Human/Mouse/Rat/Hamster ACE-2 Antibody Summary
Gln18-Ser740
Accession # Q9BYF1
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
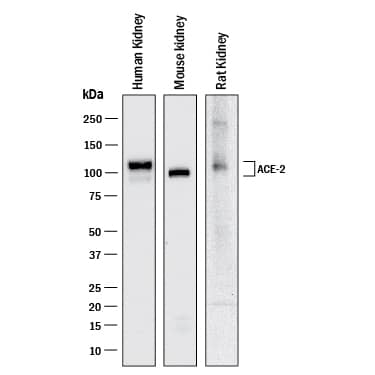 View Larger
View Larger
Detection of Human, Mouse, and Rat ACE‑2 by Western Blot. Western blot shows lysates of human kidney tissue, mouse kidney tissue, and rat kidney tissue. PVDF membrane was probed with 1 µg/mL of Goat Anti-Human/Mouse/Rat/Hamster ACE-2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF933) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for ACE-2 at approximately 100 and 110 kDa (as indicated). This experiment was conducted under reducing conditions and using Western Blot Buffer Group 1.
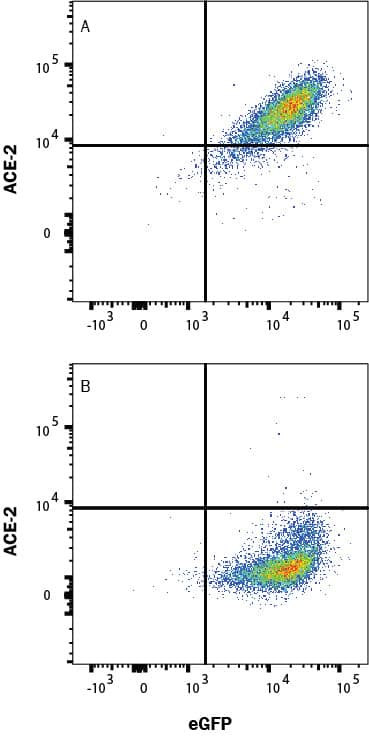 View Larger
View Larger
Detection of ACE-2 in HEK293 Human Cell Line Transfected with Human ACE-2 and eGFP by Flow Cytometry. HEK293 human embryonic kidney cell line transfected with (A) human ACE-2 or (B) irrelevant protein, and eGFP was stained with Goat Anti-Human/Mouse/Rat/Hamster ACE-2 Affinity Purified Polyclonal Antibody (Catalog # AF933) followed by Allophycocyanin-conjugated Anti-Goat IgG Secondary Antibody (F0108). Quadrant markers were set based on Goat IgG control antibody (AB-108-C, data not shown). Staining was performed using our Staining Membrane-associated Proteins protocol.
 View Larger
View Larger
ACE‑2 in Human Kidney. ACE-2 was detected in immersion fixed paraffin-embedded sections of human kidney using Goat Anti-Human/Mouse/Rat/Hamster ACE-2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF933) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
ACE‑2 in Human Kidney. ACE-2 was detected in immersion fixed paraffin-embedded sections of human kidney using Goat Anti-Human/Mouse/Rat/Hamster ACE-2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF933) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). Lower panel shows a lack of labeling if primary antibodies are omitted and tissue is stained only with secondary antibody followed by incubation with detection reagents. View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
ACE‑2 in Hamster Lung. ACE‑2 was detected in immersion fixed paraffin-embedded sections of hamster lung using Goat Anti-Human/Mouse/Rat/Hamster ACE‑2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF933) at 3 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (VC004). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to respiratory bronchioles. Staining was performed using our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
ACE‑2 in Rat Lung. ACE‑2 was detected in immersion fixed frozen sections of rat lung using Goat Anti-Human/Mouse/Rat/Hamster ACE‑2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF933) at 10 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (VC004). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cell surface in eputhelial cells in bronchioles. Staining was performed using our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
ACE‑2 in Rat Lung. ACE‑2 was detected in immersion fixed paraffin-embedded sections of rat lung using Goat Anti-Human/Mouse/Rat/Hamster ACE‑2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF933) at 10 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (VC004). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Staining was performed using our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
ACE‑2 in Rat Kidney. ACE‑2 was detected in immersion fixed paraffin-embedded sections of rat kidney using Goat Anti-Human/Mouse/Rat/Hamster ACE‑2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF933) at 10 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (VC004). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cell surface in convoluted tubules. Staining was performed using our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
Detection of Human ACE‑2 by Simple WesternTM. Simple Western lane view shows lysates of human kidney tissue, loaded at 0.2 mg/mL. A specific band was detected for ACE-2 at approximately 155 kDa (as indicated) using 10 µg/mL of Goat Anti-Human/Mouse/Rat/Hamster ACE-2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF933) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
Detection of Human ACE-2 by Western Blot (A) Transduction of pLV pseudotyped with VSV-G (A,C) or CoV-2 Spike glycoprotein (B,D) in HEK293T (A,B) or Vero E6 (C,D) cells. The lentiviral backbone incorporates enhanced green fluorescent protein (eGFP) that is expressed upon integration into target cells. The fluorescence was recorded at 48 h post transduction. Magnification 4X. (E) Transduction efficiency of pLV pseudotyped with CoV-2 Spike glycoprotein in Vero E6, hACE2-HEK293T and 293T cells. The fluorescence was recorded at 48 h post transduction. The experiments were done in triplicates and standard error of mean was plotted as error bars. (F) Whole cell lysates from Vero E6, hACE2-293T and 293T cells were run on SDS-PAGE and probed with anti ACE2 antibody. Beta-actin was used as a loading control. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33154514), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse ACE-2 by Western Blot Immunoblot analysis of ACE2 protein in the media from high glucose- or Ang II-stimulated mouse PT cells.(A) Mouse PT cells were incubated for 72 hrs in normal media (C, 7.8 mM D-glucose), with or without Ang II (10−7 M), high D-glucose (D-G, 25 mM), or high L-glucose (25 mM). Above graph is representative immunoblot for ACE2 in the media, showing bands at ∼90 kDa and ∼70 kDa. (B) Graphical representation of densitometry analysis of two ACE2 bands on immunoblots. For the ∼90 kDa band, *p<0.05 vs C, **p<0.001 vs C, **p<0.003 vs L-G; n = 5. For the ∼70 kDa band, *p<0.04 vs C; **p<0.001 vs C or L-G, **p<0.03 vs Ang II; n = 5. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085958), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse ACE-2 by Western Blot Deglycosylation of ACE2 protein in media and cell lysates from mouse PT cells.Representative immunoblot for ACE2 treated without (−) or with (+) deglycosylation with PNGase F in the media (Lanes 1–2) and cell lysates (Lanes 3–4). Lanes 1 and 3: wildtype PT cells, Lanes 2 and 4: ACE2 knockout (KO) PT cells transfected with a human ACE2 vector, Lane 5: mouse kidney cortex. Lanes 1+ and 2+ show a reduction in the sizes of ACE2 fragments in media fractions to ∼75 kDa and ∼60 kDa for mouse ACE2, and to ∼80 kDa and ∼65 kDa for human ACE2, respectively. Lanes 3+ and 4+ show a reduction in the sizes of ACE2 in cell lysates to ∼85 kDa for both mouse and human ACE2 treated with the PNGase F, respectively. Lane 5+ shows a reduction in size of ACE2 in mouse cortex from ∼100 kDa to ∼85 kDa after treatment with PNGase F. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085958), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse ACE-2 by Western Blot Immunoblot analysis of ACE2 protein in media and cell lysates from mouse PT cells.(A) Representative immunoblot for ACE2 protein in concentrated media (Lanes 1–3) and cell lysates (Lanes 4–6) from mouse PT cells. Lanes 1 and 4: wildtype cells, Lanes 2 and 5: ACE2 knockout (KO) cells, Lanes 3 and 6: ACE2 KO cells transfected with a human ACE2 expression vector, Lane 7: mouse kidney cortex showing a band at ∼100 kDa, used as a positive control. Lane 1 shows two bands in the media at ∼90 kDa and ∼70 kDa for mouse ACE2. Lane 3 shows two bands in the media for human ACE2 in transfected cells, at ∼110 kDa and ∼95 kDa. Lanes 4 and 6 show a single band in cell lysates at ∼100 kDa for mouse ACE2, and ∼120 kDa for human ACE2, respectively. Lanes 2 and 5 show no ACE2 bands detected on immunoblots of both media and cell lysates from untransfected ACE2 KO cells. (B) Increased ACE2 activity in the media from ACE2 KO cells transfected with a human ACE2 expression vector (HA-hACE2, 3.75 µg on 35 mm culture dishes). Untransfected cells and cells transfected with an empty pcDNA3 vector had no detectable ACE2 activity in the media. Numbers in parentheses represent mean values for ACE2 activity. *P<0.001 vs untransfected control or empty pcDNA3 vector, n = 4. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085958), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Simple Western: ACE-2 Antibody [Unconjugated] [AF933] - Simple Western: ACE-2 Antibody [Unconjugated] [AF933] - Design for a membrane-localized ACE2 expression system. (A) Our ACE2 construct is driven by a CMV promoter followed by the first 25 residues of ACE2 containing the leader sequence that direct ACE2 to the plasma membrane. This is followed by a 3xHA tag linked to the remainder of ACE2 (20-805) and a C-terminal sfGFP. Both 3xHA and sfGFP fusions are separated from ACE2 by flexible 3xGGGGS linkers. (B) The ACE2 fusion protein is designed to be embedded in the plasma membrane where it can perform extracellular carboxypeptidase-mediated metabolism and its levels can be detected by cell staining with antibodies to HA. (C) Lysates from untransfected or 3xHA-ACE2-sfGFP-transfected HEK293 cells were analyzed by automated Jess capillary immunoassay using antibodies to HA, GFP, and two ACE2 antibodies. (D) Confocal fluorescence microscopy of HEK cells transfected with 3xHA-ACE2-sfGFP and stained with HA and the nuclear stain DAPI. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/37644110), licensed under a CC-BY license. Not internally tested by R&D Systems.
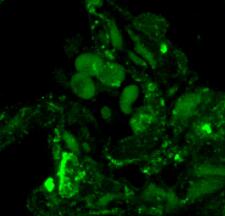
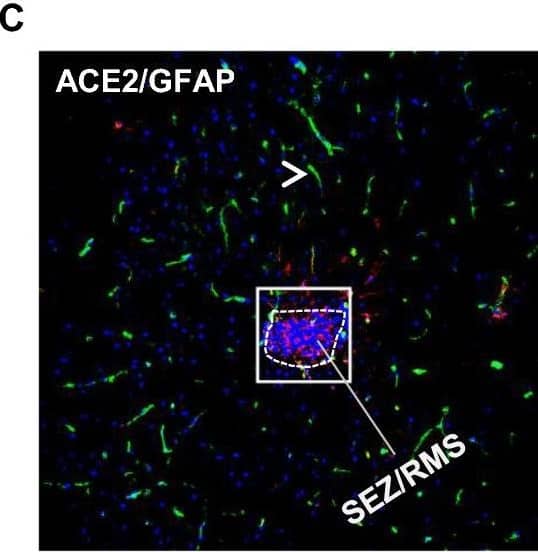 View Larger
View Larger
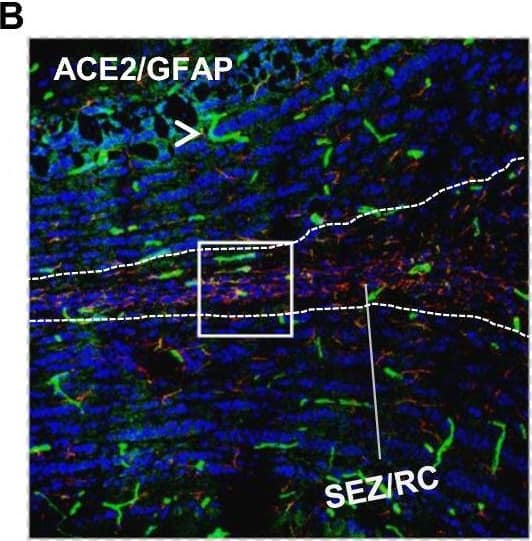
Detection of Mouse ACE-2 by Immunohistochemistry Distribution of ACE2 along the olfactory retrograde route. A Schematic elucidation of a gross view of mouse olfactory retrograde route comprised by OB surface, SEZ/RC, SEZ in the rostral migratory stream (SEZ/RMS) and SVZ of the LV (top panel) and a microscopic view of SEZ/RC and SEZ/RMS (bottom panel). B–E Co-staining of ACE2 (green) with astrocyte marker GFAP (red) in SEZ/RC and SEZ/RMS. B-C show the low magnitude images. D–E show the enlarged images of the indicated regions in B and C, respectively. DAPI (blue) was used for nuclear staining. Arrow: indicates astrocyte. Dash arrow: indicates neuroblast. Arrowhead: indicates microvessel. The dash lines in all images outline the border of SEZ/RC and SEZ/RMS, respectively. Image magnitude B-C: 20X, D-E: 100X Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35672716), licensed under a CC-BY license. Not internally tested by R&D Systems.
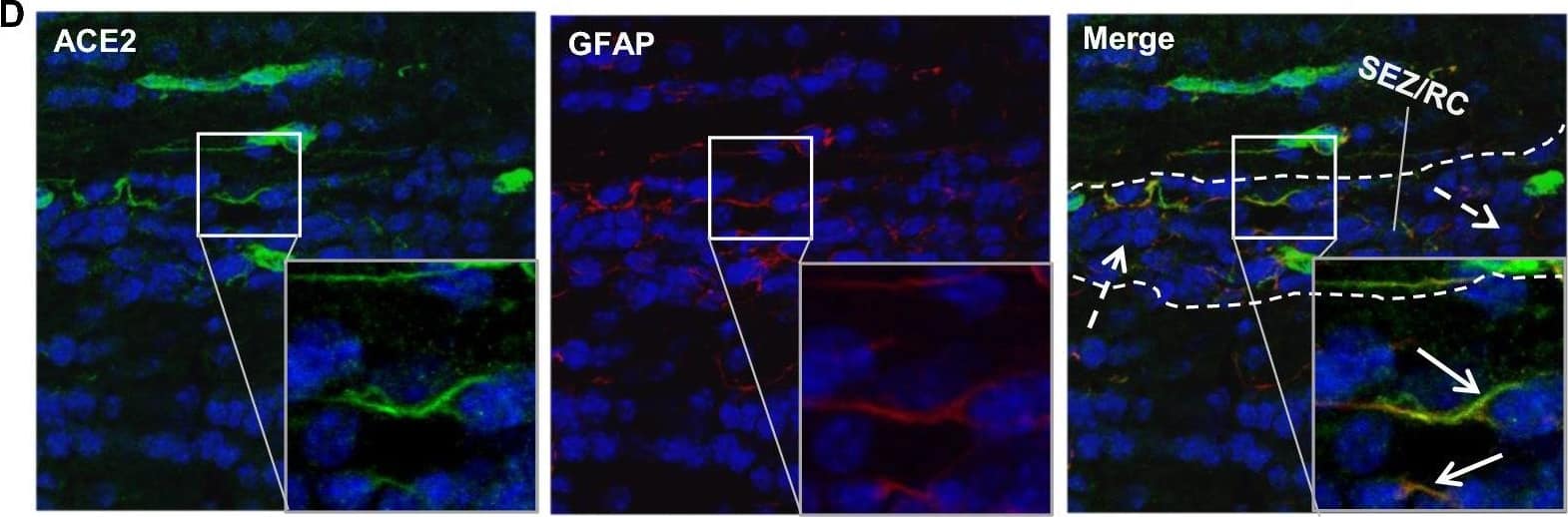 View Larger
View Larger
Detection of Mouse ACE-2 by Immunohistochemistry Distribution of ACE2 along the olfactory retrograde route. A Schematic elucidation of a gross view of mouse olfactory retrograde route comprised by OB surface, SEZ/RC, SEZ in the rostral migratory stream (SEZ/RMS) and SVZ of the LV (top panel) and a microscopic view of SEZ/RC and SEZ/RMS (bottom panel). B–E Co-staining of ACE2 (green) with astrocyte marker GFAP (red) in SEZ/RC and SEZ/RMS. B-C show the low magnitude images. D–E show the enlarged images of the indicated regions in B and C, respectively. DAPI (blue) was used for nuclear staining. Arrow: indicates astrocyte. Dash arrow: indicates neuroblast. Arrowhead: indicates microvessel. The dash lines in all images outline the border of SEZ/RC and SEZ/RMS, respectively. Image magnitude B-C: 20X, D-E: 100X Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35672716), licensed under a CC-BY license. Not internally tested by R&D Systems.
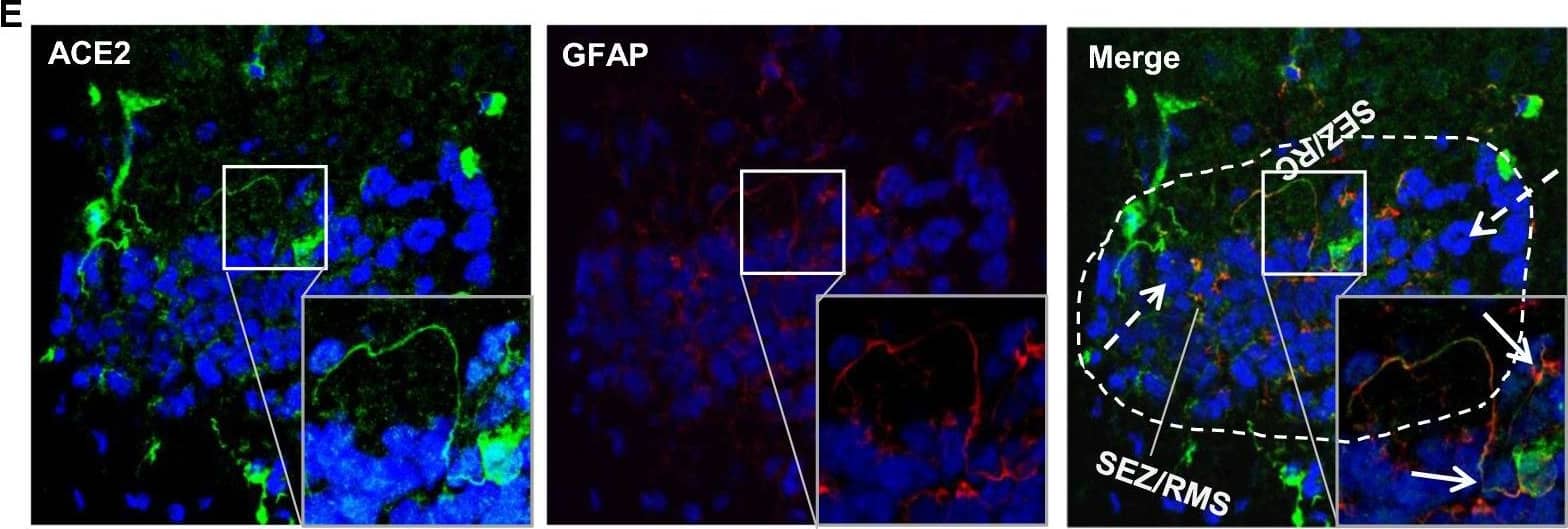 View Larger
View Larger
Detection of Mouse ACE-2 by Immunohistochemistry Distribution of ACE2 along the olfactory retrograde route. A Schematic elucidation of a gross view of mouse olfactory retrograde route comprised by OB surface, SEZ/RC, SEZ in the rostral migratory stream (SEZ/RMS) and SVZ of the LV (top panel) and a microscopic view of SEZ/RC and SEZ/RMS (bottom panel). B–E Co-staining of ACE2 (green) with astrocyte marker GFAP (red) in SEZ/RC and SEZ/RMS. B-C show the low magnitude images. D–E show the enlarged images of the indicated regions in B and C, respectively. DAPI (blue) was used for nuclear staining. Arrow: indicates astrocyte. Dash arrow: indicates neuroblast. Arrowhead: indicates microvessel. The dash lines in all images outline the border of SEZ/RC and SEZ/RMS, respectively. Image magnitude B-C: 20X, D-E: 100X Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35672716), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
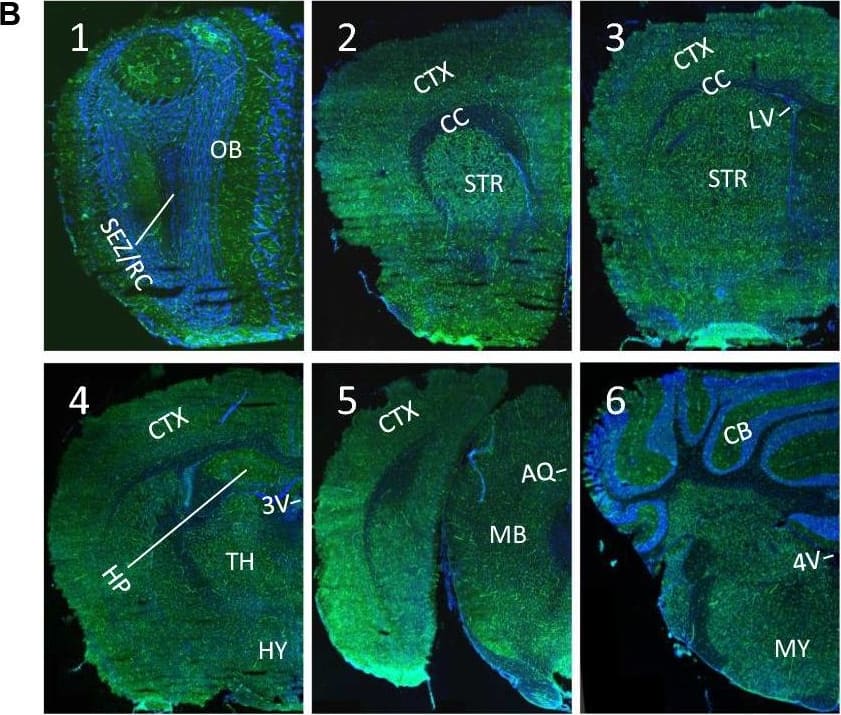
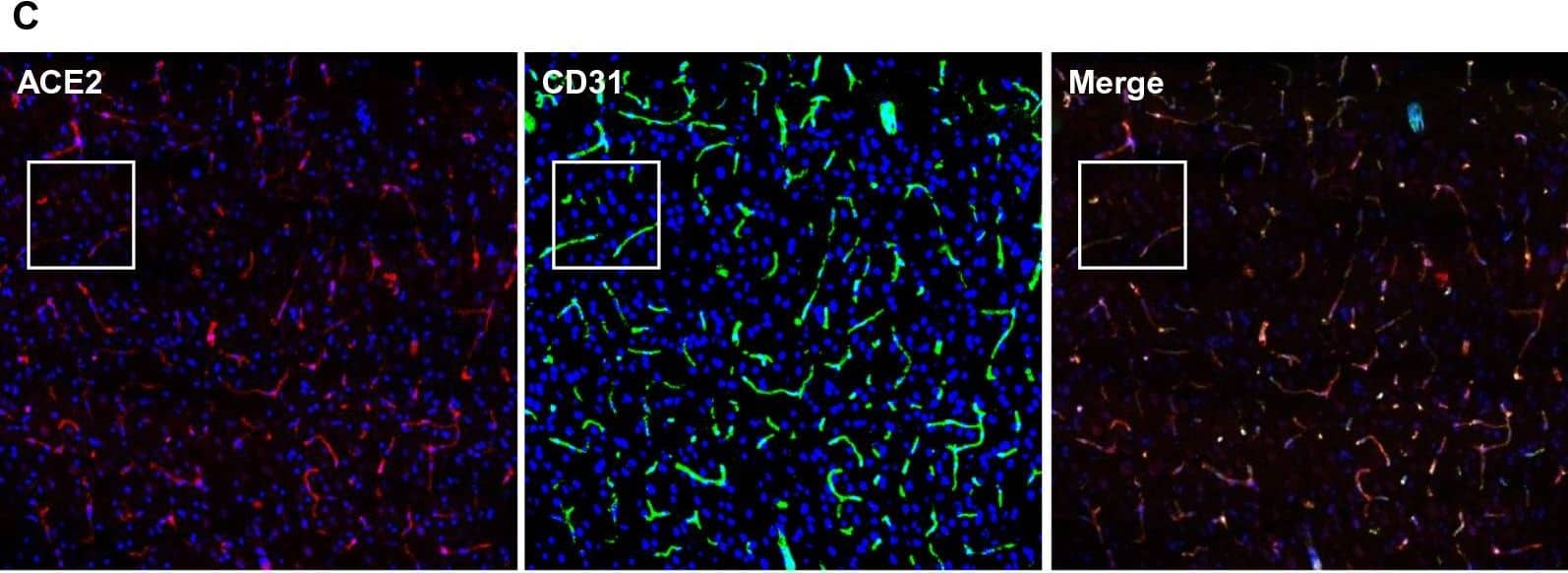
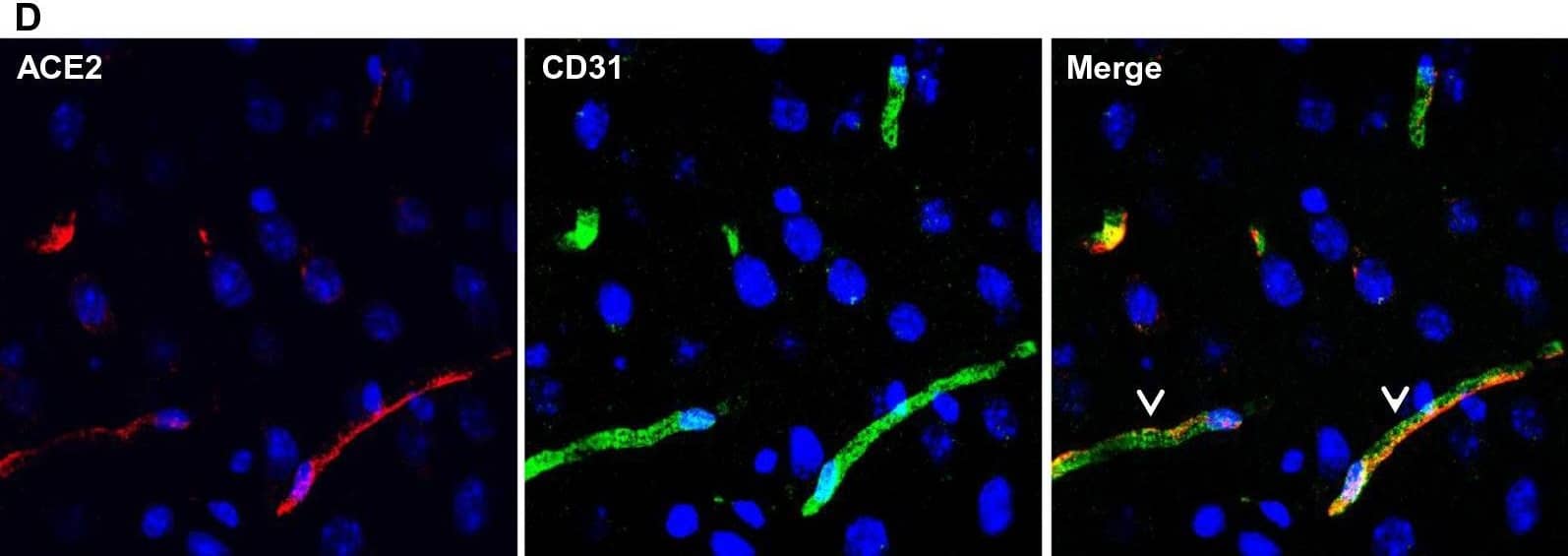
Detection of Mouse ACE-2 by Immunohistochemistry Ubiquitous expression of ACE2 in cerebral microvascular pericytes. A Schematic elucidation of sectioning plates and main brain regions of a mouse brain. OB: olfactory bulb, SEZ/RC: subependymal zone (SEZ) in the rhinocele, CTX: cerebral cortex, CC: corpus callosum, STR: striatum, LV: lateral ventricle, HP: hippocampus, TH: thalamus, 3 V: third ventricle, HY: hypothalamus, AQ: cerebral aqueduct, MB: midbrain, CB: cerebellum, 4 V: fourth ventricle, MY: medulla oblongata. B Scanning images show ACE2 (green) distribution in the hemisphere sections. C–F ACE2 (red) ubiquitously distributes in the cerebral microvessels labeled by endothelial cell marker CD31 (green) and pericyte marker PDGFR beta (green), respectively. ACE2 overlapped with pericyte marker PDGFR beta (F) but not endothelial cell marker CD31 (D). C and E showed the low magnitude images. D and F showed the enlarged images of the indicated regions in C and E, respectively. G ACE2 (red) does not distribute in large blood vessels marked by CD31 (green) but is detectable in the meninges wrapping HY. DAPI (blue) was used for nuclear staining. *: indicates large blood vessels. #: indicates meninges. Arrowhead: indicates microvessel. Image magnitude B: 10X; C, E and G: 20X; D and F: 100X Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35672716), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
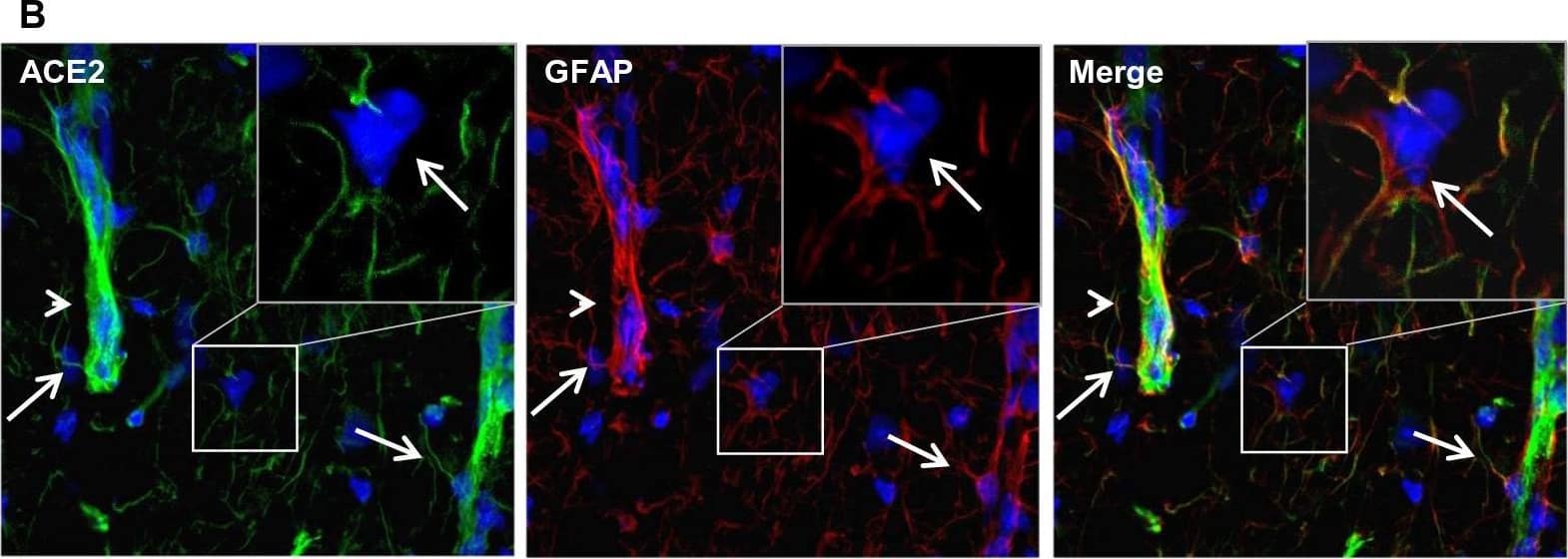
Detection of Mouse ACE-2 by Immunohistochemistry Differential distribution of ACE2 in brain parenchyma. A Uneven distribution of ACE2 (green) in indicated brain regions. Each insert shows a comparable high magnitude image of the corresponding region. B Enlarged images of the indicated region in the MY panel in A with co-staining of ACE2 (green) and astrocyte marker GFAP (red). C Co-staining of ACE2 (red) and neuron marker NeuN (green) in the MY. Cii-Civ show the high magnitude image of the comparable regions of Ci. D Quantitation of the relative expression of ACE2 in astrocytes and neurons. Values are presented as mean ± SEM. n = 60. ***: p < 0.001. DAPI (blue) was used for nuclear staining. Arrowhead: indicates microvessel. Arrow: indicates astrocyte. Dash arrow: indicates neuron. #indicates meninges wrapping MY. Image magnitude A and Ci: 20X, B and Cii-iv: 100X Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35672716), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse ACE-2 by Immunohistochemistry Ubiquitous expression of ACE2 in cerebral microvascular pericytes. A Schematic elucidation of sectioning plates and main brain regions of a mouse brain. OB: olfactory bulb, SEZ/RC: subependymal zone (SEZ) in the rhinocele, CTX: cerebral cortex, CC: corpus callosum, STR: striatum, LV: lateral ventricle, HP: hippocampus, TH: thalamus, 3 V: third ventricle, HY: hypothalamus, AQ: cerebral aqueduct, MB: midbrain, CB: cerebellum, 4 V: fourth ventricle, MY: medulla oblongata. B Scanning images show ACE2 (green) distribution in the hemisphere sections. C–F ACE2 (red) ubiquitously distributes in the cerebral microvessels labeled by endothelial cell marker CD31 (green) and pericyte marker PDGFR beta (green), respectively. ACE2 overlapped with pericyte marker PDGFR beta (F) but not endothelial cell marker CD31 (D). C and E showed the low magnitude images. D and F showed the enlarged images of the indicated regions in C and E, respectively. G ACE2 (red) does not distribute in large blood vessels marked by CD31 (green) but is detectable in the meninges wrapping HY. DAPI (blue) was used for nuclear staining. *: indicates large blood vessels. #: indicates meninges. Arrowhead: indicates microvessel. Image magnitude B: 10X; C, E and G: 20X; D and F: 100X Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35672716), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse ACE-2 by Immunohistochemistry Distribution of ACE2 along the olfactory retrograde route. A Schematic elucidation of a gross view of mouse olfactory retrograde route comprised by OB surface, SEZ/RC, SEZ in the rostral migratory stream (SEZ/RMS) and SVZ of the LV (top panel) and a microscopic view of SEZ/RC and SEZ/RMS (bottom panel). B–E Co-staining of ACE2 (green) with astrocyte marker GFAP (red) in SEZ/RC and SEZ/RMS. B-C show the low magnitude images. D–E show the enlarged images of the indicated regions in B and C, respectively. DAPI (blue) was used for nuclear staining. Arrow: indicates astrocyte. Dash arrow: indicates neuroblast. Arrowhead: indicates microvessel. The dash lines in all images outline the border of SEZ/RC and SEZ/RMS, respectively. Image magnitude B-C: 20X, D-E: 100X Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35672716), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse ACE-2 by Immunohistochemistry Ubiquitous expression of ACE2 in cerebral microvascular pericytes. A Schematic elucidation of sectioning plates and main brain regions of a mouse brain. OB: olfactory bulb, SEZ/RC: subependymal zone (SEZ) in the rhinocele, CTX: cerebral cortex, CC: corpus callosum, STR: striatum, LV: lateral ventricle, HP: hippocampus, TH: thalamus, 3 V: third ventricle, HY: hypothalamus, AQ: cerebral aqueduct, MB: midbrain, CB: cerebellum, 4 V: fourth ventricle, MY: medulla oblongata. B Scanning images show ACE2 (green) distribution in the hemisphere sections. C–F ACE2 (red) ubiquitously distributes in the cerebral microvessels labeled by endothelial cell marker CD31 (green) and pericyte marker PDGFR beta (green), respectively. ACE2 overlapped with pericyte marker PDGFR beta (F) but not endothelial cell marker CD31 (D). C and E showed the low magnitude images. D and F showed the enlarged images of the indicated regions in C and E, respectively. G ACE2 (red) does not distribute in large blood vessels marked by CD31 (green) but is detectable in the meninges wrapping HY. DAPI (blue) was used for nuclear staining. *: indicates large blood vessels. #: indicates meninges. Arrowhead: indicates microvessel. Image magnitude B: 10X; C, E and G: 20X; D and F: 100X Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35672716), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
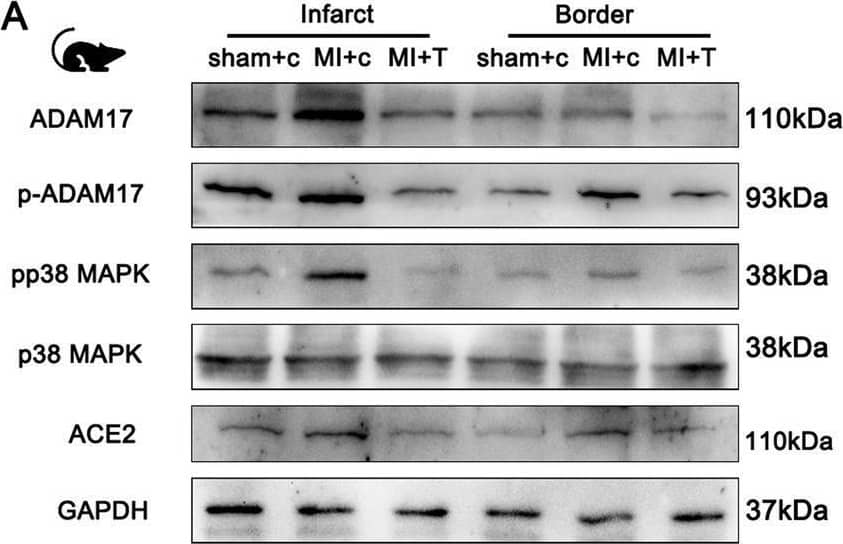
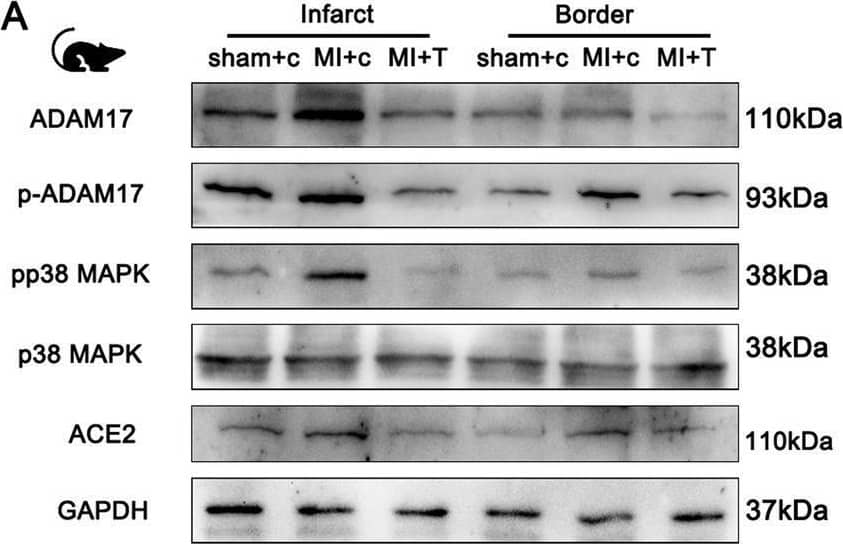
Detection of ACE-2 by Western Blot ADAM17 deletion reduces ACE2 shedding in post-MI mice. Knockout of ADAM17 in the myocardium of MI mice using TAPI-1, the same as before. A Western blot analysis of the expression of ADAM17, p-ADAM17, pP38MAPK, total P38MAPK, ACE2 protein in different position myocardium of 30 days post-MI mice. B Quantification of pP38MAPK levels in different position of myocardium. C Quantification of ACE2 levels in cardiomyocytes. D Detection of ACE2 activity in cardiomyocyte lysates by ACE2 activity fluorometric assay. E Detection of pADAM17 Thr735 expression in peripheral blood of patients with or without HF after MI. F Detection of ACE2 expression in peripheral blood of patients with or without HF after MI. N = 6 biological replicates. Data shown as mean ± SEM. Derived by two-sample t-test, *P < 0.05; **P < 0.01, ***P < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37046278), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
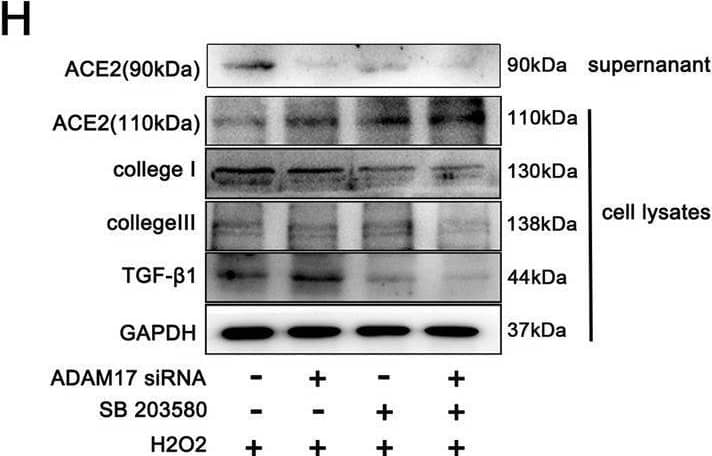
Detection of ACE-2 by Western Blot Activated P38MAPK induced phosphorylation of ADAM17 in injured cardiomyocyte. A–C Representative western blot (A) and quantitative results depicting the phosphorylation vs. total protein levels of P38 (B) and ERK1/2 (C) in H9C2 cardiomyocytes treated with 600 μM hydrogen peroxide for 12 h (n = 3). D Phosphorylated ADAM17 (p-ADAM17) was assessed by western blot with anti-phospho-ADAM17 (pThr735) antibody after transfection with ADAM17 siRNA or P38MAPK inhibitor SB203580 as indicated (n = 3). E Flow cytometric analysis of annexin V/PI double stained cardiomyocyte after treatment as indicated (right). Histogram indicating the percentage of later apoptosis cells in 4 groups (left) (n = 3). F The cell viability was detected by CCK8 assay (n = 4). G The live (green) and dead (red) cells were observed by calcein-AM/PI double staining kit after treatment as indicated (right). Histogram showing percentage of viable cells(left) (n = 3). H Protein levels of ACE2 (110 kDa), collagen I, collagen III TGF-beta 1 in cardiomyocytes and released ACE2 protein (90 kDa) in the supernatant were detected by western blotting(n = 3). I, J ACE2 activity in cardiomyocyte lysates (I) and cardiomyocyte supernatant (J) was detected by the ACE2 Activity Fluorometric Assay(n = 4). Data shown as mean ± SEM. Derived by two-sample t-test, *P < 0.05; **P < 0.01, ***P < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37046278), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of ACE-2 by Western Blot ADAM17 deletion reduces ACE2 shedding in post-MI mice. Knockout of ADAM17 in the myocardium of MI mice using TAPI-1, the same as before. A Western blot analysis of the expression of ADAM17, p-ADAM17, pP38MAPK, total P38MAPK, ACE2 protein in different position myocardium of 30 days post-MI mice. B Quantification of pP38MAPK levels in different position of myocardium. C Quantification of ACE2 levels in cardiomyocytes. D Detection of ACE2 activity in cardiomyocyte lysates by ACE2 activity fluorometric assay. E Detection of pADAM17 Thr735 expression in peripheral blood of patients with or without HF after MI. F Detection of ACE2 expression in peripheral blood of patients with or without HF after MI. N = 6 biological replicates. Data shown as mean ± SEM. Derived by two-sample t-test, *P < 0.05; **P < 0.01, ***P < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37046278), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
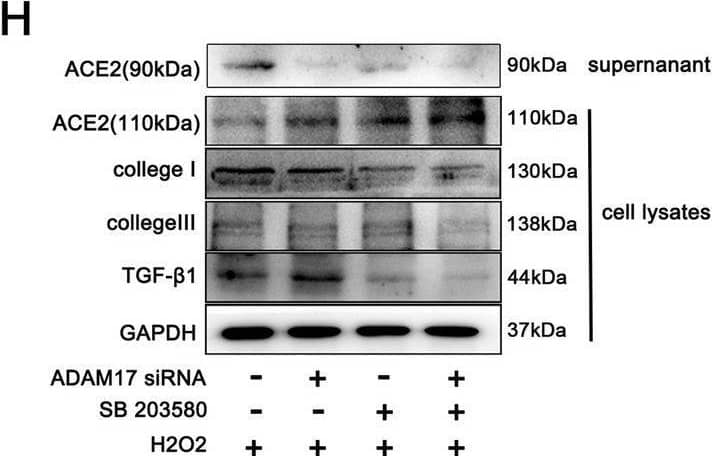
Detection of ACE-2 by Western Blot Activated P38MAPK induced phosphorylation of ADAM17 in injured cardiomyocyte. A–C Representative western blot (A) and quantitative results depicting the phosphorylation vs. total protein levels of P38 (B) and ERK1/2 (C) in H9C2 cardiomyocytes treated with 600 μM hydrogen peroxide for 12 h (n = 3). D Phosphorylated ADAM17 (p-ADAM17) was assessed by western blot with anti-phospho-ADAM17 (pThr735) antibody after transfection with ADAM17 siRNA or P38MAPK inhibitor SB203580 as indicated (n = 3). E Flow cytometric analysis of annexin V/PI double stained cardiomyocyte after treatment as indicated (right). Histogram indicating the percentage of later apoptosis cells in 4 groups (left) (n = 3). F The cell viability was detected by CCK8 assay (n = 4). G The live (green) and dead (red) cells were observed by calcein-AM/PI double staining kit after treatment as indicated (right). Histogram showing percentage of viable cells(left) (n = 3). H Protein levels of ACE2 (110 kDa), collagen I, collagen III TGF-beta 1 in cardiomyocytes and released ACE2 protein (90 kDa) in the supernatant were detected by western blotting(n = 3). I, J ACE2 activity in cardiomyocyte lysates (I) and cardiomyocyte supernatant (J) was detected by the ACE2 Activity Fluorometric Assay(n = 4). Data shown as mean ± SEM. Derived by two-sample t-test, *P < 0.05; **P < 0.01, ***P < 0.001 Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37046278), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
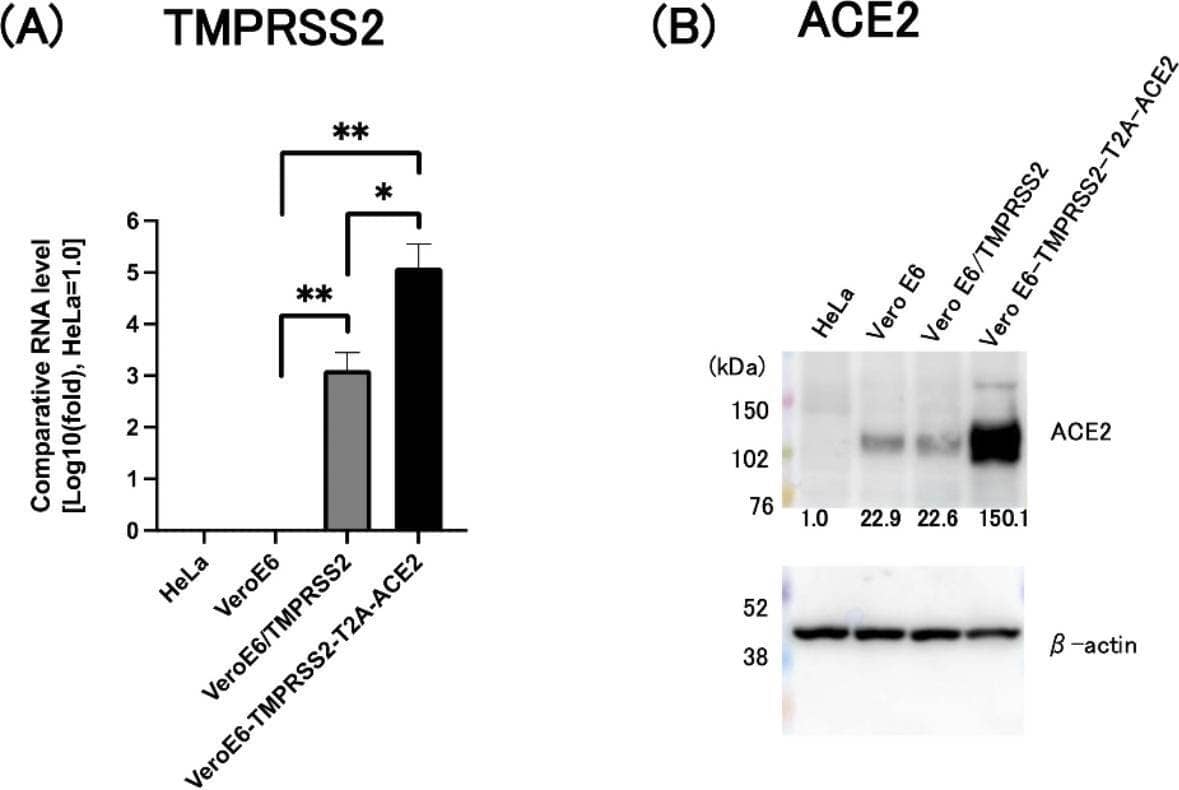
Detection of ACE-2 by Western Blot Increased expression of TMPRSS2 and ACE2 by Vero E6-TMPRSS2-T2A-ACE2 cells. (A) Analysis of TMPRSS2 mRNA levels by qRT-PCR. mRNA levels (-fold) in Vero E6, VeroE6/TMPRSS2, and Vero E6-TMPRSS2-T2A-ACE2 cells were compared with those in HeLa229 cells (set to 1.0). *: p < 0.005, **: p < 0.0001. (B) Western blot analysis with an anti-human ACE2 antibody was performed to detect ACE2 protein in HeLa229, Vero E6, VeroE6/TMPRSS2, and Vero E6-TMPRSS2-T2A-ACE2 cells. beta -actin was used as a loading control. The amount of ACE2 protein detected in each cells was normalized with the that of beta -actin and expressed as a fold change (the amount in HeLa cells was set to 1.0). The transferred membrane was cut prior to hybridization with anti-ACE2 or anti-beta -actin. The original of blots with membrane edges visible was shown in the Supplementary figure S4. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/39438626), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: ACE-2
ACE-2, also called ACEH (ACE homolog), is an integral membrane protein and a zinc metalloprotease of the ACE family that also includes somatic and germinal ACE (1). Human ACE-2 has about 40% amino acid identity to the N- and C-terminal domains of human somatic ACE. The predicted human ACE-2 protein sequence consists of 805 amino acids, including a N-terminal signal peptide, a single catalytic domain, a C-terminal membrane anchor, and a short cytoplasmic tail. ACE-2 cleaves angiotensins I and II as a carboxypeptidase. ACE-2 mRNA is found at high levels in testis, kidney, and heart and at moderate levels in colon, small intestine, and ovary. Classical ACE inhibitors such as captopril and lisinopril do not inhibit ACE-2 activity. Novel peptide inhibitors of ACE-2 do not inhibit ACE activity (2). Genetic data from Drosophila, mice and rats show that ACE-2 is an essential regulator of heart function in vivo (3).
ACE2 has been shown to be a functional receptor of the human coronaviruses SARS-CoV and SARS-CoV-2 (4, 5). This Human anti-ACE2 antibody (catalog # AF933) was used to block the variant SARS-CoV-2 and ACE2 interaction to elucidate viral transmission and potential therapeutic strategies. (5)
- Tipnis, S.R. et al. (2000) J. Biol. Chem. 275:33238.
- Crackower, M.A. et al. (2002) Nature 417:822.
- Huang, L. et al. (2003) J. Biol. Chem. 278:15532.
- Li, W. et al. (2003) Nature 426:450.
- Hoffmann, M. et al. (2020) Cell. DOI: 10.1016/j.cell.2020.02.052.
Product Datasheets
Citations for Human/Mouse/Rat/Hamster ACE-2 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
334
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Decrease in Angiotensin-Converting Enzyme activity but not concentration in plasma/lungs in COVID-19 patients offers clues for diagnosis/treatment
Authors: Henry Daniell, Smruti K. Nair, Yao Shi, Ping Wang, Kathleen T. Montone, Pamela A. Shaw et al.
Molecular Therapy - Methods & Clinical Development
-
The Upper Respiratory Tract of Felids Is Highly Susceptible to SARS-CoV-2 Infection
Authors: Nadine Krüger, Cheila Rocha, Sandra Runft, Johannes Krüger, Iris Färber, Federico Armando et al.
International Journal of Molecular Sciences
-
Characterization of CCoV-HuPn-2018 spike protein-mediated viral entry
Authors: Yongmei Liu, Danying Chen, Yuanyuan Wang, Xinglin Li, Yaruo Qiu, Mei Zheng et al.
J Virol
-
A CRISPR/Cas9 genetically engineered organoid biobank reveals essential host factors for coronaviruses
Authors: Joep Beumer, Maarten H. Geurts, Mart M. Lamers, Jens Puschhof, Jingshu Zhang, Jelte van der Vaart et al.
Nature Communications
-
Identification of potent inhibitors of SARS-CoV-2 infection by combined pharmacological evaluation and cellular network prioritization
Authors: J.J. Patten, Patrick T. Keiser, Deisy Morselli-Gysi, Giulia Menichetti, Hiroyuki Mori, Callie J. Donahue et al.
iScience
-
Long-COVID cognitive impairments and reproductive hormone deficits in men may stem from GnRH neuronal death
Authors: Sauve F, Nampoothiri S, Clarke SA et al.
EBioMedicine
-
Identification of FDA-approved bifonazole as a SARS-CoV-2 blocking agent following a bioreporter drug screen
Authors: Zaid Taha, Rozanne Arulanandam, Glib Maznyi, Elena Godbout, Madalina E. Carter-Timofte, Naziia Kurmasheva et al.
Molecular Therapy
-
ZMPSTE24 Regulates SARS-CoV-2 Spike Protein–enhanced Expression of Endothelial PAI-1
Authors: Mingming Han, Deepesh Pandey
American Journal of Respiratory Cell and Molecular Biology
-
Clinical and in Vitro Evidence against Placenta Infection at Term by Severe Acute Respiratory Syndrome Coronavirus 2
Authors: Arthur Colson, Christophe L. Depoix, Géraldine Dessilly, Pamela Baldin, Olivier Danhaive, Corinne Hubinont et al.
The American Journal of Pathology
-
ACE2 downregulation in olfactory mucosa: Eosinophilic rhinosinusitis as COVID‐19 protective factor?
Authors: Concepció Marin, Valeria Tubita, Cristóbal Langdon, Mireya Fuentes, María Jesús Rojas‐Lechuga, Antonio Valero et al.
Allergy
-
A Replication-Competent Vesicular Stomatitis Virus for Studies of SARS-CoV-2 Spike-Mediated Cell Entry and Its Inhibition
Authors: M. Eugenia Dieterle, Denise Haslwanter, Robert H. Bortz, Ariel S. Wirchnianski, Gorka Lasso, Olivia Vergnolle et al.
Cell Host & Microbe
-
Goblet Cell Hyperplasia Increases SARS-CoV-2 Infection in Chronic Obstructive Pulmonary Disease
Authors: Jaspreet Osan, Sattya N. Talukdar, Friederike Feldmann, Beth Ann DeMontigny, Kailey Jerome, Kristina L. Bailey et al.
Microbiology Spectrum
-
Broad and Differential Animal Angiotensin-Converting Enzyme 2 Receptor Usage by SARS-CoV-2
Authors: Xuesen Zhao, Danying Chen, Robert Szabla, Mei Zheng, Guoli Li, Pengcheng Du et al.
Journal of Virology
-
SARS-CoV-2 infection of human iPSC-derived cardiac cells predicts novel cytopathic features in hearts of COVID-19 patients
Authors: Juan A. Pérez-Bermejo, Serah Kang, Sarah J. Rockwood, Camille R. Simoneau, David A. Joy, Gokul N. Ramadoss et al.
bioRxiv
-
Carbohydrate-binding protein from stinging nettle as fusion inhibitor for SARS-CoV-2 variants of concern
Authors: Emiel Vanhulle, Thomas D’huys, Becky Provinciael, Joren Stroobants, Anita Camps, Sam Noppen et al.
Frontiers in Cellular and Infection Microbiology
-
TMEM106B is a receptor mediating ACE2-independent SARS-CoV-2 cell entry
Authors: Jim Baggen, Maarten Jacquemyn, Leentje Persoons, Els Vanstreels, Valerie E. Pye, Antoni G. Wrobel et al.
Cell
-
A cell-based assay for rapid assessment of ACE2 catalytic function
Authors: Warren M. Meyers, Ryan J. Hong, Wun Chey Sin, Christine S. Kim, Kurt Haas
Scientific Reports
-
ACE2-targeting monoclonal antibody as potent and broad-spectrum coronavirus blocker
Authors: Yuning Chen, Ya-Nan Zhang, Renhong Yan, Guifeng Wang, Yuanyuan Zhang, Zhe-Rui Zhang et al.
Signal Transduction and Targeted Therapy
-
CD169-mediated restrictive SARS-CoV-2 infection of macrophages induces pro-inflammatory responses
Authors: Sallieu Jalloh, Judith Olejnik, Jacob Berrigan, Annuurun Nisa, Ellen L Suder, Hisashi Akiyama et al.
bioRxiv
-
The RNA Interference Effector Protein Argonaute 2 Functions as a Restriction Factor Against SARS-CoV-2
Authors: Joaquin Lopez-Orozco, Nawell Fayad, Juveriya Qamar Qamar Khan, Alberto Felix-Lopez, Mohamed Elaish, Megha Rohamare et al.
Journal of Molecular Biology
-
Nonproductive exposure of PBMCs to SARS‐CoV ‐2 induces cell‐intrinsic innate immune responses
Authors: Julia Kazmierski, Kirstin Friedmann, Dylan Postmus, Jackson Emanuel, Cornelius Fischer, Jenny Jansen et al.
Molecular Systems Biology
-
Unbiased interrogation of memory B cells from convalescent COVID-19 patients reveals a broad antiviral humoral response targeting SARS-CoV-2 antigens beyond the spike protein
Authors: Jillian M. DiMuzio, Baron C. Heimbach, Raymond J. Howanski, John P. Dowling, Nirja B. Patel, Noeleya Henriquez et al.
Vaccine: X
-
A novel method for high-throughput screening to quantify antiviral activity against viruses that induce limited CPE
Authors: Dirk Jochmans, Pieter Leyssen, Johan Neyts
Journal of Virological Methods
-
Sec61 Inhibitor Apratoxin S4 Potently Inhibits SARS-CoV-2 and Exhibits Broad-Spectrum Antiviral Activity
Authors: Marie O. Pohl, Laura Martin-Sancho, Ranjala Ratnayake, Kris M. White, Laura Riva, Qi-Yin Chen et al.
ACS Infectious Diseases
-
SARS-CoV-2 Infects Endothelial Cells In Vivo and In Vitro
Authors: Fengming Liu, Kun Han, Robert Blair, Kornelia Kenst, Zhongnan Qin, Berin Upcin et al.
Frontiers in Cellular and Infection Microbiology
-
Human embryonic stem cell-derived cardiomyocyte platform screens inhibitors of SARS-CoV-2 infection
Authors: Thomas L. Williams, Maria T. Colzani, Robyn G. C. Macrae, Emma L. Robinson, Stuart Bloor, Edward J. D. Greenwood et al.
Communications Biology
-
Species-Specific Molecular Barriers to SARS-CoV-2 Replication in Bat Cells
Authors: Sophie-Marie Aicher, Felix Streicher, Maxime Chazal, Delphine Planas, Dongsheng Luo, Julian Buchrieser et al.
Journal of Virology
-
SARS-CoV-2 Poorly Replicates in Cells of the Human Blood-Brain Barrier Without Associated Deleterious Effects
Authors: Orianne Constant, Jonathan Barthelemy, Karine Bolloré, Edouard Tuaillon, Fabien Gosselet, Christine Chable-Bessia et al.
Frontiers in Immunology
-
Low-Density Lipoprotein Receptor (LDLR) Is Involved in Internalization of Lentiviral Particles Pseudotyped with SARS-CoV-2 Spike Protein in Ocular Cells
Authors: Uppal S, Postnikova O, Villasmil R et al.
International journal of molecular sciences
-
Quinolines-Based SARS-CoV-2 3CLpro and RdRp Inhibitors and Spike-RBD-ACE2 Inhibitor for Drug-Repurposing Against COVID-19: An in silico Analysis
Authors: Rajaiah Alexpandi, Joelma Freire De Mesquita, Shunmugiah Karutha Pandian, Arumugam Veera Ravi
Frontiers in Microbiology
-
LY6E impairs coronavirus fusion and confers immune control of viral disease
Authors: Stephanie Pfaender, Katrina B. Mar, Eleftherios Michailidis, Annika Kratzel, Ian N. Boys, Philip V’kovski et al.
Nature Microbiology
-
ACE2 overexpression inhibits acquired platinum resistance-induced tumor angiogenesis in NSCLC
Oncol Rep, 2016-07-22;36(3):1403-10.
-
SuPAR mediates viral response proteinuria by rapidly changing podocyte function
Authors: Wei, C;Datta, PK;Siegerist, F;Li, J;Yashwanth, S;Koh, KH;Kriho, NW;Ismail, A;Luo, S;Fischer, T;Amber, KT;Cimbaluk, D;Landay, A;Endlich, N;Rappaport, J;Michigan Medicine COVID−19 Investigators, ;Hayek, SS;Reiser, J;
Nature communications
-
Development of an efficient reproducible cell-cell transmission assay for rapid quantification of SARS-CoV-2 spike interaction with hACE2
Authors: George Ssenyange, Maya Kerfoot, Min Zhao, Shelli Farhadian, Sidi Chen, Lei Peng et al.
Cell Reports Methods
-
VE607 stabilizes SARS-CoV-2 Spike in the “RBD-up” conformation and inhibits viral entry
Authors: Shilei Ding, Irfan Ullah, Shang Yu Gong, Jonathan R. Grover, Mohammadjavad Mohammadi, Yaozong Chen et al.
iScience
-
SARS-CoV-2 disrupts proximal elements in the JAK-STAT pathway
Authors: Chen Dy, Khan N, Close Bj Et Al.
Journal of virology
-
Low-density lipoprotein receptor–related protein 1 (LRP1) as an auxiliary host factor for RNA viruses
Authors: Stephanie Devignot, Tim Wai Sha, Thomas R Burkard, Patrick Schmerer, Astrid Hagelkruys, Ali Mirazimi et al.
Life Science Alliance
-
SARS-CoV-2 Permissive glioblastoma cell line for high throughput antiviral screening
Authors: Emiel Vanhulle, Joren Stroobants, Becky Provinciael, Anita Camps, Sam Noppen, Piet Maes et al.
Antiviral Research
-
Discovery of Small Anti‐ACE2 Peptides to Inhibit SARS‐CoV‐2 Infectivity
Authors: Pratik Adhikary, Sashi Kandel, Umar‐Farouk Mamani, Bahaa Mustafa, Siyuan Hao, Jianming Qiu et al.
Advanced Therapeutics
-
Influenza virus infection increases ACE2 expression and shedding in human small airway epithelial cells
Authors: Kelly S. Schweitzer, Taylor Crue, Jordan M. Nall, Daniel Foster, Satria Sajuthi, Kelly A. Correll et al.
European Respiratory Journal
-
SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity
Authors: Chihiro Motozono, Mako Toyoda, Jiri Zahradnik, Akatsuki Saito, Hesham Nasser, Toong Seng Tan et al.
Cell Host & Microbe
-
Human iPSC-Derived Cardiomyocytes Are Susceptible to SARS-CoV-2 Infection
Authors: Arun Sharma, Gustavo Garcia, Yizhou Wang, Jasmine T. Plummer, Kouki Morizono, Vaithilingaraja Arumugaswami et al.
Cell Reports Medicine
-
Potential role of astrocyte angiotensin converting enzyme 2 in the neural transmission of COVID-19 and a neuroinflammatory state induced by smoking and vaping
Authors: Zhang Y, Archie SR, Ghanwatkar Y et al.
Fluids and Barriers of the CNS
-
ADAM10 and ADAM17 promote SARS‐CoV‐2 cell entry and spike protein‐mediated lung cell fusion
Authors: Georg Jocher, Vincent Grass, Sarah K Tschirner, Lydia Riepler, Stephan Breimann, Tuğberk Kaya et al.
EMBO reports
-
ACE2-Independent Interaction of SARS-CoV-2 Spike Protein with Human Epithelial Cells Is Inhibited by Unfractionated Heparin
Authors: Lynda J. Partridge, Lucy Urwin, Martin J. H. Nicklin, David C. James, Luke R. Green, Peter N. Monk
Cells
-
IMMUNO-COV v2.0: Development and Validation of a High-Throughput Clinical Assay for Measuring SARS-CoV-2-Neutralizing Antibody Titers
Authors: Rianna Vandergaast, Timothy Carey, Samantha Reiter, Chase Lathrum, Patrycja Lech, Clement Gnanadurai et al.
mSphere
-
Increased colonic expression of ACE2 associates with poor prognosis in Crohn’s disease
Authors: Takahiko Toyonaga, Kenza C. Araba, Meaghan M. Kennedy, Benjamin P. Keith, Elisabeth A. Wolber, Caroline Beasley et al.
Scientific Reports
-
SARS-CoV-2 infects an upper airway model derived from induced pluripotent stem cells
Authors: Ivo Djidrovski, Maria Georgiou, Grant L. Hughes, Edward I. Patterson, Aitor Casas-Sanchez, Shaun H. Pennington et al.
Stem Cells
-
A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids
Authors: L Yang, Y Han, BE Nilsson-Pa, V Gupta, P Wang, X Duan, X Tang, J Zhu, Z Zhao, F Jaffré, T Zhang, TW Kim, O Harschnitz, D Redmond, S Houghton, C Liu, A Naji, G Ciceri, S Guttikonda, Y Bram, DT Nguyen, M Cioffi, V Chandar, DA Hoagland, Y Huang, J Xiang, H Wang, D Lyden, A Borczuk, HJ Chen, L Studer, FC Pan, DD Ho, BR tenOever, T Evans, RE Schwartz, S Chen
Cell Stem Cell, 2020-06-19;27(1):125-136.e7.
-
Overcoming Culture Restriction for SARS-CoV-2 in Human Cells Facilitates the Screening of Compounds Inhibiting Viral Replication
Authors: Santseharay Ramirez, Carlota Fernandez-Antunez, Andrea Galli, Alexander Underwood, Long V. Pham, Line A. Ryberg et al.
Antimicrobial Agents and Chemotherapy
-
Fetal brain vulnerability to SARS-CoV-2 infection
Authors: McMahon CL, Castro J, Silvas J et al.
Brain, behavior, and immunity
-
Heparan Sulfate Facilitates Spike Protein-Mediated SARS-CoV-2 Host Cell Invasion and Contributes to Increased Infection of SARS-CoV-2 G614 Mutant and in Lung Cancer
Authors: Jingwen Yue, Weihua Jin, Hua Yang, John Faulkner, Xuehong Song, Hong Qiu et al.
Frontiers in Molecular Biosciences
-
Immunosuppressant Treatment in Rheumatic Musculoskeletal Diseases Does Not Inhibit Elicitation of Humoral Response to SARS-CoV-2 Infection and Preserves Effector Immune Cell Populations
Authors: Andrea Favalli, Ennio Giulio Favalli, Andrea Gobbini, Elena Zagato, Mauro Bombaci, Gabriella Maioli et al.
Frontiers in Immunology
-
Viral infiltration of pancreatic islets in patients with COVID-19
Authors: C Steenblock, S Richter, I Berger, M Barovic, J Schmid, U Schubert, N Jarzebska, A von Mässen, A Linkermann, A Schürmann, J Pablik, T Dienemann, K Evert, RN Rodionov, NY Semenova, VA Zinserling, RR Gainetdino, G Baretton, D Lindemann, M Solimena, B Ludwig, SR Bornstein
Nature Communications, 2021-06-10;12(1):3534.
-
The Priming Potential of Interferon Lambda-1 for Antiviral Defense in the Oral Mucosa
Authors: Yosuke Shikama, Mie Kurosawa, Masae Furukawa, Yasusei Kudo, Naozumi Ishimaru, Kenji Matsushita
Inflammation
-
Biomimetic SARS-CoV-2 Spike Protein Nanoparticles
Authors: Alvin Phan, Hugo Avila, J. Andrew MacKay
Biomacromolecules
-
Human Immunodeficiency Viruses Pseudotyped with SARS-CoV-2 Spike Proteins Infect a Broad Spectrum of Human Cell Lines through Multiple Entry Mechanisms
Authors: C Xu, A Wang, K Geng, W Honnen, X Wang, N Bruiners, S Singh, F Ferrara, S D'Angelo, ARM Bradbury, ML Gennaro, D Liu, A Pinter, TL Chang
Viruses, 2021-05-21;13(6):.
-
Synthetic carbohydrate-binding agents neutralize SARS-CoV-2 by inhibiting binding of the spike protein to ACE2
Authors: Oscar Francesconi, Lorena Donnici, Marco Fragai, Elisa Pesce, Mauro Bombaci, Alessandra Fasciani et al.
iScience
-
Cell culture systems for isolation of SARS-CoV-2 clinical isolates and generation of recombinant virus
Authors: Da-Yuan Chen, Jacquelyn Turcinovic, Shuchen Feng, Devin J. Kenney, Chue Vin Chin, Manish C. Choudhary et al.
iScience
-
ACE2‐enriched extracellular vesicles enhance infectivity of live SARS‐CoV‐2 virus
Authors: Sze Keong Tey, Hoiyan Lam, Samuel Wan Ki Wong, Hanjun Zhao, Kelvin Kai‐Wang To, Judy Wai Ping Yam
Journal of Extracellular Vesicles
-
Structural insights of a highly potent pan-neutralizing SARS-CoV-2 human monoclonal antibody
Authors: Jonathan L. Torres, Gabriel Ozorowski, Emanuele Andreano, Hejun Liu, Jeffrey Copps, Giulia Piccini et al.
Proceedings of the National Academy of Sciences
-
SARS-CoV-2 and SARS-CoV Spike-Mediated Cell-Cell Fusion Differ in Their Requirements for Receptor Expression and Proteolytic Activation
Authors: Hornich BF, Grobkopf AK, Schlagowski S et al.
Journal of Virology
-
Insect cell expression and purification of recombinant SARS‐COV‐2 spike proteins that demonstrate ACE2 binding
Authors: Lucas R. Struble, Audrey L. Smith, William E. Lutz, Gabrielle Grubbs, Satish Sagar, Kenneth W. Bayles et al.
Protein Science
-
Interferon-alpha or -beta facilitates SARS-CoV-2 pulmonary vascular infection by inducing ACE2
Authors: Timothy Klouda, Yuan Hao, Hyunbum Kim, Jiwon Kim, Judith Olejnik, Adam J. Hume et al.
Angiogenesis
-
Inhibition of SARS-CoV-2 replication in the lung with siRNA/VIPER polyplexes
Authors: Domizia Baldassi, Shubhankar Ambike, Martin Feuerherd, Cho-Chin Cheng, David J. Peeler, Daniel P. Feldmann et al.
Journal of Controlled Release
-
Susceptibility to SARS-CoV-2 of Cell Lines and Substrates Commonly Used to Diagnose and Isolate Influenza and Other Viruses
Authors: Li Wang, Xiaoyu Fan, Gaston Bonenfant, Dan Cui, Jaber Hossain, Nannan Jiang et al.
Emerging Infectious Diseases
-
SARS-CoV-2 infection induces beta cell transdifferentiation
Authors: Tang X, Uhl S, Zhang T et al.
Cell Metabolism
-
A tissue- and gender-specific regulation of the SARS-CoV-2 receptor ACE2 by p53 in pigs
Authors: Yue Zhang, Guanglin Niu, Tatiana Flisikowska, Angelika Schnieke, Krzysztof Flisikowski
Biochemical and Biophysical Research Communications
-
SARS-CoV-2 induces human plasmacytoid predendritic cell diversification via UNC93B and IRAK4
Authors: Fanny Onodi, Lucie Bonnet-Madin, Laurent Meertens, Léa Karpf, Justine Poirot, Shen-Ying Zhang et al.
Journal of Experimental Medicine
-
Term Human Placental Trophoblasts Express SARS-CoV-2 Entry Factors ACE2, TMPRSS2, and Furin
Authors: Yingshi Ouyang, Tarique Bagalkot, Wendy Fitzgerald, Elena Sadovsky, Tianjiao Chu, Ana Martínez-Marchal et al.
mSphere
-
SARS-CoV-2 Infection of Human Neurons Is TMPRSS2 Independent, Requires Endosomal Cell Entry, and Can Be Blocked by Inhibitors of Host Phosphoinositol-5 Kinase
Authors: Pinja Kettunen, Angelina Lesnikova, Noora Räsänen, Ravi Ojha, Leena Palmunen, Markku Laakso et al.
Journal of Virology
-
ORF10–Cullin-2–ZYG11B complex is not required for SARS-CoV-2 infection
Authors: Elijah L. Mena, Callie J. Donahue, Laura Pontano Vaites, Jie Li, Gergely Rona, Colin O’Leary et al.
Proceedings of the National Academy of Sciences
-
Systematic analysis of SARS-CoV-2 infection of an ACE2-negative human airway cell
Authors: Maritza Puray-Chavez, Kyle M. LaPak, Travis P. Schrank, Jennifer L. Elliott, Dhaval P. Bhatt, Megan J. Agajanian et al.
bioRxiv
-
Immediate myeloid depot for SARS-CoV-2 in the human lung
Authors: Mélia Magnen, Ran You, Arjun A. Rao, Ryan T. Davis, Lauren Rodriguez, Camille R. Simoneau et al.
Research Square
-
A replication-competent vesicular stomatitis virus for studies of SARS-CoV-2 spike-mediated cell entry and its inhibition
Authors: M. Eugenia Dieterle, Denise Haslwanter, Robert H. Bortz, Ariel S. Wirchnianski, Gorka Lasso, Olivia Vergnolle et al.
bioRxiv
-
SARS-CoV-2 Employ BSG/CD147 and ACE2 Receptors to Directly Infect Human Induced Pluripotent Stem Cell-Derived Kidney Podocytes
Authors: Titilola D. Kalejaiye, Rohan Bhattacharya, Morgan A. Burt, Tatianna Travieso, Arinze E. Okafor, Xingrui Mou et al.
Frontiers in Cell and Developmental Biology
-
Identification of Human Host Substrates of the SARS-CoV-2 Mpro and PLpro Using Subtiligase N-Terminomics
Authors: Shu Y. Luo, Eman W. Moussa, Joaquin Lopez-Orozco, Alberto Felix-Lopez, Ray Ishida, Nawell Fayad et al.
ACS Infectious Diseases
-
CDK4/6 inhibitor palbociclib promotes SARS-CoV-2 cell entry by down-regulating SKP2 dependent ACE2 degradation
Authors: Yingzi Xiao, Ying Yan, Le Chang, Huimin Ji, Huizhen Sun, Shi Song et al.
Antiviral Research
-
Histology and cytokine levels in hepatic injury accompanying a case of non-severe COVID-19
Authors: Hidetaka Matsuda, Takuto Nosaka, Katsushi Hiramatsu, Kazuto Takahashi, Tatsushi Naito, Kazuya Ofuji et al.
Clinical Journal of Gastroenterology
-
Acute Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Pregnancy Is Associated with Placental Angiotensin-Converting Enzyme 2 Shedding
Authors: Elizabeth S. Taglauer, Elisha M. Wachman, Lillian Juttukonda, Timothy Klouda, Jiwon Kim, Qiong Wang et al.
The American Journal of Pathology
-
Protocols for SARS-CoV-2 infection in primary ocular cells and eye organoids
Authors: Eriksen A, Moller R, Markovoz B et al.
STAR Protocols
-
SARS‐CoV‐2 infection activates dendritic cells via cytosolic receptors rather than extracellular TLRs
Authors: Lieve E.H. van der Donk, Julia Eder, John L. van Hamme, Philip J. M. Brouwer, Mitch Brinkkemper, Ad C. van Nuenen et al.
European Journal of Immunology
-
BET inhibition blocks inflammation-induced cardiac dysfunction and SARS-CoV-2 infection
Authors: Richard J. Mills, Sean J. Humphrey, Patrick R.J. Fortuna, Mary Lor, Simon R. Foster, Gregory A. Quaife-Ryan et al.
Cell
-
The angiotensin-converting enzyme 2 in tumor growth and tumor-associated angiogenesis in non-small cell lung cancer
Authors: Yun Feng, Huanying Wan, Jialin Liu, Ruifeng Zhang, Qinyun Ma, Bing Han et al.
Oncology Reports
-
Severe Acute Respiratory Syndrome Coronavirus 2–Induced Immune Activation and Death of Monocyte-Derived Human Macrophages and Dendritic Cells
Authors: Jian Zheng, Yuhang Wang, Kun Li, David K Meyerholz, Chantal Allamargot, Stanley Perlman
The Journal of Infectious Diseases
-
Molecular consequences of SARS-CoV-2 liver tropism
Authors: Nicola Wanner, Geoffroy Andrieux, Pau Badia-i-Mompel, Carolin Edler, Susanne Pfefferle, Maja T. Lindenmeyer et al.
Nature Metabolism
-
Selection and Validation of siRNAs Preventing Uptake and Replication of SARS-CoV-2
Authors: Maik Friedrich, Gabriele Pfeifer, Stefanie Binder, Achim Aigner, Philippe Vollmer Barbosa, Gustavo R. Makert et al.
Frontiers in Bioengineering and Biotechnology
-
Exploring Zebrafish Larvae as a COVID-19 Model: Probable Abortive SARS-CoV-2 Replication in the Swim Bladder
Authors: Valerio Laghi, Veronica Rezelj, Laurent Boucontet, Maxence Frétaud, Bruno Da Costa, Pierre Boudinot et al.
Frontiers in Cellular and Infection Microbiology
-
ACE2-independent infection of T lymphocytes by SARS-CoV-2
Authors: Xu-Rui Shen, Rong Geng, Qian Li, Ying Chen, Shu-Fen Li, Qi Wang et al.
Signal Transduction and Targeted Therapy
-
Limited extent and consequences of pancreatic SARS-CoV-2 infection
Authors: Verena van der Heide, Sonia Jangra, Phillip Cohen, Raveen Rathnasinghe, Sadaf Aslam, Teresa Aydillo et al.
Cell Reports
-
Therapeutic activity of an inhaled potent SARS-CoV-2 neutralizing human monoclonal antibody in hamsters
Authors: Michael S. Piepenbrink, Jun-Gyu Park, Fatai S. Oladunni, Ashlesha Deshpande, Madhubanti Basu, Sanghita Sarkar et al.
Cell Reports Medicine
-
Neuroinvasion of SARS-CoV-2 in human and mouse brain
Authors: Song E, Zhang C, Israelow B et al.
Journal of Experimental Medicine
-
Integration of biomimetic organoid-on-chip and 2D models advances the mechanistic Understanding of STEAP3-mediated regulation in intestinal viral infection
Authors: Chen, YW;Chiang, HJ;Liu, KT;Kao, CW;Xie, SR;Su, CM;Shih, YY;
Scientific reports
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Western Blot, Immunocytochemistry -
Spike substitutions E484D, P812R and Q954H mediate ACE2-independent entry of SARS-CoV-2 across different cell lines
Authors: Vizgirda, G;Underwood, AP;Fahnøe, U;Weis, N;Ramirez, S;Bukh, J;
PloS one
Species: Human, Primate - Chlorocebus aethiops (African Green Monkey)
Sample Types: Whole Cells
Applications: Neutralization -
Coronavirus protein interaction mapping in bat and human cells identifies molecular and genetic switches for immune evasion and replication
Authors: Batra, J;Rutkowska, M;Zhou, Y;Ye, C;Adavikolanu, R;Young, JM;Anand, D;Verma, S;Gordon, M;Malpotra, S;Cupic, A;Kehrer, T;Moen, JM;Winters, DM;Rojc, A;Mena, I;Aslam, S;Martinez-Romero, C;Conde Viñas, I;Khalil, Z;Farrugia, K;Banerjee, A;Tussia-Cohen, D;Santos, MD;Maji, S;Muralidharan, M;Foussard, H;Chen, IP;Fuchs, R;Felipe, CS;Zuliani-Alvarez, L;Choudhury, P;Obernier, K;Gracias, S;Suryawanshi, R;Ibáñez, C;Juste, J;Pache, L;Taha, TY;Jouvenet, N;Verba, KA;Fraser, JS;Demeret, C;Stroud, RM;van Bakel, H;Ott, M;Hagai, T;Polacco, B;Swaney, DL;Echeverria, I;Bouhaddou, M;Eckhardt, M;Malik, HS;Martinez-Sobrido, L;Miorin, L;García-Sastre, A;Krogan, NJ;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Synergistic activation of bat SARS-like coronaviruses spike protein by elastase and TMPRSS2
Authors: Yamamoto, Y;Inoue, T;Sugiyama, N;Furukawa, M;Sato, K;Onodera, T;Takahashi, Y;Wakita, T;Fukasawa, M;Noguchi, K;
Scientific reports
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Pathology-oriented multiplexing enables integrative disease mapping
Authors: Kuehl, M;Okabayashi, Y;Wong, MN;Gernhold, L;Gut, G;Kaiser, N;Schwerk, M;Gräfe, SK;Ma, FY;Tanevski, J;Schäfer, PSL;Mezher, S;Sarabia Del Castillo, J;Goldbeck-Strieder, T;Zolotareva, O;Hartung, M;Delgado Chaves, FM;Klinkert, L;Gnirck, AC;Spehr, M;Fleck, D;Joodaki, M;Parra, V;Shaigan, M;Diebold, M;Prinz, M;Kranz, J;Kux, JM;Braun, F;Kretz, O;Wu, H;Grahammer, F;Heins, S;Zimmermann, M;Haas, F;Kylies, D;Wanner, N;Czogalla, J;Dumoulin, B;Zolotarev, N;Lindenmeyer, M;Karlson, P;Nyengaard, JR;Sebode, M;Weidemann, S;Wiech, T;Groene, HJ;Tomas, NM;Meyer-Schwesinger, C;Kuppe, C;Kramann, R;Karras, A;Bruneval, P;Tharaux, PL;Pastene, D;Yard, B;Schaub, JA;McCown, PJ;Pyle, L;Choi, YJ;Yokoo, T;Baumbach, J;Sáez, PJ;Costa, I;Turner, JE;Hodgin, JB;Saez-Rodriguez, J;Huber, TB;Bjornstad, P;Kretzler, M;Lenoir, O;Nikolic-Paterson, DJ;Pelkmans, L;Bonn, S;Puelles, VG;
Nature
Species: Mouse
Sample Types: Whole Tissue
Applications: IF/IHC -
Starve a cold or feed a fever? Identifying cellular metabolic changes following infection and exposure to SARS-CoV-2
Authors: Loveday, EK;Welhaven, H;Erdogan, AE;Hain, KS;Domanico, LF;Chang, CB;June, RK;Taylor, MP;
PloS one
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Characterizing neuroinvasion and neuropathology of SARS-CoV-2 by using AC70 human ACE2 transgenic mice
Authors: Hsu, JC;Saenkham-Huntsinger, P;Huang, P;Octaviani, CP;Drelich, AK;Peng, BH;Tseng, CK;
Frontiers in microbiology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Immediate myeloid depot for SARS-CoV-2 in the human lung
Authors: Magnen, M;You, R;Rao, AA;Davis, RT;Rodriguez, L;Bernard, O;Simoneau, CR;Hysenaj, L;Hu, KH;Maishan, M;Conrad, C;Gbenedio, OM;Samad, B;Consortium, TUC;Love, C;Woodruff, PG;Erle, DJ;Hendrickson, CM;Calfee, CS;Matthay, MA;Roose, JP;Sil, A;Ott, M;Langelier, CR;Krummel, MF;Looney, MR;
Science advances
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Proteolytic cleavage and inactivation of the TRMT1 tRNA modification enzyme by SARS-CoV-2 main protease
Authors: Zhang, K;Eldin, P;Ciesla, JH;Briant, L;Lentini, JM;Ramos, J;Cobb, J;Munger, J;Fu, D;
eLife
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Murine alveolar macrophages rapidly accumulate intranasally administered SARS-CoV-2 Spike protein leading to neutrophil recruitment and damage
Authors: Park, C;Hwang, IY;Yan, SL;Vimonpatranon, S;Wei, D;Van Ryk, D;Girard, A;Cicala, C;Arthos, J;Kehrl, JH;
eLife
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, Neutralization -
A Gamma-adapted subunit vaccine induces broadly neutralizing antibodies against SARS-CoV-2 variants and protects mice from infection
Authors: Coria, LM;Rodriguez, JM;Demaria, A;Bruno, LA;Medrano, MR;Castro, CP;Castro, EF;Del Priore, SA;Hernando Insua, AC;Kaufmann, IG;Saposnik, LM;Stone, WB;Prado, L;Notaro, US;Amweg, AN;Diaz, PU;Avaro, M;Ortega, H;Ceballos, A;Krum, V;Zurvarra, FM;Sidabra, JE;Drehe, I;Baqué, JA;Li Causi, M;De Nichilo, AV;Payes, CJ;Southard, T;Vega, JC;Auguste, AJ;Álvarez, DE;Flo, JM;Pasquevich, KA;Cassataro, J;
Nature communications
Species: Human
Sample Types: Recombinant Protein
Applications: ELISA Capture -
Stabilization of the Metastable Pre-Fusion Conformation of the SARS-CoV-2 Spike Glycoprotein through N-Linked Glycosylation of the S2 Subunit
Authors: Zan, F;Zhou, Y;Chen, T;Chen, Y;Mu, Z;Qian, Z;Ou, X;
Viruses
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
A basally active cGAS-STING pathway limits SARS-CoV-2 replication in a subset of ACE2 positive airway cell models
Authors: Puray-Chavez, M;LaPak, KM;Jasuja, R;Pan, J;Xu, J;Eschbach, JE;Mohammed, S;Lawson, DQ;Wang, Q;Brody, SL;Major, MB;Goldfarb, D;Kutluay, SB;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Tubeimosides are pan-coronavirus and filovirus inhibitors that can block their fusion protein binding to Niemann-Pick C1
Authors: Khan, I;Li, S;Tao, L;Wang, C;Ye, B;Li, H;Liu, X;Ahmad, I;Su, W;Zhong, G;Wen, Z;Wang, J;Hua, RH;Ma, A;Liang, J;Wan, XP;Bu, ZG;Zheng, YH;
Nature communications
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Integrated multi-omics analyses identify anti-viral host factors and pathways controlling SARS-CoV-2 infection
Authors: Hou, J;Wei, Y;Zou, J;Jaffery, R;Sun, L;Liang, S;Zheng, N;Guerrero, AM;Egan, NA;Bohat, R;Chen, S;Zheng, C;Mao, X;Yi, SS;Chen, K;McGrail, DJ;Sahni, N;Shi, PY;Chen, Y;Xie, X;Peng, W;
Nature communications
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Decoy peptides effectively inhibit the binding of SARS-CoV-2 to ACE2 on oral epithelial cells
Authors: Loi, LK;Yang, CC;Lin, YC;Su, YF;Juan, YC;Chen, YH;Chang, HC;
Heliyon
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Antiviral responses in a Jamaican fruit bat intestinal organoid model of SARS-CoV-2 infection
Authors: Hashimi, M;Sebrell, TA;Hedges, JF;Snyder, D;Lyon, KN;Byrum, SD;Mackintosh, SG;Crowley, D;Cherne, MD;Skwarchuk, D;Robison, A;Sidar, B;Kunze, A;Loveday, EK;Taylor, MP;Chang, CB;Wilking, JN;Walk, ST;Schountz, T;Jutila, MA;Bimczok, D;
Nature communications
Species: Mouse
Sample Types: Organoids
Applications: IHC -
Regulatory T cell-like response to SARS-CoV-2 in Jamaican fruit bats (Artibeus jamaicensis) transduced with human ACE2
Authors: Burke, B;Rocha, SM;Zhan, S;Eckley, M;Reasoner, C;Addetia, A;Lewis, J;Fagre, A;Charley, PA;Richt, JA;Weiss, SR;Tjalkens, RB;Veesler, D;Aboellail, T;Schountz, T;
PLoS pathogens
Species: Bat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Pharmacological inhibition of bromodomain and extra-terminal proteins induces an NRF-2-mediated antiviral state that is subverted by SARS-CoV-2 infection
Authors: Mhlekude, B;Postmus, D;Stenzel, S;Weiner, J;Jansen, J;Zapatero-Belinchón, FJ;Olmer, R;Richter, A;Heinze, J;Heinemann, N;Mühlemann, B;Schroeder, S;Jones, TC;Müller, MA;Drosten, C;Pich, A;Thiel, V;Martin, U;Niemeyer, D;Gerold, G;Beule, D;Goffinet, C;
PLoS pathogens
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Long-COVID cognitive impairments and reproductive hormone deficits in men may stem from GnRH neuronal death
Authors: Sauve F, Nampoothiri S, Clarke SA et al.
EBioMedicine
-
A cell-based assay for rapid assessment of ACE2 catalytic function
Authors: Warren M. Meyers, Ryan J. Hong, Wun Chey Sin, Christine S. Kim, Kurt Haas
Scientific Reports
Species: Human
Sample Types: Cell Lysates
Applications: Simple Western -
TMEM106B is a receptor mediating ACE2-independent SARS-CoV-2 cell entry
Authors: Jim Baggen, Maarten Jacquemyn, Leentje Persoons, Els Vanstreels, Valerie E. Pye, Antoni G. Wrobel et al.
Cell
Species: Human, Primate - Chlorocebus aethiops (African Green Monkey)
Sample Types: Cell Lysates
Applications: Simple Western -
Genetically diverse mouse models of SARS-CoV-2 infection reproduce clinical variation in type I interferon and cytokine responses in COVID-19
Authors: Robertson, SJ;Bedard, O;McNally, KL;Shaia, C;Clancy, CS;Lewis, M;Broeckel, RM;Chiramel, AI;Shannon, JG;Sturdevant, GL;Rosenke, R;Anzick, SL;Forte, E;Preuss, C;Baker, CN;Harder, JM;Brunton, C;Munger, S;Bruno, DP;Lack, JB;Leung, JM;Shamsaddini, A;Gardina, P;Sturdevant, DE;Sun, J;Martens, C;Holland, SM;Rosenthal, NA;Best, SM;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Proteolytic cleavage and inactivation of the TRMT1 tRNA modification enzyme by SARS-CoV-2 main protease
Authors: Zhang, K;Eldin, P;Ciesla, JH;Briant, L;Lentini, JM;Ramos, J;Cobb, J;Munger, J;Fu, D;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Effective SARS-CoV-2 replication of monolayers of intestinal epithelial cells differentiated from human induced pluripotent stem cells
Authors: Minami, S;Matsumoto, N;Omori, H;Nakamura, Y;Tamiya, S;Nouda, R;Nurdin, JA;Yamasaki, M;Kotaki, T;Kanai, Y;Okamoto, T;Tachibana, T;Ushijima, H;Kobayashi, T;Sato, S;
Scientific reports
Species: Human
Sample Types: Whole Cells
Applications: ICC -
PLSCR1 is a cell-autonomous defence factor against SARS-CoV-2 infection
Authors: Xu, D;Jiang, W;Wu, L;Gaudet, RG;Park, ES;Su, M;Cheppali, SK;Cheemarla, NR;Kumar, P;Uchil, PD;Grover, JR;Foxman, EF;Brown, CM;Stansfeld, PJ;Bewersdorf, J;Mothes, W;Karatekin, E;Wilen, CB;MacMicking, JD;
Nature
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
An inducible hACE2 transgenic mouse model recapitulates SARS-CoV-2 infection and pathogenesis in vivo
Authors: Liu, K;Tang, M;Xu, W;Meng, X;Jin, H;Han, M;Pu, J;Li, Y;Jiao, F;Sun, R;Shen, R;Lui, KO;Lu, L;Zhou, B;
Proceedings of the National Academy of Sciences of the United States of America
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Fetal brain vulnerability to SARS-CoV-2 infection
Authors: McMahon CL, Castro J, Silvas J et al.
Brain, behavior, and immunity
-
Generation of a SARS-CoV-2 Reverse Genetics System and Novel Human Lung Cell Lines That Exhibit High Virus-Induced Cytopathology
Authors: Khan, JQ;Rohamare, M;Rajamanickam, K;Bhanumathy, KK;Lew, J;Kumar, A;Falzarano, D;Vizeacoumar, FJ;Wilson, JA;
Viruses
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
COVID-19-related hyperglycemia is associated with infection of hepatocytes and stimulation of gluconeogenesis
Authors: Barreto, EA;Cruz, AS;Veras, FP;Martins, R;Bernardelli, RS;Paiva, IM;Lima, TM;Singh, Y;Guimar�es, RC;Damasceno, S;Pereira, N;Alves, JM;Gon�alves, TT;Forato, J;Muraro, SP;Souza, GF;Batah, SS;Proenca-Modena, JL;Mori, MA;Cunha, FQ;Louzada-Junior, P;Cunha, TM;Nakaya, HI;Fabro, A;de Oliveira, RDR;Arruda, E;R�a, R;R�a Neto, �;Fernandes da Silva, MM;Leiria, LO;
Proceedings of the National Academy of Sciences of the United States of America
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Cell culture systems for isolation of SARS-CoV-2 clinical isolates and generation of recombinant virus
Authors: Da-Yuan Chen, Jacquelyn Turcinovic, Shuchen Feng, Devin J. Kenney, Chue Vin Chin, Manish C. Choudhary et al.
iScience
Species: Human, Primate - Chlorocebus aethiops (African Green Monkey)
Sample Types: Transduced Whole Cells
Applications: Flow Cytometry -
Neutrophil Elastase decreases SARS-CoV-2 Spike protein binding to human bronchial epithelia by clipping ACE-2 ectodomian from the epithelial surface
Authors: Kummarapurugu, AB;Hawkridge, AM;Ma, J;Osei, S;Martin, RK;Zheng, S;Voynow, JA;
The Journal of biological chemistry
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
A replication-competent vesicular stomatitis virus for studies of SARS-CoV-2 spike-mediated cell entry and its inhibition.
Authors: M. Eugenia Dieterle, Denise Haslwanter, Robert H. Bortz, Ariel S. Wirchnianski, Gorka Lasso, Olivia Vergnolle et al.
bioRxiv
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Histology and cytokine levels in hepatic injury accompanying a case of non-severe COVID-19
Authors: Hidetaka Matsuda, Takuto Nosaka, Katsushi Hiramatsu, Kazuto Takahashi, Tatsushi Naito, Kazuya Ofuji et al.
Clinical Journal of Gastroenterology
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Acute Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Pregnancy Is Associated with Placental Angiotensin-Converting Enzyme 2 Shedding
Authors: Elizabeth S. Taglauer, Elisha M. Wachman, Lillian Juttukonda, Timothy Klouda, Jiwon Kim, Qiong Wang et al.
The American Journal of Pathology
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Characterisation of the Antibody Response in Sinopharm (BBIBP-CorV) Recipients and COVID-19 Convalescent Sera from the Republic of Moldova
Authors: M Ulinici, A Sulji?, M Poggianell, R Milan Bono, K Resman Rus, A Paraschiv, AM Bonetti, M Todiras, A Corlateanu, S Groppa, E Ceban, M Petrovec, A Marcello
Vaccines, 2023-03-13;11(3):.
Species: Human
Sample Types: Whole Cells
Applications: FLOW -
Development and validation of a rapid and easy-to-perform point-of-care lateral flow immunoassay (LFIA) for the detection of SARS-CoV-2 spike protein
Authors: Shamim Mohammad, Yuxia Wang, John Cordero, Christopher Watson, Robert Molestina, Sujatha Rashid et al.
Frontiers in Immunology
Species: N/A
Sample Types: Protein
Applications: Western Blot -
Uptake of severe acute respiratory syndrome coronavirus 2 spike protein mediated by angiotensin converting enzyme 2 and ganglioside in human cerebrovascular cells
Authors: Conor McQuaid, Alexander Solorzano, Ian Dickerson, Rashid Deane
Frontiers in Neuroscience
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Cell-autonomous requirement for ACE2 across organs in lethal mouse SARS-CoV-2 infection
Authors: AT Tang, DW Buchholz, KM Szigety, B Imbiakha, S Gao, M Frankfurte, M Wang, J Yang, P Hewins, P Mericko-Is, NA Leu, S Sterling, IA Monreal, J Sahler, A August, X Zhu, KA Jurado, M Xu, EE Morrisey, SE Millar, HC Aguilar, ML Kahn
PloS Biology, 2023-02-06;21(2):e3001989.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Repeated ethanol exposure and withdrawal alters angiotensin-converting enzyme 2 expression in discrete brain regions: Implications for SARS-CoV-2 neuroinvasion
Authors: Balasubramanian N, James TD, Selvakumar GP et al.
Alcoholism: Clinical and Experimental Research
-
Mapping of functional SARS-CoV-2 receptors in human lungs establishes differences in variant binding and SLC1A5 as a viral entry modulator of hACE2
Authors: A Miluzio, A Cuomo, C Cordiglier, L Donnici, E Pesce, M Bombaci, M Conti, A Fasciani, L Terraccian, L Manganaro, M Toccafondi, A Scagliola, S Oliveto, S Ricciardi, R Grifantini, R De Frances, S Abrignani, N Manfrini, S Biffo
EBioMedicine, 2022-12-28;87(0):104390.
Species: Human
Sample Types: Cell Lysates, Whole Cells, Whole Tissue
Applications: Co-Immunoprecipitation, Flow Cytometry, IHC, Western Blot -
Generation and Characterization of a SARS-CoV-2-Susceptible Mouse Model Using Adeno-Associated Virus (AAV6.2FF)-Mediated Respiratory Delivery of the Human ACE2 Gene
Authors: N Tailor, BM Warner, BD Griffin, K Tierney, E Moffat, K Frost, R Vendramell, A Leung, M Willman, SP Thomas, Y Pei, SA Booth, C Embury-Hya, SK Wootton, D Kobasa
Viruses, 2022-12-28;15(1):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Control of SARS-CoV-2 infection by MT1-MMP-mediated shedding of ACE2
Authors: X Guo, J Cao, JP Cai, J Wu, J Huang, P Asthana, SKK Wong, ZW Ye, S Gurung, Y Zhang, S Wang, Z Wang, X Ge, HY Kwan, A Lyu, KM Chan, N Wong, J Huang, Z Zhou, ZX Bian, S Yuan, HLX Wong
Nature Communications, 2022-12-23;13(1):7907.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
TRPC3-Nox2 Protein Complex Formation Increases the Risk of SARS-CoV-2 Spike Protein-Induced Cardiomyocyte Dysfunction through ACE2 Upregulation
Authors: Y Kato, K Nishiyama, J Man Lee, Y Ibuki, Y Imai, T Noda, N Kamiya, T Kusakabe, Y Kanda, M Nishida
International Journal of Molecular Sciences, 2022-12-21;24(1):.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
SARS-CoV-2 Spike triggers barrier dysfunction and vascular leak via integrins and TGF-beta signaling
Authors: SB Biering, FT Gomes de S, LV Tjang, F Pahmeier, C Zhu, R Ruan, SF Blanc, TS Patel, CM Worthingto, DR Glasner, B Castillo-R, V Servellita, NTN Lo, MP Wong, CM Warnes, DR Sandoval, TM Clausen, YA Santos, DM Fox, V Ortega, AM Näär, RS Baric, SA Stanley, HC Aguilar, JD Esko, CY Chiu, JE Pak, PR Beatty, E Harris
Nature Communications, 2022-12-09;13(1):7630.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Severe acute respiratory disease in American mink experimentally infected with SARS-CoV-2
Authors: DR Adney, J Lovaglio, JE Schulz, CK Yinda, VA Avanzato, E Haddock, JR Port, MG Holbrook, PW Hanley, G Saturday, D Scott, C Shaia, AM Nelson, JR Spengler, C Tansey, CM Cossaboom, NM Wendling, C Martens, J Easley, SW Yap, SN Seifert, VJ Munster
JCI Insight, 2022-11-22;7(22):.
Species: Mink
Sample Types: Whole Tissue
Applications: IHC -
ACE2-Independent Bat Sarbecovirus Entry and Replication in Human and Bat Cells
Authors: H Guo, A Li, TY Dong, J Su, YL Yao, Y Zhu, ZL Shi, M Letko
MBio, 2022-11-21;0(0):e0256622.
Species: Bat, Human
Sample Types: Whole Cells
Applications: Neutralization -
CD169-mediated restrictive SARS-CoV-2 infection of macrophages induces pro-inflammatory responses
Authors: Jalloh S, Olejnik J, Berrigan J et al.
PLOS Pathogens
-
LRRC15 inhibits SARS-CoV-2 cellular entry in trans
Authors: J Song, RD Chow, M Pena-Herna, L Zhang, SA Loeb, EY So, OD Liang, P Ren, S Chen, CB Wilen, S Lee
PloS Biology, 2022-10-13;20(10):e3001805.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
The Integral Membrane Protein ZMPSTE24 Protects Cells from SARS-CoV-2 Spike-Mediated Pseudovirus Infection and Syncytia Formation
Authors: K Shilagardi, ED Spear, R Abraham, DE Griffin, S Michaelis
MBio, 2022-10-05;0(0):e0254322.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
SARS-CoV-2 infects adipose tissue in a fat depot- and viral lineage-dependent manner
Authors: TD Saccon, F Mousovich-, RG Ludwig, VC Carregari, AB Dos Anjos, ASC Dos Passos, MC Martini, PP Barbosa, GF de Souza, SP Muraro, J Forato, MR Amorim, RE Marques, FP Veras, E Barreto, TT Gonçalves, IM Paiva, NPB Fazolini, CMK Onodera, RB Martins Ju, PHC de Araújo, SS Batah, RMM Viana, DM de Melo, AT Fabro, E Arruda, F Queiroz Cu, TM Cunha, MAM Pretti, BJ Smith, H Marques-So, TL Knittel, GP Ruiz, GS Profeta, TCM Fontes-Cal, M Boroni, MAR Vinolo, AS Farias, PMM Moraes-Vie, JMA Bizzacchi, T Teesalu, FDM Chaim, E Cazzo, EA Chaim, JL Proença-Mó, D Martins-de, MK Osako, LO Leiria, MA Mori
Nature Communications, 2022-09-29;13(1):5722.
Species: African Green Monkey
Sample Types: Whole Cells
Applications: ICC -
Memory CD8+ T cell diversity and B cell responses correlate with protection against SARS-CoV-2 following mRNA vaccination
Authors: N Brasu, I Elia, V Russo, G Montacchie, SA Stabile, C De Intinis, F Fesi, K Gizzi, M Macagno, M Montone, B Mussolin, A Grifoni, S Faravelli, S Marchese, F Forneris, R De Frances, A Sette, V Barnaba, A Sottile, A Sapino, L Pace
Nature Immunology, 2022-09-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Metalloprotease-Dependent S2'-Activation Promotes Cell-Cell Fusion and Syncytiation of SARS-CoV-2
Authors: JV Harte, SL Wakerlin, AJ Lindsay, JV McCarthy, C Coleman-Va
Viruses, 2022-09-21;14(10):.
-
SARS-CoV-2 can infect human embryos
Authors: M Montano, AR Victor, DK Griffin, T Duong, N Bolduc, A Farmer, V Garg, AK Hadjantona, A Coates, FL Barnes, CG Zouves, WC Greene, M Viotti
Scientific Reports, 2022-09-14;12(1):15451.
Species: Human
Sample Types: Whole Cells
Applications: Control, ICC -
Surface translocation of ACE2 and TMPRSS2 upon TLR4/7/8 activation is required for SARS-CoV-2 infection in circulating monocytes
Authors: Y Yao, K Subedi, T Liu, N Khalasawi, CD Pretto-Ker, JW Wotring, J Wang, C Yin, A Jiang, C Fu, P Dimitrion, J Li, J Veenstra, Q Yi, K McKinnon, JE McKinnon, JZ Sexton, L Zhou, QS Mi
Cell Discovery, 2022-09-09;8(1):89.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Decrease in Angiotensin-Converting Enzyme activity but not concentration in plasma/lungs in COVID-19 patients offers clues for diagnosis/treatment
Authors: Henry Daniell, Smruti K. Nair, Yao Shi, Ping Wang, Kathleen T. Montone, Pamela A. Shaw et al.
Molecular Therapy - Methods & Clinical Development
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Integrated multi-omics analyses identify key anti-viral host factors and pathways controlling SARS-CoV-2 infection
Authors: J Hou, Y Wei, J Zou, R Jaffery, S Liang, C Zheng, K Chen, PY Shi, Y Chen, X Xie, W Peng
Research square, 2022-08-15;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Development of a novel peptide to prevent entry of SARS-CoV-2 into lung and olfactory bulb cells of hACE2 expressing mice
Authors: P Su, D Zhai, AHC Wong, F Liu
Molecular Brain, 2022-08-09;15(1):71.
Species: Human, Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: Co-Immunoprecipitation, IHC, Western Blot -
SARS-CoV-2 spike N-terminal domain modulates TMPRSS2-dependent viral entry and fusogenicity
Authors: B Meng, R Datir, J Choi, CITIID-NIH, JR Bradley, KGC Smith, JH Lee, RK Gupta
Cell Reports, 2022-08-03;40(7):111220.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Ancestral SARS-CoV-2, but not Omicron, replicates less efficiently in primary pediatric nasal epithelial cells
Authors: Y Zhu, KY Chew, M Wu, AC Karawita, G McCallum, LE Steele, A Yamamoto, LI Labzin, T Yarlagadda, AA Khromykh, X Wang, JDJ Sng, CJ Stocks, Y Xia, TR Kollmann, D Martino, M Joensuu, FA Meunier, G Balistreri, H Bielefeldt, AC Bowen, A Kicic, PD Sly, KM Spann, KR Short
PloS Biology, 2022-08-01;20(8):e3001728.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Distinct airway epithelial immune responses after infection with SARS-CoV-2 compared to H1N1
Authors: H Stölting, L Baillon, R Frise, K Bonner, RJ Hewitt, PL Molyneaux, ML Gore, Breathing, WS Barclay, S Saglani, CM Lloyd
Mucosal Immunology, 2022-07-15;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Sec61 Inhibitor Apratoxin S4 Potently Inhibits SARS-CoV-2 and Exhibits Broad-Spectrum Antiviral Activity
Authors: Marie O. Pohl, Laura Martin-Sancho, Ranjala Ratnayake, Kris M. White, Laura Riva, Qi-Yin Chen et al.
ACS Infectious Diseases
Species: Human, Primate - Chlorocebus aethiops (African Green Monkey)
Sample Types: Cell Lysates
Applications: Western Blot -
A Newly Engineered A549 Cell Line Expressing ACE2 and TMPRSS2 Is Highly Permissive to SARS-CoV-2, Including the Delta and Omicron Variants
Authors: CW Chang, KM Parsi, M Somasundar, E Vanderleed, P Liu, J Cruz, A Cousineau, RW Finberg, EA Kurt-Jones
Viruses, 2022-06-23;14(7):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Potential role of astrocyte angiotensin converting enzyme 2 in the neural transmission of COVID-19 and a neuroinflammatory state induced by smoking and vaping
Authors: Zhang Y, Archie SR, Ghanwatkar Y et al.
Fluids and Barriers of the CNS
-
A diabetic milieu increases ACE2 expression and cellular susceptibility to SARS-CoV-2 infections in human kidney organoids and patient cells
Authors: Garreta E, Prado P, Stanifer ML et al.
Cell Metabolism
-
A diabetic milieu increases ACE2 expression and cellular susceptibility to SARS-CoV-2 infections in human kidney organoids and patient cells
Authors: Garreta E, Prado P, Stanifer ML et al.
Cell Metabolism
-
A diabetic milieu increases ACE2 expression and cellular susceptibility to SARS-CoV-2 infections in human kidney organoids and patient cells
Authors: Garreta E, Prado P, Stanifer ML et al.
Cell Metabolism
-
IgM anti-ACE2 autoantibodies in severe COVID-19 activate complement and perturb vascular endothelial function
Authors: L Casciola-R, DR Thiemann, F Andrade, MI Trejo-Zamb, EK Leonard, JB Spangler, NE Skinner, J Bailey, S Yegnasubra, R Wang, AM Vaghasia, A Gupta, AL Cox, SC Ray, RM Linville, Z Guo, PC Searson, CE Machamer, S Desiderio, LM Sauer, O Laeyendeck, BT Garibaldi, L Gao, M Damarla, PM Hassoun, JE Hooper, CA Mecoli, L Christophe, L Gutierrez-, Q Yang, D Hines, WA Clarke, RE Rothman, A Pekosz, KZ Fenstermac, Z Wang, SL Zeger, A Rosen
JCI Insight, 2022-05-09;7(9):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
FcgammaR-mediated SARS-CoV-2 infection of monocytes activates inflammation
Authors: C Junqueira, Â Crespo, S Ranjbar, LB de Lacerda, M Lewandrows, J Ingber, B Parry, S Ravid, S Clark, MR Schrimpf, F Ho, C Beakes, J Margolin, N Russell, K Kays, J Boucau, U Das Adhika, SM Vora, V Leger, L Gehrke, L Henderson, E Janssen, D Kwon, C Sander, J Abraham, MB Goldberg, H Wu, G Mehta, S Bell, AE Goldfeld, MR Filbin, J Lieberman
Nature, 2022-04-06;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, Neutralization -
Two Different Therapeutic Approaches for SARS-CoV-2 in hiPSCs-Derived Lung Organoids
Authors: P Spitalieri, F Centofanti, M Murdocca, MG Scioli, A Latini, S Di Cesare, G Citro, A Rossi, A Orlandi, S Miersch, SS Sidhu, PP Pandolfi, A Botta, F Sangiuolo, G Novelli
Cells, 2022-04-05;11(7):.
Species: Human
Sample Types: Organoid
Applications: IHC -
Molecular consequences of SARS-CoV-2 liver tropism
Authors: Nicola Wanner; Geoffroy Andrieux; Pau Badia-i-Mompel; Carolin Edler; Susanne Pfefferle; Maja T. Lindenmeyer et al.
Nature Metabolism
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
ACE2 Receptor and Its Isoform Short-ACE2 Are Expressed on Human Spermatozoa
Authors: M Ramal-Sanc, C Castellini, C Cimini, A Taraschi, L Valbonetti, A Barbonetti, N Bernabò, B Barboni
International Journal of Molecular Sciences, 2022-03-28;23(7):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
SARS-CoV-2 infects and replicates in photoreceptor and retinal ganglion cells of human retinal organoids
Authors: Y Menuchin-L, A Schreiber, A Lecanda, A Mecate-Zam, L Brunotte, OE Psathaki, S Ludwig, T Rauen, HR Schöler
Stem Cell Reports, 2022-03-24;0(0):.
Species: Human
Sample Types: Organoid
Applications: Neutralization -
Diltiazem inhibits SARS-CoV-2 cell attachment and internalization and decreases the viral infection in mouse lung
Authors: X Wang, J Luo, Z Wen, L Shuai, C Wang, G Zhong, X He, H Cao, R Liu, J Ge, R Hua, Z Sun, X Wang, J Wang, Z Bu
PloS Pathogens, 2022-02-17;18(2):e1010343.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
SARS-CoV-2 infection triggers paracrine senescence and leads to a sustained senescence-associated inflammatory response
Authors: Shunya Tsuji, Shohei Minami, Rina Hashimoto, Yusuke Konishi, Tatsuya Suzuki, Tamae Kondo et al.
Nature Aging
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Western Blot, Immunocytochemistry -
Circulating ACE2-expressing extracellular vesicles block broad strains of SARS-CoV-2
Authors: L El-Shennaw, AD Hoffmann, NK Dashzeveg, KM McAndrews, PJ Mehl, D Cornish, Z Yu, VL Tokars, V Nicolaescu, A Tomatsidou, C Mao, CJ Felicelli, CF Tsai, C Ostiguin, Y Jia, L Li, K Furlong, J Wysocki, X Luo, CF Ruivo, D Batlle, TJ Hope, Y Shen, YK Chae, H Zhang, VS LeBleu, T Shi, S Swaminatha, Y Luo, D Missiakas, GC Randall, AR Demonbreun, MG Ison, R Kalluri, D Fang, H Liu
Nature Communications, 2022-01-20;13(1):405.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2
Authors: S Krasemann, U Haferkamp, S Pfefferle, MS Woo, F Heinrich, M Schweizer, A Appelt-Men, A Cubukova, J Barenberg, J Leu, K Hartmann, E Thies, JL Littau, D Sepulveda-, L Zhang, K Ton, Y Liang, J Matschke, F Ricklefs, T Sauvigny, J Sperhake, A Fitzek, A Gerhartl, A Brachner, N Geiger, EM König, J Bodem, S Franzenbur, A Franke, S Moese, FJ Müller, G Geisslinge, C Claussen, A Kannt, A Zaliani, P Gribbon, B Ondruschka, W Neuhaus, MA Friese, M Glatzel, O Pless
Stem Cell Reports, 2022-01-20;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Evaluation of SARS-CoV-2 entry, inflammation and new therapeutics in human lung tissue cells
Authors: J Grau-Expós, D Perea, M Suppi, N Massana, A Vergara, MJ Soler, B Trinite, J Blanco, J García-Pér, J Alcamí, A Serrano-Mo, J Rosado, V Falcó, M Genescà, MJ Buzon
PloS Pathogens, 2022-01-13;18(1):e1010171.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Cross-validation of SARS-CoV-2 responses in kidney organoids and clinical populations
Authors: L Helms, S Marchiano, IB Stanaway, TY Hsiang, BA Juliar, S Saini, YT Zhao, A Khanna, R Menon, F Alakwaa, C Mikacenic, ED Morrell, MM Wurfel, M Kretzler, JL Harder, CE Murry, J Himmelfarb, H Ruohola-Ba, PK Bhatraju, M Gale, BS Freedman
JCI Insight, 2021-12-22;0(0):.
Species: Human
Sample Types: Organoids
Applications: IHC -
SARS-CoV-2 uses metabotropic glutamate receptor subtype 2 as an internalization factor to infect cells
Authors: J Wang, G Yang, X Wang, Z Wen, L Shuai, J Luo, C Wang, Z Sun, R Liu, J Ge, X He, R Hua, X Wang, X Yang, W Chen, G Zhong, Z Bu
Cell Discovery, 2021-12-14;7(1):119.
Species: Human, Primate - C. aethiops
Sample Types: Whole Cells
Applications: Flow Cytometry, Neutralization -
SARS-CoV-2 Spike triggers barrier dysfunction and vascular leak via integrins and TGF-beta signaling
Authors: SB Biering, FTG de Sousa, LV Tjang, F Pahmeier, R Ruan, SF Blanc, TS Patel, CM Worthingto, DR Glasner, B Castillo-R, V Servellita, NTN Lo, MP Wong, CM Warnes, DR Sandoval, TM Clausen, YA Santos, V Ortega, HC Aguilar, JD Esko, CY Chui, JE Pak, PR Beatty, E Harris
bioRxiv : the preprint server for biology, 2021-12-13;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Receptor-Binding Domain Proteins of SARS-CoV-2 Variants Elicited Robust Antibody Responses Cross-Reacting with Wild-Type and Mutant Viruses in Mice
Authors: J Shi, X Jin, Y Ding, X Liu, A Shen, Y Wu, M Peng, C Shen
Vaccines, 2021-11-24;9(12):.
Species: Human
Sample Types: Recombinant Protein
Applications: ELISA Detection -
Effective chimeric antigen receptor T cells against SARS-CoV-2
Authors: Xueyang Guo, Alexandra Kazanova, Stephanie Thurmond, H. Uri Saragovi, Christopher E. Rudd
iScience
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Sialic acid-containing glycolipids mediate binding and viral entry of SARS-CoV-2
Authors: L Nguyen, KA McCord, DT Bui, KM Bouwman, EN Kitova, M Elaish, D Kumawat, GC Daskhan, I Tomris, L Han, P Chopra, TJ Yang, SD Willows, AL Mason, LK Mahal, TL Lowary, LJ West, SD Hsu, T Hobman, SM Tompkins, GJ Boons, RP de Vries, MS Macauley, JS Klassen
Nature Chemical Biology, 2021-11-09;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Inhibition of IRGM establishes a robust antiviral immune state to restrict pathogenic viruses
Authors: Parej Nath, Nishant Ranjan Chauhan, Kautilya Kumar Jena, Ankita Datey, Nilima Dinesh Kumar, Subhash Mehto et al.
EMBO reports
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Brain Cross-Protection against SARS-CoV-2 Variants by a Lentiviral Vaccine in New Transgenic Mice
Authors: MW Ku, P Authié, M Bourgine, F Anna, A Noirat, F Moncoq, B Vesin, F Nevo, J Lopez, P Souque, C Blanc, I Fert, S Chardenoux, L Lafosse, D Cussigh, D Hardy, K Nemirov, F Guinet, F Langa Vive, L Majlessi, P Charneau
Embo Molecular Medicine, 2021-10-25;0(0):e14459.
Species: Transgenic Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
An airway organoid-based screen identifies a role for the HIF1alpha-glycolysis axis in SARS-CoV-2 infection
Authors: X Duan, X Tang, MS Nair, T Zhang, Y Qiu, W Zhang, P Wang, Y Huang, J Xiang, H Wang, RE Schwartz, DD Ho, T Evans, S Chen
Cell Reports, 2021-10-15;37(6):109920.
Species: Human
Sample Types: Organoids
Applications: IHC -
SARS-CoV-2 and its variants of concern infect human conjunctival epithelial cells and induce differential antiviral innate immune response
Authors: S Singh, G Garcia, R Shah, AA Kramerov, RE Wright, TM Spektor, AV Ljubimov, V Arumugaswa, A Kumar
The ocular surface, 2021-09-25;0(0):.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
BRD2 inhibition blocks SARS-CoV-2 infection in vitro by reducing transcription of the host cell receptor ACE2
Authors: R Tian, AJ Samelson, VV Rezelj, M Chen, GN Ramadoss, X Guo, AM Kain, QD Tran, SA Lim, I Lui, J Nunez, SJ Rockwood, N Liu, J Carlson-St, J Oki, T Maures, K Holden, JS Weissman, JA Wells, B Conklin, M Vignuzzi, M Kampmann
bioRxiv : the preprint server for biology, 2021-09-20;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
"Molecular Masks" for ACE2 to Effectively and Safely Block SARS-CoV-2 Virus Entry
Authors: SP Shukla, KB Cho, V Rustagi, X Gao, X Fu, SX Zhang, B Guo, DG Udugamasoo
International Journal of Molecular Sciences, 2021-08-20;22(16):.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Flow Cytometry, Western Blot -
Signatures in SARS-CoV-2 spike protein conferring escape to neutralizing antibodies
Authors: M Alenquer, F Ferreira, D Lousa, M Valério, M Medina-Lop, ML Bergman, J Gonçalves, J Demengeot, RB Leite, J Lilue, Z Ning, C Penha-Gonç, H Soares, CM Soares, MJ Amorim
PloS Pathogens, 2021-08-05;17(8):e1009772.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
ACE2 protein expression within isogenic cell lines is heterogeneous and associated with distinct transcriptomes
Authors: EJ Sherman, BT Emmer
Scientific Reports, 2021-08-05;11(1):15900.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Western Blot, Flow Cytometry -
SARS-CoV-2 Poorly Replicates in Cells of the Human Blood-Brain Barrier Without Associated Deleterious Effects
Authors: Orianne Constant, Jonathan Barthelemy, Karine Bolloré, Edouard Tuaillon, Fabien Gosselet, Christine Chable-Bessia et al.
Frontiers in Immunology
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
L-SIGN is a receptor on liver sinusoidal endothelial cells for SARS-CoV-2 virus
Authors: Y Kondo, JL Larabee, L Gao, H Shi, B Shao, CM Hoover, JM McDaniel, YC Ho, R Silasi-Man, SA Archer-Har, P Azadi, RS Srinivasan, AR Rezaie, A Borczuk, JC Laurence, F Lupu, J Ahamed, RP McEver, JF Papin, Z Yu, L Xia
JCI Insight, 2021-07-22;6(14):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Cigarette Smoke Stimulates SARS-CoV-2 Internalization by Activating AhR and Increasing ACE2 Expression in Human Gingival Epithelial Cells
Authors: CLC Almeida-da, H Matshik Da, K Liu, DM Ojcius
International Journal of Molecular Sciences, 2021-07-18;22(14):.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
A human three-dimensional neural-perivascular 'assembloid' promotes astrocytic development and enables modeling of SARS-CoV-2 neuropathology
Authors: L Wang, D Sievert, AE Clark, S Lee, H Federman, BD Gastfriend, EV Shusta, SP Palecek, AF Carlin, JG Gleeson
Nature Medicine, 2021-07-09;0(0):.
Species: Human
Sample Types: Organoid
Applications: IHC -
Influenza virus infection increases ACE2 expression and shedding in human small airway epithelial cells
Authors: Kelly S. Schweitzer, Taylor Crue, Jordan M. Nall, Daniel Foster, Satria Sajuthi, Kelly A. Correll et al.
European Respiratory Journal
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity
Authors: Chihiro Motozono, Mako Toyoda, Jiri Zahradnik, Akatsuki Saito, Hesham Nasser, Toong Seng Tan et al.
Cell Host & Microbe
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Rapid, reliable, and reproducible cell fusion assay to quantify SARS-Cov-2 spike interaction with hACE2
Authors: M Zhao, PY Su, DA Castro, TN Tripler, Y Hu, M Cook, AI Ko, SF Farhadian, B Israelow, CS Dela Cruz, Y Xiong, RE Sutton, Yale IMPAC
PloS Pathogens, 2021-06-24;17(6):e1009683.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Deletion of ER-retention motif on SARS-CoV-2 spike protein reduces cell hybrid during cell-cell fusion
Authors: X Wang, CH Chen, S Badeti, JH Cho, A Naghizadeh, Z Wang, D Liu
Cell & bioscience, 2021-06-23;11(1):114.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Systematic analysis of SARS-CoV-2 infection of an ACE2-negative human airway cell
Authors: M Puray-Chav, KM LaPak, TP Schrank, JL Elliott, DP Bhatt, MJ Agajanian, R Jasuja, DQ Lawson, K Davis, PW Rothlauf, Z Liu, H Jo, N Lee, K Tenneti, JE Eschbach, C Shema Mugi, EM Cousins, EW Cloer, HR Vuong, LA VanBlargan, AL Bailey, P Gilchuk, JE Crowe, MS Diamond, DN Hayes, SPJ Whelan, A Horani, SL Brody, D Goldfarb, MB Major, SB Kutluay
Cell Reports, 2021-06-23;0(0):109364.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
SARS-CoV-2 infects an upper airway model derived from induced pluripotent stem cells
Authors: Ivo Djidrovski, Maria Georgiou, Grant L. Hughes, Edward I. Patterson, Aitor Casas-Sanchez, Shaun H. Pennington et al.
Stem Cells
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
ACE2-Independent Interaction of SARS-CoV-2 Spike Protein with Human Epithelial Cells Is Inhibited by Unfractionated Heparin
Authors: Lynda J. Partridge, Lucy Urwin, Martin J. H. Nicklin, David C. James, Luke R. Green, Peter N. Monk
Cells
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Androgen regulation of pulmonary AR, TMPRSS2 and ACE2 with implications for sex-discordant COVID-19 outcomes
Authors: M Baratchian, JM McManus, MP Berk, F Nakamura, S Mukhopadhy, W Xu, S Erzurum, J Drazba, J Peterson, EA Klein, B Gaston, N Sharifi
Scientific Reports, 2021-05-27;11(1):11130.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Direct derivation of human alveolospheres for SARS-CoV-2 infection modeling and drug screening
Authors: T Ebisudani, S Sugimoto, K Haga, A Mitsuishi, R Takai-Toda, M Fujii, K Toshimitsu, J Hamamoto, K Sugihara, T Hishida, H Asamura, K Fukunaga, H Yasuda, K Katayama, T Sato
Cell Reports, 2021-05-19;0(0):109218.
Species: Human
Sample Types: Whole Tissue
Applications: ICC -
SARS-CoV-2 infects human adult donor eyes and hESC-derived ocular epithelium
Authors: AZ Eriksen, R Møller, B Makovoz, SA Uhl, BR tenOever, TA Blenkinsop
Cell Stem Cell, 2021-05-17;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Susceptibility to SARS-CoV-2 of Cell Lines and Substrates Commonly Used to Diagnose and Isolate Influenza and Other Viruses
Authors: Li Wang, Xiaoyu Fan, Gaston Bonenfant, Dan Cui, Jaber Hossain, Nannan Jiang et al.
Emerging Infectious Diseases
Species: Canine, Human, Primate - Chlorocebus aethiops (African Green Monkey)
Sample Types: Cell Lysates
Applications: Western Blot -
Term Human Placental Trophoblasts Express SARS-CoV-2 Entry Factors ACE2, TMPRSS2, and Furin
Authors: Yingshi Ouyang, Tarique Bagalkot, Wendy Fitzgerald, Elena Sadovsky, Tianjiao Chu, Ana Martínez-Marchal et al.
mSphere
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
ORF10–Cullin-2–ZYG11B complex is not required for SARS-CoV-2 infection
Authors: Elijah L. Mena, Callie J. Donahue, Laura Pontano Vaites, Jie Li, Gergely Rona, Colin O’Leary et al.
Proceedings of the National Academy of Sciences
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Deletion of ER-retention Motif on SARS-CoV-2 Spike Protein Reduces Cell Hybrid During Cell-cell Fusion
Authors: CH Chen, S Badeti, JH Cho, A Naghizadeh, X Wang, D Liu
Research square, 2021-04-09;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
SARS-CoV-2 induces human plasmacytoid predendritic cell diversification via UNC93B and IRAK4
Authors: Fanny Onodi, Lucie Bonnet-Madin, Laurent Meertens, Léa Karpf, Justine Poirot, Shen-Ying Zhang et al.
Journal of Experimental Medicine
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-Cov-2
Authors: M Dittmar, JS Lee, K Whig, E Segrist, M Li, B Kamalia, L Castellana, K Ayyanathan, FL Cardenas-D, EE Morrisey, R Truitt, W Yang, K Jurado, K Samby, H Ramage, DC Schultz, S Cherry
Cell Reports, 2021-03-23;35(1):108959.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Characterisation of B.1.1.7 and Pangolin coronavirus spike provides insights on the evolutionary trajectory of SARS-CoV-2
Authors: SJ Dicken, MJ Murray, LG Thorne, AK Reuschl, C Forrest, M Ganeshalin, L Muir, MD Kalemera, M Palor, LE McCoy, C Jolly, GJ Towers, MB Reeves, J Grove
bioRxiv : the preprint server for biology, 2021-03-22;0(0):.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Flow Cytometry, Western Blot -
SARS-CoV-2 hijacks folate and one-carbon metabolism for viral replication
Authors: Y Zhang, R Guo, SH Kim, H Shah, S Zhang, JH Liang, Y Fang, M Gentili, CNO Leary, SJ Elledge, DT Hung, VK Mootha, BE Gewurz
Nature Communications, 2021-03-15;12(1):1676.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
MRC5 cells engineered to express ACE2 serve as a model system for the discovery of antivirals targeting SARS-CoV-2
Authors: K Uemura, M Sasaki, T Sanaki, S Toba, Y Takahashi, Y Orba, WW Hall, K Maenaka, H Sawa, A Sato
Scientific Reports, 2021-03-08;11(1):5376.
Species: Human, Primate
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Genome-wide CRISPR screening identifies TMEM106B as a proviral host factor for SARS-CoV-2
Authors: J Baggen, L Persoons, E Vanstreels, S Jansen, D Van Loover, B Boeckx, V Geudens, J De Man, D Jochmans, J Wauters, E Wauters, BM Vanaudenae, D Lambrechts, J Neyts, K Dallmeier, HJ Thibaut, M Jacquemyn, P Maes, D Daelemans
Nature Genetics, 2021-03-08;0(0):.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Flow Cytometry, Simple Western -
Pulmonary, cardiac and renal distribution of ACE2, furin, TMPRSS2 and ADAM17 in rats with heart failure: Potential implication for COVID-19 disease
Authors: EE Khoury, Y Knaney, A Fokra, S Kinaneh, Z Azzam, SN Heyman, Z Abassi
Journal of Cellular and Molecular Medicine, 2021-03-04;0(0):.
Species: Rat
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC -
Neuroinvasion of SARS-CoV-2 in human and mouse brain
Authors: Song E, Zhang C, Israelow B et al.
Journal of Experimental Medicine
-
An organoid-based organ-repurposing approach to treat short bowel syndrome
Authors: S Sugimoto, E Kobayashi, M Fujii, Y Ohta, K Arai, M Matano, K Ishikawa, K Miyamoto, K Toshimitsu, S Takahashi, K Nanki, Y Hakamata, T Kanai, T Sato
Nature, 2021-02-24;0(0):.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Multimerization- and glycosylation-dependent receptor binding of SARS-CoV-2 spike proteins
Authors: Kim M. Bouwman, Ilhan Tomris, Hannah L. Turner, Roosmarijn van der Woude, Tatiana M. Shamorkina, Gerlof P. Bosman et al.
PLOS Pathogens
Species: Ferret, Mouse, Syrian Hamster
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Human Erythroid Progenitors Are Directly Infected by SARS-CoV-2: Implications for Emerging Erythropoiesis in Severe COVID-19 Patients
Authors: H Huerga Enc, W Grey, M Garcia-Alb, H Wood, R Ulferts, IV Aramburu, AG Kulasekara, G Mufti, V Papayannop, R Beale, D Bonnet
Stem Cell Reports, 2021-02-05;16(3):428-436.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
An Immunological Perspective: What Happened to Pregnant Women After Recovering From COVID-19?
Authors: Sijia Zhao, Ting Xie, Li Shen, Hong Liu, Liling Wang, Xixiang Ma et al.
Frontiers in Immunology
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models
Authors: Min-Wen Ku, Maryline Bourgine, Pierre Authié, Jodie Lopez, Kirill Nemirov, Fanny Moncoq et al.
Cell Host & Microbe
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Identification of SARS-CoV-2 inhibitors using lung and colonic organoids
Authors: Yuling Han, Xiaohua Duan, Liuliu Yang, Benjamin E. Nilsson-Payant, Pengfei Wang, Fuyu Duan et al.
Nature
Species: Human, Xenograft
Sample Types: Whole Cells, Whole Tissue
Applications: Immunocytochemistry -
MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells
Authors: X Yin, L Riva, Y Pu, L Martin-San, J Kanamune, Y Yamamoto, K Sakai, S Gotoh, L Miorin, PD De Jesus, CC Yang, KM Herbert, S Yoh, JF Hultquist, A García-Sas, SK Chanda
Cell Reports, 2021-01-12;34(2):108628.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
A novel ACE2 isoform is expressed in human respiratory epithelia and is upregulated in response to interferons and RNA respiratory virus infection
Authors: C Blume, CL Jackson, CM Spalluto, J Legebeke, L Nazlamova, F Conforti, JM Perotin, M Frank, J Butler, M Crispin, J Coles, J Thompson, RA Ridley, LSN Dean, M Loxham, S Reikine, A Azim, K Tariq, DA Johnston, PJ Skipp, R Djukanovic, D Baralle, CJ McCormick, DE Davies, JS Lucas, G Wheway, V Mennella
Nature Genetics, 2021-01-11;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
SARS-CoV-2 induces human plasmacytoid pre-dendritic cell diversification via UNC93B and IRAK4
Authors: F Onodi, L Bonnet-Mad, L Meertens, L Karpf, J Poirot, SY Zhang, C Picard, A Puel, E Jouanguy, Q Zhang, J Le Goff, JM Molina, C Delaugerre, JL Casanova, A Amara, V Soumelis
bioRxiv : the preprint server for biology, 2021-01-08;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Host and viral determinants for efficient SARS-CoV-2 infection of the human lung
Authors: H Chu, B Hu, X Huang, Y Chai, D Zhou, Y Wang, H Shuai, D Yang, Y Hou, X Zhang, TT Yuen, JP Cai, AJ Zhang, J Zhou, S Yuan, KK To, IH Chan, KY Sit, DC Foo, IY Wong, AT Ng, TT Cheung, SY Law, WK Au, MA Brindley, Z Chen, KH Kok, JF Chan, KY Yuen
Nature Communications, 2021-01-08;12(1):134.
Species: Hamster - Mesocricetus auratus (Golden Hamster)
Sample Types: Whole Cells
Applications: Flow Cytometry -
SARS-CoV-2 entry into human airway organoids is serine protease-mediated and facilitated by the multibasic cleavage site
Authors: AZ Mykytyn, TI Breugem, S Riesebosch, D Schipper, PB van den Do, RJ Rottier, MM Lamers, BL Haagmans
Elife, 2021-01-04;10(0):.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Long-Term Modeling of SARS-CoV-2 Infection of In Vitro Cultured Polarized Human Airway Epithelium
Authors: Siyuan Hao, Kang Ning, Cagla Aksu Kuz, Kai Vorhies, Ziying Yan, Jianming Qiu
mBio
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Expression of SARS-CoV-2 Entry Factors in the Pancreas of Normal Organ Donors and Individuals with COVID-19
Authors: Kusmartseva I, Wu W, Syed F et al.
Cell Metabolism
-
Data, Reagents, Assays and Merits of Proteomics for SARS-CoV-2 Research and Testing
Authors: Jana Zecha, Chien-Yun Lee, Florian P. Bayer, Chen Meng, Vincent Grass, Johannes Zerweck et al.
Molecular & Cellular Proteomics
Species: Human, Primate - Chlorocebus aethiops (African Green Monkey)
Sample Types: Protein
Applications: Western Blot -
Androgen Signaling Regulates SARS-CoV-2 Receptor Levels and Is Associated with Severe COVID-19 Symptoms in Men
Authors: RM Samuel, H Majd, MN Richter, Z Ghazizadeh, SM Zekavat, A Navickas, JT Ramirez, H Asgharian, CR Simoneau, LR Bonser, KD Koh, M Garcia-Kni, M Tassetto, S Sunshine, S Farahvashi, A Kalantari, W Liu, R Andino, H Zhao, P Natarajan, DJ Erle, M Ott, H Goodarzi, F Fattahi
Cell Stem Cell, 2020-11-17;27(6):876-889.e12.
Species: Human
Sample Types: Organoid
Applications: ICC -
Goblet Cell Hyperplasia Increases SARS-CoV-2 Infection in COPD
Authors: JK Osan, SN Talukdar, F Feldmann, B Ann DeMont, K Jerome, KL Bailey, H Feldmann, M Mehedi
bioRxiv, 2020-11-12;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Effective screening of SARS-CoV-2 neutralizing antibodies in patient serum using lentivirus particles pseudotyped with SARS-CoV-2 spike glycoprotein
Authors: R Tandon, D Mitra, P Sharma, MG McCandless, SJ Stray, JT Bates, GD Marshall
Sci Rep, 2020-11-05;10(1):19076.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs
Authors: IT Lee, T Nakayama, CT Wu, Y Goltsev, S Jiang, PA Gall, CK Liao, LC Shih, CM Schürch, DR McIlwain, P Chu, NA Borchard, D Zarabanda, SS Dholakia, A Yang, D Kim, H Chen, T Kanie, CD Lin, MH Tsai, KM Phillips, R Kim, JB Overdevest, MA Tyler, CH Yan, CF Lin, YT Lin, DT Bau, GJ Tsay, ZM Patel, YA Tsou, A Tzankov, MS Matter, CJ Tai, TH Yeh, PH Hwang, GP Nolan, JV Nayak, PK Jackson
Nat Commun, 2020-10-28;11(1):5453.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
SARS-CoV-2 Mpro inhibitors and activity-based probes for patient-sample imaging
Authors: W Rut, K Groborz, L Zhang, X Sun, M Zmudzinski, B Pawlik, X Wang, D Jochmans, J Neyts, W M?ynarski, R Hilgenfeld, M Drag
Nat Chem Biol, 2020-10-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
IgM autoantibodies recognizing ACE2 are associated with severe COVID-19
Authors: L Casciola-R, DR Thiemann, F Andrade, MI Trejo Zamb, JE Hooper, E Leonard, J Spangler, AL Cox, C Machamer, L Sauer, O Laeyendeck, BT Garibaldi, SC Ray, C Mecoli, L Christophe, L Gutierrez-, Q Yang, D Hines, W Clarke, RE Rothman, A Pekosz, K Fenstermac, Z Wang, SL Zeger, A Rosen
medRxiv, 2020-10-15;0(0):.
Species: Human
Sample Types: Protein
Applications: Western Blot -
A nanoluciferase SARS-CoV-2 for rapid neutralization testing and screening of anti-infective drugs for COVID-19
Authors: X Xie, AE Muruato, X Zhang, KG Lokugamage, CR Fontes-Gar, J Zou, J Liu, P Ren, M Balakrishn, T Cihlar, CK Tseng, S Makino, VD Menachery, JP Bilello, PY Shi
Nat Commun, 2020-10-15;11(1):5214.
Species: Primate
Sample Types: Whole Cells
Applications: ICC, Neutralization -
Sex, androgens and regulation of pulmonary AR, TMPRSS2 and ACE2
Authors: M Baratchian, JM McManus, M Berk, F Nakamura, S Mukhopadhy, W Xu, S Erzurum, J Drazba, J Peterson, EA Klein, B Gaston, N Sharifi
bioRxiv, 2020-10-14;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids
Authors: L Pellegrini, A Albecka, DL Mallery, MJ Kellner, D Paul, AP Carter, LC James, MA Lancaster
Cell Stem Cell, 2020-10-13;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium
Authors: SP Sajuthi, P DeFord, Y Li, ND Jackson, MT Montgomery, JL Everman, CL Rios, E Pruesse, JD Nolin, EG Plender, ME Wechsler, ACY Mak, C Eng, S Salazar, V Medina, EM Wohlford, S Huntsman, DA Nickerson, S Germer, MC Zody, G Abecasis, HM Kang, KM Rice, R Kumar, S Oh, J Rodriguez-, EG Burchard, MA Seibold
Nat Commun, 2020-10-12;11(1):5139.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Cross-Sectional Evaluation of Humoral Responses against SARS-CoV-2 Spike.
Authors: Jérémie Prévost, Romain Gasser, Guillaume Beaudoin-Bussières, Jonathan Richard, Ralf Duerr, Annemarie Laumaea et al.
Cell Reports Medicine
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Functional genomic screens identify human host factors for SARS-CoV-2 and common cold coronaviruses
Authors: R Wang, CR Simoneau, J Kulsuptrak, M Bouhaddou, K Travisano, JM Hayashi, J Carlson-St, J Oki, K Holden, NJ Krogan, M Ott, AS Puschnik
bioRxiv : the preprint server for biology, 2020-09-24;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
SARS-CoV-2 Infection of Pluripotent Stem Cell-Derived Human Lung Alveolar Type 2 Cells Elicits a Rapid Epithelial-Intrinsic Inflammatory Response
Authors: J Huang, AJ Hume, KM Abo, RB Werder, C Villacorta, KD Alysandrat, ML Beermann, C Simone-Roa, J Lindstrom-, J Olejnik, EL Suder, E Bullitt, A Hinds, A Sharma, M Bosmann, R Wang, F Hawkins, EJ Burks, M Saeed, AA Wilson, E Mühlberger, DN Kotton
Cell Stem Cell, 2020-09-18;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Modeling COVID-19 with Human Pluripotent Stem Cell-Derived Cells Reveals Synergistic Effects of Anti-inflammatory Macrophages with ACE2 Inhibition Against SARS-CoV-2
Authors: F Duan, L Guo, L Yang, Y Han, A Thakur, BE Nilsson-Pa, P Wang, Z Zhang, CY Ma, X Zhou, T Han, T Zhang, X Wang, D Xu, X Duan, J Xiang, HF Tse, C Liao, W Luo, FP Huang, YW Chen, T Evans, RE Schwartz, B tenOever, DD Ho, S Chen, Q Lian, HJ Chen
Res Sq, 2020-09-15;0(0):.
Species: Human, Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: IHC, Neutralization -
Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2
Authors: I Busnadiego, S Fernbach, MO Pohl, U Karakus, M Huber, A Trkola, S Stertz, BG Hale
MBio, 2020-09-10;11(5):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Neuroinvasion of SARS-CoV-2 in human and mouse brain
Authors: E Song, C Zhang, B Israelow, A Lu-Culliga, AV Prado, S Skriabine, P Lu, OE Weizman, F Liu, Y Dai, K Szigeti-Bu, Y Yasumoto, G Wang, C Castaldi, J Heltke, E Ng, J Wheeler, MM Alfajaro, E Levavasseu, B Fontes, NG Ravindra, D Van Dijk, S Mane, M Gunel, A Ring, SA Jaffar Kaz, K Zhang, CB Wilen, TL Horvath, I Plu, S Haik, JL Thomas, A Louvi, SF Farhadian, A Huttner, D Seilhean, N Renier, K Bilguvar, A Iwasaki
bioRxiv : the preprint server for biology, 2020-09-08;0(0):.
Species: Human
Sample Types: Organoid
Applications: Neutralization -
Implications for SARS-CoV-2 Vaccine Design: Fusion of Spike Glycoprotein Transmembrane Domain to Receptor-Binding Domain Induces Trimerization
Authors: T Azad, R Singaravel, MJF Crupi, T Jamieson, J Dave, EEF Brown, R Rezaei, Z Taha, S Boulton, NT Martin, A Surendran, J Poutou, M Ghahremani, K Nouri, JT Whelan, J Duong, S Tucker, JS Diallo, JC Bell, CS Ilkow
Membranes (Basel), 2020-08-30;10(9):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
SARS-CoV-2 Infection of Pluripotent Stem Cell-derived Human Lung Alveolar Type 2 Cells Elicits a Rapid Epithelial-Intrinsic Inflammatory Response
Authors: J Huang, AJ Hume, KM Abo, RB Werder, C Villacorta, KD Alysandrat, ML Beermann, C Simone-Roa, J Olejnik, EL Suder, E Bullitt, A Hinds, A Sharma, M Bosmann, R Wang, F Hawkins, EJ Burks, M Saeed, AA Wilson, E Mühlberger, DN Kotton
bioRxiv, 2020-08-06;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Cross-sectional evaluation of humoral responses against SARS-CoV-2 Spike
Authors: J Prévost, R Gasser, G Beaudoin-B, J Richard, R Duerr, A Laumaea, SP Anand, G Goyette, S Ding, H Medjahed, A Lewin, J Perreault, T Tremblay, G Gendron-Le, N Gauthier, M Carrier, D Marcoux, A Piché, M Lavoie, A Benoit, V Loungnarat, G Brochu, M Desforges, PJ Talbot, GT Gould Maul, M Côté, C Therrien, B Serhir, R Bazin, M Roger, A Finzi
bioRxiv : the preprint server for biology, 2020-07-30;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Comparison of Transgenic and Adenovirus hACE2 Mouse Models for SARS-CoV-2 Infection
Authors: R Rathnasing, S Strohmeier, F Amanat, VL Gillespie, F Krammer, A García-Sas, L Coughlan, M Schotsaert, M Uccellini
bioRxiv, 2020-07-06;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Co-expression of SARS-CoV-2 entry genes in the superficial adult human conjunctival, limbal and corneal epithelium suggests an additional route of entry via the ocular surface
Authors: J Collin, R Queen, D Zerti, B Dorgau, M Georgiou, I Djidrovski, R Hussain, JM Coxhead, A Joseph, P Rooney, S Lisgo, F Figueiredo, L Armstrong, M Lako
Ocul Surf, 2020-06-03;0(0):.
Species: Human
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Infection of bat and human intestinal organoids by SARS-CoV-2
Authors: J Zhou, C Li, X Liu, MC Chiu, X Zhao, D Wang, Y Wei, A Lee, AJ Zhang, H Chu, JP Cai, CC Yip, IH Chan, KK Wong, OT Tsang, KH Chan, JF Chan, KK To, H Chen, KY Yuen
Nat. Med., 2020-05-13;0(0):.
Species: Bat, Human
Sample Types: Whole Cells
Applications: ICC -
Robust ACE2 protein expression localizes to the motile cilia of the respiratory tract epithelia and is not increased by ACE inhibitors or angiotensin receptor blockers
Authors: IT Lee, T Nakayama, CT Wu, Y Goltsev, S Jiang, PA Gall, CK Liao, LC Shih, CM Schurch, DR McIlwain, P Chu, NA Borchard, D Zarabanda, SS Dholakia, A Yang, D Kim, T Kanie, CD Lin, MH Tsai, KM Phillips, R Kim, JB Overdevest, MA Tyler, CH Yan, CF Lin, YT Lin, DT Bau, GJ Tsay, ZM Patel, YA Tsou, CJ Tai, TH Yeh, PH Hwang, GP Nolan, JV Nayak, PK Jackson
medRxiv, 2020-05-12;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Protocol and Reagents for Pseudotyping Lentiviral Particles with SARS-CoV-2 Spike Protein for Neutralization Assays
Authors: KHD Crawford, R Eguia, AS Dingens, AN Loes, KD Malone, CR Wolf, HY Chu, MA Tortorici, D Veesler, M Murphy, D Pettie, NP King, AB Balazs, JD Bloom
Viruses, 2020-05-06;12(5):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Identification of Candidate COVID-19 Therapeutics using hPSC-derived Lung Organoids
Authors: Y Han, L Yang, X Duan, F Duan, BE Nilsson-Pa, TM Yaron, P Wang, X Tang, T Zhang, Z Zhao, Y Bram, D Redmond, S Houghton, D Nguyen, D Xu, X Wang, S Uhl, Y Huang, JL Johnson, J Xiang, H Wang, FC Pan, LC Cantley, BR tenOever, DD Ho, T Evans, RE Schwartz, HJ Chen, S Chen
bioRxiv, 2020-05-05;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Liraglutide Attenuates Non-Alcoholic Fatty Liver Disease in Mice by Regulating the Local Renin-Angiotensin System
Authors: M Yang, X Ma, X Xuan, H Deng, Q Chen, L Yuan
Front Pharmacol, 2020-04-08;11(0):432.
Species: Human, Mouse
Sample Types: Cell Lysates, Tissue Homogenates
Applications: Western Blot -
SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor.
Authors: Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens T, Herrler G, Wu N, Nitsche A, Muller M, Drosten C, Pohlmann S
Cell, 2020-03-05;181(0):1-10.
Species: Primate - Chlorocebus aethiops (African Green Monkey)
Sample Types: Whole Cells
Applications: Neutralization -
Membrane Protein of Human Coronavirus NL63 Is Responsible for Interaction with the Adhesion Receptor
Authors: Antonina Naskalska, Agnieszka Dabrowska, Artur Szczepanski, Aleksandra Milewska, Krzysztof Piotr Jasik, Krzysztof Pyrc
Journal of Virology
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Protective effects of the Francisella tularensis δpdpC mutant against its virulent parental strain SCHU P9 in Cynomolgus macaques
Authors: D Tian, A Uda, Y Ami, A Hotta, ES Park, N Nagata, N Iwata-Yosh, A Yamada, K Hirayama, K Miura, Y Koyama, M Azaki, S Morikawa
Sci Rep, 2019-06-24;9(1):9193.
Species: Rhesus Macaca (Macaque)
Sample Types: Whole Tissue
Applications: IHC-P -
Translation of Angiotensin-Converting Enzyme 2 upon Liver- and Lung-Targeted Delivery of Optimized Chemically Modified mRNA
Authors: E Schrom, M Huber, M Aneja, C Dohmen, D Emrich, J Geiger, G Hasenpusch, A Herrmann-J, V Kretzschma, O Mykhailyk, T Pasewald, P Oak, A Hilgendorf, D Wohlleber, HG Hoymann, D Schaudien, C Plank, C Rudolph, R Kubisch-Do
Mol Ther Nucleic Acids, 2017-04-13;7(0):350-365.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Flow Cytometry, Western Blot -
HTCC: Broad Range Inhibitor of Coronavirus Entry
Authors: Aleksandra Milewska
PLoS ONE, 2016-06-01;11(6):e0156552.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: IHC -
Protein Kinase C-? Mediates Shedding of Angiotensin-Converting Enzyme 2 from Proximal Tubular Cells.
Authors: Fengxia Xiao, Joseph Zimpelman, Dylan Burger, Christopher Kennedy, Richard L Hébert, Kevin D Burns
Frontiers in Pharmacology, 2016-06-01;0(0):1663-9812.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: Western Blot -
Surface vimentin is critical for the cell entry of SARS-CoV.
Authors: Yu Y, Chien S, Chen I, Lai C, Tsay Y, Chang S, Chang M
J Biomed Sci, 2016-01-22;23(1):14.
Species: Primate - Chlorocebus pygerythrus (Vervet Monkey)
Sample Types: Whole Cells
Applications: Neutralization -
Characterization of angiotensin-converting enzyme 2 ectodomain shedding from mouse proximal tubular cells.
Authors: Xiao F, Zimpelmann J, Agaybi S, Gurley S, Puente L, Burns K
PLoS ONE, 2014-01-15;9(1):e85958.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East Respiratory Syndrome (MERS) Coronavirus infection.
Authors: Eckerle I, Muller M, Kallies S, Gotthardt D, Drosten C
Virol J, 2013-12-23;10(0):359.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein.
Authors: Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pohlmann S
J Virol, 2013-11-13;88(2):1293-307.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor.
Authors: Ge, Xing-Yi, Li, Jia-Lu, Yang, Xing-Lou, Chmura, Aleksei, Zhu, Guangjia, Epstein, Jonathan, Mazet, Jonna K, Hu, Ben, Zhang, Wei, Peng, Cheng, Zhang, Yu-Ji, Luo, Chu-Ming, Tan, Bing, Wang, Ning, Zhu, Yan, Crameri, Gary, Zhang, Shu-Yi, Wang, Lin-Fa, Daszak, Peter, Shi, Zheng-Li
Nature, 2013-10-30;503(7477):535-8.
Species: Bat, Civet, Human
Sample Types: Whole Cells
Applications: IHC -
Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts.
Authors: Bertram S, Heurich A, Lavender H, Gierer S, Danisch S, Perin P, Lucas JM, Nelson PS, Pohlmann S, Soilleux EJ
PLoS ONE, 2012-04-30;7(4):e35876.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron.
Authors: Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F
J. Biol. Chem., 2010-10-21;285(53):41935-46.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC-Fr, Western Blot -
Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2.
Authors: Glende J, Schwegmann-Wessels C, Al-Falah M, Pfefferle S, Qu X, Deng H, Drosten C, Naim HY, Herrler G
Virology, 2008-09-23;381(2):215-21.
Species: Primate - Chlorocebus pygerythrus (Vervet Monkey)
Sample Types: Cell Lysates
Applications: Western Blot -
Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system.
Authors: Epelman S, Tang WH, Chen SY, Van Lente F, Francis GS, Sen S
J. Am. Coll. Cardiol., 2008-08-26;52(9):750-4.
Species: Human
Sample Types: Plasma
Applications: Neutralization -
Lipid rafts are involved in SARS-CoV entry into Vero E6 cells.
Authors: Lu Y, Liu DX, Tam JP
Biochem. Biophys. Res. Commun., 2008-02-13;369(2):344-9.
Species: Primate - Chlorocebus pygerythrus (Vervet Monkey)
Sample Types: Whole Cells
Applications: Flow Cytometry -
Difference in receptor usage between severe acute respiratory syndrome (SARS) coronavirus and SARS-like coronavirus of bat origin.
Authors: Ren W, Qu X, Li W, Han Z, Yu M, Zhou P, Zhang SY, Wang LF, Deng H, Shi Z
J. Virol., 2007-12-12;82(4):1899-907.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted.
Authors: Inoue Y, Tanaka N, Tanaka Y, Inoue S, Morita K, Zhuang M, Hattori T, Sugamura K
J. Virol., 2007-05-23;81(16):8722-9.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Cell Lysates
Applications: Western Blot -
Synthetic reconstruction of zoonotic and early human severe acute respiratory syndrome coronavirus isolates that produce fatal disease in aged mice.
Authors: Rockx B, Sheahan T, Donaldson E, Harkema J, Sims A, Heise M, Pickles R, Cameron M, Kelvin D, Baric R
J. Virol., 2007-05-16;81(14):7410-23.
Species: Primate - Chlorocebus pygerythrus (Vervet Monkey)
Sample Types: Whole Cells
Applications: Neutralization -
Interferon-gamma and interleukin-4 downregulate expression of the SARS coronavirus receptor ACE2 in Vero E6 cells.
Authors: de Lang A, Osterhaus AD, Haagmans BL
Virology, 2006-07-24;353(2):474-81.
Species: Primate - Chlorocebus pygerythrus (Vervet Monkey)
Sample Types: Whole Cells
Applications: ICC -
Apical entry and release of severe acute respiratory syndrome-associated coronavirus in polarized Calu-3 lung epithelial cells.
Authors: Tseng CT, Tseng J, Perrone L, Worthy M, Popov V, Peters CJ
J. Virol., 2005-08-01;79(15):9470-9.
Species: Human
Sample Types: Whole Cells
Applications: ICC, Neutralization -
Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor.
Authors: Hofmann H, Geier M, Marzi A, Krumbiegel M, Peipp M, Fey GH, Gramberg T, Pohlmann S
Biochem. Biophys. Res. Commun., 2004-07-09;319(4):1216-21.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Multimerization- and glycosylation-dependent receptor binding of SARS-CoV-2 spike proteins
Authors: Kim M. Bouwman, Ilhan Tomris, Hannah L. Turner, Roosmarijn van der Woude, Tatiana M. Shamorkina, Gerlof P. Bosman et al.
PLOS Pathogens
-
SARS-CoV-2 Spike protein suppresses CTL-mediated killing by inhibiting immune synapse assembly
Authors: Anna Onnis, Emanuele Andreano, Chiara Cassioli, Francesca Finetti, Chiara Della Bella, Oskar Staufer et al.
Journal of Experimental Medicine
-
An Immunological Perspective: What Happened to Pregnant Women After Recovering From COVID-19?
Authors: Sijia Zhao, Ting Xie, Li Shen, Hong Liu, Liling Wang, Xixiang Ma et al.
Frontiers in Immunology
-
Development and validation of a rapid and easy-to-perform point-of-care lateral flow immunoassay (LFIA) for the detection of SARS-CoV-2 spike protein
Authors: Shamim Mohammad, Yuxia Wang, John Cordero, Christopher Watson, Robert Molestina, Sujatha Rashid et al.
Frontiers in Immunology
-
A Hybrid Soluble gp130/Spike-Nanobody Fusion Protein Simultaneously Blocks Interleukin-6 trans -Signaling and Cellular Infection with SARS-CoV-2
Authors: Julia Ettich, Julia Werner, Hendrik T. Weitz, Eva Mueller, Roland Schwarzer, Philipp A. Lang et al.
Journal of Virology
-
Attenuation of SARS-CoV-2 infection by losartan in human kidney organoids
Authors: Waleed Rahmani, Hyunjae Chung, Sarthak Sinha, Maxwell P. Bui-Marinos, Rohit Arora, Arzina Jaffer et al.
iScience
-
Uptake of severe acute respiratory syndrome coronavirus 2 spike protein mediated by angiotensin converting enzyme 2 and ganglioside in human cerebrovascular cells
Authors: Conor McQuaid, Alexander Solorzano, Ian Dickerson, Rashid Deane
Frontiers in Neuroscience
-
A Novel Glucocorticoid and Androgen Receptor Modulator Reduces Viral Entry and Innate Immune Inflammatory Responses in the Syrian Hamster Model of SARS-CoV-2 Infection
Authors: Savannah M. Rocha, Anna C. Fagre, Amanda S. Latham, Jason E. Cummings, Tawfik A. Aboellail, Philip Reigan et al.
Frontiers in Immunology
-
SARS-CoV-2 triggers pericyte-mediated cerebral capillary constriction
Authors: Chanawee Hirunpattarasilp, Greg James, Jaturon Kwanthongdee, Felipe Freitas, Jiandong Huo, Huma Sethi et al.
Brain
-
Tissue distribution of ACE2 protein in Syrian golden hamster (Mesocricetus auratus) and its possible implications in SARS-CoV-2 related studies
Authors: Suresh V, Parida D, Minz A, Senapati S
Front Pharmacol
-
Repeated ethanol exposure and withdrawal alters angiotensin-converting enzyme 2 expression in discrete brain regions: Implications for SARS-CoV-2 neuroinvasion
Authors: Balasubramanian N, James TD, Selvakumar GP et al.
Alcoholism: Clinical and Experimental Research
-
A Novel Soluble ACE2 Variant with Prolonged Duration of Action Neutralizes SARS-CoV-2 Infection in Human Kidney Organoids
Authors: Wysocki J, Ye M, Hassler L et al.
Journal of the American Society of Nephrology : JASN
-
Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models
Authors: Min-Wen Ku, Maryline Bourgine, Pierre Authié, Jodie Lopez, Kirill Nemirov, Fanny Moncoq et al.
Cell Host & Microbe
-
In well-differentiated primary human bronchial epithelial cells, TGF-beta 1 and TGF-beta 2 induce expression of furin
Authors: Michael J. O’Sullivan, Jennifer A. Mitchel, Chimwemwe Mwase, Maureen McGill, Phyllis Kanki, Jin-Ah Park
American Journal of Physiology-Lung Cellular and Molecular Physiology
-
Genetic Screens Identify Host Factors for SARS-CoV-2 and Common Cold Coronaviruses
Authors: Wang R, Simoneau CR, Kulsuptrakul J et al.
Cell
-
Flagellin From Pseudomonas aeruginosa Modulates SARS-CoV-2 Infectivity in Cystic Fibrosis Airway Epithelial Cells by Increasing TMPRSS2 Expression
Authors: Manon Ruffin, Jeanne Bigot, Claire Calmel, Julia Mercier, Maëlle Givelet, Justine Oliva et al.
Frontiers in Immunology
-
SARS-CoV-2 pseudovirus enters the host cells through spike protein-CD147 in an Arf6-dependent manner
Authors: Yun-Qi Zhou, Ke Wang, Xue-Yan Wang, Hong-Yong Cui, Yongxiang Zhao, Ping Zhu et al.
Emerging Microbes & Infections
-
Platelets mediate inflammatory monocyte activation by SARS-CoV-2 Spike protein
Authors: Li T, Yang Y, Li Y et al.
The Journal of clinical investigation
-
SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids
Authors: J Jansen, KC Reimer, JS Nagai, FS Varghese, GJ Overheul, M de Beer, R Roverts, D Daviran, LAS Fermin, B Willemsen, M Beukenboom, S Djudjaj, S von Stillf, LE van Eijk, M Mastik, M Bulthuis, WD Dunnen, H van Goor, JL Hillebrand, SH Triana, T Alexandrov, MC Timm, BT van den Be, M van den Br, Q Nlandu, J Heijnert, EMJ Bindels, RM Hoogenboez, F Mooren, C Kuppe, P Miesen, K Grünberg, T Ijzermans, EJ Steenberge, J Czogalla, MF Schreuder, N Sommerdijk, A Akiva, P Boor, VG Puelles, J Floege, TB Huber, COVID Moon, RP van Rij, IG Costa, RK Schneider, B Smeets, R Kramann
Cell Stem Cell, 2021-12-25;29(2):217-231.e8.
-
Long-Term Modeling of SARS-CoV-2 Infection of In Vitro Cultured Polarized Human Airway Epithelium
Authors: Siyuan Hao, Kang Ning, Cagla Aksu Kuz, Kai Vorhies, Ziying Yan, Jianming Qiu
mBio
-
Impact of COVID‐19 on thrombus composition and response to thrombolysis: Insights from a monocentric cohort population of COVID‐19 patients with acute ischemic stroke
Authors: Jean‐Philippe Desilles, Mialitiana Solo Nomenjanahary, Arturo Consoli, Véronique Ollivier, Dorothée Faille, Marie‐Charlotte Bourrienne et al.
Journal of Thrombosis and Haemostasis
-
Secretory IgA and T cells targeting SARS-CoV-2 spike protein are transferred to the breastmilk upon mRNA vaccination
Authors: Juliana Gonçalves, A. Margarida Juliano, Nádia Charepe, Marta Alenquer, Diogo Athayde, Filipe Ferreira et al.
Cell Reports Medicine
-
Effective vaccination strategy using SARS-CoV-2 spike cocktail against Omicron and other variants of concern
Authors: Juan Shi, Gang Wang, Jian Zheng, Abhishek K. Verma, Xiaoqing Guan, Moffat M. Malisheni et al.
npj Vaccines
-
SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury
Authors: Meng-Li Wu, Feng-Liang Liu, Jing Sun, Xin Li, Xiao-Yan He, Hong-Yi Zheng et al.
Signal Transduction and Targeted Therapy
-
Protocol for SARS-CoV-2 infection of kidney organoids derived from human pluripotent stem cells
Authors: Elena Garreta, Daniel Moya-Rull, Megan L. Stanifer, Vanessa Monteil, Patricia Prado, Andrés Marco et al.
STAR Protocols
-
LRRC15 is an inhibitory receptor blocking SARS-CoV-2 spike-mediated entry in trans
Authors: Jaewon Song, Ryan D. Chow, Mario Pena-Hernandez, Li Zhang, Skylar A. Loeb, Eui-Young So et al.
bioRxiv
-
Increased circulating microparticles contribute to severe infection and adverse outcomes of COVID-19 in patients with diabetes
Authors: Haoyu Sun, Yong Du, Rinki Kumar, Nicholas Buchkovich, Pingnian He
American Journal of Physiology-Heart and Circulatory Physiology
-
Comparison of transgenic and adenovirus hACE2 mouse models for SARS-CoV-2 infection
Authors: Rathnasinghe R, Strohmeier S, Amanat F et al.
Emerg Microbes Infect
-
Expression of SARS-CoV-2 Entry Factors in the Pancreas of Normal Organ Donors and Individuals with COVID-19
Authors: Kusmartseva I, Wu W, Syed F et al.
Cell Metabolism
-
Dynamics of ADAM17-Mediated Shedding of ACE2 Applied to Pancreatic Islets of Male db/db Mice
Authors: Kim Brint Pedersen, Harshita Chodavarapu, Constance Porretta, Leonie K. Robinson, Eric Lazartigues
Endocrinology
-
Inhibition of IRGM establishes a robust antiviral immune state to restrict pathogenic viruses
Authors: Parej Nath, Nishant Ranjan Chauhan, Kautilya Kumar Jena, Ankita Datey, Nilima Dinesh Kumar, Subhash Mehto et al.
EMBO reports
-
Data, Reagents, Assays and Merits of Proteomics for SARS-CoV-2 Research and Testing
Authors: Jana Zecha, Chien-Yun Lee, Florian P. Bayer, Chen Meng, Vincent Grass, Johannes Zerweck et al.
Molecular & Cellular Proteomics
-
Highly Specific Sigma Receptor Ligands Exhibit Anti-Viral Properties in SARS-CoV-2 Infected Cells
Authors: David A. Ostrov, Andrew P. Bluhm, Danmeng Li, Juveriya Qamar Khan, Megha Rohamare, Karthic Rajamanickam et al.
Pathogens
-
Functional differences among the spike glycoproteins of multiple emerging severe acute respiratory syndrome coronavirus 2 variants of concern
Authors: Qian Wang, Manoj S. Nair, Saumya Anang, Shijian Zhang, Hanh Nguyen, Yaoxing Huang et al.
iScience
-
Effective chimeric antigen receptor T cells against SARS-CoV-2
Authors: Xueyang Guo, Alexandra Kazanova, Stephanie Thurmond, H. Uri Saragovi, Christopher E. Rudd
iScience
-
Effect of Angiotensin-Converting-Enzyme Inhibitor and Angiotensin II Receptor Antagonist Treatment on ACE2 Expression and SARS-CoV-2 Replication in Primary Airway Epithelial Cells
Authors: Oghenemega Okoloko, Elizabeth R. Vanderwall, Lucille M. Rich, Maria P. White, Stephen R. Reeves, Whitney E. Harrington et al.
Frontiers in Pharmacology
-
Rhesus angiotensin converting enzyme 2 supports entry of severe acute respiratory syndrome coronavirus in Chinese macaques
Authors: Yunxin Chen, Li Liu, Qiang Wei, Hua Zhu, Hong Jiang, Xinming Tu et al.
Virology
-
SARS-CoV-2 infects neurons and induces neuroinflammation in a non-human primate model of COVID-19
Authors: Beckman D, Bonillas A, Diniz GB et al.
Cell reports
-
Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb
Authors: Khan M, Yoo SJ, Clijsters M et al.
Cell
-
Role of angiotensin-converting enzyme 2 and pericytes in cardiac complications of COVID-19 infection
Authors: Fulton A. Robinson, Ryan. P. Mihealsick, Brant M. Wagener, Peter Hanna, Megan D. Poston, Igor R. Efimov et al.
American Journal of Physiology-Heart and Circulatory Physiology
-
ACE2 and SCARF expression in human dorsal root ganglion nociceptors: implications for SARS-CoV-2 virus neurological effects
Authors: Stephanie Shiers, Pradipta R. Ray, Andi Wangzhou, Ishwarya Sankaranarayanan, Claudio Esteves Tatsui, Laurence D. Rhines et al.
Pain
-
Evolutionary remodelling of N‐terminal domain loops fine‐tunes SARS‐CoV‐2 spike
Authors: Diego Cantoni, Matthew J Murray, Mphatso D Kalemera, Samuel J Dicken, Lenka Stejskal, Georgina Brown et al.
EMBO reports
-
SARS-CoV-2 Omicron variant is attenuated for replication in a polarized human lung epithelial cell model
Authors: Christin Mache, Jessica Schulze, Gudrun Holland, Daniel Bourquain, Jean-Marc Gensch, Djin-Ye Oh et al.
Communications Biology
-
Cytoplasmic Tail Truncation of SARS-CoV-2 Spike Protein Enhances Titer of Pseudotyped Vectors but Masks the Effect of the D614G Mutation
Authors: Hsu-Yu Chen, Chun Huang, Lu Tian, Xiaoli Huang, Chennan Zhang, George N. Llewellyn et al.
Journal of Virology
-
Inhibition of SARS-CoV-2 viral entry upon blocking N- and O-glycan elaboration
Authors: Qi Yang, Thomas A Hughes, Anju Kelkar, Xinheng Yu, Kai Cheng, Sheldon Park et al.
eLife
-
CD169-mediated restrictive SARS-CoV-2 infection of macrophages induces pro-inflammatory responses
Authors: Jalloh S, Olejnik J, Berrigan J et al.
PLOS Pathogens
-
Endothelial cells are not productively infected by SARS‐CoV‐2
Authors: Lilian Schimmel, Keng Yih Chew, Claudia J Stocks, Teodor E Yordanov, Patricia Essebier, Arutha Kulasinghe et al.
Clinical & Translational Immunology
-
Cross-Sectional Evaluation of Humoral Responses against SARS-CoV-2 Spike
Authors: Jérémie Prévost, Romain Gasser, Guillaume Beaudoin-Bussières, Jonathan Richard, Ralf Duerr, Annemarie Laumaea et al.
Cell Reports Medicine
-
Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads
Authors: Elizabeth Taglauer, Yoel Benarroch, Kevin Rop, Elizabeth Barnett, Vishakha Sabharwal, Christina Yarrington et al.
Placenta
-
Membrane Protein of Human Coronavirus NL63 Is Responsible for Interaction with the Adhesion Receptor
Authors: Antonina Naskalska, Agnieszka Dabrowska, Artur Szczepanski, Aleksandra Milewska, Krzysztof Piotr Jasik, Krzysztof Pyrc
Journal of Virology
-
Angiotensin II-regulated microRNA 483-3p directly targets multiple components of the renin–angiotensin system
Authors: Jacqueline R. Kemp, Hamiyet Unal, Russell Desnoyer, Hong Yue, Anushree Bhatnagar, Sadashiva S. Karnik
Journal of Molecular and Cellular Cardiology
-
Prostaglandin E2 promotes intestinal repair through an adaptive cellular response of the epithelium
Authors: Hiroyuki Miyoshi, Kelli L VanDussen, Nicole P Malvin, Stacy H Ryu, Yi Wang, Naomi M Sonnek et al.
The EMBO Journal
-
Functional Effects of Cardiomyocyte Injury in COVID-19
Authors: Mustafa M. Siddiq, Angel T. Chan, Lisa Miorin, Arjun S. Yadaw, Kristin G. Beaumont, Thomas Kehrer et al.
Journal of Virology
-
SARS-CoV-2 infection triggers paracrine senescence and leads to a sustained senescence-associated inflammatory response
Authors: Shunya Tsuji, Shohei Minami, Rina Hashimoto, Yusuke Konishi, Tatsuya Suzuki, Tamae Kondo et al.
Nature Aging
-
Compound screen identifies the small molecule Q34 as an inhibitor of SARS-CoV-2 infection
Authors: Qi Cui, Gustavo Garcia, Mingzi Zhang, Cheng Wang, Hongzhi Li, Tao Zhou et al.
iScience
-
COVID-19–Associated Nonocclusive Fibrin Microthrombi in the Heart
Authors: Melanie C. Bois, Nicholas A. Boire, Andrew J. Layman, Marie-Christine Aubry, Mariam P. Alexander, Anja C. Roden et al.
Circulation
-
Generation of SARS-CoV-2 Spike Pseudotyped Virus for Viral Entry and Neutralization Assays: A 1-Week Protocol
Authors: Jose Manuel Condor Capcha, Guerline Lambert, Derek M. Dykxhoorn, Alessandro G. Salerno, Joshua M. Hare, Michael A. Whitt et al.
Frontiers in Cardiovascular Medicine
-
Identification of SARS-CoV-2 inhibitors using lung and colonic organoids
Authors: Yuling Han, Xiaohua Duan, Liuliu Yang, Benjamin E. Nilsson-Payant, Pengfei Wang, Fuyu Duan et al.
Nature
-
Immediate myeloid depot for SARS-CoV-2 in the human lung
Authors: Mélia Magnen, Ran You, Arjun A. Rao, Ryan T. Davis, Lauren Rodriguez, Camille R. Simoneau et al.
bioRxiv
-
DNA aptamers masking angiotensin converting enzyme 2 as an innovative way to treat SARS-CoV-2 pandemic
Authors: Alessandro Villa, Electra Brunialti, Jessica Dellavedova, Clara Meda, Monica Rebecchi, Matteo Conti et al.
Pharmacological Research
-
Identification of Required Host Factors for SARS-CoV-2 Infection in Human Cells
Authors: Zharko Daniloski, Tristan X. Jordan, Hans-Hermann Wessels, Daisy A. Hoagland, Silva Kasela, Mateusz Legut et al.
Cell
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse/Rat/Hamster ACE-2 Antibody
Average Rating: 5 (Based on 3 Reviews)
Have you used Human/Mouse/Rat/Hamster ACE-2 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: