Mouse VEGFR2/KDR/Flk-1 Antibody Summary
Ala20-Glu762
Accession # P35918
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
VEGFR2/KDR/Flk‑1 in Mouse Embryo. VEGFR2/KDR/Flk-1 was detected in immersion fixed frozen sections of mouse embryo (14 d.p.c.) using 15 µg/mL Goat Anti-Mouse VEGFR2/KDR/Flk-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF644) overnight at 4 °C. Tissue was stained with the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). Specific labeling was localized to mesenchymal cells. View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
VEGFR2 in Mouse Kidney Tissue. VEGFR2 was detected in acetone fixed cryosections of mouse kidney tissue using Goat Anti-Mouse VEGFR2/KDR/Flk-1 Polyclonal Antibody (Catalog # AF644) for 50 minutes at room temperature. Tissues were stained with rabbit anti-goat secondary antibody and HRP polymer-conjugated anti-rabbit IgG followed by AEC+Substrate Chromogen (red) followed by counterstaining with hematoxylin (blue). Experiments were carried out and the image was provided by Dr. Grietje Molema, University Medical Center Groningen, The Netherlands.
 View Larger
View Larger
VEGFR2/KDR/Flk‑1 Inhibition of VEGF-dependent Cell Proliferation and Neutralization by Mouse VEGFR2/KDR/Flk‑1 Antibody. Recombinant Mouse VEGFR2/KDR/Flk-1 Fc Chimera (Catalog # 443-KD) inhibits Recombinant Mouse VEGF164(Catalog # 493-MV) induced proliferation in HUVEC human umbilical vein endothelial cells in a dose-dependent manner (orange line). Inhibition of Recombinant Mouse VEGF164(5 ng/mL) activity elicited by Recombinant Mouse VEGFR2/KDR/Flk-1 Fc Chimera (50 ng/mL) is neutralized (green line) by increasing concentrations of Goat Anti-Mouse VEGFR2/KDR/Flk-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF644). The ND50 is typically 0.1-0.3 µg/mL.
 View Larger
View Larger
Detection of Mouse VEGFR2/KDR/Flk-1 by Immunocytochemistry/Immunofluorescence BM-PC express Notch pathway ligands/receptors and show increased expression of notch downstream targets during endothelial differentiation.A. Expression of Notch receptors and ligands in BM-PC was detected by RT-PCR. B. Expression of Notch downstream targets (Hes 1, Hey 1 and 2) was detected at different time points during BM-PC endothelial differentiation by quantitative real-time PCR. C. Representative images (×200) of BM-PC at day 20 of culture showing positivity for endothelial lineage specific markers, acetylated LDL, CD31, Flk-1 and vWF with DAPI nuclear counterstaining in blue. D. Quantification of BM-PC positive cells for acetylated LDL, CD31, Flk-1 and VWF after 20 days of culture. Each experiment was performed in triplicate and the mean presented (n = 3). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0003752), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VEGFR2/KDR/Flk-1 by Immunohistochemistry Vascular alterations after intraocular VEGF-A injection. a Morphology of IB4-stained P6 wild-type retinal vessels at 4 h after administration of human VEGF-A165 (0.5 µl at a concentration of 5 μg μl−1). Note blunt appearance of the vessel front after VEGF-A injection but not for vehicle (PBS) control. Scale bar, 200 µm. b Quantitation of sprouts and filopodia at the front of the P6 vessel plexus after injection of VEGF-A165 or vehicle control. Error bars, s.e.m. p-values, Student’s t-test. c PDGFR beta + (green) pericytes are unaffected by short-term VEGF-A administration, whereas VEGFR2 immunosignals (white) are increased in IB4+ (red) ECs (arrowheads). Images shown correspond to insets in a. Scale bar, 100 µm. d Quantitation of VEGFR2 immunosignals intensity in the peripheral plexus of P6 retinas after injection of VEGF-A165 or vehicle control. Error bars, s.e.m. p-values, Student’s t-test. e Confocal images showing increased Esm1 immunostaining (white) in IB4+ (red) ECs in the peripheral plexus (arrowheads) after VEGF-A injection in P6 pups. Scale bar, 200 µm. f VEGF-A165 injection-mediated increase of Esm1 immunosignals (normalized to IB4+ EC area) in the peripheral capillary plexus but not at the edge of the angiogenic front in comparison to PBS-injected controls at P6. Error bars, s.e.m. p-values, Student’s t-test. NS, not statistically significant. g Short-term VEGF-A165 administration leads to clustering of Erg1+ (green) and IB4+ (red) ECs, as indicated, in thick sprout-like structures of P6 retinas. Panels in the center and on the right (scale bar, 20 µm) show higher magnification of the insets on the left (scale bar, 100 µm). Dashed lines in panels on the right outline IB4+ vessels. h Quantitation of EC density in the leading front vessel and emerging sprouts of the P6 angiogenic front after injection of VEGF-A165 or vehicle control. Error bars, s.e.m. p-values, Student’s t-test Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29146905), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VEGFR2/KDR/Flk-1 by Immunocytochemistry/Immunofluorescence Inactivation of Flt1 in PDGFR beta + cells. a Experimental scheme of tamoxifen administration for the generation of Flt1iPC mutants. b P6 control, Flt1iPC/+ and Flt1iPC retinas stained with isolectin B4 (IB4). Dashed circles indicate vessel-covered (yellow) and peripheral avascular (white) areas in the overview pictures (top). Scale bar, 500 µm. c Quantitation of body weight and radial outgrowth of the retinal vasculature in control, Flt1iPC/+ and Flt1iPC P6 pups. Error bars, s.e.m. p-values, one-way ANOVA. NS, not statistically significant. d Confocal images of the IB4-stained P6 control, Flt1iPC/+ and Flt1iPC retinal angiogenic front illustrating differences in sprout number and morphology. Scale bar, 100 µm. e Quantitation of sprouts and filopodia in P6 control, Flt1iPC/+ and Flt1iPC retinas. Error bars, s.e.m. p-values, one-way ANOVA and Tukey’s multiple comparison test. NS, not statistically significant. f Confocal images of IB4 (red), Erg1 (green) and VEGFR2 (white) stained P6 retinas highlighting the accumulation of EC nuclei and enhanced VEGFR2 immunosignals (arrowheads) in Flt1iPC sprouts. Vessels are outlined by dashed lines on the right panel. Scale bar, 100 µm. g Quantitation of EC proliferation (EdU+ Erg1+) at the angiogenic front, EC density in sprouts and leading front vessel and VEGFR2 immunosignals intensity in the angiogenic front of control and Flt1iPC P6 retinas. Error bars, s.e.m. p-values, Student’s t-test. h Esm1 (white) expression (arrowheads) in the angiogenic front (IB4+, red, first two columns) and detection of desmin+ pericytes (green, third column) in P6 control and Flt1iPC retinas. Scale bar, 100 µm. i Quantitation of Esm1+ proportion relative to vascular area (IB4+) in the angiogenic front of control and Flt1iPC P6 retinas. Error bars, s.e.m. p-values, Student’s t-test. j Confocal images of P6 retinas stained for NG2 (green) and IB4 (red) showing no significant changes in pericyte coverage in the front (first two columns) or the remodeling plexus around veins (v) or arteries (a) (last two columns). Scale bar, 100 µm. k, l Quantitation of pericyte coverage k and relative gene expression by qPCR on whole lysates l in control and Flt1iPC P6 retinas. Error bars, s.e.m. p-values, Student’s t-test. NS, not statistically significant Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29146905), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of VEGFR2/KDR/Flk-1 in Mouse Kidney. Formalin-fixed paraffin-embedded tissue sections of mouse kidney were probed for VEGFR2 mRNA (ACD RNAScope Probe, catalog #414818, Fast Red chromogen, ACD catalog # 322750). Adjacent tissue section was processed for immunohistochemistry using goat anti-mouse VEGFR2 polyclonal antibody (R&D Systems catalog # AF644) at 1.7ug/mL with 1 hour incubation at room temperature followed by incubation with anti-goat IgG VisUCyte HRP Polymer Antibody (Catalog # VC004) and DAB chromogen (yellow-brown). Tissue was counterstained with hematoxylin (blue). Specific staining was localized to glomeruli and fibroblasts.
 View Larger
View Larger
Detection of Mouse Mouse VEGFR2/KDR/Flk-1 Antibody by Immunocytochemistry/ Immunofluorescence BM-PC express Notch pathway ligands/receptors and show increased expression of notch downstream targets during endothelial differentiation.A. Expression of Notch receptors and ligands in BM-PC was detected by RT-PCR. B. Expression of Notch downstream targets (Hes 1, Hey 1 and 2) was detected at different time points during BM-PC endothelial differentiation by quantitative real-time PCR. C. Representative images (×200) of BM-PC at day 20 of culture showing positivity for endothelial lineage specific markers, acetylated LDL, CD31, Flk-1 and vWF with DAPI nuclear counterstaining in blue. D. Quantification of BM-PC positive cells for acetylated LDL, CD31, Flk-1 and VWF after 20 days of culture. Each experiment was performed in triplicate and the mean presented (n = 3). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/19015735), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
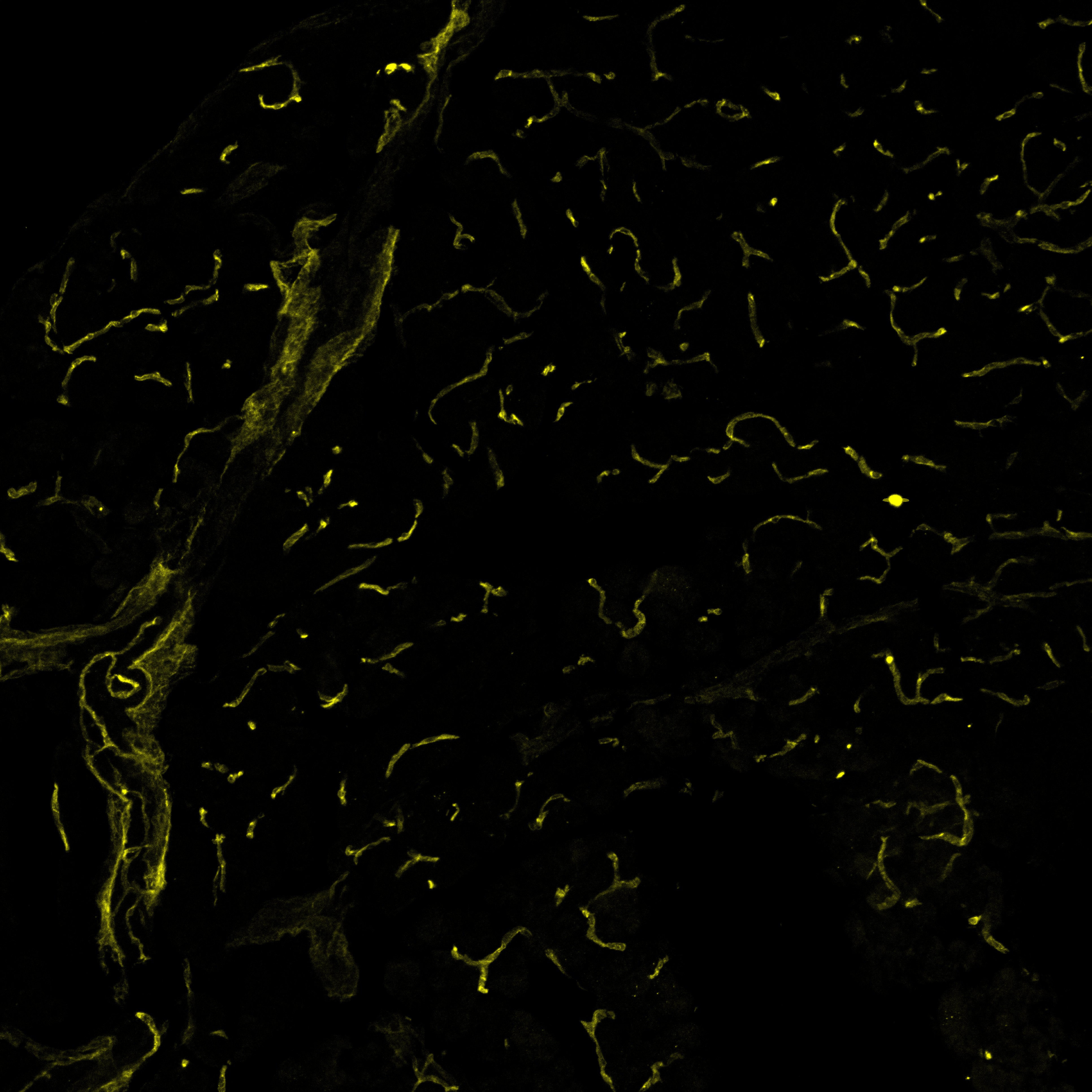
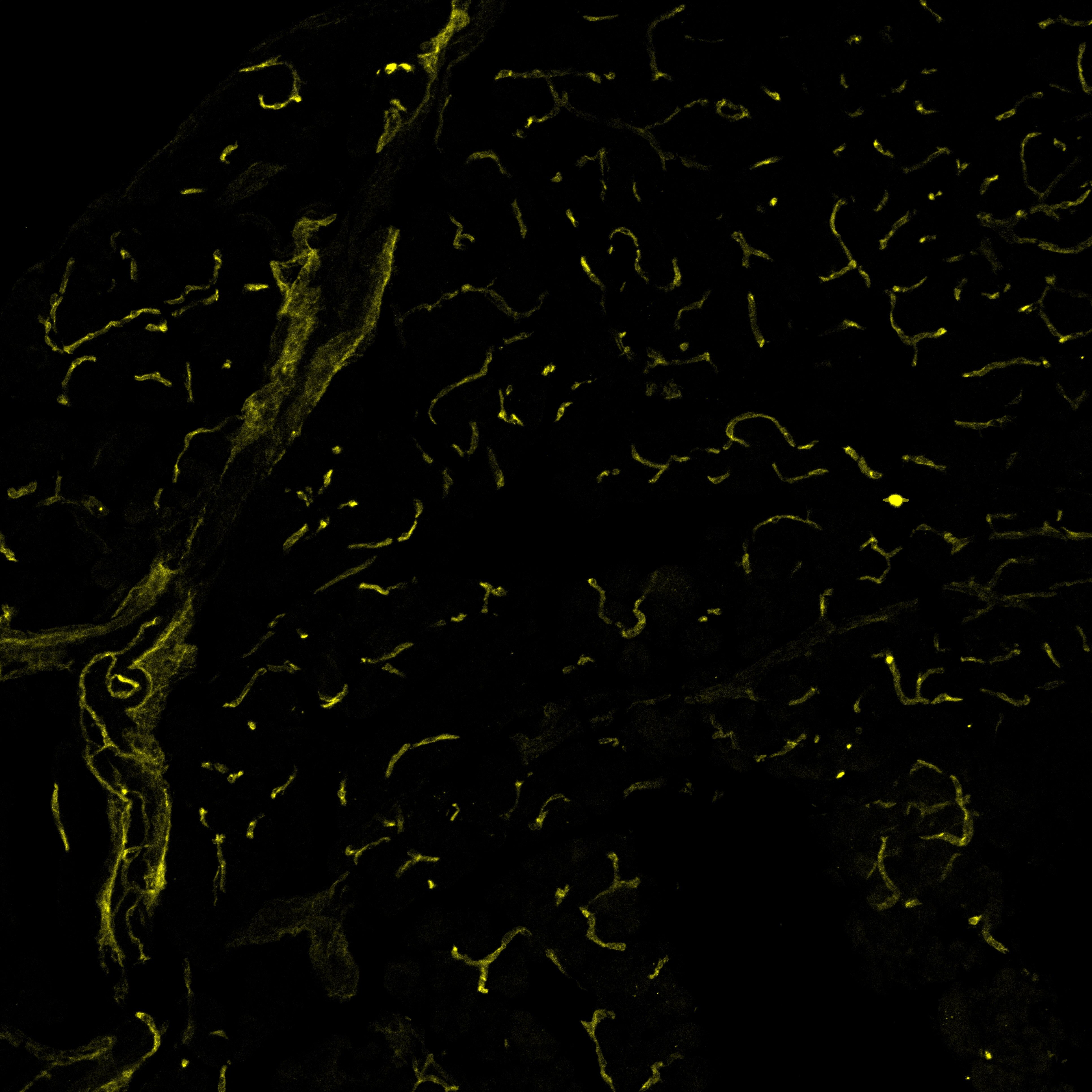
VEGFR2/KDR/Flk‑1 in Mouse Pancreas. Mouse pancreas cryosections stained with VEGFR2 (yellow), labelling blood vessels. Image from a verified customer review.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: VEGFR2/KDR/Flk-1
VEGFR2 (KDR/Flk-1), VEGFR1 (Flt-1), and VEGFR3 (Flt-4) belong to the class III subfamily of receptor tyrosine kinases (RTKs). All three receptors contain seven immunoglobulin-like repeats in their extracellular domains and kinase insert domains in their intracellular regions. The expression of VEGFR1, 2, and 3 is almost exclusively restricted to the endothelial cells. These receptors are likely to play essential roles in vasculogenesis and angiogenesis.
Mouse VEGFR2 cDNA encodes a 1367 amino acid (aa) residue precursor protein with a 19 aa residue signal peptide. Mature VEGFR2 is composed of a 743 aa residue extracellular domain, a 22 aa residue transmembrane domain and a 583 aa residue cytoplasmic domain. In contrast to VEGFR1 which binds both PlGF and VEGF with high affinity, VEGFR2 binds VEGF but not PlGF with high affinity. The recombinant soluble VEGFR2/Fc chimera binds VEGF with high affinity and is a potent VEGF antagonist.
- Ferra, N. and R. Davis-Smyth (1997) Endocrine Reviews 18:4.
- Achen, M.G. et al. (1998) Proc. Natl. Acad. Sci. USA 95:548.
Product Datasheets
Citations for Mouse VEGFR2/KDR/Flk-1 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
102
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Imaging Blood Vessels and Lymphatics in Mouse Trachea Wholemounts
Authors: Peter Baluk, Donald M. McDonald
Methods in Molecular Biology
-
Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation
Authors: Reto Huggenberger, Stefan Ullmann, Steven T. Proulx, Bronislaw Pytowski, Kari Alitalo, Michael Detmar
Journal of Experimental Medicine
-
Elevated Vascular Endothelial Growth Factor Receptor-2 Abundance Contributes to Increased Angiogenesis in Vascular Endothelial Growth Factor Receptor-1–Deficient Mice
Authors: Vivienne C. Ho, Li-Juan Duan, Chunxia Cronin, Bruce T. Liang, Guo-Hua Fong
Circulation
-
VEGFR-2 redirected CAR-T cells are functionally impaired by soluble VEGF-A competition for receptor binding
Authors: Evripidis Lanitis, Paris Kosti, Catherine Ronet, Elisabetta Cribioli, Giorgia Rota, Aodrenn Spill et al.
Journal for ImmunoTherapy of Cancer
-
Dedifferentiation and Proliferation of Artery Endothelial Cells Drive Coronary Collateral Development in Mice
Authors: Gauri Arolkar, Sneha K. Krishna Kumar, Hanjay Wang, Karen M. Gonzalez, Suraj Kumar, Bhavnesh Bishnoi et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Negative pressure wound therapy induces early wound healing by increased and accelerated expression of vascular endothelial growth factor receptors
Authors: Tsuruhito Tanaka, Nirmal Panthee, Yoshifumi Itoda, Naoko Yamauchi, Masashi Fukayama, Minoru Ono
European Journal of Plastic Surgery
-
DACH1 stimulates shear stress-guided endothelial cell migration and coronary artery growth through the CXCL12–CXCR4 signaling axis
Authors: Andrew H. Chang, Brian C. Raftrey, Gaetano D'Amato, Vinay N. Surya, Aruna Poduri, Heidi I. Chen et al.
Genes & Development
-
Semaphorin 3E–Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism
Authors: Jiha Kim, Won-Jong Oh, Nicholas Gaiano, Yutaka Yoshida, Chenghua Gu
Genes & Development
-
A Functional Role for VEGFR1 Expressed in Peripheral Sensory Neurons in Cancer Pain
Authors: Deepitha Selvaraj, Vijayan Gangadharan, Christoph W. Michalski, Martina Kurejova, Sebastian Stösser, Kshitij Srivastava et al.
Cancer Cell
-
Liquid-crystal organization of liver tissue.
Authors: Morales-Navarrete H, Nonaka H et al.
Elife
-
Bacterial meningitis in the early postnatal mouse studied at single-cell resolution
Authors: Wang J, Rattner A, Nathans J
eLife
-
Dysfunctional SEMA3E signaling underlies gonadotropin-releasing hormone neuron deficiency in Kallmann syndrome
Authors: Anna Cariboni, Valentina André, Sophie Chauvet, Daniele Cassatella, Kathryn Davidson, Alessia Caramello et al.
Journal of Clinical Investigation
-
Vascular Endothelial Growth Factor Promotes Pericyte Coverage of Brain Capillaries, Improves Cerebral Blood Flow During Subsequent Focal Cerebral Ischemia, and Preserves the Metabolic Penumbra
Authors: Anil Zechariah, Ayman ElAli, Thorsten R. Doeppner, Fengyan Jin, Mohammad R. Hasan, Iris Helfrich et al.
Stroke
-
ADAM10 controls the differentiation of the coronary arterial endothelium
Authors: Gregory Farber, Matthew M. Parks, Nicole Lustgarten Guahmich, Yi Zhang, Sébastien Monette, Scott C. Blanchard et al.
Angiogenesis
-
Vascular Endothelial Growth Factors A and C are Induced in the SVZ Following Neonatal Hypoxia–Ischemia and Exert Different Effects on Neonatal Glial Progenitors
Authors: Jennifer M. Bain, Lisamarie Moore, Zhihua Ren, Sophia Simonishvili, Steven W. Levison
Translational Stroke Research
-
The transcription factor Rreb1 regulates epithelial architecture, invasiveness, and vasculogenesis in early mouse embryos
Authors: Sophie M Morgani, Jie Su, Jennifer Nichols, Joan Massagué, Anna-Katerina Hadjantonakis
eLife
-
Heterogeneity in VEGFR3 levels drives lymphatic vessel hyperplasia through cell-autonomous and non-cell-autonomous mechanisms
Authors: Y Zhang, MH Ulvmar, L Stanczuk, I Martinez-C, M Frye, K Alitalo, T Mäkinen
Nat Commun, 2018-04-03;9(1):1296.
-
Imaging Lymphatics in Mouse Lungs
Authors: Peter Baluk, Donald M. McDonald
Methods in Molecular Biology
-
The Neuropilin 1 Cytoplasmic Domain Is Required for VEGF-A-Dependent Arteriogenesis
Authors: Anthony Lanahan, Xi Zhang, Alessandro Fantin, Zhen Zhuang, Felix Rivera-Molina, Katherine Speichinger et al.
Developmental Cell
-
VEGF receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts
Authors: Ingrid Nilsson, Fuad Bahram, Xiujuan Li, Laura Gualandi, Sina Koch, Malin Jarvius et al.
The EMBO Journal
-
Interplay of vascular endothelial growth factor receptors in organ-specific vessel maintenance
Authors: Sinem Karaman, Satu Paavonsalo, Krista Heinolainen, Madeleine H. Lackman, Amanda Ranta, Karthik A. Hemanthakumar et al.
Journal of Experimental Medicine
-
Smad4-mediated angiogenesis facilitates the beiging of white adipose tissue in mice
Authors: Chenguang Wang, Yalan Wu, Yangxian Li, Yang Zhang, Yee Lok Fung, Ka Kui Tong et al.
iScience
-
Specialized post-arterial capillaries facilitate adult bone remodelling
Authors: Mohanakrishnan, V;Sivaraj, KK;Jeong, HW;Bovay, E;Dharmalingam, B;Bixel, MG;Dinh, VV;Petkova, M;Paredes Ugarte, I;Kuo, YT;Gurusamy, M;Raftrey, B;Chu, NTL;Das, S;Rios Coronado, PE;Stehling, M;Sävendahl, L;Chagin, AS;Mäkinen, T;Red-Horse, K;Adams, RH;
Nature cell biology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Helminth infection driven gastrointestinal hypermotility is independent of eosinophils and mediated by alterations in smooth muscle instead of enteric neurons
Authors: Wang, H;Barry, K;Zaini, A;Coakley, G;Moyat, M;Daunt, CP;Wickramasinghe, LC;Azzoni, R;Chatzis, R;Yumnam, B;Camberis, M;Le Gros, G;Perdijk, O;Foong, JPP;Bornstein, JC;Marsland, BJ;Harris, NL;
PLoS pathogens
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
VEGFR3 is required for button junction formation in lymphatic vessels
Authors: Jannaway, M;Iyer, D;Mastrogiacomo, DM;Li, K;Sung, DC;Yang, Y;Kahn, ML;Scallan, JP;
Cell reports
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Molecular Ultrasound Imaging Depicts the Modulation of Tumor Angiogenesis by Acetylsalicylic Acid
Authors: F Mueller-Di, W Lederle, A Rix, S Koletnik, D Doleschel, M Snelting, F Gremse, F Kiessling
International Journal of Molecular Sciences, 2023-04-11;24(8):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
An agonistic anti-Tie2 antibody suppresses the normal-to-tumor vascular transition in the glioblastoma invasion zone
Authors: E Lee, EA Lee, E Kong, H Chon, M Llaiqui-Co, CH Park, BY Park, NR Kang, JS Yoo, HS Lee, HS Kim, SH Park, SW Choi, D Vestweber, JH Lee, P Kim, WS Lee, I Kim
Experimental & Molecular Medicine, 2023-02-24;55(2):470-484.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Low-flow intussusception and metastable VEGFR2 signaling launch angiogenesis in ischemic muscle
Authors: JM Arpino, H Yin, EK Prescott, SCR Staples, Z Nong, F Li, J Chevalier, B Balint, C O'Neil, R Mortuza, S Milkovich, JJ Lee, D Lorusso, M Sandig, DW Hamilton, DW Holdsworth, TL Poepping, CG Ellis, JG Pickering
Science Advances, 2021-11-26;7(48):eabg9509.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Regulation of intestinal immunity and tissue repair by enteric glia
Authors: F Progatzky, M Shapiro, SH Chng, B Garcia-Cas, CH Classon, S Sevgi, A Laddach, AC Bon-Frauch, R Lasrado, M Rahim, EM Amaniti, S Boeing, K Shah, LJ Entwistle, A Suárez-Bon, MS Wilson, B Stockinger, V Pachnis
Nature, 2021-10-20;599(7883):125-130.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Paladin is a phosphoinositide phosphatase regulating endosomal VEGFR2 signalling and angiogenesis
Authors: Anja Nitzsche, Riikka Pietilä, Dominic T Love, Chiara Testini, Takeshi Ninchoji, Ross O Smith et al.
EMBO reports
Species: Human, Mouse
Sample Types: Tissue Homogenates, Whole Cells
Applications: Immunoprecipitation, Neutralization, Immunocytochemistry -
ABL001, a Bispecific Antibody Targeting VEGF and DLL4, with Chemotherapy, Synergistically Inhibits Tumor Progression in Xenograft Models
Authors: DH Yeom, YS Lee, I Ryu, S Lee, B Sung, HB Lee, D Kim, JH Ahn, E Ha, YS Choi, SH Lee, WK You
International Journal of Molecular Sciences, 2020-12-29;22(1):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Distinct fibroblast subsets regulate lacteal integrity through YAP/TAZ-induced VEGF-C in intestinal villi
Authors: SP Hong, MJ Yang, H Cho, I Park, H Bae, K Choe, SH Suh, RH Adams, K Alitalo, D Lim, GY Koh
Nat Commun, 2020-08-14;11(1):4102.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
PRDM16 orchestrates angiogenesis via neural differentiation in the developing brain
Authors: L Su, X Lei, H Ma, C Feng, J Jiang, J Jiao
Cell Death Differ., 2020-02-03;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
EphrinB2 regulates VEGFR2 during dendritogenesis and hippocampal circuitry development
Authors: E Harde, L Nicholson, B Furones Cu, D Bissen, S Wigge, S Urban, M Segarra, C Ruiz de Al, A Acker-Palm
Elife, 2019-12-23;8(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Disrupting Myelin-Specific Th17 Cell Gut Homing Confers Protection in an Adoptive Transfer Experimental Autoimmune Encephalomyelitis
Authors: D Duc, S Vigne, J Bernier-La, Y Yersin, F Ruiz, N Gaïa, S Leo, V Lazarevic, J Schrenzel, TV Petrova, C Pot
Cell Rep, 2019-10-08;29(2):378-390.e4.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
ADAM10 controls the differentiation of the coronary arterial endothelium
Authors: Gregory Farber, Matthew M. Parks, Nicole Lustgarten Guahmich, Yi Zhang, Sébastien Monette, Scott C. Blanchard et al.
Angiogenesis
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Endothelial Calcineurin Signaling Restrains Metastatic Outgrowth by Regulating Bmp2
Authors: S Hendrikx, S Coso, B Prat-Luri, L Wetterwald, A Sabine, CA Franco, S Nassiri, N Zangger, H Gerhardt, M Delorenzi, TV Petrova
Cell Rep, 2019-01-29;26(5):1227-1241.e6.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Erythro-myeloid progenitors contribute endothelial cells to blood vessels
Authors: Alice Plein, Alessandro Fantin, Laura Denti, Jeffrey W. Pollard, Christiana Ruhrberg
Nature
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Deletion of Nkx2-5 in trabecular myocardium reveals the developmental origins of pathological heterogeneity associated with ventricular non-compaction cardiomyopathy
Authors: C Choquet, THM Nguyen, P Sicard, E Buttigieg, TT Tran, F Kober, I Varlet, R Sturny, MW Costa, RP Harvey, C Nguyen, P Rihet, S Richard, M Bernard, RG Kelly, N Lalevée, L Miquerol
PLoS Genet., 2018-07-06;14(7):e1007502.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
In utero electroporation induces cell death and alters embryonic microglia morphology and expression signatures in the developing hypothalamus
Authors: JM Rosin, DM Kurrasch
J Neuroinflammation, 2018-06-12;15(1):181.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Sox7 promotes high-grade glioma by increasing VEGFR2-mediated vascular abnormality
Authors: IK Kim, K Kim, E Lee, DS Oh, CS Park, S Park, JM Yang, JH Kim, HS Kim, DT Shima, JH Kim, SH Hong, YH Cho, YH Kim, JB Park, GY Koh, YS Ju, HK Lee, S Lee, I Kim
J. Exp. Med., 2018-02-14;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC-P -
Endothelial deletion of Ino80 disrupts coronary angiogenesis and causes congenital heart disease
Authors: S Rhee, JI Chung, DA King, G D'amato, DT Paik, A Duan, A Chang, D Nagelberg, B Sharma, Y Jeong, M Diehn, JC Wu, AJ Morrison, K Red-Horse
Nat Commun, 2018-01-25;9(1):368.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Angiogenic factor imbalance precedes complement deposition in placentae of the BPH/5 model of preeclampsia
Authors: JL Sones, AA Merriam, A Seffens, DA Brown-Gran, SD Butler, AM Zhao, X Xu, CJ Shawber, JK Grenier, NC Douglas
FASEB J., 2018-01-08;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Hedgehog Pathway Drives Fusion-Negative Rhabdomyosarcoma Initiated From Non-myogenic Endothelial Progenitors
Authors: CJ Drummond, JA Hanna, MR Garcia, DJ Devine, AJ Heyrana, D Finkelstei, JE Rehg, ME Hatley
Cancer Cell, 2018-01-08;33(1):108-124.e5.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Generation of iPSC-derived limb progenitor-like cells for stimulating phalange regeneration in the adult mouse
Authors: Y Chen, H Xu, G Lin
Cell Discov, 2017-12-19;3(0):17046.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Impaired angiopoietin/Tie2 signaling compromises Schlemm's canal integrity and induces glaucoma
Authors: J Kim, DY Park, H Bae, DY Park, D Kim, CK Lee, S Song, TY Chung, DH Lim, Y Kubota, YK Hong, Y He, HG Augustin, G Oliver, GY Koh
J. Clin. Invest., 2017-09-18;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Retinoic acid-induced upregulation of miR-219 promotes the differentiation of embryonic stem cells into neural cells
Authors: H Wu, J Zhao, B Fu, S Yin, C Song, J Zhang, S Zhao, Y Zhang
Cell Death Dis, 2017-07-27;8(7):e2953.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling
Authors: K Heinolaine, S Karaman, G D'Amico, T Tammela, R Sormunen, L Eklund, K Alitalo, G Zarkada
Circ. Res, 2017-03-15;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Cell-matrix signals specify bone endothelial cells during developmental osteogenesis
Authors: UH Langen, ME Pitulescu, JM Kim, R Enriquez-G, KK Sivaraj, AP Kusumbe, A Singh, J Di Russo, MG Bixel, B Zhou, L Sorokin, JM Vaquerizas, RH Adams
Nat. Cell Biol, 2017-02-20;19(3):189-201.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Pdgfrb-Cre targets lymphatic endothelial cells of both venous and non-venous origins
Authors: MH Ulvmar, I Martinez-C, L Stanczuk, T Mäkinen
Genesis, 2016-04-21;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Neural crest-derived SEMA3C activates endothelial NRP1 for cardiac outflow tract septation.
Authors: Plein A, Calmont A, Fantin A, Denti L, Anderson N, Scambler P, Ruhrberg C
J Clin Invest, 2015-06-08;125(7):2661-76.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Slit2 signaling through Robo1 and Robo2 is required for retinal neovascularization.
Authors: Rama N, Dubrac A, Mathivet T, Ni Charthaigh R, Genet G, Cristofaro B, Pibouin-Fragner L, Ma L, Eichmann A, Chedotal A
Nat Med, 2015-04-20;21(5):483-91.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Semaphorin3A elevates vascular permeability and contributes to cerebral ischemia-induced brain damage.
Authors: Hou, Sheng Ta, Nilchi, Ladan, Li, Xuesheng, Gangaraju, Sandhya, Jiang, Susan X, Aylsworth, Amy, Monette, Robert, Slinn, Jacqueli
Sci Rep, 2015-01-20;5(0):7890.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
The Schlemm's canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel.
Authors: Aspelund A, Tammela T, Antila S, Nurmi H, Leppanen V, Zarkada G, Stanczuk L, Francois M, Makinen T, Saharinen P, Immonen I, Alitalo K
J Clin Invest, 2014-07-25;124(9):3975-86.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Lenalidomide inhibits lymphangiogenesis in preclinical models of mantle cell lymphoma.
Authors: Song K, Herzog B, Sheng M, Fu J, McDaniel J, Chen H, Ruan J, Xia L
Cancer Res, 2013-10-24;73(24):7254-64.
Species: Human
Sample Types: Tissue Homogenates
Applications: Western Blot -
KDR identifies a conserved human and murine hepatic progenitor and instructs early liver development.
Authors: Goldman O, Han S, Sourisseau M, Dziedzic N, Hamou W, Corneo B, D'Souza S, Sato T, Kotton D, Bissig K, Kalir T, Jacobs A, Evans T, Evans M, Gouon-Evans V
Cell Stem Cell, 2013-06-06;12(6):748-60.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
The role of IL-1beta in the early tumor cell-induced angiogenic response.
Authors: Carmi Y, Dotan S, Rider P, Kaplanov I, White M, Baron R, Abutbul S, Huszar M, Dinarello C, Apte R, Voronov E
J Immunol, 2013-03-08;190(7):3500-9.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
The embryonic mouse hindbrain as a qualitative and quantitative model for studying the molecular and cellular mechanisms of angiogenesis.
Authors: Fantin A, Vieira J, Plein A, Maden C, Ruhrberg C
Nat Protoc, 2013-02-01;8(2):418-29.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd.
Authors: Sun Z, Li X, Massena S, Kutschera S, Padhan N, Gualandi L, Sundvold-Gjerstad V, Gustafsson K, Choy WW, Zang G, Quach M, Jansson L, Phillipson M, Abid MR, Spurkland A, Claesson-Welsh L
J. Exp. Med., 2012-06-11;209(7):1363-77.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling.
Authors: Benedito R, Rocha S, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams R
Nature, 2012-03-18;484(7392):110-4.
Species: Mouse
Sample Types: Cell Lysates
Applications: ELISA Development -
The adaptation of the blood-brain barrier to vascular endothelial growth factor and placental growth factor during pregnancy.
Authors: Schreurs MP, Houston EM, May V, Cipolla MJ
FASEB J., 2011-09-12;26(1):355-62.
Species: Rat
Sample Types: In Vivo
Applications: Neutralization -
Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy.
Authors: Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S
Nat. Cell Biol., 2011-01-30;13(3):215-22.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis.
Authors: Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A
Nature, 2010-05-05;465(7297):487-91.
Species: Mouse
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Immunoprecipitation -
Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues.
Authors: Lohela M, Helotera H, Haiko P, Dumont DJ, Alitalo K
Am. J. Pathol., 2008-11-06;173(6):1891-901.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation.
Authors: Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Yla-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K
Nature, 2008-06-25;454(7204):656-60.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Tumor necrosis factor-alpha and endothelial cells modulate Notch signaling in the bone marrow microenvironment during inflammation.
Authors: Fernandez L, Rodriguez S, Huang H, Chora A, Fernandes J, Mumaw C, Cruz E, Pollok K, Cristina F, Price JE, Ferkowicz MJ, Scadden DT, Clauss M, Cardoso AA, Carlesso N
Exp. Hematol., 2008-05-01;36(5):545-558.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Chronic hydrocephalus-induced hypoxia: increased expression of VEGFR-2+ and blood vessel density in hippocampus.
Authors: Dombrowski SM, Deshpande A, Dingwall C, Leichliter A, Leibson Z, Luciano MG
Neuroscience, 2007-12-14;152(2):346-59.
Species: Canine
Sample Types: Whole Tissue
Applications: IHC-Fr -
Anthrax lethal toxin inhibits growth of and vascular endothelial growth factor release from endothelial cells expressing the human herpes virus 8 viral G protein coupled receptor.
Authors: Depeille P, Young JJ, Boguslawski EA, Berghuis BD, Kort EJ, Resau JH, Frankel AE, Duesbery NS
Clin. Cancer Res., 2007-10-01;13(19):5926-34.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Lentiviral rescue of vascular endothelial growth factor receptor-2 expression in flk1-/- embryonic stem cells shows early priming of endothelial precursors.
Authors: Li X, Edholm D, Lanner F, Breier G, Farnebo F, Dimberg A, Claesson-Welsh L
Stem Cells, 2007-08-16;25(12):2987-95.
Species: Mouse
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Immunoprecipitation -
Effects of sustained antiangiogenic therapy in multistage prostate cancer in TRAMP model.
Authors: Isayeva T, Chanda D, Kallman L, Eltoum IE, Ponnazhagan S
Cancer Res., 2007-06-15;67(12):5789-97.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting.
Authors: Wirzenius M, Tammela T, Uutela M, He Y, Odorisio T, Zambruno G, Nagy JA, Dvorak HF, Yla-Herttuala S, Shibuya M, Alitalo K
J. Exp. Med., 2007-05-29;204(6):1431-40.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Inhibition of prostate tumor growth and bone remodeling by the vascular targeting agent VEGF121/rGel.
Authors: Mohamedali KA, Poblenz AT, Sikes CR, Navone NM, Thorpe PE, Darnay BG, Rosenblum MG
Cancer Res., 2006-11-15;66(22):10919-28.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Hypoxia-induced mitogenic factor has proangiogenic and proinflammatory effects in the lung via VEGF and VEGF receptor-2.
Authors: Yamaji-Kegan K, Su Q, Angelini DJ, Champion HC, Johns RA
Am. J. Physiol. Lung Cell Mol. Physiol., 2006-08-04;291(6):L1159-68.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Neutralization -
Antagonists to human and mouse vascular endothelial growth factor receptor 2 generated by directed protein evolution in vitro.
Authors: Getmanova EV, Chen Y, Bloom L, Gokemeijer J, Shamah S, Warikoo V, Wang J, Ling V, Sun L
Chem. Biol., 2006-05-01;13(5):549-56.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Increased VEGF levels induced by anti-VEGF treatment are independent of tumor burden in colorectal carcinomas in mice.
Authors: Schmitz V, Vilanueva H, Raskopf E, Hilbert T, Barajas M, Dzienisowicz C, Gorschluter M, Strehl J, Rabe C, Sauerbruch T, Prieto J, Caselmann WH, Qian C
Gene Ther., 2006-04-13;13(16):1198-205.
Species: Mouse
Sample Types: Serum
Applications: Western Blot -
Loss of SPARC-mediated VEGFR-1 suppression after injury reveals a novel antiangiogenic activity of VEGF-A.
Authors: Nozaki M, Sakurai E, Raisler BJ, Baffi JZ, Witta J, Ogura Y, Brekken RA, Sage EH, Ambati BK, Ambati J
J. Clin. Invest., 2006-02-01;116(2):422-9.
Species: Mouse
Sample Types: Cell Lysates, In Vivo
Applications: Immunoprecipitation, Neutralization -
Effective angiostatic treatment in a murine metastatic and orthotopic hepatoma model.
Authors: Raskopf E, Dzienisowicz C, Hilbert T, Rabe C, Leifeld L, Wernert N, Sauerbruch T, Prieto J, Qian C, Caselmann WH, Schmitz V
Hepatology, 2005-06-01;41(6):1233-40.
Species: Mouse
Sample Types: Serum
Applications: ELISA Development, Western Blot -
Antagonism of vascular endothelial growth factor results in microvessel attrition and disorganization of wound tissue.
Authors: Gudehithlu KP, Ahmed N, Wu H, Litbarg NO, Garber SL, Arruda JA, Dunea G, Singh AK
J. Lab. Clin. Med., 2005-04-01;145(4):194-203.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC-P -
Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation.
Authors: Dikov MM, Ohm JE, Ray N, Tchekneva EE, Burlison J, Moghanaki D, Nadaf S, Carbone DP
J. Immunol., 2005-01-01;174(1):215-22.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
All three receptors for vascular endothelial growth factor (VEGF) are expressed on B-chronic lymphocytic leukemia (CLL) cells.
Authors: Bairey O, Boycov O, Kaganovsky E, Zimra Y, Shaklai M, Rabizadeh E
Leuk. Res., 2004-03-01;28(3):243-8.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia.
Authors: Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C
J. Cell Biol., 2003-06-16;161(6):1163-77.
Species: Rat
Sample Types: In Vivo, Whole Tissue
Applications: IHC-Fr, Neutralization -
Vascular endothelial growth factor-B promotes in vivo angiogenesis.
Authors: Silvestre JS, Tamarat R, Ebrahimian TG, Le-Roux A, Clergue M, Emmanuel F, Duriez M, Schwartz B, Branellec D, Levy BI
Circ. Res., 2003-06-12;93(2):114-23.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Lack of alpha2-antiplasmin promotes pulmonary heart failure via overrelease of VEGF after acute myocardial infarction.
Authors: Kozawa O, Yoshimi N, Akamatsu S, Hara A, Mori H, Uematsu T
Blood, 2002-10-01;100(7):2487-93.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Paladin is a phosphoinositide phosphatase regulating endosomal VEGFR2 signalling and angiogenesis
Authors: Anja Nitzsche, Riikka Pietilä, Dominic T Love, Chiara Testini, Takeshi Ninchoji, Ross O Smith et al.
EMBO reports
-
Preclinical Characterization of XL092, a Novel Receptor Tyrosine Kinase Inhibitor of MET, VEGFR2, AXL, and MER
Authors: Jeff Hsu, Colin Chong, Jeffrey Serrill, Levina Goon, Joan Balayan, Eric N. Johnson et al.
Molecular Cancer Therapeutics
-
Plumbagin inhibits tumor angiogenesis of gastric carcinoma in mice by modulating nuclear factor-kappa B pathway
Authors: Chengqian Yang, Xingbo Feng, Zengxian Li, Qingsi He
Translational Cancer Research
-
A Unique Collateral Artery Development Program Promotes Neonatal Heart Regeneration
Authors: Soumyashree Das, Andrew B. Goldstone, Hanjay Wang, Justin Farry, Gaetano D’Amato, Michael J. Paulsen et al.
Cell
-
VEGF/VEGFR2 signaling regulates hippocampal axon branching during development
Authors: Robert Luck, Severino Urban, Andromachi Karakatsani, Eva Harde, Sivakumar Sambandan, LaShae Nicholson et al.
eLife
-
Relationship between impaired BMP signalling and clinical risk factors at early-stage vascular injury in the preterm infant
Authors: Motaharehsadat Heydarian, Prajakta Oak, Xin Zhang, Nona Kamgari, Alida Kindt, Markus Koschlig et al.
Thorax
-
Anti-metastatic action of FAK inhibitor OXA-11 in combination with VEGFR-2 signaling blockade in pancreatic neuroendocrine tumors
Authors: Ingrid Moen, Matthew Gebre, Vanesa Alonso-Camino, Debbie Chen, David Epstein, Donald M. McDonald
Clinical & Experimental Metastasis
-
The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis
Authors: Heidi I. Chen, Bikram Sharma, Brynn N. Akerberg, Harri J. Numi, Riikka Kivelä, Pipsa Saharinen et al.
Development
-
VEGF-C and aortic cardiomyocytes guide coronary artery stem development
Authors: Heidi I. Chen, Aruna Poduri, Harri Numi, Riikka Kivela, Pipsa Saharinen, Andrew S. McKay et al.
Journal of Clinical Investigation
-
Pericytes are progenitors for coronary artery smooth muscle
Authors: Katharina S Volz, Andrew H Jacobs, Heidi I Chen, Aruna Poduri, Andrew S McKay, Daniel P Riordan et al.
eLife
-
Erythro-myeloid progenitors contribute endothelial cells to blood vessels
Authors: Alice Plein, Alessandro Fantin, Laura Denti, Jeffrey W. Pollard, Christiana Ruhrberg
Nature
-
Adrb2 controls glucose homeostasis by developmental regulation of pancreatic islet vasculature
Authors: Alexis M Ceasrine, Eugene E Lin, David N Lumelsky, Radhika Iyer, Rejji Kuruvilla
eLife
-
Notch regulates the angiogenic response via induction of VEGFR-1
Authors: Yasuhiro Funahashi, Carrie J Shawber, Marina Vorontchikhina, Anshula Sharma, Hasina H Outtz, Jan Kitajewski
Journal of Angiogenesis Research
-
Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner
Authors: Morgane Sonia Thion, Donovan Low, Aymeric Silvin, Jinmiao Chen, Pauline Grisel, Jonas Schulte-Schrepping et al.
Cell
-
Genetic deficiency and pharmacological modulation of ROR alpha regulate laser-induced choroidal neovascularization
Authors: Chi-Hsiu Liu, Felix Yemanyi, Kiran Bora, Neetu Kushwah, Alexandra K. Blomfield, Theodore M. Kamenecka et al.
Aging (Albany NY)
-
Direct Activation of NADPH Oxidase 2 by 2-Deoxyribose-1-Phosphate Triggers Nuclear Factor Kappa B-Dependent Angiogenesis
Authors: Dina Vara, Joanna M. Watt, Tiago M. Fortunato, Harry Mellor, Matthew Burgess, Kate Wicks et al.
Antioxidants & Redox Signaling
-
Potential functions of embryonic cardiac macrophages in angiogenesis, lymphangiogenesis and extracellular matrix remodeling
Authors: Grzegorz Gula, Sławomir Rumiński, Justyna Niderla-Bielińska, Agnieszka Jasińska, Ewelina Kiernozek, Ewa Jankowska-Steifer et al.
Histochemistry and Cell Biology
-
Vascular Endothelial Growth Factor C for Polycystic Kidney Diseases
Authors: Jennifer L. Huang, Adrian S. Woolf, Maria Kolatsi-Joannou, Peter Baluk, Richard N. Sandford, Dorien J.M. Peters et al.
Journal of the American Society of Nephrology
-
Mesodermal retinoic acid signaling regulates endothelial cell coalescence in caudal pharyngeal arch artery vasculogenesis
Authors: Peng Li, Mohammad Pashmforoush, Henry M. Sucov
Developmental Biology
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse VEGFR2/KDR/Flk-1 Antibody
Average Rating: 4.7 (Based on 3 Reviews)
Have you used Mouse VEGFR2/KDR/Flk-1 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Mouse pancreas cryosections stained with VEGFR2 (yellow), labelling blood vessels.