Mouse Osteopontin/OPN Antibody Summary
Leu17-Asn294 (Glu99Gly)
Accession # Q547B5
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Mouse Osteopontin/OPN Sandwich Immunoassay
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
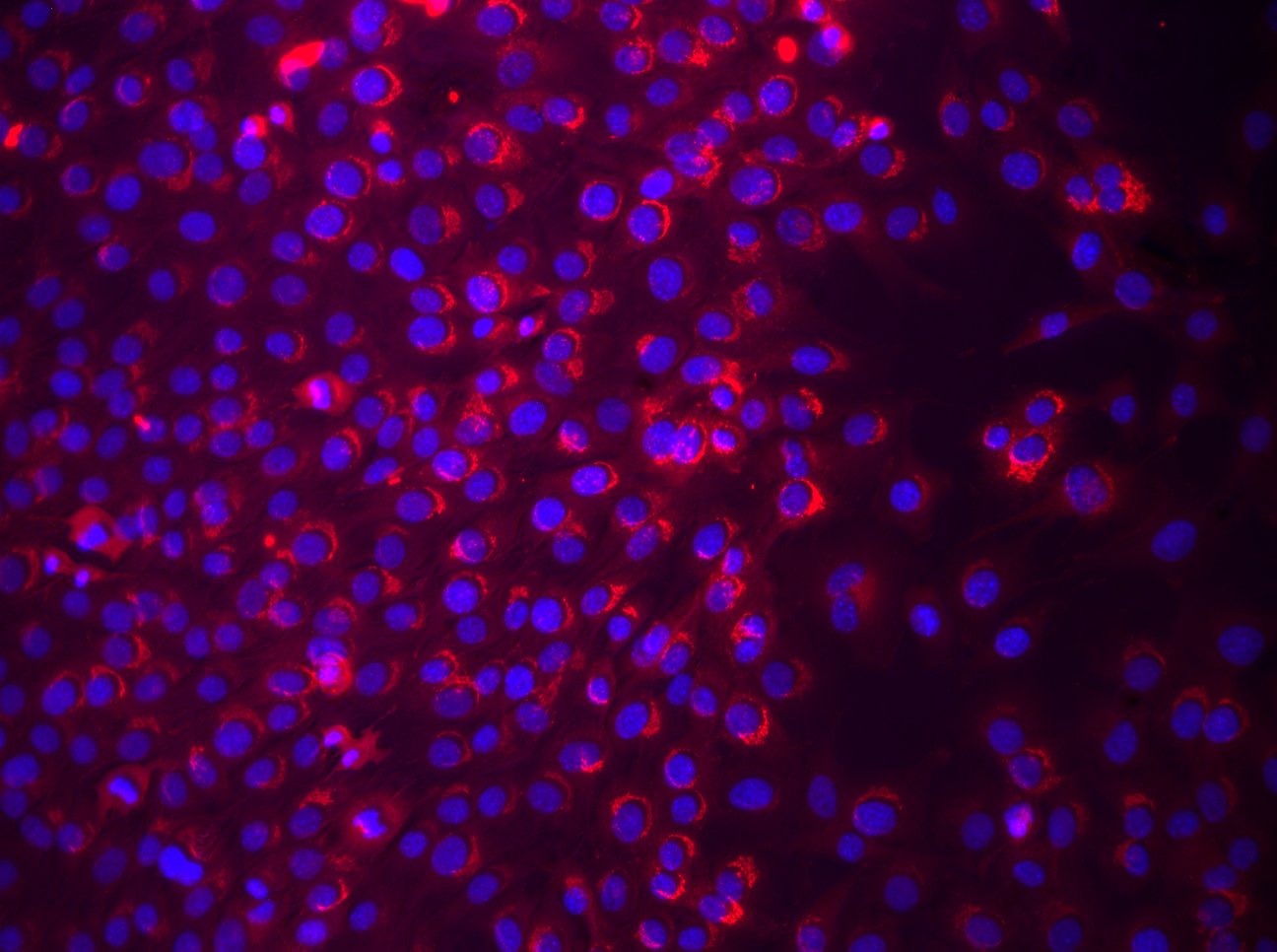
Osteopontin/OPN in C2C12 Mouse Cell Line and Mouse Splenocytes. Osteopontin/OPN was detected in immersion fixed C2C12 mouse myoblast cell line (left panel, positive stain) and mouse splenocytes (right panel, negative stain) using Goat Anti-Mouse Osteopontin/OPN Antigen Affinity-purified Polyclonal Antibody (Catalog # AF808) at 5 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; NL001) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm and secreted molecule. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
Osteopontin/OPN in Mouse Thymus. Osteopontin/OPN was detected in perfusion fixed frozen sections of mouse thymus using Mouse Osteopontin/OPN Antigen Affinity-purified Polyclonal Antibody (Catalog # AF808) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; CTS008) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
Cell Adhesion Mediated by Osteopontin/OPN and Neutralization by Mouse Osteopontin/OPN Antibody. Recombinant Mouse Osteopontin/OPN (441-OP), immobilized onto a microplate, supports the adhesion of the HEK293 human embryonic kidney cell line in a dose-dependent manner (orange line). Adhesion elicited by Recombinant Mouse Osteopontin/OPN (2 µg/mL) is neutralized (green line) by increasing concentrations of Mouse Osteopontin/OPN Antigen Affinity-purified Polyclonal Antibody (Catalog # AF808). The ND50 is typically 1-3 µg/mL.
 View Larger
View Larger
Detection of Mouse Osteopontin/OPN by Immunocytochemistry/Immunofluorescence Notch ligands Jag1 and Dll1 are both required for segregation of hepatocytic fate centrally and biliary fate peripherally.(A) Immunolabeling for OPN and HNF4A of BMEL cells presented with DLL4 on 30 kPa substrates. Control cells were transduced with an shRNA vector coding for a non-mammalian target. shJag1 and shDll1 cells were transduced with shRNA vectors targeting Jag1 and Dll1, respectively. Scale bar is 150 µm. (B) Confocal imaging of immunolabeled SOX9 and HNF4A in control, shJag1, and shDll1 cells presented with DLL4 on 30 kPa substrates. Scale bar is 75 µm. (C) Quantification of OPN+ cell counts of control, shJag1, and shDll1 cells presented with DLL4 on 30 kPa substrates. (D) Quantification of SOX9 and HNF4A intensity of control, shJag1, and shDll1 cells presented with DLL4 on 30 kPa substrates. (C, D) Mean ± 95% CI.10.7554/eLife.38536.024Figure 7—source data 1.Summary table for OPN data in Figure 7C.10.7554/eLife.38536.025Figure 7—source data 2.Summary table for SOX9 and HNF4A data in Figure 7D.Summary table for OPN data in Figure 7C.Summary table for SOX9 and HNF4A data in Figure 7D.Regression analysis of OPN+ cell counts.Data in Figure 7B were separated into peripheral and central subsets for which dimensionless radius was greater than 0.75 (R>0.75) and less than 0.75 (R<0.75). Separate multiple regression models were generated for each data subset for which coefficient estimates (corresponding to mean change in cell counts) and 95% CI were plotted. For each factor, 95% CI that do not intersect with the dashed line indicate regression coefficient estimates for which P<0.05. Control, shJag1, and shDll1 cells were presented with DLL4 on 30 kPa substrates. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30589410), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Osteopontin/OPN by Western Blot Altered expression of ECM proteins in Cyp1b1-/- LSEC.Western blot analysis of ECM proteins in the conditioned medium (CM) and cell lysates from LSEC was performed. (A) The levels of SPARC, Tenascin-C, TSP2, and Osteopontin were below the level of detection. Cyp1b1 LSEC produced TSP1, periostin, and fibronectin. (B) The quantitative assessment of the data. TSP1 level was significantly decreased in lysates from Cyp1b1-/- LSEC (***P< 0.001; n = 3), while periostin level was increased in lysates from Cyp1b1-/- LSEC. The level of fibronectin secreted into the conditioned medium was decreased in Cyp1b1-/- LSEC. These experiments were repeated with two isolation of LSEC with similar results. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30372497), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Osteopontin/OPN by Immunocytochemistry/Immunofluorescence Peripheral biliary differentiation is dependent on both Notch signaling and substrate stiffness.(A) Immunolabeling for OPN of BMEL cells presented with DLL4 on 30 kPa and 4 kPa substrates. Cells were treated with vehicle control (DMSO) or an inhibitor of Notch signaling ( gamma -secretase inhibitor X, GSI, 5 µM). (B) Quantification of OPN+ cell counts on 30 kPa and 4 kPa substrates after treatment with DMSO or GSI. (C) Immunolabeling for SOX9 and HNF4A of BMEL cells on 30 kPa and 4 kPa substrates. (D) Quantification of SOX9 and HNF4A intensity on 30 kPa and 4 kPa substrates. (E) RNA in situ hybridization for Jag1, Dll1, and Notch2 on 30 kPa and 4 kPa substrates. Cells were exogenously presented with IgG or DLL4. (A, C, E) Scale bars indicate 150 µm. (B, D) Mean ± 95% CI.10.7554/eLife.38536.013Figure 2—source data 1.Summary table for OPN data in Figure 2B and Figure 2—figure supplement 1.10.7554/eLife.38536.014Figure 2—source data 2.Summary table for SOX9 and HNF4A data in Figure 2D.Summary table for OPN data in Figure 2B and Figure 2—figure supplement 1.Summary table for SOX9 and HNF4A data in Figure 2D.Quantification of OPN+ cell counts in arrayed patterns.Cells were cultured on 30 kPa and 4 kPa substrates and presented with IgG, DLL1, DLL4, and JAG1. Treatments included vehicle control (DMSO) or an inhibitor of Notch signaling ( gamma -secretase inhibitor X, GSI, 5 µM).Regression analysis of OPN+ and ALB+ cell counts.Data in Figure 2B were separated into peripheral and central subsets for which dimensionless radius was greater than 0.75 (R>0.75) and less than 0.75 (R<0.75). Separate multiple regression models were generated for each data subset for which coefficient estimates (corresponding to mean change in cell counts) and 95% CI were plotted for OPN+ (A) and ALB+ (B) cells. For each factor, 95% CI that do not intersect with the dashed line indicate regression coefficient estimates for which P<0.05. Cells were cultured on 30 kPa and 4 kPa substrates and presented with IgG, DLL1, DLL4, and JAG1. Treatments included vehicle control (DMSO) or an inhibitor of Notch signaling ( gamma -secretase inhibitor X, GSI, 5 µM).Quantification of ALB+ cell counts in arrayed patterns.Cells were cultured on 30 kPa substrates and presented with IgG, DLL1, DLL4, and JAG1. Treatments included vehicle control (DMSO) or an inhibitor of Notch signaling ( gamma -secretase inhibitor X, GSI, 5 µM). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30589410), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Osteopontin/OPN by Immunocytochemistry/Immunofluorescence Characterisation of mouse liver progenitor cell lines.(A) Mouse liver progenitor cell (LPC) lines (BMOL1.2, BMOL-TAT) were stained using immunofluorescence to determine expression of LPC marker Osteopontin (OPN) (red). IgG isotype control images obtained using identical imaging conditions. DAPI, blue. (B) LPCs (BMOL1.2) were shown to express full-length primary cilium (Pc) structures by immunofluorescence detection of axoneme ( alpha -acetylated tubulin, green) and basal body ( gamma -tubulin, red) markers. This was also confirmed by scanning electron microscopy (SEM). BMOL-TAT cells were also confirmed to express Pc via immunofluorescence and EM studies (data not shown). (C) Nuclear GLI2 (red) expression in LPC lines (BMOL1.2, BMOL-TAT) via immunofluorescence staining. DAPI, blue. Confocal microscopy, 63x objective. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0171480), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Osteopontin/OPN by Western Blot Osteopontin (OPN) drives enhancement in macrophage (Mφ) M2 polarization and angiogenic capacity. (A) Representative images of protein expression profiles obtained by comprehensive protein array in each Mφ subset. Red arrowheads indicate OPN. (B) The mRNA expression level of Spp1 relative to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was analyzed by real-time reverse transcription polymerase chain reaction in each Mφ subset and was normalized to Mφ (–), n = 6 [***p < 0.001 vs. untreated, #p < 0.05 vs. interleukin (IL)-10 alone]. (C) The protein expression level of OPN relative to GAPDH was measured by western blotting and was normalized to Mφ (–), n = 10. Lower panels are typical images of each protein (***p < 0.001 vs. untreated, #p < 0.05 vs. IL-10 alone). (D) Representative confocal laser scanning immunofluorescence overlay images of OPN (red) and DAPI (blue) in each Mφ subset. Scale bar represents 20 µm. Images in the right row are magnified regions from white or yellow rectangles in the panels of corresponding groups. Scale bar represents 10 µm. (E) Relative mean fluorescence intensity (MFI) of CD163 was measured by FACS analysis in each Mφ subset. An anti-OPN antibody (Ab) and its isotype-matched control Ab were used at 3 µg/mL, n = 4 (***p < 0.001 vs. untreated, ##p < 0.01, #p < 0.05 vs. IL-10 alone, †††p < 0.001 vs. IL-10 + IL-18). (F) The total areas and lengths of tube-like structures were determined by the Matrigel tube formation assay where b.End5 was cocultured with each Mφ subset, n = 12 (***p < 0.001, **p < 0.01, *p < 0.05 vs. untreated, #p < 0.05 vs. IL-10 alone, †††p < 0.001 vs. IL-10 + IL-18). All data are expressed as means ± SEM and were analyzed by a one-way ANOVA followed by Tukey’s test. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29559970), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Osteopontin/OPN by Immunocytochemistry/Immunofluorescence Thrombin contributes to macrophage (Mφ) M2 polarization and angiogenic capacity through proteolytic modification for osteopontin (OPN). (A) The mRNA expression level of Prothrombin relative to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was analyzed by reverse transcription polymerase chain reaction in each Mφ subset and were normalized to Mφ (–), n = 7 [***p < 0.001, **p < 0.01 vs. untreated, ##p < 0.01 vs. interleukin (IL)-10 alone]. (B,C) The protein expression levels of (B) thrombin or (C) OPN N-Half relative to GAPDH were measured by western blotting in each Mφ subset and were normalized to Mφ (–). Lower panels are typical images of each protein. (B)n = 8 (***p < 0.001, *p < 0.05 vs. untreated, #p < 0.05 vs. IL-10 alone). (C)n = 16 (***p < 0.001, *p < 0.05 vs. untreated, ##p < 0.01, #p < 0.05 vs. IL-10 alone, †††p < 0.001 vs. IL-10 + IL-18). (D) Representative confocal laser scanning immunofluorescence images of OPN (red), thrombin (green), and their merge with DAPI (blue) in each Mφ subset. Scale bar represents 20 µm. Higher magnification images are from the white rectangle region in merged panel of Mφ (IL-10 + IL-18). Scale bar represents 10 µm. (E) Relative mean fluorescence intensity (MFI) of CD163 was measured by FACS analysis in each Mφ subset. Hirudin, a specific thrombin inhibitor, was used at 1 µg/mL, n = 3 (***p < 0.001 vs. untreated, ###p < 0.001 vs. IL-10 alone, †††p < 0.001 vs. IL-10 + IL-18). (F) The total areas and lengths of tube-like structures were determined by the Matrigel tube formation assay where b.End5 were cocultured with each Mφ subset. Hirudin was used at 1 µg/mL, n = 6 (***p < 0.001, **p < 0.01 vs. untreated, ##p < 0.01, #p < 0.05 vs. IL-10 alone, †††p < 0.001 vs. IL-10 + IL-18). All data are expressed as means ± SEM and were analyzed by a one-way ANOVA followed by Tukey’s test. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29559970), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Osteopontin/OPN by Immunocytochemistry/Immunofluorescence Osteopontin (OPN) drives enhancement in macrophage (Mφ) M2 polarization and angiogenic capacity. (A) Representative images of protein expression profiles obtained by comprehensive protein array in each Mφ subset. Red arrowheads indicate OPN. (B) The mRNA expression level of Spp1 relative to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was analyzed by real-time reverse transcription polymerase chain reaction in each Mφ subset and was normalized to Mφ (–), n = 6 [***p < 0.001 vs. untreated, #p < 0.05 vs. interleukin (IL)-10 alone]. (C) The protein expression level of OPN relative to GAPDH was measured by western blotting and was normalized to Mφ (–), n = 10. Lower panels are typical images of each protein (***p < 0.001 vs. untreated, #p < 0.05 vs. IL-10 alone). (D) Representative confocal laser scanning immunofluorescence overlay images of OPN (red) and DAPI (blue) in each Mφ subset. Scale bar represents 20 µm. Images in the right row are magnified regions from white or yellow rectangles in the panels of corresponding groups. Scale bar represents 10 µm. (E) Relative mean fluorescence intensity (MFI) of CD163 was measured by FACS analysis in each Mφ subset. An anti-OPN antibody (Ab) and its isotype-matched control Ab were used at 3 µg/mL, n = 4 (***p < 0.001 vs. untreated, ##p < 0.01, #p < 0.05 vs. IL-10 alone, †††p < 0.001 vs. IL-10 + IL-18). (F) The total areas and lengths of tube-like structures were determined by the Matrigel tube formation assay where b.End5 was cocultured with each Mφ subset, n = 12 (***p < 0.001, **p < 0.01, *p < 0.05 vs. untreated, #p < 0.05 vs. IL-10 alone, †††p < 0.001 vs. IL-10 + IL-18). All data are expressed as means ± SEM and were analyzed by a one-way ANOVA followed by Tukey’s test. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29559970), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Osteopontin/OPN by Immunocytochemistry/Immunofluorescence Localized differentiation of liver progenitors in arrayed patterns.(A) Immunolabeling of BMEL cells for the biliary marker OPN and hepatocyte marker ALB on arrayed collagen I patterns with control IgG or Fc-recombinant Notch ligands DLL1, DLL4, and JAG1. (B) Quantification of OPN+ cell counts as a function of radial distance from the centroid of each island. (C) Quantification of ALB+ cell counts as a function of radial distance from the centroid of each island. (D) Immunolabeling of BMEL cells presented with DLL4 for the biliary transcription factor SOX9 and hepatocyte transcription factor HNF4A. Arrow in each image indicates the same SOX9+/HNF4A− cell. Scale bar indicates 75 µm. (E, F) Regression analysis of OPN+ and ALB+ cell counts. Data in Figure 1B and Figure 1C were separated into peripheral and central subsets for which dimensionless radius was greater than 0.75 (R>0.75) and less than 0.75 (R<0.75). Separate multiple regression models were generated for each data subset for which coefficient estimates (corresponding to mean change in cell counts) and 95% CI were plotted for OPN+ (E) and ALB+ (F) cells. For each factor, 95% CI that do not intersect with the dashed line indicate regression coefficient estimates for which P<0.05. (A, E) Scale bars indicate 150 µm.10.7554/eLife.38536.007Figure 1—source data 1.Summary table for OPN data in Figure 1B.10.7554/eLife.38536.008Figure 1—source data 2.Summary table for ALB data in Figure 1C.Summary table for OPN data in Figure 1B.Summary table for ALB data in Figure 1C.Immunolabeling and quantification of CK19.(A) Immunolabeling of BMEL cells presented with IgG for the biliary marker CK19. (B) Quantification of CK19 intensity in BMEL cells presented with IgG as a function of radial distance from the centroid of each island. Asterisk (*) indicates P<0.001 for peak intensity (R>0.9) compared with central intensity (R<0.1) using Welch’s t-test.Immunolabeling of OPN and CK19 at t=24h and cell density with radius at t=72h.(A) Immunolabeling at t=24h of BMEL cells on 30 kPa substrates presented with IgG and DLL4 for the biliary markers OPN and CK19. Scale bar indicates 75 µm. (B) Measurement of cell density with radius for 30 kPa and 4 kPa substrates at t=72h. Vertical black bars indicate computed mean radius using the most central 95% of cells within the data set.Immunolabeling for OPN with 300, 600, and 1000 µm diameter patterns.(A) Immunolabeling for OPN of BMEL cells presented with DLL4 on 30 kPa substrates at t=72h. Both 300 µm and 1000 µm pattern diameters were included in this experiment in addition to the 600 µm pattern diameter. Scale bars indicate 150 µm. (B) Quantification of peak OPN+ cell counts for 300, 600, and 1000 µm diameter pattern. Boxplots show median, 25th and 75th percentiles (hinges), and 1.5 × IQR (whiskers). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30589410), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
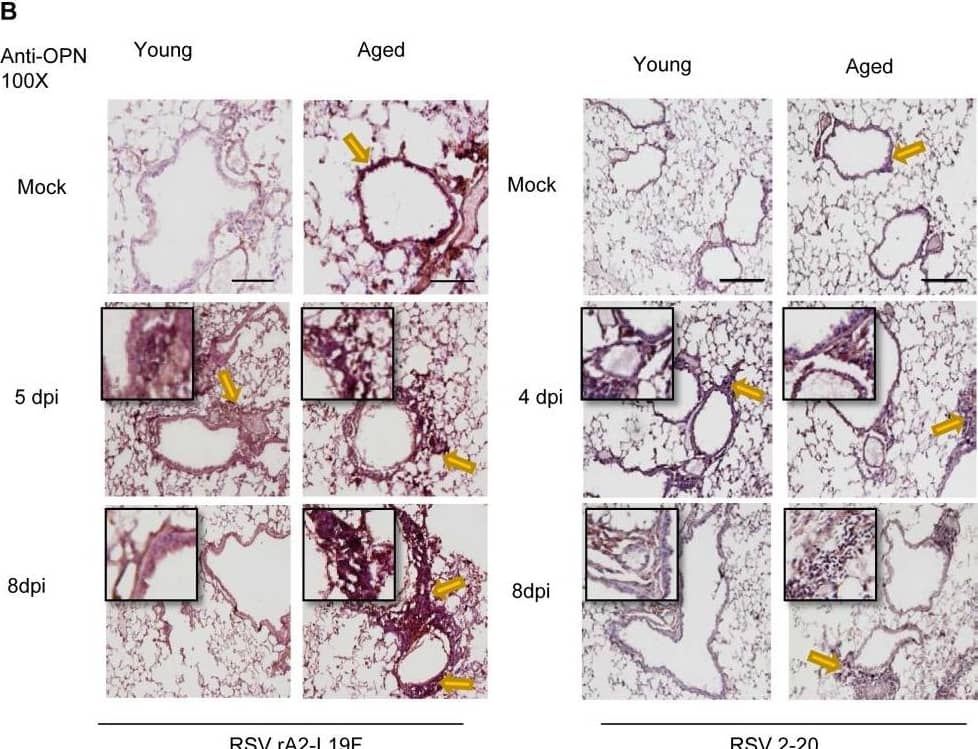
Detection of Osteopontin/OPN by Immunohistochemistry Aging results in diminished OPN production in response to 2-20 RSV infection.Young and aged BALB/c mice (n = 4/group) were intranasally infected with a dose of 106 pfu/mouse of A2 or 105 pfu/mouse of 2-20) and total lung RNA was collected on time points indicated. (A-B) RNA transcripts of OPN were analyzed with qRT-PCR and represented as a relative ratio of target gene expression to endogenous mouse HPRT and examined with rA2-L19F, 2-20, or A2. (B) 5 µm lung sections obtained at 4, 5 and 8 dpi with either rA2-L19F, 2-20, or A2 infected young and aged mice. Lung sections were immunostained for anti-mouse OPN and nickel-DAB reagent before counterstaining with hematoxylin and eosin. Representative images shown are at 100x magnification with inset [400x] displaying nickel-DAB (dark brown/black staining) positive cells and contrast the hematoxylin (light blue) nuclear stain. Representative images are shown with scale bar indicating 100 µm. (C) Enumeration of OPN-positive cells was performed with ImmunoRatio ImageJ analysis on 200X magnified lung sections from 8 dpi and values are shown as a percentage of total hematoxylin-stained cells in an individual box plot with mean interval bars. Within a single frame, at least 5 frames per mouse (n = 4/group) were collected and individual dot plots are shown of either aged or young mice with interval bars and significance, determined with ANOVA and Fisher's test (p<0.05). (D) qRT-PCR was performed on total lung RNA from young and aged 2–20 RSV infected mice for mRNA expression of OPN receptor CD44. Statistical significance was determine with ANOVA 2-way analysis with p<0.05. All experiments were performed in triplicate. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/24558422), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
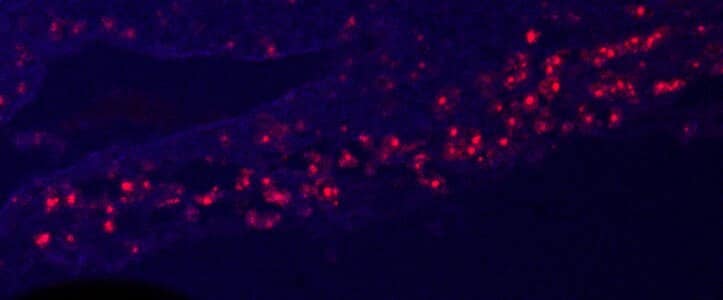
Osteopontin/OPN in Mouse Placenta. Murine placenta cryosections stained with osteopontin (red). Tissue autofluorescence in blue. Image from a verified customer review.
 View Larger
View Larger
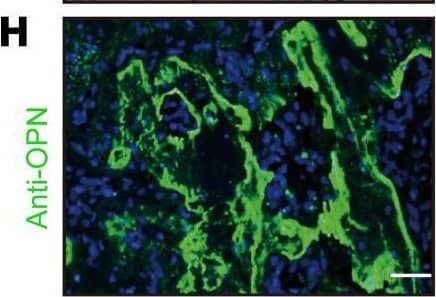
Detection of Osteopontin/OPN by Immunohistochemistry Lkb1 deletion in Ctsk-Cre–expressing cells leads to increased bone formation.(A) μCT analysis of femurs from 5-week-old female Ctsk-Ctrl and Ctsk-CKO mice. (B) Ct.Th and BV/TV of the cortical bones of femurs from 5-week-old female Ctsk-Ctrl and Ctsk-CKO mice (n = 5 for each group). (C) von Kossa staining of the tibiae from 5-week-old female Ctsk-Ctrl and Ctsk-CKO mice. Scale bar: 100 μm. (D and E) Calcein–alizarin red double-labeled fluorescence of the tibiae from 5-week-old female Ctsk-Ctrl and Ctsk-CKO mice showing MAR and BFR of the periosteum (n = 3 for each group). Scale bars: 100 μm (left); 10 μm (right). (F) Gene expression of markers for osteoblast progenitor (Runx2 and Osx), preosteoblast (Alp and Col1a1), and mature osteoblast (Opn, Bsp, and Ocn) of the cortical bones of 20-week-old female Ctsk-CKO mice (n = 3) compared with Ctsk-Ctrl mice (n = 4) tibiae. (G and H) Immunostaining of OSX (G) and OPN (H) in the tumor osteoid of Ctsk-CKO tibiae. Scale bars: 50 μm. Data are represented as mean ± SEM. *P < 0.05; **P < 0.01, unpaired Student’s t test. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/30830877), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
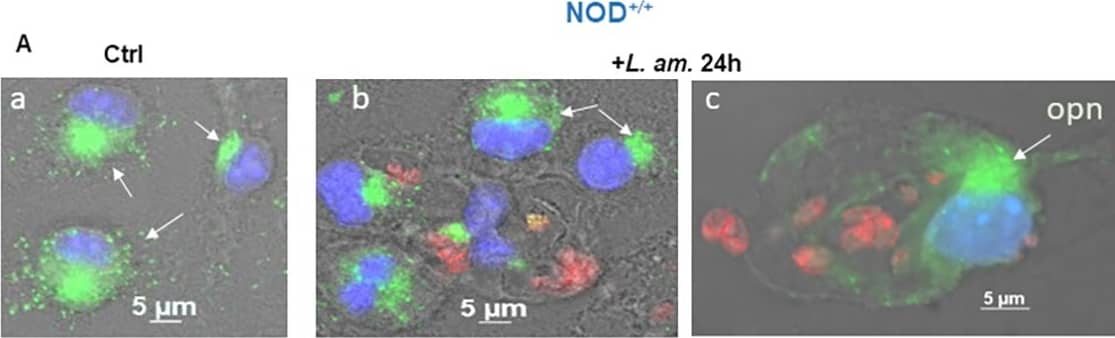
Detection of Mouse Osteopontin/OPN by Immunocytochemistry/ Immunofluorescence OPN expression in the BMF of NOD+/+ mice in the presence of Leishmania amazonensis.(A) Immunofluorescence staining of BMFs. a & d: non-infected control at 24 h and 48 h, respectively. b & c: BMFs infected with LV79 at 24 h and e & f at 48 h post-infection. Green: OPN, red: LV79; blue: nuclei. (B) qRT‒PCR of OPN transcripts (*P = 0.0294). (C) OPN protein quantification in control non-infected (ctl) and infected +LV79 BMFs. NOD ctl 24 h vs NOD+lv79 24 h (*P = 0.0454); NOD ctl 48 h vs NOD+lv79 48 h (**P = 0.0091). Two-tailed Mann‒Whitney test. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0308868), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
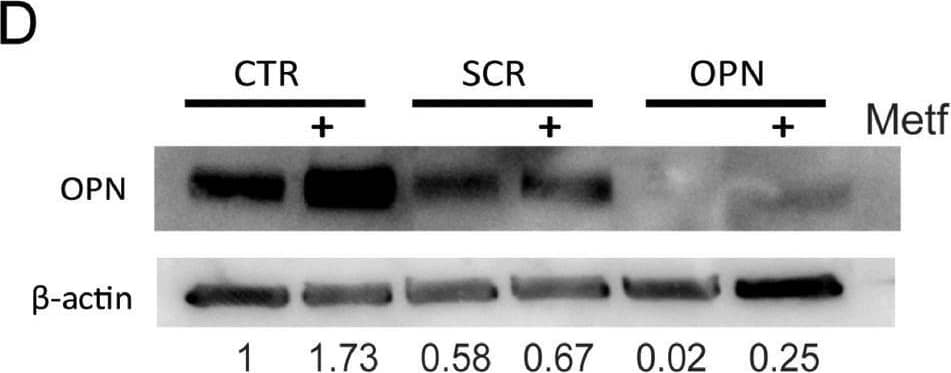
Detection of Osteopontin/OPN by Western Blot Metformin treatment increases OPN expression in preosteoblasts, contributing to an increase in adhesion of myeloma cells. (A) 2T3, MC3T3 and ST2 were treated with 5 mM metformin for 48 h and osteopontin gene expression quantified (** p < 0.01 as compared to control, n = 3–7). (B) Osteopontin protein expression following metformin treatment for 48 h in 2T3 preosteoblasts and ST2 stromal cells. (C) OPN gene expression in 2T3 preosteoblasts after transfection with either scrambled (SCR) or OPN siRNA (** p < 0.01 as compared to scrambled transfection, n = 3). (D) OPN protein expression in 2T3 preosteoblasts following transfection with OPN siRNA or scrambled control SCR and treatment with metformin. CTR = untransfected. 2T3 preosteoblasts (scrambled control (SCR) or osteopontin siRNA (OPN)) were pretreated with metformin and adhesion imaged and quantified. (E) Representative image and quantification of specific image. (F) Quantification of myeloma cell adhesion (* p < 0.05 as compared to control, n = 4). Data are presented as mean ± SEM. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/34890968), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
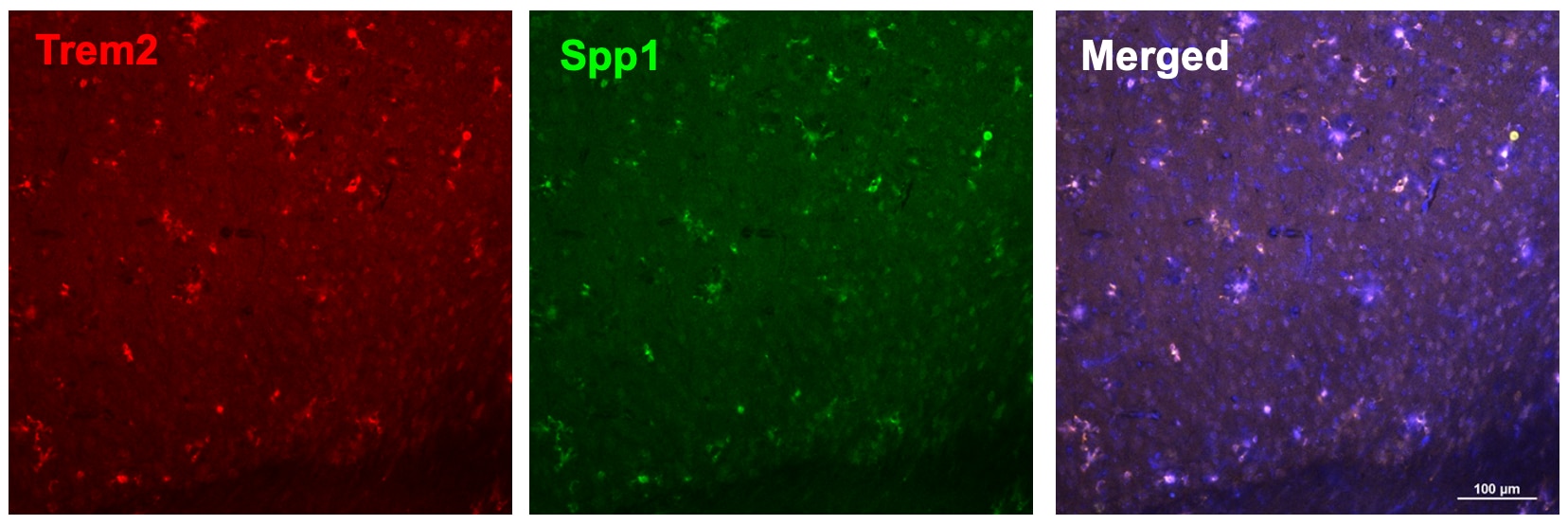
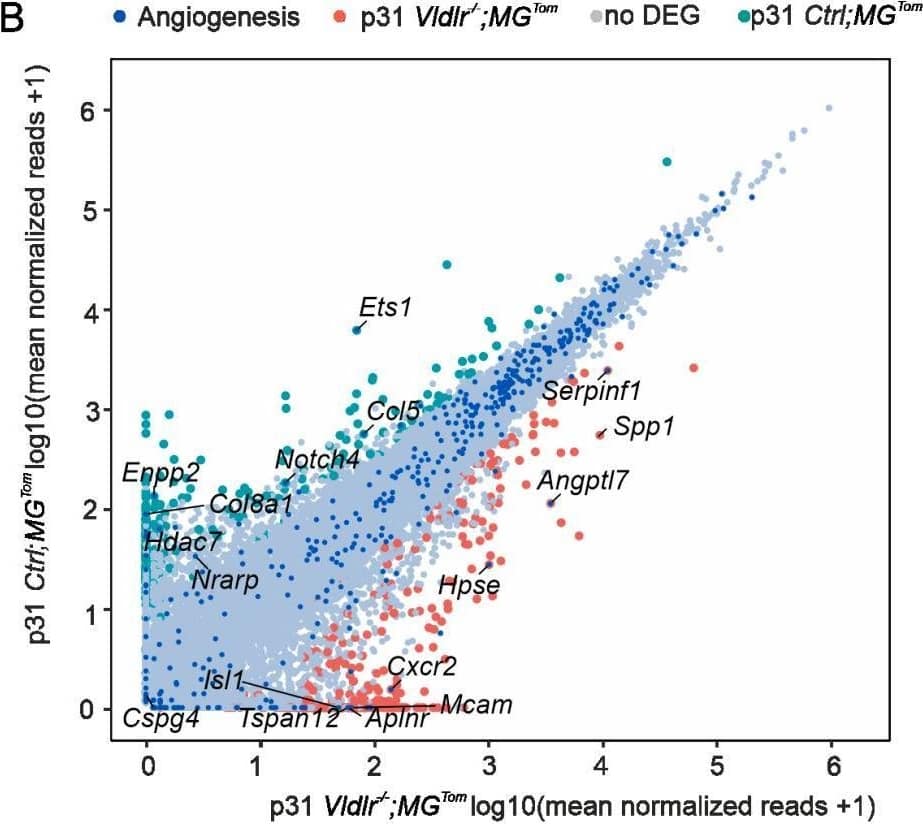
Detection of Osteopontin/OPN by Flow Cytometry Angiogenic potential of retinal microglia during RAP. (A,B) Readplot using the log2 transformation of normalized reads visualizing differentially expressed genes between microglia from Ctrl; MGTom mice (y-axis) and Vldlr−/−; MGTom mice (x-axis) on postnatal day (p)17 (A) and p31 (B). Genes associated with angiogenesis are labeled blue. (C,D) Comparison of RNA Seq results of isolated microglia and protein analysis of retinal tissue lysates from Vldlr−/−; MGTom and Ctrl; MGTom mice at postnatal day (p)17 and p31 for Vegf (C) and Ccl12 (D). mRNA: n = 5–7 samples per group, protein n = 5 samples per group. (E,F) Intravitreal injection of anti-SPP1 ((E), n = 5) or anti-CCL12 ((F), n = 10) in comparison to an IgG injection (*, p < 0.05; **, p < 0.01; ***, p < 0.001). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35408803), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
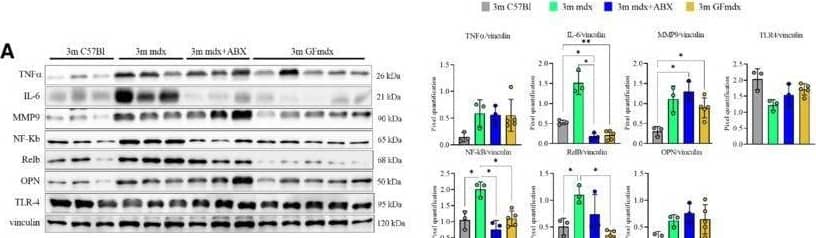
Detection of Osteopontin/OPN by Western Blot Gene and protein expression in muscles from 3m mdx, mdx+ABX, and GFmdxA–KCropped images of representative WB and RT‐qPCR analysis of TA muscle of 3m mdx (n = 3/4), 3m mdx+ABX (n = 3/4), and 3m GFmdx (n = 5) showing the expression of the proteins specifically involved in inflammation/fibrosis (A), skeletal muscle metabolism (B–E), mitochondrial biogenesis (F and G), calcium conducting channels (H and I), autophagy (J), and nicotinic acetylcholine receptors (K). Densitometric data were normalized on vinculin and expressed as mean ± SD. Data are presented as mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001; ****P < 0.0001, ordinary one‐way ANOVA, Tukey's multiple‐comparison test for WB and non‐parametric test followed by Kruskal–Wallis test for RT‐qPCR).Source data are available online for this figure. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36533294), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
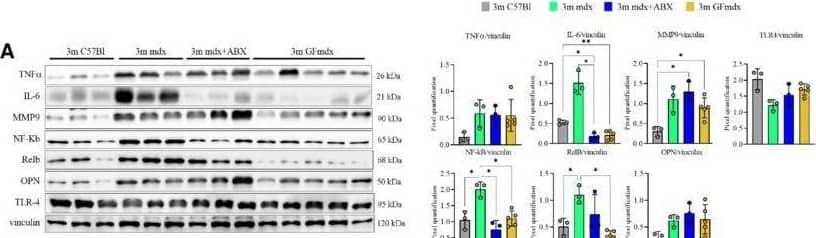
Detection of Osteopontin/OPN by Western Blot Gene and protein expression in muscles from 3m mdx, mdx+ABX, and GFmdxA–KCropped images of representative WB and RT‐qPCR analysis of TA muscle of 3m mdx (n = 3/4), 3m mdx+ABX (n = 3/4), and 3m GFmdx (n = 5) showing the expression of the proteins specifically involved in inflammation/fibrosis (A), skeletal muscle metabolism (B–E), mitochondrial biogenesis (F and G), calcium conducting channels (H and I), autophagy (J), and nicotinic acetylcholine receptors (K). Densitometric data were normalized on vinculin and expressed as mean ± SD. Data are presented as mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001; ****P < 0.0001, ordinary one‐way ANOVA, Tukey's multiple‐comparison test for WB and non‐parametric test followed by Kruskal–Wallis test for RT‐qPCR).Source data are available online for this figure. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36533294), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
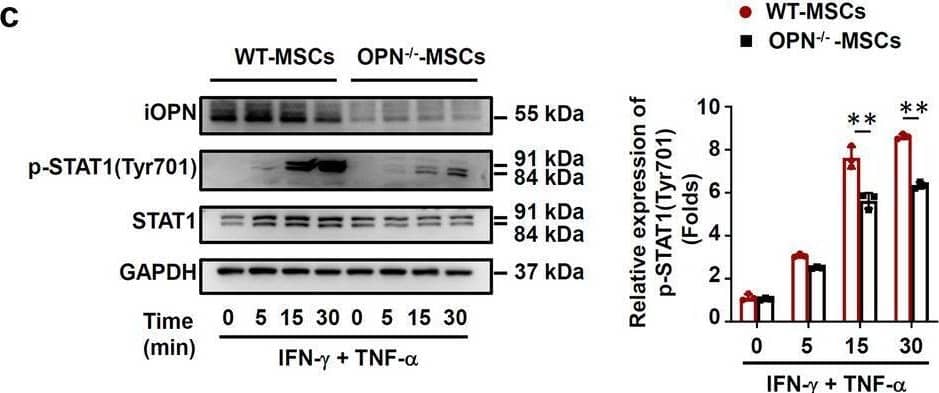
Detection of Mouse Osteopontin/OPN by Western Blot iOPN promoted STAT1-mediated immunosuppression in MSCs. a–d Vector-MSCs and iOPN-MSCs or WT-MSCs and OPN−/−-MSCs were treated with or without TNF-alpha plus IFN-gamma (10 ng/mL) for the indicated times. Cells were harvested, and OPN, NF-kappa B p65, STAT1, IKB alpha, phosphorylation of NF-kappa B p65, phosphorylation of STAT1 at Tyr701 and phosphorylation of IKB alpha were analyzed by immunoblotting analysis. Full-length blots are presented in Additional file 1: Fig. 5a-d. e–g Vector-MSCs and iOPN-MSCs were stimulated with TNF-alpha plus IFN-gamma (10 ng/mL) for 12 h. The STAT1 inhibitor fludarabine (Flu, 2 μM) was then added to the culture medium of Vector-MSCs and iOPN-MSCs. The expression of iNOS was determined by quantitative real-time PCR (e), immunoblotting analysis (f) and flow cytometry (g). Full-length blots are presented in Additional file 1: Fig. 5f. h Vector-MSCs and iOPN-MSCs were pretreated with DMSO or fludarabine (Flu, 2 μM) for 6 h and then irradiated and cocultured with CFSE-labeled splenocytes activated by anti-CD3/CD28 antibodies for 3 days at a ratio of 1:20. CD4+ T cells and CD8+ T cells were stained for proliferation analysis by flow cytometry at the end of coculture, and the percentages of proliferating T cells are shown. The results are representative of three to six independent experiments. Values are shown as the mean ± SEM and statistical significance is indicated as *P < 0.05, **P < 0.01 and ***P < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/39407354), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
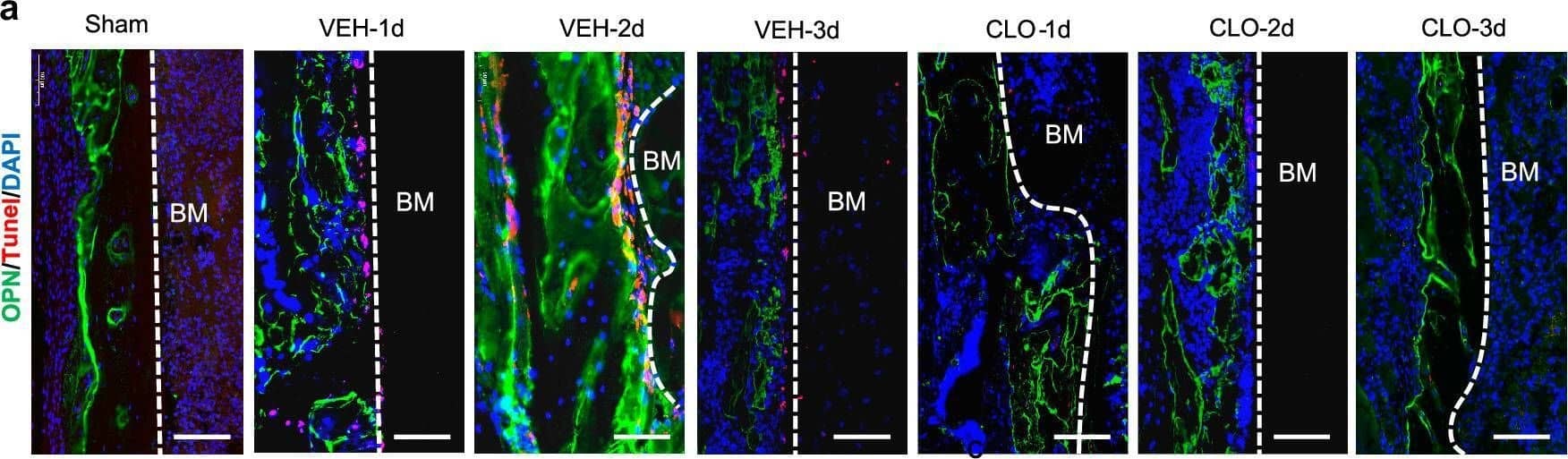
Detection of Mouse Osteopontin/OPN by Immunohistochemistry Lymphatic drainage supports osteoblast survival and BMSC proliferation. A Representative immunofluorescence staining on fracture sides using an anti-OPN antibody to label OBs (green) and an anti-tunel antibody for cell apoptosis (red). Scale bars, 50 um. b Quantitative analysis of OBs apoptosis at fracture sides (n = 5/group, one-way ANOVA). c Scheme of the experimental procedure in vitro for OBs and BMSCs treated by bone marrow or hematoma CM. Bone marrow of sham group and hematoma of fractured group were collected to generate hematoma CM. Rat BMSCs and OBs were cultured with hematoma CM for 24 h and subjected to growth and proliferation analyses. The scheme was modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 4.0 Generic License (https://creativecommons.org/licenses/by/4.0/). d Rat OBs were intervened with hematoma CM for 1 h and OB apoptosis was evaluated by flow cytometry with FITC-Annexin V and PI double staining (n = 5/group, one-way ANOVA). e Rat OBs were cultured with hematoma CM for 1 h and the growth of rat OBs was observed under a microscope. Scale bars, 50 um. f The cell count of rat OBs cultured with hematoma CM for 24 h. g Rat OBs were cultured with hematoma CM for 24 h and the cell proliferation was evaluated using a CCK8 kit. h Rat BMSCs were cultured with hematoma CM for 1 h and the growth of rat BMSCs was observed under a microscope. Scale bars, 50 um. i The cell count of rat BMSCs cultured with hematoma CM for 24 h. j Rat BMSCs, were cultured with hematoma CM for 24 h and the cell proliferation was evaluated using a CCK8 kit. Data are means ± SD. In f,g,i and j, n = 3 wells with biological replicates in the control, sham, VEH and CLO groups, one-way ANOVA. Source data are provided as a Source Data file. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/39827193), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Osteopontin/OPN
Osteopontin (OPN, previously also referred to as transformation-associated secreted phosphoprotein, bone sialoprotein I, 2ar, 2B7, early T lymphocyte activation 1 protein, minopotin, calcium oxalate crystal growth inhibitor protein), is a secreted, highly acidic, calcium-binding, RGD-containing, phosphorylated glycoprotein originally isolated from bone matrix. Subsequently, OPN has been found in kidney, placenta, blood vessels and various tumor tissues. Many cell types (including macrophages, osteoclasts, activated T cells, fibroblasts, epithelial cells, vascular smooth muscle cells, and natural killer cells) can express OPN in response to activation by cytokines, growth factors or inflammatory mediators. Elevated expression of OPN has also been associated with numerous pathobiological conditions such as atherosclerotic plaques, renal tubulointerstitial fibrosis, granuloma formations in tuberculosis and silicosis, neointimal formation associated with balloon catheterization, metastasizing tumors, and cerebral ischemia. Mouse OPN cDNA encodes a 294 amino acid (aa) residue precursor protein with a 16 aa residue predicted signal peptide that is cleaved to yield a 278 aa residue mature protein with an integrin binding sequence (RGD), and N- and O-glycosylation sites. OPN has been shown to bind to different cell types through RGD-mediated interaction with the integrins alpha v beta 1, alpha v beta 3, alpha v beta 5, and non-RGD-mediated interaction with CD44 and the integrins alpha 8 beta 1 or alpha 9 beta 1. Functionally, OPN is chemotactic for macrophages, smooth muscle cells, endothelial cells and glial cells. OPN has also been shown to inhibit nitric oxide production and cytotoxicity by activated macrophages. Human, mouse, rat, pig and bovine OPN share from approximately 40-80% amino acid sequence identity. Osteopontin is a substrate for proteolytic cleavage by thrombin, enterokinase, MMP-3 and MMP-7. The functions of OPN in a variety of cell types were shown to be modified as a result of proteolytic cleavage (2, 3).
- Ann. N.Y. Acad. Sci., vol. 760, 1995, Apr. 21.
- Senger, D.R. et al. (1996) Biochim. Biophys. Acta. 1314:13.
- Agnihotri, R. et al. (2001) J. Biol. Chem. 276:28261.
Product Datasheets
Citations for Mouse Osteopontin/OPN Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
216
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Impaired disassembly of the axon initial segment restricts mitochondrial entry into damaged axons
Authors: Kiryu-Seo S, Matsushita R, Tashiro Y et al.
The EMBO journal
-
Stress-Induced Changes in Bone Marrow Stromal Cell Populations Revealed through Single-Cell Protein Expression Mapping
Authors: N Severe, NM Karabacak, K Gustafsson, N Baryawno, G Courties, Y Kfoury, KD Kokkaliari, C Rhee, D Lee, EW Scadden, JE Garcia-Rob, T Brouse, M Nahrendorf, M Toner, DT Scadden
Cell Stem Cell, 2019-07-03;0(0):.
-
Histone H2A ubiquitination resulting from Brap loss of function connects multiple aging hallmarks and accelerates neurodegeneration
Authors: Guo Y, Chomiak AA, Hong Y et al.
iScience
-
A rapidly fabricated bone marrow enrichment material with a controllable pore size promotes osteogenesis close to autogenous bone graft in clinic
Authors: Yang, Q;Yu, B;Liu, C;Zhou, J;Zhang, Y;Yang, M;He, S;Cai, J;Dai, Q;Tang, Z;Xu, J;Zhang, Z;Hou, T;
Journal of advanced research
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Piezo1 balances the osteogenic-tenogenic plasticity of periosteal progenitor cells through the YAP pathway
Authors: Wang, L;Ren, Q;Chen, S;Lou, L;Hu, X;Xing, W;Suo, J;Sun, J;Greenblatt, MB;Feng, H;Zou, W;
Cell reports
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Overcoming myeloid-driven resistance to CAR T therapy by targeting SPP1
Authors: Gholamin, S;Natri, HM;Zhao, Y;Xu, S;Aftabizadeh, M;Comin-Anduix, B;Saravanakumar, S;Masia, C;Wong, RA;Peter, L;Chung, MI;Mee, ED;Aguilar, B;Starr, R;Torrejon, DY;Alizadeh, D;Wu, X;Kalbasi, A;Ribas, A;Forman, S;Badie, B;Banovich, NE;Brown, CE;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Sox9 inhibits Activin A to promote biliary maturation and branching morphogenesis
Authors: Hrncir, HR;Goodloe, B;Bombin, S;Hogan, CB;Jadi, O;Gracz, AD;
Nature communications
Species: Mouse
Sample Types: Organoid
Applications: Immunohistochemistry -
Cognitive decline and neuroinflammation in a mouse model of obesity: An accelerating role of ageing
Authors: Rajput, M;Malik, IA;Methi, A;Cortés Silva, JA;Fey, D;Wirths, O;Fischer, A;Wilting, J;von Arnim, CAF;
Brain, behavior, and immunity
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Molecular and spatial analysis of ganglion cells on retinal flatmounts: diversity, topography, and perivascularity
Authors: Tsai, NY;Nimkar, K;Zhao, M;Lum, MR;Yi, Y;Garrett, TR;Wang, Y;Toma, K;Caval-Holme, F;Reddy, N;Ehrlich, AT;Kriegstein, AR;Do, MTH;Sivyer, B;Shekhar, K;Duan, X;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
The pivotal role of osteopontin in UV-induced skin inflammation in a mouse model
Authors: Kim, H;Shin, CY;Park, CH;Lee, DH;Lee, SH;Chung, JH;
Open biology
Species: Mouse
Sample Types: Tissue Homogenates
Applications: ELISA Capture -
Osteopontin is a therapeutic target that drives breast cancer recurrence
Authors: Gu, Y;Taifour, T;Bui, T;Zuo, D;Pacis, A;Poirier, A;Attalla, S;Fortier, AM;Sanguin-Gendreau, V;Pan, TC;Papavasiliou, V;Lin, NU;Hughes, ME;Smith, K;Park, M;Tremblay, ML;Chodosh, LA;Jeselsohn, R;Muller, WJ;
Nature communications
Species: Transgenic Mouse
Sample Types: In Vivo
Applications: Neutralization -
Contributions of mirror-image hair cell orientation to mouse otolith organ and zebrafish neuromast function
Authors: Ono, K;Jarysta, A;Hughes, NC;Jukic, A;Chang, HHV;Deans, MR;Eatock, RA;Cullen, KE;Kindt, K;Tarchini, B;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Osteopontin deletion attenuates cyst growth but exacerbates fibrosis in mice with cystic kidney disease
Authors: Jansson, KP;Kuluva, J;Zhang, S;Swanson, T;Zhang, Y;Zimmerman, KA;Fields, TA;Wallace, DP;Rowe, PS;Stubbs, JR;
Physiological reports
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
CD36+ pro-inflammatory macrophages interact with ZCCHC12+ tumor cells in papillary thyroid cancer promoting tumor progression and recurrence
Authors: Zhang, X;Guo, L;Tian, W;Yang, Y;Yin, Y;Qiu, Y;Wang, W;Li, Y;Zhang, G;Zhao, X;Wang, G;Lin, Z;Yang, M;Zhao, W;Lu, D;
Cancer immunology research
Species: Human
Sample Types: Cell Culture Supernates, Whole Tissue
Applications: Immunohistochemistry, Western Blot -
Modulating amacrine cell-derived dopamine signaling promotes optic nerve regeneration and preserves visual function
Authors: Zhang, Q;Xue, J;Tang, J;Wu, S;Liu, Z;Wu, C;Liu, C;Liu, Y;Lin, J;Han, J;Liu, L;Chen, Y;Yang, J;Li, Z;Zhao, L;Wei, Y;Li, Y;Zhuo, Y;
Science advances
Species: Mouse, Transgenic Mouse
Sample Types: Whole Cells
Applications: Immunohistochemistry -
Spike desensitisation as a mechanism for high-contrast selectivity in retinal ganglion cells
Authors: Chang, L;Ran, Y;Yang, M;Auferkorte, O;Butz, E;Hüser, L;Haverkamp, S;Euler, T;Schubert, T;
Frontiers in cellular neuroscience
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Mitochondrial reverse electron transport in myeloid cells perpetuates neuroinflammation
Authors: Peruzzotti-Jametti, L;Willis, CM;Hamel, R;Krzak, G;Reisz, JA;Prag, HA;Wu, V;Xiang, Y;van den Bosch, AMR;Nicaise, AM;Roth, L;Bates, GR;Huang, H;Vincent, AE;Frezza, C;Viscomi, C;Marioni, JC;D'Alessandro, A;Takats, Z;Murphy, MP;Pluchino, S;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
A transcriptomic taxonomy of mouse brain-wide spinal projecting neurons
Authors: Winter, CC;Jacobi, A;Su, J;Chung, L;van Velthoven, CTJ;Yao, Z;Lee, C;Zhang, Z;Yu, S;Gao, K;Duque Salazar, G;Kegeles, E;Zhang, Y;Tomihiro, MC;Zhang, Y;Yang, Z;Zhu, J;Tang, J;Song, X;Donahue, RJ;Wang, Q;McMillen, D;Kunst, M;Wang, N;Smith, KA;Romero, GE;Frank, MM;Krol, A;Kawaguchi, R;Geschwind, DH;Feng, G;Goodrich, LV;Liu, Y;Tasic, B;Zeng, H;He, Z;
Nature
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Multisensory gaze stabilization in response to subchronic alteration of vestibular type I hair cells
Authors: Schenberg, L;Palou, A;Simon, F;Bonnard, T;Barton, CE;Fricker, D;Tagliabue, M;Llorens, J;Beraneck, M;
eLife
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Bioactive glasses promote rapid pre-osteoblastic cell migration in contrast to hydroxyapatite, while carbonated apatite shows migration inhibiting properties
Authors: Kajander, K;Sirkiä, SV;Vallittu, PK;Heino, TJ;Määttä, JA;
Scientific reports
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Lymphatic platelet thrombosis limits bone repair by precluding lymphatic transporting DAMPs
Authors: Wang, YJ;Zheng, Y;Cong, L;Wang, P;Zhao, L;Xing, L;Liu, J;Xu, H;Li, N;Zhao, Y;Shi, Q;Liang, Q;
Research square
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Decoding muscle-resident Schwann cell dynamics during neuromuscular junction remodeling
Authors: Guzman, SD;Abu-Mahfouz, A;Davis, CS;Ruiz, LP;Macpherson, PC;Brooks, SV;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
TM4SF5-mediated abnormal food-intake behavior and apelin expression facilitate non-alcoholic fatty liver disease features
Authors: Yangie Dwi Pinanga, Han Ah Lee, Eun-Ae Shin, Haesong Lee, Kyung-hee Pyo, Ji Eon Kim et al.
iScience
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Coordination between ECM and cell-cell adhesion regulates the development of islet aggregation, architecture, and functional maturation
Authors: Tixi W, Maldonado M, Chang YT et al.
eLife
-
Osteopontin drives retinal ganglion cell resiliency in glaucomatous optic neuropathy
Authors: Zhao, M;Toma, K;Kinde, B;Li, L;Patel, AK;Wu, KY;Lum, MR;Tan, C;Hooper, JE;Kriegstein, AR;La Torre, A;Liao, YJ;Welsbie, DS;Hu, Y;Han, Y;Duan, X;
Cell reports
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Different niches for stem cells carrying the same oncogenic driver affect pathogenesis and therapy response in myeloproliferative neoplasms
Authors: Elodie Grockowiak, Claudia Korn, Justyna Rak, Veronika Lysenko, Adrien Hallou, Francesca M. Panvini et al.
Nature Cancer
-
Signalling by senescent melanocytes hyperactivates hair growth
Authors: Wang, X;Ramos, R;Phan, AQ;Yamaga, K;Flesher, JL;Jiang, S;Oh, JW;Jin, S;Jahid, S;Kuan, CH;Nguyen, TK;Liang, HY;Shettigar, NU;Hou, R;Tran, KH;Nguyen, A;Vu, KN;Phung, JL;Ingal, JP;Levitt, KM;Cao, X;Liu, Y;Deng, Z;Taguchi, N;Scarfone, VM;Wang, G;Paolilli, KN;Wang, X;Guerrero-Juarez, CF;Davis, RT;Greenberg, EN;Ruiz-Vega, R;Vasudeva, P;Murad, R;Widyastuti, LHP;Lee, HL;McElwee, KJ;Gadeau, AP;Lawson, DA;Andersen, B;Mortazavi, A;Yu, Z;Nie, Q;Kunisada, T;Karin, M;Tuckermann, J;Esko, JD;Ganesan, AK;Li, J;Plikus, MV;
Nature
Species: Transgenic Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: Immunohistochemistry, Western Blot -
Interleukin-15 deficient rats have reduced osteopontin at the maternal-fetal interface
Authors: Kelly J. Baines, Michelle S. Klausner, Violet S. Patterson, Stephen J. Renaud
Frontiers in Cell and Developmental Biology
-
Peroxisomal defects in microglial cells induce a disease-associated microglial signature
Authors: Quentin Raas, Ali Tawbeh, Mounia Tahri-Joutey, Catherine Gondcaille, Céline Keime, Romain Kaiser et al.
Frontiers in Molecular Neuroscience
-
NOX4 as a critical effector mediating neuroinflammatory cytokines, myeloperoxidase and osteopontin, specifically in astrocytes in the hippocampus in Parkinson's disease
Authors: N Boonpraman, S Yoon, CY Kim, JS Moon, SS Yi
Redox Biology, 2023-04-10;62(0):102698.
Species: Mouse
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Hepatic lipid overload triggers biliary epithelial cell activation via E2Fs
Authors: E Yildiz, G El Alam, A Perino, A Jalil, PD Denechaud, K Huber, L Fajas, J Auwerx, G Sorrentino, K Schoonjans
Elife, 2023-03-06;12(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Kupffer-cell-derived IL-6 is repurposed for hepatocyte dedifferentiation via activating progenitor genes from injury-specific enhancers
Authors: L Li, L Cui, P Lin, Z Liu, S Bao, X Ma, H Nan, W Zhu, J Cen, Y Mao, X Ma, L Jiang, Y Nie, F Ginhoux, Y Li, H Li, L Hui
Cell Stem Cell, 2023-02-13;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer's disease
Authors: S De Scheppe, JZ Ge, G Crowley, LSS Ferreira, D Garceau, CE Toomey, D Sokolova, J Rueda-Carr, SH Shin, JS Kim, T Childs, T Lashley, JJ Burden, M Sasner, C Sala Frige, S Jung, S Hong
Nature Neuroscience, 2023-02-06;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Definition of the contribution of an Osteopontin-producing CD11c+ microglial subset to Alzheimer's disease
Authors: Y Qiu, X Shen, O Ravid, D Atrakchi, D Rand, AE Wight, HJ Kim, S Liraz-Zalt, I Cooper, M Schnaider, H Cantor
Proceedings of the National Academy of Sciences of the United States of America, 2023-02-02;120(6):e2218915120.
Species: Mouse
Sample Types: Recombinant Protein, Whole Tissue
Applications: Bioassay, IHC -
Vitamin D Receptor Expression Limits the Angiogenic and Inflammatory Properties of Retinal Endothelial Cells
Authors: YS Song, N Jamali, CM Sorenson, N Sheibani
Cells, 2023-01-16;12(2):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Microglia-Derived Spp1 Promotes Pathological Retinal Neovascularization via Activating Endothelial Kit/Akt/mTOR Signaling
Authors: Q Bai, X Wang, H Yan, L Wen, Z Zhou, Y Ye, Y Jing, Y Niu, L Wang, Z Zhang, J Su, T Chang, G Dou, Y Wang, J Sun
Journal of personalized medicine, 2023-01-11;13(1):.
Species: Mouse
Sample Types: In Vivo, Whole Tissue
Applications: IHC, Neutralization -
Single cell RNA sequencing analysis of mouse cochlear supporting cell transcriptomes with activated ERBB2 receptor indicates a cell-specific response that promotes CD44 activation
Authors: D Piekna-Prz, D Na, J Zhang, C Baker, JM Ashton, PM White
Frontiers in Cellular Neuroscience, 2023-01-06;16(0):1096872.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Secreted phosphoprotein 1 slows neurodegeneration and rescues visual function in mouse models of aging and glaucoma
Authors: S Li, TC Jakobs
Cell Reports, 2022-12-27;41(13):111880.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Human Recombinant Lactoferrin Promotes Differentiation and Calcification on MC3T3-E1 Cells
Authors: D Nagashima, Y Ishibashi, S Kawaguchi, M Furukawa, M Toho, M Ohno, T Nitto, N Izumo
Pharmaceutics, 2022-12-25;15(1):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC/IF -
Discoidin domain receptor 2 regulates aberrant mesenchymal lineage cell fate and matrix organization
Authors: CA Pagani, AC Bancroft, RJ Tower, N Livingston, Y Sun, JY Hong, RN Kent, AL Strong, JH Nunez, JMR Medrano, N Patel, BA Nanes, KM Dean, Z Li, C Ge, BM Baker, AW James, SJ Weiss, RT Franceschi, B Levi
Science Advances, 2022-12-21;8(51):eabq6152.
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Anti-osteopontin therapy leads to improved edema and infarct size in a murine model of ischemic stroke
Authors: D Spitzer, T Puetz, M Armbrust, M Dunst, J Macas, F Croll, KH Plate, Y Reiss, S Liebner, PN Harter, S Guérit, K Devraj
Scientific Reports, 2022-12-03;12(1):20925.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Cell Culture, In Vivo -
cis interaction of CD153 with TCR/CD3 is crucial for the pathogenic activation of senescence-associated T�cells
Authors: Y Fukushima, K Sakamoto, M Matsuda, Y Yoshikai, H Yagita, D Kitamura, M Chihara, N Minato, M Hattori
Cell Reports, 2022-09-20;40(12):111373.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Transcriptional dynamics of murine motor neuron maturation in vivo and in vitro
Authors: T Patel, J Hammelman, S Aziz, S Jang, M Closser, TL Michaels, JA Blum, DK Gifford, H Wichterle
Nature Communications, 2022-09-15;13(1):5427.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC/IF -
Regulating microglial miR-155 transcriptional phenotype alleviates Alzheimer's-induced retinal vasculopathy by limiting Clec7a/Galectin-3+ neurodegenerative microglia
Authors: H Shi, Z Yin, Y Koronyo, DT Fuchs, J Sheyn, MR Davis, JW Wilson, MA Margeta, KM Pitts, S Herron, S Ikezu, T Ikezu, SL Graham, VK Gupta, KL Black, M Mirzaei, O Butovsky, M Koronyo-Ha
Acta neuropathologica communications, 2022-09-08;10(1):136.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Cilostazol Attenuates AngII-Induced Cardiac Fibrosis in apoE Deficient Mice
Authors: Y Hada, HA Uchida, R Umebayashi, M Yoshida, J Wada
International Journal of Molecular Sciences, 2022-08-13;23(16):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
TET enzymes regulate skeletal development through increasing chromatin accessibility of RUNX2 target genes
Authors: L Wang, X You, D Ruan, R Shao, HQ Dai, W Shen, GL Xu, W Liu, W Zou
Nature Communications, 2022-08-11;13(1):4709.
Species: Mouse
Sample Types: Whole Tissue
Applications: IF -
Profiling the neurovascular unit unveils detrimental effects of osteopontin on the blood–brain barrier in acute ischemic stroke
Authors: Daniel Spitzer, Sylvaine Guérit, Tim Puetz, Maryam I. Khel, Moritz Armbrust, Maika Dunst et al.
Acta Neuropathologica
-
INZ‐701, a recombinant ENPP1 enzyme, prevents ectopic calcification in an Abcc6−/− mouse model of pseudoxanthoma elasticum
Authors: Ida Joely Jacobs, Zhiliang Cheng, Douglas Ralph, Kevin O'Brien, Lisa Flaman, Jennifer Howe et al.
Experimental Dermatology
-
Intermittent parathyroid hormone increases stability and improves osseointegration of initially unstable implants
Authors: Kevin Staats, Branden R. Sosa, Emile-Victor Kuyl, Yingzhen Niu, Vincentius Suhardi, Kathleen Turajane et al.
Bone & Joint Research
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Loss of mouse Stmn2 function causes motor neuropathy
Authors: Irune Guerra Guerra San Juan, Leslie A. Nash, Kevin S. Smith, Marcel F. Leyton-Jaimes, Menglu Qian, Joseph R. Klim et al.
Neuron
-
Vertebrate lonesome kinase modulates the hepatocyte secretome to prevent perivascular liver fibrosis and inflammation
Authors: S Pantasis, J Friemel, SM Brütsch, Z Hu, S Krautbauer, G Liebisch, J Dengjel, A Weber, S Werner, MR Bordoli
Journal of Cell Science, 2022-04-12;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Orchestration of energy metabolism and osteogenesis by Mg2+ facilitates low-dose BMP-2-driven regeneration
Authors: S Lin, S Yin, J Shi, G Yang, X Wen, W Zhang, M Zhou, X Jiang
Bioactive materials, 2022-03-24;18(0):116-127.
Species: Rat
Sample Types: Whole Cells
Applications: ICC -
Transcriptional and Distributional Profiling of Microglia in Retinal Angiomatous Proliferation
Authors: A Schlecht, J Wolf, S Boneva, G Prinz, BM Braunger, P Wieghofer, H Agostini, G Schlunck, C Lange
International Journal of Molecular Sciences, 2022-03-22;23(7):.
Species: Mouse
Sample Types: In Vivo
-
Identification of kidney injury released circulating osteopontin as causal agent of respiratory failure
Authors: FZ Khamissi, L Ning, E Kefaloyian, H Dun, A Arthanaris, A Keller, JJ Atkinson, W Li, B Wong, S Dietmann, K Lavine, D Kreisel, A Herrlich
Science Advances, 2022-02-25;8(8):eabm5900.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Hypertrophic chondrocytes serve as a reservoir for marrow-associated skeletal stem and progenitor cells, osteoblasts, and adipocytes during skeletal development
Authors: Jason T Long, Abigail Leinroth, Yihan Liao, Yinshi Ren, Anthony J Mirando, Tuyet Nguyen et al.
eLife
-
Induction of osteogenesis by bone-targeted Notch activation
Authors: C Xu, VV Dinh, K Kruse, HW Jeong, EC Watson, S Adams, F Berkenfeld, M Stehling, SJ Rasouli, R Fan, R Chen, I Bedzhov, Q Chen, K Kato, ME Pitulescu, RH Adams
Elife, 2022-02-04;11(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Increased expression of osteopontin in subchondral bone promotes bone turnover and remodeling, and accelerates the progression of OA in a mouse model
Authors: Chuangxin Lin, Zhong Chen, Dong Guo, Laixi Zhou, Sipeng Lin, Changchuan Li et al.
Aging (Albany NY)
-
Secreted osteopontin from CD4+ T�cells limits acute graft-versus-host disease
Authors: N Aggarwal, ME Deerhake, D DiPalma, SK Shahi, MR Gaggioli, AK Mangalam, ML Shinohara
Cell Reports, 2021-12-28;37(13):110170.
Species: Mouse, Transgenic Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Capture -
STAT3 is critical for skeletal development and bone homeostasis by regulating osteogenesis
Authors: S Zhou, Q Dai, X Huang, A Jin, Y Yang, X Gong, H Xu, X Gao, L Jiang
Nature Communications, 2021-11-25;12(1):6891.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Osteopontin Deficiency Ameliorates Prostatic Fibrosis and Inflammation
Authors: P Popovics, A Jain, KO Skalitzky, E Schroeder, H Ruetten, M Cadena, KS Uchtmann, CM Vezina, WA Ricke
International Journal of Molecular Sciences, 2021-11-18;22(22):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Serotonin transporter-mediated molecular axis regulates regional retinal ganglion cell vulnerability and axon regeneration after nerve injury
Authors: R Kingston, D Amin, S Misra, JM Gross, T Kuwajima
PloS Genetics, 2021-11-04;17(11):e1009885.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Development and Characterization of Alkaline Phosphatase-Positive Human Umbilical Cord Perivascular Cells
Authors: S Nonoyama, T Karakida, R Chiba-Ohku, R Yamamoto, Y Ujiie, T Nagano, Y Yamakoshi, K Gomi
Cells, 2021-11-04;10(11):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
7,8-Dihydroxiflavone Maintains Retinal Functionality and Protects Various Types of RGCs in Adult Rats with Optic Nerve Transection
Authors: Alejandro Gallego-Ortega, Beatriz Vidal-Villegas, María Norte-Muñoz, Manuel Salinas-Navarro, Marcelino Avilés-Trigueros, María Paz Villegas-Pérez et al.
International Journal of Molecular Sciences
-
Creation of X-linked Alport syndrome rat model with Col4a5 deficiency
Authors: M Namba, T Kobayashi, M Kohno, T Koyano, T Hirose, M Fukushima, M Matsuyama
Scientific Reports, 2021-10-21;11(1):20836.
Species: Rat
Sample Types: Tissue Lysates
Applications: Western Blot -
Quantitative Expression of Key Cancer Markers in the AS-30D Hepatocarcinoma Model
Authors: Marco A. Briones-Orta, Blanca Delgado-Coello, Roxana Gutiérrez-Vidal, Marcela Sosa-Garrocho, Marina Macías-Silva, Jaime Mas-Oliva
Frontiers in Oncology
-
IER2-induced senescence drives melanoma invasion through osteopontin
Authors: L Kyjacova, R Saup, K Rönsch, S Wallbaum, S Dukowic-Sc, A Foss, SD Scherer, M Rothley, A Neeb, N Grau, W Thiele, S Thaler, N Cremers, C Sticht, N Gretz, BK Garvalov, J Utikal, JP Sleeman
Oncogene, 2021-10-05;0(0):.
Species: Mouse
Sample Types: CellLysates, Whole Tissue
Applications: IHC, Western Blot -
Differential Response of M�ller Cells and Microglia in a Mouse Retinal Detachment Model and Its Implications in Detached and Non-Detached Regions
Authors: SH Lee, YS Park, SS Paik, IB Kim
Cells, 2021-08-03;10(8):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Dynamic cell contacts between periportal mesenchyme and ductal epithelium act as a rheostat for liver cell proliferation
Authors: L Cordero-Es, AM Dowbaj, TN Kohler, B Strauss, O Sarlidou, G Belenguer, C Pacini, NP Martins, R Dobie, JR Wilson-Kan, R Butler, N Prior, P Serup, F Jug, NC Henderson, F Hollfelder, M Huch
Cell Stem Cell, 2021-08-02;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
SLITRK5 is a negative regulator of hedgehog signaling in osteoblasts
Authors: J Sun, DY Shin, M Eiseman, AR Yallowitz, N Li, S Lalani, Z Li, M Cung, S Bok, S Debnath, SJ Marquez, TE White, AG Khan, IC Lorenz, JH Shim, FS Lee, R Xu, MB Greenblatt
Nature Communications, 2021-07-29;12(1):4611.
Species: Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: IHC, Western Blot -
WDR5-H3K4me3 epigenetic axis regulates OPN expression to compensate PD-L1 function to promote pancreatic cancer immune escape
Authors: Chunwan Lu, Zhuoqi Liu, John D Klement, Dafeng Yang, Alyssa D Merting, Dakota Poschel et al.
Journal for ImmunoTherapy of Cancer
-
Regional specialization and fate specification of bone stromal cells in skeletal development
Authors: KK Sivaraj, HW Jeong, B Dharmaling, D Zeuschner, S Adams, M Potente, RH Adams
Cell Reports, 2021-07-13;36(2):109352.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Spatiotemporal dynamics of inner ear sensory and non-sensory cells revealed by single-cell transcriptomics
Authors: TA Jan, Y Eltawil, AH Ling, L Chen, DC Ellwanger, S Heller, AG Cheng
Cell Reports, 2021-07-13;36(2):109358.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC, RNAScope -
Meflin defines mesenchymal stem cells and/or their early progenitors with multilineage differentiation capacity
Authors: Akitoshi Hara, Katsuhiro Kato, Toshikazu Ishihara, Hiroki Kobayashi, Naoya Asai, Shinji Mii et al.
Genes to Cells
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Microtissue Geometry and Cell‐Generated Forces Drive Patterning of Liver Progenitor Cell Differentiation in 3D
Authors: Ian C. Berg, Erfan Mohagheghian, Krista Habing, Ning Wang, Gregory H. Underhill
Advanced Healthcare Materials
-
EMX2-GPR156-G&alphai reverses hair cell orientation in mechanosensory epithelia
Authors: KS Kindt, A Akturk, A Jarysta, M Day, A Beirl, M Flonard, B Tarchini
Nature Communications, 2021-05-17;12(1):2861.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
MyD88 in myofibroblasts enhances colitis-associated tumorigenesis via promoting macrophage M2 polarization
Authors: Q Yuan, J Gu, J Zhang, S Liu, Q Wang, T Tian, Z Chen, J Zhang
Cell Reports, 2021-02-02;34(5):108724.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Secreted Phosphoprotein 1 Expression in Retinal Mononuclear Phagocytes Links Murine to Human Choroidal Neovascularization
Authors: Anja Schlecht, Peipei Zhang, Julian Wolf, Adrian Thien, Dennis-Dominik Rosmus, Stefaniya Boneva et al.
Frontiers in Cell and Developmental Biology
-
The role of extracellular matrix phosphorylation on energy dissipation in bone
Authors: Stacyann Bailey, Grazyna E Sroga, Betty Hoac, Orestis L Katsamenis, Zehai Wang, Nikolaos Bouropoulos et al.
eLife
-
Secreted protein acidic and rich in cysteine (SPARC) knockout mice have greater outflow facility
Authors: L Yu, Y Zheng, BJ Liu, MH Kang, JC Millar, DJ Rhee
PLoS ONE, 2020-11-04;15(11):e0241294.
Species: Mouse, Transgenic Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: IHC, Western Blot -
Tendon-derived cathepsin K–expressing progenitor cells activate Hedgehog signaling to drive heterotopic ossification
Authors: Heng Feng, Wenhui Xing, Yujiao Han, Jun Sun, Mingxiang Kong, Bo Gao et al.
Journal of Clinical Investigation
-
Microglia-organized scar-free spinal cord repair in neonatal mice
Authors: Y Li, X He, R Kawaguchi, Y Zhang, Q Wang, A Monavarfes, Z Yang, B Chen, Z Shi, H Meng, S Zhou, J Zhu, A Jacobi, V Swarup, PG Popovich, DH Geschwind, Z He
Nature, 2020-10-07;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Ectopic bone formation and systemic bone loss in a transmembrane TNF-driven model of human spondyloarthritis
Authors: E Christodou, C Geka, L Ntari, K Kranidioti, E Argyropoul, F Meier, M Armaka, I Mourouzis, C Pantos, M Rouchota, G Loudos, MC Denis, N Karagianni, G Kollias
Arthritis Res Ther, 2020-10-06;22(1):232.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Painting the Pancreas in Three Dimensions: Whole-Mount Immunofluorescence Method
Authors: Maricela Maldonado, Jeffrey D. Serrill, Hung-Ping Shih
Stem Cells and Tissue Repair
-
Osteopontin/secreted phosphoprotein-1 behaves as a molecular brake regulating the neuroinflammatory response to chronic viral infection
Authors: FJ Mahmud, Y Du, E Greif, T Boucher, RF Dannals, WB Mathews, MG Pomper, P Sysa-Shah, KA Metcalf Pa, C Lyons, B Carlson, M Chacona, AM Brown
Journal of Neuroinflammation, 2020-09-17;17(1):273.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Osteopontin Expression Identifies a Subset of Recruited Macrophages Distinct from Kupffer Cells in the Fatty Liver
Authors: Anneleen Remmerie, Liesbet Martens, Tinne Thoné, Angela Castoldi, Ruth Seurinck, Benjamin Pavie et al.
Immunity
-
Loss of Osteopontin Expression Reduces HSV-1-Induced Corneal Opacity
Authors: A Filiberti, GB Gmyrek, ML Montgomery, R Sallack, DJJ Carr
Invest. Ophthalmol. Vis. Sci., 2020-08-03;61(10):24.
Species: Mouse, Transgenic Mouse
Sample Types: In Vivo
Applications: In Vivo -
Integration of Hydrogel Microparticles With Three-Dimensional Liver Progenitor Cell Spheroids
Authors: Stefan D. Gentile, Andreas P. Kourouklis, Hyeon Ryoo, Gregory H. Underhill
Frontiers in Bioengineering and Biotechnology
Species: Mouse
Sample Types: Spheroid
Applications: Immunocytochemistry -
Modular output circuits of the fastigial nucleus for diverse motor and nonmotor functions of the cerebellar vermis
Authors: Hirofumi Fujita, Takashi Kodama, Sascha du Lac
eLife
-
Impact of osteopontin on the development of non‐alcoholic liver disease and related hepatocellular carcinoma
Authors: Alexander D. Nardo, Nicole G. Grün, Maximilian Zeyda, Monika Dumanic, Georg Oberhuber, Elisa Rivelles et al.
Liver International
-
Snai2 Maintains Bone Marrow Niche Cells by Repressing Osteopontin Expression
Authors: Qiaozhi Wei, Fumio Nakahara, Noboru Asada, Dachuan Zhang, Xin Gao, Chunliang Xu et al.
Developmental Cell
-
Fgf8 genetic labeling reveals the early specification of vestibular hair cell type in mouse utricle
Authors: Evan M. Ratzan, Anne M. Moon, Michael R. Deans
Development
-
Bim expression modulates the pro-inflammatory phenotype of retinal astroglial cells
Authors: J Falero-Per, N Sheibani, CM Sorenson
PLoS ONE, 2020-05-04;15(5):e0232779.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Adeno-Associated Virus Serotype 8-Mediated Genetic Labeling of Cholangiocytes in the Neonatal Murine Liver
Authors: Sanghoon Lee, Ping Zhou, Senyo Whyte, Soona Shin
Pharmaceutics
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Myofibroblast induces hepatocyte-to-ductal metaplasia via laminin-?v&beta6 integrin in liver fibrosis
Authors: T Xu, Z Lu, Z Xiao, F Liu, Y Chen, Z Wang, S Zhu, Y Song
Cell Death Dis, 2020-03-23;11(3):199.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-beta -catenin
Authors: Taifeng Zhou, Bo Gao, Yi Fan, Yuchen Liu, Shuhao Feng, Qian Cong et al.
eLife
-
Hox genes maintain critical roles in the adult skeleton
Authors: JY Song, KM Pineault, JM Dones, RT Raines, DM Wellik
Proc. Natl. Acad. Sci. U.S.A., 2020-03-13;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Vascular endothelial growth factor pathway promotes osseointegration and CD31hiEMCNhi endothelium expansion in a mouse tibial implant model: an animal study
Authors: G. Ji, R. Xu, Y. Niu, N. Li, L. Ivashkiv, M. P. G. Bostrom et al.
The Bone & Joint Journal
-
Activating Transcription Factor 3 (ATF3) Protects Retinal Ganglion Cells and Promotes Functional Preservation After Optic Nerve Crush
Authors: C Kole, B Brommer, N Nakaya, M Sengupta, L Bonet-Ponc, T Zhao, C Wang, W Li, Z He, S Tomarev
Invest. Ophthalmol. Vis. Sci., 2020-02-07;61(2):31.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Gastric squamous-columnar junction contains a large pool of cancer-prone immature osteopontin responsive Lgr5-CD44+ cells
Authors: DJ Fu, L Wang, FK Chouairi, IM Rose, DA Abetov, AD Miller, RJ Yamulla, JC Schimenti, A Flesken-Ni, AY Nikitin
Nat Commun, 2020-01-03;11(1):84.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Retinoic acid degradation shapes zonal development of vestibular organs and sensitivity to transient linear accelerations
Authors: Kazuya Ono, James Keller, Omar López Ramírez, Antonia González Garrido, Omid A. Zobeiri, Hui Ho Vanessa Chang et al.
Nature Communications
-
Osteopontin and its spatiotemporal relationship with glial cells in the striatum of rats treated with mitochondrial toxin 3-nitropropionic acid: possible involvement in phagocytosis
Authors: Tae-Ryong Riew, Soojin Kim, Xuyan Jin, Hong Lim Kim, Jeong-Hwa Lee, Mun-Yong Lee
Journal of Neuroinflammation
-
Single-Cell Profiles of Retinal Ganglion Cells Differing in Resilience to Injury Reveal Neuroprotective Genes
Authors: Nicholas M. Tran, Karthik Shekhar, Irene E. Whitney, Anne Jacobi, Inbal Benhar, Guosong Hong et al.
Neuron
-
Characterization of Matricellular Protein Expression Signatures in Mechanistically Diverse Mouse Models of Kidney Injury
Authors: D Feng, C Ngov, N Henley, N Boufaied, C Gerarduzzi
Sci Rep, 2019-11-13;9(1):16736.
Species: Mouse
Sample Types: Protein
Applications: Western Blot -
Inactivation of mTORC2 in macrophages is a signature of colorectal cancer that promotes tumorigenesis
Authors: Karl Katholnig, Birgit Schütz, Stephanie D. Fritsch, David Schörghofer, Monika Linke, Nyamdelger Sukhbaatar et al.
JCI Insight
-
In Vivo Activation and Pro-Fibrotic Function of NF-kappaB in Fibroblastic Cells During Pulmonary Inflammation and Fibrosis Induced by Carbon Nanotubes
Authors: J Dong, Q Ma
Front Pharmacol, 2019-10-02;10(0):1140.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
ATRQ β-001 Vaccine Prevents Experimental Abdominal Aortic Aneurysms
Authors: H Zhang, M Liao, M Cao, Z Qiu, X Yan, Y Zhou, H Wu, Y Wang, J Zheng, J Ding, M Wang, Y Liao, X Chen
J Am Heart Assoc, 2019-09-12;8(18):e012341.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Survival of Alpha and Intrinsically Photosensitive Retinal Ganglion Cells in NMDA-Induced Neurotoxicity and a Mouse Model of Normal Tension Glaucoma
Authors: S Honda, K Namekata, A Kimura, X Guo, C Harada, A Murakami, A Matsuda, T Harada
Invest. Ophthalmol. Vis. Sci., 2019-09-03;60(12):3696-3707.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Thrombospondin-1 Mediates Axon Regeneration in Retinal Ganglion Cells
Authors: Eric R. Bray, Benjamin J. Yungher, Konstantin Levay, Marcio Ribeiro, Gennady Dvoryanchikov, Ana C. Ayupe et al.
Neuron
-
Excessive exosome release is the pathogenic pathway linking a lysosomal deficiency to generalized fibrosis
Authors: D van de Vle, J Demmers, XX Nguyen, Y Campos, E Machado, I Annunziata, H Hu, E Gomero, X Qiu, A Bongiovann, CA Feghali-Bo, A d'Azzo
Sci Adv, 2019-07-17;5(7):eaav3270.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC-P, Western Blot -
Atoh1 Directs Regeneration and Functional Recovery of the Mature Mouse Vestibular System
Authors: ZN Sayyid, T Wang, L Chen, SM Jones, AG Cheng
Cell Rep, 2019-07-09;28(2):312-324.e4.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Pancreatic Ductal Deletion of Hnf1b Disrupts Exocrine Homeostasis, Leads to Pancreatitis, and Facilitates Tumorigenesis
Authors: E Quilichini, M Fabre, T Dirami, A Stedman, M De Vas, O Ozguc, RC Pasek, S Cereghini, L Morillon, C Guerra, A Couvelard, M Gannon, C Haumaitre
Cell Mol Gastroenterol Hepatol, 2019-06-21;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Single-Cell Analysis of the Liver Epithelium Reveals Dynamic Heterogeneity and an Essential Role for YAP in Homeostasis and Regeneration
Authors: BJ Pepe-Moone, MT Dill, A Alemany, J Ordovas-Mo, Y Matsushita, A Rao, A Sen, M Miyazaki, S Anakk, PA Dawson, N Ono, AK Shalek, A van Oudena, FD Camargo
Cell Stem Cell, 2019-05-09;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation
Authors: SG Romeo, KM Alawi, J Rodrigues, A Singh, AP Kusumbe, SK Ramasamy
Nat. Cell Biol., 2019-04-01;21(4):430-441.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Pulmonary arterial remodelling by deficiency of peroxisome proliferator-activated receptor-? in murine vascular smooth muscle cells occurs independently of obesity-related pulmonary hypertension
Authors: E Caglayan, M Trappiel, A Behringer, EM Berghausen, M Odenthal, E Wellnhofer, K Kappert
Respir. Res., 2019-02-27;20(1):42.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Normal inflammation and regeneration of muscle following injury require osteopontin from both muscle and non-muscle cells
Authors: DK Wasgewatte, EJ Mackie, CN Pagel
Skelet Muscle, 2019-02-26;9(1):6.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Osteopontin in the host response to Leishmania amazonensis
Authors: E Giraud, E Rouault, L Fiette, JH Colle, D Smirlis, E Melanitou
BMC Microbiol., 2019-02-08;19(1):32.
Species: Mouse
Sample Types: Cell Lysates, Whole Cells, Whole Tissue
Applications: ICC, IHC, Western Blot -
N-Cadherin-Expressing Bone and Marrow Stromal Progenitor Cells Maintain Reserve Hematopoietic Stem Cells
Authors: M Zhao, F Tao, A Venkatrama, Z Li, SE Smith, J Unruh, S Chen, C Ward, P Qian, JM Perry, H Marshall, J Wang, XC He, L Li
Cell Rep, 2019-01-15;26(3):652-669.e6.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Correlation of OPN gene expression with proliferation and apoptosis of ovarian cancer cells and prognosis of patients
Authors: H Hu, Z Liu, C Liu
Oncol Lett, 2019-01-07;17(3):2788-2794.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing
Authors: Qingyun Li, Zuolin Cheng, Lu Zhou, Spyros Darmanis, Norma F. Neff, Jennifer Okamoto et al.
Neuron
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Spatial patterning of liver progenitor cell differentiation mediated by cellular contractility and Notch signaling
Authors: KB Kaylan, IC Berg, MJ Biehl, A Brougham-C, I Jain, SM Jamil, LH Sargeant, NJ Cornell, LT Raetzman, GH Underhill
Elife, 2018-12-27;7(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Characterization of spatial and temporal development of Type I and Type II hair cells in the mouse utricle using new cell-type-specific markers
Authors: Stephen McInturff, Joseph C. Burns, Matthew W. Kelley
Biology Open
-
H3K36 trimethylation mediated by SETD2 regulates the fate of bone marrow mesenchymal stem cells
Authors: L Wang, N Niu, L Li, R Shao, H Ouyang, W Zou
PLoS Biol., 2018-11-13;16(11):e2006522.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells via increasing osteopontin expression
Authors: MN Lee, HS Hwang, SH Oh, A Roshanzade, JW Kim, JH Song, ES Kim, JT Koh
Exp. Mol. Med., 2018-11-05;50(11):142.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Molecular fingerprinting of On-Off direction selective retinal ganglion cells across species and relevance to primate visual circuits
Authors: OS Dhande, BK Stafford, K Franke, R El-Danaf, KA Percival, AH Phan, P Li, BJ Hansen, PL Nguyen, P Berens, WR Taylor, E Callaway, T Euler, AD Huberman
J. Neurosci., 2018-10-30;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Cyp1b1 expression impacts the angiogenic and inflammatory properties of liver sinusoidal endothelial cells
Authors: J Falero-Per, YS Song, Y Zhao, L Teixeira, CM Sorenson, N Sheibani
PLoS ONE, 2018-10-29;13(10):e0206756.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Distinct Bone Marrow Sources of Pleiotrophin Control Hematopoietic Stem Cell Maintenance and Regeneration
Authors: HA Himburg, CM Termini, L Schlussel, J Kan, M Li, L Zhao, T Fang, JP Sasine, VY Chang, JP Chute
Cell Stem Cell, 2018-08-09;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Combinatorial genetics in liver repopulation and carcinogenesis with a novel in vivo CRISPR activation platform
Authors: Kirk J. Wangensteen, Yue J. Wang, Zhixun Dou, Amber W. Wang, Elham Mosleh‐Shirazi, Max A. Horlbeck et al.
Hepatology
-
Leptin/Osteopontin Axis Regulated Type 2T Helper Cell Response in Allergic Rhinitis with Obesity
Authors: Q Zeng, X Luo, M Han, W Liu, H Li
EBioMedicine, 2018-06-07;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Gankyrin Promotes Tumor-Suppressor Protein Degradation to Drive Hepatocyte Proliferation
Authors: AM D'Souza, Y Jiang, A Cast, L Valanejad, M Wright, K Lewis, M Kumbaji, S Shah, D Smithrud, R Karns, S Shin, N Timchenko
Cell Mol Gastroenterol Hepatol, 2018-05-24;6(3):239-255.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Universality of clone dynamics during tissue development
Authors: Steffen Rulands, Fabienne Lescroart, Samira Chabab, Christopher J. Hindley, Nicole Prior, Magdalena K. Sznurkowska et al.
Nature Physics
-
Metabolic modulation of acetaminophen-induced hepatotoxicity by osteopontin
Authors: Y Wen, C Wang, J Gu, C Yu, K Wang, X Sun, Y Sun, H Wu, Y Tong, Q Xia, X Kong
Cell. Mol. Immunol., 2018-05-07;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
HMGB1 links chronic liver injury to progenitor responses and hepatocarcinogenesis
Authors: C Hernandez, P Huebener, JP Pradere, DJ Antoine, RA Friedman, RF Schwabe
J. Clin. Invest., 2018-05-07;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Growth differentiation factor 15 ameliorates nonalcoholic steatohepatitis and related metabolic disorders in mice
Authors: KH Kim, SH Kim, DH Han, YS Jo, YH Lee, MS Lee
Sci Rep, 2018-05-01;8(1):6789.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Tbr1 instructs laminar patterning of retinal ganglion cell dendrites
Authors: J Liu, JDS Reggiani, MA Laboulaye, S Pandey, B Chen, JLR Rubenstein, A Krishnaswa, JR Sanes
Nat. Neurosci., 2018-04-09;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Osteopontin deficiency ameliorates Alport pathology by preventing tubular metabolic deficits
Authors: W Ding, K Yousefi, S Goncalves, BJ Goldstein, AL Sabater, A Kloosterbo, P Ritter, G Lambert, AJ Mendez, LA Shehadeh
JCI Insight, 2018-03-22;3(6):.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC-Fr, Western Blot -
Interleukin-18 Amplifies Macrophage Polarization and Morphological Alteration, Leading to Excessive Angiogenesis
Authors: T Kobori, S Hamasaki, A Kitaura, Y Yamazaki, T Nishinaka, A Niwa, S Nakao, H Wake, S Mori, T Yoshino, M Nishibori, H Takahashi
Front Immunol, 2018-03-06;9(0):334.
Species: Mouse
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Neutralization, Western Blot -
Cardiac macrophages promote diastolic dysfunction
Authors: M Hulsmans, HB Sager, JD Roh, M Valero-Muñ, NE Houstis, Y Iwamoto, Y Sun, RM Wilson, G Wojtkiewic, B Tricot, MT Osborne, J Hung, C Vinegoni, K Naxerova, DE Sosnovik, MR Zile, AD Bradshaw, R Liao, A Tawakol, R Weissleder, A Rosenzweig, FK Swirski, F Sam, M Nahrendorf
J. Exp. Med., 2018-01-16;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Type 2 diabetes impairs the ability of skeletal muscle pericytes to augment postischemic neovascularization in db/db mice
Authors: KL Hayes, LM Messina, LM Schwartz, J Yan, AS Burnside, S Witkowski
Am. J. Physiol., Cell Physiol., 2018-01-10;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Multicolor quantitative confocal imaging cytometry
Authors: DL Coutu, KD Kokkaliari, L Kunz, T Schroeder
Nat. Methods, 2017-11-13;15(1):39-46.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
PEDF expression affects retinal endothelial cell proangiogenic properties through alterations in cell adhesive mechanisms
Authors: Juliana Falero-Perez, SunYoung Park, Christine M. Sorenson, Nader Sheibani
American Journal of Physiology-Cell Physiology
-
3D Visualization of Individual Regenerating Retinal Ganglion Cell Axons Reveals Surprisingly Complex Growth Paths
Authors: Eric R. Bray, Markus Noga, Kinjal Thakor, Yunfang Wang, Vance P. Lemmon, Kevin K. Park et al.
eNeuro
-
Bone marrow-derived monocyte infusion improves hepatic fibrosis by decreasing osteopontin, TGF-?1, IL-13 and oxidative stress
Authors: VCA de Souza, TA Pereira, VW Teixeira, H Carvalho, MCAB de Castro, CG D'assunção, AF de Barros, CL Carvalho, VMB de Lorena, VMA Costa, ÁAC Teixeira, RCBQ Figueiredo, SA de Oliveir
World J. Gastroenterol., 2017-07-28;23(28):5146-5157.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Four alpha ganglion cell types in mouse retina: Function, structure, and molecular signatures
Authors: B Krieger, M Qiao, DL Rousso, JR Sanes, M Meister
PLoS ONE, 2017-07-28;12(7):e0180091.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Sox11 Expression Promotes Regeneration of Some Retinal Ganglion Cell Types but Kills Others
Authors: MW Norsworthy, F Bei, R Kawaguchi, Q Wang, NM Tran, Y Li, B Brommer, Y Zhang, C Wang, JR Sanes, G Coppola, Z He
Neuron, 2017-06-21;94(6):1112-1120.e4.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Monocyte Chemotactic Protein-1-Interleukin-6-Osteopontin Pathway of Intra-Aneurysmal Tissue Healing
Authors: K Hosaka, K Rojas, HZ Fazal, MB Schneider, J Shores, V Federico, M McCord, L Lin, B Hoh
Stroke, 2017-03-14;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Osteopontin attenuates aging-associated phenotypes of hematopoietic stem cells
Authors: N Guidi, M Sacma, L Ständker, K Soller, G Marka, K Eiwen, JM Weiss, F Kirchhoff, T Weil, JA Cancelas, MC Florian, H Geiger
EMBO J, 2017-03-02;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development (Capture) -
Cell-matrix signals specify bone endothelial cells during developmental osteogenesis
Authors: UH Langen, ME Pitulescu, JM Kim, R Enriquez-G, KK Sivaraj, AP Kusumbe, A Singh, J Di Russo, MG Bixel, B Zhou, L Sorokin, JM Vaquerizas, RH Adams
Nat. Cell Biol, 2017-02-20;19(3):189-201.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Dentin Sialoprotein is a Novel Substrate of Matrix Metalloproteinase 9 in vitro and in vivo
Authors: G Yuan, L Chen, J Feng, G Yang, Q Ni, X Xu, C Wan, M Lindsey, KJ Donly, M MacDougall, Z Chen, S Chen
Sci Rep, 2017-02-14;7(0):42449.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Oligo-fucoidan prevents renal tubulointerstitial fibrosis by inhibiting the CD44 signal pathway
Authors: CH Chen, YM Sue, CY Cheng, YC Chen, CT Liu, YH Hsu, PA Hwang, NJ Huang, TH Chen
Sci Rep, 2017-01-18;7(0):40183.
Species: Rat
Sample Types: Cell Lysates
Applications: Western Blot -
Osteopontin Is a Blood Biomarker for Microglial Activation and Brain Injury in Experimental Hypoxic-Ischemic Encephalopathy
Authors: Yikun Li, Eric B. Dammer, Xiaohui Zhang-Brotzge, Scott Chen, Duc M. Duong, Nicholas T. Seyfried et al.
eNeuro
-
Osteopontin Blockade Attenuates Renal Injury After Ischemia Reperfusion by Inhibiting NK Cell Infiltration
Authors: Cindy Cen, Monowar Aziz, Weng-Lang Yang, Jeffrey M. Nicastro, Gene F. Coppa, Ping Wang
Shock
-
Disease-modifying effects of orally bioavailable NF-?B inhibitors in dystrophin-deficient muscle
JCI Insight, 2016-12-22;1(21):e90341.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Osteopontin regulates proliferation, apoptosis, and migration of murine claudin-low mammary tumor cells
Authors: S. Saleh, D. E. Thompson, J. McConkey, P. Murray, R. A. Moorehead
BMC Cancer
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Neutralization of Osteopontin Ameliorates Acute Lung Injury Induced by Intestinal Ischemia-Reperfusion
Authors: Yohei Hirano, Monowar Aziz, Weng-Lang Yang, Mahendar Ochani, Ping Wang
Shock
-
Vav1 Regulates Mesenchymal Stem Cell Differentiation Decision Between Adipocyte and Chondrocyte via Sirt1
Authors: Peng Qu, Lizhen Wang, Yongfen Min, Lois McKennett, Jonathan R. Keller, P. Charles Lin
Stem Cells
-
Selective Expression of Osteopontin in ALS-resistant Motor Neurons is a Critical Determinant of Late Phase Neurodegeneration Mediated by Matrix Metalloproteinase-9
Authors: Yuta Morisaki
Sci Rep, 2016-06-06;6(0):27354.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The P2X4 receptor is required for neuroprotection via ischemic preconditioning
Sci Rep, 2016-05-13;6(0):25893.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
High fat diet increases melanoma cell growth in the bone marrow by inducing osteopontin and interleukin 6
Authors: Guang-Liang Chen, Yubin Luo, Daniel Eriksson, Xianyi Meng, Cheng Qian, Tobias Bäuerle et al.
Oncotarget
-
Alternatively activated macrophages determine repair of the infarcted adult murine heart
J Clin Invest, 2016-05-03;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Osteopontin protects against high phosphate-induced nephrocalcinosis and vascular calcification
Authors: Neil J. Paloian, Elizabeth M. Leaf, Cecilia M. Giachelli
Kidney International
-
Osteopontin ablation ameliorates muscular dystrophy by shifting macrophages to a pro-regenerative phenotype
Authors: Joana Capote, Irina Kramerova, Leonel Martinez, Sylvia Vetrone, Elisabeth R. Barton, H. Lee Sweeney et al.
Journal of Cell Biology
-
The transcription factor c-JUN/AP-1 promotes HBV-related liver tumorigenesis in mice
Authors: C Trierweiler, B Hockenjos, K Zatloukal, R Thimme, H E Blum, E F Wagner et al.
Cell Death & Differentiation
Species: Transgenic Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: Immunohistochemistry, Western Blot -
Inhibition of hedgehog signaling ameliorates hepatic inflammation in mice with nonalcoholic fatty liver disease (NAFLD)
Authors: Hyunjoo Kwon, Kyoungsub Song, Chang Han, Weina Chen, Ying Wang, Srikanta Dash et al.
Hepatology
-
Combinatorial microenvironmental regulation of liver progenitor differentiation by Notch ligands, TGF beta, and extracellular matrix
Authors: Kerim B. Kaylan, Viktoriya Ermilova, Ravi Chandra Yada, Gregory H. Underhill
Scientific Reports
-
Intracellular osteopontin stabilizes TRAF3 to positively regulate innate antiviral response
Authors: K Zhao, M Zhang, L Zhang, P Wang, G Song, B Liu, H Wu, Z Yin, C Gao
Sci Rep, 2016-03-30;6(0):23771.
Species: Mouse
Sample Types: Recombinant Protein
Applications: Neutralization -
Pannexin 3 and connexin 43 modulate skeletal development through their distinct functions and expression patterns
Authors: Masaki Ishikawa, Geneva L. Williams, Tomoko Ikeuchi, Kiyoshi Sakai, Satoshi Fukumoto, Yoshihiko Yamada
Journal of Cell Science
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Slc20a2 deficiency results in fetal growth restriction and placental calcification associated with thickened basement membranes and novel CD13 and laminin alpha 1 expressing cells
Authors: Mary C. Wallingford, Hilary S. Gammill, Cecilia M. Giachelli
Reproductive Biology
-
Extracellular Adenosine Production by ecto-5'-Nucleotidase (CD73) Enhances Radiation-Induced Lung Fibrosis
Authors: F Wirsdörfer, S de Leve, F Cappuccini, T Eldh, AV Meyer, E Gau, LF Thompson, NY Chen, H Karmouty-Q, U Fischer, M Kasper, D Klein, JW Ritchey, MR Blackburn, AM Westendorf, M Stuschke, V Jendrossek
Cancer Res., 2016-02-26;76(10):3045-56.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury
Authors: Yohei Hirano, Monowar Aziz, Weng-Lang Yang, Zhimin Wang, Mian Zhou, Mahendar Ochani et al.
Critical Care
-
Inhibition of osteoblast mineralization by phosphorylated phage-derived apatite-specific peptide
Authors: Janani Ramaswamy, Hwa Kyung Nam, Harsha Ramaraju, Nan E. Hatch, David H. Kohn
Biomaterials
-
Hepatocellular carcinoma originates from hepatocytes and not from the progenitor/biliary compartment.
Authors: Mu X, Espanol-Suner R, Mederacke I, Affo S, Manco R, Sempoux C, Lemaigre F, Adili A, Yuan D, Weber A, Unger K, Heikenwalder M, Leclercq I, Schwabe R
J Clin Invest, 2015-09-08;125(10):3891-903.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Cutting edge: Role of osteopontin and integrin alphav in T cell-mediated anti-inflammatory responses in endotoxemia.
Authors: Inoue M, Shinohara M
J Immunol, 2015-05-13;194(12):5595-8.
Species: Mouse
Sample Types: Cell Culture Supernates, In Vivo
Applications: ELISA Development (Capture), Neutralization -
Identification of therapeutic targets for glioblastoma by network analysis.
Authors: Friedmann-Morvinski D, Bhargava V, Gupta S, Verma I, Subramaniam S
Oncogene, 2015-05-11;35(5):608-20.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Osteopontin depletion decreases inflammation and gastric epithelial proliferation during Helicobacter pylori infection in mice.
Authors: Park J, Lee S, Go D, Kim H, Kwon H, Kim D
Lab Invest, 2015-04-13;95(6):660-71.
Species: Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: IHC-P, Western Blot -
Loss of JUNB/AP-1 promotes invasive prostate cancer.
Authors: Thomsen M, Bakiri L, Hasenfuss S, Wu H, Morente M, Wagner E
Cell Death Differ, 2014-12-19;22(4):574-82.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing.
Authors: Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko P, Linnarsson S, Ernfors P
Nat Neurosci, 2014-11-24;18(1):145-53.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
PGC-1alpha induces SPP1 to activate macrophages and orchestrate functional angiogenesis in skeletal muscle.
Authors: Rowe G, Raghuram S, Jang C, Nagy J, Patten I, Goyal A, Chan M, Liu L, Jiang A, Spokes K, Beeler D, Dvorak H, Aird W, Arany Z
Circ Res, 2014-07-09;115(5):504-17.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Endothelial Notch activity promotes angiogenesis and osteogenesis in bone.
Authors: Ramasamy S, Kusumbe A, Wang L, Adams R
Nature, 2014-03-12;507(7492):376-80.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone.
Authors: Kusumbe A, Ramasamy S, Adams R
Nature, 2014-03-12;507(7492):323-8.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Colony-stimulating factor-1 signaling suppresses renal crystal formation.
Authors: Taguchi K, Okada A, Kitamura H, Yasui T, Naiki T, Hamamoto S, Ando R, Mizuno K, Kawai N, Tozawa K, Asano K, Tanaka M, Miyoshi I, Kohri K
J Am Soc Nephrol, 2014-02-27;25(8):1680-97.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Osteopontin expression by CD103- dendritic cells drives intestinal inflammation.
Authors: Kourepini E, Aggelakopoulou M, Alissafi T, Paschalidis N, Simoes D, Panoutsakopoulou V
Proc Natl Acad Sci U S A, 2014-02-18;111(9):E856-65.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
An osteopontin-integrin interaction plays a critical role in directing adipogenesis and osteogenesis by mesenchymal stem cells.
Authors: Chen Q, Shou P, Zhang L, Xu C, Zheng C, Han Y, Li W, Huang Y, Zhang X, Shao C, Roberts A, Rabson A, Ren G, Zhang Y, Wang Y, Denhardt D, Shi Y
Stem Cells, 2014-02-01;32(2):327-37.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice
Authors: Min Yang, Anup Ramachandran, Hui-Min Yan, Benjamin L. Woolbright, Bryan L. Copple, Peter Fickert et al.
Toxicology Letters
-
Hydrogen peroxide regulates osteopontin expression through activation of transcriptional and translational pathways.
Authors: Lyle A, Remus E, Fan A, Lassegue B, Walter G, Kiyosue A, Griendling K, Taylor W
J Biol Chem, 2013-11-18;289(1):275-85.
Species: Rat
Sample Types: Cell Lysates
Applications: Western Blot -
Impaired hepatocyte maturation, abnormal expression of biliary transcription factors and liver fibrosis in C/EBPalpha(Cebpa)-knockout mice.
Authors: Akai Y, Oitate T, Koike T, Shiojiri N
Histol Histopathol, 2013-07-18;29(1):107-25.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Endoglin regulates the activation and quiescence of endothelium by participating in canonical and non-canonical TGF-beta signaling pathways.
Authors: Park S, Dimaio T, Liu W, Wang S, Sorenson C, Sheibani N
J Cell Sci, 2013-02-15;126(0):1392-405.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: Western Blot -
Focal adhesion kinase regulates the localization and retention of pro-B cells in bone marrow microenvironments.
Authors: Park S, Wolfram P, Canty K, Harley B, Nombela-Arrieta C, Pivarnik G, Manis J, Beggs H, Silberstein L
J Immunol, 2012-12-21;190(3):1094-102.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Identification of a clonally expanding haematopoietic compartment in bone marrow.
Authors: Wang L, Benedito R, Bixel M, Zeuschner D, Stehling M, Savendahl L, Haigh J, Snippert H, Clevers H, Breier G, Kiefer F, Adams R
EMBO J, 2012-11-27;32(2):219-30.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Direction-selective retinal ganglion cells arise from molecularly specified multipotential progenitors
Authors: Irina De la Huerta, In-Jung Kim, P. Emanuela Voinescu, Joshua R. Sanes
Proceedings of the National Academy of Sciences
-
Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet.
Authors: Lau, Wei Ling, Leaf, Elizabet, Hu, Ming Cha, Takeno, Marc M, Kuro-o, Makoto, Moe, Orson W, Giachelli, Cecilia
Kidney Int, 2012-08-29;82(12):1261-70.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Loss of MMP-2 in murine osteoblasts upregulates osteopontin and bone sialoprotein expression in a circuit regulating bone homeostasis
Authors: Rebecca A. Mosig, John A. Martignetti
Disease Models & Mechanisms
Species: Mouse
Sample Types: Serum, Whole Tissue
Applications: Immunohistochemistry, Western Blot -
Repression of osteocyte Wnt/β-catenin signaling is an early event in the progression of renal osteodystrophy.
Authors: Sabbagh Y, Graciolli FG, O'Brien S, Tang W, Dos Reis LM, Ryan S, Phillips L, Boulanger J, Song W, Bracken C, Liu S, Ledbetter S, Dechow P, Canziani ME, Carvalho AB, Jorgetti V, Moyses RM, Schiavi SC
J. Bone Miner. Res., 2012-08-01;27(8):1757-72.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Neuronal Classification and Marker Gene Identification via Single-Cell Expression Profiling of Brainstem Vestibular Neurons Subserving Cerebellar Learning
Authors: Takashi Kodama, Shiloh Guerrero, Minyoung Shin, Seti Moghadam, Michael Faulstich, Sascha du Lac
Journal of Neuroscience
-
Cellular basis of tissue regeneration by omentum.
Authors: Shah S, Lowery E, Braun RK
PLoS ONE, 2012-06-06;7(6):e38368.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Proteomic Characterization of the Cellular Response to Nitrosative Stress Mediated by S-Nitrosoglutathione Reductase Inhibition
Authors: Matthew W. Foster, Zhonghui Yang, David M. Gooden, J. Will Thompson, Carol H. Ball, Meredith E. Turner et al.
Journal of Proteome Research
-
The critical role of agrin in the hematopoietic stem cell niche.
Authors: Mazzon C, Anselmo A, Cibella J, Soldani C, Destro A, Kim N, Roncalli M, Burden SJ, Dustin ML, Sarukhan A, Viola A
Blood, 2011-06-07;118(10):2733-42.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe–/– mice during disease regression
Authors: Stephane Potteaux, Emmanuel L. Gautier, Susan B. Hutchison, Nico van Rooijen, Daniel J. Rader, Michael J. Thomas et al.
Journal of Clinical Investigation
-
Cutting Edge: Critical Role of Intracellular Osteopontin in Antifungal Innate Immune Responses
Authors: Makoto Inoue, Yasuhiro Moriwaki, Tomohiro Arikawa, Yu-Hsun Chen, Young Joo Oh, Timothy Oliver et al.
The Journal of Immunology
-
T-Lymphocyte Responses to Intestinally Absorbed Antigens Can Contribute to Adipose Tissue Inflammation and Glucose Intolerance during High Fat Feeding
Authors: Yuehui Wang, Jianing Li, Lihua Tang, Yu Wang, Richard Charnigo, Willem de Villiers et al.
PLoS ONE
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance.
Authors: Kiefer FW, Zeyda M, Gollinger K, Pfau B, Neuhofer A, Weichhart T, Saemann MD, Geyeregger R, Schlederer M, Kenner L, Stulnig TM
Diabetes, 2010-01-27;59(4):935-46.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Adenosine and osteopontin contribute to the development of chronic obstructive pulmonary disease.
Authors: Schneider DJ, Lindsay JC, Zhou Y, Molina JG, Blackburn MR
FASEB J., 2009-08-31;24(1):70-80.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr -
Therapeutic silencing of an endogenous gene by siRNA cream in an arthritis model mouse.
Authors: Takanashi M, Oikawa K, Sudo K, Tanaka M, Fujita K, Ishikawa A, Nakae S, Kaspar RL, Matsuzaki M, Kudo M, Kuroda M
Gene Ther., 2009-05-28;16(8):982-9.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Phosphate feeding induces arterial medial calcification in uremic mice: role of serum phosphorus, fibroblast growth factor-23, and osteopontin.
Authors: El-Abbadi MM, Pai AS, Leaf EM, Yang HY, Bartley BA, Quan KK, Ingalls CM, Liao HW, Giachelli CM
Kidney Int., 2009-03-25;75(12):1297-307.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis.
Authors: Murugaiyan G, Mittal A, Weiner HL
J. Immunol., 2008-12-01;181(11):7480-8.
Species: Mouse
Sample Types: Cell Lysates, In Vivo
Applications: Neutralization, Western Blot -
Fibroblast growth factor-2 regulates expression of osteopontin in periodontal ligament cells.
Authors: Terashima Y, Shimabukuro Y, Terashima H, Ozasa M, Terakura M, Ikezawa K, Hashikawa T, Takedachi M, Oohara H, Yamada S, Murakami S
J. Cell. Physiol., 2008-09-01;216(3):640-50.
Species: Mouse
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
In vivo osteopontin-induced macrophage accumulation is dependent on CD44 expression.
Authors: Marcondes MC, Poling M, Watry DD, Hall D, Fox HS
Cell. Immunol., 2008-08-03;254(1):56-62.
Species: Mouse
Sample Types: Recombinant Protein
Applications: Western Blot -
Polyomavirus middle T antigen induces the transcription of osteopontin, a gene important for the migration of transformed cells.
Authors: Whalen KA, Weber GF, Benjamin TL, Schaffhausen BS
J. Virol., 2008-03-12;82(10):4946-54.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets.
Authors: Xanthou G, Alissafi T, Semitekolou M, Simoes DC, Economidou E, Gaga M, Lambrecht BN, Lloyd CM, Panoutsakopoulou V
Nat. Med., 2007-04-15;13(5):570-8.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Inhalation of ultrafine carbon particles triggers biphasic pro-inflammatory response in the mouse lung.
Authors: Andre E, Stoeger T, Takenaka S, Bahnweg M, Ritter B, Karg E, Lentner B, Reinhard C, Schulz H, Wjst M
Eur. Respir. J., 2006-04-26;28(2):275-85.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Aggregation of embryonic stem cells induces Nanog repression and primitive endoderm differentiation.
Authors: Hamazaki T, Oka M, Yamanaka S, Terada N
J. Cell. Sci., 2004-10-19;117(0):5681-6.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Lack of requirement of osteopontin for inflammation, bone erosion, and cartilage damage in the K/BxN model of autoantibody-mediated arthritis.
Authors: Jacobs JP, Pettit AR, Shinohara ML, Jansson M, Cantor H, Gravallese EM, Mathis D, Benoist C
Arthritis Rheum., 2004-08-01;50(8):2685-94.
Species: Mouse
Sample Types: Serum
Applications: ELISA Development -
Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo
Authors: Mei Y. Speer, Marc D. McKee, Robert E. Guldberg, Lucy Liaw, Hsueh-Ying Yang, Elyse Tung et al.
The Journal of Experimental Medicine
-
Retinal patterns and the cellular repertoire of neuropsin (Opn5) retinal ganglion cells
Authors: D'Souza SP, Swygart DI, Wienbar SR Et al.
The Journal of comparative neurology
-
Spatiotemporal gene expression patterns reveal molecular relatedness between retinal laminae
Authors: Jiang D, Burger CA, Casasent AK et al.
J. Comp. Neurol.
-
YAP1 and TAZ negatively control bone angiogenesis by limiting hypoxia-inducible factor signaling in endothelial cells
Authors: Sivaraj KK, Dharmalingam B, Mohanakrishnan V et al.
Elife
-
PEDF Expression Affects the Oxidative and Inflammatory State of Choroidal Endothelial Cells
Authors: M Farnoodian, CM Sorenson, N Sheibani
Am. J. Physiol., Cell Physiol., 2018-01-10;0(0):.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse Osteopontin/OPN Antibody
Average Rating: 4.1 (Based on 12 Reviews)
Have you used Mouse Osteopontin/OPN Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Murine placenta cryosections stained with osteopontin (red). Tissue autofluorescence in blue.
Reason for Rating: The antibody works excellently. It labels Spp1 on microglia in 5xFAD mouse model of Alzheimer's disease Sample Tested: Alzheimer's disease brain Species: Mouse
BSA 4% as blocking solution. Negative control with secondary only confirmed that the staining is specific.
No positive IF signal in fresh frozen skeletal muscle section.
Try it for flow cytometry
Osteopontin (in white) was stained 1:200 on cryostat sections together with CD324-AF488 (in green). Ducts are clearly stained as expected.
Antibody reacts with BSA and some of the ladder proteins from Biorad. Unusable for WB since BSA size is too close from the OSTP size.
***Bio-Techne Response: Thank you for reviewing our product. We are sorry to hear that this product did not perform as expected. We have been in touch with the customer to resolve this issue according to our Product Guarantee and to the customer’s satisfaction.*** Update from Tech Support Team: Possible customer error, no response from customer.***