Rat IL-1 beta/IL-1F2 Quantikine ELISA Kit Summary
Product Summary
Precision
Cell Culture Supernates, Serum, EDTA Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 83.4 | 253 | 610 | 82.3 | 249 | 626 |
| Standard Deviation | 7.3 | 9.9 | 24 | 4.7 | 10.3 | 27 |
| CV% | 8.8 | 3.9 | 3.9 | 5.7 | 4.1 | 4.4 |
Recovery
The recovery of rat IL-1 beta spiked to three levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Supernates (n=5) | 100 | 89-110 |
| EDTA Plasma (n=5) | 98 | 89-111 |
| Serum (n=5) | 108 | 97-120 |
Linearity
Scientific Data
 View Larger
View Larger
Rat IL-1 beta/IL-1F2 Quantikine ELISA Kit Platelet caspase 1 activity and IL‐1 beta secretion in response to CLP. Caspase 1 activity (A) was measured in platelet homogenates obtained from CLP and Sham rats. IL‐1 beta concentration (B) was measured in conditioned media from CLP and Sham rat platelets via ELISA. *P < 0.05 versus Sham. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31054188), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
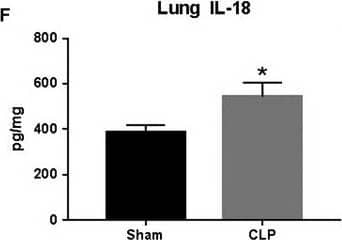
Detection of Rat IL-1 beta/IL-1F2 IL‐1 beta and IL‐18 expression in response to CLP. Plasma (A and D), kidney (B and E), and lung (C and F) IL‐1 beta and IL‐18 were measured in Sham and CLP rats (n = 8/group) via ELISA. *P < 0.05 versus Sham Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31054188), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
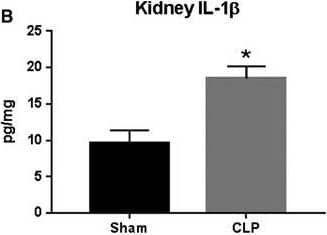
Detection of Rat IL-1 beta/IL-1F2 IL‐1 beta and IL‐18 expression in response to CLP. Plasma (A and D), kidney (B and E), and lung (C and F) IL‐1 beta and IL‐18 were measured in Sham and CLP rats (n = 8/group) via ELISA. *P < 0.05 versus Sham Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31054188), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
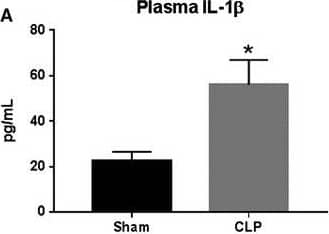
Detection of Rat IL-1 beta/IL-1F2 IL‐1 beta and IL‐18 expression in response to CLP. Plasma (A and D), kidney (B and E), and lung (C and F) IL‐1 beta and IL‐18 were measured in Sham and CLP rats (n = 8/group) via ELISA. *P < 0.05 versus Sham Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31054188), licensed under a CC-BY license. Not internally tested by R&D Systems.
Product Datasheets
Preparation and Storage
Background: IL-1 beta/IL-1F2
The Interleukin 1 (IL-1) family of proteins consists of the classic members IL-1 alpha, IL-1 beta, and IL-1ra, plus IL-18, IL-33, and IL-1F5-10. IL-1 alpha and IL-1 beta bind to the same cell surface receptors and share biological functions (1). IL-1 is not produced by unstimulated cells of healthy mice with the exception of skin keratinocytes, some epithelial cells, and certain cells of the central nervous system. In response to inflammatory agents, infections, or microbial endotoxins, however, a dramatic increase in the production of IL-1 by macrophages and various other cell types is observed. IL-1 beta plays a central role in immune and inflammatory responses, bone remodeling, fever, carbohydrate metabolism, and GH/IGF-I physiology. Inappropriate or prolonged production of IL-1 has been implicated in a variety of pathological conditions including sepsis, rheumatoid arthritis, inflammatory bowel disease, acute and chronic myelogenous leukemia, insulin-dependent diabetes mellitus, atherosclerosis, neuronal injury, and aging-related diseases (2-5).
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 50 µL of Assay Diluent to each well.
- Add 50 µL of Standard, Control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process 4 times for a total of 5 washes.
- Add 100 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours.
- Aspirate and wash 5 times.
- Add 100 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes. PROTECT FROM LIGHT.
- Add 100 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Rat IL-1 beta/IL-1F2 Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
172
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Tabernaemontana stapfiana Britten (Apocynaceae) Stem Bark Prevents Alcl3-Induced Cognitive Disability: Antioxidant and Anti-Inflammatory Activities in Wistar Rats
Authors: Ngenteh, ML;Sanda, KA;Neng, TP;Fubi, BBL;Nchang, NR;Nsah, KB;Mahamat, O;
Oxidative medicine and cellular longevity
Species: Rat
Sample Types: Serum
-
Nitazoxanide mitigates methotrexate hepatotoxicity in rats: role in inhibiting apoptosis and regulating endoplasmic reticulum stress
Authors: Mahmoud, NM;Elshazly, SM;El-Shaarawy, F;Zaitone, SA;Aldahish, AA;Ahmed, GA;Fawzy, MS;Aloyouni, SY;Abed, SY;Saeedi, T;El-Sayed, SS;
Frontiers in pharmacology
Species: Rat
Sample Types: Tissue Homogenates
-
IL-1 Blockade Mitigates Autism and Cerebral Palsy Traits in Offspring In-Utero Exposed to Group B Streptococcus Chorioamnionitis
Authors: Ayash, TA;Allard, MJ;Chevin, M;Sébire, G;
International journal of molecular sciences
Species: Rat
Sample Types: Serum
-
Deep brain stimulation mitigates memory deficits in a rodent model of traumatic brain injury
Authors: Rabelo, TK;Campos, ACP;Almeida Souza, TH;Mahmud, F;Popovic, MR;Covolan, L;Betta, VC;DaCosta, L;Lipsman, N;Diwan, M;Hamani, C;
Brain stimulation
Species: Rat
Sample Types: Tissue Homogenates
-
Development of a proliposomal pretomanid dry powder inhaler as a novel alternative approach for combating pulmonary tuberculosis
Authors: Aekwattanaphol, N;Das, SC;Khadka, P;Nakpheng, T;Ali Khumaini Mudhar Bintang, M;Srichana, T;
International journal of pharmaceutics
Species: Rat
Sample Types: Cell Culture Supernates
-
Pioglitazone treatment mitigates cardiovascular bioprosthetic degeneration in a chronic kidney disease model
Authors: Katahira, S;Barth, M;Döpp, R;Sugimura, Y;Schmidt, V;Selig, JI;Saiki, Y;Jankowski, J;Marx, N;Jahnen-Dechent, W;Lichtenberg, A;Akhyari, P;
Frontiers in pharmacology
Species: Rat
Sample Types: Plasma
-
Geranylgeranylacetone mitigates sepsis-associated intestinal injury through CHIP-dependent anti-inflammation and anti-oxidative effect
Authors: Liu, X;Liu, Y;Su, X;Jiang, L;Tang, G;Wang, Y;
International immunopharmacology
Species: Rat
Sample Types: Cell Culture Supernates
-
The Effect of Pregabalin on Microglia Differentiation in Rat with Neuropathic pain: A Preliminary Study
Authors: Hong, SW;Piao, L;Cho, EH;Seo, EH;Kim, SH;
International journal of medical sciences
Species: Rat
Sample Types: Tissue Homogenates
-
Asiatic acid rescues intestinal tissue by suppressing molecular, biochemical, and histopathological changes associated with the development of ulcerative colitis
Authors: Lokman, MS;Kassab, R;Salem, FAM;Elshopakey, GE;Hussein, A;Aldarmahi, AA;Theyab, A;Alzahrani, KJ;Hassan, KE;Alsharif, KF;Albrakati, A;Tayyeb, JZ;El-Khadragy, MF;Alkhateeb, MA;Al-Ghamdy, AO;Althagafi, HA;Abdel Moneim, A;El-Hennamy, RE;
Bioscience reports
Species: Rat
Sample Types: Tissue Homogenates
-
Neuroprotective effects of a novel peptide through the Rho-integrin-Tie2 and PI3K/Akt pathways in experimental autoimmune encephalomyelitis model
Authors: Zhou, W;Qu, H;Fu, XX;Xu, MM;Li, Q;Jiang, Y;Han, S;
Frontiers in pharmacology
Species: Rat
Sample Types: Plasma
-
Development and Characterization of a Cancer Cachexia Rat Model Transplanted with Cells of the Rat Lung Adenocarcinoma Cell Line Sato Lung Cancer (SLC)
Authors: Kasumi, E;Chiba, M;Kuzumaki, Y;Kuzuoka, H;Sato, N;Takahashi, B;
Biomedicines
Species: Rat
Sample Types: Serum
-
A novel modified-curcumin 2.24 resolves inflammation by promoting M2 macrophage polarization
Authors: Deng, J;Golub, LM;Lee, HM;Bhatt, HD;Johnson, F;Xu, TM;Gu, Y;
Scientific reports
Species: Rat
Sample Types: Cell Culture Supernates
-
Sex differences in microglia function in aged rats underlie vulnerability to cognitive decline
Authors: Ince, LM;Darling, JS;Sanchez, K;Bell, KS;Melbourne, JK;Davis, LK;Nixon, K;Gaudet, AD;Fonken, LK;
Brain, behavior, and immunity
Species: Rat
Sample Types: Tissue Homogenates
-
Melatonin Alleviates Intestinal Barrier Damaging Effects Induced by Polyethylene Microplastics in Albino Rats
Authors: El Gazzar, WB;Sliem, RE;Bayoumi, H;Nasr, HE;Shabanah, M;Elalfy, A;Radwaan, SE;Gebba, MA;Mansour, HM;Badr, AM;Amer, MF;Ashour, SS;Morsi, H;Aboelkomsan, ESAF;Baioumy, B;Sayed, AEH;Farag, AA;
International journal of molecular sciences
Species: Rat
Sample Types: Cell Culture Supernates
-
Allicin protects against LPS-induced cardiomyocyte injury by activating Nrf2-HO-1 and inhibiting NLRP3 pathways
Authors: Sun, F;Xu, K;Zhou, J;Zhang, W;Duan, G;Lei, M;
BMC cardiovascular disorders
Species: Rat
Sample Types: Cell Culture Supernates
-
Hydrogen alleviated cognitive impairment and blood‒brain barrier damage in sepsis-associated encephalopathy by regulating ABC efflux transporters in a PPAR alpha -dependent manner
Authors: Bai, Y;Mi, W;Meng, X;Dong, B;Jiang, Y;Lu, Y;Yu, Y;
BMC neuroscience
Species: Mouse
Sample Types: Tissue Homogenates
-
Therapeutic Effect of Enzymatically Hydrolyzed Cervi Cornu Collagen NP-2007 and Potential for Application in Osteoarthritis Treatment
Authors: Kim, HR;Lee, SH;Noh, EM;Choi, B;Seo, HY;Jang, H;Kim, SY;Park, MH;
International journal of molecular sciences
Species: Rat
Sample Types: Cell Culture Supernates, Serum
-
Aqueous cannabidiol ?-cyclodextrin complexed polymeric micelle nasal spray to attenuate in vitro and ex vivo SARS-CoV-2-induced cytokine storms
Authors: Changsan, N;Sawatdee, S;Suedee, R;Chunhachaichana, C;Srichana, T;
International journal of pharmaceutics
Species: Mouse
Sample Types: Cell Culture Supernates
-
Natural antioxidant formula ameliorates lipopolysaccharide-induced impairment of hippocampal neurogenesis and contextual fear memory through suppression of neuroinflammation in rats
Authors: Zeng, W;Takashima, K;Tang, Q;Zou, X;Ojiro, R;Ozawa, S;Jin, M;Ando, Y;Yoshida, T;Shibutani, M;
Journal of chemical neuroanatomy
Species: Rat
Sample Types: Tissue Homogenates
-
Artificial nerve graft constructed by coculture of activated Schwann cells and human hair keratin for repair of peripheral nerve defects
Authors: HJ Qin, H Li, JZ Chen, KR Zhang, XQ Zhao, JQ Qin, B Yu, J Yang
Neural regeneration research, 2023-05-01;18(5):1118-1123.
Species: Rat
Sample Types: Cell Culture Supernates
-
Ameliorative effect of cheqianzi decoction on hyperuricemia and kidney injury and underlying mechanism in rats
Authors: J Meng, J Tian, Y Zhao, C Li, Y Yi, Y Zhang, J Han, L Wang, C Pan, S Liu, C Liu, F Wang, X Tang, D Wang, S Qin, A Liang
Heliyon, 2023-04-11;9(4):e15333.
Species: Rat
Sample Types: Tissue Homogenates
-
Hemoglobin Derived from Subarachnoid Hemorrhage-Induced Pyroptosis of Neural Stem Cells via ROS/NLRP3/GSDMD Pathway
Authors: T Yue, X Li, X Chen, T Zhu, W Li, B Wang, C Hang
Oxidative Medicine and Cellular Longevity, 2023-01-16;2023(0):4383332.
Species: Rat
Sample Types: Cell Culture Supernates
-
Yiyi Fuzi Baijiang Powder Alleviates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Rats via Inhibiting the TLR4/NF-kappaB/NLRP3 Inflammasome Signaling Pathway to Repair the Intestinal Epithelial Barrier, and Modulating Intestinal Microbiota
Authors: J Yang, L Miao, Y Xue, X Wang
Oxidative Medicine and Cellular Longevity, 2023-01-14;2023(0):3071610.
Species: Rat
Sample Types: Tissue Homogenates
-
Ameliorative effect of cheqianzi decoction on hyperuricemia and kidney injury and underlying mechanism in rats
Authors: J Meng, J Tian, Y Zhao, C Li, Y Yi, Y Zhang, J Han, L Wang, C Pan, S Liu, C Liu, F Wang, X Tang, D Wang, S Qin, A Liang
Heliyon, 2023;9(4):e15333.
Species: Rat
Sample Types: Tissue Homogenates
-
A Comparative Study of Two Models of Intraluminal Filament Middle Cerebral Artery Occlusion in Rats: Long-Lasting Accumulation of Corticosterone and Interleukins in the Hippocampus and Frontal Cortex in Koizumi Model
Authors: MV Onufriev, MY Stepaniche, YV Moiseeva, MY Zhanina, OA Nedogreeva, PA Kostryukov, NA Lazareva, NV Gulyaeva
Biomedicines, 2022-12-02;10(12):.
Species: Rat
Sample Types: Serum
-
Black Garlic and Thiosulfinate-Enriched Extracts as Adjuvants to Ceftriaxone Treatment in a Rat Peritonitis Model of Sepsis
Authors: FJ Redondo-Ca, N Bejarano-R, V Baladrón, O Montenegro, LA Gómez, R Velasco, N Villasanti, S Illescas, MT Franco-Ser, I Gracia, JF Rodríguez, JR Muñoz-Rodr, JM Pérez-Orti
Biomedicines, 2022-12-01;10(12):.
Species: Rat
Sample Types: Serum
-
Targeting caveolae to pump bispecific antibody to TGF-beta into diseased lungs enables ultra-low dose therapeutic efficacy
Authors: AH Kadam, K Kandasamy, T Buss, B Cederstrom, C Yang, S Narayanapi, J Rodriguez, MD Levin, J Koziol, B Olenyuk, Z Borok, A Chrastina, JE Schnitzer
PLoS ONE, 2022-11-22;17(11):e0276462.
Species: Rat
Sample Types: BALF
-
Assessing the In Vivo Biocompatibility of Molecularly Imprinted Polymer Nanoparticles
Authors: S Kassem, SS Piletsky, H Yesilkaya, O Gazioglu, M Habtom, F Canfarotta, E Piletska, AC Spivey, EO Aboagye, SA Piletsky
Polymers, 2022-10-28;14(21):.
Species: Rat
Sample Types: Serum
-
MicroRNA-25-5p negatively regulates TXNIP expression and relieves inflammatory responses of brain induced by lipopolysaccharide
Authors: J Wang, Z Ye, Y Chen, X Qiao, Y Jin
Scientific Reports, 2022-10-26;12(1):17915.
Species: Rat
Sample Types: Plasma
-
DUSP8/TAK1 signaling mediates neuropathic pain through regulating neuroinflammation and neuron death in a spinal nerve ligation (SNL) rat model
Authors: C Liao, H Zhou, H Chen, G Cheng, S Li, F Ma, X Yang, B Xie, W Zhang
International immunopharmacology, 2022-10-21;113(0):109284.
Species: Rat
Sample Types: Cell Culture Supernates
-
Sacubitril/valsartan (LCZ696) ameliorates hyperthyroid-induced cardiac hypertrophy in male rats through modulation of miR-377, let-7 b, autophagy, and fibrotic signaling pathways
Authors: T Khamis, AE Alsemeh, DM Abdullah
Scientific Reports, 2022-08-27;12(1):14654.
Species: Rat
Sample Types: Serum
-
Rhabdomyolysis-induced acute kidney injury and concomitant apoptosis induction via ROS-mediated ER stress is efficaciously counteracted by epigallocatechin gallate
Authors: SN Chang, M Haroon, DK Dey, SC Kang
The Journal of nutritional biochemistry, 2022-08-24;0(0):109134.
Species: Rat
Sample Types: Tissue Homogenates
-
Transient neuroinflammation following surgery contributes to long-lasting cognitive decline in elderly rats via dysfunction of synaptic NMDA receptor
Authors: B Chen, G Qin, J Xiao, X Deng, A Lin, H Liu
Journal of Neuroinflammation, 2022-07-13;19(1):181.
Species: Rat
Sample Types: Serum
-
N-3 PUFA Ameliorates the Gut Microbiota, Bile Acid Profiles, and Neuropsychiatric Behaviours in a Rat Model of Geriatric Depression
Authors: TH Tung, YC Chen, YT Lin, SY Huang
Biomedicines, 2022-07-04;10(7):.
Species: Rat
Sample Types: Plasma
-
PPARalpha contributes to the therapeutic effect of hydrogen gas against sepsis-associated encephalopathy with the regulation to the CREB-BDNF signaling pathway and hippocampal neuron plasticity-related gene expression
Authors: Y Bai, Q Han, B Dong, H Lin, Y Jiang, X Zhang, H Chen, Y Yu
Brain research bulletin, 2022-03-30;184(0):56-67.
Species: Mouse
Sample Types: Tissue Homogenates
-
Piceatannol suppresses inflammation and promotes apoptosis in rheumatoid arthritis?fibroblast?like synoviocytes by inhibiting the NF?kappaB and MAPK signaling pathways
Authors: X Gao, X Kang, H Lu, E Xue, R Chen, J Pan, J Ma
Molecular Medicine Reports, 2022-03-24;25(5):.
Species: Rat
Sample Types: Serum
-
A Comparative Study of Koizumi and Longa Methods of Intraluminal Filament Middle Cerebral Artery Occlusion in Rats: Early Corticosterone and Inflammatory Response in the Hippocampus and Frontal Cortex
Authors: MV Onufriev, YV Moiseeva, MY Zhanina, NA Lazareva, NV Gulyaeva
International Journal of Molecular Sciences, 2021-12-17;22(24):.
Species: Rat
Sample Types: Serum
-
SARS-CoV-2 spike S1 subunit induces neuroinflammatory, microglial and behavioral sickness responses: Evidence of PAMP-like properties
Authors: MG Frank, KH Nguyen, JB Ball, S Hopkins, T Kelley, MV Baratta, M Fleshner, SF Maier
Brain, Behavior, and Immunity, 2021-12-13;100(0):267-277.
Species: Rat
Sample Types: Tissue Homogenates
-
Protective effects of endothelial progenitor cell microvesicles on Ang�II?induced rat kidney cell injury
Authors: Y Song, Z Bai, Y Zhang, J Chen, M Chen, Y Zhang, X Zhang, H Mai, B Wang, Y Lin, S Gu
Molecular Medicine Reports, 2021-11-05;25(1):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Endogenous tRNA-derived small RNA (tRF3-Thr-AGT) inhibits ZBP1/NLRP3 pathway-mediated cell pyroptosis to attenuate acute pancreatitis (AP)
Authors: B Sun, Z Chen, Q Chi, Y Zhang, B Gao
Journal of Cellular and Molecular Medicine, 2021-10-13;0(0):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Loganin Ameliorates Painful Diabetic Neuropathy by Modulating Oxidative Stress, Inflammation and Insulin Sensitivity in Streptozotocin-Nicotinamide-Induced Diabetic Rats
Authors: YC Cheng, YM Chiu, ZK Dai, BN Wu
Cells, 2021-10-08;10(10):.
Species: Rat
Sample Types: Plasma
-
Protective effect of parecoxib sodium against ischemia reperfusion?induced intestinal injury
Authors: M Li, Z Zheng
Molecular Medicine Reports, 2021-09-09;24(5):.
Species: Rat
Sample Types: Tissue Homogenates
-
GPER and IGF-1R mediate the anti-inflammatory effect of genistein against lipopolysaccharide (LPS)-induced nigrostriatal injury in rats
Authors: ZR Du, Y Gu, XM Xie, M Zhang, GY Jiang, WF Chen
The Journal of steroid biochemistry and molecular biology, 2021-08-31;214(0):105989.
Species: Rat
Sample Types: Tissue Homogenates
-
Prenatal Hyperhomocysteinemia Induces Glial Activation and Alters Neuroinflammatory Marker Expression in Infant Rat Hippocampus
Authors: AD Shcherbits, DS Vasilev, YP Milyutina, NL Tumanova, AV Mikhel, IV Zalozniaia, AV Arutjunyan
Cells, 2021-06-18;10(6):.
Species: Rat
Sample Types: Tissue Homogenates
-
Down-regulation of SNHG16 alleviates the acute lung injury in sepsis rats through miR-128-3p/HMGB3 axis
Authors: J Sun, K Xin, C Leng, J Ge
BMC pulmonary medicine, 2021-06-06;21(1):191.
Species: Rat
Sample Types: Tissue Homogenates
-
MiR-128-3p alleviates TNBS-induced colitis through inactivating TRAF6/NF-&kappaB signaling pathway in rats
Authors: LL Huo, ZR Sun
The Kaohsiung journal of medical sciences, 2021-05-27;0(0):.
Species: Rat
Sample Types: Tissue Homogenates
-
Neuroprotective Effects of VEGF-A Nanofiber Membrane and FAAH Inhibitor URB597 Against Oxygen-Glucose Deprivation-Induced Ischemic Neuronal Injury
Authors: DP Wang, KY Jin, P Zhao, Q Lin, K Kang, J Hai
International Journal of Nanomedicine, 2021-05-27;16(0):3661-3678.
Species: Rat
Sample Types: Cell Culture Supernates
-
Long non-coding RNA GAS5 aggravates myocardial depression in mice with sepsis via the microRNA-449b/HMGB1 axis and the NF-&kappaB signaling pathway
Authors: H Gao, H Ma, M Gao, A Chen, S Zha, J Yan
Bioscience Reports, 2021-04-30;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Protective effects of curcumin against rat intestinal inflammation?related motility disorders
Authors: Y Yao, R Luo, S Xiong, C Zhang, Y Zhang
Molecular Medicine Reports, 2021-03-24;23(5):.
Species: Rat
Sample Types: Serum
-
Long-Term Dipeptidyl Peptidase 4 Inhibition Worsens Hypertension and Renal and Cardiac Abnormalities in Obese Spontaneously Hypertensive Heart Failure Rats
Authors: EK Jackson, Z Mi, DG Gillespie, D Cheng, SP Tofovic
Journal of the American Heart Association, 2021-03-08;10(6):e020088.
Species: Rat
Sample Types: Plasma
-
Vagus nerve stimulation ameliorates L-NAME-induced preeclampsia-like symptoms in rats through inhibition of the inflammatory response
Authors: L Zheng, R Tang, L Shi, M Zhong, Z Zhou
BMC pregnancy and childbirth, 2021-03-04;21(1):177.
Species: Rat
Sample Types: Serum
-
Sericin-chitosan-glycosaminoglycans hydrogels incorporated with growth factors for in vitro and in vivo skin repair
Authors: S Sapru, S Das, M Mandal, AK Ghosh, SC Kundu
Carbohydrate polymers, 2021-01-29;258(0):117717.
Species: Rat
Sample Types: Serum
-
Resuscitation from hemorrhagic shock after traumatic brain injury with polymerized hemoglobin
Authors: CR Muller, V Courelli, A Lucas, AT Williams, JB Li, F Dos Santos, CT Cuddington, SR Moses, AF Palmer, EB Kistler, P Cabrales
Scientific Reports, 2021-01-28;11(1):2509.
Species: Rat
Sample Types: Plasma
-
P2X7 Receptor Induces Pyroptotic Inflammation and Cartilage Degradation in Osteoarthritis via NF-&kappaB/NLRP3 Crosstalk
Authors: Z Li, Z Huang, H Zhang, J Lu, Y Tian, Y Wei, Y Yang, L Bai
Oxidative Medicine and Cellular Longevity, 2021-01-16;2021(0):8868361.
Species: Rat
Sample Types: Cell Culture Supernates
-
Central Administration of Angiotensin-(1-7) Improves Vasopressin Impairment and Hypotensive Response in Experimental Endotoxemia
Authors: P Passaglia, F de Lima Fa, ME Batalhão, AM Stabile, LM Bendhack, J Antunes-Ro, R Lacchini, E Capellari
Cells, 2021-01-08;10(1):.
Species: Rat
Sample Types: Serum
-
Isoflurane reduces septic neuron injury by HO?1?mediated abatement of inflammation and apoptosis
Authors: L Zhang, X Zhang, T Wu, X Pan, Z Wang
Molecular Medicine Reports, 2020-12-23;23(2):.
Species: Mouse
Sample Types: Serum
-
Continuous administration of a p38&alpha inhibitor during the subacute phase after transient ischemia-induced stroke in the rat promotes dose-dependent functional recovery accompanied by increase in brain BDNF protein level
Authors: JJ Alam, M Krakovsky, U Germann, A Levy
PLoS ONE, 2020-12-04;15(12):e0233073.
Species: Rat
Sample Types: Tissue Culture Supernates
-
Sex Differences in Vagus Nerve Stimulation Effects on Rat Cardiovascular and Immune Systems
Authors: F Yaghouby, K Jang, U Hoang, S Asgari, S Vasudevan
Front Neurosci, 2020-11-06;14(0):560668.
Species: Rat
Sample Types: Plasma
-
Substrate Elasticity Exerts Functional Effects on Primary Microglia
Authors: SJ Blaschke, S Demir, A König, JA Abraham, SU Vay, M Rabenstein, DN Olschewski, C Hoffmann, M Hoffmann, N Hersch, R Merkel, B Hoffmann, M Schroeter, GR Fink, MA Rueger
Front Cell Neurosci, 2020-11-05;14(0):590500.
Species: Rat
Sample Types: Cell Culture Supernates
-
SNHG16 aggravates chronic constriction injury-induced neuropathic pain in rats via binding with miR-124-3p and miR-141-3p to upregulate JAG1
Authors: H Li, L Fan, Y Zhang, Y Cao, X Liu
Brain Res Bull, 2020-10-10;165(0):228-237.
Species: Rat
Sample Types: Cell Culture Supernates
-
Anticolitic Effect of Berberine in Rat Experimental Model: Impact of PGE2/p38 MAPK Pathways
Authors: L Jia, K Xue, J Liu, OA Habotta, L Hu, AE Abdel Mone
Mediators Inflamm, 2020-09-29;2020(0):9419085.
Species: Rat
Sample Types: Cell Culture Supernates
-
Methane Ameliorates Lipopolysaccharide-Induced Acute Orchitis by Anti-inflammatory, Antioxidative, and Antiapoptotic Effects via Regulation of the PK2/PKR1 Pathway
Authors: C Huang, W Zhang, A Sun, X Zhang, J Guo, R Ji, L Qiao, X Sun, D Zhao
Oxid Med Cell Longev, 2020-08-18;2020(0):7075836.
Species: Rat
Sample Types: Cell Culture Supernates
-
N?acetyl cysteine inhibits the lipopolysaccharide?induced inflammatory response in bone marrow mesenchymal stem cells by suppressing the TXNIP/NLRP3/IL?1&beta signaling pathway
Authors: X Wang, M Jiang, X He, B Zhang, W Peng, L Guo
Molecular Medicine Reports, 2020-08-13;0(0):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Inhibitor of RAGE and glucose?induced inflammation in bone marrow mesenchymal stem cells: Effect and mechanism of action
Authors: M Jiang, X Wang, P Wang, W Peng, B Zhang, L Guo
Molecular Medicine Reports, 2020-08-07;0(0):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Inhibition of HtrA2 alleviates inflammatory response and cell apoptosis in lipopolysaccharide?induced acute pneumonia in rats
Authors: X Wang
Molecular Medicine Reports, 2020-08-04;0(0):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Histamine 2/3 receptor agonists alleviate perioperative neurocognitive disorders by inhibiting microglia activation through the PI3K/AKT/FoxO1 pathway in aged rats
Authors: YN Chen, HH Sha, YW Wang, Q Zhou, P Bhuiyan, NN Li, YN Qian, HQ Dong
J Neuroinflammation, 2020-07-22;17(1):217.
Species: Rat
Sample Types: Cell Culture Supernates
-
Dexmedetomidine alleviates blunt chest trauma and hemorrhagic shock?resuscitation?induced acute lung injury through inhibiting the NLRP3 inflammasome
Authors: T Ming, M Yuan, Q Kong, Q Huang, Z Xia, X Wu
Mol Med Rep, 2020-07-10;22(3):2507-2515.
Species: Rat
Sample Types: Serum
-
PGI2 Analog Attenuates Salt-Induced Renal Injury through the Inhibition of Inflammation and Rac1-MR Activation
Authors: D Hirohama, W Kawarazaki, M Nishimoto, N Ayuzawa, T Marumo, S Shibata, T Fujita
Int J Mol Sci, 2020-06-22;21(12):.
Species: Rat
Sample Types: Tissue Homogenates
-
Anti?inflammatory activity of 3,5,6,7,3',4'?hexamethoxyflavone via repression of the NF?&kappaB and MAPK signaling pathways in LPS?stimulated RAW264.7 cells
Authors: ES Son, JW Park, SH Kim, HR Park, W Han, OC Kwon, JY Nam, SH Jeong, CS Lee
Mol Med Rep, 2020-06-18;22(3):1985-1993.
Species: Mouse
Sample Types: Cell Culture Supernates
-
NLRP3 inflammasome inhibition attenuates sepsis-induced platelet activation and prevents multi-organ injury in cecal-ligation puncture
Authors: DC Cornelius, OK Travis, RW Tramel, M Borges-Rod, CH Baik, M Greer, CA Giachelli, GA Tardo, JM Williams
PLoS ONE, 2020-06-17;15(6):e0234039.
Species: Rat
Sample Types: Plasma
-
Activation of FoxO1/SIRT1/RANKL/OPG pathway may underlie the therapeutic effects of resveratrol on aging-dependent male osteoporosis
Authors: O Ameen, RI Yassien, YM Naguib
BMC Musculoskelet Disord, 2020-06-12;21(1):375.
Species: Rat
Sample Types: Serum
-
Long non-coding RNA ANRIL knockdown suppresses apoptosis and pro-inflammatory cytokines while enhancing neurite outgrowth via binding microRNA-125a in a cellular model of Alzheimer's disease
Authors: B Zhou, L Li, X Qiu, J Wu, L Xu, W Shao
Mol Med Rep, 2020-06-02;22(2):1489-1497.
Species: Rat
Sample Types: Cell Culture Supernates
-
Dietary Saturated Fatty Acids Modulate Pain Behaviour in Trauma-Induced Osteoarthritis in Rats
Authors: S Sekar, SK Panchal, NK Ghattamane, L Brown, R Crawford, Y Xiao, I Prasadam
Nutrients, 2020-02-18;12(2):.
Species: Rat
Sample Types: Plasma
-
Comparison of the acute toxicity, analgesic and anti-inflammatory activities and chemical composition changes in Rhizoma anemones Raddeanae caused by vinegar processing
Authors: SS Wang, SY Zhou, XY Xie, L Zhao, Y Fu, GZ Cai, JY Gong
BMC Complement Med Ther, 2020-01-15;20(1):7.
Species: Rat
Sample Types: Serum
-
Identification of microRNA?363?3p as an essential regulator of chondrocyte apoptosis in osteoarthritis by targeting NRF1 through the p53?signaling pathway
Authors: M Zhang, Z Wang, B Li, F Sun, A Chen, M Gong
Mol Med Rep, 2020-01-14;21(3):1077-1088.
Species: Rat
Sample Types: Synovial Fluid
-
Moderate-intensity physical activity reduces systemic inflammation and maintains cardiorespiratory function following chronic particulate matter2.5 exposure in rats
Authors: A van Wavere, MJ Duncan, F Coulson, A Fenning
Toxicol Rep, 2019-12-17;7(0):93-100.
Species: Rat
Sample Types: Serum
-
Hemorrhagic Shock Sensitized the Diaphragm to Ventilator-Induced Dysfunction through the Activation of IL-6/JAK/STAT Signaling-Mediated Autophagy in Rats
Authors: LJ Zhang, SZ Ni, XL Zhou, Y Zhao
Mediators Inflamm., 2019-11-14;2019(0):3738409.
Species: Rat
Sample Types: Tissue Homogenates
-
Neutrophil activation causes tumor regression in Walker 256 tumor-bearing rats
Authors: WMT Kuwabara, J Andrade-Si, JNB Pereira, JH Scialfa, J Cipolla-Ne
Sci Rep, 2019-11-11;9(1):16524.
Species: Rat
Sample Types: BALF
-
Low-Energy Extracorporeal Shock Wave Ameliorates Streptozotocin Induced Diabetes and Promotes Pancreatic Beta Cells Regeneration in a Rat Model
Authors: CC Hsiao, CC Lin, YS Hou, JY Ko, CJ Wang
Int J Mol Sci, 2019-10-05;20(19):.
Species: Rat
Sample Types: Tissue Lysates
-
Nrf2 Suppression Delays Diabetic Wound Healing Through Sustained Oxidative Stress and Inflammation
Authors: M Li, H Yu, H Pan, X Zhou, Q Ruan, D Kong, Z Chu, H Li, J Huang, X Huang, A Chau, W Xie, Y Ding, P Yao
Front Pharmacol, 2019-09-20;10(0):1099.
Species: Rat
Sample Types: Serum
-
Low-Energy Extracorporeal Shock Wave Therapy Ameliorates Kidney Function in Diabetic Nephropathy
Authors: CC Hsiao, WH Huang, KH Cheng, CT Lee
Oxid Med Cell Longev, 2019-07-04;2019(0):8259645.
Species: Rat
Sample Types: Tissue Lysate
-
Sex-specific maternofetal innate immune responses triggered by group B Streptococci
Authors: MJ Allard, A Giraud, M Segura, G Sebire
Sci Rep, 2019-06-13;9(1):8587.
Species: Rat
Sample Types: Protein
-
Ascorbic acid deficiency increases hepatic expression of acute phase proteins through the intestine-derived IL-6 and hepatic STAT3 pathway in ODS rats
Authors: N Kawade, A Murai, W Suzuki, Y Tokuda, M Kobayashi, F Horio
J. Nutr. Biochem., 2019-05-14;70(0):116-124.
Species: Rat
Sample Types: Serum
-
NLRP3 inflammasome activation in platelets in response to sepsis
Authors: DC Cornelius, CH Baik, OK Travis, DL White, CM Young, W Austin Pie, CA Shields, B Poudel, JM Williams
Physiol Rep, 2019-05-01;7(9):e14073.
Species: Rat
Sample Types: Plasma
-
Atractylenolide III Attenuates Muscle Wasting in Chronic Kidney Disease via the Oxidative Stress-Mediated PI3K/AKT/mTOR Pathway
Authors: M Wang, R Hu, Y Wang, L Liu, H You, J Zhang, X Wu, T Pei, F Wang, L Lu, W Xiao, L Wei
Oxid Med Cell Longev, 2019-04-18;2019(0):1875471.
Species: Rat
Sample Types: Serum
-
Anti-inflammatory effect of afatinib (an EGFR-TKI) on OGD-induced neuroinflammation
Authors: YJ Chen, CC Hsu, YJ Shiao, HT Wang, YL Lo, AMY Lin
Sci Rep, 2019-02-21;9(1):2516.
Species: Rat
Sample Types: Cell Culture Supernates
-
Neuroinflammation induced by the peptide amyloid-? (25-35) increase the presence of galectin-3 in astrocytes and microglia and impairs spatial memory
Authors: E Ramírez, C Sánchez-Ma, MA Mayoral, L Mendieta, V Alatriste, A Patricio-M, ID Limón
Neuropeptides, 2019-02-14;0(0):.
Species: Rat
Sample Types: Tissue Homogenates
-
Hepatokine ?1-Microglobulin Signaling Exacerbates Inflammation and Disturbs Fibrotic Repair in Mouse Myocardial Infarction
Authors: D Hakuno, M Kimura, S Ito, J Satoh, Y Nakashima, T Horie, Y Kuwabara, M Nishiga, Y Ide, O Baba, H Nishi, T Nakao, T Nishino, F Nakazeki, S Koyama, R Hanada, RR Randolph, J Endo, T Kimura, K Ono
Sci Rep, 2018-11-13;8(1):16749.
Species: Rat
Sample Types: Cell Culture Supernates
-
Valproate reduces neuroinflammation and neuronal death in a rat chronic constriction injury model
Authors: JY Chen, LW Chu, KI Cheng, SL Hsieh, YS Juan, BN Wu
Sci Rep, 2018-11-07;8(1):16457.
Species: Rat
Sample Types: Tissue Homogenates
-
MiR-10 targets NgR to modulate the proliferation of microglial cells and the secretion of inflammatory cytokines
Authors: W Wang, R Zhan, J Zhou, J Wang, S Chen
Exp. Mol. Pathol., 2018-10-09;0(0):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Local Delivery of ?-Elemene Improves Locomotor Functional Recovery by Alleviating Endoplasmic Reticulum Stress and Reducing Neuronal Apoptosis in Rats with Spinal Cord Injury
Authors: J Wang, H Li, Y Ren, Y Yao, J Hu, M Zheng, Y Ding, YY Chen, Y Shen, LL Wang, Y Zhu
Cell. Physiol. Biochem., 2018-08-30;49(2):595-609.
Species: Rat
Sample Types: Tissue Homogenates
-
Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury
Authors: D Li, W Ren, Z Jiang, L Zhu
Mol Med Rep, 2018-08-24;0(0):.
Species: Mouse
Sample Types: BALF
-
Levistilide A Ameliorates NLRP3 Expression Involving the Syk-p38/JNK Pathway and Peripheral Obliterans in Rats
Authors: H Guo, L Sun, S Ling, JW Xu
Mediators Inflamm., 2018-08-12;2018(0):7304096.
Species: Rat
Sample Types: Serum
-
Protective Role of UCP2 in Oxidative Stress and Apoptosis during the Silent Phase of an Experimental Model of Epilepsy Induced by Pilocarpine
Authors: MRH Dutra, RDS Feliciano, KR Jacinto, TLF Gouveia, E Brigidio, AJ Serra, M Morris, MDG Naffah-Maz, JA Silva
Oxid Med Cell Longev, 2018-08-06;2018(0):6736721.
Species: Rat
Sample Types: Tissue Homogenates
-
Cobra Venom Factor-induced complement depletion protects against lung ischemia reperfusion injury through alleviating blood-air barrier damage
Authors: C Haihua, W Wei, H Kun, L Yuanli, L Fei
Sci Rep, 2018-07-09;8(1):10346.
Species: Rat
Sample Types: BALF
-
Autophagy mediates tumor necrosis factor-?-induced phenotype switching in vascular smooth muscle A7r5 cell line
Authors: M García-Mig, JA Riquelme, I Norambuena, PE Morales, F Sanhueza-O, C Nuñez-Soto, D Mondaca-Ru, N Cancino-Ar, A San Martín, M Chiong
PLoS ONE, 2018-05-11;13(5):e0197210.
Species: Rat
Sample Types: Cell Culture Supernates
-
Chronic agomelatine treatment prevents comorbid depression in the post-status epilepticus model of acquired epilepsy through suppression of inflammatory signaling
Authors: J Tchekalaro, D Atanasova, L Kortenska, M Atanasova, N Lazarov
Neurobiol. Dis., 2018-04-10;115(0):127-144.
Species: Rat
Sample Types: Plasma
-
Anti-inflammatory effect of Mongolian drug Naru-3 on traumatic spinal cord injury and its mechanism of action
Authors: B Baiyila, B He, G He, T Long
J. Int. Med. Res., 2018-04-03;0(0):3000605187601.
Species: Rat
Sample Types: Serum
-
The local cytokine and growth factor response to rhBMP-2 after spinal fusion
Authors: JD Koerner, DZ Markova, GD Schroeder, BP Calio, A Shah, CW Brooks, AR Vaccaro, DG Anderson, CK Kepler
Spine J, 2018-03-14;0(0):.
Species: Rat
Sample Types: Tissue Lysates
Applications: ELISA (Standard) -
Narrow Spectrum Kinase Inhibitors Demonstrate Promise for the Treatment of Dry Eye Disease and Other Ocular Inflammatory Disorders
Authors: S Hagan, MCT Fyfe, B Ofori-Frim, K Oliver, MR Foster, S Sirohi, Y Solanke, M Doughty, A Rowley, M Taylor, S Webber, CA Walshe
Invest. Ophthalmol. Vis. Sci., 2018-03-01;59(3):1443-1453.
Species: Rat
Sample Types: Tissue Homogenates
-
Short-term inhalation study of graphene oxide nanoplates
Authors: YH Kim, MS Jo, JK Kim, JH Shin, JE Baek, HS Park, HJ An, JS Lee, BW Kim, HP Kim, KH Ahn, K Jeon, SM Oh, JH Lee, T Workman, EM Faustman, IJ Yu
Nanotoxicology, 2018-02-01;0(0):1-15.
Species: Rat
Sample Types: Whole Cells
Applications: ELISA Capture -
Ablation of the Right Cardiac Vagus Nerve Reduces Acetylcholine Content without Changing the Inflammatory Response during Endotoxemia
Authors: K Plaschke, TQM Do, F Uhle, T Brenner, MA Weigand, J Kopitz
Int J Mol Sci, 2018-02-01;19(2):.
Species: Rat
Sample Types: Plasma
-
Green tea extract attenuates LPS-induced retinal inflammation in rats
Authors: JL Ren, QX Yu, WC Liang, PY Leung, TK Ng, WK Chu, CP Pang, SO Chan
Sci Rep, 2018-01-11;8(1):429.
Species: Rat
Sample Types: Vitreous Humor
-
Ferulaldehyde Improves the Effect of Methotrexate in Experimental Arthritis
Authors: L Slovák, K Švík, D Mihalová, J Tóth, S Czigle, ? Pašková, F Bilka, K Bauerová
Molecules, 2017-11-06;22(11):.
Species: Rat
Sample Types: Plasma
-
Electroacupuncture serum inhibits TNF???mediated chondrocyte inflammation via the Ras?Raf?MEK1/2?ERK1/2 signaling pathway
Authors: H Chen, X Shao, L Li, C Zheng, X Xu, X Hong, X Li, M Wu
Mol Med Rep, 2017-08-28;0(0):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Short-term treatment with taurolidine is associated with liver injury
Authors: R Fahrner, A Möller, AT Press, A Kortgen, M Kiehntopf, F Rauchfuss, U Settmacher, AS Mosig
BMC Pharmacol Toxicol, 2017-08-11;18(1):61.
Species: Rat
Sample Types: Serum
-
Oral Administration of Red Ginseng Extract Promotes Neurorestoration after Compressive Spinal Cord Injury in Rats
Authors: P Zhu, K Samukawa, H Fujita, H Kato, M Sakanaka
Evid Based Complement Alternat Med, 2017-07-30;2017(0):1265464.
Species: Rat
Sample Types: Tissue Homogenates
-
Aliskiren has chondroprotective efficacy in a rat model of osteoarthritis through suppression of the local renin-angiotensin system
Authors: K Yan, Y Shen
Mol Med Rep, 2017-07-28;0(0):.
Species: Rat
Sample Types: Serum
-
Mori Folium water extract alleviates articular cartilage damages and inflammatory responses in monosodium iodoacetate?induced osteoarthritis rats
Authors: JW Jeong, HH Lee, J Kim, EO Choi, H Hwang-Bo, HJ Kim, MY Kim, KI Ahn, GY Kim, KW Lee, KY Kim, SG Kim, SH Hong, C Park, HJ Cha, YH Choi
Mol Med Rep, 2017-07-21;0(0):.
Species: Rat
Sample Types: Serum
-
Innate And Adaptive Immunity are Progressively Activated in Parallel with Renal Injury in the 5/6 Renal Ablation Model
Authors: C Fanelli, SCA Arias, FG Machado, JK Okuma, DMAC Malheiros, H Azevedo, CA Moreira-Fi, NOS Camara, CK Fujihara, R Zatz
Sci Rep, 2017-06-09;7(1):3192.
Species: Rat
Sample Types: Tissue Homogenates
-
Neuroprotective effects of food restriction on autonomic innervation of the lacrimal gland in rat
Authors: HZ Nooh, NH El-Saify, NMN Eldien
Ann. Anat., 2017-05-24;0(0):.
Species: Rat
Sample Types: Tissue Homogenates
-
Pharmacologic targeting ERK1/2 attenuates the development and progression of hyperuricemic nephropathy in rats
Authors: N Liu, L Xu, Y Shi, L Fang, H Gu, H Wang, X Ding, S Zhuang
Oncotarget, 2017-05-16;8(20):33807-33826.
Species: Rat
Sample Types: Tissue Culture Supernates
-
Sodium tanshinone IIA sulfonate improves inflammation, aortic endothelial cell apoptosis, disseminated intravascular coagulation and multiple organ damage in a rat heat stroke model
Authors: F Chen, H Li, G Zhu, X Chen, Z Tang
Mol Med Rep, 2017-05-11;0(0):.
Species: Rat
Sample Types: Serum
-
Anti-aging effect and gene expression profiling of dung beetle glycosaminoglycan in aged rats
Authors: MY Ahn, BJ Kim, HJ Kim, JS Hwang, YS Jung, KK Park
Biomater Res, 2017-04-21;21(0):5.
Species: Rat
Sample Types: Serum
-
Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences
Authors: JE Finnell, CM Lombard, AR Padi, CM Moffitt, LB Wilson, CS Wood, SK Wood
PLoS ONE, 2017-02-27;12(2):e0172868.
Species: Rat
Sample Types: Tissue Homogenates
-
Investigation of the effect of phlomisoside F on complete Freund's adjuvant-induced arthritis
Authors: X Zhang, Y Dong, H Dong, W Zhang, F Li
Exp Ther Med, 2016-12-22;13(2):710-716.
Species: Rat
Sample Types: Serum
-
The analgesic effect of rolipram is associated with the inhibition of the activation of the spinal astrocytic JNK/CCL2 pathway in bone cancer pain
Authors: Chi-Hua Guo
Int. J. Mol. Med, 2016-09-30;38(5):1433-1442.
Species: Rat
Sample Types: Tissue Homogenates
-
Pyrrolidine Dithiocarbamate Prevents Neuroinflammation and Cognitive Dysfunction after Endotoxemia in Rats
Authors: Min Hui Kan, Ting Yang, Hui Qun Fu, Long Fan, Yan Wu, Niccolò Terrando et al.
Frontiers in Aging Neuroscience
Species: Rat
Sample Types: Tissue Homogenates
-
Chronic Cognitive Dysfunction after Traumatic Brain Injury Is Improved with a Phosphodiesterase 4B Inhibitor
J Neurosci, 2016-07-06;36(27):7095-108.
Species: Rat
Sample Types: Tissue Homogenates
-
Spatiotemporal Cadence of Macrophage Polarisation in a Model of Light-Induced Retinal Degeneration.
Authors: Jiao H, Natoli R, Valter K, Provis J, Rutar M
PLoS ONE, 2015-12-02;10(12):e0143952.
Species: Rat
Sample Types: Tissue Homogenates
-
Inhibition of the spinal astrocytic JNK/MCP-1 pathway activation correlates with the analgesic effects of tanshinone IIA sulfonate in neuropathic pain.
Authors: Tang J, Zhu C, Li Z, Liu X, Sun S, Zhang T, Luo Z, Zhang H, Li W
J Neuroinflammation, 2015-03-25;12(0):57.
Species: Rat
Sample Types: Tissue Homogenates
-
Amantadine Alleviates Postoperative Cognitive Dysfunction Possibly by Increasing Glial Cell Line-derived Neurotrophic Factor in Rats
Authors: Junfeng Zhang, Hongying Tan, Wei Jiang, Zhiyi Zuo
Anesthesiology
Species: Rat
Sample Types: Cell Culture Supernates
-
Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats.
Authors: Fu H, Yang T, Xiao W, Fan L, Wu Y, Terrando N, Wang T
PLoS ONE, 2014-08-29;9(8):e106331.
Species: Rat
Sample Types: Serum
-
Catalpol ameliorates sodium taurocholate-induced acute pancreatitis in rats via inhibiting activation of nuclear factor kappa B.
Authors: Xiao W, Yin G, Fan Y, Qiu L, Cang X, Yu G, Hu Y, Xing M, Wu D, Wang X, Hu G, Wan R
Int J Mol Sci, 2014-07-04;15(7):11957-72.
Species: Rat
Sample Types: Serum
-
Atrial fibrosis in a chronic murine model of obstructive sleep apnea: mechanisms and prevention by mesenchymal stem cells.
Authors: Ramos P, Rubies C, Torres M, Batlle M, Farre R, Brugada J, Montserrat J, Almendros I, Mont L
Respir Res, 2014-04-28;15(0):54.
Species: Rat
Sample Types: Plasma
-
Intrathecal antagonism of microglial TLR4 reduces inflammatory damage to blood-spinal cord barrier following ischemia/reperfusion injury in rats.
Authors: Li X, Wang J, Fang B, Tan W, Ma H
Mol Brain, 2014-04-21;7(0):28.
Species: Rat
Sample Types: Tissue Homogenates
-
Epidermal growth factor receptor inhibitor ameliorates excessive astrogliosis and improves the regeneration microenvironment and functional recovery in adult rats following spinal cord injury.
Authors: Li Z, Li J, Wang L, Zhang J, Wu J, Mao X, Shi G, Wang Q, Wang F, Zou J
J Neuroinflammation, 2014-04-05;11(0):71.
Species: Rat
Sample Types: Cell Culture Supernates
-
Perioperative aspirin improves neurological outcome after focal brain ischemia possibly via inhibition of Notch 1 in rat.
Authors: Wang Z, Huang W, Zuo Z
J Neuroinflammation, 2014-03-25;11(0):56.
Species: Rat
Sample Types: Whole Tissue
-
Rhamnogalacturonan from Acmella oleracea (L.) R.K. Jansen: gastroprotective and ulcer healing properties in rats.
Authors: Maria-Ferreira D, da Silva L, Mendes D, Cabrini D, Nascimento A, Iacomini M, Cipriani T, Santos A, Werner M, Baggio C
PLoS ONE, 2014-01-08;9(1):e84762.
Species: Rat
Sample Types: Tissue Culture Supernates
-
Mechanical ventilation enhances HMGB1 expression in an LPS-induced lung injury model.
Authors: Ding N, Wang F, Xiao H, Xu L, She S
PLoS ONE, 2013-09-10;8(9):e74633.
Species: Rat
Sample Types: BALF
-
A short course of infusion of a hydrogen sulfide-donor attenuates endotoxemia induced organ injury via stimulation of anti-inflammatory pathways, with no additional protection from prolonged infusion.
Cytokine, 2012-12-23;61(2):614-21.
Species: Rat
Sample Types: BALF
-
Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain.
J Neuroinflammation, 2012-07-23;9(0):181.
Species: Rat
Sample Types: Tissue Homogenates
-
Anthrax lethal toxin activates the inflammasome in sensitive rat macrophages
Authors: Zachary L. Newman, Devorah Crown, Stephen H. Leppla, Mahtab Moayeri
Biochemical and Biophysical Research Communications
Species: Rat
Sample Types: Cell Culture Supernates
-
Identification of a serum-induced transcriptional signature associated with type 1 diabetes in the BioBreeding rat.
Authors: Kaldunski M, Jia S, Geoffrey R, Basken J, Prosser S, Kansra S, Mordes JP, Lernmark A, Wang X, Hessner MJ
Diabetes, 2010-08-03;59(10):2375-85.
Species: Rat
Sample Types: Cell Culture Supernates
-
Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury.
Authors: Calvo M, Zhu N, Tsantoulas C, Ma Z, Grist J, Loeb JA, Bennett DL
J. Neurosci., 2010-04-14;30(15):5437-50.
Species: Rat
Sample Types: Cell Culture Supernates
-
Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta.
Authors: Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, Rice KC, Watkins LR
Neuroscience, 2010-01-20;165(2):569-83.
Species: Rat
Sample Types: CSF
-
House dust mite induces direct airway inflammation in vivo: implications for future disease therapy?
Authors: De Alba J, Raemdonck K, Dekkak A, Collins M, Wong S, Nials AT, Knowles RG, Belvisi MG, Birrell MA
Eur. Respir. J., 2009-10-19;35(6):1377-87.
Species: Rat
Sample Types: BALF
-
Peripheral osmotic stimulation inhibits the brain's innate immune response to microdialysis of acidic perfusion fluid adjacent to supraoptic nucleus.
Authors: Summy-Long JY, Hu S
Am. J. Physiol. Regul. Integr. Comp. Physiol., 2009-09-16;297(5):R1532-45.
Species: Rat
Sample Types: Plasma
-
In vivo Angiotensin II AT1 receptor blockade selectively inhibits LPS-induced innate immune response and ACTH release in rat pituitary gland.
Authors: Sanchez-Lemus E, Benicky J, Pavel J, Saavedra JM
Brain Behav. Immun., 2009-05-07;23(7):945-57.
Species: Rat
Sample Types: Tissue Homogenates
-
Role of sensory innervation in the rat pulmonary neutrophil recruitment induced by staphylococcal enterotoxins type A and B.
Authors: Desouza IA, Camargo EA, Mariano NS, Optiz-Neto JB, Resende JS, Mello GC, Costa SK, De Nucci G, Antunes E
Eur. J. Pharmacol., 2009-04-16;613(1):128-34.
Species: Rat
Sample Types: BALF
-
Spinal leptin contributes to the pathogenesis of neuropathic pain in rodents.
Authors: Lim G, Wang S, Zhang Y, Tian Y, Mao J
J. Clin. Invest., 2009-01-12;119(2):295-304.
Species: Rat
Sample Types: Cell Culture Supernates
-
Human interleukin-10 gene transfer is protective in a rat model of Parkinson's disease.
Authors: Johnston LC, Su X, Maguire-Zeiss K, Horovitz K, Ankoudinova I, Guschin D, Hadaczek P, Federoff HJ, Bankiewicz K, Forsayeth J
Mol. Ther., 2008-06-10;16(8):1392-9.
Species: Rat
Sample Types: Tissue Homogenates
-
Role of central beta-adrenergic receptors in regulating proinflammatory cytokine responses to a peripheral bacterial challenge.
Authors: Johnson JD, Cortez V, Kennedy SL, Foley TE, Hanson H, Fleshner M
Brain Behav. Immun., 2008-05-12;22(7):1078-86.
Species: Rat
Sample Types: Plasma
-
Substance P acts via the neurokinin receptor 1 to elicit bronchoconstriction, oxidative stress, and upregulated ICAM-1 expression after oil smoke exposure.
Authors: Li PC, Chen WC, Chang LC, Lin SC
Am. J. Physiol. Lung Cell Mol. Physiol., 2008-03-07;294(5):L912-20.
Species: Rat
Sample Types: BALF
-
IL-1beta promotes neurite outgrowth by deactivating RhoA via p38 MAPK pathway.
Authors: Temporin K, Tanaka H, Kuroda Y, Okada K, Yachi K, Moritomo H, Murase T, Yoshikawa H
Biochem. Biophys. Res. Commun., 2007-11-08;365(2):375-80.
Species: Rat
Sample Types: Tissue Homogenates
-
Comprehensive gene expression profiling of rat lung reveals distinct acute and chronic responses to cigarette smoke inhalation.
Authors: Stevenson CS, Docx C, Webster R, Battram C, Hynx D, Giddings J, Cooper PR, Chakravarty P, Rahman I, Marwick JA, Kirkham PA, Charman C, Richardson DL, Nirmala NR, Whittaker P, Butler K
Am. J. Physiol. Lung Cell Mol. Physiol., 2007-08-24;293(5):L1183-93.
Species: Rat
Sample Types: BALF
-
Cellular recruitment and cytokine generation in a rat model of allergic lung inflammation are differentially modulated by progesterone and estradiol.
Authors: de Oliveira AP, Domingos HV, Cavriani G, Damazo AS, Dos Santos Franco AL, Oliani SM, Oliveira-Filho RM, Vargaftig BB, de Lima WT
Am. J. Physiol., Cell Physiol., 2007-07-18;293(3):C1120-8.
Species: Rat
Sample Types: BALF
-
Expression of the mu opioid receptor in the human immunodeficiency virus type 1 transgenic rat model.
Authors: Chang SL, Beltran JA, Swarup S
J. Virol., 2007-06-06;81(16):8406-11.
Species: Rat
Sample Types: Serum
-
Gene transfer to interfere with TNFalpha signaling in neuropathic pain.
Authors: Hao S, Mata M, Glorioso JC, Fink DJ
Gene Ther., 2007-04-19;14(13):1010-6.
Species: Rat
Sample Types: Tissue Homogenates
-
Changes of gene expression of iron regulatory proteins during turpentine oil-induced acute-phase response in the rat.
Authors: Sheikh N, Dudas J, Ramadori G
Lab. Invest., 2007-04-09;87(7):713-25.
Species: Rat
Sample Types: Serum
-
Prothymosin alpha lacking the nuclear localization signal as an effective gene therapeutic strategy in collagen-induced arthritis.
Authors: Shiau AL, Chen SY, Chang MY
J. Immunol., 2007-04-01;178(7):4688-94.
Species: Rat
Sample Types: Tissue Homogenates
-
Cell-based angiopoietin-1 gene therapy for acute lung injury.
Authors: McCarter SD, Mei SH, Lai PF, Zhang QW, Parker CH, Suen RS, Hood RD, Zhao YD, Deng Y, Han RN, Dumont DJ, Stewart DJ
Am. J. Respir. Crit. Care Med., 2007-02-22;175(10):1014-26.
Species: Rat
Sample Types: BALF
-
Interleukin-6 mediates low-threshold mechanical allodynia induced by intrathecal HIV-1 envelope glycoprotein gp120.
Authors: Schoeniger-Skinner DK, Ledeboer A, Frank MG, Milligan ED, Poole S, Martin D, Maier SF, Watkins LR
Brain Behav. Immun., 2007-01-03;21(5):660-7.
Species: Rat
Sample Types: Tissue Homogenates
-
The age-related attenuation in long-term potentiation is associated with microglial activation.
Authors: Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA
J. Neurochem., 2006-09-18;99(4):1263-72.
Species: Rat
Sample Types: Tissue Homogenates
-
Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation.
Authors: Barkho BZ, Song H, Aimone JB
Stem Cells Dev., 2006-06-01;15(3):407-21.
Species: Rat
Sample Types: Cell Culture Supernates
-
Interleukin-10 aerosol reduces proinflammatory mediators in bronchoalveolar fluid of endotoxemic rat.
Authors: Hofstetter C, Flondor M, Hoegl S, Muhl H, Zwissler B
Crit. Care Med., 2005-10-01;33(10):2317-22.
Species: Rat
Sample Types: BALF
-
Prophylactic adenovirus-mediated human kallistatin gene therapy suppresses rat arthritis by inhibiting angiogenesis and inflammation.
Authors: Wang CR, Chen SY, Wu CL, Liu MF, Jin YT, Chao L, Chao J
Arthritis Rheum., 2005-04-01;52(4):1319-24.
Species: Rat
Sample Types: Tissue Homogenates
-
Developmental changes induced by graded prenatal systemic hypoxic-ischemic insults in rats.
Authors: Robinson S, Petelenz K, Li Q, Cohen ML, Dechant A, Tabrizi N, Bucek M, Lust D, Miller RH
Neurobiol. Dis., 2005-04-01;18(3):568-81.
Species: Rat
Sample Types: Amniotic Fluid
-
Cytokine profiles in the testes of rats treated with lipopolysaccharide reveal localized suppression of inflammatory responses.
Authors: O&apos;Bryan MK
Am. J. Physiol. Regul. Integr. Comp. Physiol., 2005-01-20;288(6):R1744-55.
Species: Rat
Sample Types: Tissue Homogenates
-
Chlorpromazine and loxapine reduce interleukin-1beta and interleukin-2 release by rat mixed glial and microglial cell cultures.
Authors: Labuzek K, Kowalski J, Gabryel B, Herman ZS
Eur Neuropsychopharmacol, 2005-01-01;15(1):23-30.
Species: Rat
Sample Types: Cell Culture Supernates
-
A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine.
Authors: Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR
J. Neurosci., 2004-08-18;24(33):7353-65.
Species: Rat
Sample Types: Tissue Homogenates
-
Insulin treatment improves hepatic morphology and function through modulation of hepatic signals after severe trauma.
Authors: Schubert T, Horch RE
Ann. Surg., 2004-08-01;240(2):340-9.
Species: Rat
Sample Types: Tissue Homogenates
-
HIV-1 gp120 stimulates proinflammatory cytokine-mediated pain facilitation via activation of nitric oxide synthase-I (nNOS).
Authors: Holguin A, O&apos;Connor KA, Biedenkapp J, Campisi J, Wieseler-Frank J, Milligan ED, Hansen MK, Spataro L, Maksimova E, Bravmann C, Martin D, Fleshner M, Maier SF, Watkins LR
Pain, 2004-08-01;110(3):517-30.
Species: Rat
Sample Types: CSF
-
Insulin attenuates the systemic inflammatory response in endotoxemic rats.
Authors: Jeschke MG, Klein D, Bolder U, Einspanier R
Endocrinology, 2004-06-10;145(9):4084-93.
Species: Mouse
Sample Types: Tissue Homogenates
-
Gene transfer of tumor necrosis factor inhibitor improves the function of lung allografts.
Authors: Tagawa T, Kozower BD, Kanaan SA, Daddi N, Muraoka M, Oka T, Ritter JH, Patterson GA
J. Thorac. Cardiovasc. Surg., 2004-06-01;127(6):1558-63.
Species: Rat
Sample Types: Tissue Homogenates
-
A method for measuring multiple cytokines from small samples.
Authors: O&apos;Connor KA
Brain Behav. Immun., 2004-05-01;18(3):274-80.
Species: Rat
Sample Types: CSF
-
Alveolar macrophage cytotoxicity for normal lung fibroblasts is mediated by nitric oxide release.
Authors: Morgan DL, Shines CJ
Toxicol In Vitro, 2004-02-01;18(1):139-46.
Species: Rat
Sample Types: Cell Culture Supernates
-
Oral (gavage), in utero and post-natal exposure of Sprague-Dawley rats to low doses of tributyltin chloride. Part II: effects on the immune system.
Authors: Tryphonas H, Cooke G, Caldwell D, Bondy G, Parenteau M, Hayward S, Pulido O
Food Chem. Toxicol., 2004-02-01;42(2):221-35.
Species: Rat
Sample Types: Serum
-
Differential cytokine response in interstitial fluid in skin and serum during experimental inflammation in rats.
Authors: Nedrebo T, Reed RK, Jonsson R, Berg A, Wiig H
J. Physiol. (Lond.), 2004-01-14;556(0):193-202.
Species: Rat
Sample Types: Interstitial Fluid
-
Differential expression of cytokine genes and inducible nitric oxide synthase induced by opacity phenotype variants of Streptococcus pneumoniae during acute otitis media in the rat.
Authors: Long JP, Tong HH, Shannon PA, DeMaria TF
Infect. Immun., 2003-10-01;71(10):5531-40.
Species: Rat
Sample Types: Tissue Secretion
-
Recipient intramuscular cotransfection of naked plasmid transforming growth factor beta1 and interleukin 10 ameliorates lung graft ischemia-reperfusion injury.
Authors: D&apos;Ovidio F
J. Thorac. Cardiovasc. Surg., 2002-08-01;124(2):259-69.
Species: Rat
Sample Types: BALF
-
Role of IL-18 in acute lung inflammation.
Authors: Jordan JA, Guo RF, Yun EC, Sarma V, Warner RL, Crouch LD, Senaldi G, Ulich TR, Ward PA
J. Immunol., 2001-12-15;167(12):7060-8.
Species: Rat
Sample Types: BALF
FAQs
-
Does this Quantikine ELISA Kit detect the pro form of the target protein?
This Quantikine ELISA kit is designed to detect both the natural and recombinant mature forms of the target protein. The pro form of the target protein has not been evaluated in this assay. To see the complete list of recombinant proteins tested for cross-reactivity or interference in this assay, please consult the "Specificity" section of the product insert.
Reviews for Rat IL-1 beta/IL-1F2 Quantikine ELISA Kit
Average Rating: 4.8 (Based on 5 Reviews)
Have you used Rat IL-1 beta/IL-1F2 Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
We tested IL-1β levels in homogenized rat spinal cord. The standard curve was excellent and the CV values were all very low. We have used kits from other companies and found RnD systems kits to be superior to all other companies. The kits from RnD systems result in much lower background levels and much lower CV levels, which really helps with consistency and repeatability. The image shows the standard curve and the green outlines the low CV values from a run containing homogenized rat spinal cord.
One of the best ELISA kits for IL-1B measurement. Used it with brain homogenates and results are very reproducible. Before this kit, i worked with IL-1B ELISA kits from 2 other vendors, however i could find lot of cross- reactivity. Based on my excellent experience with RnD's mice IL-1B ELISA kits i tried rat kits and they did not disappoint me. I definitely recommend this kit for all your IL-1B related research.
Among best ELISAs I've worked with. Great consistency lot to lot, high sensitivity, easy to work with small amount of samples (50ul total, but samples can be diluted a number of times due to high sensitivity). Appropriate for concentration response curves. Linear standard curve for easy extrapolation of concentration values.

















