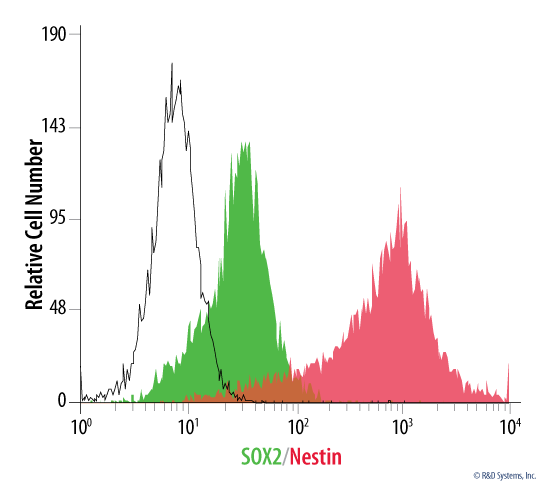
Neural stem cells provide an excellent model for research focused on neural development and neurological disorders. R&D Systems offers ready-to-use primary cortical stem cells isolated from E14.5 Sprague-Dawley rats. In addition, primary mouse cortical stem cells isolated from E14.5 CD-1 mice are available. Every lot of R&D Systems Cortical Stem Cells is validated for a high level of Nestin expression and the capacity for multi-lineage differentiation into astrocytes, neurons, and oligodendrocytes. Our cortical stem cells are tested to ensure highest quality and lot to lot consistency. Both rat and mouse Cortical Stem Cells can be optimally expanded as monolayers or neurospheres.
To complement the use of primary neural stem cells, we offer a range of supportive products, including culture media which is specifically optimized for use with neural stem cells. We offer kits to promote the in vitro proliferation of neural precursors, and kits to differentiate neural stem cells into dopaminergic neurons or oligodendrocytes. In addition, kits are available which contain panels of antibodies designed to monitor the differentiation and identification of neural precursors, astrocytes, neurons, and oligodendrocytes.