Virus Detection and Induction of Antiviral Signaling Pathways by RIG-I-like Receptors RLR
E3 Ub Ligases
Pro-Caspase-10
Homodimer
Homodimer
IFN-inducible genes
IFN-alpha
IFN-alpha 1
IFN-alpha 2
IFN-alpha 4
IFN-alpha A
IFN-alpha B2
IFN-alpha C
IFN-alpha D
IFN-alpha F
IFN-alpha H2
IFN-alpha I
IFN-alpha J1
IFN-alpha K
IFN-alpha WA
IFN-alpha 11
IFN-alpha 13
IFN-beta
IFN-kappa
IFN-omega
IL-28A/IFN-lambda 2
IL-28B/IFN-lambda 3
IL-28A/B (IFN-lambda 2/3)
IL-29/IFN-lambda 1
IL-29/IL-28B (IFN-lambda 1/3)
Limitin/IFN-zeta
Reticulum
Due to the lack of the N-terminal CARD domains required for signaling, LGP2 is thought to function as either a positive or negative regulator of RIG-I and MDA5 signaling depending on the virus and the cell type involved.
Viruses Detected by LGP2
- Encephalomyocarditis virus
- Mengovirus
- Newcastle disease virus
- Reovirus
- Sendai virus
- Vesicular stomatitis virus
Long double-stranded RNA
Viruses Detected by MDA5
- Encephalomyocarditis virus
- Mengovirus
- Murine Hepatitis virus
- Murine Norovirus-1
- Theiler's Murine Encephalomyelitis virus
- Vaccinia virus
- Dengue virus
- Rotavirus
- West Nile virus
Short single- stranded RNA containing some double-stranded regions and a 5' triphosphate Short blunt-end, double-stranded RNA with a 5' triphosphate Short double-stranded RNA Viruses Detected by RIG-I
- Ebola virus
- Epstein-Barr virus
- Hepatitis C virus
- Influenza virus A
- Influenza virus B
- Japanese encephalitis virus
- Lassa virus
- Lymphocytic choriomeningitis virus
- Measles
- Myxoma virus
- Newcastle disease virus
- Nipah virus
- Rabies virus
- Respiratory syncytial virus
- Rift Valley fever virus
- Sendai virus
- Vesicular stomatitis virus
- Dengue virus
- Rotavirus
- West Nile virus
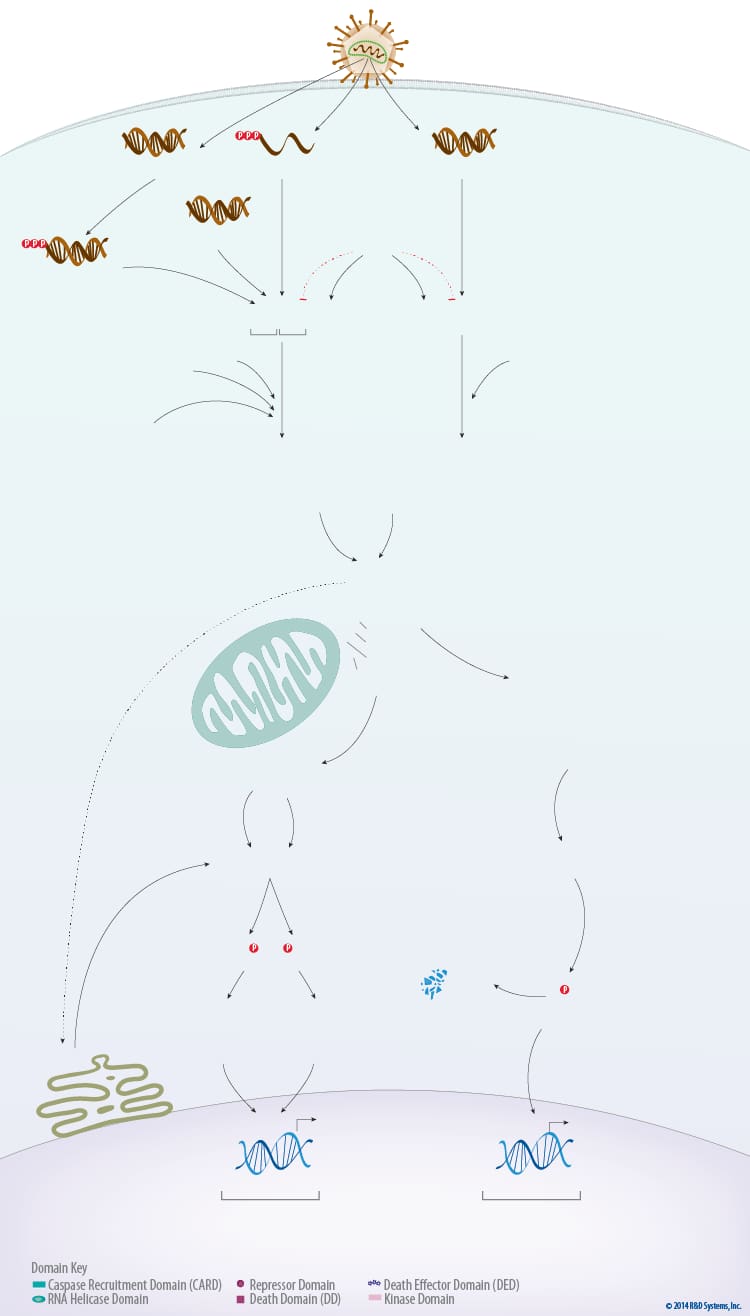
Overview of RIG-I-like Receptors
The RIG-I-like receptors (RLRs) are a family of cytosolic pattern recognition receptors that are essential for detecting viral RNA and initiating the innate immune response. The RLR family includes three members: Retinoic acid-inducible gene I (RIG-I), Melanoma differentiation-associated gene 5 (MDA5), and Laboratory of genetics and physiology 2 (LGP2). These receptors are expressed in both immune and non-immune cell types and regulate signaling pathways that promote the IRF3-, IRF7-dependent expression of type I and type III interferons (IFNs), and the NF-kappa B-dependent expression of pro-inflammatory cytokines.
All three RLR family receptors have a DExD/H box RNA helicase domain with ATPase activity. This domain along with the adjacent C-terminal domain is required for RNA binding. In addition, the C-terminal domains of RIG-I and LGP2 have been shown to act as repressor domains, ensuring that the receptors remain in an inactive conformation until they are bound by an activating RNA. Upstream of the RNA helicase domain, both RIG-I and MDA5 have two N-terminal caspase recruitment domains (CARDs) that mediate signaling by interacting with the CARD domain of the mitochondrial membrane-associated protein, Interferon-beta promoter stimulator 1 (IPS-1), also known as Mitochondrial antiviral signaling protein (MAVS). LGP2 lacks an N-terminal CARD domain and is therefore unable to interact with IPS-1/MAVS. For this reason, LGP2 is thought to positively or negatively regulate RIG-I and MDA5 signaling rather than functioning as a signaling receptor on its own.
RIG-I and MDA5 recognize different types of viral RNA, but use common signaling pathways to respond to a variety of different viruses. RIG-I preferentially binds to short 5’ triphosphorylated double-stranded RNA molecules (<300 bp) or short 5’ triphosphorylated single-stranded RNA that contains some double-stranded regions. Additionally, RIG-I is capable of binding to specific double-stranded RNAs lacking a 5’ phosphate or containing a 5’ monophosphate. In contrast, MDA5 binds internally to longer double-stranded RNA molecules (>1 kb). RNA binding by RIG-I or MDA5 is followed by the non-covalent or covalent attachment of unanchored lysine 63-linked polyubiquitin chains to the receptors, which promotes their homotetramerization and stabilization. For RIG-I, covalent attachment of the K63-linked polyubiquitin chains has been suggested to involve the E3 ubiquitin ligases, TRIM25 and Riplet. Less is known about the factors required for the addition of polyubiquitin chains to MDA5, but like
RIG-I, research suggests that this modification is essential for the proper activation of downstream signaling pathways. Following oligomerization, RIG-I and MDA5 activate IPS-1/MAVS on the mitochondrial membrane. Dimerization of IPS-1/MAVS allows it to associate with multiple adaptor proteins including TRAF-2, TRAF-6, and TRADD, which recruits TRAF-3 and TRAF family member-associated NF-kappa B activator (TANK) to trigger the activation of Tank binding kinase-1 (TBK1) and I kappa B kinase epsilon (IKK epsilon). TBK1 and IKK epsilon phosphorylate IRF3 and IRF7, which then homodimerize and translocate to the nucleus to promote the expression of type I and type III IFNs. At the same time, the IPS-1/MAVS-TRADD complex recruits FADD and RIP1 leading to the activation of IKK alpha, beta, and gamma and the phosphorylation of I kappa B. This phosphorylation promotes the ubiquitin-dependent proteasomal degradation of I kappa B, allowing NF-kappa B to translocate to the nucleus and induce the expression of its target genes. In addition to the TRAFs and TRADD, two other IPS-1/MAVS-interacting proteins may also be involved in facilitating RIG-I signaling. IPS-1/MAVS has been shown to associate with both Translocase of the outer membrane 70 (TOM70) in the mitochondria and Stimulator of interferon genes (STING), a four transmembrane protein that localizes to the outer membrane of the endoplasmic reticulum. Overexpression and siRNA knockdown studies suggest that both TOM70 and STING are required for IRF3-dependent type I IFN production following infection with specific RIG-I-activating viruses.
To learn more, please visit our RIG-I-like Receptors Research Area.



