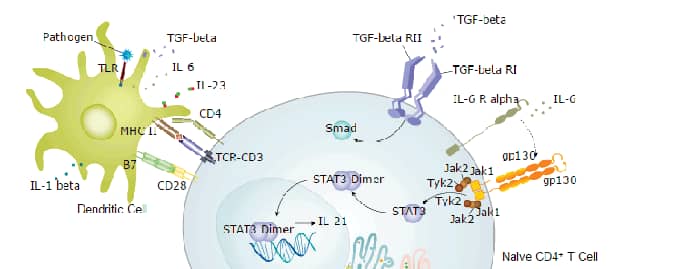
The multi-functional factor interleukin 6 (IL-6) exerts its activities through binding to a high-affinity receptor complex consisting of two membrane glycoproteins: an 80 kDa component receptor that binds IL-6 with low affinity (IL-6 R alpha ) and a signal-transducing component of 130 kDa (gp130) that does not bind IL-6 by itself, but is required for high-affinity binding of IL-6 by the complex (1‑4). Within the extracellular domain, human IL‑6 R alpha shares 52% aa sequence identity with mouse and rat IL‑6 R alpha.
A soluble form of the IL-6 R alpha has been found in the urine of healthy adult humans (5). This soluble receptor apparently arises from proteolytic cleavage of membrane-bound IL-6 R alpha. No naturally-occurring mRNA encoding a truncated form of the IL-6 R alpha has been reported. Soluble forms of human and murine IL-6 R alpha s have been constructed, however, by insertion of termination codons into the regions of the IL-6 R alpha cDNAs encoding the external portions of the receptors and prior to the transmembrane domains. These soluble receptors have been expressed in COS-7 and CHO cells and have been shown to bind to IL-6 in solution and to augment the activity of IL-6 as a result of the binding of the IL‑6/IL‑6 R alpha complex to membrane-bound gp130 (6, 7).