Mouse IL-17 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant mouse IL-17. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
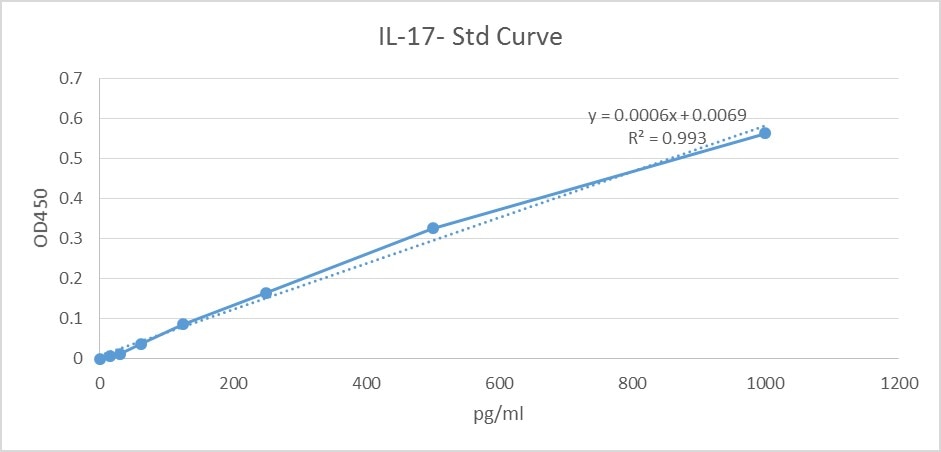
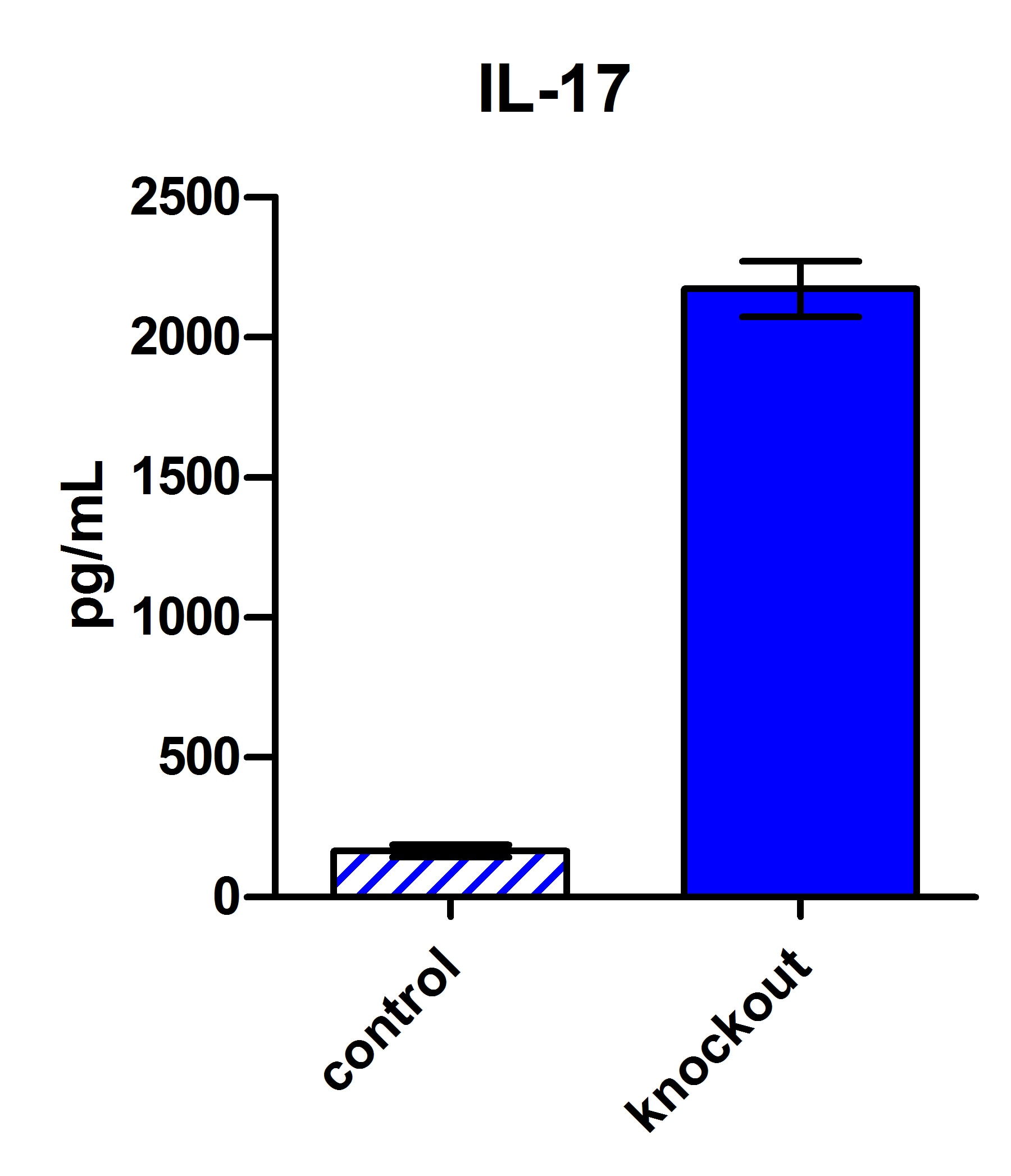
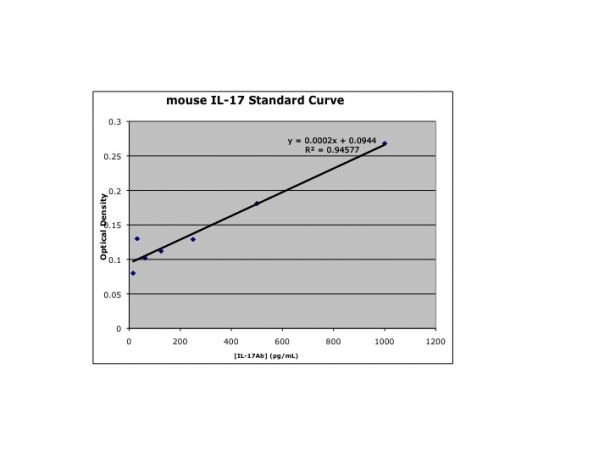
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-17/IL-17A
Mouse Interleukin 17 (IL-17; also known as IL-17A and CTLA-8) is a 21 kDa, variably glycosylated polypeptide that belongs to the IL-17 family of cytokines containing a cysteine-knot fold (1-3). Its sequence was originally isolated from an activated hybridoma created from the fusion of a mouse cytotoxic and rat T cell lymphoma cell line (2-5). It is synthesized as a 158 amino acid (aa) precursor that contains a 25 aa signal sequence and a 15 kDa, 133 aa mature segment (5). In both mouse and human, there is one conserved N-linked glycosylation site that likely contributes 5 kDa to its native molecular weight. IL-17A forms both a 35-38 kDa homodimer, and a 45-48 kDa heterodimer with IL-17F (6, 7). Mature mouse IL-17A is 61% and 89% aa identical to human and rat IL-17A, respectively (4, 5, 8). While rodent and human mature sequences show modest aa sequence identity, human IL-17 is active on both mouse and rat cells (5, 9). Cells known to produce IL-17 are the CD4+ Th17 T cells, Paneth cells, GR1+CD11b+ myeloid suppressor cells, CD27-gamma δ T cells, CD1+NK1.1- iNKT cells and CD3- CD4+ LTi-like cells (3, 5, 6, 10-12).
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse IL-17 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
197
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Characterizing the mechanisms underpinning interleukin-15R?-mediated protection against sepsis and candidiasis
Authors: Yang, J;Xu, B;Cao, J;Liu, Y;Tang, L;Zhao, P;Li, S;Li, X;Liu, J;Yu, R;Tang, Y;Tan, W;Ding, H;Li, J;Liu, Y;
Cytokine
Species: Mouse, Transgenic Mouse
Sample Types: Serum, Tissue Homogenates
-
Laquinimod treatment attenuates EAU by inhibiting both the inductive and effector phases in an APC-dependent manner
Authors: Xu, B;Mattapallil, MJ;Jia, X;Tang, J;Horai, R;Caspi, RR;Gery, I;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
An unconventional T cell nexus drives HCK-mediated chronic obstructive pulmonary disease in mice
Authors: Hsu, AT;O'Donoghue, RJJ;Tsantikos, E;Gottschalk, TA;Borger, JG;Gherardin, NA;Xu, C;Koay, HF;Godfrey, DI;Ernst, M;Anderson, GP;Hibbs, ML;
EBioMedicine
Species: Transgenic Mouse
Sample Types: BALF
-
NET formation-mediated in situ protein delivery to the inflamed central nervous system
Authors: Wu, Y;Park, J;Le, Q;Byun, J;Choi, J;Xu, H;Lee, J;Oh, Y;
Nature communications
Species: Mouse
Sample Types: Plasma, Tissue Homogenates
-
Cav3.2 channel regulates cerebral ischemia/reperfusion injury: a promising target for intervention
Authors: Dai, F;Hu, C;Li, X;Zhang, Z;Wang, H;Zhou, W;Wang, J;Geng, Q;Dong, Y;Tang, C;
Neural regeneration research
Species: Mouse
Sample Types: Tissue Homogenates
-
Intermittent ozone inhalation during house dust mite-induced sensitization primes for adverse asthma phenotype
Authors: Hussain, S;Majumder, N;Mazumder, MHH;Lewis, SE;Olapeju, O;Velayutham, M;Amin, MS;Brundage, K;Kelley, EE;Vanoirbeek, J;
Redox biology
Species: Mouse
Sample Types: Tissue Homogenates, BALF
-
Mitigated toxicity of polystyrene nanoplastics in combination exposure with copper ions by transformation into copper (I) oxide: Inhibits the oxidative potential of nanoplastics
Authors: Maruthupandy, M;Jeon, JH;Noh, J;Yang, SI;Cho, WS;
Chemosphere
Species: Mouse
Sample Types: BALF
-
Cell-intrinsic regulation of phagocyte function by interferon lambda during pulmonary viral, bacterial super-infection
Authors: Antos, D;Parks, OB;Duray, AM;Abraham, N;Michel, JJ;Kupul, S;Westcott, R;Alcorn, JF;
PLoS pathogens
Species: Mouse
Sample Types: BALF
-
IL-10 inhibition during immunization improves vaccine-induced protection against Staphylococcus aureus infection
Authors: Kelly, AM;McCarthy, KN;Claxton, TJ;Carlile, SR;O'Brien, EC;Vozza, EG;Mills, KH;McLoughlin, RM;
JCI insight
Species: Mouse
Sample Types: Cell Culture Supernates, Tissue Homogenates
-
Polydopamine-based nano adjuvant as a promising vaccine carrier induces significant immune responses against Acinetobacter baumannii-associated pneumonia
Authors: Sabzi, S;Habibi, M;Badmasti, F;Shahbazi, S;Asadi Karam, MR;Farokhi, M;
International journal of pharmaceutics
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-17A + group 2 innate lymphoid cells elicit mixed airway inflammation in chronic obstructive pulmonary disease
Authors: Flayer, CH;Linderholm, AL;Ge, MQ;Juarez, M;Franzi, L;Tham, T;Teuber, M;Liao, SY;Schivo, M;Kuhn, B;Zeki, A;Haczku, A;
medRxiv : the preprint server for health sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Design and fabrication of a vaccine candidate based on rOmpA from Klebsiella pneumoniae encapsulated in silk fibroin-sodium alginate nanoparticles against pneumonia infection
Authors: Shahbazi, S;Habibi, M;Badmasti, F;Sabzi, S;Farokhi, M;Asadi Karam, MR;
International immunopharmacology
Species: Mouse
Sample Types: Cell Culture Supernates
-
A 5-Lipoxygenase Inhibitor, Zileuton, Modulates Host Immune Responses and Improves Lung Function in a Model of Severe Acute Respiratory Syndrome (SARS) Induced by Betacoronavirus
Authors: Pereira, RDD;Rabelo, RAN;Oliveira, NFM;Porto, SLT;Andrade, ACDSP;Queiroz-Junior, CM;Barbosa, CLN;de Souza-Costa, LP;Santos, FRDS;Oliveira, FBR;da Silva, BLV;Umezu, HL;Ferreira, R;da Silva, GSF;Cruz, JS;Teixeira, MM;Costa, VV;Machado, FS;
Viruses
Species: Mouse
Sample Types: Tissue Homogenates
-
LncRNA Neat1 targets NonO and miR-128-3p to promote antigen-specific Th17 cell responses and autoimmune inflammation
Authors: Chen, S;Wang, J;Zhang, K;Ma, B;Li, X;Wei, R;Nian, H;
Cell death & disease
Species: Mouse
Sample Types: Cell Culture Supernates
-
Therapeutic potential of ozone water treatment in alleviating atopic dermatitis symptoms in mouse models: Exploring its bactericidal and direct anti-inflammatory properties
Authors: Kaneki, M;Ohira, C;Takahashi, M;Iwashita, N;Takagi, Y;Nagane, M;Uchiyama, J;Fukuyama, T;
International immunopharmacology
Species: Mouse
Sample Types: Cell Culture Supernates
-
3,3-dimethyl-1-butanol and its metabolite 3,3-dimethylbutyrate ameliorate collagen-induced arthritis independent of choline trimethylamine lyase activity
Authors: Fechtner, S;Allen, BE;Chriswell, ME;Jubair, WK;Robertson, CE;Kofonow, JN;Frank, DN;Holers, VM;Kuhn, KA;
Research square
Species: Mouse
Sample Types: Cell Culture Supernates
-
TRAF4 is crucial for ST2+ memory Th2 cell expansion in IL-33-driven airway inflammation
Authors: Xiao, J;Chen, X;Liu, W;Qian, W;Bulek, K;Hong, L;Miller-Little, W;Li, X;Liu, C;
JCI insight
Species: Transgenic Mouse
Sample Types: BALF
-
Pharmacologic inhibition of NLRP3 reduces the levels of ?-synuclein and protects dopaminergic neurons in a model of Parkinson's disease
Authors: Amo-Aparicio, J;Daly, J;Højen, JF;Dinarello, CA;
Journal of neuroinflammation
Species: Mouse
Sample Types: Tissue Homogenates
-
Role of the IL-33/ST2 Activation Pathway in the Development of the Hepatic Fibrosis Induced by Schistosoma mansoni Granulomas in Mice
Authors: Maggi, L;Camelo, GMA;Rocha, IC;Pereira Alves, W;Moreira, JMP;Almeida Pereira, T;Tafuri, WL;Rabelo, ÉML;Correa, A;Ecco, R;Negrão-Corrêa, DA;
International journal of molecular sciences
Species: Mouse
Sample Types: Tissue Homogenates
-
Human Wharton's jelly mesenchymal stem cells derived-exosomes enriched by miR-124 promote an anti-fibrotic response in an experimental model of liver fibrosis
Authors: Niknam, B;Baghaei, K;Mahmoud Hashemi, S;Hatami, B;Reza Zali, M;Amani, D;
International immunopharmacology
Species: Mouse
Sample Types: Serum
-
EC-18 prevents autoimmune arthritis by suppressing inflammatory cytokines and osteoclastogenesis
Authors: JS Park, SC Yang, HY Jeong, SY Lee, JG Ryu, JW Choi, HY Kang, SM Kim, SH Hwang, ML Cho, SH Park
Arthritis Research & Therapy, 2022-11-17;24(1):254.
Species: Mouse
Sample Types: Serum
-
Inhaled delivery of recombinant interferon-lambda restores allergic inflammation after development of asthma by controlling Th2- and Th17-cell-mediated immune responses
Authors: J Won, A Jo, CH Gil, S Kim, H Shin, H Jik Kim
International immunopharmacology, 2022-08-27;112(0):109180.
Species: Mouse
Sample Types: BALF
-
Lithocholic acid inhibits dendritic cell activation by reducing intracellular glutathione via TGR5 signaling
Authors: J Hu, Y Zhang, S Yi, C Wang, X Huang, S Pan, J Yang, G Yuan, S Tan, H Li
International journal of biological sciences, 2022-07-11;18(11):4545-4559.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Phosphatidylinositol 3-Kinase (PI3K) Orchestrates Aspergillus fumigatus-Induced Eosinophil Activation Independently of Canonical Toll-Like Receptor (TLR)/C-Type-Lectin Receptor (CLR) Signaling
Authors: A Dietschman, S Schruefer, S Westermann, F Henkel, K Castiglion, R Willebrand, J Adam, J Ruland, R Lang, DC Sheppard, J Esser-von-, D Radtke, S Krappmann, D Voehringer
MBio, 2022-06-13;0(0):e0123922.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Pretreatment with 6-Gingerol Ameliorates Sepsis-Induced Immune Dysfunction by Regulating the Cytokine Balance and Reducing Lymphocyte Apoptosis
Authors: SA Ju, QT Nguyen, TT Nguyen, JH Suh, WG An, Z Callaway, Y Joe, HT Chung, BS Kim
Oxidative Medicine and Cellular Longevity, 2021-12-29;2021(0):5427153.
Species: Mouse
Sample Types: BALF
-
Interleukin-17 affects synaptic plasticity and cognition in an experimental model of multiple sclerosis
Authors: M Di Filippo, A Mancini, L Bellingacc, L Gaetani, P Mazzocchet, T Zelante, L La Barbera, A De Luca, M Tantucci, A Tozzi, V Durante, M Sciaccalug, A Megaro, D Chiasserin, N Salvadori, V Lisetti, E Portaccio, C Costa, P Sarchielli, MP Amato, L Parnetti, MT Viscomi, L Romani, P Calabresi
Cell Reports, 2021-12-07;37(10):110094.
Species: Mouse
Sample Types: Tissue Homogenates
-
Storage conditions of high-fat diets affect pulmonary inflammation
Authors: M Kokoszynsk, ND Ubags, JJ Bivona, S Ventrone, LF Reed, AE Dixon, MJ Wargo, ME Poynter, BT Suratt
Physiological Reports, 2021-11-01;9(22):e15116.
Species: Mouse
Sample Types: Tissue Homogenates
-
Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling
Authors: J Hu, C Wang, X Huang, S Yi, S Pan, Y Zhang, G Yuan, Q Cao, X Ye, H Li
Cell Reports, 2021-09-21;36(12):109726.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Diesel exhaust particles increase nasal symptoms and IL-17A in house dust mite-induced allergic mice
Authors: HJ Jung, YK Ko, WS Shim, HJ Kim, DY Kim, CS Rhee, MK Park, DH Han
Scientific Reports, 2021-08-11;11(1):16300.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Geographic differences in gut microbiota composition impact susceptibility to enteric infection
Authors: AM Porras, Q Shi, H Zhou, R Callahan, G Montenegro, N Solomons, IL Brito
Cell Reports, 2021-07-27;36(4):109457.
Species: Mouse
Sample Types: Tissue Supernates
-
Lung-resident memory B cells protect against bacterial pneumonia
Authors: Kimberly A. Barker, Neelou S. Etesami, Anukul T. Shenoy, Emad I. Arafa, Carolina Lyon Lyon de Ana, Nicole M.S. Smith et al.
Journal of Clinical Investigation
Species: Mouse
Sample Types: Tissue Homogenates
-
NLRP1 acts as a negative regulator of Th17 cell programming in mice and humans with autoimmune diabetes
Authors: FRC Costa, JA Leite, DM Rassi, JF da Silva, J Elias-Oliv, JB Guimarães, MC Foss-Freit, NOS Câmara, A Pontillo, RC Tostes, JS Silva, D Carlos
Cell Reports, 2021-05-25;35(8):109176.
Species: Mouse
Sample Types: Tissue Homogenates
-
The Adjuvants Polyphosphazene (PCEP) and a Combination of Curdlan Plus Leptin Promote a Th17-Type Immune Response to an Intramuscular Vaccine in Mice
Authors: A Chaffey, G Hamonic, D Chand, GK Mutwiri, HL Wilson
Vaccines, 2021-05-14;9(5):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Moderately pathogenic maternal influenza A virus infection disrupts placental integrity but spares the fetal brain
Authors: AM Antonson, AD Kenney, HJ Chen, KN Corps, JS Yount, TL Gur
Brain, Behavior, and Immunity, 2021-05-12;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
PDE9 Inhibitor PF-04447943 Attenuates DSS-Induced Colitis by Suppressing Oxidative Stress, Inflammation, and Regulating T-Cell Polarization
Authors: MN Rana, J Lu, E Xue, J Ruan, Y Liu, L Zhang, R Dhar, Y Li, Z Hu, J Zhou, W Ma, H Tang
Frontiers in Pharmacology, 2021-04-08;12(0):643215.
Species: Mouse
Sample Types: Tissue Homogenates
-
Hepatoprotective Effect of Mixture of Dipropyl Polysulfides in Concanavalin A-Induced Hepatitis
Authors: D Arsenijevi, B Stojanovic, J Milovanovi, A Arsenijevi, M Simic, M Pergal, I Kodranov, O Cvetkovic, D Vojvodic, E Ristanovic, D Manojlovic, M Milovanovi, N Arsenijevi
Nutrients, 2021-03-22;13(3):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Bifidobacterium adolescentis Isolated from Different Hosts Modifies the Intestinal Microbiota and Displays Differential Metabolic and Immunomodulatory Properties in Mice Fed a High-Fat Diet
Authors: B Wang, Q Kong, S Cui, X Li, Z Gu, J Zhao, H Zhang, W Chen, G Wang
Nutrients, 2021-03-21;13(3):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Wood emissions and asthma development: Results from an experimental mouse model and a prospective cohort study
Authors: KM Junge, L Buchenauer, E Elter, K Butter, T Kohajda, G Herberth, S Röder, M Borte, W Kiess, M von Bergen, JC Simon, UE Rolle-Kamp, I Lehmann, R Gminski, M Ohlmeyer, T Polte
Environment international, 2021-02-18;151(0):106449.
Species: Mouse
Sample Types: Tissue Homogenates
-
Stem cell transplantation uncovers TDO-AHR regulation of lung dendritic cells in herpesvirus-induced pathology
Authors: SJ Gurczynski, NL Pereira, SM Hrycaj, C Wilke, RL Zemans, BB Moore
JCI Insight, 2021-01-25;6(2):.
Species: Mouse, Transgenic Mouse
Sample Types: Cell Culture Supernates
-
TARM1 contributes to development of arthritis by activating dendritic cells through recognition of collagens
Authors: R Yabe, SH Chung, MA Murayama, S Kubo, K Shimizu, Y Akahori, T Maruhashi, A Seno, T Kaifu, S Saijo, Y Iwakura
Nature Communications, 2021-01-04;12(1):94.
Species: Mouse, Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Diet Rich in Simple Sugars Promotes Pro-Inflammatory Response via Gut Microbiota Alteration and TLR4 Signaling
Authors: A Fajstova, N Galanova, S Coufal, J Malkova, M Kostovcik, M Cermakova, H Pelantova, M Kuzma, B Sediva, T Hudcovic, T Hrncir, H Tlaskalova, M Kverka, K Kostovciko
Cells, 2020-12-16;9(12):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Myeloid-derived suppressor cells therapy enhance immunoregulatory properties in acute graft versus host disease with combination of regulatory T cells
Authors: MJ Park, JA Baek, SY Kim, KA Jung, JW Choi, SH Park, SK Kwok, ML Cho
Journal of Translational Medicine, 2020-12-14;18(1):483.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Disrupting Bordetella Immunosuppression Reveals a Role for Eosinophils in Coordinating the Adaptive Immune Response in the Respiratory Tract
Authors: MC Gestal, U Blas-Macha, HM Johnson, LN Rubin, KK Dewan, C Bryant, M Tiemeyer, ET Harvill
Microorganisms, 2020-11-17;8(11):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Anti-Axl antibody treatment reduces the severity of experimental autoimmune encephalomyelitis
Authors: JC DuBois, AK Ray, P Davies, B Shafit-Zag
J Neuroinflammation, 2020-10-29;17(1):324.
Species: Mouse
Sample Types: Cell Culture Supernates
-
T cell-intrinsic role for Nod2 in protection against Th17-mediated uveitis
Authors: RJ Napier, EJ Lee, MP Davey, EE Vance, JM Furtado, PE Snow, KA Samson, SJ Lashley, BR Brown, R Horai, MJ Mattapalli, B Xu, MC Callegan, LS Uebelhoer, CL Lancioni, RK Vehe, BA Binstadt, JR Smith, RR Caspi, HL Rosenzweig
Nat Commun, 2020-10-26;11(1):5406.
Species: Mouse
Sample Types: Cell Lysates
-
Cystatin C Plays a Sex-Dependent Detrimental Role in Experimental Autoimmune Encephalomyelitis
Authors: V Hoghooghi, AL Palmer, A Frederick, Y Jiang, JE Merkens, A Balakrishn, TM Finlay, A Grubb, E Levy, P Gordon, FR Jirik, MD Nguyen, C Schuurmans, F Visser, SE Dunn, SS Ousman
Cell Rep, 2020-10-06;33(1):108236.
Species: Mouse
Sample Types: Cell Culture Supernates
-
In silico analysis and in vivo assessment of a novel epitope-based vaccine candidate against uropathogenic Escherichia coli
Authors: S Hasanzadeh, M Habibi, MA Shokrgozar, R Ahangari C, K Ahmadi, MR Asadi Kara, S Bouzari
Sci Rep, 2020-10-01;10(1):16258.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Combination of Innate Immune Modulators as Vaccine Adjuvants in Mice
Authors: A Haddadi, A Chaffey, SH Ng, D Yalamati, HL Wilson
Vaccines (Basel), 2020-10-01;8(4):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Th17/Treg imbalance in COPD development: suppressors of cytokine signaling and signal transducers and activators of transcription proteins
Authors: LEF Silva, JD Lourenço, KR Silva, FPR Santana, JB Kohler, AR Moreira, APP Velosa, CM Prado, RP Vieira, MV Aun, IFLC Tibério, JT Ito, FDTQS Lopes
Scientific Reports, 2020-09-17;10(1):15287.
Species: Mouse
Sample Types: Tissue Homogenates
-
Standardized herbal extract PM014 alleviates fine dust-induced lung inflammation in mice
Authors: YS Lee, D Min, SY Park, J Lee, H Bae
BMC Complement Med Ther, 2020-09-07;20(1):270.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Strain-Specific Differences in House Dust Mite (Dermatophagoides farinae)-Induced Mouse Models of Allergic Rhinitis
Authors: KI Lee, JS Bae, EH Kim, JH Kim, L Lyu, YJ Chung, JH Mo
Clin Exp Otorhinolaryngol, 2020-05-15;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lactobacillus reuteri attenuated allergic inflammation induced by HDM in the mouse and modulated gut microbes
Authors: L Li, Z Fang, X Liu, W Hu, W Lu, YK Lee, J Zhao, H Zhang, W Chen
PLoS ONE, 2020-04-21;15(4):e0231865.
Species: Mouse
Sample Types: BALF
-
Herbal Combinational Medication of Glycyrrhiza glabra, Agastache rugosa Containing Glycyrrhizic Acid, Tilianin Inhibits Neutrophilic Lung Inflammation by Affecting CXCL2, Interleukin-17/STAT3 Signal Pathways in a Murine Model of COPD
Authors: SH Kim, JH Hong, WK Yang, JH Geum, HR Kim, SY Choi, YM Kang, HJ An, YC Lee
Nutrients, 2020-03-27;12(4):.
Species: Mouse
Sample Types: BALF
-
Lactobacillus reuteri 5454 and Bifidobacterium animalis ssp. lactis 5764 improve colitis while differentially impacting dendritic cells maturation and antimicrobial responses
Authors: J Hrdý, J Alard, A Couturier-, O Boulard, D Boutillier, M Delacre, C Lapadatesc, A Cesaro, P Blanc, B Pot, B Ryffel, M Chamaillar, C Grangette
Sci Rep, 2020-03-24;10(1):5345.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Calcitriol Prevents Neuroinflammation and Reduces Blood-Brain Barrier Disruption and Local Macrophage/Microglia Activation
Authors: LRC de Oliveir, LAN Mimura, TFC Fraga-Silv, LLW Ishikawa, AAH Fernandes, SFG Zorzella-P, A Sartori
Front Pharmacol, 2020-03-12;11(0):161.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immunomodulatory effect of Syphacia obvelata in treatment of experimental DSS-induced colitis in mouse model
Authors: N Taghipour, N Mosaffa, HA Aghdaei, M Rostami-Ne, JV Weinstock, S Shahnavaz, MR Zali
Sci Rep, 2019-12-13;9(1):19127.
Species: Mouse
Sample Types: Cell Culture Supernates
-
DeSUMOylase SENP7-Mediated Epithelial Signaling Triggers Intestinal Inflammation via Expansion of Gamma-Delta T Cells
Authors: A Suhail, ZA Rizvi, P Mujagond, SA Ali, P Gaur, M Singh, V Ahuja, A Awasthi, CV Srikanth
Cell Rep, 2019-12-10;29(11):3522-3538.e7.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Strongyloides venezuelensis-infection alters the profile of cytokines and liver inflammation in mice co-infected with Schistosoma mansoni
Authors: MC de Rezende, JMP Moreira, LLM Fernandes, VF Rodrigues, D Negrão-Cor
Cytokine, 2019-11-26;127(0):154931.
Species: Mouse
Sample Types: Tissue Homogenates
-
Generation of protective pneumococcal-specific nasal resident memory CD4+ T cells via parenteral immunization
Authors: JM O'Hara, NS Redhu, E Cheung, NG Robertson, I Patik, SE Sayed, CM Thompson, M Herd, KB Lucas, E Conaway, CC Morton, DL Farber, R Malley, BH Horwitz
Mucosal Immunol, 2019-10-28;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Inhibition of Rho-Kinase Downregulates Th17 Cells and Ameliorates Hepatic Fibrosis by Schistosoma japonicum Infection
Authors: W Zhou, Y Yang, C Mei, P Dong, S Mu, H Wu, Y Zhou, Y Zheng, F Guo, JQ Yang
Cells, 2019-10-16;8(10):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Circulating Picomolar Levels of CCL2 Downregulate Ongoing Chronic Experimental Autoimmune Encephalomyelitis by Induction of Regulatory Mechanisms
Authors: N Kaushansky, E Bakos, S Becker-Her, I Shachar, A Ben-Nun
J. Immunol., 2019-09-04;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Frontline Science: Abnormalities in the gut mucosa of non-obese diabetic mice precede the onset of type 1 diabetes
Authors: MCG Miranda, RP Oliveira, L Torres, SLF Aguiar, N Pinheiro-R, L Lemos, MA Guimarães, D Reis, T Silveira, Ê Ferreira, TG Moreira, DC Cara, TU Maioli, BL Kelsall, D Carlos, AMC Faria
J. Leukoc. Biol., 2019-07-16;0(0):.
Species: Mouse
Sample Types: Tissue Culture Supernates
-
Ablation of RhoA impairs Th17 cell differentiation and alleviates house dust mite-triggered allergic airway inflammation
Authors: JQ Yang, KW Kalim, Y Li, Y Zheng, F Guo
J. Leukoc. Biol., 2019-07-01;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
In-depth characterization of congenital Zika syndrome in immunocompetent mice: Antibody-dependent enhancement and an antiviral peptide therapy
Authors: VN Camargos, G Foureaux, DC Medeiros, VT da Silveir, CM Queiroz-Ju, ALB Matosinhos, AFA Figueiredo, CDF Sousa, TP Moreira, VF Queiroz, ACF Dias, KTO Santana, I Passos, ALCV Real, LC Silva, FAG Mourão, NT Wnuk, MAP Oliveira, S Macari, T Silva, GP Garlet, JA Jackman, FM Soriani, MFD Moraes, EMAM Mendes, FM Ribeiro, GMJ Costa, AL Teixeira, NJ Cho, ACP Oliveira, MM Teixeira, VV Costa, DG Souza
EBioMedicine, 2019-05-23;0(0):.
Species: Mouse
Sample Types: Plasma
-
The immunoreceptor CD300a controls the intensity of inflammation and dysfunction in a model of Ag-induced arthritis in mice
Authors: BVS Valiate, RU Alvarez, L Karra, CM Queiroz-Jú, FA Amaral, F Levi-Schaf, MM Teixeira
J. Leukoc. Biol., 2019-05-20;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
DNA threads released by activated CD4+ T lymphocytes provide autocrine costimulation
Authors: M Costanza, PL Poliani, P Portararo, B Cappetti, S Musio, F Pagani, L Steinman, MP Colombo, R Pedotti, S Sangaletti
Proc. Natl. Acad. Sci. U.S.A., 2019-04-15;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Alpha-galactosylceramide enhances mucosal immunity to oral whole-cell cholera vaccines
Authors: CJH Davitt, S Longet, A Albutti, V Aversa, S Nordqvist, B Hackett, CP McEntee, M Rosa, IS Coulter, M Lebens, J Tobias, J Holmgren, EC Lavelle
Mucosal Immunol, 2019-04-05;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Inhibition of IL-17 ameliorates systemic lupus erythematosus in Roquinsan/san mice through regulating the balance of TFH cells, GC B cells, Treg and Breg
Authors: SY Lee, SH Lee, HB Seo, JG Ryu, K Jung, JW Choi, J Jhun, JS Park, JY Kwon, SK Kwok, J Youn, SH Park, ML Cho
Sci Rep, 2019-03-26;9(1):5227.
Species: Mouse
Sample Types: Serum
-
Naja annulifera Snake: New insights into the venom components and pathogenesis of envenomation
Authors: F Silva-de-F, IM Villas-Boa, SMT Serrano, B Cogliati, SAA Chudzinski, PH Lopes, ES Kitano, CK Okamoto, DV Tambourgi
PLoS Negl Trop Dis, 2019-01-18;13(1):e0007017.
Species: Mouse
Sample Types: Plasma
-
Excessive neutrophil levels in the lung underlie the age-associated increase in influenza mortality
Authors: U Kulkarni, RL Zemans, CA Smith, SC Wood, JC Deng, DR Goldstein
Mucosal Immunol, 2019-01-07;12(2):545-554.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Inhalation of the prodrug PI3K inhibitor CL27c improves lung function in asthma and fibrosis
Authors: CC Campa, RL Silva, JP Margaria, T Pirali, MS Mattos, LR Kraemer, DC Reis, G Grosa, F Copperi, EM Dalmarco, RCP Lima-Júnio, S Aprile, V Sala, F Dal Bello, DS Prado, JC Alves-Filh, C Medana, GD Cassali, GC Tron, MM Teixeira, E Ciraolo, RC Russo, E Hirsch
Nat Commun, 2018-12-12;9(1):5232.
Species: Mouse
Sample Types:
-
IL-23R Signaling Plays No Role in Myocardial Infarction
Authors: E Engelowski, NF Modares, S Gorressen, P Bouvain, D Semmler, C Alter, Z Ding, U Flögel, J Schrader, H Xu, PA Lang, J Fischer, DM Floss, J Scheller
Sci Rep, 2018-11-20;8(1):17078.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lipoteichoic acid anchor triggers Mincle to drive protective immunity against invasive group A Streptococcus infection
Authors: T Imai, T Matsumura, S Mayer-Lamb, CA Wells, E Ishikawa, SK Butcher, TC Barnett, MJ Walker, A Imamura, H Ishida, T Ikebe, T Miyamoto, M Ato, S Ohga, B Lepenies, NM van Sorge, S Yamasaki
Proc. Natl. Acad. Sci. U.S.A., 2018-10-23;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Insufficient IL-10 Production as a Mechanism Underlying the Pathogenesis of Systemic Juvenile Idiopathic Arthritis
Authors: M Imbrechts, A Avau, J Vandenhaut, B Malengier-, K Put, T Mitera, N Berghmans, O Burton, S Junius, A Liston, L de Somer, C Wouters, P Matthys
J. Immunol., 2018-09-28;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
PrtA immunization fails to protect against pulmonary and invasive infection by Streptococcus pneumoniae
Authors: CF Hsu, CH Hsiao, SF Tseng, JR Chen, YJ Liao, SJ Chen, CS Lin, HK Sytwu, YP Chuang
Respir. Res., 2018-09-25;19(1):187.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Engineered DNA plasmid reduces immunity to dystrophin while improving muscle force in a model of gene therapy of Duchenne dystrophy
Authors: PP Ho, LJ Lahey, F Mourkioti, PE Kraft, A Filareto, M Brandt, KEG Magnusson, EE Finn, JS Chamberlai, WH Robinson, HM Blau, L Steinman
Proc. Natl. Acad. Sci. U.S.A., 2018-09-04;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Low dose of chlorine exposure exacerbates nasal and pulmonary allergic inflammation in mice
Authors: IS de Genaro, FM de Almeida, DC Hizume-Kun, HT Moriya, RA Silva, JCG Cruz, RB Lopes, RF Righetti, R de Paula V, M Saiki, MA Martins, IFLC Tibério, FM Arantes-Co, BM Saraiva-Ro
Sci Rep, 2018-08-22;8(1):12636.
Species: Mouse
Sample Types: Tissue Homogenates
-
Integration of T Cell Receptor, Notch and Cytokine Signals Programs in Mouse ?? T Cell Effector Differentiation
Authors: P Zarin, TSH In, ELY Chen, J Singh, GW Wong, M Mohtashami, DL Wiest, MK Anderson, JC Zúñiga-Pfl
Immunol. Cell Biol., 2018-06-28;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Repeated Allergen Exposure in A/J Mice Causes Steroid-Insensitive Asthma via a Defect in Glucocorticoid Receptor Bioavailability
Authors: MF Serra, AC Cotias, CRR Pão, JB Daleprane, PB Jurgilas, GC Couto, EA Anjos-Valo, RSB Cordeiro, VF Carvalho, PMR Silva, MA Martins
J. Immunol., 2018-06-18;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota
Authors: C Tang, S Kakuta, K Shimizu, M Kadoki, T Kamiya, T Shimazu, S Kubo, S Saijo, H Ishigame, S Nakae, Y Iwakura
Nat. Immunol., 2018-06-18;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lung Epithelial Cells Coordinate Innate Lymphocytes and Immunity against Pulmonary Fungal Infection
Authors: N Hernández-, DL Wiesner, JS Fites, AJ McDermott, T Warner, M Wüthrich, BS Klein
Cell Host Microbe, 2018-03-22;0(0):.
Species: Mouse
Sample Types: BALF
-
ATX-MS-1467 Induces Long-Term Tolerance to Myelin Basic Protein in (DR2?�?Ob1)F1 Mice by Induction of IL-10-Secreting iTregs
Authors: ALS De Souza, S Rudin, R Chang, K Mitchell, T Crandall, S Huang, JK Choi, SL Okitsu, DL Graham, B Tomkinson, T Dellovade
Neurol Ther, 2018-03-14;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Fraxinellone Attenuates Rheumatoid Inflammation in Mice
Authors: SM Jung, J Lee, SY Baek, J Lee, SG Jang, SM Hong, JS Park, ML Cho, SH Park, SK Kwok
Int J Mol Sci, 2018-03-13;19(3):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A novel multi-peptide subunit vaccine admixed with AddaVax adjuvant produces significant immunogenicity and protection against Proteus mirabilis urinary tract infection in mice model
Authors: E Choubini, M Habibi, A Khorshidi, A Ghasemi, MR Asadi Kara, S Bouzari
Mol. Immunol., 2018-03-09;96(0):88-97.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Standard -
STAT-3-independent production of IL-17 by mouse innate-like ?? T cells controls ocular infection
Authors: AJ St Leger, AM Hansen, H Karauzum, R Horai, CR Yu, A Laurence, KD Mayer-Barb, P Silver, R Villasmil, C Egwuagu, SK Datta, RR Caspi
J. Exp. Med., 2018-02-28;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Respiratory Disease following Viral Lung Infection Alters the Murine Gut Microbiota
Authors: HT Groves, L Cuthbertso, P James, MF Moffatt, MJ Cox, JS Tregoning
Front Immunol, 2018-02-12;9(0):182.
Species: Mouse
Sample Types: Airway Lavage Fluid
-
Lactobacillus fermentum HP3-Mediated Fermented Hericium erinaceus Juice as a Health Promoting Food Supplement to Manage Diabetes Mellitus
Authors: C Chaiyasut, S Woraharn, BS Sivamaruth, N Lailerd, P Kesika, S Peerajan
J Evid Based Integr Med, 2018-01-01;23(0):2515690X18765.
Species: Rat
Sample Types: Serum
-
Influence of hydrocarbon oil structure on adjuvanticity and autoimmunity
Authors: ACY Yau, E Lönnblom, J Zhong, R Holmdahl
Sci Rep, 2017-11-08;7(1):14998.
Species: Rat
Sample Types: Cell Culture Supernates
-
Thymic-Specific Serine Protease Limits Central Tolerance and Exacerbates Experimental Autoimmune Encephalomyelitis
Authors: L Serre, M Girard, A Ramadan, P Menut, N Rouquié, LE Lucca, K Mahiddine, B Leobon, LT Mars, S Guerder
J. Immunol., 2017-10-23;0(0):.
Species: Mouse
Sample Types: Cell Lysates
-
Impact of combined sodium chloride and saturated long-chain fatty acid challenge on the differentiation of T helper cells in neuroinflammation
Authors: A Hammer, A Schliep, S Jörg, A Haghikia, R Gold, M Kleinewiet, DN Müller, RA Linker
J Neuroinflammation, 2017-09-12;14(1):184.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lamtor1 Is Critically Required for CD4(+) T Cell Proliferation and Regulatory T Cell Suppressive Function
Authors: T Hosokawa, T Kimura, S Nada, T Okuno, D Ito, S Kang, S Nojima, K Yamashita, T Nakatani, Y Hayama, Y Kato, Y Kinehara, M Nishide, N Mikami, S Koyama, H Takamatsu, D Okuzaki, N Ohkura, S Sakaguchi, M Okada, A Kumanogoh
J. Immunol., 2017-08-02;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Inducing maternal inflammation promotes leptin production in offspring but does not improve allergic symptoms in a mouse model of allergic rhinitis
Authors: A Imai, K Satoi, E Fujimoto, K Sato
Heliyon, 2017-06-22;3(6):e00327.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Schistosoma japonicum infection downregulates house dust mite-induced allergic airway inflammation in mice
Authors: S Qiu, X Fan, Y Yang, P Dong, W Zhou, Y Xu, Y Zhou, F Guo, Y Zheng, JQ Yang
PLoS ONE, 2017-06-14;12(6):e0179565.
Species: Mouse
Sample Types: BALF
-
IRF5 distinguishes severe asthma in humans and drives Th1 phenotype and airway hyperreactivity in mice
Authors: TB Oriss, M Raundhal, C Morse, RE Huff, S Das, R Hannum, MC Gauthier, KL Scholl, K Chakrabort, SM Nouraie, SE Wenzel, P Ray, A Ray
JCI Insight, 2017-05-18;2(10):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Th17 cells are refractory to senescence and retain robust antitumor activity after long-term ex vivo expansion
Authors: JS Bowers, MH Nelson, K Majchrzak, SR Bailey, B Rohrer, AD Kaiser, C Atkinson, L Gattinoni, CM Paulos
JCI Insight, 2017-03-09;2(5):e90772.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Intravitreal injection of anti-Interleukin (IL)-6 antibody attenuates experimental autoimmune uveitis in mice
Authors: J Tode, E Richert, S Koinzer, A Klettner, U Pickhinke, C Garbers, S Rose-John, B Nölle, J Roider
Cytokine, 2017-03-06;96(0):8-15.
Species: Mouse
Sample Types: Tissue Homogenates
-
Toll-Like Receptor 2 Is Required for Inflammatory Process Development during Leishmania infantum Infection
Authors: LA Sacramento, JL da Costa, MH de Lima, PA Sampaio, RP Almeida, FQ Cunha, JS Silva, V Carregaro
Front Microbiol, 2017-02-23;8(0):262.
Species: Mouse
Sample Types: Cell Culture Supernates
-
T-bet overexpression regulates AHR-mediated Th-17 differentiation through an IFN?-independent pathway
Authors: Masahiro Yokosawa
Clin. Exp. Immunol, 2017-01-31;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon
Authors: S Bettini, E Boutet-Rob, C Cartier, C Com‚ra, E Gaultier, J Dupuy, N Naud, S Tach‚, P Grysan, S Reguer, N Thieriet, M R‚fr‚giers, D ThiaudiŠre, JP Cravedi, M CarriŠre, JN Audinot, FH Pierre, L Guzylack-P, E Houdeau
Sci Rep, 2017-01-20;7(0):40373.
Species: Rat
Sample Types: Tissue Homogenates
-
Dysfunction of hepatic regulatory T cells in experimental sclerosing cholangitis is related with IL-12 signaling
Authors: Dorothee Schwinge
J. Hepatol, 2016-12-10;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Advax, a Delta Inulin Microparticle, Potentiates In-built Adjuvant Property of Co-administered Vaccines
EBioMedicine, 2016-12-01;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Metabolic and adaptive immune responses induced in mice infected with tissue-dwelling nematode Trichinella zimbabwensis
Authors: S Mukaratirw
Open Vet J, 2016-11-04;6(3):178-184.
Species: Mouse
Sample Types: Serum
-
DUOX1 mediates persistent epithelial EGFR activation, mucous cell metaplasia, and airway remodeling during allergic asthma
JCI Insight, 2016-11-03;1(18):e88811.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Poly-N-acetyl glucosamine production by Staphylococcus epidermidis cells increases their in vivo pro-inflammatory effect
Infect Immun, 2016-09-19;0(0):.
Species: Mouse
Sample Types: Tissue Secretion
-
Interleukin 7 immunotherapy improves host immunity and survival in a two-hit model of Pseudomonas aeruginosa pneumonia
J Leukoc Biol, 2016-09-14;0(0):.
Species: Mouse
Sample Types: Plasma
-
Allergen-Induced IL-6 Regulates IL-9/IL-17A Balance in CD4+ T Cells in Allergic Airway Inflammation
J Immunol, 2016-08-29;197(7):2653-64.
Species: Mouse
Sample Types: Cell Culture Supernates
-
AAV8-Mediated Angiotensin-Converting Enzyme 2 Gene Delivery Prevents Experimental Autoimmune Uveitis by Regulating MAPK, NF-?B and STAT3 Pathways
Sci Rep, 2016-08-25;6(0):31912.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The circadian clock regulates inflammatory arthritis
FASEB J, 2016-08-03;0(0):.
Species: Mouse
Sample Types: Serum
-
Interleukin-27 Mediates Susceptibility to Visceral Leishmaniasis by Suppressing the IL-17-Neutrophil Response
Authors: Gustavo F S Quirino
Infect Immun, 2016-07-21;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Myelin Oligodendrocyte Glycoprotein induces incomplete tolerance of CD4(+) T cells specific for both a myelin and a neuronal self antigen in mice
Authors: Liliana E Lucca
Eur J Immunol, 2016-07-21;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Novel Function of Extracellular Matrix Protein 1 in Suppressing Th17 Cell Development in Experimental Autoimmune Encephalomyelitis
Authors: Pan Su
J Immunol, 2016-06-17;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Impact of Histone H1 on the Progression of Allergic Rhinitis and Its Suppression by Neutralizing Antibody in Mice
Authors: T Nakano, R Kamei, T Fujimura, Y Takaoka, A Hori, CY Lai, KC Chiang, Y Shimada, N Ohmori, T Goto, K Ono, CL Chen, S Goto, S Kawamoto
PLoS ONE, 2016-04-18;11(4):e0153630.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Infection-Mediated Priming of Phagocytes Protects against Lethal Secondary Aspergillus fumigatus Challenge
Authors: A Savers, O Rasid, M Parlato, M Brock, G Jouvion, B Ryffel, JM Cavaillon, G Eberl, O Ibrahim-Gr
PLoS ONE, 2016-04-14;11(4):e0153829.
Species: Mouse
Sample Types: Tissue Homogenates
-
IL-22 Restrains Tapeworm-Mediated Protection against Experimental Colitis via Regulation of IL-25 Expression
Authors: JL Reyes, MR Fernando, F Lopes, G Leung, NL Mancini, CE Matisz, A Wang, DM McKay
PLoS Pathog, 2016-04-07;12(4):e1005481.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Digoxin Inhibits Induction of Experimental Autoimmune Uveitis in Mice, but Causes Severe Retinal Degeneration
Invest. Ophthalmol. Vis. Sci., 2016-03-01;57(3):1441-7.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Repeated Activation of Lung Invariant NKT Cells Results in Chronic Obstructive Pulmonary Disease-Like Symptoms.
Authors: Tsao C, Tsao P, Chen Y, Chuang Y
PLoS ONE, 2016-01-26;11(1):e0147710.
Species: Mouse
Sample Types: BALF
-
Obeticholic acid, a synthetic bile acid agonist of the farnesoid X receptor, attenuates experimental autoimmune encephalomyelitis.
Authors: Ho P, Steinman L
Proc Natl Acad Sci U S A, 2016-01-25;113(6):1600-5.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Memory Th1 Cells Are Protective in Invasive Staphylococcus aureus Infection.
Authors: Brown A, Murphy A, Lalor S, Leech J, O'Keeffe K, Mac Aogain M, O'Halloran D, Lacey K, Tavakol M, Hearnden C, Fitzgerald-Hughes D, Humphreys H, Fennell J, van Wamel W, Foster T, Geoghegan J, Lavelle E, Rogers T, McLoughlin R
PLoS Pathog, 2015-11-05;11(11):e1005226.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Cooperation between IL-7 Receptor and Integrin alpha2beta1 (CD49b) Drives Th17-Mediated Bone Loss.
Authors: El Azreq M, Arseneault C, Boisvert M, Page N, Allaeys I, Poubelle P, Tessier P, Aoudjit F
J Immunol, 2015-09-25;195(9):4198-209.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Prostaglandin E2 Production and T Cell Function in Mouse Adenovirus Type 1 Infection following Allogeneic Bone Marrow Transplantation.
Authors: McCarthy M, Procario M, Wilke C, Moore B, Weinberg J
PLoS ONE, 2015-09-25;10(9):e0139235.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Low dietary iron intake restrains the intestinal inflammatory response and pathology of enteric infection by food-borne bacterial pathogens.
Authors: Kortman G, Mulder M, Richters T, Shanmugam N, Trebicka E, Boekhorst J, Timmerman H, Roelofs R, Wiegerinck E, Laarakkers C, Swinkels D, Bolhuis A, Cherayil B, Tjalsma H
Eur J Immunol, 2015-06-23;45(9):2553-67.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Unexpected Role for Adaptive alphabetaTh17 Cells in Acute Respiratory Distress Syndrome.
Authors: Li J, Melton A, Su G, Hamm D, LaFemina M, Howard J, Fang X, Bhat S, Huynh K, O'Kane C, Ingram R, Muir R, McAuley D, Matthay M, Sheppard D
J Immunol, 2015-05-22;195(1):87-95.
Species: Mouse
Sample Types: BALF
-
De novo-induced self-antigen-specific Foxp3+ regulatory T cells impair the accumulation of inflammatory dendritic cells in draining lymph nodes.
Authors: Alissafi T, Hatzioannou A, Ioannou M, Sparwasser T, Grun J, Grutzkau A, Verginis P
J Immunol, 2015-05-06;194(12):5812-24.
Species: Mouse
Sample Types: Cell Culture Supernates
-
DCIR maintains bone homeostasis by regulating IFN-gamma production in T cells.
Authors: Maruhashi T, Kaifu T, Yabe R, Seno A, Chung S, Fujikado N, Iwakura Y
J Immunol, 2015-04-29;194(12):5681-91.
Species: Mouse
Sample Types: Tissue Homogenates
-
Role of a Novel Human Leukocyte Antigen-DQA1*01:02;DRB1*15:01 Mixed Isotype Heterodimer in the Pathogenesis of "Humanized" Multiple Sclerosis-like Disease.
Authors: Kaushansky N, Eisenstein M, Boura-Halfon S, Hansen B, Nielsen C, Milo R, Zeilig G, Lassmann H, Altmann D, Ben-Nun A
J Biol Chem, 2015-04-24;290(24):15260-78.
Species: Mouse
Sample Types: Cell Culture Supernates
-
RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets.
Authors: Knudson C, Hartwig S, Meyerholz D, Varga S
PLoS Pathog, 2015-03-13;11(3):e1004757.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mesenchymal stem/stromal cells protect against autoimmunity via CCL2-dependent recruitment of myeloid-derived suppressor cells.
Authors: Lee H, Ko J, Jeong H, Ko A, Kim M, Wee W, Yoon S, Oh J
J Immunol, 2015-03-13;194(8):3634-45.
Species: Mouse
Sample Types: Tissue Homogenates
-
Positional identification of RT1-B (HLA-DQ) as susceptibility locus for autoimmune arthritis.
Authors: Haag S, Tuncel J, Thordardottir S, Mason D, Yau A, Dobritzsch D, Backlund J, Peters E, Holmdahl R
J Immunol, 2015-02-11;194(6):2539-50.
Species: Rat
Sample Types: Cell Culture Supernates
-
Genome-wide transcriptional analyses of islet-specific CD4+ T cells identify Idd9 genes controlling diabetogenic T cell function.
Authors: Berry G, Frielle C, Luu T, Salzberg A, Rainbow D, Wicker L, Waldner H
J Immunol, 2015-02-11;194(6):2654-63.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Testosterone suppresses hepatic inflammation by the downregulation of IL-17, CXCL-9, and CXCL-10 in a mouse model of experimental acute cholangitis.
Authors: Schwinge D, Carambia A, Quaas A, Krech T, Wegscheid C, Tiegs G, Prinz I, Lohse A, Herkel J, Schramm C
J Immunol, 2015-02-11;194(6):2522-30.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge.
Authors: Monin L, Griffiths K, Slight S, Lin Y, Rangel-Moreno J, Khader S
Mucosal Immunol, 2015-01-28;8(5):1099-109.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A critical role for the TLR signaling adapter Mal in alveolar macrophage-mediated protection against Bordetella pertussis.
Authors: Bernard N, Finlay C, Tannahill G, Cassidy J, O'Neill L, Mills K
Mucosal Immunol, 2014-12-17;8(5):982-92.
Species: Mouse
Sample Types: Tissue Homogenates
-
Cholera toxin adjuvant promotes a balanced Th1/Th2/Th17 response independently of IL-12 and IL-17 by acting on Gsalpha in CD11b(+) DCs.
Authors: Mattsson J, Schon K, Ekman L, Fahlen-Yrlid L, Yrlid U, Lycke N
Mucosal Immunol, 2014-11-26;8(4):815-27.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The aryl hydrocarbon receptor: differential contribution to T helper 17 and T cytotoxic 17 cell development.
Authors: Hayes M, Ovcinnikovs V, Smith A, Kimber I, Dearman R
PLoS ONE, 2014-09-09;9(9):e106955.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A ThPOK-LRF transcriptional node maintains the integrity and effector potential of post-thymic CD4+ T cells.
Authors: Vacchio M, Wang L, Bouladoux N, Carpenter A, Xiong Y, Williams L, Wohlfert E, Song K, Belkaid Y, Love P, Bosselut R
Nat Immunol, 2014-08-17;15(10):947-56.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1.
Authors: Heinemann C, Heink S, Petermann F, Vasanthakumar A, Rothhammer V, Doorduijn E, Mitsdoerffer M, Sie C, Prazeres da Costa O, Buch T, Hemmer B, Oukka M, Kallies A, Korn T
Nat Commun, 2014-05-06;5(0):3770.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-17 contributes to neutrophil recruitment but not to control of viral replication during acute mouse adenovirus type 1 respiratory infection.
Authors: McCarthy M, Zhu L, Procario M, Weinberg J
Virology, 2014-04-19;456(0):259-67.
Species: Mouse
Sample Types: BALF
-
Pulmonary immune responses to 2009 pandemic influenza A (H1N1) virus in mice.
Authors: Lv J, Wang D, Hua Y, Pei S, Wang J, Hu W, Wang X, Jia N, Jiang Q
BMC Infect Dis, 2014-04-12;14(0):197.
Species: Mouse
Sample Types: Tissue Homogenates
-
Protease-activated receptor-2 activation contributes to house dust mite-induced IgE responses in mice.
Authors: Post, Sijranke, Heijink, Irene H, Petersen, Arjen H, de Bruin, Harold G, van Oosterhout, Antoon J, Nawijn, Martijn
PLoS ONE, 2014-03-20;9(3):e91206.
Species: Mouse
Sample Types: Tissue Homogenates
-
Staphylococcus aureus infection of mice expands a population of memory gammadelta T cells that are protective against subsequent infection.
Authors: Murphy A, O'Keeffe K, Lalor S, Maher B, Mills K, McLoughlin R
J Immunol, 2014-03-12;192(8):3697-708.
Species: Mouse
Sample Types: Peritoneal Lavage Fluid
-
Differential IL-10 production by DCs determines the distinct adjuvant effects of LPS and PTX in EAE induction.
Authors: Zhou H, Wang Y, Lian Q, Yang B, Ma Y, Wu X, Sun S, Liu Y, Sun B
Eur J Immunol, 2014-03-07;44(5):1352-62.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Unique macrophages different from M1/M2 macrophages inhibit T cell mitogenesis while upregulating Th17 polarization.
Authors: Tatano Y, Shimizu T, Tomioka H
Sci Rep, 2014-02-20;4(0):4146.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Signaling via tumor necrosis factor receptor 1 but not Toll-like receptor 2 contributes significantly to hydrosalpinx development following Chlamydia muridarum infection.
Authors: Dong X, Liu Y, Chang X, Lei L, Zhong G
Infect Immun, 2014-02-18;82(5):1833-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dichloroacetate modulates cytokines toward T helper 1 function via induction of the interleukin-12-interferon-gamma pathway.
Authors: Badr M, Qinna N, Qadan F, Matalka K
Onco Targets Ther, 2014-02-07;7(0):193-201.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Delineating the role of histamine-1- and -4-receptors in a mouse model of Th2-dependent antigen-specific skin inflammation.
Authors: Mahapatra S, Albrecht M, Behrens B, Jirmo A, Behrens G, Hartwig C, Neumann D, Raap U, Bahre H, Herrick C, Dittrich A
PLoS ONE, 2014-02-04;9(2):e87296.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IFN-gamma-producing and IL-17-producing gammadelta T cells differentiate at distinct developmental stages in murine fetal thymus.
Authors: Shibata K, Yamada H, Nakamura M, Hatano S, Katsuragi Y, Kominami R, Yoshikai Y
J Immunol, 2014-01-31;192(5):2210-8.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Subcomponent vaccine based on CTA1-DD adjuvant with incorporated UreB class II peptides stimulates protective Helicobacter pylori immunity.
Authors: Nedrud J, Bagheri N, Schon K, Xin W, Bergroth H, Eliasson D, Lycke N
PLoS ONE, 2013-12-31;8(12):e83321.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis.
Authors: Hams, Emily, Armstrong, Michelle, Barlow, Jillian, Saunders, Sean P, Schwartz, Christia, Cooke, Gordon, Fahy, Ruairi J, Crotty, Thomas B, Hirani, Nikhil, Flynn, Robin J, Voehringer, David, McKenzie, Andrew N, Donnelly, Seamas C, Fallon, Padraic
Proc Natl Acad Sci U S A, 2013-12-16;111(1):367-72.
Species: Mouse
Sample Types: Tissue Homogenates
-
Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor.
Authors: van Voorhis, Michael, Knopp, Samantha, Julliard, Walker, Fechner, John H, Zhang, Xiaoji, Schauer, James J, Mezrich, Joshua D
PLoS ONE, 2013-12-11;8(12):e82545.
Species: Mouse
Sample Types: Cell Culture Supernates
-
MyD88 signaling regulates both host defense and immunopathogenesis during pneumocystis infection.
Authors: Bello-Irizarry S, Wang J, Johnston C, Gigliotti F, Wright T
J Immunol, 2013-11-29;192(1):282-92.
Species: Mouse
Sample Types: BALF
-
The activating protein 1 transcription factor basic leucine zipper transcription factor, ATF-like (BATF), regulates lymphocyte- and mast cell-driven immune responses in the setting of allergic asthma.
Authors: Ubel C, Sopel N, Graser A, Hildner K, Reinhardt C, Zimmermann T, Rieker R, Maier A, Neurath M, Murphy K, Finotto S
J Allergy Clin Immunol, 2013-11-28;133(1):198-206.e1-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Preconditioning of microglia by alpha-synuclein strongly affects the response induced by toll-like receptor (TLR) stimulation.
Authors: Roodveldt C, Labrador-Garrido A, Gonzalez-Rey E, Lachaud C, Guilliams T, Fernandez-Montesinos R, Benitez-Rondan A, Robledo G, Hmadcha A, Delgado M, Dobson C, Pozo D
PLoS ONE, 2013-11-13;8(11):e79160.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Epithelial NF-kappaB orchestrates house dust mite-induced airway inflammation, hyperresponsiveness, and fibrotic remodeling.
Authors: Tully J, Hoffman S, Lahue K, Nolin J, Anathy V, Lundblad L, Daphtary N, Aliyeva M, Black K, Dixon A, Poynter M, Irvin C, Janssen-Heininger Y
J Immunol, 2013-11-13;191(12):5811-21.
Species: Mouse
Sample Types: Tissue Homogenates
-
Rac2-deficiency leads to exacerbated and protracted colitis in response to Citrobacter rodentium infection.
Authors: Fattouh R, Guo C, Lam G, Gareau M, Ngan B, Glogauer M, Muise A, Brumell J
PLoS ONE, 2013-04-16;8(4):e61629.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A novel murine model of rhinoscleroma identifies Mikulicz cells, the disease signature, as IL-10 dependent derivatives of inflammatory monocytes.
Authors: Fevre C, Almeida A, Taront S, Pedron T, Huerre M, Prevost M, Kieusseian A, Cumano A, Brisse S, Sansonetti P, Tournebize R
EMBO Mol Med, 2013-04-01;5(4):516-30.
Species: Mouse
Sample Types: Tissue Homogenates
-
1,25-Dihydroxyvitamin D3 suppresses TLR8 expression and TLR8-mediated inflammatory responses in monocytes in vitro and experimental autoimmune encephalomyelitis in vivo.
Authors: Li B, Baylink D, Deb C, Zannetti C, Rajaallah F, Xing W, Walter M, Lau K, Qin X
PLoS ONE, 2013-03-14;8(3):e58808.
Species: Mouse
Sample Types: Serum
-
Nanogel-based PspA intranasal vaccine prevents invasive disease and nasal colonization by Streptococcus pneumoniae.
Authors: Kong, Il Gyu, Sato, Ayuko, Yuki, Yoshikaz, Nochi, Tomonori, Takahashi, Haruko, Sawada, Shinichi, Mejima, Mio, Kurokawa, Shiho, Okada, Kazunari, Sato, Shintaro, Briles, David E, Kunisawa, Jun, Inoue, Yusuke, Yamamoto, Masafumi, Akiyoshi, Kazunari, Kiyono, Hiroshi
Infect Immun, 2013-03-04;81(5):1625-34.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Immune Modulation Mediated by Cryptococcal Laccase Promotes Pulmonary Growth and Brain Dissemination of Virulent Cryptococcus neoformans in Mice.
Authors: Qiu Y, Davis M, Dayrit J, Hadd Z, Meister D, Osterholzer J, Williamson P, Olszewski M
PLoS ONE, 2012-10-22;7(10):e47853.
Species: Mouse
Sample Types: Cell Culture Supernates
-
LXR-mediated inhibition of CD4+ T helper cells.
Authors: Solt L, Kamenecka T, Burris T
PLoS ONE, 2012-09-28;7(9):e46615.
Species: Mouse
Sample Types: Cell Culture Supernates
-
T1/ST2 promotes T helper 2 cell activation and polyfunctionality in bronchopulmonary mycosis.
Authors: Piehler D, Grahnert A, Eschke M, Richter T, Kohler G, Stenzel W, Alber G
Mucosal Immunol, 2012-09-19;6(2):405-14.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Macrophage Dectin-1 Expression Is Controlled by Leukotriene B4 via a GM-CSF/PU.1 Axis.
Authors: Serezani CH, Kane S, Collins L
J. Immunol., 2012-06-13;189(2):906-15.
Species: Mouse
Sample Types: BALF
-
IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs.
Authors: Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H
J. Immunol., 2011-12-23;188(3):1503-13.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin-23-mediated inflammation in Pseudomonas aeruginosa pulmonary infection.
Authors: Dubin PJ, Martz A, Eisenstatt JR, Fox MD, Logar A, Kolls JK
Infect. Immun., 2011-10-24;80(1):398-409.
Species: Mouse
Sample Types: BALF
-
Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection.
Authors: Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, Riva F, Garlanda C
Infect. Immun., 2011-10-24;80(1):100-9.
Species: Mouse
Sample Types: Serum
-
Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not interleukin-17, mediate host resistance.
Authors: Breslow JM, Meissler JJ, Hartzell RR
Infect. Immun., 2011-05-16;79(8):3317-27.
Species: Mouse
Sample Types: Tissue Secretion
-
The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF.
Authors: El-Behi M, Ciric B, Dai H
Nat. Immunol., 2011-04-24;12(6):568-75.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Therapeutic Potential of the Humoral Pattern Recognition Molecule PTX3 in Chronic Lung Infection Caused by Pseudomonas aeruginosa.
Authors: Moalli F, Paroni M, Veliz Rodriguez T, Riva F, Polentarutti N, Bottazzi B, Valentino S, Mantero S, Nebuloni M, Mantovani A, Bragonzi A, Garlanda C
J. Immunol., 2011-03-25;186(9):5425-34.
Species: Mouse
Sample Types: Tissue Homogenates
-
IL-27 mediates the response to IFN-beta therapy in multiple sclerosis patients by inhibiting Th17 cells.
Authors: Sweeney CM, Lonergan R, Basdeo SA, Kinsella K, Dungan LS, Higgins SC, Kelly PJ, Costelloe L, Tubridy N, Mills KH, Fletcher JM
Brain Behav. Immun., 2011-03-21;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Impaired basophil induction leads to an age-dependent innate defect in type 2 immunity during helminth infection in mice.
Authors: Nel HJ, Hams E, Saunders SP
J. Immunol., 2011-03-11;186(8):4631-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells.
Authors: Notley CA, Brown MA, Wright GP, Ehrenstein MR
J. Immunol., 2011-03-07;186(8):4967-72.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition.
Authors: Barber DL, Mayer-Barber KD, Feng CG
J. Immunol., 2010-12-20;186(3):1598-607.
Species: Mouse
Sample Types: BALF
-
A critical role for C5L2 in the pathogenesis of experimental allergic asthma.
Authors: Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, Konig P, Gerard NP, Gerard C, Wills-Karp M, Kohl J
J. Immunol., 2010-10-25;185(11):6741-52.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Naive and activated T cells display differential responsiveness to TL1A that affects Th17 generation, maintenance, and proliferation.
Authors: Jones GW, Stumhofer JS, Foster T, Twohig JP, Hertzog P, Topley N, Williams AS, Hunter CA, Jenkins BJ, Wang EC, Jones SA
FASEB J., 2010-09-08;25(1):409-19.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Importance of TLR2 in the direct response of T lymphocytes to Schistosoma mansoni antigens.
Authors: Burton OT, Gibbs S, Miller N, Jones FM, Wen L, Dunne DW, Cooke A, Zaccone P
Eur. J. Immunol., 2010-08-01;40(8):2221-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice.
Authors: Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS
J. Clin. Invest., 2010-04-01;120(5):1762-73.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Chronic cigarette smoke exposure generates pathogenic T cells capable of driving COPD-like disease in Rag2-/- mice.
Authors: Motz GT, Eppert BL, Wesselkamper SC, Flury JL, Borchers MT
Am. J. Respir. Crit. Care Med., 2010-02-04;181(11):1223-33.
Species: Mouse
Sample Types: BALF
-
Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity.
Authors: Sellge G, Magalhaes JG, Konradt C, Fritz JH, Salgado-Pabon W, Eberl G, Bandeira A, Di Santo JP, Sansonetti PJ, Phalipon A
J. Immunol., 2010-01-20;184(4):2076-85.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells.
Authors: Jones GW, McLoughlin RM, Hammond VJ, Parker CR, Williams JD, Malhotra R, Scheller J, Williams AS, Rose-John S, Topley N, Jones SA
J. Immunol., 2010-01-18;184(4):2130-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CCR5 is involved in resolution of inflammation in proteoglycan-induced arthritis.
Authors: Doodes PD, Cao Y, Hamel KM, Wang Y, Rodeghero RL, Kobezda T, Finnegan A
Arthritis Rheum., 2009-10-01;60(10):2945-53.
Species: Mouse
Sample Types: Synovial Fluid
-
Differential IL-23 requirement for IL-22 and IL-17A production during innate immunity against Salmonella enterica serovar Enteritidis.
Authors: Siegemund S, Schutze N, Schulz S, Wolk K, Nasilowska K, Straubinger RK, Sabat R, Alber G
Int. Immunol., 2009-03-18;21(5):555-65.
Species: Mouse
Sample Types: Peritoneal Lavage Fluid
-
Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses.
Authors: Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y
Immunity, 2009-01-16;30(1):108-19.
Species: Mouse
Sample Types: Whole Tissue
-
CD69+ CD4+ CD25- T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1.
Authors: Han Y, Guo Q, Zhang M, Chen Z, Cao X
J. Immunol., 2009-01-01;182(1):111-20.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17.
Authors: Schulz SM, Kohler G, Schutze N, Knauer J, Straubinger RK, Chackerian AA, Witte E, Wolk K, Sabat R, Iwakura Y, Holscher C, Muller U, Kastelein RA, Alber G
J. Immunol., 2008-12-01;181(11):7891-901.
Species: Mouse
Sample Types: Serum
-
Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis.
Authors: Kaya Z, Goser S, Buss SJ, Leuschner F, Ottl R, Li J, Volkers M, Zittrich S, Pfitzer G, Rose NR, Katus HA
Circulation, 2008-10-27;118(20):2063-72.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities.
Authors: Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL
J. Immunol., 2008-09-15;181(6):4299-310.
Species: Mouse
Sample Types: BALF
-
Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by gammadelta T cells.
Authors: Nakamura R, Shibata K, Yamada H, Shimoda K, Nakayama K, Yoshikai Y
J. Immunol., 2008-08-01;181(3):2071-5.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Amelioration of delayed-type hypersensitivity responses by IL-27 administration.
Authors: Miyazaki Y, Shimanoe Y, Wang S, Yoshida H
Biochem. Biophys. Res. Commun., 2008-06-20;373(3):397-402.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interferon-gamma regulates idiopathic pneumonia syndrome, a Th17+CD4+ T-cell-mediated graft-versus-host disease.
Authors: Mauermann N, Burian J, von Garnier C, Dirnhofer S, Germano D, Schuett C, Tamm M, Bingisser R, Eriksson U, Hunziker L
Am. J. Respir. Crit. Care Med., 2008-05-29;178(4):379-88.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection.
Authors: Bozza S, Zelante T, Moretti S, Bonifazi P, DeLuca A, D'Angelo C, Giovannini G, Garlanda C, Boon L, Bistoni F, Puccetti P, Mantovani A, Romani L
J. Immunol., 2008-03-15;180(6):4022-31.
Species: Mouse
Sample Types: Cell Culture Supernates
-
IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice.
Authors: Smith E, Zarbock A, Stark MA, Burcin TL, Bruce AC, Foley P, Ley K
J. Immunol., 2007-12-15;179(12):8274-9.
Species: Mouse
Sample Types: Serum
-
Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells.
Authors: Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A
Nat. Immunol., 2007-11-11;8(12):1372-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis.
Authors: Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A
J. Immunol., 2007-09-01;179(5):3268-75.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CD8+ T cells are not required for vaccine-induced immunity against Leishmania amazonensis in IL-12/23P40(-/-) C57BL/6 mice.
Authors: Hernandez Sanabria MX, Afonso LC, Golgher D, Tafuri WL, Vieira LQ
Microbes Infect., 2007-05-18;9(9):1124-34.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity.
Authors: Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, Collins C, Viala J, Ferrero RL, Girardin SE, Philpott DJ
Immunity, 2007-04-12;26(4):445-59.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production.
Authors: Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y
J. Immunol., 2007-04-01;178(7):4466-72.
Species: Mouse
Sample Types: Peritoneal Fluid
-
T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity.
Authors: Gocke AR, Cravens PD, Ben LH, Hussain RZ, Northrop SC, Racke MK, Lovett-Racke AE
J. Immunol., 2007-02-01;178(3):1341-8.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin-17 is a negative regulator of established allergic asthma.
Authors: Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B
J. Exp. Med., 2006-11-13;203(12):2715-25.
Species: Mouse
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse IL-17 DuoSet ELISA
Average Rating: 4.9 (Based on 16 Reviews)
Have you used Mouse IL-17 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
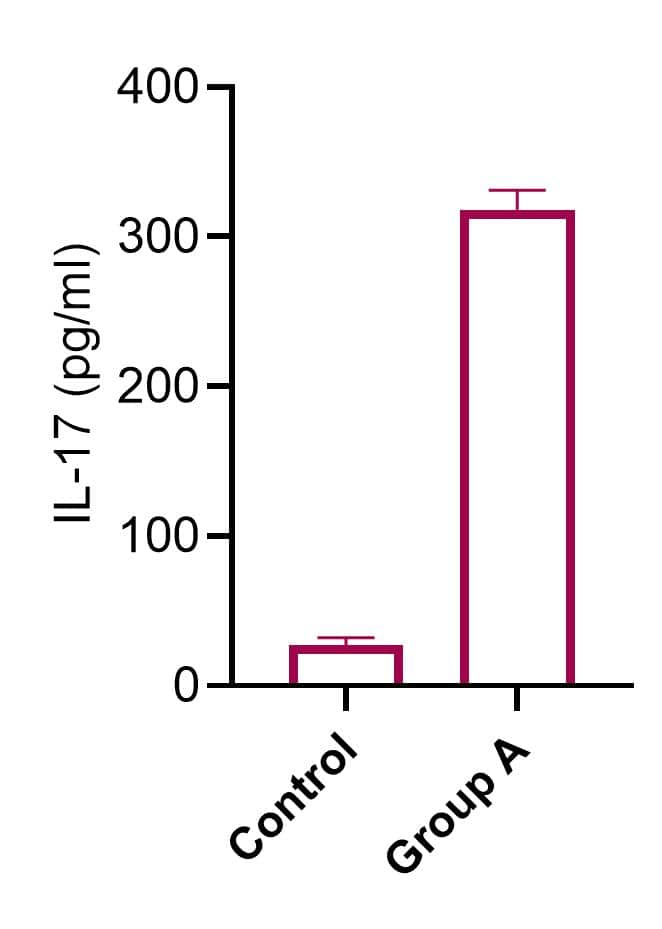
The levels of IL-17 from culture supernatants of mouse spleen cells incubated with or without A reagent (group A) were measured with IL-17
happy with product - consistent results, easy to use
Specificity: Specific
Sensitivity: Reasonably sensitive
Buffer: Reagent diluent
Dilution: 400 ng/mL (detection)