Mouse TGF-beta 1 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant TGF-ß1. The Reagent Diluent recommended may be suitable for most cell culture supernate, serum, and plasma samples. The Reagent Diluent selected for use can alter the performance of an immunoassay. Reagent Diluent optimization for samples with complex matrices such as serum and plasma, may improve their performance in this assay.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 1 (5 plates): (Catalog # DY007) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 1.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY997), or 1.4% delipidized bovine serum, 0.05% Tween 20 in PBS, pH 7.2-7.4, 0.2 μm filtered.
Blocking Buffer: (Catalog # DY004), or 5% Tween 20 in PBS, pH 7.2-7.4, 0.2 μm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Activation Reagents Required:
Sample Activation Kit 1: 3 vials (10 mL/vial) of 1N HCL and 3 vials (10 mL/vial) of 1.2 N NaOH/1M HEPES (R&D Systems, Catalog # DY010).
Scientific Data
Product Datasheets
Preparation and Storage
Background: TGF-beta 1
Transforming Growth Factor Beta 1, 2, and 3 (TGF-beta 1, TGF-beta 2, and TGF-beta 3) are highly pleiotropic cytokines that virtually all cell types secrete. TGF-beta molecules are proposed to act as cellular switches that regulate processes such as immune function, proliferation, and epithelial-mesenchymal transition. Targeted deletions of these genes in mice show that each TGF-beta isoform has some non-redundant functions: TGF-beta 1 is involved in hematopoiesis and endothelial differentiation; TGF-beta 2 affects development of cardiac, lung, craniofacial, limb, eye, ear, and urogenital systems; and TGF-beta 3 influences palatogenesis and pulmonary development. The full range of in vitro biological activities of TGF-beta 5 has not yet been explored. However, TGF-beta 1, TGF-beta 2, TGF-beta 3, and TGF-beta 5 have been found to be largely interchangeable in an inhibitory bioassay, and it is anticipated that TGF-beta 5 will show a spectrum of activities similar to the other TGF-beta family members. To date, the production of TGF-beta 5 has only been demonstrated in Xenopus.
TGF-beta ligands are initially synthesized as precursor proteins that undergo proteolytic cleavage. The mature segments form active ligand dimers via a disulfide-rich core consisting of the characteristic 'cysteine knot'. TGF-beta signaling begins with binding to a complex of the accessory receptor betaglycan (also known as TGF-beta RIII) and a type II serine/threonine kinase receptor termed TGF-beta RII. This receptor then phosphorylates and activates a type I serine/threonine kinase receptor, either ALK-1 or TGF-beta RI (also called ALK-5). The activated type I receptor phosphorylates and activates Smad proteins that regulate transcription. Use of other signaling pathways that are Smad-independent allows for distinct actions observed in response to TGF-beta in different contexts.
Assay Procedure
ACTIVATION REAGENT PREPARATION
To activate latent TGF-ß1 to the immunoreactive form, prepare the following solutions for acid activation and neutralization. The solutions may be stored in polypropylene bottles at room temperature for up to one month.
Caution: Wear protective clothing and safety glasses during preparation or use of these reagents. Refer to the appropriate MSDS before use.
1 N HCl (100 mL) - To 91.67 mL of deionized water, slowly add 8.33 mL of 12 N HCl. Mix well.
1.2 N NaOH/0.5 M HEPES (100 mL) - To 75 mL of deionized water, slowly add 12 mL of 10 N NaOH. Mix well. Add 11.9 g of HEPES. Mix well. Bring final volume to 100 mL with deionized water.
TGF-β1 SAMPLE ACTIVATION
To activate latent TGF-β1 to immunoreactive TGF-β1, follow the activation procedure outlined below. Assay samples after neutralization (pH 7.2-7.6). Use polypropylene test tubes.
Note: Do not activate the kit standards. The kit standards contain active recombinant TGF-β1.
| Cell Culture Supernates | Serum/Plasma |
|---|---|
| To 100 μL of cell culture supernate, add 20 μL of 1 N HCI. | To 40 μL of serum/plasma, add 10 μL of 1 N HCI. |
| Mix well. | Mix well. |
| Incubate 10 minutes at room temperature. | Incubate 10 minutes at room temperature. |
| Neutralize the acidified sample by adding 20 μL of 1.2 N NaOH/0.5 M HEPES. | Neutralize the acidified sample by adding 10 μL of 1.2 N NaOH/0.5 M HEPES. |
| Mix well. | Mix well. |
| Assay immediately. | Prior to the assay, dilute the activated sample 60-fold with Reagent Diluent.* |
| The concentration read off the standard curve must be multiplied by the dilution factor, 1.4. | The concentration read off the standard curve must be multiplied by the appropriate dilution factor, 90. |
*A suggested 60-fold dilution is 10 μL of activated sample + 590 μL of Reagent Diluent.
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody (to the working concentration stated in the product datasheet ) in PBS without carrier protein. Immediately coat a 96-well microplate with 100 µL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 µL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block each well of the microplate as recommended in the product datasheet. Incubate at room temperature for a minimum of 1 hour.
Note: The recommended Reagent Diluent typically contains 1% BSA. Some DuoSet Development Kits require alternative blocking agents, or for plates to be blocked overnight with a higher percentage of BSA, please see the product datasheet for details.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
PRECAUTION
The Stop Solution suggested for use with this kit is an acid solution. Wear eye, hand, face and clothing protection when using this material.
Assay Procedure
- Add 100 µL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 µL of the Detection Antibody, diluted in Reagent Diluent (as recommended in the product datasheet), to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 µL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 µL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 µL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse TGF-beta 1 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
169
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Aberrant intermediate alveolar epithelial cells promote pathogenic activation of lung fibroblasts in preclinical fibrosis models
Authors: Hoffman, ET;Shah, A;Barboza, WR;Rodriguez, LR;Dherwani, R;Dooley, PE;Minakin, K;Tomer, Y;Ayers, LJ;Jones, D;Murthy, A;Bennett, A;Lange, AN;Bawa, PS;Wang, F;Babu, A;Chavez, K;Nakamoto, RS;Cooper, CH;Basil, MC;Raredon, MSB;Kotton, DN;Alysandratos, KD;Katzen, J;
Nature communications
Species: Mouse
Sample Types: Cell Culture Supernates
-
Exosomal MicroRNA let-7 Modulates Lipid Metabolism and Inflammation in Foamy Macrophages of Chronic Obstructive Pulmonary Disease
Authors: Hsieh, MH;Lai, PF;Chen, PC;Liu, XL;Chen, WL;Kuo, WS;Wang, SD;Kao, HF;Lin, LJ;Wu, LS;Wang, JY;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates, Exosomes
-
Male mice treated with combined anti-fibrotic therapeutics, IPW5371 and tadalafil, are predisposed to adverse cardiovascular events
Authors: Stephens, JQ;Blas-Machado, U;Sherrill, C;Caudell, D;Kock, N;Davis, AM;Whitfield, JM;Hart, B;Kavanagh, K;
Frontiers in pharmacology
Species: Mouse
Sample Types: Plasma, Tissue Homogenates
-
Lung-delivered IL-10 mitigates Lung inflammation induced by repeated endotoxin exposures in male mice
Authors: Schwab, AD;Wyatt, TA;Schanze, OW;Nelson, AJ;Gleason, AM;Duryee, MJ;Mosley, DD;Thiele, GM;Mikuls, TR;Poole, JA;
Physiological reports
Species: Mouse
Sample Types: Tissue Homogenates
-
Prevention of radiotherapy-induced pro-tumorigenic microenvironment by SFK inhibitors
Authors: Choi, YJ;Kim, MJ;Lee, YJ;Choi, M;Shim, WS;Park, M;Kim, YC;Kang, KW;
Theranostics
Species: Mouse
Sample Types: Tissue Homogenates
-
Inhibition of RhoA-mediated secretory autophagy in megakaryocytes mitigates myelofibrosis in mice
Authors: Becker, IC;Barrachina, MN;Lykins, J;Camacho, V;Stone, AP;Chua, BA;Signer, RAJ;Machlus, KR;Whiteheart, SW;Roweth, HG;Italiano, JE;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates, Bone Marrow Fluid
-
Intermittent ozone inhalation during house dust mite-induced sensitization primes for adverse asthma phenotype
Authors: Hussain, S;Majumder, N;Mazumder, MHH;Lewis, SE;Olapeju, O;Velayutham, M;Amin, MS;Brundage, K;Kelley, EE;Vanoirbeek, J;
Redox biology
Species: Mouse
Sample Types: BALF
-
Acquired Immunostimulatory Phenotype of Migratory CD103+ Dendritic Cells Promotes Alloimmunity Following Corneal Transplantation
Authors: Blanco, T;Nakagawa, H;Musayeva, A;Krauthammer, M;Singh, RB;Narimatsu, A;Ge, H;Shoushtari, SI;Dana, R;
JCI insight
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Sigma-1 Receptor Exacerbates Cardiac Dysfunction Induced by Obstructive Nephropathy: A Role for Sexual Dimorphism
Authors: Munguia-Galaviz, FJ;Miranda-Diaz, AG;Gutierrez-Mercado, YK;Ku-Centurion, M;Gonzalez-Gonzalez, RA;Portilla-de Buen, E;Echavarria, R;
Biomedicines
Species: Mouse
Sample Types: Serum
-
H7N7 viral infection elicits pronounced, sex-specific neuroinflammatory responses in vitro
Authors: Gabele, L;Bochow, I;Rieke, N;Sieben, C;Michaelsen-Preusse, K;Hosseini, S;Korte, M;
Frontiers in cellular neuroscience
Species: Mouse
Sample Types: Cell Culture Supernates
-
The integrin receptor beta7 subunit mediates airway remodeling and hyperresponsiveness in allergen exposed mice
Authors: Assayag, M;Obedeyah, T;Abutbul, A;Berkman, N;
Respiratory research
Species: Mouse
Sample Types: Tissue Homogenates, BALF
-
High-fat and high-carbohydrate diets increase bone fragility through TGF-?-dependent control of osteocyte function
Authors: Dole, NS;Betancourt-Torres, A;Kaya, S;Obata, Y;Schurman, CA;Yoon, J;Yee, CS;Khanal, V;Luna, CA;Carroll, M;Salinas, JJ;Miclau, E;Acevedo, C;Alliston, T;
JCI insight
Species: Transgenic Mouse
Sample Types: Serum
-
Macrophage MCT4 inhibition activates reparative genes and protects from atherosclerosis by histone H3 lysine 18 lactylation
Authors: Zhang, Y;Jiang, H;Dong, M;Min, J;He, X;Tan, Y;Liu, F;Chen, M;Chen, X;Yin, Q;Zheng, L;Shao, Y;Li, X;Chen, H;
Cell reports
Species: Mouse
Sample Types: Serum
-
Statin administration or blocking PCSK9 alleviates airway hyperresponsiveness and lung fibrosis in high-fat diet-induced obese mice
Authors: Liang, L;Chung, SI;Guon, TE;Park, KH;Lee, JH;Park, JW;
Respiratory research
Species: Mouse
Sample Types: Serum, Tissue Homogenates
-
Tumor Cell-Associated IL-1? Affects Breast Cancer Progression and Metastasis in Mice through Manipulation of the Tumor Immune Microenvironment
Authors: Krishnamohan, M;Kaplanov, I;Maudi-Boker, S;Yousef, M;Machluf-Katz, N;Cohen, I;Elkabets, M;Titus, J;Bersudsky, M;Apte, RN;Voronov, E;Braiman, A;
International journal of molecular sciences
Species: Mouse, Transgenic Mouse
Sample Types: Tissue Homogenates
-
Benzimidazole-oxindole hybrids as multi-kinase inhibitors targeting melanoma
Authors: Allam, RM;El Kerdawy, AM;Gouda, AE;Ahmed, KA;Abdel-Mohsen, HT;
Bioorganic chemistry
Species: Mouse
Sample Types: Cell Lysates
-
Eosinophils preserve bone homeostasis by inhibiting excessive osteoclast formation and activity via eosinophil peroxidase
Authors: Andreev, D;Kachler, K;Liu, M;Chen, Z;Krishnacoumar, B;Ringer, M;Frey, S;Krönke, G;Voehringer, D;Schett, G;Bozec, A;
Nature communications
Species: Transgenic Mouse
Sample Types: Cell Culture Supernates
-
ADAMTSL2 mutations determine the phenotypic severity in Geleophysic Dysplasia
Authors: Camarena, V;Williams, MM;Morales, AA;Zafeer, MF;Kilic, OV;Kamiar, A;Abad, C;Rasmussen, MA;Briski, LM;Peart, L;Bademci, G;Barbouth, DS;Smithson, S;Wang, G;Shehadeh, LA;Walz, K;Tekin, M;
JCI insight
Species: Mouse
Sample Types: Cell Culture Supernates, Cell Lysates
-
Evaluating the Effects of Chronic Oral Exposure to the Food Additive Silicon Dioxide on Oral Tolerance Induction and Food Sensitivities in Mice
Authors: Lamas, B;Martins Breyner, N;Malaisé, Y;Wulczynski, M;Galipeau, HJ;Gaultier, E;Cartier, C;Verdu, EF;Houdeau, E;
Environmental health perspectives
Species: Mouse
Sample Types: Cell Culture Supernates
-
An NFAT1-C3a-C3aR Positive Feedback Loop in Tumor-Associated Macrophages Promotes a Glioma Stem Cell Malignant Phenotype
Authors: Zhang, Y;Song, Y;Wang, X;Shi, M;Lin, Y;Tao, D;Han, S;
Cancer immunology research
Species: Mouse
Sample Types: Cell Culture Supernates
-
Central 5-HTergic hyperactivity induces myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-like pathophysiology
Authors: Lee, JS;Kang, JY;Park, SY;Hwang, SJ;Bae, SJ;Son, CG;
Journal of translational medicine
Species: Mouse
Sample Types: Plasma
-
MCPIP1 Inhibits Hepatic Stellate Cell Activation in Autocrine and Paracrine Manners, Preventing Liver Fibrosis
Authors: Pydyn, N;Ferenc, A;Trzos, K;Po?piech, E;Wilamowski, M;Mucha, O;Major, P;Kad?uczka, J;Rodrigues, P;Bañales, J;Herranz, J;Ávila, M;Hutsch, T;Ma?czak, P;Radkowiak, D;Budzy?ski, A;Jura, J;Kotlinowski, J;
Cellular and Molecular Gastroenterology and Hepatology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Repolarizing Tumor-Associated Macrophages and inducing immunogenic cell Death: A targeted liposomal strategy to boost cancer immunotherapy
Authors: Li, C;Wang, L;Li, Z;Li, Z;Zhang, K;Cao, L;Wang, Z;Shen, C;Chen, L;
International journal of pharmaceutics
Species: Mouse
Sample Types: Cell Culture Supernates
-
NLRX1 Prevents M2 Macrophage Polarization and Excessive Renal Fibrosis in Chronic Obstructive Nephropathy
Authors: Liu, Y;Kors, L;Butter, LM;Stokman, G;Claessen, N;Zuurbier, CJ;Girardin, SE;Leemans, JC;Florquin, S;Tammaro, A;
Cells
Species: Mouse
Sample Types: Cell Culture Supernates, Tissue Homogenates
-
The long-lasting Ascaris suum antigens in the lungs shapes the tissue adaptation modifying the pulmonary architecture and immune response after infection in mice
Authors: Oliveira, FMS;Kraemer, L;Vieira-Santos, F;Leal-Silva, T;Gazzinelli-Guimarães, AC;Lopes, CA;Amorim, CCO;Pinheiro, GRG;Moura, MS;Matias, PHP;Barbosa, FS;Caliari, MV;Weatherhead, JE;Bueno, LL;Russo, RC;Fujiwara, RT;
Microbial pathogenesis
Species: Mouse
Sample Types: Tissue Homogenates
-
Daphnetin Alleviates Bleomycin-Induced Pulmonary Fibrosis through Inhibition of Epithelial-to-Mesenchymal Transition and IL-17A
Authors: Park, SJ;Ryu, HW;Kim, JH;Hahn, HJ;Jang, HJ;Ko, SK;Oh, SR;Lee, HJ;
Cells
Species: Mouse
Sample Types: Tissue Homogenates
-
Hypoxia-inducible factor 1? modulates interstitial pneumonia-mediated lung cancer progression
Authors: Shimoji, K;Nakashima, T;Masuda, T;Namba, M;Sakamoto, S;Yamaguchi, K;Horimasu, Y;Mimae, T;Miyamoto, S;Iwamoto, H;Fujitaka, K;Hamada, H;Okada, M;Hattori, N;
Journal of translational medicine
Species: Mouse
Sample Types: Serum
-
Efficacy of combined tumor irradiation and KCa3.1-targeting with TRAM-34 in a syngeneic glioma mouse model
Authors: Stransky, N;Ganser, K;Quintanilla-Martinez, L;Gonzalez-Menendez, I;Naumann, U;Eckert, F;Koch, P;Huber, SM;Ruth, P;
Scientific reports
Species: Mouse
Sample Types: Cell Culture Supernates
-
PGF2? signaling drives fibrotic remodeling and fibroblast population dynamics in mice
Authors: Rodriguez, LR;Tang, SY;Roque Barboza, W;Murthy, A;Tomer, Y;Cai, TQ;Iyer, S;Chavez, K;Das, US;Ghosh, S;Cooper, C;Dimopoulos, TT;Babu, A;Connelly, CF;FitzGerald, GA;Beers, MF;
JCI insight
Species: Mouse
Sample Types: BALF
-
Isthmin-1 attenuates allergic Asthma by stimulating adiponectin expression and alveolar macrophage efferocytosis in mice
Authors: Tee, JH;Vijayakumar, U;Shanmugasundaram, M;Lam, TYW;Liao, W;Yang, Y;Wong, WSF;Ge, R;
Respiratory research
Species: Mouse, Transgenic Mouse
Sample Types: BALF
-
Insulin-degrading enzyme (IDE) as a modulator of microglial phenotypes in the context of Alzheimer's disease and brain aging
Authors: Corraliza-Gomez, M;Bermejo, T;Lilue, J;Rodriguez-Iglesias, N;Valero, J;Cozar-Castellano, I;Arranz, E;Sanchez, D;Ganfornina, MD;
Journal of neuroinflammation
Species: Transgenic Mouse, Mouse
Sample Types: Cell Culture Supernates
-
Both C57BL/KsJ (H2d haplotype) and CB10-H2 (H2b haplotype) mice are highly susceptible to congenital toxoplasmosis
Authors: Coutinho, LB;de Oliveira, MC;Araujo, ECB;França, FBF;Almeida, MPO;Cariaco, Y;Czarnewski, P;Silva, NM;
Acta tropica
Species: Mouse
Sample Types: Serum
-
PPARG stimulation restored lung mRNA expression of core clock, inflammation- and metabolism-related genes disrupted by reversed feeding in male mice
Authors: Shlykova, O;Izmailova, O;Kabaliei, A;Palchyk, V;Shynkevych, V;Kaidashev, I;
Physiological reports
Species: Mouse
Sample Types:
-
Thiostrepton-Nanomedicine, a TLR9 Inhibitor, Attenuates Sepsis-Induced Inflammation in Mice
Authors: Esparza, K;Oliveira, SD;Castellon, M;Minshall, RD;Onyuksel, H;
Mediators of inflammation
Species: Mouse
Sample Types: Plasma
-
Hydroxyfasudil regulates immune balance and suppresses inflammatory responses in the treatment of experimental autoimmune encephalomyelitis
Authors: Chu, GG;Wang, J;Ding, ZB;Yin, JZ;Song, LJ;Wang, Q;Huang, JJ;Xiao, BG;Ma, CG;
International immunopharmacology
Species: Mouse
Sample Types: Cell Culture Supernates
-
CFTR dysfunction in smooth muscle drives TGF? dependent airway hyperreactivity
Authors: Kramer, EL;Hudock, KM;Davidson, CR;Clancy, JP;
Respiratory research
Species: Mouse
Sample Types: BALF
-
Composite Hydrogels of Ultrasound-Assisted-Digested Formic Acid-Decellularized Extracellular Matrix and Sacchachitin Nanofibers Incorporated with Platelet-Rich Plasma for Diabetic Wound Treatment
Authors: Lin, CJ;Lin, HL;You, WC;Ho, HO;Sheu, MT;Chen, LC;Cheng, WJ;
Journal of functional biomaterials
Species: Rat
Sample Types: Platelet-Rich Plasma
-
Anti-Fibrotic and Anti-Inflammatory Role of NO-Sensitive Guanylyl Cyclase in Murine Lung
Authors: Englert, N;Burkard, P;Aue, A;Rosenwald, A;Nieswandt, B;Friebe, A;
International journal of molecular sciences
Species: Mouse
Sample Types: BALF
-
Polysialylation controls immune function of myeloid cells in murine model of pneumococcal pneumonia
Authors: Shinde, P;Kiepas, A;Zhang, L;Sudhir, S;Konstantopoulos, K;Stamatos, NM;
Cell reports
Species: Mouse
Sample Types: Tissue Homogenates
-
Role of the IL-33/ST2 Activation Pathway in the Development of the Hepatic Fibrosis Induced by Schistosoma mansoni Granulomas in Mice
Authors: Maggi, L;Camelo, GMA;Rocha, IC;Pereira Alves, W;Moreira, JMP;Almeida Pereira, T;Tafuri, WL;Rabelo, ÉML;Correa, A;Ecco, R;Negrão-Corrêa, DA;
International journal of molecular sciences
Species: Mouse
Sample Types: Serum
-
Obesity-induced hyperglycemia impairs oral tolerance induction and aggravates food allergy
Authors: Torres, L;Camila Gonçalves Miranda, M;Dantas Martins, V;Caixeta, F;de Almeida Oliveira, M;Martins Trindade, L;Carvalho de Assis, H;Nascimento, V;Pinheiro Rosa, N;Gomes, E;Oliveira Almeida, S;Marquet, F;Genser, L;Marcelin, G;Clément, K;Russo, M;Maria Caetano Faria, A;Uceli Maioli, T;
Mucosal immunology
Species: Mouse
Sample Types: Feces
-
Antidiabetic and Immunoregulatory Activities of Extract of Phyllanthus emblica L. in NOD with Spontaneous and Cyclophosphamide-Accelerated Diabetic Mice
Authors: Lin, CH;Kuo, YH;Shih, CC;
International journal of molecular sciences
Species: Mouse
Sample Types:
-
Disruption of Prostaglandin F 2? Receptor Signaling Attenuates Fibrotic Remodeling and Alters Fibroblast Population Dynamics in A Preclinical Murine Model of Idiopathic Pulmonary Fibrosis
Authors: Rodriguez, LR;Tang, SY;Barboza, WR;Murthy, A;Tomer, Y;Cai, TQ;Iyer, S;Chavez, K;Das, US;Ghosh, S;Dimopoulos, T;Babu, A;Connelly, C;FitzGerald, GA;Beers, MF;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: BALF
-
Overexpression of fatty acid synthase attenuates bleomycin induced lung fibrosis by restoring mitochondrial dysfunction in mice
Authors: Shin, H;Park, S;Hong, J;Baek, AR;Lee, J;Kim, DJ;Jang, AS;Chin, SS;Jeong, SH;Park, SW;
Scientific reports
Species: Mouse
Sample Types: Tissue Homogenates
-
TREM2 expression on the microglia resolved lead exposure-induced neuroinflammation by promoting anti-inflammatory activities
Authors: Su, P;Zhang, J;Wu, J;Chen, H;Luo, W;Hu, M;
Ecotoxicology and environmental safety
Species: Mouse
Sample Types: Cell Culture Supernates
-
Human Wharton's jelly mesenchymal stem cells derived-exosomes enriched by miR-124 promote an anti-fibrotic response in an experimental model of liver fibrosis
Authors: Niknam, B;Baghaei, K;Mahmoud Hashemi, S;Hatami, B;Reza Zali, M;Amani, D;
International immunopharmacology
Species: Mouse
Sample Types: Serum
-
Metabololipidomic and proteomic profiling reveals aberrant macrophage activation and interrelated immunomodulatory mediator release during aging
Authors: P Schädel, A Czapka, N Gebert, ID Jacobsen, A Ori, O Werz
Aging Cell, 2023-04-26;0(0):e13856.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Knocking-Down CD147/EMMPRIN Expression in CT26 Colon Carcinoma Forces the Cells into Cellular and Angiogenic Dormancy That Can Be Reversed by Interactions with Macrophages
Authors: G Feigelman, E Simanovich, P Brockmeyer, MA Rahat
Biomedicines, 2023-03-02;11(3):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TGFbeta1+CCR5+ neutrophil subset increases in bone marrow and causes age-related osteoporosis in male mice
Authors: J Li, Z Yao, X Liu, R Duan, X Yi, A Ayoub, JO Sanders, A Mesfin, L Xing, BF Boyce
Nature Communications, 2023-01-11;14(1):159.
Species: Mouse
Sample Types: Tissue Homogenates
-
A Near-Infrared Mechanically Switchable Elastomeric Film as a Dynamic Cell Culture Substrate
Authors: G Spiaggia, P Taladriz-B, S Hengsberge, D Septiadi, C Geers, A Lee, B Rothen-Rut, A Petri-Fink
Biomedicines, 2022-12-22;11(1):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The lymphocyte-specific protein tyrosine kinase-specific inhibitor A-770041 attenuates lung fibrosis via the suppression of TGF-beta production in regulatory T-cells
Authors: K Kagawa, S Sato, K Koyama, T Imakura, K Murakami, Y Yamashita, N Naito, H Ogawa, H Kawano, Y Nishioka
PLoS ONE, 2022-10-27;17(10):e0275987.
Species: Mouse
Sample Types: BALF
-
Metformin Mitigated Obesity-Driven Cancer Aggressiveness in Tumor-Bearing Mice
Authors: CJ Chen, CC Wu, CY Chang, JR Li, YC Ou, WY Chen, SL Liao, JD Wang
International Journal of Molecular Sciences, 2022-08-15;23(16):.
Species: Mouse
Sample Types: Plasma
-
The role of PP2A /NLRP3 signaling pathway in ambient particulate matter 2.5 induced lung injury
Authors: B Han, Q Liu, X Su, L Zhou, B Zhang, H Kang, J Ning, C Li, B Zhao, Y Niu, W Chen, L Chen, R Zhang
Chemosphere, 2022-08-01;307(0):135794.
Species: Mouse
Sample Types: BALF
-
Complement activation contributes to subretinal fibrosis through the induction of epithelial-to-mesenchymal transition (EMT) in retinal pigment epithelial cells
Authors: M Llorián-Sa, EM Byrne, M Szczepan, K Little, M Chen, H Xu
Journal of Neuroinflammation, 2022-07-14;19(1):182.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Neuroprotective effects of ex vivo-expanded regulatory T cells on trimethyltin-induced neurodegeneration in mice
Authors: SY Park, H Yang, M Ye, X Liu, I Shim, YT Chang, H Bae
Journal of Neuroinflammation, 2022-06-11;19(1):143.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Transforming growth factor-beta signaling modifies the hematopoietic acute inflammatory response to drive bone marrow failure
Authors: J Javier, A Hinge, J Bartram, J Xu, MD Filippi
Haematologica, 2022-06-01;107(6):1323-1334.
Species: Mouse
Sample Types: Bone Marrow Fluid
-
Exogenous leptin enhances markers of airway fibrosis in a mouse model of chronic allergic airways disease
Authors: MD Ihrie, VL McQuade, JT Womble, A Hegde, MS McCravy, CVG Lacuesta, RM Tighe, LG Que, JKL Walker, JL Ingram
Respiratory Research, 2022-05-24;23(1):131.
Species: Mouse
Sample Types: BALF
-
Ethanol extract of Pharbitis nil ameliorates liver fibrosis through regulation of the TGFbeta1-SMAD2/3 pathway
Authors: HJ Jung, K Cho, SY Kim, JK Seong, SH Oh
Journal of ethnopharmacology, 2022-05-11;294(0):115370.
Species: Mouse
Sample Types: Tissue Homogenates
-
Aspirin-Triggered Resolvin D1 Reduces Chronic Dust-Induced Lung Pathology without Altering Susceptibility to Dust-Enhanced Carcinogenesis
Authors: EC Dominguez, R Phandthong, M Nguyen, A Ulu, S Guardado, S Sveiven, P Talbot, TM Nordgren
Cancers, 2022-04-09;14(8):.
Species: Mouse
Sample Types: Bronchoalveolar lavage fluid
-
Isolation and characterization of anti-inflammatory and anti-proliferative compound, for B-cell Non-Hodgkin lymphoma, from Nyctanthes arbor-tristis Linn
Authors: T Sana, S Qayyum, A Jabeen, BS Siddiqui, S Begum, RA Siddiqui, TB Hadda
Journal of ethnopharmacology, 2022-04-07;0(0):115267.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-CD80/86 antibodies inhibit inflammatory reaction and improve graft survival in a high-risk murine corneal transplantation rejection model
Authors: J Zhu, T Inomata, M Nakamura, K Fujimoto, Y Akasaki, K Fujio, A Yanagawa, K Uchida, J Sung, N Negishi, K Nagino, Y Okumura, M Miura, H Shokirova, M Kuwahara, K Hirosawa, A Midorikawa, A Eguchi, T Huang, H Yagita, S Habu, K Okumura, A Murakami
Scientific Reports, 2022-03-22;12(1):4853.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Expression and Function of Nicotinic Acetylcholine Receptors in Induced Regulatory T Cells
Authors: Y Nakata, K Miura, N Yamasaki, S Ogata, S Miura, N Hosomi, O Kaminuma
International Journal of Molecular Sciences, 2022-02-04;23(3):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of Secretoglobin+ (club cell) NFkappaB/RelA-TGFbeta signaling in aero-allergen-induced epithelial plasticity and subepithelial myofibroblast transdifferentiation
Authors: ME Skibba, X Xu, K Weiss, J Huisken, AR Brasier
Respiratory Research, 2021-12-20;22(1):315.
Species: Mouse, Transgenic Mouse
Sample Types: BALF
-
Intestinal CD11b+ B Cells Ameliorate Colitis by Secreting Immunoglobulin A
Authors: Ying Fu, Zhiming Wang, Baichao Yu, Yuli Lin, Enyu Huang, Ronghua Liu et al.
Frontiers in Immunology
Species: Mouse
Sample Types: Tissue Homogenates, Fecal Extract, Mucus
-
Lung Fibrosis Is Improved by Extracellular Vesicles from IFNgamma-Primed Mesenchymal Stromal Cells in Murine Systemic Sclerosis
Authors: P Rozier, M Maumus, ATJ Maria, K Toupet, C Jorgensen, P Guilpain, D Noël
Cells, 2021-10-13;10(10):.
Species: Mouse
Sample Types: Cell Lysates
-
Jararhagin-C, a disintegrin-like protein, improves wound healing in mice through stimulation of M2-like macrophage, angiogenesis and collagen deposition
Authors: BA Ferreira, FBR De Moura, TC Tomiosso, NCR Corrêa, LR Goulart, LS Barcelos, PB Clissa, FA Araújo
International immunopharmacology, 2021-10-13;101(0):108224.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Loss of miR-29a/b1 promotes inflammation and fibrosis in acute pancreatitis
Authors: S Dey, LM Udari, P RiveraHern, JJ Kwon, B Willis, JJ Easler, EL Fogel, S Pandol, J Kota
JCI Insight, 2021-10-08;0(0):.
Species: Mouse
Sample Types: Serum
-
Targeting Cpt1a-Bcl-2 interaction modulates apoptosis resistance and fibrotic remodeling
Authors: L Gu, R Surolia, JL Larson-Cas, C He, D Davis, J Kang, VB Antony, AB Carter
Cell Death and Differentiation, 2021-08-20;0(0):.
Species: Mouse
Sample Types: BALF
-
Collagen-Containing Fish Sidestream-Derived Protein Hydrolysates Support Skin Repair via Chemokine Induction
Authors: I Lapi, O Kolliniati, T Aspevik, EE Deiktakis, K Axarlis, MG Daskalaki, E Dermitzaki, M Tzardi, SC Kampranis, ZE Marsni, KC Kousoulaki, C Tsatsanis, M Venihaki
Marine Drugs, 2021-07-15;19(7):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Ex Vivo-Induced Bone Marrow-Derived Myeloid Suppressor Cells Prevent Corneal Allograft Rejection in Mice
Authors: J Zhu, T Inomata, K Fujimoto, K Uchida, K Fujio, K Nagino, M Miura, N Negishi, Y Okumura, Y Akasaki, K Hirosawa, M Kuwahara, A Eguchi, H Shokirova, A Yanagawa, A Midorikawa, A Murakami
Investigative Ophthalmology & Visual Science, 2021-06-01;62(7):3.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Opisthorchis viverrini antigens up-regulates the expression of CD80 and MHC class II in JAWSII mouse dendritic cells and promotes IL-10 and TGF-&beta secretions
Authors: S Jittimanee, S Wongratana, C Kaewraemru, J Jittimanee
Parasitology international, 2021-05-31;84(0):102401.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Adjuvants Polyphosphazene (PCEP) and a Combination of Curdlan Plus Leptin Promote a Th17-Type Immune Response to an Intramuscular Vaccine in Mice
Authors: A Chaffey, G Hamonic, D Chand, GK Mutwiri, HL Wilson
Vaccines, 2021-05-14;9(5):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
STAT6-Dependent Exacerbation of House Dust Mite-Induced Allergic Airway Disease in Mice by Multi-Walled Carbon Nanotubes
Authors: MD Ihrie, KS Duke, KA Shipkowski, DJ You, HY Lee, AJ Taylor-Jus, JC Bonner
NanoImpact, 2021-03-13;22(0):.
Species: Mouse
Sample Types: BALF
-
Isoliquiritigenin Reverses Epithelial-Mesenchymal Transition Through Modulation of the TGF-&beta/Smad Signaling Pathway in Endometrial Cancer
Authors: HY Chen, YF Chiang, JS Huang, TC Huang, YH Shih, KL Wang, M Ali, YH Hong, TM Shieh, SM Hsia
Cancers, 2021-03-11;13(6):.
Species: Mouse
Sample Types: Serum
-
Physalis angulata reduces the progression of chronic experimental periodontitis by immunomodulatory mechanisms
Authors: PS Vieceli, PJ Lima Juiz, PS Sales Laur, RD Couto, TC Barbosa To, IM Ribeiro, MB Pereira So, CF Villarreal
Journal of ethnopharmacology, 2021-03-03;0(0):113986.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Fatty Acids and a High-Fat Diet Induce Epithelial-Mesenchymal Transition by Activating TGF&beta and &beta-Catenin in Liver Cells
Authors: O Kwapisz, J Górka, A Korlatowic, J Kotlinowsk, A Waligórska, P Marona, N Pydyn, JW Dobrucki, J Jura, K Miekus
International Journal of Molecular Sciences, 2021-01-28;22(3):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Exposure to p40 in early life prevents intestinal inflammation in adulthood through inducing a long-lasting epigenetic imprint on TGF&beta: Sustained TGF&beta production by neonatal p40 exposure
Authors: Y Deng, OG McDonald, AL Means, RM Peek, MK Washington, SA Acra, DB Polk, F Yan
Cellular and Molecular Gastroenterology and Hepatology, 2021-01-19;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Spermidine attenuates bleomycin-induced lung fibrosis by inducing autophagy and inhibiting endoplasmic reticulum stress (ERS)-induced cell death in mice
Authors: AR Baek, J Hong, KS Song, AS Jang, DJ Kim, SS Chin, SW Park
Experimental & Molecular Medicine, 2020-12-14;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Th17/Treg imbalance in COPD development: suppressors of cytokine signaling and signal transducers and activators of transcription proteins
Authors: LEF Silva, JD Lourenço, KR Silva, FPR Santana, JB Kohler, AR Moreira, APP Velosa, CM Prado, RP Vieira, MV Aun, IFLC Tibério, JT Ito, FDTQS Lopes
Scientific Reports, 2020-09-17;10(1):15287.
Species: Mouse
Sample Types: Tissue Homogenates
-
Glutaredoxin deficiency promotes activation of the transforming growth factor beta pathway in airway epithelial cells, in association with fibrotic airway remodeling
Authors: SB Chia, JD Nolin, R Aboushoush, C Erikson, CG Irvin, ME Poynter, J van der Ve, DJ Taatjes, A van der Vl, V Anathy, YMW Janssen-He
Redox Biol, 2020-09-14;37(0):101720.
Species: Mouse
Sample Types: BALF
-
Overexpression of MicroRNA-16 Alleviates Atherosclerosis by Inhibition of Inflammatory Pathways
Authors: M Wang, J Li, J Cai, L Cheng, X Wang, P Xu, G Li, X Liang
Biomed Res Int, 2020-07-21;2020(0):8504238.
Species: Transgenic Mouse
Sample Types: Plasma
-
IL-7/alphaIL-7 mAb M25 immunocomplexes expand CD8+ T cells but paradoxically abrogate the antitumor activity of CTLA-4 and PD-1 blockage
Authors: D Hrabos, T Hnizdilova, J Tomala, J Uhlik, M Kovar
Cytokine, 2020-06-26;133(0):155174.
Species: Mouse
Sample Types: Serum
-
Immune modulation by complement receptor 3-dependent human monocyte TGF-beta1-transporting vesicles
Authors: LD Halder, EAH Jo, MZ Hasan, M Ferreira-G, T Krüger, M Westermann, DI Palme, G Rambach, N Beyersdorf, C Speth, ID Jacobsen, O Kniemeyer, B Jungnickel, PF Zipfel, C Skerka
Nat Commun, 2020-05-11;11(1):2331.
Species: Human, Mouse
Sample Types: Extracellular Vesicles
-
Deletion of SOCS2 Reduces Post-Colitis Fibrosis via Alteration of the TGFbeta Pathway
Authors: A Al-Araimi, A Al Kharusi, A Bani Oraba, MM Al-Maney, S Al Sinawi, I Al-Haddabi, F Zadjali
Int J Mol Sci, 2020-04-27;21(9):.
Species: Mouse, Transgenic Mouse
Sample Types: Plasma
-
Myeloid cell-derived TGF-beta signaling regulates ECM deposition in mammary carcinoma via adenosine-dependent mechanisms
Authors: G Vasiukov, T Novitskaya, A Zijlstra, P Owens, F Ye, Z Zhao, HL Moses, T Blackwell, I Feoktistov, SV Novitskiy
Cancer Res., 2020-04-20;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Temporal and spatial modulation of the tumor and systemic immune response in the murine Gl261 glioma model
Authors: KJ McKelvey, AL Hudson, R Prasanna K, JS Wilmott, GH Attrill, GV Long, RA Scolyer, SJ Clarke, HR Wheeler, CI Diakos, VM Howell
PLoS ONE, 2020-04-02;15(4):e0226444.
Species: Mouse
Sample Types: Plasma
-
An Adrenalectomy Mouse Model Reflecting Clinical Features for Chronic Fatigue Syndrome
Authors: JS Lee, YJ Jeon, SY Park, CG Son
Biomolecules, 2020-01-01;10(1):.
Species: Mouse
Sample Types: Serum
-
Maternal Obesity in Mice Exacerbates the Allergic Inflammatory Response in the Airways of Male Offspring
Authors: RR E-Lacerda, CJ Teixeira, S Bordin, E Antunes, GF Anhê
Nutrients, 2019-12-01;11(12):.
Species: Mouse
Sample Types: BALF
-
Strongyloides venezuelensis-infection alters the profile of cytokines and liver inflammation in mice co-infected with Schistosoma mansoni
Authors: MC de Rezende, JMP Moreira, LLM Fernandes, VF Rodrigues, D Negrão-Cor
Cytokine, 2019-11-26;127(0):154931.
Species: Mouse
Sample Types: Tissue Homogenates
-
Cellular immune response against NTHi infecting the pre-inflamed middle ear of the Junbo mouse
Authors: PP Vikhe, T Purnell, SDM Brown, DW Hood
Infect. Immun., 2019-11-18;0(0):.
Species: Mouse
Sample Types: Middle Ear Fluid
-
Roxatidine inhibits fibrosis by inhibiting NF?kappaB and MAPK signaling in macrophages sensing breast implant surface materials
Authors: L Ji, T Wang, L Tian, H Song, M Gao
Mol Med Rep, 2019-11-12;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Smad7 Controls Immunoregulatory PDL2/1-PD1 Signaling in Intestinal Inflammation and Autoimmunity
Authors: LP Garo, AK Ajay, M Fujiwara, V Beynon, C Kuhn, G Gabriely, S Sadhukan, R Raheja, S Rubino, HL Weiner, G Murugaiyan
Cell Rep, 2019-09-24;28(13):3353-3366.e5.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TGFbeta-induced degradation of TRAF3 in mesenchymal progenitor cells causes age-related osteoporosis
Authors: J Li, A Ayoub, Y Xiu, X Yin, JO Sanders, A Mesfin, L Xing, Z Yao, BF Boyce
Nat Commun, 2019-06-26;10(1):2795.
Species: Mouse
Sample Types: Serum
-
Role of adipose tissue inflammation in fat pad loss induced by fasting in lean and mildly obese mice
Authors: DR Lacerda, KA Costa, ALM Silveira, DF Rodrigues, AN Silva, JL Sabino, V Pinho, GB Menezes, DD Soares, MM Teixeira, AVM Ferreira
J. Nutr. Biochem., 2019-06-21;72(0):108208.
Species: Mouse
Sample Types: Tissue Homogenates
-
Extracellular vesicle-mediated macrophage activation: An insight into the mechanism of thioredoxin-mediated immune activation
Authors: C Yarana, H Thompson, L Chaiswing, DA Butterfiel, H Weiss, S Bondada, S Alhakeem, S Sukati, DK St Clair
Redox Biol, 2019-06-05;26(0):101237.
Species: Mouse
Sample Types: Cell Culture Supernates
-
In-depth characterization of congenital Zika syndrome in immunocompetent mice: Antibody-dependent enhancement and an antiviral peptide therapy
Authors: VN Camargos, G Foureaux, DC Medeiros, VT da Silveir, CM Queiroz-Ju, ALB Matosinhos, AFA Figueiredo, CDF Sousa, TP Moreira, VF Queiroz, ACF Dias, KTO Santana, I Passos, ALCV Real, LC Silva, FAG Mourão, NT Wnuk, MAP Oliveira, S Macari, T Silva, GP Garlet, JA Jackman, FM Soriani, MFD Moraes, EMAM Mendes, FM Ribeiro, GMJ Costa, AL Teixeira, NJ Cho, ACP Oliveira, MM Teixeira, VV Costa, DG Souza
EBioMedicine, 2019-05-23;0(0):.
Species: Mouse
Sample Types: Plasma
-
Combined Inhibition of TGF-beta Signaling and the PD-L1 Immune Checkpoint Is Differentially Effective in Tumor Models
Authors: HS Sow, J Ren, M Camps, F Ossendorp, P Ten Dijke
Cells, 2019-04-05;8(4):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
NLRX1 does not play a role in diabetes nor the development of diabetic nephropathy induced by multiple low doses of streptozotocin
Authors: AML Scantleber, M Uil, LM Butter, R Poelman, N Claessen, SE Girardin, S Florquin, JJTH Roelofs, JC Leemans
PLoS ONE, 2019-03-25;14(3):e0214437.
Species: Mouse
Sample Types: Tissue Homogenates
-
Inhibition of profibrotic microRNA-21 affects platelets and their releasate
Authors: T Barwari, S Eminaga, U Mayr, R Lu, PC Armstrong, MV Chan, M Sahraei, M Fernández-, T Moreau, J Barallobre, M Lynch, X Yin, C Schulte, F Baig, R Pechlaner, SR Langley, A Zampetaki, P Santer, M Weger, R Plasenzott, M Schosserer, J Grillari, S Kiechl, J Willeit, AM Shah, C Ghevaert, TD Warner, C Fernández-, Y Suárez, M Mayr
JCI Insight, 2018-11-02;3(21):.
Species: Mouse
Sample Types: Plasma
-
IGF-1-Overexpressing Mesenchymal Stem/Stromal Cells Promote Immunomodulatory and Proregenerative Effects in Chronic Experimental Chagas Disease
Authors: DN Silva, BSF Souza, CM Azevedo, JF Vasconcelo, PG de Jesus, MS Feitoza, CS Meira, GB Carvalho, BR Cavalcante, R Ribeiro-Do, MBP Soares
Stem Cells Int, 2018-07-24;2018(0):9108681.
Species: Mouse
Sample Types: Serum
-
miR-130a and miR-145 reprogram Gr-1+CD11b+ myeloid cells and inhibit tumor metastasis through improved host immunity
Authors: H Ishii, SK Vodnala, BR Achyut, JY So, MC Hollander, TF Greten, A Lal, L Yang
Nat Commun, 2018-07-04;9(1):2611.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of integrin alpha8 in murine model of lung fibrosis
Authors: CF Hung, CL Wilson, YH Chow, LM Schnapp
PLoS ONE, 2018-05-29;13(5):e0197937.
Species: Mouse
Sample Types: BALF
-
Activation of M1 macrophages play a critical role in the initiation of acute lung injury
Authors: HL Lu, XY Huang, YF Luo, WP Tan, PF Chen, YB Guo
Biosci. Rep., 2018-04-27;0(0):.
Species: Mouse
Sample Types: Serum
-
Calcineurin-mediated IL-2 production by CD11chighMHCII+myeloid cells is crucial for intestinal immune homeostasis
Authors: A Mencarelli, HJ Khameneh, J Fric, M Vacca, S El Daker, B Janela, JP Tang, S Nabti, A Balachande, TS Lim, F Ginhoux, P Ricciardi-, A Mortellaro
Nat Commun, 2018-03-16;9(1):1102.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Identification of periplakin as a major regulator of lung injury and repair in mice
Authors: V Besnard, R Dagher, T Madjer, A Joannes, M Jaillet, M Kolb, P Bonniaud, LA Murray, MA Sleeman, B Crestani
JCI Insight, 2018-03-08;3(5):.
Species: Mouse
Sample Types: BALF
-
Neuronal Transforming Growth Factor beta Signaling via SMAD3 Contributes to Pain in Animal Models of Chronic Pancreatitis
Authors: L Liu, Y Zhu, M Noë, Q Li, PJ Pasricha
Gastroenterology, 2018-03-02;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
TRAIL-Dependent Resolution of Pulmonary Fibrosis
Authors: DM Habiel, AP Moreira, UB Ismailoglu, MP Dunleavy, KA Cavassani, N van Rooije, AL Coelho, CM Hogaboam
Mediators Inflamm., 2018-01-24;2018(0):7934362.
Species: Mouse
Sample Types: Tissue Homogenates
-
Candesartan ameliorates brain inflammation associated with Alzheimer's disease
Authors: N Torika, K Asraf, RN Apte, S Fleisher-B
CNS Neurosci Ther, 2018-01-24;0(0):.
Species: Mouse, Rat
Sample Types: Cell Culture Supernates
-
The reduced osteogenic potential of Nf1-deficient osteoprogenitors is EGFR-independent
Authors: S.E. Tahaei, G. Couasnay, Y. Ma, N. Paria, J. Gu, B. F. Lemoine et al.
Bone
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lactobacillus fermentum HP3-Mediated Fermented Hericium erinaceus Juice as a Health Promoting Food Supplement to Manage Diabetes Mellitus
Authors: C Chaiyasut, S Woraharn, BS Sivamaruth, N Lailerd, P Kesika, S Peerajan
J Evid Based Integr Med, 2018-01-01;23(0):2515690X18765.
Species: Rat
Sample Types: Serum
-
HIV protease inhibitor-induced cardiac dysfunction and fibrosis is mediated by platelet-derived TGF-?1 and can be suppressed by exogenous carbon monoxide
Authors: J Laurence, S Elhadad, T Robison, H Terry, R Varshney, S Woolington, S Ghafoory, ME Choi, J Ahamed
PLoS ONE, 2017-10-31;12(10):e0187185.
Species: Mouse
Sample Types: Plasma
-
Overexpression of OSM and IL-6 impacts the polarization of pro-fibrotic macrophages and the development of bleomycin-induced lung fibrosis
Authors: EA Ayaub, A Dubey, J Imani, F Botelho, MRJ Kolb, CD Richards, K Ask
Sci Rep, 2017-10-16;7(1):13281.
Species: Mouse
Sample Types: BALF
-
IRF-8 regulates expansion of myeloid-derived suppressor cells and Foxp3+ regulatory T cells and modulates Th2 immune responses to gastrointestinal nematode infection
Authors: RM Valanparam, M Tam, PP Gros, JP Auger, M Segura, P Gros, A Jardim, TG Geary, K Ozato, MM Stevenson
PLoS Pathog., 2017-10-02;13(10):e1006647.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Ileocolic resection is associated with increased susceptibility to injury in a murine model of colitis
Authors: T Perry, M Laffin, RN Fedorak, A Thiesen, B Dicken, KL Madsen
PLoS ONE, 2017-09-18;12(9):e0184660.
Species: Mouse
Sample Types: Tissue Homogenates
-
Somatic extracts of Marshallagia marshalli downregulate the Th2 associated immune responses in ovalbumin-induced airway inflammation in BALB/c mice
Authors: S Parande Sh, A Ebrahimby, A Dousty, M Maleki, A Movassaghi, H Borji, A Haghparast
Parasit Vectors, 2017-05-12;10(1):233.
Species: Mouse
Sample Types: BALF
-
Hypercholesterolemia Induces Differentiation of Regulatory T Cells in the Liver
Authors: RK Mailer, A Gisterå, KA Polyzos, DF Ketelhuth, GK Hansson
Circ. Res., 2017-04-18;0(0):.
Species: Mouse
Sample Types: Plasma
-
Distinctive role of efferocytosis in dendritic cell maturation and migration in sterile or infectious conditions
Authors: L de Aquino, NN Dejani, FF Verdan, AB Orlando, V Ninõ, F De Nuzzi D, AC Salina, AI Medeiros
Immunology, 2017-04-10;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Brazilian red propolis improves cutaneous wound healing suppressing inflammation-associated transcription factor NF?B
Authors: Flavia Regina Sobreira Corrêa
Biomed. Pharmacother, 2016-12-12;86(0):162-171.
Species: Mouse
Sample Types: Plasma
-
Neuronal Protein 3.1 Deficiency Leads to Reduced Cutaneous Scar Collagen Deposition and Tensile Strength due to Impaired Transforming Growth Factor-?1 to -?3 Translation
Authors: Tao Cheng
Am. J. Pathol, 2016-12-08;0(0):.
Species: Mouse
Sample Types: Cell Lysates
-
The phytogestrogenic stilbenes, arachidin-1 and resveratrol, modulate regulatory T�cell functions responsible for successful aging in aged ICR mice
Authors: Robin Yih-Yuan Chiou
Int. J. Mol. Med., 2016-10-27;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Induced regulatory T cells are phenotypically unstable and do not protect mice from rapidly progressive glomerulonephritis
Immunology, 2016-10-10;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Polarization of macrophages towards M2 phenotype is favored by reduction in iPLA2?
J Biol Chem, 2016-09-20;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Proinflammatory M1 macrophages inhibit RANKL-induced osteoclastogenesis
Infect Immun, 2016-09-19;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Regulation of eosinophilia and allergic airway inflammation by the glycan-binding protein galectin-1
Proc Natl Acad Sci USA, 2016-07-25;0(0):.
Species: Mouse
Sample Types: BALF
-
High endogenous activated protein C levels attenuates bleomycin-induced pulmonary fibrosis
Authors: Cong Lin
J Cell Mol Med, 2016-06-14;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Gene Therapy Induces Antigen-Specific Tolerance in Experimental Collagen-Induced Arthritis
PLoS ONE, 2016-05-09;11(5):e0154630.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Induction of Siglec-1 by Endotoxin Tolerance Suppresses the Innate Immune Response by Promoting TGF-?1 Production
Authors: Y Wu, C Lan, D Ren, GY Chen
J. Biol. Chem., 2016-04-18;291(23):12370-82.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Protease activated receptor-1 regulates macrophage-mediated cellular senescence: a risk for idiopathic pulmonary fibrosis
Authors: C Lin, F Rezaee, M Waasdorp, K Shi, T van der Po, K Borensztaj, CA Spek
Oncotarget, 2015-11-03;6(34):35304-14.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat Feeding.
Authors: Jovicic N, Jeftic I, Jovanovic I, Radosavljevic G, Arsenijevic N, Lukic M, Pejnovic N
PLoS ONE, 2015-07-28;10(7):e0134089.
Species: Mouse
Sample Types: Tissue Homogenates
-
Influenza A virus-dependent remodeling of pulmonary clock function in a mouse model of COPD.
Authors: Sundar, Isaac K, Ahmad, Tanveer, Yao, Hongwei, Hwang, Jae-woon, Gerloff, Janice, Lawrence, B Paige, Sellix, Michael, Rahman, Irfan
Sci Rep, 2015-04-29;4(0):9927.
Species: Mouse
Sample Types: BALF
-
IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis.
Authors: Coffelt S, Kersten K, Doornebal C, Weiden J, Vrijland K, Hau C, Verstegen N, Ciampricotti M, Hawinkels L, Jonkers J, de Visser K
Nature, 2015-03-30;522(7556):345-8.
Species: Mouse
Sample Types: Tissue Homogenates
-
Intestinal helminths regulate lethal acute graft-versus-host disease and preserve the graft-versus-tumor effect in mice.
Authors: Li Y, Chen H, Bannick N, Henry M, Holm A, Metwali A, Urban J, Rothman P, Weiner G, Blazar B, Elliott D, Ince M
J Immunol, 2014-12-19;194(3):1011-20.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The glycosylated Rv1860 protein of mycobacterium tuberculosis inhibits dendritic cell mediated TH1 and TH17 polarization of T cells and abrogates protective immunity conferred by BCG.
Authors: Satchidanandam V, Kumar N, Jumani R, Challu V, Elangovan S, Khan N
PLoS Pathog, 2014-06-12;10(6):e1004176.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mesenchymal stem cells recruited by active TGFbeta contribute to osteogenic vascular calcification.
Authors: Wang W, Li C, Pang L, Shi C, Guo F, Chen A, Cao X, Wan M
Stem Cells Dev, 2014-03-20;23(12):1392-404.
Species: Mouse
Sample Types: Plasma
-
Genetic suppression of inflammation blocks the tumor-promoting effects of TGF-beta in gastric tissue.
Authors: Ota M, Horiguchi M, Fang V, Shibahara K, Kadota K, Loomis C, Cammer M, Rifkin D
Cancer Res, 2014-03-03;74(9):2642-51.
Species: Mouse
Sample Types: Serum
-
Unique macrophages different from M1/M2 macrophages inhibit T cell mitogenesis while upregulating Th17 polarization.
Authors: Tatano Y, Shimizu T, Tomioka H
Sci Rep, 2014-02-20;4(0):4146.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dichloroacetate modulates cytokines toward T helper 1 function via induction of the interleukin-12-interferon-gamma pathway.
Authors: Badr M, Qinna N, Qadan F, Matalka K
Onco Targets Ther, 2014-02-07;7(0):193-201.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The monocyte to macrophage transition in the murine sterile wound.
Authors: Crane, Meredith, Daley, Jean M, van Houtte, Olivier, Brancato, Samielle, Henry, William, Albina, Jorge E
PLoS ONE, 2014-01-22;9(1):e86660.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Hyperactive transforming growth factor-beta1 signaling potentiates skeletal defects in a neurofibromatosis type 1 mouse model.
Authors: Rhodes S, Wu X, He Y, Chen S, Yang H, Staser K, Wang J, Zhang P, Jiang C, Yokota H, Dong R, Peng X, Yang X, Murthy S, Azhar M, Mohammad K, Xu M, Guise T, Yang F
J Bone Miner Res, 2013-12-01;28(12):2476-89.
Species: Mouse
Sample Types: Serum
-
Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation.
Authors: Horton J, Hudak K, Chung E, White A, Scroggins B, Burkeen J, Citrin D
Stem Cells, 2013-10-01;31(10):2231-41.
Species: Mouse
Sample Types: Tissue Homogenates
-
Toll-like Receptor 2 (TLR2), Transforming Growth Factor-beta, Hyaluronan (HA), and Receptor for HA-mediated Motility (RHAMM) Are Required for Surfactant Protein A-stimulated Macrophage Chemotaxis.
Authors: Foley J, Lam D, Jiang H, Liao J, Cheong N, McDevitt T, Zaman A, Wright J, Savani R
J Biol Chem, 2012-09-04;287(44):37406-19.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection.
Authors: Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, Riva F, Garlanda C
Infect. Immun., 2011-10-24;80(1):100-9.
Species: Mouse
Sample Types: Serum
-
High expression of IL-22 suppresses antigen-induced immune responses and eosinophilic airway inflammation via an IL-10-associated mechanism.
Authors: Nakagome K, Imamura M, Kawahata K
J. Immunol., 2011-10-12;187(10):5077-89.
Species: Mouse
Sample Types: BALF
-
The Therapeutic Potential of the Humoral Pattern Recognition Molecule PTX3 in Chronic Lung Infection Caused by Pseudomonas aeruginosa.
Authors: Moalli F, Paroni M, Veliz Rodriguez T, Riva F, Polentarutti N, Bottazzi B, Valentino S, Mantero S, Nebuloni M, Mantovani A, Bragonzi A, Garlanda C
J. Immunol., 2011-03-25;186(9):5425-34.
Species: Mouse
Sample Types: Tissue Homogenates
-
Phosphoinositide 3-kinase gamma plays a critical role in bleomycin-induced pulmonary inflammation and fibrosis in mice.
Authors: Russo RC, Garcia CC, Barcelos LS, Rachid MA, Guabiraba R, Roffe E, Souza AL, Sousa LP, Mirolo M, Doni A, Cassali GD, Pinho V, Locati M, Teixeira MM
J. Leukoc. Biol., 2010-11-02;89(2):269-82.
Species: Mouse
Sample Types: Tissue Homogenates
-
A critical role for C5L2 in the pathogenesis of experimental allergic asthma.
Authors: Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, Konig P, Gerard NP, Gerard C, Wills-Karp M, Kohl J
J. Immunol., 2010-10-25;185(11):6741-52.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Importance of TLR2 in the direct response of T lymphocytes to Schistosoma mansoni antigens.
Authors: Burton OT, Gibbs S, Miller N, Jones FM, Wen L, Dunne DW, Cooke A, Zaccone P
Eur. J. Immunol., 2010-08-01;40(8):2221-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
T-cell polarization depends on concentration of the danger signal used to activate dendritic cells.
Authors: Peters M, Dudziak K, Stiehm M
Immunol. Cell Biol., 2010-02-02;88(5):537-44.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity.
Authors: Sellge G, Magalhaes JG, Konradt C, Fritz JH, Salgado-Pabon W, Eberl G, Bandeira A, Di Santo JP, Sansonetti PJ, Phalipon A
J. Immunol., 2010-01-20;184(4):2076-85.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Latent herpesvirus infection augments experimental pulmonary fibrosis.
Authors: Vannella KM, Luckhardt TR, Wilke CA, van Dyk LF, Toews GB, Moore BB
Am. J. Respir. Crit. Care Med., 2009-12-10;181(5):465-77.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1.
Authors: Zhou G, Dada LA, Wu M, Kelly A, Trejo H, Zhou Q, Varga J, Sznajder JI
Am. J. Physiol. Lung Cell Mol. Physiol., 2009-10-02;297(6):L1120-30.
Species: Rat
Sample Types: Cell Culture Supernates
-
BCL9 promotes tumor progression by conferring enhanced proliferative, metastatic, and angiogenic properties to cancer cells.
Authors: Mani M, Carrasco DE, Zhang Y, Takada K, Gatt ME, Dutta-Simmons J, Ikeda H, Diaz-Griffero F, Pena-Cruz V, Bertagnolli M, Myeroff LL, Markowitz SD, Anderson KC, Carrasco DR
Cancer Res., 2009-09-08;69(19):7577-86.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mesenchymal stem cells produce Wnt isoforms and TGF-beta1 that mediate proliferation and procollagen expression by lung fibroblasts.
Authors: Salazar KD, Lankford SM, Brody AR
Am. J. Physiol. Lung Cell Mol. Physiol., 2009-09-04;297(5):L1002-11.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mucosal immunity in mice induced by orally administered transgenic rice.
Authors: Zhang X, Yuan Z, Duan Q, Zhu H, Yu H, Wang Q
Vaccine, 2009-01-13;27(10):1596-600.
Species: Mouse
Sample Types: Cell Culture Supernates
-
CD69+ CD4+ CD25- T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1.
Authors: Han Y, Guo Q, Zhang M, Chen Z, Cao X
J. Immunol., 2009-01-01;182(1):111-20.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Coexpression of TGF-beta1 and IL-10 enables regulatory T cells to completely suppress airway hyperreactivity.
Authors: Presser K, Schwinge D, Wegmann M, Huber S, Schmitt S, Quaas A, Maxeiner JH, Finotto S, Lohse AW, Blessing M, Schramm C
J. Immunol., 2008-12-01;181(11):7751-8.
Species: Mouse
Sample Types: BALF
-
Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy.
Authors: Jaffre F, Bonnin P, Callebert J, Debbabi H, Setola V, Doly S, Monassier L, Mettauer B, Blaxall BC, Launay JM, Maroteaux L
Circ. Res., 2008-11-20;104(1):113-23.
Species: Mouse
Sample Types: Plasma
-
Methylprednisolone improves lung mechanics and reduces the inflammatory response in pulmonary but not in extrapulmonary mild acute lung injury in mice.
Authors: Leite-Junior JH, Garcia CS, Souza-Fernandes AB, Silva PL, Ornellas DS, Larangeira AP, Castro-Faria-Neto HC, Morales MM, Negri EM, Capelozzi VL, Zin WA, Pelosi P, Bozza PT, Rocco PR
Crit. Care Med., 2008-09-01;36(9):2621-8.
Species: Mouse
Sample Types: BALF
-
Expression of inflammatory mediators in the otitis media induced by Helicobacter pylori antigen in mice.
Authors: Kariya S, Okano M, Fukushima K, Nomiya S, Kataoka Y, Nomiya R, Akagi H, Nishizaki K
Clin. Exp. Immunol., 2008-08-22;154(1):134-40.
Species: Mouse
Sample Types: Middle Ear Lavage Fluid
-
In vivo investigations on anti-fibrotic potential of proteasome inhibition in lung and skin fibrosis.
Authors: Fineschi S, Bongiovanni M, Donati Y, Djaafar S, Naso F, Goffin L, Argiroffo CB, Pache JC, Dayer JM, Ferrari-Lacraz S, Chizzolini C
Am. J. Respir. Cell Mol. Biol., 2008-05-05;39(4):458-65.
Species: Mouse
Sample Types: BALF
-
Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection.
Authors: Bozza S, Zelante T, Moretti S, Bonifazi P, DeLuca A, D'Angelo C, Giovannini G, Garlanda C, Boon L, Bistoni F, Puccetti P, Mantovani A, Romani L
J. Immunol., 2008-03-15;180(6):4022-31.
Species: Mouse
Sample Types: Tissue Homogenates
-
Transforming growth factor-beta regulates house dust mite-induced allergic airway inflammation but not airway remodeling.
Authors: Fattouh R, Midence NG, Arias K, Johnson JR, Walker TD, Goncharova S, Souza KP, Gregory RC, Lonning S, Gauldie J, Jordana M
Am. J. Respir. Crit. Care Med., 2008-01-03;177(6):593-603.
Species: Mouse
Sample Types: BALF
-
CD40L disruption enhances Abeta vaccine-mediated reduction of cerebral amyloidosis while minimizing cerebral amyloid angiopathy and inflammation.
Authors: Obregon D, Hou H, Bai Y, Nikolic WV, Mori T, Luo D, Zeng J, Ehrhart J, Fernandez F, Morgan D, Giunta B, Town T, Tan J
Neurobiol. Dis., 2007-10-16;29(2):336-53.
Species: Mouse
Sample Types: Plasma
-
Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis.
Authors: Ephrem A, Chamat S, Miquel C, Fisson S, Mouthon L, Caligiuri G, Delignat S, Elluru S, Bayry J, Lacroix-Desmazes S, Cohen JL, Salomon BL, Kazatchkine MD, Kaveri SV, Misra N
Blood, 2007-10-11;111(2):715-22.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Poly(ADP-ribose) polymerase-1 (PARP-1) controls lung cell proliferation and repair after hyperoxia-induced lung damage.
Authors: Pagano A, Metrailler-Ruchonnet I, Aurrand-Lions M, Lucattelli M, Donati Y, Argiroffo CB
Am. J. Physiol. Lung Cell Mol. Physiol., 2007-06-15;293(3):L619-29.
Species: Mouse
Sample Types: BALF
-
Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity.
Authors: Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, Collins C, Viala J, Ferrero RL, Girardin SE, Philpott DJ
Immunity, 2007-04-12;26(4):445-59.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Induction of prolonged infiltration of T lymphocytes and transient T lymphocyte-dependent collagen deposition in mouse lungs following adenoviral gene transfer of CCL18.
Authors: Luzina IG, Papadimitriou JC, Anderson R, Pochetuhen K, Atamas SP
Arthritis Rheum., 2006-08-01;54(8):2643-55.
Species: Mouse
Sample Types: BALF
-
Inhibition of angiogenesis by interleukin-4 gene therapy in rat adjuvant-induced arthritis.
Authors: Haas CS, Amin MA, Allen BB, Ruth JH, Haines GK, Woods JM, Koch AE
Arthritis Rheum., 2006-08-01;54(8):2402-14.
Species: Rat
Sample Types: Tissue Homogenates
-
Hierarchical suppression of asthma-like responses by mucosal tolerance.
Authors: Keller AC, Mucida D, Gomes E, Faquim-Mauro E, Faria AM, Rodriguez D, Russo M
J. Allergy Clin. Immunol., 2006-02-01;117(2):283-90.
Species: Mouse
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse TGF-beta 1 DuoSet ELISA
Average Rating: 4.5 (Based on 15 Reviews)
Have you used Mouse TGF-beta 1 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
very economic and stable kit
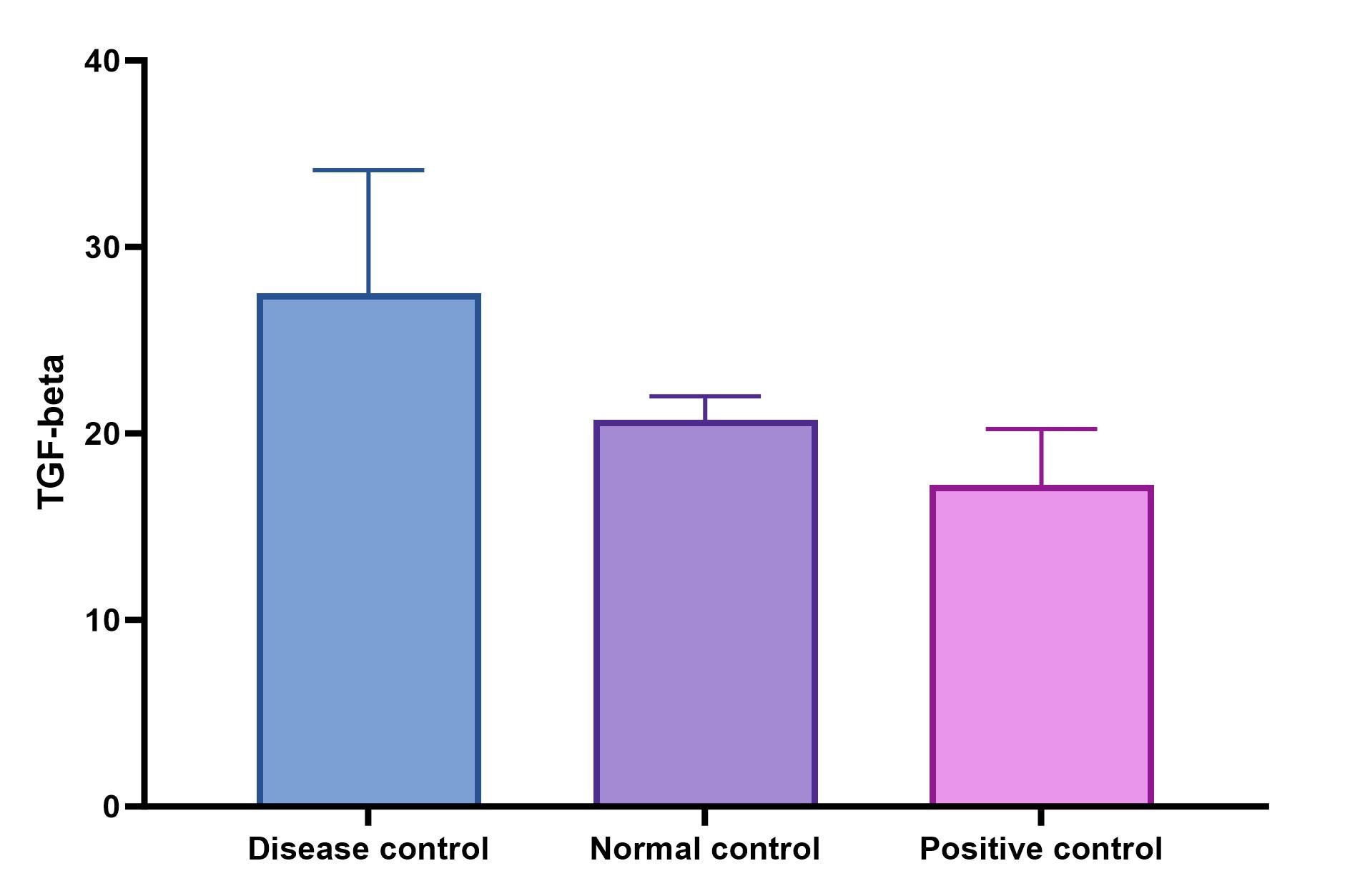
we used this kit to quantify Mouse TGF beta 1 mouse sera, it produces a very good standard curve, levels of TGF beta in control B6 mice is very low.
TGF-1 in cell culture supernatants was measured after converting latent TGF-1 to active TGF-1 by acidification (10-min incubation at room temperature with 0.2 volume of 1 N HCl for cell culture supernatants, followed by neutralization by adding the same volume
of 1.2 N NaOH in 0.5 M HEPES) with a ELISA assay.
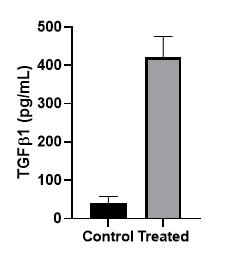
Followed the kit directions for Serum/Plasma TGF-b activation. Our serum needed to be diluted due to the high BCA levels. We were able to see a difference on our samples and the standard curve looked great.
I tested mouse serum with acid activation using RnD System's sample activation kit. Serum was tested at 1:100 which produced OD values within the linear range of the standard curve.