to prepare stock solutions.
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 0.25 mM | 18.76 mL | 93.80 mL | 187.59 mL |
| 1.25 mM | 3.75 mL | 18.76 mL | 37.52 mL |
| 2.5 mM | 1.88 mL | 9.38 mL | 18.76 mL |
| 12.5 mM | 0.38 mL | 1.88 mL | 3.75 mL |
Calculators
Background References
References are publications that support the biological activity of the product. See our Citations tab to view 77 publications citing the usage of this product.
- Watkins Excitatory amino acids. Kainic acid as a Tool in Neurobiology. Edited by E
- Ourdev Kainate receptor activation enhances amyloidogenic processing of APP in astrocytes. Mol.Neurobiol. 2019 PMID: 30484111
- Watkins and Evans Excitatory amino acid transmitters. Annu.Rev.Pharmacol.Toxicol. 1981 PMID: 6112965
Product Documents for Kainic acid
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Product Specific Notices for Kainic acid
For research use only
Citations for Kainic acid
Customer Reviews for Kainic acid (1)
Have you used Kainic acid?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
-
Species: RatVerified Customer | Posted 05/17/2019Rats were given KA to produce a spontaneous seizing animal to experiment with epileptic compounds.
There are no reviews that match your criteria.
