The term 'mesenchymal stem cells' (MSCs) is most commonly used to describe multipotent self-renewing cells that can be differentiated in vitro to generate adipocytes, chondrocytes, and osteoblasts. However, because these biological properties and hierarchical relationships remain to be clearly demonstrated in vivo, the term 'multipotent mesenchymal stromal cells' is often used to distinguish cultured cells from their in vivo precursors. Originally discovered in mouse bone marrow, multipotent mesenchymal stromal cells cultured from a variety of species and tissue types, have been shown to differentiate into progeny of additional lineages including, cardiomyocytes, endothelial cells, hepatocytes, and neural cells. Again, the physiological relevance of these findings remains to be determined.
Human Mesenchymal Stem Cell Functional Identification Kit
R&D Systems | Catalog # SC006

Key Product Details
Species
Product Summary for Human Mesenchymal Stem Cell Functional Identification Kit
Kit Summary
To verify multipotency of human mesenchymal stem cells by in vitro functional differentiation.
Key Benefits
- Confirms that the starting MSC population is multipotent
- Can be used with the Human MSC Verification Flow Kit to define MSCs according to the ISCT recommendations
- Reliably induces MSC trilineage differentiation using defined supplements
- Includes premium quality antibodies to confirm differentiation
Why Funtionally Verify Human MSC Multipotency In Vitro?
Mesenchymal stem/stromal cells (MSCs) can be characterized based on the expression of specific cell surface markers, the absence of hematopoietic markers, and adherence to plastic in vitro.
To more rigorously determine if a cell is truly an MSC, it is important to also verify its ability to differentiate into adipocytes, chondrocytes, and osteocytes.
Functional verification of MSC multipotency in vitro:
- Uses defined supplements to drive reproducible trilineage differentiation.
- Verifies a healthy, multipotent starting MSC population to increase consistency between studies and reduce unwanted experimental variability.
- Meets one of the three recommended minimal criteria for MSC identification.
Mesenchymal Stromal Cells or Mesenchymal Stem Cells?
The term ‘mesenchymal stromal cells’ is commonly used to describe a heterogeneous population of cultured cells that are adherent to plastic, have a distinct morphology, and express a specific set of marker proteins. Within this heterogeneous population are cells referred to as ‘mesenchymal stem cells.’
Mesenchymal stem cells are multipotent, self-renewing cells that have the ability to differentiate into adipocytes, chondrocytes, and osteoblasts when cultured in vitro. Read More about MSC Nomenclature
Kit Components
This kit contains the following reagents to drive MSC differentiation and a marker to analyze each of the three differentiated derivatives:
- Adipogenic Supplement
- Chondrogenic Supplement
- Osteogenic Supplement
- ITS Supplement
- Adipocyte marker: Goat Anti-Mouse FABP4 Antigen Affinity-purified Polyclonal Antibody
- Chondrocyte marker: Goat Anti-Human Aggrecan Antigen Affinity-purified Polyclonal Antibody
- Osteocyte marker: Mouse Anti-Human Osteocalcin Monoclonal Antibody
This kit requires media (not included), such as Human/Mouse/Rat StemXVivo Osteogenic/Adipogenic Base Media (CCM007) or equivalent.
The quantity of each media supplement in this kit is sufficient to make 50 mL of media for differentiation. This is enough media for the differentiation of 16 wells of a 24-well plate for osteogenic and adipogenic lineages and 10 chondrocyte pellets.
Precautions
- The Adipogenic Supplement contains 95% ethanol and is highly flammable. Keep the container tightly closed, and keep it away from sources of ignition.
- The acute and chronic effects of over-exposure to the reagents in this kit are unknown. Safe laboratory handling procedures should be followed and protective clothing should be worn when handling kit reagents.
- The ITS Supplement contains human transferrin. This transferrin was purified from donor plasma and tested at the donor level using an FDA licensed method and found to be non-reactive for anti-HIV-1/2 and Hepatitis B surface antigen.
2006 Proposed Change to MSC Nomenclature
Although mesenchymal stromal cells were once referred to as ‘mesenchymal stem cells’, a change to ‘mesenchymal stromal cells’ was proposed by the International Society for Cellular Therapy in 2006.1
The change in nomenclature originates from two important factors:
- Methods used to isolate mesenchymal stem cells yield a heterogeneous population of cells with only a fraction of these cells demonstrating multipotency.
- The absence of direct evidence that mesenchymal stem cells can self-renew and differentiate in vivo.
Use of Mesenchymal Stem and Stromal Cell Terminology
Data supporting MSC self-renewal and multipotency have been obtained using in vitro conditions, which does not adequately reflect the in vivo environment. The lack of in vivo data has led some researchers to question the validity of the term ‘mesenchymal stem cell’ providing further support for the use of ‘mesenchymal stromal cells’ to describe MSCs.2 While ‘mesenchymal stromal cells’ may be the more scientifically accurate term for MSCs, the two terms are often used interchangeably in the literature. R&D Systems recognizes the use of both mesenchymal stem cells and mesenchymal stromal cells and uses ‘MSC’ to indicate mesenchymal stem/stromal cells to account for both designations.
Definitions of Mesenchymal Stromal Cells and Mesenchymal Stem Cells
- Mesenchymal Stromal Cells – A heterogeneous population of cultured cells with similar characteristics such as the ability to adhere to plastic and the expression of specific marker proteins.
- Mesenchymal Stem Cells – A subpopulation of mesenchymal stromal cells that have the capacity to self-renew and differentiate into mesodermal lineages when cultured in vitro. The capacity to self-renew and differentiate in vivo has yet to be clearly demonstrated for mesenchymal stem cells.
References
- Dominici, M. et al. (2006) Cytotherapy 8:315.
- Keating, A. (2012) Cell Stem Cell 10:709.
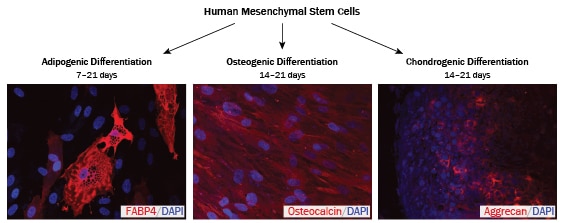
Scientific Data Images for Human Mesenchymal Stem Cell Functional Identification Kit
Verification of Multipotency using the Human Mesenchymal Stem Cell Functional Identification Kit.
Human mesenchymal stem cells were cultured in StemXVivo®Mesenchymal Stem Cell Expansion Media (Catalog # CCM004) and differentiation was induced as indicated using the media supplements included in the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006). The kit also contains a Goat Anti-Mouse FABP-4 Antigen Affinity-purified Polyclonal Antibody (adipocytes), a Goat Anti-Human Aggrecan Antigen Affinity-purified Polyclonal Antibody (chondrocytes), and a Mouse Anti-Human Osteocalcin Monoclonal Antibody (osteocytes) for the confirmation of differentiation status. The cells were stained using the NorthernLightsTM 557-conjugated Donkey Anti-Goat (Catalog # NL001; red) or Anti-Mouse (Catalog # NL007; red) IgG Secondary Antibodies, and the nuclei were counterstained with DAPI (blue).
Formulation, Preparation, and Storage
Shipping
Storage
Background: Mesenchymal Stem Cells
Alternate Names
Additional Mesenchymal Stem Cells Products
Product Documents for Human Mesenchymal Stem Cell Functional Identification Kit
Certificate of Analysis
To download a Certificate of Analysis, please enter a lot or batch number in the search box below.
Note: Certificate of Analysis not available for kit components.
Product Specific Notices for Human Mesenchymal Stem Cell Functional Identification Kit
For research use only
Citations for Human Mesenchymal Stem Cell Functional Identification Kit
Customer Reviews for Human Mesenchymal Stem Cell Functional Identification Kit (1)
Have you used Human Mesenchymal Stem Cell Functional Identification Kit?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Customer Images
-
Verified Customer | Posted 10/17/2016The MSC’s were generated from induced pluripotent stem cells and then tested using your kit for its potential to differentiate along the adipogenic and chondrogenic lineages.
There are no reviews that match your criteria.
Protocols
View specific protocols for Human Mesenchymal Stem Cell Functional Identification Kit (SC006):
Refer to the product datasheet for complete product details.
Briefly, human MSC multipotency is verified using the following in vitro differentiation procedure:
- Culture multipotent cells of interest
- Induce adipocyte, chondrocyte, and osteocyte differentiation using media supplements
- Evaluate differentiation using mature phenotype marker antibodies and fluorescent ICC
Reagents Provided
Reagents supplied in the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006):
- Adipogenic Supplement
- Chondrogenic Supplement
- Osteogenic Supplement
- ITS Supplement
- Adipocyte marker: Goat Anti-Mouse FABP4 Antigen-affinity Purified Polyclonal Antibody
- Chondrocyte marker: Goat Anti-Human Aggrecan Antigen-affinity Purified Polyclonal Antibody
- Osteocyte marker: Mouse Anti-Human Osteocalcin Antigen-affinity Purified Monoclonal Antibody
Note: The quantity of each media supplement in this kit is sufficient to make 50 mL of media for differentiation. 50 mL can be used for 16 wells of a 24-well plate for osteogenic and adipogenic lineages and 10 chondrocyte pellets.
Other Supplies Required
Reagents
- StemXVivo® Osteogenic/Adipogenic Base Media (Catalog # CCM007 or equivalent)
- D-MEM/F-12 (1X)
- Phosphate Buffered Saline (PBS)
- Penicillin-Streptomycin-Glutamate (100X)
- 4% Paraformaldehyde in PBS
- 1% BSA in PBS
- Triton® X-100
- 10% normal donkey serum
- Fibronectin (optional; Human Fibronectin, Catalog # 1918-FN, Bovine Fibronectin, Catalog # 1030-FN, or equivalent)
- Mounting medium (Catalog # CTS011 or equivalent)
- NorthernLightsTM 557-conjugated Donkey Anti-Goat IgG Secondary Antibody (Catalog # NL001 or equivalent)
- Deionized or distilled water
Materials
- Human MSCs
- 24-well culture plates
- 12 mm coverslips (Carolina Biologicals, Catalog # 633009 or equivalent)
- 15 mL centrifuge tubes
- Pipettes and pipette tips
- Serological pipettes
- Glass slides
- Fine pointed curved forceps
- Liquid barrier pen
Equipment
- 37 °C and 5% CO2 incubator
- Centrifuge
- Hemocytometer
- Inverted microscope
- 2 °C to 8 °C refrigerator
- 37 °C water bath
- Fluorescence microscope
- Cryostat
Procedure Overview
This protocol has been tested using bone marrow- and/or adipose tissue-derived MSCs. If using a different tissue source or cell line, the protocol below may need to be optimized.
Adipogenic Differentiation
Plate 2.1 x 104 MSCs/cm2 in StemXVivo® Osteogenic/Adipogenic Base Media.
Culture cells to 100% confluency.

Replace the medium with Adipogenic Differentiation Medium to induce adipogenesis.

Every 2-3 days, replace with fresh Adipogenic Differentiation Medium.
After 14-21 days, adipocytes can be fixed.
ICC detection of FABP4.

Osteogenic Differentiation
Plate 4.2 x 103 MSCs/cm2 in StemXVivo® Osteogenic/Adipogenic Base Media.
Culture cells to 50-70% confluency.

Replace the medium with Osteogenic Differentiation Medium to induce osteogenesis.

Every 3-4 days, replace with fresh Osteogenic Differentiation Medium.
After 14-21 days, osteocytes can be fixed.
ICC detection of Osteocalcin.

Chondrogenic Differentiation
Transfer 2.5 x 104 MSCs to a 15 mL conical tube.
Centrifuge and resuspend the cells in Chondrogenic Differentiation Media.
Centrifuge the cells but do not remove the medium.

Every 2-3 days, replace with fresh Chondrogenic Differentiation Media.
After 14-21 days, the chondrogenic pellet can be fixed.

Cryosection the chondrogenic pellet.
ICC detection of Aggrecan.

FAQs for Human Mesenchymal Stem Cell Functional Identification Kit
-
Q: Are there any experimental tips/hints for successful chondrogenic differentiation of mesenchymal stem cells?
A: The following tips/hints are useful for chondrogenic differentiation: a) The mesenchymal stem cells (MSCs) should not be from a late passage (passage 8 or less), b) if using the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006) or the StemXVivo® Chondrogenic Supplement (Catalog # CCM006), use the starting MSC cell number that is indicated in the protocol, c) Early during chondrogenic differentiation a pellet should form. As differentiation progresses, the pellet will grow and take up a ball-like appearance. d) The pellet should not attach to the tube, therefore care should be taken to not dislodge it while changing media.
-
Q: Can the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006) be used with non-human primate mesenchymal stem cells?
A: It is likely that the antibodies included in the kit are cross-reactive to other primates. The supplements included in the kit are not intended to be species-specific. However, the kit has not been tested with primate mesenchymal stem cells
-
Q: For the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006), how can induction of differentiation be monitored?
A: For adipogenic differentiation, the appearance of vacuoles in cells after 5-7 days is a sign of differentiation and can be monitored by microscopic examination of the cells. For osteogenic differentiation, the beginning of cell detachment after about 14 days is a sign of differentiation. Cell detachment should be monitored in this case. For chondrogenic differentiation, there isn't an exact marker to look for other than fixing and staining the frozen pellet between differentiation days 14 - 21. The exact choice of time may take some empirical testing.
-
Q: In the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006), are Part #'s 90415, 390416, and 390417 the same as the StemXVivo® Human Adipogenic Supplement (Catalog # CCM011), StemXVivo® Human Osteogenic Supplement (Catalog # CCM008), and StemXVivo® Human Chondrogenic Supplement (Catalog # CCM006), respectively?
A: Yes, the StemXVivo® Human Adipogenic Supplement (Catalog # CCM011), StemXVivo® Human Osteogenic Supplement (Catalog # CCM008), and StemXVivo® Human Chondrogenic Supplement (Catalog # CCM006) are the same as Part #'s 390415, 390416, and 390417, respectively, in the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006).
-
Q: Are there any experimental tips/hints for successful chondrogenic differentiation of mesenchymal stem cells?
A: The following tips/hints are useful for chondrogenic differentiation: a) The mesenchymal stem cells (MSCs) should not be from a late passage (passage 8 or less), b) if using the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006) or the StemXVivo® Chondrogenic Supplement (Catalog # CCM006), use the starting MSC cell number that is indicated in the protocol, c) Early during chondrogenic differentiation a pellet should form. As differentiation progresses, the pellet will grow and take up a ball-like appearance. d) The pellet should not attach to the tube, therefore care should be taken to not dislodge it while changing media.
-
Q: Can the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006) be used with non-human primate mesenchymal stem cells?
A: It is likely that the antibodies included in the kit are cross-reactive to other primates. The supplements included in the kit are not intended to be species-specific. However, the kit has not been tested with primate mesenchymal stem cells
-
Q: For the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006), how can induction of differentiation be monitored?
A: For adipogenic differentiation, the appearance of vacuoles in cells after 5-7 days is a sign of differentiation and can be monitored by microscopic examination of the cells. For osteogenic differentiation, the beginning of cell detachment after about 14 days is a sign of differentiation. Cell detachment should be monitored in this case. For chondrogenic differentiation, there isn't an exact marker to look for other than fixing and staining the frozen pellet between differentiation days 14 - 21. The exact choice of time may take some empirical testing.
-
Q: In the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006), are Part #'s 90415, 390416, and 390417 the same as the StemXVivo® Human Adipogenic Supplement (Catalog # CCM011), StemXVivo® Human Osteogenic Supplement (Catalog # CCM008), and StemXVivo® Human Chondrogenic Supplement (Catalog # CCM006), respectively?
A: Yes, the StemXVivo® Human Adipogenic Supplement (Catalog # CCM011), StemXVivo® Human Osteogenic Supplement (Catalog # CCM008), and StemXVivo® Human Chondrogenic Supplement (Catalog # CCM006) are the same as Part #'s 390415, 390416, and 390417, respectively, in the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006).
-
Q: Are there any experimental tips/hints for successful chondrogenic differentiation of mesenchymal stem cells?
A: The following tips/hints are useful for chondrogenic differentiation: a) The mesenchymal stem cells (MSCs) should not be from a late passage (passage 8 or less), b) if using the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006) or the StemXVivo® Chondrogenic Supplement (Catalog # CCM006), use the starting MSC cell number that is indicated in the protocol, c) Early during chondrogenic differentiation a pellet should form. As differentiation progresses, the pellet will grow and take up a ball-like appearance. d) The pellet should not attach to the tube, therefore care should be taken to not dislodge it while changing media.
-
Q: Can the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006) be used with non-human primate mesenchymal stem cells?
A: It is likely that the antibodies included in the kit are cross-reactive to other primates. The supplements included in the kit are not intended to be species-specific. However, the kit has not been tested with primate mesenchymal stem cells
-
Q: For the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006), how can induction of differentiation be monitored?
A: For adipogenic differentiation, the appearance of vacuoles in cells after 5-7 days is a sign of differentiation and can be monitored by microscopic examination of the cells. For osteogenic differentiation, the beginning of cell detachment after about 14 days is a sign of differentiation. Cell detachment should be monitored in this case. For chondrogenic differentiation, there isn't an exact marker to look for other than fixing and staining the frozen pellet between differentiation days 14 - 21. The exact choice of time may take some empirical testing.
-
Q: In the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006), are Part #'s 90415, 390416, and 390417 the same as the StemXVivo® Human Adipogenic Supplement (Catalog # CCM011), StemXVivo® Human Osteogenic Supplement (Catalog # CCM008), and StemXVivo® Human Chondrogenic Supplement (Catalog # CCM006), respectively?
A: Yes, the StemXVivo® Human Adipogenic Supplement (Catalog # CCM011), StemXVivo® Human Osteogenic Supplement (Catalog # CCM008), and StemXVivo® Human Chondrogenic Supplement (Catalog # CCM006) are the same as Part #'s 390415, 390416, and 390417, respectively, in the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006).
-
Q: Are there any experimental tips/hints for successful chondrogenic differentiation of mesenchymal stem cells?
A: The following tips/hints are useful for chondrogenic differentiation: a) The mesenchymal stem cells (MSCs) should not be from a late passage (passage 8 or less), b) if using the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006) or the StemXVivo® Chondrogenic Supplement (Catalog # CCM006), use the starting MSC cell number that is indicated in the protocol, c) Early during chondrogenic differentiation a pellet should form. As differentiation progresses, the pellet will grow and take up a ball-like appearance. d) The pellet should not attach to the tube, therefore care should be taken to not dislodge it while changing media.
-
Q: Can the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006) be used with non-human primate mesenchymal stem cells?
A: It is likely that the antibodies included in the kit are cross-reactive to other primates. The supplements included in the kit are not intended to be species-specific. However, the kit has not been tested with primate mesenchymal stem cells
-
Q: For the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006), how can induction of differentiation be monitored?
A: For adipogenic differentiation, the appearance of vacuoles in cells after 5-7 days is a sign of differentiation and can be monitored by microscopic examination of the cells. For osteogenic differentiation, the beginning of cell detachment after about 14 days is a sign of differentiation. Cell detachment should be monitored in this case. For chondrogenic differentiation, there isn't an exact marker to look for other than fixing and staining the frozen pellet between differentiation days 14 - 21. The exact choice of time may take some empirical testing.
-
Q: In the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006), are Part #'s 90415, 390416, and 390417 the same as the StemXVivo® Human Adipogenic Supplement (Catalog # CCM011), StemXVivo® Human Osteogenic Supplement (Catalog # CCM008), and StemXVivo® Human Chondrogenic Supplement (Catalog # CCM006), respectively?
A: Yes, the StemXVivo® Human Adipogenic Supplement (Catalog # CCM011), StemXVivo® Human Osteogenic Supplement (Catalog # CCM008), and StemXVivo® Human Chondrogenic Supplement (Catalog # CCM006) are the same as Part #'s 390415, 390416, and 390417, respectively, in the Human Mesenchymal Stem Cell Functional Identification Kit (Catalog # SC006).


