Human/Mouse Myeloperoxidase/MPO Antibody Summary
Met16-Thr718
Accession # P11247
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
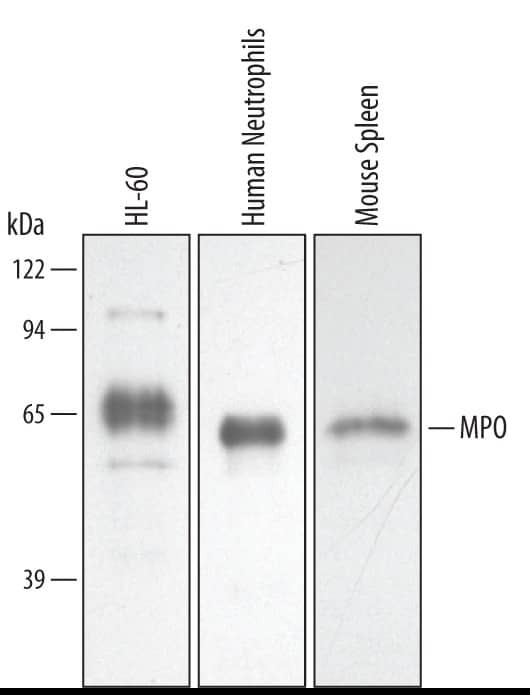
Detection of Human/Mouse Myeloperoxidase/MPO by Western Blot. Western blot shows lysates of HL-60 human acute promyelocytic leukemia cell line, human neutrophil cells, and mouse spleen tissue. PVDF membrane was probed with 0.5 µg/mL of Goat Anti-Human/Mouse Myeloperoxidase/MPO Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3667) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF019). A specific band was detected for Myeloperoxidase/MPO at approximately 60 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 2.
 View Larger
View Larger
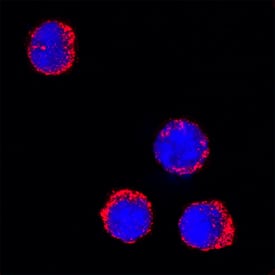
Myeloperoxidase/MPO in Mouse Splenocytes. Myeloperoxidase/MPO was detected in immersion fixed mouse splenocytes using Goat Anti-Human/Mouse Myeloperoxidase/MPO Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3667) at 15 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; NL001) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. View our protocol for Fluorescent ICC Staining of Non-adherent Cells.
 View Larger
View Larger
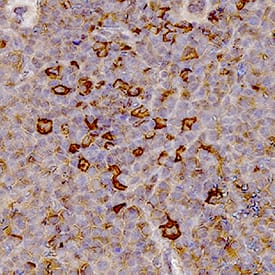
Myeloperoxidase/MPO in Mouse Spleen Tissue. Myeloperoxidase/MPO was detected in immersion fixed paraffin-embedded sections of mouse spleen tissue using Goat Anti-Human/Mouse Myeloperoxidase/MPO Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3667) at 1 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (VC004). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to lymphocytes. Staining was performed using our IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
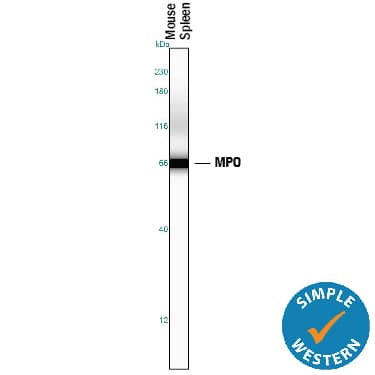
Detection of Mouse Myeloperoxidase/MPO by Simple WesternTM. Simple Western lane view shows lysates of mouse spleen tissue, loaded at 0.2 mg/mL. A specific band was detected for Myeloperoxidase/MPO at approximately 67 kDa (as indicated) using 5 µg/mL of Goat Anti-Human/Mouse Myeloperoxidase/MPO Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3667) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
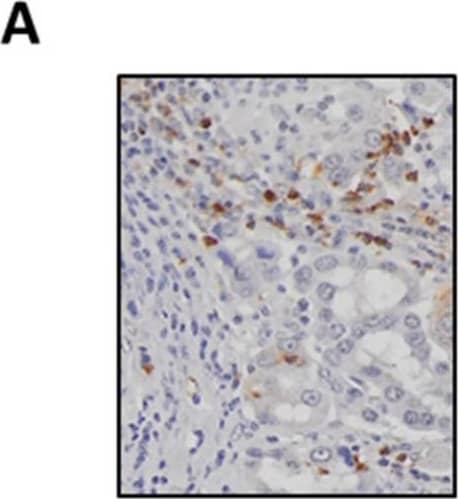
Detection of Human Myeloperoxidase/MPO by Immunohistochemistry SAA levels positively correlate with tumour-associated neutrophil infiltration. (A) Representative images of MPO-positive neutrophils in a lung adenocarcinoma tumour section (original magnification ×200). (B) Neutrophils are significantly elevated in tumours compared to control tissue biopsies, which was confirmed by (C) paired analysis of tumour and adjacent control lung tissue. (D) Circulating SAA levels were elevated in lung adenocarcinoma patients as determined by ELISA. (E) SAA transcript levels were not increased in tumours as measured by RTqPCR and confirmed by (F) sub-analysis of the paired from the tumour and adjacent control tissue samples. (G) Tissue sections were stained for SAA, which identified positive staining within seromucinous glands and tumour-associated macrophages (original magnification ×200). (H) Spearman correlation was used to assess associations between SAA transcript levels and tumour infiltrating neutrophil density (r = 0.51, *** p < 0.001). **** p < 0.0001, ** p < 0.01, ## p < 0.01. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/34203378), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
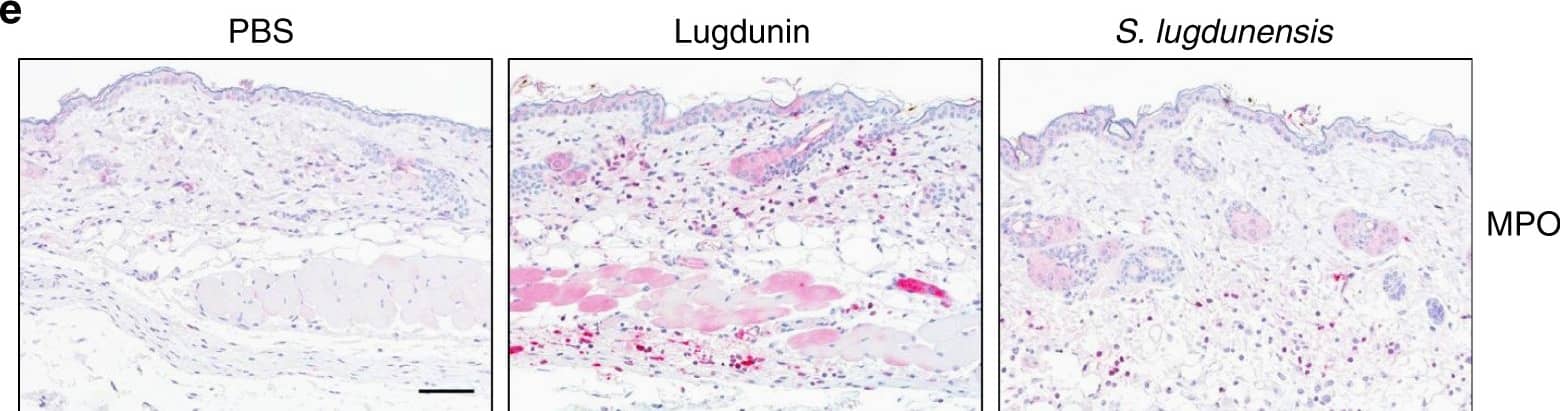
Detection of Mouse Myeloperoxidase/MPO by Immunocytochemistry/Immunofluorescence Epicutaneous lugdunin recruits phagocytic cells. a Schematic overview of the mouse experiments: 6–8-week-old female C5BL/6 wild-type (WT), MyD88-ko, or 5xTLR-ko mice were epicutaneously treated with 1.5 µg lugdunin or phosphate-buffered saline (PBS) as a control. After 24 h, mice were euthanized, immune cells were isolated from treated skin areas, and immune cell composition was analyzed by flow cytometry. b Shown is the mean percentage of CD45+ live cells in mouse skin of 10 C57BL/6 WT mice ± s.e.m. One mouse is represented as two dots analyzed by two different stainings. c Pie charts show the mean percentage of the different immune cell subsets in the skin of 10 WT mice after 24 h of PBS or lugdunin treatment. d Shown are representative flow cytometry data (left panel) and the mean percentage of neutrophils (Ly6C+Ly6G+) and monocytes (Ly6C+Ly6G−) pregated on CD11b+CD45+ live cells (see Supplementary Fig. 3a, f for the gating strategy) in mouse skin ± s.e.m. One dot represents one mouse. *P < 0.05. e Representative myeloperoxidase (MPO)-stained paraffin-embedded mouse skin sections. Scale bar, 100 µM. Source data are provided as a Source Data file Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31227691), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
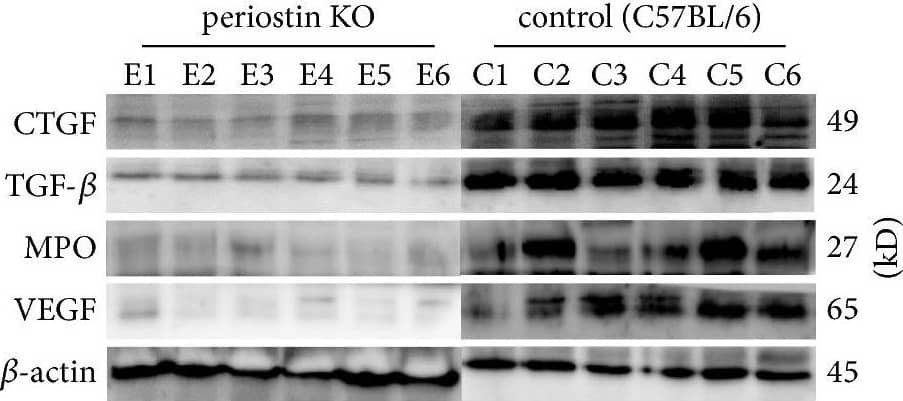
Detection of Mouse Myeloperoxidase/MPO by Western Blot Levels of CTGF, TGF-beta, MPO, and VEGF in silicone implant-induced capsular tissues determined by western blotting (a). A low signal was obtained for CTGF (b) and TGF-beta (c) protein in PN-KO mice (n = 6), whereas a strong signal was detected in the C57BL/6 mice (n = 6). Compared with the control group (n = 6), the levels of MPO (d) and VEGF (e) protein were downregulated in the PN-KO group (n = 6). Relative expression levels normalized to the housekeeping gene beta -actin. ∗∗p < 0.001. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29854742), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
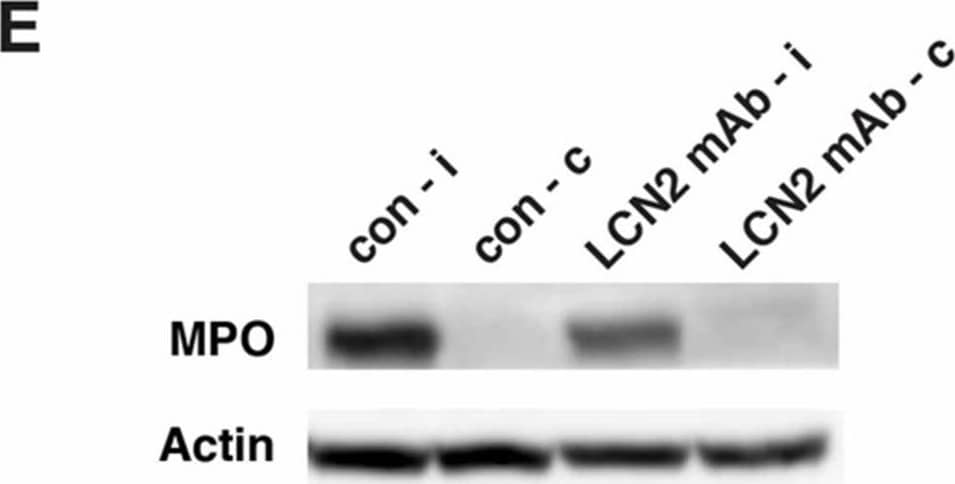
Detection of Mouse Myeloperoxidase/MPO by Western Blot LCN2 mAb limited blood–brain barrier leakage and infiltration of neutrophils after tMCAo. Representative images (A) and quantification (B) of Evans blue extravasation in the ipsilateral hemispheres of mice treated with control IgG (con) and LCN2 mAb (n = 5 per group) after one hour of tMCAo and 23 h after reperfusion. The concentration of Evans blue dye in the ipsilateral hemispheres of mice treated with LCN2 mAb was significantly decreased (** p < 0.01) as compared with that in the ipsilateral hemispheres of mice treated with control IgG (two-tailed, unpaired t test); (C) The expression level of the tight junction protein claudin-5 was analyzed after treatments with control IgG and LCN2 mAb (n = 4 per group). The ipsilateral (i) and contralateral (c) hemispheres isolated at 23 h after tMCAo were analyzed by Western blotting using antibodies against claudin-5. Representative Western blot showing the expression of claudin-5 (~22 kDa) in brain homogenates. beta -actin served as a loading control; (D) The level of claudin-5 immunoreactivity normalized to beta -actin (claudin-5/actin) in the ipsilateral hemispheres in mice treated with LCN2 mAb was significantly higher than that in the ipsilateral hemispheres of mice that received the control IgG (* p < 0.05, one-tailed, unpaired t test); (E,F) Neutrophil infiltration was analyzed by measuring the levels of MPO in brain homogenates. The ipsilateral (i) and contralateral (c) hemispheres of mice treated with control IgG (con) and LCN2 mAb (n = 4 per group) isolated at 23 h after tMCAo were analyzed by Western blotting using antibodies against MPO; (E) Representative Western blots show the expression of MPO heavy chain (~55 kD) in brain homogenates; (F) The level of MPO immunoreactivity normalized to beta -actin (MPO/actin) was significantly reduced in the ipsilateral hemispheres of mice treated with LCN2 mAb (* p < 0.05, one-tailed, unpaired t test) as compared with that in the ipsilateral hemisphere of mice that received control IgG. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32872405), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
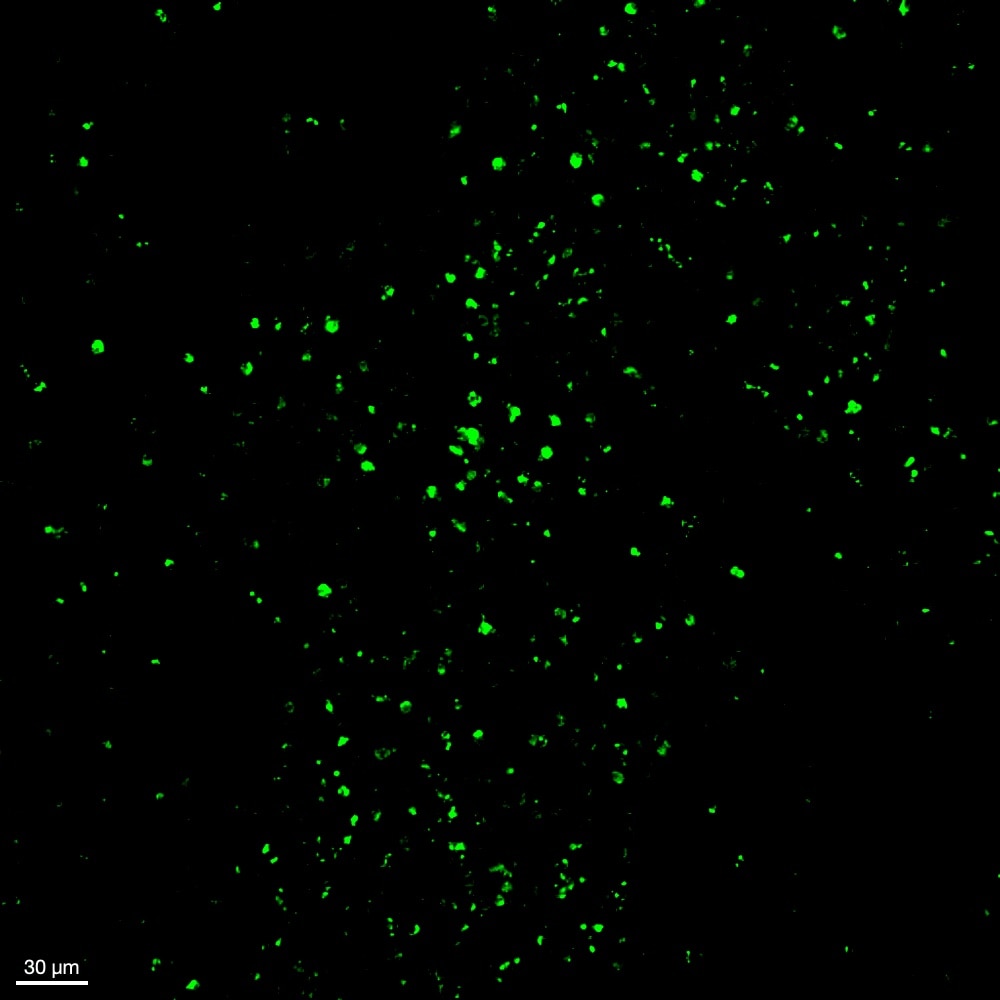
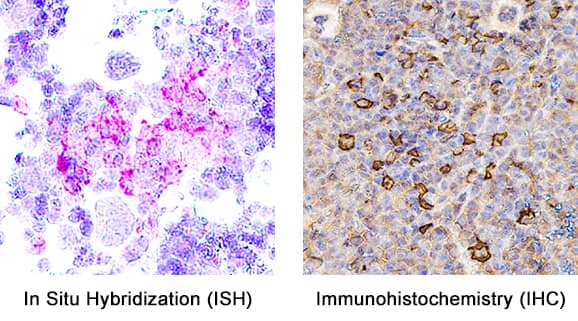
Detection of Myeloperoxidase/MPO in Mouse Spleen. Formalin-fixed paraffin-embedded tissue sections of mouse spleen were probed for MPO mRNA (ACD RNAScope Probe, catalog #603091; Fast Red chromogen, ACD catalog # 322750). Adjacent tissue section was processed for immunohistochemistry using goat anti-mouse MPO polyclonal antibody (R&D Systems catalog # AF3667) at 1ug/mL with overnight incubation at 4 degrees Celsius followed by incubation with anti-goat IgG VisUCyte HRP Polymer Antibody (Catalog # VC004) and DAB chromogen (yellow-brown). Tissue was counterstained with hematoxylin (blue). Specific staining was localized to cell surface.
 View Larger
View Larger
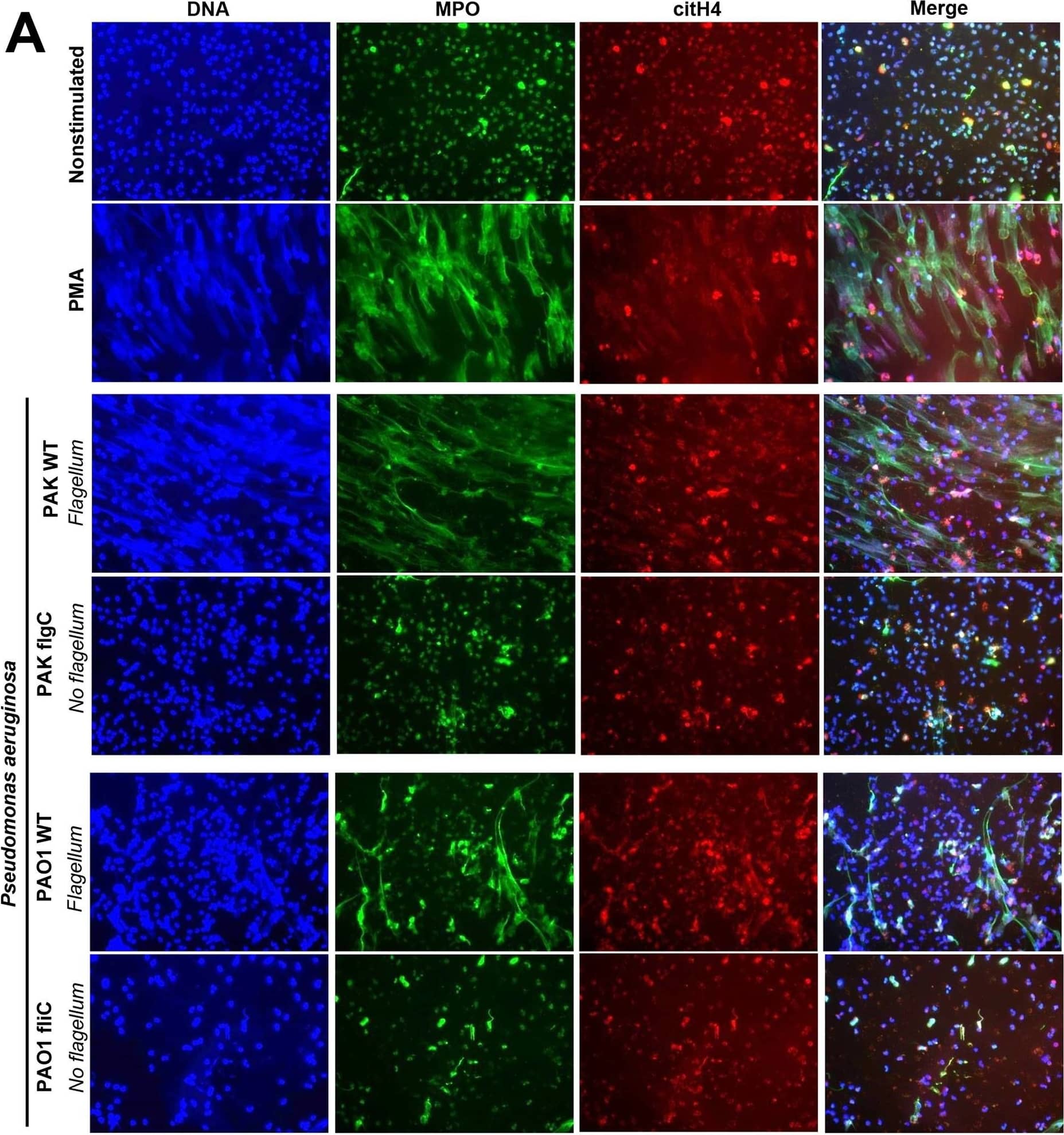
Detection of Human Human/Mouse Myeloperoxidase/MPO Antibody by Immunohistochemistry Immunofluorescence staining reveals that flagellum-deficiency abolishes P. aeruginosa-induced NET formation.Human PMNs were exposed to wild-type (WT) or flagellum-deficient strains of P. aeruginosa PAK flgC and PAO1 fliC, and NET release was documented. (A) Immunofluorescence staining of myeloperoxidase (MPO, green), citrullinated histone H4 (citH4, red), extracellular DNA (DAPI, blue) and their merged images are shown. Representative results, n = 3. (B) Quantitation of immunofluorescence images shown in panel (A). Based on nuclear morphology and co-staining with MPO and citH4, NET-forming PMNs were identified and their numbers quantitated compared to the total cell population (expressed as percentage of total). Mean+/-S.E.M., n = 3. One-way ANOVA, Tukey’s post hoc test. *, p<0.05; **, p<0.01. Nonstim., nonstimulated; WT, wild-type. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27855208), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
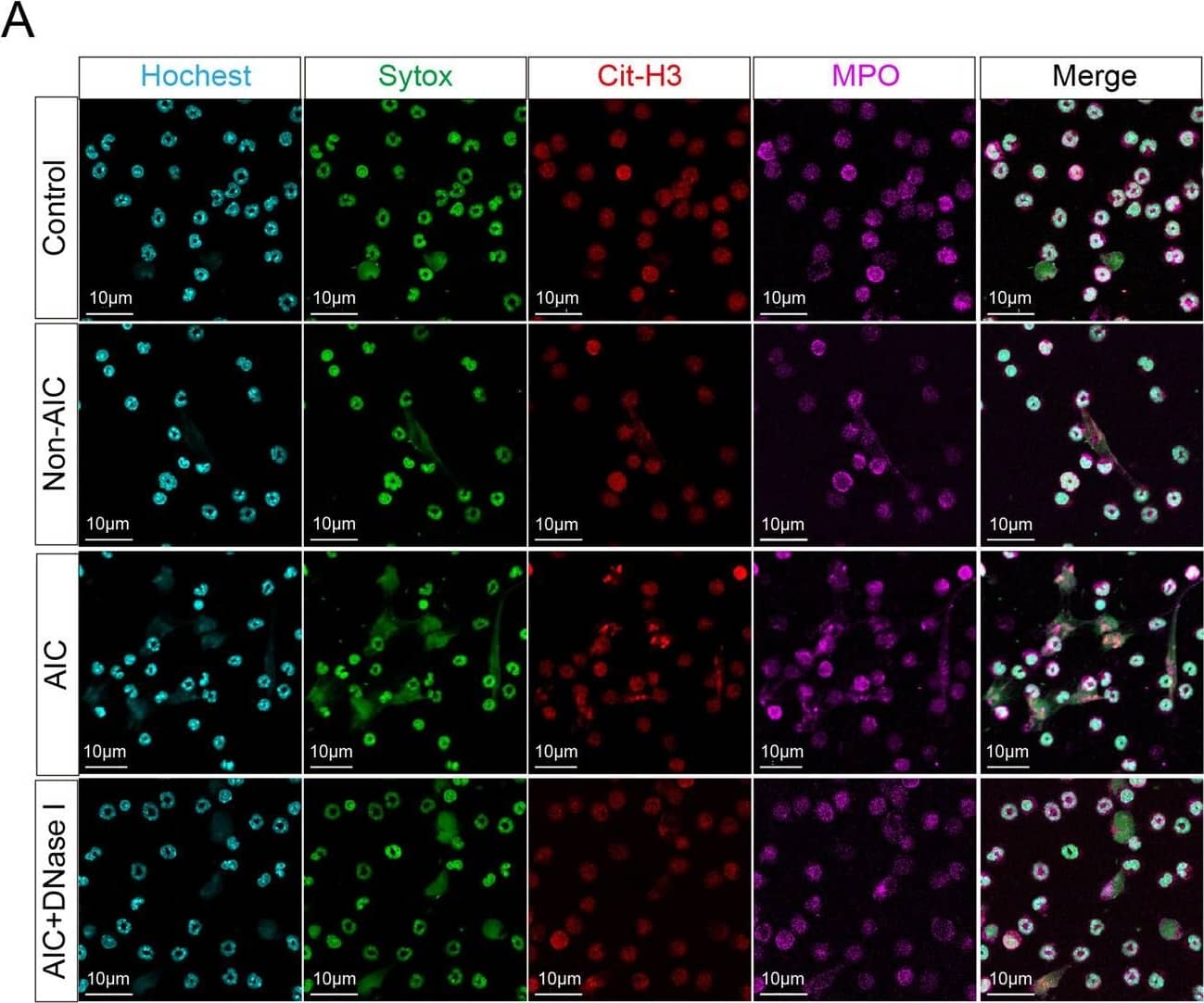
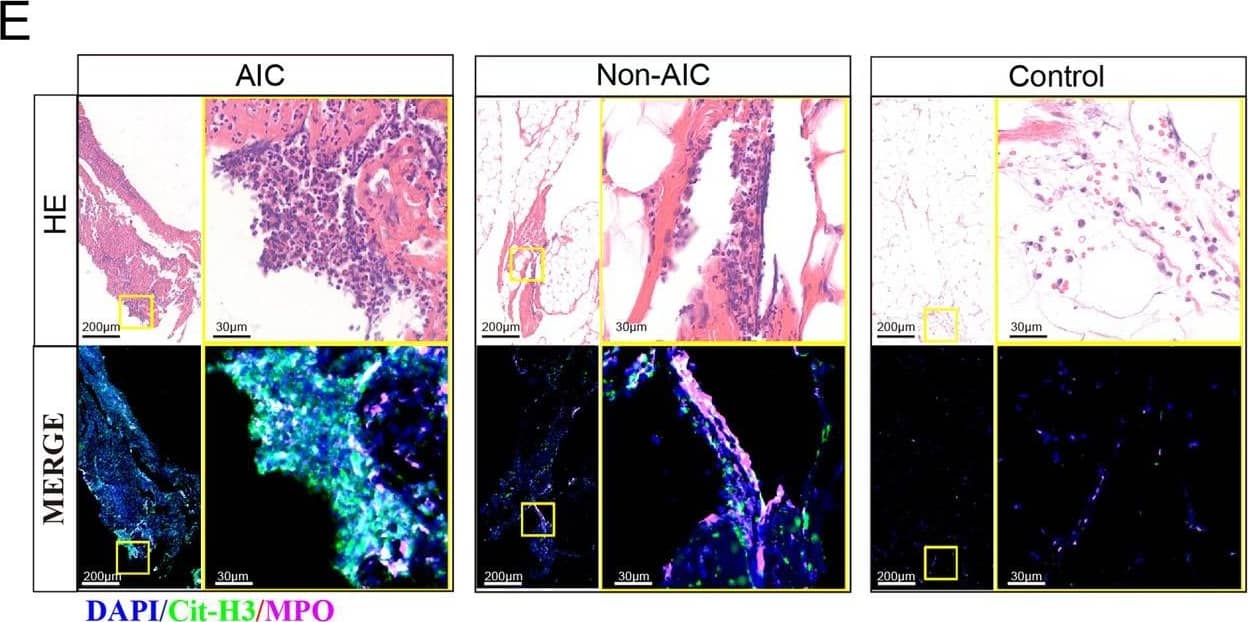
Detection of Human Myeloperoxidase/MPO by Immunohistochemistry Postoperative AIC stimulates NETs release both in peripheral blood and abdominal infectious site.A Left panel: representative immunofluorescence co-staining of DNA (Hochest and Sytox), Cit-H3 (citrullinated histone-3) and MPO (myeloperoxidase) to assess NETs formation in the neutrophils isolated from peripheral blood of control, Non-AIC, AIC and AIC + DNase I groups; Right panel: plasma and serum levels of MPO–DNA in GC patients with control, Non-AIC and AIC; B Preoperative and postoperative serum MPO–DNA levels between Non-AIC and AIC groups; C Representative SEM (scan electron microscopy) of neutrophils isolated from preoperative and postoperative ascites fluid between Non-AIC and AIC groups. Green arrows point to extracellular meshes of NETs and white arrows point to neutrophils; D Preoperative and postoperative ascites fluid MPO–DNA levels between Non-AIC and AIC groups; E Representative images of HE and immunofluorescence staining of DNA, Cit-H3, and MPO in omental tissues among AIC, Non-AIC, and control groups; F Quantification of NETs in omental tissues among AIC, Non-AIC, and control groups. Data represent the mean ± S.D. in A, F (n = 10 per group); one-way ANOVA with Tukey test was used in A, F; paired and unpaired Student’s t-tests were used in B, D (n = 10 per group). Source data are provided as a Source Data file. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35197446), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Myeloperoxidase/MPO by Immunohistochemistry Postoperative AIC stimulates NETs release both in peripheral blood and abdominal infectious site.A Left panel: representative immunofluorescence co-staining of DNA (Hochest and Sytox), Cit-H3 (citrullinated histone-3) and MPO (myeloperoxidase) to assess NETs formation in the neutrophils isolated from peripheral blood of control, Non-AIC, AIC and AIC + DNase I groups; Right panel: plasma and serum levels of MPO–DNA in GC patients with control, Non-AIC and AIC; B Preoperative and postoperative serum MPO–DNA levels between Non-AIC and AIC groups; C Representative SEM (scan electron microscopy) of neutrophils isolated from preoperative and postoperative ascites fluid between Non-AIC and AIC groups. Green arrows point to extracellular meshes of NETs and white arrows point to neutrophils; D Preoperative and postoperative ascites fluid MPO–DNA levels between Non-AIC and AIC groups; E Representative images of HE and immunofluorescence staining of DNA, Cit-H3, and MPO in omental tissues among AIC, Non-AIC, and control groups; F Quantification of NETs in omental tissues among AIC, Non-AIC, and control groups. Data represent the mean ± S.D. in A, F (n = 10 per group); one-way ANOVA with Tukey test was used in A, F; paired and unpaired Student’s t-tests were used in B, D (n = 10 per group). Source data are provided as a Source Data file. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35197446), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Rat Myeloperoxidase/MPO by Immunohistochemistry CXCR4 expressed at neutrophils mediates NET formation in rats. (D) Representative images of immunolabeling with CitH3, MPO,&DAPI at 20 mm distal to the injury site 12 h after injury. Dashed lines indicate the border of the epineurium&the parenchyma. * are high-magnification images of boxed areas in the epineurium. Arrows indicate triple immunolabeled NETs. Scale bar: 10 μm. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35961782), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
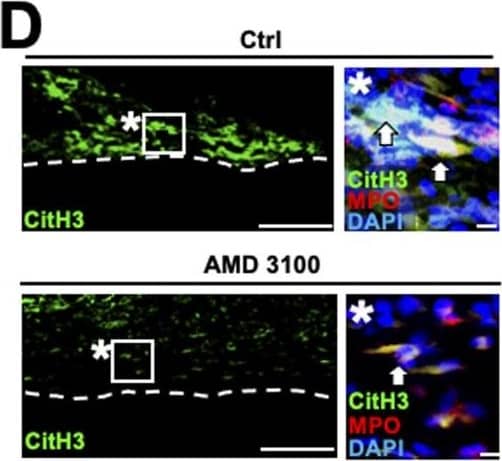
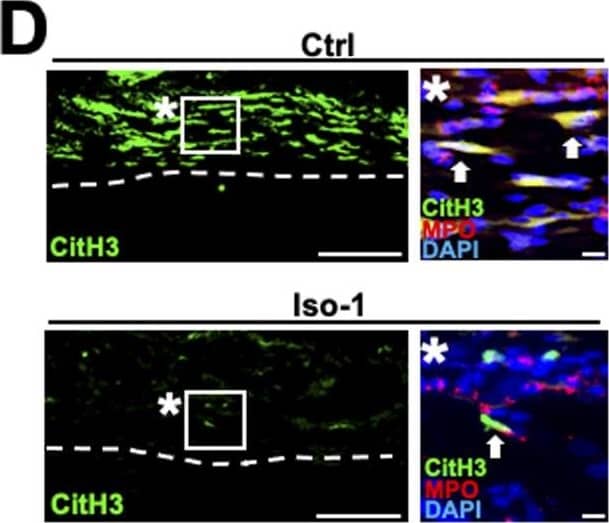
Detection of Rat Myeloperoxidase/MPO by Immunohistochemistry Migration inhibitory factor (MIF) secreted from neutrophils promotes NET formation in rats. (D) Representative images of immunolabeling with CitH3, MPO,&DAPI at 20 mm distal to the injury site 12 h after injury. Dashed lines indicate the border of the epineurium&the parenchyma. Images marked with * are high-magnification images of boxed areas in the epineurium. Arrows indicate triple immunolabeled NETs. Treatment with iso-1 dramatically inhibited NET formation. Scale bar: 10 μm. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35961782), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
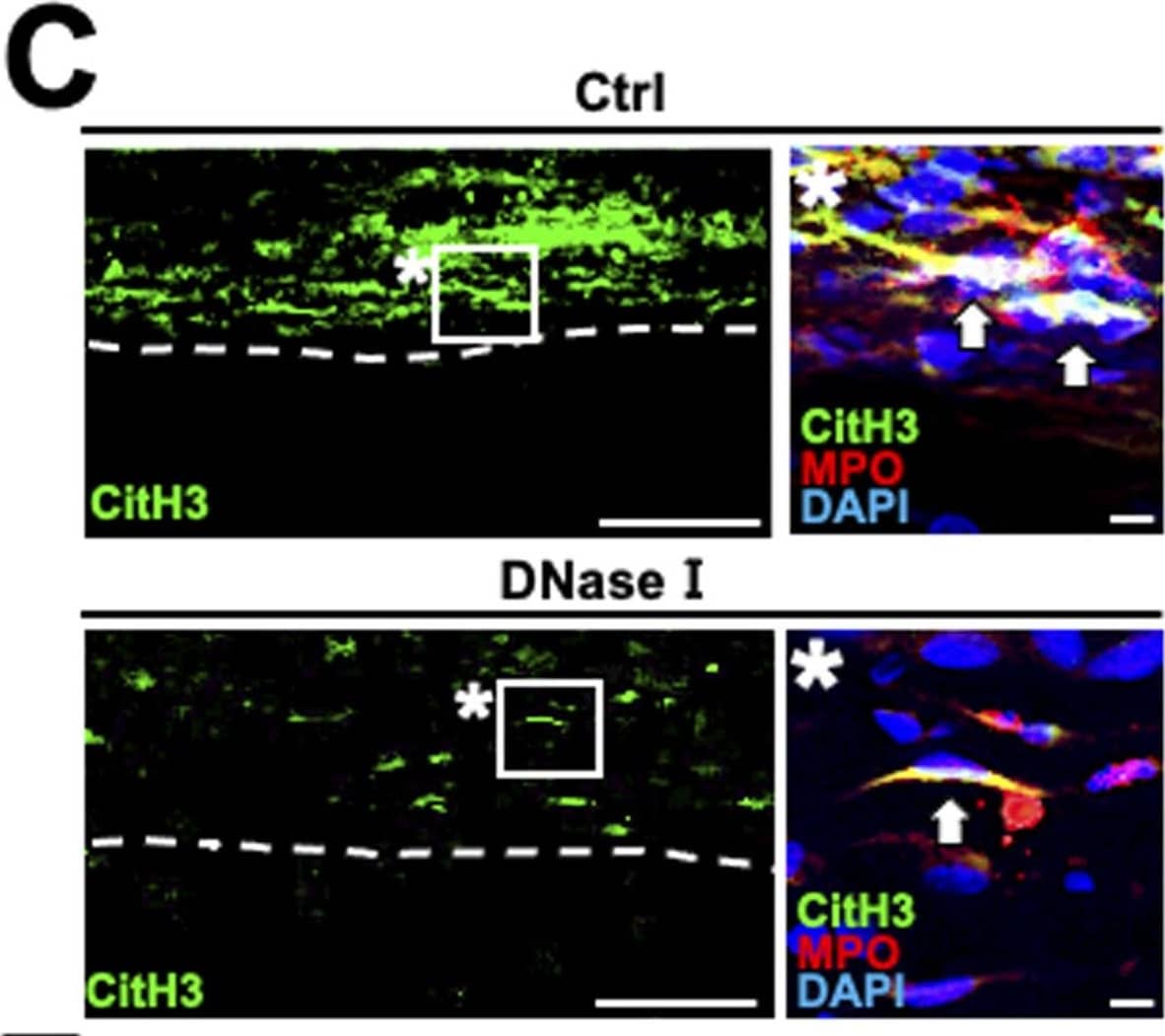
Detection of Rat Myeloperoxidase/MPO by Immunohistochemistry Inhibition of NET formation promotes the macrophage infiltration from the epineurium into the parenchyma in rats. (C) Representative images of immunolabeling with CitH3, MPO,&DAPI at 20 mm distal to the injury site. Dashed lines indicate the border of the epineurium&the parenchyma. Images marked with * are high-magnification views of the boxed areas at the epineurium. Arrows indicate neutrophils releasing NETs. Treatment with Cl-amidine or DNase I inhibited NET formation. Scale bars: 100&10 μm in low-&high-magnification images, respectively. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35961782), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
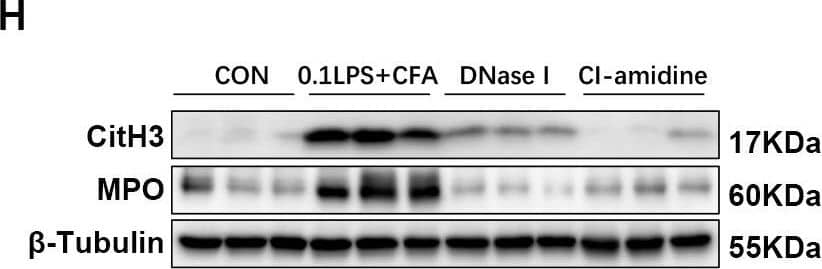
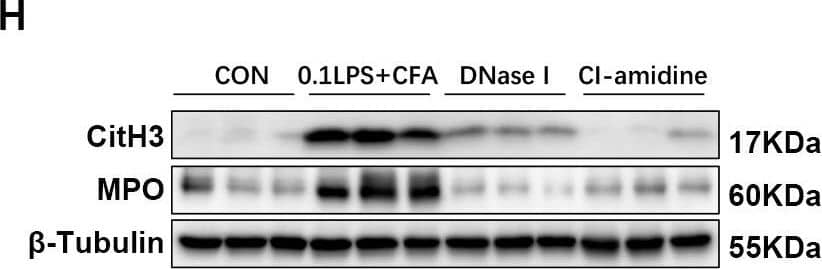
Detection of Myeloperoxidase/MPO by Western Blot DNase I or CI-amidine administration reduced airway hyperresponsiveness and alleviated airway inflammation (A) Experimental assay schematic for in vivo experiments. (B) AHR was measured 24 h after the last challenge, enhanced pause (Penh) values were used as an indicator of lung function. (C) Hematoxylin and eosin (H&E) staining of lung tissue. Scale bar = 50 μm. (D) Paraffin acid-Schiff (PAS) staining of lung. Scale bar = 20 μm. (E) Quantification of inflammation infiltration score of the H&E staining. (F) Quantification of mucus-producing goblet cells of the PAS staining. (G) The total number of cells and the differential number of cells (eosinophils and neutrophils) were quantified 48 h after the last challenge in the BALF. (H, I) Western blot analysis to measure MPO and CitH3 protein expression level in lung tissue of four groups of mice. Expression is relative to beta -Tubulin. Cropped blots are shown, and supplementary Fig. S3 and S6 presents the full-length blots. Data were shown as mean ± SEM; n = 4–6. Significance between groups was calculated using one-way ANOVA with Tukey’s post hoc method. *p < 0.05, **p < 0.01 and ***p < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36271366), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Myeloperoxidase/MPO by Western Blot DNase I or CI-amidine administration reduced airway hyperresponsiveness and alleviated airway inflammation (A) Experimental assay schematic for in vivo experiments. (B) AHR was measured 24 h after the last challenge, enhanced pause (Penh) values were used as an indicator of lung function. (C) Hematoxylin and eosin (H&E) staining of lung tissue. Scale bar = 50 μm. (D) Paraffin acid-Schiff (PAS) staining of lung. Scale bar = 20 μm. (E) Quantification of inflammation infiltration score of the H&E staining. (F) Quantification of mucus-producing goblet cells of the PAS staining. (G) The total number of cells and the differential number of cells (eosinophils and neutrophils) were quantified 48 h after the last challenge in the BALF. (H, I) Western blot analysis to measure MPO and CitH3 protein expression level in lung tissue of four groups of mice. Expression is relative to beta -Tubulin. Cropped blots are shown, and supplementary Fig. S3 and S6 presents the full-length blots. Data were shown as mean ± SEM; n = 4–6. Significance between groups was calculated using one-way ANOVA with Tukey’s post hoc method. *p < 0.05, **p < 0.01 and ***p < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36271366), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
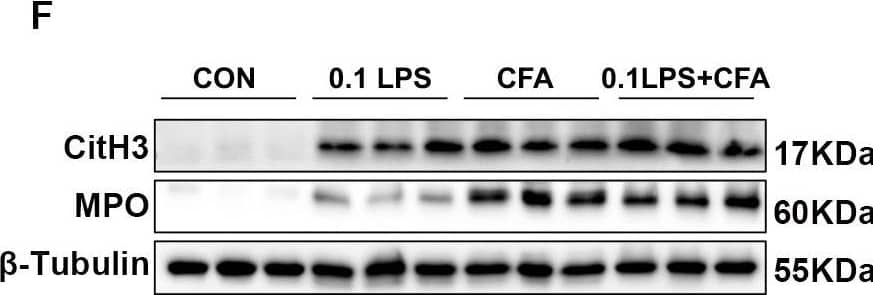
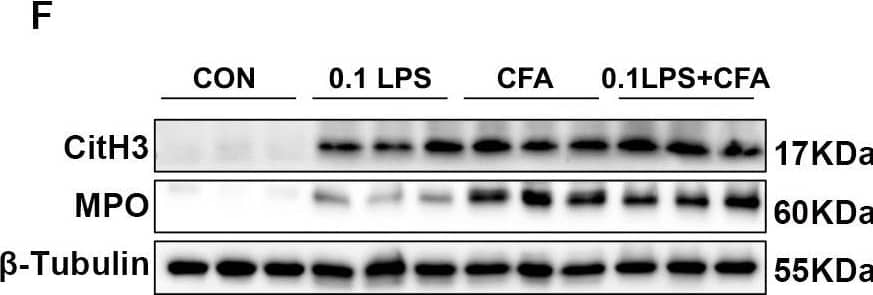
Detection of Myeloperoxidase/MPO by Western Blot Neutrophilic mouse model induced by CFA combined with LPS showed enhanced NETs formation capacity. (A) At 48 h after the last challenge, the percentage of neutrophil populations in CD45(+) leukocytes in mouse peripheral blood and bone marrow were determined by flow cytometry. The representative images in each group are shown. (B) Statistical analysis of the percentage of CD11b(+)Ly6G(+) neutrophils in CD45(+) leukocyte gate of mouse peripheral blood and bone marrow by flow cytometry. (C) Neutrophils were purified from mouse bone marrow and stimulated with PMA (100 nM) or vehicle control for 4 h. Then, neutrophils were stained for myeloperoxidase (MPO, red), citrullinated histone 3 (CitH3, green), and DNA (DAPI, blue) and confocal by immunofluorescence microscope for analysis. Representative images of NETs immunofluorescence. Scale bar = 20 μm. (D) Percentage of NETs area normalized to MPO positive signal in mouse bone marrow neutrophils after PMA stimulation. (E) Representative z-axis images of the NETs immunofluorescence in 0.1LPS + CFA group. Scale bar = 10 μm. (F, G) Western blot analysis the protein expression level of MPO and CitH3 in lung tissue of four groups of mice. Expression is relative to beta -Tubulin. Cropped blots are shown, and supplementary Fig. S2 and S5 presents the full-length blots. Data were shown as mean ± SEM; n = 4 or 6. Significance between groups was calculated using one-way ANOVA with Tukey’s post hoc method. *p < 0.05, **p < 0.01 and ***p < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36271366), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Myeloperoxidase/MPO by Western Blot Abundant NETs occur in neutrophilic asthma models. (A) Neutrophil expression in CD45(+) leucocytes in mouse peripheral blood and bone marrow were determined by flow cytometry within 48 h after the last challenge. The representative images in each group are shown. (B) Quantification: the percentage of CD11b(+)Ly6G(+) neutrophils in the CD45(+) leucocytes gate of mouse peripheral blood and bone marrow by flow cytometry. (C) In vitro NET-formation assays with purified neutrophils of mouse bone marrow neutrophils stimulated with PMA (100 nM) or RPMI 1640 for 4 h. Then the neutrophils were stained PI and analyzed by immunofluorescence confocal microscopy. Representative immunofluorescence images of NETs, Scale bar = 20 μm. (D) Comparison of the percentage of NETosis cells in each group upon PMA stimulation and control (Ctrl) stimulation. (E) After stimulated with PMA, the neutrophils were stained for myeloperoxidase (MPO, red), citrullinated histone 3 (CitH3, green) and DAPI (nuclear staining, blue) and analyzed by immunofluorescence confocal microscopy. Representative immunofluorescence images of NETs, Scale bar = 20 μm. (F) Percentage of NETs area normalized to MPO positive signal in mouse bone marrow neutrophils after PMA stimulation. (G, H) Western blot was used to detect the levels of MPO and CitH3 protein in lung tissue of five groups of mice. Expression is relative to beta -Tubulin. Cropped blots are shown, and supplementary Fig. S1 and S4 presents the full-length blots. Data were shown as mean ± SEM; n = 4. Significance between groups was calculated using one-way ANOVA with Tukey’s post hoc method. *p < 0.05, **p < 0.01 and ***p < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36271366), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Myeloperoxidase/MPO by Western Blot Neutrophilic mouse model induced by CFA combined with LPS showed enhanced NETs formation capacity. (A) At 48 h after the last challenge, the percentage of neutrophil populations in CD45(+) leukocytes in mouse peripheral blood and bone marrow were determined by flow cytometry. The representative images in each group are shown. (B) Statistical analysis of the percentage of CD11b(+)Ly6G(+) neutrophils in CD45(+) leukocyte gate of mouse peripheral blood and bone marrow by flow cytometry. (C) Neutrophils were purified from mouse bone marrow and stimulated with PMA (100 nM) or vehicle control for 4 h. Then, neutrophils were stained for myeloperoxidase (MPO, red), citrullinated histone 3 (CitH3, green), and DNA (DAPI, blue) and confocal by immunofluorescence microscope for analysis. Representative images of NETs immunofluorescence. Scale bar = 20 μm. (D) Percentage of NETs area normalized to MPO positive signal in mouse bone marrow neutrophils after PMA stimulation. (E) Representative z-axis images of the NETs immunofluorescence in 0.1LPS + CFA group. Scale bar = 10 μm. (F, G) Western blot analysis the protein expression level of MPO and CitH3 in lung tissue of four groups of mice. Expression is relative to beta -Tubulin. Cropped blots are shown, and supplementary Fig. S2 and S5 presents the full-length blots. Data were shown as mean ± SEM; n = 4 or 6. Significance between groups was calculated using one-way ANOVA with Tukey’s post hoc method. *p < 0.05, **p < 0.01 and ***p < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36271366), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Myeloperoxidase/MPO by Western Blot Abundant NETs occur in neutrophilic asthma models. (A) Neutrophil expression in CD45(+) leucocytes in mouse peripheral blood and bone marrow were determined by flow cytometry within 48 h after the last challenge. The representative images in each group are shown. (B) Quantification: the percentage of CD11b(+)Ly6G(+) neutrophils in the CD45(+) leucocytes gate of mouse peripheral blood and bone marrow by flow cytometry. (C) In vitro NET-formation assays with purified neutrophils of mouse bone marrow neutrophils stimulated with PMA (100 nM) or RPMI 1640 for 4 h. Then the neutrophils were stained PI and analyzed by immunofluorescence confocal microscopy. Representative immunofluorescence images of NETs, Scale bar = 20 μm. (D) Comparison of the percentage of NETosis cells in each group upon PMA stimulation and control (Ctrl) stimulation. (E) After stimulated with PMA, the neutrophils were stained for myeloperoxidase (MPO, red), citrullinated histone 3 (CitH3, green) and DAPI (nuclear staining, blue) and analyzed by immunofluorescence confocal microscopy. Representative immunofluorescence images of NETs, Scale bar = 20 μm. (F) Percentage of NETs area normalized to MPO positive signal in mouse bone marrow neutrophils after PMA stimulation. (G, H) Western blot was used to detect the levels of MPO and CitH3 protein in lung tissue of five groups of mice. Expression is relative to beta -Tubulin. Cropped blots are shown, and supplementary Fig. S1 and S4 presents the full-length blots. Data were shown as mean ± SEM; n = 4. Significance between groups was calculated using one-way ANOVA with Tukey’s post hoc method. *p < 0.05, **p < 0.01 and ***p < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36271366), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Myeloperoxidase/MPO
Myeloperoxidase (MPO) is a hemeprotein that belongs to the XPO subfamily of the heme peroxidase superfamily. MPO is synthesized as a preproprotein that undergoes proteolytic processing to generate a disulfide-linked heterodimer of the N-terminal beta -subunit (12 kDa) and C-terminal alpha subunit (60 kDa). Active MPO is a tetramer of two beta -subunits and two alpha -subunits that are also disulfide-linked through the two alpha -subunits. MPO is stored in granules and is an abundant protein in neutrophils and monocytes. MPO is released upon activation to catalyze the formation of powerful oxidants such as hypochlorous acid, which kills microbes. Unprocessed pro-MPO can also be released. Mouse MPO shares 87% amino acid sequence identity with that of human MPO.
Product Datasheets
Citations for Human/Mouse Myeloperoxidase/MPO Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
200
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Oxidized Phospholipids and Neutrophil Elastase Coordinately Play Critical Roles in NET Formation
Authors: Takuto Tokuhiro, Akane Ishikawa, Haruka Sato, Shunya Takita, Ayuri Yoshikawa, Ryoko Anzai et al.
Frontiers in Cell and Developmental Biology
-
DDR1-induced neutrophil extracellular traps drive pancreatic cancer metastasis
Authors: Jenying Deng, Yaan Kang, Chien-Chia Cheng, Xinqun Li, Bingbing Dai, Matthew H. Katz et al.
JCI Insight
-
Self-sustaining IL-8 loops drive a prothrombotic neutrophil phenotype in severe COVID-19
Authors: Rainer Kaiser, Alexander Leunig, Kami Pekayvaz, Oliver Popp, Markus Joppich, Vivien Polewka et al.
JCI Insight
-
Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy
Authors: Leo Nicolai, Alexander Leunig, Sophia Brambs, Rainer Kaiser, Tobias Weinberger, Michael Weigand et al.
Circulation
-
Neutrophils homing into the retina trigger pathology in early age-related macular degeneration
Authors: Sayan Ghosh, Archana Padmanabhan, Tanuja Vaidya, Alan M. Watson, Imran A. Bhutto, Stacey Hose et al.
Communications Biology
-
METTL3-mediated N6-methyladenosine exacerbates ferroptosis via m6A-IGF2BP2-dependent mitochondrial metabolic reprogramming in sepsis-induced acute lung injury
Authors: Hao Zhang, Dan Wu, Yanghanzhao Wang, Kefang Guo, Charles B. Spencer, Lilibeth Ortoga et al.
Clin Transl Med
-
Cathepsin E in neutrophils contributes to the generation of neuropathic pain in experimental autoimmune encephalomyelitis
Authors: Harada Y, Zhang J, Imari K et al.
Pain
-
The Absence of Extracellular Cold-Inducible RNA-Binding Protein (eCIRP) Promotes Pro-Angiogenic Microenvironmental Conditions and Angiogenesis in Muscle Tissue Ischemia
Authors: Matthias Kübler, Sebastian Beck, Lisa Lilian Peffenköver, Philipp Götz, Hellen Ishikawa-Ankerhold, Klaus T. Preissner et al.
International Journal of Molecular Sciences
-
Neutrophil extracellular traps-triggered impaired autophagic flux via METTL3 underlies sepsis-associated acute lung injury
Authors: Mengdi Qu, Zhaoyuan Chen, Zhiyun Qiu, Ke Nan, Yanghanzhao Wang, Yuxin Shi et al.
Cell Death Discovery
-
Age-related decline in the resistance of mice to bacterial infection and in LPS/TLR4 pathway-dependent neutrophil responses
Authors: Kirsti Hornigold, Julia Y. Chu, Stephen A. Chetwynd, Polly A. Machin, Laraine Crossland, Chiara Pantarelli et al.
Frontiers in Immunology
-
Age-Dependent Changes of Adipokine and Cytokine Secretion From Rat Adipose Tissue by Endogenous and Exogenous Toll-Like Receptor Agonists
Authors: Verena Peek, Elena Neumann, Tomohiro Inoue, Sandy Koenig, Fabian Johannes Pflieger, Rüdiger Gerstberger et al.
Frontiers in Immunology
-
A TLR–CXCL1 pathway in DRG neurons induces neutrophil accumulation in the DRG and mechanical allodynia in EAE mice
Authors: Jing Zhang, Yuka Harada, Yoshinori Hayashi
Scientific Reports
-
Causal Role for Neutrophil Elastase in Thoracic Aortic Dissection in Mice
Authors: Mei Yang, Xinmiao Zhou, Stuart W.A. Pearce, Zhisheng Yang, Qishan Chen, Kaiyuan Niu et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Therapeutic Ablation of Gain-of-Function Mutant p53 in Colorectal Cancer Inhibits Stat3-Mediated Tumor Growth and Invasion
Authors: Ramona Schulz-Heddergott, Nadine Stark, Shelley J. Edmunds, Jinyu Li, Lena-Christin Conradi, Hanibal Bohnenberger et al.
Cancer Cell
-
Chronic pulmonary bacterial infection facilitates breast cancer lung metastasis by recruiting tumor-promoting MHCII(hi) neutrophils
Authors: Ma T, Tang Y, Wang T et al.
Signal Transduction and Targeted Therapy
-
FGF19‐Induced Inflammatory CAF Promoted Neutrophil Extracellular Trap Formation in the Liver Metastasis of Colorectal Cancer
Authors: Chen Li, Tianli Chen, Jialiang Liu, Yue Wang, Chunhuan Zhang, Lu Guo et al.
Advanced Science
-
Lipopolysaccharide-Stimulated Human Fetal Membranes Induce Neutrophil Activation and Release of Vital Neutrophil Extracellular Traps
Authors: Mancy Tong, Julie A. Potter, Gil Mor, Vikki M. Abrahams
The Journal of Immunology
-
Neutrophil Extracellular Traps Are Found in Bronchoalveolar Lavage Fluids of Horses With Severe Asthma and Correlate With Asthma Severity
Authors: Pierre Janssen, Irene Tosi, Alexandre Hego, Pauline Maréchal, Thomas Marichal, Coraline Radermecker
Frontiers in Immunology
-
CLEC5A and TLR2 are critical in SARS-CoV-2-induced NET formation and lung inflammation
Authors: Sung PS, Yang SP, Peng YC et al.
Journal of biomedical science
-
Inhibition of MPO (Myeloperoxidase) Attenuates Endothelial Dysfunction in Mouse Models of Vascular Inflammation and Atherosclerosis
Authors: David Cheng, Jihan Talib, Christopher P. Stanley, Imran Rashid, Erik Michaëlsson, Eva-Lotte Lindstedt et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Immunoregulatory Monocyte Subset Promotes Metastasis Associated With Therapeutic Intervention for Primary Tumor
Authors: Takumi Shibuya, Asami Kamiyama, Hirotaka Sawada, Kenta Kikuchi, Mayu Maruyama, Rie Sawado et al.
Frontiers in Immunology
-
Aspirin-Triggered Resolvin D1 Reduces Proliferation and the Neutrophil to Lymphocyte Ratio in a Mutant KRAS-Driven Lung Adenocarcinoma Model
Authors: Amanda Vannitamby, Mohamed I. Saad, Christian Aloe, Hao Wang, Beena Kumar, Ross Vlahos et al.
Cancers (Basel)
-
C5aR1 signaling triggers lung immunopathology in COVID-19 through neutrophil extracellular traps
Authors: Bruna M. Silva, Giovanni F. Gomes, Flavio P. Veras, Seppe Cambier, Gabriel V.L. Silva, Andreza U. Quadros et al.
Journal of Clinical Investigation
-
Infection with hypervirulent Mycobacterium tuberculosis triggers emergency myelopoiesis but not trained immunity
Authors: Ana Raquel Maceiras, Diogo Silvério, Rute Gonçalves, Marcos S. Cardoso, Margarida Saraiva
Frontiers in Immunology
-
C3 Deficiency Leads to Increased Angiogenesis and Elevated Pro-Angiogenic Leukocyte Recruitment in Ischemic Muscle Tissue
Authors: Philipp Götz, Anna Braumandl, Matthias Kübler, Konda Kumaraswami, Hellen Ishikawa-Ankerhold, Manuel Lasch et al.
International Journal of Molecular Sciences
-
Neutrophil extracellular traps regulate ischemic stroke brain injury
Authors: Frederik Denorme, Irina Portier, John L. Rustad, Mark J. Cody, Claudia V. de Araujo, Chieko Hoki et al.
Journal of Clinical Investigation
-
Local Administration of Interleukin-1 Receptor Antagonist Improves Diabetic Wound Healing
Authors: David P. Perrault, Athanasios Bramos, Xingtian Xu, Songtao Shi, Alex K. Wong
Annals of Plastic Surgery
-
In vitro Detection of Neutrophil Traps and Post-attack Cell Wall Changes in Candida Hyphae
Authors: Alex Hopke, Robert T. Wheeler
BIO-PROTOCOL
-
Squamous trans-differentiation of pancreatic cancer cells promotes stromal inflammation
Authors: Tim DD Somerville, Giulia Biffi, Juliane Da beta ler-Plenker, Stella K Hur, Xue-Yan He, Krysten E Vance et al.
eLife
-
Endovascular mechanical thrombectomy in a child with COVID-19: Clot analysis reveals a novel pathway in the neuroinflammatory cascade resulting in large-vessel occlusion
Authors: Vijay M M. Ravindra, Frederik Denorme, Matthew D. Alexander, Robert A. Campbell, Ramesh Grandhi
Interv Neuroradiol
-
Heterozygosity in the glutathione synthesis gene Gclm increases sensitivity to diesel exhaust particulate induced lung inflammation in mice
Authors: Chad S. Weldy, Collin C. White, Hui-Wen Wilkerson, Timothy V. Larson, James A. Stewart, Sean E. Gill et al.
Inhalation Toxicology
-
Inhibition of neutrophil extracellular trap formation attenuates NLRP1-dependent neuronal pyroptosis via STING/IRE1 alpha pathway after traumatic brain injury in mice
Authors: Yiyao Cao, Mingming Shi, Liang Liu, Yan Zuo, Haoran Jia, Xiaobin Min et al.
Frontiers in Immunology
-
Combined protein and nucleic acid imaging reveals virus-dependent B cell and macrophage immunosuppression of tissue microenvironments
Authors: Jiang S, Chan CN, Rovira-ClavE X et al.
Immunity
-
CD11c regulates neutrophil maturation
Authors: Lifei Hou, Richard A. Voit, Miho Shibamura-Fujiogi, Sophia Koutsogiannaki, Yunan Li, Yue Chen et al.
Blood Advances
-
Inflammation and neutrophil extracellular traps in cerebral cavernous malformation
Authors: Anthony C. Y. Yau, Maria Ascencion Globisch, Favour Chinyere Onyeogaziri, Lei L. Conze, Ross Smith, Suvi Jauhiainen et al.
Cellular and Molecular Life Sciences
-
Targeting neutrophils extracellular traps (NETs) reduces multiple organ injury in a COVID-19 mouse model
Authors: FP Veras, GF Gomes, BMS Silva, DB Caetité, CJLR Almeida, CMS Silva, AH Schneider, ES Corneo, CS Bonilha, SS Batah, R Martins, E Arruda, AT Fabro, JC Alves-Filh, TM Cunha, FQ Cunha
Respiratory Research, 2023-03-02;24(1):66.
-
Influenza Virus Z-RNAs Induce ZBP1-Mediated Necroptosis
Authors: Zhang T, Yin C, Boyd D et al.
Cell
-
Neutrophilia in severe asthma is reduced in Ormdl3 overexpressing mice
Authors: Briana N. James, Cynthia Weigel, Christopher D. Green, Ryan D. R. Brown, Elisa N. D. Palladino, Anuj Tharakan et al.
The FASEB Journal
-
Neutrophil extracellular traps induce IL-1 beta production by macrophages in combination with lipopolysaccharide
Authors: Zhongshuang Hu, Taisuke Murakami, Hiroshi Tamura, Johannes Reich, Kyoko Kuwahara-Arai, Toshiaki Iba et al.
International Journal of Molecular Medicine
-
Single Cell Sequencing Identifies Distinct Cellular Alterations in Impaired Aged and Diabetic Wounds
Authors: Rojas Cortez, L;Afzali, H;Lyu, Z;Liu, M;Rojas, A;Yang, J;Gonzalez, MV;Armingol, E;Troka, M;Maracaja-Coutinho, V;Smith, P;Ko, KI;Graves, DT;
Aging cell
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Paraventricular nucleus CRH neurons regulate acute lung injury via sympathetic nerve-neutrophil axis
Authors: Li, H;Liu, T;Wang, Y;Miao, XM;Xiong, YY;Zhao, Q;Shen, WY;Su, FH;Chen, K;Dai, RP;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Modular inflammation network discovery from large-scale phenotypic screening in genetically heterogeneous mouse brains
Authors: Xiong, M;Miosge, LA;Correa-Ospina, C;Yan, CMY;Cripps, T;Bauernfried, S;Wang, Y;Crow, M;Morris, LX;Andrews, TD;Trujillo, A;Rezzonico, MG;Liang, Y;Bei, Q;Modrusan, Z;Stark, KL;Yuen, TJ;Friedman, BA;Hanson, JE;Bertram, EM;Bohlen, CJ;
Journal of neuroinflammation
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Oncometabolite 5-IP7 inhibits inositol 5-phosphatase to license E-cadherin endocytosis
Authors: Zhang, H;Zhang, B;Zhao, Y;Su, Y;Peng, Y;Yang, X;Zhao, H;Liu, H;Feng, J;Pei, H;Zhang, W;Huang, N;Jiang, K;Ito, M;Liu, G;Jork, N;Anderson, KE;Zhao, L;Nagata, E;Jessen, HJ;Hawkins, PT;Du, C;Rao, F;
Nature chemical biology
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
ATG7 in innate immune cells is required for host defense against nontuberculous mycobacterial pulmonary infections
Authors: Jeon, SM;Lee, YJ;Lee, SH;Kim, SI;Lee, B;Roh, T;Kim, YJ;Kim, HJ;Kim, IS;Whang, J;Kim, SY;Jhun, BW;Chung, C;Kang, DH;Yeo, MK;Kim, JM;Jang, J;Min, JJ;Komatsu, M;Kim, JK;Park, WY;Jo, EK;
Nature communications
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Transcriptomic analysis reveals shared deregulated neutrophil responses in COVID-19 and idiopathic pulmonary fibrosis
Authors: Divolis, G;Synolaki, E;Tringidou, R;Tzouvelekis, A;Boumpas, DT;Skendros, P;Galani, IE;
Respiratory research
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Matrix-producing neutrophils populate and shield the skin
Authors: Vicanolo, T;Özcan, A;Li, JL;Huerta-López, C;Ballesteros, I;Rubio-Ponce, A;Dumitru, AC;Nicolás-Ávila, JÁ;Molina-Moreno, M;Reyes-Gutierrez, P;Johnston, AD;Martone, C;Greto, E;Quílez-Alvarez, A;Calvo, E;Bonzon-Kulichenko, E;Álvarez-Velez, R;Chooi, MY;Kwok, I;González-Bermúdez, B;Malleret, B;Espinosa, FM;Zhang, M;Wang, YL;Sun, D;Zhen Chong, S;El-Armouche, A;Kim, KK;Udalova, IA;Greco, V;Garcia, R;Vázquez, J;Dopazo, A;Plaza, GR;Alegre-Cebollada, J;Uderhardt, S;Ng, LG;Hidalgo, A;
Nature
Species: Mouse, Transgenic Mouse
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Anti-IL-5 treatment, but not neutrophil interference, attenuates inflammation in a mixed granulocytic asthma mouse model, elicited by air pollution
Authors: De Volder, J;Bontinck, A;Haelterman, V;Boon, L;Joos, GF;Brusselle, GG;Maes, T;
Respiratory research
Species: Mouse
Sample Types:
Applications: Immunohistochemistry -
Neutrophil extracellular traps potentiate effector T cells via endothelial senescence in uveitis
Authors: Li, Z;Li, Z;Hu, Y;Xie, Y;Shi, Y;Chen, G;Huang, J;Xiao, Z;Zhu, W;Huang, H;Wang, M;Chen, J;Chen, X;Liang, D;
JCI insight
Species: Human
Sample Types:
Applications: ELISA, Immunocytochemistry -
Progesterone suppresses rhinovirus-induced airway inflammation by inhibiting neutrophil infiltration and extracellular traps formation
Authors: Dai, SZ;Wu, RH;Chen, H;Chen, MH;Xie, W;Zheng, WP;Tan, GH;Huang, FY;
International immunopharmacology
Species: Human, Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Immunohistochemistry, Immunocytochemistry -
Tamm-Horsfall protein augments neutrophil NETosis during urinary tract infection
Authors: Mercado-Evans, V;Branthoover, H;Chew, C;Serchejian, C;Saltzman, AB;Mejia, ME;Zulk, JJ;Cornax, I;Nizet, V;Patras, KA;
JCI insight
Species: Mouse, Transgenic Mouse
Sample Types: Urine, Whole Cells
Applications: Immunocytochemistry -
Lack of TYK2 signaling enhances host resistance to Candida albicans skin infection
Authors: Miranda, S;Lassnig, C;Schmidhofer, K;Kjartansdottir, H;Vogl, C;Tangermann, S;Tsymala, I;Babl, V;Müller, M;Kuchler, K;Strobl, B;
Nature communications
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Recombinant thrombomodulin and recombinant antithrombin attenuate pulmonary endothelial glycocalyx degradation and neutrophil extracellular trap formation in ventilator-induced lung injury in the context of endotoxemia
Authors: Kikuchi, K;Kazuma, S;Yamakage, M;
Respiratory research
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Cathepsin C inhibition reduces neutrophil serine protease activity and improves activated neutrophil-mediated disorders
Authors: Nishibata, Y;Arai, S;Taniguchi, M;Nakade, I;Ogawa, H;Kitano, S;Hosoi, Y;Shindo, A;Nishiyama, R;Masuda, S;Nakazawa, D;Tomaru, U;Shimizu, T;Sinko, W;Nagakura, T;Terada, Y;Ishizu, A;
Nature communications
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Progesterone modulates the immune microenvironment to suppress ovalbumin-induced airway inflammation by inhibiting NETosis
Authors: Wang, L;Huang, FY;Dai, SZ;Fu, Y;Zhou, X;Wang, CC;Tan, GH;Li, Q;
Scientific reports
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Sclerostin antibody corrects periodontal disease in type 2 diabetic mice
Authors: Turkkahraman, H;Flanagan, S;Zhu, T;Akel, N;Marino, S;Ortega-Gonzalez, D;Yuan, X;Bellido, T;
JCI insight
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Sex-dependent APOE4 neutrophil-microglia interactions drive cognitive impairment in Alzheimer's disease
Authors: Rosenzweig, N;Kleemann, KL;Rust, T;Carpenter, M;Grucci, M;Aronchik, M;Brouwer, N;Valenbreder, I;Cooper-Hohn, J;Iyer, M;Krishnan, RK;Sivanathan, KN;Brandão, W;Yahya, T;Durao, A;Yin, Z;Chadarevian, JP;Properzi, MJ;Nowarski, R;Davtyan, H;Weiner, HL;Blurton-Jones, M;Yang, HS;Eggen, BJL;Sperling, RA;Butovsky, O;
Nature medicine
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Oncogenic KRAS-dependent stromal interleukin-33 directs the pancreatic microenvironment to promote tumor growth
Authors: Donahue, KL;Watkoske, HR;Kadiyala, P;Du, W;Brown, K;Scales, MK;Elhossiny, AM;Espinoza, CE;Lasse Opsahl, EL;Griffith, BD;Wen, Y;Sun, L;Velez-Delgado, A;Renollet, NM;Morales, J;Nedzesky, NM;Baliira, RK;Menjivar, RE;Medina-Cabrera, PI;Rao, A;Allen, B;Shi, J;Frankel, TL;Carpenter, ES;Bednar, F;Zhang, Y;Pasca di Magliano, M;
Cancer discovery
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
The Elevated Inflammatory Status of Neutrophils Is Related to In-Hospital Complications in Patients with Acute Coronary Syndrome and Has Important Prognosis Value for Diabetic Patients
Authors: Barbu, E;Mihaila, AC;Gan, AM;Ciortan, L;Macarie, RD;Tucureanu, MM;Filippi, A;Stoenescu, AI;Petrea, SV;Simionescu, M;Balanescu, SM;Butoi, E;
International journal of molecular sciences
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Western Blot, Immunocytochemistry -
Modelling lung infection with Klebsiella pneumoniae after murine traumatic brain injury
Authors: Shad, A;Rewell, SSJ;Macowan, M;Gandasasmita, N;Wang, J;Chen, K;Marsland, B;O'Brien, TJ;Li, J;Semple, BD;
Journal of neuroinflammation
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Adjudin protects blood-brain barrier integrity and attenuates neuroinflammation following intracerebral hemorrhage in mice
Authors: Su, Q;Su, C;Zhang, Y;Guo, Y;Liu, Y;Liu, Y;Yong, VW;Xue, M;
International immunopharmacology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Tunable PhenoCycler imaging of the murine pre-clinical tumour microenvironments
Authors: Abraham, MJ;Goncalves, C;McCallum, P;Gupta, V;Preston, SEJ;Huang, F;Chou, H;Gagnon, N;Johnson, NA;Miller, WH;Mann, KK;Del Rincon, SV;
Cell & bioscience
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) deletion in myeloid cells augments cholestatic liver injury
Authors: Krishnan, A;Ozturk, NB;Cutshaw, KA;Guicciardi, ME;Kitagataya, T;Olson, KE;Pavelko, KD;Sherman, W;Wixom, AQ;Jalan-Sakrikar, N;Baez-Faria, M;Gutierrez, F;Gores, GJ;
Scientific reports
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Identifying genetic determinants of Streptococcus pyogenes-host interactions in a murine intact skin infection model
Authors: Wilkening, RV;Langouët-Astrié, C;Severn, MM;Federle, MJ;Horswill, AR;
Cell reports
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A dual role of lysophosphatidic acid type 2 receptor (LPAR2) in nonsteroidal anti-inflammatory drug-induced mouse enteropathy
Authors: Hutka, B;Várallyay, A;László, SB;Tóth, AS;Scheich, B;Paku, S;Vörös, I;Pós, Z;Varga, ZV;Norman, DD;Balogh, A;Benyó, Z;Tigyi, G;Gyires, K;Zádori, ZS;
Acta pharmacologica Sinica
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: Western Blot, IHC -
Inhibition of neutrophil extracellular trap formation ameliorates neuroinflammation and neuronal apoptosis via STING-dependent IRE1?/ASK1/JNK signaling pathway in mice with traumatic brain injury
Authors: Shi, G;Liu, L;Cao, Y;Ma, G;Zhu, Y;Xu, J;Zhang, X;Li, T;Mi, L;Jia, H;Zhang, Y;Liu, X;Zhou, Y;Li, S;Yang, G;Liu, X;Chen, F;Wang, B;Deng, Q;Zhang, S;Zhang, J;
Journal of neuroinflammation
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
An unexpected role of neutrophils in clearing apoptotic hepatocytes in vivo
Authors: Cao, L;Ma, L;Zhao, J;Wang, X;Fang, X;Li, W;Qi, Y;Tang, Y;Liu, J;Peng, S;Yang, L;Zhou, L;Li, L;Hu, X;Ji, Y;Hou, Y;Zhao, Y;Zhang, X;Zhao, YY;Zhao, Y;Wei, Y;Malik, AB;Saiyin, H;Xu, J;
eLife
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Intravenous Administration of Human Umbilical Cord Mesenchymal Stromal Cells Leads to an Inflammatory Response in the Lung
Authors: Hernandez Pichardo, A;Wilm, B;Liptrott, NJ;Murray, P;
Stem cells international
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Chronic pulmonary bacterial infection facilitates breast cancer lung metastasis by recruiting tumor-promoting MHCII(hi) neutrophils
Authors: Ma T, Tang Y, Wang T et al.
Signal Transduction and Targeted Therapy
-
CD47 blockade ameliorates autoimmune vasculitis via efferocytosis of neutrophil extracellular traps
Authors: Shiratori-Aso, S;Nakazawa, D;Kudo, T;Kanda, M;Ueda, Y;Watanabe-Kusunoki, K;Nishio, S;Iwasaki, S;Tsuji, T;Masuda, S;Tomaru, U;Ishizu, A;Atsumi, T;
JCI insight
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Neutrophils exacerbate acetaminophen-induced liver injury by producing cytotoxic interferon-?
Authors: Wu, H;Guo, C;Liu, Z;Cai, J;Wang, C;Yi, H;Sanyal, A;Puri, P;Zhou, H;Wang, XY;
International immunopharmacology
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
SARS-CoV-2 spike protein induces lung endothelial cell dysfunction and thrombo-inflammation depending on the C3a/C3a receptor signalling
Authors: Perico, L;Morigi, M;Pezzotta, A;Locatelli, M;Imberti, B;Corna, D;Cerullo, D;Benigni, A;Remuzzi, G;
Scientific reports
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Immune Cells Localize to Sites of Corneal Erosions in C57BL/6 Mice
Authors: Le, PM;Pal-Ghosh, S;Menko, AS;Stepp, MA;
Biomolecules
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Loss of TET2 in human hematopoietic stem cells alters the development and function of neutrophils
Authors: Huerga Encabo, H;Aramburu, IV;Garcia-Albornoz, M;Piganeau, M;Wood, H;Song, A;Ferrelli, A;Sharma, A;Minutti, CM;Domart, MC;Papazoglou, D;Gurashi, K;Llorian Sopena, M;Goldstone, R;Fallesen, T;Wang, Q;Ariza-McNaughton, L;Wiseman, DH;Batta, K;Gupta, R;Papayannopoulos, V;Bonnet, D;
Cell stem cell
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Inhibition of SARS-CoV-2-mediated thromboinflammation by CLEC2.Fc
Authors: Sung, PS;Sun, CP;Tao, MH;Hsieh, SL;
EMBO molecular medicine
Species: Human, Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Immunohistochemistry, Immunocytochemistry -
Inhibition of neutrophil extracellular trap formation attenuates NLRP1-dependent neuronal pyroptosis via STING/IRE1 alpha pathway after traumatic brain injury in mice
Authors: Yiyao Cao, Mingming Shi, Liang Liu, Yan Zuo, Haoran Jia, Xiaobin Min et al.
Frontiers in Immunology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
NOX4 as a critical effector mediating neuroinflammatory cytokines, myeloperoxidase and osteopontin, specifically in astrocytes in the hippocampus in Parkinson's disease
Authors: N Boonpraman, S Yoon, CY Kim, JS Moon, SS Yi
Redox Biology, 2023-04-10;62(0):102698.
Species: Mouse
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Blockade of neutrophil extracellular traps ameliorates toluene diisocyanate-induced steroid-resistant asthma
Authors: X Peng, Y Li, W Zhao, S Yang, J Huang, Y Chen, Y Wang, Z Gong, X Chen, C Yu, S Cai, H Zhao
International immunopharmacology, 2023-02-22;117(0):109719.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Mice inflammatory responses to inhaled aerosolized LPS: effects of various forms of human alpha1-antitrypsin
Authors: K Sivaraman, S Wrenger, B Liu, D Schaudien, C Hesse, G Gomez-Mari, S Perez-Luz, K Sewald, D DeLuca, MJ Wurm, P Pino, T Welte, B Martinez-D, S Janciauski
Journal of leukocyte biology, 2023-01-10;113(1):58-70.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
IL-1beta neutralization prevents diastolic dysfunction development, but lacks hepatoprotective effect in an aged mouse model of NASH
Authors: D Kucsera, VE Tóth, NV Sayour, T Kovács, TG Gergely, M Ruppert, T Radovits, A Fábián, A Kovács, B Merkely, P Ferdinandy, ZV Varga
Scientific Reports, 2023-01-07;13(1):356.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Functional proteomic profiling links deficient DNA clearance with increased mortality in individuals with severe COVID-19 pneumonia
Authors: Iker Valle Aramburu, Dennis Hoving, Spyros I. Vernardis, Martha C.F. Tin, Marianna Ioannou, Mia I. Temkin et al.
Immunity
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Inhibition of neutrophil elastase prevents cigarette smoke exposure-induced formation of neutrophil extracellular traps and improves lung function in a mouse model of chronic obstructive pulmonary disease
Authors: K Wang, Y Liao, X Li, R Wang, Z Zeng, M Cheng, L Gao, D Xu, F Wen, T Wang, J Chen
International immunopharmacology, 2022-12-07;114(0):109537.
Species: Human
Sample Types: Whole Cell
Applications: ICC -
A novel phosphocholine-mimetic inhibits a pro-inflammatory conformational change in C-reactive protein
Authors: J Zeller, KS Cheung Tun, TL Nero, JD McFadyen, G Krippner, B Bogner, S Kreuzaler, J Kiefer, VK Horner, D Braig, H Danish, S Baratchi, M Fricke, X Wang, MG Kather, B Kammerer, KJ Woollard, P Sharma, CJ Morton, G Pietersz, MW Parker, K Peter, SU Eisenhardt
Embo Molecular Medicine, 2022-12-05;0(0):e16236.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Scavenging dicarbonyls with 5'-O-pentyl-pyridoxamine increases HDL net cholesterol efflux capacity and attenuates atherosclerosis and insulin resistance
Authors: J Huang, H Tao, PG Yancey, Z Leuthner, LS May-Zhang, JY Jung, Y Zhang, L Ding, V Amarnath, D Liu, S Collins, SS Davies, MF Linton
Molecular Metabolism, 2022-12-05;67(0):101651.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Investigation of Neutrophil Extracellular Traps as Potential Mediators in the Pathogenesis of Non-Acute Subdural Hematomas: A Pilot Study
Authors: Michael T. Bounajem, Frederik Denorme, John L. Rustad, Robert A. Campbell, Ramesh Grandhi
Diagnostics (Basel)
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Platelet-Neutrophil Association in NETs-Rich Areas in the Retrieved AIS Patient Thrombi
Authors: GJ Pir, A Parray, R Ayadathil, SV Pananchikk, FA Mir, I Muhammad, A Abubakar, N Amir, S Hussain, KH Haroon, A Muhammad, Y Imam, SN Patro, N Akhtar, A Zakaria, S Kamran
International Journal of Molecular Sciences, 2022-11-21;23(22):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
A prospective marker for the prediction of postoperative deep venous thrombosis: Neutrophil extracellular traps
Authors: Yin Li, Qinyi Jiang, Xiaohua Zhou, Mengyuan Wu, Jian Chen, Hao Liu et al.
Frontiers in Cell and Developmental Biology
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
KDM6A Loss Recruits Tumor-Associated Neutrophils and Promotes Neutrophil Extracellular Trap Formation in Pancreatic Cancer
Authors: J Yang, L Jin, HS Kim, F Tian, Z Yi, K Bedi, M Ljungman, M Pasca di M, H Crawford, J Shi
Cancer Research, 2022-11-15;0(0):OF1-OF14.
Species: Human, Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
CLEC5A is critical in Pseudomonas aeruginosa-induced acute lung injury
Authors: PS Sung, YC Peng, SP Yang, CH Chiu, SL Hsieh
JCI Insight, 2022-09-01;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Epigallocatechin-3-gallate reduces neutrophil extracellular trap formation and tissue injury in severe acute pancreatitis
Authors: H Li, C Qiao, L Zhao, Q Jing, D Xue, Y Zhang
Journal of leukocyte biology, 2022-08-19;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Integrating network pharmacology and transcriptomic validation to investigate the efficacy and mechanism of Mufangji decoction preventing lung cancer
Authors: F Gao, Y Niu, L Sun, W Li, H Xia, Y Zhang, S Geng, Z Guo, H Lin, G Du
Journal of ethnopharmacology, 2022-07-30;0(0):115573.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC/IF -
Dysfunctional ERG signaling drives pulmonary vascular aging and persistent fibrosis
Authors: N Caporarell, J Lee, TX Pham, DL Jones, J Guan, PA Link, JA Meridew, G Marden, T Yamashita, CA Osborne, AV Bhagwate, SK Huang, RF Nicosia, DJ Tschumperl, M Trojanowsk, G Ligresti
Nature Communications, 2022-07-25;13(1):4170.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Neutrophil Extracellular Traps Promote NLRP3 Inflammasome Activation and Glomerular Endothelial Dysfunction in Diabetic Kidney Disease
Authors: A Gupta, K Singh, S Fatima, S Ambreen, S Zimmermann, R Younis, S Krishnan, R Rana, I Gadi, C Schwab, R Biemann, K Shahzad, V Rani, S Ali, PR Mertens, S Kohli, B Isermann
Nutrients, 2022-07-20;14(14):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
High PANX1 Expression Leads to Neutrophil Recruitment and the Formation of a High Adenosine Immunosuppressive Tumor Microenvironment in Basal-like Breast Cancer
Authors: W Chen, B Li, F Jia, J Li, H Huang, C Ni, W Xia
Cancers, 2022-07-11;14(14):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Deficiency of leukocyte-specific protein 1 (LSP1) alleviates asthmatic inflammation in a mouse model
Authors: NPK Le, AF do Nascime, D Schneberge, CC Quach, X Zhang, GK Aulakh, W Dawicki, L Liu, JR Gordon, B Singh
Respiratory Research, 2022-06-22;23(1):165.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Neutrophil Extracellular Traps Mediate Acute Liver Failure in Regulation of miR-223/neutrophil Elastase Signaling in Mice
Authors: D Ye, J Yao, W Du, C Chen, Y Yang, K Yan, J Li, Y Xu, S Zang, Y Zhang, X Rong, R Zhang, A Xu, J Guo
Cellular and Molecular Gastroenterology and Hepatology, 2022-06-02;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Cells, Whole Tissue
Applications: ICC, IHC, Western Blot -
Neutrophil extracellular traps regulate ischemic stroke brain injury
Authors: Frederik Denorme, Irina Portier, John L. Rustad, Mark J. Cody, Claudia V. de Araujo, Chieko Hoki et al.
Journal of Clinical Investigation
Species: Human, Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Depletion of gammadelta T Cells Leads to Reduced Angiogenesis and Increased Infiltration of Inflammatory M1-like Macrophages in Ischemic Muscle Tissue
Authors: C Arnholdt, K Kumaraswam, P Götz, M Kübler, M Lasch, E Deindl
Cells, 2022-04-29;11(9):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Endovascular mechanical thrombectomy in a child with COVID-19: Clot analysis reveals a novel pathway in the neuroinflammatory cascade resulting in large-vessel occlusion
Authors: Vijay M M. Ravindra, Frederik Denorme, Matthew D. Alexander, Robert A. Campbell, Ramesh Grandhi
Interv Neuroradiol
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Inflammation and neutrophil extracellular traps in cerebral cavernous malformation
Authors: Anthony C. Y. Yau, Maria Ascencion Globisch, Favour Chinyere Onyeogaziri, Lei L. Conze, Ross Smith, Suvi Jauhiainen et al.
Cellular and Molecular Life Sciences
Species: Human, Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Disulfiram inhibits neutrophil extracellular trap formation protecting rodents from acute lung injury and SARS-CoV-2 infection
Authors: JM Adrover, L Carrau, J Da beta ler-Ple, Y Bram, V Chandar, S Houghton, D Redmond, JR Merrill, M Shevik, BR tenOever, SK Lyons, RE Schwartz, M Egeblad
JCI Insight, 2022-03-08;0(0):.
Species: Hamster
Sample Types: Whole Tissue
Applications: IHC -
Blocking the human common beta subunit of the GM-CSF, IL-5 and IL-3 receptors markedly reduces hyperinflammation in ARDS models
Authors: H Wang, DJ Tumes, TR Hercus, KH Yip, C Aloe, R Vlahos, AF Lopez, N Wilson, CM Owczarek, S Bozinovski
Cell Death & Disease, 2022-02-10;13(2):137.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
ADAM8 signaling drives neutrophil migration and ARDS severity
Authors: C Conrad, D Yildiz, SJ Cleary, A Margraf, L Cook, U Schlomann, B Panaretou, JL Bowser, H Karmouty-Q, J Li, NK Berg, SC Martin, A Aljohmani, SF Moussavi-H, KM Wang, JJ Tian, M Magnen, C Valet, L Qiu, JP Singer, HK Eltzschig, CAPSys Stu, W Bertrams, S Herold, N Suttorp, B Schmeck, ZT Ball, A Zarbock, MR Looney, JW Bartsch
JCI Insight, 2022-02-08;7(3):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Neutrophil depletion enhanced the Clostridium novyi-NT therapy in mouse and rabbit tumor models
Authors: Verena Staedtke, Tyler Gray-Bethke, Guanshu Liu, Eleni Liapi, Gregory J Riggins, Ren-Yuan Bai
Neuro-Oncology Advances
Species: Rabbit
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
CXCR4 and CXCR7 Inhibition Ameliorates the Formation of Platelet-Neutrophil Complexes and Neutrophil Extracellular Traps through Adora2b Signaling
Authors: KC Ngamsri, RA Putri, C Jans, K Schindler, A Fuhr, Y Zhang, J Gamper-Tsi, S Ehnert, FM Konrad
International Journal of Molecular Sciences, 2021-12-17;22(24):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Modulation of Cellular NAD+ Attenuates Cancer-Associated Hypercoagulability and Thrombosis via the Inhibition of Tissue Factor and Formation of Neutrophil Extracellular Traps
Authors: W Cao, MY Zhu, SH Lee, SB Lee, HJ Kim, BO Park, CH Yoon, D Khadka, GS Oh, H Shim, TH Kwak, HS So
International Journal of Molecular Sciences, 2021-11-08;22(21):.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC -
Eosinophil extracellular traps drive asthma progression through neuro-immune signals
Authors: Y Lu, Y Huang, J Li, J Huang, L Zhang, J Feng, J Li, Q Xia, Q Zhao, L Huang, S Jiang, S Su
Nature Cell Biology, 2021-10-06;23(10):1060-1072.
Species: Mouse
Sample Types: BALF, Whole Cells
Applications: ICC -
Neutrophil-specific gain-of-function mutations in Nlrp3 promote development of cryopyrin-associated periodic syndrome
Authors: Julien Stackowicz, Nicolas Gaudenzio, Nadine Serhan, Eva Conde, Ophélie Godon, Thomas Marichal et al.
Journal of Experimental Medicine
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Sprague Dawley Rats Gaining Weight on a High Energy Diet Exhibit Damage to Taste Tissue Even after Return to a Healthy Diet
Authors: F Harnischfe, F O'Connell, M Weiss, B Axelrod, A Hajnal, K Czaja, PM Di Lorenzo, R Dando
Nutrients, 2021-08-31;13(9):.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
The Absence of Extracellular Cold-Inducible RNA-Binding Protein (eCIRP) Promotes Pro-Angiogenic Microenvironmental Conditions and Angiogenesis in Muscle Tissue Ischemia
Authors: Matthias Kübler, Sebastian Beck, Lisa Lilian Peffenköver, Philipp Götz, Hellen Ishikawa-Ankerhold, Klaus T. Preissner et al.
International Journal of Molecular Sciences
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
ELMO1 signaling is a promoter of osteoclast function and bone loss
Authors: S Arandjelov, JSA Perry, M Zhou, A Ceroi, I Smirnov, SF Walk, LS Shankman, I Cambré, S Onengut-Gu, D Elewaut, TP Conrads, KS Ravichandr
Nature Communications, 2021-08-17;12(1):4974.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Evidence of a Myenteric Plexus Barrier and Its Macrophage-Dependent Degradation During Murine Colitis: Implications in Enteric Neuroinflammation
Authors: D Dora, S Ferenczi, R Stavely, VE Toth, ZV Varga, T Kovacs, I Bodi, R Hotta, KJ Kovacs, AM Goldstein, N Nagy
Cellular and Molecular Gastroenterology and Hepatology, 2021-07-08;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
AMPK hyperactivation promotes dendrite retraction, synaptic loss, and neuronal dysfunction in glaucoma
Authors: N Belforte, J Agostinone, L Alarcon-Ma, D Villafranc, F Dotigny, JL Cueva Varg, A Di Polo
Molecular Neurodegeneration, 2021-06-29;16(1):43.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
C3 Deficiency Leads to Increased Angiogenesis and Elevated Pro-Angiogenic Leukocyte Recruitment in Ischemic Muscle Tissue
Authors: Philipp Götz, Anna Braumandl, Matthias Kübler, Konda Kumaraswami, Hellen Ishikawa-Ankerhold, Manuel Lasch et al.
International Journal of Molecular Sciences
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
NETosis in the pathogenesis of acute lung injury following cutaneous chemical burns
Authors: R Surolia, FJ Li, Z Wang, M Kashyap, RK Srivastava, AM Traylor, P Singh, KG Dsouza, H Kim, JF Pittet, JW Zmijewski, A Agarwal, M Athar, A Ahmad, VB Antony
JCI Insight, 2021-05-24;6(10):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Absence of Cold-Inducible RNA-Binding Protein (CIRP) Promotes Angiogenesis and Regeneration of Ischemic Tissue by Inducing M2-Like Macrophage Polarization
Authors: M Kübler, S Beck, S Fischer, P Götz, K Kumaraswam, H Ishikawa-A, M Lasch, E Deindl
Biomedicines, 2021-04-07;9(4):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Hsp90-stabilized MIF supports tumor progression via macrophage recruitment and angiogenesis in colorectal cancer
Authors: L Klemke, T De Oliveir, D Witt, N Winkler, H Bohnenberg, R Bucala, LC Conradi, R Schulz-Hed
Cell Death & Disease, 2021-02-04;12(2):155.
Species: Mouse
Sample Types: Organoid
Applications: IHC -
Immunomodulatory role of reactive oxygen species and nitrogen species during T cell-driven neutrophil-enriched acute and chronic cutaneous delayed-type hypersensitivity reactions
Authors: R Mehling, J Schwenck, C Lemberg, C Trautwein, L Zizmare, D Kramer, A Müller, B Fehrenbach, I Gonzalez-M, L Quintanill, K Schröder, RP Brandes, M Schaller, W Ruf, M Eichner, K Ghoreschi, M Röcken, BJ Pichler, M Kneilling
Theranostics, 2021-01-01;11(2):470-490.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Suppression of Metastatic Melanoma Growth in Lung by Modulated Electro-Hyperthermia Monitored by a Minimally Invasive Heat Stress Testing Approach in Mice
Authors: MJ Thomas, E Major, A Benedek, I Horváth, D Máthé, R Bergmann, AM Szász, T Krenács, Z Benyó
Cancers, 2020-12-21;12(12):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Alveolar barrier disruption in varicella pneumonia is associated with neutrophil extracellular trap formation
Authors: WJ Ouwendijk, HJ van den Ha, MW Delany, JJ van Kampen, GP van Nierop, T Mehraban, F Zaaraoui-B, WF van IJcken, JM van den Br, RD De Vries, AC Andeweg, GM Verjans
JCI Insight, 2020-11-05;0(0):.
Species: Primate
Sample Types: Whole Tissue
Applications: IHC -
Type I IFN exacerbates disease in tuberculosis-susceptible mice by inducing neutrophil-mediated lung inflammation and NETosis
Authors: L Moreira-Te, PJ Stimpson, E Stavropoul, S Hadebe, P Chakravart, M Ioannou, IV Aramburu, E Herbert, SL Priestnall, A Suarez-Bon, J Sousa, KL Fonseca, Q Wang, S Vashakidze, P Rodríguez-, C Vilaplana, M Saraiva, V Papayannop, A O'Garra
Nat Commun, 2020-11-04;11(1):5566.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Immunohistological Analysis of Neutrophils and Neutrophil Extracellular Traps in Human Thrombemboli Causing Acute Ischemic Stroke
Authors: F Essig, AM Kollikowsk, M Pham, L Solymosi, G Stoll, KG Haeusler, P Kraft, MK Schuhmann
Int J Mol Sci, 2020-10-07;21(19):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
The Metalloproteinase ADAMTS5 Is Expressed by Interstitial Inflammatory Cells in IgA Nephropathy and Is Proteolytically Active on the Kidney Matrix
Authors: S Taylor, M Whitfield, J Barratt, A Didangelos
J. Immunol., 2020-09-11;0(0):.
Species: Human, Rat
Sample Types: Cell Lysates, Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Neutralization of Lipocalin-2 Diminishes Stroke-Reperfusion Injury
Authors: Guona Wang, Yi-Chinn Weng, I-Chen Chiang, Yu-Ting Huang, Yi-Chu Liao, Yi-Chun Chen et al.
International Journal of Molecular Sciences
Species: Transgenic Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Age-Dependent Changes of Adipokine and Cytokine Secretion From Rat Adipose Tissue by Endogenous and Exogenous Toll-Like Receptor Agonists
Authors: Verena Peek, Elena Neumann, Tomohiro Inoue, Sandy Koenig, Fabian Johannes Pflieger, Rüdiger Gerstberger et al.
Frontiers in Immunology
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Neutrophil Extracellular Traps Impair Intestinal Barrier Function during Experimental Colitis
Authors: EY Lin, HJ Lai, YK Cheng, KQ Leong, LC Cheng, YC Chou, YC Peng, YH Hsu, HS Chiang
Biomedicines, 2020-08-05;8(8):.
Species: Mouse
Sample Types: Cell Lysates, Tissue Homogenates, Whole Tissue
Applications: ELISA Capture, IHC -
Neutrophil extracellular traps exacerbate neurological deficits after traumatic brain injury
Authors: K Vaibhav, M Braun, K Alverson, H Khodadadi, A Kutiyanawa, A Ward, C Banerjee, T Sparks, A Malik, MH Rashid, MB Khan, MF Waters, DC Hess, AS Arbab, JR Vender, N Hoda, B Baban, KM Dhandapani
Sci Adv, 2020-05-29;6(22):eaax8847.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Histones, DNA, and Citrullination Promote Neutrophil Extracellular Trap Inflammation by Regulating the Localization and Activation of TLR4
Authors: TD Tsouroukts, A Warnatsch, M Ioannou, D Hoving, Q Wang, V Papayannop
Cell Rep, 2020-05-05;31(5):107602.
Species: Human, Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Therapeutic ACPA inhibits NET formation: a potential therapy for neutrophil-mediated inflammatory diseases
Authors: RGS Chirivi, JWG van Rosmal, M van der Li, M Euler, G Schmets, G Bogatkevic, K Kambas, J Hahn, Q Braster, O Soehnlein, MH Hoffmann, HHGV Es, JMH Raats
Cell. Mol. Immunol., 2020-03-20;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Deficiency in the glycosyltransferase Gcnt1 increases susceptibility to tuberculosis through a mechanism involving neutrophils
Authors: KL Fonseca, AR Maceiras, R Matos, L Simoes-Cos, J Sousa, B Cá, L Barros, AI Fernandes, S Mereiter, R Reis, J Gomes, G Tapia, P Rodríguez-, M Martín-Cés, S Vashakidze, S Gogishvili, K Nikolaishv, R Appelberg, F Gärtner, PNS Rodrigues, C Vilaplana, CA Reis, A Magalhães, M Saraiva
Mucosal Immunol, 2020-03-13;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Programmed 'disarming' of the neutrophil proteome reduces the magnitude of inflammation
Authors: JM Adrover, A Aroca-Crev, G Crainiciuc, F Ostos, Y Rojas-Vega, A Rubio-Ponc, C Cilloniz, E Bonzón-Kul, E Calvo, D Rico, MA Moro, C Weber, I Lizasoaín, A Torres, J Ruiz-Cabel, J Vázquez, A Hidalgo
Nat. Immunol., 2020-01-13;21(2):135-144.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Keratinocyte-derived I&kappaB? drives psoriasis and associated systemic inflammation
Authors: S Lorscheid, A Müller, J Löffler, C Resch, P Bucher, FC Kurschus, A Waisman, K Schäkel, S Hailfinger, K Schulze-Os, D Kramer
JCI Insight, 2019-11-14;4(22):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Acute Liver Injury after CCl4 Administration is Independent of Smad7 Expression in Myeloid Cells
Authors: J Endig, L Unrau, P Sprezyna, S Rading, M Karsak, D Goltz, LC Heukamp, G Tiegs, L Diehl
Int J Mol Sci, 2019-11-06;20(22):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Locally instructed CXCR4hi neutrophils trigger environment-driven allergic asthma through the release of neutrophil extracellular traps
Authors: C Radermecke, C Sabatel, C Vanwinge, C Ruscitti, P Maréchal, F Perin, J Schyns, N Rocks, M Toussaint, D Cataldo, SL Johnston, F Bureau, T Marichal
Nat. Immunol., 2019-10-07;0(0):.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Neutrophils homing into the retina trigger pathology in early age-related macular degeneration
Authors: Sayan Ghosh, Archana Padmanabhan, Tanuja Vaidya, Alan M. Watson, Imran A. Bhutto, Stacey Hose et al.
Communications Biology
Species: Mouse
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Lugdunin amplifies innate immune responses in the skin in synergy with host- and microbiota-derived factors
Authors: K Bitschar, B Sauer, J Focken, H Dehmer, S Moos, M Konnerth, NA Schilling, S Grond, H Kalbacher, FC Kurschus, F Götz, B Krismer, A Peschel, B Schittek
Nat Commun, 2019-06-21;10(1):2730.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2
Authors: PS Sung, TF Huang, SL Hsieh
Nat Commun, 2019-06-03;10(1):2402.
Species: Human, Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Tofacitinib enhances delivery of antibody-based therapeutics to tumor cells through modulation of inflammatory cells
Authors: N Simon, A Antignani, SM Hewitt, M Gadina, C Alewine, D FitzGerald
JCI Insight, 2019-03-07;0(0):.
Species: Xenograft
Sample Types: Whole Tissue
Applications: IHC-P -
Neutrophils escort circulating tumour cells to enable cell cycle progression
Authors: BM Szczerba, F Castro-Gin, M Vetter, I Krol, S Gkountela, J Landin, MC Scheidmann, C Donato, R Scherrer, J Singer, C Beisel, C Kurzeder, V Heinzelman, C Rochlitz, WP Weber, N Beerenwink, N Aceto
Nature, 2019-02-06;0(0):.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Qingnao dripping pills mediate immune-inflammatory response and MAPK signaling pathway after acute ischemic stroke in rats
Authors: Z Zeng, Y Zhang, X Liang, F Wang, J Zhao, Z Xu, X Liu, X Liu
J. Pharmacol. Sci., 2019-01-22;0(0):.
Species: Rat
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Matrix metalloproteinase-9 deficiency protects mice from severe influenza A viral infection
Authors: J Rojas-Quin, X Wang, J Tipper, PR Burkett, J Zuñiga, AR Ashtekar, F Polverino, A Rout, I Yambayev, C Hernández, L Jimenez, G Ramírez, KS Harrod, CA Owen
JCI Insight, 2018-12-20;3(24):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
GWAS for Interleukin-1? levels in gingival crevicular fluid identifies IL37 variants in periodontal inflammation
Authors: S Offenbache, Y Jiao, SJ Kim, J Marchesan, KL Moss, L Jing, K Divaris, S Bencharit, CS Agler, T Morelli, S Zhang, L Sun, WT Seaman, D Cowley, SP Barros, JD Beck, M Munz, AS Schaefer, KE North
Nat Commun, 2018-09-11;9(1):3686.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Therapeutic Ablation of Gain-of-Function Mutant p53 in Colorectal Cancer Inhibits Stat3-Mediated Tumor Growth and Invasion
Authors: Ramona Schulz-Heddergott, Nadine Stark, Shelley J. Edmunds, Jinyu Li, Lena-Christin Conradi, Hanibal Bohnenberger et al.
Cancer Cell
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice
Authors: Z Cheng, L Wang, M Qu, H Liang, W Li, Y Li, L Deng, Z Zhang, GY Yang
J Neuroinflammation, 2018-05-03;15(1):135.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Local Administration of Interleukin-1 Receptor Antagonist Improves Diabetic Wound Healing
Authors: David P. Perrault, Athanasios Bramos, Xingtian Xu, Songtao Shi, Alex K. Wong
Annals of Plastic Surgery
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Reg3? is associated with cardiac inflammation and provides prognostic information in patients with acute coronary syndrome
Authors: H Lörchner, C Widera, Y Hou, A Elsässer, H Warnecke, E Giannitsis, JS Hulot, T Braun, KC Wollert, J Pöling
Int. J. Cardiol., 2018-05-01;258(0):7-13.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Cell-penetrating interactomic inhibition of nuclear factor-kappa B in a mouse model of postoperative cognitive dysfunction
Authors: SY Cheon, JM Kim, EH Kam, CC Ho, EJ Kim, S Chung, JH Jeong, DD Lee, SW Lee, BN Koo
Sci Rep, 2017-10-18;7(1):13482.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Topical Fibronectin Improves Wound Healing of Irradiated Skin
Authors: MB Johnson, B Pang, DJ Gardner, S Niknam-Ben, V Soundaraja, A Bramos, DP Perrault, K Banks, GK Lee, RY Baker, GH Kim, S Lee, Y Chai, M Chen, W Li, L Kwong, YK Hong, AK Wong
Sci Rep, 2017-06-20;7(1):3876.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The blood-borne sialyltransferase ST6Gal-1 is a negative systemic regulator of granulopoiesis
Authors: CWL Dougher, A Buffone, MJ Nemeth, M Nasirikena, EE Irons, PN Bogner, JTY Lau
J. Leukoc. Biol., 2017-05-26;0(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation
Authors: M Toussaint, DJ Jackson, D Swieboda, A Guedán, TD Tsouroukts, YM Ching, C Radermecke, H Makrinioti, J Aniscenko, MR Edwards, R Solari, F Farnir, V Papayannop, F Bureau, T Marichal, SL Johnston
Nat. Med., 2017-05-01;23(6):681-691.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Obesity promotes prolonged ovalbumin-induced airway inflammation modulating Th1, Th2 and Th17 immune responses in BALB/c mice
Authors: FM Silva, EE Oliveira, AC Gouveia, AS Brugiolo, CC Alves, JO Correa, J Gameiro, J Mattes, HC Teixeira, AP Ferreira
Clin. Exp. Immunol, 2017-03-31;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Immunodetection of NETs in Paraffin-Embedded Tissue
Authors: Volker Brinkmann, Ulrike Abu Abed, Christian Goosmann, Arturo Zychlinsky
Frontiers in Immunology
Species: Human, Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Swimming Motility Mediates the Formation of Neutrophil Extracellular Traps Induced by Flagellated Pseudomonas aeruginosa
PLoS Pathog., 2016-11-17;12(11):e1005987.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC -
PAD4 Deficiency Decreases Inflammation and Susceptibility to Pregnancy Loss in a Mouse Model
Authors: Luise Erpenbeck
Biol. Reprod, 2016-11-09;95(6):132.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Beneficial Effects of Sodium Phenylbutyrate Administration During Infection with Salmonella enterica serovar Typhimurium
Infect Immun, 2016-08-19;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Neutrophil Attack Triggers Extracellular Trap-Dependent Candida Cell Wall Remodeling and Altered Immune Recognition
PLoS Pathog, 2016-05-25;12(5):e1005644.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC -
Rhadinovirus host entry by co-operative infection.
Authors: Lawler C, Milho R, May J, Stevenson P
PLoS Pathog, 2015-03-19;11(3):e1004761.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Correction: A chitin-like component on sclerotic cells of fonsecaea pedrosoi inhibits dectin-1-mediated murine Th17 development by masking beta-glucans.
PLoS ONE, 2015-03-18;10(3):e0119244.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Lactobacillus rhamnosus GG and Bifidobacterium longum attenuate lung injury and inflammatory response in experimental sepsis.
Authors: Khailova L, Petrie B, Baird C, Dominguez Rieg J, Wischmeyer P
PLoS ONE, 2014-05-15;9(5):e97861.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria.
Authors: Behnsen J, Jellbauer S, Wong C, Edwards R, George M, Ouyang W, Raffatellu M
Immunity, 2014-02-06;40(2):262-73.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice.
Authors: Guabiraba R, Besnard A, Menezes G, Secher T, Jabir M, Amaral S, Braun H, Lima-Junior R, Ribeiro R, Cunha F, Teixeira M, Beyaert R, Graham G, Liew F
Mucosal Immunol, 2014-01-15;7(5):1079-93.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Age-associated pro-inflammatory adaptations of the mouse thoracic aorta
Authors: Bianca Hemmeryckx, Marc F. Hoylaerts, Eveline Deloose, Cor E. Van Hove, Paul Fransen, Hidde Bult et al.
Thrombosis and Haemostasis
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Role of the Yersinia pestis Ail Protein in Preventing a Protective Polymorphonuclear Leukocyte Response during Bubonic Plague
Authors: B. Joseph Hinnebusch, Clayton O. Jarrett, Julie A. Callison, Donald Gardner, Susan K. Buchanan, Gregory V. Plano
Infection and Immunity
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Transcriptomic analysis of host immune and cell death responses associated with the influenza A virus PB1-F2 protein.
Authors: Le Goffic R, Leymarie O, Chevalier C, Rebours E, Da Costa B, Vidic J, Descamps D, Sallenave JM, Rauch M, Samson M, Delmas B
PLoS Pathog., 2011-08-25;7(8):e1002202.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Tissue inhibitor of metalloproteinases 3 regulates resolution of inflammation following acute lung injury.
Authors: Gill SE, Huizar I, Bench EM
Am. J. Pathol., 2009-12-11;176(1):64-73.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: ELISA Development -
Murine scald models characterize the role of neutrophils and neutrophil extracellular traps in severe burns
Authors: Julia Elrod, Moritz Lenz, Antonia Kiwit, Lina Armbrust, Lavinia Schönfeld, Konrad Reinshagen et al.
Frontiers in Immunology
-
Senescence rewires microenvironment sensing to facilitate anti-tumor immunity
Authors: Hsuan-An Chen, Yu-Jui Ho, Riccardo Mezzadra, Jose M. Adrover, Ryan Smolkin, Changyu Zhu et al.
Cancer Discovery
-
Neutrophil depletion enhanced the Clostridium novyi-NT therapy in mouse and rabbit tumor models
Authors: Verena Staedtke, Tyler Gray-Bethke, Guanshu Liu, Eleni Liapi, Gregory J Riggins, Ren-Yuan Bai
Neuro-Oncology Advances
-
Midkine drives cardiac inflammation by promoting neutrophil trafficking and NETosis in myocarditis
Authors: Ludwig T. Weckbach, Ulrich Grabmaier, Andreas Uhl, Sebastian Gess, Felicitas Boehm, Annette Zehrer et al.
Journal of Experimental Medicine
-
Unique Angiogenesis From Cardiac Arterioles During Pericardial Adhesion Formation
Authors: Kenji Namiguchi, Tomohisa Sakaue, Mikio Okazaki, Kaho Kanno, Yuhei Komoda, Fumiaki Shikata et al.
Frontiers in Cardiovascular Medicine
-
Characterization of the CDAA Diet-Induced Non-alcoholic Steatohepatitis Model: Sex-Specific Differences in Inflammation, Fibrosis, and Cholesterol Metabolism in Middle-Aged Mice
Authors: Dániel Kucsera, Viktória E. Tóth, Dorottya Gergő, Imre Vörös, Zsófia Onódi, Anikó Görbe et al.
Frontiers in Physiology
-
Inhibition of myeloperoxidase enhances immune checkpoint therapy for melanoma
Authors: Tracy W Liu, Seth T Gammon, Ping Yang, Wencai Ma, Jing Wang, David Piwnica-Worms
Journal for ImmunoTherapy of Cancer
-
Neutrophils and neutrophil serine proteases are increased in the spleens of estrogen-treated C57BL/6 mice and several strains of spontaneous lupus-prone mice
Authors: Rujuan Dai, Catharine Cowan, Bettina Heid, Deena Khan, Zhihong Liang, Christine T. N. Pham et al.
PLOS ONE
-
A Chitin-Like Component on Sclerotic Cells of Fonsecaea pedrosoi Inhibits Dectin-1-Mediated Murine Th17 Development by Masking beta -Glucans
Authors: Bilin Dong, Dongsheng Li, Ruoyu Li, Sharon C.-A. Chen, Weihuang Liu, Wei Liu et al.
PLoS ONE
-
Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19
Authors: Coraline Radermecker, Nancy Detrembleur, Julien Guiot, Etienne Cavalier, Monique Henket, Céline d’Emal et al.
Journal of Experimental Medicine
-
Neutrophil extracellular traps promote liver micrometastasis in pancreatic ductal adenocarcinoma via the activation of cancer‑associated fibroblasts
Authors: Shin Takesue, Kenoki Ohuchida, Tomohiko Shinkawa, Yoshiki Otsubo, Sokichi Matsumoto, Akiko Sagara et al.
International Journal of Oncology
-
Interleukin-17E, inducible nitric oxide synthase and arginase1 as new biomarkers in the identification of neutrophilic dermatoses
Authors: R. Stalder, N. Brembilla, C. Conrad, N. Yawalkar, A. Navarini, W H Boehncke et al.
Clin Exp Dermatol
-
In Silico Sperm Proteome Analysis to Investigate DNA Repair Mechanisms in Varicocele Patients
Authors: Renata Finelli, Sara Darbandi, Peter Natesan Pushparaj, Ralf Henkel, Edmund Ko, Ashok Agarwal
Front Endocrinol (Lausanne)
-
Functional proteomic profiling links deficient DNA clearance with increased mortality in individuals with severe COVID-19 pneumonia
Authors: Iker Valle Aramburu, Dennis Hoving, Spyros I. Vernardis, Martha C.F. Tin, Marianna Ioannou, Mia I. Temkin et al.
Immunity
-
PDX Models: A Versatile Tool for Studying the Role of Myeloid-Derived Suppressor Cells in Breast Cancer
Authors: Morten F. Gjerstorff, Sofie Traynor, Odd L. Gammelgaard, Simone Johansen, Christina B. Pedersen, Henrik J. Ditzel et al.
Cancers (Basel)
-
Astrocyte-derived microparticles initiate a neuroinflammatory cycle due to carbon monoxide poisoning
Authors: Ruhela D, Bhopale VM, Kalakonda S et al.
Brain Behav Immun Health
-
Role of the Yersinia pestis Ail Protein in Preventing a Protective Polymorphonuclear Leukocyte Response during Bubonic Plague
Authors: B. Joseph Hinnebusch, Clayton O. Jarrett, Julie A. Callison, Donald Gardner, Susan K. Buchanan, Gregory V. Plano
Infection and Immunity
-
Interleukin-1 mediates ischaemic brain injury via distinct actions on endothelial cells and cholinergic neurons.
Authors: Wong R, Lenart N, Hill L et al.
Flow Cytometry Basics for the Non-Expert.
-
RNase A Treatment Interferes With Leukocyte Recruitment, Neutrophil Extracellular Trap Formation, and Angiogenesis in Ischemic Muscle Tissue
Authors: Manuel Lasch, Konda Kumaraswami, Simona Nasiscionyte, Susanna Kircher, Dominic van den Heuvel, Sarah Meister et al.
Frontiers in Physiology
-
Investigation of Neutrophil Extracellular Traps as Potential Mediators in the Pathogenesis of Non-Acute Subdural Hematomas: A Pilot Study
Authors: Michael T. Bounajem, Frederik Denorme, John L. Rustad, Robert A. Campbell, Ramesh Grandhi
Diagnostics (Basel)
-
Immunodetection of NETs in Paraffin-Embedded Tissue
Authors: Volker Brinkmann, Ulrike Abu Abed, Christian Goosmann, Arturo Zychlinsky
Frontiers in Immunology
-
A prospective marker for the prediction of postoperative deep venous thrombosis: Neutrophil extracellular traps
Authors: Yin Li, Qinyi Jiang, Xiaohua Zhou, Mengyuan Wu, Jian Chen, Hao Liu et al.
Frontiers in Cell and Developmental Biology
-
The Importance of Hypoxia-Inducible Factors (HIF-1 and HIF-2) for the Pathophysiology of Inflammatory Bowel Disease.
Authors: Kerber, E L, Padberg, C Et al.
Int J Mol Sci
-
Functional Blockage of S100A8/A9 Ameliorates Ischemia–Reperfusion Injury in the Lung
Authors: Kentaro Nakata, Mikio Okazaki, Tomohisa Sakaue, Rie Kinoshita, Yuhei Komoda, Dai Shimizu et al.
Bioengineering (Basel)
-
Neutrophil-specific gain-of-function mutations in Nlrp3 promote development of cryopyrin-associated periodic syndrome
Authors: Julien Stackowicz, Nicolas Gaudenzio, Nadine Serhan, Eva Conde, Ophélie Godon, Thomas Marichal et al.
Journal of Experimental Medicine
-
Impact of C57BL/6J and SV-129 Mouse Strain Differences on Ischemia-Induced Postnatal Angiogenesis and the Associated Leukocyte Infiltration in a Murine Hindlimb Model of Ischemia
Authors: Matthias Kübler, Philipp Götz, Anna Braumandl, Sebastian Beck, Hellen Ishikawa-Ankerhold, Elisabeth Deindl
International Journal of Molecular Sciences
-
Neutrophils delay repair process in Wallerian degeneration by releasing NETs outside the parenchyma
Authors: Yamamoto Y, Kadoya K, Terkawi MA et al.
Life science alliance
-
Discovery of 1-((6-Aminopyridin-3-yl)Methyl)-3-(4-Bromophenyl)Urea as a Potent, Irreversible Myeloperoxidase Inhibitor
Authors: Martin L. Marro, Andrew W. Patterson, Lac Lee, Lin Deng, Aimee Reynolds, Xianglin Ren et al.
Journal of Pharmacology and Experimental Therapeutics
-
Neutrophil inhibition improves acute inflammation in a murine model of viral myocarditis
Authors: Paolo Carai, Laura Florit Florit González, Stijn Van Bruggen, Valerie Spalart, Daria De Giorgio, Nadéche Geuens et al.
Cardiovascular Research
-
Curcumin Ameliorates Particulate Matter-Induced Pulmonary Injury through Bimodal Regulation of Macrophage Inflammation via NF-kappa B and Nrf2
Authors: Min Kook Lee, Hyo Dam Kim, Suk Hee Lee, Jin Hyup Lee
International Journal of Molecular Sciences
-
Cell-Type-Specific Responses to Interleukin-1 Control Microbial Invasion and Tumor-Elicited Inflammation in Colorectal Cancer
Authors: Oxana Dmitrieva-Posocco, Amiran Dzutsev, David F. Posocco, Vivianty Hou, Wuxing Yuan, Vishal Thovarai et al.
Immunity
-
Fc gamma Receptor IIB Controls Skin Inflammation in an Active Model of Epidermolysis Bullosa Acquisita
Authors: Balint Kovacs, Jenny Tillmann, Lisa-Christin Freund, Falk Nimmerjahn, Christian D. Sadik, Katja Bieber et al.
Frontiers in Immunology
-
Age-associated pro-inflammatory adaptations of the mouse thoracic aorta
Authors: Bianca Hemmeryckx, Marc F. Hoylaerts, Eveline Deloose, Cor E. Van Hove, Paul Fransen, Hidde Bult et al.
Thrombosis and Haemostasis
-
Enhancing Techniques for Determining Inflammatory Edema Formation and Neutrophil Accumulation in Murine Skin
Authors: Ali A. Zarban, Hiba Chaudhry, Davide Maselli, Xenia Kodji, Joao de Sousa Valente, Justin Joachim et al.
JID Innovations
-
Tlr2/4‐Mediated Hyperinflammation Promotes Cherubism‐Like Jawbone Expansion in Sh3bp2 (P416R) Knockin Mice
Authors: Yasuyuki Fujii, Nelson Monteiro, Shyam Kishor Sah, Homan Javaheri, Yasuyoshi Ueki, Zhichao Fan et al.
JBMR Plus
-
Early and Sustained Increases in Leukotriene B4 Levels Are Associated with Poor Clinical Outcome in Ischemic Stroke Patients
Authors: Su Jing Chan, Mary P. E. Ng, Hui Zhao, Geelyn J. L. Ng, Chuan De Foo, Peter T.-H. Wong et al.
Neurotherapeutics
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse Myeloperoxidase/MPO Antibody
Average Rating: 5 (Based on 4 Reviews)
Have you used Human/Mouse Myeloperoxidase/MPO Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
DAPI-blue
MPO-green