Human ICAM-1/CD54 Antibody Summary
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
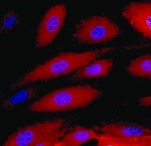
ICAM‑1/CD54 in C32 Human Cell Line. ICAM‑1/CD54 was detected in live unfixed C32 human melanoma cell line using Mouse Anti-Human ICAM‑1/CD54 Monoclonal Antibody (Catalog # BBA3) at 5 µg/mL for 1 hour at 2° - 8° C. Cells were then fixed with 4% formaldehyde followed by incubation with the NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (red; NL007) and counterstained with DAPI (blue). Specific staining was localized to cell surface and cytoplasm.
 View Larger
View Larger
Detection of Human ICAM‑1/CD54 by Western Blot. Western blot shows lysates of C32. PVDF membrane was probed with 2 µg/mL of Mouse Anti-Human ICAM‑1/CD54 Monoclonal Antibody (Catalog # BBA3) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF018). A specific band was detected for ICAM‑1/CD54 at approximately 85 kDa (as indicated). This experiment was conducted under non-reducing conditions and using Western Blot Buffer Group 1.
 View Larger
View Larger
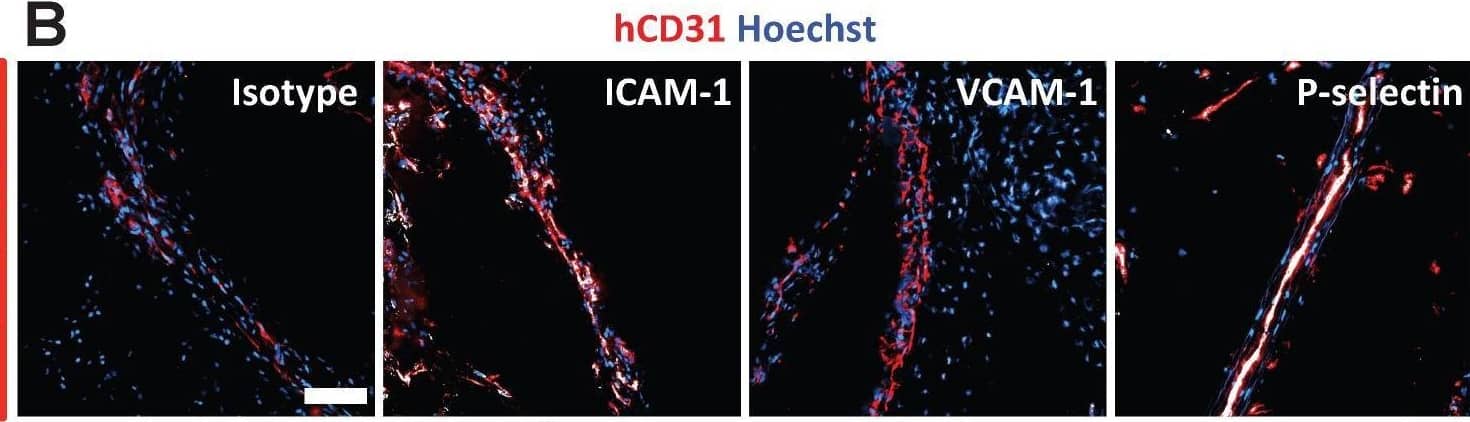
Detection of ICAM-1/CD54 by Immunohistochemistry SERS-BFNP molecular imaging of atherosclerotic coronary arteries. A single human coronary artery was isolated from the heart of a patient undergoing heart transplantation surgery. The lumen of the artery segment was then injected with a mixture of anti-ICAM-1, anti-VCAM-1, anti-P-selectin, and isotype control BFNP, sutured closed, and incubated at 37 °C/5% CO2 for 12 h. Sutures were then removed and the artery segment was thoroughly washed prior to SERS spectroscopy and subsequent analysis of morphology, expression of adhesion molecules and SERS mapping. (B) Immunofluorescence staining for CD31, and expression of (C) ICAM-1, VCAM-1 and P-selectin are shown in red. Nuclei were counterstained using Hoechst 33342 (blue). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/30613292), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of ICAM‑1/CD54 in Human PBMCs by Flow Cytometry. Human peripheral blood mononuclear cells (PBMCs) were stained with (A) Mouse Anti-Human ICAM-1/CD54 Monoclonal Antibody (Catalog # BBA3) or (B) Mouse IgG1 Isotype Control (MAB002) followed by anti-Mouse IgG PE-conjugated Secondary Antibody (F0102B) and Mouse Anti-Human CD14 APC-conjugated Monoclonal Antibody (FAB3832A). Staining was performed using our Staining Membrane-associated Proteins protocol.
 View Larger
View Larger
Detection of ICAM-1/CD54 by Immunohistochemistry In vivo SERS-BFNP molecular imaging of adhesion molecules. Following engraftment of human adipose, HANSG mice were allowed to recover for 3 weeks. Mice were then injected intravenously with 5 μg of human recombinant TNF-alpha 4 h prior to receiving an intravenous injection of BFNP. (A) Following SERS-BFNP molecular imaging, adipose grafts were excised and immunofluorescently stained for human (red) and murine (green) CD31. (B) Isotype control, ICAM-1, VCAM-1, and P-selectin staining are also shown in white counterstained with human CD31 (red). Nuclei were counterstained using Hoechst 33342 (blue). (C) To conduct SERS-BFNP molecular imaging, HANSG mice were anaesthetized and their adipose grafts non-invasively analyzed in vivo using SERS spectroscopy. (D) SERS spectra were acquired from mice that received a mixture of isotype-PPY, -BPE and -PYOT (blue spectra), or anti-P-selectin-PPY, anti-ICAM-1-BPE, anti-VCAM-1-PYOT BFNP (red spectra). The spectra shown are from 5 isotype vs. 5 targeted mice, with each spectrum acquired from a different mouse. (E) In addition to immunofluorescence microscopy, excised adipose grafts were analyzed using SERS microscopy. Detection of BFNP from sections of adipose tissue isolated from HANSG mice that received anti-ICAM-1 (purple), anti-VCAM-1 (red), and anti-P-selectin (blue) (upper panels) or Isotype-BPE (purple), Isotype-PYOT (red), and Isotype-PPY (blue) (lower panels) are shown superimposed on darkfield tissue images alongside a magnified image of Raman maps from the scanned areas (black boxes). The colored circles in the Raman map ((E) upper panel) correlate to the acquired spectra shown in (F) above their respective reference spectra. The optical image in (E) is a darkfield image. Scale bars: (A) = 1000 μm; (B) = 100 μm; (E) = 20 μm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/30613292), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
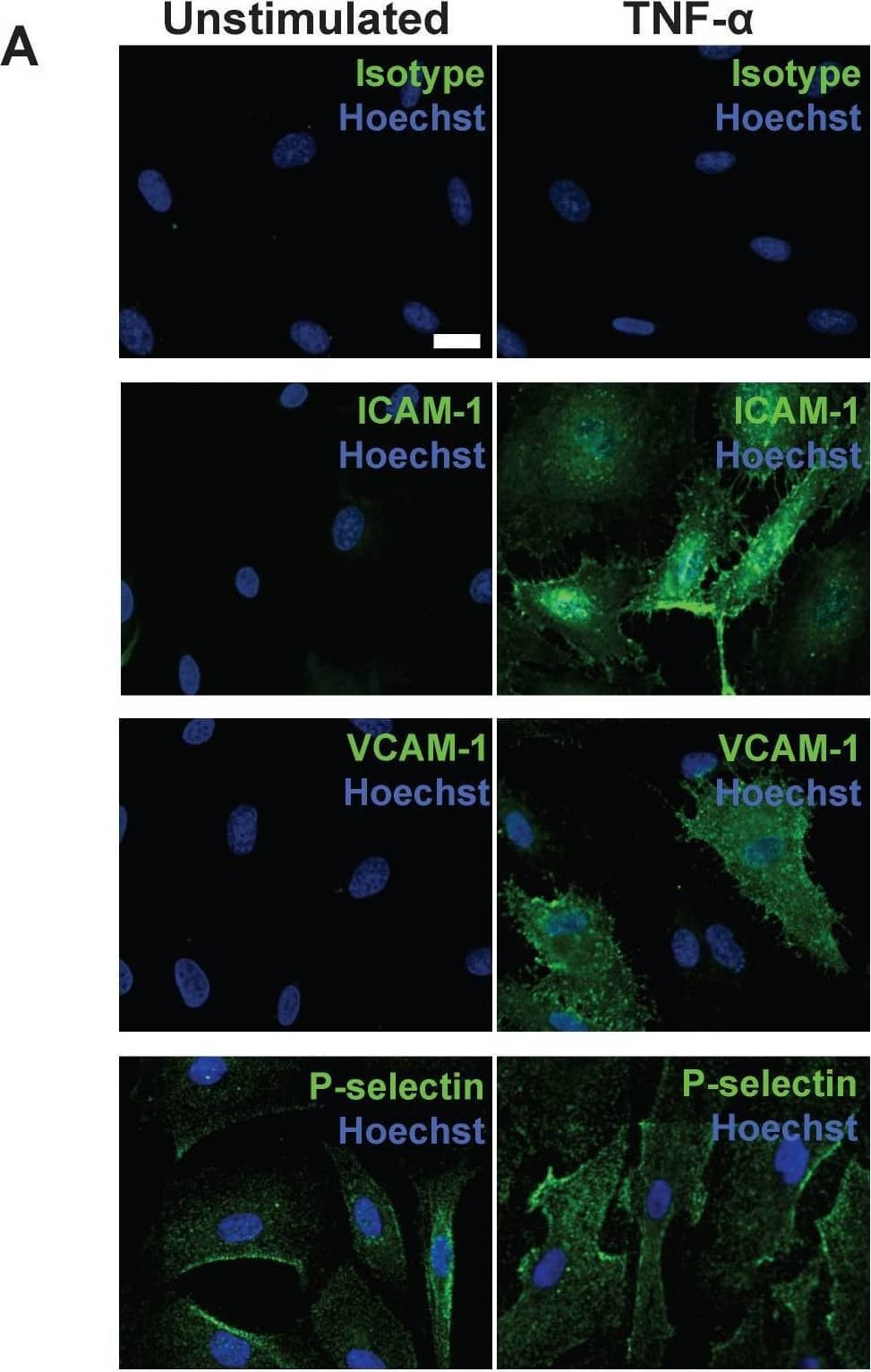
Detection of ICAM-1/CD54 by Immunocytochemistry/ Immunofluorescence Following stimulation, coronary artery endothelial cells (CAEC) express adhesion molecules detectable via immuno-SERS imaging in single and multiplex formats. (A) Fluorescence images of immunohistochemical staining of ICAM-1, VCAM-1 and P-selectin on CAEC in unstimulated and 10 ng/mL TNF-alpha -stimulated conditions. Isotype control, ICAM-1, VCAM-1 and P-selectin staining shown in green; nuclei were counterstained using Hoechst 33342 (blue). (B) CAEC were stimulated with 10 ng/mL TNF-alpha for 24 h, fixed in acetone, and incubated with isotype control, anti-ICAM-1, anti-VCAM-1 or anti-P-selectin BFNP or (C) with all BFNP simultaneously before being subjected to SERS mapping. (D) Representative spectra from anti-ICAM-1 (purple), anti-VCAM-1 (red) and anti-P-selectin (blue) BFNP acquired from the color-matched circles in (C) are shown above their respective reference spectra. Optical images in (B-C) are darkfield images. Scale bars = 20 μm. Results are representative of 3 independent experiments. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/30613292), licensed under a CC-BY license. Not internally tested by R&D Systems.
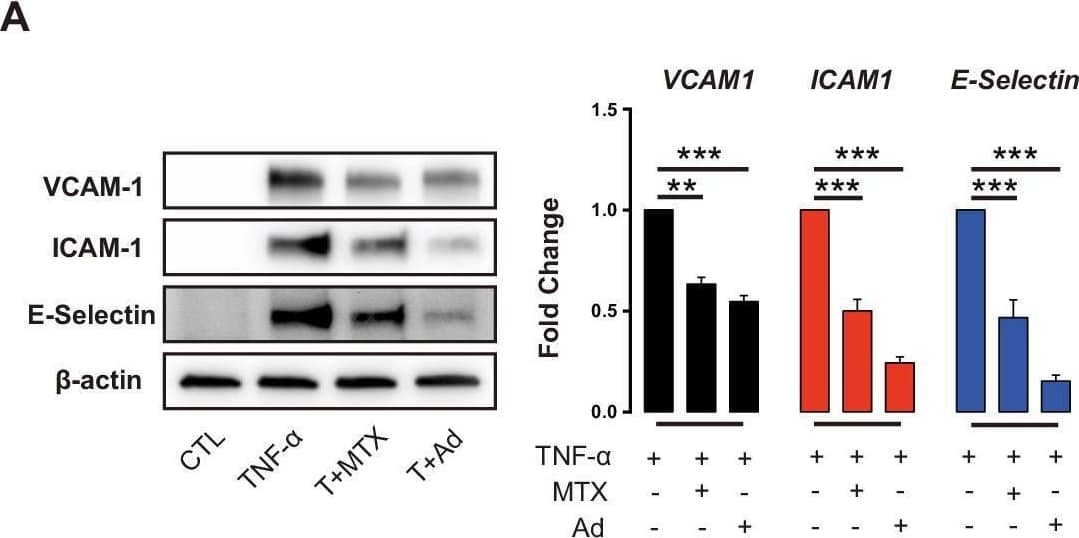 View Larger
View Larger
Detection of ICAM-1/CD54 by Western Blot Methotrexate (MTX) and Ad repress TNF-alpha -induced pro-inflammatory genes without affecting primary MIR181A-1 and MIR181B-1 expression.(A) Western blot analysis of VCAM-1, ICAM-1, and E-Selectin in HUVECs treated with or without MTX (10 µM) or Ad (50 µM), after stimulation of TNF-alpha (10 ng/ml) for 8 hr. Quantification of n = 3 independent experiments. (B) Real-time qPCR analysis of VCAM-1, ICAM-1, and E-Selectin in HUVECs treated with or without MTX (10 µM) or Ad (50 µM), after treatment with TNF-alpha (10 ng/ml) for 4 hr. Real-time qPCR analysis of (C) primary transcript of MIR181A1 or (D) primary transcript of MIR181B-1 in HUVECs treated with or without MTX (10 µM) or Ad (50 µM), after treatment with TNF-alpha (10 ng/ml) for 4 hr. (A–D), n = 3–6. *p<0.05; **p<0.01; ***p<0.001; ***p<0.0001. All values represent mean ± SEM. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/33416495), licensed under a CC-BY license. Not internally tested by R&D Systems.
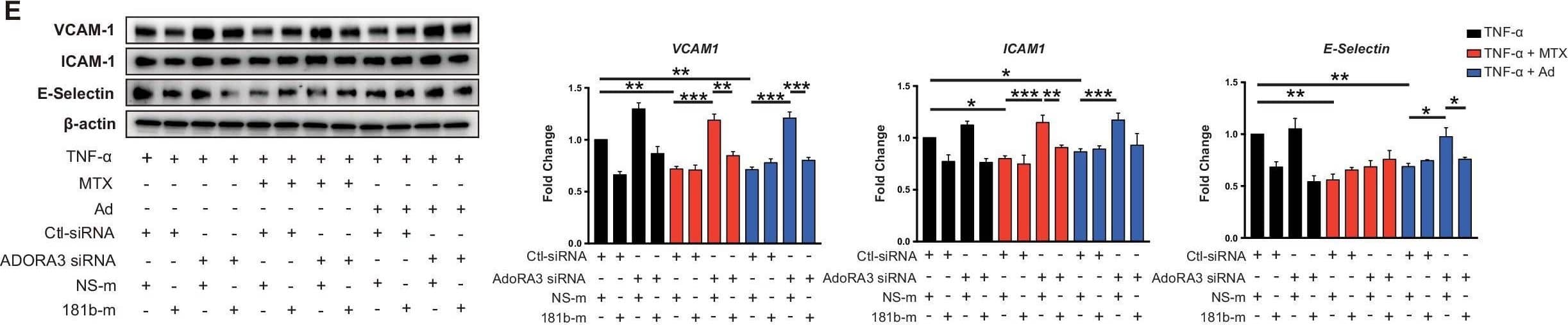 View Larger
View Larger
Detection of ICAM-1/CD54 by Western Blot Induction of MIR181B expression by methotrexate (MTX) or adenosine is adenosine receptor A3 (ADORA3) dependent.(A) Knockdown for adenosine receptors A1, A2A, A2B, and A3 in HUVECs was performed to analyze MIR181B expression. three biological replicates. One-way ANOVA. (B) MIR181B expression in HUVECs transfected with Ctl-siRNA or ADORA3 siRNA after treatment with MTX (10 µM) or Ad (50 µM) or (C) treatment with TNF-alpha (10 ng/ml) alone or in combination MTX (10 µM) or Ad (50 µM). Three biological replicates. One-way ANOVA and Unpaired two-tailed Student t test. (D) Western blot analyses of VCAM-1, ICAM-1, and E-Selectin expression in HUVECs transfected with Ctl-siRNA or ADORA3 siRNA in the presence of TNF-alpha (10 ng/ml) in combination with either MTX (10 µM) or Ad (50 µM). Three biological replicates. Unpaired two-tailed Student t test. (E) in the presence of miRNA negative control (NS-m) or MIR181B mimics (181b-m) stimulated with TNF-alpha (10 ng/ml) or in combination with MTX (10 µM) or Ad (50 µM). Please see Figure 2—source data 1. Three biological replicates. Unpaired two-tailed Student t test. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. n.s. indicated non significance. All values represent mean ± SEM.Figure 2—source data 1.Knockdown efficiency for adenosine receptor siRNAs.mRNA, and protein expression analysis for (A, B) ADORA3 siRNA, (C, D) ADORA1 siRNA, (E, F) ADORA2A siRNA and (G, H) ADORA2B siRNA compared to control siRNA in HUVECs transfected for 36 hr. (A–H), n = 3. ***p<0.001. All values represent mean ± SEM. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/33416495), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: ICAM-1/CD54
ICAM-1 is a member of the immunoglobulin superfamily whose expression is upregulated on leukocytes, epithelial cells and resting endothelial cells in response to inflammatory signals. ICAM-1 binds the leukocyte integrins LFA-1 and Mac-1.
Product Datasheets
Citations for Human ICAM-1/CD54 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
81
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Modeling lung endothelial dysfunction in sepsis-associated ARDS using a microphysiological system
Authors: Liang, NW;Wilson, C;Davis, B;Wolf, I;Qyli, T;Moy, J;Beebe, DJ;Schnapp, LM;Kerr, SC;Faust, HE;
Physiological reports
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Paracrinal regulation of neutrophil functions by coronaviral infection in iPSC-derived alveolar type II epithelial cells
Authors: Chien, Y;Huang, XY;Yarmishyn, AA;Chien, CS;Liu, YH;Hsiao, YJ;Lin, YY;Lai, WY;Huang, SC;Lee, MS;Chiou, SH;Yang, YP;Chiou, GY;
Virus research
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
ICAM-1 nanoclusters regulate hepatic epithelial cell polarity by leukocyte adhesion-independent control of apical actomyosin
Authors: Cacho-Navas, C;López-Pujante, C;Reglero-Real, N;Colás-Algora, N;Cuervo, A;Conesa, JJ;Barroso, S;de Rivas, G;Ciordia, S;Paradela, A;D'Agostino, G;Manzo, C;Feito, J;Andrés, G;Molina-Jiménez, F;Majano, P;Correas, I;Carazo, JM;Nourshargh, S;Huch, M;Millán, J;
eLife
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Immunoprecipitation, Immunocytochemistry -
Identification of new multi-substituted 1H-pyrazolo[3,4-c]pyridin-7(6H)-ones as soluble guanylyl cyclase (sGC) stimulators with vasoprotective and anti-inflammatory activities
Authors: Kintos, DP;Salagiannis, K;Sgouros, A;Nikolaropoulos, SS;Topouzis, S;Fousteris, MA;
Bioorganic chemistry
Species: Rat
Sample Types: Whole Cells
Applications: ELISA Capture -
Differential effects of Usutu and West Nile viruses on neuroinflammation, immune cell recruitment and blood–brain barrier integrity
Authors: Orianne Constant, Ghizlane Maarifi, Jonathan Barthelemy, Marie-France Martin, Bachirou Tinto, Giovanni Savini et al.
Emerging Microbes & Infections
Species: Insect - Aedes albopictus (Asian tiger mosquito)
Sample Types: Whole Cells
Applications: Flow Cytometry -
Probing cerebral malaria inflammation in 3D human brain microvessels
Authors: Howard, C;Joof, F;Hu, R;Smith, JD;Zheng, Y;
Cell reports
Species: Human
Sample Types: Whole Cells
Applications: ICC -
The tumor stroma influences immune cell distribution and recruitment in a PDAC-on-a-chip model
Authors: Marlene Geyer, Lisa-Marie Gaul, Sabrina Luigia D`Agosto, Vincenzo Corbo, Karla Queiroz
Frontiers in Immunology
-
Vascular inflammation on a chip: A scalable platform for trans-endothelial electrical resistance and immune cell migration
Authors: Haley Ehlers, Arnaud Nicolas, Frederik Schavemaker, Jeroen P. M. Heijmans, Martin Bulst, Sebastiaan J. Trietsch et al.
Frontiers in Immunology
-
IL-33 Induces an Antiviral Signature in Mast Cells but Enhances Their Permissiveness for Human Rhinovirus Infection
Authors: C Akoto, A Willis, CF Banas, JA Bell, D Bryant, C Blume, DE Davies, EJ Swindle
Viruses, 2022-11-01;14(11):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
The impact of macrophages on endothelial cells is potentiated by cycling hypoxia: Enhanced tumor inflammation and metastasis
Authors: Victor Delprat, Camille Huart, Olivier Feron, Fabrice Soncin, Carine Michiels
Frontiers in Oncology
-
A microengineered Brain-Chip to model neuroinflammation in humans
Authors: Iosif Pediaditakis, Konstantia R. Kodella, Dimitris V. Manatakis, Christopher Y. Le, Sonalee Barthakur, Alexander Sorets et al.
iScience
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
GAS6/TAM signaling pathway controls MICA expression in multiple myeloma cells
Authors: Andrea Kosta, Abdelilah Mekhloufi, Lorenzo Lucantonio, Alessandra Zingoni, Alessandra Soriani, Marco Cippitelli et al.
Frontiers in Immunology
-
IFNgamma-primed periodontal ligament cells regulate T-cell responses via IFNgamma-inducible mediators and ICAM-1-mediated direct cell contact
Authors: W Singhatana, S Kitpakorns, M Toso, P Pavasant
Royal Society open science, 2022-07-27;9(7):220056.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Plasmolipin regulates basolateral-to-apical transcytosis of ICAM-1 and leukocyte adhesion in polarized hepatic epithelial cells
Authors: Cristina Cacho-Navas, Natalia Reglero-Real, Natalia Colás-Algora, Susana Barroso, Gema de Rivas, Kostantinos Stamatakis et al.
Cellular and Molecular Life Sciences
-
Endothelial cells are not productively infected by SARS‐CoV‐2
Authors: Lilian Schimmel, Keng Yih Chew, Claudia J Stocks, Teodor E Yordanov, Patricia Essebier, Arutha Kulasinghe et al.
Clinical & Translational Immunology
-
Shear forces induce ICAM-1 nanoclustering on endothelial cells that impact on T-cell migration
Authors: Izabela K. Piechocka, Sarah Keary, Alberto Sosa-Costa, Lukas Lau, Nitin Mohan, Jelena Stanisavljevic et al.
Biophysical Journal
-
Plasmacytoid dendritic cells have divergent effects on HIV infection of initial target cells and induce a pro-retention phenotype
Authors: O Tong, G Duette, TR O'Neil, CM Royle, H Rana, B Johnson, N Popovic, S Dervish, MAE Brouwer, H Baharlou, E Patrick, G Ctercteko, S Palmer, E Lee, E Hunter, AN Harman, AL Cunningham, N Nasr
PloS Pathogens, 2021-04-19;17(4):e1009522.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
CD112 Regulates Angiogenesis and T Cell Entry into the Spleen
Authors: E Russo, P Runge, NH Jahromi, H Naboth, A Landtwing, R Montecchi, N Leicht, MC Hunter, Y Takai, C Halin
Cells, 2021-01-15;10(1):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Methotrexate attenuates vascular inflammation through an adenosine-microRNA-dependent pathway
Authors: Dafeng Yang, Stefan Haemmig, Haoyang Zhou, Daniel Pérez-Cremades, Xinghui Sun, Lei Chen et al.
eLife
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Human CD4+ T cell subsets differ in their abilities to cross endothelial and epithelial brain barriers in vitro
Authors: Hideaki Nishihara, Sasha Soldati, Adrien Mossu, Maria Rosito, Henriette Rudolph, William A. Muller et al.
Fluids and Barriers of the CNS
-
Neutrophils interact with cholangiocytes to cause cholestatic changes in alcoholic hepatitis
Authors: Masahiro Takeuchi, Paula T Vidigal, Mateus T Guerra, Melanie A Hundt, Marie E Robert, Maria Olave-Martinez et al.
Gut
-
Caspase-1 has a critical role in blood-brain barrier injury and its inhibition contributes to multifaceted repair
Authors: H Israelov, O Ravid, D Atrakchi, D Rand, S Elhaik, Y Bresler, R Twitto-Gre, L Omesi, S Liraz-Zalt, F Gosselet, M Schnaider, I Cooper
J Neuroinflammation, 2020-09-09;17(1):267.
Species: Human
Sample Types: Whole Cells
Applications: ICC, Western Blot -
Zika Virus Infection Promotes Local Inflammation, Cell Adhesion Molecule Upregulation, and Leukocyte Recruitment at the Blood-Brain Barrier
Authors: M Clé, C Desmetz, J Barthelemy, MF Martin, O Constant, G Maarifi, V Foulongne, K Bolloré, Y Glasson, F De Bock, M Blaquiere, L Dehouck, N Pirot, E Tuaillon, S Nisole, F Najioullah, P Van de Per, A Cabié, N Marchi, F Gosselet, Y Simonin, S Salinas
MBio, 2020-08-04;11(4):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
hnRNPA2/B1 Ameliorates LPS-Induced Endothelial Injury through NF-&kappaB Pathway and VE-Cadherin/&beta-Catenin Signaling Modulation In Vitro
Authors: Y Chen, D Tang, L Zhu, T Yuan, Y Jiao, H Yan, W Yu
Mediators Inflamm., 2020-05-30;2020(0):6458791.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Hepatocytes Delete Regulatory T Cells by Enclysis, a CD4+ T Cell Engulfment Process
Authors: SP Davies, GM Reynolds, AL Wilkinson, X Li, R Rose, M Leekha, YS Liu, R Gandhi, E Buckroyd, J Grove, NM Barnes, RC May, SG Hubscher, DH Adams, Y Huang, O Qureshi, Z Stamataki
Cell Rep, 2019-11-05;29(6):1610-1620.e4.
Species: Human
Sample Types: Whole Cells
Applications: IF -
Dual complementary liposomes inhibit triple-negative breast tumor progression and metastasis
Authors: P Guo, J Yang, D Liu, L Huang, G Fell, J Huang, MA Moses, DT Auguste
Sci Adv, 2019-03-20;5(3):eaav5010.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
3D human microvessel-on-a-chip model for studying monocyte-to-endothelium adhesion under flow – application in systems toxicology
Authors: Carine Poussin, Bart Kramer, Henriette L Lanz, Angelique Van den Heuvel, Alexandra Laurent, Thomas Olivier et al.
ALTEX
-
Hypoxia and matrix viscoelasticity sequentially regulate endothelial progenitor cluster-based vasculogenesis
Authors: MR Blatchley, F Hall, S Wang, HC Pruitt, S Gerecht
Sci Adv, 2019;5(3):eaau7518.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Blood outgrowth endothelial cells (BOECs) as a novel tool for studying adhesion of Plasmodium falciparum-infected erythrocytes
Authors: G Ecklu-Mens, RW Olsen, A Bengtsson, MF Ofori, L Hviid, ATR Jensen, Y Adams
PLoS ONE, 2018-10-09;13(10):e0204177.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Immune complexes containing scleroderma-specific autoantibodies induce a profibrotic and proinflammatory phenotype in skin fibroblasts
Authors: E Raschi, CB Chighizola, L Cesana, D Privitera, F Ingegnoli, C Mastaglio, PL Meroni, MO Borghi
Arthritis Res. Ther., 2018-08-29;20(1):187.
Species: Human
Sample Types: Whole Cells
Applications: Inhibition -
Protective effect of KLF15 on vascular endothelial dysfunction induced by TNF??.
Authors: Bing Liu, Lili Xu, Xinming Yu, Wei Li, Xiaozhi Sun, Shun Xiao, Mingjin Guo, Haofu Wang
Molecular Medicine Reports, 2018-06-20;0(0):1791-3004.
Species: Human
Sample Types: Cell Lysate, Cell Lysates
Applications: Western Blot -
Pinocembrin protects endothelial cells from oxidized LDL-induced injury
Authors: Q Su, Y Sun, Z Ye, H Yang, B Kong, L Li
Cytokine, 2018-06-18;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Augmented reduction in colonic inflammatory markers of dextran sulfate sodium-induced colitis with a combination of 5-aminosalicylic acid and AD-lico™ from Glycyrrhiza inflata
Authors: Jaeyoung Cho, Hyuck-Se Kweon, Sung-Oh Huh, Ali Sadra
Animal Cells and Systems
-
Matrix metalloproteinase-9 (MMP9) is involved in the TNF-α-induced fusion of human M13SV1-Cre breast epithelial cells and human MDA-MB-435-pFDR1 cancer cells
Authors: J Weiler, M Mohr, KS Zänker, T Dittmar
Cell Commun. Signal, 2018-04-10;16(1):14.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Immunoprecipitation, Neutralization -
Inflammation-Sensitive Myosin-X Functionally Supports Leukocyte Extravasation by Cdc42-Mediated ICAM-1-Rich Endothelial Filopodia Formation
Authors: J Kroon, A Schaefer, J van Rijsse, M Hoogenboez, F van Alphen, P Hordijk, ESG Stroes, S Strömblad, J van Rheene, JD van Buul
J. Immunol., 2018-01-31;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bead-based Bioassay, ICC -
DL-3-n-butylphthalide protects endothelial cells against advanced glycation end product-induced injury by attenuating oxidative stress and inflammation responses
Authors: Chang-Yun Liu, Zhen-Hua Zhao, Zhi-Ting Chen, Chun-Hui Che, Zhang-Yu Zou, Xiao-Min Wu et al.
Experimental and Therapeutic Medicine
-
ALCAM (CD166) is involved in extravasation of monocytes rather than T cells across the blood–brain barrier
Authors: Ruth Lyck, Marc-André Lécuyer, Michael Abadier, Christof B Wyss, Christoph Matti, Maria Rosito et al.
Journal of Cerebral Blood Flow & Metabolism
-
Endothelial CD2AP Binds the Receptor ICAM-1 To Control Mechanosignaling, Leukocyte Adhesion, and the Route of Leukocyte Diapedesis In Vitro
Authors: A Schaefer, TJ van Duijn, J Majolee, K Burridge, PL Hordijk
J. Immunol., 2017-05-08;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay, ICC -
CD54-Mediated Interaction with Pro-inflammatory Macrophages Increases the Immunosuppressive Function of Human Mesenchymal Stromal Cells
Authors: Nicolas Espagnolle, Adélie Balguerie, Emmanuelle Arnaud, Luc Sensebé, Audrey Varin
Stem Cell Reports
-
A quantitative method for screening and identifying molecular targets for nanomedicine
Authors: P Guo, J Yang, DR Bielenberg, D Dillon, D Zurakowski, MA Moses, DT Auguste
J Control Release, 2017-03-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
A possible role for neutrophils in allergic rhinitis revealed after cellular subclassification
Authors: J Arebro, S Ekstedt, E Hjalmarsso, O Winqvist, S Kumlien Ge, LO Cardell
Sci Rep, 2017-03-08;7(0):43568.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
3D co-culture model to analyze the crosstalk between endothelial and smooth muscle cells
Authors: Minu Karthika Ganesan
Tissue Eng Part C Methods, 2017-01-01;0(0):.
Species: Human
Sample Types: Complex Sample Type
Applications: ICC -
A novel immune resistance mechanism of melanoma cells controlled by the ADAR1 enzyme
Authors: Gilli Galore-Haskel, Yael Nemlich, Eyal Greenberg, Shira Ashkenazi, Motti Hakim, Orit Itzhaki et al.
Oncotarget
-
C6-ceramide nanoliposome suppresses tumor metastasis by eliciting PI3K and PKCzeta tumor-suppressive activities and regulating integrin affinity modulation.
Authors: Zhang P, Fu C, Hu Y, Dong C, Song Y, Song E
Sci Rep, 2015-03-20;5(0):9275.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Role of cortactin homolog HS1 in transendothelial migration of natural killer cells.
Authors: Mukherjee S, Kim J, Mooren O, Shahan S, Cohan M, Cooper J
PLoS ONE, 2015-02-27;10(2):e0118153.
Species: Human
Sample Types: Whole Cells
Applications: IHC -
Cycling Hypoxia Induces a Specific Amplified Inflammatory Phenotype in Endothelial Cells and Enhances Tumor-Promoting Inflammation In Vivo12
Authors: Céline Tellier, Déborah Desmet, Laurenne Petit, Laure Finet, Carlos Graux, Martine Raes et al.
Neoplasia
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Soluble CD54 induces human endothelial cells ex vivo expansion useful for cardiovascular regeneration and tissue engineering application.
Authors: Malara N, Trunzo V, Musolino G, Aprigliano S, Rotta G, Macrina L, Limongi T, Gratteri S, Di Fabrizio E, Renzulli A, Fini M, Mollace V
Int J Cardiol Heart Vasc, 2015-01-09;6(0):48-53.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Uveal melanoma cells utilize a novel route for transendothelial migration.
Authors: Onken, Michael, Li, Jinmei, Cooper, John A
PLoS ONE, 2014-12-15;9(12):e115472.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Endothelial cells use dynamic actin to facilitate lymphocyte transendothelial migration and maintain the monolayer barrier.
Authors: Mooren O, Li J, Nawas J, Cooper J
Mol Biol Cell, 2014-10-29;25(25):4115-29.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
West Nile virus-induced cell adhesion molecules on human brain microvascular endothelial cells regulate leukocyte adhesion and modulate permeability of the in vitro blood-brain barrier model.
Authors: Roe, Kelsey, Orillo, Beverly, Verma, Saguna
PLoS ONE, 2014-07-18;9(7):e102598.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece.
Authors: Spilioti E, Jaakkola M, Tolonen T, Lipponen M, Virtanen V, Chinou I, Kassi E, Karabournioti S, Moutsatsou P
PLoS ONE, 2014-04-21;9(4):e94860.
Species: Human
Sample Types: Whole Cells
Applications: ELISA Development (Capture) -
Inflammation converts human mesoangioblasts into targets of alloreactive immune responses: implications for allogeneic cell therapy of DMD.
Authors: Noviello M, Tedesco F, Bondanza A, Tonlorenzi R, Rosaria Carbone M, Gerli M, Marktel S, Napolitano S, Cicalese M, Ciceri F, Peretti G, Cossu G, Bonini C
Mol Ther, 2014-04-16;22(7):1342-52.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Enzyme-free passage of human pluripotent stem cells by controlling divalent cations.
Authors: Ohnuma, Kiyoshi, Fujiki, Ayaka, Yanagihara, Kana, Tachikawa, Saoko, Hayashi, Yohei, Ito, Yuzuru, Onuma, Yasuko, Chan, Techuan, Michiue, Tatsuo, Furue, Miho K, Asashima, Makoto
Sci Rep, 2014-04-11;4(0):4646.
Species: Human
Sample Types: Whole Cells
Applications: IHC -
Interaction of mesenchymal stem cells with fibroblast-like synoviocytes via cadherin-11 promotes angiogenesis by enhanced secretion of placental growth factor.
Authors: Park, Su-Jung, Kim, Ki-Jo, Kim, Wan-Uk, Cho, Chul-Soo
J Immunol, 2014-02-26;192(7):3003-10.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
GroEL1, a heat shock protein 60 of Chlamydia pneumoniae, impairs neovascularization by decreasing endothelial progenitor cell function.
Authors: Lin, Yi-Wen, Huang, Chun-Yao, Chen, Yung-Hsi, Shih, Chun-Min, Tsao, Nai-Wen, Lin, Cheng-Ye, Chang, Nen-Chun, Tsai, Chien-Su, Tsai, Hsiao-Ya, Tsai, Jui-Chi, Huang, Po-Hsun, Li, Chi-Yuan, Lin, Feng-Yen
PLoS ONE, 2013-12-23;8(12):e84731.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
A composite model of the human postcapillary venule for investigation of microvascular leukocyte recruitment.
Authors: Lauridsen H, Pober J, Gonzalez A
FASEB J, 2013-12-02;28(3):1166-80.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Zoledronate attenuates angiotensin II-induced abdominal aortic aneurysm through inactivation of Rho/ROCK-dependent JNK and NF-kappaB pathway.
Authors: Tsai S, Huang P, Peng Y, Chang W, Tsai H, Leu H, Chen J, Lin S
Cardiovasc Res, 2013-12-01;100(3):501-10.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Tumor-associated fibroblasts isolated from colorectal cancer tissues exhibit increased ICAM-1 expression and affinity for monocytes.
Authors: Schellerer V, Langheinrich M, Hohenberger W, Croner R, Merkel S, Rau T, Sturzl M, Naschberger E
Oncol Rep, 2013-11-20;31(1):255-61.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr -
Loss of p53 in stromal fibroblasts promotes epithelial cell invasion through redox-mediated ICAM1 signal
Authors: Dunyaporn Trachootham, Gang Chen, Wan Zhang, Weiqin Lu, Hui Zhang, Jinsong Liu et al.
Free Radical Biology and Medicine
-
Macrophages induce differentiation of plasma cells through CXCL10/IP-10.
J. Exp. Med., 2012-09-17;209(10):1813-23.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Activation of endothelial TLR2 by bacterial lipoprotein upregulates proteins specific for the neutrophil response
Authors: Kevin Wilhelmsen, Kailin R Mesa, Arun Prakash, Fengyun Xu, Judith Hellman
Innate Immunity
-
OxLDL and substrate stiffness promote neutrophil transmigration by enhanced endothelial cell contractility and ICAM-1
Authors: Kimberly M. Stroka, Irena Levitan, Helim Aranda-Espinoza
Journal of Biomechanics
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
ERK5 protein promotes, whereas MEK1 protein differentially regulates, the Toll-like receptor 2 protein-dependent activation of human endothelial cells and monocytes.
Authors: Wilhelmsen K, Mesa K, Lucero J, Xu F, Hellman J
J Biol Chem, 2012-06-15;287(32):26478-94.
Species: Human
Sample Types: Whole Cells
Applications: Cell-based ELISA -
Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction.
Authors: Stroka KM, Aranda-Espinoza H
Blood, 2011-06-07;118(6):1632-40.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Deficiency of the NR4A Orphan Nuclear Receptor NOR1 Decreases Monocyte Adhesion and Atherosclerosis
Authors: Yue Zhao, Deborah A. Howatt, Florence Gizard, Takashi Nomiyama, Hannes M. Findeisen, Elizabeth B. Heywood et al.
Circulation Research
-
IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes.
Authors: Pennino D, Eyerich K, Scarponi C, Carbone T, Eyerich S, Nasorri F, Garcovich S, Traidl-Hoffmann C, Albanesi C, Cavani A
J. Immunol., 2010-03-31;184(9):4880-8.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Adhesion of human haematopoietic (CD34+) stem cells to human liver compartments is integrin and CD44 dependent and modulated by CXCR3 and CXCR4.
Authors: Crosby HA, Lalor PF, Ross E, Newsome PN, Adams DH
J. Hepatol., 2009-07-30;51(4):734-49.
Species: Human
Sample Types: Whole Tissue
Applications: IHC, Neutralization -
Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption.
Authors: Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Drel VR, Obrosova IG, Pacher P
Am. J. Physiol. Heart Circ. Physiol., 2007-03-23;293(1):H610-9.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Glucocorticoid-induced tumour necrosis factor receptor-related protein-mediated macrophage stimulation may induce cellular adhesion and cytokine expression in rheumatoid arthritis.
Authors: Bae E, Kim WJ, Kang YM, Suk K, Koh EM, Cha HS, Ahn KS, Huh TL, Lee WH
Clin. Exp. Immunol., 2007-03-15;148(3):410-8.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
SPARC is a VCAM-1 counter-ligand that mediates leukocyte transmigration.
Authors: Kelly KA, Allport JR, Yu AM, Sinh S, Sage EH, Gerszten RE, Weissleder R
J. Leukoc. Biol., 2006-12-18;81(3):748-56.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Differential survival of leukocyte subsets mediated by synovial, bone marrow, and skin fibroblasts: site-specific versus activation-dependent survival of T cells and neutrophils.
Authors: Filer A, Parsonage G, Smith E, Osborne C, Thomas AM, Curnow SJ, Rainger GE, Raza K, Nash GB, Lord J, Salmon M, Buckley CD
Arthritis Rheum., 2006-07-01;54(7):2096-108.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Proteinase-activated receptor2 agonists upregulate granulocyte colony-stimulating factor, IL-8, and VCAM-1 expression in human bronchial fibroblasts.
Authors: Ramachandran R, Morice AH, Compton SJ
Am. J. Respir. Cell Mol. Biol., 2006-02-23;35(1):133-41.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Migration inhibitory factor up-regulates vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 via Src, PI3 kinase, and NFkappaB.
Authors: Amin MA, Haas CS, Zhu K, Mansfield PJ, Kim MJ, Lackowski NP, Koch AE
Blood, 2005-11-29;107(6):2252-61.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Cell expression of MMP-1 and TIMP-1 in co-cultures of human gingival fibroblasts and monocytes: the involvement of ICAM-1.
Authors: Domeij H, Modeer T, Quezada HC, Yucel-Lindberg T
Biochem. Biophys. Res. Commun., 2005-11-02;338(4):1825-33.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Requirement for intercellular adhesion molecule 1 and caveolae in invasion of human oral epithelial cells by Porphyromonas gingivalis.
Authors: Tamai R, Asai Y, Ogawa T
Infect. Immun., 2005-10-01;73(10):6290-8.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Differential effects of orbital and laminar shear stress on endothelial cells.
Authors: Frattini J, Kudo FA
J. Vasc. Surg., 2005-05-01;41(5):869-80.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Persistent cytomegalovirus infection is associated with increased expression of TGF-beta1, PDGF-AA and ICAM-1 and arterial intimal thickening in kidney allografts.
Authors: Helantera I, Loginov R, Koskinen P, Tornroth T, Gronhagen-Riska C, Lautenschlager I
Nephrol. Dial. Transplant., 2005-02-16;20(4):790-6.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
CC chemokines and transmigration of eosinophils in the presence of vascular cell adhesion molecule 1.
Authors: Yamamoto H, Nagata M, Sakamoto Y
Ann. Allergy Asthma Immunol., 2005-02-01;94(2):292-300.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Apical topography and modulation of ICAM-1 expression on activated endothelium.
Authors: Almenar-Queralt A, Duperray A, Miles LA, Felez J, Altieri DC
Am. J. Pathol., 1995-11-01;147(5):1278-88.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Circulating tumor cells from prostate cancer patients interact with E-selectin under physiologic blood flow.
Authors: Gakhar, Gunjan, Navarro, Vicente, Jurish, Madelyn, Lee, Guang Yu, Tagawa, Scott T, Akhtar, Naveed H, Seandel, Marco, Geng, Yue, Liu, He, Bander, Neil H, Giannakakou, Paraskev, Christos, Paul J, King, Michael, Nanus, David M
PLoS ONE, 2013-12-27;8(12):e85143.
-
LncRNA VINAS regulates atherosclerosis by modulating NF-&kappaB and MAPK signaling
Authors: V Simion, H Zhou, JB Pierce, D Yang, S Haemmig, Y Tesmenitsk, G Sukhova, PH Stone, P Libby, MW Feinberg
JCI Insight, 2020-11-05;0(0):.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human ICAM-1/CD54 Antibody
Average Rating: 5 (Based on 3 Reviews)
Have you used Human ICAM-1/CD54 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: