Cultrex Reduced Growth Factor Basement Membrane Extract, Type 2, Pathclear
Try it on your cultures! Request a Sample of Cultrex RGF BME, Type 2
Cultrex Reduced Growth Factor Basement Membrane Extract, Type 2, Pathclear Summary
Cultrex Reduced Growth Factor Basement Membrane Extract (RGF BME ),Type 2 is specifically qualified to support the establishment and expansion of robust organoid cultures. It's composition mimics the in vivo microenvironment to improve take rate and growth of organoids.Key Benefits
• Qualified for use in organoid cell culture
• Commonly used robust and established organoid systems
• Reduced growth factor formulation provides a more defined culture system
• Quality controlled for performance consistency
Why Use Cultrex RGF Basement Membrane Extract, Type 2?
Cultrex Reduced Growth Factor Basement Membrane Extract (RGF BME), Type 2 is a soluble form of basement membrane purified from Engelbreth-Holm-Swarm (EHS) tumor. This extract provides a natural extracellular matrix hydrogel that polymerizes at 37°C to form a reconstituted basement membrane. Basement membranes are continuous sheets of specialized extracellular matrix that form an interface between endothelial, epithelial, muscle, or neuronal cells and their adjacent stroma and that play an essential role in tissue organization by influencing cell adhesion, migration, proliferation, and differentiation. The major components of BME include laminin, collagen IV, entactin, and heparan sulfate proteoglycans.
Cultrex RGF BME, Type 2 provides a proprietary formulation that has a high storage modulus and is designed for use in robust tissue organoid culture as well as other applications requiring an extracellular matrix scaffold.
Protocols Utilizing Cultrex RGF Basement Membrane Extract, Type 2 for Organoid Cell Culture.
Cultrex RGF BME, Type 2 is ideal for use as a scaffold for organoid and 3D cell culture. Listed below are protocols designed by our research and development groups for different types of organoids featuring Cultrex RGF BME, Type 2 as well as growth factors, media supplements, and small molecules from Bio-Techne.
Video of Cultrex BME Best Practices and Protocols.
Protocol for Mouse Enteric Organoid Culture.
Protocol for Human Gastric Organoid Culture.
Protocol for Human Liver Organoid Culture.
Protocol for Human Lung Organoid Culture.
Protocol for Human Intestinal Organoid Culture.
Protocol for Harvesting Organoids for Biochemical Analysis.
Specifications
Gelling Assay - Cultrex RGF BME, Type 2 gels in less than 30 minutes at 37 °C, and maintains the gelled form in culture medium for a minimum of 7 days at 37 °C.
Dome Assay Cultrex RGF BME, Type 2 forms and maintains stable 3-D dome structures on cell culture plates.
Limitations
For research use only. Not for diagnostic use.
Product Datasheets
Scientific Data
 View Larger
View Larger
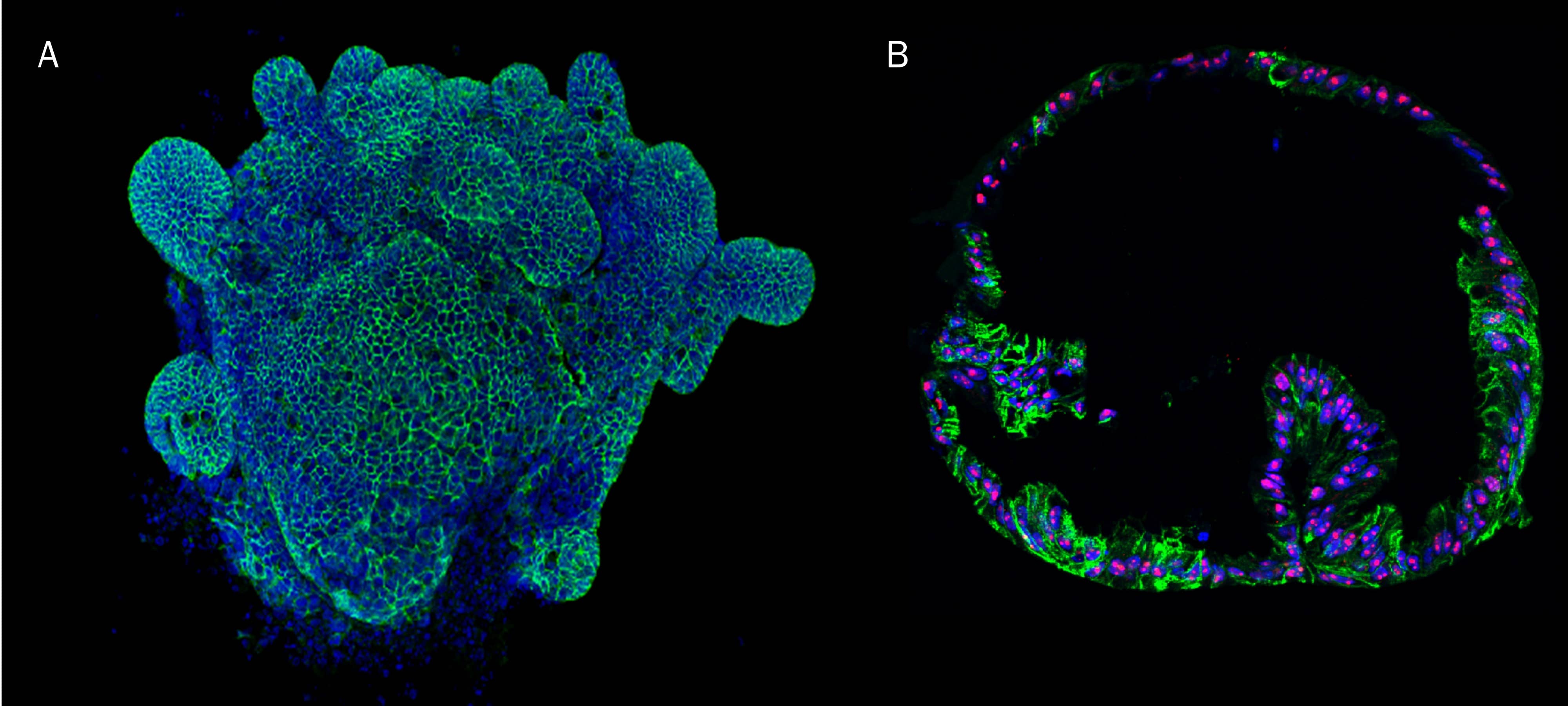
Mouse Intestinal Organoids Cultured in Cultrex RGF BME, Type 2. Mouse intestinal organoids cultured in Cultrex RGF BME, Type 2 were immersion fixed and processed for whole mount staining or paraffin embedding and sectioning for immunocytochemistry. A) Whole mount mouse intestinal organoids were stained using Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (green; Catalog # AF748) at 10 µg/mL for 3 hours at room temperature. Cells were counterstained with DAPI (blue). B) Paraffin-embedded mouse intestinal tissue stained for Human Cadherin‑17 Antibody (green; Catalog # MAB1032), Human Ki67/MKI67 Antibody (red; Catalog # AF7617), and counterstained with DAPI (blue).
 View Larger
View Larger
Human Lung Organoids Cultured in Cultrex RGF BME, Type 2. A) Representative brightfield image of human lung organoids cultured using Cultrex RGF BME, Type 2. B) Expression of Sox2 (green; Catalog # AF2018) and Acetylated Tubulin (red; Novus Biologicals, Catalog # NB600-567). C) Expression of p63/TP73L (green; Catalog # AF1916) and Cytokeratin 10 (red; Novus Biologicals, Catalog # NBP2-61736). D) Expression of Podoplanin (green; Catalog # AF3670) as a marker of type 1 alveolar cells.
 View Larger
View Larger
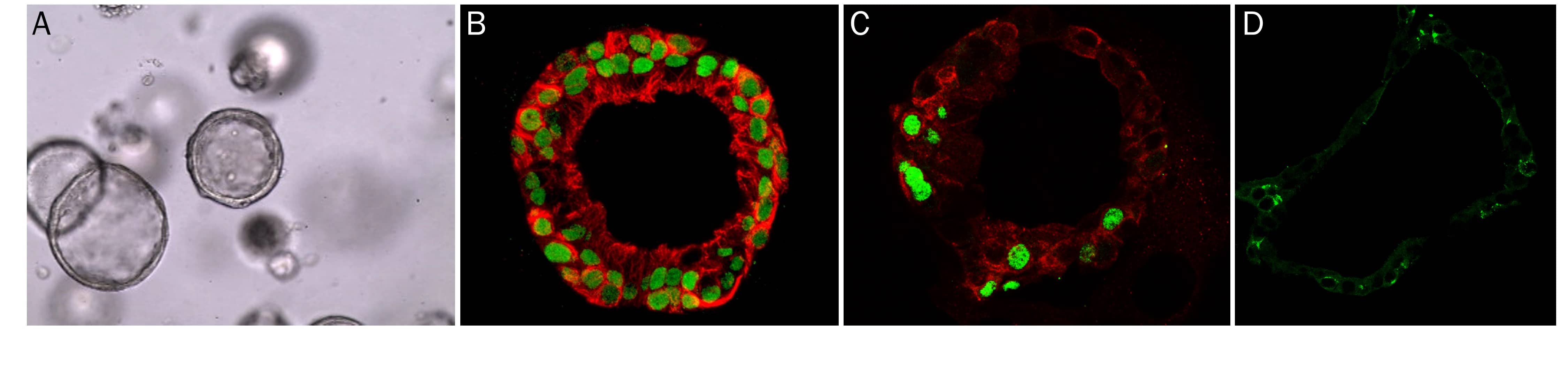
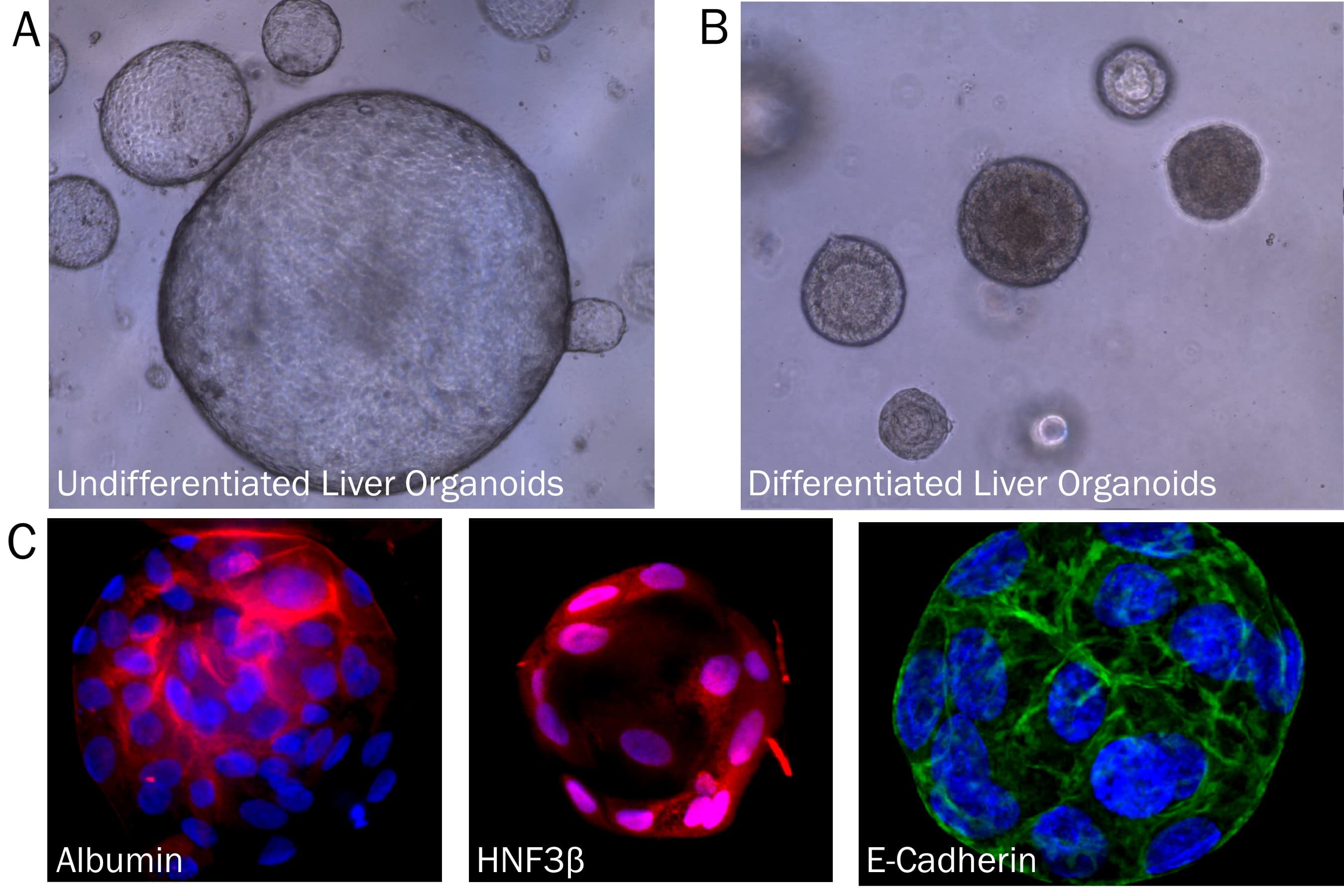
Liver Organoid Formation and Differentiation in Cultrex RGF BME, Type 2. Human Liver organoids were derived from human biopsy tissue. Undifferentiated organoids were formed by embedding dissociated tissue in Cultrex RGF BME, Type 2 and culturing in specialized media. The organoids were differentiated using media containing Recombinant Human FGF-19 (Catalog # 969-FG), DAPT (Catalog # 2634), and Dexamethasone (Catalog # 1126). A) Undifferentiated liver organoids. B) Liver organoids shrink as they differentiate. C) Representative images of differentiated liver organoids expression hepatocyte markers, Albumin and HNF3 beta, as well as E-Cadherin.
 View Larger
View Larger
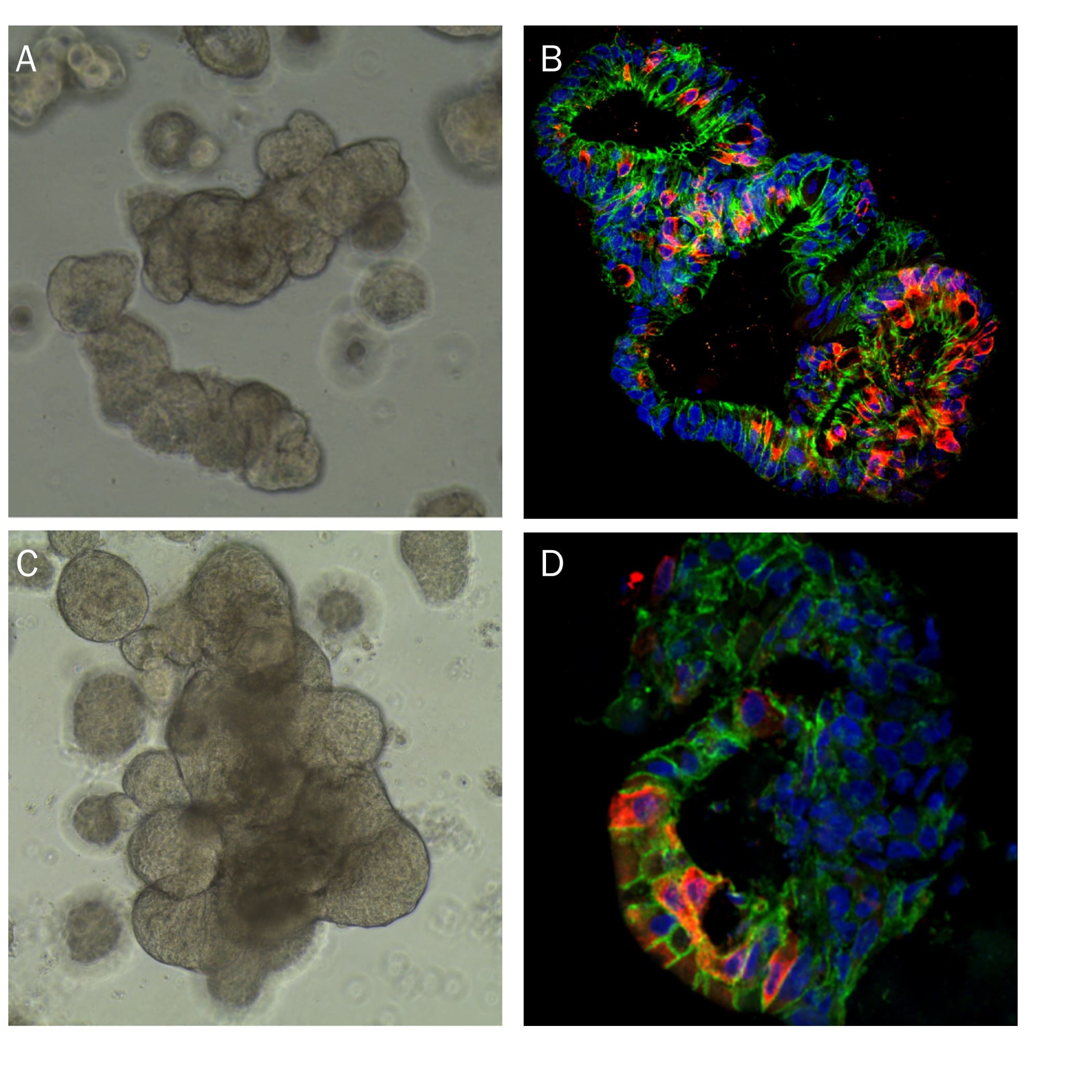
Human Intestinal Organoids Cultured using Cultrex RGF BME, Type 2. Human transverse colon organoids (A,B) and human ileum organoids (C, D) were grown using cells isolated from transverse colon and ileum biopsy tissue, respectively. Organoids were embedded in Cultrex RGF BME, Type 2 as a scaffold matrix. A) Brightfield image of human transverse organoids. B) Human transverse organoid stained using Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (green; Catalog # AF748), a MUC2 Antibody (red; Catalog # NBP2-44431), and DAPI (blue). C) Brightfield image of human ileum organoids. D) Human ileum organoid stained using a Aldolase B Antibody (red; Catalog # NBP2-15345), a Human Cadherin-17 Antibody (green; Catalog # MAB1032, and DAPI (blue).
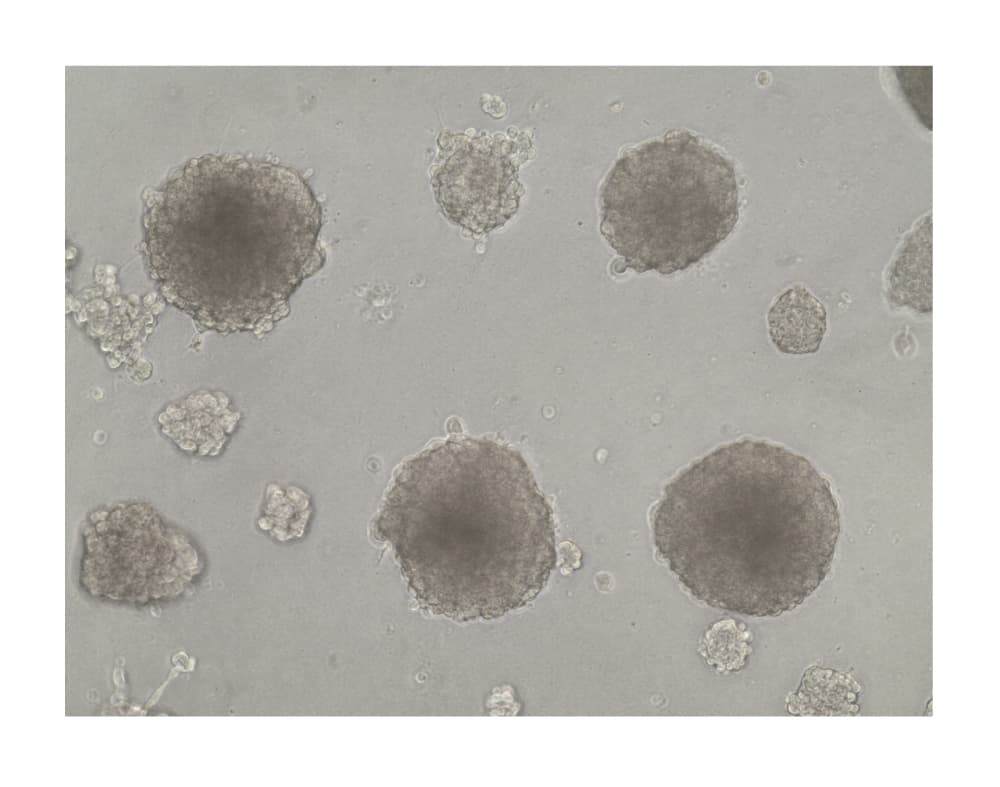 View Larger
View Larger
Formation of HepG2 Spheroids in Cultrex RGF BME, Type 2. Hepatocyte spheroids were formed by plating HepG2 liver hepatocellular carcinoma cells (10,000 cells per well) in a 24-well plate coated with 5 mg/mL of Cultrex RGF BME, Type 2. Spheroids were cultured for 21 days prior imaging by brightfield microscopy.
Citations for Cultrex Reduced Growth Factor Basement Membrane Extract, Type 2, Pathclear
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
88
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Mechanosensitive calcium channels and integrins coordinate the reprogramming of colorectal cancer cells into a fetal-like state
Authors: van der Net, MC;Vliem, MJ;Kemp, LJS;Perez-Gonzalez, C;Haddad, TS;Strating, EA;Krotenberg-Garcia, A;Houtekamer, RM;Pannekoek, WJ;van den Anker, KB;Zwakenberg, S;Monster, JL;van der Horst, SEM;Snippert, HJG;Khalil, AA;van Rheenen, J;Suijkerbuijk, SJE;Kranenburg, O;Simmer, F;Nagtegaal, ID;Vignjevic, DM;Gloerich, M;
Cell reports 2025-09-22
-
Tumor nutrient stress gives rise to a drug tolerant cell state in pancreatic cancer
Authors: Sheehan, C;Hu, L;Cognet, G;Croley, G;Nguyen, TT;Thomas-Toth, A;Agovino, D;Jonker, PB;Sadullozoda, M;Ziolkowski, LM;Martin, JK;Beutel, AK;Dano, R;Khan, MA;Halbrook, CJ;Macleod, KF;Weber, CR;LaBelle, JL;Muir, A;
bioRxiv : the preprint server for biology 2025-09-08
-
Defining the antitumor mechanism of action of a clinical-stage compound as a selective degrader of the nuclear pore complex
Authors: Yuan, L;Ji, W;Dwyer, BG;Lu, J;Bian, J;Colombo, GM;Martinez, MJ;Fernandez, D;Phillips, NA;Tang, MT;Zhou, CW;Quispe Calla, NE;Guzman Huancas, C;Eckart, M;Tran, J;Jones, HM;Qiu, T;Doench, JG;Rees, MG;Roth, JA;Cameron, MD;Charville, GW;Kuo, CJ;Dixon, SJ;Zhang, T;Hinshaw, SM;Gray, NS;Corsello, SM;
Cancer discovery 2025-09-02
-
TCR-engineered T cells targeting a shared ?-catenin mutation eradicate solid tumors
Authors: Eggebø, MS;Heinzelbecker, J;Palashati, H;Chandler, N;Tran, TT;Li, Y;Yang, W;Laos, M;Blaas, I;Rustad, EH;Bollineni, RC;Delic-Sarac, M;Lund-Johansen, F;Nielsen, MM;Olweus, J;
Nature immunology 2025-08-27
-
EMT-ciliary signaling in quasi-mesenchymal-stem-like cells drives therapeutic resistance and is a druggable vulnerability in triple-negative breast cancer
Authors: Tessier, CE;Derrien, J;Dupuy, AMM;Pelé, T;Moquet, M;Roul, J;Douillard, E;El Harrif, C;Pinson, X;Le Gallo, M;Godey, F;Tas, P;Viel, R;Grasset, E;Prigent, C;Letouzé, É;Suzanne, P;Dallemagne, P;Campone, M;Weinberg, RA;Lees, JA;Juin, PP;Guen, VJ;
EMBO molecular medicine 2025-08-26
-
Small round cell sarcoma tumoroid biobank reveals CIC::DUX4 sarcoma vulnerability to MCL-1 inhibition
Authors: Ringnalda, FCAS;van Son, GJF;Verweij, LHG;Kim, SY;Amo-Addae, V;Flucke, UE;Hiemcke-Jiwa, LS;Langenberg, KPS;Bramer, JAM;Heimans, L;van de Sande, MAJ;van Houdt, WJ;van Noesel, MM;Kerstens, HHD;Santoso, M;Seifert, G;Delattre, O;Scotlandi, K;Geoerger, B;Merks, JHM;Molenaar, JJ;van Boxtel, R;van de Wetering, M;Sanders, K;Clevers, H;
Nature communications 2025-08-21
-
TRIM24 as a therapeutic target in endocrine treatment-resistant breast cancer
Authors: Padrão, N;Gregoricchio, S;Eickhoff, N;Dong, J;Luzietti, L;Bossi, D;Severson, TM;Siefert, J;Calcinotto, A;Buluwela, L;Donaldson Collier, M;Ali, S;Young, L;Theurillat, JP;Varelija, D;Zwart, W;
Proceedings of the National Academy of Sciences of the United States of America 2025-08-19
-
Bacterial ADP-heptose triggers stem cell regeneration in the intestinal epithelium following injury
Authors: Goyal, S;Guo, CX;Singh, O;Ranger, A;Harrigan, CF;Meade, J;Luchak, A;Tsang, DK;Gaisano, HY;Gao, N;Yuzwa, SA;Wrana, JL;Philpott, DJ;Gray-Owen, SD;Girardin, SE;
Cell stem cell 2025-08-07
-
Targeting the NAT10/XIST/YAP1 Axis-Mediated Vascular Abnormalization Enhances Immune Checkpoint Blockade in Gastric Cancer
Authors: Lei, X;Zheng, B;Peng, Y;Zhang, G;Cheng, X;Li, W;He, J;Li, F;Ling, R;Fu, Z;Yang, Q;Ye, G;Li, G;
International journal of biological sciences 2025-07-28
-
hnRNPL phase separation activates PIK3CB transcription and promotes glycolysis in ovarian cancer
Authors: Qin, F;Wang, Y;Yang, C;Ren, Y;Wei, Q;Tang, Y;Xu, J;Wang, H;Luo, F;Luo, Q;Luo, X;Liu, X;Yang, D;Zuo, X;Yang, Y;Cheng, C;Xu, J;Wang, W;Liu, T;Yi, P;
Nature communications 2025-05-24
-
A patient-derived ovarian cancer organoid platform to study susceptibility to natural killer cells
Authors: Mercadante, M;Scheben, A;Estrada, J;Savas-Carstens, J;Sullivan, W;Housel, N;Volpari, T;Hebner, J;Sapar, M;Rusielewicz, T;Monsma, FJ;Semrau, S;Wang, Y;Martin, LA;
bioRxiv : the preprint server for biology 2025-03-07
-
Enhanced fluorescence lifetime imaging microscopy denoising via principal component analysis
Authors: Soltani, S;Paulson, JG;Fong, EJ;Mumenthaler, SM;Armani, AM;
bioRxiv : the preprint server for biology 2025-03-02
-
Individualized Pooled CRISPR/Cas9 Screenings Identify CDK2 as a Druggable Vulnerability in a Canine Mammary Carcinoma Patient
Authors: Inglebert, M;Dettwiler, M;He, C;Markkanen, E;Opitz, L;Naguleswaran, A;Rottenberg, S;
Veterinary sciences 2025-02-18
-
Enhanced viability and functional maturity of iPSC-derived islet organoids by collagen-VI-enriched ECM scaffolds
Authors: Zhu, D;Chen, Z;Guo, K;Xie, Q;Zou, Y;Mou, Q;Zhou, Z;Jin, G;
Cell stem cell 2025-02-15
-
Sox9 inhibits Activin A to promote biliary maturation and branching morphogenesis
Authors: Hrncir, HR;Goodloe, B;Bombin, S;Hogan, CB;Jadi, O;Gracz, AD;
Nature communications 2025-02-15
-
Dual RNA sequencing of a co-culture model of Pseudomonas aeruginosa and human 2D upper airway organoids
Authors: Pleguezuelos-Manzano, C;Beenker, WAG;van Son, GJF;Begthel, H;Amatngalim, GD;Beekman, JM;Clevers, H;den Hertog, J;
Scientific reports 2025-01-17
-
miR-449a/miR-340 reprogram cell identity and metabolism in fusion-negative rhabdomyosarcoma
Authors: Pozzo, E;Yedigaryan, L;Giarratana, N;Wang, CC;Garrido, GM;Degreef, E;Marini, V;Rinaldi, G;van der Veer, BK;Sassi, G;Eelen, G;Planque, M;Fanzani, A;Koh, KP;Carmeliet, P;Yustein, JT;Fendt, SM;Uyttebroeck, A;Sampaolesi, M;
Cell reports 2025-01-11
-
Establishment of Stable Knockdown of MACC1 Oncogene in Patient-Derived Ovarian Cancer Organoids
Authors: Hierlmayer, S;Hladchenko, L;Reichenbach, J;Klein, C;Mahner, S;Trillsch, F;Kessler, M;Chelariu-Raicu, A;
Methods and protocols 2024-12-20
-
PALS1-dependent modulations of mRNA profiles in MDCK II cells grown in non-confluent monolayers and three-dimensional cysts
Authors: Schughart, K;Möller-Kerutt, A;Höffken, V;Nedvetsky, P;Groh, AC;Braun, DA;Pavenstädt, H;Weide, T;
BMC genomic data 2024-11-29
-
KANK1 promotes breast cancer development by compromising Scribble-mediated Hippo activation
Authors: Guo, SS;Liu, Z;Wang, GM;Sun, Z;Yu, K;Fawcett, JP;Buettner, R;Gao, B;Fässler, R;
Nature communications 2024-11-29
-
Carbonic anhydrase 2 facilitates sorafenib resistance by counteracting MCT4-mediated intracellular pH dysregulation in HCC
Authors: Lu, H;Liu, H;Yan, R;Ma, W;Liu, H;Liu, R;Sun, Y;Ye, L;Gao, P;Jia, W;Zhang, P;Zhang, H;
Cell reports 2024-11-27
-
Establishment and characterization of mouse metabolic dysfunction-associated steatohepatitis-related hepatocellular carcinoma organoids
Authors: Kim, S;Jeong, N;Park, J;Noh, H;Lee, JO;Yu, SJ;Ku, JL;
Scientific reports 2024-11-10
-
Differential stiffness between brain vasculature and parenchyma promotes metastatic infiltration through vessel co-option
Authors: Uroz, M;Stoddard, AE;Sutherland, BP;Courbot, O;Oria, R;Li, L;Ravasio, CR;Ngo, MT;Yang, J;Tefft, JB;Eyckmans, J;Han, X;Elosegui-Artola, A;Weaver, VM;Chen, CS;
Nature cell biology 2024-10-24
-
Systemic and local chronic inflammation and hormone disposition promote a tumor-permissive environment for breast cancer in older women
Authors: Carleton, N;Lee, S;Li, R;Zou, J;Brown, DD;Hooda, J;Chang, A;Kumar, R;Klei, LR;Rigatti, LH;Newsome, J;John Mary, DJS;Atkinson, JM;West, RE;Nolin, TD;Oberly, PJ;Huang, Z;Poirier, D;Diego, EJ;Lucas, PC;Tseng, G;Lotze, MT;McAuliffe, PF;Zervantonakis, IK;Oesterreich, S;Lee, AV;
bioRxiv : the preprint server for biology 2024-10-21
-
m6A-driven NAT10 translation facilitates fatty acid metabolic rewiring to suppress ferroptosis and promote ovarian tumorigenesis through enhancing ACOT7 mRNA acetylation
Authors: Liu, Y;Li, J;Xu, J;Long, Y;Wang, Y;Liu, X;Hu, J;Wei, Q;Luo, Q;Luo, F;Qin, F;Yi, Q;Yang, Y;Dang, Y;Xu, J;Liu, T;Yi, P;
Oncogene 2024-10-10
-
Transient splicing inhibition causes persistent DNA damage and chemotherapy vulnerability in triple-negative breast cancer
Authors: Caggiano, C;Petrera, V;Ferri, M;Pieraccioli, M;Cesari, E;Di Leone, A;Sanchez, MA;Fabi, A;Masetti, R;Naro, C;Sette, C;
Cell reports 2024-09-13
-
NUAK1 activates STAT5/GLI1/SOX2 signaling to enhance cancer cell expansion and drives chemoresistance in gastric cancer
Authors: Cao, L;Lin, G;Fan, D;Weng, K;Chen, Y;Wang, J;Li, P;Zheng, C;Huang, C;Xie, J;
Cell reports 2024-07-23
-
A YAP-centered mechanotransduction loop drives collective breast cancer cell invasion
Authors: Khalil, AA;Smits, D;Haughton, PD;Koorman, T;Jansen, KA;Verhagen, MP;van der Net, M;van Zwieten, K;Enserink, L;Jansen, L;El-Gammal, AG;Visser, D;Pasolli, M;Tak, M;Westland, D;van Diest, PJ;Moelans, CB;Roukens, MG;Tavares, S;Fortier, AM;Park, M;Fodde, R;Gloerich, M;Zwartkruis, FJT;Derksen, PW;de Rooij, J;
Nature communications 2024-06-07
-
H2AX promotes replication fork degradation and chemosensitivity in BRCA-deficient tumours
Authors: Dibitetto, D;Liptay, M;Vivalda, F;Dogan, H;Gogola, E;González Fernández, M;Duarte, A;Schmid, JA;Decollogny, M;Francica, P;Przetocka, S;Durant, ST;Forment, JV;Klebic, I;Siffert, M;de Bruijn, R;Kousholt, AN;Marti, NA;Dettwiler, M;Sørensen, CS;Tille, JC;Undurraga, M;Labidi-Galy, I;Lopes, M;Sartori, AA;Jonkers, J;Rottenberg, S;
Nature communications 2024-05-24
-
Molecular Characterization and Therapeutic Opportunities in KRAS Wildtype Pancreatic Ductal Adenocarcinoma
Authors: Desai, A;Xiao, AH;Choi, D;Toruner, MD;Walden, D;Halfdanarson, TR;Alberts, S;McWilliams, RR;Mahipal, A;Ahn, D;Babiker, H;Stybayeva, G;Revzin, A;Kizilbash, S;Adjei, A;Bekaii-Saab, T;Mansfield, AS;Carr, RM;Ma, WW;
Cancers 2024-05-13
-
Enhancing therapeutic efficacy in luminal androgen receptor triple-negative breast cancer: exploring chidamide and enzalutamide as a promising combination strategy
Authors: Zhao, YX;Wang, H;Zhang, SW;Zhang, WX;Jiang, YZ;Shao, ZM;
Cancer cell international 2024-04-09
-
Renin-Angiotensin Inhibitor, Captopril, Attenuates Growth of Patient-Derived Colorectal Liver Metastasis Organoids
Authors: Riddiough, GE;Fifis, T;Muralidharan, V;Christophi, C;Tran, BM;Perini, MV;Vincan, E;
International journal of molecular sciences 2024-03-14
-
Sox9 links biliary maturation to branching morphogenesis
Authors: Hrncir, HR;Bombin, S;Goodloe, B;Hogan, CB;Jadi, O;Gracz, AD;
bioRxiv : the preprint server for biology 2024-01-16
-
Urine-derived bladder cancer organoids (urinoids) as a tool for cancer longitudinal response monitoring and therapy adaptation
Authors: Viergever, BJ;Raats, DAE;Geurts, V;Mullenders, J;Jonges, TN;van der Heijden, MS;van Es, JH;Kranenburg, O;Meijer, RP;
British journal of cancer 2023-12-15
-
STAT2 Controls Colorectal Tumorigenesis and Resistance to Anti-Cancer Drugs
Authors: Chiriac, MT;Hracsko, Z;Becker, C;Neurath, MF;
Cancers 2023-11-15
-
Functional screening of amplification outlier oncogenes in organoid models of early tumorigenesis
Authors: Salahudeen, AA;Seoane, JA;Yuki, K;Mah, AT;Smith, AR;Kolahi, K;De la O, SM;Hart, DJ;Ding, J;Ma, Z;Barkal, SA;Shukla, ND;Zhang, CH;Cantrell, MA;Batish, A;Usui, T;Root, DE;Hahn, WC;Curtis, C;Kuo, CJ;
Cell reports 2023-11-01
-
Luminal breast cancer identity is determined by loss of glucocorticoid receptor activity
Authors: Prekovic, S;Chalkiadakis, T;Roest, M;Roden, D;Lutz, C;Schuurman, K;Opdam, M;Hoekman, L;Abbott, N;Tesselaar, T;Wajahat, M;Dwyer, AR;Mayayo-Peralta, I;Gomez, G;Altelaar, M;Beijersbergen, R;Gy?rffy, B;Young, L;Linn, S;Jonkers, J;Tilley, W;Hickey, T;Vareslija, D;Swarbrick, A;Zwart, W;
EMBO molecular medicine 2023-10-30
-
Single-nucleus RNA sequencing reveals heterogenous microenvironments and specific drug response between cervical squamous cell carcinoma and adenocarcinoma
Authors: Lin, S;Sun, Y;Cao, C;Zhu, Z;Xu, Y;Liu, B;Hu, B;Peng, T;Zhi, W;Xu, M;Ding, W;Ren, F;Ma, D;Li, G;Wu, P;
EBioMedicine 2023-10-23
-
KLHL29-mediated DDX3X degradation promotes chemosensitivity by abrogating cell cycle checkpoint in triple-negative breast cancer
Authors: Yao, L;Hao, Q;Wang, M;Chen, Y;Cao, H;Zhang, Q;Yu, K;Jiang, Y;Shao, Z;Zhou, X;Xu, Y;
Oncogene 2023-10-16
-
Patient-derived organoid culture in epithelial ovarian cancers-Techniques, applications, and future perspectives
Authors: Chan, WS;Mo, X;Ip, PPC;Tse, KY;
Cancer medicine 2023-09-30
-
Jaw Bone Invasion of Oral Squamous Cell Carcinoma Is Associated with Osteoclast Count and Expression of Its Regulating Proteins in Patients and Organoids
Authors: de Kort, WWB;Haakma, WE;van Es, RJJ;Gawlitta, D;Driehuis, E;Gansevoort, M;Willems, SM;
Journal of clinical medicine 2023-09-18
-
Activation of invasion by oncogenic reprogramming of cholesterol metabolism via increased NPC1 expression and macropinocytosis
Authors: Skorda, A;Lauridsen, AR;Wu, C;Huang, J;Mrackova, M;Winther, NI;Jank, V;Sztupinszki, Z;Strauss, R;Bilgin, M;Maeda, K;Liu, B;Luo, Y;Jäättelä, M;Kallunki, T;
Oncogene 2023-07-07
-
Immune regulatory function of cancer-associated fibroblasts in non-small cell lung cancer
Authors: Lee, H;Hwang, M;Jang, S;Um, SW;
Tuberculosis and respiratory diseases 2023-06-22
-
Creation of EGD-Derived Gastric Cancer Organoids to Predict Treatment Responses
Authors: McDonald, HG;Harper, MM;Hill, K;Gao, A;Solomon, AL;Bailey, CJ;Lin, M;Barry-Hundeyin, M;Cavnar, MJ;Mardini, SH;Pandalai, PJ;Patel, RA;Kolesar, JM;Rueckert, JA;Hookey, L;Ropeleski, M;Merchant, SJ;Kim, J;Gao, M;
Cancers 2023-06-02
-
A histone deacetylase 3 and mitochondrial complex I axis regulates toxic formaldehyde production
Authors: Wit, N;Gogola, E;West, JA;Vornb�umen, T;Seear, RV;Bailey, PSJ;Burgos-Barragan, G;Wang, M;Krawczyk, P;Huberts, DHEW;Gergely, F;Matheson, NJ;Kaser, A;Nathan, JA;Patel, KJ;
Science advances 2023-05-19
-
Multi-omics analysis reveals distinct non-reversion mechanisms of PARPi resistance in BRCA1- versus BRCA2-deficient mammary tumors
Authors: Bhin, J;Paes Dias, M;Gogola, E;Rolfs, F;Piersma, SR;de Bruijn, R;de Ruiter, JR;van den Broek, B;Duarte, AA;Sol, W;van der Heijden, I;Andronikou, C;Kaiponen, TS;Bakker, L;Lieftink, C;Morris, B;Beijersbergen, RL;van de Ven, M;Jimenez, CR;Wessels, LFA;Rottenberg, S;Jonkers, J;
Cell reports 2023-05-19
-
ONECUT2 regulates RANKL-dependent enterocyte and microfold cell differentiation in the small intestine; a multi-omics study
Authors: MV Luna Velez, HK Neikes, RR Snabel, Y Quint, C Qian, A Martens, GJC Veenstra, MR Freeman, SJ van Heerin, M Vermeulen
Nucleic Acids Research, 2023-02-22;0(0):. 2023-02-22
-
Mutated axon guidance gene PLXNB2 sustains growth and invasiveness of stem cells isolated from cancers of unknown primary
Authors: S Brundu, V Napolitano, G Franzolin, E Lo Cascio, R Mastranton, G Sardo, E Cascardi, F Verginelli, S Sarnataro, G Gambardell, A Pisacane, A Arcovito, C Boccaccio, PM Comoglio, E Giraudo, L Tamagnone
Embo Molecular Medicine, 2023-02-01;0(0):e16104. 2023-02-01
-
Hypoxia-Driven Changes in a Human Intestinal Organoid Model and the Protective Effects of Hydrolyzed Whey
Authors: IH de Lange, C van Gorp, KRI Massy, L Kessels, N Kloosterbo, A Bjørnshave, M Stampe Ost, JGMC Damoiseaux, JPM Derikx, WG van Gemert, TGAM Wolfs
Nutrients, 2023-01-12;15(2):. 2023-01-12
-
Metabolic Activation of Benzo[a]pyrene by Human Tissue Organoid Cultures
Authors: AL Caipa Garc, JE Kucab, H Al-Serori, RSS Beck, F Fischer, M Hufnagel, A Hartwig, A Floeder, S Balbo, H Francies, M Garnett, M Huch, J Drost, M Zilbauer, VM Arlt, DH Phillips
International Journal of Molecular Sciences, 2022-12-29;24(1):. 2022-12-29
-
AKTIP loss is enriched in ERalpha-positive breast cancer for tumorigenesis and confers endocrine resistance
Authors: ASN Ng, S Zhang, VCY Mak, Y Zhou, Y Yuen, R Sharma, Y Lu, G Zhuang, W Zhao, HH Pang, LWT Cheung
Cell Reports, 2022-12-13;41(11):111821. 2022-12-13
-
Metastatic recurrence in colorectal cancer arises from residual EMP1+ cells
Authors: A Cañellas-S, C Cortina, X Hernando-M, S Palomo-Pon, EJ Mulholland, G Turon, L Mateo, S Conti, O Roman, M Sevillano, F Slebe, D Stork, A Caballé-Me, A Berenguer-, A Álvarez-Va, N Fenderico, L Novellasde, L Jiménez-Gr, T Sipka, L Bardia, P Lorden, J Colombelli, H Heyn, X Trepat, S Tejpar, E Sancho, DVF Tauriello, S Leedham, CS Attolini, E Batlle
Nature, 2022-11-09;611(7936):603-613. 2022-11-09
-
Computational pharmacogenomic screen identifies drugs that potentiate the anti-breast cancer activity of statins
Authors: JE van Leeuwe, W Ba-Alawi, E Branchard, J Cruickshan, W Schormann, J Longo, J Silvester, PL Gross, DW Andrews, DW Cescon, B Haibe-Kain, LZ Penn, DMA Gendoo
Nature Communications, 2022-10-24;13(1):6323. 2022-10-24
-
Division of labor within the DNA damage tolerance system reveals non-epistatic and clinically actionable targets for precision cancer medicine
Authors: A Spanjaard, R Shah, D de Groot, OA Buoninfant, B Morris, C Lieftink, C Pritchard, LM Zürcher, S Ormel, JJI Catsman, R de Korte-G, B Siteur, N Proost, T Boadum, M van de Ven, JY Song, M Kreft, PCM van den Be, RL Beijersber, H Jacobs
Nucleic Acids Research, 2022-07-22;50(13):7420-7435. 2022-07-22
-
The metastatic spread of breast cancer accelerates during sleep
Authors: Z Diamantopo, F Castro-Gin, FD Schwab, C Foerster, M Saini, S Budinjas, K Strittmatt, I Krol, B Seifert, V Heinzelman, C Kurzeder, C Rochlitz, M Vetter, WP Weber, N Aceto
Nature, 2022-06-22;607(7917):156-162. 2022-06-22
-
RNA splicing is a key mediator of tumour cell plasticity and a therapeutic vulnerability in colorectal cancer
Authors: AE Hall, SÖ Pohl, P Cammareri, S Aitken, NT Younger, M Raponi, CV Billard, AB Carrancio, A Bastem, P Freile, F Haward, IR Adams, JF Caceres, P Preyzner, A von Kriegs, MG Dunlop, FV Din, KB Myant
Nature Communications, 2022-05-19;13(1):2791. 2022-05-19
-
Human branching cholangiocyte organoids recapitulate functional bile duct formation
Authors: FJM Roos, GS van Tiende, H Wu, I Bordeu, D Vinke, LM Albarinos, K Monfils, S Niesten, R Smits, J Willemse, O Rosmark, G Westergren, DJ Kunz, M de Wit, PJ French, L Vallier, JNM IJzermans, R Bartfai, H Marks, BD Simons, ME van Royen, MMA Verstegen, LJW van der La
Cell Stem Cell, 2022-05-05;29(5):776-794.e13. 2022-05-05
-
Cell-intrinsic Aryl Hydrocarbon Receptor signalling is required for the resolution of injury-induced colonic stem cells
Authors: K Shah, MR Maradana, M Joaquina D, A Metidji, F Graelmann, M Llorian, P Chakravart, Y Li, M Tolaini, M Shapiro, G Kelly, C Cheshire, D Bhurta, SB Bharate, B Stockinger
Nature Communications, 2022-04-05;13(1):1827. 2022-04-05
-
Copy number amplification of ENSA promotes the progression of triple-negative breast cancer via cholesterol biosynthesis
Authors: YY Chen, JY Ge, SY Zhu, ZM Shao, KD Yu
Nature Communications, 2022-02-10;13(1):791. 2022-02-10
-
Comprehensive metabolomics expands precision medicine for triple-negative breast cancer
Authors: Y Xiao, D Ma, YS Yang, F Yang, JH Ding, Y Gong, L Jiang, LP Ge, SY Wu, Q Yu, Q Zhang, F Bertucci, Q Sun, X Hu, DQ Li, ZM Shao, YZ Jiang
Cell Research, 2022-02-01;0(0):. 2022-02-01
-
Efficient and error-free fluorescent gene tagging in human organoids without double-strand DNA cleavage
Authors: Y Bollen, JH Hageman, P van Leenen, LLM Derks, B Ponsioen, JR Buissant d, I Verlaan-Kl, M van den Bo, LWMM Terstappen, R van Boxtel, HJG Snippert
PloS Biology, 2022-01-28;20(1):e3001527. 2022-01-28
-
Lineage-specific silencing of PSAT1 induces serine auxotrophy and sensitivity to dietary serine starvation in luminal breast tumors
Authors: BH Choi, V Rawat, J Högström, PA Burns, KO Conger, ME Ozgurses, JM Patel, TS Mehta, A Warren, LM Selfors, T Muranen, JL Coloff
Cell Reports, 2022-01-18;38(3):110278. 2022-01-18
-
Air-Liquid-Interface Differentiated Human Nose Epithelium: A Robust Primary Tissue Culture Model of SARS-CoV-2 Infection
Authors: BM Tran, SL Grimley, JL McAuley, A Hachani, L Earnest, SL Wong, L Caly, J Druce, DFJ Purcell, DC Jackson, M Catton, CJ Nowell, L Leonie, G Deliyannis, SA Waters, J Torresi, E Vincan
International Journal of Molecular Sciences, 2022-01-13;23(2):. 2022-01-13
-
Adult mouse and human organoids derived from thyroid follicular cells and modeling of Graves' hyperthyroidism
Authors: J van der Va, L Bosmans, SF Sijbesma, K Knoops, WJ van de Wet, HG Otten, H Begthel, IHM Borel Rink, J Korving, EGWM Lentjes, C Lopez-Igle, PJ Peters, HM van Santen, MR Vriens, H Clevers
Proceedings of the National Academy of Sciences of the United States of America, 2021-12-21;118(51):. 2021-12-21
-
A Bioluminescent 3CLPro Activity Assay to Monitor SARS-CoV-2 Replication and Identify Inhibitors
Authors: C Mathieu, F Touret, C Jacquemin, YL Janin, A Nougairède, M Brailly, M Mazelier, D Décimo, V Vasseur, A Hans, JC Valle-Casu, X de Lamball, B Horvat, P André, M Si-Tahar, V Lotteau, PO Vidalain
Viruses, 2021-09-12;13(9):. 2021-09-12
-
Organoid-based drug screening reveals neddylation as therapeutic target for malignant rhabdoid tumors
Authors: C Calandrini, SR van Hooff, I Paassen, D Ayyildiz, S Derakhshan, MEM Dolman, KPS Langenberg, M van de Ven, C de Heus, N Liv, M Kool, RR de Krijger, GAM Tytgat, MM van den He, JJ Molenaar, J Drost
Cell Reports, 2021-08-24;36(8):109568. 2021-08-24
-
Ultrastructural analysis of breast cancer patient-derived organoids
Authors: L Signati, R Allevi, F Piccotti, S Albasini, L Villani, M Sevieri, A Bonizzi, F Corsi, S Mazzucchel
Cancer Cell International, 2021-08-10;21(1):423. 2021-08-10
-
ERBB3 overexpression due to miR-205 inactivation confers sensitivity to FGF, metabolic activation, and liability to ERBB3 targeting in glioblastoma
Authors: F De Bacco, F Orzan, J Erriquez, E Casanova, L Barault, R Albano, A D'Ambrosio, V Bigatto, G Reato, M Patanè, B Pollo, G Kuesters, C Dell'Aglio, L Casorzo, S Pellegatta, G Finocchiar, PM Comoglio, C Boccaccio
Cell Reports, 2021-07-27;36(4):109455. 2021-07-27
-
RAC1B modulates intestinal tumourigenesis via modulation of WNT and EGFR signalling pathways
Authors: V Gudiño, SÖ Pohl, CV Billard, P Cammareri, A Bolado, S Aitken, D Stevenson, AE Hall, M Agostino, J Cassidy, C Nixon, A von Kriegs, P Freile, L Popplewell, G Dickson, L Murphy, A Wheeler, M Dunlop, F Din, D Strathdee, OJ Sansom, KB Myant
Nature Communications, 2021-04-20;12(1):2335. 2021-04-20
-
Culture and analysis of kidney tubuloids and perfused tubuloid cells-on-a-chip
Authors: L Gijzen, FA Yousef Yen, F Schutgens, MK Vormann, CME Ammerlaan, A Nicolas, D Kurek, P Vulto, MB Rookmaaker, HL Lanz, MC Verhaar, H Clevers
Nature Protocols, 2021-03-05;0(0):. 2021-03-05
-
Inhibition of mitochondrial function by metformin increases glucose uptake, glycolysis and GDF-15 release from intestinal cells
Authors: M Yang, T Darwish, P Larraufie, D Rimmington, I Cimino, DA Goldspink, B Jenkins, A Koulman, CA Brighton, M Ma, BYH Lam, AP Coll, S O'Rahilly, F Reimann, FM Gribble
Scientific Reports, 2021-01-28;11(1):2529. 2021-01-28
-
Sox9EGFP defines biliary epithelial heterogeneity downstream of Yap activity
Authors: DY Tulasi, DM Castaneda, K Wager, CB Hogan, KP Alcedo, JR Raab, AD Gracz
Cellular and Molecular Gastroenterology and Hepatology, 2021-01-23;0(0):. 2021-01-23
-
Loss of sphingosine 1-phosphate receptor 3 gene function impairs injury-induced stromal angiogenesis in mouse cornea
Authors: S Yasuda, T Sumioka, H Iwanishi, Y Okada, M Miyajima, K Ichikawa, PS Reinach, S Saika
Lab Invest, 2020-11-16;0(0):. 2020-11-16
-
Single-cell derived tumor organoids display diversity in HLA class I peptide presentation
Authors: LC Demmers, K Kretzschma, A Van Hoeck, YE Bar-Epraïm, HWP van den To, M Koomen, G van Son, J van Gorp, A Pronk, N Smakman, E Cuppen, H Clevers, AJR Heck, W Wu
Nat Commun, 2020-10-21;11(1):5338. 2020-10-21
-
Hypoxia Triggers the Intravasation of Clustered Circulating Tumor Cells
Authors: C Donato, L Kunz, F Castro-Gin, A Paasinen-S, K Strittmatt, BM Szczerba, R Scherrer, N Di Maggio, W Heusermann, O Biehlmaier, C Beisel, M Vetter, C Rochlitz, WP Weber, A Banfi, T Schroeder, N Aceto
Cell Rep, 2020-09-08;32(10):108105. 2020-09-08
-
Functional Radiogenetic Profiling Implicates ERCC6L2 in Non-homologous End Joining
Authors: P Francica, M Mutlu, VA Blomen, C Oliveira, Z Nowicka, A Trenner, NM Gerhards, P Bouwman, E Stickel, ML Hekkelman, L Lingg, I Klebic, M van de Ven, R de Korte-G, D Howald, J Jonkers, AA Sartori, W Fendler, JR Chapman, T Brummelkam, S Rottenberg
Cell Rep, 2020-08-25;32(8):108068. 2020-08-25
-
Patient-derived oral mucosa organoids as an in vitro model for methotrexate induced toxicity in pediatric acute lymphoblastic leukemia
Authors: E Driehuis, N Oosterom, SG Heil, IB Muller, M Lin, S Kolders, G Jansen, R de Jonge, R Pieters, H Clevers, MM van den He
PLoS ONE, 2020-05-18;15(5):e0231588. 2020-05-18
-
Slug-expressing mouse prostate epithelial cells have increased stem cell potential
Authors: Z Kahounová, J Remšík, R Fedr, J Bouchal, A Mi?ková, E Slabáková, L Binó, A Hampl, K Sou?ek
Stem Cell Res, 2020-05-12;46(0):101844. 2020-05-12
-
Organoid cultures from normal and cancer-prone human breast tissues preserve complex epithelial lineages
Authors: JM Rosenbluth, RCJ Schackmann, GK Gray, LM Selfors, CM Li, M Boedicker, HJ Kuiken, A Richardson, J Brock, J Garber, D Dillon, N Sachs, H Clevers, JS Brugge
Nat Commun, 2020-04-06;11(1):1711. 2020-04-06
-
An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity
Authors: C Calandrini, F Schutgens, R Oka, T Margaritis, T Candelli, L Mathijsen, C Ammerlaan, RL van Inevel, S Derakhshan, S de Haan, E Dolman, P Lijnzaad, L Custers, H Begthel, HHD Kerstens, LL Visser, M Rookmaaker, M Verhaar, GAM Tytgat, P Kemmeren, RR de Krijger, R Al-Saadi, K Pritchard-, M Kool, AC Rios, MM van den He, JJ Molenaar, R van Boxtel, FCP Holstege, H Clevers, J Drost
Nat Commun, 2020-03-11;11(1):1310. 2020-03-11
-
Pancreatic cancer organoids recapitulate disease and allow personalized drug screening
Authors: E Driehuis, A van Hoeck, K Moore, S Kolders, HE Francies, MC Gulersonme, ECA Stigter, B Burgering, V Geurts, A Gracanin, G Bounova, FH Morsink, R Vries, S Boj, J van Es, GJA Offerhaus, O Kranenburg, MJ Garnett, L Wessels, E Cuppen, LAA Brosens, H Clevers
Proc. Natl. Acad. Sci. U.S.A., 2019-12-09;0(0):. 2019-12-09
-
Oral mucosal organoids as a potential platform for personalized cancer therapy
Authors: E Driehuis, S Kolders, S Spelier, K Lohmussaar, SM Willems, LA Devriese, R de Bree, EJ de Ruiter, J Korving, H Begthel, JH Van Es, V Geurts, GW He, RH van Jaarsv, R Oka, MJ Muraro, J Vivie, MMJM Zandvliet, APA Hendrickx, N Iakobachvi, P Sridevi, O Kranenburg, R van Boxtel, GJPL Kops, DA Tuveson, PJ Peters, A van Oudena, H Clevers
Cancer Discov, 2019-05-03;0(0):. 2019-05-03
-
SH3BP4 Regulates Intestinal Stem Cells and Tumorigenesis by Modulating ?-Catenin Nuclear Localization
Authors: P Antas, L Novellasde, A Kucharska, I Massie, J Carvalho, D Oukrif, E Nye, M Novelli, VSW Li
Cell Rep, 2019-02-26;26(9):2266-2273.e4. 2019-02-26
-
Neutrophils escort circulating tumour cells to enable cell cycle progression
Authors: BM Szczerba, F Castro-Gin, M Vetter, I Krol, S Gkountela, J Landin, MC Scheidmann, C Donato, R Scherrer, J Singer, C Beisel, C Kurzeder, V Heinzelman, C Rochlitz, WP Weber, N Beerenwink, N Aceto
Nature, 2019-02-06;0(0):. 2019-02-06
-
Novel Chimeric Gene Therapy Vectors Based on Adeno-Associated Virus and Four Different Mammalian Bocaviruses
Authors: J Fakhiri, MA Schneider, J Puschhof, M Stanifer, V Schildgen, S Holderbach, Y Voss, J El Andari, O Schildgen, S Boulant, M Meister, H Clevers, Z Yan, J Qiu, D Grimm
Mol Ther Methods Clin Dev, 2019-01-18;12(0):202-222. 2019-01-18
-
Release of transcriptional repression via ErbB2-induced, SUMO-directed phosphorylation of myeloid zinc finger-1 serine 27 activates lysosome redistribution and invasion
Authors: DM Brix, SA Tvingsholm, MB Hansen, KB Clemmensen, T Ohman, V Siino, M Lambrughi, K Hansen, P Puustinen, I Gromova, P James, E Papaleo, M Varjosalo, J Moreira, M Jäättelä, T Kallunki
Oncogene, 2019-01-08;0(0):. 2019-01-08
-
A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity.
Authors: Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind A, Wind K, Gracanin A, Begthel H, Korving J, van Boxtel R, Duarte A, Lelieveld D, van Hoeck A, Ernst R, Blokzijl F, Nijman I, Hoogstraat M, van de Ven M, Egan D, Zinzalla V, Moll J, Boj S, Voest E, Wessels L, van Diest P, Rottenberg S, Vries R, Cuppen E, Clevers H
Cell, 2017-12-07;172(1):373-386.e10. 2017-12-07
-
AGE-RAGE interaction in the TGF?2-mediated epithelial to mesenchymal transition of human lens epithelial cells.
Authors: Cibin T Raghavan, Ram H Nagaraj
Glycoconjugate Journal, 2016-06-04;0(0):1573-4986. 2016-06-04
FAQs
-
What kinds of tumor cells or biopsy specimens grow in vivo with Cultrex® BME?
Many cell lines and tumor biopsy specimens (usually cut into small fragments) have been found to grow in vivo when implanted with Cultrex® BME. These include melanoma, intestinal, prostate, breast, lung, renal, and liver cancers as well as the 3T3 mouse embryonic fibroblast cell line.
-
How does Cultrex® Basement Membrane Extract (BME) promote cell differentiation?
All epithelial and endothelial cells are in contact with a basement membrane matrix on at least one of their surfaces. By providing them with their natural matrix in vitro as a substrate for the cells that provides biological cues, the cells can assume a more physiological morphology (i.e. correct shape) and begin expression of cell-lineage specific proteins. Two-dimensional plastic surfaces, in combination with serum-containing media, cause cells to flatten, proliferate and de-differentiate.
-
How should Cultrex Basement Membrane Extract (BME) be stored and handled?
For best practices for handling our BME products, please refer to our helpful guide at the following link: https://www.bio-techne.com/reagents/cell-culture-reagents/cultrex-bme-and-ecm-proteins/cell-culture-matrix-for-your-research
-
What is the Tube Formation Assay?
The Tube Formation Assay is based on the ability of endothelial cells to form three-dimensional capillary-like tubular structures when cultured on a hydrogel of reconstituted basement membrane, such as Cultrex Basement Membrane Extract (BME).
-
What are the advantages of the Tube Formation Assay?
The Tube Formation Assay is the most widely used in vitro angiogenesis assay. The assay is rapid, inexpensive and quantifiable. It can be used to identify potentially angiogenic and anti-angiogenic factors, to determine endothelial cell phenotype, and to study pathways and mechanisms involved in angiogenesis. It can be performed in a high throughput mode to screen for a large number of compounds.
-
What cell types can be used in the Tube Formation Assay?
The Tube Formation Assay is specific for endothelial cells, either primary cells or immortalized cell lines. Only endothelial cells form capillary-like structures with a lumen inside. Other endothelial cell types form other structures.
-
What are the variables associated with the Tube Formation Assay?
The major variables associated with tube formation are composition of the Cultrex Basement Membrane Extract (BME) hydrogel, thickness of the hydrogel, cell density, composition of angiogenic factors in the assay medium, and assay period.
-
Which Cultrex Basement Membrane Extract (BME) should I use for the Tube Formation Assay?
Cultrex Reduced Growth Factor BME (RGF BME) is generally used for testing compounds that promote angiogenesis because formation of capillary-like structures (tubes) is significantly less compared to non-growth factor reduced varieties of Cultrex BME. The Cultrex In Vitro Angiogeneis Assay (Tube Formation) includes a qualified production lot of Cultrex RGF BME that exhibits reduced background tube formation in the absence of angiogenic factors.
-
How do I reduce spontaneous formation of tubular structures on Cultrex BME in the absence of angiogenic factors?
Primary endothelial cells, such as Human Umbilical Vein Endothelial Cells (HUVECs) form capillary-like structures in the absence of added angiogenic factors less often than immortalized endothelial cells. Generally, reducing the number of cells per cm2 plated onto Cultrex BME will result in less background or spontaneous tube formation. Titrate the number of cells and find optimal conditions for your specific cell line. When endothelial cells fully form capillary structures in response to angiogenic activators, but not in their absence, you may proceed.
-
Does Cultrex BME, Catalog # 3533-005-02, affect fluorescence readings when Alamar Blue is used for final readout of assay?
BME is known to have autofluorescence, but if appropriate controls are evaluated, background can be successfully subtracted. A BME only control well with no cells should be used to subtract the background fluorescence.
Reviews for Cultrex Reduced Growth Factor Basement Membrane Extract, Type 2, Pathclear
Average Rating: 5 (Based on 5 Reviews)
Have you used Cultrex Reduced Growth Factor Basement Membrane Extract, Type 2, Pathclear?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
dissolve it on ice, and don't put it outside for too not time
Aortic sprouting assay: mouse aortic ring cultured in a 48-well plate and imaged in an inverted microscope after 5 days.
Results are very consistent with this BME (used one layer under and another on top of the aortic ring).
We are using the BME at a final dilution of 1/30 for the maintenance of iPSC and differentiation into endoderm as well as mesoderm lineages. Attachment, growth, and differentiation of our iPSC is consistent, and we observed only minor variations between different lots.




