Search ELISA Kits
Search ELISA Kits
Table of Contents
Table of Contents
Which ELISA Kit is Right for Me?
Which ELISA Kit is Right for Me?
Quantikine vs. DuoSet ELISAs
Quantikine vs. DuoSet ELISAs
Ella™ Automated ELISA
Ella™ Automated ELISA
Compact ELISA Plate Reader
Compact ELISA Plate Reader
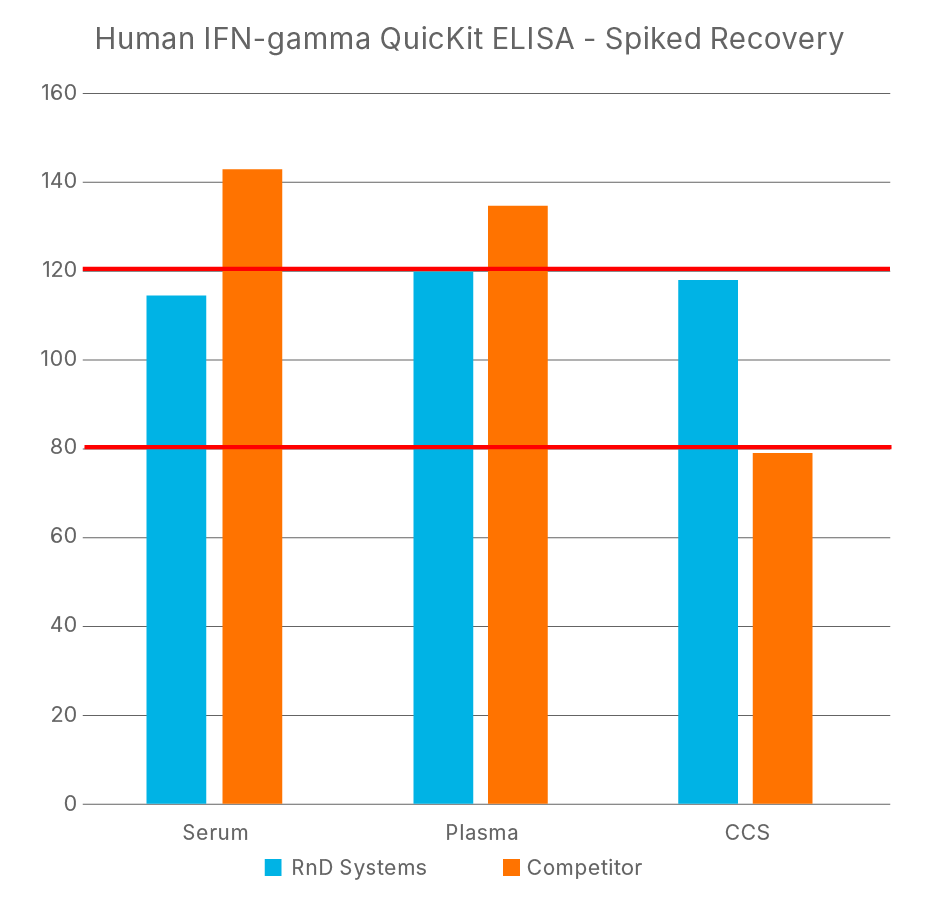
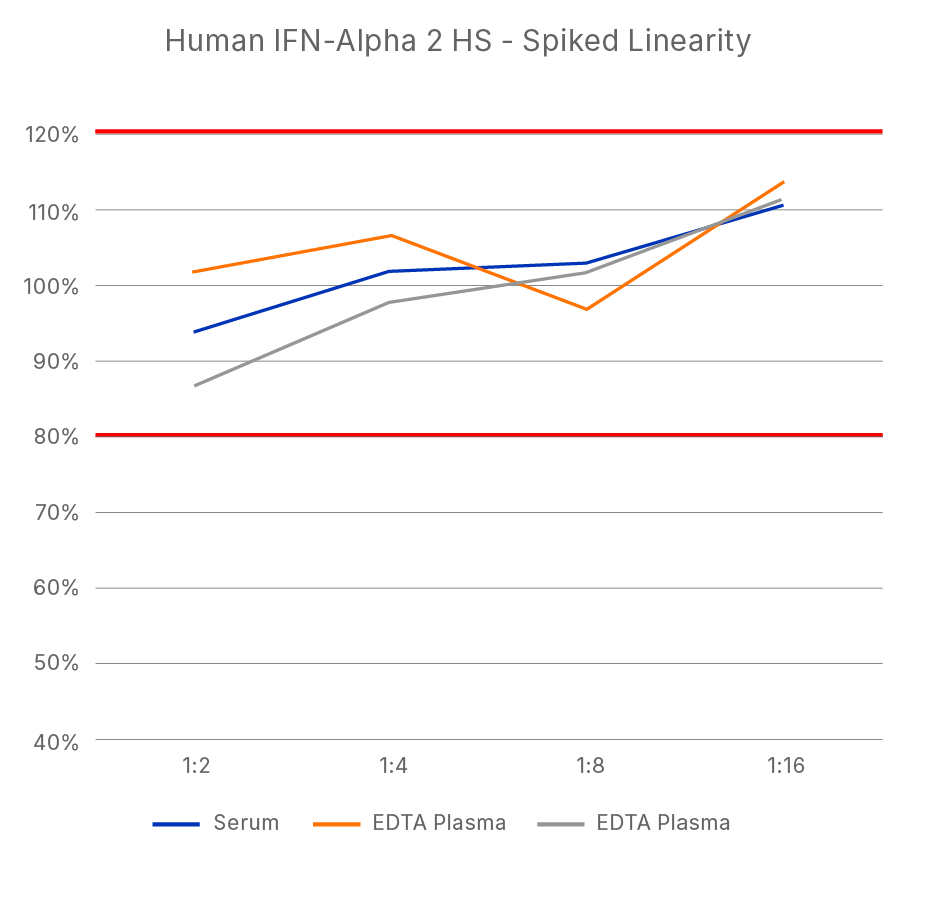
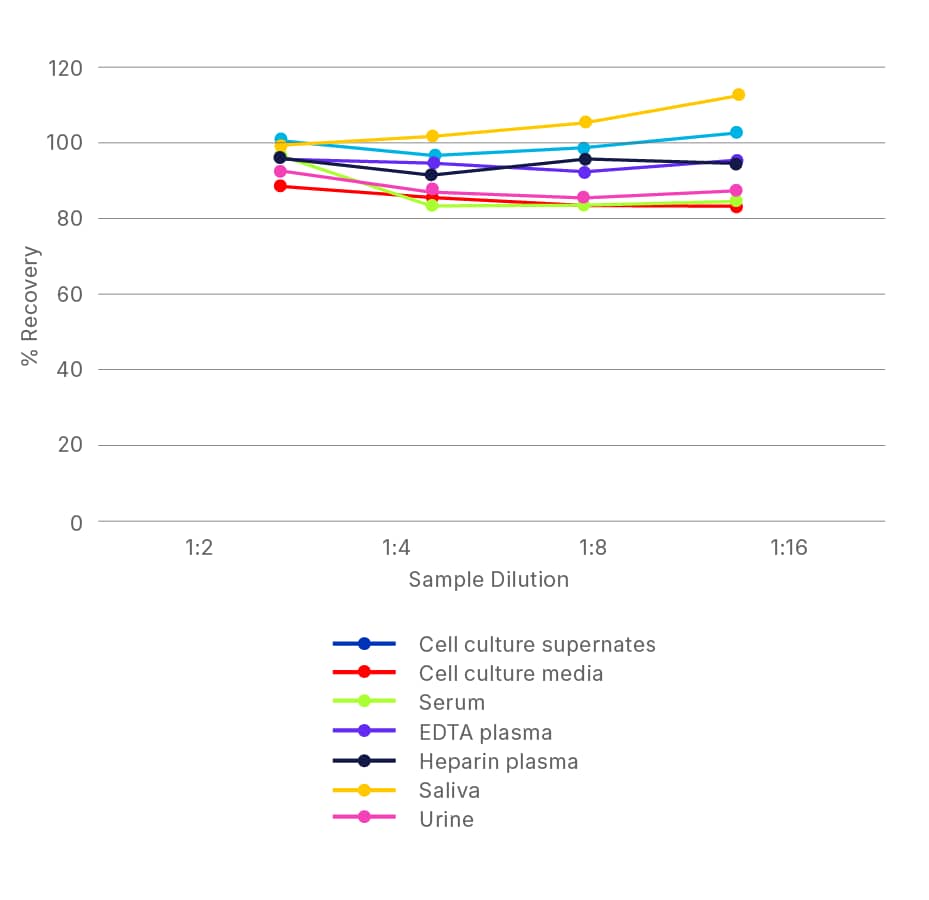
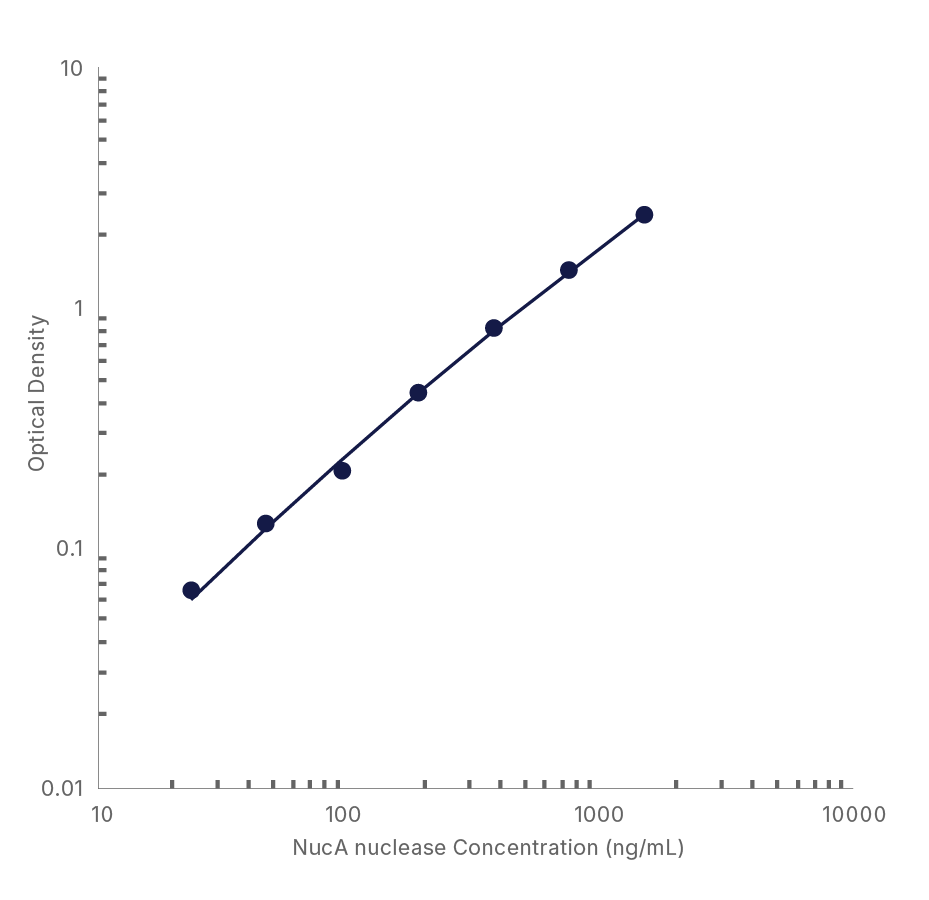
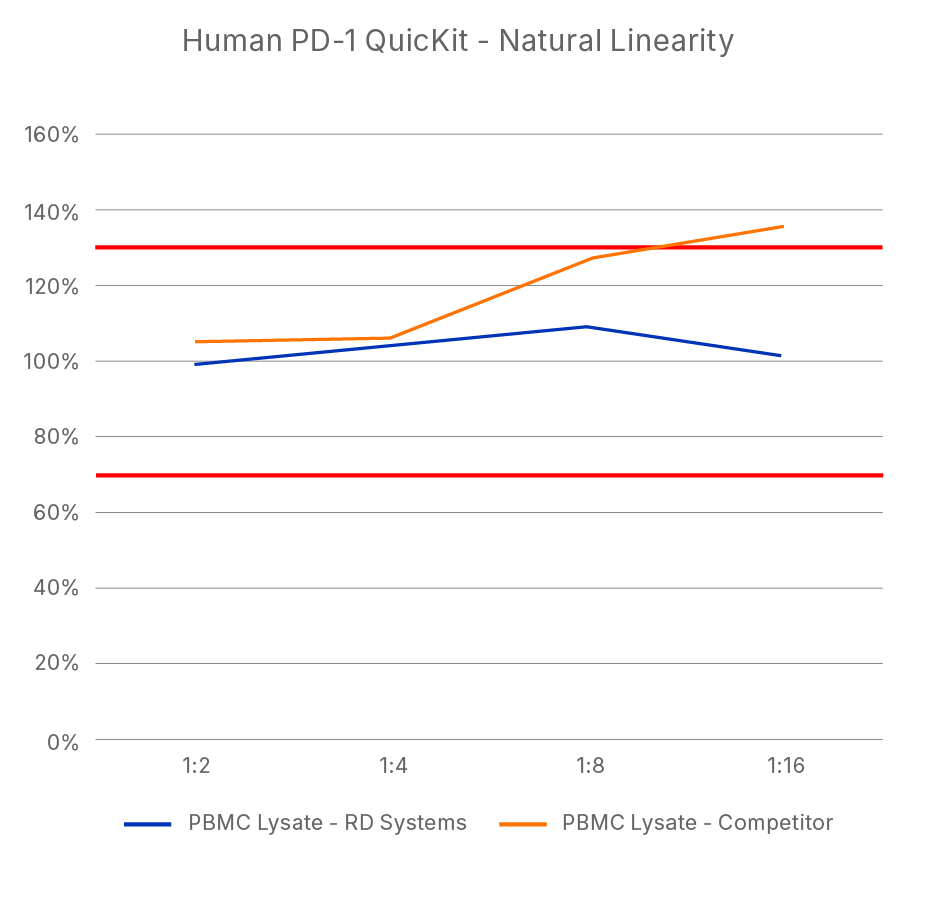
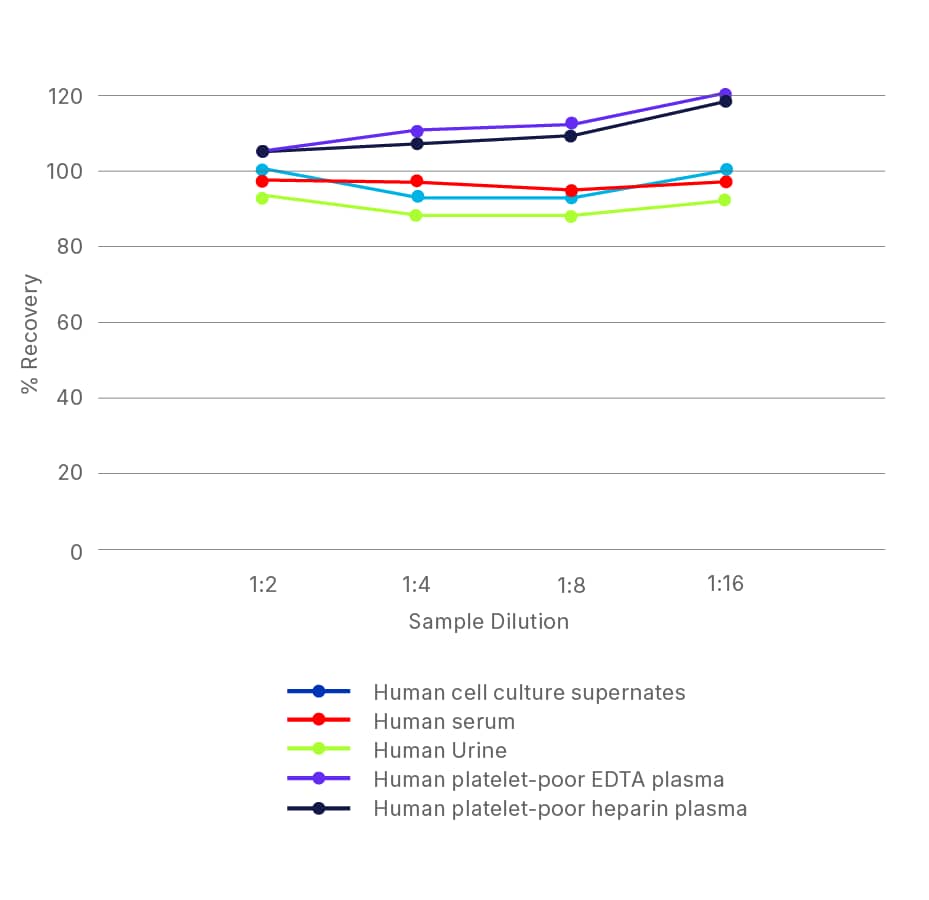
ELISA Kit - Quality Testing Examples
ELISA Kit - Quality Testing Examples
Custom and Bulk ELISA Services
Custom and Bulk ELISA Services
ELISA Resources
ELISA Resources
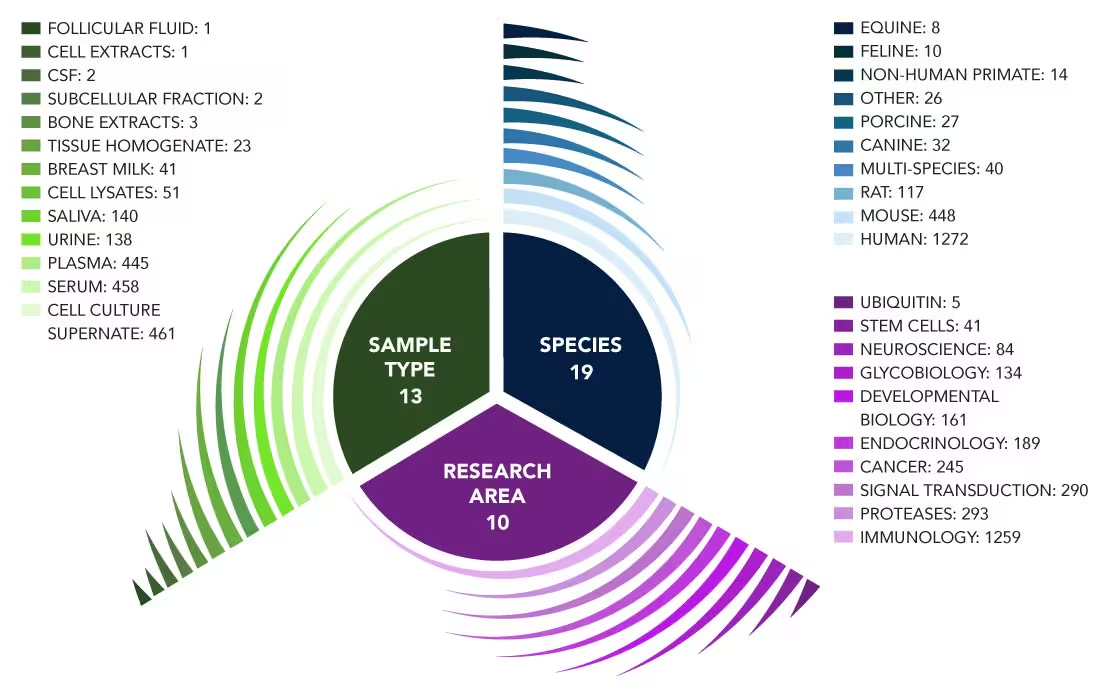
Research Areas
Research Areas
title and description wrapper
ELISA Kit Supplier of the Year
ELISA Kit Supplier of the Year
What Is an ELISA?
What Is an ELISA?
Related Products
Related Products
Featured Content
Featured Content
Featured Content

A clear, comprehensive guide to ELISAs. Learn about different ELISA formats, controls, best practices, and troubleshooting.
Featured Content

Save space and costs with Simple Reader™, a compact, cost-effective plate reader offering precise results.
Featured Content

Hands-free, built-in standard curve, results in less than 90 minutes.
Featured Content

Learn about the in-house testing processes used to ensure our ELISAs deliver the highest levels of performance and consistency.
Featured Content

Read this App Note to learn how our proprietary blocking reagents prevent false positive ELISA data arising from matrix effects.
Featured Content

Discover the top 10 best practices and techniques that can help even the experienced ELISA user get the most from their assay.