Human IL-2 Antibody Summary
Ala21-Thr153
Accession # NP_000577
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
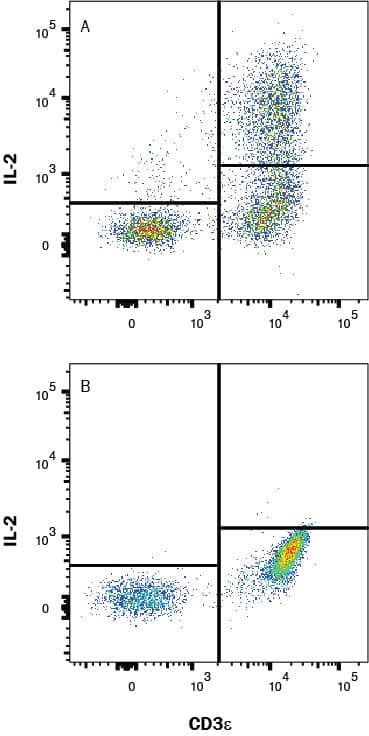
Detection of IL‑2 in PBMC's treated with PMA and Ca2+ ionomycin vs untreated PBMC's by Flow Cytometry PBMC's treated with PMA (50ng/mL) and Ca2+ ionomycin (200ng/mL) overnight (A) vs untreated PBMC's (B) were stained with Mouse Anti-Human IL‑2 Monoclonal Antibody (Catalog # mab202) and Mouse Anti-Human CD3 epsilon APC‑conjugated Monoclonal Antibody (Catalog # FAB100A) followed by Phycoerythrin-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # F0102B). To facilitate intracellular staining, cells were fixed with Flow Cytometry Fixation Buffer (Catalog # FC004) and permeabilized with Flow Cytometry Permeabilization/Wash Buffer I (Catalog # FC005). View our protocol for Staining Intracellular Molecules.
 View Larger
View Larger
Cell Proliferation Induced by IL‑2 and Neutralization by Human IL‑2 Antibody. Human IL 2 Antibody (Catalog # MAB202) neutralizes IL-2-induced proliferation in the CTLL-2 mouse cytotoxic T cell line. The Neuralization Dose (ND50) is typically 5.00-60.0 ng/mL.
 View Larger
View Larger
IL‑2 in Human PBMCs. IL-2 was detected in immersion fixed human peripheral blood mononuclear cells (PBMCs) treated with calcium ionomycin and PMA using Mouse Anti-Human IL-2 Monoclonal Antibody (Catalog # MAB202) at 8 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (red; Catalog # NL007) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. View our protocol for Fluorescent ICC Staining of Non-adherent Cells.
 View Larger
View Larger
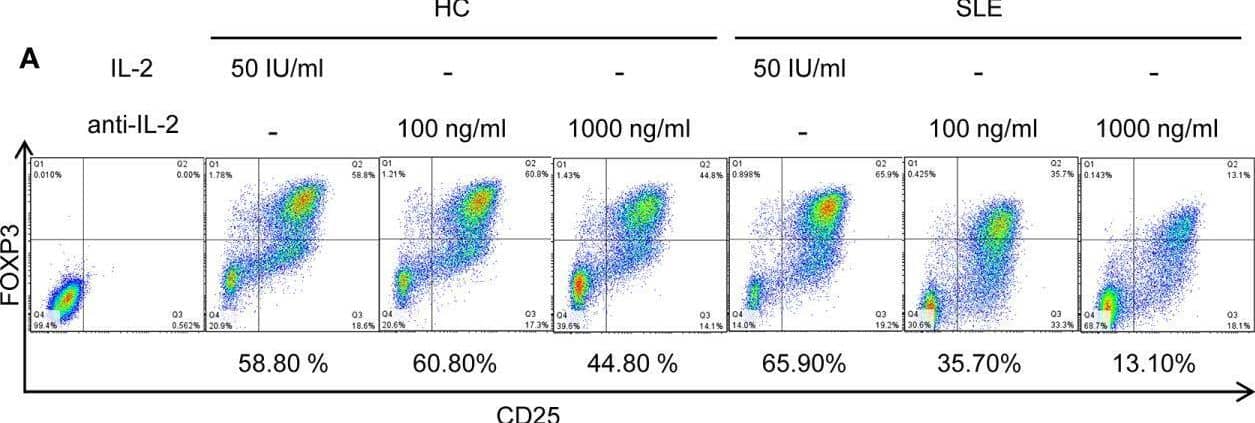
Detection of Human IL-2 by Flow Cytometry SLE CD4+ T cells are poised to activate IL-2 signaling during Treg differentiation. (A) Naïve CD4+ T cells were isolated from a systemic lupus erythematosus (SLE) patient & matched healthy control (HC) subject, & cultured for 3 days in the presence of anti-CD3/CD28 & TGF-beta (5 ng/ml) with IL-2 (50 IU/ml) or anti-IL-2 (100 or 1,000 ng/ml). The frequency of CD4+CD25+FOXP3+ cells was determined by flow cytometry. Numbers below the plots represent the frequency of CD4+CD25+FOXP3+ Tregs. The dot plots on the left end represent isotype control staining. (B) CD4+ T cells isolated from matched SLE & HC subjects were cultured for 3 days in the presence of anti-CD3/CD28 & TGF-beta (20 ng/ml) with or without IL-2 (100 IU/ml) or anti-IL-2 (100 ng/ml). Total STAT5 & its phosphorylation at tyrosine 694 were detected by immunoblotting. Representative immunoblot staining (left panel). The signal intensity of phospho-STAT5 & total STAT5 was normalized to that of actin. The normalized pSTAT5 signal intensity (middle panel) & the ratio of normalized pSTAT5 signal intensity over normalized STAT5 signal intensity (right panel) from 3 pairs of matched HC & SLE subjects. (C) Untouched T cells from matched SLE & HC subjects were cultured for 3 days without anti-CD3/CD28 stimulation. Expression of CD25 & FOXP3 in CD4+ cells were determined by flow cytometry. Representative flow cytometry dot plots are shown (left panel). Cumulative data of frequency of CD4+CD25+FOXP3+ & CD4+CD25+ cells, mean fluorescence intensity (MFI) of CD25 expression in CD4+ T cells, & the proportion of CD4+CD25+FOXP3+ cells among CD4+CD25+ cells from 17 pairs of matched SLE & HC subjects (right panel). Data were analyzed by a paired two-tailed t-test (*p<0.05, **p<0.01, ****p<0.0001). Image collected & cropped by CiteAb from the following open publication (https://www.frontiersin.org/articles/10.3389/fimmu.2021.635531/full), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
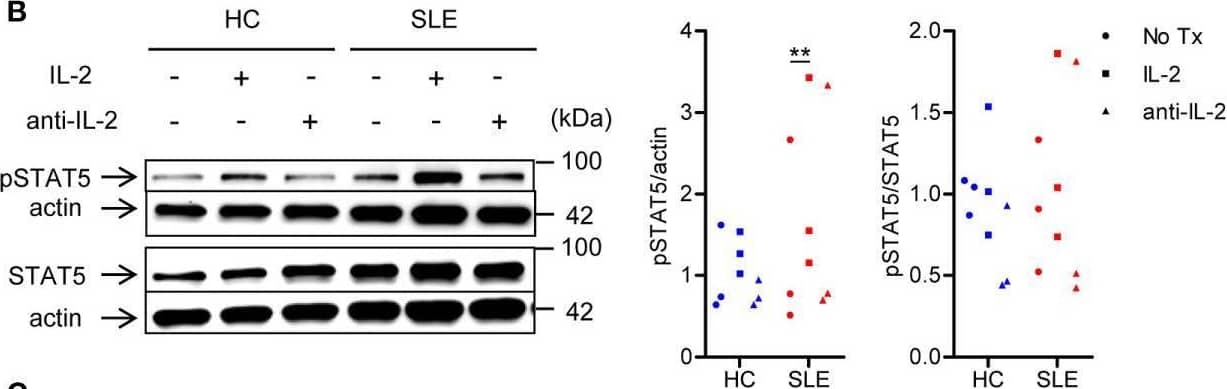
Detection of Human IL-2 by Western Blot SLE CD4+ T cells are poised to activate IL-2 signaling during Treg differentiation. (A) Naïve CD4+ T cells were isolated from a systemic lupus erythematosus (SLE) patient & matched healthy control (HC) subject, & cultured for 3 days in the presence of anti-CD3/CD28 & TGF-beta (5 ng/ml) with IL-2 (50 IU/ml) or anti-IL-2 (100 or 1,000 ng/ml). The frequency of CD4+CD25+FOXP3+ cells was determined by flow cytometry. Numbers below the plots represent the frequency of CD4+CD25+FOXP3+ Tregs. The dot plots on the left end represent isotype control staining. (B) CD4+ T cells isolated from matched SLE & HC subjects were cultured for 3 days in the presence of anti-CD3/CD28 & TGF-beta (20 ng/ml) with or without IL-2 (100 IU/ml) or anti-IL-2 (100 ng/ml). Total STAT5 & its phosphorylation at tyrosine 694 were detected by immunoblotting. Representative immunoblot staining (left panel). The signal intensity of phospho-STAT5 & total STAT5 was normalized to that of actin. The normalized pSTAT5 signal intensity (middle panel) & the ratio of normalized pSTAT5 signal intensity over normalized STAT5 signal intensity (right panel) from 3 pairs of matched HC & SLE subjects. (C) Untouched T cells from matched SLE & HC subjects were cultured for 3 days without anti-CD3/CD28 stimulation. Expression of CD25 & FOXP3 in CD4+ cells were determined by flow cytometry. Representative flow cytometry dot plots are shown (left panel). Cumulative data of frequency of CD4+CD25+FOXP3+ & CD4+CD25+ cells, mean fluorescence intensity (MFI) of CD25 expression in CD4+ T cells, & the proportion of CD4+CD25+FOXP3+ cells among CD4+CD25+ cells from 17 pairs of matched SLE & HC subjects (right panel). Data were analyzed by a paired two-tailed t-test (*p<0.05, **p<0.01, ****p<0.0001). Image collected & cropped by CiteAb from the following open publication (https://www.frontiersin.org/articles/10.3389/fimmu.2021.635531/full), licensed under a CC-BY license. Not internally tested by R&D Systems.
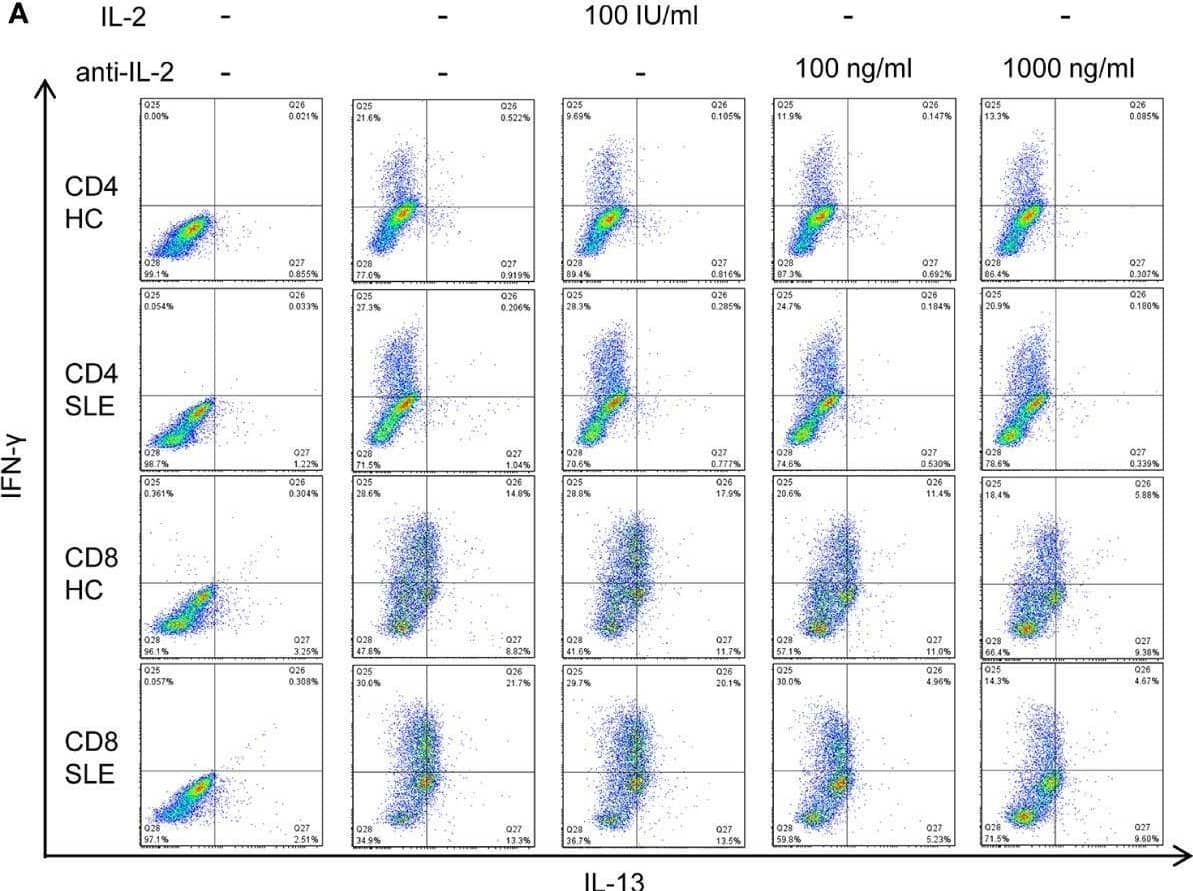 View Larger
View Larger
Detection of Human IL-2 by Flow Cytometry IL-2 expands IL-13+IFN-gamma + CD8+ T cells in systemic lupus erythematosus (SLE). (A) CD4+ and CD8+ T cells from matched SLE and health control (HC) subjects were cultured as described in Figure 2, and IL-13 and IFN-gamma expression was determined by flow cytometry. (B) Cumulative data of mean fluorescence intensity (MFI) of IFN-gamma and the frequency of IFN-gamma + and IL-13+IFN-gamma + cells. Statistical analysis was made by two-way ANOVA followed by Bonferroni’s correction for multiple comparisons (*p<0.05, **p<0.01, ****p<0.0001). Image collected and cropped by CiteAb from the following open publication (https://www.frontiersin.org/articles/10.3389/fimmu.2021.635531/full), licensed under a CC-BY license. Not internally tested by R&D Systems.
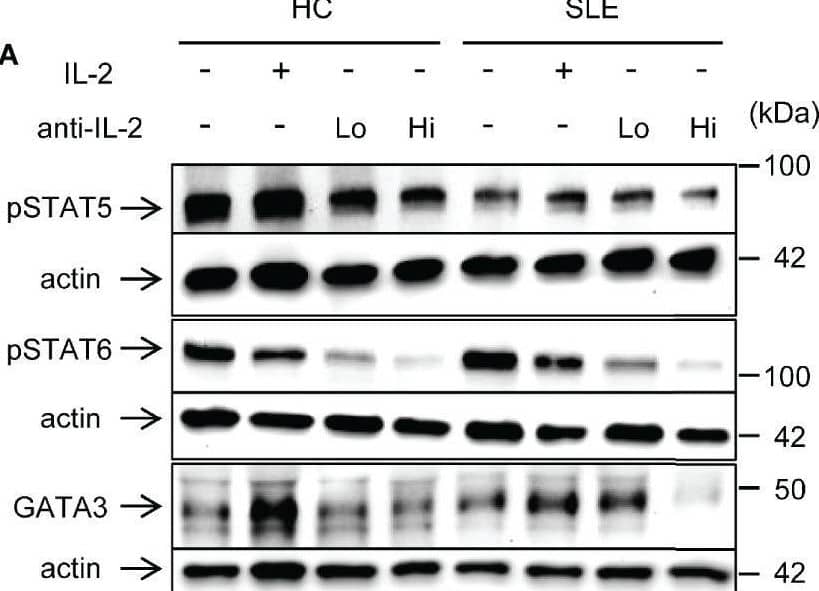 View Larger
View Larger
Detection of Human IL-2 by Western Blot IL-2 induces STAT6 phosphorylation and GATA3 expression in systemic lupus erythematosus (SLE) CD8+ T cells. (A) CD8+ T cells from matched SLE and health control (HC) subjects were cultured as described in Figure 2. Expression of GATA-3 and phosphorylation of STAT5 at tyrosine 694 and STAT6 at tyrosine 641 were detected by immunoblotting. Representative immunoblot staining was presented. Lo and Hi concentrations of anti-IL-2 denote 100 and 1,000 ng/ml, respectively. (B) The signal intensity of phospho-STAT5, phospho-STAT6, and GATA-3 were normalized to that of actin. Cumulative data from 9 pairs of matched HC and SLE subjects. Data were analyzed by a two-tailed t-test (*p<0.05, **p<0.01, ***p<0.001). (C) Pearson’s and Spearman’s correlation analyses were performed to determine the association between the expression of cytokines (IL-13, IL-5, and IFN-gamma ) and transcription factors (phospho-STAT5, phospho-STAT6, and GATA-3). The blue and red plots represent data from HC and SLE patients, respectively. Spearman correlation coefficient was presented for the association between IL-13 and phospho-STAT5, IL-5 and phospho-STAT-5, and IFN-gamma and GATA-3. Pearson correlation coefficient was presented for the remainder of associations. Image collected and cropped by CiteAb from the following open publication (https://www.frontiersin.org/articles/10.3389/fimmu.2021.635531/full), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
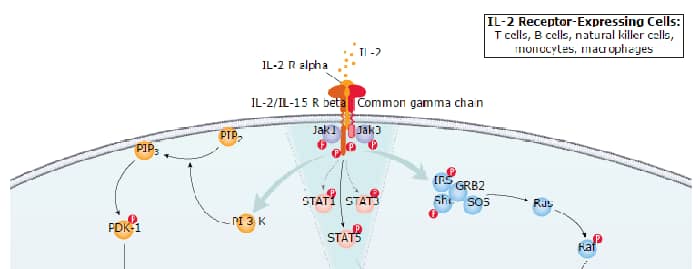
Background: IL-2
Interleukin 2 (IL-2) is a cytokine that stimulates the growth and differentiation of B cells, T cells, NK cells, and monocyte/macrophages. It functions through the heterotrimeric IL-2 receptor comprising alpha, beta, and gamma chains.
Product Datasheets
Citations for Human IL-2 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
26
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Induction of tumor cell autosis by myxoma virus-infected CAR-T and TCR-T cells to overcome primary and acquired resistance
Authors: Zheng N, Fang J, Xue G et al.
Cancer cell
-
A B7-H3-targeted CD28 bispecific antibody enhances the activity of anti-PD1 and CD3 T-cell engager immunotherapies
Authors: Moore, GL;Zeng, VG;Diaz, JE;Bonzon, C;Avery, KN;Rashid, R;Qi, J;Nam, DH;Jacinto, J;Dragovich, MA;Kim, YK;Balcazar, KP;Bakhit, CG;Eivazi, A;Nguyen, H;Muchhal, US;Szymkowski, DE;Desjarlais, JR;Hedvat, M;
Molecular cancer therapeutics
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
CXCL13-producing CD4+ T cells accumulate in early phase of tertiary lymphoid structures in ovarian cancer
Authors: M Ukita, J Hamanishi, H Yoshitomi, K Yamanoi, S Takamatsu, A Ueda, H Suzuki, Y Hosoe, Y Furutake, M Taki, K Abiko, K Yamaguchi, H Nakai, T Baba, N Matsumura, A Yoshizawa, H Ueno, M Mandai
JCI Insight, 2022-06-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Ectopic Lymphoid Follicle Formation and Human Seasonal Influenza Vaccination Responses Recapitulated in an Organ‐on‐a‐Chip
Authors: Girija Goyal, Pranav Prabhala, Gautam Mahajan, Bruce Bausk, Tal Gilboa, Liangxia Xie et al.
Advanced Science
-
Fine-tuned long-acting interleukin-2 superkine potentiates durable immune responses in mice and non-human primate
Authors: Rosemina Merchant, Carole Galligan, Manjunatha Ankathatti Munegowda, L Bruce Pearce, Peter Lloyd, Paul Smith et al.
Journal for ImmunoTherapy of Cancer
-
Immunogenicity profile in African green monkeys of a vaccine candidate based on a mutated form of human Interleukin-15
Authors: Y Rodríguez-, LG Batista-Ro, A Llopiz-Arz, P Puente-Pér, R Martínez-C, J Castro-Vel, A Santos-Sav
BMC immunology, 2021-12-18;22(1):79.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Double-Edged Sword: Interleukin-2 Promotes T Regulatory Cell Differentiation but Also Expands Interleukin-13- and Interferon-gamma -Producing CD8+ T Cells via STAT6-GATA-3 Axis in Systemic Lupus Erythematosus
Authors: Hiroshi Kato, Andras Perl
Frontiers in Immunology
-
An IL-2-grafted antibody immunotherapy with potent efficacy against metastatic cancer
Authors: D Sahin, N Arenas-Ram, M Rath, U Karakus, M Hümbelin, M van Gogh, L Borsig, O Boyman
Nature Communications, 2020-12-22;11(1):6440.
Species: Human, Mouse
Sample Types: Recombinant Protein, Serum
Applications: ELISA Detection -
Hypothermic Ex Situ Perfusion of Human Limbs With Acellular Solution for 24 Hours
Authors: Valentin Haug, Branislav Kollar, Sotirios Tasigiorgos, Yori Endo, Martin Kauke, Ali-Farid Safi et al.
Transplantation
-
Suppressor of cytokine signaling 3 is crucial for interleukin-7 receptor re-expression after T-cell activation and interleukin-7 dependent proliferation
Authors: A Güler, ML Venegas, E Adankwah, E Mayatepek, N Nausch, M Jacobsen
Eur. J. Immunol., 2019-11-14;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Effects of pepsin and pepstatin on reflux tonsil hypertrophy in vitro
Authors: JH Kim, SJ Jang, JW Yun, MH Jung, SH Woo
PLoS ONE, 2018-11-08;13(11):e0207090.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Blocking IL-2 Signal In Vivo with an IL-2 Antagonist Reduces Tumor Growth through the Control of Regulatory T Cells
Authors: T Carmenate, Y Ortíz, M Enamorado, K García-Mar, J Avellanet, E Moreno, L Graça, K León
J. Immunol., 2018-04-04;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
IL-23 and IL-1? Drive Human Th17 Cell Differentiation and Metabolic Reprogramming in Absence of CD28 Costimulation
Authors: S Revu, J Wu, M Henkel, N Rittenhous, A Menk, GM Delgoffe, AC Poholek, MJ McGeachy
Cell Rep, 2018-03-06;22(10):2642-2653.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Rescue of Tolerant CD8+ T Cells during Cancer Immunotherapy with IL2:Antibody Complexes
Authors: Lauryn E. Klevorn, Melissa M. Berrien-Elliott, Jinyun Yuan, Lindsey M. Kuehm, Gregory D. Felock, Sean A. Crowe et al.
Cancer Immunology Research
Species: Xenograft
Sample Types: In Vivo
Applications: In vivo assay -
Enhanced immune response of MAIT cells in tuberculous pleural effusions depends on cytokine signaling
Sci Rep, 2016-09-02;6(0):32320.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, Neutralization -
Activin A programs the differentiation of human TFH cells
Nat Immunol, 2016-07-04;17(8):976-84.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A systemic defect in Toll-like receptor 4 signaling increases lipopolysaccharide-induced suppression of IL-2-dependent T-cell proliferation in COPD
Authors: Jürgen Knobloch, Sarah-Jane Chikosi, Sarah Yanik, Jan Rupp, David Jungck, Andrea Koch
American Journal of Physiology-Lung Cellular and Molecular Physiology
-
Targeting the binding interface on a shared receptor subunit of a cytokine family enables the inhibition of multiple member cytokines with selectable target spectrum.
Authors: Nata T, Basheer A, Cocchi F, van Besien R, Massoud R, Jacobson S, Azimi N, Tagaya Y
J Biol Chem, 2015-07-16;290(37):22338-51.
Species: Human
Sample Types: Protein
Applications: Neutralization -
Interleukin-2 alters distribution of CD144 (VE-cadherin) in endothelial cells.
Authors: Kim, Dae Won, Zloza, Andrew, Broucek, Joseph, Schenkel, Jason M, Ruby, Carl, Samaha, Georges, Kaufman, Howard L
J Transl Med, 2014-05-06;12(0):113.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
ICOS-LICOS interaction is critically involved in TGN1412-mediated T-cell activation.
Blood, 2012-05-10;119(26):6268-77.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Aptamer-Functionalized Microgel Particles for Protein Detection
Authors: Rathi L. Srinivas, Stephen C. Chapin, Patrick S. Doyle
Analytical Chemistry
-
Adenovirus-specific human T cells are pervasive, polyfunctional, and cross-reactive.
Authors: Hutnick NA, Carnathan D, Demers K, Makedonas G, Ertl HC, Betts MR
Vaccine, 2010-02-23;28(8):1932-41.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
T cell-dependent survival of CD20+ and CD20- plasma cells in human secondary lymphoid tissue.
Authors: Withers DR, Fiorini C, Fischer RT, Ettinger R, Lipsky PE, Grammer AC
Blood, 2007-02-13;109(11):4856-64.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Primary cutaneous T-cell lymphomas show a deletion or translocation affecting NAV3, the human UNC-53 homologue.
Authors: Karenko L, Hahtola S, Paivinen S, Karhu R, Syrja S, Kahkonen M, Nedoszytko B, Kytölä S, Zhou Y, Blazevic V, Pesonen M, Nevala H, Nupponen N, Sihto H, Krebs I, Poustka A, Roszkiewicz J, Saksela K, Peterson P, Visakorpi T, Ranki A
Cancer Res., 2005-09-15;65(18):8101-10.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Aberration of CCR7 CD8 memory T cells from patients with systemic lupus erythematosus: an inducer of T helper type 2 bias of CD4 T cells.
Authors: Sen Y, Chunsong H, Baojun H, Linjie Z, Qun L, San J, Qiuping Z, Junyan L, Zhang X, Jinquan T
Immunology, 2004-06-01;112(2):274-89.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Reprogramming of IL-10 activity and signaling by IFN-gamma.
Authors: Herrero C, Hu X, Li WP, Samuels S, Sharif MN, Kotenko S, Ivashkiv LB
J. Immunol., 2003-11-15;171(10):5034-41.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization
FAQs
-
Can Catalog # MAB202 (anti-Human IL-2 antibody) be used for neutralization of mouse IL-2?
No. MAB202 is not cross-reactive to mouse IL-2 and so cannot be used for neutralization of mouse IL-2.
Reviews for Human IL-2 Antibody
Average Rating: 4.5 (Based on 2 Reviews)
Have you used Human IL-2 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: