Human/Mouse/Rat CD31/PECAM-1 Antibody Summary
Glu18-Lys590
Accession # Q08481
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Mouse CD31/PECAM‑1 by Western Blot. Western blot shows lysates of bEnd.3 mouse endothelioma cell line. PVDF membrane was probed with 0.5 µg/mL of Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF017). A specific band was detected for CD31/PECAM-1 at approximately 130 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Detection of Mouse CD31/PECAM‑1 by Simple WesternTM. Simple Western shows lysates of bEnd.3 mouse endothelioma cell line, loaded at 0.5 mg/ml. A specific band was detected for CD31/PECAM‑1 at approximately 165 kDa (as indicated) using 10 µg/mL of Goat Anti-Human/Mouse/Rat CD31/PECAM‑1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628). This experiment was conducted under reducing conditions and using the 12-230kDa separation system.
 View Larger
View Larger
Detection of CD31/PECAM‑1 in Mouse Splenocytes by Flow Cytometry. Mouse splenocytes were stained with Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628, filled histogram) or control antibody (AB-108-C, open histogram), followed by Allophycocyanin-conjugated Anti-Goat IgG Secondary Antibody (F0108).
 View Larger
View Larger
Detection of CD31/PECAM‑1 in Rat Splenocytes by Flow Cytometry. Rat splenocytes were stained with Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628, filled histogram) or isotype control antibody (AB-108-C, open histogram), followed by Allophycocyanin-conjugated Anti-Goat IgG Secondary Antibody (F0108).
 View Larger
View Larger
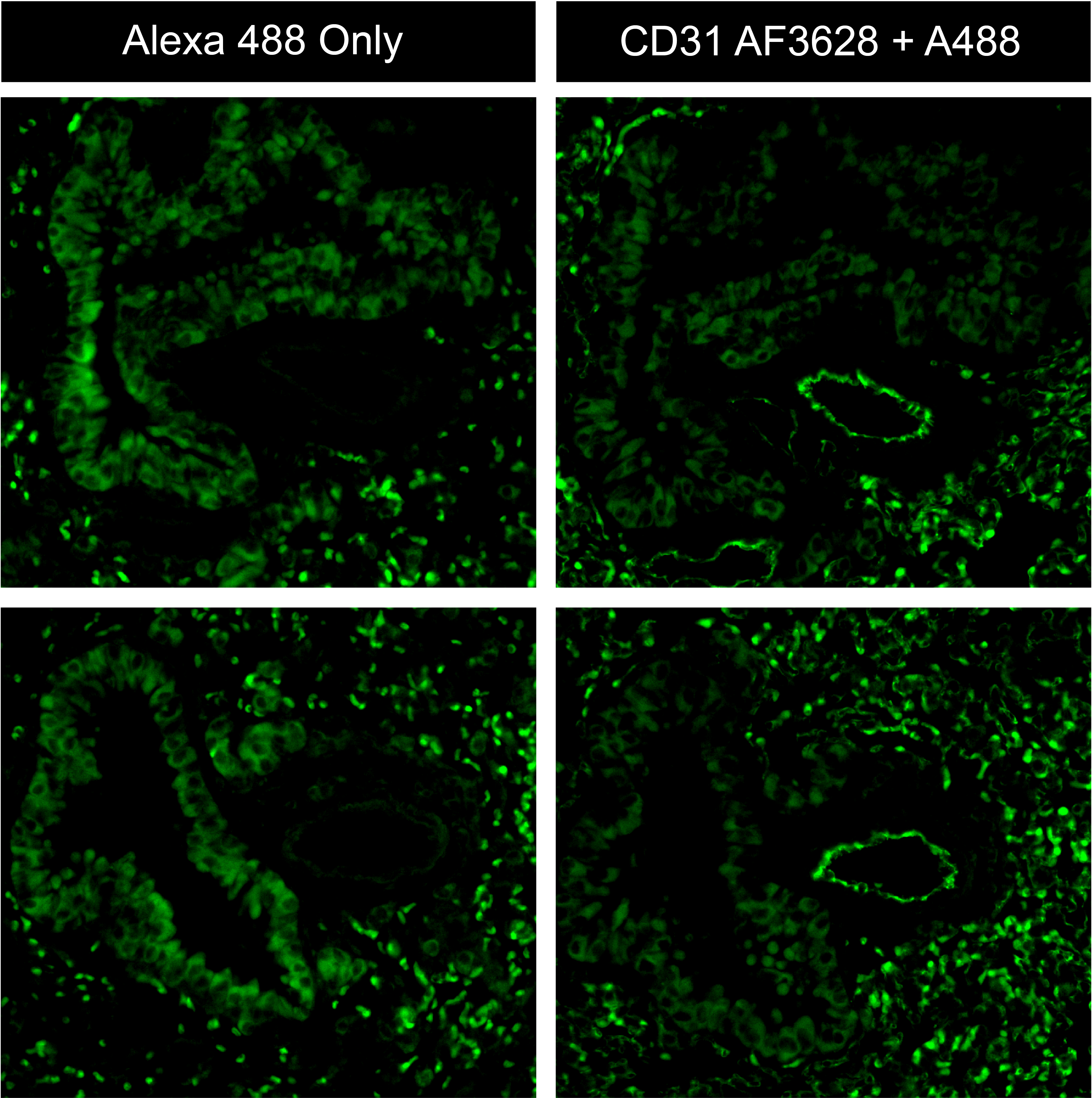
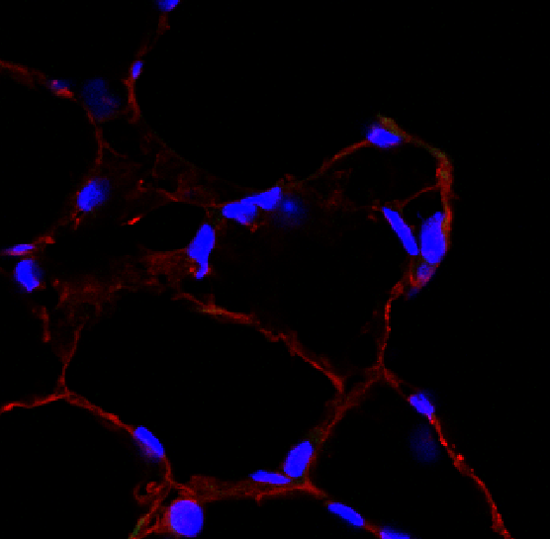
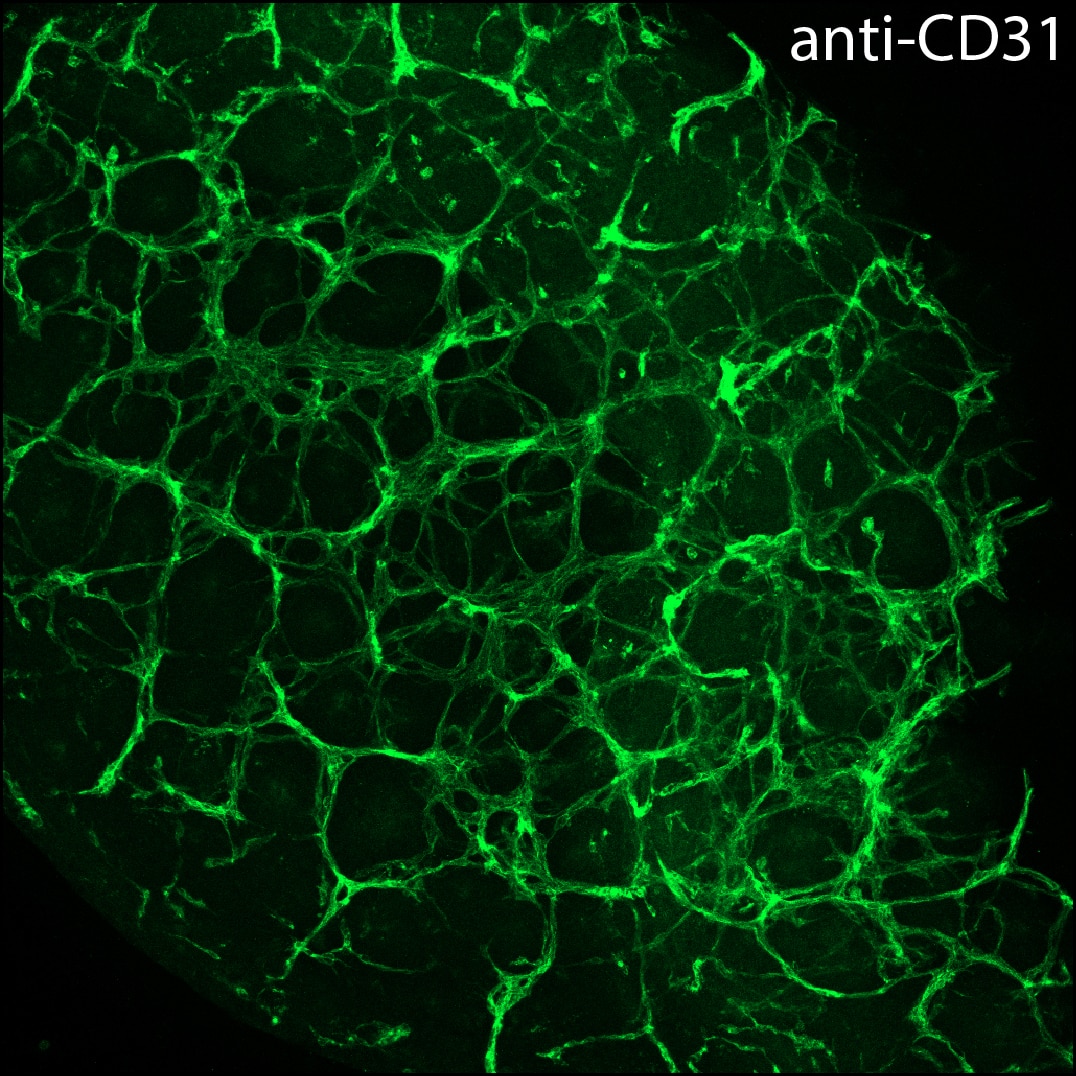
CD31/PECAM‑1 in bEnd.3 Mouse Cell Line. CD31/PECAM-1 was detected in immersion fixed bEnd.3 mouse endothelioma cell line using Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 493-conjugated Anti-Goat IgG Secondary Antibody (green; NL003) and counterstained with DAPI (blue). Specific staining was localized to cell membrane. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
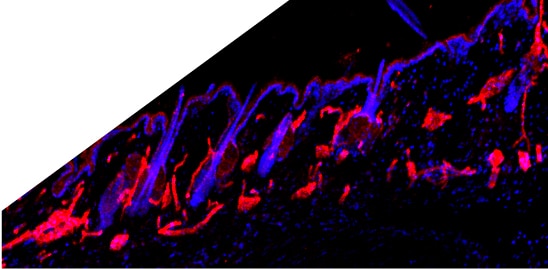
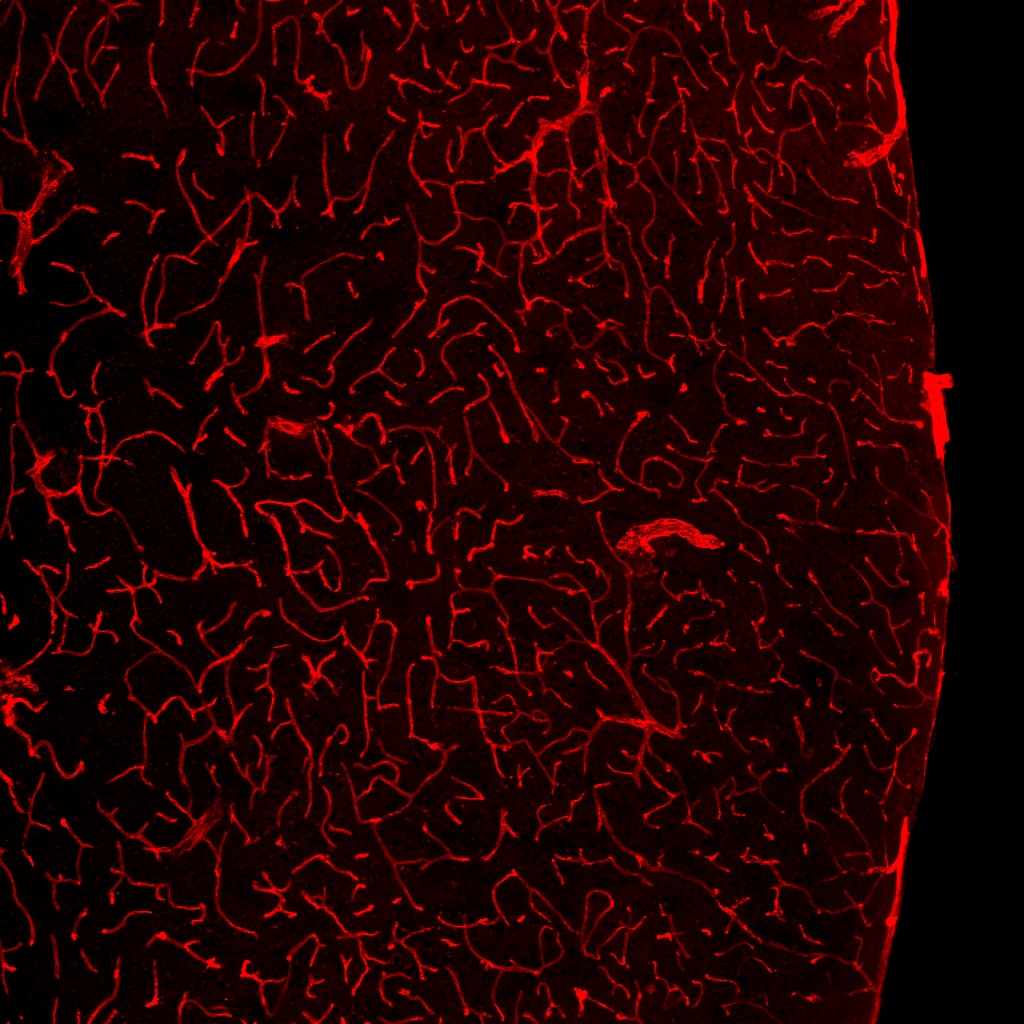
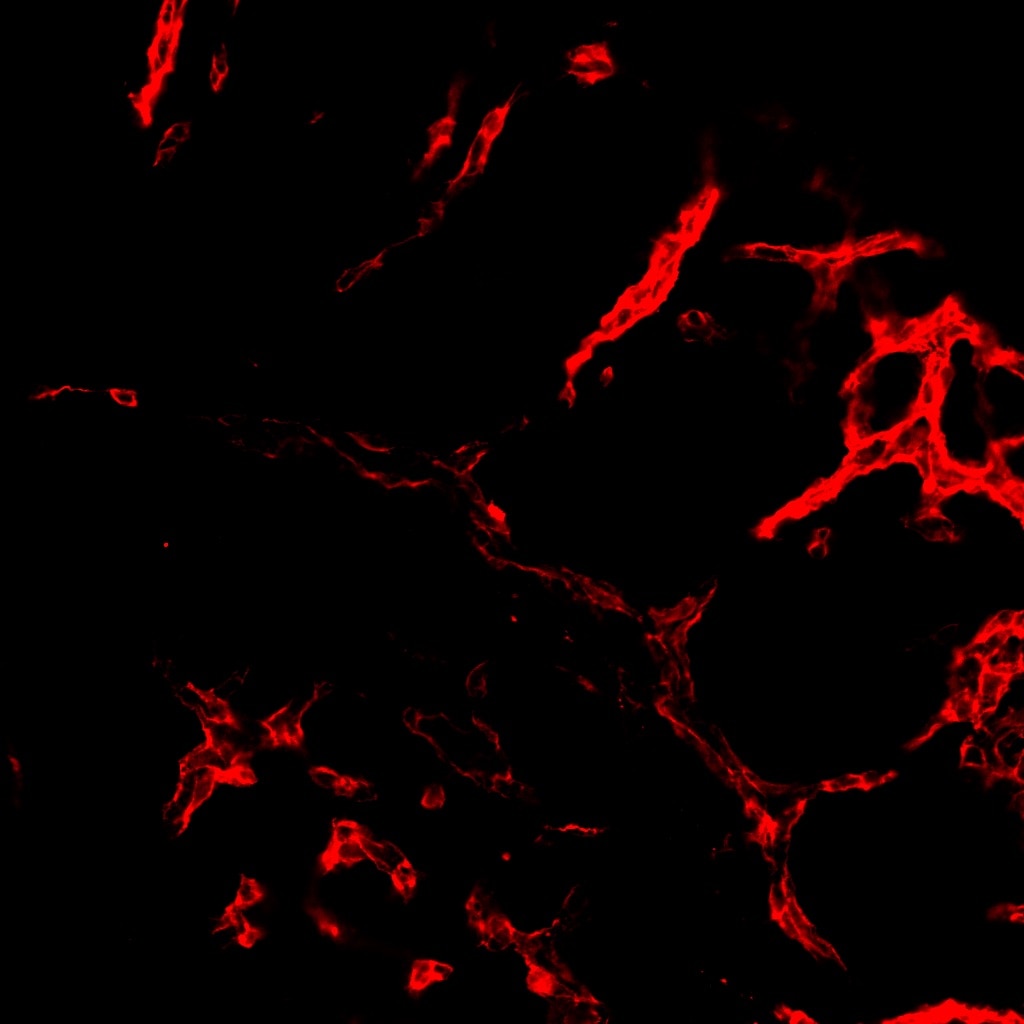
CD31/PECAM‑1 in Mouse Embryo. CD31/PECAM-1 was detected in immersion fixed frozen sections of mouse embryo (E13.5) using Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628) at 10 µg/mL overnight at 4 °C. Tissue was stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (yellow; NL001) and counterstained with DAPI (blue). Specific staining was localized to developing endothelium. View our protocol for Fluorescent IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
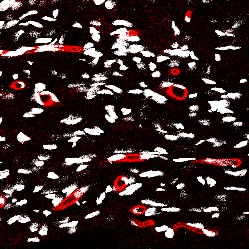
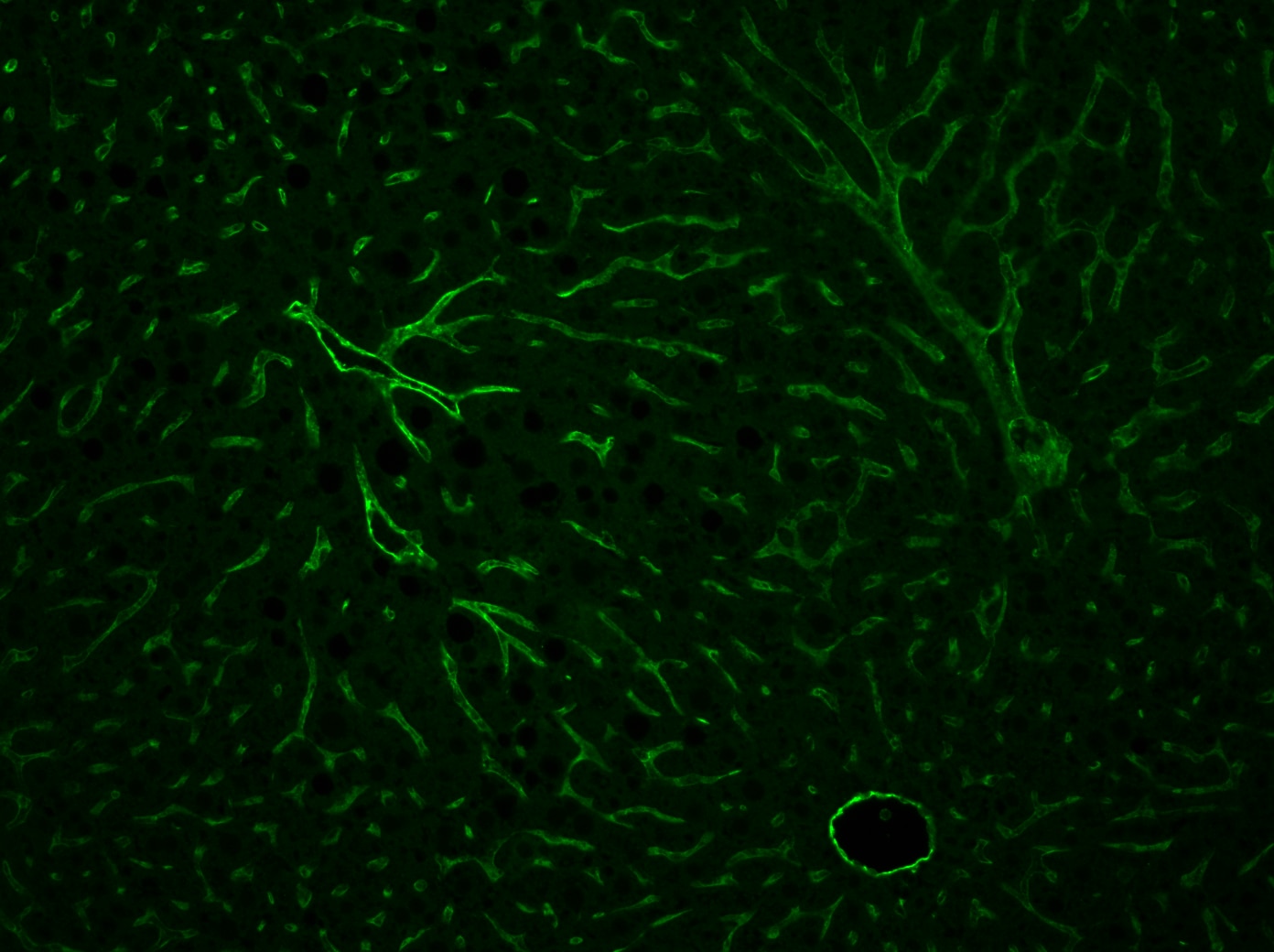
CD31/PECAM‑1 in Mouse Embryo. CD31/PECAM-1 was detected in immersion fixed frozen sections of mouse embryo (14 d.p.c.) using Goat Anti-Human/Mouse/Rat CD31/PECAM-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628) at 10 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (VC004). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to developing guts. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
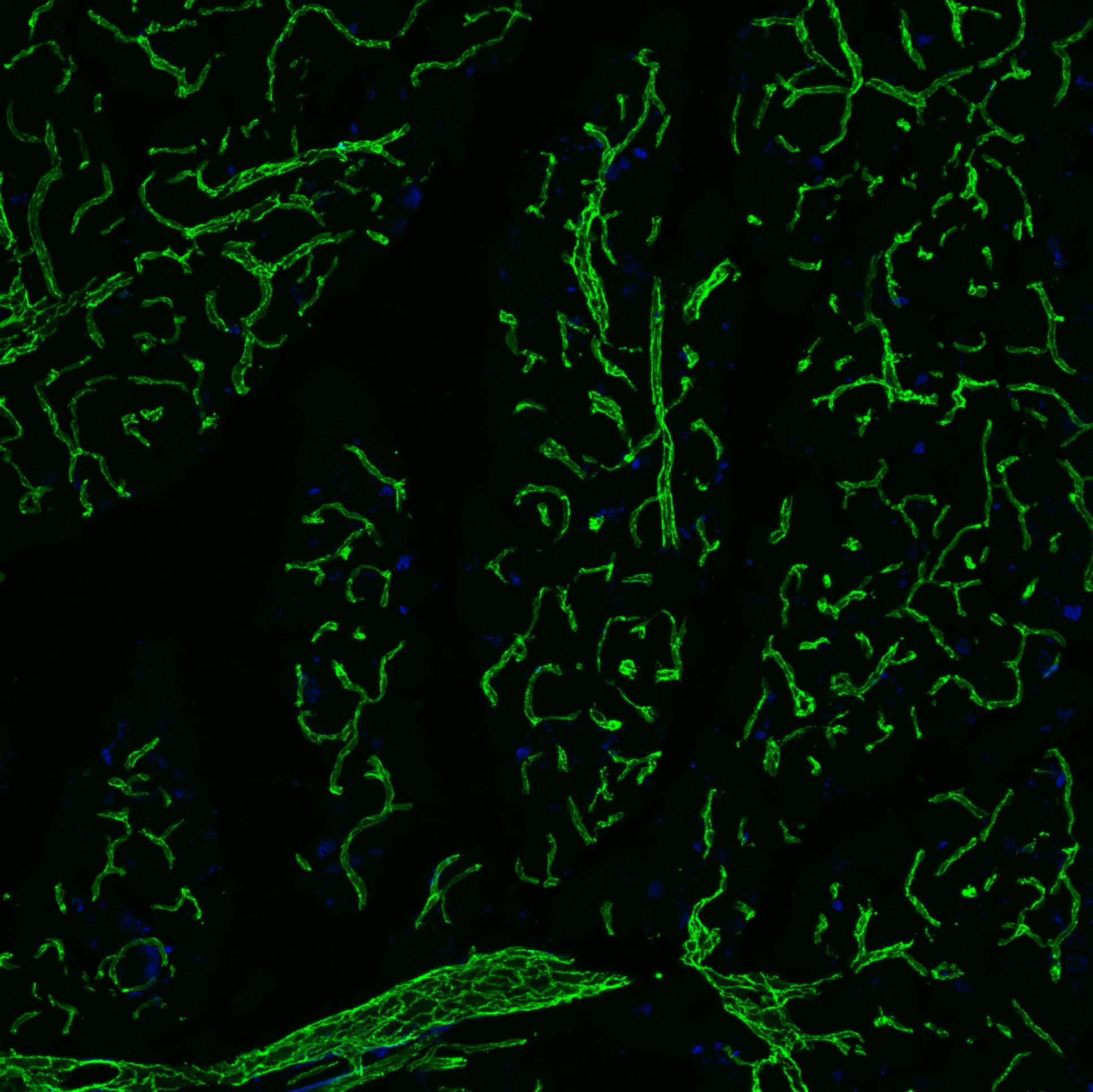
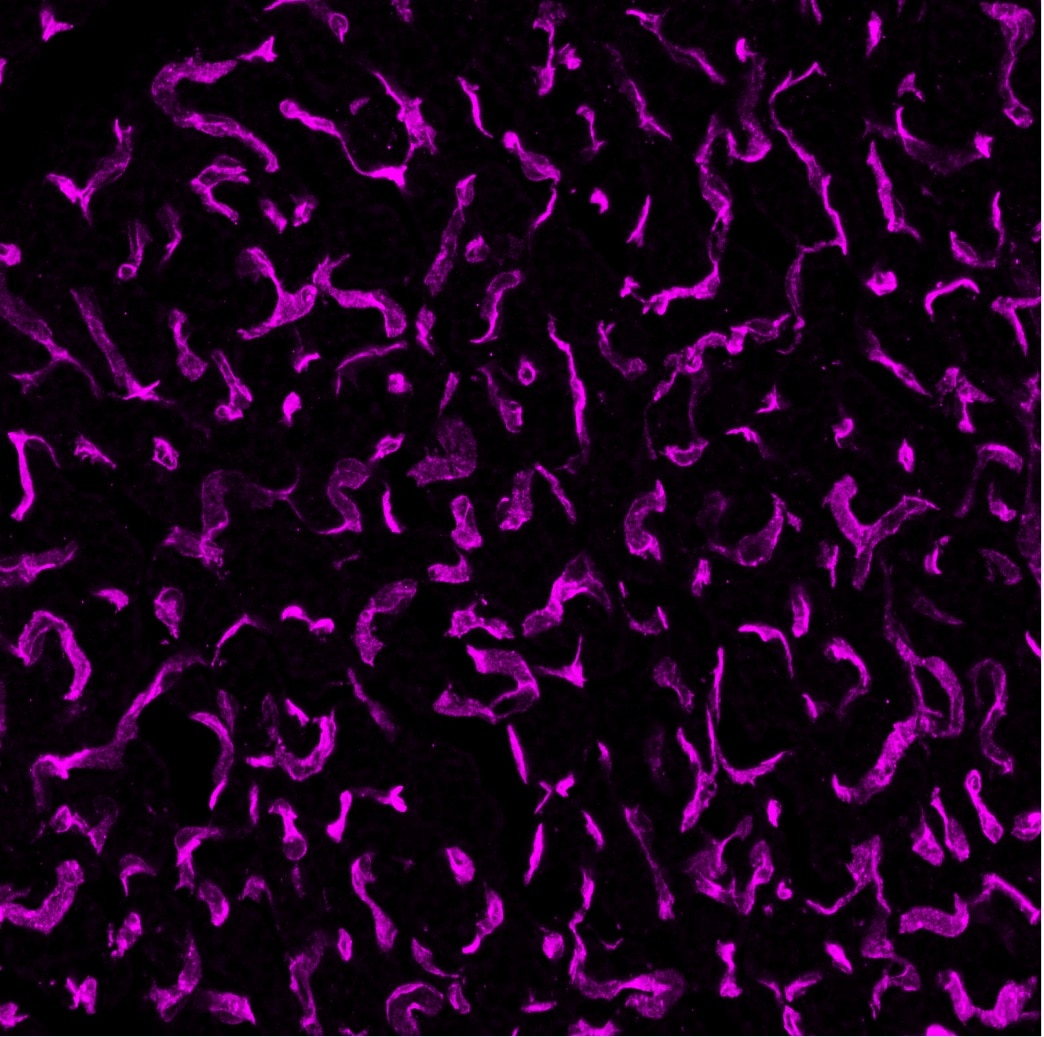
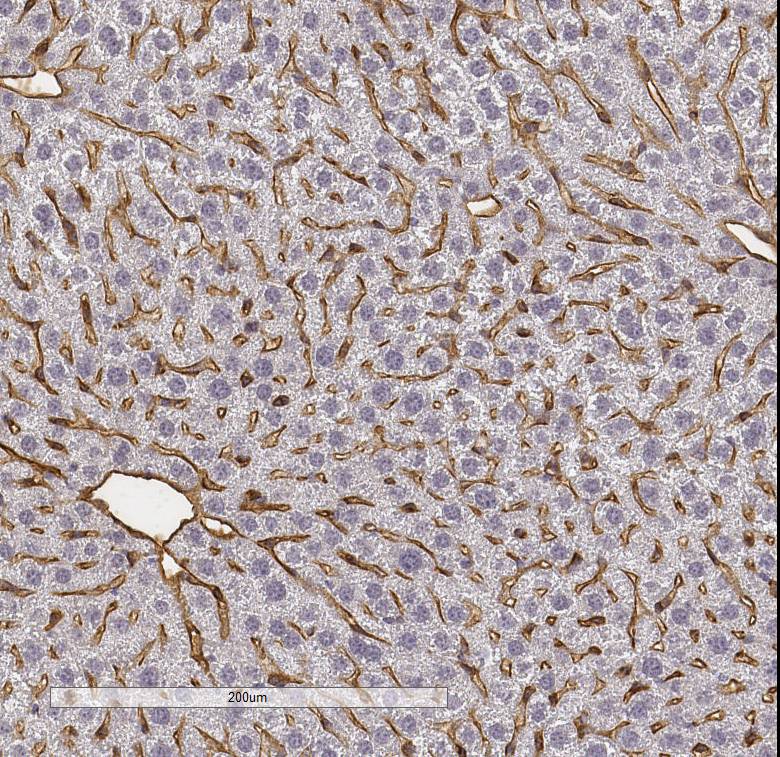
CD31/PECAM‑1 in Rat Heart. CD31/PECAM‑1 was detected in immersion fixed paraffin-embedded sections of rat heart using Goat Anti-Human/Mouse/Rat CD31/PECAM‑1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628) at 3 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Mouse IgG VisUCyte™ HRP Polymer Antibody (VC001). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to endothelial cells in vasculature.
 View Larger
View Larger
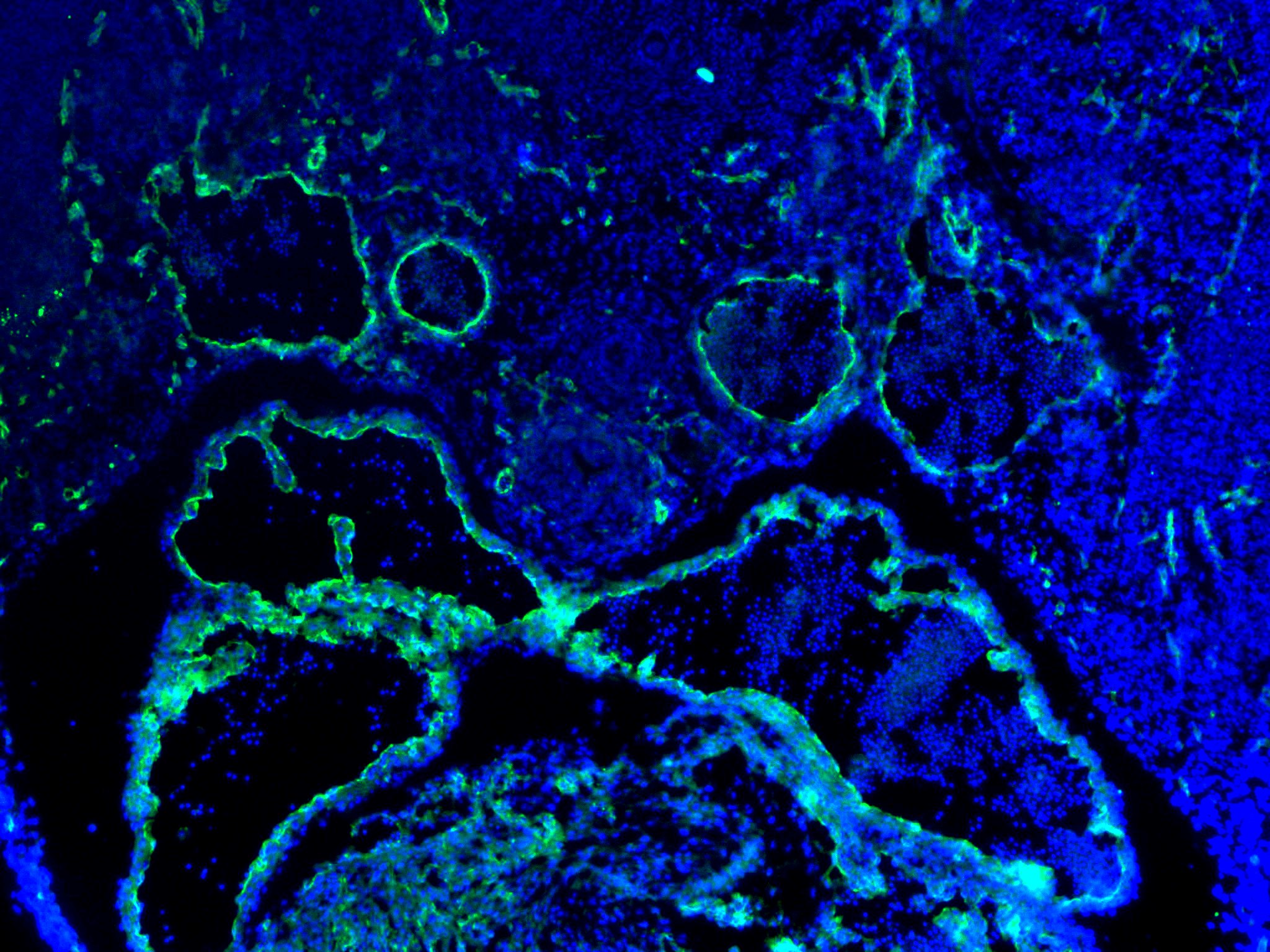
Detection of CD31/PECAM‑1 in Human Placenta. CD31/PECAM‑1 was detected in immersion fixed paraffin-embedded sections of Human Placenta using Goat Anti-Human/Mouse/Rat CD31/PECAM‑1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628) at 15 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC004). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using VisUCyte Antigen Retrieval Reagent-Basic (Catalog # VCTS021). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to endothelial cells in chorionic villi. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
Detection of CD31/PECAM‑1 in HUVEC cells by Flow Cytometry. HUVEC cells were stained with Goat Anti-Human/Mouse/Rat CD31/PECAM‑1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628, filled histogram) or isotype control antibody (Catalog # AB-108-C, open histogram), followed by Phycoerythrin-conjugated Anti-Goat IgG Secondary Antibody (Catalog # F0107). View our protocol for Staining Membrane-associated Proteins.
 View Larger
View Larger
Detection of Human CD31/PECAM‑1 by Western Blot. Western blot shows lysates of U937 human histiocytic lymphoma cell line. PVDF membrane was probed with 0.5 µg/ml of Goat Anti-Human/Mouse/Rat CD31/PECAM‑1 Antigen Affinity-purified Polyclonal Antibody (AF3628) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for CD31/PECAM‑1 at approximately 130 kDa (as indicated). This experiment was conducted under reducing conditions and using Western Blot Buffer Group 1.
 View Larger
View Larger
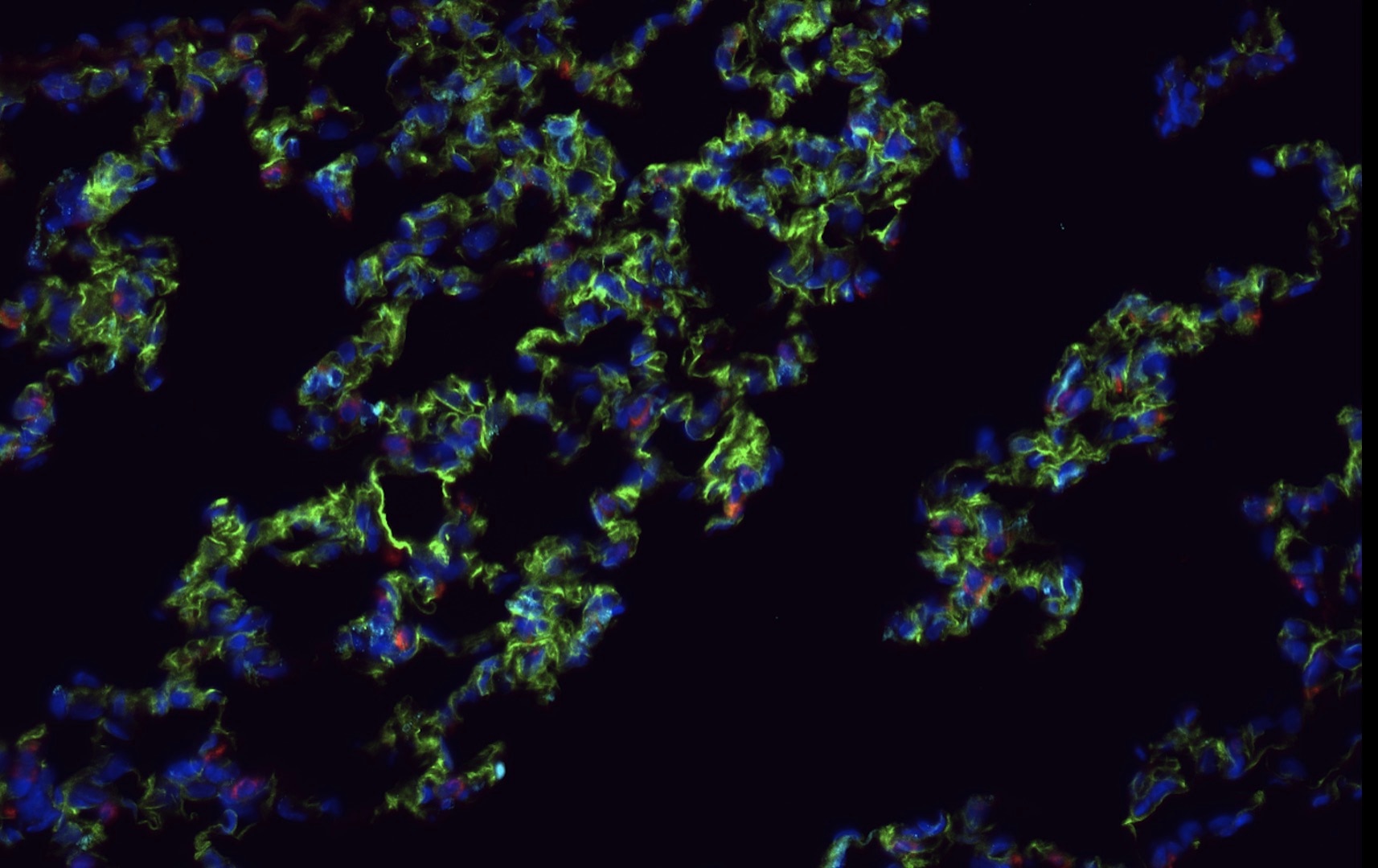
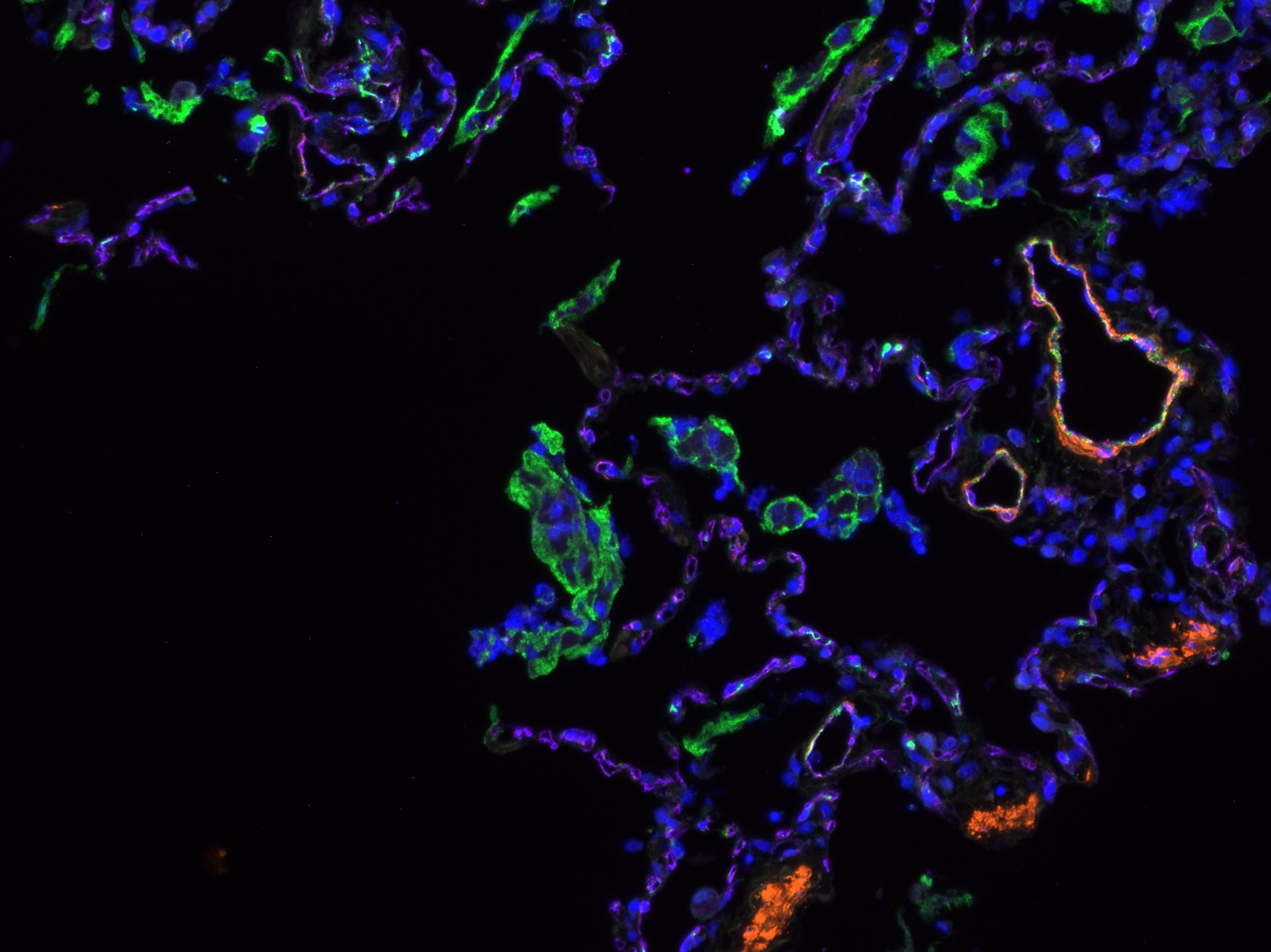
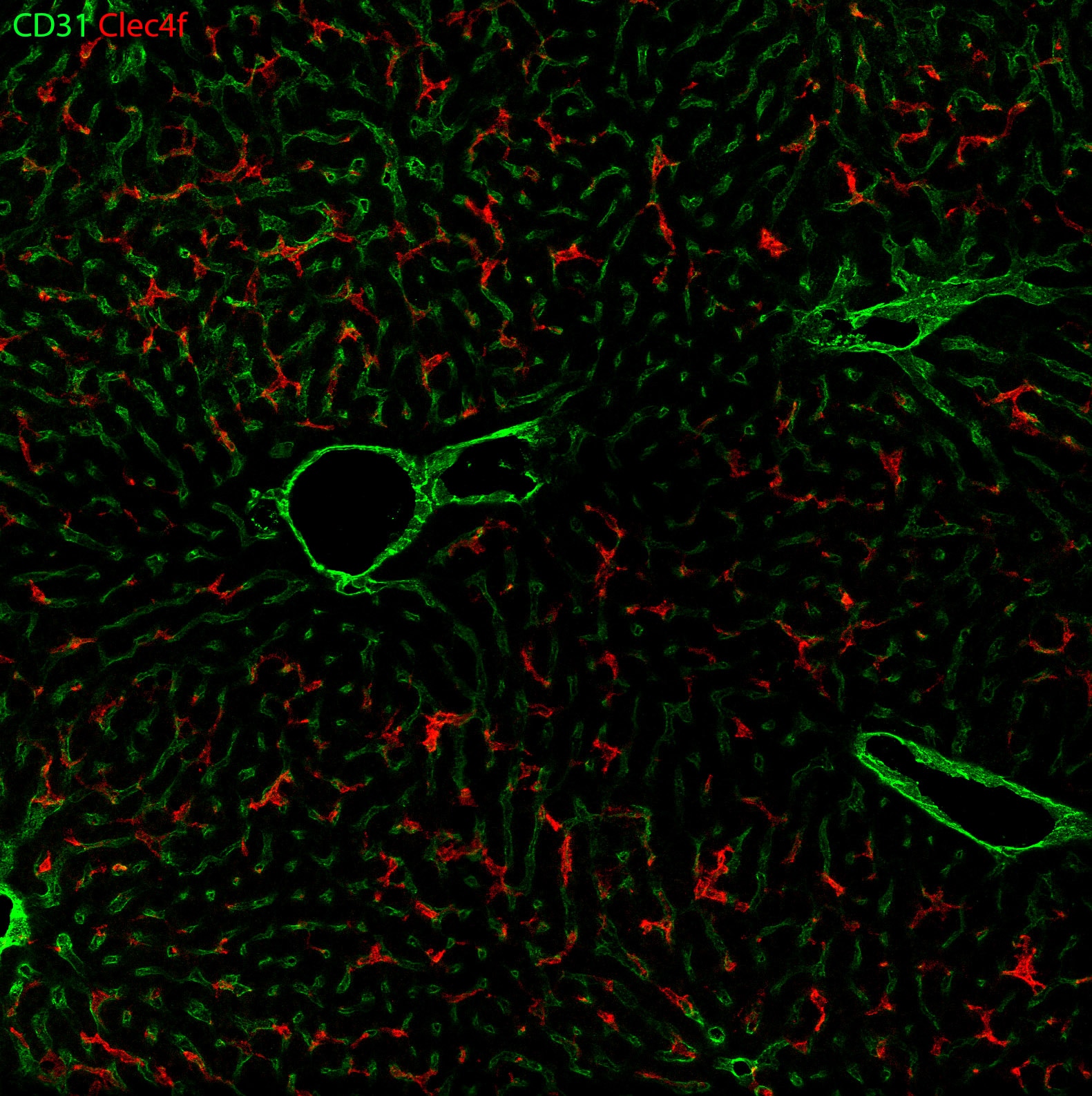
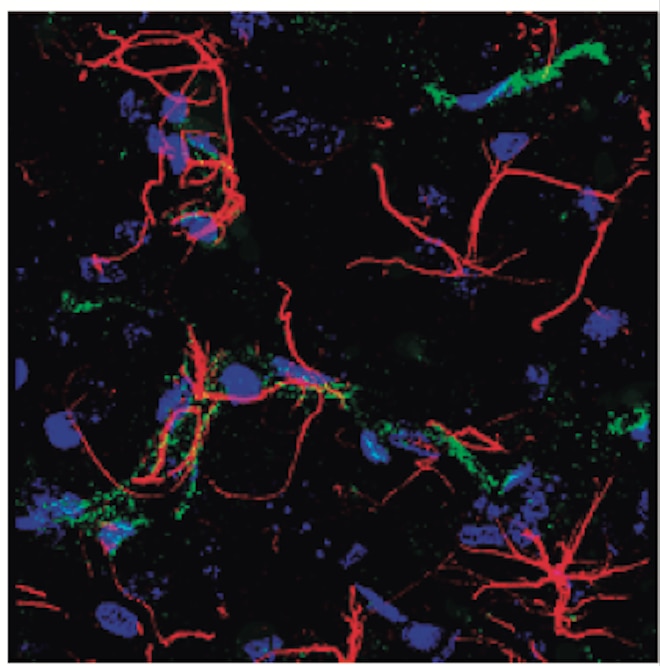
Detection of Mouse CD31/PECAM-1 by Immunohistochemistry Representative image of immunostaining corresponding to the glucose transporter protein SLC2A1 (GLUT1) in 30 week-old Ins2AKITA and WT cerebral cortex. Mural cells (ANPEP, green), glucose transporter 1 (GLUT1, red), endothelium (PECAM1, cyan). n = 2, scale bar 50 µm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30498224), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human CD31/PECAM-1 by Western Blot AcSDKP suppresses TGF beta /smad signaling and EndMT through the FGFR1/FRS2 pathway. (a) HMVECs were treated with N-FGFR1 for 48 h, and the FGFR1, TGF beta R1 and TGF beta R2 protein levels were analyzed by western blot. (b) HMVECs were treated with TGF beta 2 in the presence or absence of N-FGFR1 for 15 min with or without AcSDKP preincubation. The p-smad3 and TGF beta R1 protein levels were analyzed by western blot. Densitometric analysis of the p-smad3/smad3 and TGF beta R1/ beta -actin levels (n=3) in each group was performed. (c) HMVECs were incubated with either N-FGFR1 in the presence or absence of TGF beta 2 for 48 h with or without preincubation with AcSDKP for 2 h or with N-FGFR1 in the presence or absence of TGF beta 2 for 48 h with or without 24 h of incubation with FGF2 (50 ng/ml). The CD31, SM22 alpha, FSP1 and alpha -SMA protein levels were analyzed by western blot. (d) HMVECs were transfected with FRS2 siRNA (100 nM) for 48 h with or without AcSDKP preincubation. The VE-cadherin, FSP1, vimentin, SM22 alpha and p-smad3 levels were analyzed by western blot. (e) HMVECs were treated with N-FGFR1 for 48 h or 15 min in the presence or absence of N-TGF beta (1, 2, 3) (1.0 μg/ml). The CD31, VE-cadherin, SM22 alpha, FSP1, TGF beta R1, TGF beta R2 and p-smad3 levels were analyzed by western blot Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28771231), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence AcSDKP inhibits TGF beta /smad signaling and EndMT and restores the FGFR1 and P-MAP4K4 levels in diabetic hearts. (a) Immunofluorescence microscopy analysis of CD31/FGFR1 and CD31/P-MAP4K4 in the heart tissues from each group of mice. The scale bar is 60 μm in each panel. The CD31 and FGFR1 double-labeled cells and the CD31 and P-MAP4K4 double-labeled cells in each visual field were assessed by fluorescence microscopy and quantified. For each section, images from six different fields of view at × 400 magnification were evaluated. (b and c) Immunofluorescence microscopy analysis of CD31/ alpha -SMA, VE-cadherin /SM22 alpha and CD31/p-smad3 expression levels in the heart tissues from each group of mice. The scale bar is 60 μm in each panel. The CD31 and alpha -SMA double-labeled cells, the VE-cadherin and SM22 alpha double-labeled cells and the CD31 and p-smad3 double-labeled cells in each visual field were analyzed by fluorescence microscopy and quantified. For each section, images from six different fields of view at × 400 magnification were evaluated. Four mice from each group were analyzed. (d) Western blot analysis of the FGFR1, P-MAP4K4, TGF beta 1, TGF beta 2 and TGF beta 3 levels in cardiac tissues. A representative blot from four independent experiments was shown. The densitometric analysis of western blot data was presented (n=4). The diabetic mice are abbreviated as DM in the figure Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28771231), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human CD31/PECAM-1 by Western Blot AcSDKP suppresses TGF beta /smad signaling and EndMT through the FGFR1/FRS2 pathway. (a) HMVECs were treated with N-FGFR1 for 48 h, and the FGFR1, TGF beta R1 and TGF beta R2 protein levels were analyzed by western blot. (b) HMVECs were treated with TGF beta 2 in the presence or absence of N-FGFR1 for 15 min with or without AcSDKP preincubation. The p-smad3 and TGF beta R1 protein levels were analyzed by western blot. Densitometric analysis of the p-smad3/smad3 and TGF beta R1/ beta -actin levels (n=3) in each group was performed. (c) HMVECs were incubated with either N-FGFR1 in the presence or absence of TGF beta 2 for 48 h with or without preincubation with AcSDKP for 2 h or with N-FGFR1 in the presence or absence of TGF beta 2 for 48 h with or without 24 h of incubation with FGF2 (50 ng/ml). The CD31, SM22 alpha, FSP1 and alpha -SMA protein levels were analyzed by western blot. (d) HMVECs were transfected with FRS2 siRNA (100 nM) for 48 h with or without AcSDKP preincubation. The VE-cadherin, FSP1, vimentin, SM22 alpha and p-smad3 levels were analyzed by western blot. (e) HMVECs were treated with N-FGFR1 for 48 h or 15 min in the presence or absence of N-TGF beta (1, 2, 3) (1.0 μg/ml). The CD31, VE-cadherin, SM22 alpha, FSP1, TGF beta R1, TGF beta R2 and p-smad3 levels were analyzed by western blot Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28771231), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence AcSDKP inhibits TGF beta /smad signaling and EndMT and restores the FGFR1 and P-MAP4K4 levels in diabetic hearts. (a) Immunofluorescence microscopy analysis of CD31/FGFR1 and CD31/P-MAP4K4 in the heart tissues from each group of mice. The scale bar is 60 μm in each panel. The CD31 and FGFR1 double-labeled cells and the CD31 and P-MAP4K4 double-labeled cells in each visual field were assessed by fluorescence microscopy and quantified. For each section, images from six different fields of view at × 400 magnification were evaluated. (b and c) Immunofluorescence microscopy analysis of CD31/ alpha -SMA, VE-cadherin /SM22 alpha and CD31/p-smad3 expression levels in the heart tissues from each group of mice. The scale bar is 60 μm in each panel. The CD31 and alpha -SMA double-labeled cells, the VE-cadherin and SM22 alpha double-labeled cells and the CD31 and p-smad3 double-labeled cells in each visual field were analyzed by fluorescence microscopy and quantified. For each section, images from six different fields of view at × 400 magnification were evaluated. Four mice from each group were analyzed. (d) Western blot analysis of the FGFR1, P-MAP4K4, TGF beta 1, TGF beta 2 and TGF beta 3 levels in cardiac tissues. A representative blot from four independent experiments was shown. The densitometric analysis of western blot data was presented (n=4). The diabetic mice are abbreviated as DM in the figure Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28771231), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
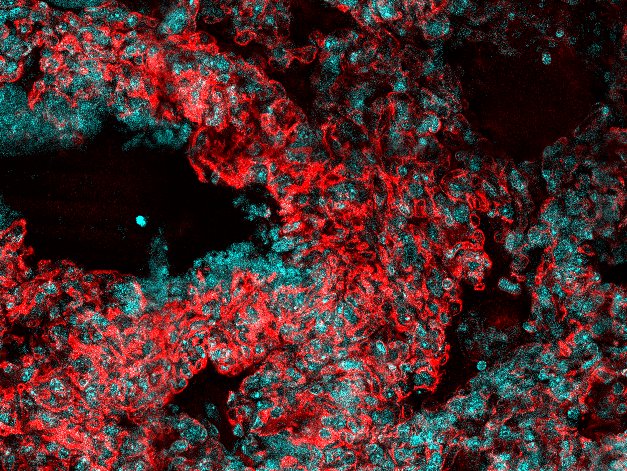
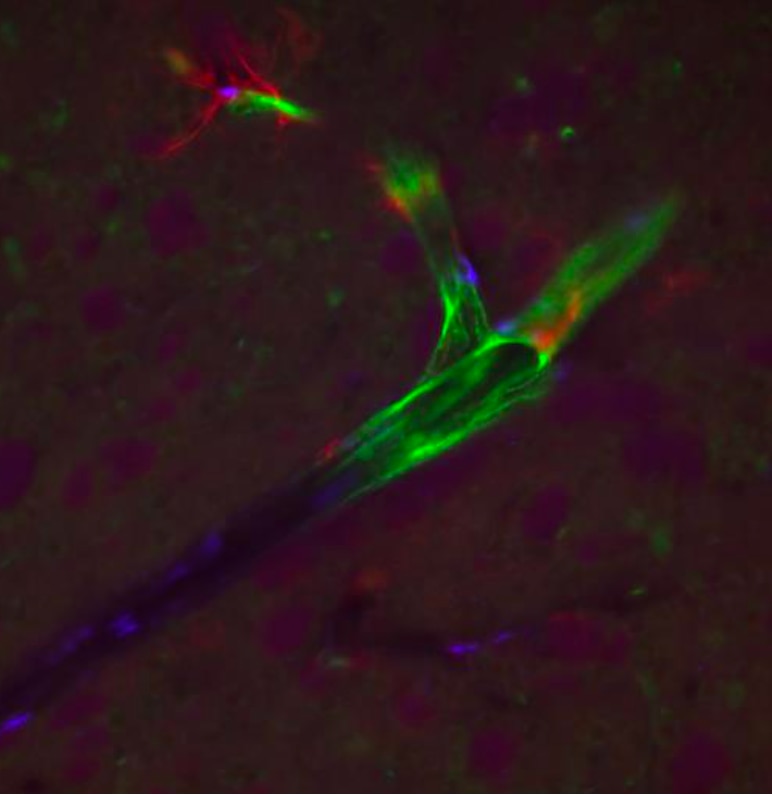
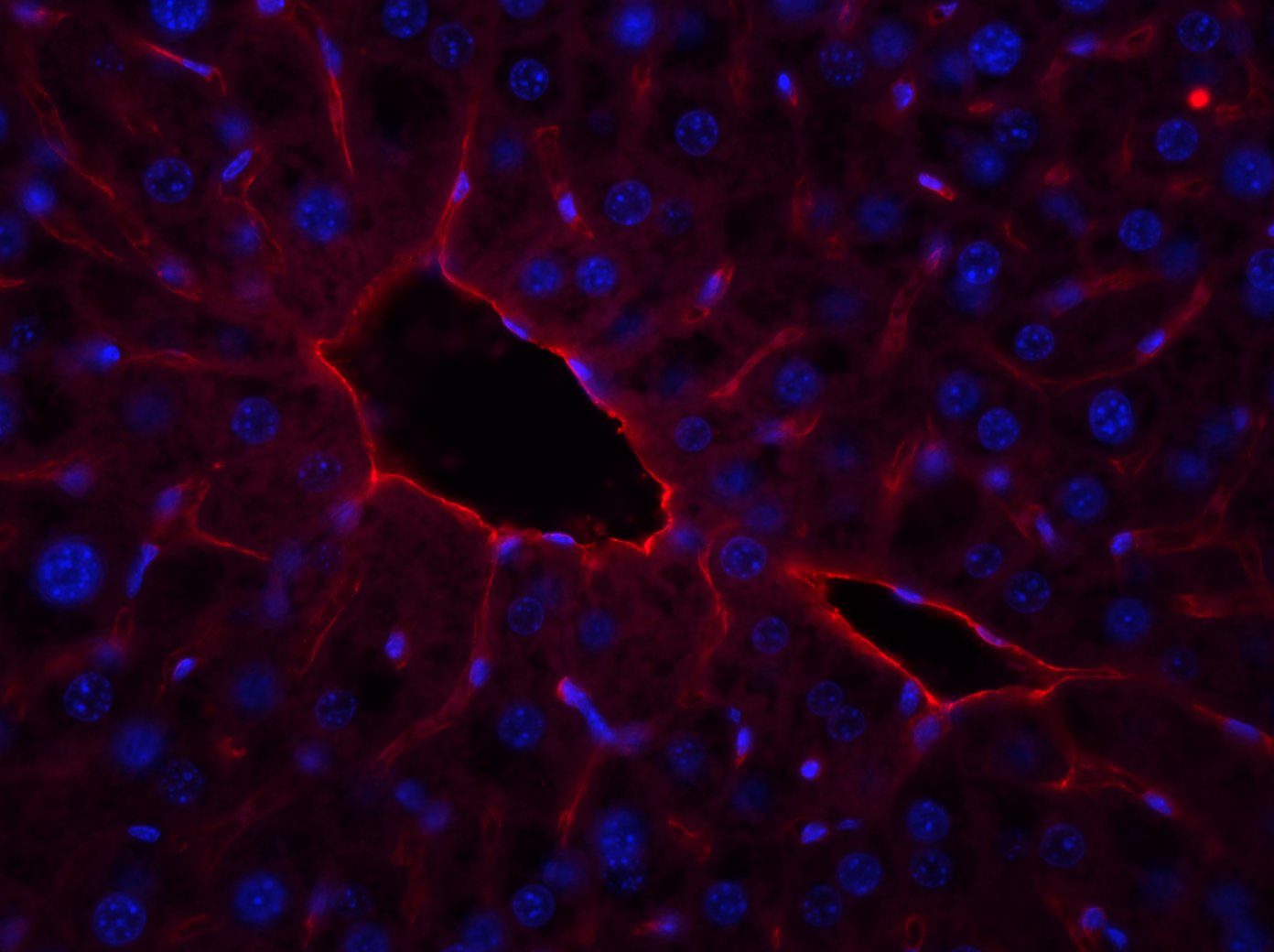
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence Diminished permeability of liver sinusoids in Plvap-deficient mice.Neither by immunohistochemistry with antibodies against CD31 (A) nor by light microscopy of 1 µm semi-thin sections (B, Richardson's stain) obvious differences are detected with regards to the overall orientation and the density of liver sinusoids between 3-week-old Plvap-/- mice and wild-type littermates. Sinusoids of Plvap-deficient mice show a higher number of macrophages in their lumen (white arrows) and focal areas with accumulations of mononuclear cells in Disse's space (black arrows). Lower panels in B show higher magnifications. C, After perfusion of a wild-type animal with FITC-dextran, a strong FITC-signal (green) throughout the liver is detected. Immunolabeling with CD31 (red) suggests that FITC-dextran molecules have accumulated in the space of Disse. In contrast, in the Plvap-deficient littermate, the signal for FITC-dextran is much weaker and barely detectable. Nuclear DNA is labeled with DAPI (blue). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0115005), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse CD31/PECAM-1 by Immunohistochemistry Blood-brain barrier permeability measurements in male Ins2AKITA and WT littermate controls. (a) Representative stereomicroscope fluorescence images of brains showing 1 kDa Alexa Fluor 555 cadaverine permeability in Ins2AKITA and WT after 2 h of dye circulation (n = 2). (b) Representative confocal images of coronal sections of 1 kDa Alexa Fluor 555 cadaverine injected mouse brains. ANPEP positive mural cells, green; PECAM1 positive vasculature, white. No Alexa Fluor 555 cadaverine leakage into brain parenchyma was observed either in Ins2AKITA or WT mice (n = 2, scale bar 30 μm). (c) Quantification of 1 kDa Alexa Fluor 555 cadaverine permeability in 26.5–32 week-old Ins2AKITA and WT mice after 2 h circulation (n = > 8, 3 independent experiments). y-axis shows the fold change in relative fluorescence units (RFU) per gram of brain tissue in relation to WT. (d) Quantification of 1 kDa Alexa Fluor 488 cadaverine permeability in 38 week-old Ins2AKITA and WT mice after 2 h circulation (n = 3). y-axis shows the fold change in relative fluorescence units (RFU) per gram of brain tissue in relation to WT. (e) Evans Blue dye permeability in 30 week-old Ins2AKITA and WT littermate control mice after overnight circulation (n = > 2). y-axis shows optical density (OD) at 620 nm per gram of tissue. PdgfbRet/Ret served as positive control for tracer leakage into the brain parenchyma. n.s., not significant, student’s t test. Data is presented as mean ± SEM. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30498224), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence Diminished permeability of liver sinusoids in Plvap-deficient mice.Neither by immunohistochemistry with antibodies against CD31 (A) nor by light microscopy of 1 µm semi-thin sections (B, Richardson's stain) obvious differences are detected with regards to the overall orientation and the density of liver sinusoids between 3-week-old Plvap-/- mice and wild-type littermates. Sinusoids of Plvap-deficient mice show a higher number of macrophages in their lumen (white arrows) and focal areas with accumulations of mononuclear cells in Disse's space (black arrows). Lower panels in B show higher magnifications. C, After perfusion of a wild-type animal with FITC-dextran, a strong FITC-signal (green) throughout the liver is detected. Immunolabeling with CD31 (red) suggests that FITC-dextran molecules have accumulated in the space of Disse. In contrast, in the Plvap-deficient littermate, the signal for FITC-dextran is much weaker and barely detectable. Nuclear DNA is labeled with DAPI (blue). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0115005), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence AcSDKP inhibits TGF beta /smad signaling and EndMT and restores the FGFR1 and P-MAP4K4 levels in diabetic hearts. (a) Immunofluorescence microscopy analysis of CD31/FGFR1 and CD31/P-MAP4K4 in the heart tissues from each group of mice. The scale bar is 60 μm in each panel. The CD31 and FGFR1 double-labeled cells and the CD31 and P-MAP4K4 double-labeled cells in each visual field were assessed by fluorescence microscopy and quantified. For each section, images from six different fields of view at × 400 magnification were evaluated. (b and c) Immunofluorescence microscopy analysis of CD31/ alpha -SMA, VE-cadherin /SM22 alpha and CD31/p-smad3 expression levels in the heart tissues from each group of mice. The scale bar is 60 μm in each panel. The CD31 and alpha -SMA double-labeled cells, the VE-cadherin and SM22 alpha double-labeled cells and the CD31 and p-smad3 double-labeled cells in each visual field were analyzed by fluorescence microscopy and quantified. For each section, images from six different fields of view at × 400 magnification were evaluated. Four mice from each group were analyzed. (d) Western blot analysis of the FGFR1, P-MAP4K4, TGF beta 1, TGF beta 2 and TGF beta 3 levels in cardiac tissues. A representative blot from four independent experiments was shown. The densitometric analysis of western blot data was presented (n=4). The diabetic mice are abbreviated as DM in the figure Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28771231), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence CL and low temperature lead to WAT browning, angiogenesis, and an increased number of myofibroblast-like cells. a, b Histological analysis of adipocyte morphology (H&E), adipocytes (PERI), mitochondria (COX4), uncoupling protein 1 (UCP1), blood vessels (CD31), and myofibroblast-like cells ( alpha SMA) in a 5-day CL-316243-treated scWAT and visWAT compared to vehicle-treated control. b Two-week 4 °C-treated scWAT and visWAT compared to 30 °C control. Double-headed arrows mark adipocyte diameter. Arrows point to respective positive signals. c–l Quantifications of adipocyte size and positive signals per field of COX4, UCP1, CD31, and alpha SMA of CL-316243- and vehicle-, and 30 °C- and 4 °C- treated scWATs and visWATs (>30 adipocytes per field; n = 10 random fields; n = 5 mice per group). PERI, perilipin; COX4, mitochondrial complex 4; UCP1, uncoupling protein 1. Scale bars, 100 μm. NS, not significant. *P < 0.05, **P < 0.01, and ***P < 0.001 by Student’s t-test. Data presented as mean ± s.e.m. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/s41467-017-02158-z), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence CL and low temperature lead to WAT browning, angiogenesis, and an increased number of myofibroblast-like cells. a, b Histological analysis of adipocyte morphology (H&E), adipocytes (PERI), mitochondria (COX4), uncoupling protein 1 (UCP1), blood vessels (CD31), and myofibroblast-like cells ( alpha SMA) in a 5-day CL-316243-treated scWAT and visWAT compared to vehicle-treated control. b Two-week 4 °C-treated scWAT and visWAT compared to 30 °C control. Double-headed arrows mark adipocyte diameter. Arrows point to respective positive signals. c–l Quantifications of adipocyte size and positive signals per field of COX4, UCP1, CD31, and alpha SMA of CL-316243- and vehicle-, and 30 °C- and 4 °C- treated scWATs and visWATs (>30 adipocytes per field; n = 10 random fields; n = 5 mice per group). PERI, perilipin; COX4, mitochondrial complex 4; UCP1, uncoupling protein 1. Scale bars, 100 μm. NS, not significant. *P < 0.05, **P < 0.01, and ***P < 0.001 by Student’s t-test. Data presented as mean ± s.e.m. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/s41467-017-02158-z), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of CD31/PECAM‑1 in Whole Blood Granulocytes by Flow Cytometry Whole blood granulocytes were stained with Goat Anti-Human/Mouse/Rat CD31/PECAM‑1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3628, filled histogram) or isotype control antibody (Catalog # AB-108-C, open histogram) followed by Phycoerythrin-conjugated Anti-Goat IgG Secondary Antibody (Catalog # F0107). View our protocol for Staining Membrane-associated Proteins.
 View Larger
View Larger
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry Neutrophil depletion reduces BBB breakdown and increases neovascularization after stroke. f, g Representative confocal images (f) and quantitative analysis of IgG extravascular deposits (g) in the peri-infarct cortex at 14 days in sham-operated mice and mice treated with control antibody or anti-Ly6G antibody (n = 6). One-way ANOVA test was applied with *P < 0.0001 (Sham vs. Isotype), *P = 0.0041 (Isotype vs. Anti-Ly6G). Bar = 15 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry Increased vascular remodeling by delayed inhibition of NET formation.a–d Representative confocal images (a, c) and quantitative analysis of IgG extravascular deposits (b, d) in the peri-infarct cortex at 14 days. Mice were subjected to stroke and treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle starting at 7 days (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.0392 (b), *P = 0.0384 (d). Bar = 10 μm. e–l Representative confocal images (e, g) of CD31-positive microvessels and in-vivo multiphoton microscopy images of perfused cortical capillaries with intravenously injected FITC-dextran (i, k) in the peri-infarct cortex at 14 days in mice treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle. Bar = 40 μm (e, g) and 100 µm (i, k). Quantification of microvascular density (f, h) and perfused capillary length (j, l) for each group (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.00378 (f), *P = 0.0364 (h), *P = 0.0026 (j), *P = 0.0006 (l). Data are presented as mean ± SD. Source data underlying graph b, d, f, h, j, and l are provided as a Source Data file. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry Increased vascular remodeling by delayed inhibition of NET formation.a–d Representative confocal images (a, c) and quantitative analysis of IgG extravascular deposits (b, d) in the peri-infarct cortex at 14 days. Mice were subjected to stroke and treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle starting at 7 days (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.0392 (b), *P = 0.0384 (d). Bar = 10 μm. e–l Representative confocal images (e, g) of CD31-positive microvessels and in-vivo multiphoton microscopy images of perfused cortical capillaries with intravenously injected FITC-dextran (i, k) in the peri-infarct cortex at 14 days in mice treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle. Bar = 40 μm (e, g) and 100 µm (i, k). Quantification of microvascular density (f, h) and perfused capillary length (j, l) for each group (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.00378 (f), *P = 0.0364 (h), *P = 0.0026 (j), *P = 0.0006 (l). Data are presented as mean ± SD. Source data underlying graph b, d, f, h, j, and l are provided as a Source Data file. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry Neutrophil depletion reduces BBB breakdown and increases neovascularization after stroke. h, j Representative confocal images (h) of CD31-positive microvessels and in-vivo multiphoton microscopy images of perfused cortical capillaries with intravenously injected FITC-dextran (MW = 2000,000 Da) (j) in the peri-infarct cortex at 14 days in mice treated with control antibody or anti-Ly6G antibody, compared with sham-operated mice. Bar = 50 μm (e) and 100 µm (g) Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry Increased vascular remodeling by delayed inhibition of NET formation.a–d Representative confocal images (a, c) and quantitative analysis of IgG extravascular deposits (b, d) in the peri-infarct cortex at 14 days. Mice were subjected to stroke and treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle starting at 7 days (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.0392 (b), *P = 0.0384 (d). Bar = 10 μm. e–l Representative confocal images (e, g) of CD31-positive microvessels and in-vivo multiphoton microscopy images of perfused cortical capillaries with intravenously injected FITC-dextran (i, k) in the peri-infarct cortex at 14 days in mice treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle. Bar = 40 μm (e, g) and 100 µm (i, k). Quantification of microvascular density (f, h) and perfused capillary length (j, l) for each group (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.00378 (f), *P = 0.0364 (h), *P = 0.0026 (j), *P = 0.0006 (l). Data are presented as mean ± SD. Source data underlying graph b, d, f, h, j, and l are provided as a Source Data file. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
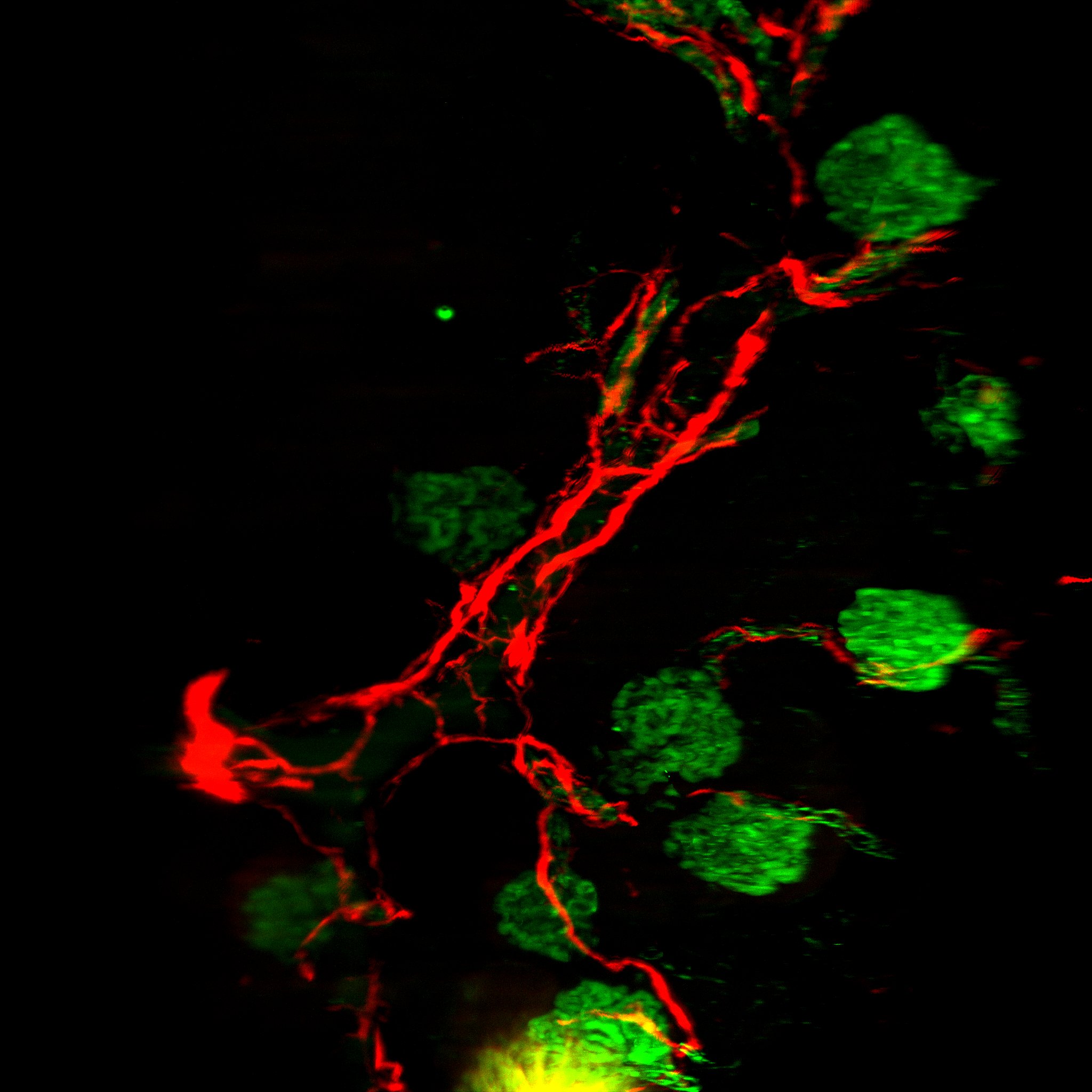
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry Neutrophils accumulate in the brain during all stages of ischemic stroke. f Representative confocal images of Ly6G-labeled neutrophils (green) and CD31-positive microvessels (white) in the peri-infarct cortex of mice at 3 days. Nuclei were visualized with Hoechst. Neutrophils were observed within brain vessels and migrated into the parenchyma. Bar = 40 μm. Independent experiments are repeated at least three times. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry Diminished permeability of liver sinusoids in Plvap-deficient mice.Neither by immunohistochemistry with antibodies against CD31 (A) nor by light microscopy of 1 µm semi-thin sections (B, Richardson's stain) obvious differences are detected with regards to the overall orientation and the density of liver sinusoids between 3-week-old Plvap-/- mice and wild-type littermates. Sinusoids of Plvap-deficient mice show a higher number of macrophages in their lumen (white arrows) and focal areas with accumulations of mononuclear cells in Disse's space (black arrows). Lower panels in B show higher magnifications. C, After perfusion of a wild-type animal with FITC-dextran, a strong FITC-signal (green) throughout the liver is detected. Immunolabeling with CD31 (red) suggests that FITC-dextran molecules have accumulated in the space of Disse. In contrast, in the Plvap-deficient littermate, the signal for FITC-dextran is much weaker and barely detectable. Nuclear DNA is labeled with DAPI (blue). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25541982), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Human/Mouse/Rat CD31/PECAM-1 Antibody by Immunohistochemistry Increased vascular remodeling by delayed inhibition of NET formation.a–d Representative confocal images (a, c) and quantitative analysis of IgG extravascular deposits (b, d) in the peri-infarct cortex at 14 days. Mice were subjected to stroke and treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle starting at 7 days (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.0392 (b), *P = 0.0384 (d). Bar = 10 μm. e–l Representative confocal images (e, g) of CD31-positive microvessels and in-vivo multiphoton microscopy images of perfused cortical capillaries with intravenously injected FITC-dextran (i, k) in the peri-infarct cortex at 14 days in mice treated with either anti-Ly6G antibody, control antibody, DNase 1, or vehicle. Bar = 40 μm (e, g) and 100 µm (i, k). Quantification of microvascular density (f, h) and perfused capillary length (j, l) for each group (n = 6), unpaired two-tailed Student’s t-test was applied with *P = 0.00378 (f), *P = 0.0364 (h), *P = 0.0026 (j), *P = 0.0006 (l). Data are presented as mean ± SD. Source data underlying graph b, d, f, h, j, and l are provided as a Source Data file. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32427863), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
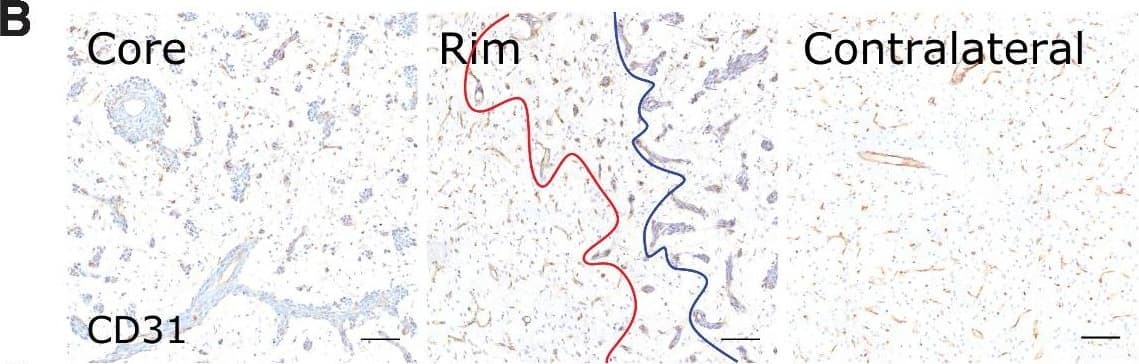
Detection of Mouse CD31/PECAM-1 by Immunohistochemistry Microvascular and tumor cell biology at the tumor rim in MDA231Br-GFP brain metastases. Representative histologic sections of rat brains, intrastriatally injected with MDA231Br-GFP tumor cells, from the tumor core, tumor rim, and the contralateral striatum, with corresponding box and whisker plots of marker expression in each region. Data shown as median ± interquartile range. Sections were immunohistochemically stained (brown) for tumor cell marker vimentin (A), endothelial marker CD31 (B), cell adhesion molecule VCAM-1 (C), cell proliferation marker Ki67 (D), and two stemness markers: SOX2 (E) and nestin (F). Scale bar = 100 μm. *, P < 0.05; ***, P < 0.001; n = 8; post hoc Bonferroni multiple comparison test for tumor cell density and microvessel density, and post hoc Dunn test for VCAM-1 expression. Tumor core was delineated from the infiltrative border as indicated by the blue and red lines, respectively. Expression of Ki67, nestin and SOX2 has been normalized to tumor area. *, P < 0.05; ***, P < 0.001; matched Wilcoxon test. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35312755), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
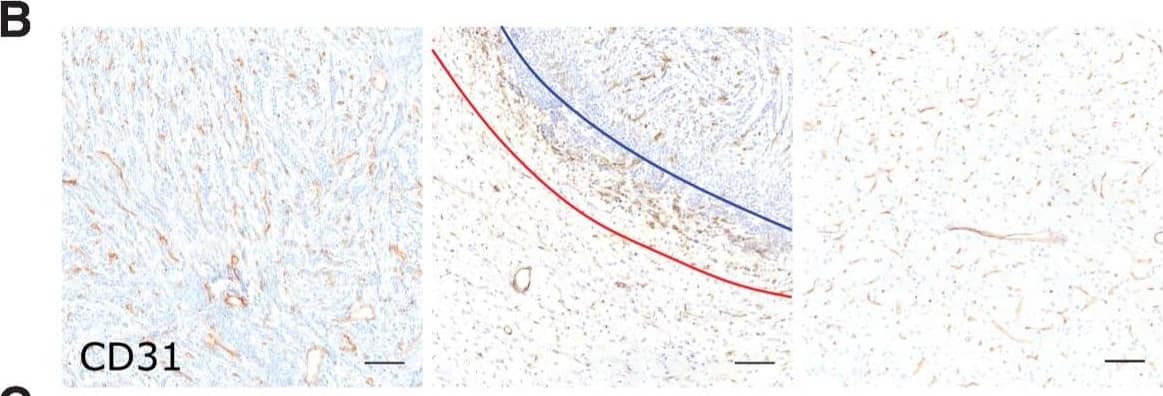
Detection of Human CD31/PECAM-1 by Immunohistochemistry Microvascular and tumor cell biology at the tumor rim in U87MG glioblastoma. Representative histologic sections of rat brains, intrastriatally injected with U87MG tumor cells, from the tumor core, tumor rim, and the contralateral striatum, with corresponding box and whisker plots of marker expression in each region. Data shown as median ± interquartile range. Sections were immunohistochemically stained (brown) for tumor cell marker vimentin (A), endothelial marker CD31 (B), cell adhesion molecule VCAM-1 (C), cell proliferation marker Ki67 (D), and two stemness markers: SOX2 (E) and nestin (F). Scale bar = 100 μm. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n = 5; post hoc Bonferroni multiple comparison test for tumor cell density and microvessel density, and post hoc Dunn test for VCAM-1 expression. Tumor core was delineated from the infiltrative border as indicated by the blue and red lines, respectively. Expression of Ki67, nestin, and SOX2 has been normalized to tumor area. **, P < 0.01; matched Wilcoxon test. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35312755), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
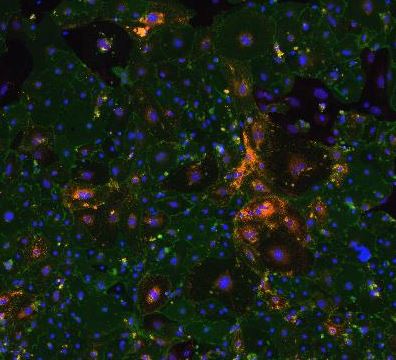
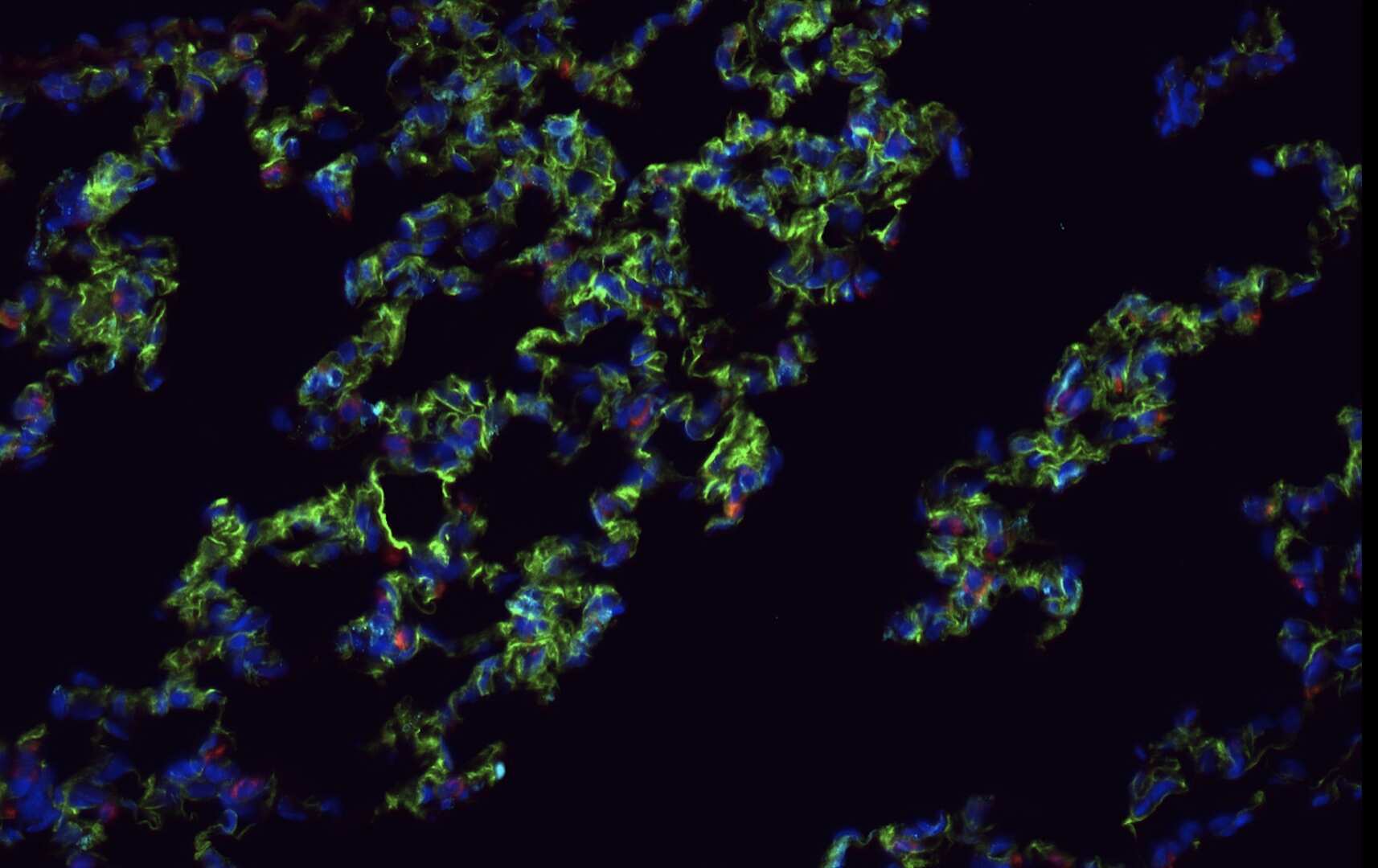
CD31/PECAM‑1 in Lung Tissue. Immunohistochemistry (paraffin-embedded) of Native Adult Rat lung tissue stained for Abca3 (red), CD31/PECAM-1 (green), and Dclk1 (cyan). Image from a verified customer review.
 View Larger
View Larger
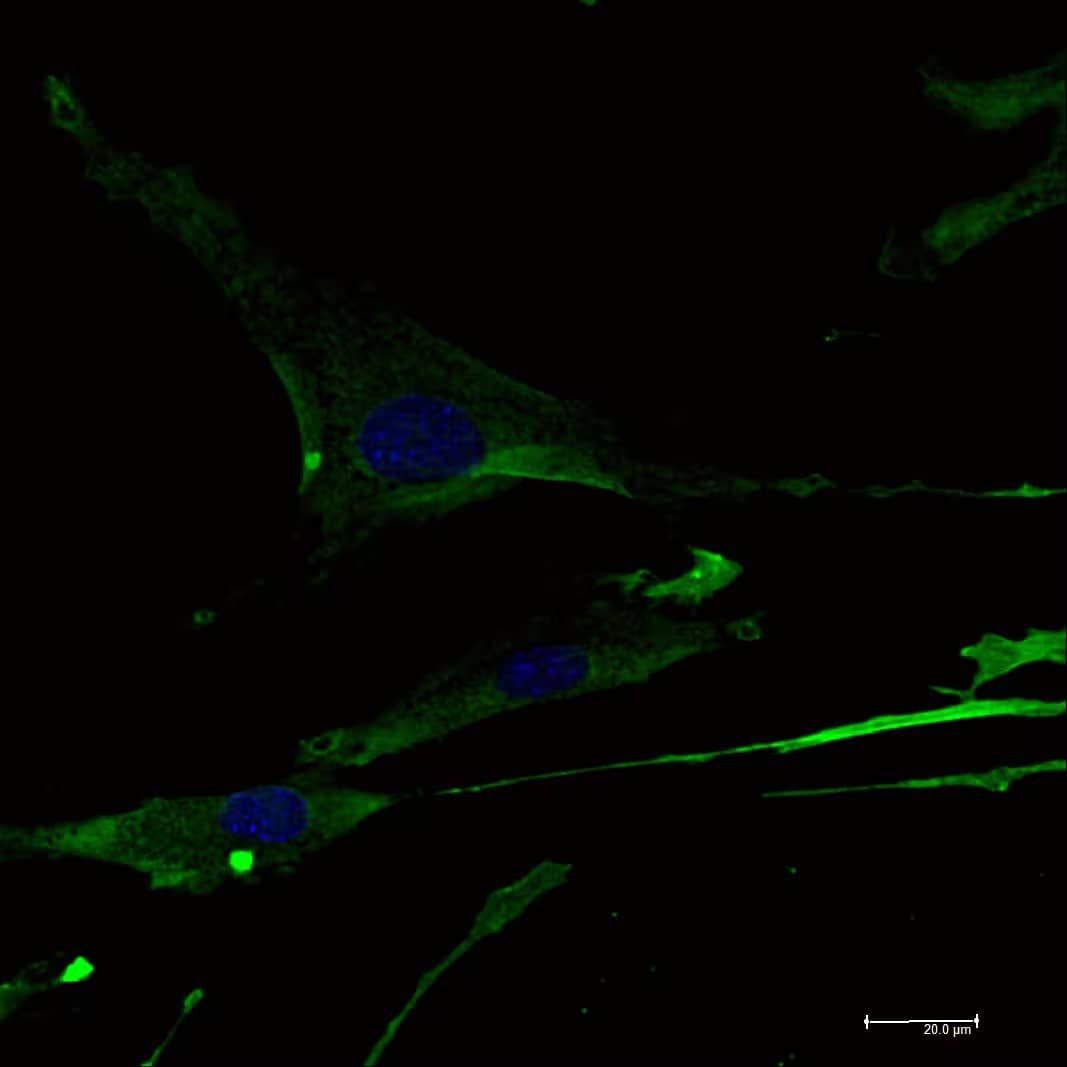
Detection of Mouse CD31/PECAM-1 by Immunocytochemistry/Immunofluorescence. IF staining of human lung endothelial cells with cd31/pecam1. Image from a verified customer review.
 View Larger
View Larger
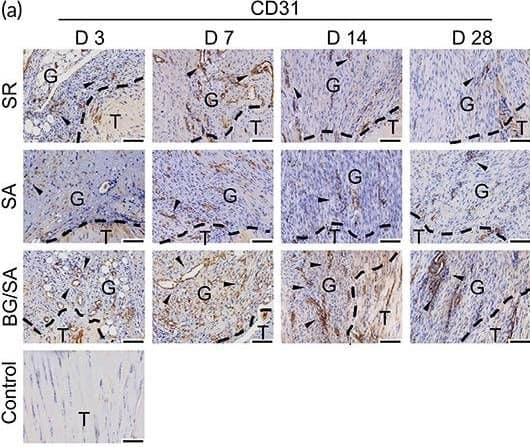
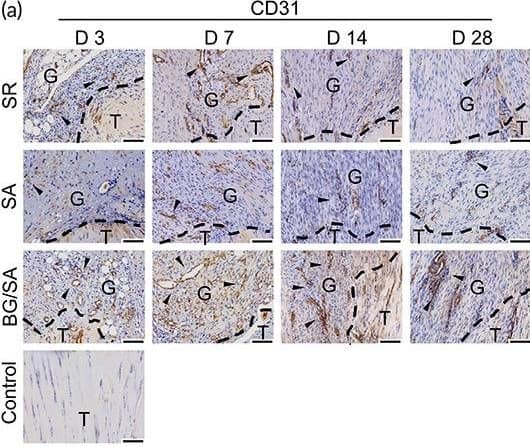
Detection of CD31/PECAM-1 by Immunohistochemistry Immunostaining analysis of angiogenic markers. (a) Immunohistochemistry (IHC) staining and semi‐quantitative analysis of CD31 in regenerated Achilles tendon of control, suture repair (SR), sodium alginate (SA), and bioactive glass (BG)/SA groups on Days 3, 7, 14, and 28 post‐surgery. Scale bar = 100 μm. T, tendon tissue; G, granulation tissue. Black arrows denote positive signals. Results of statistical analysis are presented as means ± SD; (n = 3; *p < 0.05). (b) Immunofluorescence (IF) staining of CD31 and alpha ‐SMA in regenerated Achilles tendon of SR, SA, and BG/SA groups on Days 7, 14, and 28 post‐surgery. Scale bar = 50 μm; Scale bar (zoom) = 20 μm Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36684098), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
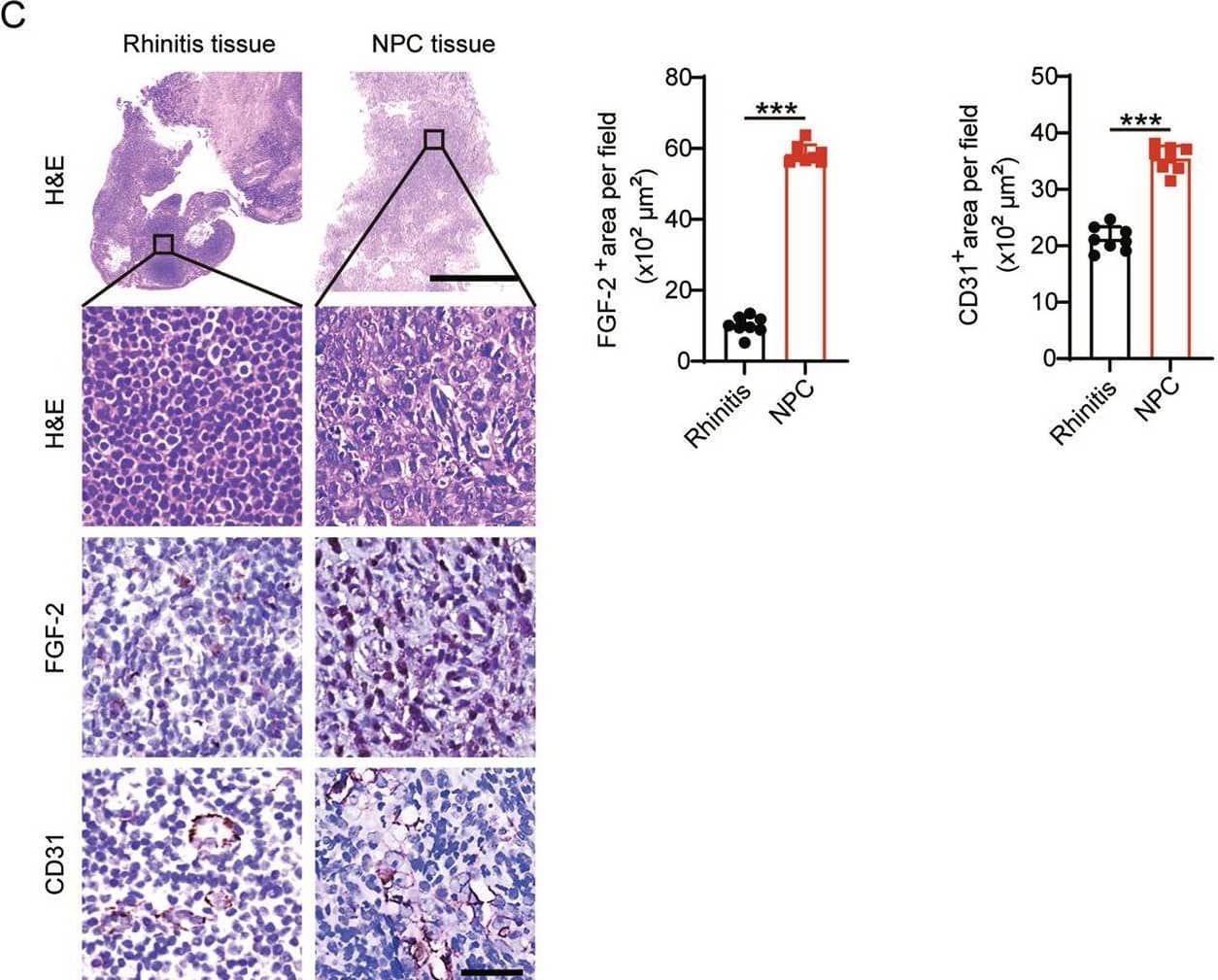
Detection of CD31/PECAM-1 by Immunohistochemistry FGF-2 expression in NPC correlates with tumor vasculature.A Transcriptomic expression levels of angiogenic factors, including VEGFA, FGF2, PDGFB, EGF, ANGPT1, EPO in human KIRC tissues, COAD tissues, NPC tissues, STAD tissues, PAAD tissues, LUAD tissues, BRCA tissues, SKCM tissues, and their adjacent healthy tissues. The red line indicates the highest expression of FGF2 in AAD-resistant NPC and the lowest expression of FGF in AAD-sensitive CRC. B Transcriptomic expression levels of FGF2 in NPC tissues, COAD tissues, and their adjacent healthy tissues (sample number: control-NPC/NPC/control-COAD/COAD = 10/31/41/290). Data were extracted from datasets GSE12452 and TCGA. C Human rhinitis tissues and NPC tissues were collected and detected for histology (H&E), FGF-2, and CD31 expression levels. Scale bar in upper panel = 500 μm, scale bar in lower three panels = 50 μm. Quantification of FGF-2+ or CD31+ signals (n = 8 random fields per group). D QPCR quantification of FGF2 and CD31 expression in freshly collected rhinitis tissues and NPC tissues (rhinitis tissue, n = 5 samples; NPC tissue, n = 6 samples). E Correlation of FGF2 and CD31 transcriptomic expression levels of human NPCs and control rhinitis tissues (Rhinitis tissue, n = 5 samples; NPC tissue, n = 6 samples). *P < 0.05; ***P < 0.001. NS not significant. Data presented as mean ± SD. KIRC kidney renal clear cell carcinoma, COAD colon adenocarcinoma, STAD stomach adenocarcinoma, PAAD pancreatic adenocarcinoma, LUAD lung adenocarcinoma, BRCA breast invasive carcinoma, SKCM skin cutaneous melanoma. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35985991), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
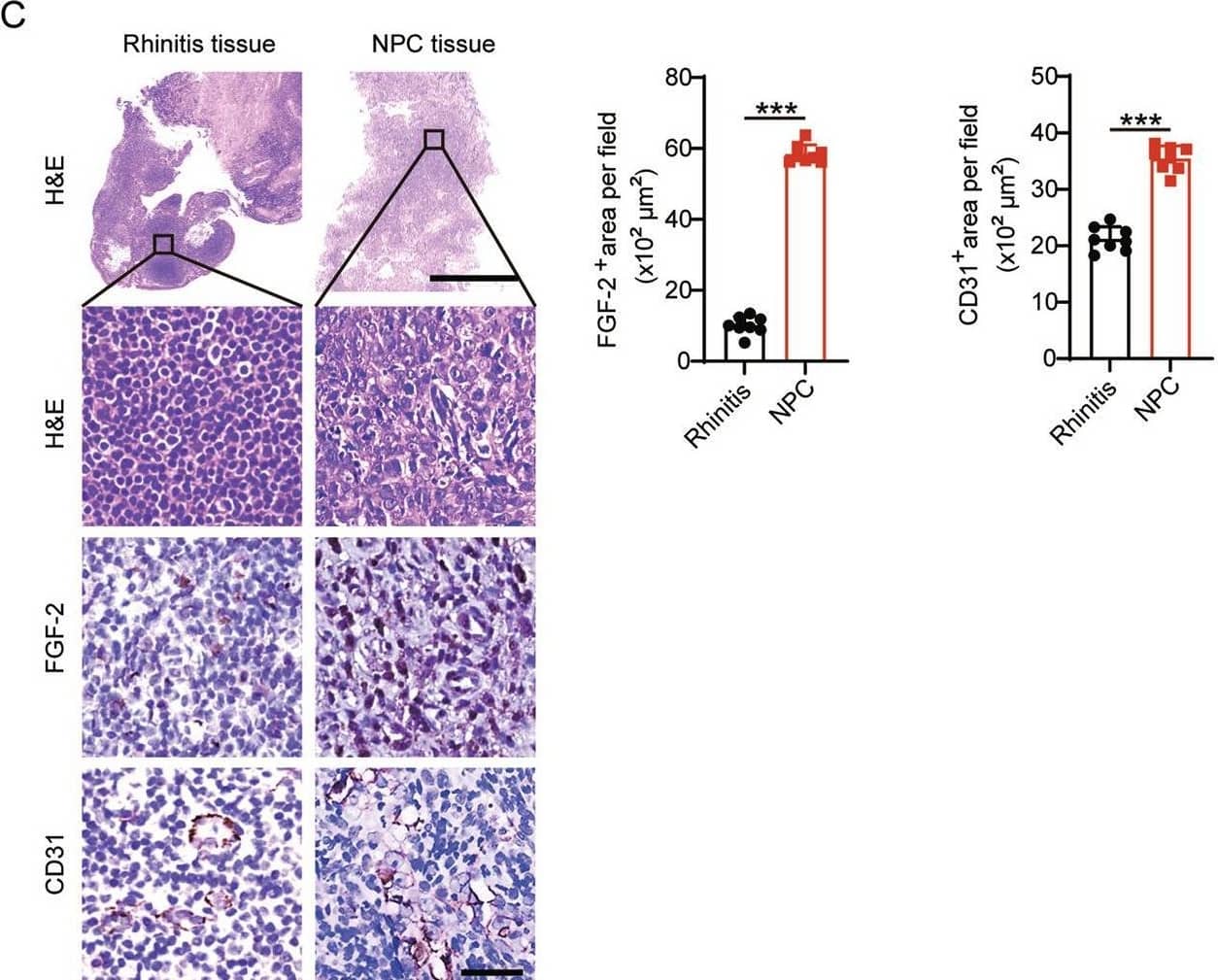
Detection of CD31/PECAM-1 by Immunohistochemistry FGF-2 expression in NPC correlates with tumor vasculature.A Transcriptomic expression levels of angiogenic factors, including VEGFA, FGF2, PDGFB, EGF, ANGPT1, EPO in human KIRC tissues, COAD tissues, NPC tissues, STAD tissues, PAAD tissues, LUAD tissues, BRCA tissues, SKCM tissues, and their adjacent healthy tissues. The red line indicates the highest expression of FGF2 in AAD-resistant NPC and the lowest expression of FGF in AAD-sensitive CRC. B Transcriptomic expression levels of FGF2 in NPC tissues, COAD tissues, and their adjacent healthy tissues (sample number: control-NPC/NPC/control-COAD/COAD = 10/31/41/290). Data were extracted from datasets GSE12452 and TCGA. C Human rhinitis tissues and NPC tissues were collected and detected for histology (H&E), FGF-2, and CD31 expression levels. Scale bar in upper panel = 500 μm, scale bar in lower three panels = 50 μm. Quantification of FGF-2+ or CD31+ signals (n = 8 random fields per group). D QPCR quantification of FGF2 and CD31 expression in freshly collected rhinitis tissues and NPC tissues (rhinitis tissue, n = 5 samples; NPC tissue, n = 6 samples). E Correlation of FGF2 and CD31 transcriptomic expression levels of human NPCs and control rhinitis tissues (Rhinitis tissue, n = 5 samples; NPC tissue, n = 6 samples). *P < 0.05; ***P < 0.001. NS not significant. Data presented as mean ± SD. KIRC kidney renal clear cell carcinoma, COAD colon adenocarcinoma, STAD stomach adenocarcinoma, PAAD pancreatic adenocarcinoma, LUAD lung adenocarcinoma, BRCA breast invasive carcinoma, SKCM skin cutaneous melanoma. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35985991), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of CD31/PECAM-1 by Immunohistochemistry Immunostaining analysis of angiogenic markers. (a) Immunohistochemistry (IHC) staining and semi‐quantitative analysis of CD31 in regenerated Achilles tendon of control, suture repair (SR), sodium alginate (SA), and bioactive glass (BG)/SA groups on Days 3, 7, 14, and 28 post‐surgery. Scale bar = 100 μm. T, tendon tissue; G, granulation tissue. Black arrows denote positive signals. Results of statistical analysis are presented as means ± SD; (n = 3; *p < 0.05). (b) Immunofluorescence (IF) staining of CD31 and alpha ‐SMA in regenerated Achilles tendon of SR, SA, and BG/SA groups on Days 7, 14, and 28 post‐surgery. Scale bar = 50 μm; Scale bar (zoom) = 20 μm Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36684098), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
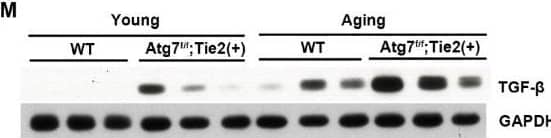
Detection of CD31/PECAM-1 by Immunohistochemistry Increase in renal fibrosis in aging kidneys of Atg7flox/flox;Tie2-Cre+ mice. (A) Representative immunoblots and densitometry for the expression of Atg7. (B) Relative protein level of Atg7 (%). (C) Representative images of H&E staining from wild-type and Atg7flox/flox;Tie2-Cre+ mice. Scale bars, 100 μm. (D) Quantification of glomeruli diameters (µm). (E) Representative 3,3′-diaminobenzidine (DAB) staining for CD31. Scale bars, 50 μm. (F) Quantification of capillary lumen diameters (µm). (G) Representative immunoblots and densitometry for the expression of CD31. (H) Relative protein levels of CD31 (%). (I) Masson’s trichrome staining from wild-type and Atg7flox/flox;Tie2-Cre mice, showing the cortex and medulla with increased extracellular matrix deposition in the aging Atg7flox/flox;Tie2-Cre+ mouse. (J) Quantification of tubulointerstitial fibrosis (%). (K) Representative DAB staining for TGF-beta. (L) Quantification of TGF-beta positive areas (%). (M) Representative immunoblots and densitometry for expression of TGF-beta. (N) Relative protein level of TGF-beta (%). (O) Representative DAB staining for alpha -SMA. (P) Quantification of alpha -SMA-positive areas (%). (Q) Representative immunoblots and densitometry for expression of alpha -SMA. (R) Relative protein levels of alpha -SMA (%). Scale bars, 50 μm for cortex and 100 μm for medulla. Values are means ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001, ns: not significant. Image collected and cropped by CiteAb from the following open publication (https://www.mdpi.com/2076-3921/13/8/886), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: CD31/PECAM-1
PECAM-1 (Platelet-Endothelial Cell Adhesion Molecule-1), also known as CD31, is a 130 kDa type I transmembrane glycoprotein adhesion molecule in the immunoglobulin superfamily (1, 2). Expression is restricted to cells involved in circulation, especially endothelial cells, platelets, monocytes, neutrophils and lymphocyte subsets. PECAM-1 is concentrated at cell-cell junctions and is required for Transendothelial Migration (TEM) (1-3). The Extracellular Domain (ECD) of PECAM-1 has ten potential N-linked glycosylation sites and six C2-type Ig-like domains, the first of which is critical for adhesion and extravasation (3, 4). The cytoplasmic domain contains Immunoregulatory Tyrosine-based Inhibitory and Switch Motifs (ITIM, ITSM) that mediate both inhibition and activation via phosphotyrosine-mediated engagement of SH2-containing signaling molecules (1, 5). Metalloproteinase-mediated ectodomain shedding occurs during apoptosis (6) but increased serum PECAM-1 ectodomain in HIV and active multiple sclerosis occurs independent of apoptosis (7, 8). In humans, expression of six isoforms with exon deletions in the cytoplasmic domain is tissue- and stage-specific, but full-length PECAM-1 is predominant. A form lacking the ITSM predominates in mouse (9). Mouse PECAM-1 ECD shows 77%, 63%, 63%, 63%, and 61% amino acid (aa) identity with rat, human, canine, porcine, and bovine PECAM-1, respectively. PECAM-1 participates with other adhesion molecules in some functions, but is the critical molecule for TEM. Homotypic PECAM-1 adhesion in trans, combined with cycling of PECAM-1 to and from surface-connected endothelial cell vesicles, leads leukocytes across endothelial tight junctions (3, 10). Homotypic adhesion and signaling functions also strongly suppress mitochondria-dependent apoptosis (11). In platelets, PECAM-1 is necessary for limiting thrombus formation (12) and promoting integrin-mediated clot retraction and platelet spreading (13), but mechanisms for these phenomena are unclear. PECAM-/- mice are deficient in chemokine-mediated chemotaxis (14).
- Ilan, N. and J.A. Madri (2003) Curr. Opin. Cell Biol. 15:515.
- Xie, Y. and W.A. Muller (1993) Proc. Natl. Acad. Sci. USA 90:5569.
- Liao, F. et al. (1997) J. Exp. Med. 185:1349.
- Nakada, M.T. et al. (2000) J. Immunol. 164:452.
- Chemnitz, J.M. et al. (2004) J. Immunol. 173:945.
- Ilan, N. et al. (2001) FASEB J. 15:362.
- Eugenin, E.A. et al. (2006) J. Leukoc. Biol. 79:444.
- Losy, J. et al. (1999) J. Neuroimmunol. 99:169.
- Wang, Y. et al. (2003) Am. J. Physiol. Heart Circ. Physiol. 284:H1008.
- Mamdouh, Z. et al. (2003) Nature 421:748.
- Gao, C. et al. (2003) Blood 102:169.
- Falati, S. et al. (2006) Blood 107:535.
- Wee, J.L. and D.E. Jackson (2005) Blood 106:3816.
- Wu, Y. et al. (2005) J. Immunol. 175:3484.
Product Datasheets
Citations for Human/Mouse/Rat CD31/PECAM-1 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
550
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Engrailed 1 coordinates cytoskeletal reorganization to induce myofibroblast differentiation
Authors: Gyorfi Ah, Matei Ae, Fuchs M Et Al.
The Journal of experimental medicine
-
TRAF3IP2 mediates high glucose-induced endothelin-1 production as well as endothelin-1-induced inflammation in endothelial cells.
Authors: Padilla J, Carpenter A, Das ND et al.
Am. J. Physiol. Heart Circ. Physiol.
-
Semiautomated pipeline for quantitative analysis of heart histopathology
Authors: Droste, P;Wong, DWL;Hohl, M;von Stillfried, S;Klinkhammer, BM;Boor, P;
Journal of translational medicine
-
Mono- and multi-nucleated ventricular cardiomyocytes constitute a transcriptionally homogenous cell population
Authors: Yekelchyk M, Guenther S, Preussner J, Braun T
Basic Res. Cardiol.
-
CD31 signaling promotes the detachment at the uropod of extravasating neutrophils allowing their migration to sites of inflammation
Authors: Andreata, F;Clément, M;Benson, RA;Hadchouel, J;Procopio, E;Even, G;Vorbe, J;Benadda, S;Ollivier, V;Ho-Tin-Noe, B;Le Borgne, M;Maffia, P;Nicoletti, A;Caligiuri, G;
eLife
-
FGFR1 is critical for the anti-endothelial mesenchymal transition effect of N-acetyl-seryl-aspartyl-lysyl-proline via induction of the MAP4K4 pathway.
Authors: Li J, Shi S, et al.
Cell Death Dis
-
Rescue of a lysosomal storage disorder caused by Grn loss of function with a brain penetrant progranulin biologic
Authors: Logan T, Simon MJ, Rana A Et al.
Cell
-
Endothelial AHR activity prevents lung barrier disruption in viral infection
Authors: Major, J;Crotta, S;Finsterbusch, K;Chakravarty, P;Shah, K;Frederico, B;D'Antuono, R;Green, M;Meader, L;Suarez-Bonnet, A;Priestnall, S;Stockinger, B;Wack, A;
Nature
-
THBS4/integrin alpha 2 axis mediates BM-MSCs to promote angiogenesis in gastric cancer associated with chronic Helicobacter pylori infection
Authors: LingNan He, WeiJun Wang, HuiYing Shi, Chen Jiang, HaiLing Yao, YuRui Zhang et al.
Aging (Albany NY)
-
Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis
Authors: Xinyu Qiu, Jin Liu, Chenxi Zheng, Yuting Su, Lili Bao, Bin Zhu et al.
Cell Proliferation
-
Intravenously-injected gold nanoparticles (AuNPs) access intracerebral F98 rat gliomas better than AuNPs infused directly into the tumor site by convection enhanced delivery
Authors: Henry M Smilowitz, Alexandria Meyers, Khalil Rahman, Nathaniel A Dyment, Dan Sasso, Crystal Xue et al.
International Journal of Nanomedicine
-
Host genetic modifiers of nonproductive angiogenesis inhibit breast cancer
Authors: MJ Flister, SW Tsaih, A Stoddard, C Plasterer, J Jagtap, AK Parchur, G Sharma, AR Prisco, A Lemke, D Murphy, M Al-Gizawiy, M Straza, S Ran, AM Geurts, MR Dwinell, AS Greene, C Bergom, PS LaViolette, A Joshi
Breast Cancer Res. Treat., 2017-05-31;0(0):.
-
Facilitation of Reparative Dentin Using a Drug Repositioning Approach With 4-Phenylbutric Acid
Authors: Lee E, Aryal Y, Kim T et al.
Front Physiol
-
A spatial vascular transcriptomic, proteomic, and phosphoproteomic atlas unveils an angiocrine Tie-Wnt signaling axis in the liver
Authors: Inverso D, Shi J, Lee KH et al.
Developmental cell
-
Design and biofabrication of dermal regeneration scaffolds: role of oligomeric collagen fibril density and architecture
Authors: David O Sohutskay, Kevin P Buno, Sunil S Tholpady, Samantha J Nier, Sherry L Voytik-Harbin
Regenerative Medicine
-
Distinct Roles for Rac1 in Sertoli Cell Function during Testicular Development and Spermatogenesis
Authors: A Heinrich, SJ Potter, L Guo, N Ratner, T DeFalco
Cell Rep, 2020-04-14;31(2):107513.
-
Aging of mouse intervertebral disc and association with back pain
Authors: Vincent K, Mohanty S, Pinelli R et al.
Bone
-
Sphingosine 1-Phosphate Receptor Signaling Establishes AP-1 Gradients to Allow for Retinal Endothelial Cell Specialization
Authors: Yanagida K, Engelbrecht E, Niaudet C et al.
Dev. Cell
-
Forced and voluntary exercise exert differential neuroprotective effects in cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and blood-brain barrier disruption
Authors: Zou, M;Wang, J;Yu, L;Bi, Y;Yan, G;Ding, H;Zhang, Y;
Brain research bulletin
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
A rapidly fabricated bone marrow enrichment material with a controllable pore size promotes osteogenesis close to autogenous bone graft in clinic
Authors: Yang, Q;Yu, B;Liu, C;Zhou, J;Zhang, Y;Yang, M;He, S;Cai, J;Dai, Q;Tang, Z;Xu, J;Zhang, Z;Hou, T;
Journal of advanced research
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Vitamin C conveys geroprotection on primate ovaries
Authors: Jing, Y;Lu, H;Li, J;He, Z;Zhao, L;Zhang, C;Huang, Z;Liu, L;Sun, S;Ma, S;Rodriguez Esteban, C;Fu, X;Zhao, G;Izpisua Belmonte, JC;Zhang, W;Qu, J;Wang, S;Liu, GH;
Cell stem cell
Species: Primate
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Single-atom Pt-doped ceria nanozymes mitigate myocardial ischemia reperfusion injury via cardiomyocyte-targeted uptake and suppression of reactive oxygen species
Authors: Pu, A;Sim, WS;Ji, Y;Kurian, AG;Lee, JH;Van Anh Bui, T;Lai, Y;Hwangbo, H;Sun, H;Kim, HW;Park, HJ;Ban, K;
Bioactive materials
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Platelet-derived growth factor-C contributes to kidney inflammation in experimental hypertension with little effect on the peritubular capillary network
Authors: Martin, IV;Dippel, C;Buhl, EM;Göllinger, R;Ermert, K;Floege, J;Stamellou, E;Raffetseder, U;Kramann, R;Ostendorf, T;
Experimental and molecular pathology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Influenza A virus infection during pregnancy increases transfer of maternal bloodborne molecules to fetal tissues
Authors: Gonzalez-Ricon, RJ;Otero, AM;Chalen, I;Savas, JN;Adetunji, S;Antonson, AM;
Brain, behavior, and immunity
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Endothelial cell supplementation promotes xenograft revascularization during short-term ovarian tissue transplantation
Authors: Spazzapan, M;Pegoraro, S;Vuerich, R;Zito, G;Balduit, A;Longo, E;Pascolo, L;Toffoli, M;Meshini, G;Mangogna, A;Ros, G;Buonomo, F;Romano, F;Lombardelli, L;Papa, G;Piccinni, MP;Zacchigna, S;Agostinis, C;Bulla, R;Ricci, G;
Bioactive materials
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Spatial Organisation of Tumour cDC1 States Correlates with Effector and Stem-Like CD8+ T Cells Location
Authors: Piot, C;Pereira da Costa, M;Biram, A;Minutti, CM;Lim, KHJ;Green, M;Mikolajczak, A;Jenkins, RP;Meader, L;Buck, MD;Cardoso, A;Rogers, N;Sahai, E;Reis E Sousa, C;
European journal of immunology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Persistent Activation of Endothelial Cells is Linked to Thrombosis and Inflammation in Cerebral Cavernous Malformation Disease
Authors: Gallego-Gutierrez, H;Frias-Anaya, E;Bui, C;Zhao, L;Hsu, E;Indralingam, HS;Körbelin, J;Trejo, J;Steinberg, J;Zemke, NR;Lopez-Ramirez, MA;
bioRxiv : the preprint server for biology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Commitment of adipose-resident c-kit+ progenitors to brown adipocytes contributes to adipose tissue homeostasis and remodeling
Authors: Chen, Q;Yu, Y;Zhang, R;Zhao, Q;Yu, D;Feng, C;Zhou, J;Luo, M;Yang, M;Sun, S;Zhang, L;Jin, M;
Nature communications
Species: Transgenic Mouse
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Impact of hypoxic versus oxic conditions on local tumor control after proton irradiation in a rat prostate carcinoma
Authors: Schmitt, M;Glowa, C;Kurth, I;Peschke, P;Brons, S;Karger, CP;
Clinical and translational radiation oncology
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Depth-Variant Deconvolution Applied to Widefield Microscopy for Rapid Large-Volume Tissue Imaging
Authors: Lee, DD;Telfer, KA;Koenis, MAJ;Lee, YK;Namink, KW;Saunders, BT;Lee, H;Kelley, HK;Ruiz, HS;Gaut, JP;Randolph, GJ;Zinselmeyer, BH;
Research square
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
An axolotl limb regeneration-inspired strategy to enhance alveolar bone regeneration
Authors: Liu, R;Wang, G;Ma, L;Yang, G;Lin, S;Sun, N;Wang, J;Ma, H;Jiang, X;Zhang, W;
Bioactive materials
Species: Human
Sample Types: Whole Cells
Applications: Immunohistochemistry, Immunocytochemistry -
Endothelial extracellular vesicle miR-423-5p regulates microvascular homeostasis and renal function after ischemia-reperfusion injury
Authors: Migneault, F;Kim, H;Doreille, A;Lan, S;Gendron, A;Normand, MH;Rimbaud, AK;Dupont, M;Bourdeau, I;Bonneil, É;Turgeon, J;Dussault, S;Thibault, P;Dieudé, M;Boilard, É;Rivard, A;Cardinal, H;Hébert, MJ;
JCI insight
Species: Human
Sample Types: Extracellular Vesicles
Applications: Western Blot -
The Duke Mouse Brain Atlas: MRI and light sheet microscopy stereotaxic atlas of the mouse brain
Authors: Mansour, H;Azrak, R;Cook, JJ;Hornburg, KJ;Qi, Y;Tian, Y;Williams, RW;Yeh, FC;White, LE;Johnson, GA;
Science advances
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
OTUD1 delays wound healing by regulating endothelial function and angiogenesis in diabetic mice
Authors: Zhang, J;Li, W;Liu, Y;Zheng, J;Liu, G;He, M;Zheng, Z;Zhu, M;Cho, N;Liang, G;Han, X;Ying, H;Shi, Q;
Journal of advanced research
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Malignant mesothelioma-associated inflammatory microenvironment promotes tumor progression via GPNMB
Authors: Belgiovine, C;Digifico, E;Erreni, M;Putignano, AR;Mannarino, L;Valentino, S;Grizzi, F;Pasqualini, F;Recordati, C;Bertola, L;Zucali, P;Pistillo, D;Paleari, V;Mantovani, A;D'Incalci, M;Marchesi, F;Allavena, P;
Journal of translational medicine
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Generation of human adult hepatocyte organoids with metabolic functions
Authors: Igarashi, R;Oda, M;Okada, R;Yano, T;Takahashi, S;Pastuhov, S;Matano, M;Masuda, N;Togasaki, K;Ohta, Y;Sato, S;Hishiki, T;Suematsu, M;Itoh, M;Fujii, M;Sato, T;
Nature
Species: Transgenic Mouse
Sample Types: Organoid, Whole Tissue
Applications: Immunohistochemistry -
Marine-derived STING inhibitors, excavatolide B promote wound repair in full-thickness-incision rats
Authors: Chang, CK;Wu, ZS;Niu, GH;Chou, YY;Tang, SH;Zhang, MM;Sung, CS;Tung, HT;Tsou, LK;Tang, CC;Sung, PJ;Lo, YH;Wen, ZH;
International immunopharmacology
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry-Paraffin -
AMPK is dispensable for physiological podocyte and glomerular functions but prevents glomerular fibrosis in experimental diabetes
Authors: Srivastava, SP;Kopasz-Gemmen, O;Kunamneni, A;Thurnman, A;Ozukan, E;Swaroop, V;Yoshida, S;Hong, S;Inoki, K;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry-Paraffin -
Compositional editing of extracellular matrices by CRISPR/Cas9 engineering of human mesenchymal stem cell lines
Authors: Prithiviraj, S;Garcia Garcia, A;Linderfalk, K;Yiguang, B;Ferveur, S;Falck, LN;Subramaniam, A;Mohlin, S;Hidalgo Gil, D;Dupard, SJ;Zacharaki, D;Raina, DB;Bourgine, PE;
eLife
Species: Chicken
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Canonical Wnt Signaling Suppresses Brain Endothelial Cell Transcytosis to Maintain Blood-Brain Barrier Integrity
Authors: du Maine, X;Gu, C;
bioRxiv : the preprint server for biology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Centripetal migration and prolonged retention of microglia promotes spinal cord injury repair
Authors: Ye, J;Shan, F;Xu, X;Liang, C;Zhang, N;Hu, H;Li, J;Ouyang, F;Wang, J;Zhao, Y;Ma, Z;Meng, C;Li, Z;Yu, S;Jing, J;Zheng, M;
Journal of neuroinflammation
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Emerging cooperativity between Oct4 and Sox2 governs the pluripotency network in early mouse embryos
Authors: Hou, Y;Nie, Z;Jiang, Q;Velychko, S;Heising, S;Bedzhov, I;Wu, G;Adachi, K;Scholer, HR;
eLife
Species: Mouse, Transgenic Mouse
Sample Types: Embryo
Applications: Immunohistochemistry -
Indigenous gut microbes modulate neural cell state and neurodegenerative disease susceptibility
Authors: Blackmer-Raynolds, L;Sampson, MM;Kozlov, A;Yang, A;Lipson, L;Hamilton, AM;Kelly, SD;Chopra, P;Chang, J;Sloan, SA;Sampson, TR;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Identification of CCL3 as a Schwann cell chemotactic factor essential for nerve regeneration
Authors: Van Emmenis, L;Mòdol-Caballero, G;Harford-Wright, E;Power, A;Cattin, AL;White, IJ;Casal, G;Boal-Carvalho, I;Bennett, CL;Lloyd, AC;
Cell reports
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Endothelial GSDMD underlies LPS-induced systemic vascular injury and lethality
Authors: Su, E;Song, X;Wei, L;Xue, J;Cheng, X;Xie, S;Jiang, H;Liu, M;
JCI insight
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Hemodynamic disturbance and mTORC1 activation: Unveiling the biomechanical pathogenesis of thoracic aortic aneurysms in Marfan syndrome
Authors: Liu, MY;Wang, M;Liu, J;Sun, AQ;He, CS;Cong, X;Kong, W;Li, W;
Journal of pharmaceutical analysis
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Reducing IgG accumulation via neonatal Fc receptor (FcRn) blockade relieves neuropathic pain
Authors: Fiore, NT;Willcox, KF;Dayani, D;Zuberi, YA;Heijnen, CJ;Grace, PM;
Brain, behavior, and immunity
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Histological effects of combined therapy involving scar resection, decellularized scaffolds, and human iPSC-NS/PCs transplantation in chronic complete spinal cord injury
Authors: Ito, K;Shinozaki, M;Hashimoto, S;Saijo, Y;Suematsu, Y;Tanaka, T;Nishi, K;Yagi, H;Shibata, S;Kitagawa, Y;Nakamura, M;Okano, H;Kohyama, J;Nagoshi, N;
Scientific reports
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Single-nucleus transcriptomics reveal the differentiation trajectories of periosteal skeletal/stem progenitor cells in bone regeneration
Authors: Perrin, S;Ethel, M;Bretegnier, V;Goachet, C;Wotawa, CA;Luka, M;Coulpier, F;Masson, C;Ménager, M;Colnot, C;
eLife
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
The Role of Endothelial L-PGDS in the Pro-Angiogenic and Anti-Inflammatory Effects of Low-Dose Alcohol Consumption
Authors: Li, J;Li, C;Subedi, U;Subedi, P;Panchatcharam, M;Sun, H;
Cells
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
In vivo, online label-free monitoring of heterogenous oxygen utilization during phototherapy with real-time ultrasound-guided photoacoustic imaging
Authors: Langley, A;Sweeney, A;Shethia, RT;Bednarke, B;Wulandana, F;Xavierselvan, M;Mallidi, S;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Polycomb Repressive Complex 2 promotes atherosclerotic plaque vulnerability
Authors: Joshi, D;Chakraborty, R;Bhogale, T;Furtado, J;Deng, H;Traylor, JG;Orr, AW;Martin, KA;Schwartz, MA;
bioRxiv : the preprint server for biology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Endothelial RIPK3 minimizes organotypic inflammation and vascular permeability in ischemia-reperfusion injury
Authors: Johnson, CF;Schafer, CM;Burge, KY;Coon, BG;Chaaban, H;Griffin, CT;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
CRISPR/CasRx suppresses KRAS-induced brain arteriovenous malformation developed in postnatal brain endothelial cells in mice
Authors: Saito, S;Nakamura, Y;Miyashita, S;Sato, T;Hoshina, K;Okada, M;Hasegawa, H;Oishi, M;Fujii, Y;Körbelin, J;Kubota, Y;Tainaka, K;Natsumeda, M;Ueno, M;
JCI insight
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Self-adhesion conductive cardiac patch based on methoxytriethylene glycol-functionalized graphene effectively improves cardiac function after myocardial infarction
Authors: Wang, X;Wang, H;Liu, X;Zhang, Y;Li, J;Liu, H;Feng, J;Jiang, W;Liu, L;Chen, Y;Li, X;Zhao, L;Guan, J;Zhang, Y;
Journal of advanced research
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Isolation and identification of patient-derived liver cancer stem cells and development of personalized treatment strategies
Authors: Guo, T;Zhang, S;Zeng, W;Liang, Y;Xie, J;Liu, S;Qiu, Y;Fu, Y;Ou, Y;Ma, K;Wang, B;Gu, W;Duan, Y;
Journal of translational medicine
Species: Human, Xenograft
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Generation of a novel constitutive smooth muscle cell-specific Myh11 -driven Cre mouse model
Authors: Dong, K;Bai, Z;He, X;Zhang, L;Hu, G;Yao, Y;Cai, CL;Zhou, J;
bioRxiv : the preprint server for biology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Human Stem Cell-Derived Cardiomyocytes Integrate Into the Heart of Monkeys With Right Ventricular Pressure Overload
Authors: Scholz, J;Secreto, FJ;Wobig, J;Kurian, J;Hagen, C;Zinnen, A;Vu, D;Johnson, SJ;Cetta, F;Qureshi, Y;Reams, R;Cannon, B;Heyer, CM;Chang, M;Fadra, N;Coonen, J;Simmons, HA;Mejia, A;Hayes, JM;Basu, P;Capuano, S;Bondarenko, V;Metzger, JM;Nelson, TJ;Emborg, ME;
Cell transplantation
Species: Rhesus Macaque
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Real-time imaging of cGMP signaling shows pronounced differences between glomerular endothelial cells and podocytes
Authors: Rutkowski, N;Görlitz, F;Wiesner, E;Binz-Lotter, J;Feil, S;Feil, R;Benzing, T;Hackl, MJ;
Scientific reports
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
IL-33/ST2 signaling in monocyte-derived macrophages maintains blood-brain barrier integrity and restricts infarctions early after ischemic stroke
Authors: Wang, M;Dufort, C;Du, Z;Shi, R;Xu, F;Huang, Z;Sigler, AR;Leak, RK;Hu, X;
Journal of neuroinflammation
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Interaction between subventricular zone microglia and neural stem cells impacts the neurogenic response in a mouse model of cortical ischemic stroke
Authors: Nath, S;Martínez Santamaría, JC;Chu, YH;Choi, JS;Conforti, P;Lin, JD;Sankowski, R;Amann, L;Galanis, C;Wu, K;Deshpande, SS;Vlachos, A;Prinz, M;Lee, JK;Schachtrup, C;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Spatiotemporal lineage tracing reveals the dynamic spatial architecture of tumor growth and metastasis
Authors: Jones, MG;Sun, D;Min, KHJ;Colgan, WN;Tian, L;Weir, JA;Chen, VZ;Koblan, LW;Yost, KE;Mathey-Andrews, N;Russell, AJC;Stickels, RR;Balderrama, KS;Rideout, WM;Chang, HY;Jacks, T;Chen, F;Weissman, JS;Yosef, N;Yang, D;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
ANXA1 Enhances the Proangiogenic Potential of Human Dental Pulp Stem Cells
Authors: Ma, X;Zhao, B;Wang, C;Sun, M;Dai, Y;E, L;Gao, M;Liu, X;Jia, Y;Yue, W;Liu, H;
Stem cells international
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Cerebrospinal fluid markers of neuroinflammation and coagulation in severe cerebral edema and chronic hydrocephalus after subarachnoid hemorrhage: a prospective study
Authors: Fang, Y;Liu, Y;Chen, L;Wang, J;Zhang, J;Zhang, H;Tian, S;Zhang, A;Zhang, J;Zhang, JH;Wang, X;Yu, J;Chen, S;
Journal of neuroinflammation
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Autologous micrografting improves regeneration of tissue-engineered urinary conduits in vivo
Authors: Juul, N;Amoushahi, M;Willacy, O;Ji, M;Villa, C;Ajalloueian, F;Chamorro, C;Fossum, M;
Scientific reports
Species: Porcine
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
GCN2 kinase activation mediates pulmonary vascular remodeling and pulmonary arterial hypertension
Authors: Zhu, MM;Dai, J;Dai, Z;Peng, Y;Zhao, YY;
JCI insight
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Optimized Enrichment of Murine Blood-Brain Barrier Vessels with a Critical Focus on Network Hierarchy in Post-Collection Analysis
Authors: Abdelazim, H;Barnes, A;Stupin, J;Hasson, R;Muñoz-Ballester, C;Young, KL;Robel, S;Smyth, JW;Lamouille, S;Chappell, JC;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Inhibiting Ca2+ channels in Alzheimer's disease model mice relaxes pericytes, improves cerebral blood flow and reduces immune cell stalling and hypoxia
Authors: Korte, N;Barkaway, A;Wells, J;Freitas, F;Sethi, H;Andrews, SP;Skidmore, J;Stevens, B;Attwell, D;
Nature neuroscience
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Translocator protein 18?kDa (TSPO) is upregulated in rat brain after peripheral nerve injury and downregulated by diroximel fumarate
Authors: Cazuza, RA;Zagrai, SM;Grieco, AR;Avery, TD;Abell, AD;Wey, HY;Loggia, ML;Grace, PM;
Brain, behavior, and immunity
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Regulation of angiogenesis by signal sequence-derived peptides
Authors: Ghim, M;Wei, L;Jung, JJ;The, E;Kukreja, G;Neishabouri, A;Ahmad, AA;Raza, MZ;Golbazi, A;Hedayatyanfard, K;Nie, L;Zhang, J;Sadeghi, MM;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Development of betabodies: the next generation of phosphatidylserine targeting agents
Authors: Phinney, NZ;Huang, X;Toombs, JE;Brekken, RA;
The Journal of biological chemistry
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Modulating amacrine cell-derived dopamine signaling promotes optic nerve regeneration and preserves visual function
Authors: Zhang, Q;Xue, J;Tang, J;Wu, S;Liu, Z;Wu, C;Liu, C;Liu, Y;Lin, J;Han, J;Liu, L;Chen, Y;Yang, J;Li, Z;Zhao, L;Wei, Y;Li, Y;Zhuo, Y;
Science advances
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Iron Chelator Deferiprone Restores Iron Homeostasis and Inhibits Retinal Neovascularization in Experimental Neovascular Age-Related Macular Degeneration
Authors: Xu, Y;Huang, S;Zhou, S;Wang, X;Wei, M;Chen, X;Zong, R;Lin, X;Li, S;Liu, Z;Chen, Q;
Investigative ophthalmology & visual science
Species: Mouse
Sample Types:
Applications: Immunohistochemistry-Frozen -
Increasing Endoglin Deletion in Endothelial Cells Exacerbates the Severity of Brain Arteriovenous Malformation in Mouse
Authors: Shabani, Z;Do Prado, LB;Zhang, R;Zhu, W;Shaligram, SS;Yadav, A;Wang, C;Su, H;
Biomedicines
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
KIT in oocytes: a key factor for oocyte survival and reproductive lifespan
Authors: Luan, Y;So, W;Dong, R;Abazarikia, A;Kim, SY;
EBioMedicine
Species: Human
Sample Types: Tissue Array
Applications: IHC-Pr -
Single nuclei transcriptomics reveal the differentiation trajectories of periosteal skeletal/stem progenitor cells in bone regeneration
Authors: Perrin, S;Ethel, M;Bretegnier, V;Goachet, C;Wotawa, CA;Luka, M;Coulpier, F;Masson, C;Ménager, M;Colnot, C;
bioRxiv : the preprint server for biology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Apelin modulates inflammation and leukocyte recruitment in experimental autoimmune encephalomyelitis
Authors: Park, H;Song, J;Jeong, HW;Grönloh, MLB;Koh, BI;Bovay, E;Kim, KP;Klotz, L;Thistlethwaite, PA;van Buul, JD;Sorokin, L;Adams, RH;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Radiation-Induced Endothelial Ferroptosis Accelerates Atherosclerosis via the DDHD2-Mediated Nrf2/GPX4 Pathway
Authors: Su, X;Liang, F;Zeng, Y;Yang, ZR;Deng, YZ;Xu, YH;Cai, XW;
Biomolecules
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Activation of a GPCR, ORL1 receptor: A novel therapy to prevent heart failure progression
Authors: Pathan, S;Pugazenthi, A;Dixon, BR;Wensel, TG;Rosengart, TK;Mathison, M;
Research square
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Auto/Paracrine C-Type Natriuretic Peptide/Cyclic GMP Signaling Prevents Endothelial Dysfunction
Authors: Werner, F;Naruke, T;Sülzenbrück, L;Schäfer, S;Rösch, M;Völker, K;Krebes, L;Abe beta er, M;Möllmann, D;Baba, HA;Schweda, F;Zernecke, A;Kuhn, M;
International journal of molecular sciences
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Tropism-shifted AAV-PHP.eB-mediated bFGF gene therapy promotes varied neurorestoration after ischemic stroke in mice
Authors: Shi, R;Ye, J;Liu, Z;Wang, C;Wu, S;Shen, H;Suo, Q;Li, W;He, X;Zhang, Z;Tang, Y;Yang, GY;Wang, Y;
Neural regeneration research
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Pilose antler extracts promotes hair growth in androgenetic alopecia mice by activating hair follicle stem cells via the AKT and Wnt pathways
Authors: Wang, F;He, G;Liu, M;Sun, Y;Ma, S;Sun, Z;Wang, Y;
Frontiers in pharmacology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Sulfur Amino Acid Restriction Enhances Exercise Capacity in Mice by Boosting Fat Oxidation in Muscle
Authors: Mann, CG;MacArthur, MR;Zhang, J;Gong, S;AbuSalim, JE;Hunter, CJ;Lu, W;Agius, T;Longchamp, A;Allagnat, F;Rabinowitz, J;Mitchell, JR;De Bock, K;Mitchell, SJ;
bioRxiv : the preprint server for biology
Species: Mouse, Transgenic Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Lactate transported by MCT1 plays an active role in promoting mitochondrial biogenesis and enhancing TCA flux in skeletal muscle
Authors: Zhang, L;Xin, C;Wang, S;Zhuo, S;Zhu, J;Li, Z;Liu, Y;Yang, L;Chen, Y;
Science advances
Species: Mouse
Sample Types: Cell Lysates, Tissue Homogenates, Whole Tissue
Applications: Immunohistochemistry, Western Blot -
Endorepellin downregulation promotes angiogenesis after experimental traumatic brain injury
Authors: Zhang, Q;Jing, Y;Gong, Q;Cai, L;Wang, R;Yang, D;Wang, L;Qu, M;Chen, H;Tang, Y;Tian, H;Ding, J;Xu, Z;
Neural regeneration research
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Vascular architecture regulates mesenchymal stromal cell heterogeneity via P53-PDGF signaling in the mouse incisor
Authors: Guo, T;Pei, F;Zhang, M;Yamada, T;Feng, J;Jing, J;Ho, TV;Chai, Y;
Cell stem cell
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Brain endothelial GSDMD activation mediates inflammatory BBB breakdown
Authors: Wei, C;Jiang, W;Wang, R;Zhong, H;He, H;Gao, X;Zhong, S;Yu, F;Guo, Q;Zhang, L;Schiffelers, LDJ;Zhou, B;Trepel, M;Schmidt, FI;Luo, M;Shao, F;
Nature
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Adjudin protects blood-brain barrier integrity and attenuates neuroinflammation following intracerebral hemorrhage in mice
Authors: Su, Q;Su, C;Zhang, Y;Guo, Y;Liu, Y;Liu, Y;Yong, VW;Xue, M;
International immunopharmacology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Osteocyte mitochondria regulate angiogenesis of transcortical vessels
Authors: Liao, P;Chen, L;Zhou, H;Mei, J;Chen, Z;Wang, B;Feng, JQ;Li, G;Tong, S;Zhou, J;Zhu, S;Qian, Y;Zong, Y;Zou, W;Li, H;Zhang, W;Yao, M;Ma, Y;Ding, P;Pang, Y;Gao, C;Mei, J;Zhang, S;Zhang, C;Liu, D;Zheng, M;Gao, J;
Nature communications
Species: Mouse, Transgenic Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Immunohistochemistry, Immunocytochemistry -
Non-endothelial expression of Endomucin in the mouse and human choroid
Authors: Brookins, E;Serrano, SE;Yacu, GS;Finer, G;Thomson, BR;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Endothelial cells regulate alveolar morphogenesis by constructing basement membranes acting as a scaffold for myofibroblasts
Authors: Watanabe-Takano, H;Kato, K;Oguri-Nakamura, E;Ishii, T;Kobayashi, K;Murata, T;Tsujikawa, K;Miyata, T;Kubota, Y;Hanada, Y;Nishiyama, K;Watabe, T;Fässler, R;Ishii, H;Mochizuki, N;Fukuhara, S;
Nature communications
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
High-throughput barcoding of nanoparticles identifies cationic, degradable lipid-like materials for mRNA delivery to the lungs in female preclinical models
Authors: Xue, L;Hamilton, AG;Zhao, G;Xiao, Z;El-Mayta, R;Han, X;Gong, N;Xiong, X;Xu, J;Figueroa-Espada, CG;Shepherd, SJ;Mukalel, AJ;Alameh, MG;Cui, J;Wang, K;Vaughan, AE;Weissman, D;Mitchell, MJ;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Morphological diversification and functional maturation of human astrocytes in glia-enriched cortical organoid transplanted in mouse brain
Authors: Wang, M;Zhang, L;Novak, SW;Yu, J;Gallina, IS;Xu, LL;Lim, CK;Fernandes, S;Shokhirev, MN;Williams, AE;Saxena, MD;Coorapati, S;Parylak, SL;Quintero, C;Molina, E;Andrade, LR;Manor, U;Gage, FH;
Nature biotechnology
Species: Human, Xenograft
Sample Types: Organoid, Whole Tissue
Applications: Immunohistochemistry -
Salidroside promotes pro-angiogenesis and repair of blood brain barrier via Notch/ITGB1 signal path in CSVD Model
Authors: Zhilan, T;Zengyu, Z;Pengpeng, J;Hualan, Y;Chao, L;Yan, X;Zimin, G;Shuangxing, H;Weiwei, L;
Journal of advanced research
Species: Rat
Sample Types: Cell Lysates, Tissue Homogenates, Whole Cells, Whole Tissue
Applications: ICC, Western Blot, IHC -
Knockdown of ANGPTL2 promotes left ventricular systolic dysfunction by upregulation of NOX4 in mice
Authors: Labbé, P;Martel, C;Shi, YF;Montezano, A;He, Y;Gillis, MA;Higgins, MÈ;Villeneuve, L;Touyz, R;Tardif, JC;Thorin-Trescases, N;Thorin, E;
Frontiers in physiology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Protection against overfeeding-induced weight gain is preserved in obesity but does not require FGF21 or MC4R
Authors: Lund, C;Ranea-Robles, P;Falk, S;Rausch, DM;Skovbjerg, G;Vibe-Petersen, VK;Krauth, N;Skytte, JL;Vana, V;Roostalu, U;Pers, TH;Lund, J;Clemmensen, C;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A clinically applicable connectivity signature for glioblastoma includes the tumor network driver CHI3L1
Authors: Hai, L;Hoffmann, DC;Wagener, RJ;Azorin, DD;Hausmann, D;Xie, R;Huppertz, MC;Hiblot, J;Sievers, P;Heuer, S;Ito, J;Cebulla, G;Kourtesakis, A;Kaulen, LD;Ratliff, M;Mandelbaum, H;Jung, E;Jabali, A;Horschitz, S;Ernst, KJ;Reibold, D;Warnken, U;Venkataramani, V;Will, R;Suvà, ML;Herold-Mende, C;Sahm, F;Winkler, F;Schlesner, M;Wick, W;Kessler, T;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
A terminal metabolite of niacin promotes vascular inflammation and contributes to cardiovascular disease risk
Authors: Ferrell, M;Wang, Z;Anderson, JT;Li, XS;Witkowski, M;DiDonato, JA;Hilser, JR;Hartiala, JA;Haghikia, A;Cajka, T;Fiehn, O;Sangwan, N;Demuth, I;König, M;Steinhagen-Thiessen, E;Landmesser, U;Tang, WHW;Allayee, H;Hazen, SL;
Nature medicine
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Impaired neuronal activity as a potential factor contributing to the underdeveloped cerebrovasculature in a young Parkinson's disease mouse model
Authors: Jeong, JY;Lee, HJ;Kim, N;Li, Y;Rah, JC;Oh, WJ;
Scientific reports
Species: Transgenic Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Crucial role for Sodium Hydrogen Exchangers in SGLT2 inhibitor-induced arterial relaxations
Authors: Forrester, EA;Benítez-Angeles, M;Redford, KE;Rosenbaum, T;Abbott, GW;Barrese, V;Dora, K;Albert, AP;Dannesboe, J;Salles-Crawley, I;Jepps, TA;Greenwood, IA;
bioRxiv : the preprint server for biology
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Successful reconstruction of the rat ureter by a syngeneic collagen tube with a cardiomyocyte sheet
Authors: Yamamoto, S;Matsui, K;Kinoshita, Y;Hiroshi Sasaki, ;Sekine, H;Saito, Y;Nakayama, Y;Kume, H;Kimura, T;Yokoo, T;Kobayashi, E;
Regenerative therapy
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Mapping cardiac remodeling in chronic kidney disease
Authors: Kaesler, N;Cheng, M;Nagai, J;O'Sullivan, J;Peisker, F;Bindels, EMJ;Babler, A;Moellmann, J;Droste, P;Franciosa, G;Dugourd, A;Saez-Rodriguez, J;Neuss, S;Lehrke, M;Boor, P;Goettsch, C;Olsen, JV;Speer, T;Lu, TS;Lim, K;Floege, J;Denby, L;Costa, I;Kramann, R;
Science advances
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Knockdown of Smox protects the integrity of the blood-brain barrier through antioxidant effect and Nrf2 pathway activation in stroke
Authors: Wang, G;Li, Z;Lin, P;Zhang, H;Wang, Y;Zhang, T;Wang, H;Li, H;Lin, L;Zhao, Y;Jia, L;Chen, Y;Ji, H;Zhao, W;Fu, Z;Zhong, Z;
International immunopharmacology
Species: Mouse
Sample Types: Whole Tissues
Applications: IHC -
A novel small molecule, CU05-1189, targeting the pleckstrin homology domain of PDK1 suppresses VEGF-mediated angiogenesis and tumor growth by blocking the Akt signaling pathway
Authors: Park, J;Zhang, H;Kwak, HJ;Gadhe, CG;Kim, Y;Kim, H;Noh, M;Shin, D;Ha, SJ;Kwon, YG;
Frontiers in pharmacology
Species: Mouse
Sample Types: Matrigel Plug
Applications: IHC -
Ultrasound-guided Photoacoustic image Annotation Toolkit in MATLAB (PHANTOM) for preclinical applications
Authors: Sweeney, A;Arora, A;Edwards, S;Mallidi, S;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Hematopoietic-specific heterozygous loss of Dnmt3a exacerbates colitis-associated colon cancer
Authors: Yang Feng, Qingchen Yuan, Rachel C. Newsome, Troy Robinson, Robert L. Bowman, Ashley N. Zuniga et al.
Journal of Experimental Medicine
-
Catalpol rescues LPS-induced cognitive impairment via inhibition of NF-?b-regulated neuroinflammation and up-regulation of TrkB-mediated BDNF secretion in mice
Authors: Hu, W;Zou, L;Yu, N;Wu, Z;Yang, W;Wu, T;Liu, Y;Pu, Y;Jiang, Y;Zhang, J;Zhu, H;Cheng, F;Feng, S;
Journal of ethnopharmacology
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
IL-1?+ macrophages fuel pathogenic inflammation in pancreatic cancer
Authors: Caronni, N;La Terza, F;Vittoria, FM;Barbiera, G;Mezzanzanica, L;Cuzzola, V;Barresi, S;Pellegatta, M;Canevazzi, P;Dunsmore, G;Leonardi, C;Montaldo, E;Lusito, E;Dugnani, E;Citro, A;Ng, MSF;Schiavo Lena, M;Drago, D;Andolfo, A;Brugiapaglia, S;Scagliotti, A;Mortellaro, A;Corbo, V;Liu, Z;Mondino, A;Dellabona, P;Piemonti, L;Taveggia, C;Doglioni, C;Cappello, P;Novelli, F;Iannacone, M;Ng, LG;Ginhoux, F;Crippa, S;Falconi, M;Bonini, C;Naldini, L;Genua, M;Ostuni, R;
Nature
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Hydrogen sulfide donor activates AKT-eNOS signaling and promotes lymphatic vessel formation
Authors: Aithabathula, RV;Pervaiz, N;Kathuria, I;Swanson, M;Singh, UP;Kumar, S;Park, F;Singla, B;
PloS one
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Structural and molecular basis of choline uptake into the brain by FLVCR2
Authors: Cater, RJ;Mukherjee, D;Iturbe, EG;Erramilli, SK;Chen, T;Koo, K;Grez, NS;Reckers, A;Kloss, B;Gawda, T;Choy, BC;Zheng, Z;Clarke, OB;Yee, SW;Kossiakoff, AA;Quick, M;Arnold, T;Mancia, F;
bioRxiv : the preprint server for biology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Semiautomated pipeline for quantitative analysis of heart histopathology
Authors: Droste, P;Wong, DWL;Hohl, M;von Stillfried, S;Klinkhammer, BM;Boor, P;
Journal of translational medicine
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
ICI 182,780 Attenuates Selective Upregulation of Uterine Artery Cystathionine ?-Synthase Expression in Rat Pregnancy
Authors: Bai, J;Li, Y;Yan, G;Zhou, J;Salmeron, AG;Fategbe, OT;Kumar, S;Chen, X;Chen, DB;
International journal of molecular sciences
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Combination human umbilical cord perivascular and endothelial colony forming cell therapy for ischemic cardiac injury
Authors: Iqbal F, Johnston A, Wyse B et al.
npj Regenerative Medicine
-
Coordination between ECM and cell-cell adhesion regulates the development of islet aggregation, architecture, and functional maturation
Authors: Tixi W, Maldonado M, Chang YT et al.
eLife
-
Astroglial Hmgb1 regulates postnatal astrocyte morphogenesis and cerebrovascular maturation
Authors: Freitas-Andrade, M;Comin, CH;Van Dyken, P;Ouellette, J;Raman-Nair, J;Blakeley, N;Liu, QY;Leclerc, S;Pan, Y;Liu, Z;Carrier, M;Thakur, K;Savard, A;Rurak, GM;Tremblay, MÈ;Salmaso, N;da F Costa, L;Coppola, G;Lacoste, B;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Characterization of choroid plexus in the preterm rabbit pup following subcutaneous administration of recombinant human IGF-1/IGFBP-3
Authors: Niklas Ortenlöf, Suvi Vallius, Helena Karlsson, Claes Ekström, Amanda Kristiansson, Bo Holmqvist et al.
Fluids and Barriers of the CNS
Species: Rabbit
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
TPL2 kinase activity regulates microglial inflammatory responses and promotes neurodegeneration in tauopathy mice
Authors: Wang, Y;Wu, T;Tsai, MC;Rezzonico, MG;Abdel-Haleem, AM;Xie, L;Gandham, VD;Ngu, H;Stark, K;Glock, C;Xu, D;Foreman, O;Friedman, BA;Sheng, M;Hanson, JE;
eLife
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Different niches for stem cells carrying the same oncogenic driver affect pathogenesis and therapy response in myeloproliferative neoplasms
Authors: Elodie Grockowiak, Claudia Korn, Justyna Rak, Veronika Lysenko, Adrien Hallou, Francesca M. Panvini et al.
Nature Cancer
-
The vascular gene Apold1 is dispensable for normal development but controls angiogenesis under pathological conditions
Authors: Fan Z, Ardicoglu R, Batavia AA et al.
Angiogenesis
-
Neuroinvasion and anosmia are independent phenomena upon infection with SARS-CoV-2 and its variants
Authors: de Melo GD, Perraud V, Alvarez F et al.
Nature communications
-
Melanocortin 1 receptor regulates cholesterol and bile acid metabolism in the liver
Authors: Thapa K, Kadiri JJ, Saukkonen K et al.
eLife
-
Proteomics identifies lipocalin-2 in neonatal inflammation associated with cerebrovascular alteration in mice and preterm infants
Authors: Giacomo Gravina, Maryam Ardalan, Tetyana Chumak, Anders K. Nilsson, Joakim C. Ek, Hanna Danielsson et al.
iScience
-
Increased beta2-adrenergic signaling is a targetable stimulus essential for bone healing by promoting callus neovascularization
Authors: Jahn, D;Knapstein, PR;Otto, E;Köhli, P;Sevecke, J;Graef, F;Graffmann, C;Fuchs, M;Jiang, S;Rickert, M;Erdmann, C;Appelt, J;Revend, L;Küttner, Q;Witte, J;Rahmani, A;Duda, G;Xie, W;Donat, A;Schinke, T;Ivanov, A;Tchouto, MN;Beule, D;Frosch, KH;Baranowsky, A;Tsitsilonis, S;Keller, J;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Ropinirole Cotreatment Prevents Perivascular Glial Recruitment in a Rat Model of L-DOPA-Induced Dyskinesia
Authors: Elabi, OF;Espa, E;Skovgård, K;Fanni, S;Cenci, MA;
Cells
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Traumatic brain injury stimulates sympathetic tone-mediated bone marrow myelopoiesis to favor fracture healing
Authors: Weijian Liu, Wei Chen, Mao Xie, Chao Chen, Zengwu Shao, Yiran Zhang et al.
Signal Transduction and Targeted Therapy
-
Torpor-like Hypothermia Induced by A1 Adenosine Receptor Agonist: A Novel Approach to Protect against Neuroinflammation
Authors: Fu, K;Hui, C;Wang, X;Ji, T;Li, X;Sun, R;Xing, C;Fan, X;Gao, Y;Su, L;
International journal of molecular sciences
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
In utero delivery of mRNA to the heart, diaphragm and muscle with lipid nanoparticles
Authors: Kewa Gao, Jie Li, Hengyue Song, Hesong Han, Yongheng Wang, Boyan Yin et al.
Bioactive Materials
-
Single‐Cell RNA Sequencing and Spatial Transcriptomics Reveal Pathogenesis of Meningeal Lymphatic Dysfunction after Experimental Subarachnoid Hemorrhage
Authors: Xiaoyu Wang, Anke Zhang, Qian Yu, Zelin Wang, Junjie Wang, Penglei Xu et al.
Advanced Science
-
Acute stress induces long-term metabolic, functional, and structural remodeling of the heart
Authors: Yoganathan, T;Perez-Liva, M;Balvay, D;Le Gall, M;Lallemand, A;Certain, A;Autret, G;Mokrani, Y;Guillonneau, F;Bruce, J;Nguyen, V;Gencer, U;Schmitt, A;Lager, F;Guilbert, T;Bruneval, P;Vilar, J;Maissa, N;Mousseaux, E;Viel, T;Renault, G;Kachenoura, N;Tavitian, B;
Nature communications
Species: Rat
Sample Types: Whole Tissues
Applications: IHC -
Three-Dimensional Histological Characterization of the Placental Vasculature Using Light Sheet Microscopy
Authors: Freise, L;Behncke, RY;Allerkamp, HH;Sandermann, TH;Chu, NH;Funk, EM;Hondrich, LJ;Riedel, A;Witzel, C;Hansmeier, NR;Danyel, M;Gellhaus, A;Dechend, R;Hägerling, R;
Biomolecules
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Disruption of the Clock Component Bmal1 in Mice Promotes Cancer Metastasis through the PAI-1-TGF-?-myoCAF-Dependent Mechanism
Authors: Wu J, Jing X, Du Q et al.
Advanced science (Weinheim, Baden-Wurttemberg, Germany)
-
Fetal brain vulnerability to SARS-CoV-2 infection
Authors: McMahon CL, Castro J, Silvas J et al.
Brain, behavior, and immunity
-
Krause corpuscles of the genitalia are vibrotactile sensors required for normal sexual behavior
Authors: Qi, L;Iskols, M;Handler, A;Ginty, DD;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
HPV-positive murine oral squamous cell carcinoma: development and characterization of a new mouse tumor model for immunological studies
Authors: Modic, Z;Cemazar, M;Markelc, B;Cör, A;Sersa, G;Kranjc Brezar, S;Jesenko, T;
Journal of translational medicine
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Endothelial progenitor cells derived from embryonic stem cells prevent alveolar simplification in a murine model of bronchopulmonary dysplasia
Authors: Olena A. Kolesnichenko, Hannah M. Flood, Yufang Zhang, Vladimir Ustiyan, Hayde K. Cuervo Jimenez, Tanya V. Kalin et al.
Frontiers in Cell and Developmental Biology
-
Cognitive Impairments, Neuroinflammation and Blood–Brain Barrier Permeability in Mice Exposed to Chronic Sleep Fragmentation during the Daylight Period
Authors: Clementine Puech, Mohammad Badran, Alexandra R. Runion, Max B. Barrow, Kylie Cataldo, David Gozal
International Journal of Molecular Sciences
-
Scalable projected Light Sheet Microscopy for high-resolution imaging of large samples
Authors: Chen, Y;Gong, C;Chauhan, S;De La Cruz, ED;Datta, MS;Tomer, R;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Tumour extracellular vesicles and particles induce liver metabolic dysfunction
Authors: Wang, G;Li, J;Bojmar, L;Chen, H;Li, Z;Tobias, GC;Hu, M;Homan, EA;Lucotti, S;Zhao, F;Posada, V;Oxley, PR;Cioffi, M;Kim, HS;Wang, H;Lauritzen, P;Boudreau, N;Shi, Z;Burd, CE;Zippin, JH;Lo, JC;Pitt, GS;Hernandez, J;Zambirinis, CP;Hollingsworth, MA;Grandgenett, PM;Jain, M;Batra, SK;DiMaio, DJ;Grem, JL;Klute, KA;Trippett, TM;Egeblad, M;Paul, D;Bromberg, J;Kelsen, D;Rajasekhar, VK;Healey, JH;Matei, IR;Jarnagin, WR;Schwartz, RE;Zhang, H;Lyden, D;
Nature
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Brain resident memory T cells rapidly expand and initiate neuroinflammatory responses following CNS viral infection
Authors: Ayasoufi, K;Wolf, DM;Namen, SL;Jin, F;Tritz, ZP;Pfaller, CK;Zheng, J;Goddery, EN;Fain, CE;Gulbicki, LR;Borchers, AL;Reesman, RA;Yokanovich, LT;Maynes, MA;Bamkole, MA;Khadka, RH;Hansen, MJ;Wu, LJ;Johnson, AJ;
Brain, behavior, and immunity
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A ZFYVE21-Rubicon-RNF34 signaling complex promotes endosome-associated inflammasome activity in endothelial cells
Authors: Li, X;Jiang, Q;Song, G;Barkestani, MN;Wang, Q;Wang, S;Fan, M;Fang, C;Jiang, B;Johnson, J;Geirsson, A;Tellides, G;Pober, JS;Jane-Wit, D;
Nature communications
Species: Human, Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Replenishable prevascularized cell encapsulation devices increase graft survival and function in the subcutaneous space
Authors: Chendke G, Kharbikar B, Ashe S et al.
Bioengineering & Translational Medicine
-
LPS-Induced Systemic Inflammation Affects the Dynamic Interactions of Astrocytes and Microglia with the Vasculature of the Mouse Brain Cortex
Authors: Xingi, E;Koutsoudaki, PN;Thanou, I;Phan, MS;Margariti, M;Scheller, A;Tinevez, JY;Kirchhoff, F;Thomaidou, D;
Cells
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
CCN2/CTGF tip the balance of growth factors towards TGF-beta 2 in primary open-angle glaucoma
Authors: Andrea E. Dillinger, Sabrina Kuespert, Amin A. Seleem, Jakob Neuendorf, Magdalena Schneider, Rudolf Fuchshofer
Frontiers in Molecular Biosciences
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Therapeutic activation of endothelial sphingosine‐1‐phosphate receptor 1 by chaperone‐bound S1P suppresses proliferative retinal neovascularization
Authors: Colin Niaudet, Bongnam Jung, Andrew Kuo, Steven Swendeman, Edward Bull, Takahiro Seno et al.
EMBO Molecular Medicine
-
Osteoprogenitor-GMP crosstalk underpins solid tumor-induced systemic immunosuppression and persists after tumor removal
Authors: Hao, X;Shen, Y;Chen, N;Zhang, W;Valverde, E;Wu, L;Chan, HL;Xu, Z;Yu, L;Gao, Y;Bado, I;Michie, LN;Rivas, CH;Dominguez, LB;Aguirre, S;Pingel, BC;Wu, YH;Liu, F;Ding, Y;Edwards, DG;Liu, J;Alexander, A;Ueno, NT;Hsueh, PR;Tu, CY;Liu, LC;Chen, SH;Hung, MC;Lim, B;Zhang, XH;
Cell stem cell
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lesion-specific suppression of YAP/TAZ by biomimetic nanodrug ameliorates atherosclerosis development
Authors: Huang, HC;Wang, TY;Rousseau, J;Mungaray, M;Michaud, C;Plaisier, C;Chen, ZB;Wang, KC;
bioRxiv : the preprint server for biology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
A Soluble Platelet-Derived Growth Factor Receptor-? Originates via Pre-mRNA Splicing in the Healthy Brain and Is Upregulated during Hypoxia and Aging
Authors: Payne LB, Abdelazim H, Hoque M et al.
Biomolecules
-
Salmonella infection induces the reorganization of follicular dendritic cell networks concomitant with the failure to generate germinal centers
Authors: Edith Marcial-Juárez, Marisol Pérez-Toledo, Saba Nayar, Elena Pipi, Areej Alshayea, Ruby Persaud et al.
iScience
-
RAB27B Drives a Cancer Stem Cell Phenotype in NSCLC Cells Through Enhanced Extracellular Vesicle Secretion
Authors: Kayleah M. Meneses, Prita Pandya, Jennifer A. Lindemann, Dania S. Al-Qasrawi, Ryan A. Argo, Celeste M. Weems et al.
Cancer Research Communications
-
Inhibition of neutrophil extracellular trap formation attenuates NLRP1-dependent neuronal pyroptosis via STING/IRE1 alpha pathway after traumatic brain injury in mice
Authors: Yiyao Cao, Mingming Shi, Liang Liu, Yan Zuo, Haoran Jia, Xiaobin Min et al.
Frontiers in Immunology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Pharmacological modulation of TSPO in microglia/macrophages and neurons in a chronic neurodegenerative model of prion disease
Authors: M Vicente-Ro, R Mancuso, A Peris-Yagu, C Simmons, NIMA Conso, D Gómez-Nico, VH Perry, F Turkheimer, S Lovestone, CA Parker, D Cash
Journal of Neuroinflammation, 2023-04-09;20(1):92.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Exogenous interleukin 33 enhances the brain's lymphatic drainage and toxic protein clearance in acute traumatic brain injury mice
Authors: M Liu, J Huang, T Liu, J Yuan, C Lv, Z Sha, C Wu, W Jiang, X Liu, M Nie, Y Chen, S Dong, Y Qian, C Gao, Y Fan, D Wu, R Jiang
Acta neuropathologica communications, 2023-04-07;11(1):61.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Cardiac Plin5 interacts with SERCA2 and promotes calcium handling and cardiomyocyte contractility
Authors: Cinato M, Mardani I, Miljanovic A et al.
Life Science Alliance
-
Immunotherapy targeting plasma ASM is protective in a mouse model of Alzheimer's disease
Authors: BJ Choi, MH Park, KH Park, WH Han, HJ Yoon, HY Jung, JY Hong, MR Chowdhury, KY Kim, J Lee, IS Song, M Pang, MK Choi, E Gulbins, M Reichel, J Kornhuber, CW Hong, C Kim, SH Kim, EH Schuchman, HK Jin, JS Bae
Nature Communications, 2023-03-24;14(1):1631.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Bitter taste cells in the ventricular walls of the murine brain regulate glucose homeostasis
Authors: Q Yu, I Gamayun, P Wartenberg, Q Zhang, S Qiao, S Kusumakshi, S Candlish, V Götz, S Wen, D Das, A Wyatt, V Wahl, F Ectors, K Kattler, D Yildiz, V Prevot, M Schwaninge, G Ternier, P Giacobini, P Ciofi, TD Müller, U Boehm
Nature Communications, 2023-03-22;14(1):1588.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Three-dimensional visualization of neural networks inside bone by Osteo-DISCO protocol and alteration of bone remodeling by surgical nerve ablation
Authors: K Utagawa, T Shin, H Yamada, H Ochi, S Sunamura, A Unno, C Akazawa, M Ema, S Takeda, A Okawa, S Sato
Scientific Reports, 2023-03-22;13(1):4674.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Sertoli cells are the unique source of stem cell factor for spermatogenesis
Authors: Yi Jacky Peng, Xinyu Thomas Tang, Hui Sophie Shu, Wenjie Dong, Hongfang Shao, Bo O. Zhou
Development
-
17 beta -Estradiol promotes sex-specific dysfunction in isolated human arterioles
Authors: Gopika SenthilKumar, Boran Katunaric, Henry Bordas-Murphy, Micaela Young, Erin L. Doren, Mary E. Schulz et al.
American Journal of Physiology-Heart and Circulatory Physiology
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Engineered multi-functional, pro-angiogenic collagen-based scaffolds loaded with endothelial cells promote large deep burn wound healing
Authors: Hengyue Song, Kewa Gao, Dake Hao, Andrew Li, Ruiwu Liu, Bryan Anggito et al.
Frontiers in Pharmacology
-
Trans-differentiation of trophoblast stem cells: implications in placental biology
Authors: Madhurima Paul, Shreeta Chakraborty, Safirul Islam, Rupasri Ain
Life Science Alliance
-
Limiting Mrs2-dependent mitochondrial Mg2+ uptake induces metabolic programming in prolonged dietary stress
Authors: TR Madaris, M Venkatesan, S Maity, MC Stein, N Vishnu, MK Venkateswa, JG Davis, K Ramachandr, S Uthayabala, C Allen, A Osidele, K Stanley, NP Bigham, TM Bakewell, M Narkunan, A Le, V Karanam, K Li, A Mhapankar, L Norton, J Ross, MI Aslam, WB Reeves, BB Singh, J Caplan, JJ Wilson, PB Stathopulo, JA Baur, M Madesh
Cell Reports, 2023-02-27;42(3):112155.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Intracapillary LPL levels in brown adipose tissue, visualized with an antibody-based approach, are regulated by ANGPTL4 at thermoneutral temperatures
Authors: W Song, Y Yang, P Heizer, Y Tu, TA Weston, JR Kim, P Munguia, H Jung, JL Fong, C Tran, M Ploug, AP Beigneux, SG Young, LG Fong
Proceedings of the National Academy of Sciences of the United States of America, 2023-02-14;120(8):e2219833120.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
SARS-CoV-2 triggers pericyte-mediated cerebral capillary constriction
Authors: Chanawee Hirunpattarasilp, Greg James, Jaturon Kwanthongdee, Felipe Freitas, Jiandong Huo, Huma Sethi et al.
Brain
-
IFN-?-STAT1-mediated CD8+ T-cell-neural stem cell cross talk controls astrogliogenesis after spinal cord injury
Authors: Wang J, Xu L, Peng D et al.
Inflammation and regeneration
-
Mitochondrial ATP Production is Required for Endothelial Cell Control of Vascular Tone
Authors: Calum Wilson, Matthew D Lee, Charlotte Buckley, Xun Zhang, John G McCarron
Function (Oxf)
-
A refined protocol for the isolation and monoculture of primary mouse renal peritubular endothelial cells
Authors: Austin D. Thompson, Jaroslav Janda, Rick G. Schnellmann
Frontiers in Cardiovascular Medicine
-
Gasdermin D inhibition ameliorates neutrophil mediated brain damage in acute ischemic stroke
Authors: Hu R, Liang J, Ding L et al.
Cell death discovery
-
Bone metastasis initiation is coupled with bone remodeling through osteogenic differentiation of NG2+ cells
Authors: W Zhang, Z Xu, X Hao, T He, J Li, Y Shen, K Liu, Y Gao, J Liu, D Edwards, AM Muscarella, L Wu, L Yu, L Xu, X Chen, YH Wu, IL Bado, Y Ding, S Aguirre, H Wang, Z Gugala, RL Satcher, ST Wong, XH Zhang
Cancer Discovery, 2023-02-06;0(0):.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Antinociceptive Effects of Aaptamine, a Sponge Component, on Peripheral Neuropathy in Rats
Authors: CS Sung, HJ Cheng, NF Chen, SH Tang, HM Kuo, PJ Sung, WF Chen, ZH Wen
Marine Drugs, 2023-02-04;21(2):.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
White-light crosslinkable milk protein bioadhesive with ultrafast gelation for first-aid wound treatment
Authors: Q Zhu, X Zhou, Y Zhang, D Ye, K Yu, W Cao, L Zhang, H Zheng, Z Sun, C Guo, X Hong, Y Zhu, Y Zhang, Y Xiao, TG Valencak, T Ren, D Ren
Biomaterials research, 2023-02-03;27(1):6.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Upadacitinib inhibits corneal inflammation and neovascularization by suppressing M1 macrophage infiltration in the corneal alkali burn model
Authors: J Yu, Y Shen, J Luo, J Jin, P Li, P Feng, H Guan
International immunopharmacology, 2023-02-03;116(0):109680.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC -
Improving anti-tumor efficacy of low-dose Vincristine in rhabdomyosarcoma via the combination therapy with FOXM1 inhibitor RCM1
Authors: Donovan J, Deng Z, Bian F et al.
Frontiers in oncology
-
A Polymeric Nanoparticle Formulation for Targeted mRNA Delivery to Fibroblasts
Authors: Artur Filipe Rodrigues, Catarina Rebelo, Susana Simões, Cristiana Paulo, Sónia Pinho, Vítor Francisco et al.
Advanced Science
-
Somatic GJA4 gain-of-function mutation in orbital cavernous venous malformations
Authors: Hiroki Hongo, Satoru Miyawaki, Yu Teranishi, Jun Mitsui, Hiroto Katoh, Daisuke Komura et al.
Angiogenesis
-
In vitro assessment of varying peptide surface density on the suppression of angiogenesis by micelles displaying alpha v beta 3 blocking peptides
Authors: Neha Phani Bhushan, Trevor Stack, Evan A. Scott, Kenneth R. Shull, Benjamin Mathew, Divya Bijukumar
Journal of Biomedical Materials Research Part B: Applied Biomaterials
-
An Engineered IFNgamma-Antibody Fusion Protein with Improved Tumor-Homing Properties
Authors: C Di Nitto, E Gilardoni, J Mock, L Nadal, T Weiss, M Weller, F Seehusen, C Libbra, E Puca, D Neri, R De Luca
Pharmaceutics, 2023-01-22;15(2):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Endothelial SMAD1/5 signaling couples angiogenesis to osteogenesis during long bone growth
Authors: Annemarie Lang, Andreas Benn, Angelique Wolter, Tim Balcaen, Joseph Collins, Greet Kerckhofs et al.
bioRxiv
-
Tocilizumab promotes repair of spinal cord injury by facilitating the restoration of tight junctions between vascular endothelial cells
Authors: Yang Luo, Fei Yao, Yi Shi, Zhenyu Zhu, Zhaoming Xiao, Xingyu You et al.
Fluids and Barriers of the CNS
-
Amyloid beta induces cardiac dysfunction and neuro-signaling impairment in the heart of an Alzheimer’s disease model
Authors: Elia, A;Parodi-Rullan, R;Vazquez-Torres, R;Carey, A;Javadov, S;Fossati, S;
bioRxiv
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Metformin promotes angiogenesis and functional recovery in aged mice after spinal cord injury by adenosine monophosphate-activated protein kinase/endothelial nitric oxide synthase pathway
Authors: Chun-Yue Duan, Jian-Zhong Sheng, Cheng-Jun Zhao, Xiao-Long Sheng, Cheng-Jun Li, Guo-Yu Qin et al.
Neural Regeneration Research
-
Knockout of Sirt2 alleviates traumatic brain injury in mice
Authors: Heng-Li Tian, Qiu-Yuan Wang, Qiu-Yuan Gong, Lin Jing, Dian-Xu Jing, Dian-Xu Yang et al.
Neural Regeneration Research
-
Inhibiting phosphatase and actin regulator 1 expression is neuroprotective in the context of traumatic brain injury
Authors: Heng-Li Tian, Zhi-Ming Zhang, Shi-Wen Ding, Yao Jing, Shi-Ming Zhang, Shi-Wen Chen et al.
Neural Regeneration Research
-
Enhanced anti-angiogenetic effect of transferrin receptor-mediated delivery of VEGF-trap in a glioblastoma mouse model
Authors: Peng Zhao, Yasuaki Anami, Peng Gao, Xuejun Fan, Leike Li, Kyoji Tsuchikama et al.
mAbs
-
Discovery and engineering of an anti-TREM2 antibody to promote amyloid plaque clearance by microglia in 5XFAD mice
Authors: Peng Zhao, Yuanzhong Xu, Xuejun Fan, Leike Li, Xin Li, Hisashi Arase et al.
mAbs
-
Extracellular vesicle-derived CircWhsc1 Promotes Cardiomyocyte Proliferation and Heart Repair by activating TRIM59/STAT3/Cyclin B2 pathway
Authors: G Wei, C Li, X Jia, J Xie, Z Tang, M Jin, Q Chen, Y Sun, S He, X Li, Y Chen, H Zheng, W Liao, Y Liao, J Bin, S Huang
Journal of advanced research, 2022-12-29;0(0):.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Immunohistochemistry, Immunocytochemistry -
Cyclin G2 in macrophages triggers CTL-mediated antitumor immunity and antiangiogenesis via interferon-gamma
Authors: Lu Liu, Jinlan Gao, Xuesha Xing, Meixi Jiang, Qi Liu, Shusen Wang et al.
Journal of Experimental & Clinical Cancer Research
-
Role of pericytes in the development of cerebral cavernous malformations
Authors: Zifeng Dai, Jingwei Li, Ying Li, Rui Wang, Huili Yan, Ziyu Xiong et al.
iScience
-
Motor neurons use push-pull signals to direct vascular remodeling critical for their connectivity
Authors: Luis F. Martins, Ilaria Brambilla, Alessia Motta, Stefano de Pretis, Ganesh Parameshwar Bhat, Aurora Badaloni et al.
Neuron
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Secretome from human adipose-derived mesenchymal stem cells promotes blood vessel formation and pericyte coverage in experimental skin repair
Authors: BM Silveira, TO Ribeiro, RS Freitas, ACO Carreira, MS Gonçalves, M Sogayar, R Meyer, A Birbrair, V Fortuna
PLoS ONE, 2022-12-19;17(12):e0277863.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Immuno-localization of definitive hematopoietic stem cells in the vascular niche of mouse fetal liver
Authors: Atreyi Biswas, Shailendra Kumar Singh, Gayathri M. Kartha, Satish Khurana
STAR Protocols
-
Piperlongumine alleviates corneal allograft rejection via suppressing angiogenesis and inflammation
Authors: Xiangyu Fan, Jini Qiu, Tianjie Yuan, Jing Zhang, Jianjiang Xu
Frontiers in Immunology
-
Microglia regulate central nervous system myelin growth and integrity
Authors: NB McNamara, DAD Munro, N Bestard-Cu, A Uyeda, JFJ Bogie, A Hoffmann, RK Holloway, I Molina-Gon, KE Askew, S Mitchell, W Mungall, M Dodds, C Dittmayer, J Moss, J Rose, S Szymkowiak, L Amann, BW McColl, M Prinz, TL Spires-Jon, W Stenzel, K Horsburgh, JJA Hendriks, C Pridans, R Muramatsu, A Williams, J Priller, VE Miron
Nature, 2022-12-14;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Three-dimensional heart extracellular matrix enhances chemically induced direct cardiac reprogramming
Authors: Y Jin, H Kim, S Min, YS Choi, SJ Seo, E Jeong, SK Kim, HA Lee, SH Jo, JH Park, BW Park, WS Sim, JJ Kim, K Ban, YG Kim, HJ Park, SW Cho
Science Advances, 2022-12-14;8(50):eabn5768.
Species: Rat
Sample Types: Tissue Homogenate
Applications: IHC -
Exosomal miR-532-5p induced by long-term exercise rescues blood-brain barrier function in 5XFAD mice via downregulation of EPHA4
Authors: X Liang, W Fa, N Wang, Y Peng, C Liu, M Zhu, N Tian, Y Wang, X Han, C Qiu, T Hou, Y Du
Aging Cell, 2022-12-09;0(0):e13748.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Conserved YKL-40 changes in mice and humans after postoperative delirium
Authors: Jennifer David-Bercholz, Leah Acker, Ana I. Caceres, Pau Yen Wu, Saanvi Goenka, Nathan O. Franklin et al.
Brain, Behavior, & Immunity - Health
-
Novel localization of folate transport systems in the murine central nervous system
Authors: Vishal Sangha, Md. Tozammel Hoque, Jeffrey T. Henderson, Reina Bendayan
Fluids and Barriers of the CNS
-
Angiopoietin-like 2 is essential to aortic valve development in mice
Authors: Pauline Labbé, Victoria Munoz Goyette, Nathalie Thorin-Trescases, Louis Villeneuve, Ines Desanlis, Constance Delwarde et al.
Communications Biology
-
Optical Tissue Clearing to Study the Intra-Pulmonary Biodistribution of Intravenously Delivered Mesenchymal Stromal Cells and Their Interactions with Host Lung Cells
Authors: Alejandra Hernandez Pichardo, Francesco Amadeo, Bettina Wilm, Raphaël Lévy, Lorenzo Ressel, Patricia Murray et al.
International Journal of Molecular Sciences
-
Impaired mitochondrial oxidative metabolism in skeletal progenitor cells leads to musculoskeletal disintegration
Authors: C Lin, Q Yang, D Guo, J Xie, YS Yang, S Chaugule, N DeSouza, WT Oh, R Li, Z Chen, AA John, Q Qiu, LJ Zhu, MB Greenblatt, S Ghosh, S Li, G Gao, C Haynes, CP Emerson, JH Shim
Nature Communications, 2022-11-11;13(1):6869.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Identification of hematopoietic stem cells residing in the meninges of adult mice at steady state
Authors: C Niu, J Yu, T Zou, Y Lu, L Deng, H Yun, CY Si, X Wu, H Jiang, T Guo, M Wu, T Kan, J Feng, C Yuan, X Yang, Q Cheng, J Dong, Q Wang, J Zhang
Cell Reports, 2022-11-08;41(6):111592.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Synaptic pruning through glial synapse engulfment upon motor learning
Authors: YM Morizawa, M Matsumoto, Y Nakashima, N Endo, T Aida, H Ishikane, K Beppu, S Moritoh, H Inada, N Osumi, E Shigetomi, S Koizumi, G Yang, H Hirai, K Tanaka, KF Tanaka, N Ohno, Y Fukazawa, K Matsui
Nature Neuroscience, 2022-11-01;25(11):1458-1469.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Ninjurin1 Deletion in NG2-Positive Pericytes Prevents Microvessel Maturation and Delays Wound Healing
Authors: Risa Matsuo, Mari Kishibe, Kiwamu Horiuchi, Kohei Kano, Takamitsu Tatsukawa, Taiki Hayasaka et al.
JID Innovations
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Continuous sensing of IFNalpha by hepatic endothelial cells shapes a vascular antimetastatic barrier
Authors: NL Tran, LM Ferreira, B Alvarez-Mo, V Buttiglion, B Ferrini, P Zordan, A Monestirol, C Fagioli, E Bezzecchi, GM Scotti, A Esposito, R Leone, C Gnasso, A Brendolan, LG Guidotti, G Sitia
Elife, 2022-10-25;11(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Copper promotes cardiac functional recovery via suppressing the transformation of fibroblasts to myofibroblasts in ischemia-infarcted monkey hearts
Authors: Y Xiao, Q Feng, L Huang, X Meng, P Han, W Zhang, YJ Kang
The Journal of nutritional biochemistry, 2022-10-12;0(0):109180.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Single-cell spatial transcriptomics reveals a dynamic control of metabolic zonation and liver regeneration by endothelial cell Wnt2 and Wnt9b
Authors: Shikai Hu, Silvia Liu, Yu Bian, Minakshi Poddar, Sucha Singh, Catherine Cao et al.
Cell Reports Medicine
-
Investigating the role of receptor interacting protein kinase 3 in venous thrombosis
Authors: Elise DeRoo, Mitri Khoury, Ting Zhou, Huan Yang, Amelia Stranz, Catherine Luke et al.
JVS-Vascular Science
-
Multiscale profiling of protease activity in cancer
Authors: AP Amini, JD Kirkpatric, CS Wang, AM Jaeger, S Su, S Naranjo, Q Zhong, CM Cabana, T Jacks, SN Bhatia
Nature Communications, 2022-10-03;13(1):5745.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A single intravenous injection of cyclosporin A-loaded lipid nanocapsules prevents retinopathy of prematurity
Authors: M Bohley, AE Dillinger, F Schweda, A Ohlmann, BM Braunger, ER Tamm, A Goepferich
Science Advances, 2022-09-23;8(38):eabo6638.
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Expanding tubular microvessels on stiff substrates with endothelial cells and pericytes from the same adult tissue
Authors: Xiuyue Song, Yali Yu, Yu Leng, Lei Ma, Jie Mu, Zihan Wang et al.
J Tissue Eng
Species: Rat
Sample Types: Whole Cells
Applications: Flow Cytometry, Immunocytochemistry -
Single-cell RNA sequencing reveals dysregulation of spinal cord cell types in a severe spinal muscular atrophy mouse model
Authors: J Sun, J Qiu, Q Yang, Q Ju, R Qu, X Wang, L Wu, L Xing
PloS Genetics, 2022-09-08;18(9):e1010392.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC fixed frozen -
Regulator of G-protein signaling 1 promotes choroidal neovascularization in age-related macular degeneration
Authors: Qi Zhang, Fengbin Zhang, Yangchen Guo, Yanyan Liu, Ningxin Pan, Hong Chen et al.
Annals of Translational Medicine
-
Biofabricated macrophage and fibroblast membranes synergistically promote skin wound healing
Authors: Dongqing Wang, Heying Chen, Li Lei, Jun Chen, Jimin Gao, Jiahe Liu et al.
Bioengineering & Translational Medicine
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
The antimicrobial peptide cathelicidin drives development of experimental autoimmune encephalomyelitis in mice by affecting Th17 differentiation
Authors: KJ Smith, D Minns, BJ McHugh, RK Holloway, R O'Connor, A Williams, L Melrose, R McPherson, VE Miron, DJ Davidson, E Gwyer Find
PloS Biology, 2022-08-26;20(8):e3001554.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Pazopanib Is a Potential Treatment for Coronavirus-Induced Lung Injuries
Authors: Yi Luan, Qianying Yuan, Qijun Wang, Susan Compton, Dianqing Wu, Wenwen Tang
The Journal of Immunology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Sourcing of human peripheral blood-derived myeloid angiogenic cells under xeno-free conditions for the treatment of critical limb ischemia
Authors: Christy Wing Tung Wong, Apurva Sawhney, Yalan Wu, Yi Wah Mak, Xiao Yu Tian, Hon Fai Chan et al.
Stem Cell Research & Therapy
-
Polyvinyl alcohol coating prevents platelet adsorption and improves mechanical property of polycaprolactone-based small-caliber vascular graft
Authors: Naohiro Wakabayashi, Takumi Yoshida, Kyohei Oyama, Daisuke Naruse, Masahiro Tsutsui, Yuta Kikuchi et al.
Frontiers in Cardiovascular Medicine
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Macrophages in close proximity to the vitreoretinal interface are potential biomarkers of inflammation during retinal vascular disease
Authors: A Rajesh, S Droho, JA Lavine
Journal of Neuroinflammation, 2022-08-08;19(1):203.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Temporal alteration of microglia to microinfarcts in rat brain induced by the vascular occlusion with fluorescent microspheres
Authors: Y Shen, J Cui, S Zhang, Y Wang, J Wang, Y Su, D Xu, Y Liu, Y Guo, W Bai
Frontiers in Cellular Neuroscience, 2022-08-03;16(0):956342.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Brown-fat-mediated tumour suppression by cold-altered global metabolism
Authors: T Seki, Y Yang, X Sun, S Lim, S Xie, Z Guo, W Xiong, M Kuroda, H Sakaue, K Hosaka, X Jing, M Yoshihara, L Qu, X Li, Y Chen, Y Cao
Nature, 2022-08-03;608(7922):421-428.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
CNS Organoid Surpasses Cell-Laden Microgel Assembly to Promote Spinal Cord Injury Repair
Authors: Zitian Wang, Haoran Zhao, Xiaowei Tang, Tianyu Meng, Davit Khutsishvili, Bing Xu et al.
Research (Wash D C)
-
Conserved meningeal lymphatic drainage circuits in mice and humans
Authors: Laurent Jacob, Jose de Brito Neto, Stephanie Lenck, Celine Corcy, Farhat Benbelkacem, Luiz Henrique Geraldo et al.
Journal of Experimental Medicine
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Claudin5 protects the peripheral endothelial barrier in an organ and vessel-type-specific manner
Authors: Mark Richards, Emmanuel Nwadozi, Sagnik Pal, Pernilla Martinsson, Mika Kaakinen, Marleen Gloger et al.
eLife
-
Direct reprogramming of adult adipose-derived regenerative cells toward cardiomyocytes using six transcriptional factors
Authors: Shingo Narita, Kazumasa Unno, Katsuhiro Kato, Yusuke Okuno, Yoshitaka Sato, Yusuke Tsumura et al.
iScience
-
Unbalanced Regulation of Sec22b and Ykt6 Blocks Autophagosome Axonal Retrograde Flux in Neuronal Ischemia–Reperfusion Injury
Authors: Haiying Li, Xiang Li, Zhongmou Xu, Jinxin Lu, Chang Cao, Wanchun You et al.
The Journal of Neuroscience
-
DOCK4 Regulation of Rho GTPases Mediates Pulmonary Vascular Barrier Function
Authors: Pascal Yazbeck, Xavier Cullere, Paul Bennett, Vijay Yajnik, Huan Wang, Kenji Kawada et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Transcriptional profiling reveals roles of intercellular Fgf9 signaling in astrocyte maturation and synaptic refinement during brainstem development
Authors: AN Brandebura, DR Kolson, EM Amick, J Ramadan, MC Kersting, RH Nichol, PS Holcomb, PH Mathers, P Stoilov, GA Spirou
The Journal of Biological Chemistry, 2022-06-23;0(0):102176.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Aquaporin 4 Depolarization-Enhanced Transferrin Infiltration Leads to Neuronal Ferroptosis after Subarachnoid Hemorrhage in Mice
Authors: Y Liu, Z Wang, C Cao, Z Xu, J Lu, H Shen, X Li, H Li, J Wu, G Chen
Oncogene, 2022-06-17;2022(0):8808677.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The myosin II inhibitor, blebbistatin, ameliorates pulmonary endothelial barrier dysfunction in acute lung injury inducedB19 by LPS via NMMHC IIA/Wnt5a/beta-catenin pathway
Authors: J Zhang, Z Pan, J Zhou, L Zhang, J Tang, S Gong, F Li, B Yu, Y Zhang, J Kou
Oncogene, 2022-06-15;0(0):116132.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Novel App knock-in mouse model shows key features of amyloid pathology and reveals profound metabolic dysregulation of microglia
Authors: D Xia, S Lianoglou, T Sandmann, M Calvert, JH Suh, E Thomsen, J Dugas, ME Pizzo, SL DeVos, TK Earr, CC Lin, S Davis, C Ha, AW Leung, H Nguyen, R Chau, E Yulyanings, I Lopez, H Solanoy, ST Masoud, CC Liang, K Lin, G Astarita, N Khoury, JY Zuchero, RG Thorne, K Shen, S Miller, JJ Palop, D Garceau, M Sasner, JD Whitesell, JA Harris, S Hummel, J Gnörich, K Wind, L Kunze, A Zatcepin, M Brendel, M Willem, C Haass, D Barnett, TS Zimmer, AG Orr, K Scearce-Le, JW Lewcock, G Di Paolo, PE Sanchez
Molecular Neurodegeneration, 2022-06-11;17(1):41.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Analysis of the expression, function and signaling of glycogen phosphorylase isoforms in hepatocellular carcinoma
Authors: L Jiang, S Liu, T Deng, Y Yang, Y Zhang
Oncology Letters, 2022-06-07;24(2):244.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Combination of Radiofrequency Ablation With Resiquimod to Treat Hepatocellular Carcinoma Via Inflammation of Tumor Immune Microenvironment and Suppression of Angiogenesis
Authors: Zhou Tian, Baojian Hong, Jianzhong Chen, Zhe Tang
Frontiers in Oncology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Organization of sympathetic innervation of interscapular brown adipose tissue in the mouse
Authors: Clara Huesing, Rui Zhang, Sanjeev Gummadi, Nathan Lee, Emily Qualls‐Creekmore, Sangho Yu et al.
Journal of Comparative Neurology
-
Ciliary IFT88 Protects Coordinated Adolescent Growth Plate Ossification From Disruptive Physiological Mechanical Forces
Authors: Clarissa R Coveney, Hasmik J Samvelyan, Jadwiga Miotla‐Zarebska, Josephine Carnegie, Emer Chang, C Jonty Corrin et al.
Journal of Bone and Mineral Research
-
VCAM-1–targeted MRI Improves Detection of the Tumor-brain Interface
Authors: Vinton W.T. Cheng, Nicholas de Pennington, Rasheed Zakaria, James R. Larkin, Sébastien Serres, Manjima Sarkar et al.
Clinical Cancer Research
-
Diabetes impairs cardioprotective function of endothelial progenitor cell-derived extracellular vesicles via H3K9Ac inhibition
Authors: G Huang, Z Cheng, A Hildebrand, C Wang, M Cimini, R Roy, AM Lucchese, C Benedict, V Mallaredy, A Magadum, D Joladarash, C Thej, C Gonzalez, M Trungcao, VNS Garikipati, JW Elrod, WJ Koch, R Kishore
Theranostics, 2022-05-21;12(9):4415-4430.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A high-efficiency AAV for endothelial cell transduction throughout the central nervous system
Authors: Trevor Krolak, Ken Y. Chan, Luke Kaplan, Qin Huang, Jason Wu, Qingxia Zheng et al.
Nature Cardiovascular Research
-
RhoBTB1 reverses established arterial stiffness in angiotensin-II hypertension by promoting actin depolymerization
Authors: S Fang, J Wu, JJ Reho, KT Lu, DT Brozoski, G Kumar, AM Werthman, SD Silva, PC Muskus Vei, KK Wackman, AJ Mathison, BQ Teng, CW Lin, FW Quelle, CD Sigmund
JCI Insight, 2022-05-09;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IF/IHC -
The transcription factor complex LMO2/TAL1 regulates branching and endothelial cell migration in sprouting angiogenesis
Authors: Y Yamada, Y Zhong, S Miki, A Taura, TH Rabbitts
Scientific Reports, 2022-05-04;12(1):7226.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Caspase-8 in endothelial cells maintains gut homeostasis and prevents small bowel inflammation in mice
Authors: N Tisch, C Mogler, A Stojanovic, R Luck, EA Korhonen, A Ellerkmann, H Adler, M Singhal, G Schermann, L Erkert, JV Patankar, A Karakatsan, AL Scherr, Y Fuchs, A Cerwenka, S Wirtz, BC Köhler, HG Augustin, C Becker, T Schmidt, C Ruiz de Al
Embo Molecular Medicine, 2022-05-02;0(0):e14121.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Fiber‐Optic Theranostics (FOT): Interstitial Fiber‐Optic Needles for Cancer Sensing and Therapy
Authors: Yang Ran, Zhiyuan Xu, Minfeng Chen, Wei Wang, Yang Wu, Jiexuan Cai et al.
Advanced Science
-
Gene therapy with Pellino-1 improves perfusion and decreases tissue loss in Flk-1 heterozygous mice but fails in MAPKAP Kinase-2 knockout murine hind limb ischemia model
Authors: Mahesh Thirunavukkarasu, Seetur R. Pradeep, Gopi Ukani, Salim Abunnaja, Mark Youssef, Diego Accorsi et al.
Microvascular Research
-
Pericyte-to-endothelial cell signaling via vitronectin-integrin regulates blood-CNS barrier
Authors: Swathi Ayloo, Christopher Gallego Lazo, Shenghuan Sun, Wei Zhang, Bianxiao Cui, Chenghua Gu
Neuron
-
Environmental eustress improves postinfarction cardiac repair via enhancing cardiac macrophage survival
Authors: PY Bai, SQ Chen, DL Jia, LH Pan, CB Liu, J Liu, W Luo, Y Yang, MY Sun, NF Wan, WW Rong, AJ Sun, JB Ge
Science Advances, 2022-04-27;8(17):eabm3436.
Species: Mouse
Sample Types: Whole Tissue
Applications: Western Blot -
Vascular derived endothelin receptor A controls endothelin-induced retinal ganglion cell death
Authors: Olivia J. Marola, Gareth R. Howell, Richard T. Libby
Cell Death Discovery
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
SU16f inhibits fibrotic scar formation and facilitates axon regeneration and locomotor function recovery after spinal cord injury by blocking the PDGFRbeta pathway
Authors: Z Li, S Yu, Y Liu, X Hu, Y Li, Z Xiao, Y Chen, D Tian, X Xu, L Cheng, M Zheng, J Jing
Journal of Neuroinflammation, 2022-04-16;19(1):95.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
2-Methoxyestradiol Inhibits Radiation-Induced Skin Injuries
Authors: JH Kim, JK Nam, AR Kim, MS Park, HJ Lee, J Park, J Kim, YJ Lee
International Journal of Molecular Sciences, 2022-04-10;23(8):.
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Quantifying Tumor Heterogeneity via MRI Habitats to Characterize Microenvironmental Alterations in HER2+ Breast Cancer
Authors: AS Kazerouni, DA Hormuth, T Davis, MJ Bloom, S Mounho, G Rahman, J Virostko, TE Yankeelov, AG Sorace
Cancers, 2022-04-06;14(7):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Rebuilding hippocampus neural circuit with hADSC-derived neuron cells for treating ischemic stroke
Authors: J Wang, R Hao, T Jiang, X Guo, F Zhou, L Cao, F Gao, G Wang, J Wang, K Ning, C Zhong, X Chen, Y Huang, J Xu, S Gao
Cell & bioscience, 2022-04-04;12(1):40.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A novel mechanism for A-to-I RNA-edited AZIN1 in promoting tumor angiogenesis in colorectal cancer
Authors: Y Wei, H Zhang, Q Feng, S Wang, Y Shao, J Wu, G Jin, W Lin, X Peng, X Xu
Cell Death & Disease, 2022-04-02;13(4):294.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Tibial cortex transverse transport accelerates wound healing via enhanced angiogenesis and immunomodulation
Authors: Yongkang Yang, Yucong Li, Qi Pan, Shanshan Bai, Haixing Wang, Xiao-hua Pan et al.
Bone & Joint Research
-
Melatonin exerts anti-angiogenic and anti-inflammatory effects in alkali-burned corneas
Authors: Meng J, Lin B, Huang S et al.
Annals of Translational Medicine
-
Orchestration of energy metabolism and osteogenesis by Mg2+ facilitates low-dose BMP-2-driven regeneration
Authors: S Lin, S Yin, J Shi, G Yang, X Wen, W Zhang, M Zhou, X Jiang
Bioactive materials, 2022-03-24;18(0):116-127.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Endothelial Transient Receptor Potential Canonical Channel Regulates Angiogenesis and Promotes Recovery After Myocardial Infarction
Authors: X Wen, Y Peng, M Gao, Y Zhu, Y Zhu, F Yu, T Zhou, J Shao, L Feng, X Ma
Journal of the American Heart Association, 2022-03-05;11(6):e023678.
Species: Mouse
Sample Types: Whole Tissue
Applications: ICC/IF -
MicroRNA-140-5p exacerbates vascular cognitive impairment by inhibiting neurogenesis in the adult mouse hippocampus after global cerebral ischemia
Authors: HB Liang, ZH Lai, XQ Tu, KQ Ding, JR He, GY Yang, H Sheng, LL Zeng
Brain research bulletin, 2022-03-04;183(0):73-83.
Species: Mouse
Sample Types: Whole Tissues
Applications: IHC -
Ionizing Radiation-Induced GDF15 Promotes Angiogenesis in Human Glioblastoma Models by Promoting VEGFA Expression Through p-MAPK1/SP1 Signaling
Authors: Hyejin Park, Ki-Seok Nam, Hae-June Lee, Kwang Seok Kim
Frontiers in Oncology
-
Transcranial focused ultrasound stimulation reduces vasogenic edema after middle cerebral artery occlusion in mice
Authors: Guo-Yuan Yang, Ji-Xian Wang, Li-Dong Deng, Sheng-Ju Qi, Muyassar Mamtilahun, Sheng-Ju Shi et al.
Neural Regeneration Research
-
Urolithin A alleviates blood-brain barrier disruption and attenuates neuronal apoptosis following traumatic brain injury in mice
Authors: Qiu-Yuan Chen, Heng-Li Tian, Qiu-Yuan Gong, Lin Wang, Dian-Xu Jing, Shi-Wen Wang et al.
Neural Regeneration Research
-
Cepharanthine Attenuates Early Brain Injury after Subarachnoid Hemorrhage in Mice via Inhibiting 15-Lipoxygenase-1-Mediated Microglia and Endothelial Cell Ferroptosis
Authors: S Gao, L Zhou, J Lu, Y Fang, H Wu, W Xu, Y Pan, J Wang, X Wang, J Zhang, A Shao
Oxidative Medicine and Cellular Longevity, 2022-02-09;2022(0):4295208.
Species: Mouse
Sample Types: Whole Tissue
Applications: IF -
ASK1 signaling regulates phase-specific glial interactions during neuroinflammation
Authors: Xiaoli Guo, Atsuko Kimura, Kazuhiko Namekata, Chikako Harada, Nobutaka Arai, Kohsuke Takeda et al.
Proceedings of the National Academy of Sciences
-
Engineered Lung Tissues Prepared from Decellularized Lung Slices
Authors: Katherine L. Leiby, Ronald Ng, Stuart G. Campbell, Laura E. Niklason
Journal of Visualized Experiments
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Customized blood-brain barrier shuttle peptide to increase AAV9 vector crossing the BBB and augment transduction in the brain
Authors: Xintao Zhang, Zheng Chai, Amanda Lee Dobbins, Michelle S. Itano, Charles Askew, Zhe Miao et al.
Biomaterials
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Normalisation of glucose metabolism by exendin‐4 in the chronic phase after stroke promotes functional recovery in male diabetic mice
Authors: Ingrid Lovise Augestad, Doortje Dekens, Dimitra Karampatsi, Osama Elabi, Alexander Zabala, Hiranya Pintana et al.
British Journal of Pharmacology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Soluble Endoglin Stimulates Inflammatory and Angiogenic Responses in Microglia That Are Associated with Endothelial Dysfunction
Authors: ES Park, S Kim, DC Yao, JPJ Savarraj, HA Choi, PR Chen, E Kim
International Journal of Molecular Sciences, 2022-01-22;23(3):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Comprehensive Expression Analysis of Cardiac Fibroblast Growth Factor 23 in Health and Pressure-induced Cardiac Hypertrophy
Authors: Fiona Eitner, Beatrice Richter, Saskia Schwänen, Malgorzata Szaroszyk, Isabel Vogt, Andrea Grund et al.
Frontiers in Cell and Developmental Biology
-
Enhanced proton treatment with a LDLR-ligand peptide-conjugated gold nanoparticles targeting the tumor microenvironment in an infiltrative brain tumor model
Authors: S Seo, EH Kim, WS Chang, WS Lee, KH Kim, JK Kim
American journal of cancer research, 2022-01-15;12(1):198-209.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Increased expression of osteopontin in subchondral bone promotes bone turnover and remodeling, and accelerates the progression of OA in a mouse model
Authors: Chuangxin Lin, Zhong Chen, Dong Guo, Laixi Zhou, Sipeng Lin, Changchuan Li et al.
Aging (Albany NY)
-
Myelinating Schwann cells and Netrin-1 control intra-nervous vascularization of the developing mouse sciatic nerve
Authors: Sonia Taïb, Noël Lamandé, Sabrina Martin, Fanny Coulpier, Piotr Topilko, Isabelle Brunet
eLife
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Stable flow-induced expression of KLK10 inhibits endothelial inflammation and atherosclerosis
Authors: Darian Williams, Marwa Mahmoud, Renfa Liu, Aitor Andueza, Sandeep Kumar, Dong-Won Kang et al.
eLife
-
Microglial replacement in the aged brain restricts neuroinflammation following intracerebral hemorrhage
Authors: X Li, X Gao, W Zhang, M Liu, Z Han, M Li, P Lei, Q Liu
Cell Death & Disease, 2022-01-10;13(1):33.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
M1 macrophages impair tight junctions between endothelial cells after spinal cord injury
Authors: Y Luo, F Yao, X Hu, Y Li, Y Chen, Z Li, Z Zhu, S Yu, D Tian, L Cheng, M Zheng, J Jing
Brain research bulletin, 2022-01-04;0(0):.
Species: Mouse
Sample Types: Whole Tissues
Applications: IHC -
3D-printed oxygen-releasing scaffolds improve bone regeneration in mice
Authors: Ashley L. Farris, Dennis Lambrechts, Yuxiao Zhou, Nicholas Y. Zhang, Naboneeta Sarkar, Megan C. Moorer et al.
Biomaterials
-
Vascular endothelial growth factor-A/vascular endothelial growth factor2 signaling in spinal neurons contributes to bone cancer pain
Authors: LJ Fan, HM Kan, XT Chen, YY Sun, LP Chen, W Shen
Molecular pain, 2022-01-01;18(0):1744806922107.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Implantable Electrical Stimulation at Dorsal Root Ganglions Accelerates Osteoporotic Fracture Healing via Calcitonin Gene‐Related Peptide
Authors: Jie Mi, Jian‐Kun Xu, Zhi Yao, Hao Yao, Ye Li, Xuan He et al.
Advanced Science
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Minocycline improves the functional recovery after traumatic brain injury via inhibition of aquaporin-4
Authors: Q Lu, J Xiong, Y Yuan, Z Ruan, Y Zhang, B Chai, L Li, S Cai, J Xiao, Y Wu, P Huang, H Zhang
International journal of biological sciences, 2022-01-01;18(1):441-458.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Inhibition of LncRNA Vof-16 expression promotes nerve regeneration and functional recovery after spinal cord injury
Authors: XM Zhang, LN Zeng, WY Yang, L Ding, KZ Chen, WJ Fu, SQ Zeng, YR Liang, GH Chen, HF Wu
Neural regeneration research, 2022-01-01;17(1):217-227.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Microglia regulate central nervous system myelin growth and integrity
Authors: NB McNamara, DAD Munro, N Bestard-Cu, A Uyeda, JFJ Bogie, A Hoffmann, RK Holloway, I Molina-Gon, KE Askew, S Mitchell, W Mungall, M Dodds, C Dittmayer, J Moss, J Rose, S Szymkowiak, L Amann, BW McColl, M Prinz, TL Spires-Jon, W Stenzel, K Horsburgh, JJA Hendriks, C Pridans, R Muramatsu, A Williams, J Priller, VE Miron
Nature, 2022;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
TREM2-independent oligodendrocyte, astrocyte, and T�cell responses to tau and amyloid pathology in mouse models of Alzheimer disease
Authors: SH Lee, MG Rezzonico, BA Friedman, MH Huntley, WJ Meilandt, S Pandey, YJ Chen, A Easton, Z Modrusan, DV Hansen, M Sheng, CJ Bohlen
Cell Reports, 2021-12-28;37(13):110158.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
E3 ubiquitin ligase mind bomb 1 overexpression reduces apoptosis and inflammation of cardiac microvascular endothelial cells in coronary microvascular dysfunction
Authors: XF Qin, YG Shan, JH Gao, FX Li, YX Guo
Cellular Signalling, 2021-12-23;91(0):110223.
Species: Rat
Sample Types: Whole Cells, Whole Tissue
Applications: Flow Cytometry, IHC -
The onset of circulation triggers a metabolic switch required for endothelial to hematopoietic transition
Authors: E Azzoni, V Frontera, G Anselmi, C Rode, C James, EM Deltcheva, AS Demian, J Brown, C Barone, A Patelli, JR Harman, M Nicholls, SJ Conway, E Morrissey, SEW Jacobsen, DB Sparrow, AL Harris, T Enver, MFTR de Bruijn
Cell Reports, 2021-12-14;37(11):110103.
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
AAV vectors accumulate in the pineal gland after injections into the brain or spinal cord
Authors: Oswald Steward, Aminata P. Coulibaly, Mariajose Metcalfe, Jamie M. Dam, Kelly M. Yee
Molecular Therapy - Methods & Clinical Development
-
Co‐delivery of fibrin‐laminin hydrogel with mesenchymal stem cell spheroids supports skeletal muscle regeneration following trauma
Authors: Peter Genovese, Anjali Patel, Natalia Ziemkiewicz, Allison Paoli, Joseph Bruns, Natasha Case et al.
Journal of Tissue Engineering and Regenerative Medicine
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Lin11-Isl1-Mec3 Domain Proteins as Mechanotransducers in Endothelial and Vascular Smooth Muscle Cells
Authors: Alexandra Sporkova, Subhajit Ghosh, Jaafar Al-Hasani, Markus Hecker
Frontiers in Physiology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Chronic Low-Dose Alcohol Consumption Promotes Cerebral Angiogenesis in Mice
Authors: Jiyu Li, Chun Li, Ethyn G. Loreno, Sumitra Miriyala, Manikandan Panchatcharam, Xiaohong Lu et al.
Frontiers in Cardiovascular Medicine
-
Lef1 restricts ectopic crypt formation and tumor cell growth in intestinal adenomas
Authors: S Heino, S Fang, M Lähde, J Högström, S Nassiri, A Campbell, D Flanagan, A Raven, M Hodder, N Nasreddin, HH Xue, M Delorenzi, S Leedham, TV Petrova, O Sansom, K Alitalo
Science Advances, 2021-11-17;7(47):eabj0512.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Adult spiny mice (Acomys) exhibit endogenous cardiac recovery in response to myocardial infarction
Authors: Hsuan Peng, Kazuhiro Shindo, Renée R. Donahue, Erhe Gao, Brooke M. Ahern, Bryana M. Levitan et al.
npj Regenerative Medicine
-
Combined whole-organ imaging at single-cell resolution and immunohistochemical analysis of prostate cancer and its liver and brain metastases
Authors: J Taranda, G Mathew, K Watrud, N El-Amine, MF Lee, C Elowsky, A Bludova, S Escobar Av, DG Nowak, TL Wee, JE Wilkinson, LC Trotman, P Osten
Cell Reports, 2021-11-16;37(7):110027.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Development of Surgical and Visualization Procedures to Analyze Vasculatures by Mouse Tail Edema Model
Authors: Shinji Kumegawa, Gen Yamada, Daiki Hashimoto, Tsuyoshi Hirashima, Mizuki Kajimoto, Kyoichi Isono et al.
Biological Procedures Online
-
Megalencephalic leukoencephalopathy with subcortical cysts is a developmental disorder of the gliovascular unit
Authors: Alice Gilbert, Xabier Elorza-Vidal, Armelle Rancillac, Audrey Chagnot, Mervé Yetim, Vincent Hingot et al.
eLife
-
Biomimetic sponges improve muscle structure and function following volumetric muscle loss
Authors: Gabriel Haas, Andrew Dunn, Josh Madsen, Peter Genovese, Hannah Chauvin, Jeffrey Au et al.
Journal of Biomedical Materials Research Part A
-
Cdc42 activity in Sertoli cells is essential for maintenance of spermatogenesis
Authors: A Heinrich, B Bhandary, SJ Potter, N Ratner, T DeFalco
Cell Reports, 2021-10-26;37(4):109885.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Monomeric C-reactive protein via endothelial CD31 for neurovascular inflammation in an ApoE genotype-dependent pattern: A risk factor for Alzheimer's disease?
Authors: Z Zhang, H Na, Q Gan, Q Tao, Y Alekseyev, J Hu, Z Yan, JB Yang, H Tian, S Zhu, Q Li, IM Rajab, JK Blusztajn, B Wolozin, A Emili, X Zhang, T Stein, LA Potempa, WQ Qiu
Aging Cell, 2021-10-23;0(0):e13501.
Species: Mouse
Sample Types: Tissue Lysates, Whole Cells
Applications: Functional Assay, Western Blot -
Radiation-Induced Fibrotic Tumor Microenvironment Regulates Anti-Tumor Immune Response
Authors: JK Nam, JH Kim, MS Park, EH Kim, J Kim, YJ Lee
Cancers, 2021-10-19;13(20):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Cellular crosstalk regulates the aqueous humor outflow pathway and provides new targets for glaucoma therapies
Authors: BR Thomson, P Liu, T Onay, J Du, SW Tompson, S Misener, RR Purohit, TL Young, J Jin, SE Quaggin
Nature Communications, 2021-10-18;12(1):6072.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Histochrome Attenuates Myocardial Ischemia-Reperfusion Injury by Inhibiting Ferroptosis-Induced Cardiomyocyte Death
Authors: Ji-Won Hwang, Jae-Hyun Park, Bong-Woo Park, Hyeok Kim, Jin-Ju Kim, Woo-Sup Sim et al.
Antioxidants (Basel)
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Differential Capability of Clinically Employed Dermal Regeneration Scaffolds to Support Vascularization for Tissue Bioengineering
Authors: C Agostinis, M Spazzapan, R Vuerich, A Balduit, C Stocco, A Mangogna, G Ricci, G Papa, S Zacchigna, R Bulla
Biomedicines, 2021-10-13;9(10):.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Targeting neurons in the tumor microenvironment with bupivacaine nanoparticles reduces breast cancer progression and metastases
Authors: M Kaduri, M Sela, S Kagan, M Poley, H Abumanhal-, P Mora-Raimu, A Ouro, N Dahan, D Hershkovit, J Shklover, J Shainsky-R, Y Buganim, A Schroeder
Science Advances, 2021-10-06;7(41):eabj5435.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The mechanistic target of rapamycin complex 1 critically regulates the function of mononuclear phagocytes and promotes cardiac remodeling in acute ischemia
Authors: GuiHao Chen, Vincent Phan, Xiang Luo, Dian J. Cao
Journal of Molecular and Cellular Cardiology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
A Protective Role of Tumor Necrosis Factor Superfamily-15 in Intracerebral Hemorrhage-Induced Secondary Brain Injury
Authors: Gui-Li Yang, Shizhao Wang, Shu Zhang, Ye Liu, Xiao Liu, Dong Wang et al.
ASN Neuro
-
Expression and related mechanisms of miR-330-3p and S100B in an animal model of cartilage injury
Authors: Wenming Wu, Dongming Liang
J Int Med Res
-
Receptor‑selective interleukin‑4 mutein attenuates laser‑induced choroidal neovascularization through the regulation of macrophage polarization in mice
Authors: Limo Gao, Wenmin Jiang, Haiying Liu, Zhiheng Chen, Yanhui Lin
Experimental and Therapeutic Medicine
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Exercise-induced angiogenesis is dependent on metabolically primed ATF3/4+ endothelial cells
Authors: Zheng Fan, Guillermo Turiel, Raphaela Ardicoglu, Moheb Ghobrial, Evi Masschelein, Tea Kocijan et al.
Cell Metabolism
-
Moringa Oleifera Seed Extract Concomitantly Supplemented with Chemotherapy Worsens Tumor Progression in Mice with Triple Negative Breast Cancer and Obesity
Authors: ERM Zunica, S Yang, A Coulter, C White, JP Kirwan, LA Gilmore
Nutrients, 2021-08-24;13(9):.
Species: Xenograft
Sample Types: Whole Tissue
Applications: IHC -
Fetal hematopoietic stem cell homing is controlled by VEGF regulating the integrity and oxidative status of the stromal-vascular bone marrow niches
Authors: M Mesnieres, AM Böhm, N Peredo, D Trompet, R Valle-Tenn, M Bajaj, N Corthout, E Nefyodova, R Cardoen, P Baatsen, S Munck, A Nagy, JJ Haigh, S Khurana, CM Verfaillie, C Maes
Cell Reports, 2021-08-24;36(8):109618.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The angiopoietin-Tie2 pathway regulates Purkinje cell dendritic morphogenesis in a cell-autonomous manner
Authors: R Luck, A Karakatsan, B Shah, G Schermann, H Adler, J Kupke, N Tisch, HW Jeong, MK Back, F Hetsch, A D'Errico, M De Palma, E Wiedtke, D Grimm, A Acker-Palm, J von Engelh, RH Adams, HG Augustin, C Ruiz de Al
Cell Reports, 2021-08-17;36(7):109522.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Efficacy and tolerability of Sorafenib plus metronomic chemotherapy S-1 for advanced hepatocellular carcinoma in preclinical and clinical assessments
Authors: H Suzuki, H Iwamoto, M Nakano, T Nakamura, A Masuda, T Sakaue, T Tanaka, D Nakano, R Kuromatsu, T Niizeki, S Okamura, S Shimose, T Shirono, Y Noda, N Kamachi, H Yano, A Kawaguchi, H Koga, T Torimura
Translational Oncology, 2021-08-11;14(11):101201.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Specialized endothelial tip cells guide neuroretina vascularization and blood-retina-barrier formation
Authors: Georgia Zarkada, Joel P. Howard, Xue Xiao, Hyojin Park, Mathilde Bizou, Severine Leclerc et al.
Developmental Cell
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
B cells modulate the expression of MHC-II on cardiac CCR2− macrophages
Authors: Cibele Rocha-Resende, Fabiana Pani, Luigi Adamo
Journal of Molecular and Cellular Cardiology
-
Regional specialization and fate specification of bone stromal cells in skeletal development
Authors: KK Sivaraj, HW Jeong, B Dharmaling, D Zeuschner, S Adams, M Potente, RH Adams
Cell Reports, 2021-07-13;36(2):109352.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges
Authors: D Schafflick, J Wolbert, M Heming, C Thomas, M Hartlehner, AL Börsch, A Ricci, S Martín-Sal, X Li, IN Lu, M Pawlak, J Minnerup, JK Strecker, T Seidenbech, SG Meuth, A Hidalgo, A Liesz, H Wiendl, G Meyer Zu H
Nature Neuroscience, 2021-07-12;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A bivalent cyclic RGD–siRNA conjugate enhances the antitumor effect of apatinib via co-inhibiting VEGFR2 in non-small cell lung cancer xenografts
Authors: Lumin Liao, Bohong Cen, Guoxian Li, Yuanyi Wei, Zhen Wang, Wen Huang et al.
Drug Delivery
-
The Organization of the Sinoatrial Node Microvasculature Varies Regionally to Match Local Myocyte Excitability
Authors: Nathan Grainger, Laura Guarina, Robert H Cudmore, L Fernando Santana
Function (Oxf)
-
Intra-vessel heterogeneity establishes enhanced sites of macromolecular leakage downstream of laminin &alpha5
Authors: M Richards, S Pal, E Sjöberg, P Martinsson, L Venkatrama, L Claesson-W
Cell Reports, 2021-06-22;35(12):109268.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lack of APOL1 in proximal tubules of normal human kidneys and proteinuric APOL1 transgenic mouse kidneys
Authors: NA Blessing, Z Wu, SM Madhavan, JW Choy, M Chen, MK Shin, M Hoek, JR Sedor, JF O'Toole, LA Bruggeman
PLoS ONE, 2021-06-17;16(6):e0253197.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
VEGFR-1/Flt-1 inhibition increases angiogenesis and improves muscle function in a mouse model of Duchenne muscular dystrophy
Authors: Jennifer Bosco, Zhiwei Zhou, Sofie Gabriëls, Mayank Verma, Nan Liu, Brian K. Miller et al.
Molecular Therapy - Methods & Clinical Development
-
An antibody against L1 cell adhesion molecule inhibits cardiotoxicity by regulating persistent DNA damage
Authors: JK Nam, AR Kim, SH Choi, JH Kim, KJ Choi, S Cho, JW Lee, HJ Cho, YW Kwon, J Cho, KS Kim, J Kim, HJ Lee, TS Lee, S Bae, HJ Hong, YJ Lee
Nature Communications, 2021-06-02;12(1):3279.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Endothelial beta 1 Integrin-Mediated Adaptation to Myocardial Ischemia
Authors: Carina Henning, Anna Branopolski, Paula Follert, Oksana Lewandowska, Aysel Ayhan, Marcel Benkhoff et al.
Thrombosis and Haemostasis
-
Loss of Transforming Growth Factor Beta Signaling in Aortic Smooth Muscle Cells Causes Endothelial Dysfunction and Aortic Hypercontractility
Authors: Jay Zhu, Stoyan Angelov, Ilkay Alp Alp Yildirim, Hao Wei, Jie Hong Hu, Mark W. Majesky et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
An Exercise Mimetic Approach to Reduce Poststroke Deconditioning and Enhance Stroke Recovery
Authors: Matthew W. McDonald, Matthew S. Jeffers, Lama Issa, Anthony Carter, Allyson Ripley, Lydia M. Kuhl et al.
Neurorehabilitation and Neural Repair
-
m7G Methyltransferase METTL1 Promotes Post-ischemic Angiogenesis via Promoting VEGFA mRNA Translation
Authors: Yongchao Zhao, Lingqiu Kong, Zhiqiang Pei, Fuhai Li, Chaofu Li, Xiaolei Sun et al.
Frontiers in Cell and Developmental Biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Perivascular cell‐derived extracellular vesicles stimulate colorectal cancer revascularization after withdrawal of antiangiogenic drugs
Authors: Maohua Huang, Minfeng Chen, Ming Qi, Geni Ye, Jinghua Pan, Changzheng Shi et al.
Journal of Extracellular Vesicles
-
Serum-Derived Small Extracellular Vesicles From Diabetic Mice Impair Angiogenic Property of Microvascular Endothelial Cells: Role of EZH2
Authors: Z Cheng, V Naga Srika, MM Truongcao, M Cimini, G Huang, C Wang, C Benedict, C Gonzalez, V Mallaredy, DA Goukassian, SK Verma, R Kishore
Journal of the American Heart Association, 2021-05-14;10(10):e019755.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Disrupting tumour vasculature and recruitment of aPDL1-loaded platelets control tumour metastasis
Authors: H Li, Z Wang, Z Chen, T Ci, G Chen, D Wen, R Li, J Wang, H Meng, R Bryan Bell, Z Gu, G Dotti, Z Gu
Nature Communications, 2021-05-13;12(1):2773.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Flow Cytometry, IHC -
Optogenetic activation of local colonic sympathetic innervations attenuates colitis by limiting immune cell extravasation
Authors: Maya Schiller, Hilla Azulay-Debby, Nadia Boshnak, Yehezqel Elyahu, Ben Korin, Tamar L. Ben-Shaanan et al.
Immunity
-
Sympathetic innervation of inguinal white adipose tissue in the mouse
Authors: Clara Huesing, Emily Qualls‐Creekmore, Nathan Lee, Marie François, Hayden Torres, Rui Zhang et al.
Journal of Comparative Neurology
-
Overexpression of circRNA SNRK targets miR-103-3p to reduce apoptosis and promote cardiac repair through GSK3 beta / beta -catenin pathway in rats with myocardial infarction
Authors: Yeqian Zhu, Pengcheng Zhao, Ling Sun, Yao Lu, Wenwu Zhu, Jian Zhang et al.
Cell Death Discovery
-
Type 2 immunity induced by bladder extracellular matrix enhances corneal wound healing
Authors: X Wang, L Chung, J Hooks, DR Maestas, A Lebid, JI Andorko, L Huleihel, AF Chin, M Wolf, NT Remlinger, MA Stepp, F Housseau, JH Elisseeff
Science Advances, 2021-04-16;7(16):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Microglia use TAM receptors to detect and engulf amyloid &beta plaques
Authors: Y Huang, KE Happonen, PG Burrola, C O'Connor, N Hah, L Huang, A Nimmerjahn, G Lemke
Nature Immunology, 2021-04-15;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Donor cell memory confers a metastable state of directly converted cells
Authors: KP Kim, C Li, D Bunina, HW Jeong, J Ghelman, J Yoon, B Shin, H Park, DW Han, JB Zaugg, J Kim, T Kuhlmann, RH Adams, KM Noh, SA Goldman, HR Schöler
Cell Stem Cell, 2021-04-12;0(0):.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
3D reconstruction and histopathological analyses on murine corporal body
Authors: Daiki Hashimoto, Mizuki Kajimoto, Yuko Ueda, Taiju Hyuga, Kota Fujimoto, Saaya Inoue et al.
Reproductive Medicine and Biology
-
The bone microenvironment invigorates metastatic seeds for further dissemination
Authors: Weijie Zhang, Igor L. Bado, Jingyuan Hu, Ying-Wooi Wan, Ling Wu, Hai Wang et al.
Cell
-
Expression and enhancement of FABP4 in septoclasts of the growth plate in FABP5-deficient mouse tibiae
Authors: Yasuhiko Bando, Nobuko Tokuda, Yudai Ogasawara, Go Onozawa, Arata Nagasaka, Koji Sakiyama et al.
Histochemistry and Cell Biology
-
The Inflammatory Profile of CTEPH-Derived Endothelial Cells Is a Possible Driver of Disease Progression
Authors: VFED Smolders, K Lodder, C Rodríguez, O Tura-Ceide, JA Barberà, JW Jukema, PHA Quax, MJ Goumans, K Kurakula
Cells, 2021-03-26;10(4):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Regulatory Function of Sympathetic Innervation on the Endo/Lysosomal Trafficking of Acetylcholine Receptor
Authors: Tatjana Straka, Charlotte Schröder, Andreas Roos, Laxmikanth Kollipara, Albert Sickmann, Marion Patrick Ivey Williams et al.
Frontiers in Physiology
-
Synergistic inflammatory signaling by cGAS may be involved in the development of atherosclerosis
Authors: Guan-Feng Lu, Sheng-Cai Chen, Yuan-Peng Xia, Zi-Ming Ye, Fei Cao, Bo Hu
Aging (Albany NY)
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
An organoid-based organ-repurposing approach to treat short bowel syndrome
Authors: S Sugimoto, E Kobayashi, M Fujii, Y Ohta, K Arai, M Matano, K Ishikawa, K Miyamoto, K Toshimitsu, S Takahashi, K Nanki, Y Hakamata, T Kanai, T Sato
Nature, 2021-02-24;0(0):.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Early postnatal interactions between beige adipocytes and sympathetic neurites regulate innervation of subcutaneous fat
Authors: Jingyi Chi, Zeran Lin, William Barr, Audrey Crane, Xiphias Ge Zhu, Paul Cohen
eLife
-
Tumor cell plasticity, heterogeneity, and resistance in crucial microenvironmental niches in glioma
Authors: E Jung, M Osswald, M Ratliff, H Dogan, R Xie, S Weil, DC Hoffmann, FT Kurz, T Kessler, S Heiland, A von Deimli, F Sahm, W Wick, F Winkler
Nature Communications, 2021-02-12;12(1):1014.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Melatonin Promotes Heterotopic Ossification Through Regulation of Endothelial-Mesenchymal Transition in Injured Achilles Tendons in Rats
Authors: Jie Zhang, Jiajun Tang, Jie Liu, Bo Yan, Bin Yan, Minjun Huang et al.
Frontiers in Cell and Developmental Biology
-
Changes of blood-brain-barrier function and transfer of amyloid beta in rats with collagen-induced arthritis
Authors: PH Lai, TH Wang, NY Zhang, KC Wu, CJ Yao, CJ Lin
Journal of Neuroinflammation, 2021-01-30;18(1):35.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Single-cell transcriptomics following ischemic injury identifies a role for B2M in cardiac repair
Authors: Bas Molenaar, Louk T. Timmer, Marjolein Droog, Ilaria Perini, Danielle Versteeg, Lieneke Kooijman et al.
Communications Biology
-
CU06-1004-Induced Vascular Normalization Improves Immunotherapy by Modulating Tumor Microenvironment via Cytotoxic T Cells
Authors: Songyi Park, Ji Hoon Oh, Dong Jin Park, Haiying Zhang, Minyoung Noh, Yeomyung Kim et al.
Frontiers in Immunology
-
DL-3n-Butylphthalide Improves Blood-Brain Barrier Integrity in Rat After Middle Cerebral Artery Occlusion
Authors: M Mamtilahun, Z Wei, C Qin, Y Wang, Y Tang, FX Shen, HL Tian, Z Zhang, GY Yang
Frontiers in Cellular Neuroscience, 2021-01-12;14(0):610714.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Carbon nano-onion-mediated dual targeting of P-selectin and P-glycoprotein to overcome cancer drug resistance
Authors: H Wang, Y Liang, Y Yin, J Zhang, W Su, AM White, Bin Jiang, J Xu, Y Zhang, S Stewart, X Lu, X He
Nature Communications, 2021-01-12;12(1):312.
Species: Mouse
Sample Types: Whole Tissues
Applications: IHC -
Cardiomyocytes stimulate angiogenesis after ischemic injury in a ZEB2-dependent manner
Authors: MM Gladka, A Kohela, B Molenaar, D Versteeg, L Kooijman, J Monshouwer, V Kremer, HR Vos, MMH Huibers, JJ Haigh, D Huylebroec, RA Boon, M Giacca, E van Rooij
Nature Communications, 2021-01-04;12(1):84.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Apolipoprotein E, low-density lipoprotein receptor, and immune cells control blood-brain barrier penetration by AAV-PHP.eB in mice
Authors: BS Xie, X Wang, YH Pan, G Jiang, JF Feng, Y Lin
Theranostics, 2021-01-01;11(3):1177-1191.
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Osteoblasts derived from mouse mandible enhance tumor growth of prostate cancer more than osteoblasts derived from long bone
Authors: MR Eber, SH Park, KF Contino, CM Patel, FC Hsu, Y Shiozawa
Journal of Bone Oncology, 2020-12-30;26(0):100346.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
An engineered 4-1BBL fusion protein with “activity on demand”
Authors: Jacqueline Mock, Marco Stringhini, Alessandra Villa, Michael Weller, Tobias Weiss, Dario Neri
Proceedings of the National Academy of Sciences
-
Hypergravity as a gravitational therapy mitigates the effects of knee osteoarthritis on the musculoskeletal system in a murine model
Authors: B Dechaumet, D Cleret, MT Linossier, A Vanden-Bos, S Chanon, E Lefai, N Laroche, MH Lafage-Pro, L Vico
PLoS ONE, 2020-12-09;15(12):e0243098.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A rapid, nondestructive method for vascular network visualization
Authors: Austin Veith, Aaron B Baker
BioTechniques
-
Islet vascularization is regulated by primary endothelial cilia via VEGF-A-dependent signaling
Authors: Y Xiong, MJ Scerbo, A Seelig, F Volta, N O'Brien, A Dicker, D Padula, H Lickert, JM Gerdes, PO Berggren
Elife, 2020-11-17;9(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Intermedin Alleviates Renal Ischemia-Reperfusion Injury and Enhances Neovascularization in Wistar Rats
Authors: Y Wang, Y Mi, J Tian, X Qiao, X Su, J Kang, Z Wu, G Wang, X Zhou, Y Zhou, R Li
Drug Des Devel Ther, 2020-11-10;14(0):4825-4834.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
The endothelial protein C receptor plays an essential role in the maintenance of pregnancy
Authors: MM Castillo, Q Yang, AS Sigala, DT McKinney, M Zhan, KL Chen, JA Jarzembows, R Sood
Sci Adv, 2020-11-06;6(45):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Histone demethylase LSD1 is critical for endochondral ossification during bone fracture healing
Authors: J Sun, H Feng, W Xing, Y Han, J Suo, AR Yallowitz, N Qian, Y Shi, MB Greenblatt, W Zou
Sci Adv, 2020-11-04;6(45):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Tendon-derived cathepsin K–expressing progenitor cells activate Hedgehog signaling to drive heterotopic ossification
Authors: Heng Feng, Wenhui Xing, Yujiao Han, Jun Sun, Mingxiang Kong, Bo Gao et al.
Journal of Clinical Investigation
-
Apolipoprotein E isoforms differentially regulate matrix metallopeptidase 9 function in Alzheimer’s disease
Authors: Charis Ringland, Jonas Elias Schweig, Daniel Paris, Ben Shackleton, Cillian E. Lynch, Maxwell Eisenbaum et al.
Neurobiology of Aging
-
Intra-cisterna magna delivery of an AAV vector with the GLUT1 promoter in a pig recapitulates the physiological expression of SLC2A1
Authors: S Nakamura, H Osaka, SI Muramatsu, N Takino, M Ito, EF Jimbo, C Watanabe, S Hishikawa, T Nakajima, T Yamagata
Gene Ther, 2020-10-19;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Microglia-organized scar-free spinal cord repair in neonatal mice
Authors: Y Li, X He, R Kawaguchi, Y Zhang, Q Wang, A Monavarfes, Z Yang, B Chen, Z Shi, H Meng, S Zhou, J Zhu, A Jacobi, V Swarup, PG Popovich, DH Geschwind, Z He
Nature, 2020-10-07;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
An injectable hydrogel‐formulated inhibitor of prolyl‐4‐hydroxylase promotes T regulatory cell recruitment and enhances alveolar bone regeneration during resolution of experimental periodontitis
Authors: Kosuke Nagai, Hidetaka Ideguchi, Tetsuhiro Kajikawa, Xiaofei Li, Triantafyllos Chavakis, Jing Cheng et al.
The FASEB Journal
-
IL-6 Induced by Periodontal Inflammation Causes Neuroinflammation and Disrupts the Blood-Brain Barrier
Authors: D Furutama, S Matsuda, Y Yamawaki, S Hatano, A Okanobu, T Memida, H Oue, T Fujita, K Ouhara, M Kajiya, N Mizuno, T Kanematsu, K Tsuga, H Kurihara
Brain Sci, 2020-09-27;10(10):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Endothelin 1-induced retinal ganglion cell death is largely mediated by JUN activation
Authors: Olivia J. Marola, Stephanie B. Syc-Mazurek, Gareth R. Howell, Richard T. Libby
Cell Death & Disease
-
Abrogation of atypical neurogenesis and vascular-derived EphA4 prevents repeated mild TBI-induced learning and memory impairments
Authors: K Greer, EKG Basso, C Kelly, A Cash, E Kowalski, S Cerna, CT Ocampo, X Wang, MH Theus
Scientific Reports, 2020-09-21;10(1):15374.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
FGFR3 in Periosteal Cells Drives Cartilage-to-Bone Transformation in Bone Repair
Authors: A Julien, S Perrin, O Duchamp de, C Carvalho, M Bensidhoum, L Legeai-Mal, C Colnot
Stem Cell Reports, 2020-09-10;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Hypoxia Triggers the Intravasation of Clustered Circulating Tumor Cells
Authors: C Donato, L Kunz, F Castro-Gin, A Paasinen-S, K Strittmatt, BM Szczerba, R Scherrer, N Di Maggio, W Heusermann, O Biehlmaier, C Beisel, M Vetter, C Rochlitz, WP Weber, A Banfi, T Schroeder, N Aceto
Cell Rep, 2020-09-08;32(10):108105.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Wnt signaling activates MFSD2A to suppress vascular endothelial transcytosis and maintain blood-retinal barrier
Authors: Zhongxiao Wang, Chi-Hsiu Liu, Shuo Huang, Zhongjie Fu, Yohei Tomita, William R. Britton et al.
Science Advances
-
Delayed metformin treatment improves functional recovery following traumatic brain injury via central AMPK-dependent brain tissue repair
Authors: YY Fan, YJ Wang, J Guo, MN Wu, MS Zhang, BL Niu, Y Li, J Zhao, CH Yang, Y Li, M Chen, XY Jiao
Brain Res. Bull., 2020-08-26;164(0):146-156.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Developmental changes in myocardial B cells mirror changes in B cells associated with different organs
Authors: C Rocha-Rese, W Yang, W Li, D Kreisel, L Adamo, DL Mann
JCI Insight, 2020-08-20;5(16):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Therapeutic paradigm of dual targeting VEGF and PDGF for effectively treating FGF-2 off-target tumors
Authors: K Hosaka, Y Yang, T Seki, Q Du, X Jing, X He, J Wu, Y Zhang, H Morikawa, M Nakamura, M Scherzer, X Sun, Y Xu, T Cheng, X Li, X Liu, Q Li, Y Liu, A Hong, Y Chen, Y Cao
Nat Commun, 2020-07-24;11(1):3704.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
PPAR-&gamma Mediates Ta-VNS-Induced Angiogenesis and Subsequent Functional Recovery after Experimental Stroke in Rats
Authors: J Li, K Zhang, Q Zhang, X Zhou, L Wen, J Ma, L Niu, C Li
Biomed Res Int, 2020-07-22;2020(0):8163789.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Co-immunostaining of ICAM-1, ICAM-2, and CD31 in Mouse Kidney Glomeruli
Authors: Sun-Sang J Sung
BIO-PROTOCOL
-
Cardiomyocyte Expression of ZO-1 Is Essential for Normal Atrioventricular Conduction but Does Not Alter Ventricular Function
Authors: Jianlin Zhang, Kevin P. Vincent, Angela K. Peter, Matthew Klos, Hongqiang Cheng, Selina Manying Huang et al.
Circulation Research
-
VEGF‐B signaling impairs endothelial glucose transcytosis by decreasing membrane cholesterol content
Authors: Christine Moessinger, Ingrid Nilsson, Lars Muhl, Manuel Zeitelhofer, Benjamin Heller Sahlgren, Josefin Skogsberg et al.
EMBO reports
-
A lineage-tracing tool to map the fate of hypoxic tumour cells
Authors: Jenny A. F. Vermeer, Jonathan Ient, Bostjan Markelc, Jakob Kaeppler, Lydie M. O. Barbeau, Arjan J. Groot et al.
Disease Models & Mechanisms
-
Zeb2 Is a Regulator of Astrogliosis and Functional Recovery after CNS Injury
Authors: AL Vivinetto, ID Kim, DC Goldberg, L Fones, E Brown, VS Tarabykin, CE Hill, S Cho, JW Cave
Cell Rep, 2020-06-30;31(13):107834.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Role of FOXM1 in vascular smooth muscle cell survival and neointima formation following vascular injury
Authors: S Franco, A Stranz, F Ljumani, G Urabe, M Chaudhary, D Stewart, VS Pilli, M Kelly, D Yamanouchi, KC Kent, B Liu
Heliyon, 2020-06-16;6(6):e04028.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Endothelial Lactate Controls Muscle Regeneration from Ischemia by Inducing M2-like Macrophage Polarization
Authors: Jing Zhang, Jonathan Muri, Gillian Fitzgerald, Tatiane Gorski, Roberto Gianni-Barrera, Evi Masschelein et al.
Cell Metabolism
-
The Paracrine Role of Endothelial Cells in Bone Formation via CXCR4/SDF-1 Pathway
Authors: T Tamari, R Kawar-Jara, O Doppelt, B Giladi, N Sabbah, H Zigdon-Gil
Cells, 2020-05-26;9(6):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Farnesoid X receptor knockout protects brain against ischemic injury through reducing neuronal apoptosis in mice
Authors: HM Shan, M Zang, Q Zhang, RB Shi, XJ Shi, M Mamtilahun, C Liu, LL Luo, X Tian, Z Zhang, GY Yang, Y Tang, J Pu, Y Wang
J Neuroinflammation, 2020-05-25;17(1):164.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke
Authors: L Kang, H Yu, X Yang, Y Zhu, X Bai, R Wang, Y Cao, H Xu, H Luo, L Lu, MJ Shi, Y Tian, W Fan, BQ Zhao
Nat Commun, 2020-05-19;11(1):2488.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Small-molecule inhibitor targeting orphan nuclear receptor COUP-TFII for prostate cancer treatment
Authors: L Wang, CM Cheng, J Qin, M Xu, CY Kao, J Shi, E You, W Gong, LP Rosa, P Chase, L Scampavia, F Madoux, T Spicer, P Hodder, HE Xu, SY Tsai, MJ Tsai
Sci Adv, 2020-04-29;6(18):eaaz8031.
Species: Xenograft
Sample Types: Whole Tissue
Applications: IHC -
Aberrant Differentiation of Human Pluripotent Stem Cell-Derived Kidney Precursor Cells inside Mouse Vascularized Bioreactors
Authors: Parisa Ranjzad, Jessica Jinks, Amir P. Salahi, Ioannis Bantounas, Brian Derby, Susan J. Kimber et al.
Nephron
-
Macrophage M2 polarization induced by exosomes from adipose-derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb
Authors: D Zhu, TK Johnson, Y Wang, M Thomas, K Huynh, Q Yang, VC Bond, YE Chen, D Liu
Stem Cell Res Ther, 2020-04-22;11(1):162.
Species: Mouse
Sample Types: Tissue
Applications: IHC-Fr -
Weekends-Off Lenvatinib for Unresectable Hepatocellular Carcinoma Improves Therapeutic Response and Tolerability toward Adverse Events
Authors: H Iwamoto, H Suzuki, S Shimose, T Niizeki, M Nakano, T Shirono, S Okamura, Y Noda, N Kamachi, T Nakamura, A Masuda, T Sakaue, T Tanaka, D Nakano, M Sakai, T Yamaguchi, R Kuromatsu, H Koga, T Torimura
Cancers (Basel), 2020-04-19;12(4):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Identification of a novel tumor angiogenesis inhibitor targeting Shh/Gli1 signaling pathway in Non-small cell lung cancer
Authors: X Lei, Y Zhong, L Huang, S Li, J Fu, L Zhang, Y Zhang, Q Deng, X Yu
Cell Death Dis, 2020-04-14;11(4):232.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Eradication of tumor growth by delivering novel photothermal selenium-coated tellurium nanoheterojunctions
Authors: S Chen, C Xing, D Huang, C Zhou, B Ding, Z Guo, Z Peng, D Wang, X Zhu, S Liu, Z Cai, J Wu, J Zhao, Z Wu, Y Zhang, C Wei, Q Yan, H Wang, D Fan, L Liu, H Zhang, Y Cao
Sci Adv, 2020-04-08;6(15):eaay6825.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Imatinib Sets Pericyte Mosaic in the Retina
Authors: T Kovács-Öll, E Ivanova, G Szarka, ÁJ Tengölics, B Völgyi, BT Sagdullaev
Int J Mol Sci, 2020-04-05;21(7):.
Species: Mouse
Sample Types: Tissue
Applications: IHC-P -
Angiopoietin-2 blockade ameliorates autoimmune neuroinflammation by inhibiting leukocyte recruitment into the CNS
Authors: Z Li, EA Korhonen, A Merlini, J Strauss, E Wihuri, H Nurmi, S Antila, J Paech, U Deutsch, B Engelhardt, S Chintharla, GY Koh, A Flügel, K Alitalo
J. Clin. Invest., 2020-04-01;0(0):.
Species: Mouse
Sample Types:
Applications: IHC-P -
Translational studies of intravenous and intracerebroventricular routes of administration for CNS cellular biodistribution for BMN 250, an enzyme replacement therapy for the treatment of Sanfilippo type B
Authors: Anita Grover, Danielle Crippen-Harmon, Lacey Nave, Jon Vincelette, Jill C. M. Wait, Andrew C. Melton et al.
Drug Delivery and Translational Research
-
Brain Endothelial Cells Are Exquisite Sensors of Age-Related Circulatory Cues
Authors: MB Chen, AC Yang, H Yousef, D Lee, W Chen, N Schaum, B Lehallier, SR Quake, T Wyss-Coray
Cell Rep, 2020-03-31;30(13):4418-4432.e4.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
High Density Display of an Anti-Angiogenic Peptide on Micelle Surfaces Enhances Their Inhibition of alpha v beta 3 Integrin-Mediated Neovascularization In Vitro
Authors: Rajini Nagaraj, Trevor Stack, Sijia Yi, Benjamin Mathew, Kenneth R Shull, Evan A Scott et al.
Nanomaterials (Basel)
-
HAND1 loss-of-function within the embryonic myocardium reveals survivable congenital cardiac defects and adult heart failure
Authors: Beth A Firulli, Rajani M George, Jade Harkin, Kevin P Toolan, Hongyu Gao, Yunlong Liu et al.
Cardiovascular Research
-
MicroRNA-29b-3p inhibits cell proliferation and angiogenesis by targeting VEGFA and PDGFB in retinal microvascular endothelial cells
Authors: W Tang, J Guo, R Gu, B Lei, X Ding, J Ma, G Xu
Mol. Vis., 2020-02-24;26(0):64-75.
Species: Rat
Sample Types: Whole Cells
Applications: ICC -
Sphingosine 1-phosphate-regulated transcriptomes in heterogenous arterial and lymphatic endothelium of the aorta
Authors: Eric Engelbrecht, Michel V Levesque, Liqun He, Michael Vanlandewijck, Anja Nitzsche, Hira Niazi et al.
eLife
-
Mouse retinal cell behaviour in space and time using light sheet fluorescence microscopy
Authors: Claudia Prahst, Parham Ashrafzadeh, Thomas Mead, Ana Figueiredo, Karen Chang, Douglas Richardson et al.
eLife
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
A mutation affecting laminin alpha 5 polymerisation gives rise to a syndromic developmental disorder
Authors: Lynelle K. Jones, Rachel Lam, Karen K. McKee, Maya Aleksandrova, John Dowling, Stephen I. Alexander et al.
Development
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Role of Endothelial ADAM17 in Early Vascular Changes Associated with Diabetic Retinopathy
Authors: L Shalaby, M Thounaojam, A Tawfik, J Li, K Hussein, WJ Jahng, M Al-Shabraw, HF Kwok, M Bartoli, D Gutsaeva
J Clin Med, 2020-02-02;9(2):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Plumbagin inhibits tumor angiogenesis of gastric carcinoma in mice by modulating nuclear factor-kappa B pathway
Authors: Chengqian Yang, Xingbo Feng, Zengxian Li, Qingsi He
Translational Cancer Research
-
Cell-specific and athero-protective roles for RIPK3 in a murine model of atherosclerosis
Authors: S Colijn, V Muthukumar, J Xie, S Gao, CT Griffin
Dis Model Mech, 2020-01-24;13(1):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
VEGF-B ablation in pancreatic beta-cells upregulates insulin expression without affecting glucose homeostasis or islet lipid uptake
Authors: FC Ning, N Jensen, J Mi, W Lindström, M Balan, L Muhl, U Eriksson, I Nilsson, D Nyqvist
Sci Rep, 2020-01-22;10(1):923.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Functional and Evolutionary Diversification of Otx2 and Crx in Vertebrate Retinal Photoreceptor and Bipolar Cell Development
Authors: H Yamamoto, T Kon, Y Omori, T Furukawa
Cell Rep, 2020-01-21;30(3):658-671.e5.
Species: Mouse
Sample Types: Whole Tissue
Applications: IF -
Inhibition of Sema4D/PlexinB1 signaling alleviates vascular dysfunction in diabetic retinopathy
Authors: JH Wu, YN Li, AQ Chen, CD Hong, CL Zhang, HL Wang, YF Zhou, PC Li, Y Wang, L Mao, YP Xia, QW He, HJ Jin, ZY Yue, B Hu
EMBO Mol Med, 2020-01-13;12(2):e10154.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Non-Invasive Monitoring of Oxygen Tension and Oxygen Transport Inside Subcutaneous Devices After H2S Treatment
Authors: A Najdahmadi, AM Smink, P de Vos, JRT Lakey, E Botvinick
Cell Transplant, 2020-01-01;29(0):9636897198939.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Blocking endothelial apoptosis revascularises the retina in a model of ischemic retinopathy
Authors: ZL Grant, L Whitehead, VHY Wong, Z He, RY Yan, AR Miles, AV Benest, DO Bates, C Prahst, K Bentley, BV Bui, RC Symons, L Coultas
J. Clin. Invest., 2020;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IIHC -
Small-molecule inhibitor targeting orphan nuclear receptor COUP-TFII for prostate cancer treatment
Authors: L Wang, CM Cheng, J Qin, M Xu, CY Kao, J Shi, E You, W Gong, LP Rosa, P Chase, L Scampavia, F Madoux, T Spicer, P Hodder, HE Xu, SY Tsai, MJ Tsai
Sci Adv, 2020;6(18):eaaz8031.
Species: Xenograft
Sample Types: Whole Tissue
Applications: IHC -
Cleaved CD31 as a target for in vivo molecular imaging of inflammation
Authors: J Vigne, S Bay, R Aid-Launai, G Pariscoat, G Rucher, J Sénémaud, A Truffier, N Anizan, G Even, C Ganneau, F Andreata, M Le Borgne, A Nicoletti, D Le Guludec, G Caligiuri, F Rouzet
Sci Rep, 2019-12-20;9(1):19560.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC-P -
Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease
Authors: N Lalaoui, SE Boyden, H Oda, GM Wood, DL Stone, D Chau, L Liu, M Stoffels, T Kratina, KE Lawlor, KJM Zaal, PM Hoffmann, N Etemadi, K Shield-Art, C Biben, WL Tsai, MD Blake, HS Kuehn, D Yang, H Anderton, N Silke, L Wachsmuth, L Zheng, NS Moura, DB Beck, G Gutierrez-, AK Ombrello, GP Pinto-Pata, AJ Kueh, MJ Herold, C Hall, H Wang, JJ Chae, NI Dmitrieva, M McKenzie, A Light, BK Barham, A Jones, TM Romeo, Q Zhou, I Aksentijev, JC Mullikin, AJ Gross, AK Shum, ED Hawkins, SL Masters, MJ Lenardo, M Boehm, SD Rosenzweig, M Pasparakis, AK Voss, M Gadina, DL Kastner, J Silke
Nature, 2019-12-11;577(7788):103-108.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Endothelial EphB4 maintains vascular integrity and transport function in adult heart
Authors: G Luxán, J Stewen, N Díaz, K Kato, SK Maney, A Aravamudha, F Berkenfeld, N Nagelmann, HC Drexler, D Zeuschner, C Faber, H Schillers, S Hermann, J Wiseman, JM Vaquerizas, ME Pitulescu, RH Adams
Elife, 2019-11-29;8(0):.
Species: Mouse
Sample Types: Tissue Lysates
Applications: IHC -
Graft immaturity and safety concerns in transplanted human kidney organoids
Authors: SA Nam, E Seo, JW Kim, HW Kim, HL Kim, K Kim, TM Kim, JH Ju, IG Gomez, K Uchimura, BD Humphreys, CW Yang, JY Lee, J Kim, DW Cho, BS Freedman, YK Kim
Exp. Mol. Med., 2019-11-28;51(11):145.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Orthopedic Surgery Triggers Attention Deficits in a Delirium-Like Mouse Model
Authors: Ravikanth Velagapudi, Saraswathi Subramaniyan, Chao Xiong, Fiona Porkka, Ramona M. Rodriguiz, William C. Wetsel et al.
Frontiers in Immunology
-
Predicting Angiogenesis by Endothelial Progenitor Cells Relying on In-Vitro Function Assays and VEGFR-2 Expression Levels
Authors: N Sabbah, T Tamari, R Elimelech, O Doppelt, U Rudich, H Zigdon-Gil
Biomolecules, 2019-11-08;9(11):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Myc‐dependent endothelial proliferation is controlled by phosphotyrosine 1212 in VEGF receptor‐2
Authors: Chiara Testini, Ross O Smith, Yi Jin, Pernilla Martinsson, Ying Sun, Marie Hedlund et al.
EMBO reports
-
Secreted protein acidic and rich in cysteine mediates active targeting of human serum albumin in U87MG xenograft mouse models
Authors: CR Park, JH Jo, MG Song, JY Park, YH Kim, H Youn, SH Paek, JK Chung, JM Jeong, YS Lee, KW Kang
Theranostics, 2019-10-11;9(24):7447-7457.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Engineering transferrable microvascular meshes for subcutaneous islet transplantation
Authors: W Song, A Chiu, LH Wang, RE Schwartz, B Li, N Bouklas, DT Bowers, D An, SH Cheong, JA Flanders, Y Pardo, Q Liu, X Wang, VK Lee, G Dai, M Ma
Nat Commun, 2019-10-10;10(1):4602.
Species: Mouse
Sample Types: Microvascular Mesh
Applications: IHC -
Mitochondrial calcium exchange links metabolism with the epigenome to control cellular differentiation
Authors: AA Lombardi, AA Gibb, E Arif, DW Kolmetzky, D Tomar, TS Luongo, P Jadiya, EK Murray, PK Lorkiewicz, G Hajnóczky, E Murphy, ZP Arany, DP Kelly, KB Margulies, BG Hill, JW Elrod
Nat Commun, 2019-10-04;10(1):4509.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
TAFI deficiency causes maladaptive vascular remodeling after hemophilic joint bleeding
Authors: T Wyseure, T Yang, JY Zhou, EJ Cooke, B Wanko, M Olmer, R Agashe, Y Morodomi, N Behrendt, M Lotz, J Morser, A von Drygal, LO Mosnier
JCI Insight, 2019-10-03;0(0):.
Species: Human, Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr, IHC-P -
Cellular Mechanisms of Angiogenesis in Neonatal Rat Models of Retinal Neurodegeneration
Authors: D Asano, M Hokazono, S Hirano, A Morita, T Nakahara
Int J Mol Sci, 2019-09-25;20(19):.
Species: Rat
Sample Types: Whole Cells
Applications: Cell Culture -
Long-term mucosal injury and repair in a murine model of pelvic radiotherapy
Authors: DK Malipatlol, P Patel, F Sjöberg, S Devarakond, M Kalm, E Angenete, EB Lindskog, R Grandér, L Persson, A Stringer, U Wilderäng, J Swanpalmer, HG Kuhn, G Steineck, C Bull
Sci Rep, 2019-09-24;9(1):13803.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF-A axis
Authors: Venkata Naga Srikanth Garikipati, Suresh Kumar Verma, Zhongjian Cheng, Dongming Liang, May M. Truongcao, Maria Cimini et al.
Nature Communications
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Cardiomyocyte d-dopachrome tautomerase protects against heart failure
Authors: Y Ma, KN Su, D Pfau, VS Rao, X Wu, X Hu, L Leng, X Du, M Piecychna, K Bedi, SG Campbell, A Eichmann, JM Testani, KB Margulies, R Bucala, LH Young
JCI Insight, 2019-09-05;4(17):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Endothelial or vascular smooth muscle cell-specific expression of human NOX5 exacerbates renal inflammation, fibrosis and albuminuria in the Akita mouse
Authors: Jay C. Jha, Aozhi Dai, Chet E. Holterman, Mark E. Cooper, Rhian M. Touyz, Chris R. Kennedy et al.
Diabetologia
-
UTX/KDM6A Deletion Promotes Recovery of Spinal Cord Injury by Epigenetically Regulating Vascular Regeneration
Authors: S Ni, Z Luo, L Jiang, Z Guo, P Li, X Xu, Y Cao, C Duan, T Wu, C Li, H Lu, J Hu
Mol. Ther., 2019-08-22;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Administration of Tramadol or Buprenorphine via the drinking water for post-operative analgesia in a mouse-osteotomy model
Authors: P Jirkof, M Durst, R Klopfleisc, R Palme, C Thöne-Rein, F Buttgereit, K Schmidt-Bl, A Lang
Sci Rep, 2019-07-24;9(1):10749.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Single-cell analysis reveals T cell infiltration in old neurogenic niches
Authors: BW Dulken, MT Buckley, P Navarro Ne, N Saligrama, R Cayrol, DS Leeman, BM George, SC Boutet, K Hebestreit, JV Pluvinage, T Wyss-Coray, IL Weissman, H Vogel, MM Davis, A Brunet
Nature, 2019-07-03;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Disruption of the Extracellular Matrix Progressively Impairs Central Nervous System Vascular Maturation Downstream of beta -Catenin Signaling
Authors: Lasse D. Jensen, Belma Hot, Daniel Ramsköld, Raoul F.V. Germano, Chika Yokota, Sarantis Giatrellis et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Loss of the transcription factor RBPJ induces disease-promoting properties in brain pericytes
Authors: R Diéguez-Hu, K Kato, BD Giaimo, M Nieminen-K, H Arf, F Ferrante, M Bartkuhn, T Zimmermann, MG Bixel, HM Eilken, S Adams, T Borggrefe, P Vajkoczy, RH Adams
Nat Commun, 2019-06-27;10(1):2817.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss
Authors: AM Nikolakopo, A Montagne, K Kisler, Z Dai, Y Wang, MT Huuskonen, AP Sagare, D Lazic, MD Sweeney, P Kong, M Wang, NC Owens, EJ Lawson, X Xie, Z Zhao, BV Zlokovic
Nat. Neurosci., 2019-06-24;22(7):1089-1098.
Species: Transgenic Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Antibody-based delivery of cytokine payloads to carbonic anhydrase IX leads to cancer cures in immunocompetent tumor-bearing mice
Authors: B Ziffels, M Stringhini, P Probst, T Fugmann, T Sturm, D Neri
Mol. Cancer Ther., 2019-06-18;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Targeted Delivery of TNF Potentiates the Antibody-Dependent Cell-Mediated Cytotoxicity of an Anti-Melanoma Immunoglobulin
Authors: Patrizia Murer, Jonathan D. Kiefer, Louis Plüss, Mattia Matasci, Sandra L. Blümich, Marco Stringhini et al.
Journal of Investigative Dermatology
-
Pinealectomy or light exposure exacerbates biliary damage and liver fibrosis in cholestatic rats through decreased melatonin synthesis
Authors: Lixian Chen, Tianhao Zhou, Nan Wu, April O'Brien, Julie Venter, Ludovica Ceci et al.
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Transfusion of Resting Platelets Reduces Brain Hemorrhage After Intracerebral Hemorrhage and tPA-Induced Hemorrhage After Cerebral Ischemia
Authors: H Luo, L Wei, L Lu, L Kang, Y Cao, X Yang, X Bai, W Fan, BQ Zhao
Front Neurosci, 2019-04-05;13(0):338.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Distinct mesoderm migration phenotypes in extra-embryonic and embryonic regions of the early mouse embryo
Authors: Bechara Saykali, Navrita Mathiah, Wallis Nahaboo, Marie-Lucie Racu, Latifa Hammou, Matthieu Defrance et al.
eLife
-
Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation
Authors: SG Romeo, KM Alawi, J Rodrigues, A Singh, AP Kusumbe, SK Ramasamy
Nat. Cell Biol., 2019-04-01;21(4):430-441.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Endothelial cells are a source of Nestin expression in Pulmonary Arterial Hypertension
Authors: AR Bhagwani, S Hultman, D Farkas, R Moncayo, K Dandamudi, AK Zadu, CD Cool, L Farkas
PLoS ONE, 2019-03-18;14(3):e0213890.
Species: Rat
Sample Types: Whole Cells
Applications: Cell Selection -
Decellularized neonatal cardiac extracellular matrix prevents widespread ventricular remodeling in adult mammals after myocardial infarction
Authors: Zhouguang Wang, Daniel W. Long, Yan Huang, William C.W. Chen, Kang Kim, Yadong Wang
Acta Biomaterialia
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Overexpression of LH3 reduces the incidence of hypertensive intracerebral hemorrhage in mice
Authors: Hao Li, Haochen Xu, Hongyan Wen, Tianlong Liu, Yingying Sun, Ning Xiao et al.
Journal of Cerebral Blood Flow & Metabolism
-
Human Neonatal Thymus Mesenchymal Stem/Stromal Cells and Chronic Right Ventricle Pressure Overload
Authors: Josue Chery, Shan Huang, Lianghui Gong, Shuyun Wang, Zhize Yuan, Joshua Wong et al.
Bioengineering (Basel)
-
PU.1 controls fibroblast polarization and tissue fibrosis
Authors: T Wohlfahrt, S Rauber, S Uebe, M Luber, A Soare, A Ekici, S Weber, AE Matei, CW Chen, C Maier, E Karouzakis, HP Kiener, E Pachera, C Dees, C Beyer, C Daniel, K Gelse, AE Kremer, E Naschberge, M Stürzl, F Butter, M Sticherlin, S Finotto, A Kreuter, MH Kaplan, A Jüngel, S Gay, SL Nutt, DW Boykin, GMK Poon, O Distler, G Schett, JHW Distler, A Ramming
Nature, 2019-01-30;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Tumour pH and protein concentration contribute to the signal of amide proton transfer magnetic resonance imaging
Authors: KJ Ray, MA Simard, JR Larkin, J Coates, P Kinchesh, SC Smart, GS Higgins, M Chappell, N Sibson
Cancer Res., 2019-01-24;0(0):.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Identification of ILK as a critical regulator of VEGFR 3 signalling and lymphatic vascular growth
Authors: Sofia Urner, Lara Planas‐Paz, Laura Sophie Hilger, Carina Henning, Anna Branopolski, Molly Kelly‐Goss et al.
The EMBO Journal
-
VCAM-1–targeted MRI Enables Detection of Brain Micrometastases from Different Primary Tumors
Authors: Vinton W.T. Cheng, Manuel Sarmiento Soto, Alexandre A. Khrapitchev, Francisco Perez-Balderas, Rasheed Zakaria, Michael D. Jenkinson et al.
Clinical Cancer Research
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Pancreatic Cell Fate Determination Relies on Notch Ligand Trafficking by NFIA
Authors: MA Scavuzzo, J Chmielowie, D Yang, K Wamble, LS Chaboub, L Duraine, B Tepe, SM Glasgow, BR Arenkiel, C Brou, B Deneen, M Borowiak
Cell Rep, 2018-12-26;25(13):3811-3827.e7.
Species: Mouse
Sample Types: Whole Tissue
Applications: Ifs -
Cardiac Sca-1 + Cells Are Not Intrinsic Stem Cells for Myocardial Development, Renewal, and Repair
Authors: Lu Zhang, Nishat Sultana, Jianyun Yan, Fan Yang, Fuxue Chen, Elena Chepurko et al.
Circulation
-
Spatially structured cell populations process multiple sensory signals in parallel in intact vascular endothelium
Authors: Matthew D. Lee, Calum Wilson, Christopher D. Saunter, Charles Kennedy, John M. Girkin, John G. McCarron
Science Signaling
-
Semaphorin-3A protects against neointimal hyperplasia after vascular injury
Authors: JH Wu, YF Zhou, CD Hong, AQ Chen, Y Luo, L Mao, YP Xia, QW He, HJ Jin, M Huang, YN Li, B Hu
EBioMedicine, 2018-12-19;39(0):95-108.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
SCL/TAL1 cooperates with Polycomb RYBP-PRC1 to suppress alternative lineages in blood-fated cells
Authors: H Chagraoui, MS Kristianse, JP Ruiz, A Serra-Barr, J Richter, E Hall-Ponse, N Gray, D Waithe, K Clark, P Hublitz, E Repapi, G Otto, P Sopp, S Taylor, S Thongjuea, P Vyas, C Porcher
Nat Commun, 2018-12-18;9(1):5375.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
An Efficient Bivalent Cyclic RGD-PIK3CB siRNA Conjugate for Specific Targeted Therapy against Glioblastoma In Vitro and In Vivo
Authors: Bohong Cen, Yuanyi Wei, Wen Huang, Muzhou Teng, Shuai He, Jianlong Li et al.
Molecular Therapy - Nucleic Acids
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Tumour-vasculature development via endothelial-to-mesenchymal transition after radiotherapy controls CD44v6+ cancer cell and macrophage polarization
Authors: SH Choi, AR Kim, JK Nam, JM Kim, JY Kim, HR Seo, HJ Lee, J Cho, YJ Lee
Nat Commun, 2018-11-30;9(1):5108.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Prolonged systemic hyperglycemia does not cause pericyte loss and permeability at the mouse blood-brain barrier
Authors: MA Mäe, T Li, G Bertuzzi, E Raschperge, M Vanlandewi, L He, K Nahar, A Dalheim, JJ Hofmann, B Laviña, A Keller, C Betsholtz, G Genové
Sci Rep, 2018-11-29;8(1):17462.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Mesenchymal Precursor Cells in Adult Nerves Contribute to Mammalian Tissue Repair and Regeneration
Authors: MJ Carr, JS Toma, APW Johnston, PE Steadman, SA Yuzwa, N Mahmud, PW Frankland, DR Kaplan, FD Miller
Cell Stem Cell, 2018-11-29;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Wnt/?-catenin signaling regulates VE-cadherin-mediated anastomosis of brain capillaries by counteracting S1pr1 signaling
Authors: K Hübner, P Cabochette, R Diéguez-Hu, C Wiesner, Y Wakayama, KS Grassme, M Hubert, S Guenther, HG Belting, M Affolter, RH Adams, B Vanhollebe, W Herzog
Nat Commun, 2018-11-19;9(1):4860.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Radiation-induced premature cellular senescence involved in glomerular diseases in rats
Authors: S Aratani, M Tagawa, S Nagasaka, Y Sakai, A Shimizu, S Tsuruoka
Sci Rep, 2018-11-14;8(1):16812.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC-P -
Genetic dissection of the miR-200-Zeb1 axis reveals its importance in tumor differentiation and invasion
Authors: AC Title, SJ Hong, ND Pires, L Hasenöhrl, S Godbersen, N Stokar-Reg, DP Bartel, M Stoffel
Nat Commun, 2018-11-07;9(1):4671.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Sensitivity of Multiphase Pseudocontinuous Arterial Spin Labelling (MP pCASL) Magnetic Resonance Imaging for Measuring Brain and Tumour Blood Flow in Mice
Authors: Jessica Buck, James R. Larkin, Manon A. Simard, Alexandre A. Khrapitchev, Michael A. Chappell, Nicola R. Sibson
Contrast Media & Molecular Imaging
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
VEGFR2 promotes central endothelial activation and the spread of pain in inflammatory arthritis
Authors: Nicholas Beazley-Long, Catherine Elizabeth Hodge, William Robert Ashby, Samuel Marcus Bestall, Fatimah Almahasneh, Alexandra Margaret Durrant et al.
Brain, Behavior, and Immunity
-
Peripheral myeloid cells contribute to brain injury in male neonatal mice
Authors: PLP Smith, A Mottahedin, P Svedin, CJ Mohn, H Hagberg, J Ek, C Mallard
J Neuroinflammation, 2018-10-30;15(1):301.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Cofilin-actin rod formation in neuronal processes after brain ischemia
Authors: Seok Joon Won, Angela M. Minnella, Long Wu, Claire H. Eun, Eric Rome, Paco S. Herson et al.
PLOS ONE
Species: Mouse
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Loss of pyruvate kinase M2 limits growth and triggers innate immune signaling in endothelial cells
Authors: OA Stone, M El-Brolosy, K Wilhelm, X Liu, AM Romão, E Grillo, JKH Lai, S Günther, S Jeratsch, C Kuenne, IC Lee, T Braun, MM Santoro, JW Locasale, M Potente, DYR Stainier
Nat Commun, 2018-10-09;9(1):4077.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Endothelial repair is dependent on CD11c+ leukocytes to establish regrowing endothelial sheets with high cellular density
Authors: U Yrlid, M Holm, M Levin, S Alsén, M Lindbom, L Glise, N Bergh, J Borén, P Fogelstran
J. Leukoc. Biol., 2018-09-28;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Angiopoietin/Tie2 axis regulates the age-at-injury cerebrovascular response to traumatic brain injury
Authors: TR Brickler, A Hazy, F Guilhaume, R Dai, EJA Kowalski, R Dickerson, J Chen, X Wang, PD Morton, A Whittingto, A Ahmed, MH Theus
J. Neurosci., 2018-09-21;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Pentoxifylline decreases post-operative intra-abdominal adhesion formation in an animal model
Authors: YL Yang, MG Lee, CC Lee, PI Su, CY Chi, CH Liu, MC Wu, ZS Yen, SC Chen
PeerJ, 2018-08-24;6(0):e5434.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The tyrosine phosphatase SHP2 controls TGF?-induced STAT3 signaling to regulate fibroblast activation and fibrosis
Authors: A Zehender, J Huang, AH Györfi, AE Matei, T Trinh-Minh, X Xu, YN Li, CW Chen, J Lin, C Dees, C Beyer, K Gelse, ZY Zhang, C Bergmann, A Ramming, W Birchmeier, O Distler, G Schett, JHW Distler
Nat Commun, 2018-08-14;9(1):3259.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Mesenchymal stem cells ameliorate hyperglycemia-induced endothelial injury through modulation of mitophagy
Authors: W Zhu, Y Yuan, G Liao, L Li, J Liu, Y Chen, J Zhang, J Cheng, Y Lu
Cell Death Dis, 2018-08-06;9(8):837.
Species: Rat
Sample Types: Whole Cells
Applications: Flow Cytometry, IHC -
Formation of the sacrum requires down-regulation of sonic hedgehog signaling in the sacral intervertebral discs
Authors: Raffaella Bonavita, Kathleen Vincent, Robert Pinelli, Chitra Lekha Dahia
Biology Open
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Growth Differentiation Factor 11 Promotes Neurovascular Recovery After Stroke in Mice
Authors: Lu Lu, Xiaofei Bai, Yongliang Cao, Haiyu Luo, Xing Yang, Lijing Kang et al.
Frontiers in Cellular Neuroscience
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Characterization of multi-cellular dynamics of angiogenesis and vascular remodelling by intravital imaging of the wounded mouse cornea
Authors: Y Wang, Y Jin, B Laviña, L Jakobsson
Sci Rep, 2018-07-13;8(1):10672.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
KSHV-induced ligand mediated activation of PDGF receptor-alpha drives Kaposi's sarcomagenesis
Authors: LE Cavallin, Q Ma, J Naipauer, S Gupta, M Kurian, P Locatelli, P Romanelli, M Nadji, PJ Goldschmid, EA Mesri
PLoS Pathog., 2018-07-09;14(7):e1007175.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Syndecan-1 induction in lung microenvironment supports the establishment of breast tumor metastases
Authors: C Chute, X Yang, K Meyer, N Yang, K O'Neil, I Kasza, K Eliceiri, C Alexander, A Friedl
Breast Cancer Res., 2018-07-05;20(1):66.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Local Arrangement of Fibronectin by Myofibroblasts Governs Peripheral Nuclear Positioning in Muscle Cells
Authors: William Roman, João P. Martins, Edgar R. Gomes
Developmental Cell
-
The beta 3‐integrin endothelial adhesome regulates microtubule‐dependent cell migration
Authors: Samuel J Atkinson, Aleksander M Gontarczyk, Abdullah AA Alghamdi, Tim S Ellison, Robert T Johnson, Wesley J Fowler et al.
EMBO reports
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Bones in human CYP26B1 deficiency and rats with hypervitaminosis A phenocopy Vegfa overexpression
Authors: T Lind, R Lugano, AM Gustafson, M Norgård, A van Haerin, A Dimberg, H Melhus, SP Robertson, G Andersson
Bone Rep, 2018-06-21;9(0):27-36.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Attenuation of Retinal Vascular Development in Neonatal Mice Subjected to Hypoxic-Ischemic Encephalopathy
Authors: IS Zaitoun, U Cikla, D Zafer, E Udho, R Almomani, A Suscha, P Cengiz, CM Sorenson, N Sheibani
Sci Rep, 2018-06-15;0(1):9166.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
cxcl12-engineered endothelial progenitor cells enhance neurogenesis and angiogenesis after ischemic brain injury in mice
Authors: Y Li, S Chang, W Li, G Tang, Y Ma, Y Liu, F Yuan, Z Zhang, GY Yang, Y Wang
Stem Cell Res Ther, 2018-05-11;9(1):139.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
UHRF1 epigenetically orchestrates smooth muscle cell plasticity in arterial disease
Authors: L Elia, P Kunderfran, P Carullo, M Vacchiano, FM Farina, IF Hall, S Mantero, C Panico, R Papait, G Condorelli, M Quintavall
J. Clin. Invest., 2018-05-07;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice
Authors: Z Cheng, L Wang, M Qu, H Liang, W Li, Y Li, L Deng, Z Zhang, GY Yang
J Neuroinflammation, 2018-05-03;15(1):135.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Gellan Gum-based luminal fillers for peripheral nerve regeneration: an in vivo study in the rat sciatic nerve repair model
Authors: CR Carvalho, S Wrobel, C Meyer, C Brandenber, IF Cengiz, R López-Cebr, J Silva-Corr, G Ronchi, RL Reis, C Grothe, JM Oliveira, K Haastert-T
Biomater Sci, 2018-05-01;0(0):.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC-P -
Nephronectin Regulates Mesangial Cell Adhesion and Behavior in Glomeruli
Authors: Susan E. Zimmerman, Chitkale Hiremath, Jun Tsunezumi, Zhufeng Yang, Bronwyn Finney, Denise K. Marciano
Journal of the American Society of Nephrology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Positive-feedback regulation of subchondral H-type vessel formation by chondrocyte promotes osteoarthritis development in mice
Authors: J Lu, H Zhang, D Cai, C Zeng, P Lai, Y Shao, H Fang, D Li, J Ouyang, C Zhao, D Xie, B Huang, J Yang, Y Jiang, X Bai
J. Bone Miner. Res., 2018-03-24;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self-renewal
Authors: SS Rayagiri, D Ranaldi, A Raven, NIF Mohamad Az, O Lefebvre, PS Zammit, AG Borycki
Nat Commun, 2018-03-14;9(1):1075.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A Bayesian Framework for Generalized Linear Mixed Modeling Identifies New Candidate Loci for Late-Onset Alzheimer's Disease
Authors: X Wang, VM Philip, G Ananda, CC White, A Malhotra, PJ Michalski, KRM Karuturi, SR Chintalapu, C Acklin, M Sasner, DA Bennett, PL De Jager, GR Howell, GW Carter
Genetics, 2018-03-05;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Intratumoral administration of IL2- and TNF-based fusion proteins cures cancer without establishing protective immunity
Authors: B Ziffels, F Pretto, D Neri
Immunotherapy, 2018-03-01;10(3):177-188.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Local delivery of tetramethylpyrazine eliminates the senescent phenotype of bone marrow mesenchymal stromal cells and creates an anti-inflammatory and angiogenic environment in aging mice
Authors: B Gao, X Lin, H Jing, J Fan, C Ji, Q Jie, C Zheng, D Wang, X Xu, Y Hu, W Lu, Z Luo, L Yang
Aging Cell, 2018-02-28;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Ablation of endothelial VEGFR1 improves metabolic dysfunction by inducing adipose tissue browning
Authors: Takahiro Seki, Kayoko Hosaka, Carina Fischer, Sharon Lim, Patrik Andersson, Mitsuhiko Abe et al.
Journal of Experimental Medicine
-
YAP and TAZ regulate adherens junction dynamics and endothelial cell distribution during vascular development
Authors: Filipa Neto, Alexandra Klaus-Bergmann, Yu Ting Ong, Silvanus Alt, Anne-Clémence Vion, Anna Szymborska et al.
eLife
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Hindlimb Ischemia Impairs Endothelial Recovery and Increases Neointimal Proliferation in the Carotid Artery
Authors: S Sorrentino, C Iaconetti, S De Rosa, A Polimeni, J Sabatino, C Gareri, F Passafaro, T Mancuso, L Tammè, C Mignogna, C Camastra, G Esposito, A Curcio, D Torella, C Indolfi
Sci Rep, 2018-01-15;8(1):761.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC-P -
Type I interferons instigate fetal demise after Zika virus infection
Authors: LJ Yockey, KA Jurado, N Arora, A Millet, T Rakib, KM Milano, AK Hastings, E Fikrig, Y Kong, TL Horvath, S Weatherbee, HJ Kliman, CB Coyne, A Iwasaki
Sci Immunol, 2018-01-05;3(19):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Dosimetric evaluation of radionuclides for VCAM-1-targeted radionuclide therapy of early brain metastases
Authors: N Falzone, NL Ackerman, LF Rosales, MA Bernal, X Liu, SG Peeters, MS Soto, A Corroyer-D, M Bernaudin, E Grimoin, O Touzani, NR Sibson, KA Vallis
Theranostics, 2018-01-01;8(1):292-303.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Visceral endoderm and the primitive streak interact to build the fetal-placental interface of the mouse gastrula
Authors: Adriana M. Rodriguez, Karen M. Downs
Developmental Biology
-
Multicolor quantitative confocal imaging cytometry
Authors: DL Coutu, KD Kokkaliari, L Kunz, T Schroeder
Nat. Methods, 2017-11-13;15(1):39-46.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Differential role of Interleukin-1 and Interleukin-6 in K-Ras-driven pancreatic carcinoma undergoing mesenchymal transition
Authors: I Siddiqui, M Erreni, MA Kamal, C Porta, F Marchesi, S Pesce, F Pasqualini, S Schiarea, C Chiabrando, A Mantovani, P Allavena
Oncoimmunology, 2017-11-01;7(2):e1388485.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Pr -
Microglial-mediated PDGF-CC activation increases cerebrovascular permeability during ischemic stroke
Authors: Enming Joseph Su, Chunzhang Cao, Linda Fredriksson, Ingrid Nilsson, Christina Stefanitsch, Tamara K. Stevenson et al.
Acta Neuropathologica
-
Smooth muscle cell recruitment to lymphatic vessels requires PDGFB and impacts vessel size but not identity
Authors: Yixin Wang, Yi Jin, Maarja Andaloussi Mäe, Yang Zhang, Henrik Ortsäter, Christer Betsholtz et al.
Development
-
PEDF expression affects retinal endothelial cell proangiogenic properties through alterations in cell adhesive mechanisms
Authors: Juliana Falero-Perez, SunYoung Park, Christine M. Sorenson, Nader Sheibani
American Journal of Physiology-Cell Physiology
-
Natural compound bavachalcone promotes the differentiation of endothelial progenitor cells and neovascularization through the ROR?-erythropoietin-AMPK axis
Authors: S Ling, RZ Ni, Y Yuan, YQ Dang, QM Zhou, S Liang, F Guo, W Feng, Y Chen, K Ikeda, Y Yamori, JW Xu
Oncotarget, 2017-09-16;8(49):86188-86205.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC-P -
Anti-angiogenic effect of arsenic trioxide in lung cancer via inhibition of endothelial cell migration, proliferation and tube formation
Authors: Meng-Hang Yang, Ke-Jie Chang, Jin-Cheng Zheng, Hai Huang, Guang-Yuan Sun, Xue-Wei Zhao et al.
Oncology Letters
-
Pericyte-targeting prodrug overcomes tumor resistance to vascular disrupting agents
Authors: M Chen, X Lei, C Shi, M Huang, X Li, B Wu, Z Li, W Han, B Du, J Hu, Q Nie, W Mai, N Ma, N Xu, X Zhang, C Fan, A Hong, M Xia, L Luo, A Ma, H Li, Q Yu, H Chen, D Zhang, W Ye
J. Clin. Invest., 2017-08-28;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Resident Arterial Cells and Circulating Bone Marrow-Derived Cells both Contribute to Intimal Hyperplasia in a Rat Allograft Carotid Transplantation Model
Authors: Y He, X Xu, T Zhu, M Tang, J Mei, Y Si
Cell. Physiol. Biochem., 2017-07-11;42(4):1303-1312.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Coronary angiogenic effect of long-term administration of Nigella sativa
Authors: LI Al Asoom
BMC Complement Altern Med, 2017-06-13;17(1):308.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Clearance of Heparan Sulfate and Attenuation of CNS Pathology by Intracerebroventricular BMN 250 in Sanfilippo Type B Mice
Authors: M Aoyagi-Sch, D Crippen-Ha, R Lawrence, J Vincelette, G Yogalingam, H Prill, BK Yip, B Baridon, C Vitelli, A Lee, O Gorostiza, EG Adintori, WC Minto, JL Van Vleet, B Yates, S Rigney, TM Christians, PMN Tiger, MJ Lo, J Holtzinger, PA Fitzpatric, JH LeBowitz, S Bullens, BE Crawford, S Bunting
Mol Ther Methods Clin Dev, 2017-06-06;6(0):43-53.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Different distributions of M1 and M2 macrophages in a mouse model of laser-induced choroidal neovascularization
Authors: Yedi Zhou, Shigeo Yoshida, Yuki Kubo, Takeru Yoshimura, Yoshiyuki Kobayashi, Takahito Nakama et al.
Molecular Medicine Reports
-
Endoglin prevents vascular malformation by regulating flow-induced cell migration and specification through VEGFR2 signalling
Authors: Y Jin, L Muhl, M Burmakin, Y Wang, AC Duchez, C Betsholtz, HM Arthur, L Jakobsson
Nat. Cell Biol., 2017-05-22;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Endoglin controls blood vessel diameter through endothelial cell shape changes in response to haemodynamic cues
Authors: WW Sugden, R Meissner, T Aegerter-W, R Tsaryk, EV Leonard, J Bussmann, MJ Hamm, W Herzog, Y Jin, L Jakobsson, C Denz, AF Siekmann
Nat. Cell Biol., 2017-05-22;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Activation of Hypoxia Signaling in Stromal Progenitors Impairs Kidney Development
Authors: K Gerl, D Steppan, M Fuchs, C Wagner, C Willam, A Kurtz, B Kurt
Am. J. Pathol., 2017-05-17;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Immunofluorescence reveals unusual patterns of labelling for connexin43 localized to calbindin-D28K-positive interstitial cells in the pineal gland
Authors: DD Tsao, SG Wang, BD Lynn, JI Nagy
Eur. J. Neurosci., 2017-05-11;0(0):.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Gli1(+) Mesenchymal Stromal Cells Are a Key Driver of Bone Marrow Fibrosis and an Important Cellular Therapeutic Target
Authors: RK Schneider, A Mullally, A Dugourd, F Peisker, R Hoogenboez, PMH Van Strien, EM Bindels, D Heckl, G Büsche, D Fleck, G Müller-New, J Wongboonsi, M Ventura Fe, VG Puelles, J Saez-Rodri, BL Ebert, BD Humphreys, R Kramann
Cell Stem Cell, 2017-04-27;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Excessive dietary intake of vitamin A reduces skull bone thickness in mice
Authors: T Lind, C Öhman, G Calounova, A Rasmusson, G Andersson, G Pejler, H Melhus
PLoS ONE, 2017-04-20;12(4):e0176217.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC-P -
Brachyury drives formation of a distinct vascular branchpoint critical for fetal-placental arterial union in the mouse gastrula
Authors: AM Rodriguez, DX Jin, AD Wolfe, MM Mikedis, L Wierenga, MP Hashmi, C Viebahn, KM Downs
Dev. Biol., 2017-04-04;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Silencing of galectin-1 inhibits retinal neovascularization and ameliorates retinal hypoxia in a murine model of oxygen-induced ischemic retinopathy
Authors: N Yang, W Zhang, T He, Y Xing
Exp. Eye Res, 2017-02-28;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Gene therapy for a mouse model of glucose transporter-1 deficiency syndrome
Authors: S Nakamura, H Osaka, SI Muramatsu, N Takino, M Ito, S Aoki, EF Jimbo, K Shimazaki, T Onaka, S Ohtsuki, T Terasaki, T Yamagata
Mol Genet Metab Rep, 2017-01-15;10(0):67-74.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Impacts of femoral artery and vein excision versus femoral artery excision on the hindlimb ischemic model in CD-1 mice
Authors: Miao Chen
Microvasc. Res, 2016-12-18;110(0):48-55.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Murine liver-resident group 1 innate lymphoid cells regulate optimal priming of anti-viral CD8+ T cells
J Leukoc Biol, 2016-08-04;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
TLR2-mediated leukocyte trafficking to the developing brain
J Leukoc Biol, 2016-08-04;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Different cyclical intermittent hypoxia severities have different effects on hippocampal microvasculature
Authors: Diane C. Lim, Daniel C. Brady, Rajath Soans, Emily Y. Kim, Laise Valverde, Brendan T. Keenan et al.
Journal of Applied Physiology
-
Osteogenic potential of alpha smooth muscle actin expressing muscle resident progenitor cells
Authors: Brya G. Matthews, Elena Torreggiani, Emilie Roeder, Igor Matic, Danka Grcevic, Ivo Kalajzic
Bone
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Ng BR is essential for endothelial cell glycosylation and vascular development
Authors: Eon Joo Park, Kariona A Grabińska, Ziqiang Guan, William C Sessa
EMBO reports
-
Vascular Defects and Spinal Cord Hypoxia in Spinal Muscular Atrophy
Authors: Eilidh Somers, Robert D. Lees, Katie Hoban, James N. Sleigh, Haiyan Zhou, Francesco Muntoni et al.
Annals of Neurology
-
FOXO1 couples metabolic activity and growth state in the vascular endothelium
Authors: Kerstin Wilhelm, Katharina Happel, Guy Eelen, Sandra Schoors, Mark F. Oellerich, Radiance Lim et al.
Nature
-
Feasibility study of the Fab fragment of a monoclonal antibody against tissue factor as a diagnostic tool
Authors: RYO TSUMURA, RYUTA SATO, FUMIAKI FURUYA, YOSHIKATSU KOGA, YOSHIYUKI YAMAMOTO, YUKI FUJIWARA et al.
International Journal of Oncology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Integrin beta1 controls VE-cadherin localization and blood vessel stability.
Authors: Yamamoto H, Ehling M, Kato K, Kanai K, van Lessen M, Frye M, Zeuschner D, Nakayama M, Vestweber D, Adams R
Nat Commun, 2015-03-10;6(0):6429.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The transcription factor Mesp1 interacts with cAMP-responsive element binding protein 1 (Creb1) and coactivates Ets variant 2 (Etv2) gene expression.
Authors: Shi X, Zirbes K, Rasmussen T, Ferdous A, Garry M, Koyano-Nakagawa N, Garry D
J Biol Chem, 2015-02-18;290(15):9614-25.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Connective Tissue Growth Factor reporter mice label a subpopulation of mesenchymal progenitor cells that reside in the trabecular bone region
Authors: Wen Wang, Sara Strecker, Yaling Liu, Liping Wang, Fayekah Assanah, Spenser Smith et al.
Bone
-
Formation of fenestrae in murine liver sinusoids depends on plasmalemma vesicle-associated protein and is required for lipoprotein passage.
Authors: Herrnberger, Leonie, Hennig, Robert, Kremer, Werner, Hellerbrand, Claus, Goepferich, Achim, Kalbitzer, Hans Rob, Tamm, Ernst R
PLoS ONE, 2014-12-26;9(12):e115005.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking alpha V beta 8-TGF beta signaling in the brain
Authors: Thomas D. Arnold, Colin Niaudet, Mei-Fong Pang, Julie Siegenthaler, Konstantin Gaengel, Bongnam Jung et al.
Development
-
The formyl peptide receptor 1 exerts a tumor suppressor function in human gastric cancer by inhibiting angiogenesis.
Authors: Prevete N, Liotti F, Visciano C, Marone G, Melillo R, de Paulis A
Oncogene, 2014-09-29;34(29):3826-38.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Peroxisome proliferator-activated receptor alpha protects capillary pericytes in the retina.
Authors: Ding L, Cheng R, Hu Y, Takahashi Y, Jenkins A, Keech A, Humphries K, Gu X, Elliott M, Xia X, Ma J
Am J Pathol, 2014-08-07;184(10):2709-20.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Periadventitial application of rapamycin-loaded nanoparticles produces sustained inhibition of vascular restenosis.
Authors: Shi, Xudong, Chen, Guojun, Guo, Lian-Wan, Si, Yi, Zhu, Men, Pilla, Srikanth, Liu, Bo, Gong, Shaoqin, Kent, K Craig
PLoS ONE, 2014-02-21;9(2):e89227.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Anticancer effects of gemcitabine are enhanced by co-administered iRGD peptide in murine pancreatic cancer models that overexpressed neuropilin-1.
Authors: Akashi Y, Oda T, Ohara Y, Miyamoto R, Kurokawa T, Hashimoto S, Enomoto T, Yamada K, Satake M, Ohkohchi N
Br J Cancer, 2014-02-20;110(6):1481-7.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
ITE and TCDD differentially regulate the vascular remodeling of rat placenta via the activation of AhR.
Authors: Wu Y, Chen X, Zhou Q, He Q, Kang J, Zheng J, Wang K, Duan T
PLoS ONE, 2014-01-24;9(1):e86549.
Species: Mouse, Rat
Sample Types: Whole Tissue
Applications: IHC, IHC-P -
Characterization of the adverse effects of nicotine on placental development: in vivo and in vitro studies.
Authors: Holloway, A C, Salomon, A, Soares, M J, Garnier, V, Raha, S, Sergent, F, Nicholson, C J, Feige, J J, Benharouga, M, Alfaidy, N
Am J Physiol Endocrinol Metab, 2013-12-24;306(4):E443-56.
Species: Rat
Sample Types: Tissue Homogenates
Applications: Western Blot -
Melanocyte-secreted fibromodulin promotes an angiogenic microenvironment.
Authors: Adini, Irit, Ghosh, Kaustabh, Adini, Avner, Chi, Zai-Long, Yoshimura, Takeru, Benny, Ofra, Connor, Kip M, Rogers, Michael, Bazinet, Lauren, Birsner, Amy E, Bielenberg, Diane R, D'Amato, Robert J
J Clin Invest, 2013-12-20;124(1):425-36.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain.
Authors: Zeng, L, He, X, Wang, Y, Tang, Y, Zheng, C, Cai, H, Liu, J, Wang, Y, Fu, Y, Yang, G-Y
Gene Ther, 2013-10-24;21(1):37-43.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The nucleus of endothelial cell as a sensor of blood flow direction
Authors: Eugene Tkachenko, Edgar Gutierrez, Semion K. Saikin, Per Fogelstrand, Chungho Kim, Alex Groisman et al.
Biology Open
-
Endoglin regulates the activation and quiescence of endothelium by participating in canonical and non-canonical TGF-beta signaling pathways.
Authors: Park S, Dimaio T, Liu W, Wang S, Sorenson C, Sheibani N
J Cell Sci, 2013-02-15;126(0):1392-405.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
TGF-beta mediates homing of bone marrow-derived human mesenchymal stem cells to glioma stem cells.
Authors: Shinojima N, Hossain A, Takezaki T, Fueyo J, Gumin J, Gao F, Nwajei F, Marini F, Andreeff M, Kuratsu J, Lang F
Cancer Res, 2013-01-30;73(7):2333-44.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The identification of different endothelial cell populations within the mouse proepicardium
Authors: Stephanie Cossette, Ravi Misra
Developmental Dynamics
-
The critical role of agrin in the hematopoietic stem cell niche.
Authors: Mazzon C, Anselmo A, Cibella J, Soldani C, Destro A, Kim N, Roncalli M, Burden SJ, Dustin ML, Sarukhan A, Viola A
Blood, 2011-06-07;118(10):2733-42.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Dysfunctional microvasculature as a consequence of shb gene inactivation causes impaired tumor growth.
Authors: Funa NS, Kriz V, Zang G, Calounova G, Akerblom B, Mares J, Larsson E, Sun Y, Betsholtz C, Welsh M
Cancer Res., 2009-02-17;69(5):2141-8.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Crosstalk between keratinocytes and adaptive immune cells in an IkappaBalpha protein-mediated inflammatory disease of the skin.
Authors: Rebholz B, Haase I, Eckelt B, Paxian S, Flaig MJ, Ghoreschi K, Nedospasov SA, Mailhammer R, Debey-Pascher S, Schultze JL, Weindl G, Forster I, Huss R, Stratis A, Ruzicka T, Rocken M, Pfeffer K, Schmid RM, Rupec RA
Immunity, 2007-08-09;27(2):296-307.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
RBFOX2 is required for establishing RNA regulatory networks essential for heart development
Authors: Verma SK, Deshmukh V, Thatcher K et al.
Nucleic acids research
-
An In Vivo CRISPR Screen Identifies Stepwise Genetic Dependencies of Metastatic Progression
Authors: Scheidmann MC, Castro-Giner F, Strittmatter K et al.
Cancer research
-
The endothelial dysfunction blocker CU06-1004 ameliorates choline-deficient L-amino acid diet-induced non-alcoholic steatohepatitis in mice
Authors: CR Bae, H Zhang, YG Kwon
PLoS ONE, 2020-12-04;15(12):e0243497.
-
Adaptable toolbox to characterize Alzheimer’s disease pathology in mouse models
Authors: Youtong H, Greg L
STAR Protocols
-
Radiation-Induced Changes in Tumor Vessels and Microenvironment Contribute to Therapeutic Resistance in Glioblastoma
Authors: Seo B, Jeon K, Moon S, et al.
Front Oncol
-
Intercrypt sentinel macrophages tune antibacterial NF-kappa B responses in gut epithelial cells via TNF
Authors: Hausmann A, Felmy B, Kunz L et al.
J Exp Med
-
CNS Pericytes Modulate Local T Cell Infiltration in EAE
Authors: K Koch, M Lindner, AK Fleck, M Liebmann, M Eschborn, L Zondler, R Diéguez-Hu, RH Adams, G Meyer Zu H, A Zarbock, T Kuhlmann, H Wiendl, L Klotz
International Journal of Molecular Sciences, 2022-10-28;23(21):.
-
Blockade of Macrophage CD147 Protects Against Foam Cell Formation in Atherosclerosis
Authors: Lv JJ, Wang H, Cui HY, et al.
Frontiers in cell and developmental biology
-
Distinct tumor architectures for metastatic colonization of the brain
Authors: S Gan, DG Macalinao, SH Shahoei, L Tian, X Jin, H Basnet, JT Muller, P Atri, E Seffar, W Chatila, AK Hadjantona, N Schultz, E Brogi, TA Bale, D Pe'er, J Massagué
bioRxiv : the preprint server for biology, 2023-01-28;0(0):.
-
YAP1 and TAZ negatively control bone angiogenesis by limiting hypoxia-inducible factor signaling in endothelial cells
Authors: Sivaraj KK, Dharmalingam B, Mohanakrishnan V et al.
Elife
-
Isolation Methods for Human CD34 Subsets Using Fluorescent and Magnetic Activated Cell Sorting: an In Vivo Comparative Study
Authors: Tripathi H, Peng H, Donahue R et al.
Stem Cell Rev Rep
-
RIPK3 modulates growth factor receptor expression in endothelial cells to support angiogenesis
Authors: Gao S, Griffin CT
Angiogenesis
-
A Type 2 Deiodinase-Dependent Increase in Vegfa Mediates Myoblast-Endothelial Cell Crosstalk During Skeletal Muscle Regeneration
Authors: Xingxing An, Ashley Ogawa-Wong, Colleen Carmody, Raffaele Ambrosio, Annunziata Gaetana Cicatiello, Cristina Luongo et al.
Thyroid
-
Macrophage Ablation Reduces M2-Like Populations and Jeopardizes Tumor Growth in a MAFIA-Based Glioma Model
Authors: Gabrusiewicz K, Hossain MB, Cortes-Santiago N et al.
Neoplasia
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse/Rat CD31/PECAM-1 Antibody
Average Rating: 4.9 (Based on 21 Reviews)
Have you used Human/Mouse/Rat CD31/PECAM-1 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Serial sections of murine lung tissue.
DAPI
GFP-vwf
RFP-alpha sma
CY5-cd31
DAPI-blue
GFP-cd31
RFP-alpha sma
Mouse lungs stained with DAPI (Cyan) and CD31 AF3628 (Red) at 1:200 dilution of primary and secondary antibodies.
PLP fixed 12 μm cryosection of mouse liver was stained 1:200 (from a stock of 0.2 mg/ml) overnight at room temperature (and with an antibody against Clec4f), followed by Donkey anti-goat CF488A @ 1:500 for 1h @ room temperature. Great staining of liver endothelium.
Adult pancreas cryostat sections stained with goat anti-CD31 and a donkey anti-goat AF488 (in green) as well as a directly conjugated rat anti-CD45-BV421 antibody (in blue). The CD31 staining works very well, clearly labelling pancreatic endothelium.
This antibody is good and easy to use!
Detection of CD31/PECAM‑1 in Mouse brown adipose tissue. 4% PFA fixed Brown adipose tissue was embedded in OCT and cryosectioned at 10µm. After permeabilization and blocking, tissue was incubated overnight at 4˚C with 2µg/ml of gt@CD31 (AF3628) and detected with Alexa fluor dk@gt 488 (1:1000) the next day. CD31 is presented in pseudo color violet here. This product is great, always very clear and bright. I often use it with 488 channel in tissues with strong autofluorescence such as hearts and quadriceps.
One of the best antibodies.
Used on E9.5-E12.5 cryo embedded mouse embryo sections (4% PFA fixed).
Attached picture is E12.5 embryo section.
Dilution used - 1:200
FFPE rat liver stained using AF3628 at 15 µg/mL overnight at 4C. Epitope retrieval was performed at 95 C for 15 minutes in citrate buffer pH 6.0. Secondary was donkey anti-goat Alexa Fluor 555 (psuedocolored red).
Optically cleared 1 mm mouse kidney section
Imaged by diSPIM in 60% TDE with 17X lens
Secondary antibody is donkey anti goat alexa 488
Red is Tubulin Beta 3 stained by alexa 568
Excellent!
FFPE mouse liver stained O/N at 4C with AF3826 at 10 ug/mL in 0.3% PBS-Tx. Secondary Donkey anti-goat Alexa Fluor 488 was applied for 1 hr RT at 2 ug/mL in PBS-Tx. Citrate buffer pH 6.0 used for epitope retrieval.
WD [1:500], HIER with DIVA; secondary ab RTU Polymer-HRP