TGF-beta 1, 2, 3 Antibody Summary
Applications
Human TGF-beta 1 Sandwich Immunoassay
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
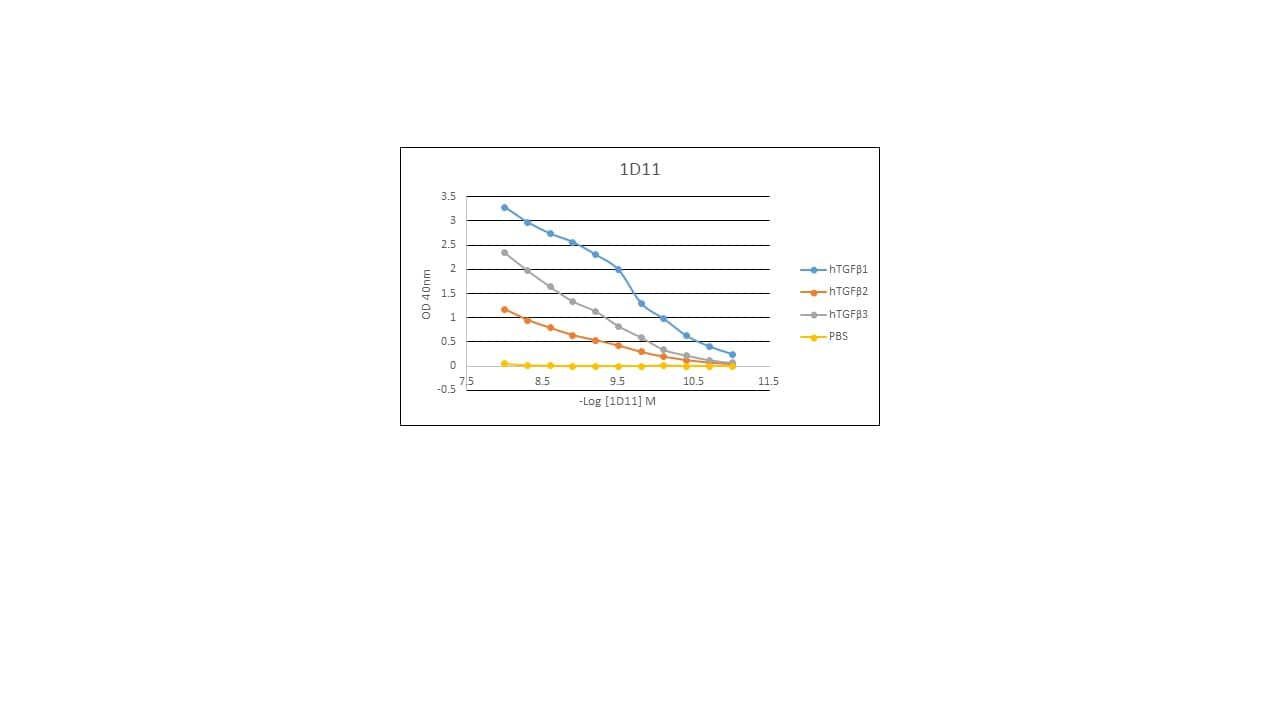
TGF‑ beta 1 Inhibition of IL‑4-dependent Cell Proliferation and Neutralization by TGF‑ beta 1, 2, 3 Antibody. Recombinant Human TGF-beta 1 (Catalog # 240-B) inhibits Recombinant Mouse IL-4 (Catalog # 404-ML) induced proliferation in the HT-2 mouse T cell line in a dose-dependent manner (orange line). Inhibition of Recombinant Mouse IL-4 (7.5 ng/mL) activity elicited by Recombinant Human TGF-beta 1 (1 ng/mL) is neutralized (green line) by increasing concentrations of Mouse Anti-TGF-beta 1, 2, 3 Monoclonal Antibody (Catalog # MAB1835). The ND50 is typically 0.25-1.25 µg/mL.
 View Larger
View Larger
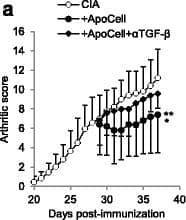
Detection of Mouse TGF beta 1/2/3 by In vivo assay Apoptotic cell-induced immunomodulation in mice with collagen-induced arthritis (CIA) is dependent on transforming growth factor (TGF)-beta. CIA mice that received or did not receive apoptotic cells, with or without anti-TGF-beta blocking antibody were scored daily (a). Data are shown as mean ± SEM of five mice per group from one of two representative experiments; *p < 0.05, **p < 0.01 (Friedman test analysis of variance (ANOVA) with Dunn's multiple comparisons test). Anti-collagen IgG2a antibodies (Ab) were quantified in plasma (b). Data from 5 to 16 mice from two to three independent experiments (no difference, Kruskal-Wallis test ANOVA with Dunn’s multiple comparisons test). Lymph node cells were collected, cultured, and T cell proliferation in response to collagen protein or CD3-specific Ab stimulations was evaluated (c). Data are shown as mean ± SEM from one of two representative experiments; *p < 0.05, **p < 0.01 (nonparametric unpaired t test). Percent and absolute number of Foxp3+ T regulatory cells (Treg) were evaluated in the spleen (d) and suppressive assays were performed by isolating and adding Treg at a different ratio into collagen-specific cultures and responder T cell proliferation was assessed (e). Data are shown as mean ± SEM from one experiment (d Kruskal-Wallis test ANOVA with Dunn’s multiple comparisons test; e Friedman test ANOVA with Dunn’s multiple comparisons test). Plasmacytoid dendritic cells (pDC), conventional dendritic cells (cDC) and macrophages (macro) were isolated and cultured with naïve allogenic T cells to determine Treg polarization (f), and their response to TLR ligand was assessed through evaluation of CD40 costimulatory molecule expression (g). Data from individual mouse plus mean (black bar) from one experiment representative of four are shown; *p < 0.05, ***p < 0.001 (f ordinary one-way ANOVA with Tukey’s multiple comparisons test; g Kruskal-Wallis test ANOVA with Dunn’s multiple comparisons test). BrDU 5-Bromo-2’-deoxyuridine Image collected and cropped by CiteAb from the following publication (https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-016-1084-0), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
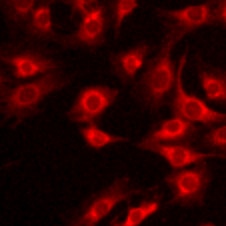
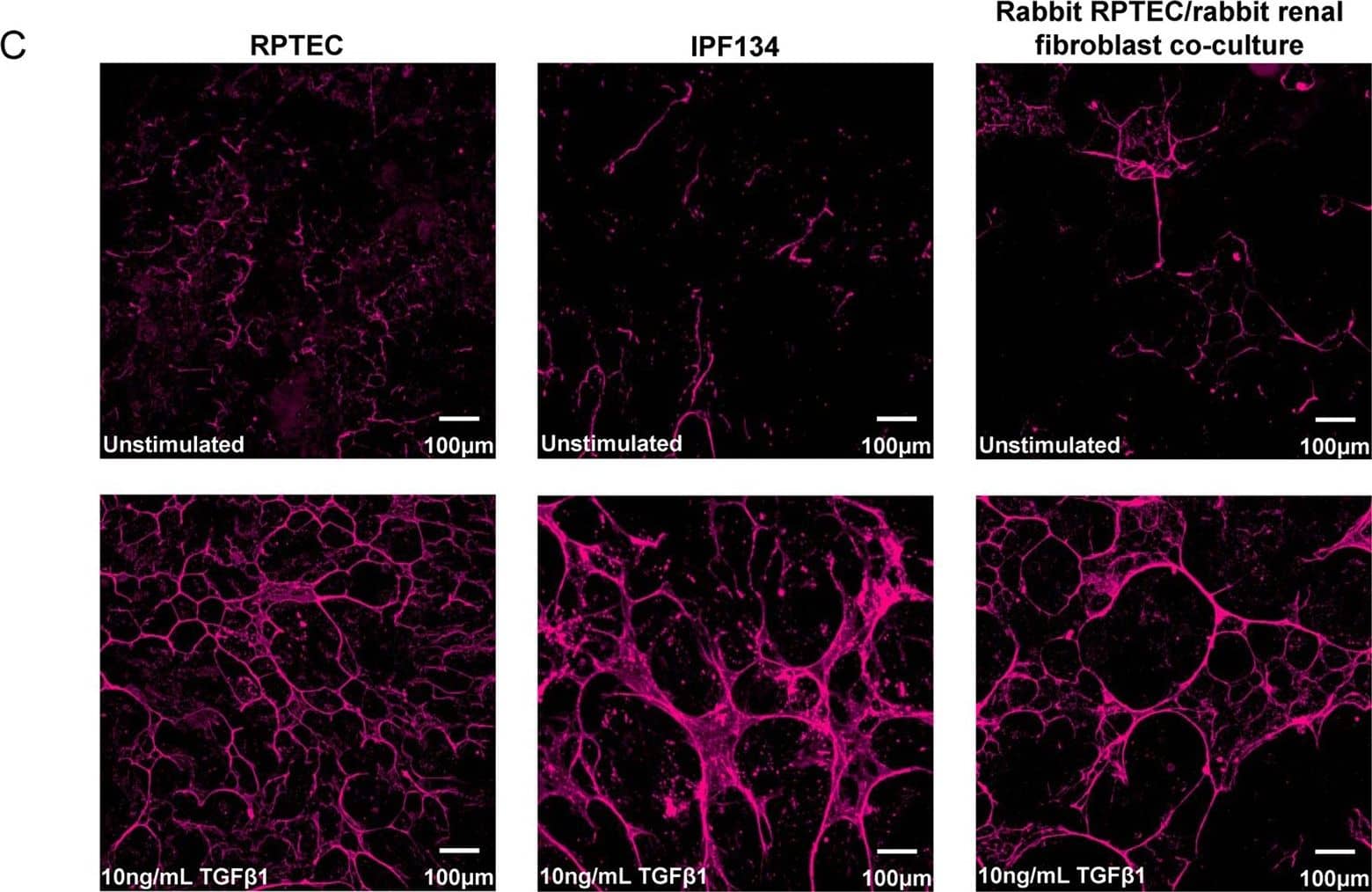
Detection of Human TGF beta 1/2/3 by Immunocytochemistry/Immunofluorescence TGF beta 1 stimulates the expression of ECM components in human and rabbit primary cells. (A) Selected ECM mRNA transcripts were measured using qRT-PCR in human RPTEC or human IPF134 following 48 hr stimulation with TGF beta 1 (10 ng/mL). Data show transcript abundance relative to the unstimulated control (indicated by the dashed line) and are shown as mean ± SD of four (RPTEC) or seven (IPF134) independent experiments. ****P < 0.0001; ****P < 0.001; **P < 0.01; Student’s t-test. (B) The accumulation of ECM in human RPTEC (i) or human IPF (ii) cells stimulated with 10ng/mL TGF beta 1 was evaluated via measurement of the incorporation of 14C-labelled amino acids into the deposited ECM. Data presented are the mean ± SD of three independent experiments. **P < 0.01; Student’s t-test. (C) Increased mature ECM accumulation following treatment of cells with a pro-fibrotic stimulus can be visualised following in-situ fluorescent staining using the Flamingo dye. Cells from three different systems (primary human RPTEC; primary human IPF134; co-culture of primary rabbit RPTEC with primary rabbit renal fibroblasts) were cultured in the absence (top) or presence (bottom) of 10 ng/ml TGF beta 1 before decellularised matrix was fixed and stained in situ with Flamingo fluorescent dye. Images show a single field. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28855577), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
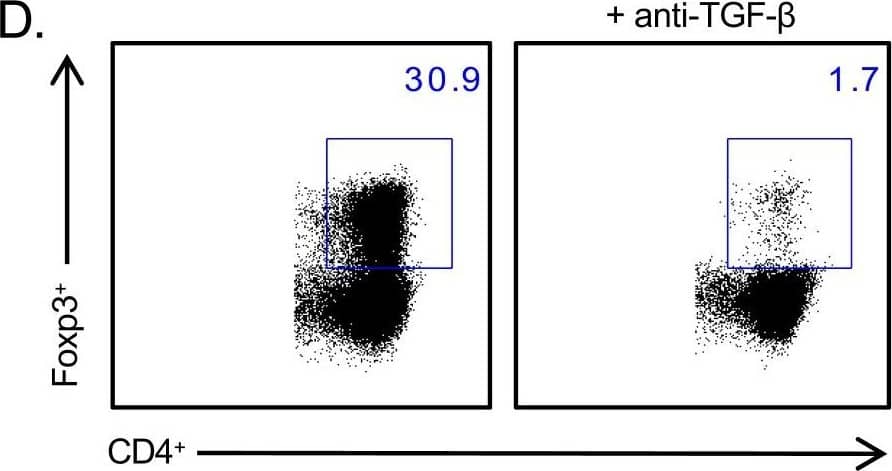
Detection of Mouse TGF-beta 1, 2, 3 Antibody by Flow Cytometry CBirTox induced expression of Foxp3 is dependent on TGF-beta but independent of RA signaling.(A) CD11c+ DCs were isolated from the spleen, MLN or LP and pulsed with 1 μg/ml of CBirTox or CBir1 peptide for 2 hours prior to co-culture with CD4+ CBir1 Tg T cells for 4 days before flow cytometry analysis. Results represent 2–3 independent experiments (B-C) CD11c+ DCs were isolated from the spleen and pulsed with 1 μg/ml of CBirTox for 1 hour before co-culture with CD4+ CBir1 Tg T cells in the presence of 1 μM of the RA inhibitor LE135 or RA for 4 days. Representative flow plots (B) of 3 independent experiments (C) are shown. (D-E) Splenic CD19+ B cells were pulsed with 2 μg of CBirTox for 2 hours before co-culture with with CD4+ CBir1 Tg T cells with or without the presence of 10 μg/ml anti-TGF-beta (1,2,3) for 4 days prior to flow cytometry analysis. Representative flow plots (D) of 4–5 independent experiments (E) are shown. Results of all experiments are expressed as the mean ± SEM. ***p<0.0005, NS, not significant. Groups of three or more were analyzed using one-way ANOVA with Bonferroni’s post test. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28750075), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
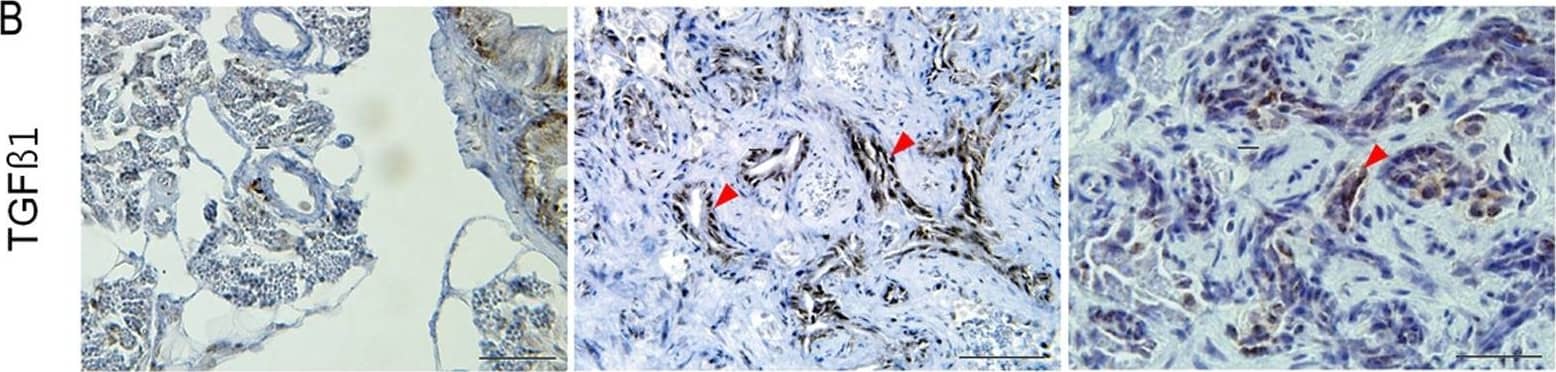
Detection of Zebrafish TGF beta 1/2/3 by Immunohistochemistry Expression of genes involved in fibrosis at 6 months.(A) IHC and ISH (inlets) showing MMP9 expression in proliferating myofibroblasts and ductular cells. (B) IHC for TGF beta 1. Contrary to MMP9, TGF beta 1 is expressed only in proliferating ductular cells which are also positive for cytokeratin. (C, D) ISH for PDGFAa and IL1b. Transcripts of both genes are detected in a small subset of proliferating myofibroblasts and ductular cells. (A–C, red arrowheads, ductular cells). Microscopic images are 400×. Bars, 50 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/22164219), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: TGF-beta 1, 2, 3
TGF-beta 1, -2, and -3 are a closely related group of proteins (70‑80% sequence homology) that are produced by many cell types and function as growth and differentiation factors. The active forms of TGF-beta 1, -2, and -3 are disulfide-linked homodimers.
- Ayala A. et al. (1992) FASEB J. 6:A1604.
- Roberts A.B. and Sporn M.B., eds. (1990) Peptide Growth Factors and Their Receptors I, Springer-Verlag, 419.
- Dasch J.R. et al. (1989) J. Immunol. 142:1536.
Product Datasheets
Citations for TGF-beta 1, 2, 3 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
227
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Neural crest derived progenitor cells contribute to tumor stroma and aggressiveness in stage 4/M neuroblastoma
Authors: P Linares-Cl, D Aguilar-Mo, I Rodríguez-, G Ramírez, C de Torres, V Santamaría, D Pascual-Va, A Colmenero-, FM Vega, J Mora, R Cabello, C Márquez, E Rivas, R Pardal
Oncotarget, 2017-09-21;8(52):89775-89792.
-
Quantitative and organisational changes in mature extracellular matrix revealed through high-content imaging of total protein fluorescently stained in situ
Authors: G Holdsworth, H Bon, M Bergin, O Qureshi, R Paveley, J Atkinson, L Huang, R Tewari, B Twomey, T Johnson
Sci Rep, 2017-08-30;7(1):9963.
-
FGFR1 is critical for the anti-endothelial mesenchymal transition effect of N-acetyl-seryl-aspartyl-lysyl-proline via induction of the MAP4K4 pathway.
Authors: Li J, Shi S, et al.
Cell Death Dis
-
Prostaglandin E2 and Transforming Growth Factor-beta Play a Critical Role in Suppression of Allergic Airway Inflammation by Adipose-Derived Stem Cells.
Authors: Cho K, Lee J, Park M, Park H, Yu H, Roh H
PLoS ONE, 2015-07-15;10(7):e0131813.
-
Suppression of latent transforming growth factor (TGF)-beta1 restores growth inhibitory TGF-beta signaling through microRNAs.
Authors: Dogar AM, Towbin H, Hall J
J. Biol. Chem., 2011-03-14;286(18):16447-58.
-
Bovine Milk‐Derived Extracellular Vesicles Inhibit Catabolic and Inflammatory Processes in Cartilage from Osteoarthritis Patients
Authors: Bartijn C. H. Pieters, Onno J. Arntz, Joyce Aarts, Anouk L. Feitsma, R. J. Joost van Neerven, Peter M. van der Kraan et al.
Molecular Nutrition & Food Research
-
BAFF promotes follicular helper T cell development and germinal center formation through BR3 signal
Authors: Chen, Y;Chen, M;Liu, Y;Li, Q;Xue, Y;Liu, L;Liang, R;Xiong, Y;Zhao, J;Chen, J;Lin, W;Wang, J;Pan, YF;Stohl, W;Zheng, SG;
JCI insight
Species: Transgenic Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Cytomegalovirus results in poor graft function via bone marrow-derived endothelial progenitor cells
Authors: Lv, W;Zhou, Y;Zhao, K;Xuan, L;Huang, F;Fan, Z;Chang, Y;Yi, Z;Jin, H;Liang, Y;Liu, Q;
Frontiers in microbiology
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Epithelial-mesenchymal transition induced by tumor cell-intrinsic PD-L1 signaling predicts a poor response to immune checkpoint inhibitors in PD-L1-high lung cancer
Authors: Jeong, H;Koh, J;Kim, S;Song, SG;Lee, SH;Jeon, Y;Lee, CH;Keam, B;Lee, SH;Chung, DH;Jeon, YK;
British journal of cancer
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Soluble NKG2DLs Are Elevated in Breast Cancer Patients and Associate with Disease Outcome
Authors: Seller, A;Tegeler, CM;Mauermann, J;Schreiber, T;Hagelstein, I;Liebel, K;Koch, A;Heitmann, JS;Greiner, SM;Hayn, C;Dannehl, D;Engler, T;Hartkopf, AD;Hahn, M;Brucker, SY;Salih, HR;Märklin, M;
International journal of molecular sciences
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Digital pathology with artificial intelligence analysis provides insight to the efficacy of anti-fibrotic compounds in human 3D MASH model
Authors: Kostadinova, R;Ströbel, S;Chen, L;Fiaschetti-Egli, K;Gadient, J;Pawlowska, A;Petitjean, L;Bieri, M;Thoma, E;Petitjean, M;
Scientific reports
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
NEDD8-activating enzyme inhibition potentiates the anti-myeloma activity of natural killer cells
Authors: Petillo, S;Sproviero, E;Loconte, L;Cuollo, L;Zingoni, A;Molfetta, R;Fionda, C;Soriani, A;Cerboni, C;Petrucci, MT;Fazio, F;Paolini, R;Santoni, A;Cippitelli, M;
Cell death & disease
Applications: Neutralization -
Synergistic inter-clonal cooperation involving crosstalk, co-option and co-dependency can enhance the invasiveness of genetically distant cancer clones
Authors: Carneiro, CS;Hapeman, JD;Nedelcu, AM;
BMC ecology and evolution
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A Reconstructed Human Melanoma-in-Skin Model to Study Immune Modulatory and Angiogenic Mechanisms Facilitating Initial Melanoma Growth and Invasion
Authors: Michielon, E;López González, M;Stolk, DA;Stolwijk, JGC;Roffel, S;Waaijman, T;Lougheed, SM;de Gruijl, TD;Gibbs, S;
Cancers
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
TGF-? Type I Receptor Signaling in Melanoma Liver Metastases Increases Metastatic Outgrowth
Authors: Marvin, DL;Dijkstra, J;Zulfiqar, RM;Vermeulen, M;Ten Dijke, P;Ritsma, L;
International journal of molecular sciences
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
The human placenta shapes the phenotype of decidual macrophages
Authors: S Vondra, AL Höbler, AI Lackner, J Raffetsede, ZN Mihalic, A Vogel, L Saleh, V Kunihs, P Haslinger, M Wahrmann, H Husslein, R Oberle, J Kargl, S Haider, P Latos, G Schabbauer, M Knöfler, J Ernerudh, J Pollheimer
Cell Reports, 2023-01-10;42(1):111977.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Fungal sensing by dectin-1 directs the non-pathogenic polarization of TH17 cells through balanced type I IFN responses in human DCs
Authors: SI Gringhuis, TM Kaptein, EBM Remmerswaa, A Drewniak, BA Wevers, B Theelen, GRAM D'Haens, T Boekhout, TBH Geijtenbee
Nature Immunology, 2022-12-01;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Capture -
CCL18 signaling from tumor-associated macrophages activates fibroblasts to adopt a chemoresistance-inducing phenotype
Authors: W Zeng, L Xiong, W Wu, S Li, J Liu, L Yang, L Lao, P Huang, M Zhang, H Chen, N Miao, Z Lin, Z Liu, X Yang, J Wang, P Wang, E Song, Y Yao, Y Nie, J Chen, D Huang
Oncogene, 2022-11-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Depletion of CD206+ M2-like macrophages induces fibro-adipogenic progenitors activation and muscle regeneration
Authors: A Nawaz, M Bilal, S Fujisaka, T Kado, MR Aslam, S Ahmed, K Okabe, Y Igarashi, Y Watanabe, T Kuwano, K Tsuneyama, A Nishimura, Y Nishida, S Yamamoto, M Sasahara, J Imura, H Mori, MM Matzuk, F Kudo, I Manabe, A Uezumi, T Nakagawa, Y Oishi, K Tobe
Nature Communications, 2022-11-21;13(1):7058.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Influence of tumor cell-derived TGF-beta on macrophage phenotype and macrophage-mediated tumor cell invasion
Authors: LAG Maldonado, CR Nascimento, NAR Fernandes, ALP Silva, NJ D'Silva, C Rossa
The international journal of biochemistry & cell biology, 2022-11-04;153(0):106330.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Roseburia intestinalis stimulates TLR5-dependent intestinal immunity against Crohn's disease
Authors: Z Shen, W Luo, B Tan, K Nie, M Deng, S Wu, M Xiao, X Wu, X Meng, T Tong, C Zhang, K Ma, Y Liao, J Xu, X Wang
EBioMedicine, 2022-09-28;85(0):104285.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Anti-TGF-beta 1 aptamer enhances therapeutic effect of tyrosine kinase inhibitor, gefitinib, on non-small cell lung cancer in xenograft model
Authors: Masaki Takahashi, Yoshifumi Hashimoto, Yoshikazu Nakamura
Molecular Therapy - Nucleic Acids
-
Twist-related protein 1 induces epithelial-mesenchymal transition and renal fibrosis through the upregulation of complement 3
Authors: T Otsuki, N Fukuda, L Chen, A Tsunemi, M Abe
PLoS ONE, 2022-08-26;17(8):e0272917.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Cell-autonomous Hedgehog signaling controls Th17 polarization and pathogenicity
Authors: J Hanna, F Beke, LM O'Brien, C Kapeni, HC Chen, V Carbonaro, AB Kim, K Kishore, TE Adolph, MO Skjoedt, K Skjoedt, M de la Roch, M de la Roch
Nature Communications, 2022-07-14;13(1):4075.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Latrophilin-2 is a novel receptor of LRG1 that rescues vascular and neurological abnormalities and restores diabetic erectile function
Authors: GN Yin, DK Kim, JI Kang, Y Im, DS Lee, AR Han, J Ock, MJ Choi, MH Kwon, A Limanjaya, SB Jung, J Yang, KW Min, J Yun, Y Koh, JE Park, D Hwang, JK Suh, JK Ryu, HM Kim
Experimental & Molecular Medicine, 2022-05-13;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Age-associated callus senescent cells produce TGF-beta 1 that inhibits fracture healing in aged mice
Authors: Jiatong Liu, Jun Zhang, Xi Lin, Brendan F. Boyce, Hengwei Zhang, Lianping Xing
Journal of Clinical Investigation
-
Platelets subvert antitumor efficacy of T cell-recruiting bispecific antibodies
Authors: Martina Svenja Lutz, Boris Klimovich, Stefanie Maurer, Jonas S Heitmann, Melanie Märklin, Latifa Zekri et al.
Journal for ImmunoTherapy of Cancer
-
Visceral fat‐specific regulation of plasminogen activator inhibitor‐1 in aged septic mice
Authors: Maria E. C. Bruno, Sujata Mukherjee, Arnold J. Stromberg, Hiroshi Saito, Marlene E. Starr
Journal of Cellular Physiology
-
Trained immunity induced by in vivo peptide-based STAT6 inhibition prevents ragweed allergy in mice
Authors: Husheem Michael, Yuanyi Li, Yufa Wang, Christine T. McCusker
Allergy, Asthma & Clinical Immunology
Species: Mouse
Sample Types: In Vivo
Applications: In vivo assay -
Reciprocal interplay between asporin and decorin: Implications in gastric cancer prognosis
Authors: D Basak, Z Jamal, A Ghosh, PK Mondal, P Dey Talukd, S Ghosh, B Ghosh Roy, R Ghosh, A Halder, A Chowdhury, GK Dhali, BK Chattopadh, ML Saha, A Basu, S Roy, C Mukherjee, NK Biswas, U Chatterji, S Datta
PLoS ONE, 2021-08-11;16(8):e0255915.
Species: Human
Sample Types: Tissue Homogenates
Applications: Co-IP, Western Blot -
Methods to improve efficacy of orally administered bioactive peptides using bovine colostrum as an exemplar
Authors: RJ Playford, MJ Weiser, T Marchbank
PLoS ONE, 2021-06-17;16(6):e0253422.
Species: Bovine
Sample Types: Milk
Applications: ELISA Capture -
Human Keratinocytes Inhibit CD4+ T-Cell Proliferation through TGFB1 Secretion and Surface Expression of HLA-G1 and PD-L1 Immune Checkpoints
Authors: G Mestrallet, F Auvré, C Schenowitz, ED Carosella, J LeMaoult, MT Martin, N Rouas-Frei, NO Fortunel
Cells, 2021-06-08;10(6):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Bone Morphogenetic Protein Antagonist Gremlin-1 Increases Myofibroblast Transition in Dermal Fibroblasts: Implications for Systemic Sclerosis
Authors: Laura Duffy, John Henderson, Max Brown, Stefan Pryzborski, Nicola Fullard, Lena Summa et al.
Frontiers in Cell and Developmental Biology
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Tumor‐associated macrophages promote the metastasis and growth of non‐small‐cell lung cancer cells through NF‐ kappa B/PP2Ac‐positive feedback loop
Authors: Zhan‐Wen Liang, Xin‐Xin Ge, Meng‐Dan Xu, Hualong Qin, Meng‐Yao Wu, Meng Shen et al.
Cancer Science
-
3D iPSC modeling of the retinal pigment epithelium-choriocapillaris complex identifies factors involved in the pathology of macular degeneration
Authors: KV Manian, CA Galloway, S Dalvi, AA Emanuel, JA Mereness, W Black, L Winschel, C Soto, Y Li, Y Song, W DeMaria, A Kumar, I Slukvin, MP Schwartz, WL Murphy, B Anand-Apte, M Chung, DSW Benoit, R Singh
Cell Stem Cell, 2021-03-29;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Astrocyte-derived small extracellular vesicles promote synapse formation via fibulin-2-mediated TGF-&beta signaling
Authors: MR Patel, AM Weaver
Cell Reports, 2021-03-09;34(10):108829.
Species: Rat
Sample Types: Cell Lysates, Whole Cells
Applications: Dot Blot, Western Blot -
Splenectomy improves liver fibrosis via tumor necrosis factor superfamily 14 (LIGHT) through the JNK/TGF-&beta1 signaling pathway
Authors: QS Liang, JG Xie, C Yu, Z Feng, J Ma, Y Zhang, D Wang, J Lu, R Zhuang, J Yin
Experimental & Molecular Medicine, 2021-03-03;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Genetically defined syngeneic mouse models of ovarian cancer as tools for the discovery of combination immunotherapy.
Authors: Sonia Iyer, Shuang Zhang, Simge Yucel, Heiko Horn, Sean G. Smith, Ferenc Reinhardt et al.
Cancer Discovery
-
RAC1B Regulation of TGFB1 Reveals an Unexpected Role of Autocrine TGF&beta1 in the Suppression of Cell Motility
Authors: H Ungefroren, H Otterbein, UF Wellner, T Keck, H Lehnert, JU Marquardt
Cancers, 2020-11-29;12(12):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
A Role for HAPLN1 During Phenotypic Modulation of Human Lung Fibroblasts In Vitro
Authors: Stephen P. Evanko, Michel D. Gooden, Inkyung Kang, Christina K. Chan, Robert B. Vernon, Thomas N. Wight
Journal of Histochemistry & Cytochemistry
-
Influence of antigen density and immunosuppressive factors on tumor-targeted costimulation with antibody-fusion proteins and bispecific antibody-mediated T cell response
Authors: Sabrina Sapski, Nadine Beha, Roland E. Kontermann, Dafne Müller
Cancer Immunology, Immunotherapy
-
Full-length IL-33 regulates Smad3 phosphorylation and gene transcription in a distinctive AP2-dependent manner
Authors: Irina G. Luzina, Rita Fishelevich, Brian S. Hampton, Jean-Paul Courneya, Francesca R. Parisella, Katerina N. Lugkey et al.
Cellular Immunology
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo
Authors: Mahmoud Elashiry, Mohamed M. Elashiry, Ranya Elsayed, Mythily Rajendran, Carol Auersvald, Rana Zeitoun et al.
Journal of Extracellular Vesicles
-
Age-induced accumulation of methylmalonic acid promotes tumour progression
Authors: AP Gomes, D Ilter, V Low, JE Endress, J Fernández-, A Rosenzweig, T Schild, D Broekaert, A Ahmed, M Planque, I Elia, J Han, C Kinzig, E Mullarky, AP Mutvei, J Asara, R de Cabo, LC Cantley, N Dephoure, SM Fendt, J Blenis
Nature, 2020-08-19;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine
Authors: LF Campesato, S Budhu, J Tchaicha, CH Weng, M Gigoux, IJ Cohen, D Redmond, L Mangarin, S Pourpe, C Liu, R Zappasodi, D Zamarin, J Cavanaugh, AC Castro, MG Manfredi, K McGovern, T Merghoub, JD Wolchok
Nat Commun, 2020-08-11;11(1):4011.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Tgf beta signaling is required for tenocyte recruitment and functional neonatal tendon regeneration
Authors: Deepak A Kaji, Kristen L Howell, Zerina Balic, Dirk Hubmacher, Alice H Huang
eLife
-
T cells expressing the lupus susceptibility allele Pbx1d enhance autoimmunity and atherosclerosis in dyslipidemic mice
Authors: W Li, AS Elshikha, C Cornaby, X Teng, G Abboud, J Brown, X Zou, L Zeumer-Spa, B Robusto, SC Choi, K Fredenburg, A Major, L Morel
JCI Insight, 2020-06-04;5(11):.
Species: Transgenic Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Variations in Circulating Active MMP-9 Levels During Renal Replacement Therapy
Authors: E Rodríguez-, JA Navarro-Ga, J Aceves-Rip, J Abarca-Zab, A Susmozas-S, T Bada-Bosch, E Hernández, E Mérida-Her, A Andrés, M Praga, M Fernández-, JM Aguado, J Segura, LM Ruilope, G Ruiz-Hurta
Biomolecules, 2020-03-26;10(4):.
Species: Human
Sample Types: Plasma
-
TGF&beta-mediated expression of TGF&beta-activating integrins in SSc monocytes: disturbed activation of latent TGF&beta?
Authors: A van Caam, J Aarts, T van Ee, E Vitters, M Koenders, F van de Loo, P van Lent, F van den Ho, R Thurlings, MC Vonk, PM van der Kr
Arthritis Res. Ther., 2020-03-06;22(1):42.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Anti-TGF-&beta attenuates tumor growth via polarization of tumor associated neutrophils towards an anti-tumor phenotype in colorectal cancer
Authors: F Qin, X Liu, J Chen, S Huang, W Wei, Y Zou, X Liu, K Deng, S Mo, J Chen, X Chen, Y Huang, W Liang
J Cancer, 2020-02-14;11(9):2580-2592.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Reversible EMT and MET mediate amnion remodeling during pregnancy and labor
Authors: Richardson LS, Taylor RN, Menon R.
Science Signaling
-
Deficiency in Dipeptidyl Peptidase-4 Promotes Chemoresistance through the CXCL12/CXCR4/mTOR/TGF&beta Signaling Pathway in Breast Cancer Cells
Authors: S Li, Y Fan, A Kumagai, E Kawakita, M Kitada, K Kanasaki, D Koya
Int J Mol Sci, 2020-01-26;21(3):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Endothelial autophagy deficiency induces IL6 - dependent endothelial mesenchymal transition and organ fibrosis
Authors: Y Takagaki, SM Lee, Z Dongqing, M Kitada, K Kanasaki, D Koya
Autophagy, 2020-01-22;0(0):1-10.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Human Cytomegalovirus miRNAs Regulate TGF-beta to Mediate Myelosuppression while Maintaining Viral Latency in CD34+ Hematopoietic Progenitor Cells
Authors: Meaghan H. Hancock, Lindsey B. Crawford, Andrew H. Pham, Jennifer Mitchell, Hillary M. Struthers, Andrew D. Yurochko et al.
Cell Host & Microbe
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
BMPR2 acts as a�gatekeeper to protect endothelial cells from increased TGF&beta�responses and altered cell mechanics
Authors: C Hiepen, J Jatzlau, S Hildebrand, B Kampfrath, M Goktas, A Murgai, JL Cuellar Ca, R Haag, C Ruppert, G Sengle, EA Cavalcanti, KG Blank, P Knaus
PLoS Biol., 2019-12-11;17(12):e3000557.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Anatomically specific reactive oxygen species production participates in Marfan syndrome aneurysm formation
Authors: Fabian Emrich, Kiril Penov, Mamoru Arakawa, Nathan Dhablania, Grayson Burdon, Albert J. Pedroza et al.
Journal of Cellular and Molecular Medicine
-
Human menstrual blood-derived stromal/stem cells modulate functional features of natural killer cells
Authors: MR Shokri, M Bozorgmehr, A Ghanavatin, R Falak, M Aleahmad, S Kazemnejad, F Shokri, AH Zarnani
Sci Rep, 2019-07-10;9(1):10007.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A PRDM16-Driven Metabolic Signal from Adipocytes Regulates Precursor Cell Fate
Authors: Wenshan Wang, Jeff Ishibashi, Sophie Trefely, Mengle Shao, Alexis J. Cowan, Alexander Sakers et al.
Cell Metabolism
-
Stromal Microenvironment Shapes the Intratumoral Architecture of Pancreatic Cancer
Authors: Matteo Ligorio, Srinjoy Sil, Jose Malagon-Lopez, Linda T. Nieman, Sandra Misale, Mauro Di Pilato et al.
Cell
-
TGF??1 upregulates the expression of lncRNA?ATB to promote atherosclerosis
Authors: H Yu, S Ma, L Sun, J Gao, C Zhao
Mol Med Rep, 2019-03-29;19(5):4222-4228.
Species: Human
Sample Types: Serum
Applications: ELISA Development (Capture) -
BMSCs pre-treatment ameliorates inflammation-related tissue destruction in LPS-induced rat DIC model
Authors: B Wang, S Wu, Z Ma, T Wang, C Yang
Cell Death Dis, 2018-10-03;9(10):1024.
Species: Rat
Sample Types: Whole Cells
Applications: Neutralization -
TGF-?1 promotes expression of fibrosis-related genes through the induction of histone variant H3.3 and histone chaperone HIRA
Authors: T Shindo, S Doi, A Nakashima, K Sasaki, K Arihiro, T Masaki
Sci Rep, 2018-09-19;8(1):14060.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Immunological Properties of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells
Authors: M Idelson, R Alper, A Obolensky, N Yachimovic, J Rachmilewi, A Ejzenberg, E Beider, E Banin, B Reubinoff
Stem Cell Reports, 2018-08-16;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Separation of cell survival, growth, migration, and mesenchymal transdifferentiation effects of fibroblast secretome on tumor cells of head and neck squamous cell carcinoma
Authors: Veronika Maria Metzler, Christian Pritz, Anna Riml, Angela Romani, Raphaela Tuertscher, Teresa Steinbichler et al.
Tumour Biol.
-
TGF-?1 Negatively Regulates the Number and Function of Hematopoietic Stem Cells
Authors: X Wang, F Dong, S Zhang, W Yang, W Yu, Z Wang, S Zhang, J Wang, S Ma, P Wu, Y Gao, J Dong, F Tang, T Cheng, H Ema
Stem Cell Reports, 2018-06-21;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Protective effect of KLF15 on vascular endothelial dysfunction induced by TNF??.
Authors: Bing Liu, Lili Xu, Xinming Yu, Wei Li, Xiaozhi Sun, Shun Xiao, Mingjin Guo, Haofu Wang
Molecular Medicine Reports, 2018-06-20;0(0):1791-3004.
Species: Human
Sample Types: Cell Lysate, Cell Lysates
Applications: Western Blot -
Metastatic Brain Tumors Disrupt the Blood-Brain Barrier and Alter Lipid Metabolism by Inhibiting Expression of the Endothelial Cell Fatty Acid Transporter Mfsd2a
Authors: S Tiwary, JE Morales, SC Kwiatkowsk, FF Lang, G Rao, JH McCarty
Sci Rep, 2018-05-29;8(1):8267.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
CD8+ T cells with high TGF??1 expression cause lymph node fibrosis following HIV infection
Authors: L Huang, J Deng, W Xu, H Wang, L Shi, F Wu, D Wu, W Nei, M Zhao, P Mao, X Zhou
Mol Med Rep, 2018-05-03;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Agonist Activity -
Bacterially activated B-cells drive T cell differentiation towards Tr1 through PD-1/PD-L1 expression
Authors: SS Said, GT Barut, N Mansur, A Korkmaz, A Sayi-Yazga
Mol. Immunol., 2018-02-26;96(0):48-60.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
RHOA G17V Induces T Follicular Helper Cell Specification and Promotes Lymphomagenesis
Authors: JR Cortes, A Ambesi-Imp, L Couronné, SA Quinn, CS Kim, AC da Silva A, Z West, L Belver, MS Martin, L Scourzic, G Bhagat, OA Bernard, AA Ferrando, T Palomero
Cancer Cell, 2018-02-02;33(2):259-273.e7.
Species: Mouse
Sample Types: Whole Cells
Applications: Activation -
Proteomic Analysis of Nucleus Pulposus Cell-derived Extracellular Matrix Niche and Its Effect on Phenotypic Alteration of Dermal Fibroblasts
Authors: M Yuan, PJ Pai, X Liu, H Lam, BP Chan
Sci Rep, 2018-01-24;8(1):1512.
Species: Rabbit
Sample Types: Whole Cells
Applications: ICC -
Downregulation of membrane trafficking proteins and lactate conditioning determine loss of dendritic cell function in lung cancer
Authors: N Caronni, F Simoncello, F Stafetta, C Guarnaccia, JS Ruiz-Moren, B Opitz, T Galli, V Proux-Gill, F Benvenuti
Cancer Res., 2018-01-23;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Connective tissue growth factor expression after angiotensin II exposure is dependent on transforming growth factor-? signaling via the canonical Smad-dependent pathway in hypertensive induced myocardial fibrosis
Authors: CKS Wong, A Falkenham, T Myers, JF Légaré
J Renin Angiotensin Aldosterone Syst, 2018-01-01;19(1):1470320318759.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Evidence of lateral transmission of aggressive features between different types of breast cancer cells
Authors: Nancy Adriana Espinoza-Sánchez, Eduardo Vadillo, Juan Carlos Balandrán, Alberto Monroy-García, Rosana Pelayo, Ezequiel M. Fuentes-Pananá
International Journal of Oncology
-
Greater transforming growth factor-beta in adult female SHR is dependent on blood pressure, but does not account for sex differences in renal T-regulatory cells
Authors: Ashlee J. Tipton, Jacqueline B. Musall, G. Ryan Crislip, Jennifer C. Sullivan
American Journal of Physiology-Renal Physiology
Species: Rat
Sample Types: Tissue Homogenates, In Vivo, Whole Tissue
Applications: Immunohistochemistry, Western Blot, In vivo assay -
Mesenteric lymph node stromal cell-derived extracellular vesicles contribute to peripheral de novo induction of Foxp3(+) regulatory T cells
Authors: M Pasztoi, J Pezoldt, M Beckstette, C Lipps, D Wirth, M Rohde, K Paloczi, EI Buzas, J Huehn
Eur. J. Immunol., 2017-09-15;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
CD206+ M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors
Authors: Allah Nawaz, Aminuddin Aminuddin, Tomonobu Kado, Akiko Takikawa, Seiji Yamamoto, Koichi Tsuneyama et al.
Nature Communications
-
Activated Tissue-Resident Mesenchymal Stromal Cells Regulate Natural Killer Cell Immune and Tissue-Regenerative Function
Authors: RM Petri, A Hackel, K Hahnel, CA Dumitru, K Bruderek, SB Flohe, A Paschen, S Lang, S Brandau
Stem Cell Reports, 2017-08-03;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Granulocytic myeloid derived suppressor cells from human cord blood modulate T-helper-cell response towards an anti-inflammatory phenotype
Authors: N Köstlin, M Vogelmann, B Spring, J Schwarz, J Feucht, C Härtel, TW Orlikowsky, CF Poets, C Gille
Immunology, 2017-06-08;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
TGF-? signaling directly regulates transcription and functional expression of the electrogenic sodium bicarbonate cotransporter 1, NBCe1 (SLC4A4), via Smad4 in mouse astrocytes
Authors: S Khakipoor, C Ophoven, M Schrödl-Hä, M Feuerstein, B Heimrich, JW Deitmer, E Roussa
Glia, 2017-06-01;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: Neutralization -
Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures
Authors: CJ Bohlen, FC Bennett, AF Tucker, HY Collins, SB Mulinyawe, BA Barres
Neuron, 2017-05-17;94(4):759-773.e8.
Species: Rat
Sample Types: Whole Cells
Applications: Neutralization -
ALS skeletal muscle shows enhanced TGF-? signaling, fibrosis and induction of fibro/adipogenic progenitor markers
Authors: D Gonzalez, O Contreras, DL Rebolledo, JP Espinoza, B van Zunder, E Brandan
PLoS ONE, 2017-05-16;12(5):e0177649.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Platelets subvert T cell immunity against cancer via GARP-TGF beta axis
Authors: Saleh Rachidi, Alessandra Metelli, Brian Riesenberg, Bill X. Wu, Michelle H. Nelson, Caroline W Wallace et al.
Science Immunology
-
Integrin ?1 activation induces an anti-melanoma host response
Authors: L Ritsma, I Dey-Guha, N Talele, X Sole, Salony, J Chowdhury, KN Ross, S Ramaswamy
PLoS ONE, 2017-04-27;12(4):e0175300.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
The therapeutic HIV Env C5/gp41 vaccine candidate Vacc-C5 induces specific T cell regulation in a phase I/II clinical study
Authors: K Brekke, M Sommerfelt, M Ökvist, AM Dyrhol-Rii, D Kvale
BMC Infect. Dis, 2017-03-24;17(1):228.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Mechanosignaling activation of TGF? maintains intervertebral disc homeostasis
Authors: Q Bian, L Ma, A Jain, JL Crane, K Kebaish, M Wan, Z Zhang, X Edward Guo, PD Sponseller, CA Séguin, LH Riley, Y Wang, X Cao
Bone Res, 2017-03-21;5(0):17008.
Species: Mouse, Rat
Sample Types: In Vivo, Whole Tissue
Applications: Bioassay, Neutralization -
A new developmental mechanism for the separation of the mammalian middle ear ossicles from the jaw
Authors: DJ Urban, N Anthwal, ZX Luo, JA Maier, A Sadier, AS Tucker, KE Sears
Proc. Biol. Sci, 2017-02-08;284(1848):.
Species: Opossum
Sample Types: In Vivo
Applications: In Vivo -
Histone Deacetylase (HDAC) Inhibition Induces I?B Kinase (IKK)-dependent Interleukin-8/CXCL8 Expression in Ovarian Cancer Cells
Authors: HR Gatla, Y Zou, MM Uddin, B Singha, P Bu, A Vancura, I Vancurova
J. Biol. Chem., 2017-02-06;292(12):5043-5054.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Examining Crosstalk among Transforming Growth Factor beta, Bone Morphogenetic Protein, and Wnt Pathways*
Authors: Adam D. Coster, Curtis A. Thorne, Lani F. Wu, Steven J. Altschuler
Journal of Biological Chemistry
-
Induction of fibroblast senescence generates a non-fibrogenic myofibroblast phenotype that differentially impacts on cancer prognosis
Authors: Massimiliano Mellone, Christopher J. Hanley, Steve Thirdborough, Toby Mellows, Edwin Garcia, Jeongmin Woo et al.
Aging (Albany NY)
-
Kluyveromyces marxianus and Saccharomyces boulardii Induce Distinct Levels of Dendritic Cell Cytokine Secretion and Significantly Different T Cell Responses In Vitro
PLoS ONE, 2016-11-29;11(11):e0167410.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Pericyte MyD88 and IRAK4 control inflammatory and fibrotic responses to tissue injury
Authors: Jeremy S Duffield
J. Clin. Invest., 2016-11-21;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Tumor-Activated Mesenchymal Stromal Cells Promote Osteosarcoma Stemness and Migratory Potential via IL-6 Secretion
PLoS ONE, 2016-11-16;11(11):e0166500.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs
Nature, 2016-11-09;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Cancer associated fibroblasts regulate keratinocyte cell-cell adhesion via TGF-�-dependent pathways in genotype-specific oral cancer
Authors: S S Prime
Carcinogenesis, 2016-11-01;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Neutralization -
Sterile Inflammation Enhances ECM Degradation in Integrin ?1 KO Embryonic Skin
Cell Rep, 2016-09-20;16(12):3334-47.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
TGF-?1 Is Present at High Levels in Wound Fluid from Breast Cancer Patients Immediately Post-Surgery, and Is Not Increased by Intraoperative Radiation Therapy (IORT)
PLoS ONE, 2016-09-02;11(9):e0162221.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
[64Cu]XYIMSR-06: A dual-motif CAIX ligand for PET imaging of clear cell renal cell carcinoma
Authors: I Minn, SM Koo, HS Lee, M Brummet, SP Rowe, MA Gorin, P Sysa-Shah, WD Lewis, HH Ahn, Y Wang, SR Banerjee, RC Mease, S Nimmagadda, ME Allaf, MG Pomper, X Yang
Oncotarget, 2016-08-30;7(35):56471-56479.
Species: Mink
Sample Types: Whole Cells
Applications: Neutralization -
Autonomous and Non-autonomous Defects Underlie Hypertrophic Cardiomyopathy in BRAF-Mutant hiPSC-Derived Cardiomyocytes
Stem Cell Reports, 2016-08-25;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Apoptotic cell infusion treats ongoing collagen-induced arthritis, even in the presence of methotrexate, and is synergic with anti-TNF therapy
Arthritis Res Ther, 2016-08-11;18(1):184.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
K+/Cl- cotransporter KCC2 membrane trafficking and functionality is regulated by transforming growth factor beta 2
J Cell Sci, 2016-08-05;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Polycomb repressive complex 2 regulates skeletal growth by suppressing Wnt and TGF-? signalling
Authors: Fatemeh Mirzamoham
Nat Commun, 2016-06-22;7(0):12047.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Loss of GFAT1 promotes epithelial-to-mesenchymal transition and predicts unfavorable prognosis in gastric cancer
Authors: Fangfang Duan, Dongwei Jia, Junjie Zhao, Weicheng Wu, Lingqiang Min, Shushu Song et al.
Oncotarget
-
Human macrophages induce CD4(+)Foxp3(+) regulatory T cells via binding and re-release of TGF-beta.
Authors: Schmidt A, Zhang X, Joshi R, Iqbal S, Wahlund C, Gabrielsson S, Harris R, Tegner J
Immunol Cell Biol, 2016-05-10;94(8):747-62.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
TGF-? Neutralization Enhances AngII-Induced Aortic Rupture and Aneurysm in Both Thoracic and Abdominal Regions
Authors: X Chen, DL Rateri, DA Howatt, A Balakrishn, JJ Moorleghen, LA Cassis, A Daugherty
PLoS ONE, 2016-04-22;11(4):e0153811.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
TNFR-Associated Factors 2 and 5 Differentially Regulate the Instructive IL-6 Receptor Signaling Required for Th17 Development
Authors: H Nagashima, Y Okuyama, T Hayashi, N Ishii, T So
J Immunol, 2016-04-13;0(0):.
-
Monocytes increase human cardiac myofibroblast-mediated extracellular matrix remodeling through TGF-beta 1
Authors: Holly E. M. Mewhort, Brodie D. Lipon, Daniyil A. Svystonyuk, Guoqi Teng, David G. Guzzardi, Claudia Silva et al.
American Journal of Physiology-Heart and Circulatory Physiology
-
Epigenetic Histone Modifications Involved in Profibrotic Gene Regulation by 12/15-Lipoxygenase and Its Oxidized Lipid Products in Diabetic Nephropathy
Authors: Hang Yuan, Marpadga A. Reddy, Supriya Deshpande, Ye Jia, Jung Tak Park, Linda L. Lanting et al.
Antioxidants & Redox Signaling
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Development and characterization of human monoclonal antibodies that neutralize multiple TGF beta isoforms
Authors: Daniel Bedinger, Llewelyn Lao, Shireen Khan, Steve Lee, Toshihiko Takeuchi, Amer M. Mirza
mAbs
-
Autoimmune regulator-overexpressing dendritic cells induce T helper 1 and T helper 17 cells by upregulating cytokine expression
Authors: HAIJUN LI, DONGBEI LI, JITONG SUN, YINAN LI, WEI YANG, YI LI
Molecular Medicine Reports
-
CD4+CD25+ Regulatory T Cells Inhibit Natural Killer Cell Hepatocytotoxicity of Hepatitis B Virus Transgenic Mice via Membrane-Bound TGF-beta and OX40
Authors: Yongyan Chen, Rui Sun, Xunyao Wu, Min Cheng, Haiming Wei, Zhigang Tian
Journal of Innate Immunity
-
Muscleblind-like 1 is required for normal heart valve development in vivo
Authors: Ryan J. Coram, Samantha J. Stillwagon, Anuradha Guggilam, Michael W. Jenkins, Maurice S. Swanson, Andrea N. Ladd
BMC Developmental Biology
-
Treatment of dextran sodium sulfate-induced experimental colitis by adoptive transfer of peritoneal cells
Authors: Ting Liu, Jun Ren, Wei Wang, Xia-wei Wei, Guo-bo Shen, Yan-tong Liu et al.
Scientific Reports
-
Commercially Available Preparations of Recombinant Wnt3a Contain Non-Wnt Related Activities Which May Activate TGF-beta Signaling.
Authors: Carthy J, Engstrom U, Heldin C, Moustakas A
J Cell Biochem, 2015-09-22;117(4):938-45.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
STAT6-Dependent Collagen Synthesis in Human Fibroblasts Is Induced by Bovine Milk.
Authors: Kippenberger S, Zoller N, Kleemann J, Muller J, Kaufmann R, Hofmann M, Bernd A, Meissner M, Valesky E
PLoS ONE, 2015-07-02;10(7):e0131783.
Species: Bovine
Sample Types: Milk
Applications: Neutralization -
Mammary epithelial cell phagocytosis downstream of TGF-beta3 is characterized by adherens junction reorganization.
Authors: Fornetti J, Flanders K, Henson P, Tan A, Borges V, Schedin P
Cell Death Differ, 2015-06-26;23(2):185-96.
Species: Rat
Sample Types: Tissue Homogenates
Applications: Western Blot -
Early matrix metalloproteinase-12 inhibition worsens post-myocardial infarction cardiac dysfunction by delaying inflammation resolution
Authors: Rugmani Padmanabhan Iyer, Nicolle L. Patterson, Fouad A. Zouein, Yonggang Ma, Vincent Dive, Lisandra E. de Castro Brás et al.
International Journal of Cardiology
-
Commercial cow milk contains physically stable extracellular vesicles expressing immunoregulatory TGF-beta.
Authors: Pieters B, Arntz O, Bennink M, Broeren M, van Caam A, Koenders M, van Lent P, van den Berg W, de Vries M, van der Kraan P, van de Loo F
PLoS ONE, 2015-03-30;10(3):e0121123.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Recruitment of Matrix Metalloproteinase-9 (MMP-9) to the Fibroblast Cell Surface by Lysyl Hydroxylase 3 (LH3) Triggers Transforming Growth Factor-beta (TGF-beta) Activation and Fibroblast Differentiation.
Authors: Dayer C, Stamenkovic I
J Biol Chem, 2015-03-30;290(22):13763-78.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Iron-induced Local Complement Component 3 (C3) Up-regulation via Non-canonical Transforming Growth Factor (TGF)-beta Signaling in the Retinal Pigment Epithelium.
Authors: Li Y, Song D, Song Y, Zhao L, Wolkow N, Tobias J, Song W, Dunaief J
J Biol Chem, 2015-03-23;290(19):11918-34.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
IL-10 inhibits neuraminidase-activated TGF-beta and facilitates Th1 phenotype during early phase of infection.
Authors: Dutta A, Huang C, Chen T, Lin C, Chiu C, Lin Y, Chang C, He Y
Nat Commun, 2015-03-02;6(0):6374.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Pluripotency gene expression and growth control in cultures of peripheral blood monocytes during their conversion into programmable cells of monocytic origin (PCMO): evidence for a regulatory role of autocrine activin and TGF-beta.
Authors: Ungefroren H, Hyder A, Hinz H, Groth S, Lange H, El-Sayed K, Ehnert S, Nussler A, Fandrich F, Gieseler F
PLoS ONE, 2015-02-23;10(2):e0118097.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Bortezomib inhibits expression of TGF-beta1, IL-10, and CXCR4, resulting in decreased survival and migration of cutaneous T cell lymphoma cells.
Authors: Chang T, Poltoratsky V, Vancurova I
J Immunol, 2015-02-13;194(6):2942-53.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Mesenchymal stromal cells express GARP/LRRC32 on their surface: effects on their biology and immunomodulatory capacity.
Authors: Carrillo-Galvez A, Cobo M, Cuevas-Ocana S, Gutierrez-Guerrero A, Sanchez-Gilabert A, Bongarzone P, Garcia-Perez A, Munoz P, Benabdellah K, Toscano M, Martin F, Anderson P
Stem Cells, 2015-01-01;33(1):183-95.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Ex vivo-expanded but not in vitro-induced human regulatory T cells are candidates for cell therapy in autoimmune diseases thanks to stable demethylation of the FOXP3 regulatory T cell-specific demethylated region.
Authors: Rossetti M, Spreafico R, Saidin S, Chua C, Moshref M, Leong J, Tan Y, Thumboo J, van Loosdregt J, Albani S
J Immunol, 2014-12-01;194(1):113-24.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Anterior gradient 2 downregulation in a subset of pancreatic ductal adenocarcinoma is a prognostic factor indicative of epithelial-mesenchymal transition.
Authors: Mizuuchi Y, Aishima S, Ohuchida K, Shindo K, Fujino M, Hattori M, Miyazaki T, Mizumoto K, Tanaka M, Oda Y
Lab Invest, 2014-11-24;95(2):193-206.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
STAT3 governs hyporesponsiveness and granzyme B-dependent suppressive capacity in human CD4+ T cells.
Authors: Schmetterer K, Neunkirchner A, Wojta-Stremayr D, Leitner J, Steinberger P, Pickl W
FASEB J, 2014-11-14;29(3):759-71.
Species: Human
Sample Types: Whole Cells
Applications: Blocking -
Influenza promotes collagen deposition via alphavbeta6 integrin-mediated transforming growth factor beta activation.
Authors: Jolly L, Stavrou A, Vanderstoken G, Meliopoulos V, Habgood A, Tatler A, Porte J, Knox A, Weinreb P, Violette S, Hussell T, Kolb M, Stampfli M, Schultz-Cherry S, Jenkins G
J Biol Chem, 2014-10-22;289(51):35246-63.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Senescent cancer-associated fibroblasts secrete active MMP-2 that promotes keratinocyte dis-cohesion and invasion.
Authors: Hassona Y, Cirillo N, Heesom K, Parkinson E, Prime S
Br J Cancer, 2014-08-12;111(6):1230-7.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Neutralization -
Bovine gammadelta T cells are a major regulatory T cell subset.
Authors: Guzman E, Hope J, Taylor G, Smith A, Cubillos-Zapata C, Charleston B
J Immunol, 2014-06-02;193(1):208-22.
Species: Bovine
Sample Types: Whole Cells
Applications: Bioassay -
Tenascin-X promotes epithelial-to-mesenchymal transition by activating latent TGF-beta.
Authors: Alcaraz L, Exposito J, Chuvin N, Pommier R, Cluzel C, Martel S, Sentis S, Bartholin L, Lethias C, Valcourt U
J Cell Biol, 2014-05-12;205(3):409-28.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Neutralization, Western Blot -
Functional effects of TGF-beta1 on mesenchymal stem cell mobilization in cockroach allergen-induced asthma.
Authors: Gao P, Zhou Y, Xian L, Li C, Xu T, Plunkett B, Huang S, Wan M, Cao X
J Immunol, 2014-04-07;192(10):4560-70.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
The adaptor TRAF5 limits the differentiation of inflammatory CD4(+) T cells by antagonizing signaling via the receptor for IL-6.
Authors: Nagashima, Hiroyuki, Okuyama, Yuko, Asao, Atsuko, Kawabe, Takeshi, Yamaki, Satoshi, Nakano, Hiroyasu, Croft, Michael, Ishii, Naoto, So, Takanori
Nat Immunol, 2014-03-30;15(5):449-56.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
CD4(+) NKG2D(+) T cells induce NKG2D down-regulation in natural killer cells in CD86-RAE-1epsilon transgenic mice.
Authors: Lin Z, Wang C, Xia H, Liu W, Xiao W, Qian L, Jia X, Ding Y, Ji M, Gong W
Immunology, 2014-03-01;141(3):401-15.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Tumor necrosis factor-related apoptosis-inducing ligand mediates the resolution of allergic airway inflammation induced by chronic allergen inhalation.
Authors: Faustino L, Fonseca D, Florsheim E, Resende R, Lepique A, Faquim-Mauro E, Gomes E, Silva J, Yagita H, Russo M
Mucosal Immunol, 2014-02-26;7(5):1199-208.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Abnormal muscle mechanosignaling triggers cardiomyopathy in mice with Marfan syndrome.
Authors: Cook J, Carta L, Benard L, Chemaly E, Chiu E, Rao S, Hampton T, Yurchenco P, Costa K, Hajjar R, Ramirez F
J Clin Invest, 2014-02-17;124(3):1329-39.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Basal localization of MT1-MMP is essential for epithelial cell morphogenesis in 3D collagen matrix.
Authors: Weaver S, Wolters B, Ito N, Woskowicz A, Kaneko K, Shitomi Y, Seiki M, Itoh Y
J Cell Sci, 2014-01-24;127(0):1203-13.
Species: Canine, Human, Mouse
Sample Types: Whole Cells
Applications: Neutralization -
The extracellular matrix protein MAGP1 supports thermogenesis and protects against obesity and diabetes through regulation of TGF-beta.
Authors: Craft C, Pietka T, Schappe T, Coleman T, Combs M, Klein S, Abumrad N, Mecham R
Diabetes, 2014-01-23;63(6):1920-32.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Chondrogenesis of Mesenchymal Stem Cells in an Osteochondral Environment Is Mediated by the Subchondral Bone
Authors: Marloes L. de Vries–van Melle, Roberto Narcisi, Nicole Kops, Wendy J.L.M. Koevoet, P. Koen Bos, J. Mary Murphy et al.
Tissue Engineering Part A
-
Biglycan and decorin differentially regulate signaling in the fetal membranes.
Authors: Wu Z, Horgan C, Carr O, Owens R, Iozzo R, Lechner B
Matrix Biol, 2013-12-25;35(0):266-75.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
A mechanism linking Id2-TGFbeta crosstalk to reversible adaptive plasticity in neuroblastoma.
Authors: Chakrabarti L, Wang B, Lee N, Sandler A
PLoS ONE, 2013-12-23;8(12):e83521.
Species: Mouse
Sample Types: Cell Culture Supernates, Whole Cells
Applications: Neutralization, Western Blot -
Angiotensin II-dependent TGF-beta signaling contributes to Loeys-Dietz syndrome vascular pathogenesis.
Authors: Gallo E, Loch D, Habashi J, Calderon J, Chen Y, Bedja D, van Erp C, Gerber E, Parker S, Sauls K, Judge D, Cooke S, Lindsay M, Rouf R, Myers L, ap Rhys C, Kent K, Norris R, Huso D, Dietz H
J Clin Invest, 2013-12-20;124(1):448-60.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
The metalloprotease ADAM12 regulates the effector function of human Th17 cells.
Authors: Zhou A, El Hed A, Mercer F, Kozhaya L, Unutmaz D
PLoS ONE, 2013-11-21;8(11):e81146.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement.
Authors: Bialas A, Stevens B
Nat Neurosci, 2013-10-27;16(12):1773-82.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: Immunodepletion -
Aminobisphosphonates prevent the inhibitory effects exerted by lymph node stromal cells on anti-tumor Vdelta 2 T lymphocytes in non-Hodgkin lymphomas.
Authors: Musso A, Catellani S, Canevali P, Tavella S, Vene R, Boero S, Pierri I, Gobbi M, Kunkl A, Ravetti J, Zocchi M, Poggi A
Haematologica, 2013-10-25;99(1):131-9.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Blood pressure homeostasis is maintained by a P311–TGF-beta axis
Authors: Kameswara Rao Badri, Ming Yue, Oscar A. Carretero, Sree Latha Aramgam, Jun Cao, Stephen Sharkady et al.
Journal of Clinical Investigation
-
Dendritic cell-derived thrombospondin-1 is critical for the generation of the ocular surface Th17 response to desiccating stress.
Authors: Gandhi N, Su Z, Zhang X, Volpe E, Pelegrino F, Rahman S, Li D, Pflugfelder S, de Paiva C
J Leukoc Biol, 2013-08-27;94(6):1293-301.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
PARP-1 regulates expression of TGF-beta receptors in T cells.
Authors: Zhang P, Nakatsukasa H, Tu E, Kasagi S, Cui K, Ishikawa M, Konkel J, Maruyama T, Wei G, Abbatiello B, Wang Z, Zhao K, Chen W
Blood, 2013-08-12;122(13):2224-32.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
TGF-beta Induces Acetylation of Chromatin and of Ets-1 to Alleviate Repression of miR-192 in Diabetic Nephropathy
Authors: Mitsuo Kato, Varun Dang, Mei Wang, Jung Tak Park, Supriya Deshpande, Swati Kadam et al.
Science Signaling
-
CD2-mediated regulation of peripheral CD4(+) CD25(+) regulatory T-cell apoptosis accompanied by down-regulation of Bim.
Authors: Kashiwakura Y, Sakurai D, Kanno Y, Hashiguchi M, Kobayashi A, Kurosu A, Tokudome S, Kobata T, Kojima H
Immunology, 2013-05-01;139(1):48-60.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-beta.
Authors: Yu C, Becker C, Wang Y, Marches F, Helft J, Leboeuf M, Anguiano E, Pourpe S, Goller K, Pascual V, Banchereau J, Merad M, Palucka K
Immunity, 2013-04-04;38(4):818-30.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Staphylococcus aureus phenol-soluble modulin peptides modulate dendritic cell functions and increase in vitro priming of regulatory T cells.
Authors: Schreiner J, Kretschmer D, Klenk J, Otto M, Buhring H, Stevanovic S, Wang J, Beer-Hammer S, Peschel A, Autenrieth S
J Immunol, 2013-03-04;190(7):3417-26.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Peyer's patch inducer cells play a leading role in the formation of B and T cell zone architecture.
Authors: Nakagawa R, Togawa A, Nagasawa T, Nishikawa S
J Immunol, 2013-03-04;190(7):3309-18.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Chronic but not acute virus infection induces sustained expansion of myeloid suppressor cell numbers that inhibit viral-specific T cell immunity.
Authors: Norris B, Uebelhoer L, Nakaya H, Price A, Grakoui A, Pulendran B
Immunity, 2013-02-21;38(2):309-21.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
A dynamic dual role of IL-2 signaling in the two-step differentiation process of adaptive regulatory T cells.
Authors: Guo Z, Khattar M, Schroder P, Miyahara Y, Wang G, He X, Chen W, Stepkowski S
J Immunol, 2013-02-20;190(7):3153-62.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Loss of muscleblind-like 1 promotes invasive mesenchyme formation in endocardial cushions by stimulating autocrine TGF beta 3
Authors: Kathryn E LeMasters, Yotam Blech-Hermoni, Samantha J Stillwagon, Natalie A Vajda, Andrea N Ladd
BMC Developmental Biology
-
Transforming Growth Factor-Beta Promotes Rhinovirus Replication in Bronchial Epithelial Cells by Suppressing the Innate Immune Response
Authors: Nicole Bedke, David Sammut, Ben Green, Valia Kehagia, Patrick Dennison, Gisli Jenkins et al.
PLoS ONE
-
TGF-beta signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation.
Authors: Zhang N, Bevan MJ
Nat. Immunol., 2012-05-27;13(7):667-73.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta.
Authors: Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F
Nature, 2012-04-26;484(7395):514-8.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Uterine Dysfunction in Biglycan and Decorin Deficient Mice Leads to Dystocia during Parturition
Authors: Zhiping Wu, Abraham W. Aron, Elyse E. Macksoud, Renato V. Iozzo, Chi-Ming Hai, Beatrice E. Lechner
PLoS ONE
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Ha-Ras stabilization mediates pro-fibrotic signals in dermal fibroblasts
Authors: Silvia Smaldone, Jacopo Olivieri, Gabriele Luca Gusella, Gianluca Moroncini, Armando Gabrielli, Francesco Ramirez
Fibrogenesis & Tissue Repair
-
Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease.
Authors: Ma W, Han W, Greer PA, Tuder RM, Toque HA, Wang KK, Caldwell RW, Su Y
J. Clin. Invest., 2011-10-17;121(11):4548-66.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr -
Prevention of murine autoimmune diabetes by CCL22-mediated Treg recruitment to the pancreatic islets.
Authors: Montane J, Bischoff L, Soukhatcheva G, Dai DL, Hardenberg G, Levings MK, Orban PC, Kieffer TJ, Tan R, Verchere CB
J. Clin. Invest., 2011-08-01;121(0):3024.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
T Cell Surveillance of Oncogene-Induced Prostate Cancer Is Impeded by T Cell-Derived TGF-beta 1 Cytokine
Authors: Moses K. Donkor, Abira Sarkar, Peter A. Savage, Ruth A. Franklin, Linda K. Johnson, Achim A. Jungbluth et al.
Immunity
-
Effect of Fluticasone Furoate on Interleukin 6 Secretion From Adenoid Tissues in Children With Obstructive Sleep Apnea
Authors: Rania Esteitie, Janaki Emani, Shilpy Sharma, Dana L. Suskind, Fuad M. Baroody
Archives of Otolaryngology–Head & Neck Surgery
-
Cutting edge: Human regulatory T cells require IL-35 to mediate suppression and infectious tolerance.
Authors: Chaturvedi V, Collison LW, Guy CS, Workman CJ, Vignali DA
J. Immunol., 2011-05-16;186(12):6661-6.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Autocrine Transforming Growth Factor-beta 1 Promotes In Vivo Th17 Cell Differentiation
Authors: Ilona Gutcher, Moses K. Donkor, Qian Ma, Alexander Y. Rudensky, Richard A. Flavell, Ming O. Li
Immunity
-
An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition.
Authors: Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, Lindeman GJ, Shannon MF, Drew PA, Khew-Goodall Y, Goodall GJ
Mol. Biol. Cell, 2011-03-16;22(10):1686-98.
Species: Canine
Sample Types: Whole Cells
Applications: Neutralization -
Epigenetic Histone Methylation Modulates Fibrotic Gene Expression
Authors: Guangdong Sun, Marpadga A. Reddy, Hang Yuan, Linda Lanting, Mitsuo Kato, Rama Natarajan
Journal of the American Society of Nephrology
-
Anti-Asthma Simplified Herbal Medicine Intervention-induced long-lasting tolerance to allergen exposure in an asthma model is interferon-gamma, but not transforming growth factor-beta dependent.
Authors: Srivastava K, Zhang T, Yang N, Sampson H, Li XM
Clin. Exp. Allergy, 2010-11-01;40(11):1678-88.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Phagocytosis of apoptotic cells modulates mesenchymal stem cells osteogenic differentiation to enhance IL-17 and RANKL expression on CD4+ T cells.
Authors: Tso GH, Law HK, Tu W
Stem Cells, 2010-05-01;28(5):939-54.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Stromal regulation of vessel stability by MMP14 and TGFbeta
Authors: Nor E. Sounni, Kerstin Dehne, Leon van Kempen, Mikala Egeblad, Nesrine I. Affara, Ileana Cuevas et al.
Disease Models & Mechanisms
Species: Mouse, Transgenic Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: Immunohistochemistry, Immunoprecipitation, ELISA Capture -
Uptake of apoptotic DC converts immature DC into tolerogenic DC that induce differentiation of Foxp3+ Treg
Authors: Rahul Kushwah, Jing Wu, Jordan R. Oliver, George Jiang, Jinyi Zhang, Katherine A. Siminovitch et al.
European Journal of Immunology
-
Treatment failure of a TLR-7 agonist occurs due to self-regulation of acute inflammation and can be overcome by IL-10 blockade.
Authors: Lu H, Wagner WM, Gad E, Yang Y, Duan H, Amon LM, Van Denend N, Larson ER, Chang A, Tufvesson H, Disis ML
J. Immunol., 2010-03-22;184(9):5360-7.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Muscleblind-like 1 is a negative regulator of TGF-β-dependent epithelialâmesenchymal transition of atrioventricular canal endocardial cells
Authors: Natalie A. Vajda, Kyle R. Brimacombe, Kathryn E. LeMasters, Andrea N. Ladd
Developmental Dynamics
-
Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior.
Authors: Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS
Proc. Natl. Acad. Sci. U.S.A., 2010-01-26;107(6):2669-74.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Epithelial overexpression of SOCS-3 in transgenic mice exacerbates wound inflammation in the presence of elevated TGF-beta1.
Authors: Linke A, Goren I, Bosl MR, Pfeilschifter J, Frank S
J. Invest. Dermatol., 2009-11-19;130(3):866-75.
Species: Mouse
Sample Types: Cell Lysates, In Vivo
Applications: Bioassay, Neutralization -
Fractional CO2 laser: a novel therapeutic device upon photobiomodulation of tissue remodeling and cytokine pathway of tissue repair.
Authors: Prignano F, Campolmi P, Bonan P
Dermatol Ther, 2009-11-01;22(0):S8-15.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10.
Authors: Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, Geffner JR, Rabinovich GA
Nat. Immunol., 2009-08-09;10(9):981-91.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Interleukin-17-producing gammadelta+ T cells protect NOD mice from type 1 diabetes through a mechanism involving transforming growth factor-beta.
Authors: Han G, Wang R, Chen G
Immunology, 2009-08-04;129(2):197-206.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
The AP-1 transcription factor Batf controls T(H)17 differentiation.
Authors: Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, Hatton RD, Stormo GD, Weaver CT, Russell JH, Murphy TL, Murphy KM
Nature, 2009-07-05;460(7253):405-9.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23.
Authors: Aliahmadi E, Gramlich R, Grutzkau A, Hitzler M, Kruger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M
Eur. J. Immunol., 2009-05-01;39(5):1221-30.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Homeostatic role of transforming growth factor-beta in the oral cavity and esophagus of mice and its expression by mast cells in these tissues.
Authors: Vitsky A, Waire J, Pawliuk R, Bond A, Matthews D, Lacasse E, Hawes ML, Nelson C, Richards S, Piepenhagen PA, Garman RD, Andrews L, Thurberg BL, Lonning S, Ledbetter S, Ruzek MC
Am. J. Pathol., 2009-04-30;174(6):2137-49.
Applications: Neutralization -
Loss of Thy-1 inhibits alveolar development in the newborn mouse lung.
Authors: Nicola T, Hagood JS, James ML, Macewen MW, Williams TA, Hewitt MM, Schwiebert L, Bulger A, Oparil S, Chen YF, Ambalavanan N
Am. J. Physiol. Lung Cell Mol. Physiol., 2009-03-06;296(5):L738-50.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Analysis of adhesion molecules, target cells, and role of IL-2 in human FOXP3+ regulatory T cell suppressor function.
Authors: Tran DQ, Glass DD, Uzel G, Darnell DA, Spalding C, Holland SM, Shevach EM
J. Immunol., 2009-03-01;182(5):2929-38.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
CD69+ CD4+ CD25- T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1.
Authors: Han Y, Guo Q, Zhang M, Chen Z, Cao X
J. Immunol., 2009-01-01;182(1):111-20.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Activation of TGF-beta within cultured hepatocytes and in liver injury leads to intracrine signaling with expression of connective tissue growth factor
Authors: Olav A. Gressner, Birgit Lahme, Monika Siluschek, Katharina Rehbein, Jens Herrmann, Ralf Weiskirchen et al.
Journal of Cellular and Molecular Medicine
Species: Rat
Sample Types: Whole Cells, Whole Tissue
Applications: Immunohistochemistry, Immunocytochemistry -
Glioblastoma-secreted factors induce IGFBP7 and angiogenesis by modulating Smad-2-dependent TGF-beta signaling.
Authors: Pen A, Moreno MJ, Durocher Y, Deb-Rinker P, Stanimirovic DB
Oncogene, 2008-08-18;27(54):6834-44.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Molecular mechanisms of TGFbeta receptor-triggered signaling cascades rapidly induced by the calcineurin inhibitors cyclosporin A and FK506.
Authors: Akool el-S, Doller A, Babelova A, Tsalastra W, Moreth K, Schaefer L, Pfeilschifter J, Eberhardt W
J. Immunol., 2008-08-15;181(4):2831-45.
Species: Rat
Sample Types: In Vivo
Applications: Neutralization -
Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells.
Authors: Carlson ME, Hsu M, Conboy IM
Nature, 2008-06-15;454(7203):528-32.
Species: Mouse
Sample Types: Whole Tissue
Applications: Neutralization -
Circulating myeloid dendritic cells of advanced cancer patients result in reduced activation and a biased cytokine profile in invariant NKT cells.
Authors: van der Vliet HJ, Wang R, Yue SC, Koon HB, Balk SP, Exley MA
J. Immunol., 2008-06-01;180(11):7287-93.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Characterization of naturally occurring CD4+CD25+ regulatory T cells in rhesus monkeys.
Authors: Haanstra KG, van der Maas MJ, 't Hart BA, Jonker M
Transplantation, 2008-04-27;85(8):1185-92.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Neutralization -
CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells.
Authors: Perruche S, Zhang P, Liu Y, Saas P, Bluestone JA, Chen W
Nat. Med., 2008-04-27;14(5):528-35.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Regulatory T cells control dendritic cell/NK cell cross-talk in lymph nodes at the steady state by inhibiting CD4+ self-reactive T cells.
Authors: Terme M, Chaput N, Combadiere B, Ma A, Ohteki T, Zitvogel L
J. Immunol., 2008-04-01;180(7):4679-86.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Induction of an epithelial integrin alphavbeta6 in human cytomegalovirus-infected endothelial cells leads to activation of transforming growth factor-beta1 and increased collagen production.
Authors: Tabata T, Kawakatsu H, Maidji E, Sakai T, Sakai K, Fang-Hoover J, Aiba M, Sheppard D, Pereira L
Am. J. Pathol., 2008-03-18;172(4):1127-40.
Species: Human, Mink
Sample Types: Whole Cells
Applications: Flow Cytometry, Neutralization -
Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge.
Authors: He R, Oyoshi MK, Jin H, Geha RS
Proc. Natl. Acad. Sci. U.S.A., 2007-09-24;104(40):15817-22.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Neutralization -
In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid.
Authors: Saurer L, McCullough KC, Summerfield A
J. Immunol., 2007-09-15;179(6):3504-14.
Species: Porcine
Sample Types: Cell Culture Supernates, Whole Cells
Applications: ELISA Development, Neutralization -
Integrin alpha3beta1 potentiates TGFbeta-mediated induction of MMP-9 in immortalized keratinocytes.
Authors: Lamar JM, Iyer V, DiPersio CM
J. Invest. Dermatol., 2007-08-30;128(3):575-86.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Hepatitis C virus (HCV)-specific CD8+ cells produce transforming growth factor beta that can suppress HCV-specific T-cell responses.
Authors: Alatrakchi N, Graham CS, van der Vliet HJ, Sherman KE, Exley MA, Koziel MJ
J. Virol., 2007-03-21;81(11):5882-92.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1.
Authors: Biancotto A, Grivel JC, Iglehart SJ, Vanpouille C, Lisco A, Sieg SF, Debernardo R, Garate K, Rodriguez B, Margolis LB, Lederman MM
Blood, 2007-02-08;109(10):4272-9.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Luminex Development -
Long-term staphylococcal enterotoxin C1 exposure induces soluble factor-mediated immunosuppression by bovine CD4+ and CD8+ T cells.
Authors: Seo KS, Lee SU, Park YH, Davis WC, Fox LK, Bohach GA
Infect. Immun., 2006-10-09;75(1):260-9.
Species: Bovine
Sample Types: Whole Cells
Applications: Neutralization -
IGF-I and IGFBP-3 augment transforming growth factor-beta actions in human renal carcinoma cells.
Authors: Rosendahl AH, Forsberg G
Kidney Int., 2006-09-13;70(9):1584-90.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Human liver sinusoidal endothelial cells induce apoptosis in activated T cells: a role in tolerance induction.
Authors: Karrar A, Broome U, Uzunel M, Qureshi AR, Sumitran-Holgersson S
Gut, 2006-07-13;56(2):243-52.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: IHC-Fr, Neutralization -
Requirement of Smad3 for mast cell growth.
Authors: Funaba M, Nakaya K, Ikeda T, Murakami M, Tsuchida K, Sugino H
Cell. Immunol., 2006-07-12;240(1):47-52.
Species: Mouse
Sample Types: Cell Lysates, Whole Cells
Applications: Neutralization, Western Blot -
Activin A is an endogenous inhibitor of ureteric bud outgrowth from the Wolffian duct.
Authors: Maeshima A, Vaughn DA, Choi Y, Nigam SK
Dev. Biol., 2006-04-27;295(2):473-85.
Species: Rat
Sample Types: Whole Tissue
Applications: Neutralization -
Transforming growth factor beta1 regulates follistatin mRNA expression during in vitro bovine granulosa cell differentiation.
Authors: Fazzini M, Vallejo G, Colman-Lerner A, Trigo R, Campo S, Baranao JL, Saragueta PE
J. Cell. Physiol., 2006-04-01;207(1):40-8.
Species: Bovine
Sample Types: Whole Cells
Applications: Neutralization -
Tumor metastasis in an orthotopic murine model of head and neck cancer: possible role of TGF-beta 1 secreted by the tumor cells.
Authors: Dasgupta S, Bhattacharya-Chatterjee M, O&apos;Malley BW, Chatterjee SK
J. Cell. Biochem., 2006-04-01;97(5):1036-51.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr, IHC-P -
Activation of transforming growth factor-beta by the integrin alphavbeta8 delays epithelial wound closure.
Authors: Neurohr C, Nishimura SL, Sheppard D
Am. J. Respir. Cell Mol. Biol., 2006-03-30;35(2):252-9.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Innate immune responses to herpes simplex virus type 2 influence skin homing molecule expression by memory CD4+ lymphocytes.
Authors: Koelle DM, Huang J, Hensel MT, McClurkan CL
J. Virol., 2006-03-01;80(6):2863-72.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Protection against autoimmunity in nonlymphopenic hosts by CD4+ CD25+ regulatory T cells is antigen-specific and requires IL-10 and TGF-beta.
Authors: Huang X, Zhu J, Yang Y
J. Immunol., 2005-10-01;175(7):4283-91.
Species: Mouse
Sample Types:
Applications: Neutralization -
Retinal neurons regulate proliferation of postnatal progenitors and Muller glia in the rat retina via TGF beta signaling.
Authors: Close JL, Gumuscu B, Reh TA
Development, 2005-07-01;132(13):3015-26.
Species: Rat
Sample Types: In Vivo
Applications: Neutralization -
Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function.
Authors: Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, June CH, Blazar BR, Porter SB
Blood, 2004-09-16;105(2):750-8.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Neutralization -
Aberration of CCR7 CD8 memory T cells from patients with systemic lupus erythematosus: an inducer of T helper type 2 bias of CD4 T cells.
Authors: Sen Y, Chunsong H, Baojun H, Linjie Z, Qun L, San J, Qiuping Z, Junyan L, Zhang X, Jinquan T
Immunology, 2004-06-01;112(2):274-89.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGF-beta 1+CD4+ T regulatory cell infiltrates.
Authors: Torrealba JR, Katayama M, Fechner JH, Jankowska-Gan E, Kusaka S, Schultz JM, Hu H, Hamawy MM, Jonker M, Wubben J, Doxiadis G, Bontrop R, Burlingham WJ, Knechtle SJ
J. Immunol., 2004-05-01;172(9):5753-64.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC-P, Western Blot -
P311 binds to the latency associated protein and downregulates the expression of TGF-beta1 and TGF-beta2.
Authors: Paliwal S, Shi J, Dhru U, Zhou Y, Schuger L
Biochem. Biophys. Res. Commun., 2004-03-19;315(4):1104-9.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures.
Authors: Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR, Porter SB
Blood, 2004-03-18;104(2):453-61.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice.
Authors: Kitani A, Fuss I, Pedersen A, Nawata H
J. Immunol., 2004-01-15;172(2):834-42.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Neutralization -
IL-15 and cognate antigen successfully expand de novo-induced human antigen-specific regulatory CD4+ T cells that require antigen-specific activation for suppression.
Authors: Koenen HJ, Fasse E, Joosten I
J. Immunol., 2003-12-15;171(12):6431-41.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3.
Authors: Chen, WanJun, Jin, Wenwen, Hardegen, Neil, Lei, Ke-Jian, Li, Li, Marinos, Nancy, McGrady, George, Wahl, Sharon M
J Exp Med, 2003-12-15;198(12):1875-86.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Marked airway eosinophilia prevents development of airway hyper-responsiveness during an allergic response in IL-5 transgenic mice.
Authors: Kobayashi T, Iijima K, Kita H
J. Immunol., 2003-06-01;170(11):5756-63.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Immunosuppression in hamsters with progressive visceral leishmaniasis is associated with an impairment of protein kinase C activity in their lymphocytes that can be partially reversed by okadaic acid or anti-transforming growth factor beta antibody.
Authors: Mookerjee A, Sen PC, Ghose AC
Infect. Immun., 2003-05-01;71(5):2439-46.
Species: Hamster
Sample Types: Whole Cells
Applications: Neutralization -
The cxc chemokine cCAF stimulates differentiation of fibroblasts into myofibroblasts and accelerates wound closure.
Authors: Feugate JE, 2019, Li Q, Wong L, Martins-Green M
e0216795, 2002-01-07;156(1):161-72.
Species: Chicken
Sample Types: Whole Cells
Applications: Neutralization -
Fortilin interacts with TGF-beta 1 and prevents TGF-beta receptor activation
Authors: Decha Pinkaew, Erik Martinez-Hackert, Wei Jia, Matthew D. King, Fei Miao, Nicole R. Enger et al.
Communications Biology
-
Stimulation of tolerogenic dendritic cells using dexamethasone and 1,25‑dihydroxyvitamin D3 represses autologous T cell activation and chondrocyte inflammation
Authors: Gaoyuan Wang, Junqiang Zhang, Yuan Fang, Wei Cao, Bin Xu, Xiaoyu Chen
Experimental and Therapeutic Medicine
-
Integration of TGF-beta -induced Smad signaling in the insulin-induced transcriptional response in endothelial cells
Authors: Erine H. Budi, Steven Hoffman, Shaojian Gao, Ying E. Zhang, Rik Derynck
Scientific Reports
-
Age-dependent NK cell dysfunctions in severe COVID-19 patients
Authors: Cinzia Fionda, Silvia Ruggeri, Giuseppe Sciumè, Mattia Laffranchi, Isabella Quinti, Cinzia Milito et al.
Frontiers in Immunology
-
Essential role of microglial transforming growth factor-beta 1 in antidepressant actions of (R)-ketamine and the novel antidepressant TGF-beta 1
Authors: Kai Zhang, Chun Yang, Lijia Chang, Akemi Sakamoto, Toru Suzuki, Yuko Fujita et al.
Translational Psychiatry
FAQs
-
Can the TGF-beta 1, 2, 3 Antibody, Cat# MAB1835, neutralize the activity of all three TGF-beta proteins?
Yes, this antibody can neutralize the activity of TGF-beta 1, 2, and 3 proteins.
Reviews for TGF-beta 1, 2, 3 Antibody
Average Rating: 4.4 (Based on 12 Reviews)
Have you used TGF-beta 1, 2, 3 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: