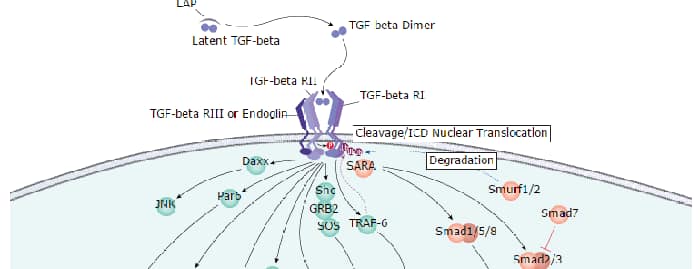
Endoglin (CD105) is a 90 kDa type I transmembrane glycoprotein of the zona pellucida (ZP) family of proteins (1-3). Endoglin and betaglycan/T beta RIII are type III receptors for TGF beta superfamily ligands, sharing 71% amino acid (aa) identity within the transmembrane (TM) and cytoplasmic domains. Endoglin is highly expressed on proliferating vascular endothelial cells, chondrocytes, and syncytiotrophoblasts of term placenta, with lower amounts on hematopoietic, mesenchymal and neural crest stem cells, activated monocytes, and lymphoid and myeloid leukemic cells (2-5). Mouse Endoglin cDNA encodes 653 aa including a 26 aa signal sequence, a 555 aa extracellular domain (ECD) with an orphan domain and a two-part ZP domain, a TM domain, and a 47 aa cytoplasmic domain (1-3). A mouse isoform with a 35 aa cytoplasmic domain (S-endoglin) can oppose effects of long (L) Endoglin (6, 7). The mouse Endoglin ECD shares 69%, 84%, 62%, 63%, and 66% aa identity with human, rat, bovine, porcine, and canine Endoglin, respectively. Endoglin homodimers interact with TGF-beta 1 and TGF-beta 3 (but not TGF-beta 2) but only after binding T beta RII (8). Similarly, they interact with activin-A and BMP-7 via activin type IIA or B receptors, and with BMP-2 via BMPR-1A/ALK-3 or BMPR-1B/ALK-6 (9). BMP-9, however, is reported to bind Endoglin directly (10). Endoglin modifies ligand-induced signaling in multiple ways. For example, expression of Endoglin can inhibit TGF-beta 1 signals but enhance BMP7 signals in the same myoblast cell line (11). In endothelial cells, Endoglin inhibits T beta RI/ALK5, but enhances ALK1-mediated activation (12). Deletion of mouse Endoglin causes lethal vascular and cardiovascular defects, and human Endoglin haploinsufficiency can a cause the vascular disorder, hereditary hemorrhagic telangiectasia type I (13, 14). These abnormalities confirm the essential function of Endoglin in differentiation of smooth muscle, angiogenesis, and neovascularization (2-4, 12-14). In preeclampsia of pregnancy, high levels of proteolytically generated soluble Endoglin and VEGF R1 (sFlt-1), along with low placental growth factor (PlGF), are pathogenic due to antiangiogenic activity (15).


Key Product Details
Validated by
Species Reactivity
Validated:
Cited:
Applications
Validated:
Cited:
Label
Antibody Source
Product Specifications
Immunogen
Glu27-Gly581
Accession # Q8K100
Specificity
Clonality
Host
Isotype
Scientific Data Images for Mouse Endoglin/CD105 Antibody
Detection of Mouse Endoglin/CD105 by Western Blot.
Western blot shows lysates of bEnd.3 mouse endothelioma cell line and MS-1 mouse pancreatic islet endothelial cell line. PVDF membrane was probed with 0.5 µg/mL of Goat Anti-Mouse Endoglin/CD105 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1320) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for Endoglin/CD105 at approximately 90-95 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
Endoglin/CD105 in MS-1 Mouse Cell Line.
Endoglin/CD105 was detected in immersion fixed MS-1 mouse pancreatic islet endothelial cell line using Goat Anti-Mouse Endoglin/CD105 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1320) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (yellow; Catalog # NL001) and counter-stained with DAPI (blue). View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
Endoglin/CD105 in Rat Mesenchymal Stem Cells.
Endoglin/CD105 was detected in immersion fixed rat mesenchymal stem cells using Goat Anti-Mouse Endoglin/CD105 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1320) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; Catalog # NL001) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
Endoglin/CD105 in Mouse Embryo.
Endoglin/CD105 was detected in immersion fixed frozen sections of mouse embryo (E13-15) using Goat Anti-Mouse Endoglin/CD105 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1320) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). Lower panel shows a lack of labeling if primary antibodies are omitted and tissue is stained only with secondary antibody followed by incubation with detection reagents. View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
Detection of Mouse Endoglin/CD105 by Simple WesternTM.
Simple Western lane view shows lysates of bEnd.3 mouse endothelioma cell line, loaded at 0.2 mg/mL. A specific band was detected for Endoglin/CD105 at approximately 121 kDa (as indicated) using 5 µg/mL of Goat Anti-Mouse Endoglin/CD105 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1320) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
Detection of Porcine Endoglin/CD105 by Immunohistochemistry
Chronic intermittent hypoxia modifies the BM vascular structure. a’–e’ Representative images of femur bone marrow stained with vWF, CD105, VE-cadherin, SMA, and CD11b counterstained with hematoxylin. a”, c”, d” BM from CIH exposed rats (n = 6) has more VE-cadherin+ vessels and SMA coverage but less vWF+ sinusoids (400×, Leica DM2500). e’, e” Representative images of CD11b immunohistochemistry in femur BM show an increase in BM monocyte count in CIH exposed animals. (400×, Leica DM2500) a’, a”’, b’, b” No changes in the total number of vessels or in megakaryocyte count were observed, as accounted by CD105 and vWF staining, respectively. Results are represented as the mean ± SD of bone marrow sections from six male Wistar rats (*p < 0.05; **p < 0.01). f Representative images of femur bone marrow fluorescently immunostained for VE-cadherin show an increase in total VE-cadherin vessels and in VE-cadherin vessel coverage. Scale bar, 50 μm (insets magnified 2.5×). Images were acquired with a Zeiss LSM 510 META microscope. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/26856724), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of Mouse Endoglin/CD105 by Immunohistochemistry
Increased tumor necrosis and reduced tumor vascularization after GET of therapeutic plasmids in vivo.Histologically stained and analyzed sections after treatments of TS/A tumors: intratumoral injection of endotoxin-free water alone (control group; CTRL) or in combination with the application of electric pulses (EP group), injection of plasmid pET-antiCD105 (TS group), pU6-antiCD105 (CON group) or pU6-SCR (SCR group) alone or combined with the application of electric pulses (GET of TS plasmid; GET of CON plasmid; GET of SCR plasmid). Triple GET of CON or TS plasmid increased necrosis (HE) and reduced the number of blood vessels (CD105) in TS/A tumors. The percentage of necrosis (upper graph) was statistically significantly increased (*p<0.05) in both of the therapeutic groups (GET of CON and TS plasmid) vs. all the pertinent control groups, with no difference between therapies, also seen in histological sections. The reduced number of blood vessels (CD105) was observed in histological sections of both therapeutic groups that were (lower graph) non-statistically significant to each other after analysis, although the reduction was statistically significant (*p<0.05) vs. all the pertinent control groups. The results represent one experiment, n = 3–4 mice for each experimental group and at least 5 analyzed fields of view for each mouse. The data represent AM ± SEM. N.S. represents statistically non-significant difference between the therapeutic groups. Scale bar = 100 μm. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0124913), licensed under a CC-BY license. Not internally tested by R&D Systems.Detection of Mouse Endoglin/CD105 by Immunohistochemistry
Increased tumor necrosis and reduced tumor vascularization after GET of therapeutic plasmids in vivo.Histologically stained and analyzed sections after treatments of TS/A tumors: intratumoral injection of endotoxin-free water alone (control group; CTRL) or in combination with the application of electric pulses (EP group), injection of plasmid pET-antiCD105 (TS group), pU6-antiCD105 (CON group) or pU6-SCR (SCR group) alone or combined with the application of electric pulses (GET of TS plasmid; GET of CON plasmid; GET of SCR plasmid). Triple GET of CON or TS plasmid increased necrosis (HE) and reduced the number of blood vessels (CD105) in TS/A tumors. The percentage of necrosis (upper graph) was statistically significantly increased (*p<0.05) in both of the therapeutic groups (GET of CON and TS plasmid) vs. all the pertinent control groups, with no difference between therapies, also seen in histological sections. The reduced number of blood vessels (CD105) was observed in histological sections of both therapeutic groups that were (lower graph) non-statistically significant to each other after analysis, although the reduction was statistically significant (*p<0.05) vs. all the pertinent control groups. The results represent one experiment, n = 3–4 mice for each experimental group and at least 5 analyzed fields of view for each mouse. The data represent AM ± SEM. N.S. represents statistically non-significant difference between the therapeutic groups. Scale bar = 100 μm. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0124913), licensed under a CC-BY license. Not internally tested by R&D Systems.Applications for Mouse Endoglin/CD105 Antibody
CyTOF-ready
Flow Cytometry
Sample: MS-1 mouse cell line
Immunocytochemistry
Sample: Immersion fixed MS-1 mouse pancreatic islet endothelial cell line and rat mesenchymal stem cells
Immunohistochemistry
Sample: Immersion fixed frozen sections of mouse embryo (E13-15)
Simple Western
Sample: bEnd.3 mouse endothelioma cell line
Western Blot
Sample: bEnd.3 mouse endothelioma cell line and MS‑1 mouse pancreatic islet endothelial cell line
Reviewed Applications
Read 4 reviews rated 4.3 using AF1320 in the following applications:
Flow Cytometry Panel Builder
Bio-Techne Knows Flow Cytometry
Save time and reduce costly mistakes by quickly finding compatible reagents using the Panel Builder Tool.
Advanced Features
- Spectra Viewer - Custom analysis of spectra from multiple fluorochromes
- Spillover Popups - Visualize the spectra of individual fluorochromes
- Antigen Density Selector - Match fluorochrome brightness with antigen density
Formulation, Preparation, and Storage
Purification
Reconstitution
Reconstitute at 0.2 mg/mL in sterile PBS. For liquid material, refer to CoA for concentration.
Formulation
Shipping
Stability & Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Calculators
Background: Endoglin/CD105
References
- Ge, A.Z. and E.C. Butcher (1994) Gene 138:201.
- ten Dijke, P. et al. (2008) Angiogenesis 11:79.
- Bernabeu, C. et al. (2007) J. Cell. Biochem. 102:1375.
- Mancini, M.L. et al. (2007) Dev. Biol. 308:520.
- Moody, J.L. et al. (2007) Stem Cells 25:2809.
- Velasco, S. et al. (2008) J. Cell Sci. 121:913.
- Perez-Gomez, E. et al. (2005) Oncogene 24:4450.
- Cheifetz, S, et al. (1992) J. Biol. Chem. 267:19027.
- Barbara, N.P. et al. (1999) J. Biol. Chem. 274:584.
- Scharpfenecker, M. et al. (2007) J. Cell Sci. 120:964.
- Scherner, O. et al. (2007) J. Biol. Chem. 282:13934.
- Pece-Barbara, N. et al. (2005) J. Biol. Chem. 280:27800.
- Arthur, H.M. et al. (2000) Dev. Biol. 217:42.
- Lebrin, F. and C.L. Mummery (2008) Trends Cardiovasc. Med. 18:25.
- Venkatesha, S. et al. (2006) Nat. Med. 12:642.
Alternate Names
Gene Symbol
UniProt
Additional Endoglin/CD105 Products
Product Documents for Mouse Endoglin/CD105 Antibody
Product Specific Notices for Mouse Endoglin/CD105 Antibody
For research use only
Citations for Mouse Endoglin/CD105 Antibody
Customer Reviews for Mouse Endoglin/CD105 Antibody (4)
Have you used Mouse Endoglin/CD105 Antibody?
Submit a review and receive an Amazon gift card!
$25/€18/£15/$25CAN/¥2500 Yen for a review with an image
$10/€7/£6/$10CAN/¥1110 Yen for a review without an image
Submit a review
Customer Images
-
Application: Western BlotSample Tested: Cell lysate from colon cancer cell lineSpecies: MouseVerified Customer | Posted 02/10/2024
-
Application: Immunocytochemistry/ImmunofluorescenceSample Tested: Embryonic heartSpecies: MouseVerified Customer | Posted 05/16/2017Block - 5% secondary specific serum + 1% BSA + 0.5% Tween-20 - 2hrs at RT Primary Ab - diluted in block - 1 in 25 - for O/N at 4'C Secondary ab - diluted in block - 2hrs at RT
-
Application: ImmunofluorescenceSample Tested: See PMID 23593103Species: MouseVerified Customer | Posted 01/05/2015
-
Application: ImmunofluorescenceSample Tested: See PMID 21060150Species: MouseVerified Customer | Posted 01/05/2015
There are no reviews that match your criteria.
Protocols
Find general support by application which include: protocols, troubleshooting, illustrated assays, videos and webinars.
- 7-Amino Actinomycin D (7-AAD) Cell Viability Flow Cytometry Protocol
- Antigen Retrieval Protocol (PIER)
- Antigen Retrieval for Frozen Sections Protocol
- Appropriate Fixation of IHC/ICC Samples
- Cellular Response to Hypoxia Protocols
- Chromogenic IHC Staining of Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Protocol
- Chromogenic Immunohistochemistry Staining of Frozen Tissue
- Detection & Visualization of Antibody Binding
- Extracellular Membrane Flow Cytometry Protocol
- Flow Cytometry Protocol for Cell Surface Markers
- Flow Cytometry Protocol for Staining Membrane Associated Proteins
- Flow Cytometry Staining Protocols
- Flow Cytometry Troubleshooting Guide
- Fluorescent IHC Staining of Frozen Tissue Protocol
- Graphic Protocol for Heat-induced Epitope Retrieval
- Graphic Protocol for the Preparation and Fluorescent IHC Staining of Frozen Tissue Sections
- Graphic Protocol for the Preparation and Fluorescent IHC Staining of Paraffin-embedded Tissue Sections
- Graphic Protocol for the Preparation of Gelatin-coated Slides for Histological Tissue Sections
- ICC Cell Smear Protocol for Suspension Cells
- ICC Immunocytochemistry Protocol Videos
- ICC for Adherent Cells
- IHC Sample Preparation (Frozen sections vs Paraffin)
- Immunocytochemistry (ICC) Protocol
- Immunocytochemistry Troubleshooting
- Immunofluorescence of Organoids Embedded in Cultrex Basement Membrane Extract
- Immunofluorescent IHC Staining of Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Protocol
- Immunohistochemistry (IHC) and Immunocytochemistry (ICC) Protocols
- Immunohistochemistry Frozen Troubleshooting
- Immunohistochemistry Paraffin Troubleshooting
- Intracellular Flow Cytometry Protocol Using Alcohol (Methanol)
- Intracellular Flow Cytometry Protocol Using Detergents
- Intracellular Nuclear Staining Flow Cytometry Protocol Using Detergents
- Intracellular Staining Flow Cytometry Protocol Using Alcohol Permeabilization
- Intracellular Staining Flow Cytometry Protocol Using Detergents to Permeabilize Cells
- Preparing Samples for IHC/ICC Experiments
- Preventing Non-Specific Staining (Non-Specific Binding)
- Primary Antibody Selection & Optimization
- Propidium Iodide Cell Viability Flow Cytometry Protocol
- Protocol for Heat-Induced Epitope Retrieval (HIER)
- Protocol for Making a 4% Formaldehyde Solution in PBS
- Protocol for VisUCyte™ HRP Polymer Detection Reagent
- Protocol for the Characterization of Human Th22 Cells
- Protocol for the Characterization of Human Th9 Cells
- Protocol for the Fluorescent ICC Staining of Cell Smears - Graphic
- Protocol for the Fluorescent ICC Staining of Cultured Cells on Coverslips - Graphic
- Protocol for the Preparation & Fixation of Cells on Coverslips
- Protocol for the Preparation and Chromogenic IHC Staining of Frozen Tissue Sections
- Protocol for the Preparation and Chromogenic IHC Staining of Frozen Tissue Sections - Graphic
- Protocol for the Preparation and Chromogenic IHC Staining of Paraffin-embedded Tissue Sections
- Protocol for the Preparation and Chromogenic IHC Staining of Paraffin-embedded Tissue Sections - Graphic
- Protocol for the Preparation and Fluorescent ICC Staining of Cells on Coverslips
- Protocol for the Preparation and Fluorescent ICC Staining of Non-adherent Cells
- Protocol for the Preparation and Fluorescent ICC Staining of Stem Cells on Coverslips
- Protocol for the Preparation and Fluorescent IHC Staining of Frozen Tissue Sections
- Protocol for the Preparation and Fluorescent IHC Staining of Paraffin-embedded Tissue Sections
- Protocol for the Preparation of Gelatin-coated Slides for Histological Tissue Sections
- Protocol for the Preparation of a Cell Smear for Non-adherent Cell ICC - Graphic
- Protocol: Annexin V and PI Staining by Flow Cytometry
- Protocol: Annexin V and PI Staining for Apoptosis by Flow Cytometry
- R&D Systems Quality Control Western Blot Protocol
- TUNEL and Active Caspase-3 Detection by IHC/ICC Protocol
- The Importance of IHC/ICC Controls
- Troubleshooting Guide: Fluorokine Flow Cytometry Kits
- Troubleshooting Guide: Immunohistochemistry
- Troubleshooting Guide: Western Blot Figures
- Western Blot Conditions
- Western Blot Protocol
- Western Blot Protocol for Cell Lysates
- Western Blot Troubleshooting
- Western Blot Troubleshooting Guide
- View all Protocols, Troubleshooting, Illustrated assays and Webinars