Mouse IL-6 Antibody Summary
Applications
Mouse IL-6 Sandwich Immunoassay
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
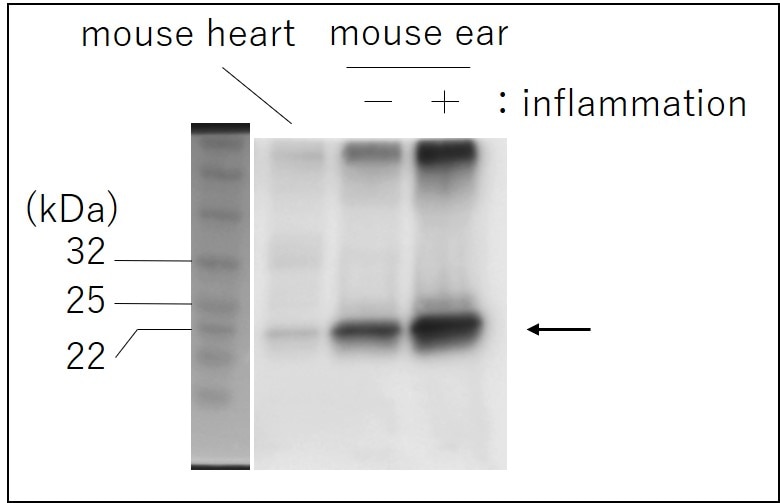
Detection of Recombinant Mouse IL‑6 by Western Blot. Western blot shows 25 ng of Recombinant Mouse IL-6 (406-ML), Recombinant Human IL-6 (206-IL) and Recombinant Rat IL-6 (506-RL). PVDF Membrane was probed with 1 µg/mL of Rat Anti-Mouse IL-6 Monoclonal Antibody (Catalog # MAB406) followed by HRP-conjugated Anti-Rat IgG Secondary Antibody (HAF005). A specific band was detected for IL-6 at approximately 18 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 3.
 View Larger
View Larger
Cell Proliferation Induced by IL‑6 and Neutralization by Mouse IL‑6 Antibody. Recombinant Mouse IL-6 (406-ML) stimulates proliferation in the T1165.85.2.1 mouse plasmacytoma cell line in a dose-dependent manner (orange line). Proliferation elicited by Recombinant Mouse IL-6 (0.25 ng/mL) is neutralized (green line) by increasing concentrations of Mouse IL-6 Monoclonal Antibody (Catalog # MAB406). The ND50 is typically 0.005-0.025 µg/mL.
 View Larger
View Larger
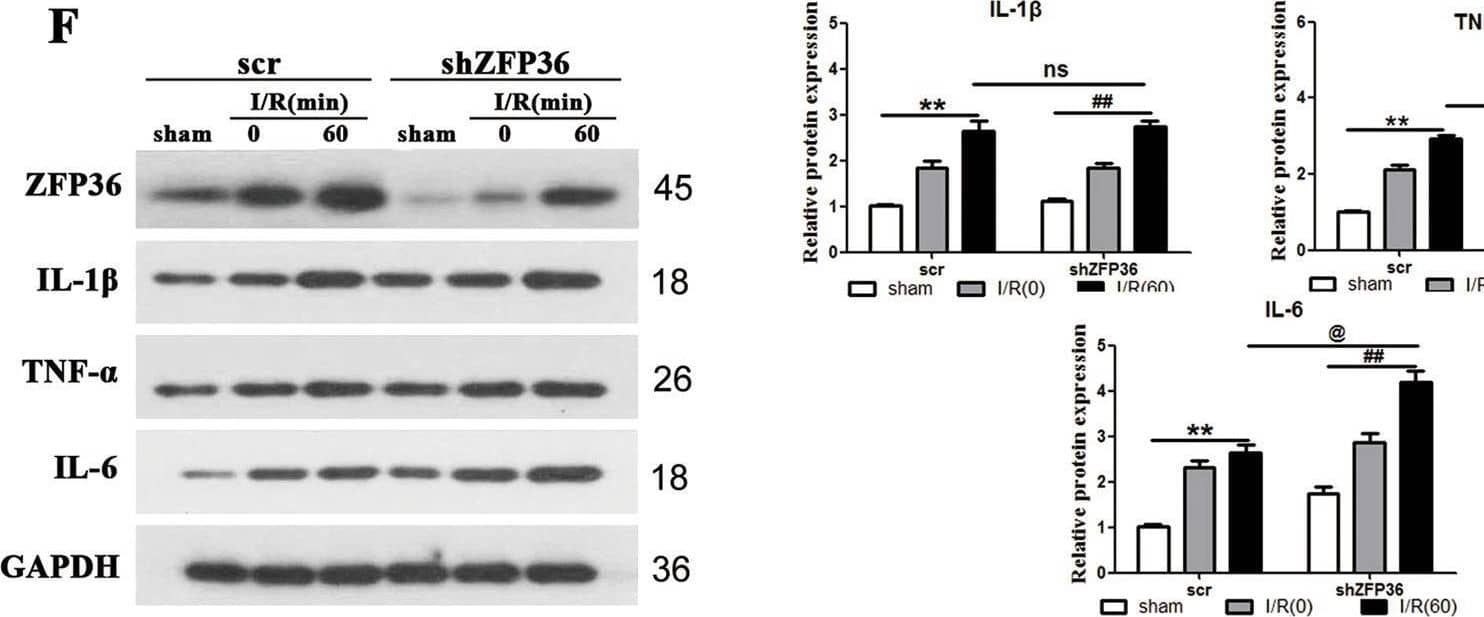
Detection of IL-6 by Western Blot Role of ZFP36 in intestinal ischemia–reperfusion (I/R)-induced acute lung injury.C57BL/6 mice were subjected to 60 min of intestinal ischemia followed by 0, 30, 60, and 90 min of reperfusion as indicated. Sham mice were included as the control. A ZFP36 mRNA and protein expression in lung tissues were analyzed by RT-qPCR and Western blot (n = 6 per group, **P < 0.01). B Immunohistochemical staining of lung tissues for ZFP36 of sham and I/R 60 min. Scale bars: 50 μm. C–F Levels of IL-1 beta (C), TNF-alpha (D), and IL-6 (E) were measured by ELISA and Western blotting (F). G H&E staining of lung tissues. Red arrows outline collapsed alveoli, blue arrows outline multiple inflammatory cells infilitration, black arrows outline bronchial hemorrhage. Scale bars: 50 μm. H–J Arterial blood PaO2 (H), lung water content (I), and BALF protein content (J) were measured. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/34238924), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
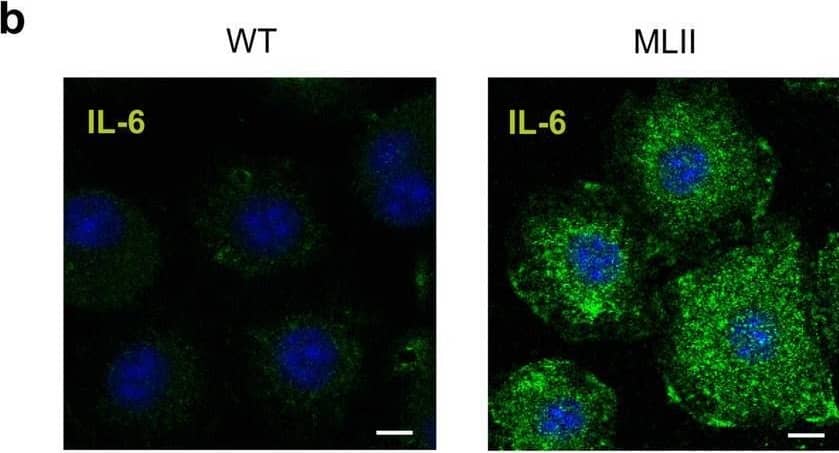
Detection of IL-6 by Immunocytochemistry/ Immunofluorescence Increased expression of IL-6 in primary cultured osteoblasts and chondrocytes from MLII mice. (a) Signal log ratio (SLR) of differentially expressed genes (SLR ≥ 2 or ≤ -2) in terminally differentiated osteoblasts from wild-type (WT) and MLII mice related to the gene ontology (GO) "Bone biological processes": "Bone mineralization" (GO 0030282) and "Osteoclast differentiation" (GO 0030316). (b) IL-6 immunostaining (green) of WT and MLII osteoblasts. Nuclei were visualized by 4′,6-diamidino-2-phenylindole (DAPI) staining (blue). Scale bar: 10 μm. (c) Concentration of IL-6 in conditioned media from WT and MLII osteoblasts (n = 5, mean ± SD, ***p ≤ 0.001). (d) Expression levels of Il6 mRNA related to Gapdh in primary osteoblasts, chondrocytes and osteoclasts from WT and MLII mice (n = 3, mean ± SD, ***p ≤ 0.001). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/33574442), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: IL-6
Interleukin-6 (IL-6) is a pleiotropic, alpha -helical, phosphorylated and variably glycosylated cytokine that plays important roles in the acute phase reaction, inflammation, hematopoiesis, bone metabolism, and cancer progression. Mature mouse IL-6 is 187 amino acids (aa) in length that is typically expressed as a 22-28 kDA molecular weight protein. Mouse IL-6 shares 39% and 85% aa sequence identity with human and rat IL-6, respectively. Alternative splicing generates several isoforms with internal deletions, some of which exhibit antagonistic properties. IL-6 induces signaling through a cell surface heterodimeric receptor complex composed of a ligand binding subunit (IL-6 R alpha) and a signal transducing subunit (gp130). IL-6 binds to IL-6 R alpha, triggering IL-6 R alpha association with gp130 and gp130 dimerization. gp130 is also a component of the receptors for CLC, CNTF, CT-1, IL-11, IL-27, LIF, and OSM. Soluble forms of IL-6 R alpha are generated by both alternative splicing and proteolytic cleavage. In a mechanism known as trans-signaling, complexes of soluble IL-6 and IL-6 R alpha elicit responses from gp130-expressing cells that lack cell surface IL-6 R alpha. Trans-signaling enables a wider range of cell types to respond to IL-6, as the expression of gp130 is ubiquitous, while that of IL-6 R alpha is predominantly restricted to hepatocytes, monocytes, and resting lymphocytes. Soluble splice forms of gp130 block trans-signaling from IL-6/IL-6 R alpha but not from other cytokines that use gp130 as a co-receptor. IL-6, along with TNF-alpha and IL-1, function to drive the acute inflammatory response and the transition from acute inflammation to either acquired immunity or chronic inflammatory disease. When dysregulated, it contributes to chronic inflammation in obesity, insulin resistance, inflammatory bowel disease, arthritis, sepsis, and atherosclerosis. IL-6 can also function as an anti-inflammatory molecule, as in skeletal muscle where it is secreted in response to exercise. In addition, it enhances hematopoietic stem cell proliferation and the differentiation of Th17 cells, memory B cells, and plasma cells.
Product Datasheets
Citations for Mouse IL-6 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
101
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
PDE11A negatively regulates lithium responsivity
Mol Psychiatry, 2016-09-20;0(0):.
-
Delayed Neutralization of Interleukin 6 Reduces Organ Injury, Selectively Suppresses Inflammatory Mediator, and Partially Normalizes Immune Dysfunction Following Trauma and Hemorrhagic Shock
Authors: Yong Zhang, Jinxiang Zhang, Sebastian Korff, Faez Ayoob, Yoram Vodovotz, Timothy R. Billiar
Shock
-
Microglia-induced IL-6 protects against neuronal loss following HSV-1 infection of neural progenitor cells
Authors: Ana J. Chucair-Elliott, Christopher Conrady, Min Zheng, Chandra M. Kroll, Thomas E. Lane, Daniel J. J. Carr
Glia
-
Class A Scavenger Receptor Exacerbates Osteoclastogenesis by an Interleukin-6-Mediated Mechanism through ERK and JNK Signaling Pathways
Authors: Shuyu Guo, Yuanyuan Ni, Jingjing Ben, Yang Xia, Tingting Zhou, Dongyue Wang et al.
International Journal of Biological Sciences
-
Histone deacetylase 3 supports endochondral bone formation by controlling cytokine signaling and matrix remodeling
Authors: Lomeli R. Carpio, Elizabeth W. Bradley, Meghan E. McGee-Lawrence, Megan M. Weivoda, Daniel D. Poston, Amel Dudakovic et al.
Science Signaling
-
Macrophages potentiate STAT3 signaling in skeletal muscles and regulate pancreatic cancer cachexia
Authors: Surendra K. Shukla, Spas D. Markov, Kuldeep S. Attri, Enza Vernucci, Ryan J. King, Aneesha Dasgupta et al.
Cancer Letters
-
A Role for Hypocretin/Orexin in Metabolic and Sleep Abnormalities in a Mouse Model of Non-metastatic Breast Cancer
Authors: Jeremy C. Borniger, William H. Walker II, Surbhi, Kathryn M. Emmer, Ning Zhang, Abigail A. Zalenski et al.
Cell Metabolism
-
Delayed activation of PPAR-beta /δ improves long-term survival in mouse sepsis: effects on organ inflammation and coagulation
Authors: Daniel Busch, Amar Kapoor, Pia Rademann, Frank Hildebrand, Soheyl Bahrami, Christoph Thiemermann et al.
Innate Immunity
-
Crosstalk between bone marrow-derived myofibroblasts and gastric cancer cells regulates cancer stemness and promotes tumorigenesis
Authors: L Zhu, X Cheng, J Shi, J Lin, G Chen, H Jin, AB Liu, H Pyo, J Ye, Y Zhu, H Wang, H Chen, J Fang, L Cai, TC Wang, CS Yang, SP Tu
Oncogene, 2016-04-25;0(0):.
-
Intercellular mitochondrial transfer alleviates pyroptosis in dental pulp damage
Authors: Konghuai Wang, Lu Zhou, Hanqing Mao, Jiayi Liu, Zhi Chen, Lu Zhang
Cell Proliferation
-
Role of high-mobility group box-1 in myocardial ischemia/reperfusion injury and the effect of ethyl pyruvate
Authors: YUNLING LIN, LIANGLONG CHEN, WEIWEI LI, JUN FANG
Experimental and Therapeutic Medicine
-
Adipocyte-Secreted IL-6 Sensitizes Macrophages to IL-4 Signaling
Authors: Luan D, Dadpey B, Zaid J et al.
Diabetes
-
Results of the preclinical multicenter randomized controlled paclitaxel-induced neuropathy prevention replication study (PINPRICS)
Authors: Boehmerle, W;Hagenacker, T;Leo, M;Schmitt, LI;Lehmann, HC;Klein, I;Stegherr, R;Konietschke, F;Endres, M;Huehnchen, P;
BMC research notes
Species: Mouse
Sample Types: In Vivo
Applications: In vivo assay -
Stress systems exacerbate the inflammatory response after corneal abrasion in sleep-deprived mice via the IL-17 signaling pathway
Authors: Xue, Y;Xu, P;Hu, Y;Liu, S;Yan, R;Liu, S;Li, Y;Liu, J;Fu, T;Li, Z;
Mucosal immunology
Species: Mouse
Sample Types: In Vivo
Applications: In vivo assay -
Nanoparticles of Lactiplantibacillus plantarum K8 Reduce Staphylococcus aureus Respiratory Infection and Tumor Necrosis Factor Alpha- and Interferon Gamma-Induced Lung Inflammation
Authors: Hong, J;Son, M;Sin, J;Kim, H;Chung, DK;
Nutrients
Species: Mouse
Sample Types: Serum
Applications: ELISA Capture -
CD1d-dependent natural killer T-cells inactivation aggravates sepsis-induced myocardial injury via T lymphocytes infiltration and IL-6 production in mice
Authors: Chen, LR;Hui-Yang, ;Lu-Liu, ;Wang, XX;Zhang, XM;Wang, HX;
International immunopharmacology
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
The fetal response to maternal inflammation is dependent upon maternal IL-6 in a murine model
Authors: Bermick, J;Watson, S;Lueschow, S;McElroy, SJ;
Cytokine
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Glycosyltransferase Extl1 promotes CCR7-mediated dendritic cell migration to restrain infection and autoimmunity
Authors: J Liu, Y Cheng, X Zhang, Y Chen, H Zhu, K Chen, S Liu, Z Li, X Cao
Cell Reports, 2023-01-18;42(1):111991.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
IL6 supports long-term expansion of hepatocytes in vitro
Authors: R Guo, M Jiang, G Wang, B Li, X Jia, Y Ai, S Chen, P Tang, A Liu, Q Yuan, X Xie
Nature Communications, 2022-11-29;13(1):7345.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Single-cell transcriptomics reveals a senescence-associated IL-6/CCR6 axis driving radiodermatitis
Authors: M Paldor, O Levkovitch, D Eidelshtei, R Adar, CD Enk, Y Marmary, S Elgavish, Y Nevo, H Benyamini, I Plaschkes, S Klein, A Mali, S Rose-John, A Peled, E Galun, JH Axelrod
Oncogene, 2022-07-04;0(0):e15653.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Hyperphosphatemia increases inflammation to exacerbate anemia and skeletal muscle wasting independently of FGF23-FGFR4 signaling
Authors: B Czaya, K Heitman, I Campos, C Yanucil, D Kentrup, D Westbrook, O Gutierrez, JL Babitt, G Jung, IB Salusky, M Hanudel, C Faul
Elife, 2022-03-18;11(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Irradiated fibroblasts increase interleukin-6 expression and induce migration of head and neck squamous cell carcinoma
Authors: S Suzuki, S Toyoma, Y Kawasaki, T Yamada
PLoS ONE, 2022-01-28;17(1):e0262549.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
M1 macrophages impair tight junctions between endothelial cells after spinal cord injury
Authors: Y Luo, F Yao, X Hu, Y Li, Y Chen, Z Li, Z Zhu, S Yu, D Tian, L Cheng, M Zheng, J Jing
Brain research bulletin, 2022-01-04;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Aging triggers an upregulation of a multitude of cytokines in the male and especially the female rodent hippocampus but more discrete changes in other brain regions
Authors: L Porcher, S Bruckmeier, SD Burbano, JE Finnell, N Gorny, J Klett, SK Wood, MP Kelly
Journal of Neuroinflammation, 2021-09-22;18(1):219.
Species: Mouse, Rat
Sample Types: Tissue Homogenates
Applications: Western Blot -
Transgenic inhibition of interleukin-6 trans-signaling does not prevent skeletal pathologies in mucolipidosis type II mice
Authors: LM Westermann, A Baranowsky, G Di Lorenzo, T Danyukova, J Soul, JM Schwartz, G Hendrickx, M Amling, S Rose-John, C Garbers, T Schinke, S Pohl
Scientific Reports, 2021-02-11;11(1):3556.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Macrophage and adipocyte interaction as a source of inflammation in kidney disease
Authors: C Martos-Rus, G Katz-Green, Z Lin, E Serrano, D Whitaker-M, M Domingo-Vi, M Roche, K Ramaswamy, DC Hooper, B Falkner, MP Martinez C
Scientific Reports, 2021-02-03;11(1):2974.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Aging Impairs Mitochondrial Function and Mitophagy and Elevates Interleukin 6 Within the Cerebral Vasculature
Authors: DJ Tyrrell, MG Blin, J Song, SC Wood, DR Goldstein
J Am Heart Assoc, 2020-11-23;9(23):e017820.
Species: Mouse
Sample Types: Whole Tissue
Applications: Isotype Control -
&beta2-Adrenergic Receptors Increase Cardiac Fibroblast Proliferation Through the G&alphas/ERK1/2-Dependent Secretion of Interleukin-6
Authors: MA Tanner, TP Thomas, CA Maitz, LA Grisanti
Int J Mol Sci, 2020-11-12;21(22):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
p38&alpha in Macrophages Aggravates Arterial Endothelium Injury by Releasing IL-6 through phosphorylating Megakaryocytic Leukemia 1
Authors: M Zhang, J Gao, X Zhao, M Zhao, D Ma, X Zhang, D Tian, B Pan, X Yan, J Wu, X Meng, H Yin, L Zheng
Redox Biology, 2020-11-01;38(0):101775.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Linking skeletal muscle aging with osteoporosis by lamin A/C deficiency
Authors: L Xiong, K Zhao, Y Cao, HH Guo, JX Pan, X Yang, X Ren, L Mei, WC Xiong
PLoS Biol., 2020-06-01;18(6):e3000731.
Species: Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: IHC, Western Blot -
Adipocytes promote tumor progression and induce PD-L1 expression via TNF-&alpha/IL-6 signaling
Authors: Z Li, C Zhang, JX Du, J Zhao, MT Shi, MW Jin, H Liu
Cancer Cell Int., 2020-05-20;20(0):179.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Inhibition of Serine Protease Activity Protects Against High Fat Diet-Induced Inflammation and Insulin Resistance
Authors: CS Kuo, JS Chen, LY Lin, GW Schmid-Sch, S Chien, PH Huang, JW Chen, SJ Lin
Sci Rep, 2020-02-03;10(1):1725.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Endothelial autophagy deficiency induces IL6 - dependent endothelial mesenchymal transition and organ fibrosis
Authors: Y Takagaki, SM Lee, Z Dongqing, M Kitada, K Kanasaki, D Koya
Autophagy, 2020-01-22;0(0):1-10.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
B cell hyperactivation in an Ackr4-deficient mouse strain is not caused by lack of ACKR4 expression
Authors: N Eckert, K Werth, S Willenzon, L Tan, R Förster
J. Leukoc. Biol., 2019-12-16;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Berberine ameliorates lipopolysaccharide?induced inflammatory responses in mouse inner medullary collecting duct?3 cells by downregulation of NF?kappaB pathway
Authors: DG Kim, JW Choi, IJ Jo, MJ Kim, HS Lee, SH Hong, HJ Song, GS Bae, SJ Park
Mol Med Rep, 2019-11-19;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Activation of JAK/STAT Signaling in Megakaryocytes Sustains Myeloproliferation In Vivo
Authors: Brittany Woods, Wei Chen, Sophia Chiu, Christian Marinaccio, Chunling Fu, Lilly Gu et al.
Clinical Cancer Research
Species: Transgenic Mouse
Sample Types: In Vivo
Applications: Neutralization -
Phosphorylation of Hsp20 Promotes Fibrotic Remodeling and Heart Failure
Authors: GT Gardner, JG Travers, J Qian, GS Liu, K Haghighi, N Robbins, M Jiang, Y Li, GC Fan, J Rubinstein, BC Blaxall, EG Kranias
JACC Basic Transl Sci, 2019-04-29;4(2):188-199.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Regulation of the bi-directional cross-talk between ovarian cancer cells and adipocytes by SPARC
Authors: B John, C Naczki, C Patel, A Ghoneum, S Qasem, Z Salih, N Said
Oncogene, 2019-02-14;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Direct Conversion of Mouse Fibroblasts into Neural Stem Cells by Chemical Cocktail Requires Stepwise Activation of Growth Factors and Nup210
Authors: Y Tang, S Xiong, P Yu, F Liu, L Cheng
Cell Rep, 2018-07-31;24(5):1355-1362.e3.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Theophylline suppresses interleukin-6 expression by inhibiting glucocorticoid receptor signaling in pre-adipocytes
Authors: T Mitani, T Takaya, N Harada, S Katayama, R Yamaji, S Nakamura, H Ashida
Arch. Biochem. Biophys., 2018-04-04;646(0):98-106.
Species: Mouse
Sample Types: Plasma
Applications: ELISA Capture -
Transcription factor 21 (Tcf21) promotes proinflammatory interleukin 6 expression and extracellular matrix remodeling in visceral adipose stem cells
Authors: T Akama, TH Chun
J. Biol. Chem., 2018-03-14;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Functional Assay, ICC -
Tumor-extrinsic discoidin domain receptor 1 promotes mammary tumor growth by regulatingadipose stromal interleukin 6 production in mice
Authors: X Sun, K Gupta, B Wu, D Zhang, B Yuan, X Zhang, HC Chiang, C Zhang, TJ Curiel, MP Bendeck, S Hursting, Y Hu, R Li
J. Biol. Chem., 2018-01-03;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The calcineurin-NFAT axis contributes to host defense during Pseudomonas aeruginosa lung infection
Authors: Z Pang, RD Junkins, AJ MacNeil, C McCormick, Z Cheng, WM Chen, TJ Lin
J. Leukoc. Biol., 2017-10-10;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: ELISA Development (Capture) -
IL-33 Attenuates Sepsis by Inhibiting IL-17 Receptor Signaling through Upregulation of SOCS3
Authors: R Lv, J Zhao, M Lei, D Xiao, Y Yu, J Xie
Cell. Physiol. Biochem., 2017-08-09;42(5):1961-1972.
Species: Mouse
Sample Types: Serum
Applications: ELISA Development (Capture) -
Notch Balances Th17 and Induced Regulatory T Cell Functions in Dendritic Cells by Regulating Aldh1a2 Expression
Authors: TS Zaman, H Arimochi, S Maruyama, C Ishifune, SI Tsukumo, A Kitamura, K Yasutomo
J. Immunol., 2017-08-04;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Augmented sphingosine 1 phosphate receptor-1 signaling in cardiac fibroblasts induces cardiac hypertrophy and fibrosis through angiotensin II and interleukin-6
Authors: SI Ohkura, S Usui, SI Takashima, N Takuwa, K Yoshioka, Y Okamoto, Y Inagaki, N Sugimoto, T Kitano, M Takamura, T Wada, S Kaneko, Y Takuwa
PLoS ONE, 2017-08-03;12(8):e0182329.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Inhibitory effect ofquercetinon titaniumparticle-inducedendoplasmic reticulum stress (ERS)-related apoptosisandin vivoosteolysis
Authors: L Zhang, Z Tian, W Li, X Wang, Z Man, S Sun
Biosci. Rep., 2017-07-31;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development (Capture) -
The human amniotic fluid stem cell secretome effectively counteracts doxorubicin-induced cardiotoxicity
Sci Rep, 2016-07-21;6(0):29994.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis
Authors: Jung-Yeon Lim
Sci Rep, 2016-06-01;6(0):26851.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development (Capture) -
IL6 Blockade Reprograms the Lung Tumor Microenvironment to Limit the Development and Progression of K-ras-Mutant Lung Cancer
Authors: MS Caetano, H Zhang, AM Cumpian, L Gong, N Unver, EJ Ostrin, S Daliri, SH Chang, CE Ochoa, S Hanash, C Behrens, II Wistuba, C Sternberg, H Kadara, CG Ferreira, SS Watowich, SJ Moghaddam
Cancer Res., 2016-04-01;76(11):3189-99.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo, Neutralization -
Radiation-driven lipid accumulation and dendritic cell dysfunction in cancer.
Authors: Gao F, Liu C, Guo J, Sun W, Xian L, Bai D, Liu H, Cheng Y, Li B, Cui J, Zhang C, Cai J
Sci Rep, 2015-04-29;5(0):9613.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Epigenetic silencing of microRNA-149 in cancer-associated fibroblasts mediates prostaglandin E2/interleukin-6 signaling in the tumor microenvironment.
Authors: Li P, Shan J, Chen X, Zhang D, Su L, Huang X, Yu B, Zhi Q, Li C, Wang Y, Tomei S, Cai Q, Ji J, Li J, Chouchane L, Yu Y, Sun F, Xu Z, Liu B, Zhu Z
Cell Res, 2015-04-28;25(5):588-603.
Species: Mouse
Sample Types: Whole Cells
Applications: Blocking -
KIM-1-mediated phagocytosis reduces acute injury to the kidney.
Authors: Yang L, Brooks C, Xiao S, Sabbisetti V, Yeung M, Hsiao L, Ichimura T, Kuchroo V, Bonventre J
J Clin Invest, 2015-03-09;125(4):1620-36.
Species: Mouse
Sample Types: Cell Lysates
Applications: ELISA Development (Capture) -
Apolipoprotein E promotes subretinal mononuclear phagocyte survival and chronic inflammation in age-related macular degeneration.
Authors: Levy O, Calippe B, Lavalette S, Hu S, Raoul W, Dominguez E, Housset M, Paques M, Sahel J, Bemelmans A, Combadiere C, Guillonneau X, Sennlaub F
EMBO Mol Med, 2015-02-01;7(2):211-26.
Species: Human, Mouse
Sample Types: Whole Organism, Whole Tissue
Applications: IHC, In Vivo -
Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling.
Authors: Chandler, Ronald L, Damrauer, Jeffrey, Raab, Jesse R, Schisler, Jonathan, Wilkerson, Matthew, Didion, John P, Starmer, Joshua, Serber, Daniel, Yee, Della, Xiong, Jessie, Darr, David B, Pardo-Manuel de Villena, Fernando, Kim, William, Magnuson, Terry
Nat Commun, 2015-01-27;6(0):6118.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes p53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6).
Authors: Volonte D, Zou H, Bartholomew J, Liu Z, Morel P, Galbiati F
J Biol Chem, 2014-12-15;290(7):4202-14.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis.
Authors: Bonapace L, Coissieux M, Wyckoff J, Mertz K, Varga Z, Junt T, Bentires-Alj M
Nature, 2014-10-22;515(7525):130-3.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Autocrine interleukin-6 drives skin-derived mesenchymal stem cell trafficking via regulating voltage-gated Ca(2+) channels.
Authors: Ke F, Zhang L, Liu Z, Liu J, Yan S, Xu Z, Bai J, Zhu H, Lou F, Wang H, Shi Y, Jiang Y, Su B, Wang H
Stem Cells, 2014-10-01;32(10):2799-810.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC-P -
Delayed Neutralization of Interleukin 6 Reduces Organ Injury, Selectively Suppresses Inflammatory Mediator, and Partially Normalizes Immune Dysfunction Following Trauma and Hemorrhagic Shock
Authors: Yong Zhang, Jinxiang Zhang, Sebastian Korff, Faez Ayoob, Yoram Vodovotz, Timothy R. Billiar
Shock
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Distinct mechanisms of induction of hepatic growth hormone resistance by endogenous IL-6, TNF-alpha, and IL-1beta.
Authors: Zhao Y, Xiao X, Frank S, Lin H, Xia Y
Am J Physiol Endocrinol Metab, 2014-06-03;307(2):E186-98.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Stabilization and augmentation of circulating AIM in mice by synthesized IgM-Fc.
Authors: Kai, Toshihir, Yamazaki, Tomoko, Arai, Satoko, Miyazaki, Toru
PLoS ONE, 2014-05-07;9(5):e97037.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Blockade of Fas signaling in breast cancer cells suppresses tumor growth and metastasis via disruption of Fas signaling-initiated cancer-related inflammation.
Authors: Liu Q, Tan Q, Zheng Y, Chen K, Qian C, Li N, Wang Q, Cao X
J Biol Chem, 2014-03-13;289(16):11522-35.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity.
Authors: Ramkhelawon B, Hennessy E, Menager M, Ray T, Sheedy F, Hutchison S, Wanschel A, Oldebeken S, Geoffrion M, Spiro W, Miller G, McPherson R, Rayner K, Moore K
Nat Med, 2014-03-02;20(4):377-84.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Microarray analyses demonstrate the involvement of type I interferons in psoriasiform pathology development in D6-deficient mice.
Authors: Baldwin H, Pallas K, King V, Jamieson T, McKimmie C, Nibbs R, Carballido J, Jaritz M, Rot A, Graham G
J Biol Chem, 2013-11-05;288(51):36473-83.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Catecholamine stress alters neutrophil trafficking and impairs wound healing by beta2-adrenergic receptor-mediated upregulation of IL-6.
Authors: Kim M, Gorouhi F, Ramirez S, Granick J, Byrne B, Soulika A, Simon S, Isseroff R
J Invest Dermatol, 2013-10-11;134(3):809-17.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
The signaling suppressor CIS controls proallergic T cell development and allergic airway inflammation
Authors: Xuexian O Yang, Huiyuan Zhang, Byung-Seok Kim, Xiaoyin Niu, Juan Peng, Yuhong Chen et al.
Nature Immunology
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Basophils control T-cell responses and limit disease activity in experimental murine colitis.
Authors: Gomez M, Talke Y, Hofmann C, Ketelsen I, Hermann F, Reich B, Goebel N, Schmidbauer K, Dunger N, Bruhl H, Renner K, Syed S, Mack M
Mucosal Immunol, 2013-06-12;7(1):188-99.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Maternal immune activation during gestation interacts with Disc1 point mutation to exacerbate schizophrenia-related behaviors in mice.
Authors: Lipina T, Zai C, Hlousek D, Roder J, Wong A
J Neurosci, 2013-05-01;33(18):7654-66.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Regulator of calcineurin 1 suppresses inflammation during respiratory tract infections.
Authors: Junkins R, MacNeil A, Wu Z, McCormick C, Lin T
J Immunol, 2013-04-15;190(10):5178-86.
Species: Mouse
Sample Types: BALF
Applications: ELISA Development (Capture) -
Loss of the transcription factor GLI1 identifies a signaling network in the tumor microenvironment mediating KRAS oncogene-induced transformation.
Authors: Mills, Lisa D, Zhang, Yaqing, Marler, Ronald J, Herreros-Villanueva, Marta, Zhang, Lizhi, Almada, Luciana, Couch, Fergus, Wetmore, Cynthia, Pasca di Magliano, Marina, Fernandez-Zapico, Martin E
J Biol Chem, 2013-03-12;288(17):11786-94.
Species: Mouse
Sample Types: Cell Lysates
Applications: Neutralization -
Response patterns of cytokines/chemokines in two murine strains after irradiation.
Authors: Zhang M, Yin L, Zhang K, Sun W, Yang S, Zhang B, Salzman P, Wang W, Liu C, Vidyasagar S, Zhang L, Ju S, Okunieff P, Zhang L
Cytokine, 2012-01-25;58(2):169-77.
Species: Mouse
Sample Types: Plasma
Applications: Luminex Development -
CXCL1 can be regulated by IL-6 and promotes granulocyte adhesion to brain capillaries during bacterial toxin exposure and encephalomyelitis.
Authors: Roy M, Richard JF, Dumas A, Vallieres L
J Neuroinflammation, 2012-01-23;9(0):18.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus.
Authors: Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, Chan YR, Ouyang W, Brown GD, Weaver CT, Steele C
Infect. Immun., 2011-10-28;80(1):410-7.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy.
Authors: Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N, Lasarte JJ, Matsuzaki G, Ikuta K, Ryffel B, Benlagha K, Tesniere A, Ibrahim N, Dechanet-Merville J, Chaput N, Smyth MJ, Kroemer G, Zitvogel L
J. Exp. Med., 2011-03-07;208(3):491-503.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Interleukin-6 enhances glucose-stimulated insulin secretion from pancreatic beta-cells: potential involvement of the PLC-IP3-dependent pathway
Authors: Toshinobu Suzuki, Junta Imai, Tetsuya Yamada, Yasushi Ishigaki, Keizo Kaneko, Kenji Uno et al.
Diabetes
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Resistance of human alveolar macrophages to Bacillus anthracis lethal toxin.
Authors: Wu W, Mehta H, Chakrabarty K, Booth JL, Duggan ES, Patel KB, Ballard JD, Coggeshall KM, Metcalf JP
J. Immunol., 2009-10-07;183(9):5799-806.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis.
Authors: Osuchowski MF, Connett J, Welch K, Granger J, Remick DG
Crit. Care Med., 2009-05-01;37(5):1567-73.
Species: Mouse
Sample Types: Plasma
Applications: ELISA Development -
ST2 deficient mice display a normal host defense against pulmonary infection with Mycobacterium tuberculosis.
Authors: Wieland CW, van der Windt GJ, Florquin S, McKenzie AN, van der Poll T
Microbes Infect., 2009-03-13;11(4):524-30.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
CD14 contributes to pulmonary inflammation and mortality during murine tuberculosis.
Authors: Wieland CW, van der Windt GJ, Wiersinga WJ, Florquin S, van der Poll T
Immunology, 2008-04-03;125(2):272-9.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: ELISA Development -
Tumor-specific Th17-polarized cells eradicate large established melanoma.
Authors: Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP
Blood, 2008-03-19;112(2):362-73.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Maternal immune activation alters fetal brain development through interleukin-6.
Authors: Smith SE, Li J, Garbett K, Mirnics K, Patterson PH
J. Neurosci., 2007-10-03;27(40):10695-702.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Comparison of the roles of IL-1, IL-6, and TNFalpha in cell culture and murine models of aseptic loosening.
Authors: Taki N, Tatro JM, Lowe R, Goldberg VM, Greenfield EM
Bone, 2006-12-21;40(5):1276-83.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
A new RXR agonist, HX630, suppresses intimal hyperplasia in a mouse blood flow cessation model.
Authors: Haraguchi G, Suzuki J, Kosuge H, Ogawa M, Koga N, Muto S, Itai A, Kagechika H, Shudo K, Isobe M
J. Mol. Cell. Cardiol., 2006-09-11;41(5):885-92.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality.
Authors: Osuchowski MF, Welch K, Siddiqui J, Remick DG
J. Immunol., 2006-08-01;177(3):1967-74.
Species: Mouse
Sample Types: Plasma
Applications: ELISA Development -
Glechoma hederacea inhibits inflammatory mediator release in IFN-gamma and LPS-stimulated mouse peritoneal macrophages.
Authors: An HJ, Jeong HJ, Um JY, Kim HM, Hong SH
J Ethnopharmacol, 2006-03-10;106(3):418-24.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Role of interleukin-6 in cardiomyocyte/cardiac fibroblast interactions during myocyte hypertrophy and fibroblast proliferation.
Authors: Fredj S, Bescond J, Louault C, Delwail A, Potreau D
J. Cell. Physiol., 2005-08-01;204(2):428-36.
Species: Mouse
Sample Types: Cell Culture Supernates, Whole Cells
Applications: ELISA Development, Neutralization -
Sequential ELISA to profile multiple cytokines from small volumes.
Authors: Osuchowski MF, Siddiqui J, Copeland S, Remick DG
J. Immunol. Methods, 2005-07-01;302(1):172-81.
Species: Mouse
Sample Types: Plasma
Applications: ELISA Development -
Selective macrophage suppression during sepsis.
Authors: Ellaban E, Bolgos G, Remick D
Cell. Immunol., 2005-02-26;231(1):103-11.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways.
Authors: Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A
J. Immunol., 2003-05-01;170(9):4665-72.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Tick salivary gland extract-activated transmission of Borrelia afzelii spirochaetes.
Authors: Pechova J, Stĕpánová G, Kovar L, Kopecky J
Folia Parasitol., 2002-01-01;49(2):153-9.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Fli1-haploinsufficient dermal fibroblasts promote skin-localized transdifferentiation of Th2-like regulatory T cells
Authors: R Saigusa, Y Asano, T Taniguchi, M Hirabayash, K Nakamura, S Miura, T Yamashita, T Takahashi, Y Ichimura, T Toyama, A Yoshizaki, M Trojanowsk, S Sato
Arthritis Res. Ther., 2018-02-07;20(1):23.
-
Melatonin induces anti-inflammatory effects via endoplasmic reticulum stress in RAW264.7 macrophages
Authors: Yina Chen, Qian Zhao, Yangjie Sun, Yin Jin, Jie Zhang, Jiansheng Wu
Molecular Medicine Reports
-
IL-1-induced JAK/STAT signaling is antagonized by TGF-beta to shape CAF heterogeneity in pancreatic ductal adenocarcinoma.
Authors: Giulia Biffi, Tobiloba E. Oni, Benjamin Spielman, Yuan Hao, Ela Elyada, Youngkyu Park et al.
Cancer Discovery
-
Interleukin-6 Is Essential for Primary Resistance to Francisella tularensis Live Vaccine Strain Infection
Authors: Sherry L. Kurtz, Oded Foreman, Catharine M. Bosio, Miriam R. Anver, Karen L. Elkins
Infection and Immunity
-
Interleukin-6 enhances glucose-stimulated insulin secretion from pancreatic beta-cells: potential involvement of the PLC-IP3-dependent pathway
Authors: Toshinobu Suzuki, Junta Imai, Tetsuya Yamada, Yasushi Ishigaki, Keizo Kaneko, Kenji Uno et al.
Diabetes
-
A microwell-based impedance sensor on an insertable microneedle for real-time in vivo cytokine detection
Authors: Naixin Song, Pengfei Xie, Wen Shen, Hanju Oh, Yejia Zhang, Flavia Vitale et al.
Microsystems & Nanoengineering
-
Spinal interleukin-6 contributes to central sensitisation and persistent pain hypersensitivity in a model of juvenile idiopathic arthritis
Authors: Charlie H.T. Kwok, Annastazia E Learoyd, Julia Canet-Pons, Tuan Trang, Maria Fitzgerald
Brain, Behavior, and Immunity
-
Activation of JAK/STAT Signaling in Megakaryocytes Sustains Myeloproliferation In Vivo
Authors: Brittany Woods, Wei Chen, Sophia Chiu, Christian Marinaccio, Chunling Fu, Lilly Gu et al.
Clinical Cancer Research
-
Fisetin inhibits pristine-induced systemic lupus erythematosus in a murine model through CXCLs regulation
Authors: Su‑Ping Xu, Yong‑Sheng Li
International Journal of Molecular Medicine
-
The role of IL-6 in neurodevelopment after prenatal stress
Authors: Serena B. Gumusoglu, Rebecca S. Fine, Samuel J. Murray, Jada L. Bittle, Hanna E. Stevens
Brain, Behavior, and Immunity
-
Considerations regarding use of solvents in in vitro cell based assays
Authors: Michael Timm, Lasse Saaby, Lise Moesby, Erik Wind Hansen
Cytotechnology
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse IL-6 Antibody
Average Rating: 4.6 (Based on 5 Reviews)
Have you used Mouse IL-6 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: