Proteome Profiler Mouse XL Cytokine Array
Proteome Profiler Mouse XL Cytokine Array Summary
A membrane-based antibody array for the parallel determination of the relative levels of selected mouse cytokines and chemokines. Validated for analyte detection in cell culture supernates, cell lysates, tissue lysates, serum, and urine.
Troubleshooting GuideKey Benefits
- Detects 111 mouse cytokines simultaneously
- Requires no specialized equipment
- Compatible with LI-COR* and chemiluminescence detection
Principle of the Assay
The Proteome Profiler Mouse XL Cytokine Array Kit is a membrane-based sandwich immunoassay. Capture antibodies spotted in duplicate on nitrocellulose membranes bind to specific target proteins present in the sample (Step 1). Captured proteins are detected with biotinylated detection antibodies (Step 2) and then visualized using chemiluminescent detection reagents (Step 3). The signal produced is proportional to the amount of analyte bound.
Why Use an Antibody Array to Detect Multiple Cytokines?
Determining the expression of multiple cytokines in a single sample can be expensive, time consuming and can require specialized equipment. Performing multiple immunoprecipitations and Western blots requires time, labor, and reagents. The use of a multiplex antibody array to detect multiple cytokines in a single sample can be cost-effective and also save time and sample.
- Rectangular 4-Well Multi-dish
- 4 Mouse XL Cytokine Array nitrocellulose membranes spotted with 111 different antibodies to mouse cytokines
- Array Buffer 4
- Array Buffer 6
- Chemi Reagent 1
- Chemi Reagent 2
- Detection Antibody Cocktail, Mouse XL Cytokine Array
- Streptavidin-HRP
- Transparency Overlay Template
- Wash Buffer Concentrate (25X)
For a complete list of the kit contents and necessary materials, please see the Materials Provided/Other Supplies Required sections of the product datasheet.
| Simultaneously detect the levels of these cytokines, chemokines, and growth factors in a single sample. | ||
| Adiponectin/Acrp30 | DPPIV/CD26 | IL-27 |
| Amphiregulin | EGF | IL-28 |
| Angiopoietin-1 | Endoglin/CD105 | IL-33 |
| Angiopoietin-2 | Endostatin | LDL R |
| Angiopoietin-like 3 | Fetuin A/AHSG | Leptin |
| BAFF/BLyS/TNFSF13B | FGF acidic | LIF |
| C1q R1/CD93 | FGF-21 | Lipocalin-2/NGAL, |
| CCL2/JE/MCP-1 | Flt-3 Ligand | LIX |
| CCL3/CCL4 MIP-1 alpha/beta | Gas6 | M-CSF |
| CCL5/RANTES | G-CSF | MMP-2 |
| CCL6/C10 | GDF-15 | MMP-3 |
| CCL11/Eotaxin | GM-CSF | MMP-9 |
| CCL12/MCP-5 | HGF | Myeloperoxidase |
| CCL17/TARC | ICAM-1/CD54 | Osteopontin (OPN) |
| CCL19/MIP-3 beta | IFN-gamma | Osteoprotegerin/TNFRSF11B |
| CCL20/MIP-3 alpha | IGFBP-1 | PD-ECGF/Thymidine phosphorylase |
| CCL21/6Ckine | IGFBP-2 | PDGF-BB |
| CCL22/MDC | IGFBP-3 | Pentraxin 2/SAP |
| CD14 | IGFBP-5 | Pentraxin 3/ TSG-14 |
| CD40/TNFRSF5 | IGFBP-6 | Periostin/OSF-2 |
| CD160 | IL-1 alpha/IL1F1 | Pref-1/DLK-1/FA1 |
| Chemerin | IL-1 beta/IL-1F2 | Proliferin |
| Chitinase 3-like 1 | IL-1ra/IL-1F3 | Proprotein Convertase 9/PCSK9 |
| Coagulation Factor III/Tissue Factor | IL-2 | RAGE |
| Complement Component C5/C5a | IL-3 | RBP4 |
| Complement Factor D | IL-4 | Reg3G |
| C-Reactive Protein/CRP | IL-5 | Resistin |
| CX3CL1/Fractalkine | IL-6 | E-Selectin/CD62E |
| CXCL1/KC | IL-7 | P-Selectin/CD62P |
| CXCL2/MIP-2 | IL-10 | Serpin E1/PAI-1 |
| CXCL9/MIG | IL-11 | Serpin F1/PEDF |
| CXCL10/IP-10 | IL-12p40 | Thrombopoietin |
| CXCL11/I-TAC | IL-13 | TIM-1/KIM-1/HAVCR |
| CXCL13/BLC/BCA-1 | IL-15 | TNF-alpha |
| CXCL16 | IL-17A | VCAM-1/CD106 |
| Cystatin C | IL-22 | VEGF |
| Dkk-1 | IL-23 | WISP-1/CCN4 |
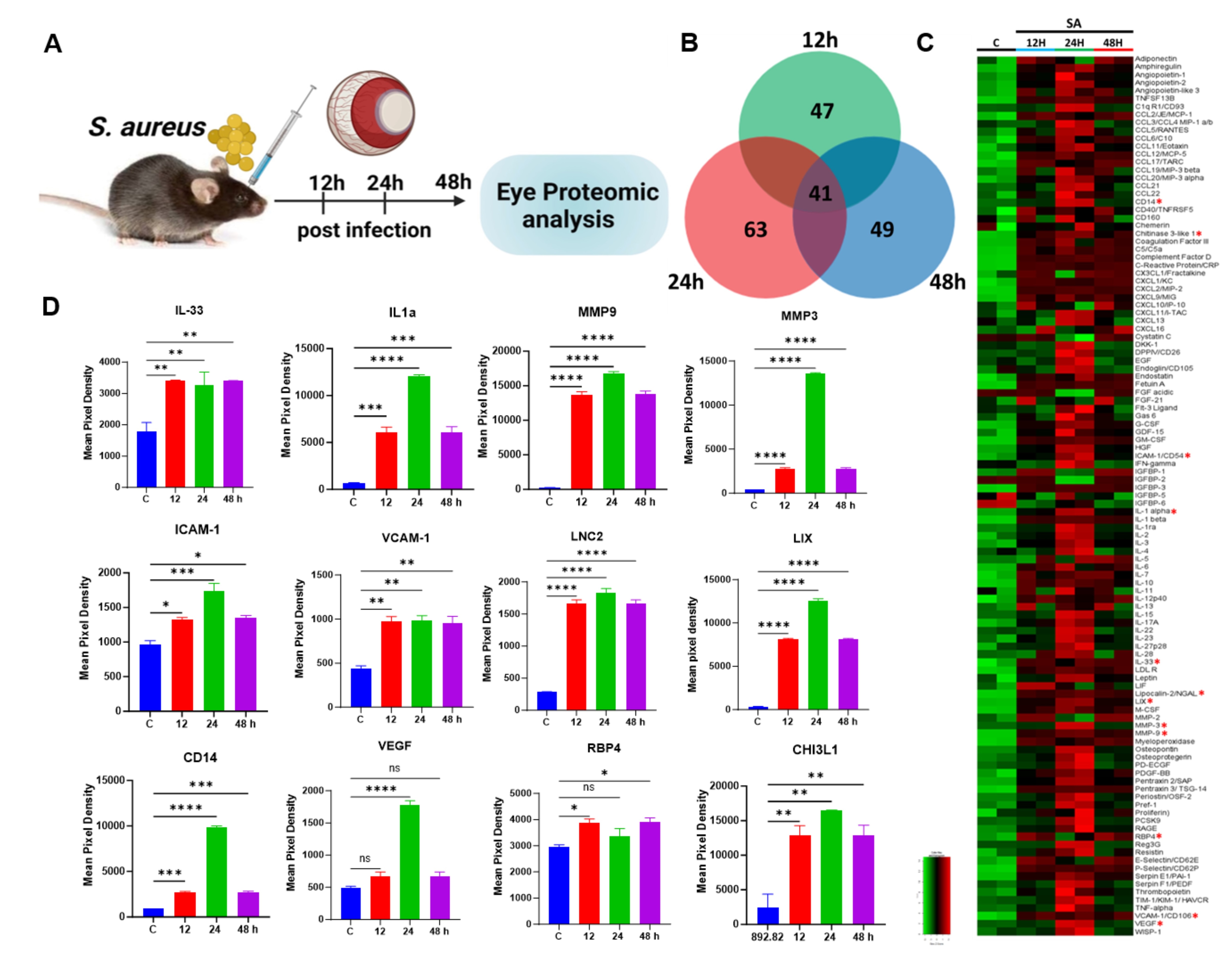
Assays for Analytes represented in the Mouse XL Cytokine Array Kit
| Analyte | Qkit | Duoset | ||
| Adiponectin/Acrp30 | MRP300 | DY1119 | ||
| Amphiregulin | DY989 | |||
| Angiopoietin-1 | ||||
| Angiopoietin-2 | ||||
| Angiopoietin-like 3 | MANL30 | DY136 | ||
| BAFF/BLyS/TNFSF13B | MBLYS0 | DY2106-05 | ||
| C1q R1/CD93 | MCD930 | |||
| CCL2/JE/MCP-1 | MJE00 | DY479 | ||
| CCL3/CCL4 MIP-1 alpha/beta | ||||
| CCL5/RANTES | MMR00 | DY478 | ||
| CCL6/C10 | DY487 | |||
| CCL11/Eotaxin | MME00 | DY420 | ||
| CCL12/MCP-5 | MCC120 | DY428 | ||
| CCL17/TARC | MCC170 | DY529 | ||
| CCL19/MIP-3 beta | DY440 | |||
| CCL20/MIP-3 alpha | MCC200 | DY760 | ||
| CCL21/6Ckine | DY457 | |||
| CCL22/MDC | MCC220 | DY439 | ||
| CD14 | MC140 | DY982 | ||
| CD40/TNFRSF5 | MCCD40 | DY097 | ||
| CD160 | MCHM00 | DY2325 | ||
| Chemerin | ||||
| Chitinase 3-like 1 | MC3L10 | DY2649 | ||
| Coagulation Factor III/Tissue Factor | ||||
| Complement Component C5/C5a | ||||
| Complement Factor D | ||||
| C-Reactive Protein/CRP | MCRP00 | DY1829 | ||
| CX3CL1/Fractalkine | MCX310 | DY472 | ||
| CXCL1/KC | MKC00B | DY453 | ||
| CXCL2/MIP-2 | MM200 | DY452 | ||
| CXCL9/MIG | MCX900 | DY492 | ||
| CXCL10/IP-10 | MCX100 | DY466 | ||
| CXCL11/I-TAC | DY572 | |||
| CXCL13/BLC/BCA-1 | MCX130 | |||
| CXCL16 | DY503 | |||
| Cystatin C | MSCTC0 | DY1238 | ||
| Dkk-1 | MKK100 | DY1765 | ||
| DPPIV/CD26 | DY954 | |||
| EGF | MEG00 | DY2028 | ||
| Endoglin/CD105 | MNDG00 | DY1320 | ||
| Endostatin | ||||
| Fetuin A/AHSG | MFTA00 | DY1563 | ||
| FGF acidic | DY4686-05 | |||
| FGF-21 | MF2100 | DY3057 | ||
| Flt-3 Ligand | MFK00 | DY427 | ||
| Gas6 | MGAS60 | DY986 | ||
| G-CSF | MCS00 | DY414 | ||
| GDF-15 | MGD150 | DY6385 | ||
| GM-CSF | MGM00 | DY415 | ||
| HGF | MHG00 | DY2207 | ||
| ICAM-1/CD54 | MIC100 | DY796 | ||
| IFN-gamma | MIF00 | DY485 | ||
| IGFBP-1 | DY1588-05 | |||
| IGFBP-2 | DY797 | |||
| IGFBP-3 | MGB300 | DY775 | ||
| IGFBP-5 | DY578 | |||
| IGFBP-6 | DY776 | |||
| IL-1 alpha/IL1F1 | MLA00 | DY400 | ||
| IL-1 beta/IL-1F2 | MLB00C | DY401 | ||
| IL-1ra/IL-1F3 | MRA00 | DY480 | ||
| IL-2 | M2000 | DY402 | ||
| IL-3 | M3000 | DY403 | ||
| IL-4 | M4000B | DY404 | ||
| IL-5 | M5000 | DY405 | ||
| IL-6 | M6000B | DY406 | ||
| IL-7 | M7000 | DY407 | ||
| IL-10 | M1000B | DY417 | ||
| IL-11 | DY418 | |||
| IL-12p40 | MP400 non-allele specific |
M1240 allele specific |
DY2398 non-allele specific |
DY499 allele specific |
| IL-13 | M1300CB | DY413 | ||
| IL-15 | DY447 | |||
| IL-17A | M1700 | DY421 | ||
| IL-22 | M2200 | DY582 | ||
| IL-23 | M2300 | DY1887 | ||
| IL-27 | DY1789B | |||
| IL-28 | ||||
| IL-33 | M3300 | DY3626 | ||
| LDL R | MLDLR0 | DY2255 | ||
| Leptin | MOB00 | DY498 | ||
| LIF | MLF00 | |||
| Lipocalin-2/NGAL, | DY1857 | |||
| LIX | MX000 | DY443 | ||
| M-CSF | MMC00 | DY416 | ||
| MMP-2 | MMP200 | |||
| MMP-3 | MMP300 | DY548 | ||
| MMP-9 | MMPT90 | MMP900B | DY6718 | DY909 |
| Myeloperoxidase | DY3667 | |||
| Osteopontin (OPN) | MOST00 | DY441 | ||
| Osteoprotegerin/TNFRSF11B | MOP00 | DY459 | ||
| PD-ECGF/Thymidine phosphorylase | ||||
| PDGF-BB | MBB00 | |||
| Pentraxin 2/SAP | MPTX20 | |||
| Pentraxin 3/ TSG-14 | MPTX30 | DY2166 | ||
| Periostin/OSF-2 | MOSF20 | DY2955 | ||
| Pref-1/DLK-1/FA1 | ||||
| Proliferin | ||||
| Proprotein Convertase 9/PCSK9 | MPC900 | DY3985 | ||
| RAGE | MRG00 | DY1179 | ||
| RBP4 | MRBP40 | DY3476 | ||
| Reg3G | ||||
| Resistin | MRSN00 | DY1069 | ||
| E-Selectin/CD62E | MES00 | DY575 | ||
| P-Selectin/CD62P | MPS00 | DY737 | ||
| Serpin E1/PAI-1 | DY3828-05 | |||
| Serpin F1/PEDF | ||||
| Thrombopoietin | MTP00 | DY488 | ||
| TIM-1/KIM-1/HAVCR | MKM100 | DY1817 | ||
| TNF-alpha | MTA00B | DY410 | ||
| VCAM-1/CD106 | MVC00 | DY643 | ||
| VEGF | MMV00 | DY493 | ||
| WISP-1/CCN4 | MWSP10 | |||
Specifications
Product Datasheets
Scientific Data
 View Larger
View Larger
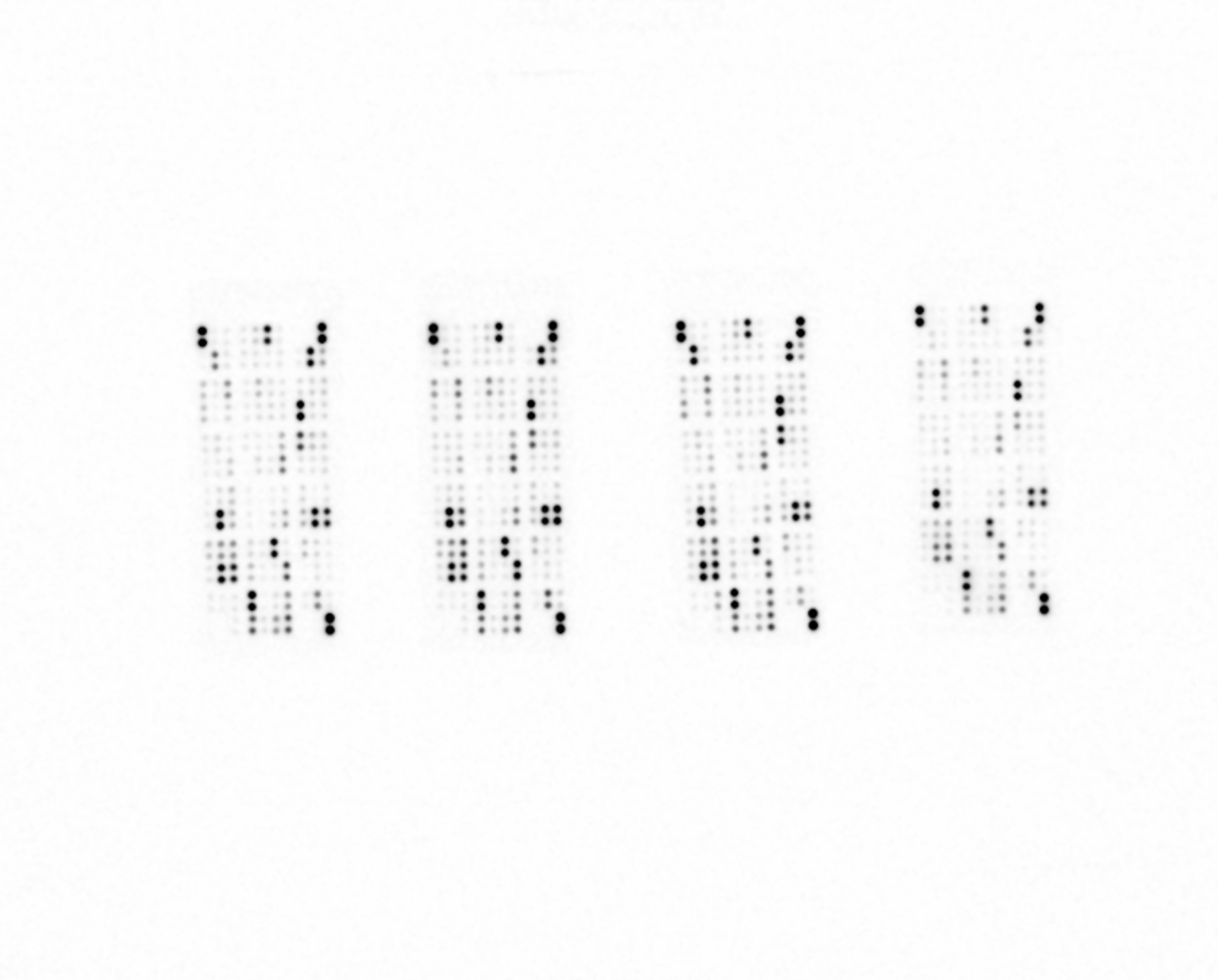
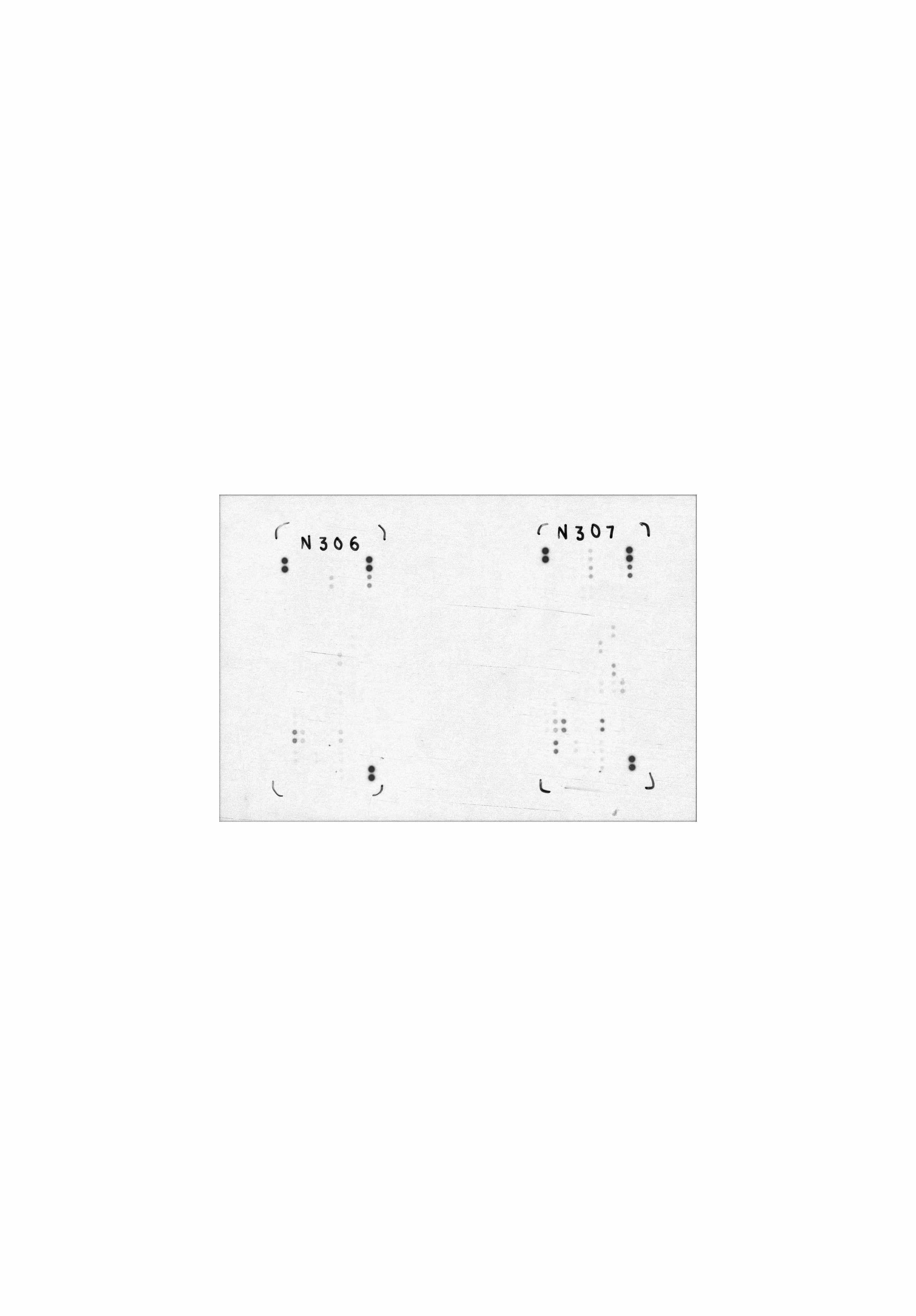
Detection of Selected Mouse Cytokines in Balb/3T3 Cell Line Lysate. Lysates from Balb/3T3 mouse embryonic fibroblast cells were untreated or treated with 100 ng/mL of recombinant mouse TNF-alpha (R&D Systems, Catalog # 410-MT) for 24 hours (200 µg lysate, 10 minute exposure).
 View Larger
View Larger
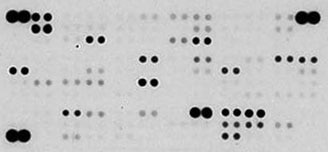
Detection of Selected Mouse Cytokines in CMT-93 Cell Line Lysate. Lysates from CMT-93 mouse rectal carcinoma cells were untreated or treated with 100 ng/mL LPS for 24 hours (200 µg lysate, 10 minute exposure).
 View Larger
View Larger
Detection of Selected Mouse Cytokines in Balb/3T3 Cell Line Supernate. Balb/3T3 mouse embryonic fibroblast cells were untreated or treated with 100 ng/mL of recombinant mouse TNF-alpha (R&D Systems, Catalog # 410-MT) for 24 hours (500 µL of cell culture supernate, 5 minute exposure).
Assay Procedure
Refer to the product datasheet for complete product details.
Briefly, relative expression levels of mouse cytokines and chemokines in samples can be determined using the following procedure:
- Prepare membrane and incubate with prepared sample
- Incubate the membrane with Detection Antibody Cocktail
- Incubate the membrane array with Streptavidin-HRP
- Develop the membrane array with Chemi Reagents 1 and 2
- Expose the membrane array to autoradiography film
View Graphic Procedure
Kit Contents
- Rectangular 4-Well Multi-dish
- 4 Mouse XL Cytokine Array nitrocellulose membranes spotted with 111 different antibodies to mouse cytokines
- Array Buffer 4
- Array Buffer 6
- Chemi Reagent 1
- Chemi Reagent 2
- Detection Antibody Cocktail, Mouse XL Cytokine Array
- Streptavidin-HRP
- Transparency Overlay Template
- Wash Buffer Concentrate (25X)
Other Supplies Required
Reagents
- Aprotinin
- Leupeptin (Catalog #EI002)
- Pepstatin (Catalog #EI003)
- Igepal® CA-630
- Deionized or distilled water
Materials
- Pipettes and pipette tips
- Gloves
- Plastic container with the capacity to hold 50 mL (for washing the arrays)
- Plastic transparent sheet protector (trimmed to 10 cm x 12 cm and open on three sides)
- Plastic wrap
- Absorbent lab wipes (KimWipes® or equivalent)
- Paper towels
- X-ray film (Kodak BioMax™ Light-1) or equivalent
- Flat-tipped tweezers
Equipment
- Rocking platform shaker
- Microcentrifuge
- Autoradiography cassette
- Film developer
- Flatbed scanner with transparency adapter capable of transmission mode
- Computer capable of running image analysis software and Microsoft Excel
Other Supplies Required for Cell Lysate Samples
- Phosphate-Buffered Saline (PBS)
- Lysis buffer (1% Igepal CA-630, 20 mM Tris-HCl (pH 8.0), 137 mM NaCl, 10% glycerol, 2 mM EDTA, 10 µg/mL Aprotinin, 10 µg/mL Leupeptin, and 10 µg/mL Pepstatin)
Other Supplies Required for Tissue Lysate Samples
- PBS with protease inhibitors (10 µg/mL Aprotinin, 10 µg/mL Leupeptin, and 10 µg/mL Pepstatin)
- Triton™ X-100
R&D Systems Protocol for Multiple Analyte Detection Using the Proteome Profiler™ Mouse XL Cytokine Array Kit, Panel A (Catalog # ARY028)
- Add 2 mL of Array Buffer 6 to each well of the supplied 4-Well Multi-dish.
- Place each array membrane in a separate well of the 4-Well Multi-dish.
- Incubate for one hour on a rocking platform shaker.
- Add 0.5 mL Array Buffer 4 to each sample.
- Adjust volume of each sample to final volume of 1.5 mL with Array Buffer 6.
- Replace the Array Buffer 6 in each well of the 4-Well Multi-dish with prepared samples.
- Incubate overnight at 2 °C to 8 °C on a rocking platform.
- Wash each array membrane 3 times with 1X Wash Buffer in a separate container.
- Wash each well of the 4-Well Multi-dish with 1X Wash Buffer.
- Add 30 µL of Detection Antibody Cocktail to 1.5 mL of Array Buffer 4/6 for each array.
- Pipette 1.5 mL diluted Detection Antibody Cocktail into each well of the 4-Well Multi-dish.
- Return each array membrane to the 4-Well Multi-dish containing diluted Detection Antibody Cocktail.
- Incubate for one hour on a rocking platform shaker.
- Wash each array membrane 3 times with 1X Wash Buffer in a separate container.
- Wash each well of the 4-Well Multi-dish with 1X Wash Buffer.
- Add 2 mL of diluted Streptavidin-HRP to each well of the 4-Well Multi-dish.
- Place the array membrane in the diluted Streptavidin-HRP solution.
- Incubate for 30 minutes on a rocking platform shaker.
- Wash each array membrane 3 times with 1X Wash Buffer in a separate container.
- Place the array membrane on a plastic sheet protector.
- Pipette 1 mL of the prepared Chemi Reagent Mix evenly onto the membrane.
- Cover the membrane with the top sheet of the plastic protector.
- Incubate for 1 minute.
- Blot off excess Chemi Reagent Mix.
- Wrap the membrane and sheet protector in plastic wrap.
- Place the wrapped array membrane in an autoradiography film cassette and expose to X-ray film.
Citations for Proteome Profiler Mouse XL Cytokine Array
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
169
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Diet-derived galactose reprograms hepatocytes to prevent T cell exhaustion and elicit antitumour immunity
Authors: Du, X;Li, W;Li, G;Guo, C;Tang, X;Bao, R;Li, R;Huang, H;Xu, S;Yu, X;Han, Q;Wan, J;Li, S;Sun, J;Zhao, R;Ye, Y;Gao, Q;Ni, ZY;Cui, X;Zou, Q;
Nature cell biology 2025-08-01
-
The CCL2-CCR2 axis drives neuromuscular denervation in amyotrophic lateral sclerosis
Authors: Nógrádi, B;Molnár, K;Kristóf, R;Horváth, O;Huang, YT;Ridgway, Z;Elicegui, A;Fuertes-Alvarez, S;Alonso-Martin, S;Szebeni, GJ;Gémes, N;Ramadan, A;Smith, HL;Krizbai, IA;Patai, R;Siklós, L;Klivényi, P;Chaytow, H;Gillingwater, TH;
Nature communications 2025-08-01
-
TET2 has endothelial-specific roles in interferon responses that are dysregulated by hyperglycaemia in vitro and in vivo
Authors: Green, HLH;Sum, H;Sinha, P;Visnagri, A;Lee, SH;Baffour-Kyei, A;Lee, H;Santos, F;Theofilatos, K;Brewer, AC;
The Journal of biological chemistry 2025-07-25
-
Temporal Regulation of Early-Stage Cytokine Expression in Diabetic Wound Healing Under Negative Pressure Wound Therapy
Authors: Chiu, HS;Huang, TS;Chen, CT;Lin, XY;Liao, PC;Liou, CC;Hsu, CC;Somvanshi, S;Sumazin, P;Hsu, PH;Sun, CC;Shyu, YC;
International journal of molecular sciences 2025-05-13
-
An atlas of ferroptosis-induced secretomes
Authors: Yapici, FI;Seidel, E;Dahlhaus, A;Weber, J;Schmidt, C;de Britto Chaves Filho, A;Yang, M;Nenchova, M;Güngör, E;Stroh, J;Kotouza, I;Beck, J;Abdallah, AT;Lackmann, JW;Bebber, CM;Androulidaki, A;Kreuzaler, P;Schulze, A;Frezza, C;von Karstedt, S;
Cell death and differentiation 2025-04-25
-
Immunomodulation by AZD1656 reverses cardiac dysfunction, metabolic remodelling and reduces infarct size in type 2 diabetic cardiomyopathy
Authors: Anderson, S;Karlstaedt, A;Chen, J;O'Riordan, C;Barnes, M;Staka, Z;Albee, L;Garrod-Ketchley, C;Vithanachchi, S;Prag, H;Cvetko, F;Thiemermann, C;Lewis, AJ;Murphy, MP;Smith, DM;Henson, S;Tyler, D;Aksentijevic, D;
bioRxiv : the preprint server for biology 2025-04-11
-
Modulation of brain immune microenvironment and cellular dynamics in systemic inflammation
Authors: Wang, J;Zhong, Z;Luo, H;Han, Q;Wu, K;Jiang, A;Chen, L;Gao, Y;Jiang, Y;
Theranostics 2025-04-09
-
The impact of a high fat diet and platelet activation on pre-metastatic niche formation
Authors: Hergueta-Redondo, M;Sánchez-Redondo, S;Hurtado, B;Santos, V;Pérez-Martínez, M;Ximénez-Embún, P;McDowell, SAC;Mazariegos, MS;Mata, G;Torres-Ruiz, R;Rodríguez-Perales, S;Martínez, L;Graña-Castro, O;Megias, D;Quail, D;Quintela-Fandino, M;Peinado, H;
Nature communications 2025-04-02
-
Restraint of inflammasome-driven cytokine responses through the mRNA stability protein TTP
Authors: O'Keefe, ME;Kondolf, HC;De Santis, S;Pizarro, TT;Abbott, DW;
Cell reports 2025-02-20
-
Oversecretion of CCL3 by Irradiation-Induced Senescent Osteocytes Mediates Bone Homeostasis Imbalance
Authors: Zhao, F;Han, H;Wang, J;Wang, J;Zhai, J;Zhu, G;
Cells 2025-02-10
-
Exosome-based targeted delivery of NF-?B ameliorates age-related neuroinflammation in the aged mouse brain
Authors: Lee, CJ;Jang, SH;Lim, J;Park, H;Ahn, SH;Park, SY;Seo, H;Song, SJ;Shin, JA;Choi, C;Gee, HY;Choi, YH;
Experimental & molecular medicine 2025-02-01
-
Aging directs the differential evolution of KRAS-driven lung adenocarcinoma
Authors: Lazure, F;Drapela, S;Liu, X;Lockhart, JH;Kashfi, H;Sarigul, N;Ilter, D;Flores, ER;Wang, X;Smalley, I;Jaeger, A;Yu, X;Gomes, AP;
bioRxiv : the preprint server for biology 2025-01-24
-
Neurotoxic effects of citronellol induced by the conversion of kynurenine to 3-hydroxykynurenine
Authors: Kim, SS;Kim, S;Kim, Y;Ha, Y;Lee, H;Im, H;Yang, JY;Shin, DS;Hwang, KS;Son, Y;Park, SB;Kim, KY;Lee, HS;Kim, KT;Cho, SH;Bae, MA;Park, HC;
Journal of hazardous materials 2024-12-24
-
CXCL1-CXCR2 signaling mediates the activation of microglia in the nucleus tractus solitarii to promote pancreatic cancer-induced pain
Authors: Chen, K;Ye, Q;Zhang, Y;Qi, Z;Huang, Y;Lu, W;Wang, X;Wang, Y;Cao, L;Qiu, S;Xu, Y;Huang, J;Xie, J;
Brain, behavior, and immunity 2024-11-10
-
Granulocyte colony-stimulating factor protects against arthritogenic alphavirus pathogenesis in a type I IFN-dependent manner
Authors: Hameed, M;Hossain, MS;Daamen, AR;Lipsky, PE;Weger-Lucarelli, J;
bioRxiv : the preprint server for biology 2024-10-13
-
CMTM6 mediates the Warburg effect and promotes the liver metastasis of colorectal cancer
Authors: Shaha, A;Wang, Y;Wang, X;Wang, D;Guinovart, D;Liu, B;Kang, N;
Experimental & molecular medicine 2024-09-02
-
Carfilzomib shows therapeutic potential for reduction of liver fibrosis by targeting hepatic stellate cell activation
Authors: Fujiwara, A;Takemura, K;Tanaka, A;Matsumoto, M;Katsuyama, M;Okanoue, T;Yamaguchi, K;Itoh, Y;Iwata, K;Amagase, K;Umemura, A;
Scientific reports 2024-08-20
-
Sensory Neurons Release Cardioprotective Factors in an In Vitro Ischemia Model
Authors: Hoebart, C;Kiss, A;Podesser, BK;Tahir, A;Fischer, MJM;Heber, S;
Biomedicines 2024-08-15
-
Adenosine triggers early astrocyte reactivity that provokes microglial responses and drives the pathogenesis of sepsis-associated encephalopathy in mice
Authors: Guo, Q;Gobbo, D;Zhao, N;Zhang, H;Awuku, NO;Liu, Q;Fang, LP;Gampfer, TM;Meyer, MR;Zhao, R;Bai, X;Bian, S;Scheller, A;Kirchhoff, F;Huang, W;
Nature communications 2024-07-27
-
Caspase-4/11 promotes hyperlipidemia and chronic kidney disease-accelerated vascular inflammation by enhancing trained immunity
Authors: Sun, Y;Lu, Y;Liu, L;Saaoud, F;Shao, Y;Xu, K;Drummer Iv, C;Cueto, R;Shan, H;Jiang, X;Zhao, H;Wang, H;Yang, X;
JCI insight 2024-07-18
-
Adeno-associated virus delivered CXCL9 sensitizes glioblastoma to anti-PD-1 immune checkpoint blockade
Authors: von Roemeling, CA;Patel, JA;Carpenter, SL;Yegorov, O;Yang, C;Bhatia, A;Doonan, BP;Russell, R;Trivedi, VS;Klippel, K;Ryu, DH;Grippin, A;Futch, HS;Ran, Y;Hoang-Minh, LB;Weidert, FL;Golde, TE;Mitchell, DA;
Nature communications 2024-07-12
-
Loss of tumor suppressors promotes inflammatory tumor microenvironment and enhances LAG3+T cell mediated immune suppression
Authors: Zahraeifard, S;Xiao, Z;So, JY;Ahad, A;Montoya, S;Park, WY;Sornapudi, T;Andohkow, T;Read, A;Kedei, N;Koparde, V;Yang, H;Lee, M;Wong, N;Cam, M;Wang, K;Ruppin, E;Luo, J;Hollander, C;Yang, L;
Nature communications 2024-07-12
-
Treatment of vascular dementia in female rats with AV-001, an Angiopoietin-1 mimetic peptide, improves cognitive function
Authors: Gao, H;Liu, X;Venkat, P;Findeis, E;Zacharek, A;Powell, B;Mccann, M;Kim, H;Zhang, Z;Chopp, M;
Frontiers in neuroscience 2024-07-10
-
Activation of the osteoblastic HIF-1? pathway partially alleviates the symptoms of STZ-induced type 1 diabetes mellitus via RegIII?
Authors: Qiu, M;Chang, L;Tang, G;Ye, W;Xu, Y;Tulufu, N;Dan, Z;Qi, J;Deng, L;Li, C;
Experimental & molecular medicine 2024-07-01
-
Multifunctional Cell Regulation Activities of the Mussel Lectin SeviL: Induction of Macrophage Polarization toward the M1 Functional Phenotype
Authors: Fujii, Y;Kamata, K;Gerdol, M;Hasan, I;Rajia, S;Kawsar, SMA;Padma, S;Chatterjee, BP;Ohkawa, M;Ishiwata, R;Yoshimoto, S;Yamada, M;Matsuzaki, N;Yamamoto, K;Niimi, Y;Miyanishi, N;Konno, M;Pallavicini, A;Kawasaki, T;Ogawa, Y;Ozeki, Y;Fujita, H;
Marine drugs 2024-06-11
-
Induced electric fields inhibit breast cancer growth and metastasis by modulating the immune tumor microenvironment
Authors: Charan, M;Jones, TH;Ahirwar, DK;Acharya, N;Subramaniam, VV;Ganju, RK;Song, JW;
bioRxiv : the preprint server for biology 2024-04-17
-
Parotid glands have a dysregulated immune response following radiation therapy
Authors: Gunning, JA;Gilman, KE;Zúñiga, TM;Simpson, RJ;Limesand, KH;
PloS one 2024-03-12
-
Mycoplasma DnaK expression increases cancer development in vivo upon DNA damage
Authors: Benedetti, F;Silvestri, G;Denaro, F;Finesso, G;Contreras-Galindo, R;Munawwar, A;Williams, S;Davis, H;Bryant, J;Wang, Y;Radaelli, E;Rathinam, CV;Gallo, RC;Zella, D;
Proceedings of the National Academy of Sciences of the United States of America 2024-03-05
-
Deciphering Early-Stage Molecular Mechanisms of Negative Pressure Wound Therapy in a Murine Model
Authors: Shyu, YC;Huang, TS;Chiu, HS;Sumazin, P;Lin, XY;Liao, PC;Liou, CC;Hsu, FC;Lin, JS;Hsu, CC;Hsu, PH;Sun, CC;Chen, CT;
International journal of molecular sciences 2024-02-17
-
Eosinophils preserve bone homeostasis by inhibiting excessive osteoclast formation and activity via eosinophil peroxidase
Authors: Andreev, D;Kachler, K;Liu, M;Chen, Z;Krishnacoumar, B;Ringer, M;Frey, S;Krönke, G;Voehringer, D;Schett, G;Bozec, A;
Nature communications 2024-02-05
-
A two-step activation mechanism enables mast cells to differentiate their response between extracellular and invasive enterobacterial infection
Authors: von Beek, C;Fahlgren, A;Geiser, P;Di Martino, ML;Lindahl, O;Prensa, GI;Mendez-Enriquez, E;Eriksson, J;Hallgren, J;Fällman, M;Pejler, G;Sellin, ME;
Nature communications 2024-01-30
-
Enhancing anti-tumor potential: low-intensity vibration suppresses osteosarcoma progression and augments MSCs' tumor-suppressive abilities
Authors: Xiong, X;Huo, Q;Li, K;Cui, C;Chang, C;Park, C;Ku, B;Hong, CS;Lim, H;Pandya, PH;Saadatzadeh, MR;Bijangi-Vishehsaraei, K;Lin, CC;Kacena, MA;Pollok, KE;Chen, A;Liu, J;Thompson, WR;Li, XL;Li, BY;Yokota, H;
Theranostics 2024-01-27
-
Activation and Functions of Col6a1+ Fibroblasts in Colitis-Associated Cancer
Authors: Chalkidi, N;Melissari, MT;Henriques, A;Stavropoulou, A;Kollias, G;Koliaraki, V;
International journal of molecular sciences 2023-12-21
-
Krüppel-like Factor 5 Plays an Important Role in the Pathogenesis of Chronic Pancreatitis
Authors: Alavi, M;Mejia-Bautista, A;Tang, M;Bandovic, J;Rosenberg, AZ;Bialkowska, AB;
Cancers 2023-11-15
-
CXCL9 recombinant adeno-associated virus (AAV) virotherapy sensitizes glioblastoma (GBM) to anti-PD-1 immune checkpoint blockade
Authors: von Roemeling, C;Yegorov, O;Yang, C;Klippel, K;Russell, R;Trivedi, V;Bhatia, A;Doonan, B;Carpenter, S;Ryu, D;Grippen, A;Futch, H;Ran, Y;Hoang-Minh, L;Weidert, F;Golde, T;Mitchell, D;
Research square 2023-11-14
-
The microRNA-mediated gene regulatory network in the hippocampus and hypothalamus of the aging mouse
Authors: Munkhzul, C;Yi, SS;Kim, J;Lee, S;Kim, H;Moon, JS;Lee, M;
PloS one 2023-11-09
-
Senescent Schwann cells induced by aging and chronic denervation impair axonal regeneration following peripheral nerve injury
Authors: Fuentes-Flores, A;Geronimo-Olvera, C;Girardi, K;Necuñir-Ibarra, D;Patel, SK;Bons, J;Wright, MC;Geschwind, D;Hoke, A;Gomez-Sanchez, JA;Schilling, B;Rebolledo, DL;Campisi, J;Court, FA;
EMBO molecular medicine 2023-10-20
-
N-acetyl cysteine mitigates lung damage and inflammation after chlorine exposure in vivo and ex vivo
Authors: Gustafson, Å;Elfsmark, L;Karlsson, T;Jonasson, S;
Toxicology and applied pharmacology 2023-10-10
-
Notch3 deletion regulates HIV-1 gene expression and systemic inflammation to ameliorate chronic kidney disease
Authors: Thornton, M;Sommer, N;McGonigle, M;Ram, AK;Yerrathota, S;Ehirim, H;Chaturvedi, A;Phan, JD;Chakravarthi, PV;Gunewardena, S;Talreja, J;Singhal, P;Fields, TA;Ray, PE;Dhillon, NK;Sharma, M;
bioRxiv : the preprint server for biology 2023-09-13
-
Heparanase 2 (Hpa2)- a new player essential for pancreatic acinar cell differentiation
Authors: Kayal, Y;Barash, U;Naroditsky, I;Ilan, N;Vlodavsky, I;
Cell death & disease 2023-07-25
-
Mechanisms of Innate Immune Injury in Arrhythmogenic Cardiomyopathy
Authors: Chelko, SP;Penna, V;Engel, M;Landim-Vieira, M;Cannon, EN;Lavine, K;Saffitz, JE;
bioRxiv : the preprint server for biology 2023-07-13
-
The SWI/SNF chromatin remodeling subunit DPF2 facilitates NRF2-dependent anti-inflammatory and anti-oxidant gene expression
Authors: Gloria Mas, Na Man, Yuichiro Nakata, Concepcion Martinez-Caja, Daniel L Karl, Felipe Beckedorff et al.
Journal of Clinical Investigation 2023-07-03
-
Loss of epidermal glucocorticoid receptor protects against whole body metabolic dysfunction upon chronic corticosterone treatment
Authors: Gallego, A;Pérez, P;
Molecular metabolism 2023-06-24
-
Aging modulates homeostatic leukocyte trafficking to the peritoneal cavity in a sex-specific manner
Authors: Hopkin, SJ;Pezhman, L;Begum, J;Kavanagh, D;McGettrick, HM;Iqbal, AJ;Chimen, M;
Journal of leukocyte biology 2023-06-13
-
CD4+ T cells aggravate hemorrhagic brain injury
Authors: Shi, SX;Xiu, Y;Li, Y;Yuan, M;Shi, K;Liu, Q;Wang, X;Jin, WN;
Science advances 2023-06-09
-
The soluble guanylyl cyclase pathway is inhibited to evade androgen deprivation-induced senescence and enable progression to castration resistance
Authors: Zhang, L;Troccoli, CI;Mateo-Victoriano, B;Lincheta, LM;Jackson, E;Shu, P;Plastini, T;Tao, W;Kwon, D;Chen, X;Sharma, J;Jorda, M;Gulley, JL;Bilusic, M;Lockhart, AC;Beuve, A;Rai, P;
bioRxiv : the preprint server for biology 2023-05-03
-
Metabololipidomic and proteomic profiling reveals aberrant macrophage activation and interrelated immunomodulatory mediator release during aging
Authors: P Schädel, A Czapka, N Gebert, ID Jacobsen, A Ori, O Werz
Aging Cell, 2023-04-26;0(0):e13856. 2023-04-26
-
NOX4 as a critical effector mediating neuroinflammatory cytokines, myeloperoxidase and osteopontin, specifically in astrocytes in the hippocampus in Parkinson's disease
Authors: N Boonpraman, S Yoon, CY Kim, JS Moon, SS Yi
Redox Biology, 2023-04-10;62(0):102698. 2023-04-10
-
Mesenchymal Stem Cells from COPD Patients Are Capable of Restoring Elastase-Induced Emphysema in a Murine Experimental Model
Authors: C Río, AK Jahn, A Martin-Med, AM Calvo Bota, MT De Francis, PJ Pont Anton, O Gigirey Ca, ÁF Carvajal, C Villena Po, C Gómez Bell, A Iglesias, J Calvo Beni, A Gayà Puig, LA Ortiz, E Sala-Llinà
International Journal of Molecular Sciences, 2023-03-18;24(6):. 2023-03-18
-
Mice with endothelial cell-selective adhesion molecule deficiency develop coronary microvascular rarefaction and left ventricle diastolic dysfunction
Authors: V Buncha, KA Fopiano, L Lang, C Williams, A Horuzsko, JA Filosa, G Kapuku, Z Bagi
Physiological Reports, 2023-03-01;11(6):e15643. 2023-03-01
-
Spectrin-Based Regulation of Cardiac Fibroblast Cell-Cell Communication
Authors: DM Nassal, R Shaheen, NJ Patel, J Yu, N Leahy, D Bibidakis, NL Parinandi, TJ Hund
Cells, 2023-02-26;12(5):. 2023-02-26
-
Paracrine Signals in Calcified Conditioned Media Elicited Differential Responses in Primary Aortic Vascular Smooth Muscle Cells and in Adventitial Fibroblasts
Authors: AM Kennon, JA Stewart
International Journal of Molecular Sciences, 2023-02-10;24(4):. 2023-02-10
-
The Effect of Super-Repressor IkB-Loaded Exosomes (Exo-srIkappaBs) in Chronic Post-Ischemia Pain (CPIP) Models
Authors: JS Chae, H Park, SH Ahn, EC Han, Y Lee, YJ Kim, EJ Ahn, HW Oh, HJ Lee, C Choi, YH Choi, WJ Kim
Pharmaceutics, 2023-02-07;15(2):. 2023-02-07
-
Chemotherapy-induced tumor immunogenicity is mediated in part by megakaryocyte-erythroid progenitors
Authors: A Vorontsova, TJ Cooper, J Haj-Shomal, M Benguigui, S Levin, B Manobla, R Menachem, M Timaner, Z Raviv, Y Shaked
Oncogene, 2023-01-16;0(0):. 2023-01-16
-
Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration
Authors: V Moiseeva, A Cisneros, V Sica, O Deryagin, Y Lai, S Jung, E Andrés, J An, J Segalés, L Ortet, V Lukesova, G Volpe, A Benguria, A Dopazo, S Aznar-Beni, Y Urano, A Del Sol, MA Esteban, Y Ohkawa, AL Serrano, E Perdiguero, P Muñoz-Cáno
Nature, 2023;613(7942):169-178. 2023
-
Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration
Authors: V Moiseeva, A Cisneros, V Sica, O Deryagin, Y Lai, S Jung, E Andrés, J An, J Segalés, L Ortet, V Lukesova, G Volpe, A Benguria, A Dopazo, S Aznar-Beni, Y Urano, A Del Sol, MA Esteban, Y Ohkawa, AL Serrano, E Perdiguero, P Muñoz-Cáno
Nature, 2022-12-21;613(7942):169-178. 2022-12-21
-
Neutrophil “plucking” on megakaryocytes drives platelet production and boosts cardiovascular disease
Authors: Tobias Petzold, Zhe Zhang, Iván Ballesteros, Inas Saleh, Amin Polzin, Manuela Thienel et al.
Immunity 2022-12-13
-
Cannabidiol and Beta-Caryophyllene in Combination: A Therapeutic Functional Interaction
Authors: H Blanton, L Yin, J Duong, K Benamar
International Journal of Molecular Sciences, 2022-12-07;23(24):. 2022-12-07
-
RNA binding protein HuD mediates the crosstalk between beta cells and islet endothelial cells by the regulation of Endostatin and Serpin E1 expression
Authors: M Jung, S Ryu, C Kim, S Cha, H Kang, E Ji, Y Hong, Y Lee, S Han, SM Jeong, W Kim, EK Lee
Cell Death & Disease, 2022-12-05;13(12):1019. 2022-12-05
-
Lcn2 mediates adipocyte-muscle-tumor communication and hypothermia in pancreatic cancer cachexia
Authors: Mengistu Lemecha, Jaya Prakash Chalise, Yuki Takamuku, Guoxiang Zhang, Takahiro Yamakawa, Garrett Larson et al.
Molecular Metabolism 2022-12-01
-
MYC promotes immune-suppression in triple-negative breast cancer via inhibition of interferon signaling
Authors: D Zimmerli, CS Brambillas, F Talens, J Bhin, R Linstra, L Romanens, A Bhattachar, SEP Joosten, AM Da Silva, N Padrao, MD Wellenstei, K Kersten, M de Boo, M Roorda, L Henneman, R de Bruijn, S Annunziato, E van der Bu, AP Drenth, C Lutz, T Endres, M van de Ven, M Eilers, L Wessels, KE de Visser, W Zwart, RSN Fehrmann, MATM van Vugt, J Jonkers
Nature Communications, 2022-11-02;13(1):6579. 2022-11-02
-
Combinatorial microRNA Loading into Extracellular Vesicles for Increased Anti-Inflammatory Efficacy
Authors: AE Pottash, D Levy, A Jeyaram, L Kuo, SM Kronstadt, W Chao, SM Jay
Non-coding RNA, 2022-10-21;8(5):. 2022-10-21
-
Pro-inflammatory megakaryocyte gene expression in murine models of breast cancer
Authors: HG Roweth, MW Malloy, GJ Goreczny, IC Becker, Q Guo, EA Mittendorf, JE Italiano, SS McAllister, EM Battinelli
Science Advances, 2022-10-12;8(41):eabo5224. 2022-10-12
-
Cancer cells produce liver metastasis via gap formation in sinusoidal endothelial cells through proinflammatory paracrine mechanisms
Authors: T Huu Hoang, M Sato-Matsu, H Yuasa, T Matsubara, LTT Thuy, H Ikenaga, DM Phuong, NV Hanh, VN Hieu, DV Hoang, H Hai, Y Okina, M Enomoto, A Tamori, A Daikoku, H Urushima, K Ikeda, NQ Dat, Y Yasui, H Shinkawa, S Kubo, R Yamagishi, N Ohtani, K Yoshizato, J Gracia-San, N Kawada
Science Advances, 2022-09-28;8(39):eabo5525. 2022-09-28
-
Metabolic improvement and liver regeneration by inhibiting CXXC5 function for non-alcoholic steatohepatitis treatment
Authors: SH Seo, E Kim, M Yoon, SH Lee, BH Park, KY Choi
Experimental & Molecular Medicine, 2022-09-16;0(0):. 2022-09-16
-
Essential Roles of the Histone Demethylase KDM4C in Renal Development and Acute Kidney Injury
Authors: HC Pan, YH Chen, WC Fang, VC Wu, CY Sun
International Journal of Molecular Sciences, 2022-08-18;23(16):. 2022-08-18
-
Sensory neuron dysfunction in orthotopic mouse models of colon cancer
Authors: M Balogh, J Zhang, CM Gaffney, N Kalakuntla, NT Nguyen, RT Trinh, C Aguilar, HV Pham, B Milutinovi, JM Nichols, R Mahalingam, AJ Shepherd
Journal of Neuroinflammation, 2022-08-12;19(1):204. 2022-08-12
-
Recombinant FGF21 Attenuates Polychlorinated Biphenyl-Induced NAFLD/NASH by Modulating Hepatic Lipocalin-2 Expression
Authors: HY Kim, YH Yoo
International Journal of Molecular Sciences, 2022-08-10;23(16):. 2022-08-10
-
Levetiracetam Suppresses the Infiltration of Neutrophils and Monocytes and Downregulates Many Inflammatory Cytokines during Epileptogenesis in Pilocarpine-Induced Status Epilepticus Mice
Authors: T Matsuo, R Komori, M Nakatani, S Ochi, A Yokota-Nak, J Matsumoto, F Takata, S Dohgu, Y Ishihara, K Itoh
International Journal of Molecular Sciences, 2022-07-12;23(14):. 2022-07-12
-
Regulation of Inflammation-Related Genes through Fosl1 Suppression in a Levetiracetam-Treated Pilocarpine-Induced Status Epilepticus Mouse Model
Authors: R Komori, T Matsuo, A Yokota-Nak, R Hashimoto, S Kubo, C Kozawa, T Kono, Y Ishihara, K Itoh
International Journal of Molecular Sciences, 2022-07-09;23(14):. 2022-07-09
-
Effects of electroacupuncture on the functionality of NG2-expressing cells in perilesional brain tissue of mice following ischemic stroke
Authors: HJ Lee, DH Jung, NK Kim, HK Shin, BT Choi
Neural regeneration research, 2022-07-01;17(7):1556-1565. 2022-07-01
-
Host CLIC4 expression in the tumor microenvironment is essential for breast cancer metastatic competence
Authors: VC Sanchez, HH Yang, A Craig-Luca, W Dubois, BL Carofino, J Lack, JE Dwyer, RM Simpson, C Cataisson, MP Lee, J Luo, KW Hunter, SH Yuspa
PloS Genetics, 2022-06-21;18(6):e1010271. 2022-06-21
-
Rationally designed bioactive milk-derived protein scaffolds enhanced new bone formation
Authors: MS Lee, J Jeon, S Park, J Lim, HS Yang
Oncogene, 2022-06-16;20(0):368-380. 2022-06-16
-
Modulation of Rxralpha Expression in Mononuclear Phagocytes Impacts on Cardiac Remodeling after Ischemia-Reperfusion Injury
Authors: S Räuber, M Fischer, D Messerer, V Wimmler, K Konda, A Todica, M Lorenz, A Titova, C Schulz, T Weinberger
Biomedicines, 2022-05-30;10(6):. 2022-05-30
-
RON (MST1R) and HGFL (MST1) Co-Overexpression Supports Breast Tumorigenesis through Autocrine and Paracrine Cellular Crosstalk
Authors: BG Hunt, A Jones, C Lester, JC Davis, NM Benight, SE Waltz
Cancers, 2022-05-19;14(10):. 2022-05-19
-
Loss of T�cell tolerance in the skin following immunopathology is linked to failed restoration of the dermal niche by recruited macrophages
Authors: HC West, J Davies, S Henderson, OK Adegun, S Ward, IR Ferrer, CA Tye, AF Vallejo, L Jardine, M Collin, ME Polak, CL Bennett
Cell Reports, 2022-05-17;39(7):110819. 2022-05-17
-
Inhibiting MNK kinases promotes macrophage immunosuppressive phenotype to limit CD8+ T cell anti-tumor immunity
Authors: TN Pham, C Spaulding, MA Shields, AE Metropulos, DN Shah, MG Khalafalla, DR Principe, DJ Bentrem, HG Munshi
JCI Insight, 2022-05-09;0(0):. 2022-05-09
-
Caspase-8 in endothelial cells maintains gut homeostasis and prevents small bowel inflammation in mice
Authors: N Tisch, C Mogler, A Stojanovic, R Luck, EA Korhonen, A Ellerkmann, H Adler, M Singhal, G Schermann, L Erkert, JV Patankar, A Karakatsan, AL Scherr, Y Fuchs, A Cerwenka, S Wirtz, BC Köhler, HG Augustin, C Becker, T Schmidt, C Ruiz de Al
Embo Molecular Medicine, 2022-05-02;0(0):e14121. 2022-05-02
-
Loss of RNA binding protein HuD facilitates the production of the senescence-associated secretory phenotype
Authors: S Ryu, M Jung, C Kim, H Kang, S Han, S Cha, SM Jeong, EK Lee
Cell Death & Disease, 2022-04-11;13(4):329. 2022-04-11
-
Transient expansion and myofibroblast conversion of adipogenic lineage precursors mediate bone marrow repair after radiation
Authors: L Zhong, L Yao, N Holdreith, W Yu, T Gui, Z Miao, Y Elkaim, M Li, Y Gong, M Pacifici, A Maity, TM Busch, KS Joeng, K Cengel, P Seale, W Tong, L Qin
JCI Insight, 2022-04-08;7(7):. 2022-04-08
-
Tumor-derived Jagged1 promotes cancer progression through immune evasion
Authors: J Meng, YZ Jiang, S Zhao, Y Tao, T Zhang, X Wang, Y Zhang, K Sun, M Yuan, J Chen, Y Wei, X Lan, M Chen, CJ David, Z Chang, X Guo, D Pan, M Chen, ZM Shao, Y Kang, H Zheng
Cell Reports, 2022-03-08;38(10):110492. 2022-03-08
-
The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas
Authors: FX Yu, PSY Lee, L Yang, N Gao, Y Zhang, AV Ljubimov, E Yang, Q Zhou, L Xie
Progress in retinal and eye research, 2022-01-04;0(0):101039. 2022-01-04
-
Ginger nanoparticles mediated induction of Foxa2 prevents high-fat diet-induced insulin resistance
Authors: A Kumar, K Sundaram, Y Teng, J Mu, MK Sriwastva, L Zhang, JL Hood, J Yan, X Zhang, JW Park, ML Merchant, HG Zhang
Theranostics, 2022-01-01;12(3):1388-1403. 2022-01-01
-
Garlic exosome-like nanoparticles reverse high-fat diet induced obesity via the gut/brain axis
Authors: K Sundaram, J Mu, A Kumar, J Behera, C Lei, MK Sriwastva, F Xu, GW Dryden, L Zhang, S Chen, J Yan, X Zhang, JW Park, ML Merchant, N Tyagi, Y Teng, HG Zhang
Theranostics, 2022-01-01;12(3):1220-1246. 2022-01-01
-
A targetable LIFR-NF-kappaB-LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis
Authors: F Yao, Y Deng, Y Zhao, Y Mei, Y Zhang, X Liu, C Martinez, X Su, RR Rosato, H Teng, Q Hang, S Yap, D Chen, Y Wang, MM Chen, M Zhang, H Liang, D Xie, X Chen, H Zhu, JC Chang, MJ You, Y Sun, B Gan, L Ma
Nature Communications, 2021-12-17;12(1):7333. 2021-12-17
-
MK2a inhibitor CMPD1 abrogates chikungunya virus infection by modulating actin remodeling pathway
Authors: P Mamidi, TK Nayak, A Kumar, S Kumar, S Chatterjee, S De, A Datey, S Ghosh, SS Keshry, S Singh, E Laha, A Ray, S Chattopadh, S Chattopadh
Oncogene, 2021-11-15;17(11):e1009667. 2021-11-15
-
Induction of IL-12p40 and type 1 immunity by Toxoplasma gondii in the absence of the TLR-MyD88 signaling cascade
Authors: LM Snyder, CM Doherty, HL Mercer, EY Denkers
PloS Pathogens, 2021-10-01;17(10):e1009970. 2021-10-01
-
Mesenchymal stromal cell aging impairs the self-organizing capacity of lung alveolar epithelial stem cells
Authors: D Chanda, M Rehan, SR Smith, KG Dsouza, Y Wang, K Bernard, D Kurundkar, V Memula, K Kojima, JA Mobley, GA Benavides, V Darley-Usm, YI Kim, JW Zmijewski, JS Deshane, S De Langhe, VJ Thannickal
Elife, 2021-09-16;10(0):. 2021-09-16
-
Trimebutine suppresses Toll-like receptor 2/4/7/8/9 signaling pathways in macrophages
Authors: N Ogawa, S Nakajima, K Tamada, N Yokoue, H Tachibana, M Okazawa, T Oyama, H Abe, H Yamazaki, A Yoshimori, A Sato, T Kamiya, T Yokomizo, F Uchiumi, T Abe, SI Tanuma
Archives of biochemistry and biophysics, 2021-09-10;711(0):109029. 2021-09-10
-
Adipsin-Dependent Secretion of Hepatocyte Growth Factor Regulates the Adipocyte-Cancer Stem Cell Interaction
Authors: M Mizuno, B Khaledian, M Maeda, T Hayashi, S Mizuno, E Munetsuna, T Watanabe, S Kono, S Okada, M Suzuki, S Takao, H Minami, N Asai, F Sugiyama, S Takahashi, Y Shimono
Cancers, 2021-08-23;13(16):. 2021-08-23
-
Innate immune regulates cutaneous sensory IL-13 receptor alpha 2 to promote atopic dermatitis
Authors: S Xiao, Z Lu, M Steinhoff, Y Li, T Buhl, M Fischer, W Chen, W Cheng, R Zhu, X Yan, H Yang, Y Liu, Y Dou, W Wang, J Wang, J Meng
Brain, Behavior, and Immunity, 2021-08-13;98(0):28-39. 2021-08-13
-
Neuroimmune mechanisms of cognitive impairment in a mouse model of Gulf War illness
Authors: JD Bryant, M Kodali, B Shuai, SS Menissy, PJ Graves, T Trong Phan, R Dantzer, AK Shetty, L Ciaccia We, AP West
Brain, Behavior, and Immunity, 2021-07-29;0(0):. 2021-07-29
-
TNFR2 Signaling Regulates the Immunomodulatory Function of Oligodendrocyte Precursor Cells
Authors: HL Desu, P Illiano, JS Choi, MC Ascona, H Gao, JK Lee, R Brambilla
Cells, 2021-07-15;10(7):. 2021-07-15
-
GIP_HUMAN[22-51] is a new proatherogenic peptide identified by native plasma peptidomics
Authors: T Masaki, Y Kodera, M Terasaki, K Fujimoto, T Hirano, M Shichiri
Scientific Reports, 2021-07-14;11(1):14470. 2021-07-14
-
Targeting senescent cells improves functional recovery after spinal cord injury
Authors: D Paramos-de, I Martins, AM Cristóvão, AF Dias, D Neves-Silv, T Pereira, D Chapela, A Farinho, A Jacinto, L Saúde
Cell Reports, 2021-07-06;36(1):109334. 2021-07-06
-
CD8+ T cell immunity blocks the metastasis of carcinogen-exposed breast cancer
Authors: K Li, T Li, Z Feng, M Huang, L Wei, Z Yan, M Long, Q Hu, J Wang, S Liu, DC Sgroi, S Demehri
Science Advances, 2021-06-18;7(25):. 2021-06-18
-
Integration of transcriptomics and system pharmacology to reveal the therapeutic mechanism underlying Qingfei Xiaoyan Wan to treat allergic asthma
Authors: JY Hou, JR Wu, D Xu, YB Chen, DD Shang, S Liu, GW Fan, YL Cui
Journal of ethnopharmacology, 2021-06-04;278(0):114302. 2021-06-04
-
WNT5A inhibition alters the malignant peripheral nerve sheath tumor microenvironment and enhances tumor growth
Authors: CS Thomson, J Pundavela, MR Perrino, RA Coover, K Choi, KE Chaney, TA Rizvi, DA Largaespad, N Ratner
Oncogene, 2021-06-02;40(24):4229-4241. 2021-06-02
-
Cell barrier function of resident peritoneal macrophages in post-operative adhesions
Authors: T Ito, Y Shintani, L Fields, M Shiraishi, MN Podaru, S Kainuma, K Yamashita, K Kobayashi, M Perretti, F Lewis-McDo, K Suzuki
Nature Communications, 2021-04-14;12(1):2232. 2021-04-14
-
JAK/STAT inhibitor therapy partially rescues the lipodystrophic autoimmune phenotype in Clec16a KO mice
Authors: R Pandey, M Bakay, BP Strenkowsk, HS Hain, H Hakonarson
Scientific Reports, 2021-04-01;11(1):7372. 2021-04-01
-
Inhibition of HIF-prolyl hydroxylases improves healing of intestinal anastomoses
Authors: MJ Strowitzki, G Kimmer, JS Wehrmann, AS Ritter, P Radhakrish, VM Opitz, C Tuffs, M Biller, J Kugler, U Keppler, JM Harnoss, J Klose, T Schmidt, A Blanco, CT Taylor, M Schneider
JCI Insight, 2021-03-30;0(0):. 2021-03-30
-
P-selectin axis plays a key role in microglia immunophenotype and glioblastoma progression
Authors: E Yeini, P Ofek, S Pozzi, N Albeck, D Ben-Shusha, G Tiram, S Golan, R Kleiner, R Sheinin, S Israeli Da, S Reich-Zeli, R Grossman, Z Ram, H Brem, TM Hyde, P Magod, D Friedmann-, A Madi, R Satchi-Fai
Nature Communications, 2021-03-26;12(1):1912. 2021-03-26
-
Preventing tumor progression to the bone by induced tumor-suppressing MSCs
Authors: X Sun, K Li, R Zha, S Liu, Y Fan, D Wu, M Hase, UK Aryal, CC Lin, BY Li, H Yokota
Theranostics, 2021-03-05;11(11):5143-5159. 2021-03-05
-
Microglia-derived interleukin-10 accelerates post-intracerebral hemorrhage hematoma clearance by regulating CD36
Authors: Q Li, X Lan, X Han, F Durham, J Wan, A Weiland, RC Koehler, J Wang
Brain, Behavior, and Immunity, 2021-02-13;94(0):437-457. 2021-02-13
-
Pharmacological normalization of pancreatic cancer-associated fibroblast secretome impairs pro-metastatic cross-talk with macrophages: Stromal CSF-1 facilitates metastasis
Authors: R Samain, A Brunel, T Douché, M Fanjul, S Cassant-So, J Rochotte, J Cros, C Neuzillet, J Raffenne, C Duluc, A Perraud, J Nigri, V Gigoux, I Bieche, M Ponzo, G Carpentier, I Cascone, R Tomasini, HA Schmid, M Mathonnet, R Nicolle, MP Bousquet, Y Martineau, S Pyronnet, C Jean, C Bousquet
Cellular and Molecular Gastroenterology and Hepatology, 2021-01-20;0(0):. 2021-01-20
-
Mechanical Loading-Driven Tumor Suppression Is Mediated by Lrp5-Dependent and Independent Mechanisms
Authors: Y Feng, S Liu, R Zha, X Sun, K Li, A Robling, B Li, H Yokota
Cancers, 2021-01-13;13(2):. 2021-01-13
-
High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance
Authors: A Kumar, K Sundaram, J Mu, GW Dryden, MK Sriwastva, C Lei, L Zhang, X Qiu, F Xu, J Yan, X Zhang, JW Park, ML Merchant, HCL Bohler, B Wang, S Zhang, C Qin, Z Xu, X Han, CJ McClain, Y Teng, HG Zhang
Nature Communications, 2021-01-11;12(1):213. 2021-01-11
-
EGFRvIII uses intrinsic and extrinsic mechanisms to reduce glioma adhesion and increase migration
Authors: A Banisadr, M Eick, P Beri, AD Parisian, B Yeoman, JK Placone, AJ Engler, F Furnari
J Cell Sci, 2020-12-24;0(0):. 2020-12-24
-
Neuroblast senescence in the aged brain augments natural killer cell cytotoxicity leading to impaired neurogenesis and cognition
Authors: WN Jin, K Shi, W He, JH Sun, L Van Kaer, FD Shi, Q Liu
Nature Neuroscience, 2020-11-30;0(0):. 2020-11-30
-
Wisp1 is a circulating factor that stimulates proliferation of adult mouse and human beta cells
Authors: R Fernandez-, A García-Ala, Y Esteban, J Mir-Coll, B Serra-Nava, M Fontcubert, C Broca, M Armanet, A Wojtuscisz, V Kram, MF Young, J Vidal, R Gomis, R Gasa
Nat Commun, 2020-11-25;11(1):5982. 2020-11-25
-
Senescence-activated enhancer landscape orchestrates the senescence-associated secretory phenotype in murine fibroblasts
Authors: Y Guan, C Zhang, G Lyu, X Huang, X Zhang, T Zhuang, L Jia, L Zhang, C Zhang, C Li, W Tao
Nucleic Acids Res, 2020-11-04;0(0):. 2020-11-04
-
Yes-associated protein (YAP) induces a secretome phenotype and transcriptionally regulates plasminogen activator Inhibitor-1 (PAI-1) expression in hepatocarcinogenesis
Authors: S Marquard, S Thomann, SME Weiler, M Bissinger, T Lutz, C Sticht, M Tóth, C de la Torr, N Gretz, BK Straub, J Marquardt, P Schirmache, K Breuhahn
Cell Commun Signal, 2020-10-23;18(1):166. 2020-10-23
-
Bisphenol A exposure increases epididymal susceptibility to infection in mice
Authors: YJ Park, WK Pang, DY Ryu, EO Adegoke, MS Rahman, MG Pang
Ecotoxicol Environ Saf, 2020-10-19;208(0):111476. 2020-10-19
-
Adaptive thermogenesis enhances the life-threatening response to heat in mice with an Ryr1 mutation
Authors: HJ Wang, CS Lee, RSZ Yee, L Groom, I Friedman, L Babcock, DK Georgiou, J Hong, AD Hanna, J Recio, JM Choi, T Chang, NH Agha, J Romero, P Sarkar, N Voermans, MW Gaber, SY Jung, ML Baker, RG Pautler, RT Dirksen, S Riazi, SL Hamilton
Nat Commun, 2020-10-09;11(1):5099. 2020-10-09
-
Hyperactivity of Innate Immunity Triggers Pain via TLR2-IL-33-Mediated Neuroimmune Crosstalk
Authors: J Huang, MA Gandini, L Chen, S M'Dahoma, PL Stemkowski, H Chung, DA Muruve, GW Zamponi
Cell Rep, 2020-10-06;33(1):108233. 2020-10-06
-
Chemerin Isoform-Specific Effects on Hepatocyte Migration and Immune Cell Inflammation
Authors: Susanne Feder, Astrid Bruckmann, Nichole McMullen, Christopher J. Sinal, Christa Buechler
International Journal of Molecular Sciences 2020-09-29
-
Dietary &alpha-Linolenic Acid Counters Cardioprotective Dysfunction in Diabetic Mice: Unconventional PUFA Protection
Authors: JS Russell, TA Griffith, S Naghipour, J Vider, EF Du Toit, HH Patel, JN Peart, JP Headrick
Nutrients, 2020-09-02;12(9):. 2020-09-02
-
Tenascin-C Orchestrates an Immune-Suppressive Tumor Microenvironment in Oral Squamous Cell Carcinoma
Authors: Caroline Spenlé, Thomas Loustau, Devadarssen Murdamoothoo, William Erne, Stephanie Beghelli-de la Forest Divonne, Romain Veber et al.
Cancer Immunology Research 2020-09-01
-
The Gustatory Sensory G-Protein GNAT3 Suppresses Pancreatic Cancer Progression in Mice
Authors: MT Hoffman, SB Kemp, DJ Salas-Esca, Y Zhang, NG Steele, S The, D Long, S Benitz, W Yan, RF Margolskee, F Bednar, M Pasca di M, HJ Wen, HC Crawford
Cell Mol Gastroenterol Hepatol, 2020-08-31;0(0):. 2020-08-31
-
Evidence of mesenchymal stromal cell adaptation to local microenvironment following subcutaneous transplantation
Authors: MB Preda, AM Lupan, CA Neculachi, LI Leti, IM Fenyo, S Popescu, EG Rusu, CI Marinescu, M Simionescu, A Burlacu
J. Cell. Mol. Med., 2020-08-12;0(0):. 2020-08-12
-
In situ neutrophil efferocytosis shapes T cell immunity to influenza infection
Authors: K Lim, TH Kim, A Trzeciak, AM Amitrano, EC Reilly, H Prizant, DJ Fowell, DJ Topham, M Kim
Nat. Immunol., 2020-08-03;0(0):. 2020-08-03
-
Lipoteichoic Acid Accelerates Bone Healing by Enhancing Osteoblast Differentiation and Inhibiting Osteoclast Activation in a Mouse Model of Femoral Defects
Authors: CC Hu, CH Chang, YM Hsiao, Y Chang, YY Wu, SWN Ueng, MF Chen
Int J Mol Sci, 2020-08-03;21(15):. 2020-08-03
-
SUMO E3 ligase PIAS1 is a potential biomarker indicating stress susceptibility
Authors: HY Lin, YS Liu, CY Huang, F Cathomas, K Liu, J Wang, HT Cheng, SW Lai, YC Liu, CJ Chen, C Lin, DY Lu
Psychoneuroendocrinology, 2020-07-11;120(0):104800. 2020-07-11
-
Triterpenoids Extracted From Antrodia cinnamomea Mycelia Attenuate Acute Alcohol-Induced Liver Injury in C57BL/6 Mice via Suppression Inflammatory Response
Authors: Y Liu, Z Wang, F Kong, L Teng, X Zheng, X Liu, D Wang
Front Microbiol, 2020-07-03;11(0):1113. 2020-07-03
-
An autophagic deficit in the uterine vessel microenvironment provokes hyperpermeability through deregulated VEGFA, NOS1, and CTNNB1
Authors: B Lee, H Shin, JE Oh, J Park, M Park, SC Yang, JH Jun, SH Hong, H Song, HJ Lim
Autophagy, 2020-06-17;0(0):1-18. 2020-06-17
-
Intramembrane proteolysis of an extracellular serine protease, epithin/PRSS14, enables its intracellular nuclear function
Authors: Y Cho, SB Kim, J Kim, AVQ Pham, MJ Yoon, JH Park, KT Hwang, D Park, Y Cho, MG Kim, C Kim
BMC Biol., 2020-06-03;18(1):60. 2020-06-03
-
Host cytosolic RNA sensing pathway promotes T Lymphocyte-mediated mycobacterial killing in macrophages
Authors: Y Cheng, NJ Kiene, A Tatarian, EF Eix, JS Schorey
PLoS Pathog., 2020-05-28;16(5):e1008569. 2020-05-28
-
NOX4 Deficiency Exacerbates the Impairment of Cystatin C-Dependent Hippocampal Neurogenesis by a Chronic High Fat Diet
Authors: P Jiranugrom, ID Yoo, MW Park, JH Ryu, JS Moon, SS Yi
Genes (Basel), 2020-05-19;11(5):. 2020-05-19
-
Toxoplasma gondii dense granule protein GRA24 drives MyD88-independent p38 MAPK activation, IL-12 production and induction of protective immunity
Authors: HL Mercer, LM Snyder, CM Doherty, BA Fox, DJ Bzik, EY Denkers
PLoS Pathog., 2020-05-15;16(5):e1008572. 2020-05-15
-
Sestrin2 modulates cardiac inflammatory response through maintaining redox homeostasis during ischemia and reperfusion
Authors: D Ren, N Quan, J Fedorova, J Zhang, Z He, J Li
Redox Biol, 2020-05-05;34(0):101556. 2020-05-05
-
Adrenergic Blockade Promotes Maintenance of Dormancy in Prostate Cancer Through Upregulation of GAS6
Authors: AM Decker, JT Decker, Y Jung, FC Cackowski, S Daignault-, TM Morgan, LD Shea, RS Taichman
Transl Oncol, 2020-04-28;13(7):100781. 2020-04-28
-
High-salt diet inhibits tumour growth in mice via regulating myeloid-derived suppressor cell differentiation
Authors: W He, J Xu, R Mu, Q Li, DL Lv, Z Huang, J Zhang, C Wang, L Dong
Nat Commun, 2020-04-07;11(1):1732. 2020-04-07
-
YAP Non-cell-autonomously Promotes Pluripotency Induction in Mouse Cells
Authors: AA Hartman, SM Scalf, J Zhang, X Hu, X Chen, AE Eastman, C Yang, S Guo
Stem Cell Reports, 2020-04-02;14(4):730-743. 2020-04-02
-
The mechanism of Tyk2 deficiency-induced immunosuppression in mice involves robust IL-10 production in macrophages
Authors: K Hirashima, R Muromoto, H Minoguchi, T Matsumoto, Y Kitai, JI Kashiwakur, K Shimoda, K Oritani, T Matsuda
Cytokine, 2020-03-21;130(0):155077. 2020-03-21
-
Cancer associated fibroblast FAK regulates malignant cell metabolism
Authors: F Demirciogl, J Wang, J Candido, ASH Costa, P Casado, B de Luxan D, LE Reynolds, J Gomez-Escu, E Newport, V Rajeeve, AM Baker, M Roy-Luzarr, TA Graham, J Foster, Y Wang, JJ Campbell, R Singh, P Zhang, TJ Schall, FR Balkwill, J Sosabowski, PR Cutillas, C Frezza, P Sancho, K Hodivala-D
Nat Commun, 2020-03-10;11(1):1290. 2020-03-10
-
Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2
Authors: X Zhou, S Wahane, MS Friedl, M Kluge, CC Friedel, K Avrampou, V Zachariou, L Guo, B Zhang, X He, RH Friedel, H Zou
Nat. Neurosci., 2020-03-01;23(3):337-350. 2020-03-01
-
beta4GALT1 controls beta1 integrin function to govern thrombopoiesis and hematopoietic stem cell homeostasis
Authors: S Giannini, MM Lee-Sundlo, L Rivadeneyr, CA Di Buduo, R Burns, JT Lau, H Falet, A Balduini, KM Hoffmeiste
Nat Commun, 2020-01-17;11(1):356. 2020-01-17
-
beta4GALT1 controls beta1 integrin function to govern thrombopoiesis and hematopoietic stem cell homeostasis
Authors: S Giannini, MM Lee-Sundlo, L Rivadeneyr, CA Di Buduo, R Burns, JT Lau, H Falet, A Balduini, KM Hoffmeiste
Nat Commun, 2020;11(1):356. 2020
-
Dual microglia effects on blood brain barrier permeability induced by systemic inflammation
Authors: K Haruwaka, A Ikegami, Y Tachibana, N Ohno, H Konishi, A Hashimoto, M Matsumoto, D Kato, R Ono, H Kiyama, AJ Moorhouse, J Nabekura, H Wake
Nat Commun, 2019-12-20;10(1):5816. 2019-12-20
-
Depletion of Bone Marrow-Derived Fibrocytes Attenuates TAA-Induced Liver Fibrosis in Mice
Authors: F Hempel, M Roderfeld, R Savai, A Sydykov, K Irungbam, R Schermuly, R Voswinckel, K Köhler, Y Churin, L Kiss, J Bier, J Pons-Kühne, E Roeb
Cells, 2019-10-07;8(10):. 2019-10-07
-
Non-classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung
Authors: J Schyns, Q Bai, C Ruscitti, C Radermecke, S De Scheppe, S Chakarov, F Farnir, D Pirottin, F Ginhoux, G Boeckxstae, F Bureau, T Marichal
Nat Commun, 2019-09-03;10(1):3964. 2019-09-03
-
Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer
Authors: A El-Kenawi, C Gatenbee, M Robertson-, R Bravo, J Dhillon, Y Balaguruna, A Berglund, N Visvakarma, A Ibrahim-Ha, J Choi, K Luddy, R Gatenby, S Pilon-Thom, A Anderson, B Ruffell, R Gillies
Br. J. Cancer, 2019-08-16;121(7):556-566. 2019-08-16
-
Tumour-Derived Laminin alpha5 (LAMA5) Promotes Colorectal Liver Metastasis Growth, Branching Angiogenesis and Notch Pathway Inhibition
Authors: A Gordon-Wee, SY Lim, A Yuzhalin, S Lucotti, JAF Vermeer, K Jones, J Chen, RJ Muschel
Cancers (Basel), 2019-05-06;11(5):. 2019-05-06
-
Platelet GPIb? is a mediator and potential interventional target for NASH and subsequent liver cancer
Authors: M Malehmir, D Pfister, S Gallage, M Szydlowska, D Inverso, E Kotsiliti, V Leone, M Peiseler, BGJ Surewaard, D Rath, A Ali, MJ Wolf, H Drescher, ME Healy, D Dauch, D Kroy, O Krenkel, M Kohlhepp, T Engleitner, A Olkus, T Sijmonsma, J Volz, C Deppermann, D Stegner, P Helbling, C Nombela-Ar, A Rafiei, M Hinterleit, M Rall, F Baku, O Borst, CL Wilson, J Leslie, T O'Connor, CJ Weston, DH Adams, L Sheriff, A Teijeiro, M Prinz, R Bogeska, N Anstee, MN Bongers, M Notohamipr, T Geisler, DJ Withers, J Ware, DA Mann, HG Augustin, A Vegiopoulo, MD Milsom, AJ Rose, PF Lalor, JM Llovet, R Pinyol, F Tacke, R Rad, M Matter, N Djouder, P Kubes, PA Knolle, K Unger, L Zender, B Nieswandt, M Gawaz, A Weber, M Heikenwald
Nat. Med., 2019-04-01;25(4):641-655. 2019-04-01
-
Liver-derived fibroblast growth factor 21 mediates effects of glucagon-like peptide-1 in attenuating hepatic glucose output
Authors: J Liu, K Yang, J Yang, W Xiao, Y Le, F Yu, L Gu, S Lang, Q Tian, T Jin, R Wei, T Hong
EBioMedicine, 2019-03-01;0(0):. 2019-03-01
-
Prostate cancer promotes a vicious cycle of bone metastasis progression through inducing osteocytes to secrete GDF15 that stimulates prostate cancer growth and invasion
Authors: W Wang, X Yang, J Dai, Y Lu, J Zhang, ET Keller
Oncogene, 2019-02-12;0(0):. 2019-02-12
-
Matrix bound nanovesicle-associated IL-33 activates a pro-remodeling macrophage phenotype via a non-canonical, ST2-independent pathway
Authors: GS Hussey, JL Dziki, YC Lee, JG Bartolacci, M Behun, HR Turnquist, SF Badylak
J Immunol Regen Med, 2019-02-01;3(0):26-35. 2019-02-01
-
T cell microvilli constitute immunological synaptosomes that carry messages to antigen-presenting cells
Authors: HR Kim, Y Mun, KS Lee, YJ Park, JS Park, JH Park, BN Jeon, CH Kim, Y Jun, YM Hyun, M Kim, SM Lee, CS Park, SH Im, CD Jun
Nat Commun, 2018-09-07;9(1):3630. 2018-09-07
-
Therapeutic Ablation of Gain-of-Function Mutant p53 in Colorectal Cancer Inhibits Stat3-Mediated Tumor Growth and Invasion
Authors: Ramona Schulz-Heddergott, Nadine Stark, Shelley J. Edmunds, Jinyu Li, Lena-Christin Conradi, Hanibal Bohnenberger et al.
Cancer Cell 2018-08-13
-
IL-23 secreted by myeloid cells drives castration-resistant prostate cancer
Authors: A Calcinotto, C Spataro, E Zagato, D Di Mitri, V Gil, M Crespo, G De Bernard, M Losa, M Mirenda, E Pasquini, A Rinaldi, S Sumanasuri, MB Lambros, A Neeb, R Lucianò, CA Bravi, D Nava-Rodri, D Dolling, T Prayer-Gal, A Ferreira, A Briganti, A Esposito, S Barry, W Yuan, A Sharp, J de Bono, A Alimonti
Nature, 2018-06-27;0(0):. 2018-06-27
-
Extracellular vesicles mediate low dose ionizing radiation-induced immune and inflammatory responses in the blood
Authors: T Szatmári, E Persa, E Kis, A Benedek, R Hargitai, G Sáfrány, K Lumniczky
Int. J. Radiat. Biol., 2018-03-29;0(0):1-31. 2018-03-29
-
Comparison of the immune response during acute and chronic Staphylococcus aureus infection
Authors: RA Brady, CP Mocca, RD Plaut, K Takeda, DL Burns
PLoS ONE, 2018-03-29;13(3):e0195342. 2018-03-29
-
Interleukin-18 Amplifies Macrophage Polarization and Morphological Alteration, Leading to Excessive Angiogenesis
Authors: T Kobori, S Hamasaki, A Kitaura, Y Yamazaki, T Nishinaka, A Niwa, S Nakao, H Wake, S Mori, T Yoshino, M Nishibori, H Takahashi
Front Immunol, 2018-03-06;9(0):334. 2018-03-06
-
Mesenchymal stem cells over-expressing cxcl12 enhance the radioresistance of the small intestine
Authors: P Chang, B Zhang, L Shao, W Song, W Shi, L Wang, T Xu, D Li, X Gao, Y Qu, L Dong, J Wang
Cell Death Dis, 2018-02-05;9(2):154. 2018-02-05
-
Conditioned Medium of Bone Marrow-Derived Mesenchymal Stromal Cells as a Therapeutic Approach to Neuropathic Pain: A Preclinical Evaluation
Authors: KB Gama, DS Santos, AF Evangelist, DN Silva, AC de Alcânta, RR Dos Santos, MBP Soares, CF Villarreal
Stem Cells Int, 2018-01-30;2018(0):8179013. 2018-01-30
-
Tumor-extrinsic discoidin domain receptor 1 promotes mammary tumor growth by regulatingadipose stromal interleukin 6 production in mice
Authors: X Sun, K Gupta, B Wu, D Zhang, B Yuan, X Zhang, HC Chiang, C Zhang, TJ Curiel, MP Bendeck, S Hursting, Y Hu, R Li
J. Biol. Chem., 2018-01-03;0(0):. 2018-01-03
-
A CREB3-regulated ER-Golgi trafficking signature promotes metastatic progression in breast cancer
Authors: BV Howley, LA Link, S Grelet, M El-Sabban, PH Howe
Oncogene, 2017-12-18;0(0):. 2017-12-18
-
Pharmacological HIF-inhibition attenuates postoperative adhesion formation
Authors: MJ Strowitzki, AS Ritter, P Radhakrish, JM Harnoss, VM Opitz, M Biller, J Wehrmann, U Keppler, J Scheer, M Wallwiener, T Schmidt, A Ulrich, M Schneider
Sci Rep, 2017-10-13;7(1):13151. 2017-10-13
-
A Jak1/2 inhibitor, baricitinib, inhibits osteoclastogenesis by suppressing RANKL expression in osteoblasts in vitro
Authors: K Murakami, Y Kobayashi, S Uehara, T Suzuki, M Koide, T Yamashita, M Nakamura, N Takahashi, H Kato, N Udagawa, Y Nakamura
PLoS ONE, 2017-07-14;12(7):e0181126. 2017-07-14
-
Map3k8 controls granulocyte colony-stimulating factor production and neutrophil precursor proliferation in lipopolysaccharide-induced emergency granulopoiesis
Authors: Á Sánchez, C Relaño, A Carrasco, C Contreras-, A Martín-Duc, A Aranda, S Alemany
Sci Rep, 2017-07-10;7(1):5010. 2017-07-10
-
Effective combinatorial immunotherapy for castration-resistant prostate cancer
Authors: X Lu, JW Horner, E Paul, X Shang, P Troncoso, P Deng, S Jiang, Q Chang, DJ Spring, P Sharma, JA Zebala, DY Maeda, YA Wang, RA DePinho
Nature, 2017-03-20;543(7647):728-732. 2017-03-20
-
Metabolic origins of spatial organization in the tumor microenvironment
Authors: C Carmona-Fo, M Deforet, L Akkari, CB Thompson, JA Joyce, JB Xavier
Proc. Natl. Acad. Sci. U.S.A, 2017-02-28;0(0):. 2017-02-28
-
Promyelocytic Leukemia Protein (PML) Controls Listeria monocytogenes Infection
Authors: D Ribet, V Lallemand-, O Ferhi, MA Nahori, H Varet, H de Th?, P Cossart
MBio, 2017-01-10;8(1):. 2017-01-10
-
Vagal Regulation of Group 3 Innate Lymphoid Cells and the Immunoresolvent PCTR1 Controls Infection Resolution
Authors: Jesmond Dalli, Romain A. Colas, Hildur Arnardottir, Charles N. Serhan
Immunity 2017-01-05
-
Depletion of microglia exacerbates postischemic inflammation and brain injury
Authors: WN Jin, SX Shi, Z Li, M Li, K Wood, RJ Gonzales, Q Liu
J. Cereb. Blood Flow Metab., 2017-01-01;0(0):271678X176941. 2017-01-01
-
Astrocyte-derived interleukin-15 exacerbates ischemic brain injury via propagation of cellular immunity
Proc. Natl. Acad. Sci. U.S.A, 2016-12-19;0(0):. 2016-12-19
-
Novel Resolvin D2 Receptor Axis in Infectious Inflammation
Authors: Nan Chiang
J. Immunol, 2016-12-19;0(0):. 2016-12-19
-
Evaluation of PLGA containing anti-CTLA4 inhibited endometriosis progression by regulating CD4+CD25+Treg cells in peritoneal fluid of mouse endometriosis model
Authors: Qi Liu
Eur J Pharm Sci, 2016-10-29;0(0):. 2016-10-29
FAQs
-
Will the Array identification number stamped on the Array membrane interfere with detection if it is not cut-off before the membrane is blocked?
The dye used for printing the Array identification number on the membranes will fluoresce and interfere with the LI-COR detection. It is critical that the number is cut off before beginning the experiment.
Reviews for Proteome Profiler Mouse XL Cytokine Array
Average Rating: 4.9 (Based on 36 Reviews)
Have you used Proteome Profiler Mouse XL Cytokine Array?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
The Proteome Profiler Mouse XL Cytokine array performed with high sensitivity and reproducibility, enabling the simultaneous detection of multiple cytokines from limited sample volumes from our mouse retinal sampes.
High sensitivity with publishable results
Performed on mammary tumor lysates.
I bought two boxes, and they're both great
This product works very well
Conditioned medium of macrophages (36h) isolated from metastatic lungs.
This kit is really great!
Secretome of 4T1 murine breast cancer cells.
Cytokine array of the secretome of MLE12 cells.
We used the array kit for profiling inflammatory cytokines in plasma samples from mice. I trust that it works very well.
Excellent antibody array to screen a multitude of cytokines, chemokines, and growth factors in one small strip of membrane. Readout is not a fluorescence so you don't need a special fluorescence reader. If you are doing Western blotting, you can do this easily.
Excellent specificity and detection for large number of cytokines. Works perfectly with little optimization.
Pancreas lysate. 750ug protein/ membrane
200 uL pooled serum from wild-type C57Bl/6 mice.
5 min exposure time
This is a great kit. It provides excellent reliability and a large number of cytokines, chemokines to screening in a single assay. I strongly recommend it.