Mouse/Rat TrkC Antibody Summary
Cys32-Thr429
Accession # Q6VNS1
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Mouse and Rat TrkC by Western Blot. Western blot shows lysates of mouse brain (cerebellum) tissue, mouse brain (cortex) tissue, and rat brain tissue. PVDF membrane was probed with 0.5 µg/mL of Goat Anti-Mouse/Rat TrkC Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1404) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). Specific bands were detected for TrkC at approximately 100 and 140 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Detection of Mouse TrkC by Simple WesternTM. Simple Western lane view shows lysates of mouse brain tissue, loaded at 0.2 mg/mL. Specific bands were detected for TrkC at approximately 111 and 159 kDa (as indicated) using 25 µg/mL of Goat Anti-Mouse/Rat TrkC Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1404) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
Detection of Mouse TrkC by Immunocytochemistry/ Immunofluorescence Cra1/+ mice show no evidence of proprioceptive sensory neuron loss at symptomatic ages.A) Proprioceptive sensory neuron labeling and quantification with parvalbumin, (B) ER81, and (C) TrkC shows no difference between of +/+ and Cra1/+ mice at 6 months of age (Scale bar = 20 µm). D) Parvalbumin labeling of proprioceptive sensory neuron fibers within the spinal cord shows the central projection of these sensory neurons is intact in Cra1/+ mice (Scale bar = 20 µm; N = 3 animals per genotype). Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0016753), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse TrkC by Immunocytochemistry/ Immunofluorescence Cra1/+ mice show no evidence of proprioceptive sensory neuron loss at symptomatic ages.A) Proprioceptive sensory neuron labeling and quantification with parvalbumin, (B) ER81, and (C) TrkC shows no difference between of +/+ and Cra1/+ mice at 6 months of age (Scale bar = 20 µm). D) Parvalbumin labeling of proprioceptive sensory neuron fibers within the spinal cord shows the central projection of these sensory neurons is intact in Cra1/+ mice (Scale bar = 20 µm; N = 3 animals per genotype). Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0016753), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
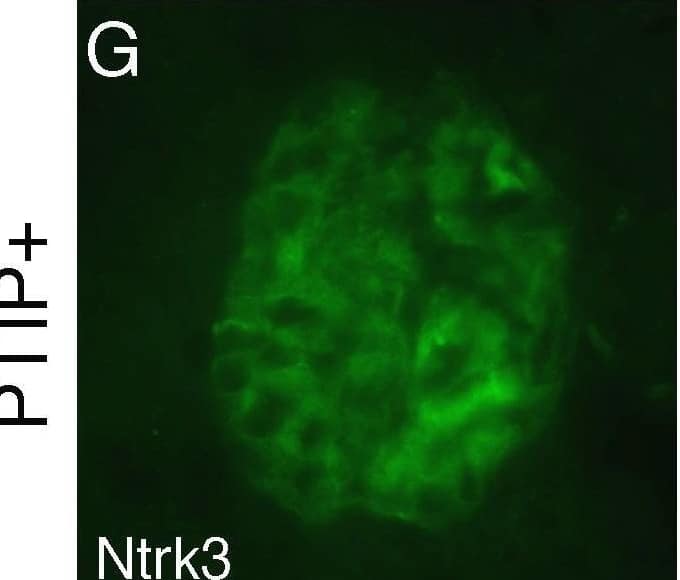
Detection of Mouse TrkC by Immunocytochemistry/ Immunofluorescence Ntrk3 in the Glomerulus. Fresh frozen tissues were sectioned and fixed in methanol followed by immunostaining with goat anti-Ntrk3, rabbit anti-WT1, or rabbit anti-Nephrin, as indicated. PTIP+ sections (A–C, G–I) showed strong Ntrk3 staining in all glomeruli, in a pattern similar to Nephrin. The PTIP− kidney sections (D–F, J–L) showed much lower levels of Ntrk3 protein in glomeruli. All micrographs were taken at manually set, equal exposures. Right panels (C, F, I, L) are overlays of Ntrk3 and WT1 or Ntrk3 and Nephrin and are counterstained with DAPI (blue) to visualize all cell nuclei. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/21060806), licensed under a CC0-1.0 license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: TrkC
The neurotrophins, including NGF, BDNF, NT‑3 and NT‑4/5, constitute a group of structurally related, secreted proteins that play an important role in the development and function of the nervous system. The biological activities of the neurotrophins are mediated by binding to and activating two unrelated receptor types: the p75 neurotrophin receptor (p75NTR) and the Trk family of receptor tyrosine kinases (1, 2). p75NTR is a member of the tumor necrosis factor receptor superfamily (TNFRSF) and has been designated TNFRSF16. It binds all neurotrophins with low affinity to transduce cellular signaling pathways that synergize or antagonize those activated by the Trk receptors. Three Trk family proteins, TrkA, TrkB, and TrkC, exhibiting different ligand specificities, have been identified. TrkA binds NGF and NT‑3, TrkB binds BDNF, NT‑3 and NT‑4/5, and TrkC only binds NT‑3 (1‑2). All Trk family proteins share a conserved, complex subdomain organization consisting of a signal peptide, two cysteine-rich domains, a cluster of three leucine-rich motifs, and two immunoglobulin-like domains in the extracellular region, as well as an intracellular region that contains the tyrosine kinase domain (3). Natural splice variants of the different Trks, lacking the first cysteine-rich domain, the first and second or all three of the leucine-rich motifs, or the tyrosine kinase domain, have been described (4). At the protein sequence level, Trks are highly conserved between species with the extracellular domains of human and mouse TrkC showing 94% amino acid sequence identity (5). The proteins also exhibit cross-species activity. The primary location of TrkC expression is in the nervous system and, specifically, in regions of the CNS. Low level TrkC expression has also been observed in a wide variety of tissues outside the nervous system (6).
- Huang, E.J. and L.F. Reichardt (2003) Annu. Rev. Biochem. 72:609.
- Dechant, G. (2001) Cell Tissue Res. 305:229.
- Schneider, R. and M. Schweiger (1991) Oncogene 6:1807.
- Ninkina, N. et al. (1997) J. Biol. Chem. 272:13019.
- Menn, B. et al. (1998) J. Comp. Neurol. 401:47.
- Shelton, D. et al. (1995) J. Neurosci. 15:477.
Product Datasheets
Citations for Mouse/Rat TrkC Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
73
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Identification of Trigeminal Sensory Neuronal Types Innervating Masseter Muscle
Authors: Karen A. Lindquist, Sergei Belugin, Anahit H. Hovhannisyan, Tatiana M. Corey, Adam Salmon, Armen N. Akopian
eNeuro
-
Targeted deletion of Atoh8 results in severe hearing loss in mice
Authors: Qi Tang, Meng‐Yao Xie, Yong‐Li Zhang, Ruo‐Yan Xue, Xiao‐Hui Zhu, Hua Yang
genesis
-
TrkB-containing exosomes promote the transfer of glioblastoma aggressiveness to YKL-40-inactivated glioblastoma cells
Authors: Sandra Pinet, Barbara Bessette, Nicolas Vedrenne, Aurélie Lacroix, Laurence Richard, Marie-Odile Jauberteau et al.
Oncotarget
-
Prolactin receptor expression in mouse dorsal root ganglia neuronal subtypes is sex‐dependent
Authors: Mayur Patil, Anahit H. Hovhannisyan, Andi Wangzhou, Jennifer Mecklenburg, Wouter Koek, Vincent Goffin et al.
Journal of Neuroendocrinology
-
The Role of TRESK in Discrete Sensory Neuron Populations and Somatosensory Processing
Authors: Greg A. Weir, Philippa Pettingill, Yukyee Wu, Galbha Duggal, Andrei-Sorin Ilie, Colin J. Akerman et al.
Frontiers in Molecular Neuroscience
-
NOP receptor agonist attenuates nitroglycerin-induced migraine-like symptoms in mice
Authors: Katarzyna M. Targowska-Duda, Akihiko Ozawa, Zachariah Bertels, Andrea Cippitelli, Jason L. Marcus, Hanna K. Mielke-Maday et al.
Neuropharmacology
-
Boundary cap neural crest stem cells homotopically implanted to the injured dorsal root transitional zone give rise to different types of neurons and glia in adult rodents.
Authors: Trolle, Carl, Konig, Niclas, Abrahamsson, Ninnie, Vasylovska, Svitlana, Kozlova, Elena N
BMC Neurosci, 2014-05-05;15(0):60.
-
The Familial Dysautonomia disease gene,Ikbkap/Elp1, is required in the developing and adult central nervous system
Authors: Marta Chaverra, Lynn George, Marc Mergy, Hannah Waller, Katharine Kujawa, Connor Murnion et al.
Disease Models & Mechanisms
-
Dynamic expression of transcription factor Brn3b during mouse cranial nerve development
Authors: Szilard Sajgo, Seid Ali, Octavian Popescu, Tudor Constantin Badea
Journal of Comparative Neurology
-
Generation of self-organized autonomic ganglion organoids from fibroblasts
Authors: Liu S, Xiang K, Yuan F, Xiang M
iScience
-
Role of DSCAM in the development of the spinal locomotor and sensorimotor circuits
Authors: Louise Thiry, Maxime Lemieux, Olivier D. Laflamme, Frédéric Bretzner
Journal of Neurophysiology
-
Anterograde trafficking of neurotrophin-3 in the adult olfactory system in vivo
Authors: Huan Liu, Michael Lu, Kathleen M. Guthrie
Experimental Neurology
-
A Spinal Circuit for Modular Gating of Organ Somatosensation
Authors: Zhang, Y;Liu, F;Yang, L;Hahm, HJ;Heitmeier, MR;Okuda, T;Kawatani, M;Harrigan, JT;Morrison-Rodriguez, EC;Petukhova, A;Lu, Z;Heitmeier, HA;Samineni, VK;
bioRxiv : the preprint server for biology
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Local keratinocyte-nociceptor interactions enhance obesity-mediated small fiber neuropathy via NGF-TrkA-PI3K signaling axis
Authors: Koui, Y;Song, S;Dong, X;Mukouyama, YS;
bioRxiv : the preprint server for biology
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Titin is a nucleolar protein in neurons
Authors: Cameron, B;Torres-Hernandez, L;Montague, VL;Lewis, KA;Smith, H;Fox, J;Guo, X;Kalb, RG;George, L;
Research square
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
c-Maf-positive spinal cord neurons are critical elements of a dorsal horn circuit for mechanical hypersensitivity in neuropathy
Authors: N Frezel, M Ranucci, E Foster, H Wende, P Pelczar, R Mendes, RP Ganley, K Werynska, S d'Aquin, C Beccarini, C Birchmeier, HU Zeilhofer, H Wildner
Cell Reports, 2023-03-21;42(4):112295.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Closing the Gap between the Auditory Nerve and Cochlear Implant Electrodes: Which Neurotrophin Cocktail Performs Best for Axonal Outgrowth and Is Electrical Stimulation Beneficial?
Authors: D Schmidbaue, S Fink, F Rousset, H Löwenheim, P Senn, R Glueckert
International Journal of Molecular Sciences, 2023-01-19;24(3):.
Species: Mouse
Sample Types: Organoid
Applications: IHC -
Exploring the potential application of dental pulp stem cells in neuroregenerative medicine
Authors: N Sultan, BA Scheven
Neural regeneration research, 2022-04-01;17(4):775-776.
Species: Rat
Sample Types: Whole Cells
Applications: Neutralization -
Nociceptor subtypes are born continuously over DRG development.
Authors: Mark A. Landy, Megan Goyal, Helen C. Lai
Developmental Biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Targeted deletion of Atoh8 results in severe hearing loss in mice
Authors: Qi Tang, Meng‐Yao Xie, Yong‐Li Zhang, Ruo‐Yan Xue, Xiao‐Hui Zhu, Hua Yang
genesis
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Dental pulp stem cells stimulate neuronal differentiation of PC12 cells
Authors: N Sultan, LE Amin, AR Zaher, ME Grawish, BA Scheven
Neural regeneration research, 2021-09-01;16(9):1821-1828.
Species: Rat
Sample Types: Whole Cells
Applications: Neutralization -
Cyclic Stretch of Either PNS or CNS Located Nerves Can Stimulate Neurite Outgrowth
Authors: V Kampanis, B Tolou-Dabb, L Zhou, W Roth, R Puttagunta
Cells, 2020-12-28;10(1):.
Species: Rat
Sample Types: Whole Cells
Applications: ICC -
Spinal Inhibitory Ptf1a-Derived Neurons Prevent Self-Generated Itch
Authors: A Escalante, R Klein
Cell Rep, 2020-11-24;33(8):108422.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Single cell RNA sequencing identifies early diversity of sensory neurons forming via bi-potential intermediates
Authors: L Faure, Y Wang, ME Kastriti, P Fontanet, KKY Cheung, C Petitpré, H Wu, LL Sun, K Runge, L Croci, MA Landy, HC Lai, GG Consalez, A de Chevign, F Lallemend, I Adameyko, S Hadjab
Nat Commun, 2020-08-21;11(1):4175.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Generation of self-organized sensory ganglion organoids and retinal ganglion cells from fibroblasts
Authors: D Xiao, Q Deng, Y Guo, X Huang, M Zou, J Zhong, P Rao, Z Xu, Y Liu, Y Hu, Y Shen, K Jin, M Xiang
Sci Adv, 2020-05-29;6(22):eaaz5858.
Species: Mouse
Sample Types: Whole Tissue
Applications: ICC, IHC -
Ameloblastomas Exhibit Stem Cell Potential, Possess Neurotrophic Properties, and Establish Connections with Trigeminal Neurons
Authors: P Pagella, J Catón, CT Meisel, TA Mitsiadis
Cells, 2020-03-06;9(3):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Loss of bhlha9 Impairs Thermotaxis and Formalin-Evoked Pain in a Sexually Dimorphic Manner
Authors: M Bohic, I Marics, C Santos, P Malapert, N Ben-Arie, C Salio, A Reynders, Y Le Feuvre, AJ Saurin, A Moqrich
Cell Rep, 2020-01-21;30(3):602-610.e6.
Species: Mouse
Sample Types: Whole Tissue
Applications: IF -
A cell fitness selection model for neuronal survival during development
Authors: Y Wang, H Wu, P Fontanet, S Codeluppi, N Akkuratova, C Petitpré, Y Xue-Franzé, K Niederreit, A Sharma, F Da Silva, G Comai, G Agirman, D Palumberi, S Linnarsson, I Adameyko, A Moqrich, A Schedl, G La Manno, S Hadjab, F Lallemend
Nat Commun, 2019-09-12;10(1):4137.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Prolactin receptor expression in mouse dorsal root ganglia neuronal subtypes is sex‐dependent
Authors: Mayur Patil, Anahit H. Hovhannisyan, Andi Wangzhou, Jennifer Mecklenburg, Wouter Koek, Vincent Goffin et al.
Journal of Neuroendocrinology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
The Role of TRESK in Discrete Sensory Neuron Populations and Somatosensory Processing
Authors: Greg A. Weir, Philippa Pettingill, Yukyee Wu, Galbha Duggal, Andrei-Sorin Ilie, Colin J. Akerman et al.
Frontiers in Molecular Neuroscience
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Spinal Neuropeptide Y1 Receptor-Expressing Neurons Form an Essential Excitatory Pathway for Mechanical Itch
Authors: D Acton, X Ren, S Di Costanz, A Dalet, S Bourane, I Bertocchi, C Eva, M Goulding
Cell Rep, 2019-07-16;28(3):625-639.e6.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Notch signalling defines dorsal root ganglia neuroglial fate choice during early neural crest cell migration
Authors: S Wiszniak, Q Schwarz
BMC Neurosci, 2019-04-29;20(1):21.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-F -
Functional Local Proprioceptive Feedback Circuits Initiate and Maintain Locomotor Recovery after Spinal Cord Injury
Authors: A Takeoka, S Arber
Cell Rep, 2019-04-02;27(1):71-85.e3.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Prdm12 Directs Nociceptive Sensory Neuron Development by Regulating the Expression of the NGF Receptor TrkA
Authors: S Desiderio, S Vermeiren, C Van Campen, S Kricha, E Malki, S Richts, EV Fletcher, T Vanwelden, BZ Schmidt, KA Henningfel, T Pieler, CG Woods, V Nagy, C Verfaillie, EJ Bellefroid
Cell Rep, 2019-03-26;26(13):3522-3536.e5.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
PRDM12 Is Required for Initiation of the Nociceptive Neuron Lineage during Neurogenesis
Authors: L Bartesaghi, Y Wang, P Fontanet, S Wanderoy, F Berger, H Wu, N Akkuratova, F Bouçanova, JJ Médard, C Petitpré, MA Landy, MD Zhang, P Harrer, C Stendel, R Stucka, M Dusl, ME Kastriti, L Croci, HC Lai, GG Consalez, A Pattyn, P Ernfors, J Senderek, I Adameyko, F Lallemend, S Hadjab, R Chrast
Cell Rep, 2019-03-26;26(13):3484-3492.e4.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Mechanically Activated Piezo Channels Mediate Touch and Suppress Acute Mechanical Pain Response in Mice
Authors: M Zhang, Y Wang, J Geng, S Zhou, B Xiao
Cell Rep, 2019-02-05;26(6):1419-1431.e4.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Characteristics of sensory neuronal groups in CGRP-cre-ER reporter mice: Comparison to Nav1.8-cre, TRPV1-cre and TRPV1-GFP mouse lines
Authors: MJ Patil, AH Hovhannisy, AN Akopian
PLoS ONE, 2018-06-04;13(6):e0198601.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Optogenetic Activation of Non-Nociceptive A? Fibers Induces Neuropathic Pain-Like Sensory and Emotional Behaviors after Nerve Injury in Rats
Authors: R Tashima, K Koga, M Sekine, K Kanehisa, Y Kohro, K Tominaga, K Matsushita, H Tozaki-Sai, Y Fukazawa, K Inoue, H Yawo, H Furue, M Tsuda
eNeuro, 2018-02-15;5(1):.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Phenotypic and Functional Characterization of Peripheral Sensory Neurons derived from Human Embryonic Stem Cells
Authors: AJ Alshawaf, S Viventi, W Qiu, G D'Abaco, B Nayagam, M Erlichster, G Chana, I Everall, J Ivanusic, E Skafidas, M Dottori
Sci Rep, 2018-01-12;8(1):603.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
The Familial Dysautonomia disease gene,Ikbkap/Elp1, is required in the developing and adult central nervous system
Authors: Marta Chaverra, Lynn George, Marc Mergy, Hannah Waller, Katharine Kujawa, Connor Murnion et al.
Disease Models & Mechanisms
Species: Mouse
Sample Types: Embryo
Applications: Immunohistochemistry -
Genetic Tracing of Cav3.2 T-Type Calcium Channel Expression in the Peripheral Nervous System
Authors: YA Bernal Sie, J Haseleu, A Kozlenkov, V Bégay, GR Lewin
Front Mol Neurosci, 2017-03-15;10(0):70.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Development of a pharmacodynamic biomarker to measure target engagement from inhibition of the NGF-TrkA pathway
Authors: EA Price, A Krasowska-, KK Nanda, SJ Stachel, DA Henze
J. Neurosci. Methods, 2017-03-07;282(0):34-42.
Species: Rat
Sample Types: Tissue Homogenates
Applications: ELISA Development (Capture) -
Genetic ablation of GINIP-expressing primary sensory neurons strongly impairs Formalin-evoked pain
Authors: L Urien, S Gaillard, L Lo Re, P Malapert, M Bohic, A Reynders, A Moqrich
Sci Rep, 2017-02-27;7(0):43493.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Tropomyosin Receptor Kinase C Targeted Delivery of a Peptidomimetic Ligand-Photosensitizer Conjugate Induces Antitumor Immune Responses Following Photodynamic Therapy
Sci Rep, 2016-11-17;6(0):37209.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Endogenous Modulation of Trkb Signaling by Treadmill Exercise After Peripheral Nerve Injury
Authors: Ariadna Arbat-Plan
Neuroscience, 2016-10-29;0(0):.
Species: Rat
Sample Types: Tissue Homogenates
Applications: Western Blot -
Merkel Cell-Driven BDNF Signaling Specifies SAI Neuron Molecular and Electrophysiological Phenotypes
Authors: EG Reed-Geagh, MC Wright, LA See, PC Adelman, KH Lee, HR Koerber, SM Maricich
J Neurosci, 2016-04-13;36(15):4362-76.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Dynamic expression of transcription factor Brn3b during mouse cranial nerve development
Authors: Szilard Sajgo, Seid Ali, Octavian Popescu, Tudor Constantin Badea
Journal of Comparative Neurology
Species: Mouse
Sample Types: Embryo
Applications: Immunohistochemistry -
Distinct adhesion-independent functions of beta-catenin control stage-specific sensory neurogenesis and proliferation.
Authors: Gay M, Valenta T, Herr P, Paratore-Hari L, Basler K, Sommer L
BMC Biol, 2015-04-11;13(0):24.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Egr3-dependent muscle spindle stretch receptor intrafusal muscle fiber differentiation and fusimotor innervation homeostasis.
Authors: Oliveira Fernandes M, Tourtellotte W
J Neurosci, 2015-04-08;35(14):5566-78.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing.
Authors: Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko P, Linnarsson S, Ernfors P
Nat Neurosci, 2014-11-24;18(1):145-53.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
ADAM metalloproteases promote a developmental switch in responsiveness to the axonal repellant Sema3A.
Authors: Romi E, Gokhman I, Wong E, Antonovsky N, Ludwig A, Sagi I, Saftig P, Tessier-Lavigne M, Yaron A
Nat Commun, 2014-06-05;5(0):4058.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Uncoupling of molecular maturation from peripheral target innervation in nociceptors expressing a chimeric TrkA/TrkC receptor.
Authors: Gorokhova S, Gaillard S, Urien L, Malapert P, Legha W, Baronian G, Desvignes J, Alonso S, Moqrich A
PLoS Genet, 2014-02-06;10(2):e1004081.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Protein tyrosine phosphatase receptor type O inhibits trigeminal axon growth and branching by repressing TrkB and Ret signaling.
Authors: Gatto G, Dudanova I, Suetterlin P, Davies A, Drescher U, Bixby J, Klein R
J Neurosci, 2013-03-20;33(12):5399-410.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Positional differences of axon growth rates between sensory neurons encoded by Runx3.
EMBO J., 2012-08-17;31(18):3718-29.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Npn-1 contributes to axon-axon interactions that differentially control sensory and motor innervation of the limb.
Authors: Huettl RE, Soellner H, Bianchi E, Novitch BG, Huber AB
PLoS Biol., 2011-02-22;9(2):e1001020.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Reduced spiral ganglion neuronal loss by adjunctive neurotrophin-3 in experimental pneumococcal meningitis.
Authors: Demel C, Hoegen T, Giese A
J Neuroinflammation, 2011-01-24;8(1):7.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Sortilin associates with Trk receptors to enhance anterograde transport and neurotrophin signaling.
Authors: Vaegter CB, Jansen P, Fjorback AW
Nat. Neurosci., 2010-11-21;14(1):54-61.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Neurotrophin-3 production promotes human neuroblastoma cell survival by inhibiting TrkC-induced apoptosis.
Authors: Bouzas-Rodriguez J, Cabrera JR, Delloye-Bourgeois C, Ichim G, Delcros JG, Raquin MA, Rousseau R, Combaret V, Benard J, Tauszig-Delamasure S, Mehlen P
J. Clin. Invest., 2010-02-15;120(3):850-8.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Runx3 is required for the specification of TrkC-expressing mechanoreceptive trigeminal ganglion neurons.
Authors: Senzaki K, Ozaki S, Yoshikawa M, Ito Y, Shiga T
Mol. Cell. Neurosci., 2009-12-23;43(3):296-307.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Immunohistochemical analysis of acid-sensing ion channel 2 expression in rat dorsal root ganglion and effects of axotomy.
Authors: Kawamata T, Ninomiya T, Toriyabe M, Yamamoto J, Niiyama Y, Omote K, Namiki A
Neuroscience, 2006-09-01;143(1):175-87.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
TRPV2, a capsaicin receptor homologue, is expressed predominantly in the neurotrophin-3-dependent subpopulation of primary sensory neurons.
Authors: Tamura S, Morikawa Y, Senba E
Neuroscience, 2005-01-01;130(1):223-8.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Conditional Deletion of Pten Leads to Defects in Nerve Innervation and Neuronal Survival in Inner Ear Development
Authors: Hyung Jin Kim, Hae-Mi Woo, Jihee Ryu, Jinwoong Bok, Jin Woo Kim, Sang Back Choi et al.
PLoS ONE
-
Neuromuscular Junction Defects in Mice with Mutation of dynein heavy chain 1
Authors: Stephanie L. Courchesne, Maria F. Pazyra-Murphy, Daniel J. Lee, Rosalind A. Segal
PLoS ONE
-
A Brainstem-Spinal Cord Inhibitory Circuit for Mechanical Pain Modulation by GABA and Enkephalins
Authors: Amaury François, Sarah A. Low, Elizabeth I. Sypek, Amelia J. Christensen, Chaudy Sotoudeh, Kevin T. Beier et al.
Neuron
-
Genetic interplay between transcription factor Pou4f1/Brn3a and neurotrophin receptor Ret in retinal ganglion cell type specification
Authors: Vladimir Vladimirovich Muzyka, Tudor Constantin Badea
Neural Development
-
A Local Source of FGF Initiates Development of the Unmyelinated Lineage of Sensory Neurons
Authors: Saïda Hadjab, Marina C. M. Franck, Yiqiao Wang, Ulrich Sterzenbach, Anil Sharma, Patrik Ernfors et al.
The Journal of Neuroscience
-
Requirement for Dicer in Maintenance of Monosynaptic Sensory-Motor Circuits in the Spinal Cord
Authors: Fumiyasu Imai, Xiaoting Chen, Matthew T. Weirauch, Yutaka Yoshida
Cell Reports
-
Nociceptor subtypes are born continuously over DRG development.
Authors: Mark A. Landy, Megan Goyal, Helen C. Lai
Developmental Biology
-
Identification of Spinal Neurons Contributing to the Dorsal Column Projection Mediating Fine Touch and Corrective Motor Movements
Authors: Sónia Paixão, Laura Loschek, Louise Gaitanos, Pilar Alcalà Alcalà Morales, Martyn Goulding, Rüdiger Klein
Neuron
-
Altering a Histone H3K4 Methylation Pathway in Glomerular Podocytes Promotes a Chronic Disease Phenotype
Authors: Gaelle M. Lefevre, Sanjeevkumar R. Patel, Doyeob Kim, Lino Tessarollo, Gregory R. Dressler
PLoS Genetics
-
Muscle-selective RUNX3 dependence of sensorimotor circuit development
Authors: Yiqiao Wang, Haohao Wu, Pavel Zelenin, Paula Fontanet, Simone Wanderoy, Charles Petitpré et al.
Development
-
A Functional Topographic Map for Spinal Sensorimotor Reflexes
Authors: Graziana Gatto, Steeve Bourane, Xiangyu Ren, Stefania Di Costanzo, Peter K. Fenton, Priyabrata Halder et al.
Neuron
-
Early ear neuronal development, but not olfactory or lens development, can proceed without SOX2
Authors: Martina Dvorakova, Iva Macova, Romana Bohuslavova, Miroslava Anderova, Bernd Fritzsch, Gabriela Pavlinkova
Developmental Biology
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse/Rat TrkC Antibody
Average Rating: 5 (Based on 2 Reviews)
Have you used Mouse/Rat TrkC Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:




