Rat TNF-alpha DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant rat TNF-alpha. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
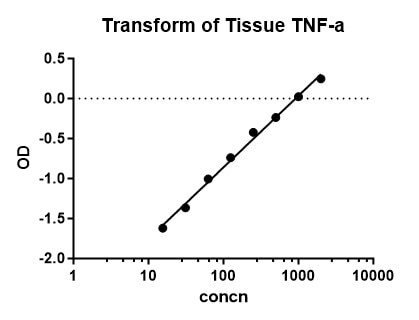
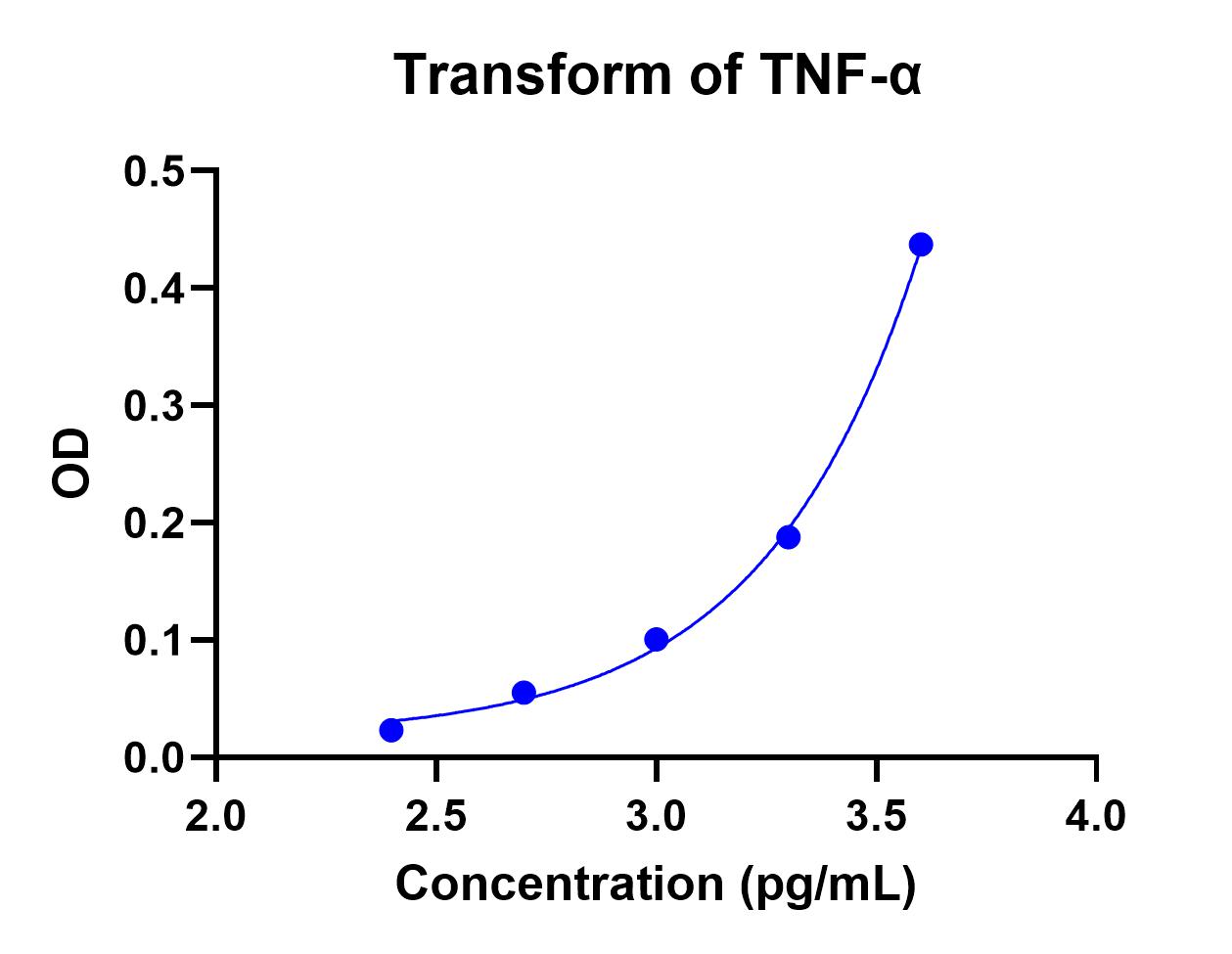
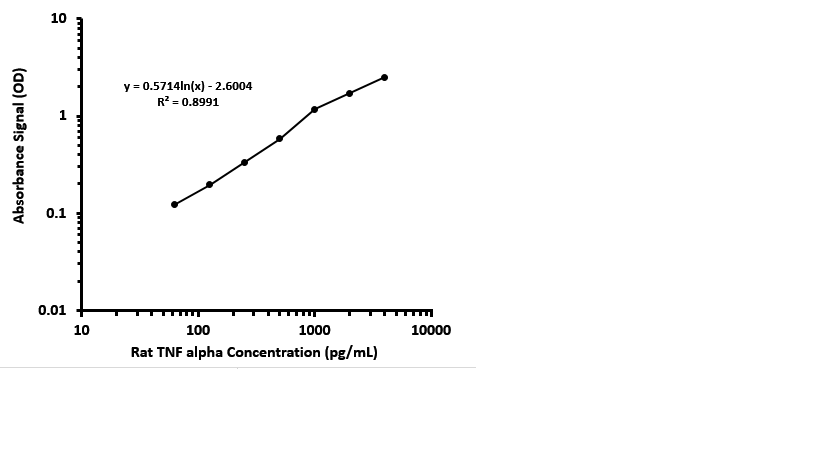
Scientific Data
Product Datasheets
Preparation and Storage
Background: TNF-alpha
Tumor necrosis factor alpha (TNF-alpha ), also known as cachectin and TNFSF1A, is the prototypic ligand of the TNF superfamily (1). It is a pleiotropic molecule that plays a central role in inflammation, immune system development, apoptosis, and lipid metabolism (2-5). TNF-alpha is also involved in a number of pathological conditions including asthma, Crohn's disease, rheumatoid arthritis, neuropathic pain, obesity, type 2 diabetes, septic shock, autoimmunity, and cancer (5-11).
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Rat TNF-alpha DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
118
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Rats Exposed to a Low Resource Environment in Early Life Display Sex Differences in Blood Pressure, Autonomic Activity, and Brain and Kidney Pro-inflammatory Markers During Adulthood
Authors: Smith, J;Smith, S;Jones, K;Castillo, A;Bolton, JL;Howard, A;Colon-Perez, L;Femi-Ogunyemi, F;Burkes, A;Cunningham, M;
bioRxiv : the preprint server for biology
Species: Rat
Sample Types: Tissue Supernates
-
Chronic Copper Overload Triggers Inflammation in Mesenteric PVAT Alongside Changes in Renin-Angiotensin System-Related Pathways
Authors: Mawandji, NBS;Lisboa, NADS;Gomes, KN;Vieira, JM;Simão, JJ;Alonso-Vale, MI;Nunes, KZ;Vassallo, DV;Bolsoni-Lopes, A;
Nutrients
Species: Rat
Sample Types: Cell Culture Supernates
-
Trichosanthes kirilowii Maxim. and Bioactive Compound Cucurbitacin D Alleviate Cisplatin-Induced Peripheral Neuropathy In Vitro and In Vivo
Authors: Kang, S;Choi, G;Kim, D;Kim, H;Cheon, C;Ko, SG;
Integrative cancer therapies
Species: Rat
Sample Types: Serum
-
Cardioprotective effect of senotherapy in chronically obese middle-aged female rats may be mediated by a MERCSs/Nrf2 interaction
Authors: Silva-Palacios, A;Zúñiga-Muñoz, AM;Soria-Castro, E;Álvarez-León, E;Nieto, M;Navarrete-Anastasio, G;Carbó, R;García-Niño, WR;López-Cervantes, PS;Salas-Venegas, V;Flores-Torres, RP;Luna-López, A;Zazueta, C;Königsberg, M;
The Journal of nutritional biochemistry
Species: Rat
Sample Types: Serum
-
A high fraction of inspired oxygen does not mitigate atelectasis-induced lung tissue hypoxia or injury in experimental acute respiratory distress syndrome
Authors: Tojo, K;Yazawa, T;
Scientific reports
Species: Rat
Sample Types: BALF
-
Diosmin and Coenzyme q10: Synergistic histopathological and functional protection against doxorubicin-induced hepatorenal injury in rats
Authors: Mansour, DF;Hashad, IM;Rady, M;Abd-El Razik, AN;Saleh, DO;
Toxicology reports
Species: Rat
Sample Types: Serum
-
Hemoadsorption improves kidney microcirculatory oxygenation and oxygen consumption, ameliorates tubular injury, and improves kidney function in a rat model of sepsis-induced AKI
Authors: Ergin, B;Kutucu, DE;Kapucu, A;van Dam, W;Moretto, L;Heyman, P;Ince, C;
Scientific reports
Species: Rat
Sample Types: Plasma
-
Periodontitis and diabetes in pregnant rats: Maternal-fetal outcomes
Authors: Souza, SS;Lopes Cruz, L;Alves-Reis, AM;Costa, VQ;Moraes-Souza, RQ;Damasceno, DC;Volpato, GT;
Heliyon
Species: Rat
Sample Types: Serum
-
The Role of p38 Mitogen-Activated Protein Kinase-Mediated F-Actin in the Acupuncture-Induced Mitigation of Inflammatory Pain in Arthritic Rats
Authors: Zhou, X;Zhang, YC;Lu, KQ;Xiao, R;Tang, WC;Wang, F;
Brain sciences
Species: Rat
Sample Types: Tissue Homogenates
-
Physical training attenuates systemic cytokine response and tissue damage triggered by apical periodontitis
Authors: Ferreira, RO;Pereira, MS;Souza-Monteiro, D;Frazão, DR;de Moura, JDM;Baia-da-Silva, DC;Bittencourt, LO;Balbinot, GS;Collares, FM;Lima, MLS;de Araújo, AA;Lima, RR;
Scientific reports
Species: Rat
Sample Types: Serum
-
Methamphetamine Enhancement of HIV-1 gp120-Mediated NLRP3 Inflammasome Activation and Resultant Proinflammatory Responses in Rat Microglial Cultures
Authors: Dutta, D;Liu, J;Xu, E;Xiong, H;
International Journal of Molecular Sciences
Species: Rat
Sample Types: Cell Culture Supernates
-
Therapeutic potential of luteolin-loaded poly(lactic-co-glycolic acid)/modified magnesium hydroxide microsphere in functional thermosensitive hydrogel for treating neuropathic pain
Authors: Park, SY;Jung, JH;Kim, DS;Lee, JK;Song, BG;Shin, HE;Jung, JW;Baek, SW;You, S;Han, I;Han, DK;
Journal of tissue engineering
Species: Rat
Sample Types: Serum
-
Oxyberberine protects middle cerebral artery occlusion triggered cerebral injury through TLR4/NLRP3 pathway in rats
Authors: Rahman, Z;Shaikh, AS;Rao, KV;Dandekar, MP;
Journal of chemical neuroanatomy
Species: Rat
Sample Types: Antibody
-
Lung molecular and histological changes in type 2 diabetic rats and its improvement by high-intensity interval training
Authors: Rajizadeh, MA;Khoramipour, K;Joukar, S;Darvishzadeh-Mahani, F;Iranpour, M;Bejeshk, MA;Zaboli, MD;
BMC pulmonary medicine
Species: Rat
Sample Types: BALF
-
MANF protein expression is upregulated in immune cells in the ischemic human brain and systemic recombinant MANF delivery in rat ischemic stroke model demonstrates anti-inflammatory effects
Authors: Anttila, JE;Mattila, OS;Liew, HK;Mätlik, K;Mervaala, E;Lindholm, P;Lindahl, M;Lindsberg, PJ;Tseng, KY;Airavaara, M;
Acta neuropathologica communications
Species: Rat
Sample Types: Serum, Tissue Homogenates
-
Analysis of anti-rheumatic activity of Nyctanthes arbor-tristis via in vivo and pharmacovigilance approaches
Authors: Sharma, A;Goel, A;Lin, Z;
Frontiers in pharmacology
Species: Rat
Sample Types: Serum
-
Partial Replacement of Diet with Dehulled Adlay Ameliorates Hepatic Steatosis, Inflammation, Oxidative Stress, and Gut Dysbiosis in Rats with Nonalcoholic Fatty Liver Disease
Authors: Huang, HC;Lee, PN;Huang, WC;Yang, HY;
Nutrients
Species: Rat
Sample Types: Tissue Homogenates
-
Prevotella histicola Transplantation Ameliorates Cognitive Impairment and Decreases Oxidative Stress in Vascular Dementia Rats
Authors: Duan, R;Hou, J;Wang, X;Huang, Z;Cao, H;Hu, J;Peng, Q;Duan, H;Wang, Q;Chen, X;
Brain sciences
Species: Rat
Sample Types: Tissue Homogenates
-
Potential Benefits of Epidermal Growth Factor for Inhibiting Muscle Degrative Markers in Rats with Alcoholic Liver Damage
Authors: Xiao, Q;Chen, YH;Chen, YL;Chien, YS;Hsieh, LH;Shirakawa, H;Yang, SC;
International journal of molecular sciences
Species: Rat
Sample Types: Tissue Homgenates
-
Investigating behavioral phenotypes related to autism spectrum disorder in a gene-environment interaction model of Cntnap2 deficiency and Poly I:C maternal immune activation
Authors: FL Haddad, C De Oliveir, S Schmid
Frontiers in Neuroscience, 2023-03-14;17(0):1160243.
Species: Rat
Sample Types: Serum
-
Efficacy and Safety Evaluation of Mometasone Furoate in Treating Ocular Inflammation
Authors: NA Lage, MRB de Paiva, DV Vasconcelo, RR Machado, SL Fialho, A Silva-Cunh
Pharmaceutics, 2023-01-05;15(1):.
Species: Rat
Sample Types: Tissue Homogenates
-
Triptolide Attenuates Muscular Inflammation and Oxidative Stress in a Delayed-Onset Muscle Soreness Animal Model
Authors: CC Hsu, CC Tsai, PY Ko, TH Kwan, MY Liu, PT Wu, IM Jou
International Journal of Environmental Research and Public Health, 2022-12-12;19(24):.
Species: Rat
Sample Types: Tissue Homogenates
-
Effect of Hydrogel Containing Achyrocline satureioides (Asteraceae) Extract-Loaded Nanoemulsions on Wound Healing Activity
Authors: LA Balestrin, PI Back, MDS Marques, GMS Araújo, MCF Carrasco, MM Batista, T Silveira, JL Rodrigues, FNS Fachel, LS Koester, VL Bassani, AP Horn, CL Dora, HF Teixeira
Pharmaceutics, 2022-12-06;14(12):.
Species: Rat
Sample Types: Tissue Homogenates
-
Mitochondrial protective effects caused by the administration of mefenamic acid in sepsis
Authors: D Dominguini, M Michels, LB Wessler, EL Streck, T Barichello, F Dal-Pizzol
Journal of Neuroinflammation, 2022-11-04;19(1):268.
Species: Rat
Sample Types: Tissue Homogenates
-
Effect of citronellol on oxidative stress, neuroinflammation and autophagy pathways in an in vivo model of Parkinson's disease
Authors: RL Jayaraj, S Azimullah, KA Parekh, SK Ojha, R Beiram
Heliyon, 2022-11-03;8(11):e11434.
Species: Rat
Sample Types: Tissue Homogenates
-
Glia from the central and peripheral nervous system are differentially affected by paclitaxel chemotherapy via modulating their neuroinflammatory and neuroregenerative properties
Authors: I Klein, J Boenert, F Lange, B Christense, MK Wassermann, MHJ Wiesen, DN Olschewski, M Rabenstein, C Müller, HC Lehmann, GR Fink, M Schroeter, MA Rueger, SU Vay
Frontiers in Pharmacology, 2022-11-02;13(0):1038285.
Species: Rat
Sample Types: Cell Culture Supernates
-
Cisplatin-induced changes in calcitonin gene-related peptide or TNF-alpha release in rat dorsal root ganglia in vitro model of neurotoxicity are not reverted by rosiglitazone
Authors: HR Oliveira, MS Coelho, FAR Neves, DB Duarte
Neurotoxicology, 2022-10-10;93(0):211-221.
Species: Rat
Sample Types: Cell Culture Supernates
-
The superior beneficial effects of exercise training versus hormone replacement therapy on skeletal muscle of ovariectomized rats
Authors: SB Silva, K Honorato-S, SP Costa, TE Domingues, TMM da Cruz, CM Rodrigues, KB Costa, JM Dos Santos, VK da Silva L, TP Gaiad, AP Santos, MF Dias-Peixo, CC Coimbra, AM Dos Reis, RE Szawka, PHS Figueiredo, HS Costa, MX Oliveira, VA Mendonça, ACR Lacerda
Scientific Reports, 2022-05-24;12(1):8764.
Species: Rat
Sample Types: Tissue Homogenates
-
Inhibitory Effects of Saururus chinensis Extract on Receptor for Advanced Glycation End-Products-Dependent Inflammation and Diabetes-Induced Dysregulation of Vasodilation
Authors: K Hayashi, K Sato, S Ochi, S Kawano, S Munesue, A Harashima, Y Oshima, K Kimura, T Kyoi, Y Yamamoto
International Journal of Molecular Sciences, 2022-05-20;23(10):.
Species: Rat
Sample Types: Plasma
-
Low-Dose Aspirin Augments the Anti-Inflammatory Effects of Low-Dose Lithium in Lipopolysaccharide-Treated Rats
Authors: R Shvartsur, G Agam, S Uzzan, AN Azab
Pharmaceutics, 2022-04-20;14(5):.
Species: Rat
Sample Types: Tissue Homogenates
-
LRRK2 Inhibition Mitigates the Neuroinflammation Caused by TLR2-Specific alpha-Synuclein and Alleviates Neuroinflammation-Derived Dopaminergic Neuronal Loss
Authors: DH Ho, D Nam, M Seo, SW Park, W Seol, I Son
Cells, 2022-03-02;11(5):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Exposure to Nickel Oxide Nanoparticles Induces Acute and Chronic Inflammatory Responses in Rat Lungs and Perturbs the Lung Microbiome
Authors: MJ Jeong, S Jeon, HS Yu, WS Cho, S Lee, D Kang, Y Kim, YJ Kim, SY Kim
International Journal of Environmental Research and Public Health, 2022-01-04;19(1):.
Species: Rat
Sample Types: BALF
-
Therapeutic Effects of Fenofibrate Nano-Emulsion Eye Drops on Retinal Vascular Leakage and Neovascularization
Authors: L Huang, W Liang, K Zhou, RA Wassel, ZD Ridge, JX Ma, B Wang
Biology, 2021-12-15;10(12):.
Species: Rat
Sample Types: Tissue Homogenates
-
Effect of Pulmonary Inflammation by Surface Functionalization of Zinc Oxide Nanoparticles
Authors: A Jung, SH Kim, JY Yang, J Jeong, JK Lee, JH Oh, JH Lee
Toxics, 2021-12-06;9(12):.
Species: Rat
Sample Types: BALF
-
Prenatal administration of IL-1Ra attenuate the neurodevelopmental impacts following non-pathogenic inflammation during pregnancy
Authors: ME Brien, K Hughes, S Girard
Scientific Reports, 2021-12-03;11(1):23404.
Species: Rat
Sample Types: Placenta
-
Moderate Physical Exercise Activates ATR2 Receptors, Improving Inflammation and Oxidative Stress in the Duodenum of 2K1C Hypertensive Rats
Authors: ACA da Silva, JS Severo, BLB Dos Santos, PHM Mendes, LMS Nobre, AP de Oliveir, FCS Ferreira, JVR Medeiros, RC Lima-Junio, A Havt, RC Palheta-Ju, AA Dos Santos, M Tolentino
Frontiers in Physiology, 2021-10-14;12(0):734038.
Species: Rat
Sample Types: Tissue Homogenates
-
DEN-Induced Rat Model Reproduces Key Features of Human Hepatocellular Carcinoma
Authors: K Kurma, O Manches, F Chuffart, N Sturm, K Gharzeddin, J Zhang, M Mercey-Res, S Rousseaux, A Millet, H Lerat, PN Marche, Z Macek Jilk, T Decaens
Cancers, 2021-10-04;13(19):.
Species: Rat
Sample Types: Whole Tissue
-
Neuron-Derived Extracellular Vesicles Modulate Microglia Activation and Function
Authors: H Peng, BT Harvey, CI Richards, K Nixon
Biology, 2021-09-22;10(10):.
Species: Rat
Sample Types: Cell Culture Supernates
-
The Prophylactic Effects of Glutamine on Muscle Protein Synthesis and Degradation in Rats with Ethanol-Induced Liver Damage
Authors: Q Xiao, YH Chen, SA Pratama, YL Chen, H Shirakawa, HC Peng, SC Yang
Nutrients, 2021-08-14;13(8):.
Species: Rat
Sample Types: Tissue Homogenates
-
Programming of intestinal homeostasis in male rat offspring after maternal exposure to chlorpyrifos and/or to a high fat diet
Authors: M Guibourden, H El Khayat, N Djekkoun, H Khorsi-Cau, V Bach, PM Anton, J Gay-Quéhei
Scientific Reports, 2021-06-01;11(1):11420.
Species: Rat
Sample Types: Plasma
-
In Vitro Evaluation of the Anti-Inflammatory Effect of KMUP-1 and In Vivo Analysis of Its Therapeutic Potential in Osteoarthritis
Authors: SE Huang, E Sulistyowa, YY Chao, BN Wu, ZK Dai, JH Hsu, JL Yeh
Biomedicines, 2021-05-28;9(6):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
TLR2- and TLR3-activated microglia induce different levels of neuronal network dysfunction in a context-dependent manner
Authors: S Schilling, B Chausse, HO Dikmen, F Almouhanna, JO Hollnagel, A Lewen, O Kann
Brain, Behavior, and Immunity, 2021-05-17;0(0):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Inflammatory Cytokine Profile and Plasticity of Brain and Spinal Microglia in Response to ATP and Glutamate
Authors: SJB Jesudasan, SJ Gupta, MA Churchward, KG Todd, IR Winship
Frontiers in Cellular Neuroscience, 2021-04-06;15(0):634020.
Species: Rat
Sample Types: Cell Culture Supernates
-
Mast cells contribute to alveolar bone loss in Spontaneously Hypertensive Rats with periodontal disease regulating cytokines production
Authors: VGB Brito, MS Patrocinio, MCL Sousa, AEA Barreto, SCT Frasnelli, VS Lara, CF Santos, SHP Oliveira
PLoS ONE, 2021-03-04;16(3):e0247372.
Species: Rat
Sample Types: Tissue Homogenates
-
Small intestine remodeling in male Goto-Kakizaki rats
Authors: JNB Pereira, GM Murata, FT Sato, AR Marosti, CRO Carvalho, R Curi
Physiological Reports, 2021-02-01;9(3):e14755.
Species: Rat
Sample Types: Tissue Homogenates
-
Allanblackia floribunda Seed Extract Attenuates the Ethanol-Induced Gastric Ulcer in Rats via the Inhibition of TNF-&alpha and INF-&gamma Levels and Modulation in the Expression of Ki67 Protein
Authors: FA Armah, IT Henneh, J Alake, W Ahlidja, B Amoani, EG Ofori, B Asante-Kye, GI Temitayo, CK Adokoh
BioMed Research International, 2021-01-11;2021(0):6694572.
Species: Rat
Sample Types: Serum
-
The Poststroke Peripheral Immune Response Is Differentially Regulated by Leukemia Inhibitory Factor in Aged Male and Female Rodents
Authors: SM Davis, LA Collier, SJ Messmer, KR Pennypacke
Oxidative Medicine and Cellular Longevity, 2020-12-10;2020(0):8880244.
Species: Rat
Sample Types: Tissue Homogenates
-
&alpha-Bisabolol, a Dietary Bioactive Phytochemical Attenuates Dopaminergic Neurodegeneration through Modulation of Oxidative Stress, Neuroinflammation and Apoptosis in Rotenone-Induced Rat Model of Parkinson's disease
Authors: H Javed, MFN Meeran, S Azimullah, L Bader Eddi, VD Dwivedi, NK Jha, S Ojha
Biomolecules, 2020-10-08;10(10):.
Species: Rat
Sample Types: Tissue Homogenates
-
Impacts of fish oil on the gut microbiota of rats with alcoholic liver damage
Authors: YL Chen, H Shirakawa, NS Lu, HC Peng, Q Xiao, SC Yang
J. Nutr. Biochem., 2020-09-11;86(0):108491.
Species: Rat
Sample Types: Cell Culture Supernates
-
Cilostazol ameliorates diabetic nephropathy by inhibiting highglucose- induced apoptosis
Authors: CW Chian, YS Lee, YJ Lee, YH Chen, CP Wang, WC Lee, HJ Lee
Korean J. Physiol. Pharmacol., 2020-09-01;24(5):403-412.
Species: Rat
Sample Types: Tissue Homogenate, Tissue Homogenates
-
T cell infiltration and upregulation of MHCII in microglia leads to accelerated neuronal loss in an alpha-synuclein rat model of Parkinson's disease
Authors: MS Subbarayan, C Hudson, LD Moss, KR Nash, PC Bickford
J Neuroinflammation, 2020-08-15;17(1):242.
Species: Rat
Sample Types: Cell Culture Supernates, Whole Cells
-
Hepcidin-to-Ferritin Ratio Is Decreased in Astrocytes With Extracellular Alpha-Synuclein and Iron Exposure
Authors: J Cui, X Guo, Q Li, N Song, J Xie
Front Cell Neurosci, 2020-03-10;14(0):47.
Species: Rat
Sample Types: Cell Culture Supernates
-
TNF-&alpha mediated upregulation of NaV1.7 currents in rat dorsal root ganglion neurons is independent of CRMP2 SUMOylation
Authors: FHP de Macedo, RD Aires, EG Fonseca, RCM Ferreira, DPD Machado, L Chen, FX Zhang, IA Souza, VS Lemos, TRL Romero, A Moutal, R Khanna, GW Zamponi, JS Cruz
Mol Brain, 2019-12-30;12(1):117.
Species: Rat
Sample Types: Plasma
-
Acute sleep deprivation during pregnancy in rats: Rapid elevation of placental and fetal inflammation and kynurenic acid
Authors: AM Baratta, NR Kanyuch, CA Cole, H Valafar, J Deslaurier, A Pocivavsek
Neurobiol Stress, 2019-12-14;12(0):100204.
Species: Rat
Sample Types: Tissue Homogenates
-
Protective effect of Platymiscium floribundum Vog. in tree extract on periodontitis inflammation in rats
Authors: JMO Freire, HV Chaves, AH Teixeira, LHT de Sousa, IR Pinto, JJDN Costa, NA de Sousa, KMA Pereira, VCC Girão, VCS Ferreira, JEÁ Dos Santos, MAS Lima, ATA Pimenta, RC Montenegro, MEA de Moraes, VPT Pinto, GC Filho, MM Bezerra
PLoS ONE, 2019-11-04;14(11):e0223800.
Species: Rat
Sample Types: Gingivial Fluid
-
The Preventive Effects of Greenshell Mussel (Perna canaliculus) on Early-Stage Metabolic Osteoarthritis in Rats with Diet-Induced Obesity
Authors: P Siriarchav, MC Kruger, MR Miller, HS Tian, FM Wolber
Nutrients, 2019-07-15;11(7):.
Species: Rat
Sample Types: Plasma
-
Hepatoprotective Effect of Jianpi Huoxue Formula on Nonalcoholic Fatty Liver Disease Induced by Methionine-Choline-Deficient Diet in Rat
Authors: Y Feng, Y Chen, B Yang, Q Lan, T Wang, G Cui, Z Ren, IC Choi, GP Leung, F Yan, D Chen, HH Yu, SMY Lee
Biomed Res Int, 2019-07-01;2019(0):7465272.
Species: Rat
Sample Types: Serum
-
A diet including xanthan gum triggers a pro-inflammatory response in Wistar rats inoculated with Walker 256 cells
Authors: AB Silva Risc, NIP Neto, K Gascho, M Carnier, DA de Miranda, FP Silva, VT Boldarine, M Seelaender, EB Ribeiro, LM Oyama, CM Oller do N
PLoS ONE, 2019-06-18;14(6):e0218567.
Species: Rat
Sample Types: Tissue Homogenates
-
Time dependent dual effect of anti-inflammatory treatments on sarin-induced brain inflammation: suggested role of prostaglandins
Authors: S Chapman, E Grauer, R Gez, I Egoz, S Lazar
Neurotoxicology, 2019-05-13;0(0):.
Species: Rat
Sample Types: Tissue Homogenates
-
Hyperbilirubinemia in Gunn Rats is Associated with Decreased Inflammatory Response in LPS-Mediated Systemic Inflammation
Authors: P Valaskova, A Dvorak, M Lenicek, K Zizalova, N Kutinova-C, J Zelenka, M Cahova, L Vitek, L Muchova
Int J Mol Sci, 2019-05-09;20(9):.
Species: Rat
Sample Types: Serum
-
Polyphenols-Rich Fruit (Euterpe edulis Mart.) Prevents Peripheral Inflammatory Pathway Activation by the Short-Term High-Fat Diet
Authors: AB Santamarin, G Jamar, LV Mennitti, DA Ribeiro, CM Cardoso, VV de Rosso, LM Oyama, LP Pisani
Molecules, 2019-04-27;24(9):.
Species: Rat
Sample Types: Tissue Homogenates
-
Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury
Authors: G Thomi, D Surbek, V Haesler, M Joerger-Me, A Schoeberle
Stem Cell Res Ther, 2019-03-21;10(1):105.
Species: Rat
Sample Types: Tissue Homogenates
-
BRD4 inhibition attenuates inflammatory response in microglia and facilitates recovery after spinal cord injury in rats
Authors: J Wang, J Chen, H Jin, D Lin, Y Chen, X Chen, B Wang, S Hu, Y Wu, Y Wu, Y Zhou, N Tian, W Gao, X Wang, X Zhang
J. Cell. Mol. Med., 2019-02-26;0(0):.
Species: Rat
Sample Types: Tissue Homogenates
-
Cholinergic Stimulation by Pyridostigmine Bromide Before Myocardial Infarction Prevent Cardiac and Autonomic Dysfunction
Authors: CA Barboza, AR Fukushima, N Carrozzi, JF Machi, PMM Dourado, CT Mostarda, MC Irigoyen, L Nathanson, M Morris, EC Caperuto, B Rodrigues
Sci Rep, 2019-02-21;9(1):2481.
Species: Rat
Sample Types: Plasma
-
Crude extract from Libidibia ferrea (Mart. ex. Tul.) L.P. Queiroz leaves decreased intra articular inflammation induced by zymosan in rats
Authors: TR Falcão, CAO Rodrigues, AA de Araújo, CACX de Medeiro, LAL Soares, MRA Ferreira, RC Vasconcelo, RF de Araújo, MLD de Sousa L, GCB Guerra
BMC Complement Altern Med, 2019-02-12;19(1):47.
Species: Rat
Sample Types: Synovial Fluid
-
Improvement of diabetic wound healing by topical application of Vicenin-2 hydrocolloid film on Sprague Dawley rats
Authors: WS Tan, P Arulselvan, SF Ng, CN Mat Taib, MN Sarian, S Fakurazi
BMC Complement Altern Med, 2019-01-17;19(1):20.
Species: Rat
Sample Types: Tissue Homogenates
-
Characterization of the Myocardial Inflammatory Response in Acute Stress-Induced (Takotsubo) Cardiomyopathy
Authors: HM Wilson, L Cheyne, PAJ Brown, K Kerr, A Hannah, J Srinivasan, N Duniak, G Horgan, DK Dawson
JACC Basic Transl Sci, 2018-12-31;3(6):766-778.
Species: Rat
Sample Types: Serum
-
Obesity and Type 2 Diabetes mellitus induce lipopolysaccharide tolerance in rat neutrophils
Authors: WMT Kuwabara, CNF Yokota, R Curi, TC Alba-Loure
Sci Rep, 2018-12-03;8(1):17534.
Species: Rat
Sample Types: BALF
-
Activation of trigeminal ganglion satellite glial cells in CFA-induced tooth pulp pain in rats
Authors: HF Filippini, PA Scalzilli, KM Costa, RDS Freitas, MM Campos
PLoS ONE, 2018-11-12;13(11):e0207411.
Species: Rat
Sample Types: Serum
-
Leukemia inhibitory factor modulates the peripheral immune response in a rat model of emergent large vessel occlusion
Authors: SM Davis, LA Collier, ED Winford, CC Leonardo, CT Ajmo, EA Foran, TJ Kopper, JC Gensel, KR Pennypacke
J Neuroinflammation, 2018-10-15;15(1):288.
Species: Rat
Sample Types: Tissue Homogenates
-
Human Mesenchymal Stem Cell Secretome from Bone Marrow or Adipose-Derived Tissue Sources for Treatment of Hypoxia-Induced Pulmonary Epithelial Injury
Authors: N Shologu, M Scully, JG Laffey, D O'Toole
Int J Mol Sci, 2018-09-30;19(10):.
Species: Rat
Sample Types: Cell Culture Supernates
-
The plasticity of primary microglia and their multifaceted effects on endogenous neural stem cells in vitro and in vivo
Authors: SU Vay, LJ Flitsch, M Rabenstein, R Rogall, S Blaschke, J Kleinhaus, N Reinert, A Bach, GR Fink, M Schroeter, MA Rueger
J Neuroinflammation, 2018-08-13;15(1):226.
Species: Rat
Sample Types: Cell Culture Supernates
-
The Use of Ju�ara (Euterpe edulis Mart.) Supplementation for Suppression of NF-?B Pathway in the Hypothalamus after High-Fat Diet in Wistar Rats
Authors: AB Santamarin, G Jamar, LV Mennitti, VV de Rosso, HC Cesar, LM Oyama, LP Pisani
Molecules, 2018-07-21;23(7):.
Species: Rat
Sample Types: Tissue Homogenates
-
Cortical stimulation in conscious rats controls joint inflammation
Authors: GS Bassi, L Ulloa, VR Santos, F Del Vecchi, P Delfino-Pe, GJ Rodrigues, JA Castania, FQ Cunha, HC Salgado, TM Cunha, N Garcia-Cai, A Kanashiro
Prog. Neuropsychopharmacol. Biol. Psychiatry, 2018-03-06;0(0):.
Species: Rat
Sample Types: Synovial Fluid
-
Early exposure to distinct sources of lipids affects differently the development and hepatic inflammatory profiles of 21-day-old rat offspring
Authors: LV Mennitti, LM Oyama, AB Santamarin, CMDPO do Nascime, LP Pisani
J Inflamm Res, 2018-01-18;11(0):11-24.
Species: Rat
Sample Types: Tissue Homogenates
-
Different Dietary Proportions of Fish Oil Regulate Inflammatory Factors but Do Not Change Intestinal Tight Junction ZO-1 Expression in Ethanol-Fed Rats
Authors: YW Chien, HC Peng, YL Chen, MH Pai, HY Wang, HL Chuang, SC Yang
Mediators Inflamm., 2017-12-13;2017(0):5801768.
Species: Rat
Sample Types: Tissue Homogenates
-
Carotid sinus nerve electrical stimulation in conscious rats attenuates systemic inflammation via chemoreceptor activation
Authors: FM Santos-Alm, G Domingos-S, CA Meschiari, LC Fávaro, C Becari, JA Castania, A Lopes, TM Cunha, DJA Moraes, FQ Cunha, L Ulloa, A Kanashiro, GCSV Tezini, HC Salgado
Sci Rep, 2017-07-24;7(1):6265.
Species: Rat
Sample Types: Plasma
-
Intestinal anti-inflammatory activity of Ground Cherry (Physalis angulata L.) standardized CO2 phytopharmaceutical preparation
Authors: LD Almeida Ju, AEV Quaglio, CAR de Almeida, LC Di Stasi
World J. Gastroenterol., 2017-06-28;23(24):4369-4380.
Species: Rat
Sample Types: Tissue Homogenates
-
Impact of gestational nicotine exposure on intrauterine and fetal infection in a rodent model
Authors: M von Chamie, L Reyes, LF Hayward, MB Brown
Biol. Reprod., 2017-05-01;96(5):1071-1084.
Species: Rat
Sample Types: Whole Tissue
Applications: ELISA Capture -
Impact of matrix metalloproteinase 9 rs3918242 genetic variant on lipid-lowering efficacy of simvastatin therapy in Chinese patients with coronary heart disease
Authors: Y Xu, Y Wang, J Zhi, L Qi, T Zhang, X Li
BMC Pharmacol Toxicol, 2017-04-08;18(1):28.
Species: Human
Sample Types: Plasma
-
The protective role of nitric oxide-dependent innate immunosuppression in the early stage of cartilage damage in rats: Role of nitric oxide in cartilage damage
Authors: CC Hsu, CL Lin, IM Jou, PH Wang, JS Lee
Bone Joint Res, 2017-04-01;6(4):253-258.
Species: Rat
Sample Types: Tissue Homogenates
-
Pyrrolidine dithiocarbamate administered during ex-vivo lung perfusion promotes rehabilitation of injured donor rat lungs obtained after prolonged warm ischemia
Authors: C Francioli, X Wang, R Parapanov, E Abdelnour, J Lugrin, F Gronchi, J Perentes, P Eckert, HB Ris, L Piquilloud, T Krueger, L Liaudet
PLoS ONE, 2017-03-21;12(3):e0173916.
Species: Rat
Sample Types: BALF
-
Original research paper. Pulmonary prophylactic impact of melatonin and/or quercetin: A novel therapy for inflammatory hypoxic stress in rats
Authors: NM Al-Rasheed, L Fadda, HA Attia, IA Sharaf, AM Mohamed, NM Al-Rasheed
Acta Pharm, 2017-03-01;67(1):125-135.
Species: Rat
Sample Types: Serum
-
Post-translational Activation of Glutamate Cysteine Ligase with Dimercaprol: A Novel Mechanism of Inhibiting Neuroinflammation in vitro
Authors: PB McElroy, A Sri Hari, BJ Day, M Patel
J. Biol. Chem, 2017-02-15;0(0):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Incline treadmill exercise suppresses pain hypersensitivity associated with the modulation of pro-inflammatory cytokines and anti-inflammatory cytokine in rats with peripheral nerve injury
Authors: KL Tsai, PC Huang, LK Wang, CH Hung, YW Chen
Neurosci. Lett, 2017-02-12;643(0):27-31.
Species: Rat
Sample Types: Tissue Homogenates
-
Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon
Authors: S Bettini, E Boutet-Rob, C Cartier, C Com‚ra, E Gaultier, J Dupuy, N Naud, S Tach‚, P Grysan, S Reguer, N Thieriet, M R‚fr‚giers, D ThiaudiŠre, JP Cravedi, M CarriŠre, JN Audinot, FH Pierre, L Guzylack-P, E Houdeau
Sci Rep, 2017-01-20;7(0):40373.
Species: Rat
Sample Types: Tissue Homogenates
-
Neuroprotection by Caffeine in Hyperoxia-Induced Neonatal Brain Injury
Authors: S Endesfelde, U Weichelt, E Strauá, A Schl”r, M Sifringer, T Scheuer, C Bhrer, T Schmitz
Int J Mol Sci, 2017-01-18;18(1):.
Species: Rat
Sample Types: Tissue Homogenates
-
Experimental Diabetes Alters the Morphology and Nano-Structure of the Achilles Tendon
Authors: RR Oliveira, R Medina de, L Magalh?es, F Guimar?es, F Tovar-Moll, L Eurico Nas, GA de Castro
PLoS ONE, 2017-01-17;12(1):e0169513.
Species: Rat
Sample Types: Tissue Homogenates
-
Propentofylline Prevents Sickness Behavior and Depressive-Like Behavior Induced by Lipopolysaccharide in Rats via Neuroinflammatory Pathway
Authors: MM Moraes, MC Galv?o, D Cabral, CP Coelho, N Queiroz-Ha, MF Martins, EF Bondan, MM Bernardi, TB Kirsten
PLoS ONE, 2017-01-05;12(1):e0169446.
Species: Rat
Sample Types: Plasma
-
The Involvement of Pial Microvessels in Leukocyte Invasion after Mild Traumatic Brain Injury
PLoS ONE, 2016-12-28;11(12):e0167677.
Species: Rat
Sample Types: Tissue Homogenates
-
Epigallocatechin-3-gallate prevents cardiac apoptosis by modulating the intrinsic apoptotic pathway in isoproterenol-induced myocardial infarction
Authors: Mohammed Dardor
Eur. J. Pharmacol., 2016-11-15;0(0):.
Species: Rat
Sample Types: Serum
-
Intra-articular blockade of P2X7 receptor reduces the articular hyperalgesia and inflammation in the knee joint synovitis especially in female rats
Authors: Cláudia Herrera Tambeli
J Pain, 2016-11-04;0(0):.
Species: Rat
Sample Types: Synovial Lavage Fluid
-
Impact of exercise training associated to pyridostigmine treatment on autonomic function and inflammatory profile after myocardial infarction in rats
Authors: Bruno Rodrigues
Int. J. Cardiol., 2016-10-26;0(0):.
Species: Rat
Sample Types: Plasma
-
Combination of methylprednisolone and rosiglitazone promotes recovery of neurological function after spinal cord injury
Neural Regen Res, 2016-10-01;11(10):1678-1684.
Species: Rat
Sample Types: Tissue Homogenates
-
Zinc prevents sickness behavior induced by lipopolysaccharides after a stress challenge in rats.
Authors: Kirsten T, Galvao M, Reis-Silva T, Queiroz-Hazarbassanov N, Bernardi M
PLoS ONE, 2015-03-16;10(3):e0120263.
Species: Rat
Sample Types: Plasma
-
S-nitrosoglutathione accelerates recovery from 5-fluorouracil-induced oral mucositis.
Authors: Skeff M, Brito G, de Oliveira M, Braga C, Cavalcante M, Baldim V, Holanda-Afonso R, Silva-Boghossian C, Colombo A, Ribeiro R, Moura-Neto V, Leitao R
PLoS ONE, 2014-12-05;9(12):e113378.
Species: Hamster
Sample Types: Tissue Homogenates
-
Muscle hyperalgesia induced by peripheral P2X3 receptors is modulated by inflammatory mediators.
Authors: Schiavuzzo J, Teixeira J, Melo B, da Silva dos Santos D, Jorge C, Oliveira-Fusaro M, Parada C
Neuroscience, 2014-11-20;285(0):24-33.
Species: Rat
Sample Types: Tissue Homogenates
-
Exploring LPS-induced sepsis in rats and mice as a model to study potential protective effects of the nociceptin/orphanin FQ system
Authors: Jonathan P Thompson
Peptides, 2014-08-23;61(0):56-60.
Species: Rat
Sample Types: Serum
-
Protective effect of hypercapnic acidosis in ischemia-reperfusion lung injury is attributable to upregulation of heme oxygenase-1.
Authors: Wu S, Li M, Ko F, Wu G, Huang K, Chu S
PLoS ONE, 2013-09-10;8(9):e74742.
Species: Rat
Sample Types: BALF
-
Oligofructose supplementation (10%) during pregnancy and lactation does not change the inflammatory effect of concurrent trans fatty acid ingestion on 21-day-old offspring.
Authors: Hachul A, Mennitti L, de Oliveira J, Moreno M, Okuda M, Dos Santos B, Oyama L, Ribeiro E, do Nascimento C, Pisani L
Lipids Health Dis, 2013-05-01;12(0):59.
Species: Rat
Sample Types: Tissue Homogenates
-
Effects of freshwater clam extract supplementation on time to exhaustion, muscle damage, pro/anti-inflammatory cytokines, and liver injury in rats after exhaustive exercise.
Authors: Huang K, Wu W, Yang F, Chiu Y, Peng T, Hsu B, Liao K, Lee R
Molecules, 2013-03-26;18(4):3825-38.
Species: Rat
Sample Types: Plasma
-
Depot-specific modulation of adipokine levels in rat adipose tissue by diet-induced obesity: the effect of aerobic training and energy restriction.
Authors: Yamashita AS, Lira FS, Rosa JC, Paulino EC, Brum PC, Negrao CE, dos Santos RV, Batista ML, do Nascimento CO, Oyama LM, Seelaender M
Cytokine, 2010-08-21;52(3):168-74.
Species: Rat
Sample Types: Tissue Homogenates
-
Supernatant of stored platelets causes lung inflammation and coagulopathy in a novel in vivo transfusion model.
Authors: Vlaar AP, Hofstra JJ, Kulik W
Blood, 2010-05-17;116(8):1360-8.
Species: Rat
Sample Types: Tissue Homogenates
-
TNF-alpha, IL-1beta, IL-6, and cinc-1 levels in rat brain after meningitis induced by Streptococcus pneumoniae.
Authors: Barichello T, dos Santos I, Savi GD, Simoes LR, Silvestre T, Comim CM, Sachs D, Teixeira MM, Teixeira AL, Quevedo J
J. Neuroimmunol., 2010-03-03;221(1):42-5.
Species: Rat
Sample Types: Tissue Homogenates
-
The lectin-like domain of tumor necrosis factor improves lung function after rat lung transplantation--potential role for a reduction in reactive oxygen species generation.
Authors: Hamacher J, Stammberger U, Roux J, Kumar S, Yang G, Xiong C, Schmid RA, Fakin RM, Chakraborty T, Hossain HM, Pittet JF, Wendel A, Black SM, Lucas R
Crit. Care Med., 2010-03-01;38(3):871-8.
Species: Rat
Sample Types: BALF
-
Effects of ciliary muscle plasmid electrotransfer of TNF-alpha soluble receptor variants in experimental uveitis.
Authors: Touchard E, Bloquel C, Bigey P, Kowalczuc L, Jonet L, Thillaye-Goldenberg B, Naud MC, Scherman D, de Kozak Y, Benezra D, Behar-Cohen F
Gene Ther., 2009-05-14;16(7):862-73.
Species: Rat
Sample Types: Aqueous Humor
-
Augmentation of endogenous cannabinoid tone modulates lipopolysaccharide-induced alterations in circulating cytokine levels in rats.
Authors: Roche M, Kelly JP, O'Driscoll M, O'Driscoll M, Finn DP
Immunology, 2008-04-03;125(2):263-71.
Species: Rat
Sample Types: Plasma
-
MAPKs are key players in mediating cytokine release and cell death induced by unconjugated bilirubin in cultured rat cortical astrocytes.
Authors: Fernandes A, Falcao AS, Silva RF, Brito MA, Brites D
Eur. J. Neurosci., 2007-02-01;25(4):1058-68.
Species: Rat
Sample Types: Cell Culture Supernates
-
Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging.
Authors: Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I
Am. J. Physiol. Lung Cell Mol. Physiol., 2006-10-13;292(2):L567-76.
Species: Rat
Sample Types: BALF
-
Utility of exhaled nitric oxide as a noninvasive biomarker of lung inflammation in a disease model.
Authors: Birrell MA, McCluskie K, Hardaker E, Knowles R, Belvisi MG
Eur. Respir. J., 2006-09-27;28(6):1236-44.
Species: Rat
Sample Types: BALF
-
Role of matrix metalloproteinases in the inflammatory response in human airway cell-based assays and in rodent models of airway disease.
Authors: Birrell MA, Wong S, Dekkak A, De Alba J, Haj-Yahia S, Belvisi MG
J. Pharmacol. Exp. Ther., 2006-05-11;318(2):741-50.
Species: Rat
Sample Types: BALF
-
Inflammatory signalling pathways involved in astroglial activation by unconjugated bilirubin.
Authors: Fernandes A, Falcao AS, Silva RF, Gordo AC, Gama MJ, Brito MA, Brites D
J. Neurochem., 2006-02-10;96(6):1667-79.
Species: Rat
Sample Types: Cell Culture Supernates
-
Cardiomyocyte function after burn injury and lipopolysaccharide exposure: single-cell contraction analysis and cytokine secretion profile.
Authors: Niederbichler AD, Westfall MV, Su GL, Donnerberg J, Usman A, Vogt PM, Ipaktchi KR, Arbabi S, Wang SC, Hemmila MR
Shock, 2006-02-01;25(2):176-83.
Species: Rat
Sample Types: Cell Culture Supernates
-
GM-CSF action in the CNS decreases food intake and body weight.
Authors: Reed JA, Clegg DJ, Smith KB, Tolod-Richer EG, Matter EK, Picard LS, Seeley RJ
J. Clin. Invest., 2005-11-01;115(11):3035-44.
Species: Rat
Sample Types: Serum
-
Effects of estradiol on lipopolysaccharide and Pam3Cys stimulation of CCL20/macrophage inflammatory protein 3 alpha and tumor necrosis factor alpha production by uterine epithelial cells in culture.
Authors: Crane-Godreau MA, Wira CR
Infect. Immun., 2005-07-01;73(7):4231-7.
Species: Human
Sample Types: Cell Culture Supernates
-
CCL20/macrophage inflammatory protein 3alpha and tumor necrosis factor alpha production by primary uterine epithelial cells in response to treatment with lipopolysaccharide or Pam3Cys.
Authors: Crane-Godreau MA, Wira CR
Infect. Immun., 2005-01-01;73(1):476-84.
Species: Rat
Sample Types: Cell Culture Supernates
-
Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat.
Authors: Gilmore JH, Jarskog LF, Vadlamudi S
J. Neuroimmunol., 2004-11-24;159(1):106-12.
Species: Rat
Sample Types: Tissue Homogenates
-
A study of the biologic activity of trauma-hemorrhagic shock mesenteric lymph over time and the relative role of cytokines.
Authors: Davidson MT, Deitch EA, Lu Q, Osband A, Feketeova E, Nemeth ZH, Hasko G, Xu DZ
Surgery, 2004-07-01;136(1):32-41.
Species: Rat
Sample Types: Lymph
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Rat TNF-alpha DuoSet ELISA
Average Rating: 4.4 (Based on 7 Reviews)
Have you used Rat TNF-alpha DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
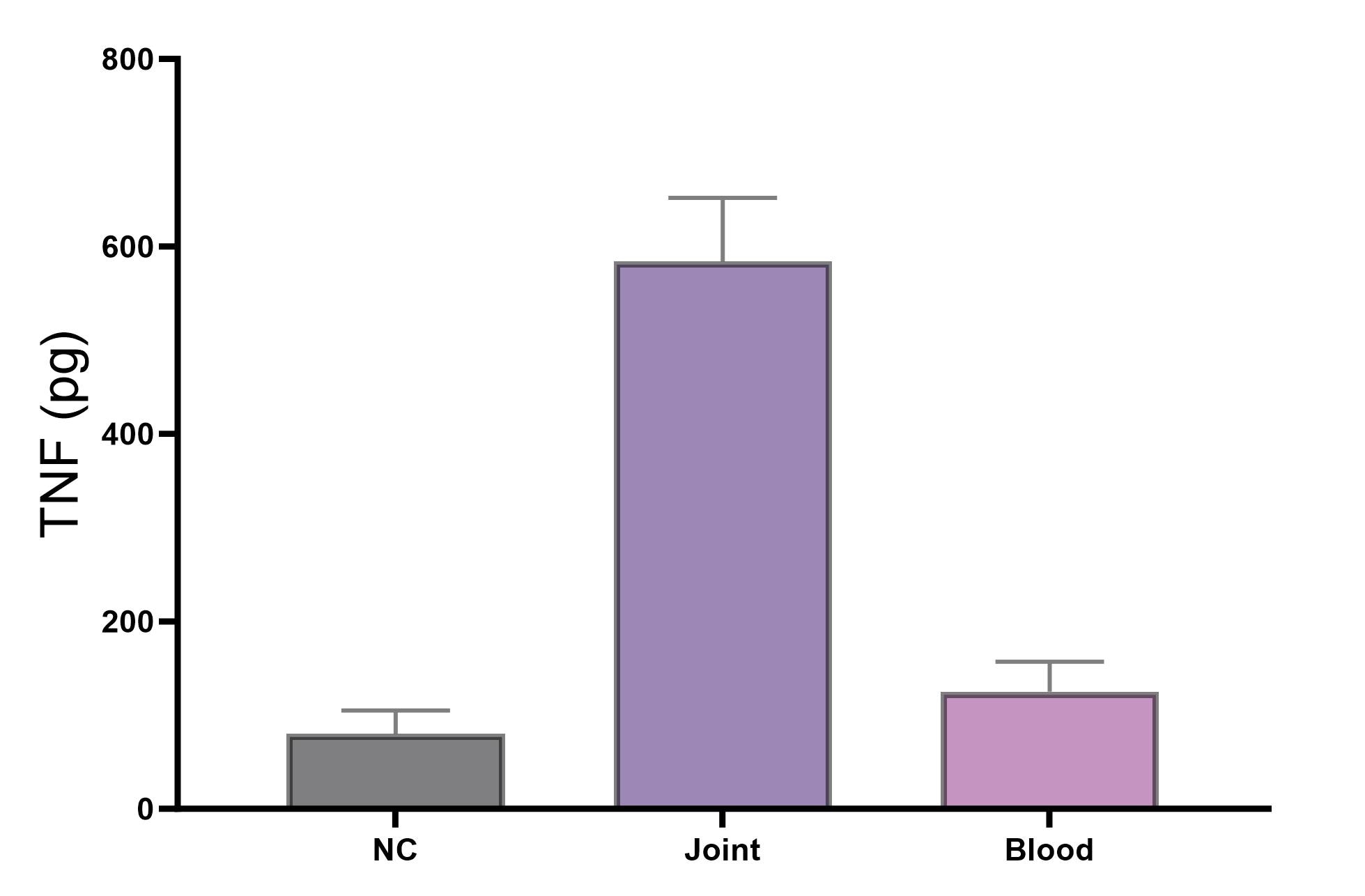
Lower STD shows less difference