Human Luminex® Discovery Assay
Build your own Luminex Assay with our Luminex Assay Customization Tool.
U.S. orders usually ship in 7-10 business days
Select the "Bulk Orders" button to request additional sizes or formulations.
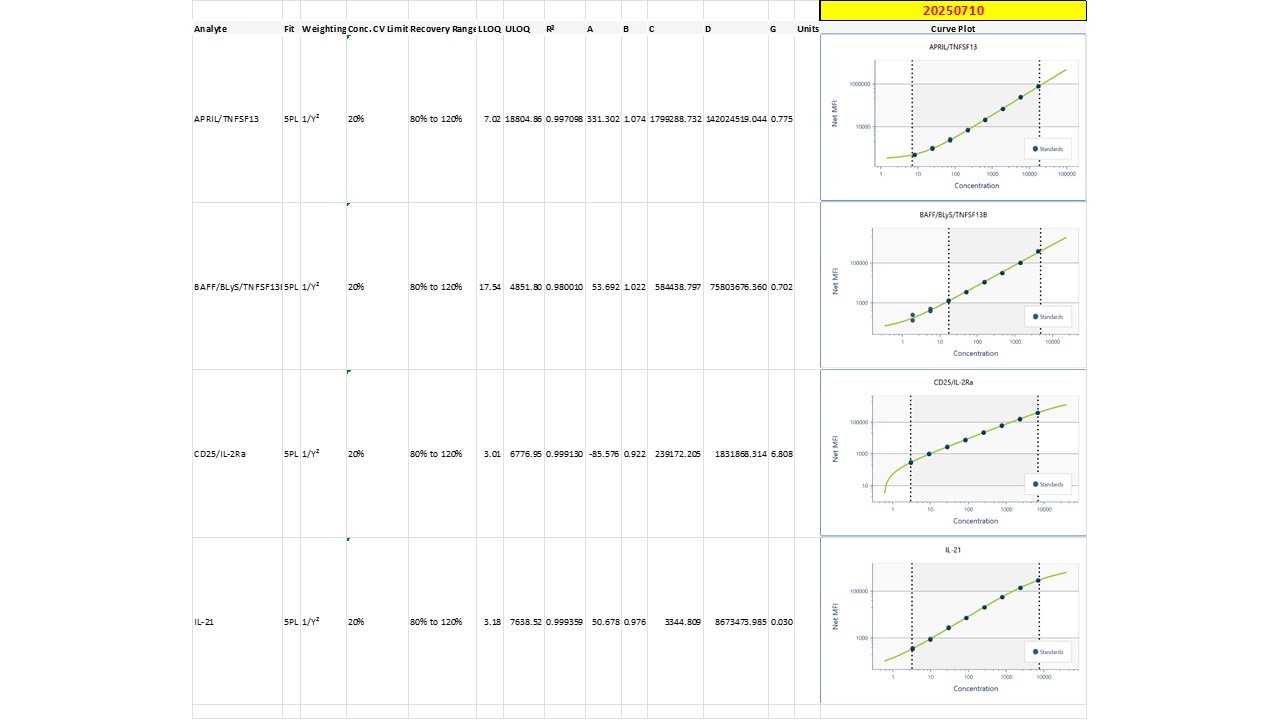
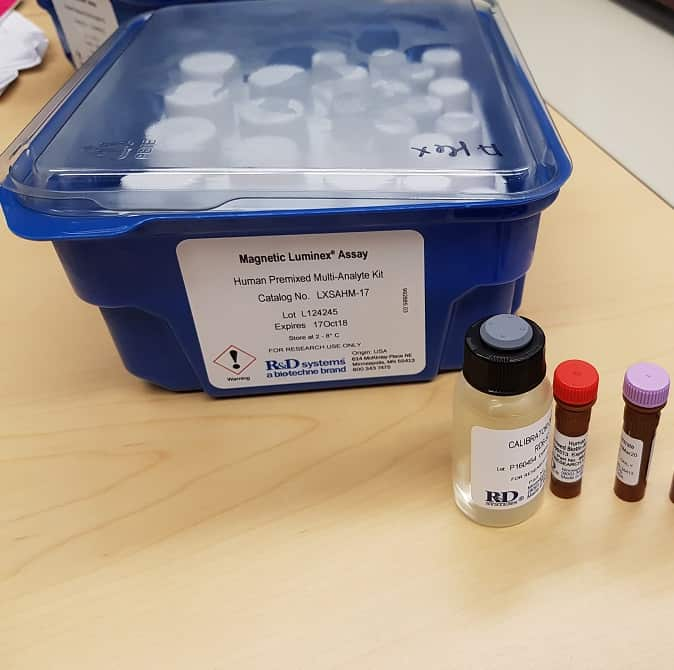
Human Luminex® Discovery Assay Summary
| Assay Type | Magnetic bead-based multiplex assay for the Luminex® platform |
| Format | 1 x 96-well microplate and Magnetic antibody-coated beads |
| Species | Human |
| Analytes Detected | Please see analyte list in assay customization tool below. |
| Performance Validation | Luminex Assays are validated for use with cell culture supernatants, plasma and serum. All Luminex assays are tested for sensitivity, intra-assay precision, inter-assay precision and to ensure assay linearity for validated sample types. Antibody pairs are selected and tested to confirm the parallel detection of natural and recombinant standard protein and to ensure the accurate determination of target analytes within biological samples. Assays for each target analyte are screened against all target analytes to confirm low antibody cross-reactivity. |
Make your selections below to configure your assay
Not finding your analyte of interest? View our complete Luminex offering in the full version of our Luminex Assay Customization Tool. We also offer Custom Luminex Services.
Magnetic Luminex Assays are the most flexible bead-based multiplex assays that we offer. They allow up to 50 user-defined target analytes to be simultaneously profiled using cell culture supernates, serum, or plasma samples.
- Simultaneously profile up to 50 analytes of your choice in one sample
- Choose from a wide menu of analytes, including many unique analytes
- Requires a small sample volume (<50 µL)
- Run your assay in just 3-4 hours
- Mass-calibrated standards for consistent results with every new lot of material
- Enough reagents for one 96-well plate
- Quick turn-around time: Kits are typically shipped within 5-10 days of ordering.*
Please allow additional time for international orders.
- Premixed cocktail of antibody-coated Magnetic beads
- Premixed cocktail of biotinylated detection antibody
- Standard Cocktail(s)
- Bead Diluent
- Biotin Antibody Diluent
- Standard/Sample Diluent
- Wash Buffer
- Streptavidin-PE
- One flat-bottom 96-well Microplate
- Foil Plate Sealers (4)
- Mixing Bottle
- Certificate of Analysis**
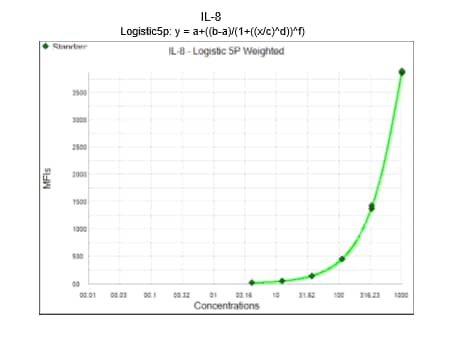
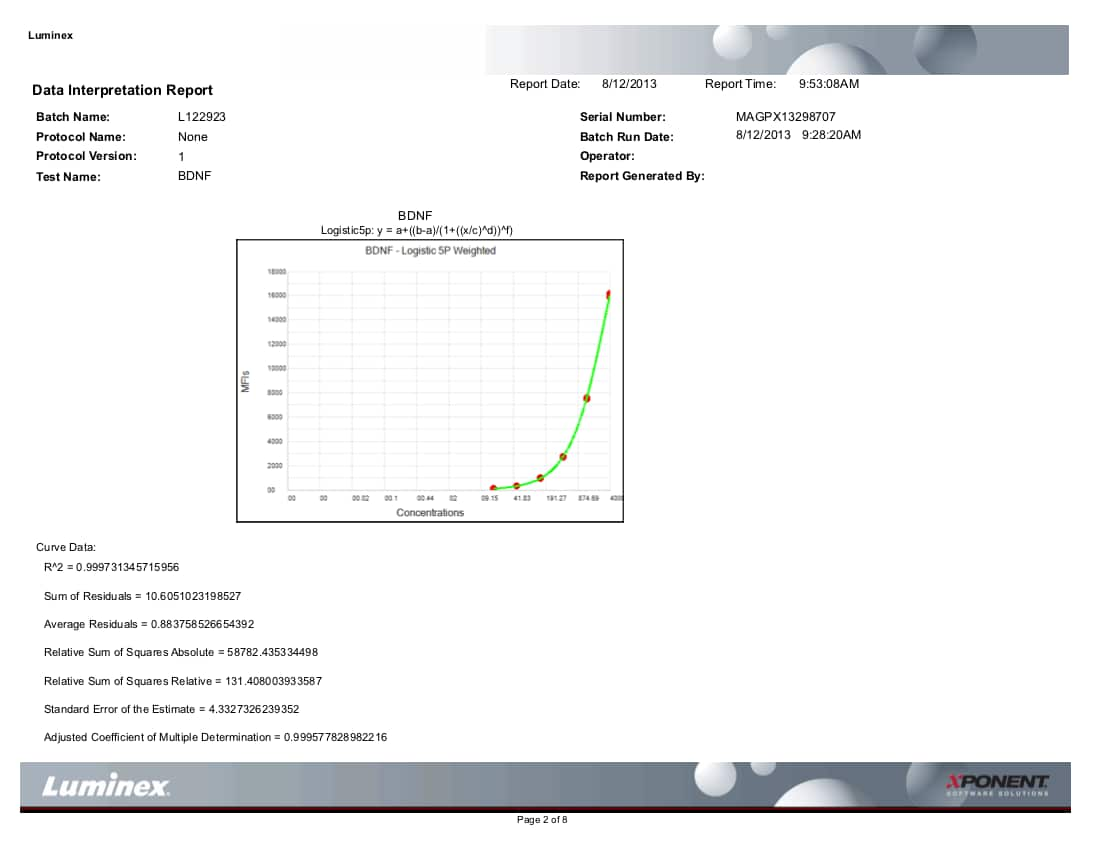
**A standard curve must be generated each time an assay is run, utilizing values from the Certificate of Analysis provided with each Luminex Assay.
Assays for the Luminex platform are offered as High Performance Assays or Assays. The Luminex High Performance Assays are fully validated panels with a focused selection of analytes. They are available in We Mix, You Mix, and Predetermined formats. The Luminex Assays allows for the maximum number of analytes in a multiplex and is supplied as a premixed kit. View a table comparing the features and benefits of these bead-based multiplex assays.
Product Datasheets
Citations for Human Luminex® Discovery Assay
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
678
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Associations of serum TNF-?, IL-8, and IL-18 levels with the clinical symptoms in acute schizophrenia: a cross-sectional study
Authors: Xu, L;Yang, H;Chen, W;Yang, M;Zhang, J;Zhang, X;Tang, X;
BMC psychiatry
Species: Human
Sample Types: Serum
-
Royal Jelly Extracellular Vesicles Enhance Diabetic Wound Healing via Inflammation Modulation, Fibroblast Migration, and Angiogenesis
Authors: Tsai, YY;Chang, LS;Lan, CW;Chen, YJ;Yang, JH;
International journal of nanomedicine
Species: Human
Sample Types: Cell Culture Supernates
-
Macrophage Migration Inhibitory Factor and Post-Discharge Inflammatory Profiles in Severe COVID-19: A Prospective Observational Study from Romania
Authors: László, N;M?rginean, C;Mátyás, BB;Man, CA;Nagy, EE;Jimborean, G;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
A novel chromen-based small molecule induces apoptosis and modulates cellular response to triple-negative breast cancer
Authors: Tuah, B;Fosu, K;Prah, DA;Hodogbe, BKY;Serwaa, A;Amon, JNK;Ayine-Tora, DM;Amewu, RK;Sarpong, KAN;Aikins, AR;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Gut commensal microbiota drive tailored macrophage responses
Authors: Jones, J;García-Martínez, KY;Lee, YY;Rhee, M;Nath, RR;Takahashi, S;Grodner, B;De Vlaminck, I;Leifer, CA;Brito, IL;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Measuring the Senescence-Associated Secretory Phenotype
Authors: Karras, A;Lioulios, G;Kantartzi, K;Fylaktou, A;Panagoutsos, S;Stangou, M;
Biomedicines
Species: Human
Sample Types: Cell Culture Supernates
-
Multivariate protein landscape of host response in hospitalised patients with suspected infection in the emergency department
Authors: Sinha, P;Spicer, AB;Bhavani, S;López-Espina, C;Watson, GL;Bhargava, A;Schmalz, L;Khan, S;Sims, MD;Palagiri, AV;Dagan, A;Iyer, KV;Crisp, MJ;DeMarco, C;Halalau, A;Maddens, N;Kurtzman, N;Sarma, D;Gosai, F;Syed, A;Azad, S;Espinosa, A;Davila, F;Davila, H;Evans, NS;Smith, S;Reddy, B;Verhoef, PA;Churpek, MM;
Nature communications
Species: Human
Sample Types: Plasma
-
Metabolic dysfunction impairs Mycobacterium tuberculosis-specific cytokine and chemokine responses in latent tuberculosis and type 2 diabetes mellitus
Authors: Ssekamatte, P;Sitenda, D;Nabatanzi, R;Nkurunungi, G;Nakibuule, M;Kibirige, D;Kyazze, AP;Kateete, DP;Bagaya, BS;Sande, OJ;van Crevel, R;Cose, S;Biraro, IA;
Scientific reports
Species: Human
Sample Types: Plasma
-
Prakriti elucidates the inter-individual variability in coronary artery disease risk-predicting biomarkers: A tertiary care hospital-based case control study
Authors: Dua, P;Prasher, B;Seth, S;Pandey, S;Maulik, SK;Reeta, KH;
Journal of Ayurveda and integrative medicine
Species: Human
Sample Types: Serum, Plasma
-
Microvascular, Biochemical, and Clinical Impact of Hyperbaric Oxygen Therapy in Recalcitrant Diabetic Foot Ulcers
Authors: Martins-Mendes, D;Costa, R;Rodrigues, I;Camacho, Ó;Coelho, PB;Paixão-Dias, V;Luís, C;Pereira, AC;Fernandes, R;Lima, J;Soares, R;
Cells
Species: Human
Sample Types: Serum
-
Development of COVID-19 Vaccine Candidates Using Attenuated Recombinant Vesicular Stomatitis Virus Vectors with M Protein Mutations
Authors: Chang, M;Huang, H;Yue, M;Jiang, Y;Yan, S;Chen, Y;Wu, W;Gao, Y;Fang, M;Yuan, Q;Xiong, H;Zhang, T;
Viruses
Species: Human
Sample Types: Cell Culture Supernates
-
Circulating Biomarkers as Potential Risk Factors for Inguinal Hernia
Authors: Baldini, E;Sorrenti, S;Lori, E;Palla, L;Cardarelli, S;Pironi, D;Tripodi, D;Pavan, A;Fakeri, A;Cobo, V;Pellegrini, C;Nardi, P;Rinaldi, V;Ulisse, S;Palumbo, P;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Metabolic imprinting drives epithelial memory during mucosal fungal infection
Authors: Sekar, J;Solis, NV;Miao, J;Millet, N;Tom, B;Quintanilla, D;Pellon, A;Moyes, DL;Gogos, JA;Rossiter, HB;Filler, SG;Netea, MG;Yee, JK;Swidergall, M;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Association between aqueous humor cytokines and posterior capsular opacification in patients with high myopia
Authors: Cheng, L;Xie, S;Jin, H;Lv, Y;Zhu, B;Zou, H;Li, B;Jin, P;
Cytokine
Species: Human
Sample Types: Aqueous Humor
-
Serum IL-18/IL-13 Ratio Predicts Super Response to Secukinumab in Patients with Psoriasis
Authors: Ziolkowska-Banasik, D;Pastuszczak, M;Zawadzinska-Halat, K;Hadas, E;Bozek, A;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
Mycobacterium tuberculosis Infection of Retinal Endothelial Cells Induces Interferon Signaling Activation: Insights Into Tubercular Retinal Vasculitis
Authors: Putera, I;Swagemakers, SMA;Nagtzaam, NMA;van Holten-Neelen, C;La Distia Nora, R;de Steenwinkel, JEM;Rombach, SM;van Hagen, PM;Dik, WA;
Investigative ophthalmology & visual science
Species: Human
Sample Types: Cell Culture Supernates
-
Interferon Gamma and Tumor Necrosis Factor Alpha Are Inflammatory Biomarkers for Major Adverse Cardiovascular Events in Patients with Peripheral Artery Disease
Authors: Li, B;Lindner, E;Abuhalimeh, R;Shaikh, F;Younes, H;Abuhalimeh, B;Zamzam, A;Abdin, R;Qadura, M;
Biomedicines
Species: Human
Sample Types: Plasma
-
HE4 as a Prognostic Biomarker of Major Adverse Cardiovascular Events in Patients with Abdominal Aortic Aneurysm: A Canadian Prospective Observational Study
Authors: Khan, H;Zamzam, A;Shaikh, F;Mamdani, M;Saposnik, G;Qadura, M;
Biomedicines
Species: Human
Sample Types: Plasma
-
Matrix Metalloproteinases 7 and 10 Are Prognostic Biomarkers for Systemic Cardiovascular Risk in Individuals with Peripheral Artery Disease
Authors: Li, B;Shaikh, F;Younes, H;Abuhalimeh, B;Zamzam, A;Abdin, R;Qadura, M;
Biomolecules
Species: Human
Sample Types: Plasma
-
Training in normobaric hypoxia induces hematological changes that affect iron metabolism and immunity
Authors: Nolte, S;Malhan, D;Klemmer, A;Kastner, T;Walter, N;Fleckenstein, D;Keck, J;Klügel, S;Maier, C;Gebhardt, K;Stauber, T;Relógio, A;Krüger, K;Hollander, K;
Scientific reports
Species: Human
Sample Types: Plasma
-
Biomarkers of Calcification, Endothelial Injury, and Platelet-Endothelial Interaction in Patients with Aortic Valve Stenosis
Authors: Ba?ka, P;M?cka, K;Berger?Kucza, A;Wrona?Kolasa, K;Rybicka-Musialik, A;Nowak, B;El?bieciak, M;Mizia?Szubryt, M;Wróbel, W;Francuz, T;Lelek, M;Kosowska, A;Garczorz, W;Bochenek, T;Swinarew, A;Paluch, J;Wybraniec, M;Mizia?Stec, K;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
Prospective evaluation of soluble CD14 as a biomarker following five aflibercept treatments in diabetic macular edema
Authors: Lee, H;Lee, M;Kim, N;Kim, N;Moon, D;Son, C;Chung, H;
Scientific reports
Species: Human
Sample Types: Aqueous Humor
-
IL-16 production is a mechanism of resistance to BTK inhibitors and R-CHOP in lymphomas
Authors: Arribas, AJ;Guidetti, F;Cannas, E;Cascione, L;Napoli, S;Sartori, G;Fuzio, F;Pesenti, E;Tarantelli, C;Spriano, F;Zucchetto, A;Rossi, FM;Bruscaggin, A;Rinaldi, A;Castro de Moura, M;Jovic, S;Raimondi, A;Pittau, RB;Terzi di Bergamo, L;Ye, X;Stathis, A;Ben-David, Y;Pan-Hammarström, Q;Simonetta, F;Stussi, G;Zucca, E;Gattei, V;Brown, JR;Esteller, M;Rossi, D;Bertoni, F;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Serum
-
Cell-free mitochondrial DNA may predict the risk of post-operative complications and outcomes in surgical aortic valve replacement patients
Authors: Hyslop, K;Ki, KK;Naidoo, R;O'Brien, D;Prabhu, A;Gill, D;Anstey, C;Rapchuk, IL;McDonald, CI;Marshall, L;Pearse, I;Fraser, JF;Suen, JY;Passmore, MR;
Scientific reports
Species: Human
Sample Types: Plasma
-
Host biomarkers and parasite biomass are associated with severe malaria in Mozambican children: a case-control study
Authors: Varo, R;Sitoe, A;Madrid, L;Aide, P;Cuamba, I;Cossa, A;Jairoce, C;Barrios, D;Martiáñez-Vendrell, X;Balanza, N;Cisteró, P;Ouchi, D;Quintó, L;Crowley, VM;Gupta, H;Kain, KC;Mayor, A;Bassat, Q;
Scientific reports
Species: Human
Sample Types: Plasma
-
Characterization of the gut microbiota in different immunological responses among PLWH
Authors: Guo, Y;Tang, G;Wang, Z;Chu, Q;Zhang, X;Xu, X;Fan, Y;
Scientific reports
Species: Human
Sample Types: Plasma
-
Determinants of retinopathy and short-term neurological outcomes after cerebral malaria
Authors: Bodeau-Livinec, F;Aubouy, A;Besnard, I;Angendu, K;Brisset, J;Royo, J;Kinkpe, E;Ayedadjou, L;Mowendabeka, A;Lathière, T;Boumediène, F;Dossou-Dagba, I;Alao, J;Faucher, JF;
Scientific reports
Species: Human
Sample Types: Plasma
-
A 23-Plex Cytokine/Chemokine Analysis Identifies TNFRII, MMP-8, and sIL-1RII as Potential Biomarkers for Systemic Sclerosis
Authors: Perricone, C;Cafaro, G;Pozzolo, RD;Bruno, L;Sasso, N;Cecchetti, R;Antonucci, M;Topini, F;Bistoni, O;Mecocci, P;Gerli, R;Bartoloni, E;
Biomedicines
Species: Human
Sample Types: Serum
-
Plasma biomarkers in patients with familial cavernous malformation and their first-degree relatives: a cross-sectional study
Authors: Li, C;Huang, S;Li, Q;Zhuo, L;Kang, Y;Liu, P;Huang, W;Ma, K;Lin, X;Zhuang, W;Chen, D;Wang, H;Yan, L;Wang, D;Lin, Y;Kang, D;Lin, F;
Scientific reports
Species: Human
Sample Types: Plasma
-
Modulating IL-21-driven B cell responses in idiopathic inflammatory myopathies via inhibition of the JAK/STAT pathway
Authors: Merino-Vico, A;Kocyigit, M;Frazzei, G;Landman, L;Boon, L;van Leeuwen, EM;Lundberg, IE;van der Kooi, AJ;Raaphorst, J;van Hamburg, JP;Tas, SW;
Arthritis research & therapy
Species: Human
Sample Types: Cell Culture Supernates
-
Soluble tumour necrosis factor receptor 1 predicts hospitalization in children and young adults with dengue virus infection in the Philippines
Authors: Li, V;Mishra, H;Ngai, M;Crowley, VM;Tran, V;Painaga, MSS;Gaite, JY;Hamilton, P;Conroy, AL;Kain, KC;Hawkes, MT;
Cytokine
Species: Human
Sample Types: Plasma
-
Orbital Clinicopathological Differences in Thyroid Eye Disease: An Analysis of Cytokines With Histopathological and Clinical Correlation
Authors: Li, Y;Tang, J;Jing, G;Li, Y;Ma, R;Kang, X;Rong, L;Liu, W;Yao, L;Lv, X;Deng, A;Wu, W;Yang, X;
Investigative ophthalmology & visual science
Species: Human
Sample Types: Tissue Homogenates
-
Cytomegalovirus reactivation and acute and chronic complications in children with cerebral malaria: a prospective cohort study
Authors: Mayhew, JA;Witten, AJ;Bond, CA;Opoka, RO;Bangirana, P;Conroy, AL;Hernandez-Alvarado, N;Schleiss, MR;John, CC;
Malaria journal
Species: Human
Sample Types: Plasma
-
Pre-Concentration Freezing Alters the Composition of Mesenchymal Stem/Stromal Cell-Conditioned Medium
Authors: Cadelano, F;Giannasi, C;Gualerzi, A;Gerli, M;Niada, S;Della Morte, E;Brini, AT;
Biology
Species: Human
Sample Types: Cell Culture Supernates
-
Inflammatory responses revealed through HIV infection of microglia-containing cerebral organoids
Authors: Narasipura, SD;Zayas, JP;Ash, MK;Reyes, AF;Shull, T;Gambut, S;Szczerkowski, JLA;McKee, C;Schneider, JR;Lorenzo-Redondo, R;Al-Harthi, L;Mamede, JI;
Journal of neuroinflammation
Species: Human
Sample Types: Cell Culture Supernates
-
Chronic alcohol consumption enhances the differentiation capacity of hematopoietic stem and progenitor cells into osteoclast precursors
Authors: Hemati, H;Blanton, MB;Koura, J;Khadka, R;Grant, KA;Messaoudi, I;
bioRxiv : the preprint server for biology
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Cell Culture Supernates
-
The role of plasma inflammatory markers in late-life depression and conversion to dementia: a 3-year follow-up study
Authors: Bocharova, M;Borza, T;Watne, LO;Engedal, K;O'Brien, JT;Selbæk, G;Idland, AV;Hodsoll, J;Young, AH;Aarsland, D;
Molecular psychiatry
Species: Human
Sample Types: Serum
-
Senescence-related cytokine levels are associated with HIV-1 serostatus and persistence.
Authors: Li, Y;Soto-Ramirez, ZN;Roscher, J;Medvec, T;Alaoui-El-Azher, M;Piazza, P;Chen, Y;Sluis-Cremer, N;Rinaldo, C;Macatangay, BJ;
medRxiv : the preprint server for health sciences
Species: Human
Sample Types: Plasma
-
Comparative Analysis of Salivary Cytokine Profiles and Oral Microbial Composition in Caries-Active and Caries-Free Children
Authors: Karched, M;Alyahya, A;Khalaf, ME;Bhardwaj, RG;Al-Sane, M;Qudeimat, MA;
Journal of dentistry
Species: Human
Sample Types: Saliva
-
Exopolysaccharides from Bifidobacterium longum subsp. infantis and Bifidobacterium adolescentis modulate Toll-like receptor signaling
Authors: Akkerman, R;Oerlemans, MMP;Ferrari, M;Fernández-Lainez, C;Walvoort, MTC;de Vos, P;
Carbohydrate polymers
Species: Human
Sample Types: Cell Culture Supernates
-
Intrapericardial injection of hydrogels with ASC and their secretome to treat dilated cardiomyopathies
Authors: Liguori, TTA;Liguori, GR;Sinkunas, V;Correia, CJ;Dos Santos Coutinho E Silva, R;Zanoni, FL;Aiello, VD;Harmsen, MC;Moreira, LFP;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Associations of the levels of adipokines and cytokines in individual follicles with in vitro fertilization outcomes in women with different ovarian reserves
Authors: Li, X;Li, C;Yang, J;Lin, M;Zhou, X;Su, Z;Zhang, Y;Li, X;Chen, X;
Journal of ovarian research
Species: Human
Sample Types: Follicular Fluid
-
Soluble Herpes Virus Entry Mediator and Type II/III Interferons Are Upregulated in Primary Biliary Cholangitis
Authors: Chung, Y;Tsou, HLP;Heneghan, MA;Chokshi, S;Riva, A;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Matched serum- and urine-derived biomarkers of interstitial cystitis/bladder pain syndrome
Authors: Kuret, T;Sterle, I;Romih, R;Verani?, P;
PloS one
Species: Human
Sample Types: Serum, Urine
-
Lactobacillus crispatus S-layer proteins modulate innate immune response and inflammation in the lower female reproductive tract
Authors: Decout, A;Krasias, I;Roberts, L;Molina, B;Charenton, C;Romero, D;Tee, Q;Marchesi, J;Ng, S;Sykes, L;Bennett, P;MacIntyre, D;
Nature communications
Species: Human
Sample Types: Cervical Fluid
-
Exploring potential multiple molecular biomarkers that predict treatment response in patients with lupus nephritis
Authors: Park, D;Joo, Y;Nam, E;Lee, J;Bang, S;Lee, H;Bae, S;
Scientific reports
Species: Human
Sample Types: Serum, Urine
-
A pilot study of a gratitude journaling intervention to enhance spiritual well-being and exercise self-efficacy in Black breast cancer survivors
Authors: Cousin, L;Braithwaite, D;Anton, S;Zhang, Z;Lee, J;Leewenburgh, C;Lyon, D;
BMC psychiatry
Species: Human
Sample Types: Plasma
-
Exogenous binding immunoglobulin protein (BiP) enhance immune regulatory phenotype in ex-vivo Mtb infected PBMCs stratified based on QuantiFERON response
Authors: Motaung, B;Snyders, C;Malherbe, S;Gutschmidt, A;van Rensburg, I;Loxton, AG;
Cytokine
Species: Human
Sample Types: Cell Culture Supernates
-
Therapeutic interventions targeting enteropathy in severe acute malnutrition modulate systemic and vascular inflammation and epithelial regeneration
Authors: Sturgeon, JP;Mutasa, K;Bwakura-Dangarembizi, M;Amadi, B;Ngosa, D;Dzikiti, A;Chandwe, K;Besa, E;Mutasa, B;Murch, SH;Hill, S;Playford, RJ;VanBuskirk, K;Kelly, P;Prendergast, AJ;TAME Trial Team, ;
EBioMedicine
Species: Human
Sample Types: Plasma
-
Describing Biological Vulnerability in Small, Vulnerable Newborns in Urban Burkina Faso (DenBalo): Gut Microbiota, Immune System, and Breastmilk Assembly
Authors: Ouédraogo, LO;Deng, L;Ouattara, CA;Compaoré, A;Ouédraogo, M;Argaw, A;Lachat, C;Houpt, ER;Saidi, Q;Haerynck, F;Sonnenburg, J;Azad, MB;Tavernier, SJ;Bastos-Moreira, Y;Toe, LC;Dailey-Chwalibóg, T;
Nutrients
Species: Human
Sample Types: Vaginal Swab
-
Mitochondrial metabolism and epigenetic crosstalk drive the SASP
Authors: Passos, J;Martini, H;Birch, J;Marques, F;Victorelli, S;Lagnado, A;Pirius, N;Franco, A;Lee, G;Han, Y;Rowsey, J;Gaspar-Maia, A;Havas, A;Murad, R;Lei, X;Porritt, R;Maddocks, O;Jurk, D;Khosla, S;Adams, P;
Research square
Species: Human
Sample Types: Cell Culture Supernates
-
Impact of suspected preterm labour in foetal cardiovascular and metabolic programming: a prospective cohort study protocol
Authors: Abadía-Cuchí, N;Clavero-Adell, M;González, J;Medel-Martinez, A;Fabre, M;Ayerza-Casas, A;Youssef, L;Lerma-Irureta, J;Maestro-Quibus, P;Rodriguez-Calvo, J;Ruiz-Martinez, S;Lerma, D;Schoolermer, J;Oros, D;Paules, C;
BMJ open
Species: Human
Sample Types: Serum
-
Variations in inflammatory regulators in male patients with chronic schizophrenia associated with psychopathology and cognitive deficits
Authors: Guo, T;Chen, L;Luan, L;Yang, M;Zhang, X;Yang, H;
BMC psychiatry
Species: Human
Sample Types: Serum
-
Age-related loss of intestinal barrier integrity plays an integral role in thymic involution and T cell ageing
Authors: Conway, J;De Jong, EN;White, AJ;Dugan, B;Rees, NP;Parnell, SM;Lamberte, LE;Sharma-Oates, A;Sullivan, J;Mauro, C;van Schaik, W;Anderson, G;Bowdish, DME;Duggal, NA;
Aging cell
Species: Human
Sample Types: Serum
-
Deciphering influence of donor age on adipose-derived stem cells: in vitro paracrine function and angiogenic potential
Authors: Trotzier, C;Bellanger, C;Abdessadeq, H;Delannoy, P;Mojallal, A;Auxenfans, C;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Clinical and biologic profiles of patients with acute respiratory distress syndrome by prevalence of chronic obstructive pulmonary disease or emphysema; a cohort study
Authors: Nath, S;Qurashi, H;Kitsios, GD;Bain, W;Aneis, H;Suber, T;Prendergast, N;Hensley, M;Schaefer, C;Zhang, Y;Bon, J;McVerry, BJ;Evankovich, J;Shah, FA;
Respiratory research
Species: Human
Sample Types: Serum
-
Effect of Statins and Renin-Angiotensin-Aldosterone System Inhibitors on IL-6 Levels in COVID-19 Patients
Authors: Pereckaite, L;Vaguliene, N;Vitkauskaite, A;Vitkauskiene, A;Urboniene, D;
Journal of clinical medicine
Species: Human
Sample Types: Plasma
-
Elevated Platelet Aggregation in Patients with Ovarian Cancer: More than Just Increased Platelet Count
Authors: Isingizwe, ZR;Meelheim, BA;Benbrook, DM;
Cancers
Species: Human
Sample Types: Plasma
-
Dynamic reciprocal interactions between activated T cells and tumor associated macrophages drive macrophage reprogramming and proinflammatory T cell migration within prostate tumor models
Authors: Heninger, E;Breneman, MT;Recchia, EE;Kerr, SC;Dogru, RE;Sharifi, MN;LeBeau, AM;Kosoff, D;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Host Response Markers of Inflammation and Endothelial Activation Associated with COVID-19 Severity and Mortality: A GeoSentinel Prospective Observational Cohort
Authors: Weckman, AM;Guagliardo, SAJ;Crowley, VM;Moro, L;Piubelli, C;Ursini, T;van Ierssel, SH;Gobbi, FG;Emetulu, H;Rizwan, A;Angelo, KM;Licitra, C;Connor, BA;Barkati, S;Ngai, M;Zhong, K;Huits, R;Hamer, DH;Libman, M;Kain, KC;
Viruses
Species: Human
Sample Types: Plasma
-
Modulation of IFN-? induced macrophage inflammatory responses via indomethacin-loaded NLCs for OA management
Authors: Martínez-Borrajo, R;Rouco, H;Virzì, NF;Diaz-Rodriguez, P;Landin, M;
International journal of pharmaceutics
Species: Human
Sample Types: Cell Culture Supernates
-
The assessment of usefulness of cytokines and other soluble mediators as the predictors of sequalae development in various forms of tick-borne encephalitis (TBE)
Authors: Czupryna, P;Moniuszko-Malinowska, A;Trojan, G;Adamczuk, J;Martonik, D;Parfieniuk-Kowerda, A;Kruszewska, E;Giecko, M;Grygorczuk, S;
Cytokine
Species: Human
Sample Types: Serum
-
HIV reservoir and premature aging: risk factors for aging-associated illness in adolescents and young adults with perinatally acquired HIV
Authors: Petrara, MR;Ruffoni, E;Carmona, F;Cavallari, I;Zampieri, S;Morello, M;Del Bianco, P;Rampon, O;Cotugno, N;Palma, P;Rossi, P;Giaquinto, C;Giunco, S;De Rossi, A;
PLoS pathogens
Species: Human
Sample Types: Plasma
-
Evaluation of the Effect of mRNA and Inactivated SARS-CoV-2 Vaccines on the Levels of Cytokines IL-2, IFN-?, and Anti-RBD Spike SARS-CoV-2 Antibodies in People Living with HIV (PLHIV)
Authors: Amanah, A;Ariyanto, IA;Bela, B;Primanagara, R;Sudarmono, P;
Biomedicines
Species: Human
Sample Types: Plasma
-
Anisakis extracellular vesicles elicit immunomodulatory and potentially tumorigenic outcomes on human intestinal organoids
Authors: Bellini, I;Scribano, D;Ambrosi, C;Chiovoloni, C;Rondón, S;Pronio, A;Palamara, AT;Pietrantoni, A;Kashkanova, A;Sandoghdar, V;D'Amelio, S;Cavallero, S;
Parasites & vectors
Species: Human
Sample Types: Cell Culture Supernates
-
Cerebrospinal fluid soluble CD27 is a sensitive biomarker of inflammation in autoimmune encephalitis
Authors: Cobanovic, S;Blaabjerg, M;Illes, Z;Nissen, MS;Nielsen, CH;Kondziella, D;Buhelt, S;Mahler, MR;Sellebjerg, F;Romme Christensen, J;
Journal of the neurological sciences
Species: Human
Sample Types: CSF
-
Long-term cellular immunity of vaccines for Zaire Ebola Virus Diseases
Authors: Wiedemann, A;Lhomme, E;Huchon, M;Foucat, E;Bérerd-Camara, M;Guillaumat, L;Yaradouno, M;Tambalou, J;Rodrigues, C;Ribeiro, A;Béavogui, AH;Lacabaratz, C;Thiébaut, R;Richert, L;Lévy, Y;Prevac study team, ;
Nature communications
Species: Human
Sample Types: Serum
-
The role of cytokines in acute and chronic postsurgical pain after major musculoskeletal surgeries in a quaternary pediatric center
Authors: Chidambaran, V;Duan, Q;Pilipenko, V;Glynn, SM;Sproles, A;Martin, LJ;Lacagnina, MJ;King, CD;Ding, L;
Brain, behavior, and immunity
Species: Human
Sample Types: Plasma
-
The Fibrotic Phenotype of Human Precision-Cut Lung Slices Is Maintained after Cryopreservation
Authors: Marimoutou, M;Patel, V;Kim, JH;Schaible, N;Alvarez, J;Hughes, J;Obermok, M;Rodríguez, CI;Kallarakal, T;Suki, B;Amin, K;Krishnan, R;Behrsing, HP;
Toxics
Species: Human
Sample Types: Cell Culture Supernates
-
Investigating the Prognostic Potential of Plasma ST2 in Patients with Peripheral Artery Disease: Identification and Evaluation
Authors: Li, B;Shaikh, F;Zamzam, A;Abdin, R;Qadura, M;
Proteomes
Species: Human
Sample Types: Plasma
-
Low-Frequency Ventilation May Facilitate Weaning in Acute Respiratory Distress Syndrome Treated with Extracorporeal Membrane Oxygenation: A Randomized Controlled Trial
Authors: Hermann, M;König, S;Laxar, D;Krall, C;Kraft, F;Krenn, K;Baumgartner, C;Tretter, V;Maleczek, M;Hermann, A;Fraunschiel, M;Ullrich, R;
Journal of clinical medicine
Species: Human
Sample Types: Serum
-
Systemic and cerebro-cardiac biomarkers following traumatic brain injury: an interim analysis of randomized controlled clinical trial of early administration of beta blockers
Authors: El-Menyar, A;Asim, M;Khan, N;Rizoli, S;Mahmood, I;Al-Ani, M;Kanbar, A;Alaieb, A;Hakim, S;Younis, B;Taha, I;Jogol, H;Siddiqui, T;Hammo, AA;Abdurraheim, N;Alabdallat, M;Bahey, AA;Ahmed, K;Atique, S;Chaudry, IH;Prabhu, KS;Uddin, S;Al-Thani, H;
Scientific reports
Species: Human
Sample Types: Serum
-
3D-Bioprinted Co-Cultures of Glioblastoma Multiforme and Mesenchymal Stromal Cells Indicate a Role for Perivascular Niche Cells in Shaping Glioma Chemokine Microenvironment
Authors: Zielniok, K;Rusinek, K;S?ysz, A;Lachota, M;B?czy?ska, E;Krata, N;Szpakowska, A;Ciepielak, M;Foroncewicz, B;Mucha, K;Zago?d?on, R;Pojda, Z;
Cells
Species: Human
Sample Types: Cell Culture Supernates
-
Serum-Derived Extracellular Vesicles for the Treatment of Severe Ocular Surface Disease
Authors: Saraf, N;Ramachandran, RA;Cao, M;Lemoff, A;Baniasadi, H;Robertson, DM;
The ocular surface
Species: Human
Sample Types: Cell Culture Supernates
-
ASSOCIATION BETWEEN CALCIUM-CHANNEL BLOCKERS AND GINGIVAL ENLARGEMENT: A CASE-CONTROL STUDY
Authors: Mainas, G;Santamaria, P;Zoheir, N;Alamri, MM;Hughes, F;Lu, EM;Nibali, L;
Journal of dentistry
Species: Human
Sample Types: Saliva
-
Elevated serum IGFBP-1 levels correlate with cognitive deficits in treatment-resistant and chronic medicated schizophrenia patients
Authors: Yang, H;Yang, M;Zhang, Y;Shi, Z;Zhang, X;Zhang, C;
Cytokine
Species: Human
Sample Types: Serum
-
Emerging multisystem biomarkers in hereditary transthyretin amyloidosis: a pilot study
Authors: Luigetti, M;Vitali, F;Romano, A;Sciarrone, MA;Guglielmino, V;Ardito, M;Sabino, A;Servidei, S;Piro, G;Carbone, C;Graziani, F;Lillo, R;Ferraro, PM;Primiano, G;
Scientific reports
Species: Human
Sample Types: Serum
-
Microplastic-induced hepatic adverse effects evaluated in advanced quadruple cell human primary models following three weeks of repeated exposure
Authors: Guraka, A;Souch, G;Duff, R;Brown, D;Moritz, W;Kermanizadeh, A;
Chemosphere
Species: Human
Sample Types: Cell Culture Supernates
-
Evaluating the modulation of peripheral immune profile in people living with HIV and (Neuro)cysticercosis
Authors: Lema, YL;Prodjinotho, UF;Makasi, C;Nanyaro, MA;Kilale, AM;Mfinanga, S;Stelzle, D;Schmidt, V;Carabin, H;Winkler, AS;Lyamuya, EF;Ngowi, BJ;Chachage, M;Prazeres da Costa, C;
PLoS neglected tropical diseases
Species: Human
Sample Types: Serum
-
Persistent low-level viraemia is associated with non-infectious comorbidities in an observational cohort in four African countries
Authors: Esber, AL;Colt, S;Jian, N;Dear, N;Slike, B;Sing'oei, V;Maswai, J;Iroezindu, M;Bahemana, E;Kibuuka, H;Polyak, CS;Streeck, H;Shah, N;Crowell, TA;Ake, JA;AFRICOS Study Group, ;
Journal of the International AIDS Society
Species: Human
Sample Types: Plasma
-
Direct effects of heroin and methadone on T cell function
Authors: Ninnemann, A;Hock, K;Luppus, S;Scherbaum, N;Temme, C;Buer, J;Westendorf, AM;Hansen, W;
International immunopharmacology
Species: Human
Sample Types: Cell Culture Supernates
-
Sustained amphiregulin expression in intermediate alveolar stem cells drives progressive fibrosis
Authors: Zhao, R;Wang, Z;Wang, G;Geng, J;Wu, H;Liu, X;Bin, E;Sui, J;Dai, H;Tang, N;
Cell stem cell
Species: Human
Sample Types: Serum
-
Elucidating the Immune Response to SARS-CoV-2: Natural Infection versus Covaxin/Covishield Vaccination in a South Indian Population
Authors: Vanamudhu, A;Devi Arumugam, R;Nancy, A;Selvaraj, N;Moiden, K;Hissar, S;Ranganathan, UD;Bethunaickan, R;Babu, S;Kumar, NP;
Viruses
Species: Human
Sample Types: Plasma
-
Concussion-Related Biomarker Variations in Retired Rugby Players and Implications for Neurodegenerative Disease Risk: The UK Rugby Health Study
Authors: Alanazi, N;Fitzgerald, M;Hume, P;Hellewell, S;Horncastle, A;Anyaegbu, C;Papini, MG;Hargreaves, N;Halicki, M;Entwistle, I;Hind, K;Chazot, P;
International journal of molecular sciences
Species: Human
Sample Types: Serum, Exosomes
-
Circulating Polymorphonuclear Myeloid-Derived Suppressor Cells (PMN-MDSCs) Have a Biological Role in Patients with Primary Myelofibrosis
Authors: Campanelli, R;Carolei, A;Catarsi, P;Abbà, C;Boveri, E;Paulli, M;Gentile, R;Morosini, M;Albertini, R;Mantovani, S;Massa, M;Barosi, G;Rosti, V;
Cancers
Species: Human
Sample Types: Platelet-poor Plasma
-
Inflammatory profiles are associated with long COVID up to 6 months after COVID-19 onset: A prospective cohort study of individuals with mild to critical COVID-19
Authors: Wynberg, E;Han, AX;van Willigen, HDG;Verveen, A;van Pul, L;Maurer, I;van Leeuwen, EM;van den Aardweg, JG;de Jong, MD;Nieuwkerk, P;Prins, M;Kootstra, NA;de Bree, GJ;RECoVERED Study Group, ;
PloS one
Species: Human
Sample Types: Serum
-
Dapagliflozin, inflammation and left ventricular remodelling in patients with type 2 diabetes and left ventricular hypertrophy
Authors: Dihoum, A;Brown, AJ;McCrimmon, RJ;Lang, CC;Mordi, IR;
BMC cardiovascular disorders
Species: Human
Sample Types: Plasma
-
Anadenanthera colubrina regulated LPS-induced inflammation by suppressing NF-?B and p38-MAPK signaling pathways
Authors: Maia, CMA;Vasconcelos, PGS;Pasetto, S;Godwin, WC;Silva, JPRE;Tavares, JF;Pardi, V;Costa, EMMB;Murata, RM;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Associations between Microglia and Astrocytic Proteins and Tau Biomarkers across the Continuum of Alzheimer's Disease
Authors: Doroszkiewicz, J;Kulczy?ska-Przybik, A;Dulewicz, M;Mroczko, J;Borawska, R;S?owik, A;Zetterberg, H;Hanrieder, J;Blennow, K;Mroczko, B;
International journal of molecular sciences
Species: Human
Sample Types: CSF
-
Epithelial cells maintain memory of prior infection with Streptococcus pneumoniae through di-methylation of histone H3
Authors: Chevalier, C;Chica, C;Matheau, J;Pain, A;Connor, MG;Hamon, MA;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Mid-Upper Arm Circumference Is a Strong Predictor of Mortality Among Ugandan Adults With HIV-Associated Cryptococcal Meningitis: A Prospective Cohort Study
Authors: Hale, G;Adzemovic, T;Huppler Hullsiek, K;Mulwana, S;Ndyetukira, JF;Sadiq, A;Kabahubya, M;Ayebare, P;Nankungu, L;Namudde, A;Namanda, S;Menya, G;Nakitto, G;Muzoora, C;Nuwagira, E;Rhein, J;Meya, DB;Boulware, DR;Ellis, J;Abassi, M;
Open forum infectious diseases
Species: Human
Sample Types: Cell Culture Supernates
-
Prevascularized spongy-like hydrogels maintain their angiogenic potential after prolonged hypothermic storage
Authors: Freitas-Ribeiro, S;Moreira, H;da Silva, LP;Noro, J;Sampaio-Marques, B;Ludovico, P;Jarnalo, M;Horta, R;Marques, AP;Reis, RL;Pirraco, RP;
Bioactive materials
Species: Human
Sample Types: Cell Culture Supernates
-
Innate lymphoid cells are activated in HFRS, and their function can be modulated by hantavirus-induced type I interferons
Authors: García, M;Carrasco García, A;Weigel, W;Christ, W;Lira-Junior, R;Wirth, L;Tauriainen, J;Maleki, K;Vanoni, G;Vaheri, A;Mäkelä, S;Mustonen, J;Nordgren, J;Smed-Sörensen, A;Strandin, T;Mjösberg, J;Klingström, J;
PLoS pathogens
Species: Human
Sample Types: Cell Culture Supernates, Plasma
-
Repeat controlled human Plasmodium falciparum infections delay bloodstream patency and reduce symptoms
Authors: Ferrer, P;Berry, AA;Bucsan, AN;Prajapati, SK;Krishnan, K;Barbeau, MC;Rickert, DM;Guerrero, SM;Usui, M;Abebe, Y;Patil, A;Chakravarty, S;Billingsley, PF;Pa'ahana-Brown, F;Strauss, K;Shrestha, B;Nomicos, E;Deye, GA;Sim, BKL;Hoffman, SL;Williamson, KC;Lyke, KE;
Nature communications
Species: Human
Sample Types: Plasma
-
Comparison of Glomerular Filtration Rate Equations in a Rural New Mexico Cohort: Results from the COMPASS Study
Authors: Balasch, MM;Roumelioti, ME;Argyropoulos, CP;
medRxiv : the preprint server for health sciences
Species: Human
Sample Types: Serum
-
Predicting Major Adverse Carotid Cerebrovascular Events in Patients with Carotid Stenosis: Integrating a Panel of Plasma Protein Biomarkers and Clinical Features-A Pilot Study
Authors: Khan, H;Zamzam, A;Shaikh, F;Saposnik, G;Mamdani, M;Qadura, M;
Journal of clinical medicine
Species: Human
Sample Types: Plasma
-
Choroid plexus defects in Down syndrome brain organoids enhance neurotropism of SARS-CoV-2
Authors: Shaker, MR;Slonchak, A;Al-Mhanawi, B;Morrison, SD;Sng, JDJ;Cooper-White, J;Khromykh, AA;Wolvetang, EJ;
Science advances
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophils and galectin-3 defend mice from lethal bacterial infection and humans from acute respiratory failure
Authors: Das, S;Kaminski, TW;Schlegel, BT;Bain, W;Hu, S;Patel, A;Kale, SL;Chen, K;Lee, JS;Mallampalli, RK;Kagan, VE;Rajasundaram, D;McVerry, BJ;Sundd, P;Kitsios, GD;Ray, A;Ray, P;
Nature communications
Species: Human
Sample Types: Endotracheal Tube Aspirates
-
The Severity of Diabetic Retinopathy Corresponds with Corneal Nerve Alterations and Ocular Discomfort of the Patient
Authors: Machali?ska, A;Kuligowska, A;Ziontkowska-Wrza?ek, A;Stroynowska, B;Pius-Sadowska, E;Safranow, K;Machali?ski, J;Mozolewska-Piotrowska, K;Machali?ski, B;
International journal of molecular sciences
Species: Human
Sample Types: Tears
-
Marine-Derived Phosphoeleganin and Its Semisynthetic Derivative Decrease IL6 Levels and Improve Insulin Signaling in Human Hepatocellular Carcinoma Cells
Authors: Agognon, AL;Casertano, M;Vito, A;Orso, S;Cabaro, S;Mormone, F;Morelli, C;Perruolo, G;Formisano, P;Menna, M;Imperatore, C;Oriente, F;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Supplementation of Seaweed Extracts to the Diet Reduces Symptoms of Alzheimers Disease in the APPswePS1?E9 Mouse Model
Authors: Martens, N;Zhan, N;Yam, S;Leijten, F;Palumbo, M;Caspers, M;Tiane, A;Friedrichs, S;Li, Y;Zee, L;Voortman, G;Zimetti, F;Jaarsma, D;Verschuren, L;Jonker, J;Kuipers, F;Lütjohann, D;Vanmierlo, T;Mulder, M;
Nutrients
Species: Human
Sample Types: Cell Culture Supernates
-
Insights into IL-1 family cytokines in kidney allograft transplantation: IL-18BP and free IL-18 as emerging biomarkers
Authors: Cecrdlova, E;Krupickova, L;Fialova, M;Novotny, M;Tichanek, F;Svachova, V;Mezerova, K;Viklicky, O;Striz, I;
Cytokine
Species: Human
Sample Types: Serum
-
Sustained IFN signaling is associated with delayed development of SARS-CoV-2-specific immunity
Authors: Brunet?Ratnasingham, E;Morin, S;Randolph, H;Labrecque, M;Bélair, J;Lima-Barbosa, R;Pagliuzza, A;Marchitto, L;Hultström, M;Niessl, J;Cloutier, R;Flores, A;Brassard, N;Benlarbi, M;Prévost, J;Ding, S;Anand, S;Sannier, G;Marks, A;Wågsäter, D;Bareke, E;Zeberg, H;Lipcsey, M;Frithiof, R;Larsson, A;Zhou, S;Nakanishi, T;Morrison, D;Vézina, D;Bourassa, C;Gendron?Lepage, G;Medjahed, H;Point, F;Richard, J;Larochelle, C;Prat, A;Cunningham, J;Arbour, N;Durand, M;Richards, J;Moon, K;Chomont, N;Finzi, A;Tétreault, M;Barreiro, L;Wolf, G;Kaufmann, D;
Nature communications
Species: Human
Sample Types: Plasma
-
Effects of Water-Based Exercise on Patients Older than 60 Years Undergoing Cardiac Rehabilitation after Coronary Intervention
Authors: Ksela, J;Kafol, J;Vasic, D;Jug, B;
Journal of cardiovascular development and disease
Species: Human
Sample Types: Plasma
-
Amphiregulin as a biomarker for monitoring lifethreatening acute graft-versus-host disease: secondary analysis of two prospective clinical trials
Authors: Holtan, SG;El Jurdi, N;Rashidi, A;Betts, BC;Demorest, C;Galvin, JP;MacMillan, ML;Weisdorf, DJ;Panoskaltsis-Mortari, A;Pratta, MA;
Haematologica
Species: Human
Sample Types: Plasma
-
A metabolic-dysfunction associated steatotic liver acinus biomimetic induces pancreatic islet dysfunction in a coupled microphysiology system
Authors: Aleman, J;Ravikumar, K;Wiegand, C;Schurdak, ME;Vernetti, L;Gavlock, D;Reese, C;DeBiasio, R;LaRocca, G;Angarita, YD;Gough, A;Soto-Gutierrez, A;Behari, J;Yechoor, V;Miedel, MT;Stern, AM;Banerjee, I;Taylor, DL;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Tissue Homogenates
-
Protein Expression of TLR2, TLR4, and TLR9 on Monocytes in TB, HIV, and TB/HIV
Authors: Tamene, W;Wassie, L;Marconi, VC;Abebe, M;Kebede, A;Sack, U;Howe, R;
Journal of immunology research
Species: Human
Sample Types: Plasma
-
Deregulation in adult IgA vasculitis skin as the basis for the discovery of novel serum biomarkers
Authors: Bajelj, M;Hladnik, M;Blagus, R;Jur?i?, V;Marke, A;Toluay, TD;Sodin-emrl, S;Ho?evar, A;Lakota, K;
Arthritis research & therapy
Species: Human
Sample Types: Serum
-
Preclinical Development of CAR T Cells with Antigen-Inducible IL18 Enforcement to Treat GD2-Positive Solid Cancers
Authors: Fischer?Riepe, L;Kailayangiri, S;Zimmermann, K;Pfeifer, R;Aigner, M;Altvater, B;Kretschmann, S;Völkl, S;Hartley, J;Dreger, C;Petry, K;Bosio, A;Döllen, A;Hartmann, W;Lode, H;Görlich, D;Mackensen, A;Jungblut, M;Schambach, A;Abken, H;Rössig, C;
Clinical Cancer Research
Species: Human
Sample Types: Cell Culture Supernates
-
Large-scale phenotyping of patients with long COVID post-hospitalization reveals mechanistic subtypes of disease
Authors: Liew, F;Efstathiou, C;Fontanella, S;Richardson, M;Saunders, R;Swieboda, D;Sidhu, JK;Ascough, S;Moore, SC;Mohamed, N;Nunag, J;King, C;Leavy, OC;Elneima, O;McAuley, HJC;Shikotra, A;Singapuri, A;Sereno, M;Harris, VC;Houchen-Wolloff, L;Greening, NJ;Lone, NI;Thorpe, M;Thompson, AAR;Rowland-Jones, SL;Docherty, AB;Chalmers, JD;Ho, LP;Horsley, A;Raman, B;Poinasamy, K;Marks, M;Kon, OM;Howard, LS;Wootton, DG;Quint, JK;de Silva, TI;Ho, A;Chiu, C;Harrison, EM;Greenhalf, W;Baillie, JK;Semple, MG;Turtle, L;Evans, RA;Wain, LV;Brightling, C;Thwaites, RS;Openshaw, PJM;PHOSP-COVID collaborative group, ;ISARIC investigators, ;
Nature immunology
Species: Human
Sample Types: Nasal Fluid
-
Effects of aflibercept and bevacizumab on cell viability, cell metabolism and inflammation in hypoxic human Müller cells
Authors: Matsuda, M;da Silva, RA;Roda, VMP;Marquezini, MV;Monteiro, MLR;Hamassaki, DE;
PloS one
Species: Human
Sample Types: Cell Culture Supernates
-
Circulatory endostatin level and risk of cardiovascular events in patients with end-stage renal disease on hemodialysis
Authors: Kim, J;Kim, M;Jeong, K;Moon, J;Lee, S;Ko, G;Lee, D;Lee, S;Kim, Y;Hwang, H;
Kidney Research and Clinical Practice
Species: Human
Sample Types: Plasma
-
Short-Term Effects of Primary and Secondary Particulate Matter on Ceramide Metabolism, Pro-Inflammatory Response, and Blood Coagulation
Authors: Zhang, B;Xu, H;He, X;Wang, T;Li, M;Shan, X;Zhu, Y;Liu, C;Zhao, Q;Song, X;Sun, Y;Zheng, L;Huang, W;
Toxics
Species: Human
Sample Types: Serum
-
Digital pathology with artificial intelligence analysis provides insight to the efficacy of anti-fibrotic compounds in human 3D MASH model
Authors: Kostadinova, R;Ströbel, S;Chen, L;Fiaschetti-Egli, K;Gadient, J;Pawlowska, A;Petitjean, L;Bieri, M;Thoma, E;Petitjean, M;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
A novel lentiviral vector-based approach to generate chimeric antigen receptor T cells targeting Aspergillus fumigatus
Authors: Kumaresan, PR;Wurster, S;Bavisi, K;da Silva, TA;Hauser, P;Kinnitt, J;Albert, ND;Bharadwaj, U;Neelapu, S;Kontoyiannis, DP;
mBio
Species: Human
Sample Types: Cell Culture Supernates
-
Intestinal stroma guides monocyte differentiation to macrophages through GM-CSF
Authors: Kvedaraite, E;Lourda, M;Mouratidou, N;Düking, T;Padhi, A;Moll, K;Czarnewski, P;Sinha, I;Xagoraris, I;Kokkinou, E;Damdimopoulos, A;Weigel, W;Hartwig, O;Santos, TE;Soini, T;Van Acker, A;Rahkonen, N;Flodström Tullberg, M;Ringqvist, E;Buggert, M;Jorns, C;Lindforss, U;Nordenvall, C;Stamper, CT;Unnersjö-Jess, D;Akber, M;Nadisauskaite, R;Jansson, J;Vandamme, N;Sorini, C;Grundeken, ME;Rolandsdotter, H;Rassidakis, G;Villablanca, EJ;Ideström, M;Eulitz, S;Arnell, H;Mjösberg, J;Henter, JI;Svensson, M;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
ANXA3-rich exosomes derived from tumor-associated macrophages regulate ferroptosis and lymphatic metastasis of laryngeal squamous cell carcinoma
Authors: Xu, L;Li, W;Liu, D;Cao, J;Ge, J;Liu, X;Wang, Y;Teng, Y;Liu, P;Guo, X;He, C;Liu, M;Tian, L;
Cancer immunology research
Species: Human
Sample Types: Cell Culture Supernates
-
Multi-modal analysis of inflammation as a potential mediator of depressive symptoms in young people with HIV: The GOLD depression study
Authors: Mudra Rakshasa-Loots, A;Naidoo, S;Hamana, T;Fanqa, B;van Wyhe, KS;Lindani, F;van der Kouwe, AJW;Glashoff, R;Kruger, S;Robertson, F;Cox, SR;Meintjes, EM;Laughton, B;
PloS one
Species: Human
Sample Types: Serum
-
The response of salivary proinflammatory biomarkers to tooth extraction in individuals with type II diabetes mellitus
Authors: Al Shehhi, YI;Elemam, NM;Alsaegh, MA;
BMC oral health
Species: Human
Sample Types: Saliva
-
Diesel exhaust particle exposure exacerbates ciliary and epithelial barrier dysfunction in the multiciliated bronchial epithelium models
Authors: Park, E;Kim, BY;Lee, S;Son, KH;Bang, J;Hong, SH;Lee, JW;Uhm, KO;Kwak, HJ;Lim, HJ;
Ecotoxicology and environmental safety
Species: Human
Sample Types: Cell Culture Supernates
-
Modeling early pathophysiological phenotypes of diabetic retinopathy in a human inner blood-retinal barrier-on-a-chip
Authors: Maurissen, TL;Spielmann, AJ;Schellenberg, G;Bickle, M;Vieira, JR;Lai, SY;Pavlou, G;Fauser, S;Westenskow, PD;Kamm, RD;Ragelle, H;
Nature communications
Species: Human
Sample Types: Adenoid Lavage Fluid
-
KDM6A Regulates Immune Response Genes in Multiple Myeloma
Authors: Dupéré-Richer, D;Riva, A;Maji, S;Barwick, BG;Román, HC;Sobh, A;Quickstad, G;Li, J;De, U;Piper, C;Kulis, M;Ezponda, T;Martin-Subero, JI;Tonon, G;Zhang, W;Mitsiades, CS;Boise, LH;Bennett, RL;Licht, JD;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Lysates
-
Quantiferon Monitor Testing Sheds Light on Immune System Disparities between Multiple Sclerosis Patients and Healthy Individuals
Authors: Sou?ková, I;Sou?ek, O;Krejsek, J;Vyata, O;Matyá, D;Peterka, M;Novotný, M;Kunc, P;Pavelek, Z;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
Relaxation of mitochondrial hyperfusion in the diabetic retina via N6-furfuryladenosine confers neuroprotection regardless of glycaemic status
Authors: Anderson, A;Alfahad, N;Wimalachandra, D;Bouzinab, K;Rudzinska, P;Wood, H;Fazey, I;Xu, H;Lyons, TJ;Barnes, NM;Narendran, P;Lord, JM;Rauz, S;Ganley, IG;Curtis, TM;Wallace, GR;Hombrebueno, JR;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Serum biomarkers in patients with hand-arm vibration injury and in controls
Authors: Tekavec, E;Nilsson, T;Dahlin, LB;Huynh, E;Axmon, A;Nordander, C;Riddar, J;Kåredal, M;
Scientific reports
Species: Human
Sample Types: Serum
-
Interleukin 6 polymorphisms are associated with cardiovascular risk factors in premature coronary artery disease patients and healthy controls of the GEA Mexican study
Authors: Posadas-Sánchez, R;López-Uribe, ÁR;Fragoso, JM;Vargas-Alarcón, G;
Experimental and molecular pathology
Species: Human
Sample Types: Plasma, Serum
-
Eicosatetraynoic Acid Regulates Pro-Fibrotic Pathways in an Induced Pluripotent Stem Cell Derived Macrophage:Human Intestinal Organoid Model of Crohn's Disease
Authors: Jurickova, I;Dreskin, BW;Angerman, E;Bonkowski, E;Tominaga, K;Iwasawa, K;Braun, T;Takebe, T;Helmrath, MA;Haberman, Y;Wells, JM;Denson, LA;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Lysates
-
Combining Classic and Novel Neutrophil-Related Biomarkers to Identify Non-Small-Cell Lung Cancer
Authors: Ren, Y;Wang, Q;Xu, C;Guo, Q;Dai, R;Xu, X;Zhang, Y;Wu, M;Wu, X;Tu, H;
Cancers
Species: Human
Sample Types: Plasma
-
Gaining Biological Insights through Supervised Data Visualization
Authors: Rhodes, JS;Aumon, A;Morin, S;Girard, M;Larochelle, C;Brunet-Ratnasingham, E;Pagliuzza, A;Marchitto, L;Zhang, W;Cutler, A;Grand'Maison, F;Zhou, A;Finzi, A;Chomont, N;Kaufmann, DE;Zandee, S;Prat, A;Wolf, G;Moon, KR;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Plasma
-
Immunomodulatory response to neoadjuvant nivolumab in non-metastatic clear cell renal cell carcinoma
Authors: Singla, N;Nirschl, TR;Obradovic, AZ;Shenderov, E;Lombardo, K;Liu, X;Pons, A;Zarif, JC;Rowe, SP;Trock, BJ;Hammers, HJ;Bivalacqua, TJ;Pierorazio, PM;Deutsch, JS;Lotan, TL;Taube, JM;Ged, YMA;Gorin, MA;Allaf, ME;Drake, CG;
Scientific reports
Species: Human
Sample Types: Serum
-
Crystal type, chain length and polydispersity impact the resistant starch type 3 immunomodulatory capacity via Toll-like receptors
Authors: Silva Lagos, L;Klostermann, CE;López-Velázquez, G;Fernández-Lainez, C;Leemhuis, H;Oudhuis, AACML;Buwalda, P;Schols, HA;de Vos, P;
Carbohydrate polymers
Species: Human
Sample Types: Cell Culture Supernates
-
Evaluation of Metabolic and Cardiovascular Risk Measured by Laboratory Biomarkers and Cardiopulmonary Exercise Test in Children and Adolescents Recovered from Brain Tumors: The CARMEP Study
Authors: Romano, A;Sollazzo, F;Rivetti, S;Morra, L;Servidei, T;Lucchetti, D;Attinà, G;Maurizi, P;Mastrangelo, S;Zovatto, IC;Monti, R;Bianco, M;Palmieri, V;Ruggiero, A;
Cancers
Species: Human
Sample Types: Serum
-
Intra-amniotic infection and/or inflammation is associated with fetal cardiac concentric hypertrophy and diastolic dysfunction in preterm labor and preterm prelabor rupture of membranes
Authors: Murillo, C;Rueda, C;Larroya, M;Boada, D;Grau, L;Ponce, J;Herranz, A;Gómez, O;Ferrero, S;Andreu-Fernández, V;Gratacós, E;Crispi, F;Palacio, M;Cobo, T;
American journal of obstetrics and gynecology
Species: Human
Sample Types: Amniotic Fluid
-
Neutrophil activation and clonal CAR-T re-expansion underpinning cytokine release syndrome during ciltacabtagene autoleucel therapy in multiple myeloma
Authors: Yang, S;Xu, J;Dai, Y;Jin, S;Sun, Y;Li, J;Liu, C;Ma, X;Chen, Z;Chen, L;Hou, J;Mi, JQ;Chen, SJ;
Nature communications
Species: Human
Sample Types: Serum
-
Haplotype of the Lipoprotein(a) Gene Variants rs10455872 and rs3798220 Is Associated with Parameters of Coagulation, Fibrinolysis, and Inflammation in Patients after Myocardial Infarction and Highly Elevated Lipoprotein(a) Values
Authors: Ugovek, S;Rehberger Likozar, A;Levstek, T;Trebuak Podkrajek, K;Zupan, J;ebetjen, M;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
Omega-3 Fatty Acids and Markers of Thrombosis in Patients with Atrial Fibrillation
Authors: Reiner, MF;Bertschi, DA;Werlen, L;Wiencierz, A;Aeschbacher, S;Lee, P;Rodondi, N;Moutzouri, E;Bonati, L;Reichlin, T;Moschovitis, G;Rutishauser, J;Kühne, M;Osswald, S;Conen, D;Beer, JH;
Nutrients
Species: Human
Sample Types: Plasma
-
Sequential immunotherapy and targeted therapy for metastatic BRAF V600 mutated melanoma: 4-year survival and biomarkers evaluation from the phase II SECOMBIT trial
Authors: Ascierto, PA;Casula, M;Bulgarelli, J;Pisano, M;Piccinini, C;Piccin, L;Cossu, A;Mandalà, M;Ferrucci, PF;Guidoboni, M;Rutkowski, P;Ferraresi, V;Arance, A;Guida, M;Maiello, E;Gogas, H;Richtig, E;Fierro, MT;Lebbe, C;Helgadottir, H;Queirolo, P;Spagnolo, F;Tucci, M;Del Vecchio, M;Cao, MG;Minisini, AM;De Placido, S;Sanmamed, MF;Mallardo, D;Paone, M;Vitale, MG;Melero, I;Grimaldi, AM;Giannarelli, D;Dummer, R;Sileni, VC;Palmieri, G;
Nature communications
Species: Human
Sample Types: Serum
-
Factors Associated With Intraocular Inflammation in Neovascular Age-Related Macular Degeneration Patients Treated With Brolucizumab
Authors: Hashimoto, Y;Inoda, S;Takahashi, H;Takahashi, R;Yoshida, H;Fujino, Y;Sakamoto, S;Kawashima, H;Yanagi, Y;
Investigative ophthalmology & visual science
Species: Human
Sample Types: Aqueous Humor, Serum
-
Human Precision-Cut Liver Slices: A Potential Platform to Study Alcohol-Related Liver Disease
Authors: Rastovic, U;Bozzano, SF;Riva, A;Simoni-Nieves, A;Harris, N;Miquel, R;Lackner, C;Zen, Y;Zamalloa, A;Menon, K;Heaton, N;Chokshi, S;Palma, E;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Pro-Resolving Mediators in Rotator Cuff Disease: How Is the Bursa Involved?
Authors: Klatte-Schulz, F;Bormann, N;Bonell, A;Al-Michref, J;Nguyen, HL;Klöckner, P;Thiele, K;Moroder, P;Seifert, M;Sawitzki, B;Wildemann, B;Duda, GN;
Cells
Species: Human
Sample Types: Tissue Culture Supernates
-
Amino acid catabolite markers for early prognostication of pneumonia in patients with COVID-19
Authors: Maeda, R;Seki, N;Uwamino, Y;Wakui, M;Nakagama, Y;Kido, Y;Sasai, M;Taira, S;Toriu, N;Yamamoto, M;Matsuura, Y;Uchiyama, J;Yamaguchi, G;Hirakawa, M;Kim, YG;Mishima, M;Yanagita, M;Suematsu, M;Sugiura, Y;
Nature communications
Species: Human
Sample Types: Serum
-
Utility of Cerebrospinal Fluid Protein Levels as a Potential Predictive Biomarker of Disease Severity in HIV-Associated Cryptococcal Meningitis
Authors: Kasibante, J;Irfanullah, E;Wele, A;Okafor, E;Ssebambulidde, K;Okurut, S;Kagimu, E;Gakuru, J;Rutakingirwa, MK;Mugabi, T;Nuwagira, E;Jjunju, S;Mpoza, E;Tugume, L;Nsangi, L;Musibire, AK;Muzoora, C;Rhein, J;Meya, DB;Boulware, DR;Abassi, M;
medRxiv : the preprint server for health sciences
Species: Human
Sample Types: Cell Culture Supernates
-
General joint hypermobility in temporomandibular joint disease; clinical characteristics, biomarkers, and surgical aspects
Authors: Ulmner, M;Sugars, R;Naimi-Akbar, A;Reseland, JE;Lund, B;
Heliyon
Species: Human
Sample Types: Tissue Homogenates
-
In vitro and in vivo evaluation of electrospun poly (?-caprolactone)/collagen scaffolds and Wharton's jelly mesenchymal stromal cells (hWJ-MSCs) constructs as potential alternative for skin tissue engineering
Authors: Lizarazo-Fonseca, L;Correa-Araujo, L;Prieto-Abello, L;Camacho-Rodríguez, B;Silva-Cote, I;
Regenerative therapy
Species: Human
Sample Types: Cell Culture Supernates
-
Cytokines in aqueous humor of patients with congenital cataract during delayed sequential bilateral cataract surgery
Authors: Hui, N;Yu, L;Qu, L;Yan, H;
BMC ophthalmology
Species: Human
Sample Types: Aqueous Humor
-
VEGFR and DPP-IV as Markers of Severe COVID-19 and Predictors of ICU Admission
Authors: Pius-Sadowska, E;Kulig, P;Nied?wied?, A;Baumert, B;?uczkowska, K;Rogi?ska, D;Sobu?, A;Ula?czyk, Z;Kawa, M;Paczkowska, E;Parczewski, M;Machali?ska, A;Machali?ski, B;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
PVB exerts anti-inflammatory effects by inhibiting the activation of MAPK and NF-?B signaling pathways and ROS generation in neutrophils
Authors: Ouyang, J;Hong, Y;Wan, Y;He, X;Geng, B;Yang, X;Xiang, J;Cai, J;Zeng, Z;Liu, Z;Peng, N;Jiang, Y;Liu, J;
International immunopharmacology
Species: Human
Sample Types: Cell Culture Supernates
-
IL-17A + group 2 innate lymphoid cells elicit mixed airway inflammation in chronic obstructive pulmonary disease
Authors: Flayer, CH;Linderholm, AL;Ge, MQ;Juarez, M;Franzi, L;Tham, T;Teuber, M;Liao, SY;Schivo, M;Kuhn, B;Zeki, A;Haczku, A;
medRxiv : the preprint server for health sciences
Species: Human
Sample Types: Sputum Supernates
-
A proinflammatory gut mucosal cytokine response is associated with mild COVID-19 disease and superior induction of serum antibodies
Authors: Costigan, D;Fenn, J;Yen, S;Ilott, N;Bullers, S;Hale, J;Greenhalf, W;Conibear, E;Koycheva, A;Madon, K;Jahan, I;Huang, M;Badhan, A;Parker, E;Rosadas, C;Jones, K;McClure, M;Tedder, R;Taylor, G;Baillie, KJ;Semple, MG;Jm Openshaw, P;Pearson, C;Johnson, J;Lalvani, A;Thornton, EE;INSTINCT Study Group, ;ISARIC4C investigators, ;
Mucosal immunology
Species: Human
Sample Types: Fecal Homogenates
-
Cytokine profile and viral diversity in the early seronegative stage of community-acquired hepatitis C virus (HCV) infection
Authors: Radkowski, M;Grabarczyk, P;Kryczka, T;Caraballo Cortès, K;Kubicka-Russel, D;Janiak, M;Osuch, S;Perlejewski, K;Laskus, T;
Scientific reports
Species: Human
Sample Types: Serum
-
DONOR VARIABILITY IN HUMAN MESENCHYMAL STEM CELL OSTEOGENIC RESPONSE AS A FUNCTION OF PASSAGE CONDITIONS AND DONOR SEX
Authors: Kolliopoulos, V;Tiffany, A;Polanek, M;Harley, BAC;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
?(2 ? 1)-?(2 ? 6) and ?(2 ? 1) fructans protect from impairment of intestinal tight junction's gene expression and attenuate human dendritic cell responses in a fructan-dependent fashion
Authors: Fernández-Lainez, C;Aan de Stegge, M;Silva-Lagos, LA;López-Velázquez, G;de Vos, P;
Carbohydrate polymers
Species: Human
Sample Types: Cell Culture Supernatants
-
Calorie restriction reduces biomarkers of cellular senescence in humans
Authors: Aversa, Z;White, TA;Heeren, AA;Hulshizer, CA;Saul, D;Zhang, X;Molina, AJA;Redman, LM;Martin, CK;Racette, SB;Huffman, KM;Bhapkar, M;Khosla, S;Das, SK;Fielding, RA;Atkinson, EJ;LeBrasseur, NK;
Aging cell
Species: Human
Sample Types: Plasma
-
Mesenchymal Stromal Cells Primed by Toll-like Receptors 3 and 4 Enhanced Anti-Inflammatory Effects against LPS-Induced Macrophages via Extracellular Vesicles
Authors: Hwang, S;Sung, DK;Kim, YE;Yang, M;Ahn, SY;Sung, SI;Chang, YS;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Correlation between salivary cytokine profiles and white spot lesions in adolescent patients receiving clear aligner orthodontic treatment
Authors: Liu, Q;Guo, T;Dang, W;Song, Z;Wen, Y;Luo, H;Wang, A;
BMC oral health
Species: Human
Sample Types: Saliva
-
A human urothelial microtissue model reveals shared colonization and survival strategies between uropathogens and commensals
Authors: Flores, C;Ling, J;Loh, A;Maset, RG;Aw, A;White, IJ;Fernando, R;Rohn, JL;
Science advances
Species: Human
Sample Types: Cell Culture Supernates
-
Disease specific urinary biomarkers in the central nervous system
Authors: Duggins-Warf, M;Ghalali, A;Sesen, J;Martinez, T;Fehnel, KP;Pineda, S;Zurakowski, D;Smith, ER;
Scientific reports
Species: Human
Sample Types: Urine
-
Association of Periodontitis and Atopic Dermatitis with the Levels of IL-13, IL-31, and TSLP in the Gingival Crevicular Fluid
Authors: Jiménez, C;Fernández, J;Aroca, M;Bordagaray, MJ;Pellegrini, E;Contador, J;Hernández, M;Valenzuela, F;Fernández, A;
International journal of molecular sciences
Species: Human
Sample Types: Gingival Crevicular Fluid (GCF)
-
Electrospun Scaffolds of Polylactic Acid, Collagen, and Amorphous Calcium Phosphate for Bone Repair
Authors: Cárdenas-Aguazaco, W;Camacho, B;Gómez-Pachón, EY;Lara-Bertrand, AL;Silva-Cote, I;
Pharmaceutics
Species: Human
Sample Types: Cell Cultue Supernates
-
Analysis of proteins released from osteoarthritic cartilage by compressive loading
Authors: Tsuno, H;Tanaka, N;Naito, M;Ohashi, S;Iwasawa, M;Kadoguchi, T;Mitomi, H;Matsui, T;Furukawa, H;Fukui, N;
Scientific reports
Species: Human
Sample Types: Tissue Homogenates
-
Global and cell type-specific immunological hallmarks of severe dengue progression identified via a systems immunology approach
Authors: Ghita, L;Yao, Z;Xie, Y;Duran, V;Cagirici, HB;Samir, J;Osman, I;Rebellón-Sánchez, DE;Agudelo-Rojas, OL;Sanz, AM;Sahoo, MK;Robinson, ML;Gelvez-Ramirez, RM;Bueno, N;Luciani, F;Pinsky, BA;Montoya, JG;Estupiñan-Cardenas, MI;Villar-Centeno, LA;Rojas-Garrido, EM;Rosso, F;Quake, SR;Zanini, F;Einav, S;
Nature immunology
Species: Human
Sample Types: Serum
-
Smoking and Diabetes Attenuate Number of CD34+ Haematopoietic Stem Cells in Peripheral Blood of Patients with Advanced Peripheral Artery Disease
Authors: Sernek, B;Kamnikar, R;Sebestjen, M;Boc, A;Boc, V;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
HHV-6A Infection of Papillary Thyroid Cancer Cells Induces Several Effects Related to Cancer Progression
Authors: Mardente, S;Romeo, MA;Asquino, A;Po, A;Gilardini Montani, MS;Cirone, M;
Viruses
Species: Human
Sample Types: Cell Culture Supernates
-
CXCL5 and CXCL14, but not CXCL16 as potential biomarkers of colorectal cancer
Authors: Zajkowska, M;Dulewicz, M;Kulczy?ska-Przybik, A;Safiejko, K;Juchimiuk, M;Konopko, M;Koz?owski, L;Mroczko, B;
Scientific reports
Species: Human
Sample Types: Serum
-
Targeting myeloid chemotaxis to reverse prostate cancer therapy resistance
Authors: Guo, C;Sharp, A;Gurel, B;Crespo, M;Figueiredo, I;Jain, S;Vogl, U;Rekowski, J;Rouhifard, M;Gallagher, L;Yuan, W;Carreira, S;Chandran, K;Paschalis, A;Colombo, I;Stathis, A;Bertan, C;Seed, G;Goodall, J;Raynaud, F;Ruddle, R;Swales, KE;Malia, J;Bogdan, D;Tiu, C;Caldwell, R;Aversa, C;Ferreira, A;Neeb, A;Tunariu, N;Westaby, D;Carmichael, J;Fenor de la Maza, MLD;Yap, C;Matthews, R;Badham, H;Prout, T;Turner, A;Parmar, M;Tovey, H;Riisnaes, R;Flohr, P;Gil, J;Waugh, D;Decordova, S;Schlag, A;Calì, B;Alimonti, A;de Bono, JS;
Nature
Species: Human
Sample Types: Serum
-
Safety and infectivity of female cercariae in Schistosoma-naïve, healthy participants: a controlled human Schistosoma mansoni infection study
Authors: Koopman, JPR;Houlder, EL;Janse, JJ;Casacuberta-Partal, M;Lamers, OAC;Sijtsma, JC;de Dood, C;Hilt, ST;Ozir-Fazalalikhan, A;Kuiper, VP;Roozen, GVT;de Bes-Roeleveld, LM;Kruize, YCM;Wammes, LJ;Smits, HH;van Lieshout, L;van Dam, GJ;van Amerongen-Westra, IM;Meij, P;Corstjens, PLAM;Jochems, SP;van Diepen, A;Yazdanbakhsh, M;Hokke, CH;Roestenberg, M;
EBioMedicine
Species: Human
Sample Types: Serum
-
Functional insights into human macrophage response against Scedosporium apiospermum and Scedosporium dehoogii
Authors: Mahmoud, DE;Hanachi, M;Yaakoub, H;Blanchard, S;Pignon, P;Souiai, O;Delneste, Y;Bouchara, JP;Papon, N;Hérivaux, A;
Cytokine
Species: Human
Sample Types: Cell Culture Supernates
-
Repurposing Metformin for periodontal disease management as a form of oral-systemic preventive medicine
Authors: Neves, VCM;Satie Okajima, L;Elbahtety, E;Joseph, S;Daly, J;Menon, A;Fan, D;Volkyte, A;Mainas, G;Fung, K;Dhami, P;Pelegrine, AA;Sharpe, P;Nibali, L;Ide, M;
Journal of translational medicine
Species: Human
Sample Types: Gingival Crevicular Fluid
-
Influence of Macrophages on Vascular Invasion of Inflammatory Breast Cancer Emboli Measured Using an In Vitro Microfluidic Multi-Cellular Platform
Authors: Gadde, M;Mehrabi-Dehdezi, M;Debeb, BG;Woodward, WA;Rylander, MN;
Cancers
Species: Human
Sample Types: Cell Culture Supernates
-
Biomarkers of cellular senescence and risk of death in humans
Authors: St Sauver, JL;Weston, SA;Atkinson, EJ;Mc Gree, ME;Mielke, MM;White, TA;Heeren, AA;Olson, JE;Rocca, WA;Palmer, AK;Cummings, SR;Fielding, RA;Bielinski, SJ;LeBrasseur, NK;
Aging cell
Species: Human
Sample Types: Plasma
-
Cytokine-Induced iNOS in A549 Alveolar Epithelial Cells: A Potential Role in COVID-19 Lung Pathology
Authors: Barilli, A;Recchia Luciani, G;Visigalli, R;Sala, R;Soli, M;Dall'Asta, V;Rotoli, BM;
Biomedicines
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated PLAUR is observed in the airway epithelium of asthma patients and blocking improves barrier integrity
Authors: Portelli, MA;Bhaker, S;Pang, V;Bates, DO;Johnson, SR;Mazar, AP;Shaw, D;Brightling, C;Sayers, I;
Clinical and translational allergy
Species: Human
Sample Types: Cell Culture Supernates
-
Fibroblast growth factors induce hepatic tumorigenesis post radiofrequency ablation
Authors: Markezana, A;Paldor, M;Liao, H;Ahmed, M;Zorde-Khvalevsky, E;Rozenblum, N;Stechele, M;Salvermoser, L;Laville, F;Goldmann, S;Rosenberg, N;Andrasina, T;Ricke, J;Galun, E;Goldberg, SN;
Scientific reports
Species: Human
Sample Types: Plasma
-
Factors associated with patent foramen ovale-related stroke: SAFAS study
Authors: Pommier, T;Lafont, A;Didier, R;Garnier, L;Duloquin, G;Meloux, A;Sagnard, A;Graber, M;Dogon, G;Laurent, G;Vergely, C;Béjot, Y;Guenancia, C;
Revue neurologique
Species: Human
Sample Types: Serum
-
Validation of sTREM-1 and IL-6 based algorithms for outcome prediction of COVID-19
Authors: Van Singer, M;Brahier, T;Koch, J;Hugli, PO;Weckman, AM;Zhong, K;Kain, TJ;Leligdowicz, A;Bernasconi, E;Ceschi, A;Parolari, S;Vuichard-Gysin, D;Kain, KC;Albrich, WC;Boillat-Blanco, N;
BMC infectious diseases
Species: Human
Sample Types: Plasma
-
Prognostic Insights from Longitudinal Multicompartment Study of Host-Microbiota Interactions in Critically Ill Patients
Authors: Kitsios, GD;Sayed, K;Fitch, A;Yang, H;Britton, N;Shah, F;Bain, W;Evankovich, JW;Qin, S;Wang, X;Li, K;Patel, A;Zhang, Y;Radder, J;Cruz, CD;Okin, DA;Huang, CY;van Tyne, D;Benos, PV;Methé, B;Lai, P;Morris, A;McVerry, BJ;
medRxiv : the preprint server for health sciences
Species: Human
Sample Types: BALF, Plasma, Endotracheal Tube Aspirates
-
Use of biomarkers for predicting a malignant course in acute ischemic stroke: an observational case-control study
Authors: Guimarães de Almeida Barros, A;Roquim E Silva, L;Pessoa, A;Eiras Falcão, A;Viana Magno, LA;Valadão Freitas Rosa, D;Aurelio Romano Silva, M;Marques de Miranda, D;Nicolato, R;
Scientific reports
-
Increased Levels of Circulating IGFBP4 and ANGPTL8 with a Prospective Role in Diabetic Nephropathy
Authors: AlMajed, HT;Abu-Farha, M;Alshawaf, E;Devarajan, S;Alsairafi, Z;Elhelaly, A;Cherian, P;Al-Khairi, I;Ali, H;Jose, RM;Thanaraj, TA;Al-Ozairi, E;Al-Mulla, F;Al Attar, A;Abubaker, J;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Plasma type I collagen ?1 chain in relation to coronary artery disease: findings from a prospective population-based cohort and an acute myocardial infarction prospective cohort in Sweden
Authors: Hammaréus, F;Nilsson, L;Ong, KL;Kristenson, M;Festin, K;Lundberg, AK;Chung, RWS;Swahn, E;Alfredsson, J;Holm Nielsen, S;Jonasson, L;
BMJ open
Species: Human
Sample Types: Plasma
-
Mild/asymptomatic COVID-19 in unvaccinated pregnant mothers impairs neonatal immune responses
Authors: Doratt, BM;Sureshchandra, S;True, H;Rincon, M;Marshall, NE;Messaoudi, I;
JCI insight
Species: Human
Sample Types: Cell Culture Supernates
-
Predicting sepsis using a combination of clinical information and molecular immune markers sampled in the ambulance
Authors: Tuerxun, K;Eklund, D;Wallgren, U;Dannenberg, K;Repsilber, D;Kruse, R;Särndahl, E;Kurland, L;
Scientific reports
Species: Human
Sample Types: Plasma
-
Irreversible cell cycle exit associated with senescence is mediated by constitutive MYC degradation
Authors: Afifi, MM;Crncec, A;Cornwell, JA;Cataisson, C;Paul, D;Ghorab, LM;Hernandez, MO;Wong, M;Kedei, N;Cappell, SD;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
Whole Blood Transcriptomics Identifies Subclasses of Pediatric Septic Shock
Authors: Yang, JO;Zinter, MS;Pellegrini, M;Wong, MY;Gala, K;Markovic, D;Nadel, B;Peng, K;Do, N;Mangul, S;Nadkarni, VM;Karlsberg, A;Deshpande, D;Butte, MJ;Asaro, L;Agus, M;Sapru, A;
Research square
Species: Human
Sample Types: Plasma
-
Belatacept inhibit human B cell germinal center development in immunodeficient mice
Authors: Samson, C;Thiolat, A;Moktefi, A;Cohen, JL;Pilon, C;Grimbert, P;
Scientific reports
Species: Mouse
Sample Types: Serum
-
Candida albicans stimulates formation of a multi-receptor complex that mediates epithelial cell invasion during oropharyngeal infection
Authors: Quynh T. Phan, Norma V. Solis, Max V. Cravener, Marc Swidergall, Jianfeng Lin, Manning Y. Huang et al.
PLOS Pathogens
Species: Mouse
Sample Types: Whole Cells
-
Characterization of the pleural microenvironment niche and cancer transition using single-cell RNA sequencing in EGFR-mutated lung cancer
Authors: Wu, YY;Hsu, YL;Huang, YC;Su, YC;Wu, KL;Chang, CY;Ong, CT;Lai, JC;Shen, TY;Lee, TH;Hung, JY;Tsai, YM;
Theranostics
Species: Human
Sample Types: Pleural Effusion
-
Heparin-Immobilized Polyethersulfone for Hemocompatibility Enhancement of Dialysis Membrane: In Situ Synchrotron Imaging, Experimental, and Ex Vivo Studies
Authors: Kalugin, D;Bahig, J;Shoker, A;Abdelrasoul, A;
Membranes
-
Distinct pulmonary and systemic effects of dexamethasone in severe COVID-19
Authors: Fragiadakis, G;Neyton, L;Patel, R;Sarma, A;Consortium, UC;Willmore, A;Haller, SC;Kangelaris, K;Eckalbar, W;Erle, D;Krummel, M;Hendrickson, C;Woodruff, P;Langelier, C;Calfee, C;
Research square
Species: Human
Sample Types: Plasma
-
Association of Growth Differentiation Factor-15 With Event Cause and Cardiovascular Failure After Pediatric Cardiac Arrest in a Multi-Institutional Trial
Authors: Herrmann, JR;Jackson, TC;Fabio, A;Clark, RSB;Berger, RP;Janesko-Feldman, KL;Kochanek, PM;Fink, EL;POCCA Investigators *, ;
Journal of the American Heart Association
Species: Human
Sample Types: Serum
-
Transient anti-cytokine autoantibodies superimpose the hyperinflammatory response in Kawasaki disease and multisystem inflammatory syndrome in children: a comparative cohort study on correlates of disease
Authors: Netea, SA;Biesbroek, G;van Stijn, D;Ijspeert, H;van der Made, CI;Jansen, MH;Geissler, J;van den Berg, JMM;van der Kuip, M;Gruppen, MP;Schonenberg-Meinema, D;Kapitein, B;van Furth, AMMT;Nagelkerke, SQ;Pajkrt, D;Plötz, FB;den Boer, MEJL;Landman, GW;van Houten, MA;Goetschalckx, I;Kawasaki Study Group, ;Toonen, EJM;van de Veerdonk, FL;Kuipers, IM;Dik, WA;Kuijpers, TW;
EBioMedicine
Species: Human
Sample Types: Plasma
-
Serum IL-23 significantly decreased in obese patients with psoriatic arthritis six months after a structured weight loss intervention
Authors: Landgren, AJ;Jonsson, CA;Bilberg, A;Eliasson, B;Torres, L;Dehlin, M;Jacobsson, LTH;Gjertsson, I;Larsson, I;Klingberg, E;
Arthritis research & therapy
Species: Human
Sample Types: Serum
-
Clinical Profile of SARS-CoV-2 Infection: Mechanisms of the Cellular Immune Response and Immunogenetic Markers in Patients from Brazil
Authors: Pacheco, V;Cuber Guimarães, R;Corrêa-Moreira, D;Magalhães, CE;Figueiredo, D;Guttmann, P;Trindade, GF;da Silva, JFA;Ano Bom, APD;de Lourdes Maia, M;Melgaço, JG;da Costa Barros, TA;da Silva, AMV;Group, C;Oliveira, MME;
Viruses
Species: Human
Sample Types: Plasma
-
Inflammatory profiles in febrile children with moderate and severe malnutrition presenting at-hospital in Uganda are associated with increased mortality
Authors: Weckman, AM;McDonald, CR;Ngai, M;Richard-Greenblatt, M;Leligdowicz, A;Conroy, AL;Kain, KC;Namasopo, S;Hawkes, MT;
EBioMedicine
Species: Human
Sample Types: Plasma
-
Predicting Lung Function Using Biomarkers in Alpha-1 Antitrypsin Deficiency
Authors: Spittle, DA;Mansfield, A;Pye, A;Turner, AM;Newnham, M;
Biomedicines
Species: Human
Sample Types: Serum
-
Pan-integrin inhibitor GLPG-0187 promotes T-cell killing of mismatch repair-deficient colorectal cancer cells by suppression of SMAD/TGF-? signaling
Authors: Verschleiser, B;MacDonald, W;Carlsen, L;Huntington, KE;Zhou, L;El-Deiry, WS;
American journal of cancer research
Species: Human
Sample Types: Cell Culture Supernates
-
Potential Utility of Cerebrospinal Fluid Glycoprotein Nonmetastatic Melanoma Protein B as a Neuroinflammatory Diagnostic Biomarker in Mild Cognitive Impairment and Alzheimer's Disease
Authors: Doroszkiewicz, J;Kulczyńska-Przybik, A;Dulewicz, M;Borawska, R;Zajkowska, M;Słowik, A;Mroczko, B;
Journal of clinical medicine
Species: Human
Sample Types: CSF
-
Screening of placenta accreta spectrum disorder using maternal serum biomarkers and clinical indicators: a case-control study
Authors: Zhou, J;Yang, S;Xu, X;Xu, X;Wang, X;Ye, A;Chen, Y;He, F;Yu, B;
BMC pregnancy and childbirth
Species: Human
Sample Types: Serum
-
Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial
Authors: Routy, B;Lenehan, JG;Miller, WH;Jamal, R;Messaoudene, M;Daisley, BA;Hes, C;Al, KF;Martinez-Gili, L;Punčochář, M;Ernst, S;Logan, D;Belanger, K;Esfahani, K;Richard, C;Ninkov, M;Piccinno, G;Armanini, F;Pinto, F;Krishnamoorthy, M;Figueredo, R;Thebault, P;Takis, P;Magrill, J;Ramsay, L;Derosa, L;Marchesi, JR;Parvathy, SN;Elkrief, A;Watson, IR;Lapointe, R;Segata, N;Haeryfar, SMM;Mullish, BH;Silverman, MS;Burton, JP;Maleki Vareki, S;
Nature medicine
Species: Human
Sample Types: Serum
-
Initial Tumor Necrosis Factor-Alpha and Endothelial Activation Are Associated with Hemorrhagic Complications during Extracorporeal Membrane Oxygenation
Authors: Jang, JH;Shin, KH;Lee, HR;Son, E;Lee, SE;Seol, HY;Yoon, SH;Kim, T;Cho, WH;Jeon, D;Kim, YS;Yeo, HJ;
Journal of clinical medicine
Species: Human
Sample Types: Serum
-
Association between Vitamin D Deficiency and Clinical Parameters in Men and Women Aged 50 Years or Older: A Cross-Sectional Cohort Study
Authors: Lee, JH;Kim, YA;Kim, YS;Lee, Y;Seo, JH;
Nutrients
Species: Human
Sample Types: Serum
-
Novel inflammatory mediator profile observed during pediatric heart surgery with cardiopulmonary bypass and continuous ultrafiltration
Authors: Bierer, J;Stanzel, R;Henderson, M;Sett, S;Sapp, J;Andreou, P;Marshall, JS;Horne, D;
Journal of translational medicine
Species: Human
Sample Types: Plasma
-
Inflammation of Dry Eye Syndrome: A Cellular Study of the Epithelial and Macrophagic Involvement of NFAT5 and RAGE
Authors: Henrioux, F;Navel, V;Belville, C;Charnay, C;Antoine, A;Chiambaretta, F;Sapin, V;Blanchon, L;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Molecular pathogenicity of 1-nonadecene and L-lactic acid, unique metabolites in radicular cysts and periapical granulomas
Authors: Altaie, AM;Mohammad, MG;Madkour, MI;AlSaegh, MA;Jayakumar, MN;K G, AR;Samsudin, AR;Halwani, R;Hamoudi, RA;Soliman, SSM;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
Tetraspanin CD9-derived peptides inhibit Pseudomonas aeruginosa corneal infection and aid in wound healing of corneal epithelial cells
Authors: Jadi, PK;Dave, A;Issa, R;Tabbasum, K;Okurowska, K;Samarth, A;Urwin, L;Green, LR;Partridge, LJ;MacNeil, S;Garg, P;Monk, PN;Roy, S;
The ocular surface
Species: Human
Sample Types: Cell Culture Supenates
-
Current status and future landscape of diagnosing tuberculosis infection
Authors: Kobashi, Y;
Respiratory investigation
Species: Human
Sample Types: Cell Culture Supernates
-
Post-Traumatic Stress Response and Appendicitis in Children-Clinical Usefulness of Selected Biomarkers
Authors: Sobczak, J;Burzyńska, M;Sikora, A;Wysocka, A;Karawani, J;Sikora, JP;
Biomedicines
Species: Human
Sample Types: Serum
-
Single-cell transcriptome analysis reveals the regulatory effects of artesunate on splenic immune cells in polymicrobial sepsis
Authors: Chen, J;He, X;Bai, Y;Liu, J;Wong, YK;Xie, L;Zhang, Q;Luo, P;Gao, P;Gu, L;Guo, Q;Cheng, G;Wang, C;Wang, J;
Journal of pharmaceutical analysis
Species: Mouse
Sample Types: Tissue Homogenates
-
Single cell transcriptomics identifies distinct profiles in pediatric acute respiratory distress syndrome
Authors: Flerlage, T;Crawford, JC;Allen, EK;Severns, D;Tan, S;Surman, S;Ridout, G;Novak, T;Randolph, A;West, AN;Thomas, PG;
Nature communications
Species: Human
Sample Types: Tracheal Aspirate
-
Inflammatory Biomarkers and Physiomarkers of Late-Onset Sepsis and Necrotizing Enterocolitis in Premature Infants
Authors: Kumar, R;Kausch, S;Gummadi, AKS;Fairchild, KD;Abhyankar, M;Petri, WA;Sullivan, BA;
medRxiv : the preprint server for health sciences
Species: Human
Sample Types: Plasma
-
Pancreatic Tumor-Derived Extracellular Vesicles Stimulate Schwann Cell Phenotype Indicative of Perineural Invasion via IL-8 Signaling
Authors: Gregory, E;Powers, I;Jamshidi-Parsian, A;Griffin, R;Song, Y;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Extracellular Vesicles
-
VCAM-1 complements CA-125 in detecting recurrent ovarian cancer
Authors: Song, J;Sokoll, LJ;Zhang, Z;Chan, DW;
Clinical proteomics
Species: Human
Sample Types: Serum
-
Investigating Mycobacterium tuberculosis sufR (rv1460) in vitro and ex vivo expression and immunogenicity
Authors: Baatjies, L;van Rensberg, IC;Snyders, C;Gutschmidt, A;Loxton, AG;Williams, MJ;
PloS one
Species: Human
Sample Types: Cell Culture Supernates
-
Cellular allostatic load is linked to increased energy expenditure and accelerated biological aging
Authors: Bobba-Alves, N;Sturm, G;Lin, J;Ware, SA;Karan, KR;Monzel, AS;Bris, C;Procaccio, V;Lenaers, G;Higgins-Chen, A;Levine, M;Horvath, S;Santhanam, BS;Kaufman, BA;Hirano, M;Epel, E;Picard, M;
Psychoneuroendocrinology
Species: Human
Sample Types: Cell Culture Supernates
-
Sarcopenic Obesity in People with Alcoholic Use Disorder: Relation with Inflammation, Vascular Risk Factors and Serum Vitamin D Levels
Authors: Martín-González, C;Fernández-Alonso, P;Pérez-Hernández, O;Abreu-González, P;Espelosín-Ortega, E;Fernández-Rodríguez, CM;Martín-Ponce, E;González-Reimers, E;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
CCX559 is a potent, orally-administered small molecule PD-L1 inhibitor that induces anti-tumor immunity
Authors: Sullivan, KMC;Vilalta, M;Ertl, LS;Wang, Y;Dunlap, C;Ebsworth, K;Zhao, BN;Li, S;Zeng, Y;Miao, Z;Fan, P;Mali, V;Lange, C;McMurtrie, D;Yang, J;Lui, R;Scamp, R;Chhina, V;Kumamoto, A;Yau, S;Dang, T;Easterday, A;Liu, S;Miao, S;Charo, I;Schall, TJ;Zhang, P;
PloS one
Species: Human
Sample Types: Cell Culture Supernates
-
Circulating and intratumoral immune determinants of response to atezolizumab plus bevacizumab in patients with variant histology or sarcomatoid renal cell carcinoma
Authors: Saliby, RM;El Zarif, T;Bakouny, Z;Shah, V;Xie, W;Flippot, R;Denize, T;Kane, MH;Madsen, KN;Ficial, M;Hirsch, L;Wei, XX;Steinarter, JA;Harshman, LC;Vaishampayan, UN;Severgnini, M;McDermott, DF;Lee, GM;Xu, W;Van Allen, EM;McGregor, BA;Signoretti, S;Choueiri, TK;McKay, RR;Braun, DA;
Cancer immunology research
Species: Human
Sample Types: Plasma
-
T Cells Expressing a Modified FcgammaRI Exert Antibody-Dependent Cytotoxicity and Overcome the Limitations of CAR T-cell Therapy against Solid Tumors
Authors: D Rasoulouni, N Santana-Ma, A Gutwillig, L Farhat-You, L Tal, S Amar, M Milyavsky, SSNA Muddineni, N Solomon, H Shpilt, S Dotan, N Pilpel, C Waskow, M Feinmesser, P Rider, Y Carmi
Cancer Immunology Research, 2023-06-02;0(0):OF1-OF18.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased Alveolar Epithelial Damage Markers and Inflammasome-Regulated Cytokines Are Associated with Pulmonary Superinfection in ARDS
Authors: Peukert, K;Sauer, A;Seeliger, B;Feuerborn, C;Fox, M;Schulz, S;Wild, L;Borger, V;Schuss, P;Schneider, M;Güresir, E;Coburn, M;Putensen, C;Wilhelm, C;Bode, C;
Journal of clinical medicine
Species: Human
Sample Types: BALF
-
Nucleocapsid-specific T cell responses associate with control of SARS-CoV-2 in the upper airways before seroconversion
Authors: Eser, TM;Baranov, O;Huth, M;Ahmed, MIM;De�k, F;Held, K;Lin, L;Pekayvaz, K;Leunig, A;Nicolai, L;Pollakis, G;Buggert, M;Price, DA;Rubio-Acero, R;Reich, J;Falk, P;Markgraf, A;Puchinger, K;Castelletti, N;Olbrich, L;Vanshylla, K;Klein, F;Wieser, A;Hasenauer, J;Kroidl, I;Hoelscher, M;Geldmacher, C;
Nature communications
Species: Human
Sample Types: Plasma
-
Association of Peripheral Inflammatory Biomarkers and Growth Factors Levels with Sex, Therapy and Other Clinical Factors in Schizophrenia and Patient Stratification Based on These Data
Authors: Ermakov, EA;Melamud, MM;Boiko, AS;Kamaeva, DA;Ivanova, SA;Nevinsky, GA;Buneva, VN;
Brain sciences
Species: Human
Sample Types: Serum
-
Clinical Profiling and Biomarkers for Post-Operative Atrial Fibrillation Prediction in Patients Undergoing Cardiac Surgery
Authors: Iglesias-�lvarez, D;Fu, X;Mart�nez-Cereijo, JM;Agra-Bermejo, RM;Veiras-Del R�o, S;Selas-Cobos, S;Rial-Munin, MV;Eiras-Mari�o, M;Mart�nez-Salgado, A;Taboada-Mu�iz, M;Reija-L�pez, L;Souaf, S;Garc�a-Carro, J;Fern�ndez-Gonz�lez, �L;Adrio-Nazar, B;Gonz�lez-Juanatey, JR;Eiras, S;Rodr�guez-Ma�ero, M;
Journal of clinical medicine
Species: Human
Sample Types: Plasma
-
Transcriptomic responses in the blood and sputum of cigarette smokers compared to e-cigarette vapers
Authors: Perez, MF;Yurieva, M;Poddutoori, S;Mortensen, EM;Crotty Alexander, LE;Williams, A;
Respiratory research
Species: Human
Sample Types: Sputum
-
A Novel Approach to Staging and Detection of Colorectal Cancer in Early Stages
Authors: Zajkowska, M;Mroczko, B;
Journal of clinical medicine
Species: Human
Sample Types: Serum
-
Multi-Omics Data Integration Reveals Key Variables Contributing to Subgingival Microbiome Dysbiosis-Induced Inflammatory Response in a Hyperglycemic Microenvironment
Authors: Lafleur, S;Bodein, A;Mbuya Mala�ka Mutombo, J;Mathieu, A;Joly Beauparlant, C;Minne, X;Chandad, F;Droit, A;Houde, VP;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
IL-6 and cfDNA monitoring throughout COVID-19 hospitalization are accurate markers of its outcomes
Authors: Bello, S;Lasierra, AB;L�pez-Vergara, L;de Diego, C;Torralba, L;de Gopegui, PR;Lahoz, R;Abad�a, C;Godino, J;Cebollada, A;Jimeno, B;Bello, C;Tejada, A;Torres, A;
Respiratory research
Species: Human
Sample Types: Serum
-
Altered Functionality of Lipoprotein(a) Impacts on Angiogenesis in Diabetic Retinopathy
Authors: Shariatzadeh, M;Nagtzaam, NMA;van Vark-van der Zee, L;van Holten-Neelen, C;Verhoeven, AJM;Dehairs, J;Swinnen, JV;Leijten, F;Ten Berge, JC;Ciriano, JPM;Wong, KT;Mulder, M;Leenen, PJM;Dik, WA;
Investigative ophthalmology & visual science
Species: Human
Sample Types: Cell Culture Supernates
-
Delayed generation of functional virus-specific circulating T follicular helper cells correlates with severe COVID-19
Authors: M Yu, A Charles, A Cagigi, W Christ, B Österberg, S Falck-Jone, L Azizmohamm, E Åhlberg, R Falck-Jone, J Svensson, M Nie, A Warnqvist, F Hellgren, K Lenart, R Arcoverde, S Ols, G Lindgren, A Lin, H Maecker, M Bell, N Johansson, J Albert, C Sundling, P Czarnewski, J Klingström, A Färnert, K Loré, A Smed-Sören
Nature Communications, 2023-04-15;14(1):2164.
Species: Human
Sample Types: Cell Culture Supernates
-
Biomarkers of cellular senescence in idiopathic pulmonary fibrosis
Authors: Z Aversa, EJ Atkinson, EM Carmona, TA White, AA Heeren, SK Jachim, X Zhang, SR Cummings, SE Chiarella, AH Limper, NK LeBrasseur
Respiratory Research, 2023-04-07;24(1):101.
Species: Human
Sample Types: Plasma
-
Alterations of adipokines, pancreatic hormones and incretins in acute and convalescent COVID-19 children
Authors: A Rajamanick, A Venkataram, NP Kumar, R Sasidaran, AN Pandiaraja, N Selvaraj, R Mittal, K Gowshika, S Putlibai, S Lakshan Ra, PV Ramanan, S Babu
BMC pediatrics, 2023-04-03;23(1):156.
Species: Human
Sample Types: Plasma
-
Frequency of Peripheral CD8+ T Cells Expressing Chemo-Attractant Receptors CCR1, 4 and 5 Increases in NPC Patients with EBV Clearance upon Radiotherapy
Authors: S Mahajan, HE Balcioglu, A Oostvogels, WA Dik, KCA Chan, KW Lo, EP Hui, A Tsang, J Tong, WKJ Lam, K Wong, ATC Chan, BBY Ma, R Debets
Cancers, 2023-03-21;15(6):.
Species: Human
Sample Types: Plasma
-
CD8+ Regulatory T Cell Deficiency in Elderly-Onset Rheumatoid Arthritis
Authors: R Watanabe, K Kadoba, A Tamamoto, K Murata, K Murakami, H Onizawa, T Fujii, A Onishi, M Tanaka, H Ito, A Morinobu, M Hashimoto
Journal of Clinical Medicine, 2023-03-17;12(6):.
Species: Human
Sample Types: Plasma
-
Dynamic bioinspired coculture model for probing ER+ breast cancer dormancy in the bone marrow niche
Authors: L Pradhan, D Moore, EM Ovadia, SL Swedzinski, T Cossette, RA Sikes, K van Golen, AM Kloxin
Science Advances, 2023-03-08;9(10):eade3186.
Species: Human
Sample Types: Cell Culture Supernates
-
Autoantibodies against chemokines post-SARS-CoV-2 infection correlate with disease course
Authors: J Muri, V Cecchinato, A Cavalli, AA Shanbhag, M Matkovic, M Biggiogero, PA Maida, J Moritz, C Toscano, E Ghovehoud, R Furlan, F Barbic, A Voza, G De Nadai, C Cervia, Y Zurbuchen, P Taeschler, LA Murray, G Danelon-Sa, S Moro, T Gong, P Piffaretti, F Bianchini, V Crivelli, L Podešvová, M Pedotti, D Jarrossay, J Sgrignani, S Thelen, M Uhr, E Bernasconi, A Rauch, A Manzo, A Ciurea, MBL Rocchi, L Varani, B Moser, B Bottazzi, M Thelen, BA Fallon, O Boyman, A Mantovani, C Garzoni, A Franzetti-, M Uguccioni, DF Robbiani
Nature Immunology, 2023-03-06;24(4):604-611.
Species: Human
Sample Types: Plasma
-
Prognostic accuracy of biomarkers of immune and endothelial activation in Mozambican children hospitalized with pneumonia
Authors: N Balanza, C Erice, M Ngai, CR McDonald, AM Weckman, J Wright, M Richard-Gr, R Varo, E López-Vare, A Sitoe, P Vitorino, J Bramugy, M Lanaspa, S Acácio, L Madrid, B Baro, KC Kain, Q Bassat
PLOS global public health, 2023-02-23;3(2):e0001553.
Species: Human
Sample Types: Plasma
-
HSPs/STAT3 Interplay Sustains DDR and Promotes Cytokine Release by Primary Effusion Lymphoma Cells
Authors: R Gonnella, A Arena, R Zarrella, MS Gilardini, R Santarelli, M Cirone
International Journal of Molecular Sciences, 2023-02-15;24(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
Gut Microbiota in Patients with Postoperative Atrial Fibrillation Undergoing Off-Pump Coronary Bypass Graft Surgery
Authors: Y Wang, Y He, R Li, H Jiang, D Tao, K Zhao, Z Yin, J Zhang, H Wang
Journal of Clinical Medicine, 2023-02-13;12(4):.
Species: Human
Sample Types: Serum
-
GSK-3 inhibitor elraglusib enhances tumor-infiltrating immune cell activation in tumor biopsies and synergizes with anti-PD-L1 in a murine model of colorectal cancer
Authors: KE Huntington, AD Louie, PR Srinivasan, C Schorl, S Lu, D Silverberg, D Newhouse, Z Wu, L Zhou, BA Borden, FJ Giles, M Dooner, BA Carneiro, WS El-Deiry
bioRxiv : the preprint server for biology, 2023-02-07;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Detrimental effects of PCSK9 loss-of-function in the pediatric host response to sepsis are mediated through independent influence on Angiopoietin-1
Authors: Atreya MR, Cvijanovich NZ, Fitzgerald JC et al.
Research square
Species: Human
Sample Types: Serum
-
Lung function and collagen 1a levels are associated with changes in 6�min walk test distance during treatment of TB among HIV-infected adults: a prospective cohort study
Authors: Y Baik, P Maenetje, D Schramm, C Tiemessen, I Ncube, G Churchyard, R Wallis, MD Vangu, H Kornfeld, Y Li, SC Auld, GP Bisson
BMC pulmonary medicine, 2023-02-03;23(1):53.
Species: Human
Sample Types: Plasma
-
A Comprehensive Analysis of Cytokine Network in Centenarians
Authors: M Pinti, L Gibellini, D Lo Tartaro, S De Biasi, M Nasi, R Borella, L Fidanza, A Neroni, L Troiano, C Franceschi, A Cossarizza
International Journal of Molecular Sciences, 2023-02-01;24(3):.
Species: Human
Sample Types: Plasma
-
Secreted proteins MDK, WFDC2, and CXCL14 as candidate biomarkers for early diagnosis of lung adenocarcinoma
Authors: J Li, J Li, H Hao, F Lu, J Wang, M Ma, B Jia, M Zhuo, J Wang, Y Chi, X Zhai, Y Wang, M Wu, T An, J Zhao, F Yang, Z Wang
BMC Cancer, 2023-01-31;23(1):110.
Species: Human
Sample Types: Plasma
-
Salivary Diagnostics in Pediatrics and the Status of Saliva-Based Biosensors
Authors: H Min, S Zhu, L Safi, M Alkourdi, BH Nguyen, A Upadhyay, SD Tran
Biosensors, 2023-01-30;13(2):.
Species: Human
Sample Types: Saliva
-
Challenges and the Way forward in Diagnosis and Treatment of Tuberculosis Infection
Authors: KL Chin, L Anibarro, ME Sarmiento, A Acosta
Tropical medicine and infectious disease, 2023-01-28;8(2):.
Species: Human
Sample Types: Serum
-
Effects of Intraoperative Support Strategies on Endothelial Injury and Clinical Lung Transplant Outcomes
Authors: JN Coster, K Noda, JP Ryan, EG Chan, M Furukawa, JD Luketich, PG Sanchez
Seminars in thoracic and cardiovascular surgery, 2023-01-28;0(0):.
Species: Human
Sample Types: Plasma
-
The Effects of HIV Infection on the Immune Response to Malaria Among Pregnant Women in Kumba, Southwest Cameroon: Protocol for a Cross-sectional Study
Authors: BN Obase, Z Francis, EL Forgu, A Honore, JD Bigoga, DS Nsagha
JMIR research protocols, 2023-01-24;12(0):e38213.
Species: Human
Sample Types: Plasma
-
Chemokine modulation in microscopic and submicroscopic Plasmodium falciparum malaria infection in women at delivery in Yaound�, Cameroon
Authors: R Megnekou, CMM Nana, JC Djontu, BMZ Bitye, BC Nana, BKT Zangue, CJ Donkeu, E Essangui, RM Salawiss, RNM Seumko'o, L Ayong, RGF Leke
PLoS ONE, 2023-01-23;18(1):e0280615.
Species: Human
Sample Types: Plasma
-
Imaging the tumour microenvironment in rectal cancer: Decline in tumour blood flow during radiotherapy predicts good outcome
Authors: KM Bakke, S Meltzer, E Grøvik, A Negård, SH Holmedal, LTG Mikalsen, AE Færden, LG Lyckander, FMI Julbø, A Bjørnerud, KI Gjesdal, AH Ree, KR Redalen
Physics and imaging in radiation oncology, 2023-01-23;25(0):100417.
Species: Human
Sample Types: Serum
-
Longitudinal efficacy and toxicity of SARS-CoV-2 vaccination in cancer patients treated with immunotherapy
Authors: P Spiliopoul, HJ Janse van, L Avery, V Kulasingam, A Razak, P Bedard, A Hansen, A Chruscinsk, B Wang, M Kulikova, R Chen, V Speers, A Nguyen, J Lee, B Coburn, A Spreafico, LL Siu
Cell Death & Disease, 2023-01-20;14(1):49.
Species: Human
Sample Types: Serum
-
Cytokine upsurge among drug-resistant tuberculosis endorse the signatures of hyper inflammation and disease severity
Authors: P Sampath, A Rajamanick, K Thiruvenga, AP Natarajan, S Hissar, M Dhanapal, B Thangavelu, L Jayabal, PM Ramesh, UD Ranganatha, S Babu, R Bethunaick
Scientific Reports, 2023-01-16;13(1):785.
Species: Human
Sample Types: Plasma
-
Plasma Cytokine Profiling Reveals Differences between Silicotic Patients with Simple Silicosis and Those with Progressive Massive Fibrosis Caused by Engineered Stone
Authors: A Campos-Car, G Jiménez-Gó, A García-Núñ, A Hidalgo-Mo, A León-Jimén
International Journal of Molecular Sciences, 2023-01-12;24(2):.
Species: Human
Sample Types: Plasma
-
The human placenta shapes the phenotype of decidual macrophages
Authors: S Vondra, AL Höbler, AI Lackner, J Raffetsede, ZN Mihalic, A Vogel, L Saleh, V Kunihs, P Haslinger, M Wahrmann, H Husslein, R Oberle, J Kargl, S Haider, P Latos, G Schabbauer, M Knöfler, J Ernerudh, J Pollheimer
Cell Reports, 2023-01-10;42(1):111977.
Species: Human
Sample Types: Cell Culture Supernates
-
Pro-Inflammatory and Cytotoxic Effects of Polystyrene Microplastics on Human and Murine Intestinal Cell Lines
Authors: V Mattioda, V Benedetti, C Tessarolo, F Oberto, A Favole, M Gallo, W Martelli, MI Crescio, E Berio, L Masoero, A Benedetto, M Pezzolato, E Bozzetta, C Grattarola, C Casalone, C Corona, F Giorda
Biomolecules, 2023-01-10;13(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Neural stem cell transplantation in patients with progressive multiple sclerosis: an open-label, phase 1 study
Authors: A Genchi, E Brambilla, F Sangalli, M Radaelli, M Bacigalupp, R Furlan, A Andolfo, D Drago, C Magagnotti, GM Scotti, R Greco, P Vezzulli, L Ottoboni, M Bonopane, D Capilupo, F Ruffini, D Belotti, B Cabiati, S Cesana, G Matera, L Leocani, V Martinelli, L Moiola, L Vago, P Panina-Bor, A Falini, F Ciceri, A Uglietti, MP Sormani, G Comi, MA Battaglia, MA Rocca, L Storelli, E Pagani, G Gaipa, G Martino
Nature Medicine, 2023-01-09;29(1):75-85.
Species: Human
Sample Types: CSF
-
The Effects of Combined Exposure to Simulated Microgravity, Ionizing Radiation, and Cortisol on the In Vitro Wound Healing Process
Authors: WE Radstake, K Gautam, S Miranda, R Vermeesen, K Tabury, E Rehnberg, J Buset, A Janssen, L Leysen, M Neefs, M Verslegers, J Claesen, MJ van Goethe, U Weber, C Fournier, A Parisi, S Brandenbur, M Durante, B Baselet, S Baatout
Cells, 2023-01-07;12(2):.
Species: Human
Sample Types: Cell Lysates
-
Spleen tyrosine kinase inhibition restores myeloid homeostasis in COVID-19
Authors: G Wigerblad, SA Warner, MJ Ramos-Beni, L Kardava, X Tian, R Miao, R Reger, M Chakrabort, S Wong, Y Kanthi, AF Suffredini, S Dell'Orso, S Brooks, C King, O Shlobin, SD Nathan, J Cohen, S Moir, RW Childs, MJ Kaplan, DS Chertow, JR Strich
Science Advances, 2023-01-04;9(1):eade8272.
Species: Human
Sample Types: Plasma
-
Conjugated Linoleic Acids Have Anti-Inflammatory Effects in Cultured Endothelial Cells
Authors: CA Valenzuela, EJ Baker, EA Miles, PC Calder
International Journal of Molecular Sciences, 2023-01-03;24(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
ERBB4-mediated signaling is a mediator of resistance to BTK and PI3K inhibitors in B cell lymphoid neoplasms
Authors: AJ Arribas, S Napoli, L Cascione, L Barnabei, G Sartori, E Cannas, E Gaudio, C Tarantelli, AA Mensah, F Spriano, A Zucchetto, FM Rossi, A Rinaldi, MC de Moura, S Jovic, RB Pittau, A Stathis, G Stussi, V Gattei, JR Brown, M Esteller, E Zucca, D Rossi, F Bertoni
bioRxiv : the preprint server for biology, 2023-01-02;0(0):.
Species: Human
Sample Types: Serum
-
Extracellular Matrix Expression in Human Pancreatic Fat Cells of Patients with Normal Glucose Regulation, Prediabetes and Type 2 Diabetes
Authors: Siegel-Axel, D;Barroso Oquendo, M;Gerst, F;Fend, F;Wagner, R;Heni, M;Königsrainer, A;Häring, H;Fritsche, A;Schleicher, E;Birkenfeld, A;Stefan, N;
International Journal of Molecular Sciences
Species: Human
Sample Types: Cell Culture Supernates
-
The ADAM9/WISP-1 axis cooperates with osteoblasts to stimulate primary prostate tumor growth and metastasis
Authors: AC Chang, LW Lin, YC Chen, PC Chen, SC Liu, HC Tai, HC Wu, SY Sung, TH Lin, CH Tang
International journal of biological sciences, 2023-01-01;19(3):760-771.
Species: Human
Sample Types: Cell Culture Supernates
-
Autoantibodies against chemokines post-SARS-CoV-2 infection correlate with disease course
Authors: J Muri, V Cecchinato, A Cavalli, AA Shanbhag, M Matkovic, M Biggiogero, PA Maida, J Moritz, C Toscano, E Ghovehoud, R Furlan, F Barbic, A Voza, G De Nadai, C Cervia, Y Zurbuchen, P Taeschler, LA Murray, G Danelon-Sa, S Moro, T Gong, P Piffaretti, F Bianchini, V Crivelli, L Podešvová, M Pedotti, D Jarrossay, J Sgrignani, S Thelen, M Uhr, E Bernasconi, A Rauch, A Manzo, A Ciurea, MBL Rocchi, L Varani, B Moser, B Bottazzi, M Thelen, BA Fallon, O Boyman, A Mantovani, C Garzoni, A Franzetti-, M Uguccioni, DF Robbiani
Nature Immunology, 2023;24(4):604-611.
Species: Human
Sample Types: Plasma
-
Alterations in Renin-Angiotensin System (RAS) Peptide Levels in Patients with HIV
Authors: I Asante, A Lu, BI Mitchell, WA Boisvert, CM Shikuma, DC Chow, SG Louie
Metabolites, 2022-12-31;13(1):.
Species: Human
Sample Types: Plasma
-
Extensively Hydrolyzed Hypoallergenic Infant Formula with Retained T Cell Reactivity
Authors: R Freidl, V Garib, B Linhart, EM Haberl, I Mader, Z Szépfalusi, K Schmidthal, N Douladiris, A Pampura, E Varlamov, T Lepeshkova, E Beltyukov, V Naumova, S Taka, D Nosova, O Guliashko, M Kundi, A Kiyamova, S Katsamaki, R Valenta
Nutrients, 2022-12-26;15(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
NKG2D receptor activation drives primary graft dysfunction severity and poor lung transplantation outcomes
Authors: DR Calabrese, T Tsao, M Magnen, C Valet, Y Gao, B Mallavia, JJ Tian, EA Aminian, KM Wang, A Shemesh, EB Punzalan, A Sarma, CS Calfee, SA Christenso, CR Langelier, SR Hays, JA Golden, LE Leard, ME Kleinhenz, NA Kolaitis, RJ Shah, A Venado, LL Lanier, JR Greenland, DM Sayah, A Ardehali, J Kukreja, SS Weigt, JA Belperio, JP Singer, MR Looney
JCI Insight, 2022-12-22;0(0):.
Species: Human
Sample Types: BALF
-
Engineering of immunoinstructive extracellular matrices for enhanced osteoinductivity
Authors: A García-Gar, S Pigeot, I Martin
Bioactive materials, 2022-12-21;24(0):174-184.
Species: Human
Sample Types: Tissue Homogenates
-
Senescence induces proteolytically-active PAPP-A secretion and association with extracellular vesicles in human pre-adipocytes
Authors: CA Conover, LK Bale
Experimental gerontology, 2022-12-20;172(0):112070.
Species: Human
Sample Types: Cell Culture Supernates
-
Distinct and dynamic activation profiles of circulating dendritic cells and monocytes in mild COVID-19 and after yellow fever vaccination
Authors: E Winheim, T Eser, F Deák, MIM Ahmed, O Baranov, L Rinke, K Eisenächer, A Santos-Per, H Karimzadeh, M Pritsch, C Scherer, M Muenchhoff, JC Hellmuth, M von Bergwe, L Olbrich, M Hoelscher, A Wieser, I Kroidl, S Rothenfuss, C Geldmacher, AB Krug
European Journal of Immunology, 2022-12-09;0(0):.
Species: Human
Sample Types: Plasma
-
Proteomics profiles of blood glucose-related proteins involved in a Chinese longevity cohort
Authors: R Zhang, F Liu, S Ye, X Du, L Ma, H Cao, Z Wang, C Li
Clinical Proteomics, 2022-12-03;19(1):45.
Species: Human
Sample Types: Plasma
-
Cytokines and Chemokines in Breastmilk of SARS-CoV-2 Infected or COVID-19 Vaccinated Mothers
Authors: F Trofin, OS Dorneanu, D Constantin, EV Nastase, C Lunc?, LS Iancu, IM Andrioaie, A Duhaniuc, CM Cianga, M Pavel-Tana, DT Anton-P?du, P Cianga
Vaccines, 2022-11-24;10(12):.
Species: Human
Sample Types: Breast Milk
-
Pro-Inflammatory Alterations of Circulating Monocytes in Latent Tuberculosis Infection
Authors: MG Feria, C Chang, E Ticona, A Moussa, B Zhang, I Ballena, R Azañero, C Ticona, CN De Cecco, CJ Fichtenbau, RE O'Donnell, A La Rosa, J Sanchez, S Andorf, L Atehortua, JD Katz, CA Chougnet, GS Deepe, MA Huaman
Open forum infectious diseases, 2022-11-19;9(12):ofac629.
Species: Human
Sample Types: Plasma
-
Adjuvants in fungicide formulations can be skin sensitizers and cause different types of cell stress responses
Authors: RI de Ávila, S Carreira S, V Siino, F Levander, M Lindstedt, KS Zeller
Toxicology reports, 2022-11-18;9(0):2030-2041.
Species: Human
Sample Types: Cell Culture Supernates
-
Comprehensive Assessment of Secreted Immuno-Modulatory Cytokines by Serum-Differentiated and Stem-like Glioblastoma Cells Reveals Distinct Differences between Glioblastoma Phenotypes
Authors: LD Robilliard, J Yu, A Anchan, G Finlay, CE Angel, ES Graham
International Journal of Molecular Sciences, 2022-11-16;23(22):.
Species: Human
Sample Types: Cell Culture Supernates
-
Clinical significance of serum and vitreous soluble interleukin-2 receptor in patients with intraocular lymphoma
Authors: K Suzuki, K Namba, S Kase, Y Ogino, K Hase, D Iwata, K Mizuuchi, M Hiraoka, N Kitaichi, S Ishida
BMC ophthalmology, 2022-11-10;22(1):428.
Species: Human
Sample Types: Vitreous Humor
-
Urinary Cysteinyl Leukotrienes as Biomarkers of Endothelial Activation, Inflammation and Oxidative Stress and Their Relationship with Organ Dysfunction in Human Septic Shock
Authors: M Reina-Cout, M Santos-Oli, P Pereira-Te, C Silva-Pere, J Quelhas-Sa, Á Duarte, S Martins, P Serrão, CC Dias, M Morato, JT Guimarães, R Roncon-Alb, JA Paiva, A Albino-Tei, T Sousa
Biomedicines, 2022-11-08;10(11):.
Species: Human
Sample Types: Serum
-
A multi-marker integrative analysis reveals benefits and risks of bariatric surgery
Authors: S Palleschi, V Guglielmi, L Nisticò, C Ferreri, C Tabolacci, F Facchiano, E Iorio, A Giuliani, S Brescianin, E Medda, C Fagnani, B Rossi, A Minoprio, M Chirico, ME Pisanu, F Di Nolfo, P Fortini, V Simonelli, S Baccarini, S Laterza, T Morretti, A Dell'Orso, F Manganello, P Gentilesch, P Sbraccia, E Dogliotti
Scientific Reports, 2022-11-07;12(1):18877.
Species: Human
Sample Types: Plasma
-
Association between Serum Biomarkers and Peripheral Neuropathy in Microscopic Polyangiitis
Authors: Y Masuda, S Matsuda, T Kotani, D Nishioka, S Ota, T Hosokawa, S Ishida, T Takeuchi
Oncogene, 2022-11-02;23(21):.
Species: Human
Sample Types: Serum
-
HFpEF and Atrial Fibrillation: The Enigmatic Interplay of Dysmetabolism, Biomarkers, and Vascular Endothelial Dysfunction
Authors: J Bosanac, L Straus, M Novakovi?, D Košuta, M Boži? Mijo, J Tasi?, B Jug
Disease Markers, 2022-10-25;2022(0):9539676.
Species: Human
Sample Types: Plasma
-
Epstein Barr virus-mediated transformation of B cells from XIAP-deficient patients leads to increased expression of the tumor suppressor CADM1
Authors: C Engelmann, P Schuhmache, H Zdimerova, S Virdi, M Hauri-Hohl, J Pachlopnik, A Grundhoff, RA Marsh, WW Wong, C Münz
Cell Death & Disease, 2022-10-22;13(10):892.
Species: Human
Sample Types: Cell Culture Supernates
-
Biological Effects of Intravenous Vitamin C on Neutrophil Extracellular Traps and the Endothelial Glycocalyx in Patients with Sepsis-Induced ARDS
Authors: X Qiao, MG Kashiouris, M L'Heureux, BJ Fisher, SW Leichtle, JD Truwit, R Nanchal, RD Hite, PE Morris, GS Martin, J Sevransky, AA Fowler
Nutrients, 2022-10-21;14(20):.
Species: Human
Sample Types: Plasma
-
Sex as a determinant of disease severity and clinical outcome in febrile children under five presenting to a regional referral hospital in Uganda
Authors: CR McDonald, AM Weckman, E Richardson, MT Hawkes, A Leligdowic, S Namasopo, RO Opoka, AL Conroy, KC Kain
PLoS ONE, 2022-10-21;17(10):e0276234.
Species: Human
Sample Types: Plasma
-
S100A8-mediated metabolic adaptation controls HIV-1 persistence in macrophages in vivo
Authors: F Real, A Zhu, B Huang, A Belmellat, A Sennepin, T Vogl, C Ransy, M Revol, R Arrigucci, A Lombès, J Roth, ML Gennaro, F Bouillaud, S Cristofari, M Bomsel
Nature Communications, 2022-10-11;13(1):5956.
Species: Human
Sample Types: Tissue Homogenates
-
Cocaine use associated gut permeability and microbial translocation in people living with HIV in the Miami Adult Study on HIV (MASH) cohort
Authors: J Hernandez, JA Tamargo, S Sales Mart, HR Martin, A Campa, RP Sékaly, R Bordi, KE Sherman, SD Rouster, HL Meeds, JH Khalsa, RN Mandler, S Lai, MK Baum
PLoS ONE, 2022-10-10;17(10):e0275675.
Species: Human
Sample Types: Plasma
-
Receptor for Advanced Glycation End-Products Promotes Activation of Alveolar Macrophages through the NLRP3 Inflammasome/TXNIP Axis in Acute Lung Injury
Authors: W Lenga Ma B, M Fournet, R Zhai, J Lutz, R Blondonnet, C Bourgne, C Leclaire, C Saint-Béat, C Theilliere, C Belville, D Bouvier, L Blanchon, M Berger, V Sapin, M Jabaudon
International Journal of Molecular Sciences, 2022-10-01;23(19):.
Species: Human
Sample Types: Cell Culture Supernates
-
The Giardial Arginine Deiminase Participates in Giardia-Host Immunomodulation in a Structure-Dependent Fashion via Toll-like Receptors
Authors: C Fernández-, I de la Mora, S Enríquez-F, I García-Tor, LA Flores-Lóp, P Gutiérrez-, P de Vos, G López-Velá
International Journal of Molecular Sciences, 2022-09-30;23(19):.
Species: Human
Sample Types: Cell Culture Supernates
-
Selenium-Stimulated Exosomes Enhance Wound Healing by Modulating Inflammation and Angiogenesis
Authors: JS Heo
International Journal of Molecular Sciences, 2022-09-29;23(19):.
Species: Human
Sample Types: Cell Culture Supernates
-
Cytokine Profile at Diagnosis Affecting Trough Concentration of Infliximab in Pediatric Crohn's Disease
Authors: Y Kwon, ES Kim, YZ Kim, YH Choe, MJ Kim
Biomedicines, 2022-09-23;10(10):.
Species: Human
Sample Types: Plasma
-
The Role of Fatty Acid-Binding Protein 4 in the Characterization of Atrial Fibrillation and the Prediction of Outcomes after Catheter Ablation
Authors: JN López-Cano, M Couselo-Se, T González-F, C Almengló, E Álvarez, A González-M, L González-M, JL Martínez-S, J García-Sea, J Fernández-, B Kreidieh, E González-B, JR González-J, S Eiras, M Rodríguez-
International Journal of Molecular Sciences, 2022-09-21;23(19):.
Species: Human
Sample Types: Plasma
-
Immediate outcome prognostic value of plasma factors in patients with acute ischemic stroke after intravenous thrombolytic treatment
Authors: H Lu, S Li, X Zhong, S Huang, X Jiao, G He, B Jiang, Y Liu, Z Gao, J Wei, Y Lin, Z Chen, Y Li
BMC neurology, 2022-09-20;22(1):359.
Species: Human
Sample Types: Plasma
-
The JAK1/2 Inhibitor Baricitinib Mitigates the Spike-Induced Inflammatory Response of Immune and Endothelial Cells In Vitro
Authors: A Barilli, R Visigalli, F Ferrari, G Recchia Lu, M Soli, V Dall'Asta, BM Rotoli
Biomedicines, 2022-09-19;10(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Clinical, metabolic, and immunological characterisation of adult Ugandan patients with new-onset diabetes and low vitamin D status
Authors: D Kibirige, I Sekitoleko, P Balungi, J Kyosiimire, W Lumu
BMC endocrine disorders, 2022-09-15;22(1):230.
Species: Human
Sample Types: Serum
-
Minimal Development of Liver Fibrosis in Adult Tolerant Liver Transplant Recipients Late After Immunosuppressive Drug Weaning and Transplantation
Authors: AA Duizendstr, RJ De Knegt, NMA Nagtzaam, MGH Betjes, WA Dik, NHR Litjens, J Kwekkeboom
Transplantation Proceedings, 2022-09-11;0(0):.
Species: Human
Sample Types: Serum
-
Amaryllidaceae Alkaloids Decrease the Proliferation, Invasion, and Secretion of Clinically Relevant Cytokines by Cultured Human Colon Cancer Cells
Authors: V Mathieu, B Laguera, M Masi, SA Dulanto, TW Bingham, LW Hernandez, D Sarlah, A Evidente, DLJ Lafontaine, A Kornienko, MA Lane
Biomolecules, 2022-09-09;12(9):.
Species: Human
Sample Types: Cell Culture Supernatants
-
Evaluation of a Supervised Adapted Physical Activity Program Associated or Not with Oral Supplementation with Arginine and Leucine in Subjects with Obesity and Metabolic Syndrome: A Randomized Controlled Trial
Authors: V Folope, C Meret, I Castres, C Tourny, E Houivet, S Grigioni, H Lelandais, A Petit, A Coquard, C Guérin, M Quillard, C Bôle-Feyso, P Déchelotte, N Achamrah, M Coëffier
Nutrients, 2022-09-08;14(18):.
Species: Human
Sample Types: Plasma
-
Integrated Antitumor Activities of Cellular Immunotherapy with CIK Lymphocytes and Interferons against KIT/PDGFRA Wild Type GIST
Authors: E Fiorino, A Merlini, L D'Ambrosio, I Cerviere, E Berrino, C Marchiò, L Giraudo, M Basiricò, A Massa, C Donini, V Leuci, R Rotolo, F Galvagno, L Vitali, A Proment, S Ferrone, A Pisacane, Y Pignochino, M Aglietta, G Grignani, G Mesiano, D Sangiolo
International Journal of Molecular Sciences, 2022-09-08;23(18):.
Species: Human
Sample Types: Cell Culture Supernates
-
Intestinal Injury Biomarkers Predict Mortality in Pediatric Severe Malaria
Authors: ML Sarangam, R Namazzi, D Datta, C Bond, CPB Vanderpool, RO Opoka, CC John, AL Conroy
MBio, 2022-09-07;0(0):e0132522.
Species: Human
Sample Types: Serum
-
Lumican is elevated in the lung in human and experimental acute respiratory distress syndrome and promotes early fibrotic responses to lung injury
Authors: K Wang, Y Wang, Y Cao, H Wang, Y Zhou, L Gao, Z Zeng, M Cheng, X Jin, J Chen, F Wen, T Wang
Journal of Translational Medicine, 2022-09-04;20(1):392.
Species: Human
Sample Types: BALF
-
"Role of kidney function and concentrations of BAFF, sPD-L1 and sCD25 on mortality in hospitalized patients with COVID-19"
Authors: L Mansouri, S Sendic, S Havervall, C Thålin, SH Jacobson, J Lundahl
Bmc Nephrology, 2022-09-02;23(1):299.
Species: Human
Sample Types: Plasma
-
The impact of gender and peripheral blood parameters on the characteristics of L-PRF membranes
Authors: C Andrade Al, F Ugarte Ame, C Inostroza, P Diaz Calde, D Rosenberg, N Pinto Carr, M Quirynen
Journal of oral biology and craniofacial research, 2022-09-01;12(6):753-759.
Species: Human
Sample Types: Cell Culture Supernates
-
Protective Immunity of COVID-19 Vaccination with ChAdOx1 nCoV-19 Following Previous SARS-CoV-2 Infection: A Humoral and Cellular Investigation
Authors: T Azamor, IS Horbach, D Brito E Cu, JG Melgaço, AMVD Silva, LN Tubarão, AS Azevedo, RT Santos, NDS Alves, TL Machado, J Silva, AF Souza, C Bayma, VP Rocha, ABT Frederico, BM Dias, BP Setatino, CB Denani, SPDC Campos, WD Schwarcz, MV Sucupira, EP Mendes, EDD Silva, SM Barbosa de, APD Ano Bom, S Missailidi
Viruses, 2022-08-30;14(9):.
Species: Human
Sample Types: Plasma
-
Receptor-kinase EGFR-MAPK adaptor proteins mediate the epithelial response to Candida albicans via the cytolytic peptide toxin, candidalysin
Authors: NO Ponde, L Lortal, A Tsavou, OW Hepworth, DN Wickramasi, J Ho, JP Richardson, DL Moyes, SL Gaffen, JR Naglik
The Journal of Biological Chemistry, 2022-08-28;0(0):102419.
Species: Human
Sample Types: Cell Culture Supenates
-
Assessing the response of human primary macrophages to defined fibrous architectures fabricated by melt electrowriting
Authors: C Mondadori, A Chandrakar, S Lopa, P Wieringa, G Talò, S Perego, G Lombardi, A Colombini, M Moretti, L Moroni
Bioactive materials, 2022-08-27;21(0):209-222.
Species: Human
Sample Types: Cell Culture Supernates
-
Utility of bile acids in large airway bronchial wash versus bronchoalveolar lavage as biomarkers of microaspiration in lung transplant recipients: a retrospective cohort study
Authors: CYK Zhang, M Ahmed, E Huszti, L Levy, SE Hunter, KM Boonstra, S Moshkelgos, AT Sage, S Azad, R Ghany, JC Yeung, OM Crespin, LG Singer, S Keshavjee, T Martinu
Respiratory Research, 2022-08-26;23(1):219.
Species: Human
Sample Types: BALF
-
Melanoma Mediated Disruption of Brain Endothelial Barrier Integrity Is Not Prevented by the Inhibition of Matrix Metalloproteinases and Proteases
Authors: A Anchan, G Finlay, CE Angel, JJW Hucklesby, SE Graham
Biosensors, 2022-08-19;12(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Characteristics of major and macular branch retinal vein occlusion
Authors: YJ Choi, D Jee, JW Kwon
Scientific Reports, 2022-08-18;12(1):14103.
Species: Human
Sample Types: Aqueous Humor
-
Sinonasal Biomarkers Defining Type 2-High and Type 2-Low Inflammation in Chronic Rhinosinusitis with Nasal Polyps
Authors: E De Corso, S Baroni, S Settimi, ME Onori, RF Mastrapasq, E Troiani, G Moretti, D Lucchetti, M Corbò, C Montuori, A Cantiani, DP Porru, S Lo Verde, GA Di Bella, C Caruso, J Galli
Journal of personalized medicine, 2022-07-29;12(8):.
Species: Human
Sample Types: Nasal Fluid
-
Serum levels of matrix metalloproteinases as prognostic markers for severe dengue with plasma leakage
Authors: S Sivasubram, S Mohandas, V Gopalan, K Govindan, P Varadaraja, K Kaveri, KM Ramkumar
Experimental and molecular pathology, 2022-07-28;128(0):104821.
Species: Human
Sample Types: Serum
-
Immune suppression is associated with enhanced systemic inflammatory, endothelial and procoagulant responses in critically ill patients
Authors: X Brands, F Uhel, LA van Vught, MA Wiewel, AJ Hoogendijk, R Lutter, MJ Schultz, BP Scicluna, T van der Po
PLoS ONE, 2022-07-25;17(7):e0271637.
Species: Human
Sample Types: Plasma
-
Comparison of Pulmonary Function and Inflammation in Children/Adolescents with New-Onset Asthma with Different Adiposity Statuses
Authors: X Ying, J Lin, S Yuan, C Pan, W Dong, J Zhang, L Zhang, J Lin, Y Yin, J Wu
Nutrients, 2022-07-20;14(14):.
Species: Human
Sample Types: Serum
-
Overnutrition, Nasopharyngeal Pathogenic Bacteria and Proinflammatory Cytokines in Infants with Viral Lower Respiratory Tract Infections
Authors: G Arias-Brav, G Valderrama, J Inostroza, C Tapia, D Toro-Ascuy, O Ramilo, P Orellana, N Cifuentes-, F Zorondo-Ro, A Mejias, LF Fuenzalida
International Journal of Environmental Research and Public Health, 2022-07-19;19(14):.
Species: Human
Sample Types: Nasal Fluid
-
Immune and endothelial activation markers and risk stratification of childhood pneumonia in Uganda: A secondary analysis of a prospective cohort study
Authors: CR McDonald, A Leligdowic, AL Conroy, AM Weckman, M Richard-Gr, M Ngai, C Erice, K Zhong, S Namasopo, RO Opoka, MT Hawkes, KC Kain
Oncogene, 2022-07-13;19(7):e1004057.
Species: Human
Sample Types: Plasma
-
Multi-omic characterization of pediatric ARDS via nasal brushings
Authors: JG Williams, R Joshi, D Haslam, N Yehya, RL Jones, A Paranjpe, M Pujato, KM Roskin, PM Lahni, HR Wong, BM Varisco
Respiratory Research, 2022-07-09;23(1):181.
Species: Human
Sample Types: Serum
-
The association between amniotic fluid-derived inflammatory mediators and the risk of retinopathy of prematurity
Authors: JH Jang, JG Kim, YH Lee, JG Bae, JH Park
Oncogene, 2022-07-08;101(27):e29368.
Species: Human
Sample Types: Amniotic Fluid
-
White Blood Cell Count and Serum Cytokine Profile in Tropical Hardwood Workers in Kumasi
Authors: IE Ennin, MA Frempong, D Dodoo, FA Yeboah, RS Maalman
Oncogene, 2022-06-27;2022(0):8245717.
Species: Human
Sample Types: Serum
-
Inflammation, Amygdala-Ventromedial Prefrontal Functional Connectivity and Symptoms of Anxiety and PTSD in African American Women Recruited from an Inner-City Hospital: Preliminary Results
Authors: ND Mehta, JS Stevens, Z Li, N Fani, CF Gillespie, M Ravi, V Michopoulo, JC Felger
Brain, Behavior, and Immunity, 2022-06-27;105(0):122-130.
Species: Human
Sample Types: Plasma
-
Increasing circulating ESM-1 and adhesion molecules are associated with earlystage atherosclerosis in OSA patients:A cross-sectional study
Authors: H Sun, Y Du, L Zhang, H Yu, X Jiao, Q Lv, F Li, Y Wang, Q Sun, C Hu, L Li, H Zhang, Z Du, Y Qin
Sleep medicine, 2022-06-23;98(0):114-120.
Species: Human
Sample Types: Plasma
-
CD96 Downregulation Promotes the Immune Response of CD4 T Cells and Associates with Ankylosing Spondylitis
Authors: F Wu, H Yang, X Xu, C Ren, Y Zheng, H Zhang, B Cai, R Qiu, W Ren, R Quan
Oncogene, 2022-06-19;2022(0):3946754.
Species: Human
Sample Types: Cell Culture Supernates
-
Analysis of hepatic fibrosis markers in the serum of chronic hepatitis B patients according to basal core promoter/precore mutants
Authors: C Lefeuvre, M Roux, S Blanchard, H Le Guillou, J Boursier, F Lunel-Fabi, P Jeannin, A Pivert, A Ducancelle
Oncogene, 2022-06-17;12(1):10261.
Species: Human
Sample Types: Serum
-
Effect of add-on hydroxychloroquine therapy on serum proinflammatory cytokine levels in patients with systemic lupus erythematosus
Authors: R Wakiya, K Ueeda, S Nakashima, H Shimada, T Kameda, MMF Mansour, M Kato, T Miyagi, K Sugihara, M Mizusaki, R Mino, N Kadowaki, H Dobashi
Scientific Reports, 2022-06-17;12(1):10175.
Species: Human
Sample Types: Serum
-
Evaluation of host biomarkers for monitoring treatment response in spinal tuberculosis: A 12-month cohort study
Authors: TN Mann, JH Davis, C Beltran, G Walzl, J du Toit, RP Lamberts, NN Chegou
Cytokine, 2022-06-17;157(0):155944.
Species: Human
Sample Types: Serum
-
Mechanisms of Sensitivity and Resistance of Primary Effusion Lymphoma to Dimethyl Fumarate (DMF)
Authors: R Gonnella, R Zarrella, R Santarelli, CA Germano, MS Gilardini, M Cirone
International Journal of Molecular Sciences, 2022-06-17;23(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
A Cease in Shift Work Reverses Arterial Stiffness but Increases Weight and Glycosylated Hemoglobin A 5-Month Follow-Up in Industry
Authors: M Skogstad, HCD Aass, LK Lunde, Ø Skare, PA Sirnes, D Matre
Oncogene, 2022-06-12;9(6):.
Species: Human
Sample Types: Plasma
-
Regulatory role of miR-146a in corneal epithelial wound healing via its inflammatory targets in human diabetic cornea
Authors: AJ Poe, R Shah, D Khare, M Kulkarni, H Phan, S Ghiam, V Punj, AV Ljubimov, M Saghizadeh
The ocular surface, 2022-06-09;25(0):92-100.
Species: Human
Sample Types: Cell Culture Supernates
-
Plasma extracellular vesicles bearing PD-L1, CD40, CD40L or TNF-RII are significantly reduced after treatment of AIDS-NHL
Authors: LE Martínez, S Lensing, D Chang, LI Magpantay, R Mitsuyasu, RF Ambinder, JA Sparano, O Martínez-M, M Epeldegui
Oncogene, 2022-06-02;12(1):9185.
Species: Human
Sample Types: Extracellular Vesicles
-
DNA damage to beta cells in culture recapitulates features of senescent beta cells that accumulate in type 1 diabetes
Authors: G Brawerman, J Pipella, PJ Thompson
Molecular Metabolism, 2022-06-02;0(0):101524.
Species: Human
Sample Types: Cell Culture Supernates
-
Cytomegalovirus proteins, maternal pregnancy cytokines, and their impact on neonatal immune cytokine profiles and acute lymphoblastic leukemogenesis in children
Authors: Joseph L. Wiemels, Rong Wang, Mi Zhou, Helen Hansen, Rachel Gallant, Junghyun Jung et al.
Haematologica
Species: Human
Sample Types: Serum
-
Lung Biomolecular Profile and Function of Grafts from Donors after Cardiocirculatory Death with Prolonged Donor Warm Ischemia Time
Authors: F Gori, J Fumagalli, C Lonati, A Carlin, P Leonardi, O Biancolill, A Rossetti, I Righi, D Tosi, A Palleschi, L Rosso, LC Morlacchi, F Blasi, L Vivona, G Florio, V Scaravilli, F Valenza, A Zanella, G Grasselli
Oncogene, 2022-05-29;11(11):.
Species: Human
Sample Types: Lung Perfusate
-
Characteristics of the gut microbiota in women with premenstrual symptoms: A cross-sectional study
Authors: T Takeda, K Yoshimi, S Kai, G Ozawa, K Yamada, K Hiramatsu
PLoS ONE, 2022-05-27;17(5):e0268466.
Species: Human
Sample Types: Serum
-
Changes in salivary matrix metalloproteinase-3, -8, and -9 concentrations after 6�weeks of non-surgical periodontal therapy
Authors: HN Kim
BMC Oral Health, 2022-05-13;22(1):175.
Species: Human
Sample Types: Saliva
-
Isatis tinctoria L. Leaf Extract Inhibits Replicative Senescence in Dermal Fibroblasts by Regulating mTOR-NF-kappaB-SASP Signaling
Authors: J Woo, S Shin, H Ji, D Ryu, E Cho, Y Kim, J Kim, D Park, E Jung
Nutrients, 2022-05-09;14(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
P. aeruginosa type III and type VI secretion systems modulate early response gene expression in type II pneumocytes in vitro
Authors: E Sen-Kilic, AB Huckaby, FH Damron, M Barbier
BMC Genomics, 2022-05-04;23(1):345.
Species: Human
Sample Types: Cell Culture Supernates
-
Longitudinal multi-omics analyses link gut microbiome dysbiosis with recurrent urinary tract infections in women
Authors: CJ Worby, HL Schreiber, TJ Straub, LR van Dijk, RA Bronson, BS Olson, JS Pinkner, CLP Obernuefem, VL Muñoz, AE Paharik, PN Azimzadeh, BJ Walker, CA Desjardins, WC Chou, K Bergeron, SB Chapman, A Klim, AL Manson, TJ Hannan, TM Hooton, AL Kau, HH Lai, KW Dodson, SJ Hultgren, AM Earl
Nature Microbiology, 2022-05-02;7(5):630-639.
Species: Human
Sample Types: Plasma
-
Autologous hematopoietic stem cell transplantation promotes connective tissue remodeling in systemic sclerosis patients
Authors: DC Zanin-Silv, M Santana-Go, MY Kawashima-, JR Lima-Júnio, JBE Dias, DA Moraes, DT Covas, KCR Malmegrim, L Ramalho, MC Oliveira
Arthritis Research & Therapy, 2022-04-29;24(1):95.
Species: Human
Sample Types: Serum
-
EGFR/MET promotes hepatocellular carcinoma metastasis by stabilizing tumor cells and resisting to RTKs inhibitors in circulating tumor microemboli
Authors: S Song, Z Yu, Y You, C Liu, X Xie, H Lv, F Xiao, Q Zhu, C Qin
Cell Death & Disease, 2022-04-15;13(4):351.
Species: Human
Sample Types: Cell Culture Supernates
-
Blood biomarkers in early bacterial infection and sepsis diagnostics in feverish young children
Authors: A Dagys, G Laucaityt?, A Volkevi?i?, S Abramavi?i, R K?valas, A Vitkauskie, L Jankauskai
International Journal of Medical Sciences, 2022-04-11;19(4):753-761.
Species: Human
Sample Types: Plasma
-
Do urinary metals associate with the homeostasis of inflammatory mediators? Results from the perspective of inflammatory signaling in middle-aged and older adults
Authors: A Li, Y Mei, M Zhao, J Xu, J Zhao, Q Zhou, X Ge, Q Xu
Environment international, 2022-04-09;163(0):107237.
Species: Human
Sample Types: Plasma
-
The role of albumin and the extracellular matrix on the pathophysiology of oedema formation in severe malnutrition
Authors: GB Gonzales, JM Njunge, BM Gichuki, B Wen, M Ngari, I Potani, J Thitiri, D Laukens, W Voskuijl, R Bandsma, J Vanmassenh, JA Berkley
EBioMedicine, 2022-04-07;79(0):103991.
Species: Human
Sample Types: Plasma
-
Low-Concentrations of Fatty Acids Induce an Early Increase in IL-8 Levels in Normal Human Astrocytes
Authors: AM Dobri, E Codrici, ID Popescu, L Albulescu, ET Fertig, AM Enciu, C Tanase, ME Hinescu
Metabolites, 2022-04-06;12(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
FcgammaR-mediated SARS-CoV-2 infection of monocytes activates inflammation
Authors: C Junqueira, Â Crespo, S Ranjbar, LB de Lacerda, M Lewandrows, J Ingber, B Parry, S Ravid, S Clark, MR Schrimpf, F Ho, C Beakes, J Margolin, N Russell, K Kays, J Boucau, U Das Adhika, SM Vora, V Leger, L Gehrke, L Henderson, E Janssen, D Kwon, C Sander, J Abraham, MB Goldberg, H Wu, G Mehta, S Bell, AE Goldfeld, MR Filbin, J Lieberman
Nature, 2022-04-06;0(0):.
Species: Human
Sample Types: Plasma
-
Tobacco Smoking and Second-Hand Smoke Exposure Impact on Tuberculosis in Children
Authors: N Altet, I Latorre, MÁ Jiménez-Fu, A Soriano-Ar, R Villar-Her, C Milà, P Rodríguez-, B Muriel-Mor, P Comella-De, P Godoy, JP Millet, ML de Souza-G, CA Jiménez-Ru, J Domínguez, On Behalf
Journal of Clinical Medicine, 2022-04-02;11(7):.
Species: Human
Sample Types: Plasma
-
RBMS1 promotes gastric cancer metastasis through autocrine IL-6/JAK2/STAT3 signaling
Authors: M Liu, H Li, H Zhang, H Zhou, T Jiao, M Feng, F Na, M Sun, M Zhao, L Xue, L Xu
Cell Death & Disease, 2022-03-31;13(3):287.
Species: Human
Sample Types: Cell Culture Supernates
-
Circulating ADAMTS13 Levels Are Associated with an Increased Occurrence of Obstructive Sleep Apnea
Authors: M Huang, S Liu, S Liu, W Wen, Y Ning, Y Jia, Y Yang, X Jiao, W Zheng, M Zhang
Disease Markers, 2022-03-29;2022(0):1504137.
Species: Human
Sample Types: Plasma
-
Blood KL-6 predicts prognosis in primary Sj�gren's syndrome-associated interstitial lung disease
Authors: YJ Kim, J Choe, SJ Moon, JW Song
Scientific Reports, 2022-03-29;12(1):5343.
Species: Human
Sample Types: Plasma
-
NADPH oxidase 4 signaling in a ventilator-induced lung injury mouse model
Authors: SH Lee, MH Shin, AY Leem, SH Lee, KS Chung, YS Kim, MS Park
Respiratory Research, 2022-03-27;23(1):73.
Species: Human
Sample Types: BALF
-
Endoglin and Other Angiogenesis Markers in Recurrent Varicose Veins
Authors: FSL Sánchez, JAC Martínez, L Méndez-Gar, MB García-Cen, M Pericacho
Journal of personalized medicine, 2022-03-25;12(4):.
Species: Human
Sample Types: Serum
-
Immune Activation in Primary Human Immunodeficiency Virus: Influence of Duration of Infection, Treatment, and Substance Use
Authors: T Gilada, SR Schnittman, E White, J Mercader, Y Wang, S Dasgupta, R Valdez, D Pinto-Sant, S Pasalar, J Sanchez, P Gonzales, JR Lama, R Bender Ign, A Duerr
Open forum infectious diseases, 2022-03-24;9(6):ofac155.
Species: Human
Sample Types: Plasma
-
Placental lesions and differential expression of pro-and anti-angiogenic growth mediators and oxidative DNA damage marker in placentae of Ghanaian suboptimal and optimal health status pregnant women who later developed preeclampsia
Authors: EO Anto, DA Coall, EA Asiamah, OO Afriyie, O Addai-Mens, YA Wiafe, W Owiredu, C Obirikoran, ME Annani-Ako, NA Titiloye, E Adua, E Acheampong, EA Adu, S Opoku, AO Anto, A Tawiah, Y Wang, W Wang
PLoS ONE, 2022-03-21;17(3):e0265717.
Species: Human
Sample Types: Serum
-
Molecular Biomarkers of Prognosis in Advanced Renal Cell Carcinoma Patients Treated With Pazopanib Plus Interferon Alpha (INF-2A) in a Phase I/II Study by the Spanish Oncology Genitourinary Group
Authors: X García-Del, I Durán, JL Perez-Grac, M Ángel Clim, B Mellado, JA Virizuela, DE Castellano, A González D, I García Car, C Álvarez-Fe, J García-Don, M Gil-Martin, AG Hernández
Clinical genitourinary cancer, 2022-03-18;0(0):.
Species: Human
Sample Types: Serum
-
3,4-Dihydroxyphenylethanol (DPE or Hydroxytyrosol) Counteracts ERK1/2 and mTOR Activation, Pro-Inflammatory Cytokine Release, Autophagy and Mitophagy Reduction Mediated by Benzo[a]pyrene in Primary Human Colonic Epithelial Cells
Authors: R Santarelli, C Pompili, MS Gilardini, L Evangelist, R Gonnella, M Cirone
Pharmaceutics, 2022-03-17;14(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Natural antibody IgG levels are associated with HBeAg-positivity and seroconversion in chronic hepatitis B patients treated with entecavir
Authors: YH Choi, HW Lee, MA Purdy
Scientific Reports, 2022-03-14;12(1):4382.
Species: Human
Sample Types: Serum
-
Resistin production does not affect outcomes in a mouse model of acute surgical sepsis
Authors: AS Bonavia, ZC Chroneos, V Ruiz-Velas, CH Lang
PLoS ONE, 2022-03-14;17(3):e0265241.
Species: Human
Sample Types: Serum
-
Cytokine responses to LPS in reprogrammed monocytes are associated with the transcription factor PU.1
Authors: K Tuerxun, K Midtbö, E Särndahl, E Vorontsov, R Karlsson, A Persson, R Kruse, D Eklund
Journal of leukocyte biology, 2022-03-13;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Immune-Mediated Inflammatory Responses of Alveolar Epithelial Cells: Implications for COVID-19 Lung Pathology
Authors: A Barilli, R Visigalli, F Ferrari, MG Bianchi, V Dall'Asta, BM Rotoli
Biomedicines, 2022-03-07;10(3):.
Species: Human
Sample Types: Conditioned Media
-
Extracellular pyruvate kinase M2 promotes osteoclastogenesis and is associated with radiographic progression in early rheumatoid arthritis
Authors: DW Han, YS Choi, HW Kim, S Shin, YJ Ha, EH Kang, JW Park, JK Park, K Shin, YW Song, YJ Lee
Scientific Reports, 2022-03-07;12(1):4024.
Species: Human
Sample Types: Serum
-
Plasma proteome changes linked to late phase response after inhaled allergen challenge in asthmatics
Authors: M Weitoft, M Kadefors, H Stenberg, E Tufvesson, Z Diamant, S Rolandsson, L Bjermer, O Rosmark, G Westergren
Respiratory Research, 2022-03-05;23(1):50.
Species: Human
Sample Types: Plasma
-
Multiplexed measurements of salivary fetuin-A, insulin, and adiponectin as potential non-invasive biomarkers in childhood obesity
Authors: V Selvaraju, JR Babu, T Geetha
Cytokine, 2022-03-04;153(0):155843.
Species: Human
Sample Types: Saliva
-
Salivary biomarkers in children with juvenile idiopathic arthritis and healthy age-matched controls: a prospective observational study
Authors: M Collin, M Ernberg, N Christidis, B Hedenberg-
Scientific Reports, 2022-02-25;12(1):3240.
Species: Human
Sample Types: Saliva
-
Dried human cultured epidermis accelerates wound healing in diabetic mouse skin defect wounds
Authors: M Sakamoto, T Nakano, I Tsuge, H Yamanaka, Y Katayama, Y Shimizu, Y Note, M Inoie, N Morimoto
Scientific Reports, 2022-02-24;12(1):3184.
Species: Human
Sample Types: Cell Culture Supernates
-
Microbial-driven preterm labour involves crosstalk between the innate and adaptive immune response
Authors: D Chan, PR Bennett, YS Lee, S Kundu, TG Teoh, M Adan, S Ahmed, RG Brown, AL David, HV Lewis, B Gimeno-Mol, JE Norman, SJ Stock, V Terzidou, P Kropf, M Botto, DA MacIntyre, L Sykes
Nature Communications, 2022-02-21;13(1):975.
Species: Human
Sample Types: Cervicovaginal Fluid
-
Serum VEGF-D level is correlated with renal dysfunction and proteinuria in patients with diabetic chronic kidney disease
Authors: TTU Nguyen, H Kim, YJ Chae, JH Jung, W Kim
Medicine, 2022-02-18;101(7):e28804.
Species: Human
Sample Types: Serum
-
The presence of serum anti-SARS-CoV-2 IgA appears to protect primary health care workers from COVID-19
Authors: V Hennings, K Thörn, S Albinsson, C Lingblom, K Andersson, C Andersson, K Järbur, R Pullerits, M Idorn, SR Paludan, K Eriksson, C Wennerås
European Journal of Immunology, 2022-02-18;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Association of significantly elevated plasma levels of NGAL and IGFBP4 in patients with diabetic nephropathy
Authors: H Ali, M Abu-Farha, E Alshawaf, S Devarajan, Y Bahbahani, I Al-Khairi, P Cherian, Z Alsairafi, V Vijayan, F Al-Mulla, A Al Attar, J Abubaker
Bmc Nephrology, 2022-02-11;23(1):64.
Species: Human
Sample Types: Plasma
-
Interleukin-6 Is a Circulating Prognostic Biomarker for Hepatocellular Carcinoma Patients Treated with Combined Immunotherapy
Authors: Y Myojin, T Kodama, R Sakamori, K Maesaka, T Matsumae, Y Sawai, Y Imai, K Ohkawa, M Miyazaki, S Tanaka, E Mita, S Tawara, T Yakushijin, Y Nozaki, H Hagiwara, Y Tahata, R Yamada, H Hikita, T Tatsumi, T Takehara
Cancers, 2022-02-10;14(4):.
Species: Human
Sample Types: Plasma
-
Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of VIS649 (Sibeprenlimab), an APRIL-Neutralizing IgG2 Monoclonal Antibody, in Healthy Volunteers
Authors: M Mathur, J Barratt, Y Suzuki, F Engler, MF Pasetti, J Yarbrough, S Sloan, D Oldach
Kidney international reports, 2022-02-08;7(5):993-1003.
Species: Human
Sample Types: Serum
-
miR-132-3p Modulates DUSP9-Dependent p38/JNK Signaling Pathways to Enhance Inflammation in the Amnion Leading to Labor
Authors: Z Zhong, Z Liu, R Zheng, J Chai, S Jiang
International Journal of Molecular Sciences, 2022-02-07;23(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Cerebrospinal Fluid EV Concentration and Size Are Altered in Alzheimer's Disease and Dementia with Lewy Bodies
Authors: A Longobardi, R Nicsanu, S Bellini, R Squitti, M Catania, P Tiraboschi, C Saraceno, C Ferrari, R Zanardini, G Binetti, G Di Fede, L Benussi, R Ghidoni
Cells, 2022-01-28;11(3):.
Species: Human
Sample Types: CSF
-
Plasma-Derived Hemopexin as a Candidate Therapeutic Agent for Acute Vaso-Occlusion in Sickle Cell Disease: Preclinical Evidence
Authors: T Gentinetta, JD Belcher, V Brügger-Ve, J Adam, T Ruthsatz, J Bain, D Schu, L Ventrici, M Edler, H Lioe, K Patel, C Chen, J Nguyen, F Abdulla, P Zhang, A Wassmer, M Jain, M Mischnik, M Pelzing, K Martin, R Davis, S Didichenko, A Schaub, N Brinkman, E Herzog, A Zürcher, GM Vercellott, GJ Kato, G Höbarth
Journal of Clinical Medicine, 2022-01-26;11(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Circulating Interleukin-6 and CD16 positive monocytes increase following angioplasty of an arteriovenous fistula
Authors: S Hakki, EJ Robinson, MG Robson
Scientific Reports, 2022-01-26;12(1):1427.
Species: Human
Sample Types: Plasma
-
Candidalysins Are a New Family of Cytolytic Fungal Peptide Toxins
Authors: JP Richardson, R Brown, N Kichik, S Lee, E Priest, S Mogavero, C Maufrais, DN Wickramasi, A Tsavou, NK Kotowicz, OW Hepworth, A Gallego-Co, NO Ponde, J Ho, DL Moyes, D Wilson, C D'Enfert, B Hube, JR Naglik
MBio, 2022-01-25;0(0):e0351021.
Species: Human
Sample Types: Cell Culture Supernates
-
Could Bone Biomarkers Predict Bone Turnover after Kidney Transplantation?-A Proof-of-Concept Study
Authors: J Magalhães, J Quelhas-Sa, L Pereira, R Neto, I Castro-Fer, S Martins, JM Frazão, C Carvalho
Journal of Clinical Medicine, 2022-01-17;11(2):.
Species: Human
Sample Types: Plasma
-
Pre-diagnostic levels of sVEGFR2, sTNFR2, sIL-2Ralpha and sIL-6R are associated with glioma risk: A nested case-control study of repeated samples
Authors: WY Wu, F Späth, C Wibom, B Björkblom, AM Dahlin, B Melin
Cancer Medicine, 2022-01-14;0(0):.
Species: Human
Sample Types: Plasma
-
Pancreatic ductal adenocarcinoma: Prognostic indicators of advanced disease
Authors: D Kruger, N Lahoud, YY Yako, J Devar, M Smith
PLoS ONE, 2022-01-12;17(1):e0262439.
Species: Human
Sample Types: Plasma
-
Role of immune mediators in predicting hospitalization of SARS-CoV-2 positive patients
Authors: S Ashrafzade, MR Campbell, JC Jara Aguir, J Walsh, A Kumanovics, G Jenkinson, P Rinaldo, MR Snyder, A Algeciras-
Cytokine, 2021-12-25;150(0):155790.
Species: Human
Sample Types: Plasma
-
Comparison between Cervical Ureaplasma spp. Colonization and the Intensity of Inflammatory Mediators in the Amniotic Fluid Retrieved during Cesarean Delivery in Preterm Birth
Authors: J Bae, S Kim, I Hwang, J Park
International Journal of Environmental Research and Public Health, 2021-12-23;19(1):.
Species: Human
Sample Types: Amniotic Fluid
-
Differential cytokine expression in direct and indirect co-culture of islets and mesenchymal stromal cells
Authors: I Dietrich, J Girdleston, H Giele
Cytokine, 2021-12-17;150(0):155779.
Species: Human
Sample Types: Cell Culture Supernates
-
Epithelial phenotype restoring drugs suppress macular degeneration phenotypes in an iPSC model
Authors: R Sharma, A George, M Nimmagadda, D Ortolan, BS Karla, Z Qureshy, D Bose, R Dejene, G Liang, Q Wan, J Chang, BS Jha, O Memon, KJ Miyagishim, A Rising, M Lal, E Hanson, R King, MM Campos, M Ferrer, J Amaral, D McGaughey, K Bharti
Nature Communications, 2021-12-15;12(1):7293.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of SARS-CoV-2 seropositivity on antigen - specific cytokine and chemokine responses in latent tuberculosis
Authors: A Rajamanick, N Pavan Kuma, P Chandrasek, A Nancy, PK Bhavani, N Selvaraj, K Karunanith, S Munisankar, R Srinivasan, R Mariam Ren, S Priya Kuma, V Venkatrama, S Babu
Cytokine, 2021-12-14;150(0):155785.
Species: Human
Sample Types: Whole Blood
-
Plasmin drives burn-induced systemic inflammatory response syndrome
Authors: BHY Gibson, CC Wollenman, SN Moore-Lotr, PR Keller, JB Summitt, AR Revenko, MJ Flick, TS Blackwell, JG Schoenecke
JCI Insight, 2021-12-08;6(23):.
Species: Human
Sample Types: Plasma
-
Higher mucosal antibody concentrations in women with genital tract inflammation
Authors: P Sobia, T Pillay, LJP Liebenberg, A Sivro, LE Mansoor, F Osman, JS Passmore, Q Abdool Kar, SS Abdool Kar, C Baxter, LR McKinnon, D Archary
Scientific Reports, 2021-12-06;11(1):23514.
Species: Human
Sample Types: Vaginal Lavage Fluid
-
Aggregated Mycobacterium tuberculosis Enhances the Inflammatory Response
Authors: HE Rodel, IATM Ferreira, CGK Ziegler, Y Ganga, M Bernstein, SH Hwa, K Nargan, G Lustig, G Kaplan, M Noursadegh, AK Shalek, AJC Steyn, A Sigal
Frontiers in Microbiology, 2021-12-02;12(0):757134.
Species: Human
Sample Types: Cell Culture Supernates
-
Severe COVID-19 infection is associated with aberrant cytokine production by infected lung epithelial cells rather than by systemic immune dysfunction
Authors: T Gajewski, S Rouhani, J Trujillo, A Pyzer, J Yu, J Fessler, A Cabanov, E Higgs, K Cron, Y Zha, Y Lu, J Bloodworth, M Abasiyanik, S Okrah, B Flood, K Hatogai, M Leung, A Pezeshk, L Kozloff, R Reschke, G Strohbehn, CS Chervin, M Kumar, S Schrantz, ML Madariaga, K Beavis, KT Yeo, R Sweis, J Segal, S Tay, E Izumchenko, J Mueller, L Chen
Research square, 2021-11-24;0(0):.
Species: Human
Sample Types: Serum
-
Identification and characterization of a novel glutaminase inhibitor
Authors: H Cederkvist, SS Kolan, JA Wik, Z Sener, BS Skålhegg
FEBS Open Bio, 2021-11-08;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
The Angiopoietin-Tie2 axis contributes to placental vascular disruption and adverse birth outcomes in malaria in pregnancy
Authors: V Tran, AM Weckman, VM Crowley, LS Cahill, K Zhong, A Cabrera, RE Elphinston, V Pearce, M Madanitsa, L Kalilani-P, V Mwapasa, C Khairallah, AL Conroy, FO Ter Kuile, JG Sled, KC Kain
EBioMedicine, 2021-11-07;73(0):103683.
Species: Human
Sample Types: Plasma
-
Study protocol: understanding the pathophysiologic mechanisms underlying delirium in older people undergoing hip fracture surgery
Authors: R Gamberale, C D'Orlando, S Brunelli, R Meneveri, P Mazzola, G Foti, G Bellani, G Zatti, D Munegato, S Volpato, A Zurlo, G Caruso, A Andreano, MG Valsecchi, G Bellelli
BMC geriatrics, 2021-11-04;21(1):633.
Species: Human
Sample Types: Plasma
-
Predictive role of endothelial cell activation in cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukaemia
Authors: F Hong, M Shi, J Cao, Y Wang, Y Gong, H Gao, Z Li, J Zheng, L Zeng, A He, K Xu
Journal of Cellular and Molecular Medicine, 2021-11-03;0(0):.
Species: Human
Sample Types: Serum
-
The Globular C1q Receptor Is Required for Epidermal Growth Factor Receptor Signaling during Candida albicans Infection
Authors: QT Phan, J Lin, NV Solis, M Eng, M Swidergall, F Wang, S Li, SL Gaffen, TF Chou, SG Filler
MBio, 2021-11-02;0(0):e0271621.
Species: Human
Sample Types: Cell Culture Supernates
-
Systems biological assessment of altered cytokine responses to bacteria and fungi reveals impaired immune functionality in schizophrenia
Authors: Y Gao, Y Fan, Z Yang, Q Ma, B Zhao, X He, F Gao, L Qian, W Wang, C Chen, Y Chen, C Gao, X Ma, F Zhu
Molecular Psychiatry, 2021-11-02;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Impaired HA-specific T follicular helper cell and antibody responses to influenza vaccination are linked to inflammation in humans
Authors: DL Hill, CE Whyte, S Innocentin, JL Lee, J Dooley, J Wang, EA James, JC Lee, WW Kwok, MS Zand, A Liston, EJ Carr, MA Linterman
Elife, 2021-11-02;10(0):.
Species: Human
Sample Types: Serum
-
Interaction between Macrophages and Human Mesenchymal Stromal Cells Derived from Bone Marrow and Wharton's Jelly-A Comparative Study
Authors: M Dymowska, A Aksamit, K Zielniok, M Kniotek, B Kaleta, A Roszczyk, M Zych, F Dabrowski, L Paczek, A Burdzinska
Pharmaceutics, 2021-11-01;13(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Diagnostic and Prognostic Utility of the Extracellular Vesicles Subpopulations Present in Pleural Effusion
Authors: J Javadi, A Görgens, H Vanky, D Gupta, A Hjerpe, S El-Andalou, D Hagey, K Dobra
Biomolecules, 2021-10-29;11(11):.
Species: Human
Sample Types: Exosomes
-
Aqueous humor analyses of diabetic macular edema patients with subretinal fluid
Authors: JW Kwon, B Kim, D Jee, YK Cho
Scientific Reports, 2021-10-25;11(1):20985.
Species: Human
Sample Types: Aqueous Humor
-
Release of infectious virus and cytokines in nasopharyngeal swabs from individuals infected with non-alpha or alpha SARS-CoV-2 variants: an observational retrospective study
Authors: B Monel, D Planas, L Grzelak, N Smith, N Robillard, I Staropoli, P Goncalves, F Porrot, F Guivel-Ben, ND Guinet, J Rodary, J Puech, V Euzen, L Bélec, G Orvoen, L Nunes, V Moulin, J Fourgeaud, M Wack, S Imbeaud, P Campagne, D Duffy, JPD Santo, T Bruel, H Péré, D Veyer, O Schwartz
EBioMedicine, 2021-10-19;73(0):103637.
Species: Human
Sample Types: Nasal Lavage Fluid
-
Host characteristics associated with serologic inflammatory biomarkers in women
Authors: SS Wang, C Zhong, M Epeldegui, S Nunes, L Magpantay, JC DeHart, S Hurley, D Goldberg, E Martinez, JV Lacey, O Martinez-M, P Reynolds
Cytokine, 2021-10-16;149(0):155726.
Species: Human
Sample Types: Serum
-
Specific Cytokine Profiles Predict the Severity of Influenza A Pneumonia: A Prospectively Multicenter Pilot Study
Authors: Y Xie, Y Yu, L Zhao, P Ning, Q Luo, Y Zhang, L Yin, Y Zheng, Z Gao
BioMed Research International, 2021-10-13;2021(0):9533044.
Species: Human
Sample Types: Saliva
-
Low bone turnover is associated with plain X-ray vascular calcification in predialysis patients
Authors: R Neto, L Pereira, J Magalhães, J Quelhas-Sa, J Frazão
PLoS ONE, 2021-10-13;16(10):e0258284.
Species: Human
Sample Types: Plasma
-
A Fatty Acid Fraction Purified From Sea Buckthorn Seed Oil Has Regenerative Properties on Normal Skin Cells
Authors: M Dudau, E Codrici, I Tarcomnicu, S Mihai, ID Popescu, L Albulescu, N Constantin, I Cucolea, T Costache, D Rambu, AM Enciu, ME Hinescu, C Tanase
Frontiers in Pharmacology, 2021-10-08;12(0):737571.
Species: Human
Sample Types: Cell Culture Supernates
-
Cancer testis antigen PRAME: An anti-cancer target with immunomodulatory potential
Authors: A Naik, R Thomas, G Al-Khadair, R Bacha, W Hendrickx, J Decock
Journal of Cellular and Molecular Medicine, 2021-10-06;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
The Potential Role of Proinflammatory Cytokines and Complement Components in the Development of Drug-Induced Neuropathy in Patients with Multiple Myeloma
Authors: K ?uczkowska, M Rutka, D Rogi?ska, E Paczkowska, B Baumert, S Milczarek, M Górska, P Kulig, B Os?kowska, M Janowski, K Safranow, K Sommerfeld, E Borowiecka, P Zawodny, A Kocl?ga, G Helbig, B Machali?sk
Journal of Clinical Medicine, 2021-10-04;10(19):.
Species: Human
Sample Types: Plasma
-
The Rise of IGFBP4 in People with Obstructive Sleep Apnea and Multilevel Sleep Surgery Recovers Its Basal Levels
Authors: A Alterki, E Al Shawaf, I Al-Khairi, P Cherian, D Sriraman, M Hammad, TA Thanaraj, MAK Ebrahim, F Al-Mulla, M Abu-Farha, J Abubaker
Disease Markers, 2021-10-04;2021(0):1219593.
Species: Human
Sample Types: Plasma
-
Predictors of low ovarian reserve in cART-treated women living with HIV
Authors: E Merlini, C Tincati, V Sacchi, M Augello, V Bono, ES Cannizzo, M Allegrini, L Gazzola, AD Monforte, AM Marconi, M Ravizza, G Marchetti
Medicine, 2021-10-01;100(39):e27157.
Species: Human
Sample Types: Plasma
-
Enhanced eosinophil-mediated inflammation associated with antibody and complement-dependent pneumonic insults in critical COVID-19
Authors: DM Kim, Y Kim, JW Seo, J Lee, U Park, NY Ha, J Koh, H Park, JW Lee, HJ Ro, NR Yun, DY Kim, SH Yoon, YS Na, DS Moon, SC Lim, CM Kim, K Jeon, JG Kang, NY Jang, H Jeong, J Kim, S Cheon, KM Sohn, JY Moon, S Kym, SR Han, MS Lee, HJ Kim, WY Park, JY Choi, HW Shin, HY Kim, CH Cho, YK Jeon, YS Kim, NH Cho
Cell Reports, 2021-09-20;0(0):109798.
Species: Human
Sample Types: BALF
-
Cytokine Response to SARS-CoV-2 Infection in Children
Authors: A Curatola, A Chiaretti, S Ferretti, G Bersani, D Lucchetti, L Capossela, A Sgambato, A Gatto
Viruses, 2021-09-18;13(9):.
Species: Human
Sample Types: Serum
-
Exposure to widespread drinking water chemicals, blood inflammation markers, and colorectal cancer
Authors: CM Villanueva, A Espinosa, E Gracia-Lav, J Vlaanderen, R Vermeulen, AJ Molina, P Amiano, I Gómez-Aceb, G Castaño-Vi, P Vineis, M Kogevinas
Environment international, 2021-09-17;157(0):106873.
Species: Human
Sample Types: Serum
-
Differential expression of tear film cytokines in Stevens-Johnson syndrome patients and comparative review of literature
Authors: MA Koduri, D Prasad, S Upadhyaya, J Jaffet, SS Shanbhag, S Basu, V Singh
Scientific Reports, 2021-09-16;11(1):18433.
Species: Human
Sample Types: Tears
-
Identification of immune correlates of fatal outcomes in critically ill COVID-19 patients
Authors: J Youngs, NM Provine, N Lim, HR Sharpe, A Amini, YL Chen, J Luo, MD Edmans, P Zacharopou, W Chen, O Sampson, R Paton, WJ Hurt, DA Duncan, AL McNaughton, VN Miao, S Leaver, DLA Wyncoll, J Ball, P Hopkins, Oxford Imm, Oxford Pro, DT Skelly, E Barnes, S Dunachie, G Ogg, T Lambe, I Pavord, AK Shalek, CP Thompson, L Xue, DC Macallan, P Goulder, P Klenerman, T Bicanic
PloS Pathogens, 2021-09-16;17(9):e1009804.
Species: Human
Sample Types: Serum
-
Effects of Caloric Restriction and Rope-Skipping Exercise on Cardiometabolic Health: A Pilot Randomized Controlled Trial in Young Adults
Authors: Z Tang, Y Ming, M Wu, J Jing, S Xu, H Li, Y Zhu
Nutrients, 2021-09-16;13(9):.
Species: Human
Sample Types:
-
Prevalence of Fatigue and Unrecognized Depression in Patients with Inflammatory Bowel Disease in Remission under Immunosuppressants and Biologicals
Authors: M Truyens, E De Ruyck, GB Gonzales, S Bos, D Laukens, M De Vos
Journal of Clinical Medicine, 2021-09-11;10(18):.
Species: Human
Sample Types: Serum
-
Modeling In Vitro Osteoarthritis Phenotypes in a Vascularized Bone Model Based on a Bone-Marrow Derived Mesenchymal Cell Line and Endothelial Cells
Authors: A Pirosa, EB Tankus, A Mainardi, P Occhetta, L Dönges, C Baum, M Rasponi, I Martin, A Barbero
International Journal of Molecular Sciences, 2021-09-03;22(17):.
Species: Human
Sample Types: Cell Culture Supernates
-
Distinct systemic and mucosal immune responses during acute SARS-CoV-2 infection
Authors: N Smith, P Goncalves, B Charbit, L Grzelak, M Beretta, C Planchais, T Bruel, V Rouilly, V Bondet, J Hadjadj, N Yatim, H Pere, SH Merkling, A Ghozlane, S Kernéis, F Rieux-Lauc, B Terrier, O Schwartz, H Mouquet, D Duffy, JP Di Santo
Nature Immunology, 2021-09-01;0(0):.
Species: Human
Sample Types: Plasma
-
Inhibition of the cGAS-STING pathway ameliorates the premature senescence hallmarks of Ataxia-Telangiectasia brain organoids
Authors: J Aguado, HK Chaggar, C Gómez-Incl, MR Shaker, HC Leeson, A Mackay-Sim, EJ Wolvetang
Aging Cell, 2021-08-30;0(0):e13468.
Species: Human
Sample Types: Organoids
-
Alteration of immune profiles is associated with pulmonary function and symptoms in patients with chronic obstructive pulmonary disease
Authors: S Li, S Zhao, Z Wu, F Wang, W Li
Molecular Medicine Reports, 2021-08-26;24(5):.
Species: Human
Sample Types: Plasma
-
Pulmonary and blood dendritic cells from sarcoidosis patients more potently induce IFNgamma-producing Th1 cells compared with monocytes
Authors: R Lepzien, M Nie, P Czarnewski, S Liu, M Yu, A Ravindran, S Kullberg, A Eklund, J Grunewald, A Smed-Sören
Journal of leukocyte biology, 2021-08-25;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Assessment and Prognosis in CSA-AKI Using Novel Kidney Injury Biomarkers: A Prospective Observational Study
Authors: J Udzik, A Waszczyk, K Safranow, A Biskupski, K Majer, S Kwiatkowsk, E Kwiatkowsk
Biology, 2021-08-24;10(9):.
Species: Human
Sample Types: Urine
-
A map of metabolic phenotypes in patients with myalgic encephalomyelitis/chronic fatigue syndrome
Authors: F Hoel, A Hoel, IK Pettersen, IG Rekeland, K Risa, K Alme, K Sørland, A Fosså, K Lien, I Herder, HL Thürmer, ME Gotaas, C Schäfer, RK Berge, K Sommerfelt, HP Marti, O Dahl, O Mella, Ø Fluge, KJ Tronstad
JCI Insight, 2021-08-23;6(16):.
Species: Human
Sample Types: Serum
-
Immune checkpoint inhibitors increase T cell immunity during SARS-CoV-2 infection
Authors: N Yatim, J Boussier, P Tetu, N Smith, T Bruel, B Charbit, L Barnabei, A Corneau, L Da Meda, C Allayous, B Baroudjian, M Jebali, F Herms, L Grzelak, I Staropoli, V Calmettes, J Hadjadj, O Peyrony, C Cassius, J LeGoff, N Kramkimel, S Aractingi, M Fontes, C Blanc, F Rieux-Lauc, O Schwartz, B Terrier, D Duffy, C Lebbé
Science Advances, 2021-08-18;7(34):.
Species: Human
Sample Types: Plasma
-
Establishment of Three-Dimensional Bioprinted Bladder Cancer-on-a-Chip with a Microfluidic System Using Bacillus Calmette-Gu�rin
Authors: JH Kim, S Lee, SJ Kang, YW Choi, SY Choi, JY Park, IH Chang
International Journal of Molecular Sciences, 2021-08-18;22(16):.
Species: Human
Sample Types: Cancer on Chip
-
Variable Changes of Circulating ANGPTL3 and ANGPTL4 in Different Obese Phenotypes: Relationship with Vasodilator Dysfunction
Authors: F Schinzari, G Vizioli, U Campia, M Tesauro, C Cardillo
Biomedicines, 2021-08-18;9(8):.
Species: Human
Sample Types: Plasma
-
Longitudinal Assessment of Cytokine Expression and Plasminogen Activation in Hantavirus Cardiopulmonary Syndrome Reveals Immune Regulatory Dysfunction in End-Stage Disease
Authors: P Simons, Y Guo, V Bondu, SL Tigert, M Harkins, S Goodfellow, C Tompkins, D Chabot-Ric, XO Yang, LG Bosc, S Bradfute, DA Lawrence, T Buranda
Viruses, 2021-08-12;13(8):.
Species: Human
Sample Types: Plasma
-
Potential effects of angiogenesis-related factors on the severity of APAC and surgical outcomes of trabeculectomy
Authors: J Wang, MS Fu, MW Zhou, BL Ke, ZH Zhang, X Xu
BMC ophthalmology, 2021-08-12;21(1):297.
Species: Human
Sample Types: Aqueous Humor
-
SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis and cytokine release
Authors: C Junqueira, Ã Crespo, S Ranjbar, M Lewandrows, J Ingber, LB de Lacerda, B Parry, S Ravid, S Clark, F Ho, SM Vora, V Leger, C Beakes, J Margolin, N Russell, K Kays, L Gehrke, UD Adhikari, L Henderson, E Janssen, D Kwon, C Sander, J Abraham, M Filbin, MB Goldberg, H Wu, G Mehta, S Bell, AE Goldfeld, J Lieberman
Research square, 2021-08-11;0(0):.
Species: Human
Sample Types: Plasma
-
Induced endothelial cells from peripheral arterial disease patients and neonatal fibroblasts have comparable angiogenic properties
Authors: JD Hywood, S Sadeghipou, ZE Clayton, J Yuan, C Stubbs, JWT Wong, JP Cooke, S Patel
PLoS ONE, 2021-08-10;16(8):e0255075.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of the Autophagy-Inhibiting Agent Chloroquine on Acute Myeloid Leukemia Cells; Characterization of Patient Heterogeneity
Authors: IS Grønningsæ, H Reikvam, E Aasebø, S Bartaula-B, M Hernandez-, F Selheim, FS Berven, TH Tvedt, Ø Bruserud, KJ Hatfield
Journal of personalized medicine, 2021-08-10;11(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of BCG vaccination on proinflammatory responses in elderly individuals
Authors: N Pavan Kuma, C Padmapriya, A Rajamanick, SB Marinaik, A Nancy, S Padmanaban, N Akbar, M Murhekar, S Babu
Science Advances, 2021-08-04;7(32):.
Species: Human
Sample Types: Plasma
-
Early Inflammatory Cytokine Expression in Cerebrospinal Fluid of Patients with Spontaneous Intraventricular Hemorrhage
Authors: WC Ziai, AR Parry-Jone, CB Thompson, LH Sansing, MT Mullen, SB Murthy, A Mould, S Nekoovaght, DF Hanley
Biomolecules, 2021-07-30;11(8):.
Species: Human
Sample Types: CSF
-
Endogenous control of inflammation characterizes pregnant women with asymptomatic or paucisymptomatic SARS-CoV-2 infection
Authors: S De Biasi, DL Tartaro, L Gibellini, A Paolini, A Quong, C Petes, G Awong, S Douglas, D Lin, J Nieto, FM Galassi, R Borella, L Fidanza, M Mattioli, C Leone, I Neri, M Meschiari, L Cicchetti, A Iannone, T Trenti, M Sarti, M Girardis, G Guaraldi, C Mussini, F Facchinett, A Cossarizza
Nature Communications, 2021-07-29;12(1):4677.
Species: Human
Sample Types: Plasma
-
Monocyte-dependent co-stimulation of cytokine induction in human gammadelta T cells by TLR8 RNA ligands
Authors: R Serrano, C Coch, C Peters, G Hartmann, D Wesch, D Kabelitz
Scientific Reports, 2021-07-27;11(1):15231.
Species: Human
Sample Types: Cell Culture Supernates
-
Establishment of a Patient-Derived, Magnetic Levitation-Based, Three-Dimensional Spheroid Granuloma Model for Human Tuberculosis
Authors: LA Kotze, CGG Beltran, D Lang, AG Loxton, S Cooper, M Meiring, CFN Koegelenbe, BW Allwood, ST Malherbe, AM Hiemstra, B Glanzmann, C Kinnear, G Walzl, N du Plessis
mSphere, 2021-07-21;0(0):e0055221.
Species: Human
Sample Types: Tissue Homogenates
-
Predictive Ability of Serum IL-27 Level for Assessing Activity of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis
Authors: T Yoon, SS Ahn, JY Pyo, LE Lee, JJ Song, YB Park, SW Lee
Mediators of Inflammation, 2021-07-19;2021(0):6668884.
Species: Human
Sample Types: Serum
-
Serum adipokine profiles in patients with microscopic polyangiitis and granulomatosis with polyangiitis: An exploratory analysis
Authors: SS Ahn, T Yoon, JJ Song, YB Park, SW Lee
PLoS ONE, 2021-07-09;16(7):e0254226.
Species: Human
Sample Types: Serum
-
Increased levels of VCAM-1 is associated with higher occurrence of coronary artery disease in adults with moderate to severe obstructive sleep apnea
Authors: Q Lv, H Sun, Z Du, X Jiao, H Yu, Q Sun, F Li, Y Wang, L Li, C Hu, Y Qin
Sleep medicine, 2021-07-09;85(0):131-137.
Species: Human
Sample Types: Plasma
-
Inhibition of histone deacetylase 6 suppresses inflammatory responses and invasiveness of fibroblast-like-synoviocytes in inflammatory arthritis
Authors: JK Park, S Shon, HJ Yoo, DH Suh, D Bae, J Shin, JH Jun, N Ha, H Song, YI Choi, T Pap, YW Song
Arthritis Research & Therapy, 2021-07-05;23(1):177.
Species: Human
Sample Types: Cell Culture Supernates
-
Vocal Fold Fibroblasts in Reinke's Edema Show Alterations Involved in Extracellular Matrix Production, Cytokine Response and Cell Cycle Control
Authors: M Grill, I Lazzeri, A Kirsch, N Steurer, T Grossmann, M Karbiener, E Heitzer, M Gugatschka
Biomedicines, 2021-06-26;9(7):.
Species: Human
Sample Types: Cell Culture Supernates
-
Diagnostic and prognostic potential of the proteomic profiling of serum-derived extracellular vesicles in prostate cancer
Authors: M Signore, R Alfonsi, G Federici, S Nanni, A Addario, L Bertuccini, A Aiello, AL Di Pace, I Sperduti, G Muto, A Giacobbe, D Collura, L Brunetto, G Simone, M Costantini, L Crinò, S Rossi, C Tabolacci, M Diociaiuti, T Merlino, M Gallucci, S Sentinelli, R Papalia, R De Maria, D Bonci
Cell Death & Disease, 2021-06-21;12(7):636.
Species: Human
Sample Types: Serum
-
Eotaxins and Their Receptor as Biomarkers of Colorectal Cancer
Authors: M Zajkowska, A Kulczy?ska, M Dulewicz, K Safiejko, M Juchimiuk, M Konopko, L Koz?owski, B Mroczko
Journal of Clinical Medicine, 2021-06-17;10(12):.
Species: Human
Sample Types: Serum
-
A combined biomarker approach for characterising extracellular matrix profiles in acute myocardial infarction
Authors: MM Brunton-O', AS Holley, KE Hally, GA Kristono, SA Harding, PD Larsen
Scientific Reports, 2021-06-16;11(1):12705.
Species: Human
Sample Types: Plasma
-
Plantaricin NC8 alphabeta prevents Staphylococcus aureus-mediated cytotoxicity and inflammatory responses of human keratinocytes
Authors: A Musa, E Wiman, R Selegård, D Aili, T Bengtsson, H Khalaf
Scientific Reports, 2021-06-15;11(1):12514.
Species: Human
Sample Types: Cell Culture Supernates
-
Neurogenic and Neuroprotective Potential of Stem/Stromal Cells Derived from Adipose Tissue
Authors: A Figiel-Dab, K Radoszkiew, P Rybkowska, NE Krzesniak, D Sulejczak, A Sarnowska
Cells, 2021-06-11;10(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
A new molecular classification to drive precision treatment strategies in primary Sj�gren's syndrome
Authors: P Soret, C Le Dantec, E Desvaux, N Foulquier, B Chassagnol, S Hubert, C Jamin, G Barturen, G Desachy, V Devauchell, C Boudjeniba, D Cornec, A Saraux, S Jousse-Jou, N Barbarroja, I Rodríguez-, E De Langhe, L Beretta, C Chizzolini, L Kovács, T Witte, PRECISESAD, PRECISESAD, E Bettacchio, A Buttgereit, Z Makowska, R Lesche, MO Borghi, J Martin, S Courtade-G, L Xuereb, M Guedj, P Moingeon, ME Alarcón-Ri, L Laigle, JO Pers
Nature Communications, 2021-06-10;12(1):3523.
Species: Human
Sample Types: Serum
-
Prospective Assessment of SARS-CoV-2 Seroconversion (PASS) study: an observational cohort study of SARS-CoV-2 infection and vaccination in healthcare workers
Authors: BM Jackson-Th, E Goguet, ED Laing, CH Olsen, S Pollett, KM Hollis-Per, SE Maiolatesi, L Illinik, KF Ramsey, AE Reyes, Y Alcorta, MA Wong, J Davies, O Ortega, E Parmelee, AR Lindrose, M Moser, E Graydon, AG Letizia, CA Duplessis, A Ganesan, KP Pratt, AM Malloy, DW Scott, SK Anderson, AL Snow, CL Dalgard, JH Powers, D Tribble, TH Burgess, CC Broder, E Mitre
BMC infectious diseases, 2021-06-09;21(1):544.
Species: Human
Sample Types: Cell Culture Supernates
-
Yeast-Host Interactions: Anadenanthera colubrina Modulates Virulence Factors of C. albicans and Inflammatory Response In Vitro
Authors: CMA Maia, S Pasetto, CFW Nonaka, EMMB Costa, RM Murata
Frontiers in Pharmacology, 2021-06-08;12(0):629778.
Species: Human
Sample Types: Cell Culture Supernates
-
Integrating longitudinal clinical laboratory tests with targeted proteomic and transcriptomic analyses reveal the landscape of host responses in COVID-19
Authors: Y Tan, W Zhang, Z Zhu, N Qiao, Y Ling, M Guo, T Yin, H Fang, X Xu, G Lu, P Zhang, S Yang, Z Fu, D Liang, Y Xie, R Zhang, L Jiang, S Yu, J Lu, F Jiang, J Chen, C Xiao, S Wang, S Chen, XW Bian, H Lu, F Liu, S Chen
Cell Discovery, 2021-06-08;7(1):42.
Species: Human
Sample Types: Serum
-
Elevated Levels of the Endothelial Molecules ICAM-1, VEGF-A, and VEGFR2 in Microscopic Asymptomatic Malaria
Authors: A Frimpong, J Amponsah, D Agyemang, AS Adjokatseh, S Eyiah-Ampa, NA Ennuson, D Obiri, LE Amoah, KA Kusi
Open forum infectious diseases, 2021-06-04;8(7):ofab302.
Species: Human
Sample Types: Plasma
-
Differential association between inflammatory cytokines and multiorgan dysfunction in COVID-19 patients with obesity
Authors: MA Dragon-Dur, X Chen, A Kirilovsky, N Ben Hamoud, C El Sissy, J Russick, E Charpentie, Y Binois, F Marliot, M Meylan, C Granier, H Pere, A Saldmann, B Rance, AS Jannot, S Baron, M Chebbi, A Fayol, N Josseaume, C Rives-Lang, PL Tharaux, B Cholley, JL Diehl, JB Arlet, M Azizi, A Karras, S Czernichow, DM Smadja, JS Hulot, I Cremer, E Tartour, E Mousseaux, F Pagès
PLoS ONE, 2021-05-26;16(5):e0252026.
Species: Human
Sample Types: Plasma
-
High plasma concentration of non-esterified polyunsaturated fatty acids is a specific feature of severe COVID-19 pneumonia
Authors: M Nguyen, A Bourredjem, L Piroth, B Bouhemad, A Jalil, G Pallot, N Le Guern, C Thomas, T Pilot, V Bergas, H Choubley, JP Quenot, PE Charles, L Lagrost, V Deckert, JP de Barros, PG Guinot, D Masson, C Binquet, T Gautier, M Blot, Lymphonie
Scientific Reports, 2021-05-24;11(1):10824.
Species: Human
Sample Types: Plasma
-
Toxoplasma gondii could have a possible role in the cancer mechanism by modulating the host's cell response
Authors: A Caner
Acta tropica, 2021-05-21;220(0):105966.
Species: Human
Sample Types: Cell Culture Supernates
-
Circulating levels of angiogenic factors and their association with preeclampsia among pregnant women at Mulago National Referral Hospital in Uganda
Authors: S Nabweyambo, OJ Sande, N McGovern, F Bwanga, A Ssekagiri, A Keesiga, M Adroma, R Wasswa, M Atuheirwe, J Namugenyi, B Castelnuov, A Nakimuli
PLoS ONE, 2021-05-19;16(5):e0251227.
Species: Human
Sample Types: Plasma
-
Evaluation of a Three-Marker Panel for the Detection of Uveal Melanoma Metastases: A Single-Center Retrospective Analysis
Authors: Z Lin, D Süsskind
Cancers, 2021-05-18;13(10):.
Species: Human
Sample Types: Serum
-
Dataset-chemokines, cytokines, and biomarkers in the saliva of children with Sj�gren's syndrome
Authors: MHH Withanage, MPG Hernandez, EE Starman, AB Davis, E Zeng, SM Lieberman, KA Brogden, EA Lanzel
Data in Brief, 2021-05-14;36(0):107139.
Species: Human
Sample Types: Saliva
-
Cytokines and Leukocytes Subpopulations Profile in SARS-CoV-2 Patients Depending on the CT Score Severity
Authors: E Rutkowska, I Kwiecie?, M ?abicka, A Maliborski, A Raniszewsk, K K?os, W Urba?ska, I Klajnowicz, P Rzepecki, A Chcia?owsk
Viruses, 2021-05-11;13(5):.
Species: Human
Sample Types: Serum
-
Sex disparities in vitamin D status and the impact on systemic inflammation and survival in rectal cancer
Authors: H Abrahamsso, S Meltzer, VN Hagen, C Johansen, PA Bousquet, KR Redalen, AH Ree
BMC Cancer, 2021-05-11;21(1):535.
Species: Human
Sample Types: Serum
-
Serum and cerebrospinal fluid host proteins indicate stroke in children with tuberculous meningitis
Authors: CM Manyelo, NN Chegou, JA Seddon, CI Snyders, H Mutavhatsi, PM Manngo, G Walzl, K Stanley, RS Solomons
PLoS ONE, 2021-04-30;16(4):e0250944.
Species: Human
Sample Types: Serum
-
Antiplatelet Therapy Combined with Anastrozole Induces Features of Partial EMT in Breast Cancer Cells and Fails to Mitigate Breast-Cancer Induced Hypercoagulation
Authors: KR Xulu, TN Augustine
International Journal of Molecular Sciences, 2021-04-16;22(8):.
Species: Human
Sample Types: Whole Blood
-
A panel of selected serum protein biomarkers for the detection of aggressive prostate cancer
Authors: J Song, S Ma, LJ Sokoll, RV Eguez, N Höti, H Zhang, P Mohr, R Dua, D Patil, KD May, S Williams, R Arnold, MG Sanda, DW Chan, Z Zhang
Theranostics, 2021-04-15;11(13):6214-6224.
Species: Human
Sample Types: Serum
-
The circulating soluble form of the CD40 costimulatory immune checkpoint receptor and liver metastasis risk in rectal cancer
Authors: S Meltzer, A Torgunrud, H Abrahamsso, AM Solbakken, K Flatmark, S Dueland, KM Bakke, PA Bousquet, A Negård, C Johansen, LG Lyckander, FO Larsen, JV Schou, KR Redalen, AH Ree
British Journal of Cancer, 2021-04-09;0(0):.
Species: Human
Sample Types: Serum
-
The Expression and Function of Metastases Associated Lung Adenocarcinoma Transcript-1 Long Non-Coding RNA in Subchondral Bone and Osteoblasts from Patients with Osteoarthritis
Authors: FA Alnajjar, A Sharma-Oat, SN Wijesinghe, H Farah, DE Nanus, T Nicholson, ET Davis, SW Jones
Cells, 2021-04-01;10(4):.
Species: Human
Sample Types: Serum
-
Urinary extracellular matrix proteins as predictors of the severity of ureteropelvic junction obstruction in children
Authors: MF Mello, S Thalita Do, EY Kondo, J de Bessa J, FT Dénes, RI Lopes
Journal of pediatric urology, 2021-03-27;0(0):.
Species: Human
Sample Types: Urine
-
Ruxolitinib before allogeneic hematopoietic transplantation in patients with myelofibrosis on behalf SFGM-TC and FIM groups
Authors: M Robin, R Porcher, C Orvain, JO Bay, F Barraco, A Huynh, A Charbonnie, E Forcade, S Chantepie, C Bulabois, I Yakoub-Agh, M Detrait, D Michonneau, P Turlure, N Raus, F Boyer, F Suarez, L Vincent, SN Guyen, J Cornillon, A Villate, B Dupriez, B Cassinat, V Rolland, MH Schlageter, G Socié, JJ Kiladjian
Bone marrow transplantation, 2021-03-25;0(0):.
Species: Human
Sample Types: Serum
-
Changes in plasma sclerostin level associated with use of a medium cut-off dialyzer in end-stage renal disease
Authors: SH Ahn, MM Ko, JH Song, JH Jung
Kidney research and clinical practice, 2021-03-22;40(1):120-134.
Species: Human
Sample Types: Plasma
-
Markers of tissue remodeling and inflammation in the white and brown adipose tissues of a model hibernator
Authors: SM Logan, KB Storey
Cellular Signalling, 2021-03-09;82(0):109975.
Species: Squirrel
Sample Types: Tissue Homogenates
-
Characterization of the perioperative changes of exosomal immune-related cytokines induced by prostatectomy in early-stage prostate cancer patients
Authors: M Macías, Á García-Cor, M Torres, J Ancizu-Mar, J Ignacio Pa, F Díez-Cabal, J Enrique Ro, D Rosell, B Miñana, B Mateos, D Ajona, R Sánchez-Ba, O Bedialaune, S Chocarro, A Navarro, MP Andueza, A Gúrpide, J Luis Perez, E Alegre, Á González
Cytokine, 2021-02-16;141(0):155471.
Species: Human
Sample Types: Serum
-
Syndecan-1 Overexpressing Mesothelioma Cells Inhibit Proliferation, Wound Healing, and Tube Formation of Endothelial Cells
Authors: J Javadi, G Heidari-Ha, A Schmalzl, T Szatmári, M Metintas, P Aspenström, A Hjerpe, K Dobra
Cancers, 2021-02-06;13(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
IRE1 Alpha/XBP1 Axis Sustains Primary Effusion Lymphoma Cell Survival by Promoting Cytokine Release and STAT3 Activation
Authors: R Gonnella, MS Gilardini, L Guttieri, MA Romeo, R Santarelli, M Cirone
Biomedicines, 2021-01-27;9(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of epidermal growth factor receptor inhibitor-induced interferon pathway signaling in the head and neck squamous cell carcinoma therapeutic response
Authors: SP Korpela, TK Hinz, A Oweida, J Kim, J Calhoun, R Ferris, RA Nemenoff, SD Karam, ET Clambey, LE Heasley
Journal of Translational Medicine, 2021-01-23;19(1):43.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of Macrophages and Osteoclasts in the Osteosarcoma Tumor Microenvironment at Diagnosis: New Perspective for Osteosarcoma Treatment?
Authors: A Gomez-Brou, J Gilhodes, NV Acker, R Brion, C Bouvier, P Assemat, N Gaspar, S Aubert, JM Guinebreti, B Marie, F Larousseri, N Entz-Werlé, G Pinieux, E Mascard, F Gouin, P Brousset, MD Tabone, M Jimenez, MC Le Deley, JY Blay, L Brugieres, S Piperno-Ne, F Rédini
Cancers, 2021-01-23;13(3):.
Species: Human
Sample Types: Serum
-
The HSP GRP94 interacts with macrophage intracellular complement C3 and impacts M2 profile during ER stress
Authors: K Chaumonnot, S Masson, H Sikner, A Bouchard, V Baverel, PS Bellaye, B Collin, C Garrido, E Kohli
Cell Death & Disease, 2021-01-22;12(1):114.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of obstructive sleep apnea on immunity in cases of chronic rhinosinusitis with nasal polyp
Authors: DK Kim, BC Lee, KJ Park, GM Son
Clinical and experimental otorhinolaryngology, 2021-01-19;0(0):.
Species: Human
Sample Types: Tissue Homogenates
-
Plasma Endocan as a Predictor of Cardiovascular Event in Patients with End-Stage Renal Disease on Hemodialysis
Authors: JS Kim, GJ Ko, YG Kim, SY Lee, DY Lee, KH Jeong, SH Lee
Journal of Clinical Medicine, 2020-12-18;9(12):.
Species: Human
Sample Types: Plasma
-
The extracellular polysaccharide matrix of Pseudomonas aeruginosa biofilms is a determinant of polymorphonuclear leukocyte responses
Authors: M Rybtke, PØ Jensen, CH Nielsen, T Tolker-Nie
Infect Immun, 2020-12-15;0(0):.
Species: Human
Sample Types: Plasma
-
Osteopontin Predicts Three-Month Outcome in Stroke Patients Treated by Reperfusion Therapies
Authors: E Meseguer, D Diallo, J Labreuche, H Charles, S Delbosc, G Mangin, L Monteiro T, G Caligiuri, A Nicoletti, P Amarenco
Journal of Clinical Medicine, 2020-12-13;9(12):.
Species: Human
Sample Types: Plasma
-
Predictive inflammatory biomarkers for change in suicidal ideation in major depressive disorder and panic disorder: A 12-week follow-up study
Authors: KW Choi, EH Jang, AY Kim, H Kim, MJ Park, S Byun, M Fava, D Mischoulon, GI Papakostas, HY Yu, HJ Jeon
Journal of psychiatric research, 2020-12-04;133(0):73-81.
Species: Human
Sample Types: Serum
-
Anti-IL-17A treatment reduces serum inflammatory, angiogenic and tissue remodeling biomarkers accompanied by less synovial high endothelial venules in peripheral spondyloarthritis
Authors: MH Kaaij, B Helder, LJJ van Mens, MGH van de San, DLP Baeten, SW Tas
Scientific Reports, 2020-12-03;10(1):21094.
Species: Human
Sample Types: Serum
-
Co-Adsorption of Synthetic Mincle Agonists and Antigen to Silica Nanoparticles for Enhanced Vaccine Activity: A Formulation Approach to Co-Delivery
Authors: WM Abdelwahab, A Riffey, C Buhl, C Johnson, K Ryter, JT Evans, DJ Burkhart
Int J Pharm, 2020-11-27;0(0):120119.
Species: Human
Sample Types: Cell Culture Supernates
-
Gingival Crevicular Fluid Zinc- and Aspartyl-Binding Protease Profile of Individuals with Moderate/Severe Atopic Dermatitis
Authors: F Valenzuela, J Fernández, M Aroca, C Jiménez, D Albers, M Hernández, A Fernández
Biomolecules, 2020-11-26;10(12):.
Species: Human
Sample Types: Gingival Crevicular Fluid
-
Temporal Telomere and DNA Damage Responses in the Space Radiation Environment
Authors: JJ Luxton, MJ McKenna, LE Taylor, KA George, SR Zwart, BE Crucian, VR Drel, FE Garrett-Ba, MJ Mackay, D Butler, J Foox, K Grigorev, D Bezdan, C Meydan, SM Smith, K Sharma, CE Mason, SM Bailey
Cell Rep, 2020-11-25;33(10):108435.
Species: Human
Sample Types: Plasma
-
In vitro mechanical vibration down-regulates pro-inflammatory and pro-fibrotic signaling in human vocal fold fibroblasts
Authors: D Hortobagyi, T Grossmann, M Tschernitz, M Grill, A Kirsch, C Gerstenber, M Gugatschka
PLoS ONE, 2020-11-19;15(11):e0241901.
Species: Human
Sample Types: Cell Culture Supernates
-
The cross-talk between STAT1/STAT3 and ROS up-regulates PD-L1 and promotes the release of pro-inflammatory/immune suppressive cytokines in primary monocytes infected by HHV-6B
Authors: MA Romeo, MS Gilardini, R Benedetti, L Giambelli, R D'Aprile, A Gaeta, A Faggioni, M Cirone
Virus Res, 2020-11-15;292(0):198231.
Species: Human
Sample Types: Cell Culture Supernates
-
IgE Activates Monocytes from Cancer Patients to Acquire a Pro-Inflammatory Phenotype
Authors: M Nakamura, EA Souri, G Osborn, R Laddach, J Chauhan, C Stavraka, S Lombardi, A Black, A Khiabany, DO Khair, M Figini, A Winship, S Ghosh, A Montes, JF Spicer, HJ Bax, DH Josephs, KE Lacy, S Tsoka, SN Karagianni
Cancers (Basel), 2020-11-15;12(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Prognostic role of follicular fluid tumor necrosis factor alpha in the risk of early ovarian hyperstimulation syndrome
Authors: MJ Alhilali, A Parham, A Attaranzad, M Amirian, M Azizzadeh
BMC Pregnancy Childbirth, 2020-11-12;20(1):691.
Species: Human
Sample Types: Cell Culture Supernates
-
Daily Viral Kinetics and Innate and Adaptive Immune Response Assessment in COVID-19: a Case Series
Authors: P Vetter, CS Eberhardt, B Meyer, PA Martinez M, G Torriani, F Pigny, S Lemeille, S Cordey, F Laubscher, DL Vu, A Calame, M Schibler, F Jacquerioz, G Blanchard-, CA Siegrist, L Kaiser, AM Didierlaur, I Eckerle
mSphere, 2020-11-11;5(6):.
Species: Human
Sample Types: Plasma
-
Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia
Authors: L Gibellini, S De Biasi, A Paolini, R Borella, F Boraldi, M Mattioli, D Lo Tartaro, L Fidanza, A Caro-Maldo, M Meschiari, V Iadisernia, E Bacca, G Riva, L Cicchetti, D Quaglino, G Guaraldi, S Busani, M Girardis, C Mussini, A Cossarizza
EMBO Mol Med, 2020-11-05;0(0):e13001.
Species: Human
Sample Types: Plasma
-
Probiotic supplementation in marathonists and its impact on lymphocyte population and function after a marathon: a randomized placebo-controlled double-blind study
Authors: H Batatinha, E Tavares-Si, GSF Leite, AS Resende, JAT Albuquerqu, C Arslanian, RA Fock, AH Lancha, FS Lira, K Krüger, R Thomatieli, JC Rosa-Neto
Sci Rep, 2020-11-02;10(1):18777.
Species: Human
Sample Types: Whole Blood
-
Vadadustat, a HIF Prolyl Hydroxylase Inhibitor, Improves Immunomodulatory Properties of Human Mesenchymal Stromal Cells
Authors: K Zielniok, A Burdzinska, B Kaleta, R Zagozdzon, L Paczek
Cells, 2020-11-01;9(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Proinflammatory and angiogenesis-related cytokines in vitreous samples of highly myopic patients
Authors: Q Wei, X Zhuang, J Fan, R Jiang, Q Chang, G Xu, Z Yu
Cytokine, 2020-10-28;137(0):155308.
Species: Human
Sample Types: Vitreous Humor
-
Circulating biomarkers and outcomes from a randomised phase 2 trial of gemcitabine versus capecitabine-based chemoradiotherapy for pancreatic cancer
Authors: F Willenbroc, CM Cox, EE Parkes, CS Wilhelm-Be, AG Abraham, R Owens, A Sabbagh, CM Jones, DLI Hughes, T Maughan, CN Hurt, EE O'Neill, S Mukherjee
Br J Cancer, 2020-10-26;0(0):.
Species: Human
Sample Types: Plasma
-
Elevated cytokine, chemokine, and growth and differentiation factor-15 levels in hemorrhagic shock and encephalopathy syndrome: A retrospective observational study
Authors: H Yamaguchi, M Nishiyama, S Tokumoto, Y Ishida, K Tomioka, K Aoki, Y Seino, D Toyoshima, H Takeda, H Kurosawa, H Sakuma, H Tada, K Nozu, A Maruyama, R Tanaka, K Iijima, H Nagase
Cytokine, 2020-10-05;137(0):155324.
Species: Human
Sample Types: Serum
-
Identification of serum biomarker panel to differentiate malignant from benign thyroid nodules using multiplex bead assay
Authors: RA Ramadan, W Ragab, RS Assaad, AE Shaaban, AI Fayad
J Egypt Natl Canc Inst, 2020-09-04;32(1):35.
Species: Human
Sample Types: Serum
-
Human CD83-targeted chimeric antigen receptor T cells prevent and treat graft-versus-host disease
Authors: B Shrestha, K Walton, J Reff, EM Sagatys, N Tu, JC Boucher, G Li, T Ghafoor, M Felices, J Miller, J Pidala, BR Blazar, C Anasetti, BC Betts, ML Davila
J. Clin. Invest., 2020-09-01;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
IKKepsilon and TBK1 in diffuse large B-cell lymphoma: A possible mechanism of action of an IKKepsilon/TBK1 inhibitor to repress NF-kappaB and IL-10 signalling
Authors: M Carr, S Mamand, KL Chapman, T Perrior, SD Wagner
J. Cell. Mol. Med., 2020-08-28;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Cytokine changes during treatment of anti-Caspr2 encephalitis: a case report
Authors: YC Wei, CL Wu, WC Weng
BMC Neurol, 2020-08-13;20(1):299.
Species: Human
Sample Types: Plasma
-
Co-expression of NMDA-receptor subunits NR1, NR2A, and NR2B in dysplastic neurons of teratomas in patients with paraneoplastic NMDA-receptor-encephalitis: a retrospective clinico-pathology study of 159 patients
Authors: XY Jiang, S Lei, L Zhang, X Liu, MT Lin, I Blumcke, YS Piao, D Zhou, JM Li
Acta Neuropathol Commun, 2020-08-08;8(1):130.
Species: Human
Sample Types: CSF
-
Inflammatory and lipid regulation by cholinergic activity in epicardial stromal cells from patients who underwent open-heart surgery
Authors: M Couselo-Se, JN Lopez-Cano, ÁL Fernandez, L González-M, LM Seoane, D Duran-Muño, A Rozados-Lu, JR González-J, M Rodríguez-, S Eiras
J. Cell. Mol. Med., 2020-08-07;0(0):.
Species: Human
Sample Types: Tissue Homogenates, Whole Tissue
-
The effect of circadian-adjusted LED-based lighting on sleep, daytime sleepiness and biomarkers of inflammation in a randomized controlled cross-over trial by pragmatic design in elderly care home dwellers
Authors: CB Linander, T Kallemose, LM Joergensen, O Andersen, JO Nehlin, BN Jawad
Arch Gerontol Geriatr, 2020-08-06;91(0):104223.
Species: Human
Sample Types: Plasma
-
Repeated Application of Autologous Bone Marrow-Derived Lineage-Negative Stem/Progenitor Cells-Focus on Immunological Pathways in Patients with ALS
Authors: B Baumert, A Sobu?, M Go??b-Jano, E Paczkowska, K ?uczkowska, D Rogi?ska, A Zawi?lak, S Milczarek, B Os?kowska, W Pawlukowsk, A Meller, K Machowska-, A We?nicka, K Safranow, P Nowacki, B Machali?sk
Cells, 2020-08-01;9(8):.
Species: Human
Sample Types: Plasma
-
Increased hepatic and circulating chemokine and osteopontin expression occurs early in human NAFLD development
Authors: M Kriss, L Golden-Mas, J Kaplan, F Mirshahi, VW Setiawan, AJ Sanyal, HR Rosen
PLoS ONE, 2020-07-30;15(7):e0236353.
Species: Human
Sample Types: Plasma
-
Mannitol and renal graft injury in patients undergoing deceased donor renal transplantation - a randomized controlled clinical trial
Authors: C Reiterer, K Hu, S Sljivic, M Falkner vo, E Fleischman, A Kainz, B Kabon
BMC Nephrol, 2020-07-28;21(1):307.
Species: Human
Sample Types: Serum
-
Long-lasting severe immune dysfunction in Ebola virus disease survivors
Authors: A Wiedemann, E Foucat, H Hocini, C Lefebvre, BP Hejblum, M Durand, M Krüger, AK Keita, A Ayouba, S Mély, JC Fernandez, A Touré, S Fourati, C Lévy-March, H Raoul, E Delaporte, L Koivogui, R Thiébaut, C Lacabaratz, Y Lévy
Nat Commun, 2020-07-24;11(1):3730.
Species: Human
Sample Types: Serum
-
Chemical modification of pro-inflammatory proteins by peroxynitrite increases activation of TLR4 and NF-&kappaB: Implications for the health effects of air pollution and oxidative stress
Authors: K Ziegler, AT Kunert, K Reinmuth-S, AL Leifke, D Widera, MG Weller, D Schuppan, J Fröhlich-N, K Lucas, U Pöschl
Redox Biol, 2020-07-20;0(0):101581.
Species: Human
Sample Types: Cell Culture Supernates
-
Modulation of the mechanical responses of synovial fibroblasts by osteoarthritis-associated inflammatory stressors
Authors: J Jamal, MM Roebuck, SY Lee, SP Frostick, AA Abbas, AM Merican, TS Hui, A Wood, SL Tan, G Bou-Ghario, PF Wong
Int. J. Biochem. Cell Biol., 2020-07-13;126(0):105800.
Species: Human
Sample Types: Tissue Culture Supernates
-
Repeated 5-day cycles of low dose aldesleukin in amyotrophic lateral sclerosis (IMODALS): A phase 2a randomised, double-blind, placebo-controlled trial
Authors: W Camu, M Mickunas, JL Veyrune, C Payan, C Garlanda, M Locati, R Juntas-Mor, N Pageot, A Malaspina, U Andreasson, J Kirby, C Suehs, S Saker, C Masseguin, J De Vos, H Zetterberg, PJ Shaw, A Al-Chalabi, PN Leigh, T Tree, G Bensimon
EBioMedicine, 2020-07-07;0(0):102844.
Species: Human
Sample Types: Plasma
-
Interleukin Gene Variability and Periodontal Bacteria in Patients with Generalized Aggressive Form of Periodontitis
Authors: P Borilova L, Z Danek, T Deissova, F Hromcik, B Lipovy, D Szaraz, J Janos, A Fassmann, J Bartova, I Drizhal, L Izakovicov
Int J Mol Sci, 2020-07-02;21(13):.
Species: Human
Sample Types: Cell Culture Supernates
-
Functional recovery in multiple sclerosis patients undergoing rehabilitation programs is associated with plasma levels of hemostasis inhibitors
Authors: Z Nicole, L Nicola, M Fabio, S Sofia, B Marcello, T Veronica, C Matteo, S Paola, B Nino, M Giovanna, B Francesco
Mult Scler Relat Disord, 2020-06-20;44(0):102319.
Species: Human
Sample Types: Plasma
-
Diminished hepatic IFN response following HCV clearance triggers HBV reactivation in coinfection
Authors: X Cheng, T Uchida, Y Xia, R Umarova, CJ Liu, PJ Chen, A Gaggar, V Suri, MM Mücke, J Vermehren, S Zeuzem, Y Teraoka, M Osawa, H Aikata, K Tsuji, N Mori, S Hige, Y Karino, M Imamura, K Chayama, TJ Liang
J. Clin. Invest., 2020-06-01;0(0):.
Species: Human
Sample Types: Plasma
-
Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients.
Authors: Smadja D, Guerin C, Chocron R, Yatim N, Boussier J, Gendron N, Khider L, Hadjadj J, Goudot G, Debuc B, Juvin P, Hauw-Berlemont C, Augy J, Peron N, Messas E, Planquette B, Sanchez O, Charbit B, Gaussem P, Duffy D, Terrier B, Mirault T, Diehl J
Angiogenesis, 2020-05-27;0(0):.
Species: Human
Sample Types: Platelet Poor Plasma
-
Neutrophil Phenotypes in Coronary Artery Disease
Authors: P Maréchal, J Tridetti, ML Nguyen, O Wéra, Z Jiang, M Gustin, AF Donneau, C Oury, P Lancellott
J Clin Med, 2020-05-25;9(5):.
Species: Human
Sample Types: Plasma
-
Metabolomic fingerprinting and systemic inflammatory profiling of asthma COPD overlap (ACO)
Authors: N Ghosh, P Choudhury, SR Kaushik, R Arya, R Nanda, P Bhattachar, S Roychowdhu, R Banerjee, K Chaudhury
Respir. Res., 2020-05-24;21(1):126.
Species: Human
Sample Types: Serum
-
Resveratrol Inhibits Particulate Matter-Induced Inflammatory Responses in Human Keratinocytes
Authors: JW Shin, HS Lee, JI Na, CH Huh, KC Park, HR Choi
Int J Mol Sci, 2020-05-13;21(10):.
Species: Human
Sample Types: Whole Cell
-
Systemic Type I IFN Inflammation in Human ISG15 Deficiency Leads to Necrotizing Skin Lesions
Authors: M Martin-Fer, M Bravo Garc, C Gruber, S Murias Loz, MNH Malik, F Alsohime, A Alakeel, R Valdez, S Buta, G Buda, MA Marti, M Larralde, B Boisson, M Feito Rodr, X Qiu, M Chrabieh, M Al Ayed, S Al Muhsen, JV Desai, EMN Ferre, SD Rosenzweig, B Amador-Bor, LY Bravo-Gall, R Olmer, S Merkert, M Bret, AK Sood, A Al-Rabiaah, MH Temsah, R Halwani, M Hernandez, F Pessler, JL Casanova, J Bustamante, MS Lionakis, D Bogunovic
Cell Rep, 2020-05-12;31(6):107633.
Species: Human
Sample Types:
-
Targeting Cellular Metabolism in Acute Myeloid Leukemia and The Role of Patient Heterogeneity
Authors: IS Grønningsæ, H Reikvam, E Aasebø, S Bartaula-B, TH Tvedt, Ø Bruserud, KJ Hatfield
Cells, 2020-05-07;9(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Expression profiles of the internal jugular and saphenous veins: Focus on hemostasis genes
Authors: N Ziliotto, S Meneghetti, E Menegatti, M Baroni, B Lunghi, F Salvi, M Ferracin, A Branchini, D Gemmati, F Mascoli, P Zamboni, F Bernardi, G Marchetti
Thromb. Res., 2020-05-03;191(0):113-124.
Species: Human
Sample Types: Plasma
-
Glutathione dynamics determine the therapeutic efficacy of mesenchymal stem cells for graft-versus-host disease via CREB1-NRF2 pathway
Authors: J Lim, J Heo, H Ju, JW Shin, Y Kim, S Lee, HY Yu, CM Ryu, H Yun, S Song, KS Hong, HM Chung, HR Kim, JS Roe, K Choi, IG Kim, EM Jeong, DM Shin
Sci Adv, 2020-04-15;6(16):eaba1334.
Species: Mouse
Sample Types: Serum
-
The Potential of Nanobody-Targeted Photodynamic Therapy to Trigger Immune Responses
Authors: I Beltrán He, ML Angelier, T Del Buono, A Di Maggio, Y Yu, S Oliveira
Cancers (Basel), 2020-04-15;12(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
Human Chorionic Gonadotropin modulates CXCL10 Expression through Histone Methylation in human decidua
Authors: M Silasi, Y You, S Simpson, J Kaislasuo, L Pal, S Guller, G Peng, R Ramhorst, E Grasso, S Etemad, S Durosier, P Aldo, G Mor
Sci Rep, 2020-04-01;10(1):5785.
Species: Human
Sample Types: Cell Culture Supernates
-
Therapeutic effects of CO-releaser/Nrf2 activator hybrids (HYCOs) in the treatment of skin wound, psoriasis and multiple sclerosis
Authors: Z El Ali, A Ollivier, S Manin, M Rivard, R Motterlini, R Foresti
Redox Biol, 2020-04-01;34(0):101521.
Species: Human
Sample Types: Whole Cells
-
Evaluation of TNF-&alpha genetic polymorphisms as predictors for sepsis susceptibility and progression
Authors: AM Georgescu, C Banescu, R Azamfirei, A Hutanu, V Moldovan, I Badea, S Voidazan, M Dobreanu, IR Chirtes, L Azamfirei
BMC Infect. Dis., 2020-03-14;20(1):221.
Species: Human
Sample Types: Plasma
-
Connexin43 Hemichannel Targeting With TAT-Gap19 Alleviates Radiation-Induced Endothelial Cell Damage
Authors: R Ramadan, E Vromans, DC Anang, I Goetschalc, D Hoorelbeke, E Decrock, S Baatout, L Leybaert, A Aerts
Front Pharmacol, 2020-03-05;11(0):212.
Species: Human
Sample Types: Cell Culture Supernates
-
Helminth infection modulates systemic pro-inflammatory cytokines and chemokines implicated in type 2 diabetes mellitus pathogenesis
Authors: A Rajamanick, S Munisankar, C Dolla, PA Menon, K Thiruvenga, TB Nutman, S Babu
PLoS Negl Trop Dis, 2020-03-03;14(3):e0008101.
Species: Human
Sample Types: Plasma
-
Effective Activation of Human Antigen-Presenting Cells and Cytotoxic CD8+ T Cells by a Calcium Phosphate-Based Nanoparticle Vaccine Delivery System
Authors: F Scheffel, T Knuschke, L Otto, S Kollenda, V Sokolova, C Cosmovici, J Buer, J Timm, M Epple, AM Westendorf
Vaccines (Basel), 2020-03-01;8(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Complex human adenoid tissue-based ex vivo culture systems reveal anti-inflammatory drug effects on germinal center T and B cells
Authors: A Schmidt, JE Huber, Ö Sercan Alp, R Gürkov, CA Reichel, M Herrmann, OT Keppler, T Leeuw, D Baumjohann
EBioMedicine, 2020-02-27;53(0):102684.
Species: Human
Sample Types: Cell Culture Supernates
-
Simultaneous kinase inhibition with ibrutinib and BCL2 inhibition with venetoclax offers a therapeutic strategy for acute myeloid leukemia
Authors: CA Eide, SE Kurtz, A Kaempf, N Long, A Agarwal, CE Tognon, M Mori, BJ Druker, BH Chang, AV Danilov, JW Tyner
Leukemia, 2020-02-24;0(0):.
Species: Human
Sample Types: blood
-
The ratio of plasma angiopoietin-2 to angiopoietin-1 as a prognostic biomarker in patients with sepsis
Authors: CH Seol, SH Yong, JH Shin, SH Lee, AY Leem, SM Park, YS Kim, KS Chung
Cytokine, 2020-02-12;129(0):155029.
Species: Human
Sample Types: Plasma
-
Elevated M2 Macrophage Markers in Epiretinal Membranes With Ectopic Inner Foveal Layers
Authors: J Baek, HY Park, JH Lee, M Choi, JH Lee, M Ha, MY Lee
Invest. Ophthalmol. Vis. Sci., 2020-02-07;61(2):19.
Species: Human
Sample Types: eye vitreous fluid
-
Plasma FABP4 is associated with liver disease recovery during treatment-induced clearance of chronic HCV infection
Authors: JB Gorin, DFG Malone, B Strunz, T Carlsson, S Aleman, NK Björkström, K Falconer, JK Sandberg
Sci Rep, 2020-02-07;10(1):2081.
Species: Human
Sample Types: Plasma
-
Remitted depression and cognition in HIV: The role of cortisol and inflammation
Authors: LH Rubin, SA Langenecke, KL Phan, SM Keating, GN Neigh, KM Weber, PM Maki
Psychoneuroendocrinology, 2020-02-07;114(0):104609.
Species: Human
Sample Types: Saliva
-
Gut permeability, inflammation, and bone density across the menopause transition
Authors: A Shieh, M Epeldegui, AS Karlamangl, GA Greendale
JCI Insight, 2020-01-30;0(0):.
Species: Human
Sample Types: Plasma
-
An IL-18-centered inflammatory network as a biomarker for cerebral white matter injury
Authors: M Altendahl, P Maillard, D Harvey, D Cotter, S Walters, A Wolf, B Singh, V Kakarla, I Azizkhania, SA Sheth, G Xiao, E Fox, M You, M Leng, D Elashoff, JH Kramer, C Decarli, F Elahi, JD Hinman
PLoS ONE, 2020-01-24;15(1):e0227835.
Species: Human
Sample Types: Serum
-
Rifaximin improves survival in cirrhotic patients with refractory ascites: A real-world study
Authors: XY Lv, HG Ding, JF Zheng, CL Fan, L Li
World J. Gastroenterol., 2020-01-14;26(2):199-218.
Species: Human
Sample Types: Plasma
-
Cytokines and Chemokines Are Detectable in Swivel-Derived Exhaled Breath Condensate (SEBC): A Pilot Study in Mechanically Ventilated Patients
Authors: P van der Ze, I van Walree, JW Fijen, AJ van Houte, H van Velzen, G Rijkers, D Gommers, H Endeman
Dis. Markers, 2020-01-11;2020(0):2696317.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated plasma and synovial fluid interleukin-8 and interleukin-18 may be associated with the pathogenesis of knee osteoarthritis
Authors: SM Koh, CK Chan, SH Teo, S Singh, A Merican, WM Ng, A Abbas, T Kamarul
Knee, 2020-01-06;0(0):.
Species: Human
Sample Types: Plasma, Synovial Fluid
-
Characterization of potential biomarkers of reactogenicity of licensed antiviral vaccines: randomized controlled clinical trials conducted by the BIOVACSAFE consortium
Authors: J Weiner, DJM Lewis, J Maertzdorf, HJ Mollenkopf, C Bodinham, K Pizzoferro, C Linley, A Greenwood, A Mantovani, B Bottazzi, P Denoel, G Leroux-Roe, KE Kester, I Jonsdottir, R van den Be, SHE Kaufmann, G Del Giudic
Sci Rep, 2019-12-30;9(1):20362.
Species: Human
Sample Types: Serum
-
Altered monocytic phenotypes are linked with systemic inflammation and may be linked to mortality in dialysis patients
Authors: S Brandt, L Ewert, FG Scurt, C Reichardt, JA Lindquist, X Gorny, B Isermann, PR Mertens
Sci Rep, 2019-12-13;9(1):19103.
Species: Human
Sample Types: Serum
-
Characterization of the local wound environment following treatment of chronic leg ulcers with SIS wound matrix
Authors: JP Hodde, MC Hiles, DW Metzger
J Tissue Viability, 2019-12-13;0(0):.
Species: Human
Sample Types: Wound Fluid
-
Multiple cytokine analyses of aqueous humor from the patients with retinitis pigmentosa
Authors: B Lu, H Yin, Q Tang, W Wang, C Luo, X Chen, X Zhang, K Lai, J Xu, X Chen, K Yao
Cytokine, 2019-12-03;127(0):154943.
Species: Human
Sample Types: Aqueous Humor
-
Plasma chemokines are biomarkers of disease severity, higher bacterial burden and delayed sputum culture conversion in pulmonary tuberculosis
Authors: NP Kumar, K Moideen, A Nancy, V Viswanatha, BS Shruthi, S Sivakumar, M Natarajan, H Kornfeld, S Babu
Sci Rep, 2019-12-03;9(1):18217.
Species: Human
Sample Types: Plasma
-
Pro-inflammatory cytokines associate with NETosis during sickle cell vaso-occlusive crises
Authors: EA Barbu, L Mendelsohn, L Samsel, SL Thein
Cytokine, 2019-11-25;127(0):154933.
Species: Human
Sample Types: Plasma
-
Tunable hydrogels for mesenchymal stem cell delivery: integrin-induced transcriptome alterations and hydrogel optimization for human wound healing
Authors: AI Marusina, AA Merleev, JI Luna, L Olney, NE Haigh, D Yoon, C Guo, EM Ovadia, M Shimoda, G Luxardi, S Boddu, NN Lal, Y Takada, KS Lam, R Liu, RR Isseroff, S Le, JA Nolta, AM Kloxin, E Maverakis
Stem Cells, 2019-11-23;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells
Authors: V Frodermann, D Rohde, G Courties, N Severe, MJ Schloss, H Amatullah, CS McAlpine, S Cremer, FF Hoyer, F Ji, ID van Koever, F Herisson, L Honold, GS Masson, S Zhang, J Grune, Y Iwamoto, SP Schmidt, GR Wojtkiewic, IH Lee, K Gustafsson, G Pasterkamp, SCA de Jager, RI Sadreyev, J MacFadyen, P Libby, P Ridker, DT Scadden, K Naxerova, KL Jeffrey, FK Swirski, M Nahrendorf
Nat. Med., 2019-11-07;25(11):1761-1771.
Species: Human
Sample Types: Plasma
-
Elevated interleukin-25 and its association to Th2 cytokines in systemic lupus erythematosus with lupus nephritis
Authors: M Selvaraja, M Abdullah, M Arip, VK Chin, A Shah, S Amin Nordi
PLoS ONE, 2019-11-07;14(11):e0224707.
Species: Human
Sample Types: Plasma
-
Endotype-driven prediction of acute exacerbations in chronic obstructive pulmonary disease (EndAECOPD): protocol for a prospective cohort study
Authors: W Xiao, LY Du, B Mao, TW Miao, JJ Fu
BMJ Open, 2019-11-04;9(11):e034592.
Species: Human
Sample Types: Saliva
-
Blood Compatibility-An Important but Often Forgotten Aspect of the Characterization of Antimicrobial Peptides for Clinical Application
Authors: S Harm, K Lohner, U Fichtinger, C Schildböck, J Zottl, J Hartmann
Int J Mol Sci, 2019-10-31;20(21):.
Species: Human
Sample Types: Whole Blood
-
Neutrophil Recruitment to Noninvasive MRSA at the Stratum Corneum of Human Skin Mediates Transient Colonization
Authors: A Schulz, L Jiang, L de Vor, M Ehrström, F Wermeling, L Eidsmo, K Melican
Cell Rep, 2019-10-29;29(5):1074-1081.e5.
Species: Human
Sample Types: Tissue Homogenates
-
Signal Integration and Transcriptional Regulation of the Inflammatory Response Mediated by the GM-/M-CSF Signaling Axis in Human Monocytes
Authors: RM Rodriguez, B Suarez-Alv, JL Lavín, AM Ascensión, M Gonzalez, JJ Lozano, AB Raneros, PD Bulnes, JR Vidal-Cast, C Huidobro, C Martin-Mar, AB Sanz, M Ruiz-Orteg, A Puig-Kröge, AL Corbí, MJ Araúzo-Bra, AM Aransay, C Lopez-Larr
Cell Rep, 2019-10-22;29(4):860-872.e5.
Species: Human
Sample Types: Cell Culture Supernates
-
Pre-treatment peripheral biomarkers associated with treatment response in panic symptoms in patients with major depressive disorder and panic disorder: A 12-week follow-up study
Authors: K Kim, EH Jang, AY Kim, M Fava, D Mischoulon, GI Papakostas, H Kim, EJ Na, HY Yu, HJ Jeon
Compr Psychiatry, 2019-10-19;95(0):152140.
Species: Human
Sample Types: Serum
-
Methylglyoxal and Glyoxal as Potential Peripheral Markers for MCI Diagnosis and Their Effects on the Expression of Neurotrophic, Inflammatory and Neurodegenerative Factors in Neurons and in Neuronal Derived-Extracellular Vesicles
Authors: M Haddad, M Perrotte, MRB Khedher, C Demongin, A Lepage, T Fülöp, C Ramassamy
Int J Mol Sci, 2019-10-03;20(19):.
Species: Human
Sample Types: Cell Lysates
-
Early malaria infection, dysregulation of angiogenesis, metabolism and inflammation across pregnancy, and risk of preterm birth in Malawi: A cohort study
Authors: RE Elphinston, AM Weckman, CR McDonald, V Tran, K Zhong, M Madanitsa, L Kalilani-P, C Khairallah, SM Taylor, SR Meshnick, V Mwapasa, FO Ter Kuile, AL Conroy, KC Kain
PLoS Med., 2019-10-01;16(10):e1002914.
Species: Human
Sample Types: Plasma
-
Serum Levels of Tumor Necrosis Factor-α and Loudness Dependence of Auditory Evoked Potentials at Pretreatment and Posttreatment in Patients with Major Depressive Disorder
Authors: BH Lee, YM Park, SH Lee, M Shim
Brain Sci, 2019-09-26;9(10):.
Species: Human
Sample Types: Serum
-
BET Proteins Are Required for Transcriptional Activation of the Senescent Islet Cell Secretome in Type 1 Diabetes
Authors: PJ Thompson, A Shah, H Apostolopo, A Bhushan
Int J Mol Sci, 2019-09-26;20(19):.
Species: Human
Sample Types: Cell Culture Supernates
-
Lower activation of CD4+ memory T cells in preeclampsia compared to healthy pregnancies persists postpartum
Authors: TEC Kieffer, SA Scherjon, MM Faas, JR Prins
J. Reprod. Immunol., 2019-09-20;136(0):102613.
Species: Human
Sample Types: Plasma
-
Urinary NGAL and RBP Are Biomarkers of Normoalbuminuric Renal Insufficiency in Type 2 Diabetes Mellitus
Authors: A Li, B Yi, Y Liu, J Wang, Q Dai, Y Huang, YC Li, H Zhang
J Immunol Res, 2019-09-15;2019(0):5063089.
Species: Human
Sample Types: Urine
-
Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson's disease
Authors: P Krashia, A Cordella, A Nobili, L La Barbera, M Federici, A Leuti, F Campanelli, G Natale, G Marino, V Calabrese, F Vedele, V Ghiglieri, B Picconi, G Di Lazzaro, T Schirinzi, G Sancesario, N Casadei, O Riess, S Bernardini, A Pisani, P Calabresi, MT Viscomi, CN Serhan, V Chiurchiù, M D'Amelio, NB Mercuri
Nat Commun, 2019-09-02;10(1):3945.
Species: Human
Sample Types: Plasma
-
Peripheral Brain-Derived Neurotrophic Factor and Contactin-1 Levels in Patients with Attention-Deficit/Hyperactivity Disorder
Authors: LJ Wang, CC Wu, MJ Lee, MC Chou, SY Lee, WJ Chou
J Clin Med, 2019-09-02;8(9):.
Species: Human
Sample Types: Plasma
-
Effect of Ionizing Radiation on Human EA.hy926 Endothelial Cells under Inflammatory Conditions and Their Interactions with A549 Tumour Cells
Authors: S Schröder, S Broese, J Baake, D Juer beta, S Kriesen, G Hildebrand, K Manda
J Immunol Res, 2019-09-02;2019(0):9645481.
Species: Human
Sample Types: Cell Culture Supernates
-
NK cells are activated and primed for skin-homing during acute dengue virus infection in humans
Authors: CL Zimmer, M Cornillet, C Solà-Riera, KW Cheung, MA Ivarsson, MQ Lim, N Marquardt, YS Leo, DC Lye, J Klingström, PA MacAry, HG Ljunggren, L Rivino, NK Björkström
Nat Commun, 2019-08-29;10(1):3897.
Species: Human
Sample Types: Plasma
-
Postmortem Retrieval Analysis of Metallosis and Periprosthetic Tissue Metal Concentrations in Total Knee Arthroplasty
Authors: CM Arnholt, JB White, JA Lowell, MR Perkins, WM Mihalko, SM Kurtz
J Arthroplasty, 2019-08-19;0(0):.
Species: Human
Sample Types: Synovial Fluid
-
The predictive value of CSF multiple assay in multiple sclerosis: A single center experience
Authors: C De Fino, M Lucchini, D Lucchetti, V Nociti, FA Losavio, A Bianco, F Colella, C Ricciardi-, A Sgambato, M Mirabella
Mult Scler Relat Disord, 2019-07-28;35(0):176-181.
Species: Human
Sample Types: Serum
-
A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer
Authors: L Zaharuddin, NM Mokhtar, KN Muhammad N, RA Raja Ali
BMC Gastroenterol, 2019-07-24;19(1):131.
Species: Human
Sample Types: Serum
-
Multilevel defects in the hematopoietic niche in essential thrombocythemia
Authors: T Sun, M Ju, X Dai, H Dong, W Gu, Y Gao, R Fu, X Liu, Y Huang, W Liu, Y Chi, W Wang, H Li, Y Zhou, L Shi, R Yang, L Zhang
Haematologica, 2019-07-09;0(0):.
Species: Human
Sample Types: Bone Marrow Fluid
-
High Constitutive Cytokine Release by Primary Human Acute Myeloid Leukemia Cells Is Associated with a Specific Intercellular Communication Phenotype
Authors: H Reikvam, E Aasebø, AK Brenner, S Bartaula-B, IS Grønningsæ, RB Forthun, R Hovland, Ø Bruserud
J Clin Med, 2019-07-04;8(7):.
Species: Human
Sample Types: Cell Culture Supernates
-
BCG Therapy of Bladder Cancer Stimulates a Prolonged Release of the Chemoattractant CXCL10 (IP10) in Patient Urine
Authors: O Ashiru, G Esteso, EM García-Cue, E Castellano, C Samba, E Escudero-L, S López-Cobo, M Álvarez-Ma, A Linares, MM Ho, A Leibar, L Martínez-P, M Valés-Góme
Cancers (Basel), 2019-07-04;11(7):.
Species: Human
Sample Types: Urine
-
Kynurenine metabolism and inflammation-induced depressed mood: A human experimental study
Authors: JL Kruse, JH Cho, R Olmstead, L Hwang, K Faull, NI Eisenberge, MR Irwin
Psychoneuroendocrinology, 2019-07-03;109(0):104371.
Species: Human
Sample Types: Plasma
-
Hyaluronic Acid (HA), Platelet-Rich Plasm and Extracorporeal Shock Wave Therapy (ESWT) promote human chondrocyte regeneration in vitro and ESWT-mediated increase of CD44 expression enhances their susceptibility to HA treatment
Authors: M Vetrano, D Ranieri, M Nanni, A Pavan, F Malisan, MC Vulpiani, V Visco
PLoS ONE, 2019-06-28;14(6):e0218740.
Species: Human
Sample Types: Cell Culture Supernates
-
Alteration of the IL-33-sST2 pathway in hypertensive patients and a mouse model
Authors: X Yin, H Cao, Y Wei, HH Li
Hypertens. Res., 2019-06-24;0(0):.
Species: Human
Sample Types: Serum
-
Biomarkers of cavernous angioma with symptomatic hemorrhage
Authors: SB Lyne, R Girard, J Koskimäki, HA Zeineddine, D Zhang, Y Cao, Y Li, A Stadnik, T Moore, R Lightle, C Shi, R Shenkar, J Carrión-Pe, SP Polster, S Romanos, A Akers, M Lopez-Rami, KJ Whitehead, ML Kahn, MH Ginsberg, DA Marchuk, IA Awad
JCI Insight, 2019-06-20;4(12):.
Species: Human
Sample Types: Plasma
-
TAZ is required for lung alveolar epithelial cell differentiation after injury
Authors: T Sun, Z Huang, H Zhang, C Posner, G Jia, TR Ramalingam, M Xu, HD Brightbill, JG Egen, A Dey, JR Arron
JCI Insight, 2019-06-18;5(0):.
Species: Human
Sample Types: Plasma
-
High-density lipoprotein cholesterol is associated with multiple sclerosis fatigue: A�fatigue-metabolism nexus?
Authors: RW Browne, D Jakimovski, N Ziliotto, J Kuhle, F Bernardi, B Weinstock-, R Zivadinov, M Ramanathan
J Clin Lipidol, 2019-06-18;0(0):.
Species: Human
Sample Types: Plasma
-
Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans
Authors: L Wu, M Xia, Y Duan, L Zhang, H Jiang, X Hu, H Yan, Y Zhang, Y Gu, H Shi, J Li, X Gao, J Li
Cell Death Dis, 2019-06-13;10(6):468.
Species: Human
Sample Types: Serum
-
T cell deletion of MyD88 connects IL-17 and IkBz to RAS oncogenesis
Authors: C Cataisson, R Salcedo, AM Michalowsk, M Klosterman, S Naik, L Li, MJ Pan, A Sweet, JQ Chen, LG Kostecka, M Karwan, L Smith, RM Dai, CA Stewart, L Lyakh, WT Hsieh, A Khan, H Yang, M Lee, G Trinchieri, SH Yuspa
Mol. Cancer Res., 2019-06-04;0(0):.
Species: Mouse, Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Serum Levels of Hepatocyte Growth Factor and CD40 Ligand Predict Radiation-Induced Liver Injury
Authors: KC Cuneo, T Devasia, Y Sun, MJ Schipper, D Karnak, MA Davis, D Owen, M Feng, I El Naqa, L Bazzi, R Ten Haken, TS Lawrence
Transl Oncol, 2019-05-09;12(7):889-894.
Species: Human
Sample Types: Serum
-
Flagellin-independent effects of a Toll-like receptor 5 polymorphism in the inflammatory response to Burkholderia pseudomallei
Authors: AK Dickey, N Chantratit, S Tandhavana, D Ducken, L Lovelace-M, S Seal, J Robertson, ND Myers, S Schwarz, MM Wurfel, S Kosamo, TE West
PLoS Negl Trop Dis, 2019-05-08;13(5):e0007354.
Species: Human
Sample Types: Plasma
-
Serum Bovine Immunoglobulins Improve Inflammation and Gut Barrier Function in Persons with HIV and Enteropathy on Suppressive ART
Authors: NS Utay, A Somasunder, JE Hinkle, BW Petschow, CJ Detzel, M Somsouk, CJ Fichtenbau, EM Weaver, AL Shaw, DM Asmuth
Pathog Immun, 2019-05-03;4(1):124-146.
Species: Human
Sample Types: Serum
-
Long-term every-other-day administration of DMAMCL has little effect on aging and age-associated physiological decline in mice
Authors: Z Sun, L Zhao, L Su, Q Fang, C Xu, Y Su, Y Liang, G Li, Y Xue, T Tong, J Chen
Aging (Albany NY), 2019-05-02;0(0):.
Species: Mouse
Sample Types: Plasma
-
Impact of HIV-1 infection on the IGF-1 axis and angiogenic factors in pregnant Cameroonian women receiving antiretroviral therapy
Authors: LF Esemu, EK Yuosembom, R Fang, S Rasay, BAY Fodjo, JT Nguasong, W Kidima, GL Ekali, JJ Chen, L Ndhlovu, JD Bigoga, DW Taylor, RGF Leke, A Babakhanya
PLoS ONE, 2019-05-01;14(5):e0215825.
Species: Human
Sample Types: Plasma
-
Pneumonitis resulting from radiation and immune checkpoint blockade illustrates characteristic clinical, radiologic and circulating biomarker features.
Authors: Schoenfeld J, Nishino M, Severgnini M, Manos M, Mak R, Hodi F
J Immunother Cancer, 2019-04-24;7(1):112.
Species: Human
Sample Types: Serum
-
A safe and potent anti-CD19 CAR T cell therapy
Authors: Z Ying, XF Huang, X Xiang, Y Liu, X Kang, Y Song, X Guo, H Liu, N Ding, T Zhang, P Duan, Y Lin, W Zheng, X Wang, N Lin, M Tu, Y Xie, C Zhang, W Liu, L Deng, S Gao, L Ping, X Wang, N Zhou, J Zhang, Y Wang, S Lin, M Mamuti, X Yu, L Fang, S Wang, H Song, G Wang, L Jones, J Zhu, SY Chen
Nat. Med., 2019-04-22;0(0):.
Species: Human
Sample Types: Plasma
Applications: CAR-T Confirmation -
Thrombin Preconditioning of Extracellular Vesicles Derived from Mesenchymal Stem Cells Accelerates Cutaneous Wound Healing by Boosting Their Biogenesis and Enriching Cargo Content
Authors: DK Sung, YS Chang, SI Sung, SY Ahn, WS Park
J Clin Med, 2019-04-18;8(4):.
Species: Human
Sample Types: Cell Lysates
-
Application of Cerebrospinal Fluid Host Protein Biosignatures in the Diagnosis of Tuberculous Meningitis in Children from a High Burden Setting
Authors: CM Manyelo, RS Solomons, CI Snyders, PM Manngo, H Mutavhatsi, B Kriel, K Stanley, G Walzl, NN Chegou
Mediators Inflamm., 2019-04-16;2019(0):7582948.
Species: Human
Sample Types: CSF
-
Adipose-derived stem cell therapy ameliorates ionizing irradiation fibrosis (RIF) via hepatocyte growth factor mediated TGF-? down regulation and recruitment of bone marrow cells
Authors: A Ejaz, MW Epperly, W Hou, JS Greenberge, PJ Rubin
Stem Cells, 2019-04-11;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Hemodynamic efficiency of hemodialysis treatment with high cut-off membrane during the early period of post-resuscitation shock: The HYPERDIA trial
Authors: G Geri, D Grimaldi, T Seguin, L Lamhaut, N Marin, JD Chiche, F Pène, A Bouglé, F Daviaud, T Morichau-B, M Arnaout, B Champigneu, L Zafrani, S Bourcier, YL Nguyen, J Charpentie, JP Mira, J Coste, C Vinsonneau, A Cariou
Resuscitation, 2019-04-08;0(0):.
Species: Human
Sample Types: Plasma
-
Effects of aqueous suppressants and prostaglandin analogues on early wound healing after glaucoma implant surgery
Authors: KI Jung, JE Woo, CK Park
Sci Rep, 2019-03-27;9(1):5251.
Species: Rabbit
Sample Types: Aqueous Humor
-
Socioeconomic status and early blood concentrations of inflammation-related and neurotrophic proteins among extremely preterm newborns
Authors: A Leviton, EN Allred, O Dammann, RM Joseph, RN Fichorova, TM O'Shea, KCK Kuban
PLoS ONE, 2019-03-26;14(3):e0214154.
Species: Human
Sample Types:
-
Plasm YKL-40 Levels Are Associated with Hypertension in Patients with Obstructive Sleep Apnea
Authors: K Li, Z Chen, Y Qin, YX Wei
Biomed Res Int, 2019-03-13;2019(0):5193597.
Species: Human
Sample Types: Plasma
-
Antimicrobial and inflammatory properties of South African clinical Lactobacillus isolates and vaginal probiotics
Authors: E Chetwin, MT Manhanzva, AG Abrahams, R Froissart, H Gamieldien, H Jaspan, SZ Jaumdally, SL Barnabas, S Dabee, AU Happel, D Bowers, L Davids, JS Passmore, L Masson
Sci Rep, 2019-02-13;9(1):1917.
Species: Human
Sample Types: Cell Culture Supernates
-
The Composition of Hyperacute Serum and Platelet-Rich Plasma Is Markedly Different despite the Similar Production Method
Authors: D Kardos, M Simon, G Vácz, A Hinsenkamp, T Holczer, D Cseh, A Sárközi, K Szenthe, F Bánáti, S Szathmary, S Nehrer, O Kuten, M Masteling, Z Lacza, I Hornyák
Int J Mol Sci, 2019-02-08;20(3):.
Species: Human
Sample Types: Serum
-
Plasma Profiles of Inflammatory Markers Associated With Active Tuberculosis in Antiretroviral Therapy-Naive Human Immunodeficiency Virus-Positive Individuals
Authors: O Olsson, P Björkman, M Jansson, TT Balcha, D Mulleta, H Yeba, C Valfridsso, F Carlsson, S Skogmar
Open Forum Infect Dis, 2019-01-31;6(2):ofz015.
Species: Human
Sample Types: Plasma
-
IFN-? and IL-17A differentially influence the response of human macrophages and neutrophils to Pseudomonas aeruginosa infection
Authors: S Muntaka, Y Almuhanna, D Jackson, S Singh, A Afryie-Asa, M Cámara, L Martínez-P
Infect. Immun., 2019-01-24;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
G-CSF administration favours SDF-1 release and activation of neutrophils and monocytes in recipients of autologous peripheral blood progenitor cells
Authors: K G?bura, A Butrym, M Chaszczews, T Wróbel, K Kuliczkows, K Bogunia-Ku
Cytokine, 2019-01-24;116(0):38-47.
Species: Human
Sample Types: Serum
-
Pioglitazone-Primed Mesenchymal Stem Cells Stimulate Cell Proliferation, Collagen Synthesis and Matrix Gene Expression in Tenocytes
Authors: W Kim, SK Lee, YW Kwon, SG Chung, S Kim
Int J Mol Sci, 2019-01-22;20(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Chronic Rhinosinusitis without Nasal Polyps in Asian Patients Shows Mixed Inflammatory Patterns and Neutrophil-Related Disease Severity
Authors: DW Kim, KM Eun, EY Roh, S Shin, DK Kim
Mediators Inflamm., 2019-01-15;2019(0):7138643.
Species: Human
Sample Types: Tissue Homogenates
-
Comparison of serum and saliva miRNAs for identification and characterization of mTBI in adult mixed martial arts fighters
Authors: D LaRocca, S Barns, SD Hicks, A Brindle, J Williams, R Uhlig, P Johnson, C Neville, FA Middleton
PLoS ONE, 2019-01-02;14(1):e0207785.
Species: Human
Sample Types: Serum
-
T cell deletion of MyD88 connects IL-17 and IkBz to RAS oncogenesis
Authors: C Cataisson, R Salcedo, AM Michalowsk, M Klosterman, S Naik, L Li, MJ Pan, A Sweet, JQ Chen, LG Kostecka, M Karwan, L Smith, RM Dai, CA Stewart, L Lyakh, WT Hsieh, A Khan, H Yang, M Lee, G Trinchieri, SH Yuspa
Mol. Cancer Res., 2019;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Marked infiltration of neutrophils at the upper palpebral conjunctiva in patients with chronic graft-versus-host disease
Authors: YS Byun, YS Yoo, MJ Kang, WJ Whang, KS Na, JW Mok, CK Joo
Ocul Surf, 2018-12-20;0(0):.
-
Mapping of ?/? T cells reveals V?2+ T cells resistance to senescence
Authors: W Xu, G Monaco, EH Wong, WLW Tan, H Kared, Y Simoni, SW Tan, WZY How, CTY Tan, BTK Lee, D Carbajo, S K G, ICH Low, EWH Mok, S Foo, J Lum, HL Tey, WP Tan, M Poidinger, E Newell, TP Ng, R Foo, AN Akbar, T Fülöp, A Larbi
EBioMedicine, 2018-12-07;39(0):44-58.
Species: Human
Sample Types: Cell Culture Supernates
-
Matrix Metalloproteinase Response of Dendritic Cell, Gingival Epithelial Keratinocyte, and T-Cell Transwell Co-Cultures Treated with Porphyromonas gingivalis Hemagglutinin-B
Authors: AM Bates, CL Fischer, VP Abhyankar, GK Johnson, JM Guthmiller, A Progulske-, KA Brogden
Int J Mol Sci, 2018-12-07;19(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential
Authors: L Wampach, A Heintz-Bus, JV Fritz, J Ramiro-Gar, J Habier, M Herold, S Narayanasa, A Kaysen, AH Hogan, L Bindl, J Bottu, R Halder, C Sjöqvist, P May, AF Andersson, C de Beaufor, P Wilmes
Nat Commun, 2018-11-30;9(1):5091.
Species: Human
Sample Types: Cell Culture Supernates
-
Cellular metabolism constrains innate immune responses in early human ontogeny
Authors: B Kan, C Michalski, H Fu, HHT Au, K Lee, EA Marchant, MF Cheng, E Anderson-B, M Aharoni-Si, P Tilley, RG Mirmira, CJ Ross, DS Luciani, E Jan, PM Lavoie
Nat Commun, 2018-11-16;9(1):4822.
Species: Human
Sample Types: Cell Culture Supernates
-
Cathepsin S Alters the Expression of Pro-Inflammatory Cytokines and MMP-9, Partially through Protease-Activated Receptor-2, in Human Corneal Epithelial Cells
Authors: W Klinngam, R Fu, SR Janga, MC Edman, SF Hamm-Alvar
Int J Mol Sci, 2018-11-09;19(11):.
Species: Human
Sample Types: Cell Lysates
-
Soluble Siglec-14 glycan-recognition protein is generated by alternative splicing and suppresses myeloid inflammatory responses
Authors: PJ Huang, PY Low, I Wang, SD Hsu, T Angata
J. Biol. Chem., 2018-10-30;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Human Induced Pluripotent Stem Cell-Derived Astrocytes Are Differentially Activated by Multiple Sclerosis-Associated Cytokines
Authors: S Perriot, A Mathias, G Perriard, M Canales, N Jonkmans, N Merienne, C Meunier, L El Kassar, AL Perrier, DA Laplaud, M Schluep, N Déglon, R Du Pasquie
Stem Cell Reports, 2018-10-25;11(5):1199-1210.
Species: Human
Sample Types: Cell Culture Supernates
-
Exosomes Secreted from Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Accelerate Skin Cell Proliferation
Authors: S Kim, SK Lee, H Kim, TM Kim
Int J Mol Sci, 2018-10-11;19(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
Viable cryopreserved umbilical tissue (vCUT) reduces post-operative adhesions in a rabbit abdominal adhesion model
Authors: S Dhall, T Coksaygan, T Hoffman, M Moorman, A Lerch, JQ Kuang, M Sathyamoor, A Danilkovit
Bioact Mater, 2018-10-10;4(1):97-106.
Species: Human
Sample Types: Tissue Homogenates
-
Increased macrophages and�changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation
Authors: HQ Cai, VS Catts, MJ Webster, C Galletly, D Liu, M O'Donnell, TW Weickert, CS Weickert
Mol. Psychiatry, 2018-09-13;0(0):.
Species: Human
Sample Types: Plasma
-
Dietary intervention with an Okinawan-based Nordic diet in type 2 diabetes renders decreased interleukin-18 concentrations and increased neurofilament light concentrations in plasma.
Authors: Nilholm C, Roth B, Hoglund P, Blennow K, Englund E, Hansson O, Zetterberg H, Ohlsson B
Nutr Res, 2018-08-19;60(0):13-25.
Species: Human
Sample Types: Plasma
-
Matricellular Protein Levels in Aqueous Humor and Surgical Outcomes of Trabeculectomy
Authors: Z Zhang, Y Miao, J Wang, M Zhou, M Fu, Y Wang
Invest. Ophthalmol. Vis. Sci., 2018-08-01;59(10):3906-3910.
Species: Human
Sample Types: Aqueous Humor
-
Loss of androgen receptor signaling in prostate cancer‐associated fibroblasts (CAFs) promotes CCL2‐ and CXCL8‐mediated cancer cell migration
Authors: Bianca Cioni, Ekaterina Nevedomskaya, Monique H. M. Melis, Johan van Burgsteden, Suzan Stelloo, Emma Hodel et al.
Molecular Oncology
Species: Human
Sample Types: Cell Culture Supernates
-
Endothelial Microvesicles and Soluble Markers of Endothelial Injury in Critically Ill Newborns
Authors: V Vítková, M Pánek, P Janec, M Šibíková, V Vobruba, M Haluzík, J Živný, J Janota
Mediators Inflamm., 2018-07-19;2018(0):1975056.
Species: Human
Sample Types: Plasma
-
Low-density lipoprotein (LDL)-dependent uptake of Gram-positive lipoteichoic acid and Gram-negative lipopolysaccharide occurs through LDL receptor
Authors: PM Grin, DJ Dwivedi, KM Chathely, BL Trigatti, A Prat, NG Seidah, PC Liaw, AE Fox-Robich
Sci Rep, 2018-07-12;8(1):10496.
Species: Human
Sample Types: Cell Culture Supernates
-
Hypoxia with Wharton's jelly mesenchymal stem cell coculture maintains stemness of umbilical cord blood-derived CD34+ cells
Authors: D Zhao, L Liu, Q Chen, F Wang, Q Li, Q Zeng, J Huang, M Luo, W Li, Y Zheng, T Liu
Stem Cell Res Ther, 2018-06-13;9(1):158.
Species: Human
Sample Types: Cell Culture Supernates
-
Macrophage Migration Inhibitory Factor and microRNA-451a in Response to Mindfulness-Based Therapy or Treatment as usual in Patients with Depression, Anxiety or Stress- and Adjustment Disorders
Authors: X Wang, K Sundquist, K Palmér, A Hedelius, A Memon, J Sundquist
Int. J. Neuropsychopharmacol., 2018-06-01;0(0):.
Species: Human
Sample Types: Plasma
-
Sequential broncho-alveolar lavages reflect distinct pulmonary compartments: clinical and research implications in lung transplantation
Authors: L Levy, SC Juvet, K Boonstra, LG Singer, S Azad, B Joe, M Cypel, S Keshavjee, T Martinu
Respir. Res., 2018-05-25;19(1):102.
Species: Human
Sample Types: BALF
-
The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment
Authors: DG Leuning, NRM Beijer, NA du Fossé, S Vermeulen, E Lievers, C van Kooten, TJ Rabelink, J Boer
Sci Rep, 2018-05-16;8(1):7716.
Species: Human
Sample Types: Cell Culture Supernates
-
Meal-exercise challenge and physical activity reduction impact on immunity and inflammation (MERIIT trial)
Authors: D Silva, R Moreira, O Sokhatska, M Beltrão, T Montanha, V Garcia-Lar, R Villegas, M Severo, A Pizarro, M Pinto, C Martins, A Duarte, L Delgado, J Rufo, I Paciência, JP Teixeira, C Costa, P Moreira, J Carvalho, A Moreira
Contemp Clin Trials Commun, 2018-05-09;10(0):177-189.
Species: Human
Sample Types: Plasma
-
A 3D Human Renal Cell Carcinoma-on-a-Chip for the Study of Tumor Angiogenesis
Authors: CP Miller, C Tsuchida, Y Zheng, J Himmelfarb, S Akilesh
Neoplasia, 2018-05-07;20(6):610-620.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum Cytokine and Growth Factor Levels in Children with Autism Spectrum Disorder
Authors: I Lochman, V Švachová, K Mílková Pa, H Med?ická, V Novák, L Trilecová, L Pavliska, V Procházka
Med. Sci. Monit., 2018-04-29;24(0):2639-2646.
Species: Human
Sample Types: Serum
-
Systemic immune response induced by oxaliplatin-based neoadjuvant therapy favours survival without metastatic progression in high-risk rectal cancer
Authors: E Kalanxhi, S Meltzer, JV Schou, FO Larsen, S Dueland, K Flatmark, BV Jensen, KH Hole, T Seierstad, KR Redalen, DL Nielsen, AH Ree
Br. J. Cancer, 2018-04-26;118(10):1322-1328.
Species: Human
Sample Types: Serum
-
A screen of Crohn's disease-associated microbial metabolites identifies ascorbate as a novel metabolic inhibitor of activated human T cells.
Authors: Chang Y, Rossetti M, Vlamakis H, Casero D, Sunga G, Harre N, Miller S, Humphries R, Stappenbeck T, Simpson K, Sartor R, Wu G, Lewis J, Bushman F, McGovern D, Salzman N, Borneman J, Xavier R, Huttenhower C, Braun J
Mucosal Immunol, 2018-04-25;12(2):457-467.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum YKL-40 as a biomarker for liver fibrosis in chronic hepatitis B patients with normal and mildly elevated ALT
Authors: L Yan, Y Deng, J Zhou, H Zhao, G Wang
Infection, 2018-03-29;0(0):.
Species: Human
Sample Types: Serum
-
A multivariate approach to investigate the combined biological effects of multiple exposures
Authors: P Jain, P Vineis, B Liquet, J Vlaanderen, B Bodinier, K van Veldho, M Kogevinas, TJ Athersuch, L Font-Riber, CM Villanueva, R Vermeulen, M Chadeau-Hy
J Epidemiol Community Health, 2018-03-21;0(0):.
Species: Human
Sample Types: Serum
-
Anti-CTLA-4 based therapy elicits humoral immunity to galectin-3 in patients with metastatic melanoma.
Authors: Wu X, Giobbie-Hurder A, Connolly E, Li J, Liao X, Severgnini M, Zhou J, Rodig S, Hodi F
Oncoimmunology, 2018-03-13;7(7):e1440930.
Species: Human
Sample Types: Serum
-
Increased plasma and adipose tissue levels of ANGPTL8/Betatrophin and ANGPTL4 in people with hypertension
Authors: M Abu-Farha, P Cherian, MG Qaddoumi, I AlKhairi, D Sriraman, M Alanbaei, J Abubaker
Lipids Health Dis, 2018-03-01;17(1):35.
Species: Human
Sample Types: Plasma
-
Uteroglobin and FLRG concentrations in aqueous humor are associated with age in primary open angle glaucoma patients
Authors: EL Ashworth B, T Toh, R Eri, AW Hewitt, AL Cook
BMC Ophthalmol, 2018-02-27;18(1):57.
Species: Human
Sample Types: Aqueous Humor
-
Effects of 4-Week Intervention with Ulmus macrocarpa Hance Extract on Immune Function Biomarkers in Healthy Adults: A Randomized Controlled Trial
Authors: AR Cho, SY Lee, YH Cho, CM Kim, SG Kim
Evid Based Complement Alternat Med, 2018-02-25;2018(0):5690816.
Species: Human
Sample Types: Serum
-
Decreased MCP-1/CCR2 axis-mediated chemotactic effect of conjunctival fibroblasts after transdifferentiation into myofibroblasts
Authors: U Tsutsumi-K, T Inoue, A Futakuchi, K Shobayashi, E Takahashi, S Kojima, M Inoue-Moch, T Fujimoto, H Tanihara
Exp. Eye Res., 2018-02-17;170(0):76-80.
Species: Human
Sample Types: Cell Culture Supernates
-
Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis
Authors: Y Han, X Xu, C Tang, P Gao, X Chen, X Xiong, M Yang, S Yang, X Zhu, S Yuan, F Liu, L Xiao, YS Kanwar, L Sun
Redox Biol, 2018-02-15;16(0):32-46.
Species: Human
Sample Types: Cell Lysates
-
Biphasic activation of complement and fibrinolysis during the human nasal allergic response
Authors: RS Thwaites, NC Gunawardan, V Broich, EH Mann, J Ahnström, GA Campbell, S Lindsley, N Singh, T Tunstall, DA Lane, PJ Openshaw, CM Hawrylowic, TT Hansel
J. Allergy Clin. Immunol., 2018-02-07;0(0):.
Species: Human
Sample Types: Nasal Fluid
-
Whole-genome transcriptomic insights into protective molecular mechanisms in metabolically healthy obese African Americans
Authors: A Gaye, AP Doumatey, SK Davis, CN Rotimi, GH Gibbons
NPJ Genom Med, 2018-01-29;3(0):4.
Species: Human
Sample Types: Serum
-
The host response in critically ill sepsis patients on statin therapy: a prospective observational study
Authors: MA Wiewel, BP Scicluna, LA van Vught, AJ Hoogendijk, AH Zwinderman, R Lutter, J Horn, OL Cremer, MJ Bonten, MJ Schultz, T van der Po
Ann Intensive Care, 2018-01-18;8(1):9.
Species: Human
Sample Types: Plasma
-
Altered B lymphocyte homeostasis and functions in systemic sclerosis
Authors: A Forestier, T Guerrier, M Jouvray, J Giovannell, G Lefèvre, V Sobanski, C Hauspie, E Hachulla, PY Hatron, H Zephir, P Vermersch, M Labalette, D Launay, S Dubucquoi
Autoimmun Rev, 2018-01-16;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Course of SP-D, YKL-40, CCL18 and CA 15-3 in adult patients hospitalised with community-acquired pneumonia and their association with disease severity and aetiology: A post-hoc analysis
Authors: SMC Spoorenber, SMT Vestjens, GP Voorn, CHM van Moorse, B Meek, P Zanen, GT Rijkers, WJW Bos, JC Grutters
PLoS ONE, 2018-01-11;13(1):e0190575.
Species: Human
Sample Types: Serum
-
Circadian clock component REV-ERB? controls homeostatic regulation of pulmonary inflammation
Authors: M Pariollaud, J Gibbs, T Hopwood, S Brown, N Begley, R Vonslow, T Poolman, B Guo, B Saer, DH Jones, JP Tellam, S Bresciani, NC Tomkinson, J Wojno-Pico, AW Cooper, DA Daniels, RP Trump, D Grant, W Zuercher, TM Willson, AS MacDonald, B Bolognese, PL Podolin, Y Sanchez, AS Loudon, DW Ray
J. Clin. Invest., 2018;0(0):.
Species: Mouse
Sample Types: BALF
-
Clinical implications of the plasma EphA2 receptor level in critically ill patients with septic shock
Authors: SH Lee, JH Shin, JH Song, AY Leem, MS Park, YS Kim, J Chang, KS Chung
Sci Rep, 2017-12-14;7(1):17612.
Species: Human
Sample Types: Plasma
-
Development of Endotoxin Tolerance Does Not Influence the Response to a Challenge with the Mucosal Live-Attenuated Influenza Vaccine in Humans In Vivo
Authors: RM Koch, M Kox, EJM Thijs, JC Rahamat-La, FL van de Vee, J Gerretsen, J Schloesser, D Diavatopou, GF Rimmelzwaa, MG Netea, JG van der Ho, MI de Jonge, P Pickkers
Front Immunol, 2017-12-11;8(0):1600.
Species: Human
Sample Types: Plasma
-
Hypoxic Lung-Cancer-Derived Extracellular Vesicle MicroRNA-103a Increases the Oncogenic Effects of Macrophages by Targeting PTEN
Authors: YL Hsu, JY Hung, WA Chang, SF Jian, YS Lin, YC Pan, CY Wu, PL Kuo
Mol. Ther., 2017-11-29;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated Markers of Death Receptor-Activated Apoptosis are Associated with Increased Risk for Development of Diabetes and Cardiovascular Disease
Authors: IY Mattisson, H Björkbacka, M Wigren, A Edsfeldt, O Melander, GN Fredrikson, E Bengtsson, I Gonçalves, M Orho-Melan, G Engström, P Almgren, J Nilsson
EBioMedicine, 2017-11-24;26(0):187-197.
Species: Human
Sample Types: Cell Culture Supernates
-
Microparticles from patients with systemic lupus erythematosus induce production of reactive oxygen species and degranulation of polymorphonuclear leukocytes
Authors: LK Winberg, S Jacobsen, CH Nielsen
Arthritis Res. Ther., 2017-10-17;19(1):230.
Species: Human
Sample Types: Cell Culture Supernates
-
Activin in acute pancreatitis: Potential risk-stratifying marker and novel therapeutic target.
Authors: Jonas J Staudacher, Cemal Yazici, Timothy Carroll, Jessica Bauer, Jingbo Pang, Nancy Krett, Yinglin Xia, Annette Wilson, Georgios Papachris, Andrea Dirmeier, Claudia Kunst, David C Whitcomb, Giamila Fantuzzi, Barbara Jung
Scientific Reports, 2017-10-06;0(0):2045-2322.
Species: Human, Mouse
Sample Types: Serum
-
Mesenchymal Stem Cell Soluble Mediators and Cystic Fibrosis
Authors: MT Sutton, D Fletcher, N Episalla, L Auster, S Kaur, MC Gwin, M Folz, D Velasquez, V Roy, R van Heecke, DP Lennon, AI Caplan, TL Bonfield
J Stem Cell Res Ther, 2017-09-22;7(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Growth Differentiation Factor 15 Is Associated With Major Amputation and Mortality in Patients With Peripheral Artery Disease
Authors: JJ De Haan, S Haitjema, HM den Ruijte, G Pasterkamp, GJ de Borst, M Teraa, MC Verhaar, H Gremmels, SCA de Jager
J Am Heart Assoc, 2017-08-30;6(9):.
Species: Human
Sample Types: Plasma
-
Estrogen receptor ?/HDAC/NFAT axis for delphinidin effects on proliferation and differentiation of T lymphocytes from patients with cardiovascular risks
Authors: O Dayoub, S Le Lay, R Soleti, N Clere, G Hilairet, S Dubois, F Gagnadoux, J Boursier, MC Martínez, R Andriantsi
Sci Rep, 2017-08-24;7(1):9378.
Species: Human
Sample Types: Cell Culture Supernates
-
Polyfunctional response by ImmTAC (IMCgp100) redirected CD8(+) and CD4(+) T cells
Authors: C Boudousqui, G Bossi, JM Hurst, KA Rygiel, BK Jakobsen, NJ Hassan
Immunology, 2017-08-02;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Involvement of Monocyte Subsets in the Immunopathology of Giant Cell Arteritis
Authors: Y van Sleen, Q Wang, KSM van der Ge, J Westra, WH Abdulahad, P Heeringa, AMH Boots, E Brouwer
Sci Rep, 2017-07-26;7(1):6553.
Species: Human
Sample Types: Serum
-
Legionella pneumophila infection activates bystander cells differentially by bacterial and host cell vesicles
Authors: AL Jung, CE Herkt, C Schulz, K Bolte, K Seidel, N Scheller, A Sittka-Sta, W Bertrams, B Schmeck
Sci Rep, 2017-07-24;7(1):6301.
Species: Human
Sample Types: Cell Culture Supernates
-
Circulating LOXL2 Levels Reflect Severity of Intestinal Fibrosis and GALT CD4(+) T Lymphocyte Depletion in Treated HIV Infection
Authors: S Seang, A Somasunder, M Nigalye, M Somsouk, TW Schacker, JL Sanchez, PW Hunt, NS Utay, JE Lake
Pathog Immun, 2017-06-21;2(2):239-252.
Species: Human
Sample Types: Plasma
-
Mechanistic Evaluation of the Impact of Smoking and Chronic Obstructive Pulmonary Disease on the Nasal Epithelium
Authors: M Talikka, F Martin, A Sewer, G Vuillaume, P Leroy, K Luettich, N Chaudhary, MJ Peck, MC Peitsch, J Hoeng
Clin Med Insights Circ Respir Pulm Med, 2017-06-05;11(0):1179548417710.
Species: Human
Sample Types: Nasal Lavage Fluid
-
Aberrant monocyte responses predict and characterize dengue virus infection in individuals with severe disease
Authors: YK Yong, HY Tan, SH Jen, EM Shankar, SK Natkunam, J Sathar, R Manikam, SD Sekaran
J Transl Med, 2017-05-31;15(1):121.
Species: Human
Sample Types: Plasma
-
Chlamydia trachomatis infection of human endometrial stromal cells induces defective decidualisation and chemokine release
Authors: S Giakoumelo, N Wheelhouse, J Brown, J Wade, I Simitsidel, D Gibson, PTK Saunders, P Horner, G Entrican, SEM Howie, AW Horne
Sci Rep, 2017-05-17;7(1):2001.
Species: Human
Sample Types: Cell Culture Supernates
-
?-Sitosterol Reduces the Expression of Chemotactic Cytokine Genes in Cystic Fibrosis Bronchial Epithelial Cells
Authors: I Lampronti, MC Dechecchi, A Rimessi, V Bezzerri, E Nicolis, A Guerrini, M Tacchini, A Tamanini, S Munari, E D'Aversa, A Santangelo, G Lippi, G Sacchetti, P Pinton, R Gambari, M Agostini, G Cabrini
Front Pharmacol, 2017-05-12;8(0):236.
Species: Human
Sample Types: Cell Culture Supernates
-
Acute changes in serum immune markers due to swimming in a chlorinated pool
Authors: J Vlaanderen, K van Veldho, L Font-Riber, CM Villanueva, M Chadeau-Hy, L Portengen, JO Grimalt, C Zwiener, D Heederik, X Zhang, P Vineis, M Kogevinas, R Vermeulen
Environ Int, 2017-05-05;105(0):1-11.
Species: Human
Sample Types: Serum
-
Malondialdehyde as a Prognostic Factor in Alcoholic Hepatitis
Authors: Onán Pérez-Hern
Alcohol Alcohol, 2017-05-01;0(0):.
Species: Human
Sample Types: Serum
-
Defining the inflammatory signature of human lung explant tissue in the presence and absence of glucocorticoid
Authors: TL Rimington, E Hodge, CK Billington, S Bhaker, B K C, I Kilty, S Jelinsky, IP Hall, I Sayers
F1000Res, 2017-04-11;6(0):460.
Species: Human
Sample Types: Tissue Culture Supernates
-
Matrix Metalloproteinase-28 Is a Key Contributor to Emphysema Pathogenesis
Authors: AM Manicone, SA Gharib, KQ Gong, WE Eddy, ME Long, CW Frevert, WA Altemeier, WC Parks, AM Houghton
Am. J. Pathol., 2017-04-08;0(0):.
Species: Human
Sample Types: BALF
-
Antecedents and correlates of blood concentrations of neurotrophic growth factors in very preterm newborns
Authors: A Leviton, EN Allred, H Yamamoto, RN Fichorova, K Kuban, TM O'Shea, O Dammann, ELGAN Stud
Cytokine, 2017-04-07;0(0):.
Species: Human
Sample Types: Complex Sample Type
-
Bilirubin Prevents Atherosclerotic Lesion Formation in Low-Density Lipoprotein Receptor-Deficient Mice by Inhibiting Endothelial VCAM-1 and ICAM-1 Signaling
Authors: ME Vogel, G Idelman, ES Konaniah, SD Zucker
J Am Heart Assoc, 2017-04-01;6(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
Molecular Mechanisms Underlying the Filtration Bleb-Maintaining Effects of Suberoylanilide Hydroxamic Acid (SAHA)
Authors: A Futakuchi, T Inoue, T Fujimoto, U Kuroda, M Inoue-Moch, E Takahashi, S Ohira, H Tanihara
Invest. Ophthalmol. Vis. Sci., 2017-04-01;58(4):2421-2429.
Species: Human
Sample Types: Cell Culture Supernates
-
Circulating GDF-15 levels predict future secondary manifestations of cardiovascular disease explicitly in women but not men with atherosclerosis
Authors: A Gohar, I Gonçalves, J Vrijenhoek, S Haitjema, I van Koever, J Nilsson, GJ de Borst, JP de Vries, G Pasterkamp, HM den Ruijte, H Björkbacka, SC de Jager
Int. J. Cardiol., 2017-03-22;0(0):.
Species: Human
Sample Types: Plasma
-
Citrulline and Monocyte-Derived Macrophage Reactivity Prior to Conditioning Predict Acute Graft-Verus-Host Disease
Authors: T Hueso, V Coiteux, M Joncquel C, J Labreuche, T Jouault, I Yakoub-Agh, D Seguy
Biol. Blood Marrow Transplant, 2017-03-02;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Aberrant SYK kinase signaling is essential for tumorigenesis induced by TSC2 inactivation
Authors: Y Cui, WK Steagall, A Lamattina, G Pacheco-Ro, M Stylianou, P Kidambi, B Stump, F Golzarri, IO Rosas, C Priolo, EP Henske, J Moss, S El-Chemaly
Cancer Res, 2017-02-15;0(0):.
Species: Human
Sample Types: Serum
-
Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals
Authors: RV Luthold, GR Fernandes, AC Franco-de-, LG Folchetti, SR Ferreira
Metab. Clin. Exp., 2017-01-13;69(0):76-86.
Species: Human
Sample Types: Plasma
-
VEGF-A is increased in exogenous endophthalmitis
Authors: ME Seamone, DR Lewis, ID Haidl, RR Gupta, DM O' Brien, J Dickinson, A Samad, JS Marshall, AF Cruess
Can. J. Ophthalmol., 2017-01-10;52(3):277-282.
Species: Human
Sample Types: Cell Culture Supernates
-
Immune adaptation to chronic intense exercise training: new microarray evidence
Authors: D Liu, R Wang, AR Grant, J Zhang, PM Gordon, Y Wei, P Chen
BMC Genomics, 2017-01-05;18(1):29.
Species: Human
Sample Types: Serum
-
Durability of the Rituximab Response in Acetylcholine Receptor Autoantibody-Positive Myasthenia Gravis
Authors: Richard J Nowak
JAMA Neurol, 2017-01-01;0(0):.
Species: Human
Sample Types: Serum
-
Assessment of the Role of Metabolic Determinants on the Relationship between Insulin Sensitivity and Secretion
PLoS ONE, 2016-12-21;11(12):e0168352.
Species: Human
Sample Types: Plasma
-
Angiopoietin-2 as a Biomarker and Target for Immune Checkpoint Therapy.
Authors: Wu X, Giobbie-Hurder A, Liao X, Connelly C, Connolly E, Li J, Manos M, Lawrence D, McDermott D, Severgnini M, Zhou J, Gjini E, Lako A, Lipschitz M, Pak C, Abdelrahman S, Rodig S, Hodi F
Cancer Immunol Res, 2016-12-21;5(1):17-28.
Species: Human
Sample Types: Serum
-
Immobilized hematopoietic growth factors onto magnetic particles offer a scalable strategy for cell therapy manufacturing in suspension cultures
Authors: Robert J Thomas
Biotechnol J, 2016-12-01;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Impact of placental Plasmodium falciparum malaria infection on the Cameroonian maternal and neonate's plasma levels of some cytokines known to regulate T cells differentiation and function
Malar. J., 2016-11-21;15(1):561.
Species: Human
Sample Types: Plasma
-
Effect of thrombopoietin-receptor agonists on circulating cytokine and chemokine levels in patients with primary immune thrombocytopenia (ITP)
Authors: James Bussel
Platelets, 2016-11-07;0(0):1-6.
Species: Human
Sample Types: Plasma
-
Increased levels of 3-hydroxykynurenine parallel disease severity in human acute pancreatitis
Sci Rep, 2016-09-27;6(0):33951.
Species: Human
Sample Types: Plasma
-
Clinical-Grade Isolated Human Kidney Perivascular Stromal Cells as an Organotypic Cell Source for Kidney Regenerative Medicine
Authors: DG Leuning, ME Reinders, J Li, AJ Peired, E Lievers, HC de Boer, WE Fibbe, P Romagnani, C van Kooten, MH Little, MA Engelse, TJ Rabelink
Stem Cells Transl Med, 2016-09-20;6(2):405-418.
Species: Human
Sample Types: Cell Culture Supernates
-
CF Lung Infection and Inflammation: IL-17 Pathophysiology and Therapeutic Intervention
Authors: Daniel Hsu
Infect Immun, 2016-08-19;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Data on serologic inflammatory biomarkers assessed using multiplex assays and host characteristics in the Multicenter AIDS Cohort Study (MACS)
Data Brief, 2016-08-16;9(0):262-70.
Species: Human
Sample Types: Serum
-
T Helper Cell Activation and Expansion Is Sensitive to Glutaminase Inhibition under Both Hypoxic and Normoxic Conditions
PLoS ONE, 2016-07-28;11(7):e0160291.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum Galectin-9 and Galectin-3-Binding Protein in Acute Dengue Virus Infection
Authors: Kuan-Ting Liu
Int J Mol Sci, 2016-05-27;17(6):.
Species: Human
Sample Types: Serum
-
In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: a comparison to bevacizumab
Br J Cancer, 2016-05-03;114(11):1212-8.
Species: Human
Sample Types: Plasma
-
Levels of Adipokines in Amniotic Fluid and Cord Blood Collected from Dichorionic-Diamniotic Twins Discordant for Fetal Growth
PLoS ONE, 2016-05-02;11(5):e0154537.
Species: Human
Sample Types: Amniotic Fluid
-
Cerebral Spinal Fluid levels of Cytokines are elevated in Patients with Metachromatic Leukodystrophy
Authors: KA Thibert, GV Raymond, J Tolar, WP Miller, PJ Orchard, TC Lund
Sci Rep, 2016-04-15;6(0):24579.
Species: Human
Sample Types: CSF
-
Inflammatory markers are associated with decreased psychomotor speed in patients with major depressive disorder
Authors: David R Goldsmith
Brain Behav. Immun, 2016-04-01;56(0):281-8.
Species: Human
Sample Types: Plasma
-
TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals.
Authors: Wang F, Hou H, Wu S, Tang Q, Liu W, Huang M, Yin B, Huang J, Mao L, Lu Y, Sun Z
Eur J Immunol, 2015-09-07;45(10):2886-97.
-
Functional and homeostatic defects of regulatory T cells in patients with coronary artery disease
Authors: L Hasib, AK Lundberg, H Zachrisson, J Ernerudh, L Jonasson
J. Intern. Med, 2015-08-11;279(1):63-77.
Species: Human
Sample Types: Cell Culture Supernates
-
Analysis of septic biomarker patterns: prognostic value in predicting septic state.
Authors: Carlyn C, Andersen N, Baltch A, Smith R, Reilly A, Lawrence D
Diagn Microbiol Infect Dis, 2015-07-11;83(3):312-8.
Species: Human
Sample Types: Serum
-
The generation of macrophages with anti-inflammatory activity in the absence of STAT6 signaling.
Authors: Fleming B, Chandrasekaran P, Dillon L, Dalby E, Suresh R, Sarkar A, El-Sayed N, Mosser D
J Leukoc Biol, 2015-06-05;98(3):395-407.
Species: Human
Sample Types: Cell Culture Supernates
-
Vitamin D limits chemokine expression in adipocytes and macrophage migration in vitro and in male mice.
Authors: Karkeni E, Marcotorchino J, Tourniaire F, Astier J, Peiretti F, Darmon P, Landrier J
Endocrinology, 2015-03-02;156(5):1782-93.
Species: Human
Sample Types: Cell Culture Supernates
-
Alpha fetoprotein DNA prime and adenovirus boost immunization of two hepatocellular cancer patients.
Authors: Butterfield L, Economou J, Gamblin T, Geller D
J Transl Med, 2014-04-05;12(0):86.
Species: Human
Sample Types: Serum
FAQs
-
Are QC controls available for both the Luminex Discovery and High Performance Assays?
Controls are not offered for Luminex Discovery Assays, but may be formulated by the end user. A general guide may be available upon request. Controls are available for select Luminex High Performance Assays and can be found under the supplemental products tab on the product webpage. Some High Performance Luminex kits include controls and the controls will be listed in the materials provided section on the product datasheet. When included in the kit, controls are not sold separately.
-
What is the reason for the recommendation to use 35 µL sample volume, instead of the default 50 µL volume, for Luminex multiplex containing > 25 analytes, when assayed on Luminex MAGPIX® analyzer?
R&D Systems recommend 35 µL sample volume for > 25 analytes to control the number of beads in the sample load when using Luminex MAGPIX® analyzer. Luminex MAGPIX® analyzer is a non-flow-based reader which utilizes a magnet to hold the magnetic microparticles in a monolayer in a chamber where the beads are imaged and then processed by the software to generate data. Since MAGPIX® system pushes the entire Sample Load Volume into the chamber, multiplex assay containing > 25 analytes may overload the chamber capacity with 50 µL sample load default setting. The captured images can have too many beads in them to give accurate results, and some of the analytes/regions may present lower than desired bead counts.
-
Does the Human Luminex Discovery Assay detect soluble form or membrane-bound analyte?
The sample types typically validated by R&D Systems for Luminex assays contain soluble form of proteins, and therefore it is expected that the soluble form of proteins are detected. It is possible there are assays that also detect the membrane form of respective target proteins, however, R&D Systems does not generally evaluate our assays against samples known to contain membrane-bound proteins.
-
What speed do you recommend centrifuging cell culture supernates samples?
For cell culture supernates, remove particulates by centrifuging for 5 minutes at 500 x g.
-
Why does the Luminex Assay Customization Tool provide the option to split based on dilution?
The feature to split based on dilution enables investigators to configure premix Luminex panels based on sample type (cell culture supernates, serum, and plasma). This keeps analytes requiring the same dilution factor within a single panel, while separating analytes requiring different dilutions. The recommended dilution factor for each analyte may be different for each sample type, and the suggested sample dilutions are built into the Luminex Customization Tool. Note that the suggested dilution factors are based on samples from healthy volunteers.
-
Is the reconstituted standard a concentrate?
For Discovery Assays, each standard cocktail is a concentrate upon reconstitution with the volume specified on the Certificate of Analysis (CoA). After creating Standard 1 following the table in the product insert, the Standard 1 value is the concentration listed on the CoA.
-
At what frequency should Calibration and Verification tests be performed?
It is recommended that a Calibration test be performed within one week prior to running an assay. A Verification test should be performed prior to data acquisition on the day of running an assay.
Reviews for Human Luminex® Discovery Assay
Average Rating: 4.5 (Based on 41 Reviews)
Have you used Human Luminex® Discovery Assay?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Wish for RDS to provide more in-house data (characterizations)
We purchased and tested a 15plex (including PSA) luminex discovery assay with 40 human serum samples. We checked the correlation of PSA measurements in 15plex and a clinical assay (Access 2 Immunoassay Analyzer (Beckman Coulter, INC.) in 40 serum samples. Attached figures show the PSA standard curve in 15plex that works well with both STD and serum samples, but the correlation of PSA measurements in both 15plex and clinical assay was bad due to a few samples jumping up in 15plex.
Easy to use 25 plex, significantly easier over ELISAS. 24/25 analytes r^2>0.999 on standard curve, and slightly improved IGFBP3 accuracy over previous kits (with r^2 = 0.987).
First time I ever used the Magnetic Luminex Assay and I had no trouble running the assay.
a target protein expressing cancer cells co-incubate with PBMC
cancer cell co-incubate with healthy donor PBMC with lead candidate mAbs.
Gave us clear readouts of the level of RANTES in our samples
the standard was great but the test did not detect TNF alpha in our material while other ELISAs did
We found that the volumes for beads, antibodies, and streptavidin biotin were higher than in previous kits. Even a couple of mls more made easier to prepare the suspension with enough volume to run a whole plate. Bubbles usually make the experiment more complicated but this time we did not find that the buffers were too foamy.
This was the first time we ran induced sputums to test these biomarkers. The values obtained were low or negative. We used the samples without dilution as we had no literature data to refer to.