IL-17 Family Signaling Pathways
IL-17 R
IL-17 R
IL-17RB
IL-17 R
Receptor
IL-17 R
IL-17RB
Ligand
have not been characterized.
- Th17 cells
- CD8+ T cells
- gamma delta T cells
- Lymphoid tissue inducer (LTi) and LTi-like cells
- Natural Killer (NK) cells
- Natural Killer T (NKT) cells
- Neutrophils
- Intestinal Paneth cells
- Endothelial cells
- Epithelial cells
- Fibroblasts
- Keratinocytess
- Macrophages
- Synoviocytes
- T cells
- Immunity to extracellular pathogens
- Pro-inflammatory cytokine production
- Anti-microbial protein production
- Chemokine production
- Neutrophil recruitment
- High levels of expression are associated with autoimmune diseases
- Th17 cells
- CD8+ T cells
- gamma delta T cells
- Lymphoid tissue inducer (LTi) and LTi-like cells
- Natural Killer (NK) cells
- Natural Killer T (NKT) cells
- Endothelial cells
- Epithelial cells
- Fibroblasts
- Keratinocytess
- Macrophages
- Synoviocytes
- Immunity to extracellular pathogens
- Pro-inflammatory cytokine production
- Anti-microbial protein production
- Neutrophil recruitment
- Chemokine production
- Chondrocytes
- Neurons
- Endothelial cells
- Monocytes
- Pro-inflammatory cytokine production
- Neutrophil recruitment
- Epithelial cells
- Keratinocytes
- Macrophages
- Tissue epithelial cells
- Keratinocytess
- Monocytes
- Th17 cells
- Pro-inflammatory cytokine production
- Anti-microbial protein production
- Neutrophil recruitment
- Chemokine production
- Resting CD4+ T cells
- Resting B cells
- Cells of the brain, heart, muscle, lung, pancreas, and adipose tissue
- Unknown - the identity of the receptor for IL-17D is unknown
- Pro-inflammatory cytokine production
- Endothelial cells
- Mucosal epithelial cells
- Intraepithelial lymphocytes
- Alveolar macrophages
- Basophils
- Eosinophils
- Mast cells
- Th2 cells
- Airway epithelial cells
- Th2 cells
- Th9 cells
- Macrophages
- Natural Killer T (NKT) cells
- Other innate immune cell types (ex. Nuocytes)
- Defense against parasitic helminths
- Stimulates Th2-, Th9-type immune responses
- Inhibits Th1-, Th17-type immune responses
- Eosinophil recruitment
- High levels of expression are associated with exacerbated allergen-induced airway inflammation
- Th17 cells
- CD8+ T cells
- gamma delta T cells
- Lymphoid tissue inducer (LTi) and LTi-like cells
- Natural Killer (NK) cells
- Natural Killer T (NKT) cells
- Lung and colonic epithelial cells
- Endothelial cells
- Epithelial cells
- Fibroblasts
- Keratinocytess
- Macrophages
- Synoviocytes
- Immunity to extracellular pathogens
- Pro-inflammatory cytokine production
- Anti-microbial protein production
- Neutrophil recruitment
- Chemokine production
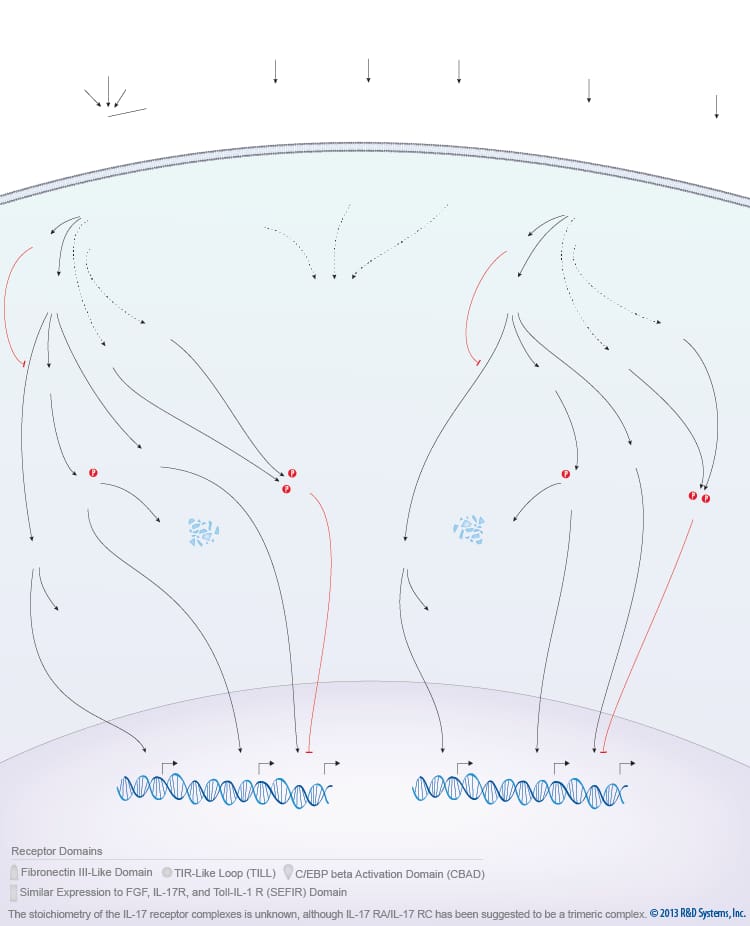
Overview of IL-17 Family Signaling
The IL-17 family of cytokines consists of six members, IL-17/IL-17A, IL-17B, IL-17C, IL-17D, IL-17E/IL-25, and IL-17F, which are produced by a number of different cell types and primarily promote pro-inflammatory immune responses. Cytokines belonging to the IL-17 family are secreted as dimers and bind to members of the IL-17 receptor family. The IL-17 receptor family includes IL-17 R/IL-17RA, IL-17B R/IL-17RB, IL-17RC, IL-17RD/SEF, and IL-17RE. All of these receptor subunits are type I transmembrane proteins that contain two fibronectin type III-like domains in their extracellular regions and a cytoplasmic SEF/IL-17 R/TIR (SEFIR) domain. Unlike the other IL-17 family receptors, IL-17RA also contains a cytoplasmic Toll/IL-1 R-like loop (TILL), and a C/EBP beta activation domain (CBAD). The IL-17 family receptors oligomerize to form functional receptor complexes. Although the stoichiometry of these complexes is not well understood, the receptor for IL-17A, IL-17F, and IL-17A/F is thought to be a heterotrimeric complex consisting of two IL-17RA subunits and one IL-17RC subunit. The IL-17RA receptor subunit also interacts with IL-17RE and IL-17B R/IL-17RB to form functional receptor complexes for IL-17C and IL-17E/IL-25, respectively. In addition to binding IL-17E/IL-25, IL-17B R/IL-17RB binds to IL-17B but little is known about the composition of the receptor complex or the signaling pathways that it activates. Similarly, the receptor for IL-17D and the ligand for IL-17RD/SEF are unknown, and the signaling pathways activated downstream of IL-17C have not been characterized.
Of the IL-17 family cytokines, the signaling pathways activated by IL-17A and IL-17E/IL-25 are the most fully characterized. Binding of IL-17A to its receptor complex leads to the recruitment of the ACT1 adaptor protein, which interacts with the IL-17 receptor through their shared SEFIR domains. Association of ACT1 with the IL-17 receptor complex leads to the recruitment of Tumor Necrosis Factor (TNF) Receptor-associated Factor 6 and 3 (TRAF-6 and TRAF-3) and activation of a series of intracellular kinases including TGF-beta-activated Kinase 1 (TAK1), I-kappa B Kinase (IKK), Extracellular Signal-regulated Kinase (ERK), Glycogen Synthase Kinase 3 beta (GSK-3 beta), and p38 MAPK. Together, these kinases promote the NF-kappa B-, AP-1-, and C/EBP-dependent expression of pro-inflammatory cytokines, chemokines, and anti-microbial peptides. Although IL-17A, IL-17F, and IL-17A/F bind to the same receptor complex and are thought to activate the same signaling pathways, IL-17A is more potent than IL-17A/F and is considerably more potent than IL-17F. This may explain why high levels of IL-17A expression are more strongly associated with the development of autoimmune disease.
IL-17E/IL-25 is the most divergent member of the IL-17 cytokine family and promotes biological effects that are distinct from those induced by other IL-17 family members. IL-17E signals through a receptor complex consisting of IL-17B R/IL-17RB and IL-17RA. Similar to IL-17A, IL-17E signaling requires ACT1, activates p38 MAPK, ERK, and NF-kappa B, and stimulates the expression of pro-inflammatory chemokines and cytokines such as IL-6 and IL-8. In contrast to IL-17A signaling, however, signaling induced by IL-17E primarily promotes Th2- and Th9-type immune responses by driving the expression of IL-4, IL-5, and IL-13 and eosinophil recruitment. As a result, IL-17E/IL-25 promotes host defense against parasitic helminths but may also contribute to the pathogenesis of allergen-induced airway inflammation. Additionally, IL-17E can act as an anti-inflammatory cytokine by inhibiting the secretion of cytokines required for Th1 and Th17 cell development.
| IL-17 Family Cytokines - Products by Molecule | |||||
| IL-17/IL-17A | IL-17B | IL-17C | IL-17D | IL-17E/IL-25 | IL-17F |
| IL-17 Family Receptors - Products by Molecule | |||||
| IL-17RA/IL-17R | IL-17RB | IL-17RC | IL-17RD/SEF | IL-17RE | |

